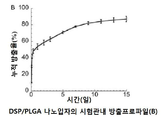
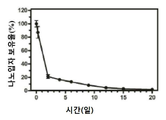
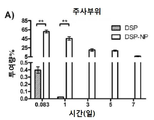
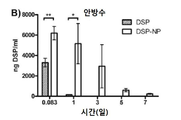
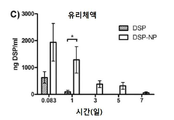
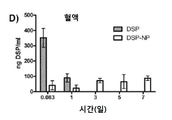
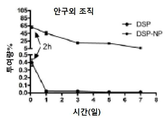
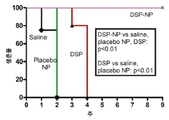
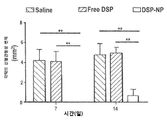
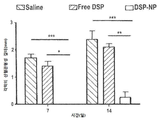
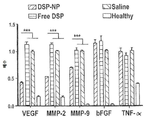
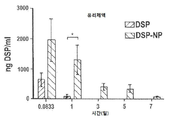
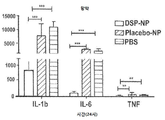
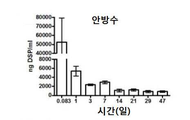
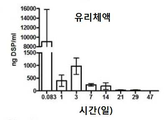
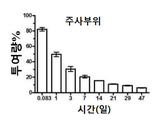
KR20170046146A - 각막 동종이식 거부 및 신혈관 형성의 예방을 위한 글루코 코르티코이드-로딩된 나노입자 - Google Patents
각막 동종이식 거부 및 신혈관 형성의 예방을 위한 글루코 코르티코이드-로딩된 나노입자 Download PDFInfo
- Publication number
- KR20170046146A KR20170046146A KR1020177006811A KR20177006811A KR20170046146A KR 20170046146 A KR20170046146 A KR 20170046146A KR 1020177006811 A KR1020177006811 A KR 1020177006811A KR 20177006811 A KR20177006811 A KR 20177006811A KR 20170046146 A KR20170046146 A KR 20170046146A
- Authority
- KR
- South Korea
- Prior art keywords
- dsp
- injection
- polymer
- nanoparticles
- glucocorticoid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 239000002105 nanoparticle Substances 0.000 title claims abstract description 163
- 239000003862 glucocorticoid Substances 0.000 title claims abstract description 76
- 206010029113 Neovascularisation Diseases 0.000 title claims description 18
- 230000002265 prevention Effects 0.000 title description 8
- 229960002344 dexamethasone sodium phosphate Drugs 0.000 claims abstract description 166
- 239000002245 particle Substances 0.000 claims abstract description 118
- 229920001477 hydrophilic polymer Polymers 0.000 claims abstract description 20
- 238000013268 sustained release Methods 0.000 claims abstract description 15
- 239000012730 sustained-release form Substances 0.000 claims abstract description 15
- PLCQGRYPOISRTQ-FCJDYXGNSA-L dexamethasone sodium phosphate Chemical compound [Na+].[Na+].C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)COP([O-])([O-])=O)(O)[C@@]1(C)C[C@@H]2O PLCQGRYPOISRTQ-FCJDYXGNSA-L 0.000 claims abstract 5
- 239000007924 injection Substances 0.000 claims description 140
- 238000002347 injection Methods 0.000 claims description 140
- 229920000642 polymer Polymers 0.000 claims description 89
- 229920001606 poly(lactic acid-co-glycolic acid) Polymers 0.000 claims description 75
- 238000000034 method Methods 0.000 claims description 44
- 206010061218 Inflammation Diseases 0.000 claims description 27
- 230000004054 inflammatory process Effects 0.000 claims description 27
- 229920001223 polyethylene glycol Polymers 0.000 claims description 17
- 150000003839 salts Chemical class 0.000 claims description 17
- 229920002988 biodegradable polymer Polymers 0.000 claims description 14
- 239000004621 biodegradable polymer Substances 0.000 claims description 14
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 14
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 13
- 206010052779 Transplant rejections Diseases 0.000 claims description 12
- 229920002732 Polyanhydride Polymers 0.000 claims description 11
- 238000000338 in vitro Methods 0.000 claims description 10
- 230000033115 angiogenesis Effects 0.000 claims description 8
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 7
- 230000000717 retained effect Effects 0.000 claims description 6
- 229910019142 PO4 Inorganic materials 0.000 claims description 5
- 229920003171 Poly (ethylene oxide) Polymers 0.000 claims description 5
- 150000002148 esters Chemical class 0.000 claims description 5
- 229910021645 metal ion Inorganic materials 0.000 claims description 5
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 claims description 4
- 239000010452 phosphate Substances 0.000 claims description 4
- 229920001400 block copolymer Polymers 0.000 claims description 3
- 230000009920 chelation Effects 0.000 claims description 3
- 239000005014 poly(hydroxyalkanoate) Substances 0.000 claims description 3
- 229920000903 polyhydroxyalkanoate Polymers 0.000 claims description 3
- 229920001273 Polyhydroxy acid Polymers 0.000 claims description 2
- 201000005111 ocular hyperemia Diseases 0.000 claims description 2
- 230000002123 temporal effect Effects 0.000 claims 1
- 238000002054 transplantation Methods 0.000 abstract description 17
- 239000011159 matrix material Substances 0.000 abstract description 14
- 201000000159 corneal neovascularization Diseases 0.000 abstract description 13
- 206010055665 Corneal neovascularisation Diseases 0.000 abstract description 12
- 229920001992 poloxamer 407 Polymers 0.000 abstract description 4
- XBBVURRQGJPTHH-UHFFFAOYSA-N 2-hydroxyacetic acid;2-hydroxypropanoic acid Chemical compound OCC(O)=O.CC(O)C(O)=O XBBVURRQGJPTHH-UHFFFAOYSA-N 0.000 abstract 1
- VQODGRNSFPNSQE-CXSFZGCWSA-N dexamethasone phosphate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)COP(O)(O)=O)(O)[C@@]1(C)C[C@@H]2O VQODGRNSFPNSQE-CXSFZGCWSA-N 0.000 description 166
- 210000001508 eye Anatomy 0.000 description 76
- 239000003814 drug Substances 0.000 description 59
- 210000001519 tissue Anatomy 0.000 description 58
- 229940079593 drug Drugs 0.000 description 52
- 229940037128 systemic glucocorticoids Drugs 0.000 description 46
- 235000002639 sodium chloride Nutrition 0.000 description 42
- 239000007943 implant Substances 0.000 description 38
- 239000000203 mixture Substances 0.000 description 33
- 241000700159 Rattus Species 0.000 description 32
- 230000002980 postoperative effect Effects 0.000 description 32
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 28
- 210000004087 cornea Anatomy 0.000 description 27
- 238000011282 treatment Methods 0.000 description 27
- 239000011780 sodium chloride Substances 0.000 description 24
- 239000000243 solution Substances 0.000 description 23
- 241001465754 Metazoa Species 0.000 description 21
- 230000014759 maintenance of location Effects 0.000 description 21
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 20
- 239000013543 active substance Substances 0.000 description 19
- 238000009472 formulation Methods 0.000 description 19
- -1 hydrocortisone Chemical class 0.000 description 19
- 230000000694 effects Effects 0.000 description 18
- 239000002202 Polyethylene glycol Substances 0.000 description 17
- 230000004410 intraocular pressure Effects 0.000 description 16
- 238000002360 preparation method Methods 0.000 description 16
- 206010030113 Oedema Diseases 0.000 description 15
- 210000004369 blood Anatomy 0.000 description 15
- 239000008280 blood Substances 0.000 description 15
- 239000003246 corticosteroid Substances 0.000 description 14
- 239000003889 eye drop Substances 0.000 description 14
- 229940012356 eye drops Drugs 0.000 description 14
- 229920000361 Poly(styrene)-block-poly(ethylene glycol) Polymers 0.000 description 13
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 13
- 238000000576 coating method Methods 0.000 description 13
- 210000001525 retina Anatomy 0.000 description 13
- 239000002904 solvent Substances 0.000 description 13
- 108020004414 DNA Proteins 0.000 description 12
- 238000011068 loading method Methods 0.000 description 12
- 206010046851 Uveitis Diseases 0.000 description 11
- 238000002513 implantation Methods 0.000 description 11
- 238000001727 in vivo Methods 0.000 description 11
- 230000004083 survival effect Effects 0.000 description 11
- 239000007864 aqueous solution Substances 0.000 description 10
- 229960001334 corticosteroids Drugs 0.000 description 10
- 229960003957 dexamethasone Drugs 0.000 description 10
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 10
- 201000010099 disease Diseases 0.000 description 10
- 208000035475 disorder Diseases 0.000 description 10
- 150000003431 steroids Chemical class 0.000 description 10
- 230000000699 topical effect Effects 0.000 description 10
- 229910052725 zinc Inorganic materials 0.000 description 10
- 239000011701 zinc Substances 0.000 description 10
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 9
- 241000699670 Mus sp. Species 0.000 description 9
- 239000000725 suspension Substances 0.000 description 9
- 239000012114 Alexa Fluor 647 Substances 0.000 description 8
- 102000004127 Cytokines Human genes 0.000 description 8
- 108090000695 Cytokines Proteins 0.000 description 8
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 8
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 8
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 8
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 8
- 230000015572 biosynthetic process Effects 0.000 description 8
- 239000003795 chemical substances by application Substances 0.000 description 8
- 239000011248 coating agent Substances 0.000 description 8
- 229920001577 copolymer Polymers 0.000 description 8
- 238000009826 distribution Methods 0.000 description 8
- 230000002209 hydrophobic effect Effects 0.000 description 8
- 229920001600 hydrophobic polymer Polymers 0.000 description 8
- 239000008194 pharmaceutical composition Substances 0.000 description 8
- 239000002504 physiological saline solution Substances 0.000 description 8
- 238000001356 surgical procedure Methods 0.000 description 8
- 150000008064 anhydrides Chemical class 0.000 description 7
- 239000010839 body fluid Substances 0.000 description 7
- 239000013522 chelant Substances 0.000 description 7
- 230000007423 decrease Effects 0.000 description 7
- 239000011859 microparticle Substances 0.000 description 7
- 230000002035 prolonged effect Effects 0.000 description 7
- 230000001225 therapeutic effect Effects 0.000 description 7
- 102100040247 Tumor necrosis factor Human genes 0.000 description 6
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 6
- 210000004204 blood vessel Anatomy 0.000 description 6
- 210000001124 body fluid Anatomy 0.000 description 6
- VHRGRCVQAFMJIZ-UHFFFAOYSA-N cadaverine Chemical compound NCCCCCN VHRGRCVQAFMJIZ-UHFFFAOYSA-N 0.000 description 6
- 150000001875 compounds Chemical class 0.000 description 6
- 230000004453 corneal transparency Effects 0.000 description 6
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 6
- 239000011521 glass Substances 0.000 description 6
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 6
- 239000003018 immunosuppressive agent Substances 0.000 description 6
- 230000002757 inflammatory effect Effects 0.000 description 6
- 239000000463 material Substances 0.000 description 6
- 238000005259 measurement Methods 0.000 description 6
- 239000002953 phosphate buffered saline Substances 0.000 description 6
- 238000012453 sprague-dawley rat model Methods 0.000 description 6
- 230000009885 systemic effect Effects 0.000 description 6
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 6
- 229910021642 ultra pure water Inorganic materials 0.000 description 6
- 239000012498 ultrapure water Substances 0.000 description 6
- 210000004127 vitreous body Anatomy 0.000 description 6
- 206010051625 Conjunctival hyperaemia Diseases 0.000 description 5
- 208000010412 Glaucoma Diseases 0.000 description 5
- 239000004793 Polystyrene Substances 0.000 description 5
- 206010060872 Transplant failure Diseases 0.000 description 5
- 210000002159 anterior chamber Anatomy 0.000 description 5
- 230000001772 anti-angiogenic effect Effects 0.000 description 5
- 230000015556 catabolic process Effects 0.000 description 5
- 210000000795 conjunctiva Anatomy 0.000 description 5
- 238000006731 degradation reaction Methods 0.000 description 5
- 238000009792 diffusion process Methods 0.000 description 5
- 239000000839 emulsion Substances 0.000 description 5
- 238000005538 encapsulation Methods 0.000 description 5
- 238000001125 extrusion Methods 0.000 description 5
- 239000007850 fluorescent dye Substances 0.000 description 5
- 229960003444 immunosuppressant agent Drugs 0.000 description 5
- 230000028709 inflammatory response Effects 0.000 description 5
- 239000003112 inhibitor Substances 0.000 description 5
- 239000007927 intramuscular injection Substances 0.000 description 5
- 238000010255 intramuscular injection Methods 0.000 description 5
- 230000007774 longterm Effects 0.000 description 5
- 108020004999 messenger RNA Proteins 0.000 description 5
- 229940023490 ophthalmic product Drugs 0.000 description 5
- 230000035515 penetration Effects 0.000 description 5
- 229920001983 poloxamer Polymers 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 238000003757 reverse transcription PCR Methods 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- IGAZHQIYONOHQN-UHFFFAOYSA-N Alexa Fluor 555 Chemical compound C=12C=CC(=N)C(S(O)(=O)=O)=C2OC2=C(S(O)(=O)=O)C(N)=CC=C2C=1C1=CC=C(C(O)=O)C=C1C(O)=O IGAZHQIYONOHQN-UHFFFAOYSA-N 0.000 description 4
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 4
- YQEZLKZALYSWHR-UHFFFAOYSA-N Ketamine Chemical compound C=1C=CC=C(Cl)C=1C1(NC)CCCCC1=O YQEZLKZALYSWHR-UHFFFAOYSA-N 0.000 description 4
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 4
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 4
- 239000004037 angiogenesis inhibitor Substances 0.000 description 4
- 229940121363 anti-inflammatory agent Drugs 0.000 description 4
- 239000002260 anti-inflammatory agent Substances 0.000 description 4
- 238000005119 centrifugation Methods 0.000 description 4
- 239000000032 diagnostic agent Substances 0.000 description 4
- 229940039227 diagnostic agent Drugs 0.000 description 4
- 239000002552 dosage form Substances 0.000 description 4
- 239000006196 drop Substances 0.000 description 4
- 238000003384 imaging method Methods 0.000 description 4
- 229960003299 ketamine Drugs 0.000 description 4
- 230000002045 lasting effect Effects 0.000 description 4
- 238000011694 lewis rat Methods 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 239000002609 medium Substances 0.000 description 4
- 238000012634 optical imaging Methods 0.000 description 4
- 230000037361 pathway Effects 0.000 description 4
- 229920001451 polypropylene glycol Polymers 0.000 description 4
- 238000001556 precipitation Methods 0.000 description 4
- 239000003755 preservative agent Substances 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- 150000003384 small molecules Chemical class 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 238000001228 spectrum Methods 0.000 description 4
- 229940124597 therapeutic agent Drugs 0.000 description 4
- BPICBUSOMSTKRF-UHFFFAOYSA-N xylazine Chemical compound CC1=CC=CC(C)=C1NC1=NCCCS1 BPICBUSOMSTKRF-UHFFFAOYSA-N 0.000 description 4
- 229960001600 xylazine Drugs 0.000 description 4
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 208000002177 Cataract Diseases 0.000 description 3
- 208000002691 Choroiditis Diseases 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- 102000003974 Fibroblast growth factor 2 Human genes 0.000 description 3
- 108090000379 Fibroblast growth factor 2 Proteins 0.000 description 3
- 206010022557 Intermediate uveitis Diseases 0.000 description 3
- FQISKWAFAHGMGT-SGJOWKDISA-M Methylprednisolone sodium succinate Chemical compound [Na+].C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@](O)(C(=O)COC(=O)CCC([O-])=O)CC[C@H]21 FQISKWAFAHGMGT-SGJOWKDISA-M 0.000 description 3
- 241000699666 Mus <mouse, genus> Species 0.000 description 3
- 239000004721 Polyphenylene oxide Substances 0.000 description 3
- 239000004743 Polypropylene Substances 0.000 description 3
- 208000003971 Posterior uveitis Diseases 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 210000001744 T-lymphocyte Anatomy 0.000 description 3
- 241000021375 Xenogenes Species 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 239000003963 antioxidant agent Substances 0.000 description 3
- 230000004888 barrier function Effects 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- 208000037976 chronic inflammation Diseases 0.000 description 3
- 230000006020 chronic inflammation Effects 0.000 description 3
- 238000013270 controlled release Methods 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 238000012377 drug delivery Methods 0.000 description 3
- 238000002296 dynamic light scattering Methods 0.000 description 3
- 210000000981 epithelium Anatomy 0.000 description 3
- 230000005284 excitation Effects 0.000 description 3
- 208000030533 eye disease Diseases 0.000 description 3
- 210000000744 eyelid Anatomy 0.000 description 3
- 238000005227 gel permeation chromatography Methods 0.000 description 3
- 229960000890 hydrocortisone Drugs 0.000 description 3
- 210000004969 inflammatory cell Anatomy 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 239000007928 intraperitoneal injection Substances 0.000 description 3
- 239000003589 local anesthetic agent Substances 0.000 description 3
- 238000012423 maintenance Methods 0.000 description 3
- 238000007726 management method Methods 0.000 description 3
- 239000004005 microsphere Substances 0.000 description 3
- 231100000252 nontoxic Toxicity 0.000 description 3
- 230000003000 nontoxic effect Effects 0.000 description 3
- 210000000056 organ Anatomy 0.000 description 3
- 239000000825 pharmaceutical preparation Substances 0.000 description 3
- 235000021317 phosphate Nutrition 0.000 description 3
- 229920000747 poly(lactic acid) Polymers 0.000 description 3
- 229920001184 polypeptide Polymers 0.000 description 3
- 229920001155 polypropylene Polymers 0.000 description 3
- 102000004196 processed proteins & peptides Human genes 0.000 description 3
- 108090000765 processed proteins & peptides Proteins 0.000 description 3
- 230000000069 prophylactic effect Effects 0.000 description 3
- 102000004169 proteins and genes Human genes 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 229920002477 rna polymer Polymers 0.000 description 3
- 238000000935 solvent evaporation Methods 0.000 description 3
- 239000012798 spherical particle Substances 0.000 description 3
- 238000007619 statistical method Methods 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 231100000419 toxicity Toxicity 0.000 description 3
- 230000001988 toxicity Effects 0.000 description 3
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 2
- UEJJHQNACJXSKW-UHFFFAOYSA-N 2-(2,6-dioxopiperidin-3-yl)-1H-isoindole-1,3(2H)-dione Chemical compound O=C1C2=CC=CC=C2C(=O)N1C1CCC(=O)NC1=O UEJJHQNACJXSKW-UHFFFAOYSA-N 0.000 description 2
- 102100026802 72 kDa type IV collagenase Human genes 0.000 description 2
- 101710151806 72 kDa type IV collagenase Proteins 0.000 description 2
- XYLJNLCSTIOKRM-UHFFFAOYSA-N Alphagan Chemical compound C1=CC2=NC=CN=C2C(Br)=C1NC1=NCCN1 XYLJNLCSTIOKRM-UHFFFAOYSA-N 0.000 description 2
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- 108010065805 Interleukin-12 Proteins 0.000 description 2
- 102000013462 Interleukin-12 Human genes 0.000 description 2
- 108010002350 Interleukin-2 Proteins 0.000 description 2
- 108090001005 Interleukin-6 Proteins 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 2
- 108010015302 Matrix metalloproteinase-9 Proteins 0.000 description 2
- 102100030412 Matrix metalloproteinase-9 Human genes 0.000 description 2
- 235000010627 Phaseolus vulgaris Nutrition 0.000 description 2
- 244000046052 Phaseolus vulgaris Species 0.000 description 2
- 102100035846 Pigment epithelium-derived factor Human genes 0.000 description 2
- 239000004952 Polyamide Substances 0.000 description 2
- 229920000954 Polyglycolide Polymers 0.000 description 2
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 description 2
- ZOIORXHNWRGPMV-UHFFFAOYSA-N acetic acid;zinc Chemical compound [Zn].CC(O)=O.CC(O)=O ZOIORXHNWRGPMV-UHFFFAOYSA-N 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 108010081667 aflibercept Proteins 0.000 description 2
- 206010064930 age-related macular degeneration Diseases 0.000 description 2
- 239000003732 agents acting on the eye Substances 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- 238000004220 aggregation Methods 0.000 description 2
- 230000002491 angiogenic effect Effects 0.000 description 2
- 230000002924 anti-infective effect Effects 0.000 description 2
- 230000003110 anti-inflammatory effect Effects 0.000 description 2
- 239000000043 antiallergic agent Substances 0.000 description 2
- 229960005475 antiinfective agent Drugs 0.000 description 2
- 239000004599 antimicrobial Substances 0.000 description 2
- 239000003443 antiviral agent Substances 0.000 description 2
- 210000001742 aqueous humor Anatomy 0.000 description 2
- 230000003143 atherosclerotic effect Effects 0.000 description 2
- 229940120638 avastin Drugs 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 229960000686 benzalkonium chloride Drugs 0.000 description 2
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 2
- 229960002537 betamethasone Drugs 0.000 description 2
- UREBDLICKHMUKA-DVTGEIKXSA-N betamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-DVTGEIKXSA-N 0.000 description 2
- 229960000397 bevacizumab Drugs 0.000 description 2
- 210000000481 breast Anatomy 0.000 description 2
- 229960003679 brimonidine Drugs 0.000 description 2
- 238000011685 brown norway rat Methods 0.000 description 2
- 150000007942 carboxylates Chemical class 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- RZEKVGVHFLEQIL-UHFFFAOYSA-N celecoxib Chemical compound C1=CC(C)=CC=C1C1=CC(C(F)(F)F)=NN1C1=CC=C(S(N)(=O)=O)C=C1 RZEKVGVHFLEQIL-UHFFFAOYSA-N 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 238000012512 characterization method Methods 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- 210000002808 connective tissue Anatomy 0.000 description 2
- 239000000599 controlled substance Substances 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 238000000502 dialysis Methods 0.000 description 2
- 235000019800 disodium phosphate Nutrition 0.000 description 2
- 239000000975 dye Substances 0.000 description 2
- 230000003511 endothelial effect Effects 0.000 description 2
- 206010015037 epilepsy Diseases 0.000 description 2
- AAKJLRGGTJKAMG-UHFFFAOYSA-N erlotinib Chemical compound C=12C=C(OCCOC)C(OCCOC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 AAKJLRGGTJKAMG-UHFFFAOYSA-N 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 230000008020 evaporation Effects 0.000 description 2
- 230000001747 exhibiting effect Effects 0.000 description 2
- 230000004438 eyesight Effects 0.000 description 2
- 238000002073 fluorescence micrograph Methods 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 239000010931 gold Substances 0.000 description 2
- 229910052737 gold Inorganic materials 0.000 description 2
- 239000003102 growth factor Substances 0.000 description 2
- 230000028993 immune response Effects 0.000 description 2
- 230000003053 immunization Effects 0.000 description 2
- 238000002649 immunization Methods 0.000 description 2
- 238000002650 immunosuppressive therapy Methods 0.000 description 2
- 229940117681 interleukin-12 Drugs 0.000 description 2
- 238000010253 intravenous injection Methods 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- GOTYRUGSSMKFNF-UHFFFAOYSA-N lenalidomide Chemical compound C1C=2C(N)=CC=CC=2C(=O)N1C1CCC(=O)NC1=O GOTYRUGSSMKFNF-UHFFFAOYSA-N 0.000 description 2
- 229960004194 lidocaine Drugs 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 238000004599 local-density approximation Methods 0.000 description 2
- 210000002540 macrophage Anatomy 0.000 description 2
- 208000002780 macular degeneration Diseases 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 239000002207 metabolite Substances 0.000 description 2
- 229960004584 methylprednisolone Drugs 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 2
- 210000001328 optic nerve Anatomy 0.000 description 2
- 239000003960 organic solvent Substances 0.000 description 2
- 239000003002 pH adjusting agent Substances 0.000 description 2
- 239000012188 paraffin wax Substances 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- 230000002085 persistent effect Effects 0.000 description 2
- 230000004962 physiological condition Effects 0.000 description 2
- 108090000102 pigment epithelium-derived factor Proteins 0.000 description 2
- 229920001281 polyalkylene Polymers 0.000 description 2
- 229920002647 polyamide Polymers 0.000 description 2
- 239000004417 polycarbonate Substances 0.000 description 2
- 229920000515 polycarbonate Polymers 0.000 description 2
- 229920000570 polyether Polymers 0.000 description 2
- 239000004626 polylactic acid Substances 0.000 description 2
- 229920002223 polystyrene Polymers 0.000 description 2
- 238000004321 preservation Methods 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 238000011321 prophylaxis Methods 0.000 description 2
- 229960003876 ranibizumab Drugs 0.000 description 2
- 102000027426 receptor tyrosine kinases Human genes 0.000 description 2
- 108091008598 receptor tyrosine kinases Proteins 0.000 description 2
- 108020003175 receptors Proteins 0.000 description 2
- 102000005962 receptors Human genes 0.000 description 2
- RZJQGNCSTQAWON-UHFFFAOYSA-N rofecoxib Chemical compound C1=CC(S(=O)(=O)C)=CC=C1C1=C(C=2C=CC=CC=2)C(=O)OC1 RZJQGNCSTQAWON-UHFFFAOYSA-N 0.000 description 2
- 239000000523 sample Substances 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- 230000003637 steroidlike Effects 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- 230000002459 sustained effect Effects 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 238000007910 systemic administration Methods 0.000 description 2
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 description 2
- 229960003433 thalidomide Drugs 0.000 description 2
- 229920000428 triblock copolymer Polymers 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- 210000003462 vein Anatomy 0.000 description 2
- 239000000080 wetting agent Substances 0.000 description 2
- 239000004246 zinc acetate Substances 0.000 description 2
- QCHFTSOMWOSFHM-WPRPVWTQSA-N (+)-Pilocarpine Chemical compound C1OC(=O)[C@@H](CC)[C@H]1CC1=CN=CN1C QCHFTSOMWOSFHM-WPRPVWTQSA-N 0.000 description 1
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 description 1
- LEBVLXFERQHONN-UHFFFAOYSA-N 1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide Chemical compound CCCCN1CCCCC1C(=O)NC1=C(C)C=CC=C1C LEBVLXFERQHONN-UHFFFAOYSA-N 0.000 description 1
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 1
- MOTOSAGBNXXRRE-UHFFFAOYSA-N 2-phenylsulfanylacetic acid Chemical compound OC(=O)CSC1=CC=CC=C1 MOTOSAGBNXXRRE-UHFFFAOYSA-N 0.000 description 1
- WNWVKZTYMQWFHE-UHFFFAOYSA-N 4-ethylmorpholine Chemical compound [CH2]CN1CCOCC1 WNWVKZTYMQWFHE-UHFFFAOYSA-N 0.000 description 1
- QTQGHKVYLQBJLO-UHFFFAOYSA-N 4-methylbenzenesulfonate;(4-methyl-1-oxo-1-phenylmethoxypentan-2-yl)azanium Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1.CC(C)CC(N)C(=O)OCC1=CC=CC=C1 QTQGHKVYLQBJLO-UHFFFAOYSA-N 0.000 description 1
- WLCZTRVUXYALDD-IBGZPJMESA-N 7-[[(2s)-2,6-bis(2-methoxyethoxycarbonylamino)hexanoyl]amino]heptoxy-methylphosphinic acid Chemical compound COCCOC(=O)NCCCC[C@H](NC(=O)OCCOC)C(=O)NCCCCCCCOP(C)(O)=O WLCZTRVUXYALDD-IBGZPJMESA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 102400000068 Angiostatin Human genes 0.000 description 1
- 108010079709 Angiostatins Proteins 0.000 description 1
- 108090000644 Angiozyme Proteins 0.000 description 1
- 108091023037 Aptamer Proteins 0.000 description 1
- MLDQJTXFUGDVEO-UHFFFAOYSA-N BAY-43-9006 Chemical compound C1=NC(C(=O)NC)=CC(OC=2C=CC(NC(=O)NC=3C=C(C(Cl)=CC=3)C(F)(F)F)=CC=2)=C1 MLDQJTXFUGDVEO-UHFFFAOYSA-N 0.000 description 1
- 108010001478 Bacitracin Proteins 0.000 description 1
- 208000035143 Bacterial infection Diseases 0.000 description 1
- 201000004569 Blindness Diseases 0.000 description 1
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 1
- VOVIALXJUBGFJZ-KWVAZRHASA-N Budesonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O VOVIALXJUBGFJZ-KWVAZRHASA-N 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- 108090000994 Catalytic RNA Proteins 0.000 description 1
- 102000053642 Catalytic RNA Human genes 0.000 description 1
- GHXZTYHSJHQHIJ-UHFFFAOYSA-N Chlorhexidine Chemical compound C=1C=C(Cl)C=CC=1NC(N)=NC(N)=NCCCCCCN=C(N)N=C(N)NC1=CC=C(Cl)C=C1 GHXZTYHSJHQHIJ-UHFFFAOYSA-N 0.000 description 1
- 208000017667 Chronic Disease Diseases 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 108020004635 Complementary DNA Proteins 0.000 description 1
- 208000028006 Corneal injury Diseases 0.000 description 1
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 1
- 229930105110 Cyclosporin A Natural products 0.000 description 1
- 108010036949 Cyclosporine Proteins 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 208000006558 Dental Calculus Diseases 0.000 description 1
- 206010012689 Diabetic retinopathy Diseases 0.000 description 1
- QXNVGIXVLWOKEQ-UHFFFAOYSA-N Disodium Chemical compound [Na][Na] QXNVGIXVLWOKEQ-UHFFFAOYSA-N 0.000 description 1
- 102000001301 EGF receptor Human genes 0.000 description 1
- 108060006698 EGF receptor Proteins 0.000 description 1
- 102400001047 Endostatin Human genes 0.000 description 1
- 108010079505 Endostatins Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 206010015943 Eye inflammation Diseases 0.000 description 1
- UUOUOERPONYGOS-CLCRDYEYSA-N Fluocinolone Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@]([C@H](O)C4)(O)C(=O)CO)[C@@H]4[C@@H]3C[C@H](F)C2=C1 UUOUOERPONYGOS-CLCRDYEYSA-N 0.000 description 1
- 206010017533 Fungal infection Diseases 0.000 description 1
- 108700039691 Genetic Promoter Regions Proteins 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 102100031181 Glyceraldehyde-3-phosphate dehydrogenase Human genes 0.000 description 1
- 102000002068 Glycopeptides Human genes 0.000 description 1
- 108010015899 Glycopeptides Proteins 0.000 description 1
- 206010053759 Growth retardation Diseases 0.000 description 1
- 206010019909 Hernia Diseases 0.000 description 1
- 102100032742 Histone-lysine N-methyltransferase SETD2 Human genes 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000654725 Homo sapiens Histone-lysine N-methyltransferase SETD2 Proteins 0.000 description 1
- 108010003272 Hyaluronate lyase Proteins 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- 206010021143 Hypoxia Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 102000006992 Interferon-alpha Human genes 0.000 description 1
- 108010047761 Interferon-alpha Proteins 0.000 description 1
- 102000000588 Interleukin-2 Human genes 0.000 description 1
- 201000006165 Kuhnt-Junius degeneration Diseases 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- 239000005551 L01XE03 - Erlotinib Substances 0.000 description 1
- 239000002147 L01XE04 - Sunitinib Substances 0.000 description 1
- 108010028921 Lipopeptides Proteins 0.000 description 1
- 108700018351 Major Histocompatibility Complex Proteins 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 102000002274 Matrix Metalloproteinases Human genes 0.000 description 1
- 108010000684 Matrix Metalloproteinases Proteins 0.000 description 1
- 208000031888 Mycoses Diseases 0.000 description 1
- 108010057466 NF-kappa B Proteins 0.000 description 1
- 102000003945 NF-kappa B Human genes 0.000 description 1
- 208000001388 Opportunistic Infections Diseases 0.000 description 1
- 208000001132 Osteoporosis Diseases 0.000 description 1
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- MKPDWECBUAZOHP-AFYJWTTESA-N Paramethasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]2(C)C[C@@H]1O MKPDWECBUAZOHP-AFYJWTTESA-N 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 1
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 1
- 229920000463 Poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) Polymers 0.000 description 1
- 229930182556 Polyacetal Natural products 0.000 description 1
- 229920000608 Polyaspartic Polymers 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 229920002413 Polyhexanide Polymers 0.000 description 1
- 229920001710 Polyorthoester Polymers 0.000 description 1
- 229920000388 Polyphosphate Polymers 0.000 description 1
- KCLANYCVBBTKTO-UHFFFAOYSA-N Proparacaine Chemical compound CCCOC1=CC=C(C(=O)OCCN(CC)CC)C=C1N KCLANYCVBBTKTO-UHFFFAOYSA-N 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 208000028017 Psychotic disease Diseases 0.000 description 1
- 108091027981 Response element Proteins 0.000 description 1
- 206010038910 Retinitis Diseases 0.000 description 1
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 1
- QCHFTSOMWOSFHM-UHFFFAOYSA-N SJ000285536 Natural products C1OC(=O)C(CC)C1CC1=CN=CN1C QCHFTSOMWOSFHM-UHFFFAOYSA-N 0.000 description 1
- 108020004459 Small interfering RNA Proteins 0.000 description 1
- UIRKNQLZZXALBI-MSVGPLKSSA-N Squalamine Chemical compound C([C@@H]1C[C@H]2O)[C@@H](NCCCNCCCCN)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@H](C)CC[C@H](C(C)C)OS(O)(=O)=O)[C@@]2(C)CC1 UIRKNQLZZXALBI-MSVGPLKSSA-N 0.000 description 1
- UIRKNQLZZXALBI-UHFFFAOYSA-N Squalamine Natural products OC1CC2CC(NCCCNCCCCN)CCC2(C)C2C1C1CCC(C(C)CCC(C(C)C)OS(O)(=O)=O)C1(C)CC2 UIRKNQLZZXALBI-UHFFFAOYSA-N 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 239000004098 Tetracycline Substances 0.000 description 1
- YZCKVEUIGOORGS-NJFSPNSNSA-N Tritium Chemical compound [3H] YZCKVEUIGOORGS-NJFSPNSNSA-N 0.000 description 1
- 238000010162 Tukey test Methods 0.000 description 1
- 108010059993 Vancomycin Proteins 0.000 description 1
- 208000000208 Wet Macular Degeneration Diseases 0.000 description 1
- 238000002679 ablation Methods 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 229960004150 aciclovir Drugs 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 239000000048 adrenergic agonist Substances 0.000 description 1
- 239000000674 adrenergic antagonist Substances 0.000 description 1
- 229940126157 adrenergic receptor agonist Drugs 0.000 description 1
- 230000001780 adrenocortical effect Effects 0.000 description 1
- 229960002833 aflibercept Drugs 0.000 description 1
- FJXOGVLKCZQRDN-PHCHRAKRSA-N alclometasone Chemical compound C([C@H]1Cl)C2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O FJXOGVLKCZQRDN-PHCHRAKRSA-N 0.000 description 1
- 229920005576 aliphatic polyanhydride Polymers 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 125000002947 alkylene group Chemical group 0.000 description 1
- 102000030484 alpha-2 Adrenergic Receptor Human genes 0.000 description 1
- 108020004101 alpha-2 Adrenergic Receptor Proteins 0.000 description 1
- 229960004821 amikacin Drugs 0.000 description 1
- LKCWBDHBTVXHDL-RMDFUYIESA-N amikacin Chemical compound O([C@@H]1[C@@H](N)C[C@H]([C@@H]([C@H]1O)O[C@@H]1[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O1)O)NC(=O)[C@@H](O)CCN)[C@H]1O[C@H](CN)[C@@H](O)[C@H](O)[C@H]1O LKCWBDHBTVXHDL-RMDFUYIESA-N 0.000 description 1
- 229940126575 aminoglycoside Drugs 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 239000002870 angiogenesis inducing agent Substances 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000003266 anti-allergic effect Effects 0.000 description 1
- 230000001384 anti-glaucoma Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 229940006133 antiglaucoma drug and miotics carbonic anhydrase inhibitors Drugs 0.000 description 1
- 229940006138 antiglaucoma drug and miotics prostaglandin analogues Drugs 0.000 description 1
- 239000003096 antiparasitic agent Substances 0.000 description 1
- 229940125687 antiparasitic agent Drugs 0.000 description 1
- 229960002610 apraclonidine Drugs 0.000 description 1
- IEJXVRYNEISIKR-UHFFFAOYSA-N apraclonidine Chemical compound ClC1=CC(N)=CC(Cl)=C1NC1=NCCN1 IEJXVRYNEISIKR-UHFFFAOYSA-N 0.000 description 1
- 239000012736 aqueous medium Substances 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 229920005578 aromatic polyanhydride Polymers 0.000 description 1
- FZCSTZYAHCUGEM-UHFFFAOYSA-N aspergillomarasmine B Natural products OC(=O)CNC(C(O)=O)CNC(C(O)=O)CC(O)=O FZCSTZYAHCUGEM-UHFFFAOYSA-N 0.000 description 1
- 229960004099 azithromycin Drugs 0.000 description 1
- MQTOSJVFKKJCRP-BICOPXKESA-N azithromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)N(C)C[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 MQTOSJVFKKJCRP-BICOPXKESA-N 0.000 description 1
- 229960003071 bacitracin Drugs 0.000 description 1
- 229930184125 bacitracin Natural products 0.000 description 1
- CLKOFPXJLQSYAH-ABRJDSQDSA-N bacitracin A Chemical compound C1SC([C@@H](N)[C@@H](C)CC)=N[C@@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]1C(=O)N[C@H](CCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2N=CNC=2)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)NCCCC1 CLKOFPXJLQSYAH-ABRJDSQDSA-N 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 229960002470 bimatoprost Drugs 0.000 description 1
- AQOKCDNYWBIDND-FTOWTWDKSA-N bimatoprost Chemical compound CCNC(=O)CCC\C=C/C[C@H]1[C@@H](O)C[C@@H](O)[C@@H]1\C=C\[C@@H](O)CCC1=CC=CC=C1 AQOKCDNYWBIDND-FTOWTWDKSA-N 0.000 description 1
- 239000000560 biocompatible material Substances 0.000 description 1
- 238000012984 biological imaging Methods 0.000 description 1
- 239000012620 biological material Substances 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 230000037058 blood plasma level Effects 0.000 description 1
- HCRKCZRJWPKOAR-JTQLQIEISA-N brinzolamide Chemical compound CCN[C@H]1CN(CCCOC)S(=O)(=O)C2=C1C=C(S(N)(=O)=O)S2 HCRKCZRJWPKOAR-JTQLQIEISA-N 0.000 description 1
- 229960000722 brinzolamide Drugs 0.000 description 1
- 229960004436 budesonide Drugs 0.000 description 1
- 210000005252 bulbus oculi Anatomy 0.000 description 1
- 229960003150 bupivacaine Drugs 0.000 description 1
- 238000010804 cDNA synthesis Methods 0.000 description 1
- 238000000237 capillary viscometry Methods 0.000 description 1
- 229960000623 carbamazepine Drugs 0.000 description 1
- FFGPTBGBLSHEPO-UHFFFAOYSA-N carbamazepine Chemical compound C1=CC2=CC=CC=C2N(C(=O)N)C2=CC=CC=C21 FFGPTBGBLSHEPO-UHFFFAOYSA-N 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 239000003489 carbonate dehydratase inhibitor Substances 0.000 description 1
- LWAFSWPYPHEXKX-UHFFFAOYSA-N carteolol Chemical compound N1C(=O)CCC2=C1C=CC=C2OCC(O)CNC(C)(C)C LWAFSWPYPHEXKX-UHFFFAOYSA-N 0.000 description 1
- 229960001222 carteolol Drugs 0.000 description 1
- 238000005266 casting Methods 0.000 description 1
- 229940047495 celebrex Drugs 0.000 description 1
- 229960000590 celecoxib Drugs 0.000 description 1
- 210000004027 cell Anatomy 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 229960005395 cetuximab Drugs 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 229960003260 chlorhexidine Drugs 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 229960002842 clobetasol Drugs 0.000 description 1
- FCSHDIVRCWTZOX-DVTGEIKXSA-N clobetasol Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CCl)(O)[C@@]1(C)C[C@@H]2O FCSHDIVRCWTZOX-DVTGEIKXSA-N 0.000 description 1
- 229960001146 clobetasone Drugs 0.000 description 1
- XXIFVOHLGBURIG-OZCCCYNHSA-N clobetasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CCl)(O)[C@@]1(C)CC2=O XXIFVOHLGBURIG-OZCCCYNHSA-N 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 238000005354 coacervation Methods 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 230000001268 conjugating effect Effects 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 239000002872 contrast media Substances 0.000 description 1
- 238000013267 controlled drug release Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- RYJIRNNXCHOUTQ-OJJGEMKLSA-L cortisol sodium phosphate Chemical compound [Na+].[Na+].O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)COP([O-])([O-])=O)[C@@H]4[C@@H]3CCC2=C1 RYJIRNNXCHOUTQ-OJJGEMKLSA-L 0.000 description 1
- 229940111134 coxibs Drugs 0.000 description 1
- 230000001186 cumulative effect Effects 0.000 description 1
- 238000011461 current therapy Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 239000003255 cyclooxygenase 2 inhibitor Substances 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000004452 decreased vision Effects 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 230000003412 degenerative effect Effects 0.000 description 1
- 239000007857 degradation product Substances 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000001212 derivatisation Methods 0.000 description 1
- 229960003662 desonide Drugs 0.000 description 1
- WBGKWQHBNHJJPZ-LECWWXJVSA-N desonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O WBGKWQHBNHJJPZ-LECWWXJVSA-N 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- KPHWPUGNDIVLNH-UHFFFAOYSA-M diclofenac sodium Chemical compound [Na+].[O-]C(=O)CC1=CC=CC=C1NC1=C(Cl)C=CC=C1Cl KPHWPUGNDIVLNH-UHFFFAOYSA-M 0.000 description 1
- 230000010339 dilation Effects 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000002224 dissection Methods 0.000 description 1
- 239000003210 dopamine receptor blocking agent Substances 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 229960002017 echothiophate Drugs 0.000 description 1
- BJOLKYGKSZKIGU-UHFFFAOYSA-N ecothiopate Chemical compound CCOP(=O)(OCC)SCC[N+](C)(C)C BJOLKYGKSZKIGU-UHFFFAOYSA-N 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 230000004406 elevated intraocular pressure Effects 0.000 description 1
- 238000004945 emulsification Methods 0.000 description 1
- 206010014801 endophthalmitis Diseases 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 230000007071 enzymatic hydrolysis Effects 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- 230000001037 epileptic effect Effects 0.000 description 1
- 229960003449 epinastine Drugs 0.000 description 1
- WHWZLSFABNNENI-UHFFFAOYSA-N epinastine Chemical compound C1C2=CC=CC=C2C2CN=C(N)N2C2=CC=CC=C21 WHWZLSFABNNENI-UHFFFAOYSA-N 0.000 description 1
- 210000005081 epithelial layer Anatomy 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- 229940051306 eylea Drugs 0.000 description 1
- 239000010419 fine particle Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 229960000676 flunisolide Drugs 0.000 description 1
- 229940043075 fluocinolone Drugs 0.000 description 1
- 229960003973 fluocortolone Drugs 0.000 description 1
- GAKMQHDJQHZUTJ-ULHLPKEOSA-N fluocortolone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@@H](C)[C@H](C(=O)CO)[C@@]2(C)C[C@@H]1O GAKMQHDJQHZUTJ-ULHLPKEOSA-N 0.000 description 1
- 238000001215 fluorescent labelling Methods 0.000 description 1
- 229960002714 fluticasone Drugs 0.000 description 1
- MGNNYOODZCAHBA-GQKYHHCASA-N fluticasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(O)[C@@]2(C)C[C@@H]1O MGNNYOODZCAHBA-GQKYHHCASA-N 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 229960002963 ganciclovir Drugs 0.000 description 1
- 229960002518 gentamicin Drugs 0.000 description 1
- 229940089161 ginsenoside Drugs 0.000 description 1
- 229930182494 ginsenoside Natural products 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 108020004445 glyceraldehyde-3-phosphate dehydrogenase Proteins 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 231100000001 growth retardation Toxicity 0.000 description 1
- 230000003394 haemopoietic effect Effects 0.000 description 1
- MCAHMSDENAOJFZ-BVXDHVRPSA-N herbimycin Chemical compound N1C(=O)\C(C)=C\C=C/[C@H](OC)[C@@H](OC(N)=O)\C(C)=C\[C@H](C)[C@@H](OC)[C@@H](OC)C[C@H](C)[C@@H](OC)C2=CC(=O)C=C1C2=O MCAHMSDENAOJFZ-BVXDHVRPSA-N 0.000 description 1
- 229930193320 herbimycin Natural products 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 238000010842 high-capacity cDNA reverse transcription kit Methods 0.000 description 1
- 238000010562 histological examination Methods 0.000 description 1
- 239000012943 hotmelt Substances 0.000 description 1
- 229920002674 hyaluronan Polymers 0.000 description 1
- 229960003160 hyaluronic acid Drugs 0.000 description 1
- 229960004204 hydrocortisone sodium phosphate Drugs 0.000 description 1
- 229960001401 hydrocortisone sodium succinate Drugs 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 230000001146 hypoxic effect Effects 0.000 description 1
- 239000012216 imaging agent Substances 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 208000026278 immune system disease Diseases 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 229940125721 immunosuppressive agent Drugs 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 238000001746 injection moulding Methods 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 230000009545 invasion Effects 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 230000006651 lactation Effects 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 229960001160 latanoprost Drugs 0.000 description 1
- GGXICVAJURFBLW-CEYXHVGTSA-N latanoprost Chemical compound CC(C)OC(=O)CCC\C=C/C[C@H]1[C@@H](O)C[C@@H](O)[C@@H]1CC[C@@H](O)CCC1=CC=CC=C1 GGXICVAJURFBLW-CEYXHVGTSA-N 0.000 description 1
- 229960004942 lenalidomide Drugs 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 238000010859 live-cell imaging Methods 0.000 description 1
- 229960005015 local anesthetics Drugs 0.000 description 1
- 229940076783 lucentis Drugs 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 229940092110 macugen Drugs 0.000 description 1
- 230000005291 magnetic effect Effects 0.000 description 1
- 238000003760 magnetic stirring Methods 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- FPYJFEHAWHCUMM-UHFFFAOYSA-N maleic anhydride Chemical compound O=C1OC(=O)C=C1 FPYJFEHAWHCUMM-UHFFFAOYSA-N 0.000 description 1
- 238000009607 mammography Methods 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- GRVDJDISBSALJP-UHFFFAOYSA-N methyloxidanyl Chemical compound [O]C GRVDJDISBSALJP-UHFFFAOYSA-N 0.000 description 1
- 229960000334 methylprednisolone sodium succinate Drugs 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 229950007856 mofetil Drugs 0.000 description 1
- 239000003068 molecular probe Substances 0.000 description 1
- 229960001664 mometasone Drugs 0.000 description 1
- QLIIKPVHVRXHRI-CXSFZGCWSA-N mometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CCl)(O)[C@@]1(C)C[C@@H]2O QLIIKPVHVRXHRI-CXSFZGCWSA-N 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 239000002077 nanosphere Substances 0.000 description 1
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 1
- 239000012457 nonaqueous media Substances 0.000 description 1
- 230000004942 nuclear accumulation Effects 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 1
- 229960004114 olopatadine Drugs 0.000 description 1
- JBIMVDZLSHOPLA-LSCVHKIXSA-N olopatadine Chemical compound C1OC2=CC=C(CC(O)=O)C=C2C(=C/CCN(C)C)\C2=CC=CC=C21 JBIMVDZLSHOPLA-LSCVHKIXSA-N 0.000 description 1
- 238000001543 one-way ANOVA Methods 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 239000013110 organic ligand Substances 0.000 description 1
- 150000002902 organometallic compounds Chemical class 0.000 description 1
- 230000001151 other effect Effects 0.000 description 1
- YOURXVGYNVXQKT-UHFFFAOYSA-N oxacycloundecane-2,11-dione Chemical compound O=C1CCCCCCCCC(=O)O1 YOURXVGYNVXQKT-UHFFFAOYSA-N 0.000 description 1
- 125000005430 oxychloro group Chemical group 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 229960001972 panitumumab Drugs 0.000 description 1
- 201000007407 panuveitis Diseases 0.000 description 1
- 230000005298 paramagnetic effect Effects 0.000 description 1
- 229960002858 paramethasone Drugs 0.000 description 1
- 230000001734 parasympathetic effect Effects 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 238000005191 phase separation Methods 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 230000000649 photocoagulation Effects 0.000 description 1
- 238000002428 photodynamic therapy Methods 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 229960001416 pilocarpine Drugs 0.000 description 1
- 239000000902 placebo Substances 0.000 description 1
- 229940068196 placebo Drugs 0.000 description 1
- 229920000071 poly(4-hydroxybutyrate) Polymers 0.000 description 1
- 229920001308 poly(aminoacid) Polymers 0.000 description 1
- 229920000117 poly(dioxanone) Polymers 0.000 description 1
- 229920000218 poly(hydroxyvalerate) Polymers 0.000 description 1
- 229920001306 poly(lactide-co-caprolactone) Polymers 0.000 description 1
- 229920000141 poly(maleic anhydride) Polymers 0.000 description 1
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 1
- 239000002745 poly(ortho ester) Substances 0.000 description 1
- 229920001583 poly(oxyethylated polyols) Polymers 0.000 description 1
- 229920002627 poly(phosphazenes) Polymers 0.000 description 1
- 229920000070 poly-3-hydroxybutyrate Polymers 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 229920001515 polyalkylene glycol Polymers 0.000 description 1
- 229920001610 polycaprolactone Polymers 0.000 description 1
- 239000004632 polycaprolactone Substances 0.000 description 1
- 229920002721 polycyanoacrylate Polymers 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920006149 polyester-amide block copolymer Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 239000004633 polyglycolic acid Substances 0.000 description 1
- 229920001855 polyketal Polymers 0.000 description 1
- 229920001444 polymaleic acid Polymers 0.000 description 1
- 238000012667 polymer degradation Methods 0.000 description 1
- 239000004926 polymethyl methacrylate Substances 0.000 description 1
- 229920006324 polyoxymethylene Polymers 0.000 description 1
- 239000001205 polyphosphate Substances 0.000 description 1
- 235000011176 polyphosphates Nutrition 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 231100000683 possible toxicity Toxicity 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 229960005205 prednisolone Drugs 0.000 description 1
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 description 1
- 229960004618 prednisone Drugs 0.000 description 1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 239000000583 progesterone congener Substances 0.000 description 1
- 229960003981 proparacaine Drugs 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 210000001747 pupil Anatomy 0.000 description 1
- MIXMJCQRHVAJIO-TZHJZOAOSA-N qk4dys664x Chemical compound O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O MIXMJCQRHVAJIO-TZHJZOAOSA-N 0.000 description 1
- 238000004445 quantitative analysis Methods 0.000 description 1
- 150000003242 quaternary ammonium salts Chemical class 0.000 description 1
- 150000007660 quinolones Chemical class 0.000 description 1
- 238000003753 real-time PCR Methods 0.000 description 1
- 239000002464 receptor antagonist Substances 0.000 description 1
- 229940044551 receptor antagonist Drugs 0.000 description 1
- 238000009877 rendering Methods 0.000 description 1
- 230000002207 retinal effect Effects 0.000 description 1
- 238000004007 reversed phase HPLC Methods 0.000 description 1
- 229940120975 revlimid Drugs 0.000 description 1
- 108091092562 ribozyme Proteins 0.000 description 1
- 229960001487 rimexolone Drugs 0.000 description 1
- QTTRZHGPGKRAFB-OOKHYKNYSA-N rimexolone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CC)(C)[C@@]1(C)C[C@@H]2O QTTRZHGPGKRAFB-OOKHYKNYSA-N 0.000 description 1
- 229960000371 rofecoxib Drugs 0.000 description 1
- 238000002390 rotary evaporation Methods 0.000 description 1
- 230000002000 scavenging effect Effects 0.000 description 1
- 239000013049 sediment Substances 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 230000000862 serotonergic effect Effects 0.000 description 1
- 238000007873 sieving Methods 0.000 description 1
- 230000011664 signaling Effects 0.000 description 1
- 230000005476 size effect Effects 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 235000011008 sodium phosphates Nutrition 0.000 description 1
- JJICLMJFIKGAAU-UHFFFAOYSA-M sodium;2-amino-9-(1,3-dihydroxypropan-2-yloxymethyl)purin-6-olate Chemical compound [Na+].NC1=NC([O-])=C2N=CN(COC(CO)CO)C2=N1 JJICLMJFIKGAAU-UHFFFAOYSA-M 0.000 description 1
- RMLUKZWYIKEASN-UHFFFAOYSA-M sodium;2-amino-9-(2-hydroxyethoxymethyl)purin-6-olate Chemical compound [Na+].O=C1[N-]C(N)=NC2=C1N=CN2COCCO RMLUKZWYIKEASN-UHFFFAOYSA-M 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 235000010356 sorbitol Nutrition 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 238000013222 sprague-dawley male rat Methods 0.000 description 1
- 238000001694 spray drying Methods 0.000 description 1
- 229950001248 squalamine Drugs 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 239000008174 sterile solution Substances 0.000 description 1
- 239000002294 steroidal antiinflammatory agent Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 150000003900 succinic acid esters Chemical class 0.000 description 1
- 229940124530 sulfonamide Drugs 0.000 description 1
- 150000003456 sulfonamides Chemical class 0.000 description 1
- WINHZLLDWRZWRT-ATVHPVEESA-N sunitinib Chemical compound CCN(CC)CCNC(=O)C1=C(C)NC(\C=C/2C3=CC(F)=CC=C3NC\2=O)=C1C WINHZLLDWRZWRT-ATVHPVEESA-N 0.000 description 1
- 229960001796 sunitinib Drugs 0.000 description 1
- 230000020382 suppression by virus of host antigen processing and presentation of peptide antigen via MHC class I Effects 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- 238000012353 t test Methods 0.000 description 1
- 229960001967 tacrolimus Drugs 0.000 description 1
- 229940120982 tarceva Drugs 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- 210000003478 temporal lobe Anatomy 0.000 description 1
- 229920001897 terpolymer Polymers 0.000 description 1
- 229960002372 tetracaine Drugs 0.000 description 1
- GKCBAIGFKIBETG-UHFFFAOYSA-N tetracaine Chemical compound CCCCNC1=CC=C(C(=O)OCCN(C)C)C=C1 GKCBAIGFKIBETG-UHFFFAOYSA-N 0.000 description 1
- 235000019364 tetracycline Nutrition 0.000 description 1
- 150000003522 tetracyclines Chemical class 0.000 description 1
- 229940040944 tetracyclines Drugs 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- RTKIYNMVFMVABJ-UHFFFAOYSA-L thimerosal Chemical compound [Na+].CC[Hg]SC1=CC=CC=C1C([O-])=O RTKIYNMVFMVABJ-UHFFFAOYSA-L 0.000 description 1
- 229940033663 thimerosal Drugs 0.000 description 1
- 229960000707 tobramycin Drugs 0.000 description 1
- NLVFBUXFDBBNBW-PBSUHMDJSA-S tobramycin(5+) Chemical compound [NH3+][C@@H]1C[C@H](O)[C@@H](C[NH3+])O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H]([NH3+])[C@H](O)[C@@H](CO)O2)O)[C@H]([NH3+])C[C@@H]1[NH3+] NLVFBUXFDBBNBW-PBSUHMDJSA-S 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 229940125379 topical corticosteroid Drugs 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 229960005294 triamcinolone Drugs 0.000 description 1
- GFNANZIMVAIWHM-OBYCQNJPSA-N triamcinolone Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@]([C@H](O)C4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 GFNANZIMVAIWHM-OBYCQNJPSA-N 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- 229910052722 tritium Inorganic materials 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 1
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 1
- 238000000825 ultraviolet detection Methods 0.000 description 1
- 229920005577 unsaturated polyanhydride Polymers 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- 230000001982 uveitic effect Effects 0.000 description 1
- MYPYJXKWCTUITO-LYRMYLQWSA-N vancomycin Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=C2C=C3C=C1OC1=CC=C(C=C1Cl)[C@@H](O)[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@H]3C(=O)N[C@H]1C(=O)N[C@H](C(N[C@@H](C3=CC(O)=CC(O)=C3C=3C(O)=CC=C1C=3)C(O)=O)=O)[C@H](O)C1=CC=C(C(=C1)Cl)O2)=O)NC(=O)[C@@H](CC(C)C)NC)[C@H]1C[C@](C)(N)[C@H](O)[C@H](C)O1 MYPYJXKWCTUITO-LYRMYLQWSA-N 0.000 description 1
- 229960003165 vancomycin Drugs 0.000 description 1
- MYPYJXKWCTUITO-UHFFFAOYSA-N vancomycin Natural products O1C(C(=C2)Cl)=CC=C2C(O)C(C(NC(C2=CC(O)=CC(O)=C2C=2C(O)=CC=C3C=2)C(O)=O)=O)NC(=O)C3NC(=O)C2NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(CC(C)C)NC)C(O)C(C=C3Cl)=CC=C3OC3=CC2=CC1=C3OC1OC(CO)C(O)C(O)C1OC1CC(C)(N)C(O)C(C)O1 MYPYJXKWCTUITO-UHFFFAOYSA-N 0.000 description 1
- 230000006444 vascular growth Effects 0.000 description 1
- 229940087652 vioxx Drugs 0.000 description 1
- 239000004034 viscosity adjusting agent Substances 0.000 description 1
- 230000004304 visual acuity Effects 0.000 description 1
- 230000004393 visual impairment Effects 0.000 description 1
- 235000012431 wafers Nutrition 0.000 description 1
- 239000002569 water oil cream Substances 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 230000029663 wound healing Effects 0.000 description 1
- YZYKBQUWMPUVEN-UHFFFAOYSA-N zafuleptine Chemical compound OC(=O)CCCCCC(C(C)C)NCC1=CC=C(F)C=C1 YZYKBQUWMPUVEN-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/56—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids
- A61K31/57—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids substituted in position 17 beta by a chain of two carbon atoms, e.g. pregnane or progesterone
- A61K31/573—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids substituted in position 17 beta by a chain of two carbon atoms, e.g. pregnane or progesterone substituted in position 21, e.g. cortisone, dexamethasone, prednisone or aldosterone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0048—Eye, e.g. artificial tears
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0048—Eye, e.g. artificial tears
- A61K9/0051—Ocular inserts, ocular implants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/5021—Organic macromolecular compounds
- A61K9/5031—Organic macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyethylene glycol, poly(lactide-co-glycolide)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/51—Nanocapsules; Nanoparticles
- A61K9/5107—Excipients; Inactive ingredients
- A61K9/513—Organic macromolecular compounds; Dendrimers
- A61K9/5146—Organic macromolecular compounds; Dendrimers obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyethylene glycol, polyamines, polyanhydrides
- A61K9/5153—Polyesters, e.g. poly(lactide-co-glycolide)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Epidemiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Ophthalmology & Optometry (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Optics & Photonics (AREA)
- Physics & Mathematics (AREA)
- Biomedical Technology (AREA)
- Nanotechnology (AREA)
- Dermatology (AREA)
- Transplantation (AREA)
- Pain & Pain Management (AREA)
- Rheumatology (AREA)
- Heart & Thoracic Surgery (AREA)
- Cardiology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Steroid Compounds (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201462037000P | 2014-08-13 | 2014-08-13 | |
| US62/037,000 | 2014-08-13 | ||
| US201562139561P | 2015-03-27 | 2015-03-27 | |
| US62/139,561 | 2015-03-27 | ||
| PCT/US2015/043478 WO2016025215A1 (en) | 2014-08-13 | 2015-08-03 | Glucocorticoid-loaded nanoparticles for prevention of corneal allograft rejection and neovascularization |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20170046146A true KR20170046146A (ko) | 2017-04-28 |
Family
ID=54011072
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177006811A Withdrawn KR20170046146A (ko) | 2014-08-13 | 2015-08-03 | 각막 동종이식 거부 및 신혈관 형성의 예방을 위한 글루코 코르티코이드-로딩된 나노입자 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US10195212B2 (enExample) |
| EP (1) | EP3193827A1 (enExample) |
| JP (1) | JP2017524712A (enExample) |
| KR (1) | KR20170046146A (enExample) |
| CN (1) | CN106794152A (enExample) |
| CA (1) | CA2957764C (enExample) |
| MX (1) | MX2017001863A (enExample) |
| WO (1) | WO2016025215A1 (enExample) |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8889193B2 (en) | 2010-02-25 | 2014-11-18 | The Johns Hopkins University | Sustained delivery of therapeutic agents to an eye compartment |
| EP3233098B1 (en) | 2014-12-15 | 2024-05-29 | The Johns Hopkins University | Cvs transplantation for treatment of bacterial vaginosis |
| CN107278151A (zh) | 2014-12-15 | 2017-10-20 | 约翰霍普金斯大学 | 舒尼替尼制剂及其在治疗青光眼中的使用方法 |
| CN107635545A (zh) | 2015-01-27 | 2018-01-26 | 约翰霍普金斯大学 | 用于增强粘膜表面处活性剂的输送的低渗水凝胶制剂 |
| KR20180058758A (ko) | 2015-09-22 | 2018-06-01 | 그레이버그 비젼, 인크. | 안구 장애의 치료를 위한 화합물 및 조성물 |
| BR112018009644A2 (pt) | 2015-11-12 | 2018-11-06 | Graybug Vision Inc | micropartículas agregantes sólidas modificadas na superfície, material injetável, processo para preparação de micropartículas agregantes sólidas modificadas na superfície, método para tratamento de um distúrbio ocular, e, uso de micropartículas agregantes sólidas modificadas na superfície |
| DE102017002454A1 (de) * | 2017-03-14 | 2018-09-20 | Friedrich-Schiller-Universität Jena | Organische Polymerpartikel enthaltend Poly(oxazolin)-Stabilisatoren und Verwendung von Poly(oxazolinen) zur Stabilisierung von organischen Polymerpartikeln |
| JP7217022B2 (ja) | 2017-03-23 | 2023-02-02 | グレイバグ ビジョン インコーポレイテッド | 眼障害の治療のための薬物及び組成物 |
| CA3057875A1 (en) | 2017-05-10 | 2018-11-15 | Graybug Vision, Inc. | Extended release microparticles and suspensions thereof for medical therapy |
| WO2019071275A1 (en) * | 2017-10-06 | 2019-04-11 | Aciont Inc. | DEVICES FOR DELIVERY OF NON-INVASIVE OCULAR MEDICINE |
| WO2020210805A1 (en) * | 2019-04-11 | 2020-10-15 | The Johns Hopkins University | Nanoparticles for drug delivery to brain |
| WO2021168239A1 (en) * | 2020-02-21 | 2021-08-26 | The Johns Hopkins University | Suprachoroidal delivery of drug particles to reduce toxicity |
Family Cites Families (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4432964A (en) * | 1981-08-24 | 1984-02-21 | Alza Corporation | Topical composition containing steroid in two forms released independently from polymeric carrier |
| US4888176A (en) | 1984-05-21 | 1989-12-19 | Massachusetts Institute Of Technology | Controlled drug delivery high molecular weight polyanhydrides |
| US4757128A (en) | 1986-08-01 | 1988-07-12 | Massachusetts Institute Of Technology | High molecular weight polyanhydride and preparation thereof |
| US4789724A (en) | 1986-10-17 | 1988-12-06 | Massachusetts Institute Of Technology | Preparation of anhydride copolymers |
| US4857311A (en) | 1987-07-31 | 1989-08-15 | Massachusetts Institute Of Technology | Polyanhydrides with improved hydrolytic degradation properties |
| US4997652A (en) | 1987-12-22 | 1991-03-05 | Visionex | Biodegradable ocular implants |
| US5932462A (en) | 1995-01-10 | 1999-08-03 | Shearwater Polymers, Inc. | Multiarmed, monofunctional, polymer for coupling to molecules and surfaces |
| WO2004084871A1 (en) * | 2003-03-26 | 2004-10-07 | Ltt Bio-Pharma Co., Ltd. | Intravenous nanoparticles for targenting drug delivery and sustained drug release |
| US20090148527A1 (en) * | 2007-12-07 | 2009-06-11 | Robinson Michael R | Intraocular formulation |
| US20050226814A1 (en) | 2004-04-13 | 2005-10-13 | Bausch & Lomb Incorporated | Diagnostic method and kit for implantation of a sustained release drug-delivery implant with a steroid |
| US20060089590A1 (en) * | 2004-10-27 | 2006-04-27 | John Higuchi | Methods and devices for sustained in-vivo release of an active agent |
| JP2007001926A (ja) * | 2005-06-24 | 2007-01-11 | Ltt Bio-Pharma Co Ltd | 吸入・噴霧用ステロイド製剤 |
| EP1985309B1 (en) * | 2005-12-26 | 2016-11-23 | LTT Bio-Pharma Co., Ltd. | Nanoparticles containing water-soluble non-peptide low-molecular weight drug |
| US20100129456A1 (en) * | 2007-05-14 | 2010-05-27 | Ltt Bio-Pharma Co., Ltd. | Sustained-release nanoparticle containing low-molecular-weight drug with negatively charged group |
| JP2011512903A (ja) * | 2008-02-25 | 2011-04-28 | アイゲート ファーマ エスエーエス | イオントフォレシスを介した眼組織への治療薬の向上した送達 |
| US20110206773A1 (en) * | 2008-05-20 | 2011-08-25 | Yale University | Sustained delivery of drugs from biodegradable polymeric microparticles |
| US9095506B2 (en) | 2008-11-17 | 2015-08-04 | Allergan, Inc. | Biodegradable alpha-2 agonist polymeric implants and therapeutic uses thereof |
| WO2011041373A1 (en) | 2009-09-29 | 2011-04-07 | Eyegate Pharmaceuticals, Inc. | Positively-charged poly (d,l-lactide-co-glycolide) nanoparticles and fabrication methods of the same |
| CA2845673A1 (en) | 2011-09-13 | 2013-03-21 | Altacor Limited | Pharmaceutical nanoparticle compositions |
| AU2013209452B2 (en) | 2012-01-19 | 2015-11-05 | The Johns Hopkins University | Nanoparticle formulations with enhanced mucosal penetration |
| NZ741873A (en) * | 2012-05-03 | 2019-04-26 | Kala Pharmaceuticals Inc | Pharmaceutical nanoparticles showing improved mucosal transport |
-
2015
- 2015-08-03 JP JP2017507390A patent/JP2017524712A/ja active Pending
- 2015-08-03 EP EP15756267.9A patent/EP3193827A1/en not_active Withdrawn
- 2015-08-03 KR KR1020177006811A patent/KR20170046146A/ko not_active Withdrawn
- 2015-08-03 WO PCT/US2015/043478 patent/WO2016025215A1/en not_active Ceased
- 2015-08-03 MX MX2017001863A patent/MX2017001863A/es unknown
- 2015-08-03 CN CN201580053822.9A patent/CN106794152A/zh active Pending
- 2015-08-03 US US15/502,732 patent/US10195212B2/en active Active
- 2015-08-03 CA CA2957764A patent/CA2957764C/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| US20170157147A1 (en) | 2017-06-08 |
| CA2957764C (en) | 2019-07-02 |
| CN106794152A (zh) | 2017-05-31 |
| MX2017001863A (es) | 2017-07-17 |
| CA2957764A1 (en) | 2016-02-18 |
| EP3193827A1 (en) | 2017-07-26 |
| WO2016025215A1 (en) | 2016-02-18 |
| US10195212B2 (en) | 2019-02-05 |
| JP2017524712A (ja) | 2017-08-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10195212B2 (en) | Glucocorticoid-loaded nanoparticles for prevention of corneal allograft rejection and neovascularization | |
| US11660349B2 (en) | Non-linear multiblock copolymer-drug conjugates for the delivery of active agents | |
| CN104363924B (zh) | 用于递送hif‑1抑制剂的控制释放调配物 | |
| JP6570513B2 (ja) | 持続的眼内放出のためのマイクロスフェア薬剤送達システム | |
| Liu et al. | Prolonged ocular retention of mucoadhesive nanoparticle eye drop formulation enables treatment of eye diseases using significantly reduced dosage | |
| Kwon et al. | Potential therapeutic usage of nanomedicine for glaucoma treatment | |
| US20100204325A1 (en) | Valproic acid drug delivery systems and intraocular therapeutic uses thereof | |
| Bravo-Osuna et al. | Pharmaceutical microscale and nanoscale approaches for efficient treatment of ocular diseases | |
| JP2015013863A (ja) | 増大した前眼部クリアランス速度を有するα−2アドレナリン受容体アゴニストを用いる眼科治療 | |
| KR20210013339A (ko) | 개선된 점막 수송을 나타내는 제약 나노입자 | |
| JP2007535367A (ja) | エストラジオール誘導体またはエストラトポン誘導体を含有する徐放性眼内インプラント、ならびに関連する製造法 | |
| US11369575B2 (en) | PPARα agonist compositions and methods of use | |
| Pillay et al. | Intraocular drug delivery technologies: advancing treatment of posterior segment disorders of the eye | |
| EP4433076A1 (en) | Compositions and methods for the treatment of ocular diseases and injuries | |
| US20190022016A1 (en) | Compositions for sustained release of anti-glaucoma agents to control intraocular pressure | |
| JP2024534403A (ja) | 眼疾患のための緑茶ベースのナノコンプレックス | |
| Kundu et al. | Emerging drug delivery strategies for glaucoma therapy: focus on nanoparticles and stimuli-responsive systems | |
| Ramesh | Ocular barriers and ocular drug delivery: Bridging the gap using nanomicelles as drug carriers | |
| Schmitt | Design and development of ocular formulations for preclinical and clinical trials | |
| Genov et al. | Innovative drug delivery in ophthalmology | |
| Virmani et al. | Eyes on the Future: Nano-medicine Innovations in Enhancing Ocular Bioavailability | |
| CN119947711A (zh) | 用于持续药物递送的有机凝胶、其制备方法和用途 | |
| Chowdhury | Development and preliminary in vitro evaluation of nanomicelles laden in situ gel of dexamethasone for ophthalmic delivery | |
| HK1241281B (en) | Sunitinib formulations and methods for use thereof in treatment of ocular disorders | |
| HK1241281A1 (en) | Sunitinib formulations and methods for use thereof in treatment of ocular disorders |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20170310 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination |