KR20110129374A - 이산화티탄-다중벽 탄소 나노 튜브를 이용한 효율 높은 염료 감응 태양전지 나노복합체 - Google Patents
이산화티탄-다중벽 탄소 나노 튜브를 이용한 효율 높은 염료 감응 태양전지 나노복합체 Download PDFInfo
- Publication number
- KR20110129374A KR20110129374A KR1020117016105A KR20117016105A KR20110129374A KR 20110129374 A KR20110129374 A KR 20110129374A KR 1020117016105 A KR1020117016105 A KR 1020117016105A KR 20117016105 A KR20117016105 A KR 20117016105A KR 20110129374 A KR20110129374 A KR 20110129374A
- Authority
- KR
- South Korea
- Prior art keywords
- tio
- nanocomposite
- solar cell
- cnt
- walled carbon
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 239000002114 nanocomposite Substances 0.000 title claims abstract description 63
- 239000002048 multi walled nanotube Substances 0.000 title claims abstract description 61
- 239000010936 titanium Substances 0.000 title claims description 18
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 title claims description 14
- 229910052719 titanium Inorganic materials 0.000 title claims description 14
- 229910010413 TiO 2 Inorganic materials 0.000 claims abstract description 70
- 238000000034 method Methods 0.000 claims description 30
- 238000001027 hydrothermal synthesis Methods 0.000 claims description 26
- 239000002243 precursor Substances 0.000 claims description 11
- 239000000758 substrate Substances 0.000 claims description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 9
- HSZCZNFXUDYRKD-UHFFFAOYSA-M lithium iodide Chemical compound [Li+].[I-] HSZCZNFXUDYRKD-UHFFFAOYSA-M 0.000 claims description 8
- 238000004519 manufacturing process Methods 0.000 claims description 8
- VXUYXOFXAQZZMF-UHFFFAOYSA-N titanium(IV) isopropoxide Chemical compound CC(C)O[Ti](OC(C)C)(OC(C)C)OC(C)C VXUYXOFXAQZZMF-UHFFFAOYSA-N 0.000 claims description 8
- 239000011244 liquid electrolyte Substances 0.000 claims description 7
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 claims description 7
- KJTLSVCANCCWHF-UHFFFAOYSA-N Ruthenium Chemical compound [Ru] KJTLSVCANCCWHF-UHFFFAOYSA-N 0.000 claims description 6
- 150000001875 compounds Chemical class 0.000 claims description 6
- 229910052707 ruthenium Inorganic materials 0.000 claims description 6
- LTNAYKNIZNSHQA-UHFFFAOYSA-L 2-(4-carboxypyridin-2-yl)pyridine-4-carboxylic acid;ruthenium(2+);dithiocyanate Chemical compound N#CS[Ru]SC#N.OC(=O)C1=CC=NC(C=2N=CC=C(C=2)C(O)=O)=C1.OC(=O)C1=CC=NC(C=2N=CC=C(C=2)C(O)=O)=C1 LTNAYKNIZNSHQA-UHFFFAOYSA-L 0.000 claims description 4
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 claims description 4
- 239000000428 dust Substances 0.000 claims description 4
- 229910052740 iodine Inorganic materials 0.000 claims description 4
- 239000011630 iodine Substances 0.000 claims description 4
- 238000012546 transfer Methods 0.000 claims description 4
- 239000011521 glass Substances 0.000 claims description 3
- 230000003301 hydrolyzing effect Effects 0.000 claims description 3
- 229910052697 platinum Inorganic materials 0.000 claims description 3
- 238000010345 tape casting Methods 0.000 claims description 3
- XOLBLPGZBRYERU-UHFFFAOYSA-N tin dioxide Chemical compound O=[Sn]=O XOLBLPGZBRYERU-UHFFFAOYSA-N 0.000 claims description 3
- 229910001887 tin oxide Inorganic materials 0.000 claims description 3
- XJDNKRIXUMDJCW-UHFFFAOYSA-J titanium tetrachloride Chemical compound Cl[Ti](Cl)(Cl)Cl XJDNKRIXUMDJCW-UHFFFAOYSA-J 0.000 claims description 3
- 238000005406 washing Methods 0.000 claims description 3
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 claims description 2
- 229910052731 fluorine Inorganic materials 0.000 claims description 2
- 239000011737 fluorine Substances 0.000 claims description 2
- 150000003609 titanium compounds Chemical class 0.000 claims description 2
- BCVXHSPFUWZLGQ-UHFFFAOYSA-N mecn acetonitrile Chemical group CC#N.CC#N BCVXHSPFUWZLGQ-UHFFFAOYSA-N 0.000 claims 1
- 239000002041 carbon nanotube Substances 0.000 abstract description 17
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 abstract description 9
- 229910021393 carbon nanotube Inorganic materials 0.000 abstract description 8
- 239000002105 nanoparticle Substances 0.000 description 8
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 6
- 239000002073 nanorod Substances 0.000 description 5
- 238000011282 treatment Methods 0.000 description 5
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- 239000002131 composite material Substances 0.000 description 4
- 239000011941 photocatalyst Substances 0.000 description 4
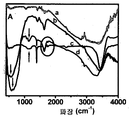
- 238000005033 Fourier transform infrared spectroscopy Methods 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- SOQBVABWOPYFQZ-UHFFFAOYSA-N oxygen(2-);titanium(4+) Chemical compound [O-2].[O-2].[Ti+4] SOQBVABWOPYFQZ-UHFFFAOYSA-N 0.000 description 3
- 230000001699 photocatalysis Effects 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 239000004408 titanium dioxide Substances 0.000 description 3
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
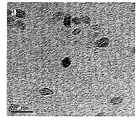
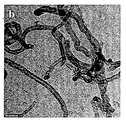
- 238000003917 TEM image Methods 0.000 description 2
- 239000004809 Teflon Substances 0.000 description 2
- 229920006362 Teflon® Polymers 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 230000021615 conjugation Effects 0.000 description 2
- 239000008367 deionised water Substances 0.000 description 2
- 229910021641 deionized water Inorganic materials 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 239000002071 nanotube Substances 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 238000003980 solgel method Methods 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 125000006850 spacer group Chemical group 0.000 description 2
- 238000004627 transmission electron microscopy Methods 0.000 description 2
- 238000001157 Fourier transform infrared spectrum Methods 0.000 description 1
- 235000000177 Indigofera tinctoria Nutrition 0.000 description 1
- 229910003077 Ti−O Inorganic materials 0.000 description 1
- 238000010306 acid treatment Methods 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 238000010351 charge transfer process Methods 0.000 description 1
- 230000000536 complexating effect Effects 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 229940097275 indigo Drugs 0.000 description 1
- COHYTHOBJLSHDF-UHFFFAOYSA-N indigo powder Natural products N1C2=CC=CC=C2C(=O)C1=C1C(=O)C2=CC=CC=C2N1 COHYTHOBJLSHDF-UHFFFAOYSA-N 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 229910044991 metal oxide Inorganic materials 0.000 description 1
- 150000004706 metal oxides Chemical class 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 239000011368 organic material Substances 0.000 description 1
- 125000002524 organometallic group Chemical group 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 238000009832 plasma treatment Methods 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000027756 respiratory electron transport chain Effects 0.000 description 1
- 238000000527 sonication Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01G—COMPOUNDS CONTAINING METALS NOT COVERED BY SUBCLASSES C01D OR C01F
- C01G23/00—Compounds of titanium
- C01G23/04—Oxides; Hydroxides
- C01G23/047—Titanium dioxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y10/00—Nanotechnology for information processing, storage or transmission, e.g. quantum computing or single electron logic
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y30/00—Nanotechnology for materials or surface science, e.g. nanocomposites
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y40/00—Manufacture or treatment of nanostructures
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B32/00—Carbon; Compounds thereof
- C01B32/15—Nano-sized carbon materials
- C01B32/158—Carbon nanotubes
- C01B32/168—After-treatment
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01G—CAPACITORS; CAPACITORS, RECTIFIERS, DETECTORS, SWITCHING DEVICES, LIGHT-SENSITIVE OR TEMPERATURE-SENSITIVE DEVICES OF THE ELECTROLYTIC TYPE
- H01G9/00—Electrolytic capacitors, rectifiers, detectors, switching devices, light-sensitive or temperature-sensitive devices; Processes of their manufacture
- H01G9/20—Light-sensitive devices
- H01G9/2027—Light-sensitive devices comprising an oxide semiconductor electrode
- H01G9/2031—Light-sensitive devices comprising an oxide semiconductor electrode comprising titanium oxide, e.g. TiO2
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/20—Carbon compounds, e.g. carbon nanotubes or fullerenes
- H10K85/221—Carbon nanotubes
- H10K85/225—Carbon nanotubes comprising substituents
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/80—Crystal-structural characteristics defined by measured data other than those specified in group C01P2002/70
- C01P2002/85—Crystal-structural characteristics defined by measured data other than those specified in group C01P2002/70 by XPS, EDX or EDAX data
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/01—Particle morphology depicted by an image
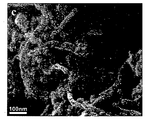
- C01P2004/03—Particle morphology depicted by an image obtained by SEM
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/01—Particle morphology depicted by an image
- C01P2004/04—Particle morphology depicted by an image obtained by TEM, STEM, STM or AFM
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/80—Particles consisting of a mixture of two or more inorganic phases
- C01P2004/82—Particles consisting of a mixture of two or more inorganic phases two phases having the same anion, e.g. both oxidic phases
- C01P2004/84—Particles consisting of a mixture of two or more inorganic phases two phases having the same anion, e.g. both oxidic phases one phase coated with the other
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01G—CAPACITORS; CAPACITORS, RECTIFIERS, DETECTORS, SWITCHING DEVICES, LIGHT-SENSITIVE OR TEMPERATURE-SENSITIVE DEVICES OF THE ELECTROLYTIC TYPE
- H01G9/00—Electrolytic capacitors, rectifiers, detectors, switching devices, light-sensitive or temperature-sensitive devices; Processes of their manufacture
- H01G9/20—Light-sensitive devices
- H01G9/2059—Light-sensitive devices comprising an organic dye as the active light absorbing material, e.g. adsorbed on an electrode or dissolved in solution
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E10/00—Energy generation through renewable energy sources
- Y02E10/50—Photovoltaic [PV] energy
- Y02E10/542—Dye sensitized solar cells
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E10/00—Energy generation through renewable energy sources
- Y02E10/50—Photovoltaic [PV] energy
- Y02E10/549—Organic PV cells
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Nanotechnology (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Power Engineering (AREA)
- Physics & Mathematics (AREA)
- Crystallography & Structural Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- General Physics & Mathematics (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Environmental & Geological Engineering (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Geology (AREA)
- Manufacturing & Machinery (AREA)
- Mathematical Physics (AREA)
- Theoretical Computer Science (AREA)
- Composite Materials (AREA)
- Inorganic Compounds Of Heavy Metals (AREA)
- Hybrid Cells (AREA)
- Photovoltaic Devices (AREA)
- Carbon And Carbon Compounds (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN48DE2009 | 2009-01-12 | ||
| IN48/DEL/2009 | 2009-01-12 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20110129374A true KR20110129374A (ko) | 2011-12-01 |
Family
ID=42108949
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020117016105A Ceased KR20110129374A (ko) | 2009-01-12 | 2010-01-12 | 이산화티탄-다중벽 탄소 나노 튜브를 이용한 효율 높은 염료 감응 태양전지 나노복합체 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20120012177A1 (enExample) |
| EP (1) | EP2376385A1 (enExample) |
| JP (1) | JP2012515132A (enExample) |
| KR (1) | KR20110129374A (enExample) |
| CN (1) | CN102292291A (enExample) |
| WO (1) | WO2010079516A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101305481B1 (ko) * | 2013-02-14 | 2013-09-06 | 광주대학교산학협력단 | 염료감응 태양전지용 고투명성 이산화티타늄 페이스트 제조방법 |
Families Citing this family (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080271739A1 (en) | 2007-05-03 | 2008-11-06 | 3M Innovative Properties Company | Maintenance-free respirator that has concave portions on opposing sides of mask top section |
| US9770611B2 (en) | 2007-05-03 | 2017-09-26 | 3M Innovative Properties Company | Maintenance-free anti-fog respirator |
| CN102151561A (zh) * | 2011-01-22 | 2011-08-17 | 浙江理工大学 | 一种纳米碳管负载二氧化钛的光催化剂及其制备方法 |
| JP5660952B2 (ja) * | 2011-03-30 | 2015-01-28 | 大阪瓦斯株式会社 | 酸化チタン−カーボン複合体の製造方法 |
| US8920767B2 (en) | 2011-08-19 | 2014-12-30 | Ut-Battelle, Llc | Array of titanium dioxide nanostructures for solar energy utilization |
| KR101328636B1 (ko) | 2011-09-26 | 2013-11-14 | 부산대학교 산학협력단 | 복합체 나노와이어 합성 방법 및 그를 이용한 염료감응형 태양전지의 제조 방법 |
| CN104350011B (zh) * | 2012-03-19 | 2016-09-28 | 香港科技大学 | 在纳米管的内表面和外表面及纳米管层间掺杂金属、金属氧化物和金属配合物及纳米管的制备方法 |
| CN102938327B (zh) * | 2012-12-04 | 2016-05-11 | 奇瑞汽车股份有限公司 | 掺杂的二氧化钛及其制备方法、该材料制备的染料敏化太阳能电池光阳极、电池 |
| JP6065600B2 (ja) * | 2013-01-18 | 2017-01-25 | 国立大学法人山口大学 | 光電極、光電変換素子及び光電極の製造方法 |
| JP2014177695A (ja) * | 2013-02-15 | 2014-09-25 | Sekisui Chem Co Ltd | 複合膜の製造方法、複合膜、光電極および色素増感太陽電池 |
| CA2917504C (en) | 2013-07-15 | 2022-06-21 | 3M Innovative Properties Company | Respirator having optically active exhalation valve |
| CO7090252A1 (es) * | 2014-10-10 | 2014-10-21 | Univ Del Valle | Sintesis de nanocompuestos que incorporan oxido de titanio fase anatasa y composición que los contienen para el tratamiento del cáncer |
| GB201508114D0 (en) | 2015-05-12 | 2015-06-24 | 3M Innovative Properties Co | Respirator tab |
| CN105527773A (zh) * | 2015-12-29 | 2016-04-27 | 江苏大学 | 二氧化钛功能化多壁碳纳米管纳米复合光限制材料及其制备方法 |
| CN108883395B (zh) * | 2016-01-11 | 2021-07-09 | 北京光合新能科技有限公司 | 用于产生长链烃分子的等离激元纳米颗粒催化剂和方法 |
| JP6757060B2 (ja) * | 2016-04-18 | 2020-09-16 | 国立研究開発法人産業技術総合研究所 | 可視光活性チタニア・炭素粒子複合体とその製造方法 |
| WO2018118851A1 (en) * | 2016-12-19 | 2018-06-28 | University Of Cincinnati | Photocatalytic carbon filter |
| CN110869110B (zh) | 2017-07-14 | 2022-11-18 | 3M创新有限公司 | 用于输送多个液体流的适配器 |
| JP7018643B2 (ja) * | 2017-10-06 | 2022-02-14 | 国立研究開発法人産業技術総合研究所 | 可視光活性修飾炭素粒子・チタニアコアシェル複合体、その製造方法 |
| JP7243999B2 (ja) * | 2017-10-20 | 2023-03-22 | 学校法人法政大学 | カーボン材料の電荷特性制御方法 |
| CN112305041B (zh) * | 2020-09-15 | 2022-05-27 | 东莞东阳光医疗智能器件研发有限公司 | 多重定量电化学免疫传感器及其构建方法 |
| CN112332025A (zh) * | 2020-11-10 | 2021-02-05 | 南京工业大学 | 一种锂硫电池用隔膜及其制备方法 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3907736B2 (ja) * | 1996-03-08 | 2007-04-18 | 独立行政法人理化学研究所 | 金属酸化物薄膜の製造方法 |
| JP3658486B2 (ja) * | 1997-03-11 | 2005-06-08 | 独立行政法人理化学研究所 | 有機/金属酸化物複合薄膜の製造方法 |
| JP4151884B2 (ja) * | 2001-08-08 | 2008-09-17 | 独立行政法人理化学研究所 | 固体表面に複合金属酸化物のナノ材料が形成された材料の製造方法 |
| CN100395896C (zh) * | 2003-12-05 | 2008-06-18 | 鸿富锦精密工业(深圳)有限公司 | 染料敏化太阳能电池及其电极 |
| KR100589323B1 (ko) * | 2004-02-03 | 2006-06-14 | 삼성에스디아이 주식회사 | 광 흡수파장대가 확장된 염료감응 태양전지 및 그 제조방법 |
| KR100554179B1 (ko) * | 2004-06-09 | 2006-02-22 | 한국전자통신연구원 | 전도성 금속 기판을 포함하는 구부림이 가능한 염료감응태양전지 |
| JP5350635B2 (ja) * | 2004-11-09 | 2013-11-27 | ボード・オブ・リージエンツ,ザ・ユニバーシテイ・オブ・テキサス・システム | ナノファイバーのリボンおよびシートならびにナノファイバーの撚り糸および無撚り糸の製造および適用 |
| JP5382756B2 (ja) * | 2005-03-09 | 2014-01-08 | 独立行政法人理化学研究所 | カーボンナノチューブ組成物およびこれを用いた製造方法 |
| JP2006130507A (ja) * | 2005-12-28 | 2006-05-25 | Yamaha Corp | 光酸化触媒 |
| KR101312269B1 (ko) * | 2007-01-05 | 2013-09-25 | 삼성전자주식회사 | 고분자 태양전지 및 그의 제조방법 |
-
2010
- 2010-01-12 KR KR1020117016105A patent/KR20110129374A/ko not_active Ceased
- 2010-01-12 US US13/143,964 patent/US20120012177A1/en not_active Abandoned
- 2010-01-12 EP EP10706760A patent/EP2376385A1/en not_active Withdrawn
- 2010-01-12 CN CN2010800044353A patent/CN102292291A/zh active Pending
- 2010-01-12 JP JP2011544971A patent/JP2012515132A/ja active Pending
- 2010-01-12 WO PCT/IN2010/000023 patent/WO2010079516A1/en not_active Ceased
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101305481B1 (ko) * | 2013-02-14 | 2013-09-06 | 광주대학교산학협력단 | 염료감응 태양전지용 고투명성 이산화티타늄 페이스트 제조방법 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20120012177A1 (en) | 2012-01-19 |
| CN102292291A (zh) | 2011-12-21 |
| EP2376385A1 (en) | 2011-10-19 |
| WO2010079516A1 (en) | 2010-07-15 |
| JP2012515132A (ja) | 2012-07-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20110129374A (ko) | 이산화티탄-다중벽 탄소 나노 튜브를 이용한 효율 높은 염료 감응 태양전지 나노복합체 | |
| Lee et al. | Fabrication of dye sensitized solar cell using TiO2 coated carbon nanotubes | |
| Chou et al. | Hierarchically Structured ZnO Film for Dye‐Sensitized Solar Cells with Enhanced Energy Conversion Efficiency | |
| Patil et al. | Single step hydrothermal synthesis of hierarchical TiO 2 microflowers with radially assembled nanorods for enhanced photovoltaic performance | |
| Habibi Jetani et al. | TiO2/GO nanocomposites: synthesis, characterization, and DSSC application | |
| Khannam et al. | A graphene oxide incorporated TiO 2 photoanode for high efficiency quasi solid state dye sensitized solar cells based on a poly-vinyl alcohol gel electrolyte | |
| Khannam et al. | An efficient quasi-solid state dye sensitized solar cells based on graphene oxide/gelatin gel electrolyte with NiO supported TiO2 photoanode | |
| Ahmad et al. | Chemical sintering of TiO2 based photoanode for efficient dye sensitized solar cells using Zn nanoparticles | |
| Shen et al. | Solar paint from TiO2 particles supported quantum dots for photoanodes in quantum dot–sensitized solar cells | |
| Mehmood et al. | Co-sensitization of graphene/TiO2 nanocomposite thin films with ruthenizer and metal free organic photosensitizers for improving the power conversion efficiency of dye-sensitized solar cells (DSSCs) | |
| KR101458759B1 (ko) | 산화티타늄/금속나노입자/탄소나노구조체를 포함하는 나노복합체, 그 제조 방법 및 이를 이용한 태양전지 전극 | |
| Amini et al. | Hybrid 1D/2D carbon nanostructure-incorporated titania photoanodes for perovskite solar cells | |
| Liu et al. | Titanium mesh supported TiO 2 nanowire arrays/Nb-doped TiO 2 nanoparticles for fully flexible dye-sensitized solar cells with improved photovoltaic properties | |
| Mehmood | Efficient and economical dye-sensitized solar cells based on graphene/TiO2 nanocomposite as a photoanode and graphene as a Pt-free catalyst for counter electrode | |
| Ahmad et al. | Effect of Nanodiamonds on the optoelectronic properties of TiO 2 Photoanode in dye-sensitized solar cell | |
| KR20170051575A (ko) | 금속 산화 수산화물의 나노입자 및 그래핀의 캡핑층을 포함하는 광전기화학전지용 광전극 및 이를 포함하는 하이브리드 유기 광전기화학전지 | |
| Jalali et al. | TiO2 surface nanostructuring for improved dye loading and light scattering in double-layered screen-printed dye-sensitized solar cells | |
| Shin et al. | Highly transparent dual-sensitized titanium dioxide nanotube arrays for spontaneous solar water splitting tandem configuration | |
| Cha et al. | Li+ doped anodic TiO2 nanotubes for enhanced efficiency of Dye-sensitized solar cells | |
| Pujiarti et al. | Enhanced efficiency in dye-sensitized solar cell by localized surface plasmon resonance effect of gold nanoparticles | |
| Drygała et al. | Carbon nanotubes counter electrode for dye-sensitized solar cells application | |
| Khorasani et al. | Electron transport engineering with different types of titanium dioxide nanostructures in perovskite solar cells | |
| Chen et al. | CdS sensitized TiO2 nanorod arrays based solar cells prepared with polymer-assisted layer-by-layer adsorption and reaction method | |
| Sani et al. | Modification of Dye-Sensitized Solar Cells by SWCNT Composition as the Active Layer and Introducing TiO 2@ SiO 2 Core–Shell Nanostructure for Light Scattering Layer: Toward Efficiency Enhancement | |
| Mahalingam et al. | Morphological and electron mobility studies in nanograss In2O3 DSSC incorporating multi-walled carbon nanotubes |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20110712 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20141216 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20160127 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20160330 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20160127 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |