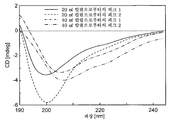
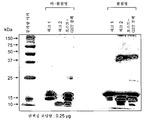
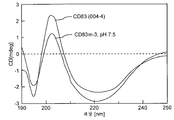
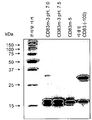
KR20160104101A - 신규 가용성 cd83 폴리펩티드, 제형 및 사용 방법 - Google Patents
신규 가용성 cd83 폴리펩티드, 제형 및 사용 방법 Download PDFInfo
- Publication number
- KR20160104101A KR20160104101A KR1020167023212A KR20167023212A KR20160104101A KR 20160104101 A KR20160104101 A KR 20160104101A KR 1020167023212 A KR1020167023212 A KR 1020167023212A KR 20167023212 A KR20167023212 A KR 20167023212A KR 20160104101 A KR20160104101 A KR 20160104101A
- Authority
- KR
- South Korea
- Prior art keywords
- leu
- thr
- glu
- ser
- pro
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/02—Muscle relaxants, e.g. for tetanus or cramps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/38—Drugs for disorders of the endocrine system of the suprarenal hormones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/14—Vasoprotectives; Antihaemorrhoidals; Drugs for varicose therapy; Capillary stabilisers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Immunology (AREA)
- Diabetes (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Cell Biology (AREA)
- Pulmonology (AREA)
- Heart & Thoracic Surgery (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- Neurology (AREA)
- Toxicology (AREA)
- Cardiology (AREA)
- Physical Education & Sports Medicine (AREA)
- Endocrinology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Obesity (AREA)
- Neurosurgery (AREA)
- Pain & Pain Management (AREA)
- Emergency Medicine (AREA)
- Hematology (AREA)
- Rheumatology (AREA)
- Transplantation (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12870908P | 2008-05-23 | 2008-05-23 | |
| US61/128,709 | 2008-05-23 | ||
| PCT/US2009/003174 WO2009142759A1 (en) | 2008-05-23 | 2009-05-22 | Novel soluble cd83 polypeptides, formulations and methods of use |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020107028713A Division KR101679084B1 (ko) | 2008-05-23 | 2009-05-22 | 신규 가용성 cd83 폴리펩티드, 제형 및 사용 방법 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20160104101A true KR20160104101A (ko) | 2016-09-02 |
Family
ID=41340432
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167023212A Ceased KR20160104101A (ko) | 2008-05-23 | 2009-05-22 | 신규 가용성 cd83 폴리펩티드, 제형 및 사용 방법 |
| KR1020107028713A Expired - Fee Related KR101679084B1 (ko) | 2008-05-23 | 2009-05-22 | 신규 가용성 cd83 폴리펩티드, 제형 및 사용 방법 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020107028713A Expired - Fee Related KR101679084B1 (ko) | 2008-05-23 | 2009-05-22 | 신규 가용성 cd83 폴리펩티드, 제형 및 사용 방법 |
Country Status (12)
| Country | Link |
|---|---|
| US (1) | US20110182903A1 (cg-RX-API-DMAC7.html) |
| EP (1) | EP2288387B1 (cg-RX-API-DMAC7.html) |
| JP (1) | JP2011520959A (cg-RX-API-DMAC7.html) |
| KR (2) | KR20160104101A (cg-RX-API-DMAC7.html) |
| CN (1) | CN102036690B (cg-RX-API-DMAC7.html) |
| AU (1) | AU2009249540B9 (cg-RX-API-DMAC7.html) |
| BR (1) | BRPI0913577B1 (cg-RX-API-DMAC7.html) |
| CA (1) | CA2725198A1 (cg-RX-API-DMAC7.html) |
| IL (1) | IL209207A (cg-RX-API-DMAC7.html) |
| MX (1) | MX2010012576A (cg-RX-API-DMAC7.html) |
| RU (1) | RU2535340C2 (cg-RX-API-DMAC7.html) |
| WO (1) | WO2009142759A1 (cg-RX-API-DMAC7.html) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20200030444A (ko) | 2018-09-12 | 2020-03-20 | 아주대학교산학협력단 | Cd83 억제제를 유효성분으로 포함하는 베체트병의 예방 또는 치료용 조성물 |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1422241A1 (en) | 2002-11-19 | 2004-05-26 | Alexander Steinkasserer | Use of soluble forms of CD83 and nucleic acids encoding them for the treatment or prevention of diseases |
| US7169898B2 (en) | 2002-12-04 | 2007-01-30 | Alexander Steinkasserer | Soluble CD83 proteins and use thereof for the treatment or prevention of a disease or medical condition caused by dysfunction or undesired function of a cellular immune response involving T cells |
| US9102726B2 (en) | 2002-12-04 | 2015-08-11 | Argos Therapeutics, Inc. | Nucleic acid of recombination expression vector encoding soluble forms of CD83, host cells transformed/transfected therewith and pharmaceutical compositions containing same |
| CN103243119B (zh) * | 2013-05-20 | 2016-03-30 | 中国科学技术大学 | 可溶性cd83的表达和制备方法 |
| CA3042683A1 (en) | 2016-11-07 | 2018-05-11 | Coimmune, Inc. | Bispecific antibodies that modulate tlr-4 signaling and uses thereof |
| BR112019020214A2 (pt) * | 2017-04-01 | 2020-04-22 | Avm Biotechnology Llc | substituição do pré-condicionamento citotóxico antes da imunoterapia celular |
| EP3895723A1 (en) | 2020-04-16 | 2021-10-20 | Friedrich-Alexander-Universität Erlangen-Nürnberg | Scd83 for wound healing, hair growth, and skin and hair care |
| DE102024110866A1 (de) | 2024-04-18 | 2025-10-23 | Christian Arnold | Pharmazeutische Zusammensetzung zur Heilung einer Wunde |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6136310A (en) * | 1991-07-25 | 2000-10-24 | Idec Pharmaceuticals Corporation | Recombinant anti-CD4 antibodies for human therapy |
| US5316920A (en) * | 1992-04-17 | 1994-05-31 | Dana-Faber Cancer Institute, Inc. | Lymphocyte activation antigen HB15, a member of the immunoglobulin superfamily |
| US5710262A (en) * | 1992-04-17 | 1998-01-20 | Dana-Faber Cancer Institute, Inc. | Nucleic acid encoding HB15 polypeptides |
| US6197930B1 (en) * | 1997-08-26 | 2001-03-06 | Zymogenetics, Inc. | Adipocyte-specific protein homologs |
| ATE297217T1 (de) | 1999-10-27 | 2005-06-15 | Alexandra Lucas | Zusammensetzung zur vorbeugung und behandlung von transplantatabstossung |
| US20020014242A1 (en) * | 2000-07-31 | 2002-02-07 | Abraham Scaria | Use of rapamycin to inhibit immune response and induce tolerance to gene therapy vector and encoded transgene products |
| US20040185040A1 (en) * | 2001-11-21 | 2004-09-23 | Celltech R & D Limited | Modulating immune responses |
| US20030219436A1 (en) * | 2002-03-15 | 2003-11-27 | Ledbetter Jeffrey A. | Compositions and methods to regulate an immune response using CD83 gene expressed in tumors and using soluble CD83-Ig fusion protein |
| AU2003282550C1 (en) * | 2002-10-09 | 2008-09-25 | Tolerx, Inc. | Molecules preferentially associated with effector T cells or regulatory T cells and methods of their use |
| EP1422241A1 (en) * | 2002-11-19 | 2004-05-26 | Alexander Steinkasserer | Use of soluble forms of CD83 and nucleic acids encoding them for the treatment or prevention of diseases |
| US9102726B2 (en) * | 2002-12-04 | 2015-08-11 | Argos Therapeutics, Inc. | Nucleic acid of recombination expression vector encoding soluble forms of CD83, host cells transformed/transfected therewith and pharmaceutical compositions containing same |
| US7169898B2 (en) * | 2002-12-04 | 2007-01-30 | Alexander Steinkasserer | Soluble CD83 proteins and use thereof for the treatment or prevention of a disease or medical condition caused by dysfunction or undesired function of a cellular immune response involving T cells |
| EP2051987B1 (en) * | 2006-08-18 | 2014-11-19 | Argos Therapeutics, Inc. | Use of cd83 in combination therapies |
-
2009
- 2009-05-22 EP EP09750990A patent/EP2288387B1/en not_active Not-in-force
- 2009-05-22 JP JP2011510515A patent/JP2011520959A/ja active Pending
- 2009-05-22 CA CA2725198A patent/CA2725198A1/en not_active Abandoned
- 2009-05-22 AU AU2009249540A patent/AU2009249540B9/en not_active Ceased
- 2009-05-22 BR BRPI0913577-4A patent/BRPI0913577B1/pt not_active IP Right Cessation
- 2009-05-22 KR KR1020167023212A patent/KR20160104101A/ko not_active Ceased
- 2009-05-22 KR KR1020107028713A patent/KR101679084B1/ko not_active Expired - Fee Related
- 2009-05-22 RU RU2010152553/10A patent/RU2535340C2/ru active
- 2009-05-22 MX MX2010012576A patent/MX2010012576A/es active IP Right Grant
- 2009-05-22 US US12/736,887 patent/US20110182903A1/en not_active Abandoned
- 2009-05-22 CN CN2009801186344A patent/CN102036690B/zh not_active Expired - Fee Related
- 2009-05-22 WO PCT/US2009/003174 patent/WO2009142759A1/en not_active Ceased
-
2010
- 2010-11-09 IL IL209207A patent/IL209207A/en active IP Right Grant
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20200030444A (ko) | 2018-09-12 | 2020-03-20 | 아주대학교산학협력단 | Cd83 억제제를 유효성분으로 포함하는 베체트병의 예방 또는 치료용 조성물 |
Also Published As
| Publication number | Publication date |
|---|---|
| BRPI0913577B1 (pt) | 2022-05-31 |
| EP2288387A4 (en) | 2011-04-27 |
| IL209207A (en) | 2014-05-28 |
| WO2009142759A1 (en) | 2009-11-26 |
| AU2009249540B9 (en) | 2014-09-18 |
| EP2288387A1 (en) | 2011-03-02 |
| KR20110018381A (ko) | 2011-02-23 |
| BRPI0913577A2 (pt) | 2020-08-18 |
| AU2009249540A8 (en) | 2011-01-20 |
| HK1155066A1 (en) | 2012-05-11 |
| AU2009249540B2 (en) | 2014-07-03 |
| RU2010152553A (ru) | 2012-06-27 |
| JP2011520959A (ja) | 2011-07-21 |
| AU2009249540A1 (en) | 2009-11-26 |
| IL209207A0 (en) | 2011-01-31 |
| US20110182903A1 (en) | 2011-07-28 |
| EP2288387B1 (en) | 2013-02-13 |
| CN102036690A (zh) | 2011-04-27 |
| CA2725198A1 (en) | 2009-11-26 |
| RU2535340C2 (ru) | 2014-12-10 |
| CN102036690B (zh) | 2013-11-06 |
| MX2010012576A (es) | 2011-04-05 |
| KR101679084B1 (ko) | 2016-11-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101679084B1 (ko) | 신규 가용성 cd83 폴리펩티드, 제형 및 사용 방법 | |
| AU2002305716B2 (en) | Methods for protecting allogeneic islet transplant using soluble CTLA4 mutant molecules | |
| JP2009240311A (ja) | 活性化されたt細胞の表面上のレセプタ:act−4 | |
| JPH09509826A (ja) | 活性化cd4▲上+▼t細胞の表層上のレセプターに対するリガンド(act−4−l) | |
| CA2658000C (en) | Use of cd83 in combination therapies | |
| JP2010279365A (ja) | 修飾したヒト胸腺ストローマ細胞リンホポエチン | |
| JP2018522547A (ja) | Il−37バリアント | |
| EP1525221A1 (en) | Conjugate of notch signalling pathway modulators and their use in medical treatment | |
| WO2006011485A1 (ja) | アミロイドペプチドの凝集を抑制する蛋白質とその作用 | |
| AU2021336259A1 (en) | Interleukin-2 muteins and uses thereof | |
| HK1155066B (en) | Novel soluble cd83 polypeptides, formulations and methods of use | |
| CN115073607A (zh) | Tnfr2与baff受体的融合蛋白 | |
| CA2332379A1 (en) | Molecules associated with apoptosis | |
| WO1998041634A1 (en) | Human growth and differentiation protein | |
| EA042572B1 (ru) | Мутеины il-2 и способы их применения | |
| HK1262138A1 (en) | Uses of soluble ctla4 mutant molecules | |
| HK1120221B (en) | Uses of soluble ctla4 mutant molecules |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20160824 Application number text: 1020107028713 Filing date: 20101221 |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20160923 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20161020 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20170425 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20161020 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |