KR20140071272A - 항 당뇨병 펩타이드로서의 아포리포프로테인 aiv - Google Patents
항 당뇨병 펩타이드로서의 아포리포프로테인 aiv Download PDFInfo
- Publication number
- KR20140071272A KR20140071272A KR1020137020637A KR20137020637A KR20140071272A KR 20140071272 A KR20140071272 A KR 20140071272A KR 1020137020637 A KR1020137020637 A KR 1020137020637A KR 20137020637 A KR20137020637 A KR 20137020637A KR 20140071272 A KR20140071272 A KR 20140071272A
- Authority
- KR
- South Korea
- Prior art keywords
- apolipoprotein
- leu
- glu
- apoa
- gln
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 108010073614 apolipoprotein A-IV Proteins 0.000 title claims abstract description 94
- 108090000765 processed proteins & peptides Proteins 0.000 title description 4
- 230000003178 anti-diabetic effect Effects 0.000 title description 3
- 239000003472 antidiabetic agent Substances 0.000 title description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 claims abstract description 103
- 239000008103 glucose Substances 0.000 claims abstract description 102
- 102100037320 Apolipoprotein A-IV Human genes 0.000 claims abstract description 81
- 238000000034 method Methods 0.000 claims abstract description 47
- 208000001072 type 2 diabetes mellitus Diseases 0.000 claims abstract description 40
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 26
- 210000004369 blood Anatomy 0.000 claims abstract description 23
- 239000008280 blood Substances 0.000 claims abstract description 23
- 241000282414 Homo sapiens Species 0.000 claims description 20
- 238000007912 intraperitoneal administration Methods 0.000 claims description 19
- 150000001413 amino acids Chemical class 0.000 claims description 16
- 239000003937 drug carrier Substances 0.000 claims description 4
- 239000007788 liquid Substances 0.000 claims description 4
- 238000007910 systemic administration Methods 0.000 claims description 4
- 102000007592 Apolipoproteins Human genes 0.000 claims description 3
- 108010071619 Apolipoproteins Proteins 0.000 claims description 3
- 238000007918 intramuscular administration Methods 0.000 claims description 3
- 238000001990 intravenous administration Methods 0.000 claims description 3
- 238000007920 subcutaneous administration Methods 0.000 claims description 3
- 239000003085 diluting agent Substances 0.000 claims description 2
- 238000002360 preparation method Methods 0.000 claims description 2
- 125000003275 alpha amino acid group Chemical group 0.000 claims 2
- 239000013011 aqueous formulation Substances 0.000 claims 2
- 239000000126 substance Substances 0.000 claims 1
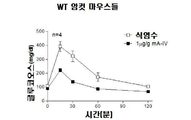
- 241000699670 Mus sp. Species 0.000 description 96
- 235000001727 glucose Nutrition 0.000 description 52
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 33
- 238000007446 glucose tolerance test Methods 0.000 description 31
- 239000011780 sodium chloride Substances 0.000 description 31
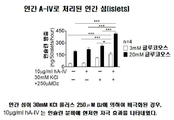
- 230000003914 insulin secretion Effects 0.000 description 18
- 238000011813 knockout mouse model Methods 0.000 description 14
- 108090000623 proteins and genes Proteins 0.000 description 14
- 206010012601 diabetes mellitus Diseases 0.000 description 12
- 235000018102 proteins Nutrition 0.000 description 12
- 102000004169 proteins and genes Human genes 0.000 description 12
- 108010027004 Apolipoproteins A Proteins 0.000 description 11
- 102000018619 Apolipoproteins A Human genes 0.000 description 11
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 11
- 238000002474 experimental method Methods 0.000 description 11
- 101000806793 Homo sapiens Apolipoprotein A-IV Proteins 0.000 description 10
- 235000001014 amino acid Nutrition 0.000 description 10
- 229940024606 amino acid Drugs 0.000 description 10
- 239000007924 injection Substances 0.000 description 10
- 229940090044 injection Drugs 0.000 description 10
- 238000002347 injection Methods 0.000 description 10
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 9
- 101000806795 Mus musculus Apolipoprotein A-IV Proteins 0.000 description 9
- YBAFDPFAUTYYRW-UHFFFAOYSA-N N-L-alpha-glutamyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCC(O)=O YBAFDPFAUTYYRW-UHFFFAOYSA-N 0.000 description 8
- 108010045517 Serum Amyloid P-Component Proteins 0.000 description 8
- 230000004071 biological effect Effects 0.000 description 8
- 235000005911 diet Nutrition 0.000 description 8
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 8
- 235000012054 meals Nutrition 0.000 description 8
- 238000012360 testing method Methods 0.000 description 8
- WSGXUIQTEZDVHJ-GARJFASQSA-N Leu-Ala-Pro Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@@H]1C(O)=O WSGXUIQTEZDVHJ-GARJFASQSA-N 0.000 description 7
- 102100036202 Serum amyloid P-component Human genes 0.000 description 7
- 108010050848 glycylleucine Proteins 0.000 description 7
- 108010059886 Apolipoprotein A-I Proteins 0.000 description 6
- 102000005666 Apolipoprotein A-I Human genes 0.000 description 6
- PKVWNYGXMNWJSI-CIUDSAMLSA-N Gln-Gln-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O PKVWNYGXMNWJSI-CIUDSAMLSA-N 0.000 description 6
- 208000002705 Glucose Intolerance Diseases 0.000 description 6
- 206010018429 Glucose tolerance impaired Diseases 0.000 description 6
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 6
- 241001465754 Metazoa Species 0.000 description 6
- 108010005233 alanylglutamic acid Proteins 0.000 description 6
- 125000001931 aliphatic group Chemical group 0.000 description 6
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 6
- 230000037213 diet Effects 0.000 description 6
- 230000000694 effects Effects 0.000 description 6
- -1 injectables Substances 0.000 description 6
- 238000011084 recovery Methods 0.000 description 6
- 238000006467 substitution reaction Methods 0.000 description 6
- HWEINOMSWQSJDC-SRVKXCTJSA-N Gln-Leu-Arg Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O HWEINOMSWQSJDC-SRVKXCTJSA-N 0.000 description 5
- MUSGDMDGNGXULI-DCAQKATOSA-N Glu-Glu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O MUSGDMDGNGXULI-DCAQKATOSA-N 0.000 description 5
- 239000004471 Glycine Substances 0.000 description 5
- 210000004027 cell Anatomy 0.000 description 5
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 5
- 235000009200 high fat diet Nutrition 0.000 description 5
- 108010003700 lysyl aspartic acid Proteins 0.000 description 5
- IVGJYOOGJLFKQE-AVGNSLFASA-N Glu-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N IVGJYOOGJLFKQE-AVGNSLFASA-N 0.000 description 4
- QXUPRMQJDWJDFR-NRPADANISA-N Glu-Val-Ser Chemical compound CC(C)[C@H](NC(=O)[C@@H](N)CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O QXUPRMQJDWJDFR-NRPADANISA-N 0.000 description 4
- 102000004877 Insulin Human genes 0.000 description 4
- 108090001061 Insulin Proteins 0.000 description 4
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical group OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 4
- CQQGCWPXDHTTNF-GUBZILKMSA-N Leu-Ala-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCC(O)=O CQQGCWPXDHTTNF-GUBZILKMSA-N 0.000 description 4
- 241000124008 Mammalia Species 0.000 description 4
- 125000000539 amino acid group Chemical group 0.000 description 4
- 239000008187 granular material Substances 0.000 description 4
- 229940125396 insulin Drugs 0.000 description 4
- 108010034529 leucyl-lysine Proteins 0.000 description 4
- 108010054155 lysyllysine Proteins 0.000 description 4
- 108010079317 prolyl-tyrosine Proteins 0.000 description 4
- 239000006188 syrup Substances 0.000 description 4
- 235000020357 syrup Nutrition 0.000 description 4
- XVZCXCTYGHPNEM-IHRRRGAJSA-N (2s)-1-[(2s)-2-[[(2s)-2-amino-4-methylpentanoyl]amino]-4-methylpentanoyl]pyrrolidine-2-carboxylic acid Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(O)=O XVZCXCTYGHPNEM-IHRRRGAJSA-N 0.000 description 3
- MCKSLROAGSDNFC-ACZMJKKPSA-N Ala-Asp-Gln Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O MCKSLROAGSDNFC-ACZMJKKPSA-N 0.000 description 3
- WDIYWDJLXOCGRW-ACZMJKKPSA-N Ala-Asp-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O WDIYWDJLXOCGRW-ACZMJKKPSA-N 0.000 description 3
- WKOBSJOZRJJVRZ-FXQIFTODSA-N Ala-Glu-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O WKOBSJOZRJJVRZ-FXQIFTODSA-N 0.000 description 3
- SGYSTDWPNPKJPP-GUBZILKMSA-N Arg-Ala-Arg Chemical compound NC(=N)NCCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O SGYSTDWPNPKJPP-GUBZILKMSA-N 0.000 description 3
- SCQIQCWLOMOEFP-DCAQKATOSA-N Asp-Leu-Arg Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O SCQIQCWLOMOEFP-DCAQKATOSA-N 0.000 description 3
- BRRPVTUFESPTCP-ACZMJKKPSA-N Asp-Ser-Glu Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O BRRPVTUFESPTCP-ACZMJKKPSA-N 0.000 description 3
- SQIARYGNVQWOSB-BZSNNMDCSA-N Asp-Tyr-Phe Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O SQIARYGNVQWOSB-BZSNNMDCSA-N 0.000 description 3
- YPMDZWPZFOZYFG-GUBZILKMSA-N Gln-Leu-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YPMDZWPZFOZYFG-GUBZILKMSA-N 0.000 description 3
- QMVCEWKHIUHTSD-GUBZILKMSA-N Gln-Met-Glu Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CCC(=O)N)N QMVCEWKHIUHTSD-GUBZILKMSA-N 0.000 description 3
- ARIORLIIMJACKZ-KKUMJFAQSA-N Glu-Pro-Tyr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ARIORLIIMJACKZ-KKUMJFAQSA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- UDLAWRKOVFDKFL-PEFMBERDSA-N Ile-Asp-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N UDLAWRKOVFDKFL-PEFMBERDSA-N 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 3
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 3
- KWTVLKBOQATPHJ-SRVKXCTJSA-N Leu-Ala-Lys Chemical compound C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(C)C)N KWTVLKBOQATPHJ-SRVKXCTJSA-N 0.000 description 3
- GRZSCTXVCDUIPO-SRVKXCTJSA-N Leu-Arg-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O GRZSCTXVCDUIPO-SRVKXCTJSA-N 0.000 description 3
- HASRFYOMVPJRPU-SRVKXCTJSA-N Leu-Arg-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(O)=O)C(O)=O HASRFYOMVPJRPU-SRVKXCTJSA-N 0.000 description 3
- FQZPTCNSNPWHLJ-AVGNSLFASA-N Leu-Gln-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(O)=O FQZPTCNSNPWHLJ-AVGNSLFASA-N 0.000 description 3
- HQUXQAMSWFIRET-AVGNSLFASA-N Leu-Glu-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CCCCN HQUXQAMSWFIRET-AVGNSLFASA-N 0.000 description 3
- WQWSMEOYXJTFRU-GUBZILKMSA-N Leu-Glu-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O WQWSMEOYXJTFRU-GUBZILKMSA-N 0.000 description 3
- XVZCXCTYGHPNEM-UHFFFAOYSA-N Leu-Leu-Pro Natural products CC(C)CC(N)C(=O)NC(CC(C)C)C(=O)N1CCCC1C(O)=O XVZCXCTYGHPNEM-UHFFFAOYSA-N 0.000 description 3
- KLSUAWUZBMAZCL-RHYQMDGZSA-N Leu-Thr-Pro Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@H]1C(O)=O KLSUAWUZBMAZCL-RHYQMDGZSA-N 0.000 description 3
- IUWMQCZOTYRXPL-ZPFDUUQYSA-N Lys-Ile-Asp Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(O)=O IUWMQCZOTYRXPL-ZPFDUUQYSA-N 0.000 description 3
- RBEATVHTWHTHTJ-KKUMJFAQSA-N Lys-Leu-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O RBEATVHTWHTHTJ-KKUMJFAQSA-N 0.000 description 3
- 241000699666 Mus <mouse, genus> Species 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- YGZWVPBHYABGLT-KJEVXHAQSA-N Thr-Pro-Tyr Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 YGZWVPBHYABGLT-KJEVXHAQSA-N 0.000 description 3
- BURPTJBFWIOHEY-UWJYBYFXSA-N Tyr-Ala-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 BURPTJBFWIOHEY-UWJYBYFXSA-N 0.000 description 3
- XLMDWQNAOKLKCP-XDTLVQLUSA-N Tyr-Ala-Gln Chemical compound C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N XLMDWQNAOKLKCP-XDTLVQLUSA-N 0.000 description 3
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 3
- 210000000683 abdominal cavity Anatomy 0.000 description 3
- 235000004279 alanine Nutrition 0.000 description 3
- 108010008685 alanyl-glutamyl-aspartic acid Proteins 0.000 description 3
- 108010039538 alanyl-glycyl-aspartyl-valine Proteins 0.000 description 3
- 108010045023 alanyl-prolyl-tyrosine Proteins 0.000 description 3
- 108010092854 aspartyllysine Proteins 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 230000037396 body weight Effects 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- 230000001684 chronic effect Effects 0.000 description 3
- FHIVAFMUCKRCQO-UHFFFAOYSA-N diazinon Chemical compound CCOP(=S)(OCC)OC1=CC(C)=NC(C(C)C)=N1 FHIVAFMUCKRCQO-UHFFFAOYSA-N 0.000 description 3
- 235000014113 dietary fatty acids Nutrition 0.000 description 3
- 239000000839 emulsion Substances 0.000 description 3
- 239000000194 fatty acid Substances 0.000 description 3
- 229930195729 fatty acid Natural products 0.000 description 3
- 239000012634 fragment Substances 0.000 description 3
- 108010020688 glycylhistidine Proteins 0.000 description 3
- 230000001965 increasing effect Effects 0.000 description 3
- 239000007928 intraperitoneal injection Substances 0.000 description 3
- 108010009298 lysylglutamic acid Proteins 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- 108010082795 phenylalanyl-arginyl-arginine Proteins 0.000 description 3
- 239000006187 pill Substances 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 239000003826 tablet Substances 0.000 description 3
- 108010005834 tyrosyl-alanyl-glycine Proteins 0.000 description 3
- 239000004474 valine Substances 0.000 description 3
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 2
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 2
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 2
- 244000215068 Acacia senegal Species 0.000 description 2
- 102000006822 Agouti Signaling Protein Human genes 0.000 description 2
- 108010072151 Agouti Signaling Protein Proteins 0.000 description 2
- ZODMADSIQZZBSQ-FXQIFTODSA-N Ala-Gln-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O ZODMADSIQZZBSQ-FXQIFTODSA-N 0.000 description 2
- MFMDKJIPHSWSBM-GUBZILKMSA-N Ala-Lys-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O MFMDKJIPHSWSBM-GUBZILKMSA-N 0.000 description 2
- ADSGHMXEAZJJNF-DCAQKATOSA-N Ala-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N ADSGHMXEAZJJNF-DCAQKATOSA-N 0.000 description 2
- CQJHFKKGZXKZBC-BPNCWPANSA-N Ala-Pro-Tyr Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 CQJHFKKGZXKZBC-BPNCWPANSA-N 0.000 description 2
- LSMDIAAALJJLRO-XQXXSGGOSA-N Ala-Thr-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(O)=O LSMDIAAALJJLRO-XQXXSGGOSA-N 0.000 description 2
- YJHKTAMKPGFJCT-NRPADANISA-N Ala-Val-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O YJHKTAMKPGFJCT-NRPADANISA-N 0.000 description 2
- MUXONAMCEUBVGA-DCAQKATOSA-N Arg-Arg-Gln Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(O)=O MUXONAMCEUBVGA-DCAQKATOSA-N 0.000 description 2
- UISQLSIBJKEJSS-GUBZILKMSA-N Arg-Arg-Ser Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CO)C(O)=O UISQLSIBJKEJSS-GUBZILKMSA-N 0.000 description 2
- VNFWDYWTSHFRRG-SRVKXCTJSA-N Arg-Gln-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O VNFWDYWTSHFRRG-SRVKXCTJSA-N 0.000 description 2
- PNIGSVZJNVUVJA-BQBZGAKWSA-N Arg-Gly-Asn Chemical compound NC(N)=NCCC[C@H](N)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O PNIGSVZJNVUVJA-BQBZGAKWSA-N 0.000 description 2
- STHNZYKCJHWULY-AVGNSLFASA-N Arg-Pro-His Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CCCN=C(N)N)N)C(=O)N[C@@H](CC2=CN=CN2)C(=O)O STHNZYKCJHWULY-AVGNSLFASA-N 0.000 description 2
- KMFPQTITXUKJOV-DCAQKATOSA-N Arg-Ser-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O KMFPQTITXUKJOV-DCAQKATOSA-N 0.000 description 2
- ZZXMOQIUIJJOKZ-ZLUOBGJFSA-N Asn-Asn-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC(N)=O ZZXMOQIUIJJOKZ-ZLUOBGJFSA-N 0.000 description 2
- VLDRQOHCMKCXLY-SRVKXCTJSA-N Asn-Ser-Phe Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O VLDRQOHCMKCXLY-SRVKXCTJSA-N 0.000 description 2
- GKWFMNNNYZHJHV-SRVKXCTJSA-N Asp-Lys-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CC(O)=O GKWFMNNNYZHJHV-SRVKXCTJSA-N 0.000 description 2
- MYLZFUMPZCPJCJ-NHCYSSNCSA-N Asp-Lys-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O MYLZFUMPZCPJCJ-NHCYSSNCSA-N 0.000 description 2
- QSFHZPQUAAQHAQ-CIUDSAMLSA-N Asp-Ser-Leu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O QSFHZPQUAAQHAQ-CIUDSAMLSA-N 0.000 description 2
- XMKXONRMGJXCJV-LAEOZQHASA-N Asp-Val-Glu Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O XMKXONRMGJXCJV-LAEOZQHASA-N 0.000 description 2
- 241000416162 Astragalus gummifer Species 0.000 description 2
- 201000004569 Blindness Diseases 0.000 description 2
- 108010074051 C-Reactive Protein Proteins 0.000 description 2
- 102100032752 C-reactive protein Human genes 0.000 description 2
- 241000484025 Cuniculus Species 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- SHERTACNJPYHAR-ACZMJKKPSA-N Gln-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCC(N)=O SHERTACNJPYHAR-ACZMJKKPSA-N 0.000 description 2
- LMPBBFWHCRURJD-LAEOZQHASA-N Gln-Asn-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCC(=O)N)N LMPBBFWHCRURJD-LAEOZQHASA-N 0.000 description 2
- UICOTGULOUGGLC-NUMRIWBASA-N Gln-Asp-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)N)N)O UICOTGULOUGGLC-NUMRIWBASA-N 0.000 description 2
- NKCZYEDZTKOFBG-GUBZILKMSA-N Gln-Gln-Arg Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O NKCZYEDZTKOFBG-GUBZILKMSA-N 0.000 description 2
- UFNSPPFJOHNXRE-AUTRQRHGSA-N Gln-Gln-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O UFNSPPFJOHNXRE-AUTRQRHGSA-N 0.000 description 2
- MAGNEQBFSBREJL-DCAQKATOSA-N Gln-Glu-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCC(=O)N)N MAGNEQBFSBREJL-DCAQKATOSA-N 0.000 description 2
- QKCZZAZNMMVICF-DCAQKATOSA-N Gln-Leu-Glu Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O QKCZZAZNMMVICF-DCAQKATOSA-N 0.000 description 2
- LURQDGKYBFWWJA-MNXVOIDGSA-N Gln-Lys-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(=O)N)N LURQDGKYBFWWJA-MNXVOIDGSA-N 0.000 description 2
- FKXCBKCOSVIGCT-AVGNSLFASA-N Gln-Lys-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O FKXCBKCOSVIGCT-AVGNSLFASA-N 0.000 description 2
- JRHPEMVLTRADLJ-AVGNSLFASA-N Gln-Lys-Lys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)N)N JRHPEMVLTRADLJ-AVGNSLFASA-N 0.000 description 2
- ZXGLLNZQSBLQLT-SRVKXCTJSA-N Gln-Met-Lys Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)N)N ZXGLLNZQSBLQLT-SRVKXCTJSA-N 0.000 description 2
- AKJRHDMTEJXTPV-ACZMJKKPSA-N Glu-Asn-Ala Chemical compound C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CCC(O)=O)C(O)=O AKJRHDMTEJXTPV-ACZMJKKPSA-N 0.000 description 2
- XHWLNISLUFEWNS-CIUDSAMLSA-N Glu-Gln-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O XHWLNISLUFEWNS-CIUDSAMLSA-N 0.000 description 2
- PVBBEKPHARMPHX-DCAQKATOSA-N Glu-Gln-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CCC(O)=O PVBBEKPHARMPHX-DCAQKATOSA-N 0.000 description 2
- AUTNXSQEVVHSJK-YVNDNENWSA-N Glu-Glu-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O AUTNXSQEVVHSJK-YVNDNENWSA-N 0.000 description 2
- YLJHCWNDBKKOEB-IHRRRGAJSA-N Glu-Glu-Phe Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O YLJHCWNDBKKOEB-IHRRRGAJSA-N 0.000 description 2
- LRPXYSGPOBVBEH-IUCAKERBSA-N Glu-Gly-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(O)=O LRPXYSGPOBVBEH-IUCAKERBSA-N 0.000 description 2
- VSRCAOIHMGCIJK-SRVKXCTJSA-N Glu-Leu-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O VSRCAOIHMGCIJK-SRVKXCTJSA-N 0.000 description 2
- IRXNJYPKBVERCW-DCAQKATOSA-N Glu-Leu-Glu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O IRXNJYPKBVERCW-DCAQKATOSA-N 0.000 description 2
- DAHLWSFUXOHMIA-FXQIFTODSA-N Glu-Ser-Gln Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O DAHLWSFUXOHMIA-FXQIFTODSA-N 0.000 description 2
- UZWUBBRJWFTHTD-LAEOZQHASA-N Glu-Val-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCC(O)=O UZWUBBRJWFTHTD-LAEOZQHASA-N 0.000 description 2
- OVSKVOOUFAKODB-UWVGGRQHSA-N Gly-Arg-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CCCN=C(N)N OVSKVOOUFAKODB-UWVGGRQHSA-N 0.000 description 2
- JVWPPCWUDRJGAE-YUMQZZPRSA-N Gly-Asn-Leu Chemical compound [H]NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O JVWPPCWUDRJGAE-YUMQZZPRSA-N 0.000 description 2
- XQHSBNVACKQWAV-WHFBIAKZSA-N Gly-Asp-Asn Chemical compound [H]NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O XQHSBNVACKQWAV-WHFBIAKZSA-N 0.000 description 2
- FZQLXNIMCPJVJE-YUMQZZPRSA-N Gly-Asp-Leu Chemical compound [H]NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O FZQLXNIMCPJVJE-YUMQZZPRSA-N 0.000 description 2
- HDNXXTBKOJKWNN-WDSKDSINSA-N Gly-Glu-Asn Chemical compound NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O HDNXXTBKOJKWNN-WDSKDSINSA-N 0.000 description 2
- LHYJCVCQPWRMKZ-WEDXCCLWSA-N Gly-Leu-Thr Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O LHYJCVCQPWRMKZ-WEDXCCLWSA-N 0.000 description 2
- GMTXWRIDLGTVFC-IUCAKERBSA-N Gly-Lys-Glu Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O GMTXWRIDLGTVFC-IUCAKERBSA-N 0.000 description 2
- ZZJVYSAQQMDIRD-UWVGGRQHSA-N Gly-Pro-His Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O ZZJVYSAQQMDIRD-UWVGGRQHSA-N 0.000 description 2
- 229920000084 Gum arabic Polymers 0.000 description 2
- 239000007995 HEPES buffer Substances 0.000 description 2
- MJNWEIMBXKKCSF-XVYDVKMFSA-N His-Ala-Asn Chemical compound C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CN=CN1)N MJNWEIMBXKKCSF-XVYDVKMFSA-N 0.000 description 2
- DCRODRAURLJOFY-XPUUQOCRSA-N His-Ala-Gly Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](C)C(=O)NCC(O)=O DCRODRAURLJOFY-XPUUQOCRSA-N 0.000 description 2
- VFBZWZXKCVBTJR-SRVKXCTJSA-N His-Leu-Asp Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CC1=CN=CN1)N VFBZWZXKCVBTJR-SRVKXCTJSA-N 0.000 description 2
- UROVZOUMHNXPLZ-AVGNSLFASA-N His-Leu-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CN=CN1 UROVZOUMHNXPLZ-AVGNSLFASA-N 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- YKZAMJXNJUWFIK-JBDRJPRFSA-N Ile-Ser-Ala Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)O)N YKZAMJXNJUWFIK-JBDRJPRFSA-N 0.000 description 2
- 206010022489 Insulin Resistance Diseases 0.000 description 2
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 2
- FADYJNXDPBKVCA-UHFFFAOYSA-N L-Phenylalanyl-L-lysin Natural products NCCCCC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FADYJNXDPBKVCA-UHFFFAOYSA-N 0.000 description 2
- WUFYAPWIHCUMLL-CIUDSAMLSA-N Leu-Asn-Ala Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(O)=O WUFYAPWIHCUMLL-CIUDSAMLSA-N 0.000 description 2
- VIWUBXKCYJGNCL-SRVKXCTJSA-N Leu-Asn-His Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 VIWUBXKCYJGNCL-SRVKXCTJSA-N 0.000 description 2
- WIDZHJTYKYBLSR-DCAQKATOSA-N Leu-Glu-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O WIDZHJTYKYBLSR-DCAQKATOSA-N 0.000 description 2
- OGUUKPXUTHOIAV-SDDRHHMPSA-N Leu-Glu-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N OGUUKPXUTHOIAV-SDDRHHMPSA-N 0.000 description 2
- VWHGTYCRDRBSFI-ZETCQYMHSA-N Leu-Gly-Gly Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)NCC(O)=O VWHGTYCRDRBSFI-ZETCQYMHSA-N 0.000 description 2
- KXODZBLFVFSLAI-AVGNSLFASA-N Leu-His-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(C)C)CC1=CN=CN1 KXODZBLFVFSLAI-AVGNSLFASA-N 0.000 description 2
- ZRHDPZAAWLXXIR-SRVKXCTJSA-N Leu-Lys-Ala Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O ZRHDPZAAWLXXIR-SRVKXCTJSA-N 0.000 description 2
- KQFZKDITNUEVFJ-JYJNAYRXSA-N Leu-Phe-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(C)C)CC1=CC=CC=C1 KQFZKDITNUEVFJ-JYJNAYRXSA-N 0.000 description 2
- XOWMDXHFSBCAKQ-SRVKXCTJSA-N Leu-Ser-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC(C)C XOWMDXHFSBCAKQ-SRVKXCTJSA-N 0.000 description 2
- IWMJFLJQHIDZQW-KKUMJFAQSA-N Leu-Ser-Phe Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 IWMJFLJQHIDZQW-KKUMJFAQSA-N 0.000 description 2
- AIMGJYMCTAABEN-GVXVVHGQSA-N Leu-Val-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O AIMGJYMCTAABEN-GVXVVHGQSA-N 0.000 description 2
- FDBTVENULFNTAL-XQQFMLRXSA-N Leu-Val-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N FDBTVENULFNTAL-XQQFMLRXSA-N 0.000 description 2
- RVOMPSJXSRPFJT-DCAQKATOSA-N Lys-Ala-Arg Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O RVOMPSJXSRPFJT-DCAQKATOSA-N 0.000 description 2
- DGAAQRAUOFHBFJ-CIUDSAMLSA-N Lys-Asn-Ala Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(O)=O DGAAQRAUOFHBFJ-CIUDSAMLSA-N 0.000 description 2
- GJJQCBVRWDGLMQ-GUBZILKMSA-N Lys-Glu-Ala Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O GJJQCBVRWDGLMQ-GUBZILKMSA-N 0.000 description 2
- DCRWPTBMWMGADO-AVGNSLFASA-N Lys-Glu-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O DCRWPTBMWMGADO-AVGNSLFASA-N 0.000 description 2
- IMAKMJCBYCSMHM-AVGNSLFASA-N Lys-Glu-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CCCCN IMAKMJCBYCSMHM-AVGNSLFASA-N 0.000 description 2
- VMTYLUGCXIEDMV-QWRGUYRKSA-N Lys-Leu-Gly Chemical compound OC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCCCN VMTYLUGCXIEDMV-QWRGUYRKSA-N 0.000 description 2
- IOQWIOPSKJOEKI-SRVKXCTJSA-N Lys-Ser-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O IOQWIOPSKJOEKI-SRVKXCTJSA-N 0.000 description 2
- KQBJYJXPZBNEIK-DCAQKATOSA-N Met-Glu-Arg Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CCCNC(N)=N KQBJYJXPZBNEIK-DCAQKATOSA-N 0.000 description 2
- XMBSYZWANAQXEV-UHFFFAOYSA-N N-alpha-L-glutamyl-L-phenylalanine Natural products OC(=O)CCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XMBSYZWANAQXEV-UHFFFAOYSA-N 0.000 description 2
- 108010002311 N-glycylglutamic acid Proteins 0.000 description 2
- 208000008589 Obesity Diseases 0.000 description 2
- KAHUBGWSIQNZQQ-KKUMJFAQSA-N Phe-Asn-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC1=CC=CC=C1 KAHUBGWSIQNZQQ-KKUMJFAQSA-N 0.000 description 2
- GPSMLZQVIIYLDK-ULQDDVLXSA-N Phe-Lys-Val Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O GPSMLZQVIIYLDK-ULQDDVLXSA-N 0.000 description 2
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 2
- DLZBBDSPTJBOOD-BPNCWPANSA-N Pro-Tyr-Ala Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C)C(O)=O DLZBBDSPTJBOOD-BPNCWPANSA-N 0.000 description 2
- 208000001647 Renal Insufficiency Diseases 0.000 description 2
- OOKCGAYXSNJBGQ-ZLUOBGJFSA-N Ser-Asn-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O OOKCGAYXSNJBGQ-ZLUOBGJFSA-N 0.000 description 2
- OJPHFSOMBZKQKQ-GUBZILKMSA-N Ser-Gln-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CO OJPHFSOMBZKQKQ-GUBZILKMSA-N 0.000 description 2
- LALNXSXEYFUUDD-GUBZILKMSA-N Ser-Glu-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O LALNXSXEYFUUDD-GUBZILKMSA-N 0.000 description 2
- FUMGHWDRRFCKEP-CIUDSAMLSA-N Ser-Leu-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O FUMGHWDRRFCKEP-CIUDSAMLSA-N 0.000 description 2
- DYEGLQRVMBWQLD-IXOXFDKPSA-N Ser-Thr-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)NC(=O)[C@H](CO)N)O DYEGLQRVMBWQLD-IXOXFDKPSA-N 0.000 description 2
- 102000054727 Serum Amyloid A Human genes 0.000 description 2
- 101710190759 Serum amyloid A protein Proteins 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- OYTNZCBFDXGQGE-XQXXSGGOSA-N Thr-Gln-Ala Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](C)C(=O)O)N)O OYTNZCBFDXGQGE-XQXXSGGOSA-N 0.000 description 2
- ZQUKYJOKQBRBCS-GLLZPBPUSA-N Thr-Gln-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N)O ZQUKYJOKQBRBCS-GLLZPBPUSA-N 0.000 description 2
- DKDHTRVDOUZZTP-IFFSRLJSSA-N Thr-Gln-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)[C@@H](C)O)C(O)=O DKDHTRVDOUZZTP-IFFSRLJSSA-N 0.000 description 2
- SHOMROOOQBDGRL-JHEQGTHGSA-N Thr-Glu-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O SHOMROOOQBDGRL-JHEQGTHGSA-N 0.000 description 2
- LXXCHJKHJYRMIY-FQPOAREZSA-N Thr-Tyr-Ala Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C)C(O)=O LXXCHJKHJYRMIY-FQPOAREZSA-N 0.000 description 2
- KPMIQCXJDVKWKO-IFFSRLJSSA-N Thr-Val-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O KPMIQCXJDVKWKO-IFFSRLJSSA-N 0.000 description 2
- 229920001615 Tragacanth Polymers 0.000 description 2
- SLLKXDSRVAOREO-KZVJFYERSA-N Val-Ala-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](C)NC(=O)[C@H](C(C)C)N)O SLLKXDSRVAOREO-KZVJFYERSA-N 0.000 description 2
- JLFKWDAZBRYCGX-ZKWXMUAHSA-N Val-Asn-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CO)C(=O)O)N JLFKWDAZBRYCGX-ZKWXMUAHSA-N 0.000 description 2
- PGBJAZDAEWPDAA-NHCYSSNCSA-N Val-Gln-Met Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCSC)C(=O)O)N PGBJAZDAEWPDAA-NHCYSSNCSA-N 0.000 description 2
- SZTTYWIUCGSURQ-AUTRQRHGSA-N Val-Glu-Glu Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O SZTTYWIUCGSURQ-AUTRQRHGSA-N 0.000 description 2
- OTJMMKPMLUNTQT-AVGNSLFASA-N Val-Leu-Arg Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](C(C)C)N OTJMMKPMLUNTQT-AVGNSLFASA-N 0.000 description 2
- PWCJARIQERIIGF-BZSNNMDCSA-N Val-Met-Trp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)N PWCJARIQERIIGF-BZSNNMDCSA-N 0.000 description 2
- 239000000205 acacia gum Substances 0.000 description 2
- 235000010489 acacia gum Nutrition 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 108010008355 arginyl-glutamine Proteins 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 230000000378 dietary effect Effects 0.000 description 2
- 239000007884 disintegrant Substances 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 108010079547 glutamylmethionine Proteins 0.000 description 2
- 108010040030 histidinoalanine Proteins 0.000 description 2
- 108010028295 histidylhistidine Proteins 0.000 description 2
- 201000001421 hyperglycemia Diseases 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 201000006370 kidney failure Diseases 0.000 description 2
- 108010044311 leucyl-glycyl-glycine Proteins 0.000 description 2
- 108010057821 leucylproline Proteins 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- 108010017391 lysylvaline Proteins 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 235000010981 methylcellulose Nutrition 0.000 description 2
- 235000020824 obesity Nutrition 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- 108010073025 phenylalanylphenylalanine Proteins 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 108091033319 polynucleotide Proteins 0.000 description 2
- 102000040430 polynucleotide Human genes 0.000 description 2
- 239000002157 polynucleotide Substances 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 235000004252 protein component Nutrition 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 239000000196 tragacanth Substances 0.000 description 2
- 235000010487 tragacanth Nutrition 0.000 description 2
- 229940116362 tragacanth Drugs 0.000 description 2
- 239000013598 vector Substances 0.000 description 2
- 238000001262 western blot Methods 0.000 description 2
- 239000000080 wetting agent Substances 0.000 description 2
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical group OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 1
- 229940058015 1,3-butylene glycol Drugs 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- 125000005274 4-hydroxybenzoic acid group Chemical class 0.000 description 1
- PXKLCFFSVLKOJM-ACZMJKKPSA-N Ala-Asn-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O PXKLCFFSVLKOJM-ACZMJKKPSA-N 0.000 description 1
- CXQODNIBUNQWAS-CIUDSAMLSA-N Ala-Gln-Arg Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N CXQODNIBUNQWAS-CIUDSAMLSA-N 0.000 description 1
- DCVYRWFAMZFSDA-ZLUOBGJFSA-N Ala-Ser-Ala Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O DCVYRWFAMZFSDA-ZLUOBGJFSA-N 0.000 description 1
- HOVPGJUNRLMIOZ-CIUDSAMLSA-N Ala-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](C)N HOVPGJUNRLMIOZ-CIUDSAMLSA-N 0.000 description 1
- WQKAQKZRDIZYNV-VZFHVOOUSA-N Ala-Ser-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WQKAQKZRDIZYNV-VZFHVOOUSA-N 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- DFCIPNHFKOQAME-FXQIFTODSA-N Arg-Ala-Asn Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(O)=O DFCIPNHFKOQAME-FXQIFTODSA-N 0.000 description 1
- NABSCJGZKWSNHX-RCWTZXSCSA-N Arg-Arg-Thr Chemical compound NC(N)=NCCC[C@@H](C(=O)N[C@@H]([C@H](O)C)C(O)=O)NC(=O)[C@@H](N)CCCN=C(N)N NABSCJGZKWSNHX-RCWTZXSCSA-N 0.000 description 1
- SQKPKIJVWHAWNF-DCAQKATOSA-N Arg-Asp-Lys Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(O)=O SQKPKIJVWHAWNF-DCAQKATOSA-N 0.000 description 1
- GIVWETPOBCRTND-DCAQKATOSA-N Arg-Gln-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O GIVWETPOBCRTND-DCAQKATOSA-N 0.000 description 1
- MZRBYBIQTIKERR-GUBZILKMSA-N Arg-Glu-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O MZRBYBIQTIKERR-GUBZILKMSA-N 0.000 description 1
- NKBQZKVMKJJDLX-SRVKXCTJSA-N Arg-Glu-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O NKBQZKVMKJJDLX-SRVKXCTJSA-N 0.000 description 1
- OGUPCHKBOKJFMA-SRVKXCTJSA-N Arg-Glu-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCCN=C(N)N OGUPCHKBOKJFMA-SRVKXCTJSA-N 0.000 description 1
- GXXWTNKNFFKTJB-NAKRPEOUSA-N Arg-Ile-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O GXXWTNKNFFKTJB-NAKRPEOUSA-N 0.000 description 1
- JEOCWTUOMKEEMF-RHYQMDGZSA-N Arg-Leu-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JEOCWTUOMKEEMF-RHYQMDGZSA-N 0.000 description 1
- HIMXTOIXVXWHTB-DCAQKATOSA-N Arg-Met-Gln Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N HIMXTOIXVXWHTB-DCAQKATOSA-N 0.000 description 1
- ULBHWNVWSCJLCO-NHCYSSNCSA-N Arg-Val-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCN=C(N)N ULBHWNVWSCJLCO-NHCYSSNCSA-N 0.000 description 1
- 239000004475 Arginine Chemical group 0.000 description 1
- RZVVKNIACROXRM-ZLUOBGJFSA-N Asn-Ala-Asp Chemical compound C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CC(=O)N)N RZVVKNIACROXRM-ZLUOBGJFSA-N 0.000 description 1
- LEFKSBYHUGUWLP-ACZMJKKPSA-N Asn-Ala-Glu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O LEFKSBYHUGUWLP-ACZMJKKPSA-N 0.000 description 1
- XYOVHPDDWCEUDY-CIUDSAMLSA-N Asn-Ala-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O XYOVHPDDWCEUDY-CIUDSAMLSA-N 0.000 description 1
- XHFXZQHTLJVZBN-FXQIFTODSA-N Asn-Arg-Asn Chemical compound C(C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)N)N)CN=C(N)N XHFXZQHTLJVZBN-FXQIFTODSA-N 0.000 description 1
- MOHUTCNYQLMARY-GUBZILKMSA-N Asn-His-Gln Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)N)N MOHUTCNYQLMARY-GUBZILKMSA-N 0.000 description 1
- NLRJGXZWTKXRHP-DCAQKATOSA-N Asn-Leu-Arg Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O NLRJGXZWTKXRHP-DCAQKATOSA-N 0.000 description 1
- YVXRYLVELQYAEQ-SRVKXCTJSA-N Asn-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)N)N YVXRYLVELQYAEQ-SRVKXCTJSA-N 0.000 description 1
- RCFGLXMZDYNRSC-CIUDSAMLSA-N Asn-Lys-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O RCFGLXMZDYNRSC-CIUDSAMLSA-N 0.000 description 1
- UYRPHDGXHKBZHJ-CIUDSAMLSA-N Asn-Met-Gln Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)N)N UYRPHDGXHKBZHJ-CIUDSAMLSA-N 0.000 description 1
- HPBNLFLSSQDFQW-WHFBIAKZSA-N Asn-Ser-Gly Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O HPBNLFLSSQDFQW-WHFBIAKZSA-N 0.000 description 1
- QUMKPKWYDVMGNT-NUMRIWBASA-N Asn-Thr-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)N)N)O QUMKPKWYDVMGNT-NUMRIWBASA-N 0.000 description 1
- ZUFPUBYQYWCMDB-NUMRIWBASA-N Asn-Thr-Glu Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O ZUFPUBYQYWCMDB-NUMRIWBASA-N 0.000 description 1
- MYRLSKYSMXNLLA-LAEOZQHASA-N Asn-Val-Glu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O MYRLSKYSMXNLLA-LAEOZQHASA-N 0.000 description 1
- SDHFVYLZFBDSQT-DCAQKATOSA-N Asp-Arg-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(=O)O)N SDHFVYLZFBDSQT-DCAQKATOSA-N 0.000 description 1
- UGKZHCBLMLSANF-CIUDSAMLSA-N Asp-Asn-Leu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O UGKZHCBLMLSANF-CIUDSAMLSA-N 0.000 description 1
- KIJLEFNHWSXHRU-NUMRIWBASA-N Asp-Gln-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KIJLEFNHWSXHRU-NUMRIWBASA-N 0.000 description 1
- PDECQIHABNQRHN-GUBZILKMSA-N Asp-Glu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC(O)=O PDECQIHABNQRHN-GUBZILKMSA-N 0.000 description 1
- OVPHVTCDVYYTHN-AVGNSLFASA-N Asp-Glu-Phe Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 OVPHVTCDVYYTHN-AVGNSLFASA-N 0.000 description 1
- CLUMZOKVGUWUFD-CIUDSAMLSA-N Asp-Leu-Asn Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O CLUMZOKVGUWUFD-CIUDSAMLSA-N 0.000 description 1
- XLILXFRAKOYEJX-GUBZILKMSA-N Asp-Leu-Gln Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O XLILXFRAKOYEJX-GUBZILKMSA-N 0.000 description 1
- YWLDTBBUHZJQHW-KKUMJFAQSA-N Asp-Lys-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(=O)O)N YWLDTBBUHZJQHW-KKUMJFAQSA-N 0.000 description 1
- DONWIPDSZZJHHK-HJGDQZAQSA-N Asp-Lys-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(=O)O)N)O DONWIPDSZZJHHK-HJGDQZAQSA-N 0.000 description 1
- QOCFFCUFZGDHTP-NUMRIWBASA-N Asp-Thr-Gln Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(O)=O QOCFFCUFZGDHTP-NUMRIWBASA-N 0.000 description 1
- MFDPBZAFCRKYEY-LAEOZQHASA-N Asp-Val-Gln Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O MFDPBZAFCRKYEY-LAEOZQHASA-N 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Chemical group OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 241000282465 Canis Species 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 208000017667 Chronic Disease Diseases 0.000 description 1
- 108091026890 Coding region Proteins 0.000 description 1
- 208000015943 Coeliac disease Diseases 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 238000008157 ELISA kit Methods 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 241001198387 Escherichia coli BL21(DE3) Species 0.000 description 1
- 241000282324 Felis Species 0.000 description 1
- NNQHEEQNPQYPGL-FXQIFTODSA-N Gln-Ala-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(O)=O NNQHEEQNPQYPGL-FXQIFTODSA-N 0.000 description 1
- RZSLYUUFFVHFRQ-FXQIFTODSA-N Gln-Ala-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O RZSLYUUFFVHFRQ-FXQIFTODSA-N 0.000 description 1
- PRBLYKYHAJEABA-SRVKXCTJSA-N Gln-Arg-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O PRBLYKYHAJEABA-SRVKXCTJSA-N 0.000 description 1
- AAOBFSKXAVIORT-GUBZILKMSA-N Gln-Asn-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O AAOBFSKXAVIORT-GUBZILKMSA-N 0.000 description 1
- RKAQZCDMSUQTSS-FXQIFTODSA-N Gln-Asp-Gln Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N RKAQZCDMSUQTSS-FXQIFTODSA-N 0.000 description 1
- XFKUFUJECJUQTQ-CIUDSAMLSA-N Gln-Gln-Glu Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O XFKUFUJECJUQTQ-CIUDSAMLSA-N 0.000 description 1
- KVXVVDFOZNYYKZ-DCAQKATOSA-N Gln-Gln-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O KVXVVDFOZNYYKZ-DCAQKATOSA-N 0.000 description 1
- SNLOOPZHAQDMJG-CIUDSAMLSA-N Gln-Glu-Glu Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O SNLOOPZHAQDMJG-CIUDSAMLSA-N 0.000 description 1
- LGIKBBLQVSWUGK-DCAQKATOSA-N Gln-Leu-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O LGIKBBLQVSWUGK-DCAQKATOSA-N 0.000 description 1
- MLSKFHLRFVGNLL-WDCWCFNPSA-N Gln-Leu-Thr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MLSKFHLRFVGNLL-WDCWCFNPSA-N 0.000 description 1
- ZEEPYMXTJWIMSN-GUBZILKMSA-N Gln-Lys-Ser Chemical compound NCCCC[C@@H](C(=O)N[C@@H](CO)C(O)=O)NC(=O)[C@@H](N)CCC(N)=O ZEEPYMXTJWIMSN-GUBZILKMSA-N 0.000 description 1
- FALJZCPMTGJOHX-SRVKXCTJSA-N Gln-Met-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(O)=O FALJZCPMTGJOHX-SRVKXCTJSA-N 0.000 description 1
- KPNWAJMEMRCLAL-GUBZILKMSA-N Gln-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)N)N KPNWAJMEMRCLAL-GUBZILKMSA-N 0.000 description 1
- MRVYVEQPNDSWLH-XPUUQOCRSA-N Gln-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H](N)CCC(N)=O MRVYVEQPNDSWLH-XPUUQOCRSA-N 0.000 description 1
- ZZLDMBMFKZFQMU-NRPADANISA-N Gln-Val-Ala Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O ZZLDMBMFKZFQMU-NRPADANISA-N 0.000 description 1
- ICRKQMRFXYDYMK-LAEOZQHASA-N Gln-Val-Asn Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O ICRKQMRFXYDYMK-LAEOZQHASA-N 0.000 description 1
- ZFBBMCKQSNJZSN-AUTRQRHGSA-N Gln-Val-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZFBBMCKQSNJZSN-AUTRQRHGSA-N 0.000 description 1
- BBFCMGBMYIAGRS-AUTRQRHGSA-N Gln-Val-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O BBFCMGBMYIAGRS-AUTRQRHGSA-N 0.000 description 1
- DYFJZDDQPNIPAB-NHCYSSNCSA-N Glu-Arg-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(O)=O DYFJZDDQPNIPAB-NHCYSSNCSA-N 0.000 description 1
- SBYVDRJAXWSXQL-AVGNSLFASA-N Glu-Asn-Phe Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O SBYVDRJAXWSXQL-AVGNSLFASA-N 0.000 description 1
- JVSBYEDSSRZQGV-GUBZILKMSA-N Glu-Asp-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CCC(O)=O JVSBYEDSSRZQGV-GUBZILKMSA-N 0.000 description 1
- OXEMJGCAJFFREE-FXQIFTODSA-N Glu-Gln-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O OXEMJGCAJFFREE-FXQIFTODSA-N 0.000 description 1
- RFDHKPSHTXZKLL-IHRRRGAJSA-N Glu-Gln-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCC(=O)O)N RFDHKPSHTXZKLL-IHRRRGAJSA-N 0.000 description 1
- ITBHUUMCJJQUSC-LAEOZQHASA-N Glu-Ile-Gly Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(O)=O ITBHUUMCJJQUSC-LAEOZQHASA-N 0.000 description 1
- XTZDZAXYPDISRR-MNXVOIDGSA-N Glu-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N XTZDZAXYPDISRR-MNXVOIDGSA-N 0.000 description 1
- NJCALAAIGREHDR-WDCWCFNPSA-N Glu-Leu-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O NJCALAAIGREHDR-WDCWCFNPSA-N 0.000 description 1
- CUPSDFQZTVVTSK-GUBZILKMSA-N Glu-Lys-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCC(O)=O CUPSDFQZTVVTSK-GUBZILKMSA-N 0.000 description 1
- BCYGDJXHAGZNPQ-DCAQKATOSA-N Glu-Lys-Glu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O BCYGDJXHAGZNPQ-DCAQKATOSA-N 0.000 description 1
- ILWHFUZZCFYSKT-AVGNSLFASA-N Glu-Lys-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O ILWHFUZZCFYSKT-AVGNSLFASA-N 0.000 description 1
- KJBGAZSLZAQDPV-KKUMJFAQSA-N Glu-Phe-Arg Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](CCC(=O)O)N KJBGAZSLZAQDPV-KKUMJFAQSA-N 0.000 description 1
- LZEUDRYSAZAJIO-AUTRQRHGSA-N Glu-Val-Glu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O LZEUDRYSAZAJIO-AUTRQRHGSA-N 0.000 description 1
- WGYHAAXZWPEBDQ-IFFSRLJSSA-N Glu-Val-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WGYHAAXZWPEBDQ-IFFSRLJSSA-N 0.000 description 1
- FMNHBTKMRFVGRO-FOHZUACHSA-N Gly-Asn-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CN FMNHBTKMRFVGRO-FOHZUACHSA-N 0.000 description 1
- JSNNHGHYGYMVCK-XVKPBYJWSA-N Gly-Glu-Val Chemical compound [H]NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O JSNNHGHYGYMVCK-XVKPBYJWSA-N 0.000 description 1
- PDAWDNVHMUKWJR-ZETCQYMHSA-N Gly-Gly-His Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CC1=CNC=N1 PDAWDNVHMUKWJR-ZETCQYMHSA-N 0.000 description 1
- TWTPDFFBLQEBOE-IUCAKERBSA-N Gly-Leu-Gln Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O TWTPDFFBLQEBOE-IUCAKERBSA-N 0.000 description 1
- LPZUKJALYGXBIE-SRVKXCTJSA-N His-Gln-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CC1=CN=CN1)N LPZUKJALYGXBIE-SRVKXCTJSA-N 0.000 description 1
- IMCHNUANCIGUKS-SRVKXCTJSA-N His-Glu-Arg Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O IMCHNUANCIGUKS-SRVKXCTJSA-N 0.000 description 1
- RNMNYMDTESKEAJ-KKUMJFAQSA-N His-Leu-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CN=CN1 RNMNYMDTESKEAJ-KKUMJFAQSA-N 0.000 description 1
- LVXFNTIIGOQBMD-SRVKXCTJSA-N His-Leu-Ser Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O LVXFNTIIGOQBMD-SRVKXCTJSA-N 0.000 description 1
- 206010060378 Hyperinsulinaemia Diseases 0.000 description 1
- 108010065920 Insulin Lispro Proteins 0.000 description 1
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical group SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical group NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- ZGUNAGUHMKGQNY-ZETCQYMHSA-N L-alpha-phenylglycine zwitterion Chemical compound OC(=O)[C@@H](N)C1=CC=CC=C1 ZGUNAGUHMKGQNY-ZETCQYMHSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical group NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical group OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical group OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical group OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical group CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical group CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical group NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical group C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 241000283953 Lagomorpha Species 0.000 description 1
- XBBKIIGCUMBKCO-JXUBOQSCSA-N Leu-Ala-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XBBKIIGCUMBKCO-JXUBOQSCSA-N 0.000 description 1
- STAVRDQLZOTNKJ-RHYQMDGZSA-N Leu-Arg-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O STAVRDQLZOTNKJ-RHYQMDGZSA-N 0.000 description 1
- PJYSOYLLTJKZHC-GUBZILKMSA-N Leu-Asp-Gln Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCC(N)=O PJYSOYLLTJKZHC-GUBZILKMSA-N 0.000 description 1
- ZTLGVASZOIKNIX-DCAQKATOSA-N Leu-Gln-Glu Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N ZTLGVASZOIKNIX-DCAQKATOSA-N 0.000 description 1
- CIVKXGPFXDIQBV-WDCWCFNPSA-N Leu-Gln-Thr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O CIVKXGPFXDIQBV-WDCWCFNPSA-N 0.000 description 1
- KVMULWOHPPMHHE-DCAQKATOSA-N Leu-Glu-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O KVMULWOHPPMHHE-DCAQKATOSA-N 0.000 description 1
- NEEOBPIXKWSBRF-IUCAKERBSA-N Leu-Glu-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O NEEOBPIXKWSBRF-IUCAKERBSA-N 0.000 description 1
- YFBBUHJJUXXZOF-UWVGGRQHSA-N Leu-Gly-Pro Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N1CCC[C@H]1C(O)=O YFBBUHJJUXXZOF-UWVGGRQHSA-N 0.000 description 1
- OHZIZVWQXJPBJS-IXOXFDKPSA-N Leu-His-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OHZIZVWQXJPBJS-IXOXFDKPSA-N 0.000 description 1
- LVTJJOJKDCVZGP-QWRGUYRKSA-N Leu-Lys-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O LVTJJOJKDCVZGP-QWRGUYRKSA-N 0.000 description 1
- ODRREERHVHMIPT-OEAJRASXSA-N Leu-Thr-Phe Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 ODRREERHVHMIPT-OEAJRASXSA-N 0.000 description 1
- MVJRBCJCRYGCKV-GVXVVHGQSA-N Leu-Val-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O MVJRBCJCRYGCKV-GVXVVHGQSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Chemical group CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- KCXUCYYZNZFGLL-SRVKXCTJSA-N Lys-Ala-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O KCXUCYYZNZFGLL-SRVKXCTJSA-N 0.000 description 1
- WSXTWLJHTLRFLW-SRVKXCTJSA-N Lys-Ala-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O WSXTWLJHTLRFLW-SRVKXCTJSA-N 0.000 description 1
- FACUGMGEFUEBTI-SRVKXCTJSA-N Lys-Asn-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CCCCN FACUGMGEFUEBTI-SRVKXCTJSA-N 0.000 description 1
- ZXEUFAVXODIPHC-GUBZILKMSA-N Lys-Glu-Asn Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O ZXEUFAVXODIPHC-GUBZILKMSA-N 0.000 description 1
- UETQMSASAVBGJY-QWRGUYRKSA-N Lys-Gly-His Chemical compound NCCCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC1=CNC=N1 UETQMSASAVBGJY-QWRGUYRKSA-N 0.000 description 1
- FHIAJWBDZVHLAH-YUMQZZPRSA-N Lys-Gly-Ser Chemical compound NCCCC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O FHIAJWBDZVHLAH-YUMQZZPRSA-N 0.000 description 1
- IVFUVMSKSFSFBT-NHCYSSNCSA-N Lys-Ile-Gly Chemical compound OC(=O)CNC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CCCCN IVFUVMSKSFSFBT-NHCYSSNCSA-N 0.000 description 1
- NJNRBRKHOWSGMN-SRVKXCTJSA-N Lys-Leu-Asn Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O NJNRBRKHOWSGMN-SRVKXCTJSA-N 0.000 description 1
- LJADEBULDNKJNK-IHRRRGAJSA-N Lys-Leu-Val Chemical compound CC(C)C[C@H](NC(=O)[C@@H](N)CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O LJADEBULDNKJNK-IHRRRGAJSA-N 0.000 description 1
- RIJCHEVHFWMDKD-SRVKXCTJSA-N Lys-Lys-Asn Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O RIJCHEVHFWMDKD-SRVKXCTJSA-N 0.000 description 1
- HVAUKHLDSDDROB-KKUMJFAQSA-N Lys-Lys-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O HVAUKHLDSDDROB-KKUMJFAQSA-N 0.000 description 1
- RPWTZTBIFGENIA-VOAKCMCISA-N Lys-Thr-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O RPWTZTBIFGENIA-VOAKCMCISA-N 0.000 description 1
- NYTDJEZBAAFLLG-IHRRRGAJSA-N Lys-Val-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(O)=O NYTDJEZBAAFLLG-IHRRRGAJSA-N 0.000 description 1
- GILLQRYAWOMHED-DCAQKATOSA-N Lys-Val-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCCN GILLQRYAWOMHED-DCAQKATOSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 229940122534 Melanocortin receptor antagonist Drugs 0.000 description 1
- YCUSPBPZVJDMII-YUMQZZPRSA-N Met-Gly-Glu Chemical compound CSCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCC(O)=O YCUSPBPZVJDMII-YUMQZZPRSA-N 0.000 description 1
- HSJIGJRZYUADSS-IHRRRGAJSA-N Met-Lys-Leu Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O HSJIGJRZYUADSS-IHRRRGAJSA-N 0.000 description 1
- HAQLBBVZAGMESV-IHRRRGAJSA-N Met-Lys-Lys Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(O)=O HAQLBBVZAGMESV-IHRRRGAJSA-N 0.000 description 1
- OBPCXINRFKHSRY-SDDRHHMPSA-N Met-Met-Pro Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCSC)C(=O)N1CCC[C@@H]1C(=O)O)N OBPCXINRFKHSRY-SDDRHHMPSA-N 0.000 description 1
- CNAGWYQWQDMUGC-IHRRRGAJSA-N Met-Phe-Asn Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(=O)N)C(=O)O)N CNAGWYQWQDMUGC-IHRRRGAJSA-N 0.000 description 1
- DBMLDOWSVHMQQN-XGEHTFHBSA-N Met-Ser-Thr Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O DBMLDOWSVHMQQN-XGEHTFHBSA-N 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- SITLTJHOQZFJGG-UHFFFAOYSA-N N-L-alpha-glutamyl-L-valine Natural products CC(C)C(C(O)=O)NC(=O)C(N)CCC(O)=O SITLTJHOQZFJGG-UHFFFAOYSA-N 0.000 description 1
- KZNQNBZMBZJQJO-UHFFFAOYSA-N N-glycyl-L-proline Natural products NCC(=O)N1CCCC1C(O)=O KZNQNBZMBZJQJO-UHFFFAOYSA-N 0.000 description 1
- 208000028389 Nerve injury Diseases 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Chemical group NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Chemical group OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- SXJGROGVINAYSH-AVGNSLFASA-N Phe-Gln-Asp Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N SXJGROGVINAYSH-AVGNSLFASA-N 0.000 description 1
- WYPVCIACUMJRIB-JYJNAYRXSA-N Phe-Gln-Lys Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCCCN)C(=O)O)N WYPVCIACUMJRIB-JYJNAYRXSA-N 0.000 description 1
- YEEFZOKPYOUXMX-KKUMJFAQSA-N Phe-Gln-Met Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O YEEFZOKPYOUXMX-KKUMJFAQSA-N 0.000 description 1
- ZLGQEBCCANLYRA-RYUDHWBXSA-N Phe-Gly-Glu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(O)=O ZLGQEBCCANLYRA-RYUDHWBXSA-N 0.000 description 1
- KDYPMIZMXDECSU-JYJNAYRXSA-N Phe-Leu-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CC=CC=C1 KDYPMIZMXDECSU-JYJNAYRXSA-N 0.000 description 1
- GPLWGAYGROGDEN-BZSNNMDCSA-N Phe-Phe-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(O)=O GPLWGAYGROGDEN-BZSNNMDCSA-N 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 229920001214 Polysorbate 60 Polymers 0.000 description 1
- WPQKSRHDTMRSJM-CIUDSAMLSA-N Pro-Asp-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1 WPQKSRHDTMRSJM-CIUDSAMLSA-N 0.000 description 1
- JFNPBBOGGNMSRX-CIUDSAMLSA-N Pro-Gln-Ala Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O JFNPBBOGGNMSRX-CIUDSAMLSA-N 0.000 description 1
- PEYNRYREGPAOAK-LSJOCFKGSA-N Pro-His-Ala Chemical compound C([C@@H](C(=O)N[C@@H](C)C([O-])=O)NC(=O)[C@H]1[NH2+]CCC1)C1=CN=CN1 PEYNRYREGPAOAK-LSJOCFKGSA-N 0.000 description 1
- JUJGNDZIKKQMDJ-IHRRRGAJSA-N Pro-His-His Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC1=CNC=N1)C(O)=O JUJGNDZIKKQMDJ-IHRRRGAJSA-N 0.000 description 1
- MCWHYUWXVNRXFV-RWMBFGLXSA-N Pro-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@@H]2CCCN2 MCWHYUWXVNRXFV-RWMBFGLXSA-N 0.000 description 1
- ULWBBFKQBDNGOY-RWMBFGLXSA-N Pro-Lys-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCCCN)C(=O)N2CCC[C@@H]2C(=O)O ULWBBFKQBDNGOY-RWMBFGLXSA-N 0.000 description 1
- XYAFCOJKICBRDU-JYJNAYRXSA-N Pro-Phe-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C(C)C)C(O)=O XYAFCOJKICBRDU-JYJNAYRXSA-N 0.000 description 1
- LZHHZYDPMZEMRX-STQMWFEESA-N Pro-Tyr-Gly Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)NCC(O)=O LZHHZYDPMZEMRX-STQMWFEESA-N 0.000 description 1
- BXHRXLMCYSZSIY-STECZYCISA-N Pro-Tyr-Ile Chemical compound CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1)C(O)=O BXHRXLMCYSZSIY-STECZYCISA-N 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 235000019485 Safflower oil Nutrition 0.000 description 1
- LVVBAKCGXXUHFO-ZLUOBGJFSA-N Ser-Ala-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(O)=O LVVBAKCGXXUHFO-ZLUOBGJFSA-N 0.000 description 1
- VAIZFHMTBFYJIA-ACZMJKKPSA-N Ser-Asp-Gln Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCC(N)=O VAIZFHMTBFYJIA-ACZMJKKPSA-N 0.000 description 1
- ULVMNZOKDBHKKI-ACZMJKKPSA-N Ser-Gln-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O ULVMNZOKDBHKKI-ACZMJKKPSA-N 0.000 description 1
- IXCHOHLPHNGFTJ-YUMQZZPRSA-N Ser-Gly-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CO)N IXCHOHLPHNGFTJ-YUMQZZPRSA-N 0.000 description 1
- GJFYFGOEWLDQGW-GUBZILKMSA-N Ser-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CO)N GJFYFGOEWLDQGW-GUBZILKMSA-N 0.000 description 1
- KCGIREHVWRXNDH-GARJFASQSA-N Ser-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CO)N KCGIREHVWRXNDH-GARJFASQSA-N 0.000 description 1
- UPLYXVPQLJVWMM-KKUMJFAQSA-N Ser-Phe-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(O)=O UPLYXVPQLJVWMM-KKUMJFAQSA-N 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Chemical group OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- VUVCRYXYUUPGSB-GLLZPBPUSA-N Thr-Gln-Glu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N)O VUVCRYXYUUPGSB-GLLZPBPUSA-N 0.000 description 1
- KGKWKSSSQGGYAU-SUSMZKCASA-N Thr-Gln-Thr Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N)O KGKWKSSSQGGYAU-SUSMZKCASA-N 0.000 description 1
- FHDLKMFZKRUQCE-HJGDQZAQSA-N Thr-Glu-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O FHDLKMFZKRUQCE-HJGDQZAQSA-N 0.000 description 1
- LHEZGZQRLDBSRR-WDCWCFNPSA-N Thr-Glu-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O LHEZGZQRLDBSRR-WDCWCFNPSA-N 0.000 description 1
- CRZNCABIJLRFKZ-IUKAMOBKSA-N Thr-Ile-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H]([C@@H](C)O)N CRZNCABIJLRFKZ-IUKAMOBKSA-N 0.000 description 1
- PRNGXSILMXSWQQ-OEAJRASXSA-N Thr-Leu-Phe Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O PRNGXSILMXSWQQ-OEAJRASXSA-N 0.000 description 1
- VGYVVSQFSSKZRJ-OEAJRASXSA-N Thr-Phe-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)[C@H](O)C)CC1=CC=CC=C1 VGYVVSQFSSKZRJ-OEAJRASXSA-N 0.000 description 1
- NHQVWACSJZJCGJ-FLBSBUHZSA-N Thr-Thr-Ile Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O NHQVWACSJZJCGJ-FLBSBUHZSA-N 0.000 description 1
- AKHDFZHUPGVFEJ-YEPSODPASA-N Thr-Val-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O AKHDFZHUPGVFEJ-YEPSODPASA-N 0.000 description 1
- SBYQHZCMVSPQCS-RCWTZXSCSA-N Thr-Val-Met Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCSC)C(O)=O SBYQHZCMVSPQCS-RCWTZXSCSA-N 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Chemical group CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Chemical group 0.000 description 1
- OFCKFBGRYHOKFP-IHPCNDPISA-N Trp-Asp-Tyr Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC3=CC=C(C=C3)O)C(=O)O)N OFCKFBGRYHOKFP-IHPCNDPISA-N 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- DLZKEQQWXODGGZ-KWQFWETISA-N Tyr-Ala-Gly Chemical compound OC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 DLZKEQQWXODGGZ-KWQFWETISA-N 0.000 description 1
- UEOOXDLMQZBPFR-ZKWXMUAHSA-N Val-Ala-Asn Chemical compound C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](C(C)C)N UEOOXDLMQZBPFR-ZKWXMUAHSA-N 0.000 description 1
- COYSIHFOCOMGCF-WPRPVWTQSA-N Val-Arg-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CCCN=C(N)N COYSIHFOCOMGCF-WPRPVWTQSA-N 0.000 description 1
- COYSIHFOCOMGCF-UHFFFAOYSA-N Val-Arg-Gly Natural products CC(C)C(N)C(=O)NC(C(=O)NCC(O)=O)CCCN=C(N)N COYSIHFOCOMGCF-UHFFFAOYSA-N 0.000 description 1
- PVPAOIGJYHVWBT-KKHAAJSZSA-N Val-Asn-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](C(C)C)N)O PVPAOIGJYHVWBT-KKHAAJSZSA-N 0.000 description 1
- CGGVNFJRZJUVAE-BYULHYEWSA-N Val-Asp-Asn Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N CGGVNFJRZJUVAE-BYULHYEWSA-N 0.000 description 1
- TZVUSFMQWPWHON-NHCYSSNCSA-N Val-Asp-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](C(C)C)N TZVUSFMQWPWHON-NHCYSSNCSA-N 0.000 description 1
- HURRXSNHCCSJHA-AUTRQRHGSA-N Val-Gln-Gln Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N HURRXSNHCCSJHA-AUTRQRHGSA-N 0.000 description 1
- QHFQQRKNGCXTHL-AUTRQRHGSA-N Val-Gln-Glu Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O QHFQQRKNGCXTHL-AUTRQRHGSA-N 0.000 description 1
- VFOHXOLPLACADK-GVXVVHGQSA-N Val-Gln-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](C(C)C)N VFOHXOLPLACADK-GVXVVHGQSA-N 0.000 description 1
- GBESYURLQOYWLU-LAEOZQHASA-N Val-Glu-Asp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CC(=O)O)C(=O)O)N GBESYURLQOYWLU-LAEOZQHASA-N 0.000 description 1
- VLDMQVZZWDOKQF-AUTRQRHGSA-N Val-Glu-Gln Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N VLDMQVZZWDOKQF-AUTRQRHGSA-N 0.000 description 1
- VVZDBPBZHLQPPB-XVKPBYJWSA-N Val-Glu-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O VVZDBPBZHLQPPB-XVKPBYJWSA-N 0.000 description 1
- YDPFWRVQHFWBKI-GVXVVHGQSA-N Val-Glu-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N YDPFWRVQHFWBKI-GVXVVHGQSA-N 0.000 description 1
- WDIGUPHXPBMODF-UMNHJUIQSA-N Val-Glu-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N WDIGUPHXPBMODF-UMNHJUIQSA-N 0.000 description 1
- OACSGBOREVRSME-NHCYSSNCSA-N Val-His-Asn Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(N)=O)C(O)=O OACSGBOREVRSME-NHCYSSNCSA-N 0.000 description 1
- IJGPOONOTBNTFS-GVXVVHGQSA-N Val-Lys-Glu Chemical compound [H]N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O IJGPOONOTBNTFS-GVXVVHGQSA-N 0.000 description 1
- DIOSYUIWOQCXNR-ONGXEEELSA-N Val-Lys-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O DIOSYUIWOQCXNR-ONGXEEELSA-N 0.000 description 1
- QIVPZSWBBHRNBA-JYJNAYRXSA-N Val-Pro-Phe Chemical compound CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O QIVPZSWBBHRNBA-JYJNAYRXSA-N 0.000 description 1
- RYHUIHUOYRNNIE-NRPADANISA-N Val-Ser-Gln Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N RYHUIHUOYRNNIE-NRPADANISA-N 0.000 description 1
- WHNSHJJNWNSTSU-BZSNNMDCSA-N Val-Val-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)C(C)C)C(O)=O)=CNC2=C1 WHNSHJJNWNSTSU-BZSNNMDCSA-N 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 238000001042 affinity chromatography Methods 0.000 description 1
- 108010044940 alanylglutamine Proteins 0.000 description 1
- 150000001408 amides Chemical group 0.000 description 1
- 150000001412 amines Chemical group 0.000 description 1
- 238000002266 amputation Methods 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Chemical group OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 108010047857 aspartylglycine Proteins 0.000 description 1
- 230000000923 atherogenic effect Effects 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 210000003050 axon Anatomy 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Chemical group OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 235000019437 butane-1,3-diol Nutrition 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical group 0.000 description 1
- 229920003123 carboxymethyl cellulose sodium Polymers 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 229940105329 carboxymethylcellulose Drugs 0.000 description 1
- 229940063834 carboxymethylcellulose sodium Drugs 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Chemical group SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 235000013681 dietary sucrose Nutrition 0.000 description 1
- 108010009297 diglycyl-histidine Proteins 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 235000004626 essential fatty acids Nutrition 0.000 description 1
- 235000019441 ethanol Nutrition 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000003629 gastrointestinal hormone Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 230000004110 gluconeogenesis Effects 0.000 description 1
- 230000014101 glucose homeostasis Effects 0.000 description 1
- 229940093181 glucose injection Drugs 0.000 description 1
- 230000010030 glucose lowering effect Effects 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 108010057083 glutamyl-aspartyl-leucine Proteins 0.000 description 1
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 1
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 description 1
- 108010015792 glycyllysine Proteins 0.000 description 1
- 108010037850 glycylvaline Proteins 0.000 description 1
- 125000002795 guanidino group Chemical group C(N)(=N)N* 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 208000019622 heart disease Diseases 0.000 description 1
- 230000002440 hepatic effect Effects 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- 108010025306 histidylleucine Proteins 0.000 description 1
- 230000003451 hyperinsulinaemic effect Effects 0.000 description 1
- 201000008980 hyperinsulinism Diseases 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 210000002490 intestinal epithelial cell Anatomy 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Chemical group CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- 229930027917 kanamycin Natural products 0.000 description 1
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 1
- 229960000318 kanamycin Drugs 0.000 description 1
- 229930182823 kanamycin A Natural products 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 239000012669 liquid formulation Substances 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 108010056582 methionylglutamic acid Proteins 0.000 description 1
- 108010034507 methionyltryptophan Proteins 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- 229920001206 natural gum Polymers 0.000 description 1
- 230000008764 nerve damage Effects 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 239000002304 perfume Substances 0.000 description 1
- 210000003200 peritoneal cavity Anatomy 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 108010051242 phenylalanylserine Proteins 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 230000003234 polygenic effect Effects 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 230000000291 postprandial effect Effects 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108010004914 prolylarginine Proteins 0.000 description 1
- 108010070643 prolylglutamic acid Proteins 0.000 description 1
- 108010090894 prolylleucine Proteins 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 235000013772 propylene glycol Nutrition 0.000 description 1
- 238000001243 protein synthesis Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 239000003813 safflower oil Substances 0.000 description 1
- 235000005713 safflower oil Nutrition 0.000 description 1
- 235000014102 seafood Nutrition 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 108010026333 seryl-proline Proteins 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 201000002859 sleep apnea Diseases 0.000 description 1
- BTURAGWYSMTVOW-UHFFFAOYSA-M sodium dodecanoate Chemical compound [Na+].CCCCCCCCCCCC([O-])=O BTURAGWYSMTVOW-UHFFFAOYSA-M 0.000 description 1
- 229940082004 sodium laurate Drugs 0.000 description 1
- WSWCOQWTEOXDQX-MQQKCMAXSA-N sorbic acid group Chemical class C(\C=C\C=C\C)(=O)O WSWCOQWTEOXDQX-MQQKCMAXSA-N 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 235000010356 sorbitol Nutrition 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 229960004793 sucrose Drugs 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/775—Apolipopeptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/1703—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- A61K38/1709—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/177—Receptors; Cell surface antigens; Cell surface determinants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/22—Hormones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/22—Hormones
- A61K38/26—Glucagons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/22—Hormones
- A61K38/28—Insulins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/12—Antidiuretics, e.g. drugs for diabetes insipidus
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/575—Hormones
- C07K14/605—Glucagons
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/575—Hormones
- C07K14/655—Somatostatins
- C07K14/6555—Somatostatins at least 1 amino acid in D-form
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K9/00—Peptides having up to 20 amino acids, containing saccharide radicals and having a fully defined sequence; Derivatives thereof
- C07K9/001—Peptides having up to 20 amino acids, containing saccharide radicals and having a fully defined sequence; Derivatives thereof the peptide sequence having less than 12 amino acids and not being part of a ring structure
- C07K9/005—Peptides having up to 20 amino acids, containing saccharide radicals and having a fully defined sequence; Derivatives thereof the peptide sequence having less than 12 amino acids and not being part of a ring structure containing within the molecule the substructure with m, n > 0 and m+n > 0, A, B, D, E being heteroatoms; X being a bond or a chain, e.g. muramylpeptides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K9/00—Peptides having up to 20 amino acids, containing saccharide radicals and having a fully defined sequence; Derivatives thereof
- C07K9/006—Peptides having up to 20 amino acids, containing saccharide radicals and having a fully defined sequence; Derivatives thereof the peptide sequence being part of a ring structure
- C07K9/008—Peptides having up to 20 amino acids, containing saccharide radicals and having a fully defined sequence; Derivatives thereof the peptide sequence being part of a ring structure directly attached to a hetero atom of the saccharide radical, e.g. actaplanin, avoparcin, ristomycin, vancomycin
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- Immunology (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Diabetes (AREA)
- Endocrinology (AREA)
- Toxicology (AREA)
- Mycology (AREA)
- Microbiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Hematology (AREA)
- Marine Sciences & Fisheries (AREA)
- Emergency Medicine (AREA)
- Obesity (AREA)
- Cell Biology (AREA)
- Crystallography & Structural Chemistry (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161434196P | 2011-01-19 | 2011-01-19 | |
| US61/434,196 | 2011-01-19 | ||
| PCT/US2012/021802 WO2012100010A1 (en) | 2011-01-19 | 2012-01-19 | Apolipoprotein aiv as an antidiabetic peptide |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20140071272A true KR20140071272A (ko) | 2014-06-11 |
Family
ID=45755499
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020137020637A Ceased KR20140071272A (ko) | 2011-01-19 | 2012-01-19 | 항 당뇨병 펩타이드로서의 아포리포프로테인 aiv |
Country Status (13)
| Country | Link |
|---|---|
| US (2) | US9051394B2 (OSRAM) |
| EP (1) | EP2665485B1 (OSRAM) |
| JP (1) | JP2014504601A (OSRAM) |
| KR (1) | KR20140071272A (OSRAM) |
| CN (1) | CN103391786B (OSRAM) |
| AU (1) | AU2012207301B2 (OSRAM) |
| CA (1) | CA2823712A1 (OSRAM) |
| EA (1) | EA028218B1 (OSRAM) |
| ES (1) | ES2620404T3 (OSRAM) |
| IL (1) | IL227207B (OSRAM) |
| MX (1) | MX342020B (OSRAM) |
| SG (1) | SG191992A1 (OSRAM) |
| WO (1) | WO2012100010A1 (OSRAM) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2010347724B2 (en) * | 2010-03-12 | 2016-06-23 | Exxonmobil Upstream Research Company | Dynamic grouping of domain objects via smart groups |
| AU2012207301B2 (en) | 2011-01-19 | 2016-05-19 | University Of Cincinnati | Apolipoprotein AIV as an antidiabetic peptide |
| AU2012366182B2 (en) | 2012-01-19 | 2017-08-17 | University Of Cincinnati | Method of treating diabetes using non-glycosylated apolipoprotein A-IV |
| JP6033433B2 (ja) * | 2012-07-25 | 2016-11-30 | ユニバーシティ・オブ・シンシナティ | アポリポタンパク質aivを用いて高血糖障害を治療する方法 |
| AU2013295721B2 (en) | 2012-07-25 | 2017-07-20 | University Of Cincinnati | Method of treating type I diabetes using apolipoprotein AIV |
| CN112546201A (zh) * | 2019-09-26 | 2021-03-26 | 中国科学院生物物理研究所 | 人工脂蛋白颗粒apoA-Ⅳ脂肪体在治疗和/或预防糖尿病中的应用 |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2686605B1 (fr) * | 1992-01-27 | 1994-03-11 | Rhone Poulenc Rorer Sa | Nouveaux polypeptides, leur preparation et leur utilisation. |
| WO1994027629A1 (en) | 1993-05-28 | 1994-12-08 | Merck & Co., Inc. | Method to control appetite and treat obesity |
| AU2001251245A1 (en) * | 2000-04-05 | 2001-10-23 | Genaissance Pharmaceuticals, Inc. | Haplotypes of the apoa4 gene |
| NZ537006A (en) | 2002-05-17 | 2008-01-31 | Esperion Therapeutics Inc | The use of apolipoprotein A-I Milano, for the manufacture ofa composition for treating preventing or reducing ischemic reperfusion injury in a tissue or organ |
| US20100267052A1 (en) * | 2006-09-01 | 2010-10-21 | American Type Culture Collection | Compositions and methods for diagnosis and treatment of type 2 diabetes |
| WO2009116861A2 (en) | 2008-03-21 | 2009-09-24 | Podiceps B.V. | Diagnostic of pre-symptomatic metabolic syndrome |
| CN103833712B (zh) | 2008-11-28 | 2016-02-03 | 中国医学科学院药物研究所 | 硝克柳胺化合物晶ii型、其制法和其药物组合物与用途 |
| AU2012207301B2 (en) | 2011-01-19 | 2016-05-19 | University Of Cincinnati | Apolipoprotein AIV as an antidiabetic peptide |
| AU2012366182B2 (en) | 2012-01-19 | 2017-08-17 | University Of Cincinnati | Method of treating diabetes using non-glycosylated apolipoprotein A-IV |
| JP6033433B2 (ja) | 2012-07-25 | 2016-11-30 | ユニバーシティ・オブ・シンシナティ | アポリポタンパク質aivを用いて高血糖障害を治療する方法 |
| AU2013295721B2 (en) | 2012-07-25 | 2017-07-20 | University Of Cincinnati | Method of treating type I diabetes using apolipoprotein AIV |
-
2012
- 2012-01-19 AU AU2012207301A patent/AU2012207301B2/en not_active Ceased
- 2012-01-19 KR KR1020137020637A patent/KR20140071272A/ko not_active Ceased
- 2012-01-19 EP EP12705517.6A patent/EP2665485B1/en active Active
- 2012-01-19 EA EA201391063A patent/EA028218B1/ru not_active IP Right Cessation
- 2012-01-19 US US13/980,749 patent/US9051394B2/en active Active
- 2012-01-19 JP JP2013550572A patent/JP2014504601A/ja active Pending
- 2012-01-19 WO PCT/US2012/021802 patent/WO2012100010A1/en not_active Ceased
- 2012-01-19 CA CA2823712A patent/CA2823712A1/en not_active Abandoned
- 2012-01-19 ES ES12705517.6T patent/ES2620404T3/es active Active
- 2012-01-19 CN CN201280005394.9A patent/CN103391786B/zh not_active Expired - Fee Related
- 2012-01-19 SG SG2013054358A patent/SG191992A1/en unknown
- 2012-01-19 MX MX2013008374A patent/MX342020B/es active IP Right Grant
-
2013
- 2013-06-26 IL IL227207A patent/IL227207B/en active IP Right Grant
-
2015
- 2015-05-05 US US14/704,418 patent/US9266941B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| US9051394B2 (en) | 2015-06-09 |
| EA201391063A1 (ru) | 2013-12-30 |
| CN103391786B (zh) | 2016-06-22 |
| MX342020B (es) | 2016-09-08 |
| EP2665485B1 (en) | 2017-01-11 |
| CN103391786A (zh) | 2013-11-13 |
| SG191992A1 (en) | 2013-08-30 |
| AU2012207301A8 (en) | 2013-08-29 |
| EP2665485A1 (en) | 2013-11-27 |
| US9266941B2 (en) | 2016-02-23 |
| AU2012207301A1 (en) | 2013-08-01 |
| AU2012207301A2 (en) | 2015-03-26 |
| US20150252095A1 (en) | 2015-09-10 |
| WO2012100010A1 (en) | 2012-07-26 |
| MX2013008374A (es) | 2013-12-06 |
| AU2012207301B2 (en) | 2016-05-19 |
| CA2823712A1 (en) | 2012-07-26 |
| ES2620404T3 (es) | 2017-06-28 |
| US20140005107A1 (en) | 2014-01-02 |
| JP2014504601A (ja) | 2014-02-24 |
| EA028218B1 (ru) | 2017-10-31 |
| IL227207B (en) | 2019-09-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN107921085B (zh) | 用于治疗衰老相关病症的方法和组合物 | |
| TW201431884A (zh) | 靶向治療性溶小體酶融合蛋白及其用途 | |
| US9266941B2 (en) | Apolipoprotein AIV as an antidiabetic peptide | |
| AU2012366182B2 (en) | Method of treating diabetes using non-glycosylated apolipoprotein A-IV | |
| KR20200024263A (ko) | 고혈당증의 치료 및 예방을 위한 펩타이드 | |
| US20230414706A1 (en) | Compositions and methods for treating metabolic diseases | |
| WO2017062693A1 (en) | Methods for treating rare genetic disorders using glucagon receptor antagonistic antibodies | |
| AU2012385960B2 (en) | Method of treating hyperglycemic disorders using apolipoprotein AIV | |
| JP2019214558A (ja) | 腎障害の予防又は治療用の医薬組成物 | |
| JP5447784B2 (ja) | マキサディランの部分ペプチドを含む神経因性疼痛の治療剤 | |
| JP2017532292A (ja) | ミリストイル化レプチン関連ペプチド及びその使用 | |
| JP2023544910A (ja) | コロナウイルス治療法 | |
| BR112014016734B1 (pt) | Composições compreendendo um peptídeo e usos de composições e peptídeos no tratamento médico |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20130805 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20161129 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20171211 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20180219 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20171211 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |