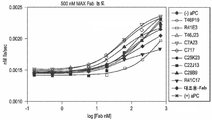
KR20150088869A - 활성화 단백질 C(aPC)에 대한 모노클로날 항체 - Google Patents
활성화 단백질 C(aPC)에 대한 모노클로날 항체 Download PDFInfo
- Publication number
- KR20150088869A KR20150088869A KR1020157017008A KR20157017008A KR20150088869A KR 20150088869 A KR20150088869 A KR 20150088869A KR 1020157017008 A KR1020157017008 A KR 1020157017008A KR 20157017008 A KR20157017008 A KR 20157017008A KR 20150088869 A KR20150088869 A KR 20150088869A
- Authority
- KR
- South Korea
- Prior art keywords
- ser
- gly
- amino acid
- seq
- acid sequence
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 108090000623 proteins and genes Proteins 0.000 title claims abstract description 56
- 102000004169 proteins and genes Human genes 0.000 title claims abstract description 29
- 241000282414 Homo sapiens Species 0.000 claims abstract description 247
- 101800004937 Protein C Proteins 0.000 claims abstract description 134
- 102000017975 Protein C Human genes 0.000 claims abstract description 134
- 101800001700 Saposin-D Proteins 0.000 claims abstract description 130
- 229960000856 protein c Drugs 0.000 claims abstract description 130
- 230000027455 binding Effects 0.000 claims abstract description 86
- 230000014508 negative regulation of coagulation Effects 0.000 claims abstract description 41
- 238000000034 method Methods 0.000 claims abstract description 37
- 206010053567 Coagulopathies Diseases 0.000 claims abstract description 28
- 108010021625 Immunoglobulin Fragments Proteins 0.000 claims abstract description 9
- 102000008394 Immunoglobulin Fragments Human genes 0.000 claims abstract description 9
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 160
- 230000004048 modification Effects 0.000 claims description 32
- 238000012986 modification Methods 0.000 claims description 32
- 150000001413 amino acids Chemical class 0.000 claims description 27
- 230000015271 coagulation Effects 0.000 claims description 25
- 238000005345 coagulation Methods 0.000 claims description 25
- 150000007523 nucleic acids Chemical class 0.000 claims description 23
- 238000006467 substitution reaction Methods 0.000 claims description 22
- 102000039446 nucleic acids Human genes 0.000 claims description 21
- 108020004707 nucleic acids Proteins 0.000 claims description 21
- 230000003213 activating effect Effects 0.000 claims description 19
- 230000000415 inactivating effect Effects 0.000 claims description 16
- 208000032843 Hemorrhage Diseases 0.000 claims description 12
- 230000000740 bleeding effect Effects 0.000 claims description 12
- 230000004913 activation Effects 0.000 claims description 11
- 102100022641 Coagulation factor IX Human genes 0.000 claims description 9
- 230000007812 deficiency Effects 0.000 claims description 9
- 239000008194 pharmaceutical composition Substances 0.000 claims description 9
- 102000015081 Blood Coagulation Factors Human genes 0.000 claims description 7
- 108010039209 Blood Coagulation Factors Proteins 0.000 claims description 7
- 108010076282 Factor IX Proteins 0.000 claims description 7
- 102220471249 M-phase inducer phosphatase 1_S82A_mutation Human genes 0.000 claims description 7
- 239000003114 blood coagulation factor Substances 0.000 claims description 7
- 229960004222 factor ix Drugs 0.000 claims description 7
- 230000002068 genetic effect Effects 0.000 claims description 7
- 102200099928 rs137853185 Human genes 0.000 claims description 7
- 102220267892 rs1555193020 Human genes 0.000 claims description 7
- 102200068694 rs281865207 Human genes 0.000 claims description 7
- 102220128896 rs376207606 Human genes 0.000 claims description 7
- 108010054218 Factor VIII Proteins 0.000 claims description 6
- 102000001690 Factor VIII Human genes 0.000 claims description 6
- 102220543692 Guanine nucleotide-binding protein G(s) subunit alpha isoforms XLas_N53G_mutation Human genes 0.000 claims description 6
- 208000015294 blood coagulation disease Diseases 0.000 claims description 6
- 229960000301 factor viii Drugs 0.000 claims description 6
- 230000009852 coagulant defect Effects 0.000 claims description 5
- 201000003542 Factor VIII deficiency Diseases 0.000 claims description 4
- 108010054265 Factor VIIa Proteins 0.000 claims description 4
- 230000023555 blood coagulation Effects 0.000 claims description 4
- 239000003937 drug carrier Substances 0.000 claims description 4
- 102220566102 Antileukoproteinase_L97G_mutation Human genes 0.000 claims description 3
- 102220545207 Carbohydrate sulfotransferase 6_S98W_mutation Human genes 0.000 claims description 3
- 102220498119 Electron transfer flavoprotein subunit beta_S96A_mutation Human genes 0.000 claims description 3
- 102220465272 Interferon alpha-8_N53K_mutation Human genes 0.000 claims description 3
- 102220618489 Isocitrate dehydrogenase [NAD] subunit gamma, mitochondrial_N54Q_mutation Human genes 0.000 claims description 3
- 102220509487 Kinesin-like protein KIF2C_S95E_mutation Human genes 0.000 claims description 3
- 102220637940 Probable cation-transporting ATPase 13A5_S96Y_mutation Human genes 0.000 claims description 3
- 102100023097 Protein S100-A1 Human genes 0.000 claims description 3
- 102220639964 Radial spoke head protein 3 homolog_S56A_mutation Human genes 0.000 claims description 3
- 102220483412 Tensin-2_G99E_mutation Human genes 0.000 claims description 3
- 102220477940 Triggering receptor expressed on myeloid cells 1_T25S_mutation Human genes 0.000 claims description 3
- 102220555263 Tumor protein D52_D52Y_mutation Human genes 0.000 claims description 3
- 102220360203 c.162C>A Human genes 0.000 claims description 3
- 102220358488 c.167G>A Human genes 0.000 claims description 3
- 102220414533 c.292A>C Human genes 0.000 claims description 3
- VTYYLEPIZMXCLO-UHFFFAOYSA-L calcium carbonate Substances [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 claims description 3
- 229940012414 factor viia Drugs 0.000 claims description 3
- 208000014674 injury Diseases 0.000 claims description 3
- 102200090659 rs137852502 Human genes 0.000 claims description 3
- 102220285537 rs1381256979 Human genes 0.000 claims description 3
- 102220112600 rs147886860 Human genes 0.000 claims description 3
- 102200104290 rs1557052294 Human genes 0.000 claims description 3
- 102220253369 rs201879717 Human genes 0.000 claims description 3
- 102200066087 rs267607277 Human genes 0.000 claims description 3
- 102220005551 rs33960522 Human genes 0.000 claims description 3
- 102200118227 rs34579351 Human genes 0.000 claims description 3
- 102200118229 rs34665886 Human genes 0.000 claims description 3
- 102220034241 rs483352780 Human genes 0.000 claims description 3
- 102220075323 rs540745201 Human genes 0.000 claims description 3
- 102200074433 rs587783537 Human genes 0.000 claims description 3
- 102220330013 rs78488639 Human genes 0.000 claims description 3
- 102220093647 rs876661194 Human genes 0.000 claims description 3
- 239000004404 sodium propyl p-hydroxybenzoate Substances 0.000 claims description 3
- 230000008733 trauma Effects 0.000 claims description 3
- 102220588467 von Willebrand factor A domain-containing protein 5B1_S96R_mutation Human genes 0.000 claims description 3
- 102220574236 Ellis-van Creveld syndrome protein_N54A_mutation Human genes 0.000 claims description 2
- 102100040418 Tumor protein D52 Human genes 0.000 claims description 2
- 102220418059 c.283A>G Human genes 0.000 claims description 2
- 102220088953 rs144098296 Human genes 0.000 claims description 2
- 102220277874 rs146221748 Human genes 0.000 claims description 2
- 230000003248 secreting effect Effects 0.000 claims description 2
- 108091007433 antigens Proteins 0.000 abstract description 39
- 102000036639 antigens Human genes 0.000 abstract description 39
- 239000000427 antigen Substances 0.000 abstract description 38
- 230000035602 clotting Effects 0.000 abstract description 22
- 238000004091 panning Methods 0.000 abstract description 15
- 239000011230 binding agent Substances 0.000 abstract description 11
- 230000000903 blocking effect Effects 0.000 abstract description 11
- 238000012216 screening Methods 0.000 abstract description 8
- 230000001225 therapeutic effect Effects 0.000 abstract description 5
- 102000014914 Carrier Proteins Human genes 0.000 abstract description 2
- 108091008324 binding proteins Proteins 0.000 abstract description 2
- 241000880493 Leptailurus serval Species 0.000 description 54
- 230000000694 effects Effects 0.000 description 52
- 210000004027 cell Anatomy 0.000 description 42
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Natural products NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 40
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 34
- 238000002965 ELISA Methods 0.000 description 33
- WLJPJRGQRNCIQS-ZLUOBGJFSA-N Ser-Ser-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O WLJPJRGQRNCIQS-ZLUOBGJFSA-N 0.000 description 29
- XXXAXOWMBOKTRN-XPUUQOCRSA-N Ser-Gly-Val Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O XXXAXOWMBOKTRN-XPUUQOCRSA-N 0.000 description 28
- 108020004414 DNA Proteins 0.000 description 26
- 239000000758 substrate Substances 0.000 description 26
- 238000003556 assay Methods 0.000 description 25
- 108010089804 glycyl-threonine Proteins 0.000 description 24
- 108090000190 Thrombin Proteins 0.000 description 23
- 108010086434 alanyl-seryl-glycine Proteins 0.000 description 23
- 235000001014 amino acid Nutrition 0.000 description 23
- 235000018102 proteins Nutrition 0.000 description 23
- 229960004072 thrombin Drugs 0.000 description 23
- BUDNAJYVCUHLSV-ZLUOBGJFSA-N Ala-Asp-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O BUDNAJYVCUHLSV-ZLUOBGJFSA-N 0.000 description 22
- 230000014509 gene expression Effects 0.000 description 22
- 108010008685 alanyl-glutamyl-aspartic acid Proteins 0.000 description 21
- 229940024606 amino acid Drugs 0.000 description 21
- 108010050848 glycylleucine Proteins 0.000 description 21
- 101000595198 Homo sapiens Podocalyxin Proteins 0.000 description 20
- UIGMAMGZOJVTDN-WHFBIAKZSA-N Ser-Gly-Ser Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O UIGMAMGZOJVTDN-WHFBIAKZSA-N 0.000 description 20
- 230000005764 inhibitory process Effects 0.000 description 20
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 19
- AXKJPUBALUNJEO-UBHSHLNASA-N Ser-Trp-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC(N)=O)C(O)=O AXKJPUBALUNJEO-UBHSHLNASA-N 0.000 description 19
- YDWLCDQXLCILCZ-BWAGICSOSA-N Thr-His-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O YDWLCDQXLCILCZ-BWAGICSOSA-N 0.000 description 19
- NZOCIWKZUVUNDW-ZKWXMUAHSA-N Ile-Gly-Ala Chemical compound CC[C@H](C)[C@H](N)C(=O)NCC(=O)N[C@@H](C)C(O)=O NZOCIWKZUVUNDW-ZKWXMUAHSA-N 0.000 description 18
- 108010069020 alanyl-prolyl-glycine Proteins 0.000 description 18
- 239000000203 mixture Substances 0.000 description 18
- 108010074105 Factor Va Proteins 0.000 description 17
- FDMKYQQYJKYCLV-GUBZILKMSA-N Pro-Pro-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 FDMKYQQYJKYCLV-GUBZILKMSA-N 0.000 description 17
- QYSFWUIXDFJUDW-DCAQKATOSA-N Ser-Leu-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O QYSFWUIXDFJUDW-DCAQKATOSA-N 0.000 description 17
- 239000012634 fragment Substances 0.000 description 17
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 17
- 108090000765 processed proteins & peptides Proteins 0.000 description 17
- YQPFCZVKMUVZIN-AUTRQRHGSA-N Glu-Val-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O YQPFCZVKMUVZIN-AUTRQRHGSA-N 0.000 description 16
- MIIVFRCYJABHTQ-ONGXEEELSA-N Gly-Leu-Val Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O MIIVFRCYJABHTQ-ONGXEEELSA-N 0.000 description 16
- PDUHNKAFQXQNLH-ZETCQYMHSA-N Gly-Lys-Gly Chemical compound NCCCC[C@H](NC(=O)CN)C(=O)NCC(O)=O PDUHNKAFQXQNLH-ZETCQYMHSA-N 0.000 description 16
- WNZOCXUOGVYYBJ-CDMKHQONSA-N Gly-Phe-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)CN)O WNZOCXUOGVYYBJ-CDMKHQONSA-N 0.000 description 16
- FBGXMKUWQFPHFB-JBDRJPRFSA-N Ile-Ser-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N FBGXMKUWQFPHFB-JBDRJPRFSA-N 0.000 description 16
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 16
- FEHQLKKBVJHSEC-SZMVWBNQSA-N Leu-Glu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC(C)C)C(O)=O)=CNC2=C1 FEHQLKKBVJHSEC-SZMVWBNQSA-N 0.000 description 16
- QNBVTHNJGCOVFA-AVGNSLFASA-N Leu-Leu-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CCC(O)=O QNBVTHNJGCOVFA-AVGNSLFASA-N 0.000 description 16
- KIZIOFNVSOSKJI-CIUDSAMLSA-N Leu-Ser-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N KIZIOFNVSOSKJI-CIUDSAMLSA-N 0.000 description 16
- LCNASHSOFMRYFO-WDCWCFNPSA-N Leu-Thr-Gln Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(N)=O LCNASHSOFMRYFO-WDCWCFNPSA-N 0.000 description 16
- YMTLKLXDFCSCNX-BYPYZUCNSA-N Ser-Gly-Gly Chemical compound OC[C@H](N)C(=O)NCC(=O)NCC(O)=O YMTLKLXDFCSCNX-BYPYZUCNSA-N 0.000 description 16
- DJDSEDOKJTZBAR-ZDLURKLDSA-N Thr-Gly-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O DJDSEDOKJTZBAR-ZDLURKLDSA-N 0.000 description 16
- MBLJBGZWLHTJBH-SZMVWBNQSA-N Trp-Val-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 MBLJBGZWLHTJBH-SZMVWBNQSA-N 0.000 description 16
- 108010008355 arginyl-glutamine Proteins 0.000 description 16
- 231100000673 dose–response relationship Toxicity 0.000 description 16
- 108010008671 glycyl-tryptophyl-methionine Proteins 0.000 description 16
- 108010020755 prolyl-glycyl-glycine Proteins 0.000 description 16
- VKKYFICVTYKFIO-CIUDSAMLSA-N Arg-Ala-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N VKKYFICVTYKFIO-CIUDSAMLSA-N 0.000 description 15
- MKJBPDLENBUHQU-CIUDSAMLSA-N Asn-Ser-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O MKJBPDLENBUHQU-CIUDSAMLSA-N 0.000 description 15
- UGXYFDQFLVCDFC-CIUDSAMLSA-N Asn-Ser-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O UGXYFDQFLVCDFC-CIUDSAMLSA-N 0.000 description 15
- JBDLMLZNDRLDIX-HJGDQZAQSA-N Asn-Thr-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O JBDLMLZNDRLDIX-HJGDQZAQSA-N 0.000 description 15
- MNQMTYSEKZHIDF-GCJQMDKQSA-N Asp-Thr-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O MNQMTYSEKZHIDF-GCJQMDKQSA-N 0.000 description 15
- OYTPNWYZORARHL-XHNCKOQMSA-N Gln-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)N)N OYTPNWYZORARHL-XHNCKOQMSA-N 0.000 description 15
- FQCILXROGNOZON-YUMQZZPRSA-N Gln-Pro-Gly Chemical compound NC(=O)CC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O FQCILXROGNOZON-YUMQZZPRSA-N 0.000 description 15
- AXZGZMGRBDQTEY-SRVKXCTJSA-N Leu-Gln-Met Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O AXZGZMGRBDQTEY-SRVKXCTJSA-N 0.000 description 15
- GQZMPWBZQALKJO-UWVGGRQHSA-N Lys-Gly-Arg Chemical compound [H]N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O GQZMPWBZQALKJO-UWVGGRQHSA-N 0.000 description 15
- FGWUALWGCZJQDJ-URLPEUOOSA-N Phe-Thr-Ile Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O FGWUALWGCZJQDJ-URLPEUOOSA-N 0.000 description 15
- QEDMOZUJTGEIBF-FXQIFTODSA-N Ser-Arg-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(O)=O QEDMOZUJTGEIBF-FXQIFTODSA-N 0.000 description 15
- 239000013604 expression vector Substances 0.000 description 15
- 108010070409 phenylalanyl-glycyl-glycine Proteins 0.000 description 15
- YOBGUCWZPXJHTN-BQBZGAKWSA-N Gly-Ser-Arg Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCCN=C(N)N YOBGUCWZPXJHTN-BQBZGAKWSA-N 0.000 description 14
- 208000009292 Hemophilia A Diseases 0.000 description 14
- OWFGFHQMSBTKLX-UFYCRDLUSA-N Val-Tyr-Tyr Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)N OWFGFHQMSBTKLX-UFYCRDLUSA-N 0.000 description 14
- 230000003024 amidolytic effect Effects 0.000 description 14
- 230000001965 increasing effect Effects 0.000 description 14
- 108010064235 lysylglycine Proteins 0.000 description 14
- 108010031719 prolyl-serine Proteins 0.000 description 14
- 238000011282 treatment Methods 0.000 description 14
- YYSWCHMLFJLLBJ-ZLUOBGJFSA-N Ala-Ala-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YYSWCHMLFJLLBJ-ZLUOBGJFSA-N 0.000 description 13
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 13
- QESXLSQLQHHTIX-RHYQMDGZSA-N Leu-Val-Thr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QESXLSQLQHHTIX-RHYQMDGZSA-N 0.000 description 13
- 108010081404 acein-2 Proteins 0.000 description 13
- 238000004925 denaturation Methods 0.000 description 13
- 230000036425 denaturation Effects 0.000 description 13
- 238000004519 manufacturing process Methods 0.000 description 13
- 241000894007 species Species 0.000 description 13
- 108010061238 threonyl-glycine Proteins 0.000 description 13
- 239000013598 vector Substances 0.000 description 13
- QLSRIZIDQXDQHK-RCWTZXSCSA-N Arg-Val-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QLSRIZIDQXDQHK-RCWTZXSCSA-N 0.000 description 12
- VAWNQIGQPUOPQW-ACZMJKKPSA-N Asp-Glu-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O VAWNQIGQPUOPQW-ACZMJKKPSA-N 0.000 description 12
- 101100112922 Candida albicans CDR3 gene Proteins 0.000 description 12
- JXFLPKSDLDEOQK-JHEQGTHGSA-N Gln-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCC(N)=O JXFLPKSDLDEOQK-JHEQGTHGSA-N 0.000 description 12
- OTQSTOXRUBVWAP-NRPADANISA-N Gln-Ser-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O OTQSTOXRUBVWAP-NRPADANISA-N 0.000 description 12
- SBQDRNOLGSYHQA-YUMQZZPRSA-N Lys-Ser-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SBQDRNOLGSYHQA-YUMQZZPRSA-N 0.000 description 12
- 101100435060 Mus musculus Apc gene Proteins 0.000 description 12
- 101100068676 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) gln-1 gene Proteins 0.000 description 12
- 241000283973 Oryctolagus cuniculus Species 0.000 description 12
- JARJPEMLQAWNBR-GUBZILKMSA-N Pro-Asp-Arg Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O JARJPEMLQAWNBR-GUBZILKMSA-N 0.000 description 12
- FWTFAZKJORVTIR-VZFHVOOUSA-N Thr-Ser-Ala Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O FWTFAZKJORVTIR-VZFHVOOUSA-N 0.000 description 12
- HTONZBWRYUKUKC-RCWTZXSCSA-N Val-Thr-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O HTONZBWRYUKUKC-RCWTZXSCSA-N 0.000 description 12
- 239000000872 buffer Substances 0.000 description 12
- 108010060199 cysteinylproline Proteins 0.000 description 12
- 108010057821 leucylproline Proteins 0.000 description 12
- MEFILNJXAVSUTO-JXUBOQSCSA-N Ala-Leu-Thr Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MEFILNJXAVSUTO-JXUBOQSCSA-N 0.000 description 11
- BTRULDJUUVGRNE-DCAQKATOSA-N Ala-Pro-Lys Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O BTRULDJUUVGRNE-DCAQKATOSA-N 0.000 description 11
- LXMKTIZAGIBQRX-HRCADAONSA-N Arg-Phe-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)NC(=O)[C@H](CCCN=C(N)N)N)C(=O)O LXMKTIZAGIBQRX-HRCADAONSA-N 0.000 description 11
- CPYHLXSGDBDULY-IHPCNDPISA-N Asn-Trp-Phe Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O CPYHLXSGDBDULY-IHPCNDPISA-N 0.000 description 11
- ALMIMUZAWTUNIO-BZSNNMDCSA-N Asp-Tyr-Tyr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ALMIMUZAWTUNIO-BZSNNMDCSA-N 0.000 description 11
- KVXVVDFOZNYYKZ-DCAQKATOSA-N Gln-Gln-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O KVXVVDFOZNYYKZ-DCAQKATOSA-N 0.000 description 11
- GWCRIHNSVMOBEQ-BQBZGAKWSA-N Gly-Arg-Ser Chemical compound [H]NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O GWCRIHNSVMOBEQ-BQBZGAKWSA-N 0.000 description 11
- RJVZMGQMJOQIAX-GJZGRUSLSA-N Gly-Trp-Met Chemical compound [H]NCC(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCSC)C(O)=O RJVZMGQMJOQIAX-GJZGRUSLSA-N 0.000 description 11
- 208000031220 Hemophilia Diseases 0.000 description 11
- FOCSWPCHUDVNLP-PMVMPFDFSA-N His-Trp-Tyr Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC3=CC=C(C=C3)O)C(=O)O)NC(=O)[C@H](CC4=CN=CN4)N FOCSWPCHUDVNLP-PMVMPFDFSA-N 0.000 description 11
- 101000924577 Homo sapiens Adenomatous polyposis coli protein Proteins 0.000 description 11
- AFXCXDQNRXTSBD-FJXKBIBVSA-N Pro-Gly-Thr Chemical compound [H]N1CCC[C@H]1C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O AFXCXDQNRXTSBD-FJXKBIBVSA-N 0.000 description 11
- DMNANGOFEUVBRV-GJZGRUSLSA-N Pro-Trp-Gly Chemical compound N([C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)NCC(=O)O)C(=O)[C@@H]1CCCN1 DMNANGOFEUVBRV-GJZGRUSLSA-N 0.000 description 11
- FUMGHWDRRFCKEP-CIUDSAMLSA-N Ser-Leu-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O FUMGHWDRRFCKEP-CIUDSAMLSA-N 0.000 description 11
- MXDOAJQRJBMGMO-FJXKBIBVSA-N Thr-Pro-Gly Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O MXDOAJQRJBMGMO-FJXKBIBVSA-N 0.000 description 11
- 108010052670 arginyl-glutamyl-glutamic acid Proteins 0.000 description 11
- RTZCUEHYUQZIDE-WHFBIAKZSA-N Ala-Ser-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O RTZCUEHYUQZIDE-WHFBIAKZSA-N 0.000 description 10
- MFORDNZDKAVNSR-SRVKXCTJSA-N Gln-Pro-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCC(N)=O MFORDNZDKAVNSR-SRVKXCTJSA-N 0.000 description 10
- PVMPDMIKUVNOBD-CIUDSAMLSA-N Leu-Asp-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O PVMPDMIKUVNOBD-CIUDSAMLSA-N 0.000 description 10
- NRFGTHFONZYFNY-MGHWNKPDSA-N Leu-Ile-Tyr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 NRFGTHFONZYFNY-MGHWNKPDSA-N 0.000 description 10
- RGUXWMDNCPMQFB-YUMQZZPRSA-N Leu-Ser-Gly Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O RGUXWMDNCPMQFB-YUMQZZPRSA-N 0.000 description 10
- SITLTJHOQZFJGG-UHFFFAOYSA-N N-L-alpha-glutamyl-L-valine Natural products CC(C)C(C(O)=O)NC(=O)C(N)CCC(O)=O SITLTJHOQZFJGG-UHFFFAOYSA-N 0.000 description 10
- MCIXMYKSPQUMJG-SRVKXCTJSA-N Phe-Ser-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O MCIXMYKSPQUMJG-SRVKXCTJSA-N 0.000 description 10
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 10
- PZTZYZUTCPZWJH-FXQIFTODSA-N Val-Ser-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PZTZYZUTCPZWJH-FXQIFTODSA-N 0.000 description 10
- 208000034158 bleeding Diseases 0.000 description 10
- 230000002401 inhibitory effect Effects 0.000 description 10
- 239000002773 nucleotide Substances 0.000 description 10
- 125000003729 nucleotide group Chemical group 0.000 description 10
- WQKAQKZRDIZYNV-VZFHVOOUSA-N Ala-Ser-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WQKAQKZRDIZYNV-VZFHVOOUSA-N 0.000 description 9
- JJGRJMKUOYXZRA-LPEHRKFASA-N Asn-Arg-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(=O)N)N)C(=O)O JJGRJMKUOYXZRA-LPEHRKFASA-N 0.000 description 9
- HAOUOFNNJJLVNS-BQBZGAKWSA-N Gly-Pro-Ser Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O HAOUOFNNJJLVNS-BQBZGAKWSA-N 0.000 description 9
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 9
- FUKDBQGFSJUXGX-RWMBFGLXSA-N Lys-Arg-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCCN)N)C(=O)O FUKDBQGFSJUXGX-RWMBFGLXSA-N 0.000 description 9
- HGNRJCINZYHNOU-LURJTMIESA-N Lys-Gly Chemical compound NCCCC[C@H](N)C(=O)NCC(O)=O HGNRJCINZYHNOU-LURJTMIESA-N 0.000 description 9
- HNDMFDBQXYZSRM-IHRRRGAJSA-N Ser-Val-Phe Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O HNDMFDBQXYZSRM-IHRRRGAJSA-N 0.000 description 9
- 230000009471 action Effects 0.000 description 9
- 239000003146 anticoagulant agent Substances 0.000 description 9
- 210000004602 germ cell Anatomy 0.000 description 9
- 102000004196 processed proteins & peptides Human genes 0.000 description 9
- 238000000746 purification Methods 0.000 description 9
- 239000000243 solution Substances 0.000 description 9
- 108010073969 valyllysine Proteins 0.000 description 9
- 102100034540 Adenomatous polyposis coli protein Human genes 0.000 description 8
- CXRCVCURMBFFOL-FXQIFTODSA-N Ala-Ala-Pro Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O CXRCVCURMBFFOL-FXQIFTODSA-N 0.000 description 8
- IOFVWPYSRSCWHI-JXUBOQSCSA-N Ala-Thr-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](C)N IOFVWPYSRSCWHI-JXUBOQSCSA-N 0.000 description 8
- CIMULJZTTOBOPN-WHFBIAKZSA-N Gly-Asn-Asn Chemical compound NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O CIMULJZTTOBOPN-WHFBIAKZSA-N 0.000 description 8
- OIARJGNVARWKFP-YUMQZZPRSA-N Leu-Asn-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O OIARJGNVARWKFP-YUMQZZPRSA-N 0.000 description 8
- UHNQRAFSEBGZFZ-YESZJQIVSA-N Leu-Phe-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N2CCC[C@@H]2C(=O)O)N UHNQRAFSEBGZFZ-YESZJQIVSA-N 0.000 description 8
- AIQWYVFNBNNOLU-RHYQMDGZSA-N Leu-Thr-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O AIQWYVFNBNNOLU-RHYQMDGZSA-N 0.000 description 8
- SIEBDTCABMZCLF-XGEHTFHBSA-N Ser-Val-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SIEBDTCABMZCLF-XGEHTFHBSA-N 0.000 description 8
- SPVHQURZJCUDQC-VOAKCMCISA-N Thr-Lys-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O SPVHQURZJCUDQC-VOAKCMCISA-N 0.000 description 8
- BKVICMPZWRNWOC-RHYQMDGZSA-N Thr-Val-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)[C@@H](C)O BKVICMPZWRNWOC-RHYQMDGZSA-N 0.000 description 8
- UABYBEBXFFNCIR-YDHLFZDLSA-N Tyr-Asp-Val Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O UABYBEBXFFNCIR-YDHLFZDLSA-N 0.000 description 8
- QHDXUYOYTPWCSK-RCOVLWMOSA-N Val-Asp-Gly Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)NCC(=O)O)N QHDXUYOYTPWCSK-RCOVLWMOSA-N 0.000 description 8
- SYSWVVCYSXBVJG-RHYQMDGZSA-N Val-Leu-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N)O SYSWVVCYSXBVJG-RHYQMDGZSA-N 0.000 description 8
- OFTXTCGQJXTNQS-XGEHTFHBSA-N Val-Thr-Ser Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](C(C)C)N)O OFTXTCGQJXTNQS-XGEHTFHBSA-N 0.000 description 8
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 8
- 229940127219 anticoagulant drug Drugs 0.000 description 8
- 238000007820 coagulation assay Methods 0.000 description 8
- 230000007547 defect Effects 0.000 description 8
- UHBYWPGGCSDKFX-VKHMYHEASA-N gamma-carboxy-L-glutamic acid Chemical group OC(=O)[C@@H](N)CC(C(O)=O)C(O)=O UHBYWPGGCSDKFX-VKHMYHEASA-N 0.000 description 8
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 8
- 108010017391 lysylvaline Proteins 0.000 description 8
- 230000001105 regulatory effect Effects 0.000 description 8
- 108010027345 wheylin-1 peptide Proteins 0.000 description 8
- PKYCWFICOKSIHZ-UHFFFAOYSA-N 1-(3,7-dihydroxyphenoxazin-10-yl)ethanone Chemical compound OC1=CC=C2N(C(=O)C)C3=CC=C(O)C=C3OC2=C1 PKYCWFICOKSIHZ-UHFFFAOYSA-N 0.000 description 7
- HHABWQIFXZPZCK-ACZMJKKPSA-N Cys-Gln-Ser Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CS)N HHABWQIFXZPZCK-ACZMJKKPSA-N 0.000 description 7
- 108010079356 FIIa Proteins 0.000 description 7
- KVBPDJIFRQUQFY-ACZMJKKPSA-N Glu-Cys-Ser Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(O)=O KVBPDJIFRQUQFY-ACZMJKKPSA-N 0.000 description 7
- ZLFNNVATRMCAKN-ZKWXMUAHSA-N Ile-Ser-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)NCC(=O)O)N ZLFNNVATRMCAKN-ZKWXMUAHSA-N 0.000 description 7
- ILDSIMPXNFWKLH-KATARQTJSA-N Leu-Thr-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O ILDSIMPXNFWKLH-KATARQTJSA-N 0.000 description 7
- 229920001213 Polysorbate 20 Polymers 0.000 description 7
- CGBYDGAJHSOGFQ-LPEHRKFASA-N Pro-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@@H]2CCCN2 CGBYDGAJHSOGFQ-LPEHRKFASA-N 0.000 description 7
- IHCXPSYCHXFXKT-DCAQKATOSA-N Pro-Arg-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O IHCXPSYCHXFXKT-DCAQKATOSA-N 0.000 description 7
- OOKCGAYXSNJBGQ-ZLUOBGJFSA-N Ser-Asn-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O OOKCGAYXSNJBGQ-ZLUOBGJFSA-N 0.000 description 7
- VGNYHOBZJKWRGI-CIUDSAMLSA-N Ser-Asn-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CO VGNYHOBZJKWRGI-CIUDSAMLSA-N 0.000 description 7
- HJWLQSFTGDQSRX-BPUTZDHNSA-N Trp-Met-Ser Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CO)C(O)=O HJWLQSFTGDQSRX-BPUTZDHNSA-N 0.000 description 7
- VFJIWSJKZJTQII-SRVKXCTJSA-N Tyr-Asp-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O VFJIWSJKZJTQII-SRVKXCTJSA-N 0.000 description 7
- NHOVZGFNTGMYMI-KKUMJFAQSA-N Tyr-Ser-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 NHOVZGFNTGMYMI-KKUMJFAQSA-N 0.000 description 7
- 108010047495 alanylglycine Proteins 0.000 description 7
- 239000011575 calcium Substances 0.000 description 7
- 108010078144 glutaminyl-glycine Proteins 0.000 description 7
- VPZXBVLAVMBEQI-UHFFFAOYSA-N glycyl-DL-alpha-alanine Natural products OC(=O)C(C)NC(=O)CN VPZXBVLAVMBEQI-UHFFFAOYSA-N 0.000 description 7
- 239000002609 medium Substances 0.000 description 7
- 239000013642 negative control Substances 0.000 description 7
- 230000036961 partial effect Effects 0.000 description 7
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 7
- 229920001184 polypeptide Polymers 0.000 description 7
- 239000006228 supernatant Substances 0.000 description 7
- OZFAFGSSMRRTDW-UHFFFAOYSA-N (2,4-dichlorophenyl) benzenesulfonate Chemical compound ClC1=CC(Cl)=CC=C1OS(=O)(=O)C1=CC=CC=C1 OZFAFGSSMRRTDW-UHFFFAOYSA-N 0.000 description 6
- XQGIRPGAVLFKBJ-CIUDSAMLSA-N Ala-Asn-Lys Chemical compound N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)O XQGIRPGAVLFKBJ-CIUDSAMLSA-N 0.000 description 6
- MNZHHDPWDWQJCQ-YUMQZZPRSA-N Ala-Leu-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O MNZHHDPWDWQJCQ-YUMQZZPRSA-N 0.000 description 6
- WQLDNOCHHRISMS-NAKRPEOUSA-N Ala-Pro-Ile Chemical compound [H]N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(O)=O WQLDNOCHHRISMS-NAKRPEOUSA-N 0.000 description 6
- FFZJHQODAYHGPO-KZVJFYERSA-N Ala-Pro-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N FFZJHQODAYHGPO-KZVJFYERSA-N 0.000 description 6
- OMSKGWFGWCQFBD-KZVJFYERSA-N Ala-Val-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OMSKGWFGWCQFBD-KZVJFYERSA-N 0.000 description 6
- PUUPMDXIHCOPJU-HJGDQZAQSA-N Asn-Thr-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)N)N)O PUUPMDXIHCOPJU-HJGDQZAQSA-N 0.000 description 6
- SNDBKTFJWVEVPO-WHFBIAKZSA-N Asp-Gly-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(O)=O SNDBKTFJWVEVPO-WHFBIAKZSA-N 0.000 description 6
- JSHWXQIZOCVWIA-ZKWXMUAHSA-N Asp-Ser-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O JSHWXQIZOCVWIA-ZKWXMUAHSA-N 0.000 description 6
- OHLLDUNVMPPUMD-DCAQKATOSA-N Cys-Leu-Val Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H](CS)N OHLLDUNVMPPUMD-DCAQKATOSA-N 0.000 description 6
- 239000012591 Dulbecco’s Phosphate Buffered Saline Substances 0.000 description 6
- DHNWZLGBTPUTQQ-QEJZJMRPSA-N Gln-Asp-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)N)N DHNWZLGBTPUTQQ-QEJZJMRPSA-N 0.000 description 6
- XKPACHRGOWQHFH-IRIUXVKKSA-N Gln-Thr-Tyr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O XKPACHRGOWQHFH-IRIUXVKKSA-N 0.000 description 6
- HNAUFGBKJLTWQE-IFFSRLJSSA-N Gln-Val-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CCC(=O)N)N)O HNAUFGBKJLTWQE-IFFSRLJSSA-N 0.000 description 6
- DNPCBMNFQVTHMA-DCAQKATOSA-N Glu-Leu-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O DNPCBMNFQVTHMA-DCAQKATOSA-N 0.000 description 6
- UQJNXZSSGQIPIQ-FBCQKBJTSA-N Gly-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)CN UQJNXZSSGQIPIQ-FBCQKBJTSA-N 0.000 description 6
- FULZDMOZUZKGQU-ONGXEEELSA-N Gly-Val-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)CN FULZDMOZUZKGQU-ONGXEEELSA-N 0.000 description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- ZPVJJPAIUZLSNE-DCAQKATOSA-N His-Arg-Ser Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O ZPVJJPAIUZLSNE-DCAQKATOSA-N 0.000 description 6
- AKEDPWJFQULLPE-IUCAKERBSA-N His-Glu-Gly Chemical compound N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O AKEDPWJFQULLPE-IUCAKERBSA-N 0.000 description 6
- JZNVOBUNTWNZPW-GHCJXIJMSA-N Ile-Ser-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(=O)O)C(=O)O)N JZNVOBUNTWNZPW-GHCJXIJMSA-N 0.000 description 6
- 108060003951 Immunoglobulin Proteins 0.000 description 6
- WSGXUIQTEZDVHJ-GARJFASQSA-N Leu-Ala-Pro Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@@H]1C(O)=O WSGXUIQTEZDVHJ-GARJFASQSA-N 0.000 description 6
- SBANPBVRHYIMRR-UHFFFAOYSA-N Leu-Ser-Pro Natural products CC(C)CC(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O SBANPBVRHYIMRR-UHFFFAOYSA-N 0.000 description 6
- BRTVHXHCUSXYRI-CIUDSAMLSA-N Leu-Ser-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O BRTVHXHCUSXYRI-CIUDSAMLSA-N 0.000 description 6
- VUBIPAHVHMZHCM-KKUMJFAQSA-N Leu-Tyr-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CC1=CC=C(O)C=C1 VUBIPAHVHMZHCM-KKUMJFAQSA-N 0.000 description 6
- MPGHETGWWWUHPY-CIUDSAMLSA-N Lys-Ala-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN MPGHETGWWWUHPY-CIUDSAMLSA-N 0.000 description 6
- XFIHDSBIPWEYJJ-YUMQZZPRSA-N Lys-Ala-Gly Chemical compound OC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN XFIHDSBIPWEYJJ-YUMQZZPRSA-N 0.000 description 6
- NTBFKPBULZGXQL-KKUMJFAQSA-N Lys-Asp-Tyr Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 NTBFKPBULZGXQL-KKUMJFAQSA-N 0.000 description 6
- OIQSIMFSVLLWBX-VOAKCMCISA-N Lys-Leu-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OIQSIMFSVLLWBX-VOAKCMCISA-N 0.000 description 6
- YTJFXEDRUOQGSP-DCAQKATOSA-N Lys-Pro-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O YTJFXEDRUOQGSP-DCAQKATOSA-N 0.000 description 6
- VHTOGMKQXXJOHG-RHYQMDGZSA-N Lys-Thr-Val Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O VHTOGMKQXXJOHG-RHYQMDGZSA-N 0.000 description 6
- AJHCSUXXECOXOY-UHFFFAOYSA-N N-glycyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)CN)C(O)=O)=CNC2=C1 AJHCSUXXECOXOY-UHFFFAOYSA-N 0.000 description 6
- 108091034117 Oligonucleotide Proteins 0.000 description 6
- 238000012408 PCR amplification Methods 0.000 description 6
- JDMKQHSHKJHAHR-UHFFFAOYSA-N Phe-Phe-Leu-Tyr Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)CC1=CC=CC=C1 JDMKQHSHKJHAHR-UHFFFAOYSA-N 0.000 description 6
- JLLJTMHNXQTMCK-UBHSHLNASA-N Phe-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC1=CC=CC=C1 JLLJTMHNXQTMCK-UBHSHLNASA-N 0.000 description 6
- QARPMYDMYVLFMW-KKUMJFAQSA-N Phe-Pro-Glu Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(O)=O)C1=CC=CC=C1 QARPMYDMYVLFMW-KKUMJFAQSA-N 0.000 description 6
- UAYHMOIGIQZLFR-NHCYSSNCSA-N Pro-Gln-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O UAYHMOIGIQZLFR-NHCYSSNCSA-N 0.000 description 6
- VDGTVWFMRXVQCT-GUBZILKMSA-N Pro-Glu-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1 VDGTVWFMRXVQCT-GUBZILKMSA-N 0.000 description 6
- HWLKHNDRXWTFTN-GUBZILKMSA-N Pro-Pro-Cys Chemical compound C1C[C@H](NC1)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CS)C(=O)O HWLKHNDRXWTFTN-GUBZILKMSA-N 0.000 description 6
- MKGIILKDUGDRRO-FXQIFTODSA-N Pro-Ser-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1 MKGIILKDUGDRRO-FXQIFTODSA-N 0.000 description 6
- 108010076504 Protein Sorting Signals Proteins 0.000 description 6
- UQFYNFTYDHUIMI-WHFBIAKZSA-N Ser-Gly-Ala Chemical compound OC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](N)CO UQFYNFTYDHUIMI-WHFBIAKZSA-N 0.000 description 6
- YUJLIIRMIAGMCQ-CIUDSAMLSA-N Ser-Leu-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YUJLIIRMIAGMCQ-CIUDSAMLSA-N 0.000 description 6
- MUJQWSAWLLRJCE-KATARQTJSA-N Ser-Leu-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MUJQWSAWLLRJCE-KATARQTJSA-N 0.000 description 6
- BYCVMHKULKRVPV-GUBZILKMSA-N Ser-Lys-Gln Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O BYCVMHKULKRVPV-GUBZILKMSA-N 0.000 description 6
- SRSPTFBENMJHMR-WHFBIAKZSA-N Ser-Ser-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SRSPTFBENMJHMR-WHFBIAKZSA-N 0.000 description 6
- CUXJENOFJXOSOZ-BIIVOSGPSA-N Ser-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CO)N)C(=O)O CUXJENOFJXOSOZ-BIIVOSGPSA-N 0.000 description 6
- OLKICIBQRVSQMA-SRVKXCTJSA-N Ser-Ser-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O OLKICIBQRVSQMA-SRVKXCTJSA-N 0.000 description 6
- SNXUIBACCONSOH-BWBBJGPYSA-N Ser-Thr-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CO)C(O)=O SNXUIBACCONSOH-BWBBJGPYSA-N 0.000 description 6
- BDMWLJLPPUCLNV-XGEHTFHBSA-N Ser-Thr-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O BDMWLJLPPUCLNV-XGEHTFHBSA-N 0.000 description 6
- PLQWGQUNUPMNOD-KKUMJFAQSA-N Ser-Tyr-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(O)=O PLQWGQUNUPMNOD-KKUMJFAQSA-N 0.000 description 6
- RCOUFINCYASMDN-GUBZILKMSA-N Ser-Val-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCSC)C(O)=O RCOUFINCYASMDN-GUBZILKMSA-N 0.000 description 6
- FIFDDJFLNVAVMS-RHYQMDGZSA-N Thr-Leu-Met Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(O)=O FIFDDJFLNVAVMS-RHYQMDGZSA-N 0.000 description 6
- MROIJTGJGIDEEJ-RCWTZXSCSA-N Thr-Pro-Pro Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 MROIJTGJGIDEEJ-RCWTZXSCSA-N 0.000 description 6
- ZESGVALRVJIVLZ-VFCFLDTKSA-N Thr-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@@H]1C(=O)O)N)O ZESGVALRVJIVLZ-VFCFLDTKSA-N 0.000 description 6
- DQDXHYIEITXNJY-BPUTZDHNSA-N Trp-Gln-Gln Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N DQDXHYIEITXNJY-BPUTZDHNSA-N 0.000 description 6
- ULHASJWZGUEUNN-XIRDDKMYSA-N Trp-Lys-Ser Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O ULHASJWZGUEUNN-XIRDDKMYSA-N 0.000 description 6
- LGEYOIQBBIPHQN-UWJYBYFXSA-N Tyr-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 LGEYOIQBBIPHQN-UWJYBYFXSA-N 0.000 description 6
- SZEIFUXUTBBQFQ-STQMWFEESA-N Tyr-Pro-Gly Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O SZEIFUXUTBBQFQ-STQMWFEESA-N 0.000 description 6
- KWKJGBHDYJOVCR-SRVKXCTJSA-N Tyr-Ser-Cys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N)O KWKJGBHDYJOVCR-SRVKXCTJSA-N 0.000 description 6
- ZLFHAAGHGQBQQN-GUBZILKMSA-N Val-Ala-Pro Natural products CC(C)[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O ZLFHAAGHGQBQQN-GUBZILKMSA-N 0.000 description 6
- VJOWWOGRNXRQMF-UVBJJODRSA-N Val-Ala-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)C(C)C)C(O)=O)=CNC2=C1 VJOWWOGRNXRQMF-UVBJJODRSA-N 0.000 description 6
- BMGOFDMKDVVGJG-NHCYSSNCSA-N Val-Asp-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N BMGOFDMKDVVGJG-NHCYSSNCSA-N 0.000 description 6
- LHADRQBREKTRLR-DCAQKATOSA-N Val-Cys-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](C(C)C)N LHADRQBREKTRLR-DCAQKATOSA-N 0.000 description 6
- XWYUBUYQMOUFRQ-IFFSRLJSSA-N Val-Glu-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](C(C)C)N)O XWYUBUYQMOUFRQ-IFFSRLJSSA-N 0.000 description 6
- UEHRGZCNLSWGHK-DLOVCJGASA-N Val-Glu-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O UEHRGZCNLSWGHK-DLOVCJGASA-N 0.000 description 6
- HGJRMXOWUWVUOA-GVXVVHGQSA-N Val-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](C(C)C)N HGJRMXOWUWVUOA-GVXVVHGQSA-N 0.000 description 6
- UMPVMAYCLYMYGA-ONGXEEELSA-N Val-Leu-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O UMPVMAYCLYMYGA-ONGXEEELSA-N 0.000 description 6
- CKTMJBPRVQWPHU-JSGCOSHPSA-N Val-Phe-Gly Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)O)N CKTMJBPRVQWPHU-JSGCOSHPSA-N 0.000 description 6
- VCIYTVOBLZHFSC-XHSDSOJGSA-N Val-Phe-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N2CCC[C@@H]2C(=O)O)N VCIYTVOBLZHFSC-XHSDSOJGSA-N 0.000 description 6
- KRAHMIJVUPUOTQ-DCAQKATOSA-N Val-Ser-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N KRAHMIJVUPUOTQ-DCAQKATOSA-N 0.000 description 6
- LLJLBRRXKZTTRD-GUBZILKMSA-N Val-Val-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)O)N LLJLBRRXKZTTRD-GUBZILKMSA-N 0.000 description 6
- 108010087924 alanylproline Proteins 0.000 description 6
- 108010050025 alpha-glutamyltryptophan Proteins 0.000 description 6
- 150000001408 amides Chemical class 0.000 description 6
- 125000000539 amino acid group Chemical group 0.000 description 6
- 108010069205 aspartyl-phenylalanine Proteins 0.000 description 6
- 108010068265 aspartyltyrosine Proteins 0.000 description 6
- 230000009260 cross reactivity Effects 0.000 description 6
- 230000001120 cytoprotective effect Effects 0.000 description 6
- 210000002889 endothelial cell Anatomy 0.000 description 6
- 108010000434 glycyl-alanyl-leucine Proteins 0.000 description 6
- 108010027668 glycyl-alanyl-valine Proteins 0.000 description 6
- 108010050475 glycyl-leucyl-tyrosine Proteins 0.000 description 6
- 108010015792 glycyllysine Proteins 0.000 description 6
- 239000001963 growth medium Substances 0.000 description 6
- 108010085325 histidylproline Proteins 0.000 description 6
- 102000018358 immunoglobulin Human genes 0.000 description 6
- 230000002779 inactivation Effects 0.000 description 6
- 239000003112 inhibitor Substances 0.000 description 6
- 108010073472 leucyl-prolyl-proline Proteins 0.000 description 6
- 108010025153 lysyl-alanyl-alanine Proteins 0.000 description 6
- 108010009298 lysylglutamic acid Proteins 0.000 description 6
- 108010024654 phenylalanyl-prolyl-alanine Proteins 0.000 description 6
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 6
- 230000003389 potentiating effect Effects 0.000 description 6
- 238000002360 preparation method Methods 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- 239000000047 product Substances 0.000 description 6
- 108010077112 prolyl-proline Proteins 0.000 description 6
- 108010090894 prolylleucine Proteins 0.000 description 6
- 239000011541 reaction mixture Substances 0.000 description 6
- 230000002829 reductive effect Effects 0.000 description 6
- 239000011550 stock solution Substances 0.000 description 6
- 108010052774 valyl-lysyl-glycyl-phenylalanyl-tyrosine Proteins 0.000 description 6
- IESDGNYHXIOKRW-YXMSTPNBSA-N (2s)-2-[[(2s)-1-[(2s)-6-amino-2-[[(2s,3r)-2-amino-3-hydroxybutanoyl]amino]hexanoyl]pyrrolidine-2-carbonyl]amino]-5-(diaminomethylideneamino)pentanoic acid Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O IESDGNYHXIOKRW-YXMSTPNBSA-N 0.000 description 5
- DIBLBAURNYJYBF-XLXZRNDBSA-N (2s)-2-[[(2s)-2-[[2-[[(2s)-6-amino-2-[[(2s)-2-amino-3-methylbutanoyl]amino]hexanoyl]amino]acetyl]amino]-3-phenylpropanoyl]amino]-3-(4-hydroxyphenyl)propanoic acid Chemical compound C([C@H](NC(=O)CNC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=CC=C1 DIBLBAURNYJYBF-XLXZRNDBSA-N 0.000 description 5
- SLKLLQWZQHXYSV-CIUDSAMLSA-N Asn-Ala-Lys Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O SLKLLQWZQHXYSV-CIUDSAMLSA-N 0.000 description 5
- LGCVSPFCFXWUEY-IHPCNDPISA-N Asn-Trp-Tyr Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC3=CC=C(C=C3)O)C(=O)O)NC(=O)[C@H](CC(=O)N)N LGCVSPFCFXWUEY-IHPCNDPISA-N 0.000 description 5
- AHWRSSLYSGLBGD-CIUDSAMLSA-N Asp-Pro-Glu Chemical compound OC(=O)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O AHWRSSLYSGLBGD-CIUDSAMLSA-N 0.000 description 5
- 102100023804 Coagulation factor VII Human genes 0.000 description 5
- ZXCAQANTQWBICD-DCAQKATOSA-N Cys-Lys-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CS)N ZXCAQANTQWBICD-DCAQKATOSA-N 0.000 description 5
- BCFXQBXXDSEHRS-FXQIFTODSA-N Cys-Ser-Arg Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O BCFXQBXXDSEHRS-FXQIFTODSA-N 0.000 description 5
- 108010023321 Factor VII Proteins 0.000 description 5
- RZSLYUUFFVHFRQ-FXQIFTODSA-N Gln-Ala-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O RZSLYUUFFVHFRQ-FXQIFTODSA-N 0.000 description 5
- GHAXJVNBAKGWEJ-AVGNSLFASA-N Gln-Ser-Tyr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O GHAXJVNBAKGWEJ-AVGNSLFASA-N 0.000 description 5
- FITIQFSXXBKFFM-NRPADANISA-N Gln-Val-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O FITIQFSXXBKFFM-NRPADANISA-N 0.000 description 5
- ITYRYNUZHPNCIK-GUBZILKMSA-N Glu-Ala-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O ITYRYNUZHPNCIK-GUBZILKMSA-N 0.000 description 5
- GLWXKFRTOHKGIT-ACZMJKKPSA-N Glu-Asn-Asn Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O GLWXKFRTOHKGIT-ACZMJKKPSA-N 0.000 description 5
- HNVFSTLPVJWIDV-CIUDSAMLSA-N Glu-Glu-Gln Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O HNVFSTLPVJWIDV-CIUDSAMLSA-N 0.000 description 5
- ALMBZBOCGSVSAI-ACZMJKKPSA-N Glu-Ser-Asn Chemical compound C(CC(=O)O)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(=O)N)C(=O)O)N ALMBZBOCGSVSAI-ACZMJKKPSA-N 0.000 description 5
- MFYLRRCYBBJYPI-JYJNAYRXSA-N Glu-Tyr-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N)O MFYLRRCYBBJYPI-JYJNAYRXSA-N 0.000 description 5
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 5
- XCLCVBYNGXEVDU-WHFBIAKZSA-N Gly-Asn-Ser Chemical compound NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O XCLCVBYNGXEVDU-WHFBIAKZSA-N 0.000 description 5
- GRIRDMVMJJDZKV-RCOVLWMOSA-N Gly-Asn-Val Chemical compound [H]NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O GRIRDMVMJJDZKV-RCOVLWMOSA-N 0.000 description 5
- PABFFPWEJMEVEC-JGVFFNPUSA-N Gly-Gln-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCC(=O)N)NC(=O)CN)C(=O)O PABFFPWEJMEVEC-JGVFFNPUSA-N 0.000 description 5
- UUYBFNKHOCJCHT-VHSXEESVSA-N Gly-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN UUYBFNKHOCJCHT-VHSXEESVSA-N 0.000 description 5
- FFJQHWKSGAWSTJ-BFHQHQDPSA-N Gly-Thr-Ala Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O FFJQHWKSGAWSTJ-BFHQHQDPSA-N 0.000 description 5
- GNNJKUYDWFIBTK-QWRGUYRKSA-N Gly-Tyr-Asp Chemical compound [H]NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(O)=O)C(O)=O GNNJKUYDWFIBTK-QWRGUYRKSA-N 0.000 description 5
- WMKXFMUJRCEGRP-SRVKXCTJSA-N His-Asn-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)N WMKXFMUJRCEGRP-SRVKXCTJSA-N 0.000 description 5
- MKWSZEHGHSLNPF-NAKRPEOUSA-N Ile-Ala-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)O)N MKWSZEHGHSLNPF-NAKRPEOUSA-N 0.000 description 5
- 108010065920 Insulin Lispro Proteins 0.000 description 5
- UGTHTQWIQKEDEH-BQBZGAKWSA-N L-alanyl-L-prolylglycine zwitterion Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O UGTHTQWIQKEDEH-BQBZGAKWSA-N 0.000 description 5
- CFVQPNSCQMKDPB-CIUDSAMLSA-N Lys-Cys-Cys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CS)C(=O)O)N CFVQPNSCQMKDPB-CIUDSAMLSA-N 0.000 description 5
- XNKDCYABMBBEKN-IUCAKERBSA-N Lys-Gly-Gln Chemical compound NCCCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCC(N)=O XNKDCYABMBBEKN-IUCAKERBSA-N 0.000 description 5
- WQDKIVRHTQYJSN-DCAQKATOSA-N Lys-Ser-Arg Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N WQDKIVRHTQYJSN-DCAQKATOSA-N 0.000 description 5
- IOQWIOPSKJOEKI-SRVKXCTJSA-N Lys-Ser-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O IOQWIOPSKJOEKI-SRVKXCTJSA-N 0.000 description 5
- DLCAXBGXGOVUCD-PPCPHDFISA-N Lys-Thr-Ile Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O DLCAXBGXGOVUCD-PPCPHDFISA-N 0.000 description 5
- OBVHKUFUDCPZDW-JYJNAYRXSA-N Met-Arg-Phe Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 OBVHKUFUDCPZDW-JYJNAYRXSA-N 0.000 description 5
- 108010079364 N-glycylalanine Proteins 0.000 description 5
- CDNPIRSCAFMMBE-SRVKXCTJSA-N Phe-Asn-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O CDNPIRSCAFMMBE-SRVKXCTJSA-N 0.000 description 5
- MQVFHOPCKNTHGT-MELADBBJSA-N Phe-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC2=CC=CC=C2)N)C(=O)O MQVFHOPCKNTHGT-MELADBBJSA-N 0.000 description 5
- QPVFUAUFEBPIPT-CDMKHQONSA-N Phe-Gly-Thr Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O QPVFUAUFEBPIPT-CDMKHQONSA-N 0.000 description 5
- MSHZERMPZKCODG-ACRUOGEOSA-N Phe-Leu-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 MSHZERMPZKCODG-ACRUOGEOSA-N 0.000 description 5
- IIEOLPMQYRBZCN-SRVKXCTJSA-N Phe-Ser-Cys Chemical compound N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O IIEOLPMQYRBZCN-SRVKXCTJSA-N 0.000 description 5
- RETPETNFPLNLRV-JYJNAYRXSA-N Pro-Asn-Trp Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O RETPETNFPLNLRV-JYJNAYRXSA-N 0.000 description 5
- VPEVBAUSTBWQHN-NHCYSSNCSA-N Pro-Glu-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O VPEVBAUSTBWQHN-NHCYSSNCSA-N 0.000 description 5
- ZLXKLMHAMDENIO-DCAQKATOSA-N Pro-Lys-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(O)=O ZLXKLMHAMDENIO-DCAQKATOSA-N 0.000 description 5
- PCWLNNZTBJTZRN-AVGNSLFASA-N Pro-Pro-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 PCWLNNZTBJTZRN-AVGNSLFASA-N 0.000 description 5
- GMJDSFYVTAMIBF-FXQIFTODSA-N Pro-Ser-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O GMJDSFYVTAMIBF-FXQIFTODSA-N 0.000 description 5
- PRKWBYCXBBSLSK-GUBZILKMSA-N Pro-Ser-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O PRKWBYCXBBSLSK-GUBZILKMSA-N 0.000 description 5
- XDKKMRPRRCOELJ-GUBZILKMSA-N Pro-Val-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C(C)C)NC(=O)[C@@H]1CCCN1 XDKKMRPRRCOELJ-GUBZILKMSA-N 0.000 description 5
- YQHZVYJAGWMHES-ZLUOBGJFSA-N Ser-Ala-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YQHZVYJAGWMHES-ZLUOBGJFSA-N 0.000 description 5
- OYEDZGNMSBZCIM-XGEHTFHBSA-N Ser-Arg-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OYEDZGNMSBZCIM-XGEHTFHBSA-N 0.000 description 5
- SFTZWNJFZYOLBD-ZDLURKLDSA-N Ser-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO SFTZWNJFZYOLBD-ZDLURKLDSA-N 0.000 description 5
- CXBFHZLODKPIJY-AAEUAGOBSA-N Ser-Gly-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CO)N CXBFHZLODKPIJY-AAEUAGOBSA-N 0.000 description 5
- LRZLZIUXQBIWTB-KATARQTJSA-N Ser-Lys-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O LRZLZIUXQBIWTB-KATARQTJSA-N 0.000 description 5
- XJDMUQCLVSCRSJ-VZFHVOOUSA-N Ser-Thr-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O XJDMUQCLVSCRSJ-VZFHVOOUSA-N 0.000 description 5
- ODSAPYVQSLDRSR-LKXGYXEUSA-N Thr-Cys-Asn Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(O)=O ODSAPYVQSLDRSR-LKXGYXEUSA-N 0.000 description 5
- ASJDFGOPDCVXTG-KATARQTJSA-N Thr-Cys-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O ASJDFGOPDCVXTG-KATARQTJSA-N 0.000 description 5
- DSLHSTIUAPKERR-XGEHTFHBSA-N Thr-Cys-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(O)=O DSLHSTIUAPKERR-XGEHTFHBSA-N 0.000 description 5
- KCRQEJSKXAIULJ-FJXKBIBVSA-N Thr-Gly-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O KCRQEJSKXAIULJ-FJXKBIBVSA-N 0.000 description 5
- NCXVJIQMWSGRHY-KXNHARMFSA-N Thr-Leu-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N)O NCXVJIQMWSGRHY-KXNHARMFSA-N 0.000 description 5
- WRQLCVIALDUQEQ-UNQGMJICSA-N Thr-Phe-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O WRQLCVIALDUQEQ-UNQGMJICSA-N 0.000 description 5
- WPSKTVVMQCXPRO-BWBBJGPYSA-N Thr-Ser-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O WPSKTVVMQCXPRO-BWBBJGPYSA-N 0.000 description 5
- KPMIQCXJDVKWKO-IFFSRLJSSA-N Thr-Val-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O KPMIQCXJDVKWKO-IFFSRLJSSA-N 0.000 description 5
- 108010079274 Thrombomodulin Proteins 0.000 description 5
- 102000012607 Thrombomodulin Human genes 0.000 description 5
- SINRIKQYQJRGDQ-MEYUZBJRSA-N Tyr-Lys-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 SINRIKQYQJRGDQ-MEYUZBJRSA-N 0.000 description 5
- RIVVDNTUSRVTQT-IRIUXVKKSA-N Tyr-Thr-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N)O RIVVDNTUSRVTQT-IRIUXVKKSA-N 0.000 description 5
- ANHVRCNNGJMJNG-BZSNNMDCSA-N Tyr-Tyr-Cys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)N[C@@H](CS)C(=O)O)N)O ANHVRCNNGJMJNG-BZSNNMDCSA-N 0.000 description 5
- ZQGPWORGSNRQLN-NHCYSSNCSA-N Val-Asp-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N ZQGPWORGSNRQLN-NHCYSSNCSA-N 0.000 description 5
- JXGWQYWDUOWQHA-DZKIICNBSA-N Val-Gln-Phe Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N JXGWQYWDUOWQHA-DZKIICNBSA-N 0.000 description 5
- AHHJARQXFFGOKF-NRPADANISA-N Val-Glu-Cys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CS)C(=O)O)N AHHJARQXFFGOKF-NRPADANISA-N 0.000 description 5
- UGFMVXRXULGLNO-XPUUQOCRSA-N Val-Ser-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O UGFMVXRXULGLNO-XPUUQOCRSA-N 0.000 description 5
- ZLNYBMWGPOKSLW-LSJOCFKGSA-N Val-Val-Asp Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O ZLNYBMWGPOKSLW-LSJOCFKGSA-N 0.000 description 5
- SSKKGOWRPNIVDW-AVGNSLFASA-N Val-Val-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N SSKKGOWRPNIVDW-AVGNSLFASA-N 0.000 description 5
- 108010013835 arginine glutamate Proteins 0.000 description 5
- 108010059459 arginyl-threonyl-phenylalanine Proteins 0.000 description 5
- 239000013078 crystal Substances 0.000 description 5
- 230000000593 degrading effect Effects 0.000 description 5
- 238000012217 deletion Methods 0.000 description 5
- 230000037430 deletion Effects 0.000 description 5
- 230000001419 dependent effect Effects 0.000 description 5
- 229940012413 factor vii Drugs 0.000 description 5
- 108010063718 gamma-glutamylaspartic acid Proteins 0.000 description 5
- 239000008103 glucose Substances 0.000 description 5
- 238000001727 in vivo Methods 0.000 description 5
- 238000003780 insertion Methods 0.000 description 5
- 230000037431 insertion Effects 0.000 description 5
- 108010070643 prolylglutamic acid Proteins 0.000 description 5
- 108010048397 seryl-lysyl-leucine Proteins 0.000 description 5
- 238000001542 size-exclusion chromatography Methods 0.000 description 5
- 239000011780 sodium chloride Substances 0.000 description 5
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 5
- 108010080629 tryptophan-leucine Proteins 0.000 description 5
- VNYMOTCMNHJGTG-JBDRJPRFSA-N Ala-Ile-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O VNYMOTCMNHJGTG-JBDRJPRFSA-N 0.000 description 4
- ATABBWFGOHKROJ-GUBZILKMSA-N Arg-Pro-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O ATABBWFGOHKROJ-GUBZILKMSA-N 0.000 description 4
- ISJWBVIYRBAXEB-CIUDSAMLSA-N Arg-Ser-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(O)=O ISJWBVIYRBAXEB-CIUDSAMLSA-N 0.000 description 4
- MRQQMVZUHXUPEV-IHRRRGAJSA-N Asp-Arg-Phe Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O MRQQMVZUHXUPEV-IHRRRGAJSA-N 0.000 description 4
- 102000009839 Endothelial Protein C Receptor Human genes 0.000 description 4
- 108010009900 Endothelial Protein C Receptor Proteins 0.000 description 4
- 102000010911 Enzyme Precursors Human genes 0.000 description 4
- 108010062466 Enzyme Precursors Proteins 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- 241000588724 Escherichia coli Species 0.000 description 4
- LJEPDHWNQXPXMM-NHCYSSNCSA-N Gln-Arg-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(O)=O LJEPDHWNQXPXMM-NHCYSSNCSA-N 0.000 description 4
- ZBKUIQNCRIYVGH-SDDRHHMPSA-N Gln-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)N)N ZBKUIQNCRIYVGH-SDDRHHMPSA-N 0.000 description 4
- WZZSKAJIHTUUSG-ACZMJKKPSA-N Glu-Ala-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCC(O)=O WZZSKAJIHTUUSG-ACZMJKKPSA-N 0.000 description 4
- JJKKWYQVHRUSDG-GUBZILKMSA-N Glu-Ala-Lys Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O JJKKWYQVHRUSDG-GUBZILKMSA-N 0.000 description 4
- KASDBWKLWJKTLJ-GUBZILKMSA-N Glu-Glu-Met Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(O)=O KASDBWKLWJKTLJ-GUBZILKMSA-N 0.000 description 4
- YWAQATDNEKZFFK-BYPYZUCNSA-N Gly-Gly-Ser Chemical compound NCC(=O)NCC(=O)N[C@@H](CO)C(O)=O YWAQATDNEKZFFK-BYPYZUCNSA-N 0.000 description 4
- NSTUFLGQJCOCDL-UWVGGRQHSA-N Gly-Leu-Arg Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N NSTUFLGQJCOCDL-UWVGGRQHSA-N 0.000 description 4
- YGHSQRJSHKYUJY-SCZZXKLOSA-N Gly-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN YGHSQRJSHKYUJY-SCZZXKLOSA-N 0.000 description 4
- 108010054964 H-hexahydrotyrosyl-alanyl-arginine-4-nitroanilide Proteins 0.000 description 4
- 208000024659 Hemostatic disease Diseases 0.000 description 4
- 101500025568 Homo sapiens Saposin-D Proteins 0.000 description 4
- SLQVFYWBGNNOTK-BYULHYEWSA-N Ile-Gly-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)NCC(=O)N[C@@H](CC(=O)N)C(=O)O)N SLQVFYWBGNNOTK-BYULHYEWSA-N 0.000 description 4
- YBKKLDBBPFIXBQ-MBLNEYKQSA-N Ile-Thr-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)O)N YBKKLDBBPFIXBQ-MBLNEYKQSA-N 0.000 description 4
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 4
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 4
- BPCLGWHVPVTTFM-QWRGUYRKSA-N Phe-Ser-Gly Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)NCC(O)=O BPCLGWHVPVTTFM-QWRGUYRKSA-N 0.000 description 4
- RMODQFBNDDENCP-IHRRRGAJSA-N Pro-Lys-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O RMODQFBNDDENCP-IHRRRGAJSA-N 0.000 description 4
- GOMUXSCOIWIJFP-GUBZILKMSA-N Pro-Ser-Arg Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O GOMUXSCOIWIJFP-GUBZILKMSA-N 0.000 description 4
- 206010040047 Sepsis Diseases 0.000 description 4
- HJEBZBMOTCQYDN-ACZMJKKPSA-N Ser-Glu-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O HJEBZBMOTCQYDN-ACZMJKKPSA-N 0.000 description 4
- 108010022999 Serine Proteases Proteins 0.000 description 4
- 102000012479 Serine Proteases Human genes 0.000 description 4
- 108010090804 Streptavidin Proteins 0.000 description 4
- KZSYAEWQMJEGRZ-RHYQMDGZSA-N Thr-Leu-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O KZSYAEWQMJEGRZ-RHYQMDGZSA-N 0.000 description 4
- BDGBHYCAZJPLHX-HJGDQZAQSA-N Thr-Lys-Asn Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O BDGBHYCAZJPLHX-HJGDQZAQSA-N 0.000 description 4
- PKZIWSHDJYIPRH-JBACZVJFSA-N Trp-Tyr-Gln Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(O)=O PKZIWSHDJYIPRH-JBACZVJFSA-N 0.000 description 4
- JWHOIHCOHMZSAR-QWRGUYRKSA-N Tyr-Asp-Gly Chemical compound OC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 JWHOIHCOHMZSAR-QWRGUYRKSA-N 0.000 description 4
- MWUYSCVVPVITMW-IGNZVWTISA-N Tyr-Tyr-Ala Chemical compound C([C@@H](C(=O)N[C@@H](C)C(O)=O)NC(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=C(O)C=C1 MWUYSCVVPVITMW-IGNZVWTISA-N 0.000 description 4
- OVBMCNDKCWAXMZ-NAKRPEOUSA-N Val-Ile-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](C(C)C)N OVBMCNDKCWAXMZ-NAKRPEOUSA-N 0.000 description 4
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 4
- 230000002776 aggregation Effects 0.000 description 4
- 238000004220 aggregation Methods 0.000 description 4
- 230000002429 anti-coagulating effect Effects 0.000 description 4
- 239000011324 bead Substances 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- 229940088598 enzyme Drugs 0.000 description 4
- 208000031169 hemorrhagic disease Diseases 0.000 description 4
- 239000000833 heterodimer Substances 0.000 description 4
- 229940100689 human protein c Drugs 0.000 description 4
- 230000007062 hydrolysis Effects 0.000 description 4
- 238000006460 hydrolysis reaction Methods 0.000 description 4
- 238000011534 incubation Methods 0.000 description 4
- 239000003550 marker Substances 0.000 description 4
- 239000008188 pellet Substances 0.000 description 4
- 108010051242 phenylalanylserine Proteins 0.000 description 4
- 238000011533 pre-incubation Methods 0.000 description 4
- 238000003259 recombinant expression Methods 0.000 description 4
- 108010051110 tyrosyl-lysine Proteins 0.000 description 4
- KWPACVJPAFGBEQ-IKGGRYGDSA-N (2s)-1-[(2r)-2-amino-3-phenylpropanoyl]-n-[(3s)-1-chloro-6-(diaminomethylideneamino)-2-oxohexan-3-yl]pyrrolidine-2-carboxamide Chemical compound C([C@@H](N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)CCl)C1=CC=CC=C1 KWPACVJPAFGBEQ-IKGGRYGDSA-N 0.000 description 3
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 3
- SGFBVLBKDSXGAP-GKCIPKSASA-N Ala-Phe-Trp Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O)N SGFBVLBKDSXGAP-GKCIPKSASA-N 0.000 description 3
- NCQMBSJGJMYKCK-ZLUOBGJFSA-N Ala-Ser-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O NCQMBSJGJMYKCK-ZLUOBGJFSA-N 0.000 description 3
- USNSOPDIZILSJP-FXQIFTODSA-N Arg-Asn-Asn Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O USNSOPDIZILSJP-FXQIFTODSA-N 0.000 description 3
- OGUPCHKBOKJFMA-SRVKXCTJSA-N Arg-Glu-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCCN=C(N)N OGUPCHKBOKJFMA-SRVKXCTJSA-N 0.000 description 3
- BSGSDLYGGHGMND-IHRRRGAJSA-N Arg-Phe-Cys Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N BSGSDLYGGHGMND-IHRRRGAJSA-N 0.000 description 3
- DNLQVHBBMPZUGJ-BQBZGAKWSA-N Arg-Ser-Gly Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O DNLQVHBBMPZUGJ-BQBZGAKWSA-N 0.000 description 3
- QEYJFBMTSMLPKZ-ZKWXMUAHSA-N Asn-Ala-Val Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O QEYJFBMTSMLPKZ-ZKWXMUAHSA-N 0.000 description 3
- JRCASHGTXZYSPW-XIRDDKMYSA-N Asn-His-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CC3=CN=CN3)NC(=O)[C@H](CC(=O)N)N JRCASHGTXZYSPW-XIRDDKMYSA-N 0.000 description 3
- UQBGYPFHWFZMCD-ZLUOBGJFSA-N Asp-Asn-Asn Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O UQBGYPFHWFZMCD-ZLUOBGJFSA-N 0.000 description 3
- PSLSTUMPZILTAH-BYULHYEWSA-N Asp-Gly-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC(O)=O PSLSTUMPZILTAH-BYULHYEWSA-N 0.000 description 3
- GWIJZUVQVDJHDI-AVGNSLFASA-N Asp-Phe-Glu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(O)=O)C(O)=O GWIJZUVQVDJHDI-AVGNSLFASA-N 0.000 description 3
- MGSVBZIBCCKGCY-ZLUOBGJFSA-N Asp-Ser-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O MGSVBZIBCCKGCY-ZLUOBGJFSA-N 0.000 description 3
- 102100030802 Beta-2-glycoprotein 1 Human genes 0.000 description 3
- 101710180007 Beta-2-glycoprotein 1 Proteins 0.000 description 3
- 102100026735 Coagulation factor VIII Human genes 0.000 description 3
- XLLSMEFANRROJE-GUBZILKMSA-N Cys-Leu-Glu Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CS)N XLLSMEFANRROJE-GUBZILKMSA-N 0.000 description 3
- 238000001712 DNA sequencing Methods 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 108010061932 Factor VIIIa Proteins 0.000 description 3
- LZRMPXRYLLTAJX-GUBZILKMSA-N Gln-Arg-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O LZRMPXRYLLTAJX-GUBZILKMSA-N 0.000 description 3
- VAIWPXWHWAPYDF-FXQIFTODSA-N Glu-Asp-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O VAIWPXWHWAPYDF-FXQIFTODSA-N 0.000 description 3
- WTMZXOPHTIVFCP-QEWYBTABSA-N Glu-Ile-Phe Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 WTMZXOPHTIVFCP-QEWYBTABSA-N 0.000 description 3
- KKBWDNZXYLGJEY-UHFFFAOYSA-N Gly-Arg-Pro Natural products NCC(=O)NC(CCNC(=N)N)C(=O)N1CCCC1C(=O)O KKBWDNZXYLGJEY-UHFFFAOYSA-N 0.000 description 3
- BYYNJRSNDARRBX-YFKPBYRVSA-N Gly-Gln-Gly Chemical compound NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O BYYNJRSNDARRBX-YFKPBYRVSA-N 0.000 description 3
- IDOGEHIWMJMAHT-BYPYZUCNSA-N Gly-Gly-Cys Chemical compound NCC(=O)NCC(=O)N[C@@H](CS)C(O)=O IDOGEHIWMJMAHT-BYPYZUCNSA-N 0.000 description 3
- POJJAZJHBGXEGM-YUMQZZPRSA-N Gly-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)CN POJJAZJHBGXEGM-YUMQZZPRSA-N 0.000 description 3
- JSLVAHYTAJJEQH-QWRGUYRKSA-N Gly-Ser-Phe Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 JSLVAHYTAJJEQH-QWRGUYRKSA-N 0.000 description 3
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 3
- VIJMRAIWYWRXSR-CIUDSAMLSA-N His-Ser-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CN=CN1 VIJMRAIWYWRXSR-CIUDSAMLSA-N 0.000 description 3
- 101000911390 Homo sapiens Coagulation factor VIII Proteins 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- UCOCBWDBHCUPQP-DCAQKATOSA-N Leu-Arg-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O UCOCBWDBHCUPQP-DCAQKATOSA-N 0.000 description 3
- IWTBYNQNAPECCS-AVGNSLFASA-N Leu-Glu-His Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 IWTBYNQNAPECCS-AVGNSLFASA-N 0.000 description 3
- BTNXKBVLWJBTNR-SRVKXCTJSA-N Leu-His-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(N)=O)C(O)=O BTNXKBVLWJBTNR-SRVKXCTJSA-N 0.000 description 3
- JFSGIJSCJFQGSZ-MXAVVETBSA-N Leu-Ile-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC(C)C)N JFSGIJSCJFQGSZ-MXAVVETBSA-N 0.000 description 3
- FAELBUXXFQLUAX-AJNGGQMLSA-N Leu-Leu-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC(C)C FAELBUXXFQLUAX-AJNGGQMLSA-N 0.000 description 3
- FBNPMTNBFFAMMH-UHFFFAOYSA-N Leu-Val-Arg Natural products CC(C)CC(N)C(=O)NC(C(C)C)C(=O)NC(C(O)=O)CCCN=C(N)N FBNPMTNBFFAMMH-UHFFFAOYSA-N 0.000 description 3
- NQCJGQHHYZNUDK-DCAQKATOSA-N Lys-Arg-Ser Chemical compound NCCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CCCN=C(N)N NQCJGQHHYZNUDK-DCAQKATOSA-N 0.000 description 3
- VMTYLUGCXIEDMV-QWRGUYRKSA-N Lys-Leu-Gly Chemical compound OC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCCCN VMTYLUGCXIEDMV-QWRGUYRKSA-N 0.000 description 3
- WBSCNDJQPKSPII-KKUMJFAQSA-N Lys-Lys-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(O)=O WBSCNDJQPKSPII-KKUMJFAQSA-N 0.000 description 3
- 101000595196 Mus musculus Podocalyxin Proteins 0.000 description 3
- 108091028043 Nucleic acid sequence Proteins 0.000 description 3
- WPTYDQPGBMDUBI-QWRGUYRKSA-N Phe-Gly-Asn Chemical compound N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O WPTYDQPGBMDUBI-QWRGUYRKSA-N 0.000 description 3
- FKKHDBFNOLCYQM-FXQIFTODSA-N Pro-Cys-Ala Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(O)=O FKKHDBFNOLCYQM-FXQIFTODSA-N 0.000 description 3
- QEWBZBLXDKIQPS-STQMWFEESA-N Pro-Gly-Tyr Chemical compound [H]N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O QEWBZBLXDKIQPS-STQMWFEESA-N 0.000 description 3
- XSXABUHLKPUVLX-JYJNAYRXSA-N Pro-Ser-Trp Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O XSXABUHLKPUVLX-JYJNAYRXSA-N 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 108010029485 Protein Isoforms Proteins 0.000 description 3
- 102000001708 Protein Isoforms Human genes 0.000 description 3
- 108010094028 Prothrombin Proteins 0.000 description 3
- KCFKKAQKRZBWJB-ZLUOBGJFSA-N Ser-Cys-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(O)=O KCFKKAQKRZBWJB-ZLUOBGJFSA-N 0.000 description 3
- KNCJWSPMTFFJII-ZLUOBGJFSA-N Ser-Cys-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(O)=O)C(O)=O KNCJWSPMTFFJII-ZLUOBGJFSA-N 0.000 description 3
- IAORETPTUDBBGV-CIUDSAMLSA-N Ser-Leu-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CO)N IAORETPTUDBBGV-CIUDSAMLSA-N 0.000 description 3
- SRKMDKACHDVPMD-SRVKXCTJSA-N Ser-Lys-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)N SRKMDKACHDVPMD-SRVKXCTJSA-N 0.000 description 3
- 229930006000 Sucrose Natural products 0.000 description 3
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 3
- UMFLBPIPAJMNIM-LYARXQMPSA-N Thr-Trp-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CC3=CC=CC=C3)C(=O)O)N)O UMFLBPIPAJMNIM-LYARXQMPSA-N 0.000 description 3
- 108010022394 Threonine synthase Proteins 0.000 description 3
- 108010000499 Thromboplastin Proteins 0.000 description 3
- 102000002262 Thromboplastin Human genes 0.000 description 3
- 208000007536 Thrombosis Diseases 0.000 description 3
- MICFJCRQBFSKPA-UMPQAUOISA-N Trp-Met-Thr Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)=CNC2=C1 MICFJCRQBFSKPA-UMPQAUOISA-N 0.000 description 3
- ZHQWPWQNVRCXAX-XQQFMLRXSA-N Val-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](C(C)C)N ZHQWPWQNVRCXAX-XQQFMLRXSA-N 0.000 description 3
- QZKVWWIUSQGWMY-IHRRRGAJSA-N Val-Ser-Phe Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 QZKVWWIUSQGWMY-IHRRRGAJSA-N 0.000 description 3
- 229930003448 Vitamin K Natural products 0.000 description 3
- 108010024078 alanyl-glycyl-serine Proteins 0.000 description 3
- 108010041407 alanylaspartic acid Proteins 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 108010047857 aspartylglycine Proteins 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 230000037396 body weight Effects 0.000 description 3
- 230000003197 catalytic effect Effects 0.000 description 3
- 239000003593 chromogenic compound Substances 0.000 description 3
- 238000003776 cleavage reaction Methods 0.000 description 3
- 239000011248 coating agent Substances 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 230000000295 complement effect Effects 0.000 description 3
- 108010004073 cysteinylcysteine Proteins 0.000 description 3
- 108010016616 cysteinylglycine Proteins 0.000 description 3
- 238000001514 detection method Methods 0.000 description 3
- 102000004419 dihydrofolate reductase Human genes 0.000 description 3
- 239000006185 dispersion Substances 0.000 description 3
- 239000003814 drug Substances 0.000 description 3
- 239000002158 endotoxin Substances 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- XBGGUPMXALFZOT-UHFFFAOYSA-N glycyl-L-tyrosine hemihydrate Natural products NCC(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 XBGGUPMXALFZOT-UHFFFAOYSA-N 0.000 description 3
- 108010084264 glycyl-glycyl-cysteine Proteins 0.000 description 3
- 108010033719 glycyl-histidyl-glycine Proteins 0.000 description 3
- 108010074027 glycyl-seryl-phenylalanine Proteins 0.000 description 3
- 108010084389 glycyltryptophan Proteins 0.000 description 3
- 208000009429 hemophilia B Diseases 0.000 description 3
- 229960002897 heparin Drugs 0.000 description 3
- 229920000669 heparin Polymers 0.000 description 3
- 102000055691 human APC Human genes 0.000 description 3
- 230000016784 immunoglobulin production Effects 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 108010044374 isoleucyl-tyrosine Proteins 0.000 description 3
- BPHPUYQFMNQIOC-NXRLNHOXSA-N isopropyl beta-D-thiogalactopyranoside Chemical compound CC(C)S[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O BPHPUYQFMNQIOC-NXRLNHOXSA-N 0.000 description 3
- 108010054155 lysyllysine Proteins 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 235000013336 milk Nutrition 0.000 description 3
- 239000008267 milk Substances 0.000 description 3
- 210000004080 milk Anatomy 0.000 description 3
- 238000005457 optimization Methods 0.000 description 3
- 230000037361 pathway Effects 0.000 description 3
- 150000003904 phospholipids Chemical class 0.000 description 3
- SHUZOJHMOBOZST-UHFFFAOYSA-N phylloquinone Natural products CC(C)CCCCC(C)CCC(C)CCCC(=CCC1=C(C)C(=O)c2ccccc2C1=O)C SHUZOJHMOBOZST-UHFFFAOYSA-N 0.000 description 3
- 239000013641 positive control Substances 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 230000007065 protein hydrolysis Effects 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 230000007017 scission Effects 0.000 description 3
- 230000028327 secretion Effects 0.000 description 3
- -1 sodium chloride Chemical compound 0.000 description 3
- 230000009870 specific binding Effects 0.000 description 3
- 238000010561 standard procedure Methods 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 239000005720 sucrose Substances 0.000 description 3
- 238000004448 titration Methods 0.000 description 3
- 238000013519 translation Methods 0.000 description 3
- 230000014621 translational initiation Effects 0.000 description 3
- 108010038745 tryptophylglycine Proteins 0.000 description 3
- 235000019168 vitamin K Nutrition 0.000 description 3
- 239000011712 vitamin K Substances 0.000 description 3
- 150000003721 vitamin K derivatives Chemical class 0.000 description 3
- 229940046010 vitamin k Drugs 0.000 description 3
- JNBCIUTTWUUDDX-LSBAASHUSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-amino-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]-4-carboxybutanoyl]amino]-4-carboxybutanoyl]amino]-4-methylpentanoic acid Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CC=CC=C1 JNBCIUTTWUUDDX-LSBAASHUSA-N 0.000 description 2
- KQFRUSHJPKXBMB-BHDSKKPTSA-N Ala-Ala-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)C)C(O)=O)=CNC2=C1 KQFRUSHJPKXBMB-BHDSKKPTSA-N 0.000 description 2
- FXKNPWNXPQZLES-ZLUOBGJFSA-N Ala-Asn-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O FXKNPWNXPQZLES-ZLUOBGJFSA-N 0.000 description 2
- WCBVQNZTOKJWJS-ACZMJKKPSA-N Ala-Cys-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(O)=O WCBVQNZTOKJWJS-ACZMJKKPSA-N 0.000 description 2
- WMYJZJRILUVVRG-WDSKDSINSA-N Ala-Gly-Gln Chemical compound C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCC(N)=O WMYJZJRILUVVRG-WDSKDSINSA-N 0.000 description 2
- RGDKRCPIFODMHK-HJWJTTGWSA-N Ala-Leu-Leu-His Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 RGDKRCPIFODMHK-HJWJTTGWSA-N 0.000 description 2
- BFMIRJBURUXDRG-DLOVCJGASA-N Ala-Phe-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)C)CC1=CC=CC=C1 BFMIRJBURUXDRG-DLOVCJGASA-N 0.000 description 2
- XWFWAXPOLRTDFZ-FXQIFTODSA-N Ala-Pro-Ser Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O XWFWAXPOLRTDFZ-FXQIFTODSA-N 0.000 description 2
- 108010032595 Antibody Binding Sites Proteins 0.000 description 2
- 108010039627 Aprotinin Proteins 0.000 description 2
- KJGNDQCYBNBXDA-GUBZILKMSA-N Arg-Arg-Cys Chemical compound C(C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CS)C(=O)O)N)CN=C(N)N KJGNDQCYBNBXDA-GUBZILKMSA-N 0.000 description 2
- JTKLCCFLSLCCST-SZMVWBNQSA-N Arg-Arg-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)N)C(O)=O)=CNC2=C1 JTKLCCFLSLCCST-SZMVWBNQSA-N 0.000 description 2
- XVLLUZMFSAYKJV-GUBZILKMSA-N Arg-Asp-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O XVLLUZMFSAYKJV-GUBZILKMSA-N 0.000 description 2
- CRCCTGPNZUCAHE-DCAQKATOSA-N Arg-His-Ser Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CC1=CN=CN1 CRCCTGPNZUCAHE-DCAQKATOSA-N 0.000 description 2
- MOGMYRUNTKYZFB-UNQGMJICSA-N Arg-Thr-Phe Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 MOGMYRUNTKYZFB-UNQGMJICSA-N 0.000 description 2
- RCENDENBBJFJHZ-ACZMJKKPSA-N Asn-Asn-Gln Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O RCENDENBBJFJHZ-ACZMJKKPSA-N 0.000 description 2
- SPIPSJXLZVTXJL-ZLUOBGJFSA-N Asn-Cys-Ser Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(O)=O SPIPSJXLZVTXJL-ZLUOBGJFSA-N 0.000 description 2
- MECFLTFREHAZLH-ACZMJKKPSA-N Asn-Glu-Cys Chemical compound C(CC(=O)O)[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(=O)N)N MECFLTFREHAZLH-ACZMJKKPSA-N 0.000 description 2
- MVXJBVVLACEGCG-PCBIJLKTSA-N Asn-Phe-Ile Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O MVXJBVVLACEGCG-PCBIJLKTSA-N 0.000 description 2
- BCADFFUQHIMQAA-KKHAAJSZSA-N Asn-Thr-Val Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O BCADFFUQHIMQAA-KKHAAJSZSA-N 0.000 description 2
- QTKYFZCMSQLYHI-UBHSHLNASA-N Asn-Trp-Asn Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC(N)=O)C(O)=O QTKYFZCMSQLYHI-UBHSHLNASA-N 0.000 description 2
- JPPLRQVZMZFOSX-UWJYBYFXSA-N Asn-Tyr-Ala Chemical compound NC(=O)C[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](C)C(O)=O)CC1=CC=C(O)C=C1 JPPLRQVZMZFOSX-UWJYBYFXSA-N 0.000 description 2
- JZLFYAAGGYMRIK-BYULHYEWSA-N Asn-Val-Asp Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O JZLFYAAGGYMRIK-BYULHYEWSA-N 0.000 description 2
- SOYOSFXLXYZNRG-CIUDSAMLSA-N Asp-Arg-Gln Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O SOYOSFXLXYZNRG-CIUDSAMLSA-N 0.000 description 2
- QRULNKJGYQQZMW-ZLUOBGJFSA-N Asp-Asn-Asp Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O QRULNKJGYQQZMW-ZLUOBGJFSA-N 0.000 description 2
- QXHVOUSPVAWEMX-ZLUOBGJFSA-N Asp-Asp-Ser Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O QXHVOUSPVAWEMX-ZLUOBGJFSA-N 0.000 description 2
- JTRDJYIZIKCIRC-AJNGGQMLSA-N Asp-Leu-Leu-Gln Chemical compound CC(C)C[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O JTRDJYIZIKCIRC-AJNGGQMLSA-N 0.000 description 2
- XXAMCEGRCZQGEM-ZLUOBGJFSA-N Asp-Ser-Asn Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O XXAMCEGRCZQGEM-ZLUOBGJFSA-N 0.000 description 2
- CUQDCPXNZPDYFQ-ZLUOBGJFSA-N Asp-Ser-Asp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O CUQDCPXNZPDYFQ-ZLUOBGJFSA-N 0.000 description 2
- KGHLGJAXYSVNJP-WHFBIAKZSA-N Asp-Ser-Gly Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O KGHLGJAXYSVNJP-WHFBIAKZSA-N 0.000 description 2
- JSNWZMFSLIWAHS-HJGDQZAQSA-N Asp-Thr-Leu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)O)NC(=O)[C@H](CC(=O)O)N)O JSNWZMFSLIWAHS-HJGDQZAQSA-N 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- 241000282465 Canis Species 0.000 description 2
- 101000595191 Canis lupus familiaris Podocalyxin Proteins 0.000 description 2
- TVYMKYUSZSVOAG-ZLUOBGJFSA-N Cys-Ala-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(O)=O TVYMKYUSZSVOAG-ZLUOBGJFSA-N 0.000 description 2
- HQZGVYJBRSISDT-BQBZGAKWSA-N Cys-Gly-Arg Chemical compound [H]N[C@@H](CS)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O HQZGVYJBRSISDT-BQBZGAKWSA-N 0.000 description 2
- WTNLLMQAFPOCTJ-GARJFASQSA-N Cys-His-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CN=CN2)NC(=O)[C@H](CS)N)C(=O)O WTNLLMQAFPOCTJ-GARJFASQSA-N 0.000 description 2
- OTXLNICGSXPGQF-KBIXCLLPSA-N Cys-Ile-Glu Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(O)=O OTXLNICGSXPGQF-KBIXCLLPSA-N 0.000 description 2
- YNJBLTDKTMKEET-ZLUOBGJFSA-N Cys-Ser-Ser Chemical compound SC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O YNJBLTDKTMKEET-ZLUOBGJFSA-N 0.000 description 2
- 241000701022 Cytomegalovirus Species 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- XOKGKOQWADCLFQ-GARJFASQSA-N Gln-Arg-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(=O)N)N)C(=O)O XOKGKOQWADCLFQ-GARJFASQSA-N 0.000 description 2
- UVAOVENCIONMJP-GUBZILKMSA-N Gln-Cys-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O UVAOVENCIONMJP-GUBZILKMSA-N 0.000 description 2
- YVYVMJNUENBOOL-KBIXCLLPSA-N Glu-Ile-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCC(=O)O)N YVYVMJNUENBOOL-KBIXCLLPSA-N 0.000 description 2
- VMKCPNBBPGGQBJ-GUBZILKMSA-N Glu-Leu-Asn Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)O)N VMKCPNBBPGGQBJ-GUBZILKMSA-N 0.000 description 2
- PJBVXVBTTFZPHJ-GUBZILKMSA-N Glu-Leu-Asp Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CCC(=O)O)N PJBVXVBTTFZPHJ-GUBZILKMSA-N 0.000 description 2
- BCYGDJXHAGZNPQ-DCAQKATOSA-N Glu-Lys-Glu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O BCYGDJXHAGZNPQ-DCAQKATOSA-N 0.000 description 2
- OFIHURVSQXAZIR-SZMVWBNQSA-N Glu-Lys-Trp Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O OFIHURVSQXAZIR-SZMVWBNQSA-N 0.000 description 2
- BGVYNAQWHSTTSP-BYULHYEWSA-N Gly-Asn-Ile Chemical compound [H]NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O BGVYNAQWHSTTSP-BYULHYEWSA-N 0.000 description 2
- CEXINUGNTZFNRY-BYPYZUCNSA-N Gly-Cys-Gly Chemical compound [NH3+]CC(=O)N[C@@H](CS)C(=O)NCC([O-])=O CEXINUGNTZFNRY-BYPYZUCNSA-N 0.000 description 2
- ADZGCWWDPFDHCY-ZETCQYMHSA-N Gly-His-Gly Chemical compound OC(=O)CNC(=O)[C@@H](NC(=O)CN)CC1=CN=CN1 ADZGCWWDPFDHCY-ZETCQYMHSA-N 0.000 description 2
- RVGMVLVBDRQVKB-UWVGGRQHSA-N Gly-Met-His Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)CN RVGMVLVBDRQVKB-UWVGGRQHSA-N 0.000 description 2
- OCPPBNKYGYSLOE-IUCAKERBSA-N Gly-Pro-Met Chemical compound CSCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)CN OCPPBNKYGYSLOE-IUCAKERBSA-N 0.000 description 2
- LCRDMSSAKLTKBU-ZDLURKLDSA-N Gly-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)CN LCRDMSSAKLTKBU-ZDLURKLDSA-N 0.000 description 2
- XHVONGZZVUUORG-WEDXCCLWSA-N Gly-Thr-Lys Chemical compound NCC(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CCCCN XHVONGZZVUUORG-WEDXCCLWSA-N 0.000 description 2
- DUAWRXXTOQOECJ-JSGCOSHPSA-N Gly-Tyr-Val Chemical compound [H]NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(O)=O DUAWRXXTOQOECJ-JSGCOSHPSA-N 0.000 description 2
- PROLDOGUBQJNPG-RWMBFGLXSA-N His-Arg-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC2=CN=CN2)N)C(=O)O PROLDOGUBQJNPG-RWMBFGLXSA-N 0.000 description 2
- 101000653189 Homo sapiens Tissue factor pathway inhibitor Proteins 0.000 description 2
- NULSANWBUWLTKN-NAKRPEOUSA-N Ile-Arg-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CO)C(=O)O)N NULSANWBUWLTKN-NAKRPEOUSA-N 0.000 description 2
- LBRCLQMZAHRTLV-ZKWXMUAHSA-N Ile-Gly-Ser Chemical compound CC[C@H](C)[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O LBRCLQMZAHRTLV-ZKWXMUAHSA-N 0.000 description 2
- UASTVUQJMLZWGG-PEXQALLHSA-N Ile-His-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)NCC(=O)O)N UASTVUQJMLZWGG-PEXQALLHSA-N 0.000 description 2
- MLSUZXHSNRBDCI-CYDGBPFRSA-N Ile-Pro-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)O)N MLSUZXHSNRBDCI-CYDGBPFRSA-N 0.000 description 2
- OMDWJWGZGMCQND-CFMVVWHZSA-N Ile-Tyr-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC(=O)O)C(=O)O)N OMDWJWGZGMCQND-CFMVVWHZSA-N 0.000 description 2
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 2
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 2
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 2
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 2
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 2
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 2
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 2
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 2
- KVRKAGGMEWNURO-CIUDSAMLSA-N Leu-Ala-Cys Chemical compound C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(C)C)N KVRKAGGMEWNURO-CIUDSAMLSA-N 0.000 description 2
- MJOZZTKJZQFKDK-GUBZILKMSA-N Leu-Ala-Gln Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCC(N)=O MJOZZTKJZQFKDK-GUBZILKMSA-N 0.000 description 2
- ZURHXHNAEJJRNU-CIUDSAMLSA-N Leu-Asp-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O ZURHXHNAEJJRNU-CIUDSAMLSA-N 0.000 description 2
- KGCLIYGPQXUNLO-IUCAKERBSA-N Leu-Gly-Glu Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCC(O)=O KGCLIYGPQXUNLO-IUCAKERBSA-N 0.000 description 2
- KOSWSHVQIVTVQF-ZPFDUUQYSA-N Leu-Ile-Asp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(O)=O KOSWSHVQIVTVQF-ZPFDUUQYSA-N 0.000 description 2
- AKVBOOKXVAMKSS-GUBZILKMSA-N Leu-Ser-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O AKVBOOKXVAMKSS-GUBZILKMSA-N 0.000 description 2
- VDIARPPNADFEAV-WEDXCCLWSA-N Leu-Thr-Gly Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O VDIARPPNADFEAV-WEDXCCLWSA-N 0.000 description 2
- FBNPMTNBFFAMMH-AVGNSLFASA-N Leu-Val-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N FBNPMTNBFFAMMH-AVGNSLFASA-N 0.000 description 2
- AAKRWBIIGKPOKQ-ONGXEEELSA-N Leu-Val-Gly Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O AAKRWBIIGKPOKQ-ONGXEEELSA-N 0.000 description 2
- VKVDRTGWLVZJOM-DCAQKATOSA-N Leu-Val-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O VKVDRTGWLVZJOM-DCAQKATOSA-N 0.000 description 2
- AIRZWUMAHCDDHR-KKUMJFAQSA-N Lys-Leu-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O AIRZWUMAHCDDHR-KKUMJFAQSA-N 0.000 description 2
- AEIIJFBQVGYVEV-YESZJQIVSA-N Lys-Phe-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)NC(=O)[C@H](CCCCN)N)C(=O)O AEIIJFBQVGYVEV-YESZJQIVSA-N 0.000 description 2
- UIJVKVHLCQSPOJ-XIRDDKMYSA-N Lys-Ser-Trp Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O UIJVKVHLCQSPOJ-XIRDDKMYSA-N 0.000 description 2
- 241000829100 Macaca mulatta polyomavirus 1 Species 0.000 description 2
- VZBXCMCHIHEPBL-SRVKXCTJSA-N Met-Glu-Lys Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CCCCN VZBXCMCHIHEPBL-SRVKXCTJSA-N 0.000 description 2
- PCTFVQATEGYHJU-FXQIFTODSA-N Met-Ser-Asn Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O PCTFVQATEGYHJU-FXQIFTODSA-N 0.000 description 2
- KSIPKXNIQOWMIC-RCWTZXSCSA-N Met-Thr-Arg Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCCNC(N)=N KSIPKXNIQOWMIC-RCWTZXSCSA-N 0.000 description 2
- WUGMRIBZSVSJNP-UHFFFAOYSA-N N-L-alanyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)C(N)C)C(O)=O)=CNC2=C1 WUGMRIBZSVSJNP-UHFFFAOYSA-N 0.000 description 2
- YBAFDPFAUTYYRW-UHFFFAOYSA-N N-L-alpha-glutamyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCC(O)=O YBAFDPFAUTYYRW-UHFFFAOYSA-N 0.000 description 2
- 230000004988 N-glycosylation Effects 0.000 description 2
- KZNQNBZMBZJQJO-UHFFFAOYSA-N N-glycyl-L-proline Natural products NCC(=O)N1CCCC1C(O)=O KZNQNBZMBZJQJO-UHFFFAOYSA-N 0.000 description 2
- 108010070519 PAR-1 Receptor Proteins 0.000 description 2
- 102000035195 Peptidases Human genes 0.000 description 2
- 108091005804 Peptidases Proteins 0.000 description 2
- NAXPHWZXEXNDIW-JTQLQIEISA-N Phe-Gly-Gly Chemical compound OC(=O)CNC(=O)CNC(=O)[C@@H](N)CC1=CC=CC=C1 NAXPHWZXEXNDIW-JTQLQIEISA-N 0.000 description 2
- 102100024078 Plasma serine protease inhibitor Human genes 0.000 description 2
- 108010077971 Plasminogen Inactivators Proteins 0.000 description 2
- 102000010752 Plasminogen Inactivators Human genes 0.000 description 2
- HFZNNDWPHBRNPV-KZVJFYERSA-N Pro-Ala-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O HFZNNDWPHBRNPV-KZVJFYERSA-N 0.000 description 2
- TYMBHHITTMGGPI-NAKRPEOUSA-N Pro-Ile-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@@H]1CCCN1 TYMBHHITTMGGPI-NAKRPEOUSA-N 0.000 description 2
- GNFHQWNCSSPOBT-ULQDDVLXSA-N Pro-Trp-Gln Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)N[C@@H](CCC(=O)N)C(=O)O GNFHQWNCSSPOBT-ULQDDVLXSA-N 0.000 description 2
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 2
- 108010001953 Protein C Inhibitor Proteins 0.000 description 2
- 229940122929 Protein C inhibitor Drugs 0.000 description 2
- 229940096437 Protein S Drugs 0.000 description 2
- 102000029301 Protein S Human genes 0.000 description 2
- 108010066124 Protein S Proteins 0.000 description 2
- 102100037136 Proteinase-activated receptor 1 Human genes 0.000 description 2
- 102100027378 Prothrombin Human genes 0.000 description 2
- WTWGOQRNRFHFQD-JBDRJPRFSA-N Ser-Ala-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O WTWGOQRNRFHFQD-JBDRJPRFSA-N 0.000 description 2
- OHKFXGKHSJKKAL-NRPADANISA-N Ser-Glu-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O OHKFXGKHSJKKAL-NRPADANISA-N 0.000 description 2
- IOVHBRCQOGWAQH-ZKWXMUAHSA-N Ser-Gly-Ile Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(O)=O IOVHBRCQOGWAQH-ZKWXMUAHSA-N 0.000 description 2
- KCNSGAMPBPYUAI-CIUDSAMLSA-N Ser-Leu-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O KCNSGAMPBPYUAI-CIUDSAMLSA-N 0.000 description 2
- ZIFYDQAFEMIZII-GUBZILKMSA-N Ser-Leu-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O ZIFYDQAFEMIZII-GUBZILKMSA-N 0.000 description 2
- WOJYIMBIKTWKJO-KKUMJFAQSA-N Ser-Phe-His Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)NC(=O)[C@H](CO)N WOJYIMBIKTWKJO-KKUMJFAQSA-N 0.000 description 2
- STIAINRLUUKYKM-WFBYXXMGSA-N Ser-Trp-Ala Chemical compound C1=CC=C2C(C[C@@H](C(=O)N[C@@H](C)C(O)=O)NC(=O)[C@@H](N)CO)=CNC2=C1 STIAINRLUUKYKM-WFBYXXMGSA-N 0.000 description 2
- MFQMZDPAZRZAPV-NAKRPEOUSA-N Ser-Val-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CO)N MFQMZDPAZRZAPV-NAKRPEOUSA-N 0.000 description 2
- ZLNWJMRLHLGKFX-SVSWQMSJSA-N Thr-Cys-Ile Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O ZLNWJMRLHLGKFX-SVSWQMSJSA-N 0.000 description 2
- KBBRNEDOYWMIJP-KYNKHSRBSA-N Thr-Gly-Thr Chemical compound C[C@H]([C@@H](C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)O)N)O KBBRNEDOYWMIJP-KYNKHSRBSA-N 0.000 description 2
- JQAWYCUUFIMTHE-WLTAIBSBSA-N Thr-Gly-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O JQAWYCUUFIMTHE-WLTAIBSBSA-N 0.000 description 2
- XYFISNXATOERFZ-OSUNSFLBSA-N Thr-Ile-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H]([C@@H](C)O)N XYFISNXATOERFZ-OSUNSFLBSA-N 0.000 description 2
- YOPQYBJJNSIQGZ-JNPHEJMOSA-N Thr-Tyr-Tyr Chemical compound C([C@H](NC(=O)[C@@H](N)[C@H](O)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 YOPQYBJJNSIQGZ-JNPHEJMOSA-N 0.000 description 2
- KZTLZZQTJMCGIP-ZJDVBMNYSA-N Thr-Val-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KZTLZZQTJMCGIP-ZJDVBMNYSA-N 0.000 description 2
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 2
- 239000004473 Threonine Substances 0.000 description 2
- VEYXZZGMIBKXCN-UBHSHLNASA-N Trp-Asp-Asp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)O)C(=O)O)N VEYXZZGMIBKXCN-UBHSHLNASA-N 0.000 description 2
- VMBBTANKMSRJSS-JSGCOSHPSA-N Trp-Glu-Gly Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O VMBBTANKMSRJSS-JSGCOSHPSA-N 0.000 description 2
- DZIKVMCFXIIETR-JSGCOSHPSA-N Trp-Gly-Glu Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(O)=O DZIKVMCFXIIETR-JSGCOSHPSA-N 0.000 description 2
- ZHZLQVLQBDBQCQ-WDSOQIARSA-N Trp-Lys-Arg Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N ZHZLQVLQBDBQCQ-WDSOQIARSA-N 0.000 description 2
- 108090000631 Trypsin Proteins 0.000 description 2
- 102000004142 Trypsin Human genes 0.000 description 2
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 2
- BSCBBPKDVOZICB-KKUMJFAQSA-N Tyr-Leu-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O BSCBBPKDVOZICB-KKUMJFAQSA-N 0.000 description 2
- ZPFLBLFITJCBTP-QWRGUYRKSA-N Tyr-Ser-Gly Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(=O)NCC(O)=O ZPFLBLFITJCBTP-QWRGUYRKSA-N 0.000 description 2
- AGDDLOQMXUQPDY-BZSNNMDCSA-N Tyr-Tyr-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(O)=O AGDDLOQMXUQPDY-BZSNNMDCSA-N 0.000 description 2
- JVYIGCARISMLMV-HOCLYGCPSA-N Val-Gly-Trp Chemical compound CC(C)[C@@H](C(=O)NCC(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)N JVYIGCARISMLMV-HOCLYGCPSA-N 0.000 description 2
- KISFXYYRKKNLOP-IHRRRGAJSA-N Val-Phe-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)O)N KISFXYYRKKNLOP-IHRRRGAJSA-N 0.000 description 2
- MJOUSKQHAIARKI-JYJNAYRXSA-N Val-Phe-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](C(C)C)C(O)=O)CC1=CC=CC=C1 MJOUSKQHAIARKI-JYJNAYRXSA-N 0.000 description 2
- VSCIANXXVZOYOC-AVGNSLFASA-N Val-Pro-His Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)N VSCIANXXVZOYOC-AVGNSLFASA-N 0.000 description 2
- VIKZGAUAKQZDOF-NRPADANISA-N Val-Ser-Glu Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O VIKZGAUAKQZDOF-NRPADANISA-N 0.000 description 2
- LCHZBEUVGAVMKS-RHYQMDGZSA-N Val-Thr-Leu Chemical compound CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)[C@@H](C)O)C(O)=O LCHZBEUVGAVMKS-RHYQMDGZSA-N 0.000 description 2
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 229960000723 ampicillin Drugs 0.000 description 2
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 2
- 230000003321 amplification Effects 0.000 description 2
- 229960004405 aprotinin Drugs 0.000 description 2
- 108010043240 arginyl-leucyl-glycine Proteins 0.000 description 2
- 108010068380 arginylarginine Proteins 0.000 description 2
- 108010040443 aspartyl-aspartic acid Proteins 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 229940019700 blood coagulation factors Drugs 0.000 description 2
- AIYUHDOJVYHVIT-UHFFFAOYSA-M caesium chloride Chemical compound [Cl-].[Cs+] AIYUHDOJVYHVIT-UHFFFAOYSA-M 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- BULLHNJGPPOUOX-UHFFFAOYSA-N chloroacetone Chemical compound CC(=O)CCl BULLHNJGPPOUOX-UHFFFAOYSA-N 0.000 description 2
- 238000011260 co-administration Methods 0.000 description 2
- 238000004440 column chromatography Methods 0.000 description 2
- 238000002648 combination therapy Methods 0.000 description 2
- 230000009918 complex formation Effects 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 230000004069 differentiation Effects 0.000 description 2
- 239000002612 dispersion medium Substances 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 239000003623 enhancer Substances 0.000 description 2
- 230000002255 enzymatic effect Effects 0.000 description 2
- 210000003527 eukaryotic cell Anatomy 0.000 description 2
- 230000001747 exhibiting effect Effects 0.000 description 2
- 238000002825 functional assay Methods 0.000 description 2
- 238000002873 global sequence alignment Methods 0.000 description 2
- 108010051307 glycyl-glycyl-proline Proteins 0.000 description 2
- 108010087823 glycyltyrosine Proteins 0.000 description 2
- 108010037850 glycylvaline Proteins 0.000 description 2
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- ZPNFWUPYTFPOJU-LPYSRVMUSA-N iniprol Chemical compound C([C@H]1C(=O)NCC(=O)NCC(=O)N[C@H]2CSSC[C@H]3C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(N[C@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC=4C=CC=CC=4)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC=4C=CC=CC=4)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]2N(CCC2)C(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N2[C@@H](CCC2)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N2[C@@H](CCC2)C(=O)N3)C(=O)NCC(=O)NCC(=O)N[C@@H](C)C(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@H](C(=O)N1)C(C)C)[C@@H](C)O)[C@@H](C)CC)=O)[C@@H](C)CC)C1=CC=C(O)C=C1 ZPNFWUPYTFPOJU-LPYSRVMUSA-N 0.000 description 2
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 2
- 229960000310 isoleucine Drugs 0.000 description 2
- 238000011031 large-scale manufacturing process Methods 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 230000002101 lytic effect Effects 0.000 description 2
- 210000004962 mammalian cell Anatomy 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 210000004379 membrane Anatomy 0.000 description 2
- 229960000485 methotrexate Drugs 0.000 description 2
- 238000002703 mutagenesis Methods 0.000 description 2
- 231100000350 mutagenesis Toxicity 0.000 description 2
- 230000035772 mutation Effects 0.000 description 2
- 238000003199 nucleic acid amplification method Methods 0.000 description 2
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 2
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 2
- 108010064486 phenylalanyl-leucyl-valine Proteins 0.000 description 2
- 239000013612 plasmid Substances 0.000 description 2
- 239000002797 plasminogen activator inhibitor Substances 0.000 description 2
- 230000008488 polyadenylation Effects 0.000 description 2
- 229920000136 polysorbate Polymers 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 238000000159 protein binding assay Methods 0.000 description 2
- 229940039716 prothrombin Drugs 0.000 description 2
- 238000011552 rat model Methods 0.000 description 2
- 230000009257 reactivity Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 238000002864 sequence alignment Methods 0.000 description 2
- 108010026333 seryl-proline Proteins 0.000 description 2
- 238000004904 shortening Methods 0.000 description 2
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 238000010254 subcutaneous injection Methods 0.000 description 2
- 239000007929 subcutaneous injection Substances 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 238000012032 thrombin generation assay Methods 0.000 description 2
- 201000005665 thrombophilia Diseases 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 230000035897 transcription Effects 0.000 description 2
- 230000002103 transcriptional effect Effects 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- 230000000472 traumatic effect Effects 0.000 description 2
- 239000012588 trypsin Substances 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- 108010077037 tyrosyl-tyrosyl-phenylalanine Proteins 0.000 description 2
- 241000701161 unidentified adenovirus Species 0.000 description 2
- 239000004474 valine Substances 0.000 description 2
- 230000003612 virological effect Effects 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 238000001262 western blot Methods 0.000 description 2
- ZXJZGWOMAFPSJH-DCAQKATOSA-N (2S)-1-[2-[[2-[[(2S)-2-[[(2S)-2-[(2-aminoacetyl)amino]-3-carboxypropanoyl]amino]-3-hydroxypropanoyl]amino]acetyl]amino]acetyl]pyrrolidine-2-carboxylic acid Chemical compound [H]NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)NCC(=O)NCC(=O)N1CCC[C@H]1C(O)=O ZXJZGWOMAFPSJH-DCAQKATOSA-N 0.000 description 1
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 1
- PGOHTUIFYSHAQG-LJSDBVFPSA-N (2S)-6-amino-2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-5-amino-2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3R)-2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S,3R)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-5-amino-2-[[(2S)-1-[(2S,3R)-2-[[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2S)-2-[[(2S)-2-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-1-[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-4-methylsulfanylbutanoyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-5-carbamimidamidopentanoyl]amino]propanoyl]pyrrolidine-2-carbonyl]amino]-3-methylbutanoyl]amino]-4-methylpentanoyl]amino]-4-methylpentanoyl]amino]acetyl]amino]-3-hydroxypropanoyl]amino]-4-methylpentanoyl]amino]-3-sulfanylpropanoyl]amino]-4-methylsulfanylbutanoyl]amino]-5-carbamimidamidopentanoyl]amino]-3-hydroxybutanoyl]pyrrolidine-2-carbonyl]amino]-5-oxopentanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-(1H-imidazol-5-yl)propanoyl]amino]-4-methylpentanoyl]amino]-3-hydroxybutanoyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-5-carbamimidamidopentanoyl]amino]-5-oxopentanoyl]amino]-3-hydroxybutanoyl]amino]-3-hydroxypropanoyl]amino]-3-carboxypropanoyl]amino]-3-hydroxypropanoyl]amino]-5-oxopentanoyl]amino]-5-oxopentanoyl]amino]-3-phenylpropanoyl]amino]-5-carbamimidamidopentanoyl]amino]-3-methylbutanoyl]amino]-4-methylpentanoyl]amino]-4-oxobutanoyl]amino]-5-carbamimidamidopentanoyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-4-carboxybutanoyl]amino]-5-oxopentanoyl]amino]hexanoic acid Chemical compound CSCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(O)=O PGOHTUIFYSHAQG-LJSDBVFPSA-N 0.000 description 1
- NNRFRJQMBSBXGO-CIUDSAMLSA-N (3s)-3-[[2-[[(2s)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]acetyl]amino]-4-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-oxobutanoic acid Chemical compound NC(N)=NCCC[C@H](N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O NNRFRJQMBSBXGO-CIUDSAMLSA-N 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- WQVFQXXBNHHPLX-ZKWXMUAHSA-N Ala-Ala-His Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O WQVFQXXBNHHPLX-ZKWXMUAHSA-N 0.000 description 1
- DVWVZSJAYIJZFI-FXQIFTODSA-N Ala-Arg-Asn Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(O)=O DVWVZSJAYIJZFI-FXQIFTODSA-N 0.000 description 1
- KRHRBKYBJXMYBB-WHFBIAKZSA-N Ala-Cys-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CS)C(=O)NCC(O)=O KRHRBKYBJXMYBB-WHFBIAKZSA-N 0.000 description 1
- CZPAHAKGPDUIPJ-CIUDSAMLSA-N Ala-Gln-Pro Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(O)=O CZPAHAKGPDUIPJ-CIUDSAMLSA-N 0.000 description 1
- NWVVKQZOVSTDBQ-CIUDSAMLSA-N Ala-Glu-Arg Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O NWVVKQZOVSTDBQ-CIUDSAMLSA-N 0.000 description 1
- KXEVYGKATAMXJJ-ACZMJKKPSA-N Ala-Glu-Asp Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O KXEVYGKATAMXJJ-ACZMJKKPSA-N 0.000 description 1
- LMFXXZPPZDCPTA-ZKWXMUAHSA-N Ala-Gly-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@H](C)N LMFXXZPPZDCPTA-ZKWXMUAHSA-N 0.000 description 1
- NBTGEURICRTMGL-WHFBIAKZSA-N Ala-Gly-Ser Chemical compound C[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O NBTGEURICRTMGL-WHFBIAKZSA-N 0.000 description 1
- ANGAOPNEPIDLPO-XVYDVKMFSA-N Ala-His-Cys Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CS)C(=O)O)N ANGAOPNEPIDLPO-XVYDVKMFSA-N 0.000 description 1
- LTSBJNNXPBBNDT-HGNGGELXSA-N Ala-His-Gln Chemical compound N[C@@H](C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(N)=O)C(=O)O LTSBJNNXPBBNDT-HGNGGELXSA-N 0.000 description 1
- LXAARTARZJJCMB-CIQUZCHMSA-N Ala-Ile-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O LXAARTARZJJCMB-CIQUZCHMSA-N 0.000 description 1
- LDLSENBXQNDTPB-DCAQKATOSA-N Ala-Lys-Arg Chemical compound NCCCC[C@H](NC(=O)[C@@H](N)C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N LDLSENBXQNDTPB-DCAQKATOSA-N 0.000 description 1
- SUHLZMHFRALVSY-YUMQZZPRSA-N Ala-Lys-Gly Chemical compound NCCCC[C@H](NC(=O)[C@@H](N)C)C(=O)NCC(O)=O SUHLZMHFRALVSY-YUMQZZPRSA-N 0.000 description 1
- AWNAEZICPNGAJK-FXQIFTODSA-N Ala-Met-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CO)C(O)=O AWNAEZICPNGAJK-FXQIFTODSA-N 0.000 description 1
- GFEDXKNBZMPEDM-KZVJFYERSA-N Ala-Met-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GFEDXKNBZMPEDM-KZVJFYERSA-N 0.000 description 1
- FEGOCLZUJUFCHP-CIUDSAMLSA-N Ala-Pro-Gln Chemical compound [H]N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(O)=O FEGOCLZUJUFCHP-CIUDSAMLSA-N 0.000 description 1
- LTTLSZVJTDSACD-OWLDWWDNSA-N Ala-Thr-Trp Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O LTTLSZVJTDSACD-OWLDWWDNSA-N 0.000 description 1
- YCTIYBUTCKNOTI-UWJYBYFXSA-N Ala-Tyr-Asp Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC(=O)O)C(=O)O)N YCTIYBUTCKNOTI-UWJYBYFXSA-N 0.000 description 1
- MUGAESARFRGOTQ-IGNZVWTISA-N Ala-Tyr-Tyr Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)N MUGAESARFRGOTQ-IGNZVWTISA-N 0.000 description 1
- XSLGWYYNOSUMRM-ZKWXMUAHSA-N Ala-Val-Asn Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O XSLGWYYNOSUMRM-ZKWXMUAHSA-N 0.000 description 1
- YJHKTAMKPGFJCT-NRPADANISA-N Ala-Val-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O YJHKTAMKPGFJCT-NRPADANISA-N 0.000 description 1
- XCIGOVDXZULBBV-DCAQKATOSA-N Ala-Val-Lys Chemical compound CC(C)[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](CCCCN)C(O)=O XCIGOVDXZULBBV-DCAQKATOSA-N 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- DFCIPNHFKOQAME-FXQIFTODSA-N Arg-Ala-Asn Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(O)=O DFCIPNHFKOQAME-FXQIFTODSA-N 0.000 description 1
- SBVJJNJLFWSJOV-UBHSHLNASA-N Arg-Ala-Phe Chemical compound NC(=N)NCCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 SBVJJNJLFWSJOV-UBHSHLNASA-N 0.000 description 1
- OTOXOKCIIQLMFH-KZVJFYERSA-N Arg-Ala-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N OTOXOKCIIQLMFH-KZVJFYERSA-N 0.000 description 1
- DPXDVGDLWJYZBH-GUBZILKMSA-N Arg-Asn-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O DPXDVGDLWJYZBH-GUBZILKMSA-N 0.000 description 1
- PQWTZSNVWSOFFK-FXQIFTODSA-N Arg-Asp-Asn Chemical compound C(C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N)CN=C(N)N PQWTZSNVWSOFFK-FXQIFTODSA-N 0.000 description 1
- OZNSCVPYWZRQPY-CIUDSAMLSA-N Arg-Asp-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O OZNSCVPYWZRQPY-CIUDSAMLSA-N 0.000 description 1
- SQKPKIJVWHAWNF-DCAQKATOSA-N Arg-Asp-Lys Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(O)=O SQKPKIJVWHAWNF-DCAQKATOSA-N 0.000 description 1
- YSUVMPICYVWRBX-VEVYYDQMSA-N Arg-Asp-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O YSUVMPICYVWRBX-VEVYYDQMSA-N 0.000 description 1
- PTVGLOCPAVYPFG-CIUDSAMLSA-N Arg-Gln-Asp Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O PTVGLOCPAVYPFG-CIUDSAMLSA-N 0.000 description 1
- DNUKXVMPARLPFN-XUXIUFHCSA-N Arg-Leu-Ile Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O DNUKXVMPARLPFN-XUXIUFHCSA-N 0.000 description 1
- VIINVRPKMUZYOI-DCAQKATOSA-N Arg-Met-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(O)=O VIINVRPKMUZYOI-DCAQKATOSA-N 0.000 description 1
- VVJTWSRNMJNDPN-IUCAKERBSA-N Arg-Met-Gly Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCSC)C(=O)NCC(O)=O VVJTWSRNMJNDPN-IUCAKERBSA-N 0.000 description 1
- DRDWXKWUSIKKOB-PJODQICGSA-N Arg-Trp-Ala Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](C)C(O)=O DRDWXKWUSIKKOB-PJODQICGSA-N 0.000 description 1
- ZUVMUOOHJYNJPP-XIRDDKMYSA-N Arg-Trp-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZUVMUOOHJYNJPP-XIRDDKMYSA-N 0.000 description 1
- UVTGNSWSRSCPLP-UHFFFAOYSA-N Arg-Tyr Natural products NC(CCNC(=N)N)C(=O)NC(Cc1ccc(O)cc1)C(=O)O UVTGNSWSRSCPLP-UHFFFAOYSA-N 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- VDCIPFYVCICPEC-FXQIFTODSA-N Asn-Arg-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(O)=O VDCIPFYVCICPEC-FXQIFTODSA-N 0.000 description 1
- GOVUDFOGXOONFT-VEVYYDQMSA-N Asn-Arg-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GOVUDFOGXOONFT-VEVYYDQMSA-N 0.000 description 1
- APHUDFFMXFYRKP-CIUDSAMLSA-N Asn-Asn-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC(=O)N)N APHUDFFMXFYRKP-CIUDSAMLSA-N 0.000 description 1
- ZWASIOHRQWRWAS-UGYAYLCHSA-N Asn-Asp-Ile Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O ZWASIOHRQWRWAS-UGYAYLCHSA-N 0.000 description 1
- WONGRTVAMHFGBE-WDSKDSINSA-N Asn-Gly-Gln Chemical compound C(CC(=O)N)[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CC(=O)N)N WONGRTVAMHFGBE-WDSKDSINSA-N 0.000 description 1
- AEZCCDMZZJOGII-DCAQKATOSA-N Asn-Met-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(O)=O AEZCCDMZZJOGII-DCAQKATOSA-N 0.000 description 1
- REQUGIWGOGSOEZ-ZLUOBGJFSA-N Asn-Ser-Asn Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(=O)N)C(=O)O)N)C(=O)N REQUGIWGOGSOEZ-ZLUOBGJFSA-N 0.000 description 1
- YSYTWUMRHSFODC-QWRGUYRKSA-N Asn-Tyr-Gly Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)NCC(O)=O YSYTWUMRHSFODC-QWRGUYRKSA-N 0.000 description 1
- QNNBHTFDFFFHGC-KKUMJFAQSA-N Asn-Tyr-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)N)N)O QNNBHTFDFFFHGC-KKUMJFAQSA-N 0.000 description 1
- DPWDPEVGACCWTC-SRVKXCTJSA-N Asn-Tyr-Ser Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(O)=O DPWDPEVGACCWTC-SRVKXCTJSA-N 0.000 description 1
- DPSUVAPLRQDWAO-YDHLFZDLSA-N Asn-Tyr-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](CC(=O)N)N DPSUVAPLRQDWAO-YDHLFZDLSA-N 0.000 description 1
- GBAWQWASNGUNQF-ZLUOBGJFSA-N Asp-Ala-Cys Chemical compound C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(=O)O)N GBAWQWASNGUNQF-ZLUOBGJFSA-N 0.000 description 1
- GVPSCJQLUGIKAM-GUBZILKMSA-N Asp-Arg-Arg Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O GVPSCJQLUGIKAM-GUBZILKMSA-N 0.000 description 1
- AXXCUABIFZPKPM-BQBZGAKWSA-N Asp-Arg-Gly Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O AXXCUABIFZPKPM-BQBZGAKWSA-N 0.000 description 1
- SBHUBSDEZQFJHJ-CIUDSAMLSA-N Asp-Asp-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(O)=O SBHUBSDEZQFJHJ-CIUDSAMLSA-N 0.000 description 1
- BFOYULZBKYOKAN-OLHMAJIHSA-N Asp-Asp-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O BFOYULZBKYOKAN-OLHMAJIHSA-N 0.000 description 1
- SMZCLQGDQMGESY-ACZMJKKPSA-N Asp-Gln-Cys Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(=O)O)N SMZCLQGDQMGESY-ACZMJKKPSA-N 0.000 description 1
- DGKCOYGQLNWNCJ-ACZMJKKPSA-N Asp-Glu-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O DGKCOYGQLNWNCJ-ACZMJKKPSA-N 0.000 description 1
- PZXPWHFYZXTFBI-YUMQZZPRSA-N Asp-Gly-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC(O)=O PZXPWHFYZXTFBI-YUMQZZPRSA-N 0.000 description 1
- YFSLJHLQOALGSY-ZPFDUUQYSA-N Asp-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)O)N YFSLJHLQOALGSY-ZPFDUUQYSA-N 0.000 description 1
- UZNSWMFLKVKJLI-VHWLVUOQSA-N Asp-Ile-Trp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O UZNSWMFLKVKJLI-VHWLVUOQSA-N 0.000 description 1
- PAYPSKIBMDHZPI-CIUDSAMLSA-N Asp-Leu-Asp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O PAYPSKIBMDHZPI-CIUDSAMLSA-N 0.000 description 1
- QNMKWNONJGKJJC-NHCYSSNCSA-N Asp-Leu-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O QNMKWNONJGKJJC-NHCYSSNCSA-N 0.000 description 1
- VSMYBNPOHYAXSD-GUBZILKMSA-N Asp-Lys-Glu Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O VSMYBNPOHYAXSD-GUBZILKMSA-N 0.000 description 1
- DPNWSMBUYCLEDG-CIUDSAMLSA-N Asp-Lys-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O DPNWSMBUYCLEDG-CIUDSAMLSA-N 0.000 description 1
- DONWIPDSZZJHHK-HJGDQZAQSA-N Asp-Lys-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(=O)O)N)O DONWIPDSZZJHHK-HJGDQZAQSA-N 0.000 description 1
- ZKAOJVJQGVUIIU-GUBZILKMSA-N Asp-Pro-Arg Chemical compound OC(=O)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O ZKAOJVJQGVUIIU-GUBZILKMSA-N 0.000 description 1
- HRVQDZOWMLFAOD-BIIVOSGPSA-N Asp-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CC(=O)O)N)C(=O)O HRVQDZOWMLFAOD-BIIVOSGPSA-N 0.000 description 1
- GWWSUMLEWKQHLR-NUMRIWBASA-N Asp-Thr-Glu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CC(=O)O)N)O GWWSUMLEWKQHLR-NUMRIWBASA-N 0.000 description 1
- ZUNMTUPRQMWMHX-LSJOCFKGSA-N Asp-Val-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(O)=O ZUNMTUPRQMWMHX-LSJOCFKGSA-N 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- 241000282836 Camelus dromedarius Species 0.000 description 1
- 102220518361 Casein kinase I isoform gamma-2_Y33A_mutation Human genes 0.000 description 1
- 241000700199 Cavia porcellus Species 0.000 description 1
- 102100025828 Centromere protein C Human genes 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 208000017667 Chronic Disease Diseases 0.000 description 1
- 108091033380 Coding strand Proteins 0.000 description 1
- 108020004705 Codon Proteins 0.000 description 1
- 241000186031 Corynebacteriaceae Species 0.000 description 1
- 241000699802 Cricetulus griseus Species 0.000 description 1
- AEJSNWMRPXAKCW-WHFBIAKZSA-N Cys-Ala-Gly Chemical compound SC[C@H](N)C(=O)N[C@@H](C)C(=O)NCC(O)=O AEJSNWMRPXAKCW-WHFBIAKZSA-N 0.000 description 1
- YFXFOZPXVFPBDH-VZFHVOOUSA-N Cys-Ala-Thr Chemical compound C[C@@H](O)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)CS)C(O)=O YFXFOZPXVFPBDH-VZFHVOOUSA-N 0.000 description 1
- GEEXORWTBTUOHC-FXQIFTODSA-N Cys-Arg-Ser Chemical compound C(C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CS)N)CN=C(N)N GEEXORWTBTUOHC-FXQIFTODSA-N 0.000 description 1
- RWAZRMXTVSIVJR-YUMQZZPRSA-N Cys-Gly-His Chemical compound [H]N[C@@H](CS)C(=O)NCC(=O)N[C@@H](CC1=CNC=N1)C(O)=O RWAZRMXTVSIVJR-YUMQZZPRSA-N 0.000 description 1
- LBOLGUYQEPZSKM-YUMQZZPRSA-N Cys-Gly-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CS)N LBOLGUYQEPZSKM-YUMQZZPRSA-N 0.000 description 1
- HBHMVBGGHDMPBF-GARJFASQSA-N Cys-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CS)N HBHMVBGGHDMPBF-GARJFASQSA-N 0.000 description 1
- LWYKPOCGGTYAIH-FXQIFTODSA-N Cys-Met-Asp Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CS)N LWYKPOCGGTYAIH-FXQIFTODSA-N 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 108010016626 Dipeptides Proteins 0.000 description 1
- 102220475128 Ectodysplasin-A_W33A_mutation Human genes 0.000 description 1
- YQYJSBFKSSDGFO-UHFFFAOYSA-N Epihygromycin Natural products OC1C(O)C(C(=O)C)OC1OC(C(=C1)O)=CC=C1C=C(C)C(=O)NC1C(O)C(O)C2OCOC2C1O YQYJSBFKSSDGFO-UHFFFAOYSA-N 0.000 description 1
- 108091029865 Exogenous DNA Proteins 0.000 description 1
- 108010029144 Factor IIa Proteins 0.000 description 1
- 108010048049 Factor IXa Proteins 0.000 description 1
- 108010014173 Factor X Proteins 0.000 description 1
- 108010074860 Factor Xa Proteins 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 102000003688 G-Protein-Coupled Receptors Human genes 0.000 description 1
- 108090000045 G-Protein-Coupled Receptors Proteins 0.000 description 1
- HHWQMFIGMMOVFK-WDSKDSINSA-N Gln-Ala-Gly Chemical compound OC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)CCC(N)=O HHWQMFIGMMOVFK-WDSKDSINSA-N 0.000 description 1
- LMPBBFWHCRURJD-LAEOZQHASA-N Gln-Asn-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCC(=O)N)N LMPBBFWHCRURJD-LAEOZQHASA-N 0.000 description 1
- UICOTGULOUGGLC-NUMRIWBASA-N Gln-Asp-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)N)N)O UICOTGULOUGGLC-NUMRIWBASA-N 0.000 description 1
- DRDSQGHKTLSNEA-GLLZPBPUSA-N Gln-Glu-Thr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O DRDSQGHKTLSNEA-GLLZPBPUSA-N 0.000 description 1
- XKBASPWPBXNVLQ-WDSKDSINSA-N Gln-Gly-Asn Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O XKBASPWPBXNVLQ-WDSKDSINSA-N 0.000 description 1
- ZEEPYMXTJWIMSN-GUBZILKMSA-N Gln-Lys-Ser Chemical compound NCCCC[C@@H](C(=O)N[C@@H](CO)C(O)=O)NC(=O)[C@@H](N)CCC(N)=O ZEEPYMXTJWIMSN-GUBZILKMSA-N 0.000 description 1
- MSHXWFKYXJTLEZ-CIUDSAMLSA-N Gln-Met-Asn Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N MSHXWFKYXJTLEZ-CIUDSAMLSA-N 0.000 description 1
- SYZZMPFLOLSMHL-XHNCKOQMSA-N Gln-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)N)N)C(=O)O SYZZMPFLOLSMHL-XHNCKOQMSA-N 0.000 description 1
- UEILCTONAMOGBR-RWRJDSDZSA-N Gln-Thr-Ile Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O UEILCTONAMOGBR-RWRJDSDZSA-N 0.000 description 1
- IRDASPPCLZIERZ-XHNCKOQMSA-N Glu-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)O)N IRDASPPCLZIERZ-XHNCKOQMSA-N 0.000 description 1
- CGYDXNKRIMJMLV-GUBZILKMSA-N Glu-Arg-Glu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O CGYDXNKRIMJMLV-GUBZILKMSA-N 0.000 description 1
- LJLPOZGRPLORTF-CIUDSAMLSA-N Glu-Asn-Met Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O LJLPOZGRPLORTF-CIUDSAMLSA-N 0.000 description 1
- CKOFNWCLWRYUHK-XHNCKOQMSA-N Glu-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)O)N)C(=O)O CKOFNWCLWRYUHK-XHNCKOQMSA-N 0.000 description 1
- XKPOCESCRTVRPL-KBIXCLLPSA-N Glu-Cys-Ile Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O XKPOCESCRTVRPL-KBIXCLLPSA-N 0.000 description 1
- HUFCEIHAFNVSNR-IHRRRGAJSA-N Glu-Gln-Tyr Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 HUFCEIHAFNVSNR-IHRRRGAJSA-N 0.000 description 1
- AUTNXSQEVVHSJK-YVNDNENWSA-N Glu-Glu-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O AUTNXSQEVVHSJK-YVNDNENWSA-N 0.000 description 1
- MTAOBYXRYJZRGQ-WDSKDSINSA-N Glu-Gly-Asp Chemical compound OC(=O)CC[C@H](N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(O)=O MTAOBYXRYJZRGQ-WDSKDSINSA-N 0.000 description 1
- VSRCAOIHMGCIJK-SRVKXCTJSA-N Glu-Leu-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O VSRCAOIHMGCIJK-SRVKXCTJSA-N 0.000 description 1
- QDMVXRNLOPTPIE-WDCWCFNPSA-N Glu-Lys-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QDMVXRNLOPTPIE-WDCWCFNPSA-N 0.000 description 1
- YHOJJFFTSMWVGR-HJGDQZAQSA-N Glu-Met-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O YHOJJFFTSMWVGR-HJGDQZAQSA-N 0.000 description 1
- QOXDAWODGSIDDI-GUBZILKMSA-N Glu-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)O)N QOXDAWODGSIDDI-GUBZILKMSA-N 0.000 description 1
- YQAQQKPWFOBSMU-WDCWCFNPSA-N Glu-Thr-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O YQAQQKPWFOBSMU-WDCWCFNPSA-N 0.000 description 1
- HQTDNEZTGZUWSY-XVKPBYJWSA-N Glu-Val-Gly Chemical compound CC(C)[C@H](NC(=O)[C@@H](N)CCC(O)=O)C(=O)NCC(O)=O HQTDNEZTGZUWSY-XVKPBYJWSA-N 0.000 description 1
- ZYRXTRTUCAVNBQ-GVXVVHGQSA-N Glu-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N ZYRXTRTUCAVNBQ-GVXVVHGQSA-N 0.000 description 1
- NTNUEBVGKMVANB-NHCYSSNCSA-N Glu-Val-Met Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCSC)C(O)=O NTNUEBVGKMVANB-NHCYSSNCSA-N 0.000 description 1
- FVGOGEGGQLNZGH-DZKIICNBSA-N Glu-Val-Phe Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 FVGOGEGGQLNZGH-DZKIICNBSA-N 0.000 description 1
- PUUYVMYCMIWHFE-BQBZGAKWSA-N Gly-Ala-Arg Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N PUUYVMYCMIWHFE-BQBZGAKWSA-N 0.000 description 1
- JRDYDYXZKFNNRQ-XPUUQOCRSA-N Gly-Ala-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)CN JRDYDYXZKFNNRQ-XPUUQOCRSA-N 0.000 description 1
- KRRMJKMGWWXWDW-STQMWFEESA-N Gly-Arg-Phe Chemical compound NC(=N)NCCC[C@H](NC(=O)CN)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 KRRMJKMGWWXWDW-STQMWFEESA-N 0.000 description 1
- VXKCPBPQEKKERH-IUCAKERBSA-N Gly-Arg-Pro Chemical compound NC(N)=NCCC[C@H](NC(=O)CN)C(=O)N1CCC[C@H]1C(O)=O VXKCPBPQEKKERH-IUCAKERBSA-N 0.000 description 1
- DTPOVRRYXPJJAZ-FJXKBIBVSA-N Gly-Arg-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CCCN=C(N)N DTPOVRRYXPJJAZ-FJXKBIBVSA-N 0.000 description 1
- XZRZILPOZBVTDB-GJZGRUSLSA-N Gly-Arg-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CN)C(O)=O)=CNC2=C1 XZRZILPOZBVTDB-GJZGRUSLSA-N 0.000 description 1
- LCNXZQROPKFGQK-WHFBIAKZSA-N Gly-Asp-Ser Chemical compound NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O LCNXZQROPKFGQK-WHFBIAKZSA-N 0.000 description 1
- XTQFHTHIAKKCTM-YFKPBYRVSA-N Gly-Glu-Gly Chemical compound NCC(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O XTQFHTHIAKKCTM-YFKPBYRVSA-N 0.000 description 1
- CUYLIWAAAYJKJH-RYUDHWBXSA-N Gly-Glu-Tyr Chemical compound NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 CUYLIWAAAYJKJH-RYUDHWBXSA-N 0.000 description 1
- HQRHFUYMGCHHJS-LURJTMIESA-N Gly-Gly-Arg Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CCCN=C(N)N HQRHFUYMGCHHJS-LURJTMIESA-N 0.000 description 1
- HHSOPSCKAZKQHQ-PEXQALLHSA-N Gly-His-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)CN HHSOPSCKAZKQHQ-PEXQALLHSA-N 0.000 description 1
- UESJMAMHDLEHGM-NHCYSSNCSA-N Gly-Ile-Leu Chemical compound NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O UESJMAMHDLEHGM-NHCYSSNCSA-N 0.000 description 1
- PAWIVEIWWYGBAM-YUMQZZPRSA-N Gly-Leu-Ala Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O PAWIVEIWWYGBAM-YUMQZZPRSA-N 0.000 description 1
- PCPOYRCAHPJXII-UWVGGRQHSA-N Gly-Lys-Met Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCSC)C(O)=O PCPOYRCAHPJXII-UWVGGRQHSA-N 0.000 description 1
- GAFKBWKVXNERFA-QWRGUYRKSA-N Gly-Phe-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CC1=CC=CC=C1 GAFKBWKVXNERFA-QWRGUYRKSA-N 0.000 description 1
- WNGHUXFWEWTKAO-YUMQZZPRSA-N Gly-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)CN WNGHUXFWEWTKAO-YUMQZZPRSA-N 0.000 description 1
- ZKJZBRHRWKLVSJ-ZDLURKLDSA-N Gly-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)CN)O ZKJZBRHRWKLVSJ-ZDLURKLDSA-N 0.000 description 1
- ZZWUYQXMIFTIIY-WEDXCCLWSA-N Gly-Thr-Leu Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O ZZWUYQXMIFTIIY-WEDXCCLWSA-N 0.000 description 1
- NIOPEYHPOBWLQO-KBPBESRZSA-N Gly-Trp-Glu Chemical compound NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(O)=O)C(O)=O NIOPEYHPOBWLQO-KBPBESRZSA-N 0.000 description 1
- PYFIQROSWQERAS-LBPRGKRZSA-N Gly-Trp-Gly Chemical compound C1=CC=C2C(C[C@H](NC(=O)CN)C(=O)NCC(O)=O)=CNC2=C1 PYFIQROSWQERAS-LBPRGKRZSA-N 0.000 description 1
- HQSKKSLNLSTONK-JTQLQIEISA-N Gly-Tyr-Gly Chemical compound OC(=O)CNC(=O)[C@@H](NC(=O)CN)CC1=CC=C(O)C=C1 HQSKKSLNLSTONK-JTQLQIEISA-N 0.000 description 1
- KBBFOULZCHWGJX-KBPBESRZSA-N Gly-Tyr-His Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)NC(=O)CN)O KBBFOULZCHWGJX-KBPBESRZSA-N 0.000 description 1
- NGBGZCUWFVVJKC-IRXDYDNUSA-N Gly-Tyr-Tyr Chemical compound C([C@H](NC(=O)CN)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 NGBGZCUWFVVJKC-IRXDYDNUSA-N 0.000 description 1
- IZVICCORZOSGPT-JSGCOSHPSA-N Gly-Val-Tyr Chemical compound [H]NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O IZVICCORZOSGPT-JSGCOSHPSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- RVKIPWVMZANZLI-UHFFFAOYSA-N H-Lys-Trp-OH Natural products C1=CC=C2C(CC(NC(=O)C(N)CCCCN)C(O)=O)=CNC2=C1 RVKIPWVMZANZLI-UHFFFAOYSA-N 0.000 description 1
- 102220576068 HLA class I histocompatibility antigen, B alpha chain_Y33D_mutation Human genes 0.000 description 1
- 102100021519 Hemoglobin subunit beta Human genes 0.000 description 1
- 108091005904 Hemoglobin subunit beta Proteins 0.000 description 1
- 208000016988 Hemorrhagic Stroke Diseases 0.000 description 1
- MAABHGXCIBEYQR-XVYDVKMFSA-N His-Asn-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC1=CN=CN1)N MAABHGXCIBEYQR-XVYDVKMFSA-N 0.000 description 1
- CMQOGWZUKPHLHL-DCAQKATOSA-N His-Cys-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CC1=CN=CN1)N CMQOGWZUKPHLHL-DCAQKATOSA-N 0.000 description 1
- HIAHVKLTHNOENC-HGNGGELXSA-N His-Glu-Ala Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O HIAHVKLTHNOENC-HGNGGELXSA-N 0.000 description 1
- JJHWJUYYTWYXPL-PYJNHQTQSA-N His-Ile-Arg Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CC1=CN=CN1 JJHWJUYYTWYXPL-PYJNHQTQSA-N 0.000 description 1
- RNMNYMDTESKEAJ-KKUMJFAQSA-N His-Leu-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CN=CN1 RNMNYMDTESKEAJ-KKUMJFAQSA-N 0.000 description 1
- GNBHSMFBUNEWCJ-DCAQKATOSA-N His-Pro-Asn Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(O)=O GNBHSMFBUNEWCJ-DCAQKATOSA-N 0.000 description 1
- HZWWOGWOBQBETJ-CUJWVEQBSA-N His-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC1=CN=CN1)N)O HZWWOGWOBQBETJ-CUJWVEQBSA-N 0.000 description 1
- CSTDQOOBZBAJKE-BWAGICSOSA-N His-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](CC2=CN=CN2)N)O CSTDQOOBZBAJKE-BWAGICSOSA-N 0.000 description 1
- 101000914241 Homo sapiens Centromere protein C Proteins 0.000 description 1
- 206010020608 Hypercoagulation Diseases 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- VAXBXNPRXPHGHG-BJDJZHNGSA-N Ile-Ala-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)O)N VAXBXNPRXPHGHG-BJDJZHNGSA-N 0.000 description 1
- SACHLUOUHCVIKI-GMOBBJLQSA-N Ile-Arg-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N SACHLUOUHCVIKI-GMOBBJLQSA-N 0.000 description 1
- YOTNPRLPIPHQSB-XUXIUFHCSA-N Ile-Arg-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)O)N YOTNPRLPIPHQSB-XUXIUFHCSA-N 0.000 description 1
- FADXGVVLSPPEQY-GHCJXIJMSA-N Ile-Cys-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(=O)N)C(=O)O)N FADXGVVLSPPEQY-GHCJXIJMSA-N 0.000 description 1
- FZWVCYCYWCLQDH-NHCYSSNCSA-N Ile-Leu-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)O)N FZWVCYCYWCLQDH-NHCYSSNCSA-N 0.000 description 1
- ADDYYRVQQZFIMW-MNXVOIDGSA-N Ile-Lys-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N ADDYYRVQQZFIMW-MNXVOIDGSA-N 0.000 description 1
- JHNJNTMTZHEDLJ-NAKRPEOUSA-N Ile-Ser-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O JHNJNTMTZHEDLJ-NAKRPEOUSA-N 0.000 description 1
- VGSPNSSCMOHRRR-BJDJZHNGSA-N Ile-Ser-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)O)N VGSPNSSCMOHRRR-BJDJZHNGSA-N 0.000 description 1
- HQLSBZFLOUHQJK-STECZYCISA-N Ile-Tyr-Arg Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N HQLSBZFLOUHQJK-STECZYCISA-N 0.000 description 1
- PRTZQMBYUZFSFA-XEGUGMAKSA-N Ile-Tyr-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)NCC(=O)O)N PRTZQMBYUZFSFA-XEGUGMAKSA-N 0.000 description 1
- 108700005091 Immunoglobulin Genes Proteins 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- RCFDOSNHHZGBOY-UHFFFAOYSA-N L-isoleucyl-L-alanine Natural products CCC(C)C(N)C(=O)NC(C)C(O)=O RCFDOSNHHZGBOY-UHFFFAOYSA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- TYYLDKGBCJGJGW-UHFFFAOYSA-N L-tryptophan-L-tyrosine Natural products C=1NC2=CC=CC=C2C=1CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 TYYLDKGBCJGJGW-UHFFFAOYSA-N 0.000 description 1
- CQQGCWPXDHTTNF-GUBZILKMSA-N Leu-Ala-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCC(O)=O CQQGCWPXDHTTNF-GUBZILKMSA-N 0.000 description 1
- BAJIJEGGUYXZGC-CIUDSAMLSA-N Leu-Asn-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CS)C(=O)O)N BAJIJEGGUYXZGC-CIUDSAMLSA-N 0.000 description 1
- RFUBXQQFJFGJFV-GUBZILKMSA-N Leu-Asn-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O RFUBXQQFJFGJFV-GUBZILKMSA-N 0.000 description 1
- KTFHTMHHKXUYPW-ZPFDUUQYSA-N Leu-Asp-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O KTFHTMHHKXUYPW-ZPFDUUQYSA-N 0.000 description 1
- GBDMISNMNXVTNV-XIRDDKMYSA-N Leu-Asp-Trp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O GBDMISNMNXVTNV-XIRDDKMYSA-N 0.000 description 1
- IIKJNQWOQIWWMR-CIUDSAMLSA-N Leu-Cys-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CC(C)C)N IIKJNQWOQIWWMR-CIUDSAMLSA-N 0.000 description 1
- DXYBNWJZJVSZAE-GUBZILKMSA-N Leu-Gln-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CS)C(=O)O)N DXYBNWJZJVSZAE-GUBZILKMSA-N 0.000 description 1
- RVVBWTWPNFDYBE-SRVKXCTJSA-N Leu-Glu-Arg Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O RVVBWTWPNFDYBE-SRVKXCTJSA-N 0.000 description 1
- LAPSXOAUPNOINL-YUMQZZPRSA-N Leu-Gly-Asp Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC(O)=O LAPSXOAUPNOINL-YUMQZZPRSA-N 0.000 description 1
- HYMLKESRWLZDBR-WEDXCCLWSA-N Leu-Gly-Thr Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O HYMLKESRWLZDBR-WEDXCCLWSA-N 0.000 description 1
- CSFVADKICPDRRF-KKUMJFAQSA-N Leu-His-Leu Chemical compound CC(C)C[C@H]([NH3+])C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C([O-])=O)CC1=CN=CN1 CSFVADKICPDRRF-KKUMJFAQSA-N 0.000 description 1
- YOKVEHGYYQEQOP-QWRGUYRKSA-N Leu-Leu-Gly Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O YOKVEHGYYQEQOP-QWRGUYRKSA-N 0.000 description 1
- KYIIALJHAOIAHF-KKUMJFAQSA-N Leu-Leu-His Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 KYIIALJHAOIAHF-KKUMJFAQSA-N 0.000 description 1
- UBZGNBKMIJHOHL-BZSNNMDCSA-N Leu-Leu-Phe Chemical compound CC(C)C[C@H]([NH3+])C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C([O-])=O)CC1=CC=CC=C1 UBZGNBKMIJHOHL-BZSNNMDCSA-N 0.000 description 1
- BMVFXOQHDQZAQU-DCAQKATOSA-N Leu-Pro-Asp Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(=O)O)C(=O)O)N BMVFXOQHDQZAQU-DCAQKATOSA-N 0.000 description 1
- UCBPDSYUVAAHCD-UWVGGRQHSA-N Leu-Pro-Gly Chemical compound CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O UCBPDSYUVAAHCD-UWVGGRQHSA-N 0.000 description 1
- ZJZNLRVCZWUONM-JXUBOQSCSA-N Leu-Thr-Ala Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O ZJZNLRVCZWUONM-JXUBOQSCSA-N 0.000 description 1
- LINKCQUOMUDLKN-KATARQTJSA-N Leu-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(C)C)N)O LINKCQUOMUDLKN-KATARQTJSA-N 0.000 description 1
- DAYQSYGBCUKVKT-VOAKCMCISA-N Leu-Thr-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(O)=O DAYQSYGBCUKVKT-VOAKCMCISA-N 0.000 description 1
- YQFZRHYZLARWDY-IHRRRGAJSA-N Leu-Val-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCCCN YQFZRHYZLARWDY-IHRRRGAJSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- KCXUCYYZNZFGLL-SRVKXCTJSA-N Lys-Ala-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O KCXUCYYZNZFGLL-SRVKXCTJSA-N 0.000 description 1
- ALSRJRIWBNENFY-DCAQKATOSA-N Lys-Arg-Asn Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(O)=O ALSRJRIWBNENFY-DCAQKATOSA-N 0.000 description 1
- YKIRNDPUWONXQN-GUBZILKMSA-N Lys-Asn-Gln Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N YKIRNDPUWONXQN-GUBZILKMSA-N 0.000 description 1
- MWVUEPNEPWMFBD-SRVKXCTJSA-N Lys-Cys-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@H](C(O)=O)CCCCN MWVUEPNEPWMFBD-SRVKXCTJSA-N 0.000 description 1
- GJJQCBVRWDGLMQ-GUBZILKMSA-N Lys-Glu-Ala Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O GJJQCBVRWDGLMQ-GUBZILKMSA-N 0.000 description 1
- ODUQLUADRKMHOZ-JYJNAYRXSA-N Lys-Glu-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCCCN)N)O ODUQLUADRKMHOZ-JYJNAYRXSA-N 0.000 description 1
- RFQATBGBLDAKGI-VHSXEESVSA-N Lys-Gly-Pro Chemical compound C1C[C@@H](N(C1)C(=O)CNC(=O)[C@H](CCCCN)N)C(=O)O RFQATBGBLDAKGI-VHSXEESVSA-N 0.000 description 1
- IZJGPPIGYTVXLB-FQUUOJAGSA-N Lys-Ile-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCCN)N IZJGPPIGYTVXLB-FQUUOJAGSA-N 0.000 description 1
- HVAUKHLDSDDROB-KKUMJFAQSA-N Lys-Lys-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O HVAUKHLDSDDROB-KKUMJFAQSA-N 0.000 description 1
- LUTDBHBIHHREDC-IHRRRGAJSA-N Lys-Pro-Lys Chemical compound NCCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O LUTDBHBIHHREDC-IHRRRGAJSA-N 0.000 description 1
- CTJUSALVKAWFFU-CIUDSAMLSA-N Lys-Ser-Cys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N CTJUSALVKAWFFU-CIUDSAMLSA-N 0.000 description 1
- MIFFFXHMAHFACR-KATARQTJSA-N Lys-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CCCCN MIFFFXHMAHFACR-KATARQTJSA-N 0.000 description 1
- YKBSXQFZWFXFIB-VOAKCMCISA-N Lys-Thr-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CCCCN)C(O)=O YKBSXQFZWFXFIB-VOAKCMCISA-N 0.000 description 1
- UGCIQUYEJIEHKX-GVXVVHGQSA-N Lys-Val-Glu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O UGCIQUYEJIEHKX-GVXVVHGQSA-N 0.000 description 1
- GILLQRYAWOMHED-DCAQKATOSA-N Lys-Val-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCCN GILLQRYAWOMHED-DCAQKATOSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- GODBLDDYHFTUAH-CIUDSAMLSA-N Met-Asp-Glu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCC(O)=O GODBLDDYHFTUAH-CIUDSAMLSA-N 0.000 description 1
- FYRUJIJAUPHUNB-IUCAKERBSA-N Met-Gly-Arg Chemical compound CSCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCNC(N)=N FYRUJIJAUPHUNB-IUCAKERBSA-N 0.000 description 1
- RBGLBUDVQVPTEG-DCAQKATOSA-N Met-Leu-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCSC)N RBGLBUDVQVPTEG-DCAQKATOSA-N 0.000 description 1
- FMYLZGQFKPHXHI-GUBZILKMSA-N Met-Met-Ala Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C)C(O)=O FMYLZGQFKPHXHI-GUBZILKMSA-N 0.000 description 1
- RKRFGIBULDYDPF-XIRDDKMYSA-N Met-Trp-Gln Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N RKRFGIBULDYDPF-XIRDDKMYSA-N 0.000 description 1
- GHQFLTYXGUETFD-UFYCRDLUSA-N Met-Tyr-Tyr Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)N GHQFLTYXGUETFD-UFYCRDLUSA-N 0.000 description 1
- LBSWWNKMVPAXOI-GUBZILKMSA-N Met-Val-Ser Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O LBSWWNKMVPAXOI-GUBZILKMSA-N 0.000 description 1
- 102000016943 Muramidase Human genes 0.000 description 1
- 108010014251 Muramidase Proteins 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 101000924587 Mus musculus Adenomatous polyposis coli protein Proteins 0.000 description 1
- 108010062010 N-Acetylmuramoyl-L-alanine Amidase Proteins 0.000 description 1
- WYBVBIHNJWOLCJ-UHFFFAOYSA-N N-L-arginyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCCN=C(N)N WYBVBIHNJWOLCJ-UHFFFAOYSA-N 0.000 description 1
- 108010087066 N2-tryptophyllysine Proteins 0.000 description 1
- 108010047562 NGR peptide Proteins 0.000 description 1
- 241001045988 Neogene Species 0.000 description 1
- 101100342977 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) leu-1 gene Proteins 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- 241000009328 Perro Species 0.000 description 1
- HTTYNOXBBOWZTB-SRVKXCTJSA-N Phe-Asn-Asn Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC(=O)N)C(=O)O)N HTTYNOXBBOWZTB-SRVKXCTJSA-N 0.000 description 1
- JOXIIFVCSATTDH-IHPCNDPISA-N Phe-Asn-Trp Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O)N JOXIIFVCSATTDH-IHPCNDPISA-N 0.000 description 1
- IQXOZIDWLZYYAW-IHRRRGAJSA-N Phe-Asp-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)N IQXOZIDWLZYYAW-IHRRRGAJSA-N 0.000 description 1
- OJUMUUXGSXUZJZ-SRVKXCTJSA-N Phe-Asp-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O OJUMUUXGSXUZJZ-SRVKXCTJSA-N 0.000 description 1
- CUMXHKAOHNWRFQ-BZSNNMDCSA-N Phe-Asp-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=CC=C1 CUMXHKAOHNWRFQ-BZSNNMDCSA-N 0.000 description 1
- FRPVPGRXUKFEQE-YDHLFZDLSA-N Phe-Asp-Val Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O FRPVPGRXUKFEQE-YDHLFZDLSA-N 0.000 description 1
- SFKOEHXABNPLRT-KBPBESRZSA-N Phe-His-Gly Chemical compound N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)NCC(O)=O SFKOEHXABNPLRT-KBPBESRZSA-N 0.000 description 1
- WEMYTDDMDBLPMI-DKIMLUQUSA-N Phe-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)N WEMYTDDMDBLPMI-DKIMLUQUSA-N 0.000 description 1
- YVXPUUOTMVBKDO-IHRRRGAJSA-N Phe-Pro-Cys Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)N)C(=O)N[C@@H](CS)C(=O)O YVXPUUOTMVBKDO-IHRRRGAJSA-N 0.000 description 1
- ZJPGOXWRFNKIQL-JYJNAYRXSA-N Phe-Pro-Pro Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(O)=O)C1=CC=CC=C1 ZJPGOXWRFNKIQL-JYJNAYRXSA-N 0.000 description 1
- YMIZSYUAZJSOFL-SRVKXCTJSA-N Phe-Ser-Asn Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O YMIZSYUAZJSOFL-SRVKXCTJSA-N 0.000 description 1
- SJRQWEDYTKYHHL-SLFFLAALSA-N Phe-Tyr-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CC3=CC=CC=C3)N)C(=O)O SJRQWEDYTKYHHL-SLFFLAALSA-N 0.000 description 1
- JTKGCYOOJLUETJ-ULQDDVLXSA-N Phe-Val-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CC1=CC=CC=C1 JTKGCYOOJLUETJ-ULQDDVLXSA-N 0.000 description 1
- 206010035226 Plasma cell myeloma Diseases 0.000 description 1
- 208000013544 Platelet disease Diseases 0.000 description 1
- 229920002562 Polyethylene Glycol 3350 Polymers 0.000 description 1
- 229920001030 Polyethylene Glycol 4000 Polymers 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- OOLOTUZJUBOMAX-GUBZILKMSA-N Pro-Ala-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O OOLOTUZJUBOMAX-GUBZILKMSA-N 0.000 description 1
- MLQVJYMFASXBGZ-IHRRRGAJSA-N Pro-Asn-Tyr Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O MLQVJYMFASXBGZ-IHRRRGAJSA-N 0.000 description 1
- ZCXQTRXYZOSGJR-FXQIFTODSA-N Pro-Asp-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O ZCXQTRXYZOSGJR-FXQIFTODSA-N 0.000 description 1
- LANQLYHLMYDWJP-SRVKXCTJSA-N Pro-Gln-Lys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCCCN)C(=O)O LANQLYHLMYDWJP-SRVKXCTJSA-N 0.000 description 1
- KIPIKSXPPLABPN-CIUDSAMLSA-N Pro-Glu-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1 KIPIKSXPPLABPN-CIUDSAMLSA-N 0.000 description 1
- HAAQQNHQZBOWFO-LURJTMIESA-N Pro-Gly-Gly Chemical compound OC(=O)CNC(=O)CNC(=O)[C@@H]1CCCN1 HAAQQNHQZBOWFO-LURJTMIESA-N 0.000 description 1
- FDINZVJXLPILKV-DCAQKATOSA-N Pro-His-Asn Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(N)=O)C(O)=O FDINZVJXLPILKV-DCAQKATOSA-N 0.000 description 1
- FYXCBXDAMPEHIQ-FHWLQOOXSA-N Pro-Trp-Lys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)N[C@@H](CCCCN)C(=O)O FYXCBXDAMPEHIQ-FHWLQOOXSA-N 0.000 description 1
- FIDNSJUXESUDOV-JYJNAYRXSA-N Pro-Tyr-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(O)=O FIDNSJUXESUDOV-JYJNAYRXSA-N 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 201000005660 Protein C Deficiency Diseases 0.000 description 1
- 239000012614 Q-Sepharose Substances 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 230000010757 Reduction Activity Effects 0.000 description 1
- 108020005091 Replication Origin Proteins 0.000 description 1
- BTKUIVBNGBFTTP-WHFBIAKZSA-N Ser-Ala-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)NCC(O)=O BTKUIVBNGBFTTP-WHFBIAKZSA-N 0.000 description 1
- JPIDMRXXNMIVKY-VZFHVOOUSA-N Ser-Ala-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JPIDMRXXNMIVKY-VZFHVOOUSA-N 0.000 description 1
- YUSRGTQIPCJNHQ-CIUDSAMLSA-N Ser-Arg-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O YUSRGTQIPCJNHQ-CIUDSAMLSA-N 0.000 description 1
- WXUBSIDKNMFAGS-IHRRRGAJSA-N Ser-Arg-Tyr Chemical compound NC(N)=NCCC[C@H](NC(=O)[C@H](CO)N)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 WXUBSIDKNMFAGS-IHRRRGAJSA-N 0.000 description 1
- COAHUSQNSVFYBW-FXQIFTODSA-N Ser-Asn-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O COAHUSQNSVFYBW-FXQIFTODSA-N 0.000 description 1
- ICHZYBVODUVUKN-SRVKXCTJSA-N Ser-Asn-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ICHZYBVODUVUKN-SRVKXCTJSA-N 0.000 description 1
- QPFJSHSJFIYDJZ-GHCJXIJMSA-N Ser-Asp-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CO QPFJSHSJFIYDJZ-GHCJXIJMSA-N 0.000 description 1
- MUARUIBTKQJKFY-WHFBIAKZSA-N Ser-Gly-Asp Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(O)=O MUARUIBTKQJKFY-WHFBIAKZSA-N 0.000 description 1
- GZFAWAQTEYDKII-YUMQZZPRSA-N Ser-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO GZFAWAQTEYDKII-YUMQZZPRSA-N 0.000 description 1
- KDGARKCAKHBEDB-NKWVEPMBSA-N Ser-Gly-Pro Chemical compound C1C[C@@H](N(C1)C(=O)CNC(=O)[C@H](CO)N)C(=O)O KDGARKCAKHBEDB-NKWVEPMBSA-N 0.000 description 1
- CICQXRWZNVXFCU-SRVKXCTJSA-N Ser-His-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(O)=O CICQXRWZNVXFCU-SRVKXCTJSA-N 0.000 description 1
- NLOAIFSWUUFQFR-CIUDSAMLSA-N Ser-Leu-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O NLOAIFSWUUFQFR-CIUDSAMLSA-N 0.000 description 1
- HDBOEVPDIDDEPC-CIUDSAMLSA-N Ser-Lys-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O HDBOEVPDIDDEPC-CIUDSAMLSA-N 0.000 description 1
- CRJZZXMAADSBBQ-SRVKXCTJSA-N Ser-Lys-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CO CRJZZXMAADSBBQ-SRVKXCTJSA-N 0.000 description 1
- FPCGZYMRFFIYIH-CIUDSAMLSA-N Ser-Lys-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O FPCGZYMRFFIYIH-CIUDSAMLSA-N 0.000 description 1
- AMRRYKHCILPAKD-FXQIFTODSA-N Ser-Met-Asn Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CO)N AMRRYKHCILPAKD-FXQIFTODSA-N 0.000 description 1
- UPLYXVPQLJVWMM-KKUMJFAQSA-N Ser-Phe-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(O)=O UPLYXVPQLJVWMM-KKUMJFAQSA-N 0.000 description 1
- HHJFMHQYEAAOBM-ZLUOBGJFSA-N Ser-Ser-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O HHJFMHQYEAAOBM-ZLUOBGJFSA-N 0.000 description 1
- KQNDIKOYWZTZIX-FXQIFTODSA-N Ser-Ser-Arg Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCCNC(N)=N KQNDIKOYWZTZIX-FXQIFTODSA-N 0.000 description 1
- GYDFRTRSSXOZCR-ACZMJKKPSA-N Ser-Ser-Glu Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O GYDFRTRSSXOZCR-ACZMJKKPSA-N 0.000 description 1
- OZPDGESCTGGNAD-CIUDSAMLSA-N Ser-Ser-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CO OZPDGESCTGGNAD-CIUDSAMLSA-N 0.000 description 1
- XQJCEKXQUJQNNK-ZLUOBGJFSA-N Ser-Ser-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O XQJCEKXQUJQNNK-ZLUOBGJFSA-N 0.000 description 1
- QNBVFKZSSRYNFX-CUJWVEQBSA-N Ser-Thr-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CO)N)O QNBVFKZSSRYNFX-CUJWVEQBSA-N 0.000 description 1
- VLMIUSLQONKLDV-HEIBUPTGSA-N Ser-Thr-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VLMIUSLQONKLDV-HEIBUPTGSA-N 0.000 description 1
- ZKOKTQPHFMRSJP-YJRXYDGGSA-N Ser-Thr-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ZKOKTQPHFMRSJP-YJRXYDGGSA-N 0.000 description 1
- FZNNGIHSIPKFRE-QEJZJMRPSA-N Ser-Trp-Gln Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(N)=O)C(O)=O FZNNGIHSIPKFRE-QEJZJMRPSA-N 0.000 description 1
- BCAVNDNYOGTQMQ-AAEUAGOBSA-N Ser-Trp-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)NCC(O)=O BCAVNDNYOGTQMQ-AAEUAGOBSA-N 0.000 description 1
- FHXGMDRKJHKLKW-QWRGUYRKSA-N Ser-Tyr-Gly Chemical compound OC[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CC=C(O)C=C1 FHXGMDRKJHKLKW-QWRGUYRKSA-N 0.000 description 1
- VVKVHAOOUGNDPJ-SRVKXCTJSA-N Ser-Tyr-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(O)=O VVKVHAOOUGNDPJ-SRVKXCTJSA-N 0.000 description 1
- BIWBTRRBHIEVAH-IHPCNDPISA-N Ser-Tyr-Trp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O BIWBTRRBHIEVAH-IHPCNDPISA-N 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 102000008847 Serpin Human genes 0.000 description 1
- 108050000761 Serpin Proteins 0.000 description 1
- 108010034546 Serratia marcescens nuclease Proteins 0.000 description 1
- 201000004283 Shwachman-Diamond syndrome Diseases 0.000 description 1
- 241000282898 Sus scrofa Species 0.000 description 1
- 239000004098 Tetracycline Substances 0.000 description 1
- 102220536450 Thiamine-triphosphatase_W53A_mutation Human genes 0.000 description 1
- LVHHEVGYAZGXDE-KDXUFGMBSA-N Thr-Ala-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C)C(=O)N1CCC[C@@H]1C(=O)O)N)O LVHHEVGYAZGXDE-KDXUFGMBSA-N 0.000 description 1
- XSLXHSYIVPGEER-KZVJFYERSA-N Thr-Ala-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O XSLXHSYIVPGEER-KZVJFYERSA-N 0.000 description 1
- XYEXCEPTALHNEV-RCWTZXSCSA-N Thr-Arg-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O XYEXCEPTALHNEV-RCWTZXSCSA-N 0.000 description 1
- CEXFELBFVHLYDZ-XGEHTFHBSA-N Thr-Arg-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O CEXFELBFVHLYDZ-XGEHTFHBSA-N 0.000 description 1
- YSXYEJWDHBCTDJ-DVJZZOLTSA-N Thr-Gly-Trp Chemical compound C[C@H]([C@@H](C(=O)NCC(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)N)O YSXYEJWDHBCTDJ-DVJZZOLTSA-N 0.000 description 1
- YUPVPKZBKCLFLT-QTKMDUPCSA-N Thr-His-Val Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](C(C)C)C(=O)O)N)O YUPVPKZBKCLFLT-QTKMDUPCSA-N 0.000 description 1
- FQPDRTDDEZXCEC-SVSWQMSJSA-N Thr-Ile-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O FQPDRTDDEZXCEC-SVSWQMSJSA-N 0.000 description 1
- IJVNLNRVDUTWDD-MEYUZBJRSA-N Thr-Leu-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O IJVNLNRVDUTWDD-MEYUZBJRSA-N 0.000 description 1
- JLNMFGCJODTXDH-WEDXCCLWSA-N Thr-Lys-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O JLNMFGCJODTXDH-WEDXCCLWSA-N 0.000 description 1
- DXPURPNJDFCKKO-RHYQMDGZSA-N Thr-Lys-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)[C@@H](C)O)C(O)=O DXPURPNJDFCKKO-RHYQMDGZSA-N 0.000 description 1
- NWECYMJLJGCBOD-UNQGMJICSA-N Thr-Phe-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C(C)C)C(O)=O NWECYMJLJGCBOD-UNQGMJICSA-N 0.000 description 1
- XKWABWFMQXMUMT-HJGDQZAQSA-N Thr-Pro-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O XKWABWFMQXMUMT-HJGDQZAQSA-N 0.000 description 1
- SGAOHNPSEPVAFP-ZDLURKLDSA-N Thr-Ser-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SGAOHNPSEPVAFP-ZDLURKLDSA-N 0.000 description 1
- YRJOLUDFVAUXLI-GSSVUCPTSA-N Thr-Thr-Asp Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC(O)=O YRJOLUDFVAUXLI-GSSVUCPTSA-N 0.000 description 1
- BKIOKSLLAAZYTC-KKHAAJSZSA-N Thr-Val-Asn Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O BKIOKSLLAAZYTC-KKHAAJSZSA-N 0.000 description 1
- VYVBSMCZNHOZGD-RCWTZXSCSA-N Thr-Val-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(O)=O VYVBSMCZNHOZGD-RCWTZXSCSA-N 0.000 description 1
- QNMIVTOQXUSGLN-SZMVWBNQSA-N Trp-Arg-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 QNMIVTOQXUSGLN-SZMVWBNQSA-N 0.000 description 1
- OBWQLWYNNZPWGX-QEJZJMRPSA-N Trp-Gln-Asp Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O OBWQLWYNNZPWGX-QEJZJMRPSA-N 0.000 description 1
- JLTQXEOXIJMCLZ-ZVZYQTTQSA-N Trp-Gln-Val Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O)=CNC2=C1 JLTQXEOXIJMCLZ-ZVZYQTTQSA-N 0.000 description 1
- YXONONCLMLHWJX-SZMVWBNQSA-N Trp-Glu-Leu Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O)=CNC2=C1 YXONONCLMLHWJX-SZMVWBNQSA-N 0.000 description 1
- OBAMASZCXDIXSS-SZMVWBNQSA-N Trp-Glu-Lys Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N OBAMASZCXDIXSS-SZMVWBNQSA-N 0.000 description 1
- UDCHKDYNMRJYMI-QEJZJMRPSA-N Trp-Glu-Ser Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O UDCHKDYNMRJYMI-QEJZJMRPSA-N 0.000 description 1
- SVGAWGVHFIYAEE-JSGCOSHPSA-N Trp-Gly-Gln Chemical compound C1=CC=C2C(C[C@H](N)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(O)=O)=CNC2=C1 SVGAWGVHFIYAEE-JSGCOSHPSA-N 0.000 description 1
- DNUJCLUFRGGSDJ-YLVFBTJISA-N Trp-Gly-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CC1=CNC2=CC=CC=C21)N DNUJCLUFRGGSDJ-YLVFBTJISA-N 0.000 description 1
- WVHUFSCKCBQKJW-HKUYNNGSSA-N Trp-Gly-Tyr Chemical compound C([C@H](NC(=O)CNC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)N)C(O)=O)C1=CC=C(O)C=C1 WVHUFSCKCBQKJW-HKUYNNGSSA-N 0.000 description 1
- MKDXQPMIQPTTAW-SIXJUCDHSA-N Trp-Ile-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC2=CNC3=CC=CC=C32)N MKDXQPMIQPTTAW-SIXJUCDHSA-N 0.000 description 1
- DDJHCLVUUBEIIA-BVSLBCMMSA-N Trp-Met-Phe Chemical compound C([C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C2=CC=CC=C2NC=1)CCSC)C(O)=O)C1=CC=CC=C1 DDJHCLVUUBEIIA-BVSLBCMMSA-N 0.000 description 1
- 102220469971 Tryptase delta_Y32A_mutation Human genes 0.000 description 1
- GAYLGYUVTDMLKC-UWJYBYFXSA-N Tyr-Asp-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 GAYLGYUVTDMLKC-UWJYBYFXSA-N 0.000 description 1
- RCLOWEZASFJFEX-KKUMJFAQSA-N Tyr-Asp-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 RCLOWEZASFJFEX-KKUMJFAQSA-N 0.000 description 1
- CTDPLKMBVALCGN-JSGCOSHPSA-N Tyr-Gly-Val Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O CTDPLKMBVALCGN-JSGCOSHPSA-N 0.000 description 1
- OGPKMBOPMDTEDM-IHRRRGAJSA-N Tyr-Met-Ser Chemical compound CSCC[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N OGPKMBOPMDTEDM-IHRRRGAJSA-N 0.000 description 1
- LMKKMCGTDANZTR-BZSNNMDCSA-N Tyr-Phe-Asp Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC(O)=O)C(O)=O)C1=CC=C(O)C=C1 LMKKMCGTDANZTR-BZSNNMDCSA-N 0.000 description 1
- ITDWWLTTWRRLCC-KJEVXHAQSA-N Tyr-Thr-Arg Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)[C@H]([C@H](O)C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 ITDWWLTTWRRLCC-KJEVXHAQSA-N 0.000 description 1
- PWKMJDQXKCENMF-MEYUZBJRSA-N Tyr-Thr-Leu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O PWKMJDQXKCENMF-MEYUZBJRSA-N 0.000 description 1
- CLEGSEJVGBYZBJ-MEYUZBJRSA-N Tyr-Thr-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H]([C@H](O)C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 CLEGSEJVGBYZBJ-MEYUZBJRSA-N 0.000 description 1
- NUQZCPSZHGIYTA-HKUYNNGSSA-N Tyr-Trp-Gly Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CC3=CC=C(C=C3)O)N NUQZCPSZHGIYTA-HKUYNNGSSA-N 0.000 description 1
- RMRFSFXLFWWAJZ-HJOGWXRNSA-N Tyr-Tyr-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 RMRFSFXLFWWAJZ-HJOGWXRNSA-N 0.000 description 1
- SQUMHUZLJDUROQ-YDHLFZDLSA-N Tyr-Val-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O SQUMHUZLJDUROQ-YDHLFZDLSA-N 0.000 description 1
- NWEGIYMHTZXVBP-JSGCOSHPSA-N Tyr-Val-Gly Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O NWEGIYMHTZXVBP-JSGCOSHPSA-N 0.000 description 1
- HZWPGKAKGYJWCI-ULQDDVLXSA-N Tyr-Val-Leu Chemical compound CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(O)=O HZWPGKAKGYJWCI-ULQDDVLXSA-N 0.000 description 1
- RVGVIWNHABGIFH-IHRRRGAJSA-N Tyr-Val-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O RVGVIWNHABGIFH-IHRRRGAJSA-N 0.000 description 1
- 108090000848 Ubiquitin Proteins 0.000 description 1
- 102000044159 Ubiquitin Human genes 0.000 description 1
- AZSHAZJLOZQYAY-FXQIFTODSA-N Val-Ala-Ser Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O AZSHAZJLOZQYAY-FXQIFTODSA-N 0.000 description 1
- SLLKXDSRVAOREO-KZVJFYERSA-N Val-Ala-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](C)NC(=O)[C@H](C(C)C)N)O SLLKXDSRVAOREO-KZVJFYERSA-N 0.000 description 1
- JYVKKBDANPZIAW-AVGNSLFASA-N Val-Arg-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C(C)C)N JYVKKBDANPZIAW-AVGNSLFASA-N 0.000 description 1
- GNWUWQAVVJQREM-NHCYSSNCSA-N Val-Asn-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N GNWUWQAVVJQREM-NHCYSSNCSA-N 0.000 description 1
- DDNIHOWRDOXXPF-NGZCFLSTSA-N Val-Asp-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N DDNIHOWRDOXXPF-NGZCFLSTSA-N 0.000 description 1
- PMDOQZFYGWZSTK-LSJOCFKGSA-N Val-Gly-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)C(C)C PMDOQZFYGWZSTK-LSJOCFKGSA-N 0.000 description 1
- URIRWLJVWHYLET-ONGXEEELSA-N Val-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)C(C)C URIRWLJVWHYLET-ONGXEEELSA-N 0.000 description 1
- YTUABZMPYKCWCQ-XQQFMLRXSA-N Val-His-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N2CCC[C@@H]2C(=O)O)N YTUABZMPYKCWCQ-XQQFMLRXSA-N 0.000 description 1
- OTJMMKPMLUNTQT-AVGNSLFASA-N Val-Leu-Arg Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](C(C)C)N OTJMMKPMLUNTQT-AVGNSLFASA-N 0.000 description 1
- FEXILLGKGGTLRI-NHCYSSNCSA-N Val-Leu-Asn Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](C(C)C)N FEXILLGKGGTLRI-NHCYSSNCSA-N 0.000 description 1
- XTDDIVQWDXMRJL-IHRRRGAJSA-N Val-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](C(C)C)N XTDDIVQWDXMRJL-IHRRRGAJSA-N 0.000 description 1
- HJSLDXZAZGFPDK-ULQDDVLXSA-N Val-Phe-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](C(C)C)N HJSLDXZAZGFPDK-ULQDDVLXSA-N 0.000 description 1
- USLVEJAHTBLSIL-CYDGBPFRSA-N Val-Pro-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)C(C)C USLVEJAHTBLSIL-CYDGBPFRSA-N 0.000 description 1
- AJNUKMZFHXUBMK-GUBZILKMSA-N Val-Ser-Arg Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N AJNUKMZFHXUBMK-GUBZILKMSA-N 0.000 description 1
- KSFXWENSJABBFI-ZKWXMUAHSA-N Val-Ser-Asn Chemical compound [H]N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O KSFXWENSJABBFI-ZKWXMUAHSA-N 0.000 description 1
- VHIZXDZMTDVFGX-DCAQKATOSA-N Val-Ser-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](C(C)C)N VHIZXDZMTDVFGX-DCAQKATOSA-N 0.000 description 1
- SDHZOOIGIUEPDY-JYJNAYRXSA-N Val-Ser-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](N)C(C)C)C(O)=O)=CNC2=C1 SDHZOOIGIUEPDY-JYJNAYRXSA-N 0.000 description 1
- NZYNRRGJJVSSTJ-GUBZILKMSA-N Val-Ser-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O NZYNRRGJJVSSTJ-GUBZILKMSA-N 0.000 description 1
- BZDGLJPROOOUOZ-XGEHTFHBSA-N Val-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](C(C)C)N)O BZDGLJPROOOUOZ-XGEHTFHBSA-N 0.000 description 1
- YQYFYUSYEDNLSD-YEPSODPASA-N Val-Thr-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O YQYFYUSYEDNLSD-YEPSODPASA-N 0.000 description 1
- BGTDGENDNWGMDQ-KJEVXHAQSA-N Val-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](C(C)C)N)O BGTDGENDNWGMDQ-KJEVXHAQSA-N 0.000 description 1
- AEFJNECXZCODJM-UWVGGRQHSA-N Val-Val-Gly Chemical compound CC(C)[C@H]([NH3+])C(=O)N[C@@H](C(C)C)C(=O)NCC([O-])=O AEFJNECXZCODJM-UWVGGRQHSA-N 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 238000000246 agarose gel electrophoresis Methods 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 108010005233 alanylglutamic acid Proteins 0.000 description 1
- 108010011559 alanylphenylalanine Proteins 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 230000000735 allogeneic effect Effects 0.000 description 1
- 230000002424 anti-apoptotic effect Effects 0.000 description 1
- 230000001567 anti-fibrinolytic effect Effects 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 230000002785 anti-thrombosis Effects 0.000 description 1
- 230000009830 antibody antigen interaction Effects 0.000 description 1
- 239000000729 antidote Substances 0.000 description 1
- 229940075522 antidotes Drugs 0.000 description 1
- 229940082620 antifibrinolytics Drugs 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 108010092854 aspartyllysine Proteins 0.000 description 1
- 239000012131 assay buffer Substances 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- PXXJHWLDUBFPOL-UHFFFAOYSA-N benzamidine Chemical compound NC(=N)C1=CC=CC=C1 PXXJHWLDUBFPOL-UHFFFAOYSA-N 0.000 description 1
- LZCZIHQBSCVGRD-UHFFFAOYSA-N benzenecarboximidamide;hydron;chloride Chemical compound [Cl-].NC(=[NH2+])C1=CC=CC=C1 LZCZIHQBSCVGRD-UHFFFAOYSA-N 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 description 1
- 230000006287 biotinylation Effects 0.000 description 1
- 238000007413 biotinylation Methods 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 239000008366 buffered solution Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 235000011148 calcium chloride Nutrition 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 229960003669 carbenicillin Drugs 0.000 description 1
- FPPNZSSZRUTDAP-UWFZAAFLSA-N carbenicillin Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)C(C(O)=O)C1=CC=CC=C1 FPPNZSSZRUTDAP-UWFZAAFLSA-N 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 239000005018 casein Substances 0.000 description 1
- BECPQYXYKAMYBN-UHFFFAOYSA-N casein, tech. Chemical compound NCCCCC(C(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(CC(C)C)N=C(O)C(CCC(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(C(C)O)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(COP(O)(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(N)CC1=CC=CC=C1 BECPQYXYKAMYBN-UHFFFAOYSA-N 0.000 description 1
- 235000021240 caseins Nutrition 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 239000013592 cell lysate Substances 0.000 description 1
- 230000011748 cell maturation Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 239000000701 coagulant Substances 0.000 description 1
- AGVAZMGAQJOSFJ-WZHZPDAFSA-M cobalt(2+);[(2r,3s,4r,5s)-5-(5,6-dimethylbenzimidazol-1-yl)-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl] [(2r)-1-[3-[(1r,2r,3r,4z,7s,9z,12s,13s,14z,17s,18s,19r)-2,13,18-tris(2-amino-2-oxoethyl)-7,12,17-tris(3-amino-3-oxopropyl)-3,5,8,8,13,15,18,19-octamethyl-2 Chemical compound [Co+2].N#[C-].[N-]([C@@H]1[C@H](CC(N)=O)[C@@]2(C)CCC(=O)NC[C@@H](C)OP(O)(=O)O[C@H]3[C@H]([C@H](O[C@@H]3CO)N3C4=CC(C)=C(C)C=C4N=C3)O)\C2=C(C)/C([C@H](C\2(C)C)CCC(N)=O)=N/C/2=C\C([C@H]([C@@]/2(CC(N)=O)C)CCC(N)=O)=N\C\2=C(C)/C2=N[C@]1(C)[C@@](C)(CC(N)=O)[C@@H]2CCC(N)=O AGVAZMGAQJOSFJ-WZHZPDAFSA-M 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 238000004883 computer application Methods 0.000 description 1
- 238000004590 computer program Methods 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- 238000013480 data collection Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 230000002357 endometrial effect Effects 0.000 description 1
- 230000008497 endothelial barrier function Effects 0.000 description 1
- 210000004954 endothelial membrane Anatomy 0.000 description 1
- 231100000284 endotoxic Toxicity 0.000 description 1
- 230000002346 endotoxic effect Effects 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 108010091897 factor V Leiden Proteins 0.000 description 1
- 230000020764 fibrinolysis Effects 0.000 description 1
- 239000003527 fibrinolytic agent Substances 0.000 description 1
- 230000003480 fibrinolytic effect Effects 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 230000005714 functional activity Effects 0.000 description 1
- 230000006251 gamma-carboxylation Effects 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 238000002523 gelfiltration Methods 0.000 description 1
- 210000002980 germ line cell Anatomy 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 108010013768 glutamyl-aspartyl-proline Proteins 0.000 description 1
- 108010049041 glutamylalanine Proteins 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- 108010062266 glycyl-glycyl-argininal Proteins 0.000 description 1
- 108010045126 glycyl-tyrosyl-glycine Proteins 0.000 description 1
- 108010077515 glycylproline Proteins 0.000 description 1
- 208000013746 hereditary thrombophilia due to congenital protein C deficiency Diseases 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 108010036413 histidylglycine Proteins 0.000 description 1
- 108010025306 histidylleucine Proteins 0.000 description 1
- 108010018006 histidylserine Proteins 0.000 description 1
- 230000013632 homeostatic process Effects 0.000 description 1
- 238000009396 hybridization Methods 0.000 description 1
- 230000005661 hydrophobic surface Effects 0.000 description 1
- 230000033444 hydroxylation Effects 0.000 description 1
- 238000005805 hydroxylation reaction Methods 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 230000028709 inflammatory response Effects 0.000 description 1
- 239000003999 initiator Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 208000020658 intracerebral hemorrhage Diseases 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 108010027338 isoleucylcysteine Proteins 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 229930027917 kanamycin Natural products 0.000 description 1
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 1
- 229960000318 kanamycin Drugs 0.000 description 1
- 229930182823 kanamycin A Natural products 0.000 description 1
- 108010053037 kyotorphin Proteins 0.000 description 1
- 238000002789 length control Methods 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 108010044311 leucyl-glycyl-glycine Proteins 0.000 description 1
- 108010030617 leucyl-phenylalanyl-valine Proteins 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 230000002934 lysing effect Effects 0.000 description 1
- 229960000274 lysozyme Drugs 0.000 description 1
- 239000004325 lysozyme Substances 0.000 description 1
- 235000010335 lysozyme Nutrition 0.000 description 1
- 108010003700 lysyl aspartic acid Proteins 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000002175 menstrual effect Effects 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- 108010034507 methionyltryptophan Proteins 0.000 description 1
- 239000004530 micro-emulsion Substances 0.000 description 1
- 238000001471 micro-filtration Methods 0.000 description 1
- 201000000050 myeloid neoplasm Diseases 0.000 description 1
- 230000037125 natural defense Effects 0.000 description 1
- 101150091879 neo gene Proteins 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 238000002515 oligonucleotide synthesis Methods 0.000 description 1
- 230000000399 orthopedic effect Effects 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 230000036470 plasma concentration Effects 0.000 description 1
- 238000002264 polyacrylamide gel electrophoresis Methods 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 230000004481 post-translational protein modification Effects 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000009117 preventive therapy Methods 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 239000003805 procoagulant Substances 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 108010053725 prolylvaline Proteins 0.000 description 1
- 230000004224 protection Effects 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 230000005892 protein maturation Effects 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 108010014806 prothrombinase complex Proteins 0.000 description 1
- 238000010926 purge Methods 0.000 description 1
- 238000002708 random mutagenesis Methods 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000000523 sample Substances 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 108010048818 seryl-histidine Proteins 0.000 description 1
- 108010071207 serylmethionine Proteins 0.000 description 1
- 238000009097 single-agent therapy Methods 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 230000000392 somatic effect Effects 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 238000012421 spiking Methods 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- 230000009469 supplementation Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 235000019364 tetracycline Nutrition 0.000 description 1
- 150000003522 tetracyclines Chemical class 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 108010071097 threonyl-lysyl-proline Proteins 0.000 description 1
- 206010043554 thrombocytopenia Diseases 0.000 description 1
- 230000001732 thrombotic effect Effects 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 230000005030 transcription termination Effects 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 230000010474 transient expression Effects 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 108010058119 tryptophyl-glycyl-glycine Proteins 0.000 description 1
- 108010044292 tryptophyltyrosine Proteins 0.000 description 1
- 108010078580 tyrosylleucine Proteins 0.000 description 1
- 238000000108 ultra-filtration Methods 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- 238000009777 vacuum freeze-drying Methods 0.000 description 1
- 108010009962 valyltyrosine Proteins 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/40—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/3955—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against proteinaceous materials, e.g. enzymes, hormones, lymphokines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/04—Antihaemorrhagics; Procoagulants; Haemostatic agents; Antifibrinolytic agents
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y304/00—Hydrolases acting on peptide bonds, i.e. peptidases (3.4)
- C12Y304/21—Serine endopeptidases (3.4.21)
- C12Y304/21069—Protein C activated (3.4.21.69)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2299/00—Coordinates from 3D structures of peptides, e.g. proteins or enzymes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/21—Immunoglobulins specific features characterized by taxonomic origin from primates, e.g. man
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/33—Crossreactivity, e.g. for species or epitope, or lack of said crossreactivity
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/55—Fab or Fab'
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Immunology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Mycology (AREA)
- Microbiology (AREA)
- Endocrinology (AREA)
- Hematology (AREA)
- Diabetes (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- General Engineering & Computer Science (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Investigating Or Analysing Biological Materials (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261731294P | 2012-11-29 | 2012-11-29 | |
| US61/731,294 | 2012-11-29 | ||
| US201361786472P | 2013-03-15 | 2013-03-15 | |
| US61/786,472 | 2013-03-15 | ||
| PCT/US2013/072243 WO2014085596A1 (en) | 2012-11-29 | 2013-11-27 | MONOCLONAL ANTIBODIES AGAISNT ACTIVATED PROTEIN C (aPC) |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20150088869A true KR20150088869A (ko) | 2015-08-03 |
Family
ID=50828462
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020157017008A Withdrawn KR20150088869A (ko) | 2012-11-29 | 2013-11-27 | 활성화 단백질 C(aPC)에 대한 모노클로날 항체 |
Country Status (18)
| Country | Link |
|---|---|
| US (1) | US20150307625A1 (enExample) |
| EP (1) | EP2925351A4 (enExample) |
| JP (1) | JP2016501230A (enExample) |
| KR (1) | KR20150088869A (enExample) |
| CN (1) | CN104812402A (enExample) |
| AR (1) | AR093671A1 (enExample) |
| AU (1) | AU2013352159A1 (enExample) |
| BR (1) | BR112015012414A2 (enExample) |
| CA (1) | CA2892750A1 (enExample) |
| HK (1) | HK1212896A1 (enExample) |
| IL (1) | IL238658A0 (enExample) |
| MX (1) | MX2015006424A (enExample) |
| RU (1) | RU2015125349A (enExample) |
| SG (1) | SG11201503719WA (enExample) |
| TW (1) | TW201429992A (enExample) |
| UY (1) | UY35154A (enExample) |
| WO (1) | WO2014085596A1 (enExample) |
| ZA (1) | ZA201504659B (enExample) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017087391A1 (en) * | 2015-11-17 | 2017-05-26 | Bayer Healthcare, Llc | Epitope of optimized humanized monoclonal antibodies against activated protein c and uses thereof |
| EP3831843A1 (en) * | 2019-12-08 | 2021-06-09 | Royal College Of Surgeons In Ireland | A hemostatic agent and uses thereof |
| CN115611986B (zh) * | 2021-07-13 | 2025-08-12 | 上海莱士血液制品股份有限公司 | 针对人活化蛋白c的单克隆抗体及其制备和应用 |
| CN116496394B (zh) * | 2022-01-26 | 2024-07-23 | 东莞市朋志生物科技有限公司 | 抗s100蛋白的抗体、检测s100蛋白的试剂和试剂盒 |
| US20250163183A1 (en) * | 2022-03-04 | 2025-05-22 | Coagulant Therapeutics Corporation | Camelid antibodies against activated protein c and uses thereof |
| CN118370814A (zh) * | 2023-01-20 | 2024-07-23 | 上海莱士血液制品股份有限公司 | 一种抗aPC单克隆抗体的制剂及其应用 |
| AU2024252640A1 (en) | 2023-04-07 | 2025-10-02 | Diagonal Therapeutics Inc. | Bispecific agonistic antibodies to activin a receptor like type 1 (alk1) |
| WO2025149667A1 (en) | 2024-01-12 | 2025-07-17 | Pheon Therapeutics Ltd | Antibody drug conjugates and uses thereof |
Family Cites Families (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1993009804A1 (en) * | 1991-11-18 | 1993-05-27 | The Scripps Research Institute | Serine protease derived-polypeptides and anti-peptide antibodies, systems and therapeutic methods for inhibiting coagulation |
| DE69735421T2 (de) * | 1996-04-24 | 2006-10-19 | The Regents Of The University Of Michigan, Ann Arbor | Gegen inaktivierung resistenter faktor viii |
| US6989241B2 (en) * | 2000-10-02 | 2006-01-24 | Oklahoma Medical Research Foundation | Assay for rapid detection of human activated protein C and highly specific monoclonal antibody therefor |
| US20030226155A1 (en) * | 2001-08-30 | 2003-12-04 | Biorexis Pharmaceutical Corporation | Modified transferrin-antibody fusion proteins |
| US20030203355A1 (en) * | 2002-04-24 | 2003-10-30 | Los Alamos National Laboratory | Fluorobodies: binding ligands with intrinsic fluorescence |
| GB0309126D0 (en) * | 2003-04-17 | 2003-05-28 | Neutec Pharma Plc | Clostridium difficile focussed antibodies |
| WO2006017538A2 (en) * | 2004-08-03 | 2006-02-16 | Dyax Corp. | Hk1-binding proteins |
| AU2005318171B2 (en) * | 2004-12-20 | 2011-09-29 | Crucell Holland B.V. | Binding molecules capable of neutralizing West Nile virus and uses thereof |
| US7939637B2 (en) * | 2005-12-13 | 2011-05-10 | Medimmune Limited | Insulin-like growth factor antibodies and uses thereof |
| US8039597B2 (en) * | 2007-09-07 | 2011-10-18 | Agensys, Inc. | Antibodies and related molecules that bind to 24P4C12 proteins |
| TWI489993B (zh) * | 2007-10-12 | 2015-07-01 | Novartis Ag | 骨硬化素(sclerostin)抗體組合物及使用方法 |
| WO2009055669A2 (en) * | 2007-10-26 | 2009-04-30 | Oklahoma Medical Research Foundation | Monoclonal antibodies against activated and unactivated protein c |
| GB0903151D0 (en) * | 2009-02-25 | 2009-04-08 | Bioinvent Int Ab | Antibody uses and methods |
| US8926976B2 (en) * | 2009-09-25 | 2015-01-06 | Xoma Technology Ltd. | Modulators |
| GB201011771D0 (en) * | 2010-07-13 | 2010-08-25 | Bioinvent Int Ab | Biological material and particular uses thereof |
| GB201013989D0 (en) * | 2010-08-20 | 2010-10-06 | Univ Southampton | Biological materials and methods of using the same |
| EP2640421A4 (en) * | 2010-11-16 | 2014-05-28 | Medimmune Llc | THERAPIES FOR TREATMENTS WITH ANTI-IGF ANTIBODIES |
| US8440797B2 (en) * | 2010-12-06 | 2013-05-14 | Dainippon Sumitomo Pharma Co., Ltd. | Human monoclonal antibody |
| GB201020995D0 (en) * | 2010-12-10 | 2011-01-26 | Bioinvent Int Ab | Biological materials and uses thereof |
-
2013
- 2013-11-27 RU RU2015125349A patent/RU2015125349A/ru not_active Application Discontinuation
- 2013-11-27 HK HK16100878.9A patent/HK1212896A1/zh unknown
- 2013-11-27 MX MX2015006424A patent/MX2015006424A/es unknown
- 2013-11-27 WO PCT/US2013/072243 patent/WO2014085596A1/en not_active Ceased
- 2013-11-27 US US14/443,710 patent/US20150307625A1/en not_active Abandoned
- 2013-11-27 CN CN201380062159.XA patent/CN104812402A/zh active Pending
- 2013-11-27 AU AU2013352159A patent/AU2013352159A1/en not_active Abandoned
- 2013-11-27 SG SG11201503719WA patent/SG11201503719WA/en unknown
- 2013-11-27 CA CA2892750A patent/CA2892750A1/en not_active Abandoned
- 2013-11-27 EP EP13857869.5A patent/EP2925351A4/en not_active Withdrawn
- 2013-11-27 JP JP2015545438A patent/JP2016501230A/ja active Pending
- 2013-11-27 BR BR112015012414A patent/BR112015012414A2/pt not_active IP Right Cessation
- 2013-11-27 KR KR1020157017008A patent/KR20150088869A/ko not_active Withdrawn
- 2013-11-28 UY UY0001035154A patent/UY35154A/es not_active Application Discontinuation
- 2013-11-28 TW TW102143366A patent/TW201429992A/zh unknown
- 2013-11-29 AR ARP130104417A patent/AR093671A1/es unknown
-
2015
- 2015-05-06 IL IL238658A patent/IL238658A0/en unknown
- 2015-06-26 ZA ZA2015/04659A patent/ZA201504659B/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| AU2013352159A1 (en) | 2015-06-04 |
| AR093671A1 (es) | 2015-06-17 |
| RU2015125349A (ru) | 2017-01-10 |
| CN104812402A (zh) | 2015-07-29 |
| ZA201504659B (en) | 2017-11-29 |
| EP2925351A1 (en) | 2015-10-07 |
| US20150307625A1 (en) | 2015-10-29 |
| BR112015012414A2 (pt) | 2017-09-12 |
| JP2016501230A (ja) | 2016-01-18 |
| HK1212896A1 (zh) | 2016-06-24 |
| UY35154A (es) | 2014-06-30 |
| IL238658A0 (en) | 2015-06-30 |
| TW201429992A (zh) | 2014-08-01 |
| EP2925351A4 (en) | 2016-08-24 |
| MX2015006424A (es) | 2015-08-14 |
| WO2014085596A1 (en) | 2014-06-05 |
| CA2892750A1 (en) | 2014-06-05 |
| SG11201503719WA (en) | 2015-06-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6684369B2 (ja) | 組織因子経路インヒビター(tfpi)に対する最適化されたモノクローナル抗体 | |
| CA2933259C (en) | Monoclonal antibodies against tissue factor pathway inhibitor (tfpi) | |
| JP6696899B2 (ja) | プラスミノーゲン活性化因子阻害剤−1(pai−1)に対する抗体及びその使用 | |
| KR20150088869A (ko) | 활성화 단백질 C(aPC)에 대한 모노클로날 항체 | |
| JP6170903B2 (ja) | 組織因子経路インヒビター(tfpi)に対するモノクローナル抗体 | |
| TW201802121A (zh) | 抗因子XI/XIa抗體之逆轉結合劑及其用途 | |
| JP6559188B2 (ja) | 組織因子経路インヒビター(tfpi)に対するモノクローナル抗体 | |
| JP2018038398A (ja) | 組織因子経路インヒビター(tfpi)に対するモノクローナル抗体 | |
| JP6419664B2 (ja) | 組織因子経路インヒビター(tfpi)に対するモノクローナル抗体 | |
| AU2016269554B2 (en) | Optimized monoclonal antibodies against tissue factor pathway inhibitor (TFPI) | |
| HK40017438A (en) | Monoclonal antibodies against tissue factor pathway inhibitor (tfpi) | |
| HK40017507A (en) | Monoclonal antibodies against tissue factor pathway inhibitor (tfpi) | |
| JP2018108089A (ja) | 組織因子経路インヒビター(tfpi)に対するモノクローナル抗体 | |
| HK1156635A (en) | Monoclonal antibodies against tissue factor pathway inhibitor (tfpi) | |
| HK1156635B (en) | Monoclonal antibodies against tissue factor pathway inhibitor (tfpi) | |
| HK1226645A1 (en) | Monoclonal antibodies against tissue factor pathway inhibitor (tfpi) | |
| HK1226645A (en) | Monoclonal antibodies against tissue factor pathway inhibitor (tfpi) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20150625 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |