KR20100121601A - 면역염증성 질환의 치료를 위한 치료요법 - Google Patents
면역염증성 질환의 치료를 위한 치료요법 Download PDFInfo
- Publication number
- KR20100121601A KR20100121601A KR1020107015745A KR20107015745A KR20100121601A KR 20100121601 A KR20100121601 A KR 20100121601A KR 1020107015745 A KR1020107015745 A KR 1020107015745A KR 20107015745 A KR20107015745 A KR 20107015745A KR 20100121601 A KR20100121601 A KR 20100121601A
- Authority
- KR
- South Korea
- Prior art keywords
- dipyridamole
- formulated
- prednisolone
- pharmaceutical composition
- dosage form
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000011282 treatment Methods 0.000 title claims abstract description 24
- 230000000495 immunoinflammatory effect Effects 0.000 title description 9
- 238000011285 therapeutic regimen Methods 0.000 title 1
- IZEKFCXSFNUWAM-UHFFFAOYSA-N dipyridamole Chemical compound C=12N=C(N(CCO)CCO)N=C(N3CCCCC3)C2=NC(N(CCO)CCO)=NC=1N1CCCCC1 IZEKFCXSFNUWAM-UHFFFAOYSA-N 0.000 claims abstract description 150
- 229960002768 dipyridamole Drugs 0.000 claims abstract description 150
- 239000011324 bead Substances 0.000 claims abstract description 100
- 238000000034 method Methods 0.000 claims abstract description 71
- 239000003246 corticosteroid Substances 0.000 claims abstract description 70
- 238000013270 controlled release Methods 0.000 claims abstract description 61
- 239000002552 dosage form Substances 0.000 claims abstract description 57
- 230000002378 acidificating effect Effects 0.000 claims abstract description 8
- 208000027866 inflammatory disease Diseases 0.000 claims abstract description 6
- 238000000576 coating method Methods 0.000 claims description 105
- 229960005205 prednisolone Drugs 0.000 claims description 103
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 claims description 103
- 239000011248 coating agent Substances 0.000 claims description 80
- 239000000203 mixture Substances 0.000 claims description 54
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 claims description 45
- 239000011975 tartaric acid Substances 0.000 claims description 45
- 235000002906 tartaric acid Nutrition 0.000 claims description 45
- 239000012729 immediate-release (IR) formulation Substances 0.000 claims description 42
- 239000008194 pharmaceutical composition Substances 0.000 claims description 42
- 238000009472 formulation Methods 0.000 claims description 31
- 238000004090 dissolution Methods 0.000 claims description 21
- 238000000338 in vitro Methods 0.000 claims description 16
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 claims description 13
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 claims description 13
- 239000002609 medium Substances 0.000 claims description 13
- 239000008363 phosphate buffer Substances 0.000 claims description 11
- 238000010521 absorption reaction Methods 0.000 claims description 10
- 229960002537 betamethasone Drugs 0.000 claims description 10
- UREBDLICKHMUKA-DVTGEIKXSA-N betamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-DVTGEIKXSA-N 0.000 claims description 10
- 229920003132 hydroxypropyl methylcellulose phthalate Polymers 0.000 claims description 8
- 229940031704 hydroxypropyl methylcellulose phthalate Drugs 0.000 claims description 8
- 229960004584 methylprednisolone Drugs 0.000 claims description 8
- VOVIALXJUBGFJZ-KWVAZRHASA-N Budesonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O VOVIALXJUBGFJZ-KWVAZRHASA-N 0.000 claims description 7
- 229960004436 budesonide Drugs 0.000 claims description 7
- 229960002714 fluticasone Drugs 0.000 claims description 7
- MGNNYOODZCAHBA-GQKYHHCASA-N fluticasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(O)[C@@]2(C)C[C@@H]1O MGNNYOODZCAHBA-GQKYHHCASA-N 0.000 claims description 7
- 235000019333 sodium laurylsulphate Nutrition 0.000 claims description 7
- 238000012360 testing method Methods 0.000 claims description 7
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 claims description 6
- 239000012738 dissolution medium Substances 0.000 claims description 6
- 229960004618 prednisone Drugs 0.000 claims description 6
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 claims description 6
- 230000000694 effects Effects 0.000 claims description 5
- 235000012054 meals Nutrition 0.000 claims description 5
- VHRSUDSXCMQTMA-PJHHCJLFSA-N 6alpha-methylprednisolone Chemical compound C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@](O)(C(=O)CO)CC[C@H]21 VHRSUDSXCMQTMA-PJHHCJLFSA-N 0.000 claims description 4
- 229920003139 Eudragit® L 100 Polymers 0.000 claims description 3
- 229960001334 corticosteroids Drugs 0.000 abstract description 21
- 239000000243 solution Substances 0.000 description 74
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 72
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 48
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 47
- 239000008188 pellet Substances 0.000 description 39
- 239000000725 suspension Substances 0.000 description 39
- 238000002360 preparation method Methods 0.000 description 37
- 239000002775 capsule Substances 0.000 description 34
- 229920003134 Eudragit® polymer Polymers 0.000 description 29
- 229940079593 drug Drugs 0.000 description 23
- 239000003814 drug Substances 0.000 description 23
- 238000004519 manufacturing process Methods 0.000 description 23
- 239000007921 spray Substances 0.000 description 23
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 22
- 230000008569 process Effects 0.000 description 19
- 238000003756 stirring Methods 0.000 description 19
- 239000000454 talc Substances 0.000 description 19
- 229910052623 talc Inorganic materials 0.000 description 19
- 230000003111 delayed effect Effects 0.000 description 15
- -1 digluconate Chemical compound 0.000 description 15
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 14
- DOOTYTYQINUNNV-UHFFFAOYSA-N Triethyl citrate Chemical compound CCOC(=O)CC(O)(C(=O)OCC)CC(=O)OCC DOOTYTYQINUNNV-UHFFFAOYSA-N 0.000 description 13
- 239000002270 dispersing agent Substances 0.000 description 13
- 239000001069 triethyl citrate Substances 0.000 description 13
- VMYFZRTXGLUXMZ-UHFFFAOYSA-N triethyl citrate Natural products CCOC(=O)C(O)(C(=O)OCC)C(=O)OCC VMYFZRTXGLUXMZ-UHFFFAOYSA-N 0.000 description 13
- 235000013769 triethyl citrate Nutrition 0.000 description 13
- 239000002253 acid Substances 0.000 description 12
- 201000010099 disease Diseases 0.000 description 12
- 239000012530 fluid Substances 0.000 description 12
- 230000004584 weight gain Effects 0.000 description 12
- 235000019786 weight gain Nutrition 0.000 description 12
- FQISKWAFAHGMGT-SGJOWKDISA-M Methylprednisolone sodium succinate Chemical compound [Na+].C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@](O)(C(=O)COC(=O)CCC([O-])=O)CC[C@H]21 FQISKWAFAHGMGT-SGJOWKDISA-M 0.000 description 10
- 229960000890 hydrocortisone Drugs 0.000 description 10
- 230000002572 peristaltic effect Effects 0.000 description 10
- 238000005507 spraying Methods 0.000 description 10
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 8
- 235000021152 breakfast Nutrition 0.000 description 8
- 150000001875 compounds Chemical class 0.000 description 8
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 8
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 8
- 239000008213 purified water Substances 0.000 description 8
- 150000003839 salts Chemical class 0.000 description 8
- 238000011068 loading method Methods 0.000 description 7
- 229960005294 triamcinolone Drugs 0.000 description 7
- GFNANZIMVAIWHM-OBYCQNJPSA-N triamcinolone Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@]([C@H](O)C4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 GFNANZIMVAIWHM-OBYCQNJPSA-N 0.000 description 7
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- DLVOSEUFIRPIRM-KAQKJVHQSA-N Hydrocortisone cypionate Chemical compound O=C([C@@]1(O)CC[C@H]2[C@H]3[C@@H]([C@]4(CCC(=O)C=C4CC3)C)[C@@H](O)C[C@@]21C)COC(=O)CCC1CCCC1 DLVOSEUFIRPIRM-KAQKJVHQSA-N 0.000 description 6
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 6
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 6
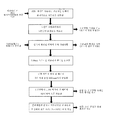
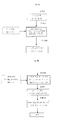
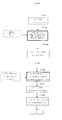
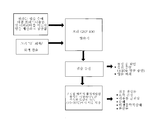
- 238000010586 diagram Methods 0.000 description 6
- 229960003331 hydrocortisone cypionate Drugs 0.000 description 6
- 230000004048 modification Effects 0.000 description 6
- 238000012986 modification Methods 0.000 description 6
- 230000001225 therapeutic effect Effects 0.000 description 6
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 5
- 201000004624 Dermatitis Diseases 0.000 description 5
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 5
- 239000004480 active ingredient Substances 0.000 description 5
- 238000001035 drying Methods 0.000 description 5
- 238000004806 packaging method and process Methods 0.000 description 5
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 5
- 230000001681 protective effect Effects 0.000 description 5
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 5
- BHDHELFREODRJK-XRYUJSLGSA-N (8s,9r,10s,13s,14s,17r)-9-fluoro-17-hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-2,6,7,8,12,14,15,16-octahydro-1h-cyclopenta[a]phenanthrene-3,11-dione Chemical compound O=C1CC[C@]2(C)[C@@]3(F)C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 BHDHELFREODRJK-XRYUJSLGSA-N 0.000 description 4
- FUFLCEKSBBHCMO-UHFFFAOYSA-N 11-dehydrocorticosterone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)C(=O)CO)C4C3CCC2=C1 FUFLCEKSBBHCMO-UHFFFAOYSA-N 0.000 description 4
- WHBHBVVOGNECLV-OBQKJFGGSA-N 11-deoxycortisol Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 WHBHBVVOGNECLV-OBQKJFGGSA-N 0.000 description 4
- MYYIMZRZXIQBGI-HVIRSNARSA-N 6alpha-Fluoroprednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3C[C@H](F)C2=C1 MYYIMZRZXIQBGI-HVIRSNARSA-N 0.000 description 4
- KUVIULQEHSCUHY-XYWKZLDCSA-N Beclometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)COC(=O)CC)(OC(=O)CC)[C@@]1(C)C[C@@H]2O KUVIULQEHSCUHY-XYWKZLDCSA-N 0.000 description 4
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 4
- HHZQLQREDATOBM-CODXZCKSSA-M Hydrocortisone Sodium Succinate Chemical compound [Na+].O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)COC(=O)CCC([O-])=O)[C@@H]4[C@@H]3CCC2=C1 HHZQLQREDATOBM-CODXZCKSSA-M 0.000 description 4
- HYRKAAMZBDSJFJ-LFDBJOOHSA-N Paramethasone acetate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)COC(C)=O)(O)[C@@]2(C)C[C@@H]1O HYRKAAMZBDSJFJ-LFDBJOOHSA-N 0.000 description 4
- RJKFOVLPORLFTN-LEKSSAKUSA-N Progesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C)CC2 RJKFOVLPORLFTN-LEKSSAKUSA-N 0.000 description 4
- RACDDTQBAFEERP-PLTZVPCUSA-N [2-[(6s,8s,9s,10r,13s,14s,17r)-6-chloro-17-hydroxy-10,13-dimethyl-3,11-dioxo-6,7,8,9,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] acetate Chemical compound C1([C@@H](Cl)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@@](C(=O)COC(=O)C)(O)[C@@]2(C)CC1=O RACDDTQBAFEERP-PLTZVPCUSA-N 0.000 description 4
- 239000000654 additive Substances 0.000 description 4
- 229920002678 cellulose Polymers 0.000 description 4
- 239000001913 cellulose Substances 0.000 description 4
- 235000010980 cellulose Nutrition 0.000 description 4
- CBGUOGMQLZIXBE-XGQKBEPLSA-N clobetasol propionate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CCl)(OC(=O)CC)[C@@]1(C)C[C@@H]2O CBGUOGMQLZIXBE-XGQKBEPLSA-N 0.000 description 4
- BMCQMVFGOVHVNG-TUFAYURCSA-N cortisol 17-butyrate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)CO)(OC(=O)CCC)[C@@]1(C)C[C@@H]2O BMCQMVFGOVHVNG-TUFAYURCSA-N 0.000 description 4
- ALEXXDVDDISNDU-JZYPGELDSA-N cortisol 21-acetate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)C)(O)[C@@]1(C)C[C@@H]2O ALEXXDVDDISNDU-JZYPGELDSA-N 0.000 description 4
- RYJIRNNXCHOUTQ-OJJGEMKLSA-L cortisol sodium phosphate Chemical compound [Na+].[Na+].O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)COP([O-])([O-])=O)[C@@H]4[C@@H]3CCC2=C1 RYJIRNNXCHOUTQ-OJJGEMKLSA-L 0.000 description 4
- 239000003085 diluting agent Substances 0.000 description 4
- 229960001067 hydrocortisone acetate Drugs 0.000 description 4
- 229960001524 hydrocortisone butyrate Drugs 0.000 description 4
- RQZAXGRLVPAYTJ-GQFGMJRRSA-N megestrol acetate Chemical compound C1=C(C)C2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)C)[C@@]1(C)CC2 RQZAXGRLVPAYTJ-GQFGMJRRSA-N 0.000 description 4
- PLBHSZGDDKCEHR-LFYFAGGJSA-N methylprednisolone acetate Chemical compound C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@](O)(C(=O)COC(C)=O)CC[C@H]21 PLBHSZGDDKCEHR-LFYFAGGJSA-N 0.000 description 4
- 239000006187 pill Substances 0.000 description 4
- ILZSJEITWDWIRX-FOMYWIRZSA-N prednisolamate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)CN(CC)CC)(O)[C@@]1(C)C[C@@H]2O ILZSJEITWDWIRX-FOMYWIRZSA-N 0.000 description 4
- 229950011122 prednisolamate Drugs 0.000 description 4
- 230000002829 reductive effect Effects 0.000 description 4
- 238000007789 sealing Methods 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 3
- 206010019233 Headaches Diseases 0.000 description 3
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 3
- 239000004698 Polyethylene Substances 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 235000010323 ascorbic acid Nutrition 0.000 description 3
- 239000011668 ascorbic acid Substances 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 3
- 239000011230 binding agent Substances 0.000 description 3
- 235000012000 cholesterol Nutrition 0.000 description 3
- 239000011094 fiberboard Substances 0.000 description 3
- 231100000869 headache Toxicity 0.000 description 3
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 3
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 239000007896 modified release capsule Substances 0.000 description 3
- 231100000252 nontoxic Toxicity 0.000 description 3
- 230000003000 nontoxic effect Effects 0.000 description 3
- 201000008482 osteoarthritis Diseases 0.000 description 3
- 229920000573 polyethylene Polymers 0.000 description 3
- 206010039073 rheumatoid arthritis Diseases 0.000 description 3
- 230000002195 synergetic effect Effects 0.000 description 3
- 239000003826 tablet Substances 0.000 description 3
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N valeric acid Chemical compound CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 3
- GNFTWPCIRXSCQF-UHFFFAOYSA-N (6alpha,11beta,17alphaOH)-6,11,17,21-Tetrahydroxypregn-4-ene-3,20-dione Natural products O=C1CCC2(C)C3C(O)CC(C)(C(CC4)(O)C(=O)CO)C4C3CC(O)C2=C1 GNFTWPCIRXSCQF-UHFFFAOYSA-N 0.000 description 2
- BSVNAPJPBOKGSU-JJKSKHOQSA-N (6r,8s,9s,10r,11s,13s,14s,17s)-6,11-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@H](CC4)C(=O)CO)[C@@H]4[C@@H]3C[C@@H](O)C2=C1 BSVNAPJPBOKGSU-JJKSKHOQSA-N 0.000 description 2
- UKVVNEHFNYKGMX-WHMNXGKUSA-N (8r,9s,10r,13s)-14-hydroxy-10,13-dimethyl-2,7,8,9,11,12,15,16-octahydro-1h-cyclopenta[a]phenanthrene-3,6,17-trione Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)C4(O)[C@@H]3CC(=O)C2=C1 UKVVNEHFNYKGMX-WHMNXGKUSA-N 0.000 description 2
- FWKZKCGCFQKDQY-KCTPXNJMSA-N (8r,9s,10r,13s,14s,15r,17r)-17-acetyl-15,17-dihydroxy-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1h-cyclopenta[a]phenanthren-3-one Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1[C@H](O)C[C@@](C(=O)C)(O)[C@@]1(C)CC2 FWKZKCGCFQKDQY-KCTPXNJMSA-N 0.000 description 2
- SHJZUHWENQCCJH-YQAXKJAASA-N (8s,9r,10s,11s,13s,14s)-9-fluoro-11-hydroxy-10,13-dimethyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthrene-3,17-dione Chemical compound O=C1CC[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CCC2=C1 SHJZUHWENQCCJH-YQAXKJAASA-N 0.000 description 2
- IKGBPSZWCRRUQS-DTAAKRQUSA-N (8s,9r,10s,11s,13s,14s,16s,17r)-17-acetyl-9-fluoro-11,17-dihydroxy-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(C)=O)(O)[C@@]1(C)C[C@@H]2O IKGBPSZWCRRUQS-DTAAKRQUSA-N 0.000 description 2
- OFSXGKOMEGSTSE-BPSSIEEOSA-N (8s,9r,10s,11s,13s,14s,17r)-17-acetyl-9-fluoro-11,17-dihydroxy-10,13-dimethyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-one Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1CC[C@@](C(=O)C)(O)[C@@]1(C)C[C@@H]2O OFSXGKOMEGSTSE-BPSSIEEOSA-N 0.000 description 2
- ZCAYUOKEIPMTMF-JPDWDDBRSA-N (8s,9s,10r,11r,13s,14s,16r,17r)-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1h-cyclopenta[a]phenanthren-3-one Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@H]2O ZCAYUOKEIPMTMF-JPDWDDBRSA-N 0.000 description 2
- IOKVWBWSLVVBRO-HXOHHMPKSA-N (8s,9s,10r,11s,13r,14s,17s)-17-acetyl-11-hydroxy-10-methyl-3-oxo-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthrene-13-carbaldehyde Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C=O)C[C@@H]2O IOKVWBWSLVVBRO-HXOHHMPKSA-N 0.000 description 2
- FPYGQPQXGSIDSF-RBWSHPMZSA-N (8s,9s,10r,13s,14s,17r)-17-hydroxy-17-(2-hydroxyacetyl)-13-(hydroxymethyl)-10-methyl-1,2,6,7,8,9,12,14,15,16-decahydrocyclopenta[a]phenanthrene-3,11-dione Chemical compound C([C@]1(CO)[C@@](O)(C(=O)CO)CC[C@H]1[C@@H]1CC2)C(=O)[C@@H]1[C@]1(C)C2=CC(=O)CC1 FPYGQPQXGSIDSF-RBWSHPMZSA-N 0.000 description 2
- OIGPMJCLTDSPPN-KAHGZSNJSA-N (8s,9s,10r,13s,14s,17r)-17-hydroxy-17-(2-hydroxyacetyl)-2,10,13-trimethyl-1,2,6,7,8,9,12,14,15,16-decahydrocyclopenta[a]phenanthrene-3,11-dione Chemical compound C([C@]1(C)[C@@](O)(C(=O)CO)CC[C@H]11)C(=O)[C@H]2[C@H]1CCC1=CC(=O)C(C)C[C@@]12C OIGPMJCLTDSPPN-KAHGZSNJSA-N 0.000 description 2
- HAKRSIFCTAKBRD-TYFJVFSVSA-N (8s,9s,10r,13s,14s,17s)-17-acetyl-1-methoxy-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one Chemical compound C1C[C@@H]2[C@@]3(C)C(OC)CC(=O)C=C3CC[C@H]2[C@@H]2CC[C@H](C(C)=O)[C@]21C HAKRSIFCTAKBRD-TYFJVFSVSA-N 0.000 description 2
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 2
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- FUFLCEKSBBHCMO-KJQYFISQSA-N 11-dehydrocorticosterone Chemical compound O=C1CC[C@]2(C)[C@H]3C(=O)C[C@](C)([C@H](CC4)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 FUFLCEKSBBHCMO-KJQYFISQSA-N 0.000 description 2
- WHBHBVVOGNECLV-UHFFFAOYSA-N 11-deoxy-17-hydroxy-corticosterone Natural products O=C1CCC2(C)C3CCC(C)(C(CC4)(O)C(=O)CO)C4C3CCC2=C1 WHBHBVVOGNECLV-UHFFFAOYSA-N 0.000 description 2
- ZESRJSPZRDMNHY-YFWFAHHUSA-N 11-deoxycorticosterone Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 ZESRJSPZRDMNHY-YFWFAHHUSA-N 0.000 description 2
- WTPMRQZHJLJSBO-XQALERBDSA-N 11-oxotestosterone Chemical compound O=C1CC[C@]2(C)[C@H]3C(=O)C[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 WTPMRQZHJLJSBO-XQALERBDSA-N 0.000 description 2
- ZHOLUHXKCIXGSR-GBHAUCNQSA-N 11alpha-Hydroxyandrosta-1,4-diene-3,17-dione Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@H](O)C[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CCC2=C1 ZHOLUHXKCIXGSR-GBHAUCNQSA-N 0.000 description 2
- JERGUCIJOXJXHF-UHFFFAOYSA-N 17alpha-Hydroxypregnenolone Natural products C1C=C2CC(O)CCC2(C)C2C1C1CCC(C(=O)C)(O)C1(C)CC2 JERGUCIJOXJXHF-UHFFFAOYSA-N 0.000 description 2
- JERGUCIJOXJXHF-TVWVXWENSA-N 17alpha-hydroxypregnenolone Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)C)(O)[C@@]1(C)CC2 JERGUCIJOXJXHF-TVWVXWENSA-N 0.000 description 2
- GCKMFJBGXUYNAG-UHFFFAOYSA-N 17alpha-methyltestosterone Natural products C1CC2=CC(=O)CCC2(C)C2C1C1CCC(C)(O)C1(C)CC2 GCKMFJBGXUYNAG-UHFFFAOYSA-N 0.000 description 2
- CCCIJQPRIXGQOE-XWSJACJDSA-N 17beta-hydroxy-17-methylestra-4,9,11-trien-3-one Chemical compound C1CC2=CC(=O)CCC2=C2[C@@H]1[C@@H]1CC[C@](C)(O)[C@@]1(C)C=C2 CCCIJQPRIXGQOE-XWSJACJDSA-N 0.000 description 2
- DBPWSSGDRRHUNT-CEGNMAFCSA-N 17α-hydroxyprogesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)C)(O)[C@@]1(C)CC2 DBPWSSGDRRHUNT-CEGNMAFCSA-N 0.000 description 2
- XUQWWIFROYJHCU-FJNAKSJRSA-N 18-Oxocortisol Chemical compound C([C@@]1([C@](O)(C(=O)CO)CC[C@H]1[C@@H]1CC2)C=O)[C@H](O)[C@@H]1[C@]1(C)C2=CC(=O)CC1 XUQWWIFROYJHCU-FJNAKSJRSA-N 0.000 description 2
- HFSXHZZDNDGLQN-ZVIOFETBSA-N 18-hydroxycorticosterone Chemical compound C([C@]1(CO)[C@@H](C(=O)CO)CC[C@H]1[C@@H]1CC2)[C@H](O)[C@@H]1[C@]1(C)C2=CC(=O)CC1 HFSXHZZDNDGLQN-ZVIOFETBSA-N 0.000 description 2
- MPDGHEJMBKOTSU-YKLVYJNSSA-N 18beta-glycyrrhetic acid Chemical compound C([C@H]1C2=CC(=O)[C@H]34)[C@@](C)(C(O)=O)CC[C@]1(C)CC[C@@]2(C)[C@]4(C)CC[C@@H]1[C@]3(C)CC[C@H](O)C1(C)C MPDGHEJMBKOTSU-YKLVYJNSSA-N 0.000 description 2
- FTMJFHVKAXPFIY-UHFFFAOYSA-N 2,2-dichloro-N-[1,3-dihydroxy-1-(3-nitrophenyl)propan-2-yl]acetamide Chemical compound OCC(NC(=O)C(Cl)Cl)C(O)c1cccc(c1)[N+]([O-])=O FTMJFHVKAXPFIY-UHFFFAOYSA-N 0.000 description 2
- PUKLDDOGISCFCP-JSQCKWNTSA-N 21-Deoxycortisone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)C)(O)[C@@]1(C)CC2=O PUKLDDOGISCFCP-JSQCKWNTSA-N 0.000 description 2
- LCZBQMKVFQNSJR-UJPCIWJBSA-N 21-deoxycortisol Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)C)(O)[C@@]1(C)C[C@@H]2O LCZBQMKVFQNSJR-UJPCIWJBSA-N 0.000 description 2
- QTQGHKVYLQBJLO-UHFFFAOYSA-N 4-methylbenzenesulfonate;(4-methyl-1-oxo-1-phenylmethoxypentan-2-yl)azanium Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1.CC(C)CC(N)C(=O)OCC1=CC=CC=C1 QTQGHKVYLQBJLO-UHFFFAOYSA-N 0.000 description 2
- VNGWBSXDGQZSFL-JMNKBGBLSA-N 6-[2-[(8s,9s,10r,11s,13s,14s,17r)-11,17-dihydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6h-cyclopenta[a]phenanthren-17-yl]-2-oxoethoxy]carbonylcyclohex-3-ene-1-carboxylic acid Chemical compound O=C([C@@]1(O)CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)[C@@H](O)C[C@@]21C)COC(=O)C1CC=CCC1C(O)=O VNGWBSXDGQZSFL-JMNKBGBLSA-N 0.000 description 2
- GNFTWPCIRXSCQF-HVIRSNARSA-N 6alpha-Hydroxycortisol Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3C[C@H](O)C2=C1 GNFTWPCIRXSCQF-HVIRSNARSA-N 0.000 description 2
- GNFTWPCIRXSCQF-UJXAPRPESA-N 6beta-hydroxycortisol Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3C[C@@H](O)C2=C1 GNFTWPCIRXSCQF-UJXAPRPESA-N 0.000 description 2
- LJGWPGVRXUUNAG-UJXAPRPESA-N 6beta-hydroxyprednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3C[C@@H](O)C2=C1 LJGWPGVRXUUNAG-UJXAPRPESA-N 0.000 description 2
- WAIJIHDWAKJCBX-BULBTXNYSA-N 9-Fluoroprednisolone Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 WAIJIHDWAKJCBX-BULBTXNYSA-N 0.000 description 2
- PQSUYGKTWSAVDQ-ZVIOFETBSA-N Aldosterone Chemical compound C([C@@]1([C@@H](C(=O)CO)CC[C@H]1[C@@H]1CC2)C=O)[C@H](O)[C@@H]1[C@]1(C)C2=CC(=O)CC1 PQSUYGKTWSAVDQ-ZVIOFETBSA-N 0.000 description 2
- PQSUYGKTWSAVDQ-UHFFFAOYSA-N Aldosterone Natural products C1CC2C3CCC(C(=O)CO)C3(C=O)CC(O)C2C2(C)C1=CC(=O)CC2 PQSUYGKTWSAVDQ-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 2
- FERIUCNNQQJTOY-UHFFFAOYSA-M Butyrate Chemical compound CCCC([O-])=O FERIUCNNQQJTOY-UHFFFAOYSA-M 0.000 description 2
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Natural products CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 2
- QWOJMRHUQHTCJG-UHFFFAOYSA-N CC([CH2-])=O Chemical compound CC([CH2-])=O QWOJMRHUQHTCJG-UHFFFAOYSA-N 0.000 description 2
- LUKZNWIVRBCLON-GXOBDPJESA-N Ciclesonide Chemical compound C1([C@H]2O[C@@]3([C@H](O2)C[C@@H]2[C@@]3(C[C@H](O)[C@@H]3[C@@]4(C)C=CC(=O)C=C4CC[C@H]32)C)C(=O)COC(=O)C(C)C)CCCCC1 LUKZNWIVRBCLON-GXOBDPJESA-N 0.000 description 2
- OMFXVFTZEKFJBZ-UHFFFAOYSA-N Corticosterone Natural products O=C1CCC2(C)C3C(O)CC(C)(C(CC4)C(=O)CO)C4C3CCC2=C1 OMFXVFTZEKFJBZ-UHFFFAOYSA-N 0.000 description 2
- VWVPRYMOFYIOOZ-KAQKJVHQSA-N Cortisol octanoate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)CCCCCCC)(O)[C@@]1(C)C[C@@H]2O VWVPRYMOFYIOOZ-KAQKJVHQSA-N 0.000 description 2
- MFYSYFVPBJMHGN-ZPOLXVRWSA-N Cortisone Chemical compound O=C1CC[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 MFYSYFVPBJMHGN-ZPOLXVRWSA-N 0.000 description 2
- MFYSYFVPBJMHGN-UHFFFAOYSA-N Cortisone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)(O)C(=O)CO)C4C3CCC2=C1 MFYSYFVPBJMHGN-UHFFFAOYSA-N 0.000 description 2
- ITRJWOMZKQRYTA-RFZYENFJSA-N Cortisone acetate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)C)(O)[C@@]1(C)CC2=O ITRJWOMZKQRYTA-RFZYENFJSA-N 0.000 description 2
- FMGSKLZLMKYGDP-UHFFFAOYSA-N Dehydroepiandrosterone Natural products C1C(O)CCC2(C)C3CCC(C)(C(CC4)=O)C4C3CC=C21 FMGSKLZLMKYGDP-UHFFFAOYSA-N 0.000 description 2
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 2
- UPEZCKBFRMILAV-JNEQICEOSA-N Ecdysone Natural products O=C1[C@H]2[C@@](C)([C@@H]3C([C@@]4(O)[C@@](C)([C@H]([C@H]([C@@H](O)CCC(O)(C)C)C)CC4)CC3)=C1)C[C@H](O)[C@H](O)C2 UPEZCKBFRMILAV-JNEQICEOSA-N 0.000 description 2
- 239000001856 Ethyl cellulose Substances 0.000 description 2
- QUSNBJAOOMFDIB-UHFFFAOYSA-N Ethylamine Chemical compound CCN QUSNBJAOOMFDIB-UHFFFAOYSA-N 0.000 description 2
- 229920003151 Eudragit® RL polymer Polymers 0.000 description 2
- 229920003152 Eudragit® RS polymer Polymers 0.000 description 2
- 229920003141 Eudragit® S 100 Polymers 0.000 description 2
- 208000007465 Giant cell arteritis Diseases 0.000 description 2
- MPDGHEJMBKOTSU-UHFFFAOYSA-N Glycyrrhetinsaeure Natural products C12C(=O)C=C3C4CC(C)(C(O)=O)CCC4(C)CCC3(C)C1(C)CCC1C2(C)CCC(O)C1(C)C MPDGHEJMBKOTSU-UHFFFAOYSA-N 0.000 description 2
- YCISZOVUHXIOFY-HKXOFBAYSA-N Halopredone acetate Chemical compound C1([C@H](F)C2)=CC(=O)C(Br)=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2CC[C@](OC(C)=O)(C(=O)COC(=O)C)[C@@]2(C)C[C@@H]1O YCISZOVUHXIOFY-HKXOFBAYSA-N 0.000 description 2
- 208000007514 Herpes zoster Diseases 0.000 description 2
- FOGXJPFPZOHSQS-AYVLZSQQSA-N Hydrocortisone butyrate propionate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)CC)(OC(=O)CCC)[C@@]1(C)C[C@@H]2O FOGXJPFPZOHSQS-AYVLZSQQSA-N 0.000 description 2
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 2
- 206010061218 Inflammation Diseases 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- UDKABVSQKJNZBH-DWNQPYOZSA-N Melengestrol acetate Chemical compound C1=C(C)C2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC(=C)[C@](OC(=O)C)(C(C)=O)[C@@]1(C)CC2 UDKABVSQKJNZBH-DWNQPYOZSA-N 0.000 description 2
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 2
- GCKMFJBGXUYNAG-HLXURNFRSA-N Methyltestosterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@](C)(O)[C@@]1(C)CC2 GCKMFJBGXUYNAG-HLXURNFRSA-N 0.000 description 2
- RXXBBHGCAXVBES-XMUHMHRVSA-N Oranabol Chemical compound C1CC2=C(O)C(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@](C)(O)[C@@]1(C)CC2 RXXBBHGCAXVBES-XMUHMHRVSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 229930186873 Ponasterone Natural products 0.000 description 2
- PJYYBCXMCWDUAZ-YKDQUOQBSA-N Ponasterone A Natural products O=C1[C@H]2[C@@](C)([C@@H]3C([C@@]4(O)[C@@](C)([C@H]([C@@](O)([C@@H](O)CCC(C)C)C)CC4)CC3)=C1)C[C@H](O)[C@H](O)C2 PJYYBCXMCWDUAZ-YKDQUOQBSA-N 0.000 description 2
- LRJOMUJRLNCICJ-JZYPGELDSA-N Prednisolone acetate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)C)(O)[C@@]1(C)C[C@@H]2O LRJOMUJRLNCICJ-JZYPGELDSA-N 0.000 description 2
- SBQAKZYUNWNIRL-WIPKXTQKSA-N Prednisolone farnesylate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)/C=C(C)/CC/C=C(C)/CCC=C(C)C)(O)[C@@]1(C)C[C@@H]2O SBQAKZYUNWNIRL-WIPKXTQKSA-N 0.000 description 2
- HUMXXHTVHHLNRO-KAJVQRHHSA-N Prednisolone tebutate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)CC(C)(C)C)(O)[C@@]1(C)C[C@@H]2O HUMXXHTVHHLNRO-KAJVQRHHSA-N 0.000 description 2
- ORNBQBCIOKFOEO-YQUGOWONSA-N Pregnenolone Natural products O=C(C)[C@@H]1[C@@]2(C)[C@H]([C@H]3[C@@H]([C@]4(C)C(=CC3)C[C@@H](O)CC4)CC2)CC1 ORNBQBCIOKFOEO-YQUGOWONSA-N 0.000 description 2
- 208000003251 Pruritus Diseases 0.000 description 2
- 206010039710 Scleroderma Diseases 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 2
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- XGMPVBXKDAHORN-RBWIMXSLSA-N Triamcinolone diacetate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](OC(C)=O)[C@@](C(=O)COC(=O)C)(O)[C@@]1(C)C[C@@H]2O XGMPVBXKDAHORN-RBWIMXSLSA-N 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- 206010047115 Vasculitis Diseases 0.000 description 2
- KSCZWFXQKITHSL-OKCNGXCSSA-N [(3s,8r,9s,10r,13s,14s,17r)-17-acetyl-17-acetyloxy-6-chloro-10,13-dimethyl-1,2,3,8,9,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-yl] acetate Chemical compound C1C[C@]2(C)[C@](C(C)=O)(OC(C)=O)CC[C@H]2[C@@H]2C=C(Cl)C3=C[C@@H](OC(=O)C)CC[C@]3(C)[C@H]21 KSCZWFXQKITHSL-OKCNGXCSSA-N 0.000 description 2
- PSJMYDLEWUWIAN-KYPKCDLESA-N [(8r,9s,10r,13s,14s,17r)-17-acetyl-6-chloro-13-methyl-3-oxo-1,2,8,9,10,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-17-yl] acetate Chemical compound C1=C(Cl)C2=CC(=O)CC[C@@H]2[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)C)[C@@]1(C)CC2 PSJMYDLEWUWIAN-KYPKCDLESA-N 0.000 description 2
- DPHFJXVKASDMBW-RQRKFSSASA-N [2-[(8s,9r,10s,11s,13s,14s,16r,17r)-9-fluoro-11,17-dihydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] acetate;hydrate Chemical compound O.C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)COC(C)=O)(O)[C@@]1(C)C[C@@H]2O DPHFJXVKASDMBW-RQRKFSSASA-N 0.000 description 2
- YZNQMPPBWQTLFJ-TUFAYURCSA-N [2-[(8s,9s,10r,11s,13s,14s,17r)-11,17-dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1h-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] butanoate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)CCC)(O)[C@@]1(C)C[C@@H]2O YZNQMPPBWQTLFJ-TUFAYURCSA-N 0.000 description 2
- AKUJBENLRBOFTD-HIBZCRSPSA-N [2-[(9r,10s,11s,13s,16r,17r)-9-fluoro-11,17-dihydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] acetate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)C1C1C[C@@H](C)[C@@](C(=O)COC(C)=O)(O)[C@@]1(C)C[C@@H]2O AKUJBENLRBOFTD-HIBZCRSPSA-N 0.000 description 2
- 229960002478 aldosterone Drugs 0.000 description 2
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 2
- UPEZCKBFRMILAV-UHFFFAOYSA-N alpha-Ecdysone Natural products C1C(O)C(O)CC2(C)C(CCC3(C(C(C(O)CCC(C)(C)O)C)CCC33O)C)C3=CC(=O)C21 UPEZCKBFRMILAV-UHFFFAOYSA-N 0.000 description 2
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 2
- 229950003339 amadinone Drugs 0.000 description 2
- AEMFNILZOJDQLW-QAGGRKNESA-N androst-4-ene-3,17-dione Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CCC2=C1 AEMFNILZOJDQLW-QAGGRKNESA-N 0.000 description 2
- 229960005471 androstenedione Drugs 0.000 description 2
- AEMFNILZOJDQLW-UHFFFAOYSA-N androstenedione Natural products O=C1CCC2(C)C3CCC(C)(C(CC4)=O)C4C3CCC2=C1 AEMFNILZOJDQLW-UHFFFAOYSA-N 0.000 description 2
- 230000003110 anti-inflammatory effect Effects 0.000 description 2
- MDJRZSNPHZEMJH-MTMZYOSNSA-N artisone acetate Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)COC(=O)C)[C@@]1(C)CC2 MDJRZSNPHZEMJH-MTMZYOSNSA-N 0.000 description 2
- 229960005070 ascorbic acid Drugs 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 208000010668 atopic eczema Diseases 0.000 description 2
- 229950000210 beclometasone dipropionate Drugs 0.000 description 2
- 229940092705 beclomethasone Drugs 0.000 description 2
- RRQAFKDLLNSONH-RIWODDSKSA-M benzyl-dodecyl-dimethylazanium;4-chlorophenol;(8s,10s,13s,14s,17r)-9-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one;5-methyl-2-propan-2-ylphenol;chloride Chemical compound [Cl-].OC1=CC=C(Cl)C=C1.CC(C)C1=CC=C(C)C=C1O.CCCCCCCCCCCC[N+](C)(C)CC1=CC=CC=C1.C1CC2=CC(=O)C=C[C@]2(C)C2(F)[C@@H]1[C@@H]1CC(C)[C@@](C(=O)CO)(O)[C@@]1(C)CC2O RRQAFKDLLNSONH-RIWODDSKSA-M 0.000 description 2
- 229960005354 betamethasone sodium phosphate Drugs 0.000 description 2
- PLCQGRYPOISRTQ-LWCNAHDDSA-L betamethasone sodium phosphate Chemical compound [Na+].[Na+].C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)COP([O-])([O-])=O)(O)[C@@]1(C)C[C@@H]2O PLCQGRYPOISRTQ-LWCNAHDDSA-L 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 2
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 229960003996 chlormadinone Drugs 0.000 description 2
- VUHJZBBCZGVNDZ-TTYLFXKOSA-N chlormadinone Chemical compound C1=C(Cl)C2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)C)(O)[C@@]1(C)CC2 VUHJZBBCZGVNDZ-TTYLFXKOSA-N 0.000 description 2
- 229950006229 chloroprednisone Drugs 0.000 description 2
- 229960003728 ciclesonide Drugs 0.000 description 2
- 235000015165 citric acid Nutrition 0.000 description 2
- 229960002842 clobetasol Drugs 0.000 description 2
- 229960004703 clobetasol propionate Drugs 0.000 description 2
- 229960004299 clocortolone Drugs 0.000 description 2
- YMTMADLUXIRMGX-RFPWEZLHSA-N clocortolone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(Cl)[C@@H]2[C@@H]2C[C@@H](C)[C@H](C(=O)CO)[C@@]2(C)C[C@@H]1O YMTMADLUXIRMGX-RFPWEZLHSA-N 0.000 description 2
- 229960001357 clocortolone pivalate Drugs 0.000 description 2
- SXYZQZLHAIHKKY-GSTUPEFVSA-N clocortolone pivalate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(Cl)[C@@H]2[C@@H]2C[C@@H](C)[C@H](C(=O)COC(=O)C(C)(C)C)[C@@]2(C)C[C@@H]1O SXYZQZLHAIHKKY-GSTUPEFVSA-N 0.000 description 2
- 229950003461 clogestone Drugs 0.000 description 2
- 229960002219 cloprednol Drugs 0.000 description 2
- YTJIBEDMAQUYSZ-FDNPDPBUSA-N cloprednol Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3C=C(Cl)C2=C1 YTJIBEDMAQUYSZ-FDNPDPBUSA-N 0.000 description 2
- 229920001577 copolymer Polymers 0.000 description 2
- OMFXVFTZEKFJBZ-HJTSIMOOSA-N corticosterone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@H](CC4)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OMFXVFTZEKFJBZ-HJTSIMOOSA-N 0.000 description 2
- FZCHYNWYXKICIO-FZNHGJLXSA-N cortisol 17-valerate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)CO)(OC(=O)CCCC)[C@@]1(C)C[C@@H]2O FZCHYNWYXKICIO-FZNHGJLXSA-N 0.000 description 2
- 229960004544 cortisone Drugs 0.000 description 2
- 229960003290 cortisone acetate Drugs 0.000 description 2
- 229960003840 cortivazol Drugs 0.000 description 2
- RKHQGWMMUURILY-UHRZLXHJSA-N cortivazol Chemical compound C([C@H]1[C@@H]2C[C@H]([C@]([C@@]2(C)C[C@H](O)[C@@H]1[C@@]1(C)C2)(O)C(=O)COC(C)=O)C)=C(C)C1=CC1=C2C=NN1C1=CC=CC=C1 RKHQGWMMUURILY-UHRZLXHJSA-N 0.000 description 2
- 229950002276 cortodoxone Drugs 0.000 description 2
- ZESRJSPZRDMNHY-UHFFFAOYSA-N de-oxy corticosterone Natural products O=C1CCC2(C)C3CCC(C)(C(CC4)C(=O)CO)C4C3CCC2=C1 ZESRJSPZRDMNHY-UHFFFAOYSA-N 0.000 description 2
- FMGSKLZLMKYGDP-USOAJAOKSA-N dehydroepiandrosterone Chemical compound C1[C@@H](O)CC[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CC=C21 FMGSKLZLMKYGDP-USOAJAOKSA-N 0.000 description 2
- ZSAMZEYLGUEVJW-TTYLFXKOSA-N delmadinone Chemical compound C1=C(Cl)C2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)C)(O)[C@@]1(C)CC2 ZSAMZEYLGUEVJW-TTYLFXKOSA-N 0.000 description 2
- 229950006309 delmadinone Drugs 0.000 description 2
- 229940119740 deoxycorticosterone Drugs 0.000 description 2
- 229960003662 desonide Drugs 0.000 description 2
- WBGKWQHBNHJJPZ-LECWWXJVSA-N desonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O WBGKWQHBNHJJPZ-LECWWXJVSA-N 0.000 description 2
- 229960002593 desoximetasone Drugs 0.000 description 2
- VWVSBHGCDBMOOT-IIEHVVJPSA-N desoximetasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@H](C(=O)CO)[C@@]1(C)C[C@@H]2O VWVSBHGCDBMOOT-IIEHVVJPSA-N 0.000 description 2
- 229960003957 dexamethasone Drugs 0.000 description 2
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 2
- 229960003657 dexamethasone acetate Drugs 0.000 description 2
- 229960002344 dexamethasone sodium phosphate Drugs 0.000 description 2
- PLCQGRYPOISRTQ-FCJDYXGNSA-L dexamethasone sodium phosphate Chemical compound [Na+].[Na+].C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)COP([O-])([O-])=O)(O)[C@@]1(C)C[C@@H]2O PLCQGRYPOISRTQ-FCJDYXGNSA-L 0.000 description 2
- YNNURTVKPVJVEI-GSLJADNHSA-N dichlorisone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)C)(O)[C@@]1(C)C[C@@H]2Cl YNNURTVKPVJVEI-GSLJADNHSA-N 0.000 description 2
- 229950009888 dichlorisone Drugs 0.000 description 2
- 229960002124 diflorasone diacetate Drugs 0.000 description 2
- BOBLHFUVNSFZPJ-JOYXJVLSSA-N diflorasone diacetate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H](C)[C@@](C(=O)COC(C)=O)(OC(C)=O)[C@@]2(C)C[C@@H]1O BOBLHFUVNSFZPJ-JOYXJVLSSA-N 0.000 description 2
- 229960004091 diflucortolone Drugs 0.000 description 2
- OGPWIDANBSLJPC-RFPWEZLHSA-N diflucortolone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@H](C(=O)CO)[C@@]2(C)C[C@@H]1O OGPWIDANBSLJPC-RFPWEZLHSA-N 0.000 description 2
- 208000035475 disorder Diseases 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- UPEZCKBFRMILAV-JMZLNJERSA-N ecdysone Chemical compound C1[C@@H](O)[C@@H](O)C[C@]2(C)[C@@H](CC[C@@]3([C@@H]([C@@H]([C@H](O)CCC(C)(C)O)C)CC[C@]33O)C)C3=CC(=O)[C@@H]21 UPEZCKBFRMILAV-JMZLNJERSA-N 0.000 description 2
- 229960003720 enoxolone Drugs 0.000 description 2
- 229920001249 ethyl cellulose Polymers 0.000 description 2
- 235000019325 ethyl cellulose Nutrition 0.000 description 2
- FSXVSUSRJXIJHB-UHFFFAOYSA-M ethyl prop-2-enoate;methyl 2-methylprop-2-enoate;trimethyl-[2-(2-methylprop-2-enoyloxy)ethyl]azanium;chloride Chemical compound [Cl-].CCOC(=O)C=C.COC(=O)C(C)=C.CC(=C)C(=O)OCC[N+](C)(C)C FSXVSUSRJXIJHB-UHFFFAOYSA-M 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 238000011049 filling Methods 0.000 description 2
- BYZCJOHDXLROEC-RBWIMXSLSA-N fluazacort Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H]3OC(C)=N[C@@]3(C(=O)COC(=O)C)[C@@]1(C)C[C@@H]2O BYZCJOHDXLROEC-RBWIMXSLSA-N 0.000 description 2
- 229950002335 fluazacort Drugs 0.000 description 2
- 229960002011 fludrocortisone Drugs 0.000 description 2
- AAXVEMMRQDVLJB-BULBTXNYSA-N fludrocortisone Chemical compound O=C1CC[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 AAXVEMMRQDVLJB-BULBTXNYSA-N 0.000 description 2
- SYWHXTATXSMDSB-GSLJADNHSA-N fludrocortisone acetate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)C)(O)[C@@]1(C)C[C@@H]2O SYWHXTATXSMDSB-GSLJADNHSA-N 0.000 description 2
- 229950010349 flugestone Drugs 0.000 description 2
- 229960003469 flumetasone Drugs 0.000 description 2
- WXURHACBFYSXBI-GQKYHHCASA-N flumethasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]2(C)C[C@@H]1O WXURHACBFYSXBI-GQKYHHCASA-N 0.000 description 2
- 229940042902 flumethasone pivalate Drugs 0.000 description 2
- JWRMHDSINXPDHB-OJAGFMMFSA-N flumethasone pivalate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)COC(=O)C(C)(C)C)(O)[C@@]2(C)C[C@@H]1O JWRMHDSINXPDHB-OJAGFMMFSA-N 0.000 description 2
- 229960000676 flunisolide Drugs 0.000 description 2
- GAKMQHDJQHZUTJ-ULHLPKEOSA-N fluocortolone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@@H](C)[C@H](C(=O)CO)[C@@]2(C)C[C@@H]1O GAKMQHDJQHZUTJ-ULHLPKEOSA-N 0.000 description 2
- 229960003336 fluorocortisol acetate Drugs 0.000 description 2
- 229960001048 fluorometholone Drugs 0.000 description 2
- FAOZLTXFLGPHNG-KNAQIMQKSA-N fluorometholone Chemical compound C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@]2(F)[C@@H](O)C[C@]2(C)[C@@](O)(C(C)=O)CC[C@H]21 FAOZLTXFLGPHNG-KNAQIMQKSA-N 0.000 description 2
- 229960001629 fluorometholone acetate Drugs 0.000 description 2
- YRFXGQHBPBMFHW-SBTZIJSASA-N fluorometholone acetate Chemical compound C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@]2(F)[C@@H](O)C[C@]2(C)[C@@](OC(C)=O)(C(C)=O)CC[C@H]21 YRFXGQHBPBMFHW-SBTZIJSASA-N 0.000 description 2
- YLRFCQOZQXIBAB-RBZZARIASA-N fluoxymesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1CC[C@](C)(O)[C@@]1(C)C[C@@H]2O YLRFCQOZQXIBAB-RBZZARIASA-N 0.000 description 2
- 229960001751 fluoxymesterone Drugs 0.000 description 2
- 229960003590 fluperolone Drugs 0.000 description 2
- HHPZZKDXAFJLOH-QZIXMDIESA-N fluperolone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1CC[C@@](C(=O)[C@@H](OC(C)=O)C)(O)[C@@]1(C)C[C@@H]2O HHPZZKDXAFJLOH-QZIXMDIESA-N 0.000 description 2
- 229960000618 fluprednisolone Drugs 0.000 description 2
- 229960000289 fluticasone propionate Drugs 0.000 description 2
- WMWTYOKRWGGJOA-CENSZEJFSA-N fluticasone propionate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(OC(=O)CC)[C@@]2(C)C[C@@H]1O WMWTYOKRWGGJOA-CENSZEJFSA-N 0.000 description 2
- 229960000671 formocortal Drugs 0.000 description 2
- QNXUUBBKHBYRFW-QWAPGEGQSA-N formocortal Chemical compound C1C(C=O)=C2C=C(OCCCl)CC[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)COC(=O)C)[C@@]1(C)C[C@@H]2O QNXUUBBKHBYRFW-QWAPGEGQSA-N 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 239000007903 gelatin capsule Substances 0.000 description 2
- 235000011187 glycerol Nutrition 0.000 description 2
- 229960002475 halometasone Drugs 0.000 description 2
- GGXMRPUKBWXVHE-MIHLVHIWSA-N halometasone Chemical compound C1([C@@H](F)C2)=CC(=O)C(Cl)=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]2(C)C[C@@H]1O GGXMRPUKBWXVHE-MIHLVHIWSA-N 0.000 description 2
- 229950008940 halopredone Drugs 0.000 description 2
- GCCIFDUTISMRTG-TUPTUZDRSA-N haloprogesterone Chemical compound C1([C@@H](F)C2)=CC(=O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@](C(=O)C)(Br)[C@@]2(C)CC1 GCCIFDUTISMRTG-TUPTUZDRSA-N 0.000 description 2
- 229950002886 haloprogesterone Drugs 0.000 description 2
- IPCSVZSSVZVIGE-UHFFFAOYSA-N hexadecanoic acid Chemical compound CCCCCCCCCCCCCCCC(O)=O IPCSVZSSVZVIGE-UHFFFAOYSA-N 0.000 description 2
- FWFVLWGEFDIZMJ-FOMYWIRZSA-N hydrocortamate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)CN(CC)CC)(O)[C@@]1(C)C[C@@H]2O FWFVLWGEFDIZMJ-FOMYWIRZSA-N 0.000 description 2
- 229950000208 hydrocortamate Drugs 0.000 description 2
- 229960002846 hydrocortisone probutate Drugs 0.000 description 2
- 229960004204 hydrocortisone sodium phosphate Drugs 0.000 description 2
- 229960001401 hydrocortisone sodium succinate Drugs 0.000 description 2
- 229950006240 hydrocortisone succinate Drugs 0.000 description 2
- 229960000631 hydrocortisone valerate Drugs 0.000 description 2
- 229960002899 hydroxyprogesterone Drugs 0.000 description 2
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 2
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 2
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 2
- 230000004054 inflammatory process Effects 0.000 description 2
- 229960002857 isoflupredone Drugs 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- WSBAGDDNVWTLOM-UHFFFAOYSA-N lesterone Natural products C1C(O)C(O)CC2(C)C(C(O)CC3(C(C(C)(O)C(O)CCC(C)(O)C)CCC33O)C)C3=CC(=O)C21 WSBAGDDNVWTLOM-UHFFFAOYSA-N 0.000 description 2
- 229960001798 loteprednol Drugs 0.000 description 2
- YPZVAYHNBBHPTO-MXRBDKCISA-N loteprednol Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)OCCl)[C@@H]4[C@@H]3CCC2=C1 YPZVAYHNBBHPTO-MXRBDKCISA-N 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 239000001630 malic acid Substances 0.000 description 2
- 235000011090 malic acid Nutrition 0.000 description 2
- 229960000606 medrogestone Drugs 0.000 description 2
- HCFSGRMEEXUOSS-JXEXPEPMSA-N medrogestone Chemical compound C1=C(C)C2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)C)(C)[C@@]1(C)CC2 HCFSGRMEEXUOSS-JXEXPEPMSA-N 0.000 description 2
- 229960001786 megestrol Drugs 0.000 description 2
- 229960004296 megestrol acetate Drugs 0.000 description 2
- 229960004805 melengestrol Drugs 0.000 description 2
- 229960001810 meprednisone Drugs 0.000 description 2
- PIDANAQULIKBQS-RNUIGHNZSA-N meprednisone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)CC2=O PIDANAQULIKBQS-RNUIGHNZSA-N 0.000 description 2
- 229910052751 metal Chemical class 0.000 description 2
- 239000002184 metal Chemical class 0.000 description 2
- 229960001293 methylprednisolone acetate Drugs 0.000 description 2
- 229960000334 methylprednisolone sodium succinate Drugs 0.000 description 2
- IMBXEJJVJRTNOW-XYMSELFBSA-N methylprednisolone succinate Chemical compound C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@](O)(C(=O)COC(=O)CCC(O)=O)CC[C@H]21 IMBXEJJVJRTNOW-XYMSELFBSA-N 0.000 description 2
- 229950009831 methylprednisolone succinate Drugs 0.000 description 2
- 229960001566 methyltestosterone Drugs 0.000 description 2
- 229950003695 metribolone Drugs 0.000 description 2
- 229960001664 mometasone Drugs 0.000 description 2
- QLIIKPVHVRXHRI-CXSFZGCWSA-N mometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CCl)(O)[C@@]1(C)C[C@@H]2O QLIIKPVHVRXHRI-CXSFZGCWSA-N 0.000 description 2
- 229960002744 mometasone furoate Drugs 0.000 description 2
- WOFMFGQZHJDGCX-ZULDAHANSA-N mometasone furoate Chemical compound O([C@]1([C@@]2(C)C[C@H](O)[C@]3(Cl)[C@@]4(C)C=CC(=O)C=C4CC[C@H]3[C@@H]2C[C@H]1C)C(=O)CCl)C(=O)C1=CC=CO1 WOFMFGQZHJDGCX-ZULDAHANSA-N 0.000 description 2
- 229960004123 mometasone furoate monohydrate Drugs 0.000 description 2
- DNKKLDKIFMDAPT-UHFFFAOYSA-N n,n-dimethylmethanamine;2-methylprop-2-enoic acid Chemical compound CN(C)C.CC(=C)C(O)=O.CC(=C)C(O)=O DNKKLDKIFMDAPT-UHFFFAOYSA-N 0.000 description 2
- 229960004911 nomegestrol Drugs 0.000 description 2
- KZUIYQJTUIACIG-YBZCJVABSA-N nomegestrol Chemical compound C1=C(C)C2=CC(=O)CC[C@@H]2[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)C)(O)[C@@]1(C)CC2 KZUIYQJTUIACIG-YBZCJVABSA-N 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 229950008280 oxymesterone Drugs 0.000 description 2
- 229960002858 paramethasone Drugs 0.000 description 2
- 229960000865 paramethasone acetate Drugs 0.000 description 2
- XNGIFLGASWRNHJ-UHFFFAOYSA-L phthalate(2-) Chemical compound [O-]C(=O)C1=CC=CC=C1C([O-])=O XNGIFLGASWRNHJ-UHFFFAOYSA-L 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 150000003102 ponasterones Chemical class 0.000 description 2
- 229960002847 prasterone Drugs 0.000 description 2
- 229960002794 prednicarbate Drugs 0.000 description 2
- FNPXMHRZILFCKX-KAJVQRHHSA-N prednicarbate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)CC)(OC(=O)OCC)[C@@]1(C)C[C@@H]2O FNPXMHRZILFCKX-KAJVQRHHSA-N 0.000 description 2
- 229960002800 prednisolone acetate Drugs 0.000 description 2
- 229950011084 prednisolone farnesylate Drugs 0.000 description 2
- JDOZJEUDSLGTLU-VWUMJDOOSA-N prednisolone phosphate Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)COP(O)(O)=O)[C@@H]4[C@@H]3CCC2=C1 JDOZJEUDSLGTLU-VWUMJDOOSA-N 0.000 description 2
- 229960002943 prednisolone sodium phosphate Drugs 0.000 description 2
- RBJROVWIRLFZFC-PNLFXGMVSA-N prednisolone steaglate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)COC(=O)CCCCCCCCCCCCCCCCC)(O)[C@@]1(C)C[C@@H]2O RBJROVWIRLFZFC-PNLFXGMVSA-N 0.000 description 2
- 229950010987 prednisolone steaglate Drugs 0.000 description 2
- APGDTXUMTIZLCJ-CGVGKPPMSA-N prednisolone succinate Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)COC(=O)CCC(O)=O)[C@@H]4[C@@H]3CCC2=C1 APGDTXUMTIZLCJ-CGVGKPPMSA-N 0.000 description 2
- 229950004597 prednisolone succinate Drugs 0.000 description 2
- WVKSUFYQOHQCMM-YGZHYJPASA-N prednisolone sulfobenzoate Chemical compound O=C([C@@]1(O)CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)[C@@H](O)C[C@@]21C)COC(=O)C1=CC=CC(S(O)(=O)=O)=C1 WVKSUFYQOHQCMM-YGZHYJPASA-N 0.000 description 2
- 229950004954 prednisolone sulfobenzoate Drugs 0.000 description 2
- 229960004259 prednisolone tebutate Drugs 0.000 description 2
- 229960001917 prednylidene Drugs 0.000 description 2
- WSVOMANDJDYYEY-CWNVBEKCSA-N prednylidene Chemical group O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](C(=C)C4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 WSVOMANDJDYYEY-CWNVBEKCSA-N 0.000 description 2
- 229960000249 pregnenolone Drugs 0.000 description 2
- ORNBQBCIOKFOEO-QGVNFLHTSA-N pregnenolone Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C)CC2 ORNBQBCIOKFOEO-QGVNFLHTSA-N 0.000 description 2
- 239000000186 progesterone Substances 0.000 description 2
- 229960003387 progesterone Drugs 0.000 description 2
- 229960001584 promegestone Drugs 0.000 description 2
- QFFCYTLOTYIJMR-XMGTWHOFSA-N promegestone Chemical compound C1CC2=CC(=O)CCC2=C2[C@@H]1[C@@H]1CC[C@@](C(=O)CC)(C)[C@@]1(C)CC2 QFFCYTLOTYIJMR-XMGTWHOFSA-N 0.000 description 2
- 230000000069 prophylactic effect Effects 0.000 description 2
- MIXMJCQRHVAJIO-TZHJZOAOSA-N qk4dys664x Chemical compound O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O MIXMJCQRHVAJIO-TZHJZOAOSA-N 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 201000000306 sarcoidosis Diseases 0.000 description 2
- 239000001632 sodium acetate Substances 0.000 description 2
- 235000017281 sodium acetate Nutrition 0.000 description 2
- WNIFXKPDILJURQ-UHFFFAOYSA-N stearyl glycyrrhizinate Natural products C1CC(O)C(C)(C)C2CCC3(C)C4(C)CCC5(C)CCC(C(=O)OCCCCCCCCCCCCCCCCCC)(C)CC5C4=CC(=O)C3C21C WNIFXKPDILJURQ-UHFFFAOYSA-N 0.000 description 2
- 150000003431 steroids Chemical class 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 2
- 206010043207 temporal arteritis Diseases 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- OGZHZYVCWDUIJV-VSXGLTOVSA-N tralonide Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(Cl)[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CF)[C@@]2(C)C[C@@H]1Cl OGZHZYVCWDUIJV-VSXGLTOVSA-N 0.000 description 2
- 229950004108 tralonide Drugs 0.000 description 2
- 229960002117 triamcinolone acetonide Drugs 0.000 description 2
- YNDXUCZADRHECN-JNQJZLCISA-N triamcinolone acetonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O YNDXUCZADRHECN-JNQJZLCISA-N 0.000 description 2
- DZQIYNZZUKIZNS-RCFDOMGHSA-N triamcinolone acetonide 21-palmitate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)COC(=O)CCCCCCCCCCCCCCC)[C@@]1(C)C[C@@H]2O DZQIYNZZUKIZNS-RCFDOMGHSA-N 0.000 description 2
- 229960004320 triamcinolone diacetate Drugs 0.000 description 2
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 2
- WSBAGDDNVWTLOM-XHZKDPLLSA-N turkesterone Chemical compound C1[C@@H](O)[C@@H](O)C[C@]2(C)[C@@H]([C@H](O)C[C@@]3([C@@H]([C@@](C)(O)[C@H](O)CCC(C)(O)C)CC[C@]33O)C)C3=CC(=O)[C@@H]21 WSBAGDDNVWTLOM-XHZKDPLLSA-N 0.000 description 2
- DXGPJKXCWRHUMH-UHFFFAOYSA-N turkesterone Natural products C1C(O)C(O)CC2(C)C(C(O)CC3(C(C(C(O)CCC(C)(C)O)C)CCC33O)C)C3=CC(=O)C21 DXGPJKXCWRHUMH-UHFFFAOYSA-N 0.000 description 2
- 229950008396 ulobetasol propionate Drugs 0.000 description 2
- BDSYKGHYMJNPAB-LICBFIPMSA-N ulobetasol propionate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H](C)[C@@](C(=O)CCl)(OC(=O)CC)[C@@]2(C)C[C@@H]1O BDSYKGHYMJNPAB-LICBFIPMSA-N 0.000 description 2
- 229940070710 valerate Drugs 0.000 description 2
- QDLHCMPXEPAAMD-QAIWCSMKSA-N wortmannin Chemical compound C1([C@]2(C)C3=C(C4=O)OC=C3C(=O)O[C@@H]2COC)=C4[C@@H]2CCC(=O)[C@@]2(C)C[C@H]1OC(C)=O QDLHCMPXEPAAMD-QAIWCSMKSA-N 0.000 description 2
- QDLHCMPXEPAAMD-UHFFFAOYSA-N wortmannin Natural products COCC1OC(=O)C2=COC(C3=O)=C2C1(C)C1=C3C2CCC(=O)C2(C)CC1OC(C)=O QDLHCMPXEPAAMD-UHFFFAOYSA-N 0.000 description 2
- TUSDEZXZIZRFGC-UHFFFAOYSA-N 1-O-galloyl-3,6-(R)-HHDP-beta-D-glucose Natural products OC1C(O2)COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC1C(O)C2OC(=O)C1=CC(O)=C(O)C(O)=C1 TUSDEZXZIZRFGC-UHFFFAOYSA-N 0.000 description 1
- 208000002874 Acne Vulgaris Diseases 0.000 description 1
- 206010000748 Acute febrile neutrophilic dermatosis Diseases 0.000 description 1
- 206010001052 Acute respiratory distress syndrome Diseases 0.000 description 1
- 208000026872 Addison Disease Diseases 0.000 description 1
- WSVLPVUVIUVCRA-KPKNDVKVSA-N Alpha-lactose monohydrate Chemical compound O.O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O WSVLPVUVIUVCRA-KPKNDVKVSA-N 0.000 description 1
- 206010002556 Ankylosing Spondylitis Diseases 0.000 description 1
- 201000001320 Atherosclerosis Diseases 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- 206010003827 Autoimmune hepatitis Diseases 0.000 description 1
- 208000006373 Bell palsy Diseases 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- 201000006474 Brain Ischemia Diseases 0.000 description 1
- 206010006811 Bursitis Diseases 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 206010008120 Cerebral ischaemia Diseases 0.000 description 1
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 208000010007 Cogan syndrome Diseases 0.000 description 1
- 206010009900 Colitis ulcerative Diseases 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- 208000014311 Cushing syndrome Diseases 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 206010012438 Dermatitis atopic Diseases 0.000 description 1
- 206010012442 Dermatitis contact Diseases 0.000 description 1
- 208000006926 Discoid Lupus Erythematosus Diseases 0.000 description 1
- 206010014954 Eosinophilic fasciitis Diseases 0.000 description 1
- 206010015150 Erythema Diseases 0.000 description 1
- 239000001263 FEMA 3042 Substances 0.000 description 1
- 208000001640 Fibromyalgia Diseases 0.000 description 1
- 208000018522 Gastrointestinal disease Diseases 0.000 description 1
- 201000005569 Gout Diseases 0.000 description 1
- 206010018634 Gouty Arthritis Diseases 0.000 description 1
- 208000009329 Graft vs Host Disease Diseases 0.000 description 1
- 206010018691 Granuloma Diseases 0.000 description 1
- 229920002907 Guar gum Polymers 0.000 description 1
- 208000035186 Hemolytic Autoimmune Anemia Diseases 0.000 description 1
- 201000004331 Henoch-Schoenlein purpura Diseases 0.000 description 1
- 206010019617 Henoch-Schonlein purpura Diseases 0.000 description 1
- 206010020112 Hirsutism Diseases 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 description 1
- 239000004354 Hydroxyethyl cellulose Substances 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 201000009794 Idiopathic Pulmonary Fibrosis Diseases 0.000 description 1
- 208000031814 IgA Vasculitis Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 1
- 206010059176 Juvenile idiopathic arthritis Diseases 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 208000005777 Lupus Nephritis Diseases 0.000 description 1
- 206010025323 Lymphomas Diseases 0.000 description 1
- 208000001344 Macular Edema Diseases 0.000 description 1
- 206010025415 Macular oedema Diseases 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-L Malonate Chemical compound [O-]C(=O)CC([O-])=O OFOBLEOULBTSOW-UHFFFAOYSA-L 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 229920000881 Modified starch Polymers 0.000 description 1
- 208000000112 Myalgia Diseases 0.000 description 1
- 201000002481 Myositis Diseases 0.000 description 1
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 208000002193 Pain Diseases 0.000 description 1
- 235000021314 Palmitic acid Nutrition 0.000 description 1
- 206010033645 Pancreatitis Diseases 0.000 description 1
- LRBQNJMCXXYXIU-PPKXGCFTSA-N Penta-digallate-beta-D-glucose Natural products OC1=C(O)C(O)=CC(C(=O)OC=2C(=C(O)C=C(C=2)C(=O)OC[C@@H]2[C@H]([C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)O2)OC(=O)C=2C=C(OC(=O)C=3C=C(O)C(O)=C(O)C=3)C(O)=C(O)C=2)O)=C1 LRBQNJMCXXYXIU-PPKXGCFTSA-N 0.000 description 1
- 206010036105 Polyneuropathy Diseases 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 229920003081 Povidone K 30 Polymers 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 201000001263 Psoriatic Arthritis Diseases 0.000 description 1
- 208000036824 Psoriatic arthropathy Diseases 0.000 description 1
- 208000013616 Respiratory Distress Syndrome Diseases 0.000 description 1
- 208000025747 Rheumatic disease Diseases 0.000 description 1
- 206010039085 Rhinitis allergic Diseases 0.000 description 1
- 241001303601 Rosacea Species 0.000 description 1
- 102100030852 Run domain Beclin-1-interacting and cysteine-rich domain-containing protein Human genes 0.000 description 1
- 101710179516 Run domain Beclin-1-interacting and cysteine-rich domain-containing protein Proteins 0.000 description 1
- 241000831652 Salinivibrio sharmensis Species 0.000 description 1
- 206010039705 Scleritis Diseases 0.000 description 1
- 206010040070 Septic Shock Diseases 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 208000021386 Sjogren Syndrome Diseases 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 208000006011 Stroke Diseases 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 208000010265 Sweet syndrome Diseases 0.000 description 1
- 201000009594 Systemic Scleroderma Diseases 0.000 description 1
- 206010042953 Systemic sclerosis Diseases 0.000 description 1
- 208000001106 Takayasu Arteritis Diseases 0.000 description 1
- 208000000491 Tendinopathy Diseases 0.000 description 1
- 206010043255 Tendonitis Diseases 0.000 description 1
- ZMZDMBWJUHKJPS-UHFFFAOYSA-M Thiocyanate anion Chemical compound [S-]C#N ZMZDMBWJUHKJPS-UHFFFAOYSA-M 0.000 description 1
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 1
- 201000006704 Ulcerative Colitis Diseases 0.000 description 1
- 208000024780 Urticaria Diseases 0.000 description 1
- 206010046851 Uveitis Diseases 0.000 description 1
- 208000036826 VIIth nerve paralysis Diseases 0.000 description 1
- 239000005862 Whey Substances 0.000 description 1
- 102000007544 Whey Proteins Human genes 0.000 description 1
- 108010046377 Whey Proteins Proteins 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 229940022663 acetate Drugs 0.000 description 1
- 239000008351 acetate buffer Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 206010000496 acne Diseases 0.000 description 1
- 210000004404 adrenal cortex Anatomy 0.000 description 1
- 201000000028 adult respiratory distress syndrome Diseases 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 208000002029 allergic contact dermatitis Diseases 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- 201000010105 allergic rhinitis Diseases 0.000 description 1
- AWUCVROLDVIAJX-UHFFFAOYSA-N alpha-glycerophosphate Natural products OCC(O)COP(O)(O)=O AWUCVROLDVIAJX-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 230000000181 anti-adherent effect Effects 0.000 description 1
- 206010003246 arthritis Diseases 0.000 description 1
- 229940072107 ascorbate Drugs 0.000 description 1
- 229940009098 aspartate Drugs 0.000 description 1
- 208000006673 asthma Diseases 0.000 description 1
- 201000008937 atopic dermatitis Diseases 0.000 description 1
- 201000000448 autoimmune hemolytic anemia Diseases 0.000 description 1
- 229940050390 benzoate Drugs 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 238000004166 bioassay Methods 0.000 description 1
- 239000000090 biomarker Substances 0.000 description 1
- 210000004958 brain cell Anatomy 0.000 description 1
- 206010006451 bronchitis Diseases 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- FATUQANACHZLRT-KMRXSBRUSA-L calcium glucoheptonate Chemical compound [Ca+2].OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)C([O-])=O.OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)C([O-])=O FATUQANACHZLRT-KMRXSBRUSA-L 0.000 description 1
- FUFJGUQYACFECW-UHFFFAOYSA-L calcium hydrogenphosphate Chemical compound [Ca+2].OP([O-])([O-])=O FUFJGUQYACFECW-UHFFFAOYSA-L 0.000 description 1
- 239000007894 caplet Substances 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 206010008118 cerebral infarction Diseases 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 239000007910 chewable tablet Substances 0.000 description 1
- 208000025302 chronic primary adrenal insufficiency Diseases 0.000 description 1
- 229940001468 citrate Drugs 0.000 description 1
- 235000009508 confectionery Nutrition 0.000 description 1
- 208000004921 cutaneous lupus erythematosus Diseases 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 235000019700 dicalcium phosphate Nutrition 0.000 description 1
- 229940095079 dicalcium phosphate anhydrous Drugs 0.000 description 1
- 208000010643 digestive system disease Diseases 0.000 description 1
- 229940020470 dipyridamole 100 mg Drugs 0.000 description 1
- 239000008298 dragée Substances 0.000 description 1
- 229940000406 drug candidate Drugs 0.000 description 1
- 239000000890 drug combination Substances 0.000 description 1
- 229940126534 drug product Drugs 0.000 description 1
- 230000008482 dysregulation Effects 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 231100000321 erythema Toxicity 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 201000005206 focal segmental glomerulosclerosis Diseases 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- 208000018685 gastrointestinal system disease Diseases 0.000 description 1
- 235000021474 generally recognized As safe (food) Nutrition 0.000 description 1
- 235000021473 generally recognized as safe (food ingredients) Nutrition 0.000 description 1
- 206010061989 glomerulosclerosis Diseases 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 229940124750 glucocorticoid receptor agonist Drugs 0.000 description 1
- 229940075507 glyceryl monostearate Drugs 0.000 description 1
- 208000024908 graft versus host disease Diseases 0.000 description 1
- 239000003979 granulating agent Substances 0.000 description 1
- 239000000665 guar gum Substances 0.000 description 1
- 235000010417 guar gum Nutrition 0.000 description 1
- 229960002154 guar gum Drugs 0.000 description 1
- 229940093915 gynecological organic acid Drugs 0.000 description 1
- 239000007902 hard capsule Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- 150000004678 hydrides Chemical class 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- ZMZDMBWJUHKJPS-UHFFFAOYSA-N hydrogen thiocyanate Natural products SC#N ZMZDMBWJUHKJPS-UHFFFAOYSA-N 0.000 description 1
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 description 1
- 229960003943 hypromellose Drugs 0.000 description 1
- 230000002519 immonomodulatory effect Effects 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 208000015446 immunoglobulin a vasculitis Diseases 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 239000003701 inert diluent Substances 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 208000036971 interstitial lung disease 2 Diseases 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 230000007803 itching Effects 0.000 description 1
- 201000002215 juvenile rheumatoid arthritis Diseases 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 229960001021 lactose monohydrate Drugs 0.000 description 1
- 201000011486 lichen planus Diseases 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 201000010230 macular retinal edema Diseases 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 239000002395 mineralocorticoid Substances 0.000 description 1
- 239000001788 mono and diglycerides of fatty acids Substances 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- 206010028417 myasthenia gravis Diseases 0.000 description 1
- WQEPLUUGTLDZJY-UHFFFAOYSA-N n-Pentadecanoic acid Natural products CCCCCCCCCCCCCCC(O)=O WQEPLUUGTLDZJY-UHFFFAOYSA-N 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 239000000123 paper Substances 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 230000007824 polyneuropathy Effects 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 201000009395 primary hyperaldosteronism Diseases 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 125000001453 quaternary ammonium group Chemical group 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- 230000000552 rheumatic effect Effects 0.000 description 1
- 201000004700 rosacea Diseases 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 210000004706 scrotum Anatomy 0.000 description 1
- 238000009517 secondary packaging Methods 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 230000036303 septic shock Effects 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- AWUCVROLDVIAJX-GSVOUGTGSA-N sn-glycerol 3-phosphate Chemical compound OC[C@@H](O)COP(O)(O)=O AWUCVROLDVIAJX-GSVOUGTGSA-N 0.000 description 1
- 235000011888 snacks Nutrition 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000007901 soft capsule Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 238000005563 spheronization Methods 0.000 description 1
- 238000001694 spray drying Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 239000003270 steroid hormone Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 235000015523 tannic acid Nutrition 0.000 description 1
- 229920002258 tannic acid Polymers 0.000 description 1
- LRBQNJMCXXYXIU-NRMVVENXSA-N tannic acid Chemical compound OC1=C(O)C(O)=CC(C(=O)OC=2C(=C(O)C=C(C=2)C(=O)OC[C@@H]2[C@H]([C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)O2)OC(=O)C=2C=C(OC(=O)C=3C=C(O)C(O)=C(O)C=3)C(O)=C(O)C=2)O)=C1 LRBQNJMCXXYXIU-NRMVVENXSA-N 0.000 description 1
- 229940033123 tannic acid Drugs 0.000 description 1
- 229960001367 tartaric acid Drugs 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 201000004415 tendinitis Diseases 0.000 description 1
- CBXCPBUEXACCNR-UHFFFAOYSA-N tetraethylammonium Chemical compound CC[N+](CC)(CC)CC CBXCPBUEXACCNR-UHFFFAOYSA-N 0.000 description 1
- QEMXHQIAXOOASZ-UHFFFAOYSA-N tetramethylammonium Chemical compound C[N+](C)(C)C QEMXHQIAXOOASZ-UHFFFAOYSA-N 0.000 description 1
- 230000003582 thrombocytopenic effect Effects 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- JUNDJWOLDSCTFK-MTZCLOFQSA-N trimegestone Chemical compound C1CC2=CC(=O)CCC2=C2[C@@H]1[C@@H]1CC[C@@](C(=O)[C@@H](O)C)(C)[C@@]1(C)CC2 JUNDJWOLDSCTFK-MTZCLOFQSA-N 0.000 description 1
- 229950008546 trimegestone Drugs 0.000 description 1
- 201000008827 tuberculosis Diseases 0.000 description 1
- 238000009423 ventilation Methods 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- XOOUIPVCVHRTMJ-UHFFFAOYSA-L zinc stearate Chemical compound [Zn+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O XOOUIPVCVHRTMJ-UHFFFAOYSA-L 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/167—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction with an outer layer or coating comprising drug; with chemically bound drugs or non-active substances on their surface
- A61K9/1676—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction with an outer layer or coating comprising drug; with chemically bound drugs or non-active substances on their surface having a drug-free core with discrete complete coating layer containing drug
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
- A61K9/1652—Polysaccharides, e.g. alginate, cellulose derivatives; Cyclodextrin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5073—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals having two or more different coatings optionally including drug-containing subcoatings
- A61K9/5078—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals having two or more different coatings optionally including drug-containing subcoatings with drug-free core
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5084—Mixtures of one or more drugs in different galenical forms, at least one of which being granules, microcapsules or (coated) microparticles according to A61K9/16 or A61K9/50, e.g. for obtaining a specific release pattern or for combining different drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/06—Antigout agents, e.g. antihyperuricemic or uricosuric agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
- A61P31/06—Antibacterial agents for tuberculosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Epidemiology (AREA)
- Immunology (AREA)
- Pulmonology (AREA)
- Rheumatology (AREA)
- Diabetes (AREA)
- Physical Education & Sports Medicine (AREA)
- Dermatology (AREA)
- Pain & Pain Management (AREA)
- Urology & Nephrology (AREA)
- Ophthalmology & Optometry (AREA)
- Cardiology (AREA)
- Endocrinology (AREA)
- Hematology (AREA)
- Obesity (AREA)
- Oncology (AREA)
- Emergency Medicine (AREA)
- Communicable Diseases (AREA)
- Vascular Medicine (AREA)
- Heart & Thoracic Surgery (AREA)
- Transplantation (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Gastroenterology & Hepatology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US1430707P | 2007-12-17 | 2007-12-17 | |
| US61/014,307 | 2007-12-17 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20100121601A true KR20100121601A (ko) | 2010-11-18 |
Family
ID=40795828
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020107015745A Withdrawn KR20100121601A (ko) | 2007-12-17 | 2008-12-17 | 면역염증성 질환의 치료를 위한 치료요법 |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US20110189293A1 (enExample) |
| EP (1) | EP2231129A4 (enExample) |
| JP (1) | JP2011506607A (enExample) |
| KR (1) | KR20100121601A (enExample) |
| CN (1) | CN101938996A (enExample) |
| AU (1) | AU2008338980A1 (enExample) |
| CA (1) | CA2709561A1 (enExample) |
| IL (1) | IL206435A0 (enExample) |
| MX (1) | MX2010006724A (enExample) |
| NZ (1) | NZ586332A (enExample) |
| WO (1) | WO2009078998A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20140121478A (ko) * | 2012-02-13 | 2014-10-15 | 다이어널 리미티드 | 제어형 하이드로코티손 방출 제형 |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB0808537D0 (en) * | 2008-05-12 | 2008-06-18 | Archimedes Dev Ltd | Compositions |
| CN115282152A (zh) * | 2015-01-28 | 2022-11-04 | 瑞采生技有限公司 | 用于增强PPARγ表现及核转位之化合物及其医疗用途 |
| CN106994128A (zh) * | 2016-01-26 | 2017-08-01 | 上海普瑞得生物技术有限公司 | 17-α羟孕酮化合物在预防和治疗中性粒细胞炎症疾病中的应用 |
| US11896719B2 (en) | 2022-01-24 | 2024-02-13 | Calliditas Therapeutics Ab | Pharmaceutical compositions |
| EP4427752A1 (en) * | 2023-03-09 | 2024-09-11 | Fundacion Instituto De Investigacion Sanitaria Fundacion Jimenez Diaz | Dipyridamole as a novel therapy for muscular myogenesis disorders and inflammatory arthritis |
Family Cites Families (53)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3031450A (en) * | 1959-04-30 | 1962-04-24 | Thomae Gmbh Dr K | Substituted pyrimido-[5, 4-d]-pyrimidines |
| AU496759B2 (en) * | 1972-12-27 | 1978-10-26 | Schering Aktiengesellschaft | New pregnan-21-oic derivatives |
| US4034087A (en) * | 1973-12-17 | 1977-07-05 | The Regents Of The University Of Michigan | Pharmaceutical composition and process of treatment |
| US4107306A (en) * | 1973-01-16 | 1978-08-15 | The Regents Of The University Of Michigan | Process for treating proliferative skin disease |
| US3934036A (en) * | 1975-01-23 | 1976-01-20 | Kyorin Seiyaku Kabushiki Kaisha | N-benzenesulfonyl-β-alanine hydrazide useful as an immunosuppressive agent |
| EP0005911B1 (en) * | 1978-05-26 | 1982-02-17 | Imperial Chemical Industries Plc | Analgesic 6-acylaminotetrahydro-1,3,5-triazine-2,4-dione derivatives, pharmaceutical compositions thereof, and process for their manufacture |
| FR2470599A1 (fr) * | 1979-12-07 | 1981-06-12 | Panoz Donald | Perfectionnements apportes aux procedes de preparation de formes galeniques a action retard et a liberation programmee et formes galeniques de medicaments ainsi obtenus |
| DE3000979A1 (de) * | 1980-01-12 | 1981-07-23 | Dr. Karl Thomae Gmbh, 7950 Biberach | Neue dipyridamol-retardformen und verfahren zu ihrer herstellung |
| BG36086A1 (en) * | 1982-01-19 | 1984-09-14 | Glbov | Method for inducing interferon |
| JPS60174716A (ja) * | 1984-02-21 | 1985-09-09 | Yamanouchi Pharmaceut Co Ltd | パツチ剤 |
| US4879119A (en) * | 1984-02-21 | 1989-11-07 | Yamanouchi Pharmaceutical Co., Ltd. | Patch |
| US4554271A (en) * | 1984-02-24 | 1985-11-19 | The Upjohn Company | Use of high doses of derivatives of 6α-methylprednisolone for the acute treatment of stroke syndrome |
| DE3627423A1 (de) * | 1986-08-13 | 1988-02-18 | Thomae Gmbh Dr K | Arzneimittel enthaltend dipyridamol oder mopidamol und o-acetylsalicylsaeure bzw. deren physiologisch vertraegliche salze, verfahren zu ihrer herstellung und ihre verwendung zur bekaempfung der thrombusbildung |
| AU618517B2 (en) * | 1986-12-23 | 1992-01-02 | Eugene J. Van Scott | Additives enhancing topical actions of therapeutic agents |
| US5668116A (en) * | 1987-03-19 | 1997-09-16 | Anthropharm Pty. Limited | Anti-inflammatory compounds and compositions |
| US5242921A (en) * | 1988-04-27 | 1993-09-07 | Yale University | Compositions and methods for treating cutaneous hyperproliferative disorders |
| US5270047A (en) * | 1991-11-21 | 1993-12-14 | Kauffman Raymond F | Local delivery of dipyridamole for the treatment of proliferative diseases |
| US5474765A (en) * | 1992-03-23 | 1995-12-12 | Ut Sw Medical Ctr At Dallas | Preparation and use of steroid-polyanionic polymer-based conjugates targeted to vascular endothelial cells |
| EG20321A (en) * | 1993-07-21 | 1998-10-31 | Otsuka Pharma Co Ltd | Medical material and process for producing the same |
| US5428040A (en) * | 1993-08-31 | 1995-06-27 | The Du Pont Merck Pharmaceutical Company | Carbocyclic fused-ring quinolinecarboxylic acids useful as immunosuppressive agents |
| DE4430128A1 (de) * | 1994-08-25 | 1996-02-29 | Hoechst Ag | Kombinationspräparat mit immunsuppressiven, kardiovaskulären und cerebralen Wirkungen |
| FR2732223B1 (fr) * | 1995-03-30 | 1997-06-13 | Sanofi Sa | Composition pharmaceutique pour administration transdermique |
| AU5772196A (en) * | 1995-05-19 | 1996-11-29 | Chiroscience Limited | 3,4-disubstituted-phenylsulphonamides and their therapeutic use |
| US6265427B1 (en) * | 1995-06-07 | 2001-07-24 | The Proctor & Gamble Company | Pharmaceutical composition for the method of treating leukemia |
| US6235706B1 (en) * | 1996-09-18 | 2001-05-22 | Merck & Co., Inc. | Combination therapy for reducing the risks associated with cardiovascular disease |
| US6331543B1 (en) * | 1996-11-01 | 2001-12-18 | Nitromed, Inc. | Nitrosated and nitrosylated phosphodiesterase inhibitors, compositions and methods of use |
| US5874437A (en) * | 1996-11-01 | 1999-02-23 | Nitromed, Inc. | Nitrosated and nitrosylated phosphodiesterase inhibitor compounds, compositions and their uses |
| US5958926A (en) * | 1996-11-01 | 1999-09-28 | Nitromed, Inc. | Nitrosated and nitrosylated phosphodiesterase inhibitor compounds, compositions and their uses |
| US5792476A (en) * | 1996-12-19 | 1998-08-11 | Abigo Medical Ab | Sustained release glucocorticoid pharmaceutical composition |
| US6054487A (en) * | 1997-03-18 | 2000-04-25 | Basf Aktiengesellschaft | Methods and compositions for modulating responsiveness to corticosteroids |
| ID23908A (id) * | 1997-06-05 | 2000-05-25 | Lilly Co Eli | Metode pengobatan dari penyakit trombosis |
| US20030077229A1 (en) * | 1997-10-01 | 2003-04-24 | Dugger Harry A. | Buccal, polar and non-polar spray or capsule containing cardiovascular or renal drugs |
| US6372254B1 (en) * | 1998-04-02 | 2002-04-16 | Impax Pharmaceuticals Inc. | Press coated, pulsatile drug delivery system suitable for oral administration |
| US6602521B1 (en) * | 1998-09-29 | 2003-08-05 | Impax Pharmaceuticals, Inc. | Multiplex drug delivery system suitable for oral administration |
| US6677326B2 (en) * | 1999-03-15 | 2004-01-13 | Arakis, Ltd. | Corticosteroid formulation comprising less than 2.5 mg prednisolone for once daily administration |
| US6383471B1 (en) * | 1999-04-06 | 2002-05-07 | Lipocine, Inc. | Compositions and methods for improved delivery of ionizable hydrophobic therapeutic agents |
| WO2001021163A2 (en) * | 1999-09-21 | 2001-03-29 | Emory University | Methods and compositions for treating platelet-related disorders using mpl pathway inhibitory agents |
| US6515010B1 (en) * | 1999-11-15 | 2003-02-04 | Smithkline Beecham Corporation | Carvedilol methanesulfonate |
| AU2599501A (en) * | 1999-12-29 | 2001-07-09 | Advanced Cardiovascular Systems Inc. | Device and active component for inhibiting formation of thrombus-inflammatory cell matrix |
| CA2439691A1 (en) * | 2001-03-02 | 2002-09-12 | Bristol-Myers Squibb Company | Co-administration of melanocortin receptor agonist and phosphodiesterase inhibitor for treatment of cyclic-amp associated disorders |
| US6960357B2 (en) * | 2001-05-25 | 2005-11-01 | Mistral Pharma Inc. | Chemical delivery device |
| DE60233414D1 (de) * | 2001-07-09 | 2009-10-01 | Combinatorx Inc | Kombinationen für die behandlung entzündlicher erkrankungen |
| GB0119848D0 (en) * | 2001-08-15 | 2001-10-10 | Univ Sheffield | Delayed and sustained drug release |
| BR0213100A (pt) * | 2001-10-05 | 2004-11-30 | Combinatorx Inc | Combinações para o tratamento de distúrbios imuniinflamatórios |
| US20040180812A1 (en) * | 2002-12-13 | 2004-09-16 | Technology Center | Methods of treating and preventing proliferative disease |
| WO2004060354A1 (en) * | 2002-12-31 | 2004-07-22 | Augsburger Larry L | Methods for making pharmaceutical dosage forms containing active cushioning components |
| US20050058688A1 (en) * | 2003-02-22 | 2005-03-17 | Lars Boerger | Device for the treatment and prevention of disease, and methods related thereto |
| JP4532868B2 (ja) * | 2003-09-22 | 2010-08-25 | キヤノン株式会社 | 放射線画像処理装置 |
| TW200517114A (en) * | 2003-10-15 | 2005-06-01 | Combinatorx Inc | Methods and reagents for the treatment of immunoinflammatory disorders |
| EP1999626A4 (en) * | 2006-01-26 | 2009-11-25 | Combinatorx Inc | METHODS, COMPOSITIONS AND NEEDS FOR TREATING MUSCULOSKELETAL DISORDERS AND SYMPTOMS ASSOCIATED THEREWITH |
| KR101489401B1 (ko) * | 2006-01-27 | 2015-02-03 | 앱탈리스 파마테크, 인코포레이티드 | 약 염기성 약물과 유기산을 포함하는 약물 전달 시스템 |
| JP2009529053A (ja) * | 2006-03-07 | 2009-08-13 | コンビナトアールエックス インコーポレーティッド | 免疫炎症性障害の処置のための組成物および方法 |
| US20090075955A1 (en) * | 2007-09-19 | 2009-03-19 | Combinatorx, Inc. | Therapeutic regimens for the treatment of immunoinflammatory disorders |
-
2008
- 2008-12-17 EP EP08861473A patent/EP2231129A4/en not_active Withdrawn
- 2008-12-17 KR KR1020107015745A patent/KR20100121601A/ko not_active Withdrawn
- 2008-12-17 CA CA2709561A patent/CA2709561A1/en not_active Abandoned
- 2008-12-17 CN CN2008801264669A patent/CN101938996A/zh active Pending
- 2008-12-17 NZ NZ586332A patent/NZ586332A/en not_active IP Right Cessation
- 2008-12-17 WO PCT/US2008/013805 patent/WO2009078998A1/en not_active Ceased
- 2008-12-17 JP JP2010539470A patent/JP2011506607A/ja active Pending
- 2008-12-17 US US12/808,477 patent/US20110189293A1/en not_active Abandoned
- 2008-12-17 AU AU2008338980A patent/AU2008338980A1/en not_active Abandoned
- 2008-12-17 MX MX2010006724A patent/MX2010006724A/es not_active Application Discontinuation
-
2010
- 2010-06-17 IL IL206435A patent/IL206435A0/en unknown
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20140121478A (ko) * | 2012-02-13 | 2014-10-15 | 다이어널 리미티드 | 제어형 하이드로코티손 방출 제형 |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2008338980A1 (en) | 2009-06-25 |
| CA2709561A1 (en) | 2009-06-25 |
| NZ586332A (en) | 2012-07-27 |
| JP2011506607A (ja) | 2011-03-03 |
| US20110189293A1 (en) | 2011-08-04 |
| EP2231129A1 (en) | 2010-09-29 |
| WO2009078998A1 (en) | 2009-06-25 |
| IL206435A0 (en) | 2010-12-30 |
| EP2231129A4 (en) | 2013-01-30 |
| CN101938996A (zh) | 2011-01-05 |
| MX2010006724A (es) | 2010-09-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| McKeage et al. | Budesonide (Entocort® EC Capsules) A Review of its Therapeutic Use in the Management of Active Crohn’s Disease in Adults | |
| EP1448205B1 (en) | Combinations for the treatment of immunoinflammatory disorders | |
| JP2022508317A (ja) | 先天性副腎過形成を処置するためのcrf1受容体アンタゴニスト、その医薬製剤および固体形態 | |
| JP2023116489A (ja) | 副腎皮質刺激ホルモン放出因子受容体拮抗薬 | |
| WO2001087284A2 (en) | Aldosterone antagonist composition for release during aldosterone acrophase | |
| KR20100121601A (ko) | 면역염증성 질환의 치료를 위한 치료요법 | |
| EP2116249B2 (de) | Pharmazeutische Zusammensetzung enthaltend Gestagene und/oder Estrogene und 5-Methyl-(6S)-tetrahydrofolat | |
| CN113329754A (zh) | 用于施用皮质类固醇的方法 | |
| KR20200040761A (ko) | 코티코트로핀 방출 인자 수용체 길항제 | |
| US20170319513A1 (en) | Delayed release cysteamine bead formulation, and methods of making and using same | |
| RU2619869C2 (ru) | Композиция гидрокортизона с контролируемым высвобождением | |
| US20120245552A1 (en) | Combination Therapy | |
| TW200529861A (en) | Methods and reagents for the treatment of inflammatory disorders | |
| JP2017200942A (ja) | 副腎機能障害の治療 | |
| TW200422042A (en) | Methods and reagents for the treatment of diseases and disorders associated with increased levels of proinflammatory cytokines | |
| US20120309722A1 (en) | Therapeutic regimens for the treatment of immunoinflammatory disorders | |
| EP2978414B1 (en) | Composition comprising hydrocortisone | |
| US20050037074A1 (en) | Delayed and sustained drug release | |
| CN107982230A (zh) | 一种醋酸乌利司他分散片及其制备方法 | |
| HK1150549A (en) | Therapeutic regimens for the treatment of immunoinflammatory disorders | |
| AU2002355903B2 (en) | Delayed and sustained drug release | |
| JP2022000473A (ja) | 先天性副腎過形成を処置するためのcrf1受容体アンタゴニスト、その医薬製剤および固体形態 | |
| McKeage et al. | Budesonide (Entocort [sup®] EC Capsules) Use of tradename is for product identification purposes only, and does not imply endorsement. Unless stated otherwise, the use of budesonide throughout this review refers to Entocort [sup®] EC... | |
| AU2002355903A1 (en) | Delayed and sustained drug release |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20100715 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |