KR20170024147A - 층형 심실 파티셔닝 디바이스의 제작 시스템 및 방법 - Google Patents
층형 심실 파티셔닝 디바이스의 제작 시스템 및 방법 Download PDFInfo
- Publication number
- KR20170024147A KR20170024147A KR1020177005196A KR20177005196A KR20170024147A KR 20170024147 A KR20170024147 A KR 20170024147A KR 1020177005196 A KR1020177005196 A KR 1020177005196A KR 20177005196 A KR20177005196 A KR 20177005196A KR 20170024147 A KR20170024147 A KR 20170024147A
- Authority
- KR
- South Korea
- Prior art keywords
- sheet
- rib
- assembly
- frame
- ribs
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000000034 method Methods 0.000 title claims abstract description 66
- 238000000638 solvent extraction Methods 0.000 title claims description 72
- 230000002861 ventricular Effects 0.000 title description 10
- 239000007943 implant Substances 0.000 claims abstract description 39
- 238000010438 heat treatment Methods 0.000 claims abstract description 21
- 239000000463 material Substances 0.000 claims description 110
- 229920000295 expanded polytetrafluoroethylene Polymers 0.000 claims description 101
- 229920001169 thermoplastic Polymers 0.000 claims description 61
- 239000012528 membrane Substances 0.000 claims description 33
- 238000003825 pressing Methods 0.000 claims description 16
- 210000005240 left ventricle Anatomy 0.000 claims description 13
- 239000004416 thermosoftening plastic Substances 0.000 claims description 13
- 230000004927 fusion Effects 0.000 claims description 11
- 239000012815 thermoplastic material Substances 0.000 claims description 11
- 230000000295 complement effect Effects 0.000 claims description 10
- 230000000379 polymerizing effect Effects 0.000 claims description 6
- 241000985665 Cecropia obtusifolia Species 0.000 claims description 5
- 230000035515 penetration Effects 0.000 claims description 5
- 238000002407 reforming Methods 0.000 claims description 5
- -1 polyethylene Polymers 0.000 description 72
- 239000004698 Polyethylene Substances 0.000 description 65
- 229920000573 polyethylene Polymers 0.000 description 65
- 210000002216 heart Anatomy 0.000 description 27
- 210000005242 cardiac chamber Anatomy 0.000 description 20
- 238000003475 lamination Methods 0.000 description 18
- 239000004744 fabric Substances 0.000 description 17
- 239000012530 fluid Substances 0.000 description 14
- 239000004810 polytetrafluoroethylene Substances 0.000 description 13
- 229940058401 polytetrafluoroethylene Drugs 0.000 description 13
- 206010007559 Cardiac failure congestive Diseases 0.000 description 12
- 230000008569 process Effects 0.000 description 12
- 206010019280 Heart failures Diseases 0.000 description 10
- 239000002355 dual-layer Substances 0.000 description 10
- 239000008280 blood Substances 0.000 description 7
- 210000004369 blood Anatomy 0.000 description 7
- 238000004891 communication Methods 0.000 description 7
- 238000001816 cooling Methods 0.000 description 7
- 230000009977 dual effect Effects 0.000 description 7
- 239000000835 fiber Substances 0.000 description 7
- 238000004519 manufacturing process Methods 0.000 description 7
- 229910052751 metal Inorganic materials 0.000 description 7
- 239000002184 metal Substances 0.000 description 7
- 201000010099 disease Diseases 0.000 description 6
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 6
- 239000011159 matrix material Substances 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 5
- 238000002513 implantation Methods 0.000 description 5
- 239000010410 layer Substances 0.000 description 5
- 238000002844 melting Methods 0.000 description 5
- 230000008018 melting Effects 0.000 description 5
- 239000011148 porous material Substances 0.000 description 5
- 239000002356 single layer Substances 0.000 description 5
- 238000011282 treatment Methods 0.000 description 5
- 229920001903 high density polyethylene Polymers 0.000 description 4
- 239000004700 high-density polyethylene Substances 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 238000012545 processing Methods 0.000 description 4
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- 230000000747 cardiac effect Effects 0.000 description 3
- 230000006870 function Effects 0.000 description 3
- 229910001000 nickel titanium Inorganic materials 0.000 description 3
- 238000005192 partition Methods 0.000 description 3
- 230000000149 penetrating effect Effects 0.000 description 3
- 229920000139 polyethylene terephthalate Polymers 0.000 description 3
- 239000005020 polyethylene terephthalate Substances 0.000 description 3
- 230000003014 reinforcing effect Effects 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- 238000002054 transplantation Methods 0.000 description 3
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- 239000004677 Nylon Substances 0.000 description 2
- 239000004696 Poly ether ether ketone Substances 0.000 description 2
- 208000007536 Thrombosis Diseases 0.000 description 2
- 229910045601 alloy Inorganic materials 0.000 description 2
- 239000000956 alloy Substances 0.000 description 2
- 238000000429 assembly Methods 0.000 description 2
- 230000000712 assembly Effects 0.000 description 2
- 238000005452 bending Methods 0.000 description 2
- 239000000560 biocompatible material Substances 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 239000000945 filler Substances 0.000 description 2
- 210000003709 heart valve Anatomy 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 230000002107 myocardial effect Effects 0.000 description 2
- HLXZNVUGXRDIFK-UHFFFAOYSA-N nickel titanium Chemical compound [Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni] HLXZNVUGXRDIFK-UHFFFAOYSA-N 0.000 description 2
- 229920001778 nylon Polymers 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 229920002530 polyetherether ketone Polymers 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 229910001220 stainless steel Inorganic materials 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 238000012800 visualization Methods 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 206010033372 Pain and discomfort Diseases 0.000 description 1
- 229920000954 Polyglycolide Polymers 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 238000004873 anchoring Methods 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 210000003323 beak Anatomy 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 229910052797 bismuth Inorganic materials 0.000 description 1
- JCXGWMGPZLAOME-UHFFFAOYSA-N bismuth atom Chemical compound [Bi] JCXGWMGPZLAOME-UHFFFAOYSA-N 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000008602 contraction Effects 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 210000004351 coronary vessel Anatomy 0.000 description 1
- 238000005336 cracking Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 238000002651 drug therapy Methods 0.000 description 1
- 230000010102 embolization Effects 0.000 description 1
- 206010016256 fatigue Diseases 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 210000001105 femoral artery Anatomy 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 230000010247 heart contraction Effects 0.000 description 1
- 208000019622 heart disease Diseases 0.000 description 1
- 230000002439 hemostatic effect Effects 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 238000009830 intercalation Methods 0.000 description 1
- 229910052741 iridium Inorganic materials 0.000 description 1
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 description 1
- 208000028867 ischemia Diseases 0.000 description 1
- 230000000302 ischemic effect Effects 0.000 description 1
- 238000010030 laminating Methods 0.000 description 1
- 210000005246 left atrium Anatomy 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 210000004165 myocardium Anatomy 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 238000001050 pharmacotherapy Methods 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 229920001610 polycaprolactone Polymers 0.000 description 1
- 239000004632 polycaprolactone Substances 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 239000004633 polyglycolic acid Substances 0.000 description 1
- 239000004626 polylactic acid Substances 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 230000002250 progressing effect Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 238000005086 pumping Methods 0.000 description 1
- 230000002787 reinforcement Effects 0.000 description 1
- 238000007634 remodeling Methods 0.000 description 1
- 229910052703 rhodium Inorganic materials 0.000 description 1
- 239000010948 rhodium Substances 0.000 description 1
- MHOVAHRLVXNVSD-UHFFFAOYSA-N rhodium atom Chemical compound [Rh] MHOVAHRLVXNVSD-UHFFFAOYSA-N 0.000 description 1
- 231100000241 scar Toxicity 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 229910001285 shape-memory alloy Inorganic materials 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 230000009772 tissue formation Effects 0.000 description 1
- 230000008467 tissue growth Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- 238000002604 ultrasonography Methods 0.000 description 1
- 210000005166 vasculature Anatomy 0.000 description 1
- 230000009385 viral infection Effects 0.000 description 1
- 239000002759 woven fabric Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/12—Surgical instruments, devices or methods for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels or umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B17/12099—Occluding by internal devices, e.g. balloons or releasable wires characterised by the location of the occluder
- A61B17/12122—Occluding by internal devices, e.g. balloons or releasable wires characterised by the location of the occluder within the heart
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/12—Surgical instruments, devices or methods for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels or umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B17/12131—Occluding by internal devices, e.g. balloons or releasable wires characterised by the type of occluding device
- A61B17/12168—Occluding by internal devices, e.g. balloons or releasable wires characterised by the type of occluding device having a mesh structure
- A61B17/12172—Occluding by internal devices, e.g. balloons or releasable wires characterised by the type of occluding device having a mesh structure having a pre-set deployed three-dimensional shape
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00526—Methods of manufacturing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00831—Material properties
- A61B2017/00955—Material properties thermoplastic
Landscapes
- Health & Medical Sciences (AREA)
- Surgery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biomedical Technology (AREA)
- Medical Informatics (AREA)
- Vascular Medicine (AREA)
- Reproductive Health (AREA)
- Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Heart & Thoracic Surgery (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Molecular Biology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Cardiology (AREA)
- Prostheses (AREA)
- External Artificial Organs (AREA)
- Surgical Instruments (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/827,927 US9332992B2 (en) | 2004-08-05 | 2013-03-14 | Method for making a laminar ventricular partitioning device |
| US13/827,927 | 2013-03-14 | ||
| PCT/US2014/027364 WO2014152461A2 (en) | 2013-03-14 | 2014-03-14 | Systems and methods for making a laminar ventricular partitioning device |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020157028718A Division KR101713607B1 (ko) | 2013-03-14 | 2014-03-14 | 층형 심실 파티셔닝 디바이스의 제작 시스템 및 방법 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20170024147A true KR20170024147A (ko) | 2017-03-06 |
Family
ID=50442723
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177005196A Withdrawn KR20170024147A (ko) | 2013-03-14 | 2014-03-14 | 층형 심실 파티셔닝 디바이스의 제작 시스템 및 방법 |
| KR1020157028718A Expired - Fee Related KR101713607B1 (ko) | 2013-03-14 | 2014-03-14 | 층형 심실 파티셔닝 디바이스의 제작 시스템 및 방법 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020157028718A Expired - Fee Related KR101713607B1 (ko) | 2013-03-14 | 2014-03-14 | 층형 심실 파티셔닝 디바이스의 제작 시스템 및 방법 |
Country Status (10)
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| BR112017006248A2 (pt) * | 2014-09-28 | 2017-12-12 | Cardiokinetix Inc | aparelhos para o tratamento de insuficiência cardíaca |
| CN104706444B (zh) * | 2015-03-03 | 2017-07-18 | 上海形状记忆合金材料有限公司 | 左心室减容装置 |
| CN105476733B (zh) * | 2016-01-27 | 2017-09-01 | 张刚成 | 心脏减容装置 |
| CN106073947A (zh) * | 2016-08-29 | 2016-11-09 | 关丽鹃 | 心脏减容系统 |
| US20200029849A1 (en) * | 2016-09-29 | 2020-01-30 | Innervate Medical, Llc | Uses of minimally invasive systems and methods for neurovascular signal management including endovascular electroencephalography and related techniques for epilepsy detection and treatment |
| EP3565506A4 (en) * | 2017-01-05 | 2020-09-23 | Harmony Development Group, Inc. | EXPANDABLE DEVICE FOR DETECTING REGURGITATIONAL JET, VOLUME AND FORCE TO INFLUENCE VENTRICULAR FUNCTION AND REMODELING |
| WO2019006152A1 (en) | 2017-06-28 | 2019-01-03 | Harmony Development Group, Inc. | INFLATABLE TRANSDUCTION IMPLANT SYSTEM COMPRISING AN ANNULAR DOUBLE FORCE TRANSDUCTION IMPLANT |
| US11167122B2 (en) | 2018-03-05 | 2021-11-09 | Harmony Development Group, Inc. | Force transducting implant system for the mitigation of atrioventricular pressure gradient loss and the restoration of healthy ventricular geometry |
| CN109820551B (zh) * | 2019-02-22 | 2022-03-01 | 北京大学深圳研究生院 | 一种充气式心室隔离装置 |
| CN110974349B (zh) * | 2019-11-25 | 2022-08-02 | 湖南瑞康通科技发展有限公司 | 取栓装置及取栓组件 |
| US20240216098A1 (en) * | 2022-12-28 | 2024-07-04 | Coherex Medical, Inc. | Cover for medical device, system and method thereof |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100030256A1 (en) * | 1997-11-12 | 2010-02-04 | Genesis Technologies Llc | Medical Devices and Methods |
| US7128073B1 (en) * | 1998-11-06 | 2006-10-31 | Ev3 Endovascular, Inc. | Method and device for left atrial appendage occlusion |
| US8257428B2 (en) * | 1999-08-09 | 2012-09-04 | Cardiokinetix, Inc. | System for improving cardiac function |
| US8529430B2 (en) * | 2002-08-01 | 2013-09-10 | Cardiokinetix, Inc. | Therapeutic methods and devices following myocardial infarction |
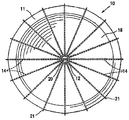
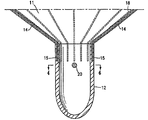
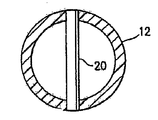
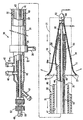
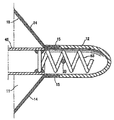
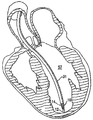
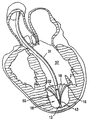
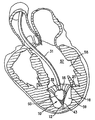
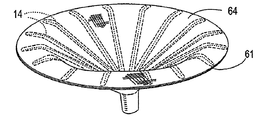
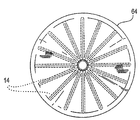
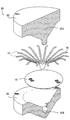
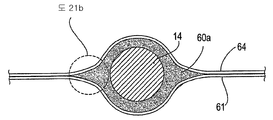
| US20060030881A1 (en) * | 2004-08-05 | 2006-02-09 | Cardiokinetix, Inc. | Ventricular partitioning device |
| US20060106420A1 (en) * | 2004-11-12 | 2006-05-18 | Medtronic Vascular, Inc. | Patch for treating a septal defect |
| US9545300B2 (en) * | 2004-12-22 | 2017-01-17 | W. L. Gore & Associates, Inc. | Filament-wound implantable devices |
| JP2010527742A (ja) * | 2007-05-31 | 2010-08-19 | レックス メディカル リミテッド パートナーシップ | 左心耳用閉鎖体デバイス |
| US9414842B2 (en) * | 2007-10-12 | 2016-08-16 | St. Jude Medical, Cardiology Division, Inc. | Multi-component vascular device |
| WO2010081033A1 (en) * | 2009-01-08 | 2010-07-15 | Coherex Medical, Inc. | Medical device for modification of left atrial appendage and related systems and methods |
| AU2009344181A1 (en) | 2009-04-10 | 2011-10-13 | Cardiokinetix, Inc. | Sealing and filling ventricular partitioning devices to improve cardiac function |
| CN102612345B (zh) * | 2009-06-17 | 2016-03-16 | 科赫里克斯医疗股份有限公司 | 用于修正左心耳的医疗装置以及相关的系统和方法 |
| US20110054515A1 (en) * | 2009-08-25 | 2011-03-03 | John Bridgeman | Device and method for occluding the left atrial appendage |
| CA2777495A1 (en) * | 2009-10-26 | 2011-05-12 | Cardiokinetix, Inc. | Ventricular volume reduction |
| KR101070811B1 (ko) * | 2011-02-18 | 2011-10-06 | 강원대학교산학협력단 | 심실구획장치 |
-
2014
- 2014-03-14 KR KR1020177005196A patent/KR20170024147A/ko not_active Withdrawn
- 2014-03-14 BR BR112015021613A patent/BR112015021613A2/pt not_active IP Right Cessation
- 2014-03-14 CN CN201410097592.3A patent/CN104042300B/zh not_active Expired - Fee Related
- 2014-03-14 RU RU2015143955A patent/RU2015143955A/ru not_active Application Discontinuation
- 2014-03-14 CA CA2901532A patent/CA2901532A1/en not_active Abandoned
- 2014-03-14 KR KR1020157028718A patent/KR101713607B1/ko not_active Expired - Fee Related
- 2014-03-14 AU AU2014239608A patent/AU2014239608A1/en not_active Abandoned
- 2014-03-14 CN CN201420118276.5U patent/CN204072201U/zh not_active Expired - Fee Related
- 2014-03-14 WO PCT/US2014/027364 patent/WO2014152461A2/en active Application Filing
- 2014-03-14 EP EP14716179.8A patent/EP2967570A2/en not_active Withdrawn
- 2014-03-14 JP JP2016502416A patent/JP2016512141A/ja active Pending
-
2015
- 2015-08-12 IL IL240520A patent/IL240520A0/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| RU2015143955A (ru) | 2017-04-20 |
| WO2014152461A3 (en) | 2014-12-04 |
| CA2901532A1 (en) | 2014-09-25 |
| CN104042300B (zh) | 2017-11-28 |
| CN204072201U (zh) | 2015-01-07 |
| KR20150127242A (ko) | 2015-11-16 |
| AU2014239608A1 (en) | 2015-08-27 |
| WO2014152461A2 (en) | 2014-09-25 |
| BR112015021613A2 (pt) | 2017-07-18 |
| KR101713607B1 (ko) | 2017-03-09 |
| JP2016512141A (ja) | 2016-04-25 |
| CN104042300A (zh) | 2014-09-17 |
| EP2967570A2 (en) | 2016-01-20 |
| IL240520A0 (en) | 2015-10-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101713607B1 (ko) | 층형 심실 파티셔닝 디바이스의 제작 시스템 및 방법 | |
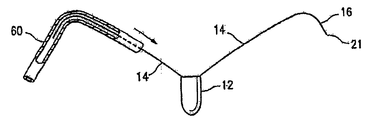
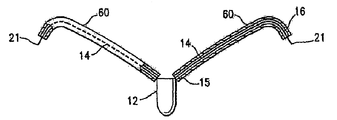
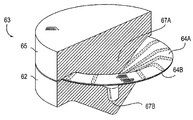
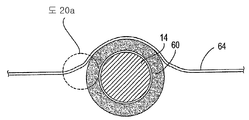
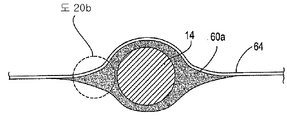
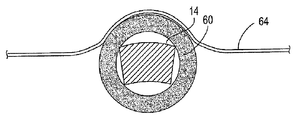
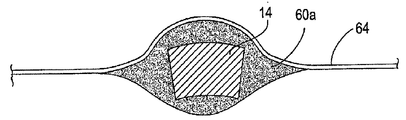
| US7897086B2 (en) | Method of making a laminar ventricular partitioning device | |
| US9332992B2 (en) | Method for making a laminar ventricular partitioning device | |
| US10064696B2 (en) | Devices and methods for delivering an endocardial device | |
| US20180256865A1 (en) | Devices and methods for treating heart failure | |
| JP4430712B2 (ja) | セメント誘導整形外科用インプラント | |
| US11813386B2 (en) | Interatrial shunt with expanded neck region | |
| CN113329717B (zh) | 用于制作封装的沙漏形支架的系统及方法 | |
| US20050015140A1 (en) | Encapsulation device and methods of use | |
| CN108024813A (zh) | 用于左心耳封堵的方法和装置 | |
| CN101917929A (zh) | 用于低型面经皮递送的模块化脉管移植物 | |
| US20230211132A1 (en) | Pulmonary arterial compliance enhancement and control device | |
| CN204169959U (zh) | 包括可扩张装置和递送导管的系统 | |
| CN112472381B (zh) | 支架 | |
| CN204193278U (zh) | 用于插入至心脏的心室中以治疗心脏衰竭的可扩张装置 | |
| CN204181750U (zh) | 植入物加载系统 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20170223 Application number text: 1020157028718 Filing date: 20151012 |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |