JP7125350B2 - ラクトース不耐性を処置する方法 - Google Patents
ラクトース不耐性を処置する方法 Download PDFInfo
- Publication number
- JP7125350B2 JP7125350B2 JP2018544455A JP2018544455A JP7125350B2 JP 7125350 B2 JP7125350 B2 JP 7125350B2 JP 2018544455 A JP2018544455 A JP 2018544455A JP 2018544455 A JP2018544455 A JP 2018544455A JP 7125350 B2 JP7125350 B2 JP 7125350B2
- Authority
- JP
- Japan
- Prior art keywords
- composition
- patient
- acid
- linoleic acid
- conjugated linoleic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/20—Carboxylic acids, e.g. valproic acid having a carboxyl group bound to a chain of seven or more carbon atoms, e.g. stearic, palmitic, arachidic acids
- A61K31/201—Carboxylic acids, e.g. valproic acid having a carboxyl group bound to a chain of seven or more carbon atoms, e.g. stearic, palmitic, arachidic acids having one or two double bonds, e.g. oleic, linoleic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
Landscapes
- Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Coloring Foods And Improving Nutritive Qualities (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662300376P | 2016-02-26 | 2016-02-26 | |
| US62/300,376 | 2016-02-26 | ||
| PCT/EP2017/054526 WO2017144725A1 (en) | 2016-02-26 | 2017-02-27 | Methods of treating lactose intolerance |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2019510745A JP2019510745A (ja) | 2019-04-18 |
| JP2019510745A5 JP2019510745A5 (enExample) | 2020-04-09 |
| JP7125350B2 true JP7125350B2 (ja) | 2022-08-24 |
Family
ID=58162625
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018544455A Active JP7125350B2 (ja) | 2016-02-26 | 2017-02-27 | ラクトース不耐性を処置する方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20190046490A1 (enExample) |
| EP (1) | EP3419616A1 (enExample) |
| JP (1) | JP7125350B2 (enExample) |
| CA (1) | CA3014575A1 (enExample) |
| MA (1) | MA43682A (enExample) |
| WO (1) | WO2017144725A1 (enExample) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2805746B1 (en) | 2009-02-16 | 2020-05-06 | Nogra Pharma Limited | Alkylamido compounds and uses thereof |
| JP6301844B2 (ja) | 2012-02-09 | 2018-03-28 | ノグラ ファーマ リミテッド | 線維症の処置方法 |
| SI3921299T1 (sl) | 2019-02-08 | 2025-03-31 | Nogra Pharma Limited | Postopek izdelave 3-(4'-aminofenil)-2-metoksipropionske kisline in njenih analogov in vmesnih proizvodov |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001525364A (ja) | 1997-12-12 | 2001-12-11 | パーデュー・リサーチ・ファンデーション | 糖尿病を治療する方法及び組成物 |
| WO2007096148A1 (en) | 2006-02-23 | 2007-08-30 | Lipid Nutrition B.V. | Immunoregulation |
| JP2010520166A (ja) | 2007-02-28 | 2010-06-10 | ジュリアーニ インターナショナル リミテッド | 免疫保護性刺激物質としてカチオン性抗菌ペプチド発現を誘導するためのPPAR−γアゴニスト |
| JP2015518483A (ja) | 2012-04-18 | 2015-07-02 | ノグラ ファーマ リミテッド | ラクトース不耐症を処置する方法 |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB8302708D0 (en) * | 1983-02-01 | 1983-03-02 | Efamol Ltd | Pharmaceutical and dietary composition |
| US7015249B1 (en) * | 1997-12-12 | 2006-03-21 | Purdue Research Foundation | Methods and compositions for treating diabetes |
| WO2003043569A2 (en) * | 2001-11-16 | 2003-05-30 | Nutrition 21, Inc. | Trans-10, cis-12 conjugated linoleic acid isomer for the treatment of diseases |
| EP1719543A1 (en) * | 2005-05-04 | 2006-11-08 | Asan Labs., Ltd. | Use of histone deacetylase inhibitors for the treatment of gastrointestinal distress |
| US20090264520A1 (en) * | 2008-04-21 | 2009-10-22 | Asha Lipid Sciences, Inc. | Lipid-containing compositions and methods of use thereof |
| RS56049B1 (sr) | 2008-11-13 | 2017-09-29 | Nogra Pharma Ltd | Antisens kompozicije i postupci za pripremu i upotrebu istih |
| JP6096179B2 (ja) * | 2012-05-10 | 2017-03-15 | 国立大学法人岩手大学 | ラクターゼ活性を有するタンパク質、該タンパク質をコードする遺伝子、該遺伝子を含有する組み換えベクター、形質転換体、及びその製造方法並びに用途 |
| WO2014154683A1 (en) * | 2013-03-26 | 2014-10-02 | Lipid Therapeutics Gmbh | Pharmaceutical formulation comprising phosphatidylcholine for the treatment of ulcerative colitis |
-
2017
- 2017-02-27 WO PCT/EP2017/054526 patent/WO2017144725A1/en not_active Ceased
- 2017-02-27 CA CA3014575A patent/CA3014575A1/en active Pending
- 2017-02-27 US US16/078,555 patent/US20190046490A1/en not_active Abandoned
- 2017-02-27 JP JP2018544455A patent/JP7125350B2/ja active Active
- 2017-02-27 EP EP17707334.3A patent/EP3419616A1/en not_active Withdrawn
- 2017-02-27 MA MA043682A patent/MA43682A/fr unknown
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001525364A (ja) | 1997-12-12 | 2001-12-11 | パーデュー・リサーチ・ファンデーション | 糖尿病を治療する方法及び組成物 |
| WO2007096148A1 (en) | 2006-02-23 | 2007-08-30 | Lipid Nutrition B.V. | Immunoregulation |
| JP2010520166A (ja) | 2007-02-28 | 2010-06-10 | ジュリアーニ インターナショナル リミテッド | 免疫保護性刺激物質としてカチオン性抗菌ペプチド発現を誘導するためのPPAR−γアゴニスト |
| JP2015518483A (ja) | 2012-04-18 | 2015-07-02 | ノグラ ファーマ リミテッド | ラクトース不耐症を処置する方法 |
| JP6211589B2 (ja) | 2012-04-18 | 2017-10-11 | ノグラ ファーマ リミテッド | ラクトース不耐症を処置する方法 |
Non-Patent Citations (4)
| Title |
|---|
| Gastroenterology,2004年,Vol.127,p.777-791 |
| JIANG J,CONJUGATED LINOLEIC ACID IN SWEDISH DAIRY PRODUCTS WITH SPECIAL REFERENCE TO THE 以下備考,INTERNATIONAL DAIRY JOURNAL,英国,1997年11月21日,VOL:7, NR:12,PAGE(S):863 - 867,https://www.sciencedirect.com/science/article/abs/pii/S0958694698000041,MANUFACTURE OF HARD CHEESES |
| LUKOVAC S,M1730 ESSENTIAL FATTY ACID (EFA) DEFICIENCY IN MICE IMPAIRS LACTOSE DIGESTION,GASTROENTEROLOGY,NL,2008年04月,VOL:134, NR:4,PAGE(S):A406-A407,https://www.gastrojournal.org/article/S0016-5085(08)61900-9/pdf |
| Nat. Struct. Mol. Biol.,2008年,Vol.15, No.8,p.1-7(p.865-867),doi:10.1038/nsmb.1447 |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2017144725A1 (en) | 2017-08-31 |
| CA3014575A1 (en) | 2017-08-31 |
| EP3419616A1 (en) | 2019-01-02 |
| US20190046490A1 (en) | 2019-02-14 |
| JP2019510745A (ja) | 2019-04-18 |
| MA43682A (fr) | 2018-11-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20240409487A1 (en) | Compounds, compositions, and methods for the treatment of inflammatory, degenerative, and neurodegenerative diseases | |
| Yao et al. | Prostaglandin‐cytokine crosstalk in chronic inflammation | |
| Shah et al. | Hypoxia-inducible factor augments experimental colitis through an MIF–dependent inflammatory signaling cascade | |
| JP2021107404A (ja) | リソソーム蓄積症治療のための組成物及び方法 | |
| JP5819065B2 (ja) | 認知機能減少の治療のためのゲノム試験およびケトン体生成化合物の使用 | |
| JP2022521454A (ja) | 加齢関連炎症および障害を治療する、予防するまたは逆転させるための組成物および方法 | |
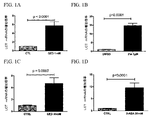
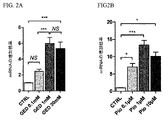
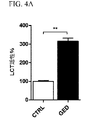
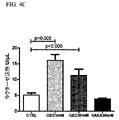
| US9682050B2 (en) | Methods of treating lactose intolerance | |
| JP7125350B2 (ja) | ラクトース不耐性を処置する方法 | |
| WO2014145970A1 (en) | Modulation of regulatory t cells via g-coupled protein receptor 43 | |
| CN104994846A (zh) | 含有羟基化脂肪酸的肠道保护剂 | |
| US20220119768A1 (en) | Method for removing senescent cell, and method for preparing senescent cell | |
| Jin et al. | Regulation of oxidative stress in the intestine of piglets after enterotoxigenic Escherichia coli (ETEC) infection | |
| Araki et al. | Genipin attenuates lipopolysaccharide-induced persistent changes of emotional behaviors and neural activation in the hypothalamic paraventricular nucleus and the central amygdala nucleus | |
| JP2016539098A (ja) | 網膜の血管障害を治療又は予防する方法 | |
| WO2023029093A1 (zh) | 青春双歧杆菌在制备用于治疗炎症相关疾病的药物中的应用 | |
| JP2008501316A (ja) | ラクトバシルスgg由来プロバイオティック化合物及びそれらの使用 | |
| Seya et al. | 1-Methyl-2-undecyl-4 (1H)-quinolone, a derivative of quinolone alkaloid evocarpine, attenuates high phosphate-induced calcification of human aortic valve interstitial cells by inhibiting phosphate cotransporter PiT-1 | |
| Yu et al. | Maintenance of glutamine synthetase expression alleviates endotoxin‐induced sepsis via alpha‐ketoglutarate‐mediated demethylation | |
| Okada et al. | Intermittent fasting prompted recovery from dextran sulfate sodium-induced colitis in mice | |
| Zhao et al. | DDAH-1 via HIF-1 target genes improves cerebral ischemic tolerance after hypoxic preconditioning and middle cerebral artery occlusion-reperfusion | |
| WO2016167622A2 (ko) | 나프토퀴논계 화합물을 유효성분으로 포함하는 췌장염 예방 및 치료용 조성물 | |
| Cavagnero et al. | Lipid-mediated innate lymphoid cell recruitment and activation in aspirin-exacerbated respiratory disease | |
| Wisutsitthiwong et al. | The plant limonoid 7-oxo-deacetoxygedunin inhibits RANKL-induced osteoclastogenesis by suppressing activation of the NF-κB and MAPK pathways | |
| Lin et al. | MiR-155 protects against sepsis-induced cardiomyocyte apoptosis via activation of NO/cGMP signaling pathway by eNOS | |
| Jiang et al. | Diabetes & Metabolism Journal |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200226 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20200226 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20210219 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20210517 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20210715 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210819 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20211124 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20220222 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20220425 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20220712 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20220812 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7125350 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |