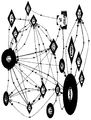
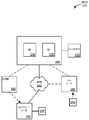
KR20120101038A - 과민성대장증후군을 진단하는 방법 - Google Patents
과민성대장증후군을 진단하는 방법 Download PDFInfo
- Publication number
- KR20120101038A KR20120101038A KR1020127013948A KR20127013948A KR20120101038A KR 20120101038 A KR20120101038 A KR 20120101038A KR 1020127013948 A KR1020127013948 A KR 1020127013948A KR 20127013948 A KR20127013948 A KR 20127013948A KR 20120101038 A KR20120101038 A KR 20120101038A
- Authority
- KR
- South Korea
- Prior art keywords
- ibs
- marker
- sample
- level
- subject
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 208000002551 irritable bowel syndrome Diseases 0.000 title claims abstract description 807
- 238000000034 method Methods 0.000 title claims abstract description 392
- 210000002966 serum Anatomy 0.000 claims abstract description 168
- 238000003745 diagnosis Methods 0.000 claims abstract description 63
- 239000000090 biomarker Substances 0.000 claims abstract description 55
- 239000000523 sample Substances 0.000 claims description 471
- 239000003550 marker Substances 0.000 claims description 456
- QZAYGJVTTNCVMB-UHFFFAOYSA-N serotonin Chemical compound C1=C(O)C=C2C(CCN)=CNC2=C1 QZAYGJVTTNCVMB-UHFFFAOYSA-N 0.000 claims description 205
- 208000024891 symptom Diseases 0.000 claims description 142
- 210000004369 blood Anatomy 0.000 claims description 132
- 239000008280 blood Substances 0.000 claims description 132
- NTYJJOPFIAHURM-UHFFFAOYSA-N Histamine Chemical compound NCCC1=CN=CN1 NTYJJOPFIAHURM-UHFFFAOYSA-N 0.000 claims description 114
- 102000004219 Brain-derived neurotrophic factor Human genes 0.000 claims description 96
- 108090000715 Brain-derived neurotrophic factor Proteins 0.000 claims description 96
- 229940077737 brain-derived neurotrophic factor Drugs 0.000 claims description 96
- 108010031374 Tissue Inhibitor of Metalloproteinase-1 Proteins 0.000 claims description 95
- 102000005353 Tissue Inhibitor of Metalloproteinase-1 Human genes 0.000 claims description 95
- 210000000440 neutrophil Anatomy 0.000 claims description 93
- 230000027455 binding Effects 0.000 claims description 92
- 102000016950 Chemokine CXCL1 Human genes 0.000 claims description 91
- 108700039882 Protein Glutamine gamma Glutamyltransferase 2 Proteins 0.000 claims description 91
- 102100038095 Protein-glutamine gamma-glutamyltransferase 2 Human genes 0.000 claims description 91
- 229940076279 serotonin Drugs 0.000 claims description 74
- 238000003018 immunoassay Methods 0.000 claims description 70
- 102000001400 Tryptase Human genes 0.000 claims description 68
- 108060005989 Tryptase Proteins 0.000 claims description 68
- 230000001965 increasing effect Effects 0.000 claims description 68
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 65
- 208000022559 Inflammatory bowel disease Diseases 0.000 claims description 59
- 229960001340 histamine Drugs 0.000 claims description 57
- 102000019298 Lipocalin Human genes 0.000 claims description 52
- 108050006654 Lipocalin Proteins 0.000 claims description 52
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 claims description 52
- 102000004190 Enzymes Human genes 0.000 claims description 51
- 108090000790 Enzymes Proteins 0.000 claims description 51
- 108010062431 Monoamine oxidase Proteins 0.000 claims description 50
- XEYBRNLFEZDVAW-ARSRFYASSA-N dinoprostone Chemical compound CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O XEYBRNLFEZDVAW-ARSRFYASSA-N 0.000 claims description 50
- 108700022763 carbohydrate-deficient transferrin Proteins 0.000 claims description 49
- 108010014419 Chemokine CXCL1 Proteins 0.000 claims description 47
- -1 eurocortin Proteins 0.000 claims description 47
- LOGFVTREOLYCPF-KXNHARMFSA-N (2s,3r)-2-[[(2r)-1-[(2s)-2,6-diaminohexanoyl]pyrrolidine-2-carbonyl]amino]-3-hydroxybutanoic acid Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H]1CCCN1C(=O)[C@@H](N)CCCCN LOGFVTREOLYCPF-KXNHARMFSA-N 0.000 claims description 46
- 102000013382 Gelatinases Human genes 0.000 claims description 46
- 108010026132 Gelatinases Proteins 0.000 claims description 46
- 102000003777 Interleukin-1 beta Human genes 0.000 claims description 46
- 108090000193 Interleukin-1 beta Proteins 0.000 claims description 46
- 230000006907 apoptotic process Effects 0.000 claims description 46
- 230000001086 cytosolic effect Effects 0.000 claims description 46
- 239000000411 inducer Substances 0.000 claims description 46
- DUUGKQCEGZLZNO-UHFFFAOYSA-N 5-hydroxyindoleacetic acid Chemical compound C1=C(O)C=C2C(CC(=O)O)=CNC2=C1 DUUGKQCEGZLZNO-UHFFFAOYSA-N 0.000 claims description 44
- 101001027327 Bos taurus Growth-regulated protein homolog alpha Proteins 0.000 claims description 44
- 230000013016 learning Effects 0.000 claims description 38
- 102000010909 Monoamine Oxidase Human genes 0.000 claims description 33
- 239000003814 drug Substances 0.000 claims description 33
- 230000008569 process Effects 0.000 claims description 33
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 claims description 32
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 claims description 30
- 229940079593 drug Drugs 0.000 claims description 30
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 30
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 claims description 28
- 238000004422 calculation algorithm Methods 0.000 claims description 28
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 claims description 28
- 229960004799 tryptophan Drugs 0.000 claims description 28
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 claims description 28
- 102100032165 Corticotropin-releasing factor-binding protein Human genes 0.000 claims description 26
- 102400000326 Glucagon-like peptide 2 Human genes 0.000 claims description 26
- 238000012896 Statistical algorithm Methods 0.000 claims description 26
- TWSALRJGPBVBQU-PKQQPRCHSA-N glucagon-like peptide 2 Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O)[C@@H](C)CC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)CC)C1=CC=CC=C1 TWSALRJGPBVBQU-PKQQPRCHSA-N 0.000 claims description 26
- 229960000890 hydrocortisone Drugs 0.000 claims description 26
- 230000001817 pituitary effect Effects 0.000 claims description 26
- 102000005962 receptors Human genes 0.000 claims description 26
- 108020003175 receptors Proteins 0.000 claims description 26
- 230000000697 serotonin reuptake Effects 0.000 claims description 26
- JFWYSGGSCOOBGK-UHFFFAOYSA-N [3-(2-azaniumylethyl)-1h-indol-5-yl] sulfate Chemical compound C1=C(OS(O)(=O)=O)C=C2C(CCN)=CNC2=C1 JFWYSGGSCOOBGK-UHFFFAOYSA-N 0.000 claims description 25
- 108010083720 corticotropin releasing factor-binding protein Proteins 0.000 claims description 25
- 102100040198 UDP-glucuronosyltransferase 1-6 Human genes 0.000 claims description 24
- 101710008381 UGT1A6 Proteins 0.000 claims description 24
- 229930182480 glucuronide Natural products 0.000 claims description 24
- 101800000399 Neurokinin A Proteins 0.000 claims description 23
- 102400000097 Neurokinin A Human genes 0.000 claims description 23
- HEAUFJZALFKPBA-YRVBCFNBSA-N Neurokinin A Chemical compound C([C@@H](C(=O)N[C@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(N)=O)C(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CC=1NC=NC=1)C(C)O)C1=CC=CC=C1 HEAUFJZALFKPBA-YRVBCFNBSA-N 0.000 claims description 23
- 102000046798 Neurokinin B Human genes 0.000 claims description 23
- NHXYSAFTNPANFK-HDMCBQFHSA-N Neurokinin B Chemical compound C([C@@H](C(=O)N[C@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(N)=O)C(C)C)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C1=CC=CC=C1 NHXYSAFTNPANFK-HDMCBQFHSA-N 0.000 claims description 23
- 101800002813 Neurokinin-B Proteins 0.000 claims description 23
- 101000830742 Homo sapiens Tryptophan 5-hydroxylase 1 Proteins 0.000 claims description 22
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 claims description 22
- 102100024971 Tryptophan 5-hydroxylase 1 Human genes 0.000 claims description 22
- 101800000221 Glucagon-like peptide 2 Proteins 0.000 claims description 21
- 239000012472 biological sample Substances 0.000 claims description 21
- 230000002792 vascular Effects 0.000 claims description 20
- 108010025020 Nerve Growth Factor Proteins 0.000 claims description 19
- 210000000936 intestine Anatomy 0.000 claims description 19
- RVWZUOPFHTYIEO-UHFFFAOYSA-N 5-hydroxyindoleacetic acid Natural products C1=C(O)C=C2C(C(=O)O)=CNC2=C1 RVWZUOPFHTYIEO-UHFFFAOYSA-N 0.000 claims description 18
- 239000003310 5-hydroxyindoleacetic acid Substances 0.000 claims description 18
- 108010078791 Carrier Proteins Proteins 0.000 claims description 18
- 206010010774 Constipation Diseases 0.000 claims description 18
- 206010012735 Diarrhoea Diseases 0.000 claims description 18
- CWWARWOPSKGELM-SARDKLJWSA-N methyl (2s)-2-[[(2s)-2-[[2-[[(2s)-2-[[(2s)-2-[[(2s)-5-amino-2-[[(2s)-5-amino-2-[[(2s)-1-[(2s)-6-amino-2-[[(2s)-1-[(2s)-2-amino-5-(diaminomethylideneamino)pentanoyl]pyrrolidine-2-carbonyl]amino]hexanoyl]pyrrolidine-2-carbonyl]amino]-5-oxopentanoyl]amino]-5 Chemical compound C([C@@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)OC)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCCN)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](N)CCCN=C(N)N)C1=CC=CC=C1 CWWARWOPSKGELM-SARDKLJWSA-N 0.000 claims description 18
- 229940053128 nerve growth factor Drugs 0.000 claims description 18
- 206010000060 Abdominal distension Diseases 0.000 claims description 16
- 238000007637 random forest analysis Methods 0.000 claims description 16
- LVRVABPNVHYXRT-BQWXUCBYSA-N 52906-92-0 Chemical group C([C@H](N)C(=O)N[C@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(O)=O)C(C)C)C1=CC=CC=C1 LVRVABPNVHYXRT-BQWXUCBYSA-N 0.000 claims description 15
- 101800002372 Motilin Proteins 0.000 claims description 15
- UDMBCSSLTHHNCD-KQYNXXCUSA-N adenosine 5'-monophosphate Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O UDMBCSSLTHHNCD-KQYNXXCUSA-N 0.000 claims description 15
- 208000024330 bloating Diseases 0.000 claims description 15
- 239000003246 corticosteroid Substances 0.000 claims description 15
- 239000003102 growth factor Substances 0.000 claims description 15
- 238000012545 processing Methods 0.000 claims description 15
- 238000013528 artificial neural network Methods 0.000 claims description 14
- 229960001334 corticosteroids Drugs 0.000 claims description 14
- 238000012544 monitoring process Methods 0.000 claims description 14
- 230000002829 reductive effect Effects 0.000 claims description 14
- 238000003118 sandwich ELISA Methods 0.000 claims description 13
- QDZOEBFLNHCSSF-PFFBOGFISA-N (2S)-2-[[(2R)-2-[[(2S)-1-[(2S)-6-amino-2-[[(2S)-1-[(2R)-2-amino-5-carbamimidamidopentanoyl]pyrrolidine-2-carbonyl]amino]hexanoyl]pyrrolidine-2-carbonyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-N-[(2R)-1-[[(2S)-1-[[(2R)-1-[[(2S)-1-[[(2S)-1-amino-4-methyl-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]pentanediamide Chemical group C([C@@H](C(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(N)=O)NC(=O)[C@@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCCN)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](N)CCCNC(N)=N)C1=CC=CC=C1 QDZOEBFLNHCSSF-PFFBOGFISA-N 0.000 claims description 12
- 101800003906 Substance P Proteins 0.000 claims description 12
- 102400000096 Substance P Human genes 0.000 claims description 12
- 102000030621 adenylate cyclase Human genes 0.000 claims description 11
- 108060000200 adenylate cyclase Proteins 0.000 claims description 11
- 208000004998 Abdominal Pain Diseases 0.000 claims description 10
- 238000002965 ELISA Methods 0.000 claims description 10
- 229940088597 hormone Drugs 0.000 claims description 10
- 239000005556 hormone Substances 0.000 claims description 10
- 235000012054 meals Nutrition 0.000 claims description 9
- 230000007514 neuronal growth Effects 0.000 claims description 8
- 230000004936 stimulating effect Effects 0.000 claims description 8
- 206010000059 abdominal discomfort Diseases 0.000 claims description 7
- 230000001919 adrenal effect Effects 0.000 claims description 7
- 230000001054 cortical effect Effects 0.000 claims description 7
- 108010088406 Glucagon-Like Peptides Proteins 0.000 claims description 6
- 108700020796 Oncogene Proteins 0.000 claims description 6
- 230000012010 growth Effects 0.000 claims description 6
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 claims description 6
- 208000002193 Pain Diseases 0.000 claims description 5
- 201000006549 dyspepsia Diseases 0.000 claims description 5
- 208000015181 infectious disease Diseases 0.000 claims description 5
- 230000036407 pain Effects 0.000 claims description 5
- 206010008479 Chest Pain Diseases 0.000 claims description 4
- 206010008469 Chest discomfort Diseases 0.000 claims description 4
- 206010058672 Negative thoughts Diseases 0.000 claims description 4
- 238000002405 diagnostic procedure Methods 0.000 claims description 4
- 208000024798 heartburn Diseases 0.000 claims description 4
- 239000003112 inhibitor Substances 0.000 claims description 4
- 230000036651 mood Effects 0.000 claims description 4
- 210000004556 brain Anatomy 0.000 claims description 2
- 229910052751 metal Inorganic materials 0.000 claims description 2
- 239000002184 metal Substances 0.000 claims description 2
- 102400001357 Motilin Human genes 0.000 claims 5
- 102000015336 Nerve Growth Factor Human genes 0.000 claims 4
- 125000000430 tryptophan group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C2=C([H])C([H])=C([H])C([H])=C12 0.000 claims 4
- 230000004218 vascular function Effects 0.000 claims 3
- 241001408449 Asca Species 0.000 claims 2
- 101710180319 Protease 1 Proteins 0.000 claims 1
- 101710137710 Thioesterase 1/protease 1/lysophospholipase L1 Proteins 0.000 claims 1
- 201000001119 neuropathy Diseases 0.000 claims 1
- 230000007823 neuropathy Effects 0.000 claims 1
- 208000033808 peripheral neuropathy Diseases 0.000 claims 1
- 230000001131 transforming effect Effects 0.000 claims 1
- 239000000101 novel biomarker Substances 0.000 abstract 1
- 238000003556 assay Methods 0.000 description 110
- 238000001514 detection method Methods 0.000 description 59
- 229940088598 enzyme Drugs 0.000 description 49
- 101000611183 Homo sapiens Tumor necrosis factor Proteins 0.000 description 42
- 102100040247 Tumor necrosis factor Human genes 0.000 description 42
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 39
- 235000018102 proteins Nutrition 0.000 description 31
- 102000004169 proteins and genes Human genes 0.000 description 31
- 108090000623 proteins and genes Proteins 0.000 description 31
- 150000001875 compounds Chemical class 0.000 description 29
- 239000000758 substrate Substances 0.000 description 29
- 230000037361 pathway Effects 0.000 description 24
- 201000010099 disease Diseases 0.000 description 21
- 229960005190 phenylalanine Drugs 0.000 description 21
- 238000004128 high performance liquid chromatography Methods 0.000 description 19
- 239000002207 metabolite Substances 0.000 description 19
- 230000035882 stress Effects 0.000 description 19
- 208000035475 disorder Diseases 0.000 description 18
- 239000003629 gastrointestinal hormone Substances 0.000 description 18
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 17
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 17
- 102100028661 Amine oxidase [flavin-containing] A Human genes 0.000 description 17
- 238000004949 mass spectrometry Methods 0.000 description 17
- 238000011282 treatment Methods 0.000 description 17
- 238000001574 biopsy Methods 0.000 description 16
- 238000003127 radioimmunoassay Methods 0.000 description 16
- 102000004127 Cytokines Human genes 0.000 description 15
- 108090000695 Cytokines Proteins 0.000 description 15
- 102100023995 Beta-nerve growth factor Human genes 0.000 description 14
- 108010074051 C-Reactive Protein Proteins 0.000 description 14
- 102100032752 C-reactive protein Human genes 0.000 description 14
- 238000004458 analytical method Methods 0.000 description 13
- 230000000968 intestinal effect Effects 0.000 description 13
- 239000000427 antigen Substances 0.000 description 12
- 108091007433 antigens Proteins 0.000 description 12
- 102000036639 antigens Human genes 0.000 description 12
- 230000000694 effects Effects 0.000 description 12
- 230000000670 limiting effect Effects 0.000 description 12
- 208000015943 Coeliac disease Diseases 0.000 description 11
- 230000002496 gastric effect Effects 0.000 description 11
- 102100021752 Corticoliberin Human genes 0.000 description 10
- 108010022152 Corticotropin-Releasing Hormone Proteins 0.000 description 10
- 239000000055 Corticotropin-Releasing Hormone Substances 0.000 description 10
- 102100033467 L-selectin Human genes 0.000 description 10
- 108010015302 Matrix metalloproteinase-9 Proteins 0.000 description 10
- 102100030412 Matrix metalloproteinase-9 Human genes 0.000 description 10
- 102000002419 Motilin Human genes 0.000 description 10
- 102100030304 Platelet factor 4 Human genes 0.000 description 10
- 150000001413 amino acids Chemical class 0.000 description 10
- 239000012634 fragment Substances 0.000 description 10
- 210000003630 histaminocyte Anatomy 0.000 description 10
- 102000010445 Lactoferrin Human genes 0.000 description 9
- 108010063045 Lactoferrin Proteins 0.000 description 9
- 102000002274 Matrix Metalloproteinases Human genes 0.000 description 9
- 108010000684 Matrix Metalloproteinases Proteins 0.000 description 9
- 238000009396 hybridization Methods 0.000 description 9
- CSSYQJWUGATIHM-IKGCZBKSSA-N l-phenylalanyl-l-lysyl-l-cysteinyl-l-arginyl-l-arginyl-l-tryptophyl-l-glutaminyl-l-tryptophyl-l-arginyl-l-methionyl-l-lysyl-l-lysyl-l-leucylglycyl-l-alanyl-l-prolyl-l-seryl-l-isoleucyl-l-threonyl-l-cysteinyl-l-valyl-l-arginyl-l-arginyl-l-alanyl-l-phenylal Chemical compound C([C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 CSSYQJWUGATIHM-IKGCZBKSSA-N 0.000 description 9
- 229940078795 lactoferrin Drugs 0.000 description 9
- 235000021242 lactoferrin Nutrition 0.000 description 9
- 238000001840 matrix-assisted laser desorption--ionisation time-of-flight mass spectrometry Methods 0.000 description 9
- 239000011859 microparticle Substances 0.000 description 9
- 102000004414 Calcitonin Gene-Related Peptide Human genes 0.000 description 8
- 108090000932 Calcitonin Gene-Related Peptide Proteins 0.000 description 8
- 102100032219 Cathepsin D Human genes 0.000 description 8
- 101000869010 Homo sapiens Cathepsin D Proteins 0.000 description 8
- 101000947178 Homo sapiens Platelet basic protein Proteins 0.000 description 8
- 108090001007 Interleukin-8 Proteins 0.000 description 8
- 102100036154 Platelet basic protein Human genes 0.000 description 8
- ORILYTVJVMAKLC-UHFFFAOYSA-N adamantane Chemical compound C1C(C2)CC3CC1CC2C3 ORILYTVJVMAKLC-UHFFFAOYSA-N 0.000 description 8
- 238000005251 capillar electrophoresis Methods 0.000 description 8
- 238000003094 enzyme-multiplied immunoassay technique Methods 0.000 description 8
- 239000002609 medium Substances 0.000 description 8
- 229920001184 polypeptide Polymers 0.000 description 8
- 230000035945 sensitivity Effects 0.000 description 8
- 239000007787 solid Substances 0.000 description 8
- 102000002572 Alpha-Globulins Human genes 0.000 description 7
- 108010068307 Alpha-Globulins Proteins 0.000 description 7
- 102100033367 Appetite-regulating hormone Human genes 0.000 description 7
- 238000009010 Bradford assay Methods 0.000 description 7
- 101800001586 Ghrelin Proteins 0.000 description 7
- 241000282414 Homo sapiens Species 0.000 description 7
- 102000004890 Interleukin-8 Human genes 0.000 description 7
- 102000001109 Leukocyte L1 Antigen Complex Human genes 0.000 description 7
- 108010069316 Leukocyte L1 Antigen Complex Proteins 0.000 description 7
- 101800001814 Neurotensin Proteins 0.000 description 7
- 102400001103 Neurotensin Human genes 0.000 description 7
- 102000013674 S-100 Human genes 0.000 description 7
- 108700021018 S100 Proteins 0.000 description 7
- 102000003141 Tachykinin Human genes 0.000 description 7
- 108010003205 Vasoactive Intestinal Peptide Proteins 0.000 description 7
- 102400000015 Vasoactive intestinal peptide Human genes 0.000 description 7
- 210000001072 colon Anatomy 0.000 description 7
- 229940041967 corticotropin-releasing hormone Drugs 0.000 description 7
- KLVRDXBAMSPYKH-RKYZNNDCSA-N corticotropin-releasing hormone (human) Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(N)=O)[C@@H](C)CC)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H]1N(CCC1)C(=O)[C@H]1N(CCC1)C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CO)[C@@H](C)CC)C(C)C)C(C)C)C1=CNC=N1 KLVRDXBAMSPYKH-RKYZNNDCSA-N 0.000 description 7
- 210000003608 fece Anatomy 0.000 description 7
- 238000002875 fluorescence polarization Methods 0.000 description 7
- 210000001035 gastrointestinal tract Anatomy 0.000 description 7
- BGHSOEHUOOAYMY-JTZMCQEISA-N ghrelin Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1N=CNC=1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)CN)C1=CC=CC=C1 BGHSOEHUOOAYMY-JTZMCQEISA-N 0.000 description 7
- VBUWHHLIZKOSMS-RIWXPGAOSA-N invicorp Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC=1NC=NC=1)C(C)C)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=C(O)C=C1 VBUWHHLIZKOSMS-RIWXPGAOSA-N 0.000 description 7
- 238000000816 matrix-assisted laser desorption--ionisation Methods 0.000 description 7
- PCJGZPGTCUMMOT-ISULXFBGSA-N neurotensin Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 PCJGZPGTCUMMOT-ISULXFBGSA-N 0.000 description 7
- 238000000746 purification Methods 0.000 description 7
- 230000004044 response Effects 0.000 description 7
- 238000012163 sequencing technique Methods 0.000 description 7
- 108060008037 tachykinin Proteins 0.000 description 7
- 238000004885 tandem mass spectrometry Methods 0.000 description 7
- QFVHZQCOUORWEI-UHFFFAOYSA-N 4-[(4-anilino-5-sulfonaphthalen-1-yl)diazenyl]-5-hydroxynaphthalene-2,7-disulfonic acid Chemical compound C=12C(O)=CC(S(O)(=O)=O)=CC2=CC(S(O)(=O)=O)=CC=1N=NC(C1=CC=CC(=C11)S(O)(=O)=O)=CC=C1NC1=CC=CC=C1 QFVHZQCOUORWEI-UHFFFAOYSA-N 0.000 description 6
- 102100025255 Haptoglobin Human genes 0.000 description 6
- 102000016387 Pancreatic elastase Human genes 0.000 description 6
- 108010067372 Pancreatic elastase Proteins 0.000 description 6
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 6
- 102000005630 Urocortins Human genes 0.000 description 6
- 108010059705 Urocortins Proteins 0.000 description 6
- 239000005557 antagonist Substances 0.000 description 6
- 230000000356 anti-lactoferrin effect Effects 0.000 description 6
- 238000011161 development Methods 0.000 description 6
- 230000018109 developmental process Effects 0.000 description 6
- 229910052709 silver Inorganic materials 0.000 description 6
- 239000004332 silver Substances 0.000 description 6
- 238000012360 testing method Methods 0.000 description 6
- 210000001519 tissue Anatomy 0.000 description 6
- 239000000777 urocortin Substances 0.000 description 6
- JWICNZAGYSIBAR-LEEGLKINSA-N (4s)-4-[[2-[[(2s)-2-[[(2s)-2-[[(2s)-2-aminopropanoyl]amino]-3-carboxypropanoyl]amino]-3-hydroxypropanoyl]amino]acetyl]amino]-5-[[2-[[(2s)-3-carboxy-1-[[(2s)-1-[[1-[[(2s)-1-[[(2s)-4-carboxy-1-[[2-[[2-[[2-[[(2s)-1-[[(1s)-1-carboxy-4-(diaminomethylideneamino Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)CNC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)C(CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)N)CC1=CC=CC=C1 JWICNZAGYSIBAR-LEEGLKINSA-N 0.000 description 5
- ZKRFOXLVOKTUTA-KQYNXXCUSA-N 9-(5-phosphoribofuranosyl)-6-mercaptopurine Chemical compound O[C@@H]1[C@H](O)[C@@H](COP(O)(O)=O)O[C@H]1N1C(NC=NC2=S)=C2N=C1 ZKRFOXLVOKTUTA-KQYNXXCUSA-N 0.000 description 5
- 102100033312 Alpha-2-macroglobulin Human genes 0.000 description 5
- 108010082161 Chemokine CCL19 Proteins 0.000 description 5
- 102000003805 Chemokine CCL19 Human genes 0.000 description 5
- 102400001368 Epidermal growth factor Human genes 0.000 description 5
- 101800003838 Epidermal growth factor Proteins 0.000 description 5
- 101800000974 Fibrinopeptide A Proteins 0.000 description 5
- 102400000525 Fibrinopeptide A Human genes 0.000 description 5
- 102400000921 Gastrin Human genes 0.000 description 5
- 108010052343 Gastrins Proteins 0.000 description 5
- 102000004878 Gelsolin Human genes 0.000 description 5
- 108090001064 Gelsolin Proteins 0.000 description 5
- 108050005077 Haptoglobin Proteins 0.000 description 5
- 101001018097 Homo sapiens L-selectin Proteins 0.000 description 5
- 101000669513 Homo sapiens Metalloproteinase inhibitor 1 Proteins 0.000 description 5
- 101000582950 Homo sapiens Platelet factor 4 Proteins 0.000 description 5
- 108010092694 L-Selectin Proteins 0.000 description 5
- 102100039364 Metalloproteinase inhibitor 1 Human genes 0.000 description 5
- 108010061952 Orosomucoid Proteins 0.000 description 5
- 102000012404 Orosomucoid Human genes 0.000 description 5
- 102100035846 Pigment epithelium-derived factor Human genes 0.000 description 5
- 108090000778 Platelet factor 4 Proteins 0.000 description 5
- 108010015078 Pregnancy-Associated alpha 2-Macroglobulins Proteins 0.000 description 5
- 102000054727 Serum Amyloid A Human genes 0.000 description 5
- 108700028909 Serum Amyloid A Proteins 0.000 description 5
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 5
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 5
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 5
- 230000003321 amplification Effects 0.000 description 5
- 150000001793 charged compounds Chemical class 0.000 description 5
- AOXOCDRNSPFDPE-UKEONUMOSA-N chembl413654 Chemical compound C([C@H](C(=O)NCC(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@H](CCSC)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC=1C=CC=CC=1)C(N)=O)NC(=O)[C@@H](C)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H]1N(CCC1)C(=O)CNC(=O)[C@@H](N)CCC(O)=O)C1=CC=C(O)C=C1 AOXOCDRNSPFDPE-UKEONUMOSA-N 0.000 description 5
- 230000013872 defecation Effects 0.000 description 5
- 229960002986 dinoprostone Drugs 0.000 description 5
- 231100000673 dose–response relationship Toxicity 0.000 description 5
- 230000002255 enzymatic effect Effects 0.000 description 5
- 229940116977 epidermal growth factor Drugs 0.000 description 5
- 239000002350 fibrinopeptide Substances 0.000 description 5
- 238000010166 immunofluorescence Methods 0.000 description 5
- 238000003364 immunohistochemistry Methods 0.000 description 5
- 230000004048 modification Effects 0.000 description 5
- 238000012986 modification Methods 0.000 description 5
- 238000003199 nucleic acid amplification method Methods 0.000 description 5
- 108090000102 pigment epithelium-derived factor Proteins 0.000 description 5
- 238000003752 polymerase chain reaction Methods 0.000 description 5
- XEYBRNLFEZDVAW-UHFFFAOYSA-N prostaglandin E2 Natural products CCCCCC(O)C=CC1C(O)CC(=O)C1CC=CCCCC(O)=O XEYBRNLFEZDVAW-UHFFFAOYSA-N 0.000 description 5
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 5
- QXNVGIXVLWOKEQ-UHFFFAOYSA-N Disodium Chemical compound [Na][Na] QXNVGIXVLWOKEQ-UHFFFAOYSA-N 0.000 description 4
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 4
- 206010061218 Inflammation Diseases 0.000 description 4
- 206010028980 Neoplasm Diseases 0.000 description 4
- 229940024606 amino acid Drugs 0.000 description 4
- 235000001014 amino acid Nutrition 0.000 description 4
- 230000000844 anti-bacterial effect Effects 0.000 description 4
- 230000000845 anti-microbial effect Effects 0.000 description 4
- 201000011510 cancer Diseases 0.000 description 4
- 210000004027 cell Anatomy 0.000 description 4
- 239000000812 cholinergic antagonist Substances 0.000 description 4
- 238000012790 confirmation Methods 0.000 description 4
- 230000002757 inflammatory effect Effects 0.000 description 4
- 230000004054 inflammatory process Effects 0.000 description 4
- 238000002493 microarray Methods 0.000 description 4
- 230000007310 pathophysiology Effects 0.000 description 4
- CMPQUABWPXYYSH-UHFFFAOYSA-N phenyl phosphate Chemical compound OP(O)(=O)OC1=CC=CC=C1 CMPQUABWPXYYSH-UHFFFAOYSA-N 0.000 description 4
- 239000000049 pigment Substances 0.000 description 4
- 210000002381 plasma Anatomy 0.000 description 4
- 239000006041 probiotic Substances 0.000 description 4
- 235000018291 probiotics Nutrition 0.000 description 4
- 238000004393 prognosis Methods 0.000 description 4
- 150000003180 prostaglandins Chemical class 0.000 description 4
- 238000012207 quantitative assay Methods 0.000 description 4
- 210000000813 small intestine Anatomy 0.000 description 4
- 238000012109 statistical procedure Methods 0.000 description 4
- 210000002700 urine Anatomy 0.000 description 4
- 230000002227 vasoactive effect Effects 0.000 description 4
- 241000283707 Capra Species 0.000 description 3
- 108010062745 Chloride Channels Proteins 0.000 description 3
- 102000011045 Chloride Channels Human genes 0.000 description 3
- 206010009900 Colitis ulcerative Diseases 0.000 description 3
- 208000018522 Gastrointestinal disease Diseases 0.000 description 3
- 108010078321 Guanylate Cyclase Proteins 0.000 description 3
- 102000014469 Guanylate cyclase Human genes 0.000 description 3
- 101000973997 Homo sapiens Nucleosome assembly protein 1-like 4 Proteins 0.000 description 3
- 108060003951 Immunoglobulin Proteins 0.000 description 3
- 238000010240 RT-PCR analysis Methods 0.000 description 3
- 102000012479 Serine Proteases Human genes 0.000 description 3
- 108010022999 Serine Proteases Proteins 0.000 description 3
- 201000006704 Ulcerative Colitis Diseases 0.000 description 3
- 230000004913 activation Effects 0.000 description 3
- 239000000556 agonist Substances 0.000 description 3
- 239000000935 antidepressant agent Substances 0.000 description 3
- 229940005513 antidepressants Drugs 0.000 description 3
- 230000001580 bacterial effect Effects 0.000 description 3
- 210000000601 blood cell Anatomy 0.000 description 3
- 229910052799 carbon Inorganic materials 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 238000010790 dilution Methods 0.000 description 3
- 239000012895 dilution Substances 0.000 description 3
- 208000037765 diseases and disorders Diseases 0.000 description 3
- 230000001747 exhibiting effect Effects 0.000 description 3
- 235000013305 food Nutrition 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 230000014509 gene expression Effects 0.000 description 3
- 230000002068 genetic effect Effects 0.000 description 3
- 230000036541 health Effects 0.000 description 3
- 102000018358 immunoglobulin Human genes 0.000 description 3
- 230000002055 immunohistochemical effect Effects 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 230000033001 locomotion Effects 0.000 description 3
- 108020004999 messenger RNA Proteins 0.000 description 3
- 210000004877 mucosa Anatomy 0.000 description 3
- 210000003097 mucus Anatomy 0.000 description 3
- 150000007523 nucleic acids Chemical class 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 230000005855 radiation Effects 0.000 description 3
- 238000011084 recovery Methods 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 210000003296 saliva Anatomy 0.000 description 3
- 230000001568 sexual effect Effects 0.000 description 3
- 238000012706 support-vector machine Methods 0.000 description 3
- 229960002876 tegaserod Drugs 0.000 description 3
- 229940124597 therapeutic agent Drugs 0.000 description 3
- 238000012549 training Methods 0.000 description 3
- 230000009466 transformation Effects 0.000 description 3
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 2
- BIAVGWDGIJKWRM-FQEVSTJZSA-N 3-hydroxy-2-phenyl-n-[(1s)-1-phenylpropyl]quinoline-4-carboxamide Chemical compound N([C@@H](CC)C=1C=CC=CC=1)C(=O)C(C1=CC=CC=C1N=1)=C(O)C=1C1=CC=CC=C1 BIAVGWDGIJKWRM-FQEVSTJZSA-N 0.000 description 2
- 108700028369 Alleles Proteins 0.000 description 2
- 102100026189 Beta-galactosidase Human genes 0.000 description 2
- 108091005471 CRHR1 Proteins 0.000 description 2
- 108091005470 CRHR2 Proteins 0.000 description 2
- 102400000739 Corticotropin Human genes 0.000 description 2
- 101800000414 Corticotropin Proteins 0.000 description 2
- 102100038018 Corticotropin-releasing factor receptor 1 Human genes 0.000 description 2
- 102100038019 Corticotropin-releasing factor receptor 2 Human genes 0.000 description 2
- 208000011231 Crohn disease Diseases 0.000 description 2
- 206010012742 Diarrhoea infectious Diseases 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N Formic acid Chemical compound OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- 206010022678 Intestinal infections Diseases 0.000 description 2
- 102000005741 Metalloproteases Human genes 0.000 description 2
- 108010006035 Metalloproteases Proteins 0.000 description 2
- 102000008109 Mixed Function Oxygenases Human genes 0.000 description 2
- 108010074633 Mixed Function Oxygenases Proteins 0.000 description 2
- 229940121948 Muscarinic receptor antagonist Drugs 0.000 description 2
- SNIXRMIHFOIVBB-UHFFFAOYSA-N N-Hydroxyl-tryptamine Chemical compound C1=CC=C2C(CCNO)=CNC2=C1 SNIXRMIHFOIVBB-UHFFFAOYSA-N 0.000 description 2
- 206010070834 Sensitisation Diseases 0.000 description 2
- 108060008539 Transglutaminase Proteins 0.000 description 2
- 108010046334 Urease Proteins 0.000 description 2
- 210000001015 abdomen Anatomy 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 208000038016 acute inflammation Diseases 0.000 description 2
- 230000006022 acute inflammation Effects 0.000 description 2
- 239000003470 adrenal cortex hormone Substances 0.000 description 2
- 230000004931 aggregating effect Effects 0.000 description 2
- 230000003872 anastomosis Effects 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 230000002391 anti-complement effect Effects 0.000 description 2
- 230000002921 anti-spasmodic effect Effects 0.000 description 2
- 229940088710 antibiotic agent Drugs 0.000 description 2
- 108010008730 anticomplement Proteins 0.000 description 2
- 229940124575 antispasmodic agent Drugs 0.000 description 2
- 239000012131 assay buffer Substances 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- 229940092732 belladonna alkaloid Drugs 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 108010005774 beta-Galactosidase Proteins 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- GERIGMSHTUAXSI-UHFFFAOYSA-N bis(8-methyl-8-azabicyclo[3.2.1]octan-3-yl) 4-phenyl-2,3-dihydro-1h-naphthalene-1,4-dicarboxylate Chemical compound CN1C(C2)CCC1CC2OC(=O)C(C1=CC=CC=C11)CCC1(C(=O)OC1CC2CCC(N2C)C1)C1=CC=CC=C1 GERIGMSHTUAXSI-UHFFFAOYSA-N 0.000 description 2
- 210000001124 body fluid Anatomy 0.000 description 2
- 239000010839 body fluid Substances 0.000 description 2
- 239000003467 chloride channel stimulating agent Substances 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 239000002299 complementary DNA Substances 0.000 description 2
- 230000001276 controlling effect Effects 0.000 description 2
- IDLFZVILOHSSID-OVLDLUHVSA-N corticotropin Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)NC(=O)[C@@H](N)CO)C1=CC=C(O)C=C1 IDLFZVILOHSSID-OVLDLUHVSA-N 0.000 description 2
- 229960000258 corticotropin Drugs 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- 208000010643 digestive system disease Diseases 0.000 description 2
- 208000007784 diverticulitis Diseases 0.000 description 2
- 208000001848 dysentery Diseases 0.000 description 2
- 230000008482 dysregulation Effects 0.000 description 2
- 238000001962 electrophoresis Methods 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 239000000284 extract Substances 0.000 description 2
- 238000000605 extraction Methods 0.000 description 2
- 238000000684 flow cytometry Methods 0.000 description 2
- 235000019253 formic acid Nutrition 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 208000021302 gastroesophageal reflux disease Diseases 0.000 description 2
- 208000018685 gastrointestinal system disease Diseases 0.000 description 2
- 238000001502 gel electrophoresis Methods 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 description 2
- 230000004179 hypothalamic–pituitary–adrenal axis Effects 0.000 description 2
- 230000001900 immune effect Effects 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 230000001976 improved effect Effects 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 208000028774 intestinal disease Diseases 0.000 description 2
- XMBWDFGMSWQBCA-YPZZEJLDSA-N iodane Chemical compound [125IH] XMBWDFGMSWQBCA-YPZZEJLDSA-N 0.000 description 2
- 229940044173 iodine-125 Drugs 0.000 description 2
- 108010024409 linaclotide Proteins 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 208000030159 metabolic disease Diseases 0.000 description 2
- 208000008275 microscopic colitis Diseases 0.000 description 2
- 230000003990 molecular pathway Effects 0.000 description 2
- 210000003205 muscle Anatomy 0.000 description 2
- 210000002569 neuron Anatomy 0.000 description 2
- 238000007899 nucleic acid hybridization Methods 0.000 description 2
- 238000005457 optimization Methods 0.000 description 2
- 229940094443 oxytocics prostaglandins Drugs 0.000 description 2
- 230000008506 pathogenesis Effects 0.000 description 2
- 238000002823 phage display Methods 0.000 description 2
- 239000013641 positive control Substances 0.000 description 2
- 238000012205 qualitative assay Methods 0.000 description 2
- 230000002285 radioactive effect Effects 0.000 description 2
- 238000007894 restriction fragment length polymorphism technique Methods 0.000 description 2
- NZCRJKRKKOLAOJ-XRCRFVBUSA-N rifaximin Chemical compound OC1=C(C(O)=C2C)C3=C4N=C5C=C(C)C=CN5C4=C1NC(=O)\C(C)=C/C=C/[C@H](C)[C@H](O)[C@@H](C)[C@@H](O)[C@@H](C)[C@H](OC(C)=O)[C@H](C)[C@@H](OC)\C=C\O[C@@]1(C)OC2=C3C1=O NZCRJKRKKOLAOJ-XRCRFVBUSA-N 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 230000008313 sensitization Effects 0.000 description 2
- 230000000405 serological effect Effects 0.000 description 2
- 239000003762 serotonin receptor affecting agent Substances 0.000 description 2
- 238000012421 spiking Methods 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 229950011332 talnetant Drugs 0.000 description 2
- IKBKZGMPCYNSLU-RGVLZGJSSA-N tegaserod Chemical compound C1=C(OC)C=C2C(/C=N/NC(=N)NCCCCC)=CNC2=C1 IKBKZGMPCYNSLU-RGVLZGJSSA-N 0.000 description 2
- 102000003601 transglutaminase Human genes 0.000 description 2
- 230000000007 visual effect Effects 0.000 description 2
- 230000001755 vocal effect Effects 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- 238000001262 western blot Methods 0.000 description 2
- DDYAPMZTJAYBOF-ZMYDTDHYSA-N (3S)-4-[[(2S)-1-[[(2S)-1-[[(2S)-5-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-4-amino-1-[[(2S,3R)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-4-amino-1-[[(2S)-1-[[(2S)-4-amino-1-[[(2S)-4-amino-1-[[(2S,3S)-1-[[(1S)-1-carboxyethyl]amino]-3-methyl-1-oxopentan-2-yl]amino]-1,4-dioxobutan-2-yl]amino]-1,4-dioxobutan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1,4-dioxobutan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-1,4-dioxobutan-2-yl]amino]-4-methylsulfanyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3R)-2-[[(2S)-2-[[(2S,3R)-2-[[2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-amino-3-(1H-imidazol-4-yl)propanoyl]amino]-3-hydroxypropanoyl]amino]-5-oxopentanoyl]amino]acetyl]amino]-3-hydroxybutanoyl]amino]-3-phenylpropanoyl]amino]-3-hydroxybutanoyl]amino]-3-hydroxypropanoyl]amino]-3-carboxypropanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-hydroxypropanoyl]amino]hexanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-methylpentanoyl]amino]-3-carboxypropanoyl]amino]-3-hydroxypropanoyl]amino]-5-carbamimidamidopentanoyl]amino]-5-carbamimidamidopentanoyl]amino]propanoyl]amino]-5-oxopentanoyl]amino]-4-oxobutanoic acid Chemical class [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(O)=O DDYAPMZTJAYBOF-ZMYDTDHYSA-N 0.000 description 1
- RUJBDQSFYCKFAA-HNNXBMFYSA-N (5r)-1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-5h-2,3-benzodiazepine Chemical compound C1([C@H](C(=NN=2)C)CC)=CC(OC)=C(OC)C=C1C=2C1=CC=C(OC)C(OC)=C1 RUJBDQSFYCKFAA-HNNXBMFYSA-N 0.000 description 1
- TWBNMYSKRDRHAT-RCWTXCDDSA-N (S)-timolol hemihydrate Chemical compound O.CC(C)(C)NC[C@H](O)COC1=NSN=C1N1CCOCC1.CC(C)(C)NC[C@H](O)COC1=NSN=C1N1CCOCC1 TWBNMYSKRDRHAT-RCWTXCDDSA-N 0.000 description 1
- IHWDSEPNZDYMNF-UHFFFAOYSA-N 1H-indol-2-amine Chemical class C1=CC=C2NC(N)=CC2=C1 IHWDSEPNZDYMNF-UHFFFAOYSA-N 0.000 description 1
- AJBZENLMTKDAEK-UHFFFAOYSA-N 3a,5a,5b,8,8,11a-hexamethyl-1-prop-1-en-2-yl-1,2,3,4,5,6,7,7a,9,10,11,11b,12,13,13a,13b-hexadecahydrocyclopenta[a]chrysene-4,9-diol Chemical compound CC12CCC(O)C(C)(C)C1CCC(C1(C)CC3O)(C)C2CCC1C1C3(C)CCC1C(=C)C AJBZENLMTKDAEK-UHFFFAOYSA-N 0.000 description 1
- FWBHETKCLVMNFS-UHFFFAOYSA-N 4',6-Diamino-2-phenylindol Chemical compound C1=CC(C(=N)N)=CC=C1C1=CC2=CC=C(C(N)=N)C=C2N1 FWBHETKCLVMNFS-UHFFFAOYSA-N 0.000 description 1
- XZKIHKMTEMTJQX-UHFFFAOYSA-N 4-Nitrophenyl Phosphate Chemical compound OP(O)(=O)OC1=CC=C([N+]([O-])=O)C=C1 XZKIHKMTEMTJQX-UHFFFAOYSA-N 0.000 description 1
- YRNWIFYIFSBPAU-UHFFFAOYSA-N 4-[4-(dimethylamino)phenyl]-n,n-dimethylaniline Chemical compound C1=CC(N(C)C)=CC=C1C1=CC=C(N(C)C)C=C1 YRNWIFYIFSBPAU-UHFFFAOYSA-N 0.000 description 1
- YPELFRMCRYSPKZ-UHFFFAOYSA-N 4-amino-5-chloro-2-ethoxy-N-({4-[(4-fluorophenyl)methyl]morpholin-2-yl}methyl)benzamide Chemical compound CCOC1=CC(N)=C(Cl)C=C1C(=O)NCC1OCCN(CC=2C=CC(F)=CC=2)C1 YPELFRMCRYSPKZ-UHFFFAOYSA-N 0.000 description 1
- 229920000936 Agarose Polymers 0.000 description 1
- 208000019901 Anxiety disease Diseases 0.000 description 1
- 108091023037 Aptamer Proteins 0.000 description 1
- 208000002109 Argyria Diseases 0.000 description 1
- 241000186000 Bifidobacterium Species 0.000 description 1
- 241000212384 Bifora Species 0.000 description 1
- 108010018054 CBir1 flagellin Proteins 0.000 description 1
- 102000055006 Calcitonin Human genes 0.000 description 1
- 108060001064 Calcitonin Proteins 0.000 description 1
- 235000003880 Calendula Nutrition 0.000 description 1
- 240000001432 Calendula officinalis Species 0.000 description 1
- 241000222122 Candida albicans Species 0.000 description 1
- 241000282465 Canis Species 0.000 description 1
- 102000014914 Carrier Proteins Human genes 0.000 description 1
- 108091006146 Channels Proteins 0.000 description 1
- 108091026890 Coding region Proteins 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 238000011537 Coomassie blue staining Methods 0.000 description 1
- 229920001393 Crofelemer Polymers 0.000 description 1
- 150000008574 D-amino acids Chemical class 0.000 description 1
- OUYCCCASQSFEME-MRVPVSSYSA-N D-tyrosine Chemical compound OC(=O)[C@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-MRVPVSSYSA-N 0.000 description 1
- 229930195709 D-tyrosine Natural products 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 238000000018 DNA microarray Methods 0.000 description 1
- 238000001712 DNA sequencing Methods 0.000 description 1
- 235000011511 Diospyros Nutrition 0.000 description 1
- 244000236655 Diospyros kaki Species 0.000 description 1
- 201000009273 Endometriosis Diseases 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 241000282324 Felis Species 0.000 description 1
- 108010040721 Flagellin Proteins 0.000 description 1
- 206010017367 Frequent bowel movements Diseases 0.000 description 1
- 208000005577 Gastroenteritis Diseases 0.000 description 1
- 206010017964 Gastrointestinal infection Diseases 0.000 description 1
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 102000001554 Hemoglobins Human genes 0.000 description 1
- 108010054147 Hemoglobins Proteins 0.000 description 1
- 108091027305 Heteroduplex Proteins 0.000 description 1
- 101000921095 Homo sapiens Corticotropin-releasing factor-binding protein Proteins 0.000 description 1
- 101001125026 Homo sapiens Nucleotide-binding oligomerization domain-containing protein 2 Proteins 0.000 description 1
- 108010048209 Human Immunodeficiency Virus Proteins Proteins 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 102100034349 Integrase Human genes 0.000 description 1
- 102000005712 Keratin-8 Human genes 0.000 description 1
- 108010070511 Keratin-8 Proteins 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- 206010023648 Lactase deficiency Diseases 0.000 description 1
- 108010092277 Leptin Proteins 0.000 description 1
- 102000016267 Leptin Human genes 0.000 description 1
- 238000012897 Levenberg–Marquardt algorithm Methods 0.000 description 1
- 206010025476 Malabsorption Diseases 0.000 description 1
- 208000004155 Malabsorption Syndromes Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 206010061296 Motor dysfunction Diseases 0.000 description 1
- 206010028116 Mucosal inflammation Diseases 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- HRNLUBSXIHFDHP-UHFFFAOYSA-N N-(2-aminophenyl)-4-[[[4-(3-pyridinyl)-2-pyrimidinyl]amino]methyl]benzamide Chemical compound NC1=CC=CC=C1NC(=O)C(C=C1)=CC=C1CNC1=NC=CC(C=2C=NC=CC=2)=N1 HRNLUBSXIHFDHP-UHFFFAOYSA-N 0.000 description 1
- 102000007072 Nerve Growth Factors Human genes 0.000 description 1
- 238000000636 Northern blotting Methods 0.000 description 1
- 102100029441 Nucleotide-binding oligomerization domain-containing protein 2 Human genes 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 108700028353 OmpC Proteins 0.000 description 1
- 229940127450 Opioid Agonists Drugs 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 208000012868 Overgrowth Diseases 0.000 description 1
- 102000004316 Oxidoreductases Human genes 0.000 description 1
- 108090000854 Oxidoreductases Proteins 0.000 description 1
- 238000012408 PCR amplification Methods 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- CXOFVDLJLONNDW-UHFFFAOYSA-N Phenytoin Chemical compound N1C(=O)NC(=O)C1(C=1C=CC=CC=1)C1=CC=CC=C1 CXOFVDLJLONNDW-UHFFFAOYSA-N 0.000 description 1
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 1
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 108010092799 RNA-directed DNA polymerase Proteins 0.000 description 1
- 108010083644 Ribonucleases Proteins 0.000 description 1
- 102000006382 Ribonucleases Human genes 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 238000012300 Sequence Analysis Methods 0.000 description 1
- 208000005718 Stomach Neoplasms Diseases 0.000 description 1
- 241000282898 Sus scrofa Species 0.000 description 1
- 229940123445 Tricyclic antidepressant Drugs 0.000 description 1
- 108010031944 Tryptophan Hydroxylase Proteins 0.000 description 1
- 102000005506 Tryptophan Hydroxylase Human genes 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- QWXOJIDBSHLIFI-UHFFFAOYSA-N [3-(1-chloro-3'-methoxyspiro[adamantane-4,4'-dioxetane]-3'-yl)phenyl] dihydrogen phosphate Chemical compound O1OC2(C3CC4CC2CC(Cl)(C4)C3)C1(OC)C1=CC=CC(OP(O)(O)=O)=C1 QWXOJIDBSHLIFI-UHFFFAOYSA-N 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 238000000246 agarose gel electrophoresis Methods 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 230000007815 allergy Effects 0.000 description 1
- JSWZEAMFRNKZNL-UHFFFAOYSA-N alosetron Chemical compound N1C=NC(CN2C(C3=C(N(C4=CC=CC=C43)C)CC2)=O)=C1C JSWZEAMFRNKZNL-UHFFFAOYSA-N 0.000 description 1
- 230000006229 amino acid addition Effects 0.000 description 1
- 229940040386 amitiza Drugs 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 230000001188 anti-phage Effects 0.000 description 1
- 239000003420 antiserotonin agent Substances 0.000 description 1
- 230000036506 anxiety Effects 0.000 description 1
- 238000000149 argon plasma sintering Methods 0.000 description 1
- JHLHNYVMZCADTC-LOSJGSFVSA-N asimadoline Chemical compound C([C@@H](N(C)C(=O)C(C=1C=CC=CC=1)C=1C=CC=CC=1)C=1C=CC=CC=1)N1CC[C@H](O)C1 JHLHNYVMZCADTC-LOSJGSFVSA-N 0.000 description 1
- 229950002202 asimadoline Drugs 0.000 description 1
- 229940125717 barbiturate Drugs 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 239000003150 biochemical marker Substances 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 210000001772 blood platelet Anatomy 0.000 description 1
- 239000004067 bulking agent Substances 0.000 description 1
- 229960004015 calcitonin Drugs 0.000 description 1
- BBBFJLBPOGFECG-VJVYQDLKSA-N calcitonin Chemical compound N([C@H](C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(N)=O)C(C)C)C(=O)[C@@H]1CSSC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1 BBBFJLBPOGFECG-VJVYQDLKSA-N 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 229940095731 candida albicans Drugs 0.000 description 1
- 230000002026 carminative effect Effects 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 150000003943 catecholamines Chemical class 0.000 description 1
- 230000020411 cell activation Effects 0.000 description 1
- 239000002771 cell marker Substances 0.000 description 1
- 230000005754 cellular signaling Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- SMNPLAKEGAEPJD-UHFFFAOYSA-N chembl34922 Chemical compound Cl.Cl.Cl.C1CN(C)CCN1C1=CC=C(NC(=N2)C=3C=C4N=C(NC4=CC=3)C=3C=CC(O)=CC=3)C2=C1 SMNPLAKEGAEPJD-UHFFFAOYSA-N 0.000 description 1
- 238000007385 chemical modification Methods 0.000 description 1
- 238000009388 chemical precipitation Methods 0.000 description 1
- 150000001805 chlorine compounds Chemical class 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 239000012504 chromatography matrix Substances 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 230000001149 cognitive effect Effects 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 238000002967 competitive immunoassay Methods 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 238000010668 complexation reaction Methods 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 230000008602 contraction Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000010485 coping Effects 0.000 description 1
- 229940047615 crofelemer Drugs 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 238000000326 densiometry Methods 0.000 description 1
- 229950009781 dextofisopam Drugs 0.000 description 1
- 230000001079 digestive effect Effects 0.000 description 1
- HSUGRBWQSSZJOP-RTWAWAEBSA-N diltiazem Chemical compound C1=CC(OC)=CC=C1[C@H]1[C@@H](OC(C)=O)C(=O)N(CCN(C)C)C2=CC=CC=C2S1 HSUGRBWQSSZJOP-RTWAWAEBSA-N 0.000 description 1
- 229960004166 diltiazem Drugs 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 239000003210 dopamine receptor blocking agent Substances 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 238000007823 electrophoretic assay Methods 0.000 description 1
- 230000002124 endocrine Effects 0.000 description 1
- 238000001839 endoscopy Methods 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 230000002550 fecal effect Effects 0.000 description 1
- 238000004401 flow injection analysis Methods 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- 238000000799 fluorescence microscopy Methods 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 206010017758 gastric cancer Diseases 0.000 description 1
- 210000005095 gastrointestinal system Anatomy 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 238000003205 genotyping method Methods 0.000 description 1
- 150000008134 glucuronides Chemical class 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 239000011544 gradient gel Substances 0.000 description 1
- 230000007149 gut brain axis pathway Effects 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 230000005934 immune activation Effects 0.000 description 1
- 230000005965 immune activity Effects 0.000 description 1
- 230000000984 immunochemical effect Effects 0.000 description 1
- 229940124541 immunological agent Drugs 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 102000010681 interleukin-8 receptors Human genes 0.000 description 1
- 108010038415 interleukin-8 receptors Proteins 0.000 description 1
- 230000008991 intestinal motility Effects 0.000 description 1
- 210000004347 intestinal mucosa Anatomy 0.000 description 1
- 230000003870 intestinal permeability Effects 0.000 description 1
- 208000028867 ischemia Diseases 0.000 description 1
- 229960000511 lactulose Drugs 0.000 description 1
- JCQLYHFGKNRPGE-FCVZTGTOSA-N lactulose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 JCQLYHFGKNRPGE-FCVZTGTOSA-N 0.000 description 1
- PFCRQPBOOFTZGQ-UHFFFAOYSA-N lactulose keto form Natural products OCC(=O)C(O)C(C(O)CO)OC1OC(CO)C(O)C(O)C1O PFCRQPBOOFTZGQ-UHFFFAOYSA-N 0.000 description 1
- 210000002429 large intestine Anatomy 0.000 description 1
- 238000001499 laser induced fluorescence spectroscopy Methods 0.000 description 1
- 239000004816 latex Substances 0.000 description 1
- 229920000126 latex Polymers 0.000 description 1
- 239000010410 layer Substances 0.000 description 1
- NRYBAZVQPHGZNS-ZSOCWYAHSA-N leptin Chemical compound O=C([C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CC(C)C)CCSC)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CS)C(O)=O NRYBAZVQPHGZNS-ZSOCWYAHSA-N 0.000 description 1
- 229940039781 leptin Drugs 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 229940060963 lotronex Drugs 0.000 description 1
- WGFOBBZOWHGYQH-MXHNKVEKSA-N lubiprostone Chemical compound O1[C@](C(F)(F)CCCC)(O)CC[C@@H]2[C@@H](CCCCCCC(O)=O)C(=O)C[C@H]21 WGFOBBZOWHGYQH-MXHNKVEKSA-N 0.000 description 1
- 238000004020 luminiscence type Methods 0.000 description 1
- 125000003588 lysine group Chemical class [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 238000010801 machine learning Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 235000013372 meat Nutrition 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 238000000329 molecular dynamics simulation Methods 0.000 description 1
- 229960004085 mosapride Drugs 0.000 description 1
- 230000004899 motility Effects 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 230000001423 neocortical effect Effects 0.000 description 1
- 238000004848 nephelometry Methods 0.000 description 1
- 210000005036 nerve Anatomy 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 210000004498 neuroglial cell Anatomy 0.000 description 1
- 230000036963 noncompetitive effect Effects 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 235000014571 nuts Nutrition 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 229940127240 opiate Drugs 0.000 description 1
- 229940005483 opioid analgesics Drugs 0.000 description 1
- 238000000399 optical microscopy Methods 0.000 description 1
- 230000037324 pain perception Effects 0.000 description 1
- 239000000123 paper Substances 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 230000008447 perception Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 229960002036 phenytoin Drugs 0.000 description 1
- 108010075437 phosphopeptidomannan Proteins 0.000 description 1
- 238000003322 phosphorimaging Methods 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 238000002264 polyacrylamide gel electrophoresis Methods 0.000 description 1
- 230000000529 probiotic effect Effects 0.000 description 1
- 229940127293 prostanoid Drugs 0.000 description 1
- 150000003814 prostanoids Chemical class 0.000 description 1
- 238000004445 quantitative analysis Methods 0.000 description 1
- NTHPAPBPFQJABD-LLVKDONJSA-N ramosetron Chemical compound C12=CC=CC=C2N(C)C=C1C(=O)[C@H]1CC(NC=N2)=C2CC1 NTHPAPBPFQJABD-LLVKDONJSA-N 0.000 description 1
- 229950001588 ramosetron Drugs 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 210000000664 rectum Anatomy 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- GZSKEXSLDPEFPT-IINYFYTJSA-N renzapride Chemical compound COC1=CC(N)=C(Cl)C=C1C(=O)N[C@H]1[C@H](C2)CCC[N@]2CC1 GZSKEXSLDPEFPT-IINYFYTJSA-N 0.000 description 1
- 229950003039 renzapride Drugs 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical compound [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 1
- 230000033764 rhythmic process Effects 0.000 description 1
- 238000002702 ribosome display Methods 0.000 description 1
- 238000007480 sanger sequencing Methods 0.000 description 1
- PGKXDIMONUAMFR-AREMUKBSSA-N saredutant Chemical compound C([C@H](CN(C)C(=O)C=1C=CC=CC=1)C=1C=C(Cl)C(Cl)=CC=1)CN(CC1)CCC1(NC(C)=O)C1=CC=CC=C1 PGKXDIMONUAMFR-AREMUKBSSA-N 0.000 description 1
- 229950004387 saredutant Drugs 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 239000000387 serotonin 5-HT4 receptor agonist Substances 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 230000011664 signaling Effects 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 201000011549 stomach cancer Diseases 0.000 description 1
- ADNPLDHMAVUMIW-CUZNLEPHSA-N substance P Chemical compound C([C@@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(N)=O)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCCN)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](N)CCCN=C(N)N)C1=CC=CC=C1 ADNPLDHMAVUMIW-CUZNLEPHSA-N 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 230000008093 supporting effect Effects 0.000 description 1
- CPDDZSSEAVLMRY-FEQFWAPWSA-N tegaserod maleate Chemical compound [H+].[H+].[O-]C(=O)\C=C/C([O-])=O.C1=C(OC)C=C2C(/C=N/NC(=N)NCCCCC)=CNC2=C1 CPDDZSSEAVLMRY-FEQFWAPWSA-N 0.000 description 1
- MPLHNVLQVRSVEE-UHFFFAOYSA-N texas red Chemical compound [O-]S(=O)(=O)C1=CC(S(Cl)(=O)=O)=CC=C1C(C1=CC=2CCCN3CCCC(C=23)=C1O1)=C2C1=C(CCC1)C3=[N+]1CCCC3=C2 MPLHNVLQVRSVEE-UHFFFAOYSA-N 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 229960004605 timolol Drugs 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 238000000844 transformation Methods 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 239000003029 tricyclic antidepressant agent Substances 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 230000009278 visceral effect Effects 0.000 description 1
- 208000009935 visceral pain Diseases 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 208000016261 weight loss Diseases 0.000 description 1
- 230000004580 weight loss Effects 0.000 description 1
- 229940064406 xifaxan Drugs 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
- 108010027843 zonulin Proteins 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6813—Hybridisation assays
- C12Q1/6827—Hybridisation assays for detection of mutation or polymorphism
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6893—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids related to diseases not provided for elsewhere
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/156—Polymorphic or mutational markers
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/06—Gastro-intestinal diseases
- G01N2800/065—Bowel diseases, e.g. Crohn, ulcerative colitis, IBS
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Molecular Biology (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Biomedical Technology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biotechnology (AREA)
- Organic Chemistry (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- General Health & Medical Sciences (AREA)
- Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Zoology (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Food Science & Technology (AREA)
- Cell Biology (AREA)
- Wood Science & Technology (AREA)
- Biophysics (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US25671709P | 2009-10-30 | 2009-10-30 | |
| US61/256,717 | 2009-10-30 | ||
| US26458809P | 2009-11-25 | 2009-11-25 | |
| US61/264,588 | 2009-11-25 | ||
| US32617610P | 2010-04-20 | 2010-04-20 | |
| US61/326,176 | 2010-04-20 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20120101038A true KR20120101038A (ko) | 2012-09-12 |
Family
ID=43922588
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020127013948A Withdrawn KR20120101038A (ko) | 2009-10-30 | 2010-10-29 | 과민성대장증후군을 진단하는 방법 |
Country Status (14)
| Country | Link |
|---|---|
| US (1) | US20120315630A1 (enExample) |
| EP (1) | EP2494074A4 (enExample) |
| JP (1) | JP2013509593A (enExample) |
| KR (1) | KR20120101038A (enExample) |
| CN (1) | CN102712956A (enExample) |
| AU (1) | AU2010313292A1 (enExample) |
| BR (1) | BR112012010108A2 (enExample) |
| CA (1) | CA2778539A1 (enExample) |
| IL (1) | IL219317A0 (enExample) |
| MX (1) | MX2012005015A (enExample) |
| PH (1) | PH12012500850A1 (enExample) |
| RU (1) | RU2012122180A (enExample) |
| WO (1) | WO2011053831A1 (enExample) |
| ZA (1) | ZA201203718B (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20190053914A (ko) * | 2016-09-16 | 2019-05-20 | 다이액스 코포레이션 | 접촉 활성화 시스템과 연관된 질환을 위한 대사물질 바이오마커 |
| KR20200090135A (ko) * | 2019-01-18 | 2020-07-28 | 주식회사 천랩 | 과민성대장증후군 특이적 미생물 바이오마커와 이를 이용하여 과민성대장증후군의 위험도를 예측하는 방법 |
Families Citing this family (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5857503B2 (ja) * | 2010-07-29 | 2016-02-10 | 小野薬品工業株式会社 | ストレス性疾患のバイオマーカー |
| WO2012158831A1 (en) | 2011-05-16 | 2012-11-22 | Prometheus Laboratories Inc. | Performance of a biomarker panel for irritable bowel syndrome |
| NZ702643A (en) * | 2012-05-19 | 2016-06-24 | Falk Pharma Gmbh | Compositions and methods for treatment of irritable bowel syndrome with 5-aminosalicylate |
| AU2013326133B2 (en) * | 2012-10-05 | 2019-07-04 | Société des Produits Nestlé S.A. | Antibodies to microbiome, stress factors and mast cell markers as diagnostic markers for IBS |
| WO2014082034A1 (en) * | 2012-11-26 | 2014-05-30 | Lexicon Pharmaceuticals, Inc. | Methods for treating irritable bowel syndrome |
| CN103175971B (zh) * | 2013-02-28 | 2016-10-05 | 苏州和锐医药科技有限公司 | 一种IgA抗体检测试剂盒 |
| EP3800470A1 (en) * | 2013-03-15 | 2021-04-07 | Sera Prognostics, Inc. | Biomarkers and methods for predicting preterm birth |
| GB201305708D0 (en) * | 2013-03-28 | 2013-05-15 | Clasado Inc | Novel use |
| RU2015155552A (ru) * | 2013-05-24 | 2017-06-27 | Нестек С.А. | Путеспецифические способы прогнозирования диагноза синдрома раздраженного кишечника |
| CA2930330A1 (en) * | 2013-12-27 | 2015-07-02 | Dignity Health | Diagnosing and treating alzheimer's disease |
| JP6713723B2 (ja) * | 2014-02-18 | 2020-06-24 | 小野薬品工業株式会社 | 過敏性腸症候群の診断用バイオマーカー |
| US10392665B2 (en) | 2015-06-19 | 2019-08-27 | Sera Prognostics, Inc. | Biomarker pairs for predicting preterm birth |
| CN106446599A (zh) * | 2015-08-11 | 2017-02-22 | 中国科学院青岛生物能源与过程研究所 | 一种筛选婴幼儿龋病的口腔致病性生物标记物的方法 |
| CN105687225B (zh) * | 2016-02-26 | 2018-07-06 | 四川好医生攀西药业有限责任公司 | 一种治疗肠易激综合征的药物组合物及其制备方法和应用 |
| GB201605110D0 (en) * | 2016-03-24 | 2016-05-11 | Mologic Ltd | Detecting sepsis |
| US10817778B2 (en) | 2016-06-27 | 2020-10-27 | International Business Machines Corporation | Customized cooking utilizing deep learning neuromorphic computing of hyperspectral input |
| RU2668496C2 (ru) * | 2017-03-16 | 2018-10-01 | Государственное бюджетное учреждение здравоохранения города Москвы Московский клинический научно-практический центр Департамента здравоохранения города Москвы | Способ диагностики тяжести течения синдрома раздраженного кишечника |
| CN106990057A (zh) * | 2017-05-05 | 2017-07-28 | 贵州普洛迈德生物工程有限公司 | 一种检测5‑羟吲哚乙酸试剂及制备方法 |
| US11662351B2 (en) | 2017-08-18 | 2023-05-30 | Sera Prognostics, Inc. | Pregnancy clock proteins for predicting due date and time to birth |
| US11488699B1 (en) | 2018-12-09 | 2022-11-01 | Cerner Innovation, Inc. | Microbiota activity sensor and decision support tool |
| US11842795B1 (en) * | 2018-12-17 | 2023-12-12 | Cerner Innovation, Inc. | Irritable bowel syndrome diagnostic sensor and decision support tool |
| US20220146527A1 (en) * | 2019-09-17 | 2022-05-12 | Chang Gung University | Method of creating characteristic profiles of mass spectra and identification model for analyzing and identifying features of microorganisms |
| US20240124524A1 (en) * | 2019-10-17 | 2024-04-18 | University Of Copenhagen | Agonist of Tacr2 |
| CN112921100A (zh) * | 2019-12-06 | 2021-06-08 | 宁波海尔施基因科技有限公司 | 一种检测人类压力敏感度基因型的试剂盒和方法 |
| EP3913371A1 (en) * | 2020-05-18 | 2021-11-24 | Neuroimmun GmbH | Method and kit for diagnosting irritable bowel syndrome and for its relief through dietary interventions |
| CN111784683B (zh) * | 2020-07-10 | 2022-05-17 | 天津大学 | 病理切片检测方法及装置、计算机设备及存储介质 |
| CN111905111B (zh) * | 2020-09-16 | 2023-03-31 | 地奥集团成都药业股份有限公司 | 一种评价复方谷氨酰胺组方对腹泻型肠易激综合征疗效的方法 |
| US20220208375A1 (en) * | 2020-12-29 | 2022-06-30 | Kpn Innovations, Llc. | System and method for generating a digestive disease functional program |
| CN115328117B (zh) * | 2022-07-15 | 2023-07-14 | 大理大学 | 基于强化学习的蛋白质动态配体通道最优路径分析方法 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1999057120A1 (en) * | 1998-05-04 | 1999-11-11 | Neotherapeutics, Inc. | Novel serotonin-like 9-substituted hypoxanthine and methods of use |
| EP1315837A2 (en) * | 2000-09-06 | 2003-06-04 | Glaxo Group Limited | 5-hydroxytryptamine receptor gene polymorphisms and response to treatment |
| US20080085524A1 (en) * | 2006-08-15 | 2008-04-10 | Prometheus Laboratories Inc. | Methods for diagnosing irritable bowel syndrome |
| EP2778685B1 (en) * | 2009-06-25 | 2017-04-26 | Nestec S.A. | Methods for diagnosing irritable bowel syndrome |
-
2010
- 2010-10-29 EP EP10827556.1A patent/EP2494074A4/en not_active Withdrawn
- 2010-10-29 CA CA2778539A patent/CA2778539A1/en not_active Abandoned
- 2010-10-29 JP JP2012537132A patent/JP2013509593A/ja not_active Withdrawn
- 2010-10-29 MX MX2012005015A patent/MX2012005015A/es unknown
- 2010-10-29 CN CN2010800605536A patent/CN102712956A/zh active Pending
- 2010-10-29 WO PCT/US2010/054810 patent/WO2011053831A1/en not_active Ceased
- 2010-10-29 RU RU2012122180/15A patent/RU2012122180A/ru unknown
- 2010-10-29 PH PH1/2012/500850A patent/PH12012500850A1/en unknown
- 2010-10-29 KR KR1020127013948A patent/KR20120101038A/ko not_active Withdrawn
- 2010-10-29 BR BR112012010108A patent/BR112012010108A2/pt not_active IP Right Cessation
- 2010-10-29 AU AU2010313292A patent/AU2010313292A1/en not_active Abandoned
-
2012
- 2012-04-06 US US13/441,658 patent/US20120315630A1/en not_active Abandoned
- 2012-04-19 IL IL219317A patent/IL219317A0/en unknown
- 2012-05-22 ZA ZA2012/03718A patent/ZA201203718B/en unknown
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20190053914A (ko) * | 2016-09-16 | 2019-05-20 | 다이액스 코포레이션 | 접촉 활성화 시스템과 연관된 질환을 위한 대사물질 바이오마커 |
| KR20220154245A (ko) * | 2016-09-16 | 2022-11-21 | 다케다 파머수티컬 컴패니 리미티드 | 접촉 활성화 시스템과 연관된 질환을 위한 대사물질 바이오마커 |
| US12188948B2 (en) | 2016-09-16 | 2025-01-07 | Takeda Pharmaceutical Company Limited | Metabolite biomarkers for diseases associated with the contact activation system |
| KR20200090135A (ko) * | 2019-01-18 | 2020-07-28 | 주식회사 천랩 | 과민성대장증후군 특이적 미생물 바이오마커와 이를 이용하여 과민성대장증후군의 위험도를 예측하는 방법 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20120315630A1 (en) | 2012-12-13 |
| ZA201203718B (en) | 2014-11-26 |
| RU2012122180A (ru) | 2013-12-10 |
| MX2012005015A (es) | 2012-06-12 |
| IL219317A0 (en) | 2012-06-28 |
| PH12012500850A1 (en) | 2013-01-07 |
| CN102712956A (zh) | 2012-10-03 |
| JP2013509593A (ja) | 2013-03-14 |
| CA2778539A1 (en) | 2011-05-05 |
| AU2010313292A1 (en) | 2012-06-07 |
| EP2494074A4 (en) | 2013-06-12 |
| BR112012010108A2 (pt) | 2016-05-31 |
| WO2011053831A1 (en) | 2011-05-05 |
| EP2494074A1 (en) | 2012-09-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20120101038A (ko) | 과민성대장증후군을 진단하는 방법 | |
| JP5571951B2 (ja) | 試料が過敏性腸症候群と関連するか否かを決定する分類方法 | |
| AU2017213566B2 (en) | Methods for diagnosing irritable bowel syndrome | |
| EP2710383B1 (en) | Performance of a biomarker panel for irritable bowel syndrome |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20120529 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| N231 | Notification of change of applicant | ||
| PN2301 | Change of applicant |
Patent event date: 20121012 Comment text: Notification of Change of Applicant Patent event code: PN23011R01D |
|
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |