JP7658917B2 - 身体機能回復促進剤 - Google Patents
身体機能回復促進剤 Download PDFInfo
- Publication number
- JP7658917B2 JP7658917B2 JP2021569757A JP2021569757A JP7658917B2 JP 7658917 B2 JP7658917 B2 JP 7658917B2 JP 2021569757 A JP2021569757 A JP 2021569757A JP 2021569757 A JP2021569757 A JP 2021569757A JP 7658917 B2 JP7658917 B2 JP 7658917B2
- Authority
- JP
- Japan
- Prior art keywords
- cerebral infarction
- physical
- subject
- intravascular
- cells
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000011084 recovery Methods 0.000 title claims description 26
- 206010008118 cerebral infarction Diseases 0.000 claims description 92
- 208000026106 cerebrovascular disease Diseases 0.000 claims description 92
- 210000001185 bone marrow Anatomy 0.000 claims description 47
- 230000003863 physical function Effects 0.000 claims description 46
- 210000005087 mononuclear cell Anatomy 0.000 claims description 45
- 230000000694 effects Effects 0.000 claims description 29
- 238000002360 preparation method Methods 0.000 claims description 13
- 210000001428 peripheral nervous system Anatomy 0.000 claims description 6
- 210000003169 central nervous system Anatomy 0.000 claims description 5
- 230000001737 promoting effect Effects 0.000 claims description 5
- 206010008088 Cerebral artery embolism Diseases 0.000 claims description 3
- 230000001269 cardiogenic effect Effects 0.000 claims description 3
- 201000010849 intracranial embolism Diseases 0.000 claims description 3
- 208000004552 Lacunar Stroke Diseases 0.000 claims description 2
- 206010051078 Lacunar infarction Diseases 0.000 claims description 2
- 230000002457 bidirectional effect Effects 0.000 claims description 2
- 238000009472 formulation Methods 0.000 claims 3
- 239000000203 mixture Substances 0.000 claims 3
- 230000003183 myoelectrical effect Effects 0.000 claims 3
- 230000007830 nerve conduction Effects 0.000 claims 2
- 210000004204 blood vessel Anatomy 0.000 claims 1
- 230000005062 synaptic transmission Effects 0.000 claims 1
- 210000004027 cell Anatomy 0.000 description 76
- 210000004556 brain Anatomy 0.000 description 46
- 102100031573 Hematopoietic progenitor cell antigen CD34 Human genes 0.000 description 36
- 101000777663 Homo sapiens Hematopoietic progenitor cell antigen CD34 Proteins 0.000 description 36
- 238000012360 testing method Methods 0.000 description 36
- 210000000578 peripheral nerve Anatomy 0.000 description 34
- 241000699670 Mus sp. Species 0.000 description 25
- 230000006870 function Effects 0.000 description 24
- 238000010172 mouse model Methods 0.000 description 24
- 238000002659 cell therapy Methods 0.000 description 15
- 230000002452 interceptive effect Effects 0.000 description 15
- 238000001356 surgical procedure Methods 0.000 description 15
- 210000000130 stem cell Anatomy 0.000 description 14
- 241000699666 Mus <mouse, genus> Species 0.000 description 13
- 238000011282 treatment Methods 0.000 description 13
- 238000001990 intravenous administration Methods 0.000 description 12
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 11
- 210000003792 cranial nerve Anatomy 0.000 description 10
- 230000007423 decrease Effects 0.000 description 10
- 230000007659 motor function Effects 0.000 description 10
- 210000005036 nerve Anatomy 0.000 description 9
- 230000001225 therapeutic effect Effects 0.000 description 7
- 230000001154 acute effect Effects 0.000 description 6
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 6
- 210000003141 lower extremity Anatomy 0.000 description 6
- 230000007658 neurological function Effects 0.000 description 6
- 208000020431 spinal cord injury Diseases 0.000 description 6
- 238000012549 training Methods 0.000 description 6
- 230000003920 cognitive function Effects 0.000 description 5
- 210000003657 middle cerebral artery Anatomy 0.000 description 5
- 238000011302 passive avoidance test Methods 0.000 description 5
- 210000005259 peripheral blood Anatomy 0.000 description 5
- 239000011886 peripheral blood Substances 0.000 description 5
- 238000010825 rotarod performance test Methods 0.000 description 5
- 230000037152 sensory function Effects 0.000 description 5
- 238000007619 statistical method Methods 0.000 description 5
- 230000000638 stimulation Effects 0.000 description 5
- 241001465754 Metazoa Species 0.000 description 4
- 239000000872 buffer Substances 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- 210000004700 fetal blood Anatomy 0.000 description 4
- 230000001771 impaired effect Effects 0.000 description 4
- 208000023589 ischemic disease Diseases 0.000 description 4
- 210000002414 leg Anatomy 0.000 description 4
- 210000002569 neuron Anatomy 0.000 description 4
- 230000002093 peripheral effect Effects 0.000 description 4
- GEHJYWRUCIMESM-UHFFFAOYSA-L sodium sulfite Chemical compound [Na+].[Na+].[O-]S([O-])=O GEHJYWRUCIMESM-UHFFFAOYSA-L 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 3
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 206010002026 amyotrophic lateral sclerosis Diseases 0.000 description 3
- 210000002798 bone marrow cell Anatomy 0.000 description 3
- 210000001168 carotid artery common Anatomy 0.000 description 3
- 238000005119 centrifugation Methods 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- 230000005484 gravity Effects 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 238000001361 intraarterial administration Methods 0.000 description 3
- 238000012423 maintenance Methods 0.000 description 3
- 230000000926 neurological effect Effects 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 230000004936 stimulating effect Effects 0.000 description 3
- 210000003462 vein Anatomy 0.000 description 3
- WRMNZCZEMHIOCP-UHFFFAOYSA-N 2-phenylethanol Chemical compound OCCC1=CC=CC=C1 WRMNZCZEMHIOCP-UHFFFAOYSA-N 0.000 description 2
- CFKMVGJGLGKFKI-UHFFFAOYSA-N 4-chloro-m-cresol Chemical compound CC1=CC(O)=CC=C1Cl CFKMVGJGLGKFKI-UHFFFAOYSA-N 0.000 description 2
- 206010012289 Dementia Diseases 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 108010025020 Nerve Growth Factor Proteins 0.000 description 2
- 102000007072 Nerve Growth Factors Human genes 0.000 description 2
- 208000012902 Nervous system disease Diseases 0.000 description 2
- 208000025966 Neurological disease Diseases 0.000 description 2
- DFPAKSUCGFBDDF-UHFFFAOYSA-N Nicotinamide Chemical compound NC(=O)C1=CC=CN=C1 DFPAKSUCGFBDDF-UHFFFAOYSA-N 0.000 description 2
- -1 PDGF Proteins 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 208000027418 Wounds and injury Diseases 0.000 description 2
- 239000000654 additive Substances 0.000 description 2
- 239000003963 antioxidant agent Substances 0.000 description 2
- 235000006708 antioxidants Nutrition 0.000 description 2
- 210000004004 carotid artery internal Anatomy 0.000 description 2
- 206010008129 cerebral palsy Diseases 0.000 description 2
- 239000002738 chelating agent Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000035475 disorder Diseases 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 230000003203 everyday effect Effects 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 208000014674 injury Diseases 0.000 description 2
- 238000000034 method Methods 0.000 description 2
- 230000009251 neurologic dysfunction Effects 0.000 description 2
- 208000015015 neurological dysfunction Diseases 0.000 description 2
- 239000003900 neurotrophic factor Substances 0.000 description 2
- 210000002475 olfactory pathway Anatomy 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 235000010265 sodium sulphite Nutrition 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 210000002385 vertebral artery Anatomy 0.000 description 2
- WWFDJIVIDXJAQR-FFWSQMGZSA-N 1-[(2R,3R,4R,5R)-4-[[(2R,3R,4R,5R)-5-(4-amino-5-methyl-2-oxopyrimidin-1-yl)-3-[[(2R,3R,4R,5R)-3-[[(2R,3R,4R,5R)-5-(4-amino-5-methyl-2-oxopyrimidin-1-yl)-3-[[(2R,3R,4R,5R)-3-[[(2R,3R,4R,5R)-3-[[(2R,3R,4R,5R)-3-[[(2R,3R,4R,5R)-5-(4-amino-5-methyl-2-oxopyrimidin-1-yl)-3-[[(2R,3R,4R,5R)-3-[[(2R,3R,4R,5R)-3-[[(2R,3R,4R,5R)-3-[[(2R,3R,4R,5R)-3-[[(2R,3R,4R,5R)-3-[[(2R,3R,4R,5R)-3-[[(2R,3R,4R,5R)-5-(4-amino-5-methyl-2-oxopyrimidin-1-yl)-3-[[(2R,3R,4R,5R)-3-[[(2R,3R,4R,5R)-5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[(2R,3R,4R,5R)-5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxy-4-(2-methoxyethoxy)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-4-(2-methoxyethoxy)oxolan-2-yl]methoxy-sulfanylphosphoryl]oxy-4-(2-methoxyethoxy)-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-4-(2-methoxyethoxy)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-5-(2-amino-6-oxo-1H-purin-9-yl)-4-(2-methoxyethoxy)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-4-(2-methoxyethoxy)-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-5-(6-aminopurin-9-yl)-4-(2-methoxyethoxy)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-5-(6-aminopurin-9-yl)-4-(2-methoxyethoxy)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-4-(2-methoxyethoxy)-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-5-(6-aminopurin-9-yl)-4-(2-methoxyethoxy)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-4-(2-methoxyethoxy)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-4-(2-methoxyethoxy)-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-4-(2-methoxyethoxy)-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-4-(2-methoxyethoxy)-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-4-(2-methoxyethoxy)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-5-(6-aminopurin-9-yl)-4-(2-methoxyethoxy)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-4-(2-methoxyethoxy)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-5-(hydroxymethyl)-3-(2-methoxyethoxy)oxolan-2-yl]-5-methylpyrimidine-2,4-dione Chemical compound COCCO[C@@H]1[C@H](O)[C@@H](COP(O)(=S)O[C@@H]2[C@@H](COP(S)(=O)O[C@@H]3[C@@H](COP(O)(=S)O[C@@H]4[C@@H](COP(O)(=S)O[C@@H]5[C@@H](COP(O)(=S)O[C@@H]6[C@@H](COP(O)(=S)O[C@@H]7[C@@H](COP(O)(=S)O[C@@H]8[C@@H](COP(O)(=S)O[C@@H]9[C@@H](COP(O)(=S)O[C@@H]%10[C@@H](COP(O)(=S)O[C@@H]%11[C@@H](COP(O)(=S)O[C@@H]%12[C@@H](COP(O)(=S)O[C@@H]%13[C@@H](COP(O)(=S)O[C@@H]%14[C@@H](COP(O)(=S)O[C@@H]%15[C@@H](COP(O)(=S)O[C@@H]%16[C@@H](COP(O)(=S)O[C@@H]%17[C@@H](COP(O)(=S)O[C@@H]%18[C@@H](CO)O[C@H]([C@@H]%18OCCOC)n%18cc(C)c(=O)[nH]c%18=O)O[C@H]([C@@H]%17OCCOC)n%17cc(C)c(N)nc%17=O)O[C@H]([C@@H]%16OCCOC)n%16cnc%17c(N)ncnc%16%17)O[C@H]([C@@H]%15OCCOC)n%15cc(C)c(N)nc%15=O)O[C@H]([C@@H]%14OCCOC)n%14cc(C)c(=O)[nH]c%14=O)O[C@H]([C@@H]%13OCCOC)n%13cc(C)c(=O)[nH]c%13=O)O[C@H]([C@@H]%12OCCOC)n%12cc(C)c(=O)[nH]c%12=O)O[C@H]([C@@H]%11OCCOC)n%11cc(C)c(N)nc%11=O)O[C@H]([C@@H]%10OCCOC)n%10cnc%11c(N)ncnc%10%11)O[C@H]([C@@H]9OCCOC)n9cc(C)c(=O)[nH]c9=O)O[C@H]([C@@H]8OCCOC)n8cnc9c(N)ncnc89)O[C@H]([C@@H]7OCCOC)n7cnc8c(N)ncnc78)O[C@H]([C@@H]6OCCOC)n6cc(C)c(=O)[nH]c6=O)O[C@H]([C@@H]5OCCOC)n5cnc6c5nc(N)[nH]c6=O)O[C@H]([C@@H]4OCCOC)n4cc(C)c(N)nc4=O)O[C@H]([C@@H]3OCCOC)n3cc(C)c(=O)[nH]c3=O)O[C@H]([C@@H]2OCCOC)n2cnc3c2nc(N)[nH]c3=O)O[C@H]1n1cnc2c1nc(N)[nH]c2=O WWFDJIVIDXJAQR-FFWSQMGZSA-N 0.000 description 1
- 208000024827 Alzheimer disease Diseases 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 206010002388 Angina unstable Diseases 0.000 description 1
- 102000009840 Angiopoietins Human genes 0.000 description 1
- 108010009906 Angiopoietins Proteins 0.000 description 1
- 208000003343 Antiphospholipid Syndrome Diseases 0.000 description 1
- 208000000575 Arteriosclerosis Obliterans Diseases 0.000 description 1
- 206010003805 Autism Diseases 0.000 description 1
- 208000020706 Autistic disease Diseases 0.000 description 1
- 206010063659 Aversion Diseases 0.000 description 1
- 208000021130 Bilirubin encephalopathy Diseases 0.000 description 1
- 208000024806 Brain atrophy Diseases 0.000 description 1
- 208000033386 Buerger disease Diseases 0.000 description 1
- 206010008025 Cerebellar ataxia Diseases 0.000 description 1
- 208000032544 Cicatrix Diseases 0.000 description 1
- 206010011086 Coronary artery occlusion Diseases 0.000 description 1
- 208000011990 Corticobasal Degeneration Diseases 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 208000012239 Developmental disease Diseases 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 201000010374 Down Syndrome Diseases 0.000 description 1
- 206010013647 Drowning Diseases 0.000 description 1
- ZGTMUACCHSMWAC-UHFFFAOYSA-L EDTA disodium salt (anhydrous) Chemical compound [Na+].[Na+].OC(=O)CN(CC([O-])=O)CCN(CC(O)=O)CC([O-])=O ZGTMUACCHSMWAC-UHFFFAOYSA-L 0.000 description 1
- 208000032027 Essential Thrombocythemia Diseases 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 101150021185 FGF gene Proteins 0.000 description 1
- 206010048744 Fear of falling Diseases 0.000 description 1
- 229920001917 Ficoll Polymers 0.000 description 1
- 201000011240 Frontotemporal dementia Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 208000035895 Guillain-Barré syndrome Diseases 0.000 description 1
- 101000707534 Homo sapiens Serine incorporator 1 Proteins 0.000 description 1
- 208000023105 Huntington disease Diseases 0.000 description 1
- 206010070511 Hypoxic-ischaemic encephalopathy Diseases 0.000 description 1
- PIWKPBJCKXDKJR-UHFFFAOYSA-N Isoflurane Chemical compound FC(F)OC(Cl)C(F)(F)F PIWKPBJCKXDKJR-UHFFFAOYSA-N 0.000 description 1
- 208000011200 Kawasaki disease Diseases 0.000 description 1
- 208000009829 Lewy Body Disease Diseases 0.000 description 1
- 201000002832 Lewy body dementia Diseases 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 102000002274 Matrix Metalloproteinases Human genes 0.000 description 1
- 108010000684 Matrix Metalloproteinases Proteins 0.000 description 1
- 206010049567 Miller Fisher syndrome Diseases 0.000 description 1
- 208000001089 Multiple system atrophy Diseases 0.000 description 1
- 206010028289 Muscle atrophy Diseases 0.000 description 1
- 206010028851 Necrosis Diseases 0.000 description 1
- 208000037212 Neonatal hypoxic and ischemic brain injury Diseases 0.000 description 1
- 208000002193 Pain Diseases 0.000 description 1
- 208000018737 Parkinson disease Diseases 0.000 description 1
- 208000030831 Peripheral arterial occlusive disease Diseases 0.000 description 1
- 108010082093 Placenta Growth Factor Proteins 0.000 description 1
- 102100035194 Placenta growth factor Human genes 0.000 description 1
- 102000001938 Plasminogen Activators Human genes 0.000 description 1
- 108010001014 Plasminogen Activators Proteins 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 238000011579 SCID mouse model Methods 0.000 description 1
- SKZKKFZAGNVIMN-UHFFFAOYSA-N Salicilamide Chemical compound NC(=O)C1=CC=CC=C1O SKZKKFZAGNVIMN-UHFFFAOYSA-N 0.000 description 1
- 102100031707 Serine incorporator 1 Human genes 0.000 description 1
- DWAQJAXMDSEUJJ-UHFFFAOYSA-M Sodium bisulfite Chemical compound [Na+].OS([O-])=O DWAQJAXMDSEUJJ-UHFFFAOYSA-M 0.000 description 1
- 208000010112 Spinocerebellar Degenerations Diseases 0.000 description 1
- 206010042928 Syringomyelia Diseases 0.000 description 1
- 206010043540 Thromboangiitis obliterans Diseases 0.000 description 1
- 201000007023 Thrombotic Thrombocytopenic Purpura Diseases 0.000 description 1
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 1
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 1
- 206010044688 Trisomy 21 Diseases 0.000 description 1
- 208000007814 Unstable Angina Diseases 0.000 description 1
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 1
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 1
- 201000004810 Vascular dementia Diseases 0.000 description 1
- 206010053648 Vascular occlusion Diseases 0.000 description 1
- 239000008351 acetate buffer Substances 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 210000002551 anterior cerebral artery Anatomy 0.000 description 1
- 230000003542 behavioural effect Effects 0.000 description 1
- UREZNYTWGJKWBI-UHFFFAOYSA-M benzethonium chloride Chemical compound [Cl-].C1=CC(C(C)(C)CC(C)(C)C)=CC=C1OCCOCC[N+](C)(C)CC1=CC=CC=C1 UREZNYTWGJKWBI-UHFFFAOYSA-M 0.000 description 1
- 229960001950 benzethonium chloride Drugs 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 1
- 239000004327 boric acid Substances 0.000 description 1
- 230000003925 brain function Effects 0.000 description 1
- 208000009973 brain hypoxia - ischemia Diseases 0.000 description 1
- 210000000133 brain stem Anatomy 0.000 description 1
- 210000001715 carotid artery Anatomy 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 229960002242 chlorocresol Drugs 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 230000015271 coagulation Effects 0.000 description 1
- 238000005345 coagulation Methods 0.000 description 1
- 230000019771 cognition Effects 0.000 description 1
- 238000007887 coronary angioplasty Methods 0.000 description 1
- 210000004351 coronary vessel Anatomy 0.000 description 1
- 210000004748 cultured cell Anatomy 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 210000005258 dental pulp stem cell Anatomy 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 229940012017 ethylenediamine Drugs 0.000 description 1
- 210000003414 extremity Anatomy 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 230000008717 functional decline Effects 0.000 description 1
- 210000001368 germline stem cell Anatomy 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 1
- 210000003714 granulocyte Anatomy 0.000 description 1
- 201000004332 intermediate coronary syndrome Diseases 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 229960002725 isoflurane Drugs 0.000 description 1
- 208000006663 kernicterus Diseases 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 238000002690 local anesthesia Methods 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 206010027175 memory impairment Diseases 0.000 description 1
- 208000001725 mucocutaneous lymph node syndrome Diseases 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- 210000001665 muscle stem cell Anatomy 0.000 description 1
- 201000006938 muscular dystrophy Diseases 0.000 description 1
- 210000002346 musculoskeletal system Anatomy 0.000 description 1
- 229940028444 muse Drugs 0.000 description 1
- 206010028417 myasthenia gravis Diseases 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 230000008035 nerve activity Effects 0.000 description 1
- 230000007383 nerve stimulation Effects 0.000 description 1
- 210000000944 nerve tissue Anatomy 0.000 description 1
- 210000000653 nervous system Anatomy 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 208000018360 neuromuscular disease Diseases 0.000 description 1
- 235000005152 nicotinamide Nutrition 0.000 description 1
- 239000011570 nicotinamide Substances 0.000 description 1
- 229950001015 nusinersen Drugs 0.000 description 1
- 230000036407 pain Effects 0.000 description 1
- 208000033300 perinatal asphyxia Diseases 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 229940127126 plasminogen activator Drugs 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 210000003388 posterior cerebral artery Anatomy 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 201000002212 progressive supranuclear palsy Diseases 0.000 description 1
- 230000001172 regenerating effect Effects 0.000 description 1
- 230000000250 revascularization Effects 0.000 description 1
- 229960000581 salicylamide Drugs 0.000 description 1
- 231100000241 scar Toxicity 0.000 description 1
- 230000037390 scarring Effects 0.000 description 1
- 230000037387 scars Effects 0.000 description 1
- 230000035939 shock Effects 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 210000003625 skull Anatomy 0.000 description 1
- 235000010378 sodium ascorbate Nutrition 0.000 description 1
- PPASLZSBLFJQEF-RKJRWTFHSA-M sodium ascorbate Substances [Na+].OC[C@@H](O)[C@H]1OC(=O)C(O)=C1[O-] PPASLZSBLFJQEF-RKJRWTFHSA-M 0.000 description 1
- 229960005055 sodium ascorbate Drugs 0.000 description 1
- WXMKPNITSTVMEF-UHFFFAOYSA-M sodium benzoate Chemical compound [Na+].[O-]C(=O)C1=CC=CC=C1 WXMKPNITSTVMEF-UHFFFAOYSA-M 0.000 description 1
- 235000010234 sodium benzoate Nutrition 0.000 description 1
- 239000004299 sodium benzoate Substances 0.000 description 1
- 229960003885 sodium benzoate Drugs 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 229940037001 sodium edetate Drugs 0.000 description 1
- 229940079827 sodium hydrogen sulfite Drugs 0.000 description 1
- 235000010267 sodium hydrogen sulphite Nutrition 0.000 description 1
- 229940001482 sodium sulfite Drugs 0.000 description 1
- AKHNMLFCWUSKQB-UHFFFAOYSA-L sodium thiosulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=S AKHNMLFCWUSKQB-UHFFFAOYSA-L 0.000 description 1
- 229940001474 sodium thiosulfate Drugs 0.000 description 1
- 235000019345 sodium thiosulphate Nutrition 0.000 description 1
- PPASLZSBLFJQEF-RXSVEWSESA-M sodium-L-ascorbate Chemical compound [Na+].OC[C@H](O)[C@H]1OC(=O)C(O)=C1[O-] PPASLZSBLFJQEF-RXSVEWSESA-M 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 208000002320 spinal muscular atrophy Diseases 0.000 description 1
- 238000005728 strengthening Methods 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 230000009182 swimming Effects 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 1
- 210000002303 tibia Anatomy 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 210000000689 upper leg Anatomy 0.000 description 1
- 208000021331 vascular occlusion disease Diseases 0.000 description 1
- 210000000216 zygoma Anatomy 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/28—Bone marrow; Haematopoietic stem cells; Mesenchymal stem cells of any origin, e.g. adipose-derived stem cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/48—Reproductive organs
- A61K35/54—Ovaries; Ova; Ovules; Embryos; Foetal cells; Germ cells
- A61K35/545—Embryonic stem cells; Pluripotent stem cells; Induced pluripotent stem cells; Uncharacterised stem cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K2035/124—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells the cells being hematopoietic, bone marrow derived or blood cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/36003—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation of motor muscles, e.g. for walking assistance
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Developmental Biology & Embryology (AREA)
- Cell Biology (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Biomedical Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Zoology (AREA)
- Virology (AREA)
- Epidemiology (AREA)
- Biotechnology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Hematology (AREA)
- Reproductive Health (AREA)
- Urology & Nephrology (AREA)
- Vascular Medicine (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Gynecology & Obstetrics (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Description
脳梗塞モデル(特許第4481706号)は下記の手法で作成した。7週齢の重症複合免疫不全マウス(SCIDマウス;C.B-17/Icr-scid/IcidJcl)をイソフルラン麻酔により全身麻酔し、左頬骨部よりアプローチして左中大脳動脈に直達できるよう頭蓋底に1.5mm程度の穿孔を行った。嗅索を通過した直後(嗅索交差部の遠位側)の左中大脳動脈を、バイポーラ電気メスを用いて凝固させ、凝固後切断することにより、左中大脳動脈を永久に閉塞し、左中大脳動脈領域の皮質に限局する脳梗塞モデルを作製した。本モデルマウスは、脳梗塞作製から4週間後の慢性期に相当する時期においても神経機能障害が残存していたことから、脳梗塞の後遺症モデルマウスとして用いた。なお、脳梗塞作製後は5匹ずつを1ケージで飼育し、軽度ではあるが互いに常に一定の神経刺激が与えられる状態を維持した。それにより筋肉の廃用性筋萎縮などが予防され、一般的な機能維持リハビリテーション治療を全てのマウスがうけていると考えられる状態で飼育した。
投与細胞の準備は以下の方法で行った。骨髄単核球については、マウス大腿骨及び脛骨より骨髄液を採取し、骨髄細胞懸濁液を比重遠心液であるFICOLL液に重層し、スイングロータ式遠心機を用いて600gで20分間遠心した。比重遠心液層の直上に観察される単核球細胞分画をパイペットで採取した。CD34陽性細胞については、GCSF動員ヒト末梢血をStem express社より購入し、CliniMACS Systemを用いて分離したCD34陽性細胞を用いた。
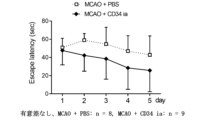
骨髄単核球細胞については、上記に記した脳梗塞後遺症モデルマウスに、1x105個のマウス由来骨髄単核球細胞を尾静脈より投与した(細胞治療群,6匹)。また、比較例として、PBSを尾静脈より投与した脳梗塞後遺症モデルマウスを用いた(PBS群,6匹)。CD34陽性細胞については、脳梗塞後遺症モデルマウスに、1x104個のヒト末梢血CD34陽性細胞を頸動脈より投与した(細胞治療群,9匹)。比較例としては、脳梗塞後遺症モデルマウスに、PBSを尾静脈より投与した群を用いた(PBS群,8匹)。
脳神経機能試験では、脳梗塞後遺症モデルマウスに骨髄細胞を静脈投与した細胞治療群と、脳梗塞後遺症モデルマウスにPBSを静脈投与したPBS群、更に無処置群 (no surgery群,6匹) を比較した。また、インタラクティブ・バイオ・フィードバック理論を具現化するHAL電子装具は、ヒト用のみが存在し、マウスに装着可能な装具が存在しないため、脳および末梢神経の両者に協調して刺激を与える身体運動を用いた。
脳神経機能試験として受動回避試験 (passive avoidance test)を行った。受動回避試験ではメルクエスト社のTMS-2装置を改良し受動的回避実験装置として使用した。明室と暗室のつながった装置であり、明室寸法及び暗室寸法はともに120(w)×120(D)×135(H)であった。動物が明室から暗室に入った際に電気刺激を与えることにより、暗室への進入とショックの恐怖を関連づけて記憶させる試験(受動的回避試験)に使用する装置である。装置の明室側にマウスを入れ、その10秒後に扉を開け、暗室への移動を可能にし、マウスが暗室に移動後に扉を閉め、10秒後に電気刺激(20mA, 3秒間)を加えた。暗室への侵入と電気刺激による痛みである恐怖を関連づけて記憶させ、動物が嫌悪刺激(電気刺激)に対する記憶を取得させた。24時間後に再び明室に入れ、明室に留まった時間(秒)を確認した。
脳神経機能試験としてワイヤハング試験 (wire hang test) を行った。ワイヤハング試験では、1cm幅の網目を有する30cm×30cmの金網の中心にマウスを乗せた。金網を逆さまにひっくり返して、床敷きを深くしたオープンケージから約40cmの高さに設置した支持台に置いた。マウスが金網から落ちるまでの時間を計測し、落ちずにしがみついたままの場合は180秒を測定値とした。この試験は落下の恐怖に関する刺激を脳に与え、金網へのしがみつき運動と強く関連づけさせることにより、脳および末梢神経の両者に同時に刺激を与えた。
試験1
脳神経機能試験として水迷路試験 (water maze test) を行った。水迷路試験は、円形のプールに水をはり、マウスが避難できる足場を水面下1cm程度の場所に作り、マウスが避難場所に到達するまでの時間 (秒) を測定することで行った。この試験では、溺水に関する刺激を脳に与え、空間認知および水泳運動と強く関連づけさせることにより、脳および末梢神経の両者に同時に刺激を与えた。
脳梗塞後遺症モデルマウスに対する、脳および末梢神経の両者に刺激を与える身体運動の効果の検討を行なった。すなわち、水迷路試験装置を用いて水の入った円形のプールで毎日水迷路試験を行い、脳梗塞により障害された運動機能及び記憶力の向上、すなわち脳および末梢神経の両者に協調した刺激を与える身体運動を2、3、4日目に行なった。
試験1
脳神経機能試験としてロータロッドテスト(回転棒テスト:rotarod test)を採用した。ロータロッドテストは、マウスの協調運動と平衡感覚を測定するためのテストである。装置はMK630A(室町機器株式会社)を用いた。300秒間で4 rpm~40 rpmにロッドの回転が加速するように装置をプログラムして、マウスを装置の回転棒に乗せてから落ちるまでの時間(秒)を記録した。
脳梗塞後遺症における脳および末梢神経の両者に協調した刺激を与える身体運動として、2日目、3日目、4日目に、全ての群においてロータロッドテストに使用する装置を用いて毎日5分間の運動訓練を実施した。すなわち、マウスを回転棒に乗せ、回転棒から落下後も再び回転棒へ戻すことにより、落下の恐怖や嫌悪感を脳に与え、協調運動及び平衡感覚の機能向上と強く関連づけさせることにより、脳および末梢神経に協調した刺激を与える運動を3日間実施した。
Claims (6)
- 脳梗塞の後遺症状態にある被験者の血管内に投与され、中枢神経と末梢神経との間の双方向性フィードバックを促す身体運動の効果を促進する身体機能回復促進のための血管内投与製剤であって、
骨髄単核球細胞を含むことを特徴とする身体機能回復促進のための血管内投与製剤。 - 前記骨髄単核球細胞は、静脈投与されることを特徴とする請求項1に記載の血管内投与製剤。
- 前記身体運動は、
前記被験者に対して動力を付与するアクチュエータを有する動作補助装着具と、
前記被験者の生体信号を検出する生体信号センサと、
前記被験者の神経伝達信号及び筋電位信号を、前記生体信号センサにより検出された生体信号から取得する生体信号処理手段と、
前記生体信号処理手段により取得された神経伝達信号及び筋電位信号を用い、前記被験者の意思に従った動力を前記アクチュエータに発生させるための指令信号を生成する随意的制御手段と、
前記随意的制御手段により生成された指令信号に基づいて、前記神経伝達信号に応じた電流及び前記筋電位信号に応じた電流をそれぞれ生成し、前記アクチュエータに供給する駆動電流生成手段と、を備える動作補助装置にて行うことを特徴とする請求項1に記載の血管内投与製剤。 - 前記動作補助装置が前記被験者の運動意図を計測し、実際の運動と理想的運動とのずれを直しながらフィードバック調整するとともに、前記被験者も実際の動作と理想的運動とのずれを感覚し、ずれの変量が最少になるように運動をフィードバック調整することを特徴とする請求項3に記載の血管内投与製剤。
- 前記血管内投与製剤は、前記身体運動の開始時の4週間前から前記身体運動開始時までの期間に前記被験者に投与されることを特徴とする請求項1に記載の血管内投与製剤。
- 前記脳梗塞は、ラクナ梗塞、アテローム血栓症脳梗塞、又は、心原性脳塞栓症の何れかであることを特徴とする請求項1に記載の血管内投与製剤。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020001657 | 2020-01-08 | ||
| JP2020001657 | 2020-01-08 | ||
| PCT/JP2020/044082 WO2021140773A1 (ja) | 2020-01-08 | 2020-11-26 | 身体機能回復促進剤 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JPWO2021140773A1 JPWO2021140773A1 (ja) | 2021-07-15 |
| JPWO2021140773A5 JPWO2021140773A5 (ja) | 2022-09-01 |
| JP7658917B2 true JP7658917B2 (ja) | 2025-04-08 |
Family
ID=76787888
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2021569757A Active JP7658917B2 (ja) | 2020-01-08 | 2020-11-26 | 身体機能回復促進剤 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20230190816A1 (ja) |
| EP (1) | EP4098266A4 (ja) |
| JP (1) | JP7658917B2 (ja) |
| CN (1) | CN115243694A (ja) |
| WO (1) | WO2021140773A1 (ja) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP4599834A1 (en) * | 2022-10-03 | 2025-08-13 | Kidswell Bio Corporation | Agent for treating or preventing cerebral palsy |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005007176A1 (ja) | 2003-06-27 | 2005-01-27 | Renomedix Institute Inc. | 間葉系細胞を有効成分とする体内投与用脳神経疾患治療薬 |
| JP2013172689A (ja) | 2012-02-27 | 2013-09-05 | Foundation For Biomedical Research & Innovation | 脳梗塞後運動機能障害モデル動物及びその使用並びに運動機能回復に対する新規治療法の有効性のスクリーニング方法 |
| JP2015159895A (ja) | 2014-02-26 | 2015-09-07 | 株式会社Clio | 脳梗塞治療のための多能性幹細胞 |
| WO2015174087A1 (ja) | 2014-05-16 | 2015-11-19 | 公益財団法人先端医療振興財団 | 脳梗塞の予防及び/又は治療の為の医薬 |
| JP2016506954A (ja) | 2013-02-06 | 2016-03-07 | Ncメディカルリサーチ株式会社 | 神経変性の治療のための細胞療法 |
| WO2018034023A1 (ja) | 2016-08-18 | 2018-02-22 | 北海道公立大学法人札幌医科大学 | 寿命延長剤 |
| JP3215859U (ja) | 2018-02-06 | 2018-04-19 | 有限会社メルクエスト | 疲労モデル動物作製装置 |
| WO2018139583A1 (ja) | 2017-01-27 | 2018-08-02 | 公益財団法人神戸医療産業都市推進機構 | 単核球分離装置及び単核球分離方法 |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0724053Y2 (ja) * | 1992-07-21 | 1995-06-05 | 有限会社シヅメメディカル | ユニット小型多連式小動物用トレッドミル |
| US20060210544A1 (en) * | 2003-06-27 | 2006-09-21 | Renomedix Institute, Inc. | Internally administered therapeutic agents for cranial nerve diseases comprising mesenchymal cells as an active ingredient |
| JP4481706B2 (ja) * | 2004-03-31 | 2010-06-16 | 独立行政法人科学技術振興機構 | 脳梗塞疾患モデルマウス |
| US20090048553A1 (en) * | 2007-08-15 | 2009-02-19 | Lixian Jiang | Bone marrow transplantation for preventing and treating neurological conditions and diabetes |
| JP5771917B2 (ja) * | 2010-08-04 | 2015-09-02 | 公益財団法人ヒューマンサイエンス振興財団 | 単核球分離管及び単核球分離システム |
| CN103826646A (zh) * | 2011-06-03 | 2014-05-28 | 麦瑟布莱斯特公司 | 治疗中风的影响的方法 |
| JP2013183720A (ja) * | 2012-03-09 | 2013-09-19 | Foundation For Biomedical Research & Innovation | 脳梗塞後うつ病モデル動物及びその使用並びにうつ状態に対する被検薬物及び移植細胞の有効性のスクリーニング方法 |
| EP3763375A4 (en) * | 2018-03-08 | 2021-09-08 | Foundation for Biomedical Research and Innovation at Kobe | CELL PREPARATION FOR THE TREATMENT AND / OR PREVENTION OF ISCHEMIC DISEASE, AND METHOD OF SCREENING A CELL PREPARATION |
-
2020
- 2020-11-26 JP JP2021569757A patent/JP7658917B2/ja active Active
- 2020-11-26 US US17/790,597 patent/US20230190816A1/en active Pending
- 2020-11-26 WO PCT/JP2020/044082 patent/WO2021140773A1/ja not_active Ceased
- 2020-11-26 EP EP20912688.7A patent/EP4098266A4/en active Pending
- 2020-11-26 CN CN202080098243.7A patent/CN115243694A/zh active Pending
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005007176A1 (ja) | 2003-06-27 | 2005-01-27 | Renomedix Institute Inc. | 間葉系細胞を有効成分とする体内投与用脳神経疾患治療薬 |
| JP2013172689A (ja) | 2012-02-27 | 2013-09-05 | Foundation For Biomedical Research & Innovation | 脳梗塞後運動機能障害モデル動物及びその使用並びに運動機能回復に対する新規治療法の有効性のスクリーニング方法 |
| JP2016506954A (ja) | 2013-02-06 | 2016-03-07 | Ncメディカルリサーチ株式会社 | 神経変性の治療のための細胞療法 |
| JP2015159895A (ja) | 2014-02-26 | 2015-09-07 | 株式会社Clio | 脳梗塞治療のための多能性幹細胞 |
| WO2015174087A1 (ja) | 2014-05-16 | 2015-11-19 | 公益財団法人先端医療振興財団 | 脳梗塞の予防及び/又は治療の為の医薬 |
| WO2018034023A1 (ja) | 2016-08-18 | 2018-02-22 | 北海道公立大学法人札幌医科大学 | 寿命延長剤 |
| WO2018139583A1 (ja) | 2017-01-27 | 2018-08-02 | 公益財団法人神戸医療産業都市推進機構 | 単核球分離装置及び単核球分離方法 |
| JP3215859U (ja) | 2018-02-06 | 2018-04-19 | 有限会社メルクエスト | 疲労モデル動物作製装置 |
Non-Patent Citations (6)
| Title |
|---|
| CHEN, Der-Cherng et al.,Intracerebral implantation of autologous peripheral blood stem cells in stroke patients: a randomize,Cell Transplantation,2014年01月29日,Vol.23,pp.1599-1612 |
| IMURA, Takeshi et al.,Interactive effects of cell therapy and rehabilitation realize the full potential of neurogenesis in,Neuroscience Letters,2013年,Vol. 555,pp. 73-78 |
| KUMAR, A. et al.,Bone marrow mononuclear cell therapy in ischaemic stroke: a systematic review,Acta Neurol Scand.,2017年,135(5):496-506,<doi: 10.1111/ane.12666> |
| TAGUCHI, A. et al.,Intravenous Autologous Bone Marrow Mononuclear Cell Transplantation for Stroke: Phase1/2a Clinical Trial in a Homogeneous Group of Stroke Patients,Stem Cells Dev.,2015年,24(19):2207-18,<doi: 10.1089/scd.2015.0160> |
| 日本臨床(増刊) 最新臨床脳卒中学(上) Vol.72, suppl.5 ,第72巻,株式会社日本臨牀社,2014年,第469-472頁 |
| 高木 康志,脳虚血における神経幹細胞研究の歴史と現状,脳循環代謝,2015年,Vol.26,pp.113-117 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP4098266A1 (en) | 2022-12-07 |
| US20230190816A1 (en) | 2023-06-22 |
| EP4098266A4 (en) | 2023-07-19 |
| WO2021140773A1 (ja) | 2021-07-15 |
| JPWO2021140773A1 (ja) | 2021-07-15 |
| CN115243694A (zh) | 2022-10-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Krucoff et al. | Enhancing nervous system recovery through neurobiologics, neural interface training, and neurorehabilitation | |
| Rossignol | Locomotion and its recovery after spinal injury | |
| Yates et al. | Organization of vestibular inputs to nucleus tractus solitarius and adjacent structures in cat brain stem | |
| Herbert et al. | Evidence for a role of the reticulospinal system in recovery of skilled reaching after cortical stroke: initial results from a model of ischemic cortical injury | |
| JP6393283B2 (ja) | 神経運動機能不全の処置のための方法及びシステム | |
| Joshua | Physiotherapy for adult neurological conditions | |
| Eschenko et al. | Behavioral, electrophysiological and histopathological consequences of systemic manganese administration in MEMRI | |
| Pazzaglia et al. | Action observation for neurorehabilitation in apraxia | |
| JP7658917B2 (ja) | 身体機能回復促進剤 | |
| Jakeman et al. | Injured mice at the gym: review, results and considerations for combining chondroitinase and locomotor exercise to enhance recovery after spinal cord injury | |
| Li et al. | Role of α7 nicotinic acetylcholine receptors in the pressor response to intracerebroventricular injection of choline: blockade by amyloid peptide Aβ1-42 | |
| JP7545714B2 (ja) | 神経障害の治療剤 | |
| JP2025092749A (ja) | 神経障害の治療剤 | |
| Schalow | Cure of urinary bladder functions in severe (95%) motoric com-plete cervical spinal cord injury in human | |
| Simcsik et al. | Effects of transcranial direct current stimulation (tdcs) during a virtual reality task in individuals with parkinson’s disease: a randomized, placebo-controlled, and triple-blind Clinical Trial | |
| Kumar et al. | Engagement-sensitive interactive neuromuscular electrical therapy system for post-stroke balance rehabilitation-a concept study | |
| Vladimirovna et al. | Post-Stroke Motor Impairments: The Possibilities of Innovative Technologies and The Results of the Own Research | |
| Danilov et al. | Effects of CN-NINM intervention on chronic stroke rehabilitation: A case study | |
| Duzhar et al. | Duchenne muscular dystrophy: Treatment with fetal progenitor cell transplant | |
| Hu et al. | Advancement in brain–machine interfaces for patients with tetraplegia: neurosurgical perspective | |
| Howell et al. | Mimicking and mitigating the cutaneous response to transcranial electrical stimulation using interferential and combinatorial techniques | |
| HK40095573A (en) | Therapeutic agent for nerve disorders | |
| Bustamante et al. | Electroconvulsive therapy in catatonic patient infected with Sars-Cov-2 | |
| Santamaria et al. | Spinal Cord Injury and Epidural Spinal Cord Stimulation | |
| HK40093348A (en) | Therapeutic agent for nerve disfunction |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20220527 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20231012 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20240827 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20240904 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20241126 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20250318 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20250327 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7658917 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |