JP6092261B2 - 抗ウイルス化合物 - Google Patents
抗ウイルス化合物 Download PDFInfo
- Publication number
- JP6092261B2 JP6092261B2 JP2014558092A JP2014558092A JP6092261B2 JP 6092261 B2 JP6092261 B2 JP 6092261B2 JP 2014558092 A JP2014558092 A JP 2014558092A JP 2014558092 A JP2014558092 A JP 2014558092A JP 6092261 B2 JP6092261 B2 JP 6092261B2
- Authority
- JP
- Japan
- Prior art keywords
- carboxylic acid
- hydroxy
- pyrrolidine
- amino
- naphthalene
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 150000001875 compounds Chemical class 0.000 title claims description 228
- 230000000840 anti-viral effect Effects 0.000 title description 4
- -1 azetidine-1-carbonyl Chemical group 0.000 claims description 84
- 241000711549 Hepacivirus C Species 0.000 claims description 71
- 125000000217 alkyl group Chemical group 0.000 claims description 66
- 238000002360 preparation method Methods 0.000 claims description 64
- SJJCQDRGABAVBB-UHFFFAOYSA-N 1-hydroxy-2-naphthoic acid Chemical compound C1=CC=CC2=C(O)C(C(=O)O)=CC=C21 SJJCQDRGABAVBB-UHFFFAOYSA-N 0.000 claims description 26
- 239000003814 drug Substances 0.000 claims description 20
- 208000015181 infectious disease Diseases 0.000 claims description 19
- 150000003839 salts Chemical class 0.000 claims description 15
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 13
- 239000008194 pharmaceutical composition Substances 0.000 claims description 13
- 125000000592 heterocycloalkyl group Chemical group 0.000 claims description 10
- 125000002950 monocyclic group Chemical group 0.000 claims description 10
- 125000001072 heteroaryl group Chemical group 0.000 claims description 9
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 9
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 9
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 9
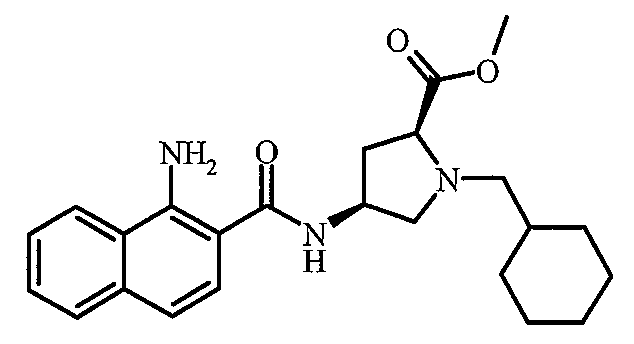
- SHYCNVQYHJEFJJ-RXVVDRJESA-N methyl (2s,4s)-1-(cyclohexylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]pyrrolidine-2-carboxylate Chemical compound C([C@H](C[C@H]1C(=O)OC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1CCCCC1 SHYCNVQYHJEFJJ-RXVVDRJESA-N 0.000 claims description 8
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 claims description 7
- OGQBWKAGSJHPHX-OFNKIYASSA-N ethyl (2s,4r)-1-(cyclohexylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]piperidine-2-carboxylate Chemical compound C([C@H](C[C@H]1C(=O)OCC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)CN1CC1CCCCC1 OGQBWKAGSJHPHX-OFNKIYASSA-N 0.000 claims description 6
- UPEHAAVGSQEUTN-UGKGYDQZSA-N ethyl (2s,4s)-1-(cyclohexylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]pyrrolidine-2-carboxylate Chemical compound C([C@H](C[C@H]1C(=O)OCC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1CCCCC1 UPEHAAVGSQEUTN-UGKGYDQZSA-N 0.000 claims description 6
- XSSMTLLPFQNVPT-UGKGYDQZSA-N n-[(3s,5s)-1-(cyclohexylmethyl)-5-(1,3-oxazol-2-yl)pyrrolidin-3-yl]-1-hydroxynaphthalene-2-carboxamide Chemical compound N1([C@@H](C[C@@H](C1)NC(=O)C1=C(C2=CC=CC=C2C=C1)O)C=1OC=CN=1)CC1CCCCC1 XSSMTLLPFQNVPT-UGKGYDQZSA-N 0.000 claims description 6
- QYUADVSMYFEEKF-REWPJTCUSA-N n-[(3s,5s)-1-(cyclohexylmethyl)-5-(5-methyl-1,3-oxazol-2-yl)pyrrolidin-3-yl]-1-hydroxynaphthalene-2-carboxamide Chemical compound O1C(C)=CN=C1[C@H]1N(CC2CCCCC2)C[C@@H](NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)C1 QYUADVSMYFEEKF-REWPJTCUSA-N 0.000 claims description 6
- WTGHUVUINCKVRI-IGKIAQTJSA-N n-[(3s,5s)-1-(cyclohexylmethyl)-5-(5-phenyl-1,3-oxazol-2-yl)pyrrolidin-3-yl]-1-hydroxynaphthalene-2-carboxamide Chemical compound N1([C@@H](C[C@@H](C1)NC(=O)C1=C(C2=CC=CC=C2C=C1)O)C=1OC(=CN=1)C=1C=CC=CC=1)CC1CCCCC1 WTGHUVUINCKVRI-IGKIAQTJSA-N 0.000 claims description 6
- JDYMJDUILPIMNV-OFVILXPXSA-N n-[(3s,5s)-5-(1,3-benzoxazol-2-yl)-1-(cyclohexylmethyl)pyrrolidin-3-yl]-1-hydroxynaphthalene-2-carboxamide Chemical compound N1([C@@H](C[C@@H](C1)NC(=O)C1=C(C2=CC=CC=C2C=C1)O)C=1OC2=CC=CC=C2N=1)CC1CCCCC1 JDYMJDUILPIMNV-OFVILXPXSA-N 0.000 claims description 6
- VHYRWKSOKODZED-REWPJTCUSA-N (2s,4s)-1-(cyclohexylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]-n-propan-2-ylpyrrolidine-2-carboxamide Chemical compound C([C@H](C[C@H]1C(=O)NC(C)C)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1CCCCC1 VHYRWKSOKODZED-REWPJTCUSA-N 0.000 claims description 5
- ARYYBHUTOQICMG-REWPJTCUSA-N (2s,4s)-1-(cyclohexylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]-n-propylpyrrolidine-2-carboxamide Chemical compound C([C@H](C[C@H]1C(=O)NCCC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1CCCCC1 ARYYBHUTOQICMG-REWPJTCUSA-N 0.000 claims description 5
- PJQOXMCBGCBSAP-PXNSSMCTSA-N (2s,4s)-1-(cyclohexylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]pyrrolidine-2-carboxamide Chemical compound C([C@H](C[C@H]1C(=O)N)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1CCCCC1 PJQOXMCBGCBSAP-PXNSSMCTSA-N 0.000 claims description 5
- IOSNPBQZLZIGPF-UGKGYDQZSA-N (2s,4s)-1-(cyclohexylmethyl)-n-ethyl-4-[(1-hydroxynaphthalene-2-carbonyl)amino]pyrrolidine-2-carboxamide Chemical compound C([C@H](C[C@H]1C(=O)NCC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1CCCCC1 IOSNPBQZLZIGPF-UGKGYDQZSA-N 0.000 claims description 5
- MUOJWWFREYCGSL-IGKIAQTJSA-N (2s,4s)-n-benzyl-1-(cyclohexylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]pyrrolidine-2-carboxamide Chemical compound N1([C@@H](C[C@@H](C1)NC(=O)C1=C(C2=CC=CC=C2C=C1)O)C(=O)NCC=1C=CC=CC=1)CC1CCCCC1 MUOJWWFREYCGSL-IGKIAQTJSA-N 0.000 claims description 5
- 125000002619 bicyclic group Chemical group 0.000 claims description 5
- GTKFJPBDXNIJIQ-PXNSSMCTSA-N ethyl (2s,4s)-1-(cyclobutylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]pyrrolidine-2-carboxylate Chemical compound C([C@H](C[C@H]1C(=O)OCC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1CCC1 GTKFJPBDXNIJIQ-PXNSSMCTSA-N 0.000 claims description 5
- QXXUJQLEZBBIAZ-RXVVDRJESA-N ethyl (2s,4s)-1-(cyclohexanecarbonyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]pyrrolidine-2-carboxylate Chemical compound C([C@H](C[C@H]1C(=O)OCC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1C(=O)C1CCCCC1 QXXUJQLEZBBIAZ-RXVVDRJESA-N 0.000 claims description 5
- VAGPJIQXEAHGMC-ICSRJNTNSA-N ethyl (2s,4s)-1-(cyclohexylmethyl)-4-[(8-hydroxyquinoline-7-carbonyl)amino]pyrrolidine-2-carboxylate Chemical compound C([C@H](C[C@H]1C(=O)OCC)NC(=O)C=2C(=C3N=CC=CC3=CC=2)O)N1CC1CCCCC1 VAGPJIQXEAHGMC-ICSRJNTNSA-N 0.000 claims description 5
- BFGIRDZVXRPASS-RXVVDRJESA-N ethyl (2s,4s)-1-(cyclopentylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]pyrrolidine-2-carboxylate Chemical compound C([C@H](C[C@H]1C(=O)OCC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1CCCC1 BFGIRDZVXRPASS-RXVVDRJESA-N 0.000 claims description 5
- GQLXXYXQTUIJMS-LPHOPBHVSA-N ethyl (2s,4s)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]-1-(2-methylpropyl)pyrrolidine-2-carboxylate Chemical compound C1N(CC(C)C)[C@H](C(=O)OCC)C[C@@H]1NC(=O)C1=CC=C(C=CC=C2)C2=C1O GQLXXYXQTUIJMS-LPHOPBHVSA-N 0.000 claims description 5
- LNPONUAIJFHMOI-KNQAVFIVSA-N methyl (2s,4r)-1-(cyclohexylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]piperidine-2-carboxylate Chemical compound C([C@H](C[C@H]1C(=O)OC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)CN1CC1CCCCC1 LNPONUAIJFHMOI-KNQAVFIVSA-N 0.000 claims description 5
- YMTRIPLCLRRTFO-LPHOPBHVSA-N methyl (2s,4s)-1-(3,3-dimethylbutyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]pyrrolidine-2-carboxylate Chemical compound C1N(CCC(C)(C)C)[C@H](C(=O)OC)C[C@@H]1NC(=O)C1=CC=C(C=CC=C2)C2=C1O YMTRIPLCLRRTFO-LPHOPBHVSA-N 0.000 claims description 5
- IPMKGDUTQHAECL-DFBJGRDBSA-N methyl (2s,4s)-1-(cyclohexylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]-2-methylpyrrolidine-2-carboxylate Chemical compound C([C@H](C[C@@]1(C)C(=O)OC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1CCCCC1 IPMKGDUTQHAECL-DFBJGRDBSA-N 0.000 claims description 5
- GSRXRCSUQTWBTL-RXVVDRJESA-N methyl (2s,4s)-1-benzyl-4-[(1-hydroxynaphthalene-2-carbonyl)amino]pyrrolidine-2-carboxylate Chemical compound C([C@H](C[C@H]1C(=O)OC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1=CC=CC=C1 GSRXRCSUQTWBTL-RXVVDRJESA-N 0.000 claims description 5
- KXOAKKQXRCJPFV-RXVVDRJESA-N methyl (2s,4s)-4-[(1-aminonaphthalene-2-carbonyl)amino]-1-(cyclohexylmethyl)pyrrolidine-2-carboxylate Chemical compound C([C@H](C[C@H]1C(=O)OC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)N)N1CC1CCCCC1 KXOAKKQXRCJPFV-RXVVDRJESA-N 0.000 claims description 5
- LCHGGMYGNYSMPC-URXFXBBRSA-N n-[(3s,5s)-1-(cyclohexylmethyl)-5-(pyrrolidine-1-carbonyl)pyrrolidin-3-yl]-1-hydroxynaphthalene-2-carboxamide Chemical compound N1([C@@H](C[C@@H](C1)NC(=O)C1=C(C2=CC=CC=C2C=C1)O)C(=O)N1CCCC1)CC1CCCCC1 LCHGGMYGNYSMPC-URXFXBBRSA-N 0.000 claims description 5
- ACYWDKZHZQVZRJ-REWPJTCUSA-N tert-butyl (2s,4s)-1-(cyclohexylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]pyrrolidine-2-carboxylate Chemical compound C([C@H](C[C@H]1C(=O)OC(C)(C)C)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1CCCCC1 ACYWDKZHZQVZRJ-REWPJTCUSA-N 0.000 claims description 5
- CUUDCLAJTOWNCK-OFNKIYASSA-N (2s,4r)-1-(cyclohexylmethyl)-n-ethyl-4-[(1-hydroxynaphthalene-2-carbonyl)amino]piperidine-2-carboxamide Chemical compound C([C@H](C[C@H]1C(=O)NCC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)CN1CC1CCCCC1 CUUDCLAJTOWNCK-OFNKIYASSA-N 0.000 claims description 4
- SHYCNVQYHJEFJJ-NQIIRXRSSA-N methyl (2s,4r)-1-(cyclohexylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]pyrrolidine-2-carboxylate Chemical compound C([C@@H](C[C@H]1C(=O)OC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1CCCCC1 SHYCNVQYHJEFJJ-NQIIRXRSSA-N 0.000 claims description 4
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 4
- WAKACCCMWRCPBH-KNQAVFIVSA-N ethyl (2s,4r)-1-benzyl-4-[(1-hydroxynaphthalene-2-carbonyl)amino]pyrrolidine-2-carboxylate Chemical compound C([C@@H](C[C@H]1C(=O)OCC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1=CC=CC=C1 WAKACCCMWRCPBH-KNQAVFIVSA-N 0.000 claims description 3
- SHYCNVQYHJEFJJ-GHTZIAJQSA-N methyl (2r,4s)-1-(cyclohexylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]pyrrolidine-2-carboxylate Chemical compound C([C@H](C[C@@H]1C(=O)OC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1CCCCC1 SHYCNVQYHJEFJJ-GHTZIAJQSA-N 0.000 claims description 3
- 230000002265 prevention Effects 0.000 claims description 3
- 239000013543 active substance Substances 0.000 claims description 2
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 63
- 238000006243 chemical reaction Methods 0.000 description 50
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 45
- 239000000203 mixture Substances 0.000 description 43
- 239000000243 solution Substances 0.000 description 35
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 30
- 239000011541 reaction mixture Substances 0.000 description 28
- 238000000034 method Methods 0.000 description 26
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 26
- YYROPELSRYBVMQ-UHFFFAOYSA-N 4-toluenesulfonyl chloride Chemical compound CC1=CC=C(S(Cl)(=O)=O)C=C1 YYROPELSRYBVMQ-UHFFFAOYSA-N 0.000 description 24
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 24
- 210000004027 cell Anatomy 0.000 description 21
- KVFDZFBHBWTVID-UHFFFAOYSA-N cyclohexanecarbaldehyde Chemical compound O=CC1CCCCC1 KVFDZFBHBWTVID-UHFFFAOYSA-N 0.000 description 19
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 18
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 17
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 17
- 150000001540 azides Chemical class 0.000 description 17
- 235000019439 ethyl acetate Nutrition 0.000 description 17
- 229910052739 hydrogen Inorganic materials 0.000 description 17
- 239000003112 inhibitor Substances 0.000 description 17
- 239000012267 brine Substances 0.000 description 16
- 239000012044 organic layer Substances 0.000 description 16
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 16
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 15
- 239000004480 active ingredient Substances 0.000 description 15
- 150000001408 amides Chemical class 0.000 description 15
- 230000008878 coupling Effects 0.000 description 15
- 238000010168 coupling process Methods 0.000 description 15
- 238000005859 coupling reaction Methods 0.000 description 15
- QQECWEJWXKGJIX-RYUDHWBXSA-N methyl (2s,4s)-4-amino-1-(cyclohexylmethyl)pyrrolidine-2-carboxylate Chemical compound COC(=O)[C@@H]1C[C@H](N)CN1CC1CCCCC1 QQECWEJWXKGJIX-RYUDHWBXSA-N 0.000 description 15
- 239000007787 solid Substances 0.000 description 15
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 14
- 229940079593 drug Drugs 0.000 description 14
- 238000009472 formulation Methods 0.000 description 14
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 14
- 239000012321 sodium triacetoxyborohydride Substances 0.000 description 13
- 108010050904 Interferons Proteins 0.000 description 12
- 102000014150 Interferons Human genes 0.000 description 12
- 238000004440 column chromatography Methods 0.000 description 12
- 239000007788 liquid Substances 0.000 description 12
- 238000006268 reductive amination reaction Methods 0.000 description 12
- 125000001424 substituent group Chemical group 0.000 description 12
- 239000007821 HATU Substances 0.000 description 11
- 238000002648 combination therapy Methods 0.000 description 11
- 239000000843 powder Substances 0.000 description 11
- 239000003826 tablet Substances 0.000 description 11
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 10
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 10
- 125000003118 aryl group Chemical group 0.000 description 10
- 229940079322 interferon Drugs 0.000 description 10
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 9
- 125000004432 carbon atom Chemical group C* 0.000 description 9
- 239000002552 dosage form Substances 0.000 description 9
- YCTNIMOEFJORHQ-KGLIPLIRSA-N (2s,4r)-4-[(2-methylpropan-2-yl)oxy]-1-phenylmethoxycarbonylpyrrolidine-2-carboxylic acid Chemical compound C1[C@H](OC(C)(C)C)C[C@@H](C(O)=O)N1C(=O)OCC1=CC=CC=C1 YCTNIMOEFJORHQ-KGLIPLIRSA-N 0.000 description 8
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 8
- 125000003545 alkoxy group Chemical group 0.000 description 8
- 239000013058 crude material Substances 0.000 description 8
- 150000004702 methyl esters Chemical class 0.000 description 8
- 230000010076 replication Effects 0.000 description 8
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 7
- 239000002253 acid Substances 0.000 description 7
- 239000003443 antiviral agent Substances 0.000 description 7
- YWSJEUCQPPMDPB-STQMWFEESA-N benzyl (2s,4s)-4-amino-2-(1,3-oxazol-2-yl)pyrrolidine-1-carboxylate Chemical compound N1([C@@H](C[C@@H](C1)N)C=1OC=CN=1)C(=O)OCC1=CC=CC=C1 YWSJEUCQPPMDPB-STQMWFEESA-N 0.000 description 7
- 239000002775 capsule Substances 0.000 description 7
- 238000010511 deprotection reaction Methods 0.000 description 7
- 239000002777 nucleoside Substances 0.000 description 7
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 7
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- 0 Oc(c(cccc1)c1cc1)c1C(*[C@@](C1)CN(CC2CCCCC2)[C@@]1c1ncc(-c2ccccc2)[o]1)=O Chemical compound Oc(c(cccc1)c1cc1)c1C(*[C@@](C1)CN(CC2CCCCC2)[C@@]1c1ncc(-c2ccccc2)[o]1)=O 0.000 description 6
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 6
- 229910052799 carbon Inorganic materials 0.000 description 6
- 239000003480 eluent Substances 0.000 description 6
- 125000005843 halogen group Chemical group 0.000 description 6
- VHHHONWQHHHLTI-UHFFFAOYSA-N hexachloroethane Chemical compound ClC(Cl)(Cl)C(Cl)(Cl)Cl VHHHONWQHHHLTI-UHFFFAOYSA-N 0.000 description 6
- 125000002768 hydroxyalkyl group Chemical group 0.000 description 6
- 238000004519 manufacturing process Methods 0.000 description 6
- 239000000463 material Substances 0.000 description 6
- 150000003833 nucleoside derivatives Chemical class 0.000 description 6
- 125000003729 nucleotide group Chemical group 0.000 description 6
- 229920006395 saturated elastomer Polymers 0.000 description 6
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 6
- 239000000725 suspension Substances 0.000 description 6
- FPBGGHWZOPEXMR-RYUDHWBXSA-N COC(=O)[C@@H]1C[C@@H](CN1CC1CCCCC1)N=[N+]=[N-] Chemical compound COC(=O)[C@@H]1C[C@@H](CN1CC1CCCCC1)N=[N+]=[N-] FPBGGHWZOPEXMR-RYUDHWBXSA-N 0.000 description 5
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 5
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 5
- IWUCXVSUMQZMFG-AFCXAGJDSA-N Ribavirin Chemical compound N1=C(C(=O)N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 IWUCXVSUMQZMFG-AFCXAGJDSA-N 0.000 description 5
- 108091027544 Subgenomic mRNA Proteins 0.000 description 5
- 125000002252 acyl group Chemical group 0.000 description 5
- 125000003710 aryl alkyl group Chemical group 0.000 description 5
- 125000004429 atom Chemical group 0.000 description 5
- 239000002585 base Substances 0.000 description 5
- 201000010099 disease Diseases 0.000 description 5
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 5
- 239000000839 emulsion Substances 0.000 description 5
- 125000005842 heteroatom Chemical group 0.000 description 5
- 125000000623 heterocyclic group Chemical group 0.000 description 5
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 5
- ACNBSHOSYNFEJQ-OLZOCXBDSA-N methyl (2s,4r)-4-amino-1-(cyclohexylmethyl)piperidine-2-carboxylate Chemical compound COC(=O)[C@@H]1C[C@H](N)CCN1CC1CCCCC1 ACNBSHOSYNFEJQ-OLZOCXBDSA-N 0.000 description 5
- 125000003884 phenylalkyl group Chemical group 0.000 description 5
- 229920001223 polyethylene glycol Polymers 0.000 description 5
- 238000000746 purification Methods 0.000 description 5
- 229960000329 ribavirin Drugs 0.000 description 5
- HZCAHMRRMINHDJ-DBRKOABJSA-N ribavirin Natural products O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1N=CN=C1 HZCAHMRRMINHDJ-DBRKOABJSA-N 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- RPDXDWLJZVPCQG-PXNSSMCTSA-N (2s,4s)-1-(cyclohexylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]pyrrolidine-2-carboxylic acid Chemical compound C([C@H](C[C@H]1C(=O)O)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1CCCCC1 RPDXDWLJZVPCQG-PXNSSMCTSA-N 0.000 description 4
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 4
- YUJOKPWXAVUIOK-WFASDCNBSA-N 1-hydroxy-n-[(3s,5s)-5-(1,3-oxazol-2-yl)pyrrolidin-3-yl]naphthalene-2-carboxamide Chemical compound C1([C@H]2NC[C@H](C2)NC(=O)C2=C(C3=CC=CC=C3C=C2)O)=NC=CO1 YUJOKPWXAVUIOK-WFASDCNBSA-N 0.000 description 4
- MZMNEDXVUJLQAF-SFYZADRCSA-N 1-o-tert-butyl 2-o-methyl (2s,4r)-4-hydroxypyrrolidine-1,2-dicarboxylate Chemical compound COC(=O)[C@@H]1C[C@@H](O)CN1C(=O)OC(C)(C)C MZMNEDXVUJLQAF-SFYZADRCSA-N 0.000 description 4
- GQHTUMJGOHRCHB-UHFFFAOYSA-N 2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepine Chemical compound C1CCCCN2CCCN=C21 GQHTUMJGOHRCHB-UHFFFAOYSA-N 0.000 description 4
- OZAIFHULBGXAKX-UHFFFAOYSA-N 2-(2-cyanopropan-2-yldiazenyl)-2-methylpropanenitrile Chemical compound N#CC(C)(C)N=NC(C)(C)C#N OZAIFHULBGXAKX-UHFFFAOYSA-N 0.000 description 4
- NHQDETIJWKXCTC-UHFFFAOYSA-N 3-chloroperbenzoic acid Chemical compound OOC(=O)C1=CC=CC(Cl)=C1 NHQDETIJWKXCTC-UHFFFAOYSA-N 0.000 description 4
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 4
- QOSSAOTZNIDXMA-UHFFFAOYSA-N Dicylcohexylcarbodiimide Chemical compound C1CCCCC1N=C=NC1CCCCC1 QOSSAOTZNIDXMA-UHFFFAOYSA-N 0.000 description 4
- GKQLYSROISKDLL-UHFFFAOYSA-N EEDQ Chemical compound C1=CC=C2N(C(=O)OCC)C(OCC)C=CC2=C1 GKQLYSROISKDLL-UHFFFAOYSA-N 0.000 description 4
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 4
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 4
- MZRVEZGGRBJDDB-UHFFFAOYSA-N N-Butyllithium Chemical compound [Li]CCCC MZRVEZGGRBJDDB-UHFFFAOYSA-N 0.000 description 4
- JRNVZBWKYDBUCA-UHFFFAOYSA-N N-chlorosuccinimide Chemical compound ClN1C(=O)CCC1=O JRNVZBWKYDBUCA-UHFFFAOYSA-N 0.000 description 4
- 101800001014 Non-structural protein 5A Proteins 0.000 description 4
- BCDGQXUMWHRQCB-UHFFFAOYSA-N aminoacetone Chemical compound CC(=O)CN BCDGQXUMWHRQCB-UHFFFAOYSA-N 0.000 description 4
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 4
- SHHGSZVXJWSCTB-KBPBESRZSA-N benzyl (2s,4s)-4-amino-2-(5-methyl-1,3-oxazol-2-yl)pyrrolidine-1-carboxylate Chemical compound O1C(C)=CN=C1[C@H]1N(C(=O)OCC=2C=CC=CC=2)C[C@@H](N)C1 SHHGSZVXJWSCTB-KBPBESRZSA-N 0.000 description 4
- 230000037396 body weight Effects 0.000 description 4
- 239000000969 carrier Substances 0.000 description 4
- 239000003153 chemical reaction reagent Substances 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- 229940126214 compound 3 Drugs 0.000 description 4
- 229940125898 compound 5 Drugs 0.000 description 4
- 125000004122 cyclic group Chemical group 0.000 description 4
- CSJLBAMHHLJAAS-UHFFFAOYSA-N diethylaminosulfur trifluoride Chemical compound CCN(CC)S(F)(F)F CSJLBAMHHLJAAS-UHFFFAOYSA-N 0.000 description 4
- 125000004438 haloalkoxy group Chemical group 0.000 description 4
- 125000001188 haloalkyl group Chemical group 0.000 description 4
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 4
- 210000000987 immune system Anatomy 0.000 description 4
- 239000004615 ingredient Substances 0.000 description 4
- 230000002401 inhibitory effect Effects 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 239000012669 liquid formulation Substances 0.000 description 4
- ZCSHNCUQKCANBX-UHFFFAOYSA-N lithium diisopropylamide Chemical compound [Li+].CC(C)[N-]C(C)C ZCSHNCUQKCANBX-UHFFFAOYSA-N 0.000 description 4
- IUYHWZFSGMZEOG-UHFFFAOYSA-M magnesium;propane;chloride Chemical compound [Mg+2].[Cl-].C[CH-]C IUYHWZFSGMZEOG-UHFFFAOYSA-M 0.000 description 4
- LAIKQFWMQSXLNL-JSGCOSHPSA-N methyl (2s,4s)-4-amino-1-(cyclohexylmethyl)-2-methylpyrrolidine-2-carboxylate Chemical compound COC(=O)[C@]1(C)C[C@H](N)CN1CC1CCCCC1 LAIKQFWMQSXLNL-JSGCOSHPSA-N 0.000 description 4
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 4
- KDLHZDBZIXYQEI-UHFFFAOYSA-N palladium Substances [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 4
- LEHBURLTIWGHEM-UHFFFAOYSA-N pyridinium chlorochromate Chemical compound [O-][Cr](Cl)(=O)=O.C1=CC=[NH+]C=C1 LEHBURLTIWGHEM-UHFFFAOYSA-N 0.000 description 4
- 230000002829 reductive effect Effects 0.000 description 4
- 125000006413 ring segment Chemical group 0.000 description 4
- 239000004055 small Interfering RNA Substances 0.000 description 4
- 239000002904 solvent Substances 0.000 description 4
- 239000007921 spray Substances 0.000 description 4
- 239000003381 stabilizer Substances 0.000 description 4
- 239000007858 starting material Substances 0.000 description 4
- 238000007920 subcutaneous administration Methods 0.000 description 4
- 239000000829 suppository Substances 0.000 description 4
- 239000000375 suspending agent Substances 0.000 description 4
- 238000013268 sustained release Methods 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- 239000002562 thickening agent Substances 0.000 description 4
- 230000029812 viral genome replication Effects 0.000 description 4
- IUSARDYWEPUTPN-OZBXUNDUSA-N (2r)-n-[(2s,3r)-4-[[(4s)-6-(2,2-dimethylpropyl)spiro[3,4-dihydropyrano[2,3-b]pyridine-2,1'-cyclobutane]-4-yl]amino]-3-hydroxy-1-[3-(1,3-thiazol-2-yl)phenyl]butan-2-yl]-2-methoxypropanamide Chemical compound C([C@H](NC(=O)[C@@H](C)OC)[C@H](O)CN[C@@H]1C2=CC(CC(C)(C)C)=CN=C2OC2(CCC2)C1)C(C=1)=CC=CC=1C1=NC=CS1 IUSARDYWEPUTPN-OZBXUNDUSA-N 0.000 description 3
- KZPYGQFFRCFCPP-UHFFFAOYSA-N 1,1'-bis(diphenylphosphino)ferrocene Chemical compound [Fe+2].C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1 KZPYGQFFRCFCPP-UHFFFAOYSA-N 0.000 description 3
- FPQQSJJWHUJYPU-UHFFFAOYSA-N 3-(dimethylamino)propyliminomethylidene-ethylazanium;chloride Chemical compound Cl.CCN=C=NCCCN(C)C FPQQSJJWHUJYPU-UHFFFAOYSA-N 0.000 description 3
- FEJUGLKDZJDVFY-UHFFFAOYSA-N 9-borabicyclo(3.3.1)nonane Chemical compound C1CCC2CCCC1B2 FEJUGLKDZJDVFY-UHFFFAOYSA-N 0.000 description 3
- 244000215068 Acacia senegal Species 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- 229920000084 Gum arabic Polymers 0.000 description 3
- 229940124683 HCV polymerase inhibitor Drugs 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 3
- 229930182555 Penicillin Natural products 0.000 description 3
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 3
- 239000002202 Polyethylene glycol Substances 0.000 description 3
- 108010052090 Renilla Luciferases Proteins 0.000 description 3
- 101800001838 Serine protease/helicase NS3 Proteins 0.000 description 3
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 3
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 241000700605 Viruses Species 0.000 description 3
- 235000010489 acacia gum Nutrition 0.000 description 3
- 239000000205 acacia gum Substances 0.000 description 3
- 125000004390 alkyl sulfonyl group Chemical group 0.000 description 3
- 150000001412 amines Chemical class 0.000 description 3
- 125000003277 amino group Chemical group 0.000 description 3
- 239000005557 antagonist Substances 0.000 description 3
- 238000003556 assay Methods 0.000 description 3
- XRWSZZJLZRKHHD-WVWIJVSJSA-N asunaprevir Chemical compound O=C([C@@H]1C[C@H](CN1C(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)(C)C)OC1=NC=C(C2=CC=C(Cl)C=C21)OC)N[C@]1(C(=O)NS(=O)(=O)C2CC2)C[C@H]1C=C XRWSZZJLZRKHHD-WVWIJVSJSA-N 0.000 description 3
- ZWBIYXANKXOQER-SJORKVTESA-N benzyl (2s,4r)-2-(2,2-dimethoxyethylcarbamoyl)-4-[(2-methylpropan-2-yl)oxy]pyrrolidine-1-carboxylate Chemical compound COC(OC)CNC(=O)[C@@H]1C[C@@H](OC(C)(C)C)CN1C(=O)OCC1=CC=CC=C1 ZWBIYXANKXOQER-SJORKVTESA-N 0.000 description 3
- XUIFFUPLSQSDAY-SJORKVTESA-N benzyl (2s,4r)-2-(5-methyl-1,3-oxazol-2-yl)-4-[(2-methylpropan-2-yl)oxy]pyrrolidine-1-carboxylate Chemical compound O1C(C)=CN=C1[C@H]1N(C(=O)OCC=2C=CC=CC=2)C[C@H](OC(C)(C)C)C1 XUIFFUPLSQSDAY-SJORKVTESA-N 0.000 description 3
- JCIGYNJCCWXVIH-CVEARBPZSA-N benzyl (2s,4r)-4-[(2-methylpropan-2-yl)oxy]-2-(1,3-oxazol-2-yl)pyrrolidine-1-carboxylate Chemical compound N1([C@@H](C[C@H](C1)OC(C)(C)C)C=1OC=CN=1)C(=O)OCC1=CC=CC=C1 JCIGYNJCCWXVIH-CVEARBPZSA-N 0.000 description 3
- SGAVICZTSXECLF-CVEARBPZSA-N benzyl (2s,4r)-4-[(2-methylpropan-2-yl)oxy]-2-(2-oxoethylcarbamoyl)pyrrolidine-1-carboxylate Chemical compound C1[C@H](OC(C)(C)C)C[C@@H](C(=O)NCC=O)N1C(=O)OCC1=CC=CC=C1 SGAVICZTSXECLF-CVEARBPZSA-N 0.000 description 3
- PGOFSXPIYREMLC-SJORKVTESA-N benzyl (2s,4r)-4-[(2-methylpropan-2-yl)oxy]-2-(2-oxopropylcarbamoyl)pyrrolidine-1-carboxylate Chemical compound CC(=O)CNC(=O)[C@@H]1C[C@@H](OC(C)(C)C)CN1C(=O)OCC1=CC=CC=C1 PGOFSXPIYREMLC-SJORKVTESA-N 0.000 description 3
- BLLOKDDEKMHUKM-KGLIPLIRSA-N benzyl (2s,4r)-4-hydroxy-2-(5-methyl-1,3-oxazol-2-yl)pyrrolidine-1-carboxylate Chemical compound O1C(C)=CN=C1[C@H]1N(C(=O)OCC=2C=CC=CC=2)C[C@H](O)C1 BLLOKDDEKMHUKM-KGLIPLIRSA-N 0.000 description 3
- FITRDRMZZWVWBP-UGKGYDQZSA-N benzyl (2s,4s)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]-2-(1,3-oxazol-2-yl)pyrrolidine-1-carboxylate Chemical compound N1([C@@H](C[C@@H](C1)NC(=O)C1=C(C2=CC=CC=C2C=C1)O)C=1OC=CN=1)C(=O)OCC1=CC=CC=C1 FITRDRMZZWVWBP-UGKGYDQZSA-N 0.000 description 3
- BOVJWRKSKBWQOQ-HOCLYGCPSA-N benzyl (2s,4s)-4-amino-2-(1,3-benzoxazol-2-yl)pyrrolidine-1-carboxylate Chemical compound N1([C@@H](C[C@@H](C1)N)C=1OC2=CC=CC=C2N=1)C(=O)OCC1=CC=CC=C1 BOVJWRKSKBWQOQ-HOCLYGCPSA-N 0.000 description 3
- KWHYCUXQQBBXOF-ROUUACIJSA-N benzyl (2s,4s)-4-amino-2-(5-phenyl-1,3-oxazol-2-yl)pyrrolidine-1-carboxylate Chemical compound N1([C@@H](C[C@@H](C1)N)C=1OC(=CN=1)C=1C=CC=CC=1)C(=O)OCC1=CC=CC=C1 KWHYCUXQQBBXOF-ROUUACIJSA-N 0.000 description 3
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 229910002091 carbon monoxide Inorganic materials 0.000 description 3
- 230000003833 cell viability Effects 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 239000003086 colorant Substances 0.000 description 3
- 229940125807 compound 37 Drugs 0.000 description 3
- 239000006071 cream Substances 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 239000002270 dispersing agent Substances 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 239000003995 emulsifying agent Substances 0.000 description 3
- 230000032050 esterification Effects 0.000 description 3
- 238000005886 esterification reaction Methods 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- QXBRYYRWIGREQG-QWRGUYRKSA-N ethyl (2s,4s)-4-amino-1-(cyclobutylmethyl)pyrrolidine-2-carboxylate Chemical compound CCOC(=O)[C@@H]1C[C@H](N)CN1CC1CCC1 QXBRYYRWIGREQG-QWRGUYRKSA-N 0.000 description 3
- GWNFQAKCJYEJEW-UHFFFAOYSA-N ethyl 3-[8-[[4-methyl-5-[(3-methyl-4-oxophthalazin-1-yl)methyl]-1,2,4-triazol-3-yl]sulfanyl]octanoylamino]benzoate Chemical compound CCOC(=O)C1=CC(NC(=O)CCCCCCCSC2=NN=C(CC3=NN(C)C(=O)C4=CC=CC=C34)N2C)=CC=C1 GWNFQAKCJYEJEW-UHFFFAOYSA-N 0.000 description 3
- 239000012091 fetal bovine serum Substances 0.000 description 3
- 239000000796 flavoring agent Substances 0.000 description 3
- 235000013355 food flavoring agent Nutrition 0.000 description 3
- 229910052736 halogen Inorganic materials 0.000 description 3
- 150000002367 halogens Chemical class 0.000 description 3
- 150000002430 hydrocarbons Chemical group 0.000 description 3
- 239000001257 hydrogen Substances 0.000 description 3
- NPZTUJOABDZTLV-UHFFFAOYSA-N hydroxybenzotriazole Substances O=C1C=CC=C2NNN=C12 NPZTUJOABDZTLV-UHFFFAOYSA-N 0.000 description 3
- 229940047124 interferons Drugs 0.000 description 3
- 239000010410 layer Substances 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- QQECWEJWXKGJIX-NWDGAFQWSA-N methyl (2r,4s)-4-amino-1-(cyclohexylmethyl)pyrrolidine-2-carboxylate Chemical compound COC(=O)[C@H]1C[C@H](N)CN1CC1CCCCC1 QQECWEJWXKGJIX-NWDGAFQWSA-N 0.000 description 3
- QQECWEJWXKGJIX-NEPJUHHUSA-N methyl (2s,4r)-4-amino-1-(cyclohexylmethyl)pyrrolidine-2-carboxylate Chemical compound COC(=O)[C@@H]1C[C@@H](N)CN1CC1CCCCC1 QQECWEJWXKGJIX-NEPJUHHUSA-N 0.000 description 3
- HHKHKEFCRREQPC-UWVGGRQHSA-N methyl (2s,4s)-4-amino-1-(3,3-dimethylbutyl)pyrrolidine-2-carboxylate Chemical compound COC(=O)[C@@H]1C[C@H](N)CN1CCC(C)(C)C HHKHKEFCRREQPC-UWVGGRQHSA-N 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- QDFOWWUSLOCTAO-REWPJTCUSA-N n-[(3s,5s)-5-(azetidine-1-carbonyl)-1-(cyclohexylmethyl)pyrrolidin-3-yl]-1-hydroxynaphthalene-2-carboxamide Chemical compound N1([C@@H](C[C@@H](C1)NC(=O)C1=C(C2=CC=CC=C2C=C1)O)C(=O)N1CCC1)CC1CCCCC1 QDFOWWUSLOCTAO-REWPJTCUSA-N 0.000 description 3
- 210000003928 nasal cavity Anatomy 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 239000002773 nucleotide Substances 0.000 description 3
- 229940049954 penicillin Drugs 0.000 description 3
- 239000003755 preservative agent Substances 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 238000006722 reduction reaction Methods 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 229960005322 streptomycin Drugs 0.000 description 3
- 239000012730 sustained-release form Substances 0.000 description 3
- DYHSDKLCOJIUFX-UHFFFAOYSA-N tert-butoxycarbonyl anhydride Chemical compound CC(C)(C)OC(=O)OC(=O)OC(C)(C)C DYHSDKLCOJIUFX-UHFFFAOYSA-N 0.000 description 3
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 3
- RWRDLPDLKQPQOW-UHFFFAOYSA-N tetrahydropyrrole Natural products C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 description 3
- 238000002560 therapeutic procedure Methods 0.000 description 3
- 239000003981 vehicle Substances 0.000 description 3
- 230000003612 virological effect Effects 0.000 description 3
- ASGMFNBUXDJWJJ-JLCFBVMHSA-N (1R,3R)-3-[[3-bromo-1-[4-(5-methyl-1,3,4-thiadiazol-2-yl)phenyl]pyrazolo[3,4-d]pyrimidin-6-yl]amino]-N,1-dimethylcyclopentane-1-carboxamide Chemical compound BrC1=NN(C2=NC(=NC=C21)N[C@H]1C[C@@](CC1)(C(=O)NC)C)C1=CC=C(C=C1)C=1SC(=NN=1)C ASGMFNBUXDJWJJ-JLCFBVMHSA-N 0.000 description 2
- UAOUIVVJBYDFKD-XKCDOFEDSA-N (1R,9R,10S,11R,12R,15S,18S,21R)-10,11,21-trihydroxy-8,8-dimethyl-14-methylidene-4-(prop-2-enylamino)-20-oxa-5-thia-3-azahexacyclo[9.7.2.112,15.01,9.02,6.012,18]henicosa-2(6),3-dien-13-one Chemical compound C([C@@H]1[C@@H](O)[C@@]23C(C1=C)=O)C[C@H]2[C@]12C(N=C(NCC=C)S4)=C4CC(C)(C)[C@H]1[C@H](O)[C@]3(O)OC2 UAOUIVVJBYDFKD-XKCDOFEDSA-N 0.000 description 2
- AOSZTAHDEDLTLQ-AZKQZHLXSA-N (1S,2S,4R,8S,9S,11S,12R,13S,19S)-6-[(3-chlorophenyl)methyl]-12,19-difluoro-11-hydroxy-8-(2-hydroxyacetyl)-9,13-dimethyl-6-azapentacyclo[10.8.0.02,9.04,8.013,18]icosa-14,17-dien-16-one Chemical compound C([C@@H]1C[C@H]2[C@H]3[C@]([C@]4(C=CC(=O)C=C4[C@@H](F)C3)C)(F)[C@@H](O)C[C@@]2([C@@]1(C1)C(=O)CO)C)N1CC1=CC=CC(Cl)=C1 AOSZTAHDEDLTLQ-AZKQZHLXSA-N 0.000 description 2
- CYPYTURSJDMMMP-WVCUSYJESA-N (1e,4e)-1,5-diphenylpenta-1,4-dien-3-one;palladium Chemical compound [Pd].[Pd].C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1 CYPYTURSJDMMMP-WVCUSYJESA-N 0.000 description 2
- ABJSOROVZZKJGI-OCYUSGCXSA-N (1r,2r,4r)-2-(4-bromophenyl)-n-[(4-chlorophenyl)-(2-fluoropyridin-4-yl)methyl]-4-morpholin-4-ylcyclohexane-1-carboxamide Chemical compound C1=NC(F)=CC(C(NC(=O)[C@H]2[C@@H](C[C@@H](CC2)N2CCOCC2)C=2C=CC(Br)=CC=2)C=2C=CC(Cl)=CC=2)=C1 ABJSOROVZZKJGI-OCYUSGCXSA-N 0.000 description 2
- SZUVGFMDDVSKSI-WIFOCOSTSA-N (1s,2s,3s,5r)-1-(carboxymethyl)-3,5-bis[(4-phenoxyphenyl)methyl-propylcarbamoyl]cyclopentane-1,2-dicarboxylic acid Chemical compound O=C([C@@H]1[C@@H]([C@](CC(O)=O)([C@H](C(=O)N(CCC)CC=2C=CC(OC=3C=CC=CC=3)=CC=2)C1)C(O)=O)C(O)=O)N(CCC)CC(C=C1)=CC=C1OC1=CC=CC=C1 SZUVGFMDDVSKSI-WIFOCOSTSA-N 0.000 description 2
- MRKKUGDWODATMF-SECBINFHSA-N (2R)-2-[[5-methoxy-2-(methylamino)pyrimidin-4-yl]amino]hexan-1-ol Chemical compound CCCC[C@H](CO)Nc1nc(NC)ncc1OC MRKKUGDWODATMF-SECBINFHSA-N 0.000 description 2
- GHYOCDFICYLMRF-UTIIJYGPSA-N (2S,3R)-N-[(2S)-3-(cyclopenten-1-yl)-1-[(2R)-2-methyloxiran-2-yl]-1-oxopropan-2-yl]-3-hydroxy-3-(4-methoxyphenyl)-2-[[(2S)-2-[(2-morpholin-4-ylacetyl)amino]propanoyl]amino]propanamide Chemical compound C1(=CCCC1)C[C@@H](C(=O)[C@@]1(OC1)C)NC([C@H]([C@@H](C1=CC=C(C=C1)OC)O)NC([C@H](C)NC(CN1CCOCC1)=O)=O)=O GHYOCDFICYLMRF-UTIIJYGPSA-N 0.000 description 2
- YJLIKUSWRSEPSM-WGQQHEPDSA-N (2r,3r,4s,5r)-2-[6-amino-8-[(4-phenylphenyl)methylamino]purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol Chemical compound C=1C=C(C=2C=CC=CC=2)C=CC=1CNC1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O YJLIKUSWRSEPSM-WGQQHEPDSA-N 0.000 description 2
- VIJSPAIQWVPKQZ-BLECARSGSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-acetamido-5-(diaminomethylideneamino)pentanoyl]amino]-4-methylpentanoyl]amino]-4,4-dimethylpentanoyl]amino]-4-methylpentanoyl]amino]propanoyl]amino]-5-(diaminomethylideneamino)pentanoic acid Chemical compound NC(=N)NCCC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(C)=O VIJSPAIQWVPKQZ-BLECARSGSA-N 0.000 description 2
- WWTBZEKOSBFBEM-SPWPXUSOSA-N (2s)-2-[[2-benzyl-3-[hydroxy-[(1r)-2-phenyl-1-(phenylmethoxycarbonylamino)ethyl]phosphoryl]propanoyl]amino]-3-(1h-indol-3-yl)propanoic acid Chemical compound N([C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)O)C(=O)C(CP(O)(=O)[C@H](CC=1C=CC=CC=1)NC(=O)OCC=1C=CC=CC=1)CC1=CC=CC=C1 WWTBZEKOSBFBEM-SPWPXUSOSA-N 0.000 description 2
- STBLNCCBQMHSRC-BATDWUPUSA-N (2s)-n-[(3s,4s)-5-acetyl-7-cyano-4-methyl-1-[(2-methylnaphthalen-1-yl)methyl]-2-oxo-3,4-dihydro-1,5-benzodiazepin-3-yl]-2-(methylamino)propanamide Chemical compound O=C1[C@@H](NC(=O)[C@H](C)NC)[C@H](C)N(C(C)=O)C2=CC(C#N)=CC=C2N1CC1=C(C)C=CC2=CC=CC=C12 STBLNCCBQMHSRC-BATDWUPUSA-N 0.000 description 2
- BWMXTCRKGOQHCO-OCCSQVGLSA-N (2s,4r)-4-(4-methylphenyl)sulfonyloxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid Chemical compound C1=CC(C)=CC=C1S(=O)(=O)O[C@H]1CN(C(=O)OC(C)(C)C)[C@H](C(O)=O)C1 BWMXTCRKGOQHCO-OCCSQVGLSA-N 0.000 description 2
- QFLWZFQWSBQYPS-AWRAUJHKSA-N (3S)-3-[[(2S)-2-[[(2S)-2-[5-[(3aS,6aR)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-[1-bis(4-chlorophenoxy)phosphorylbutylamino]-4-oxobutanoic acid Chemical compound CCCC(NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)C(C)C)P(=O)(Oc1ccc(Cl)cc1)Oc1ccc(Cl)cc1 QFLWZFQWSBQYPS-AWRAUJHKSA-N 0.000 description 2
- FNHHVPPSBFQMEL-KQHDFZBMSA-N (3S)-5-N-[(1S,5R)-3-hydroxy-6-bicyclo[3.1.0]hexanyl]-7-N,3-dimethyl-3-phenyl-2H-1-benzofuran-5,7-dicarboxamide Chemical compound CNC(=O)c1cc(cc2c1OC[C@@]2(C)c1ccccc1)C(=O)NC1[C@H]2CC(O)C[C@@H]12 FNHHVPPSBFQMEL-KQHDFZBMSA-N 0.000 description 2
- IWZSHWBGHQBIML-ZGGLMWTQSA-N (3S,8S,10R,13S,14S,17S)-17-isoquinolin-7-yl-N,N,10,13-tetramethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-amine Chemical compound CN(C)[C@H]1CC[C@]2(C)C3CC[C@@]4(C)[C@@H](CC[C@@H]4c4ccc5ccncc5c4)[C@@H]3CC=C2C1 IWZSHWBGHQBIML-ZGGLMWTQSA-N 0.000 description 2
- UDQTXCHQKHIQMH-KYGLGHNPSA-N (3ar,5s,6s,7r,7ar)-5-(difluoromethyl)-2-(ethylamino)-5,6,7,7a-tetrahydro-3ah-pyrano[3,2-d][1,3]thiazole-6,7-diol Chemical compound S1C(NCC)=N[C@H]2[C@@H]1O[C@H](C(F)F)[C@@H](O)[C@@H]2O UDQTXCHQKHIQMH-KYGLGHNPSA-N 0.000 description 2
- OOKAZRDERJMRCJ-KOUAFAAESA-N (3r)-7-[(1s,2s,4ar,6s,8s)-2,6-dimethyl-8-[(2s)-2-methylbutanoyl]oxy-1,2,4a,5,6,7,8,8a-octahydronaphthalen-1-yl]-3-hydroxy-5-oxoheptanoic acid Chemical compound C1=C[C@H](C)[C@H](CCC(=O)C[C@@H](O)CC(O)=O)C2[C@@H](OC(=O)[C@@H](C)CC)C[C@@H](C)C[C@@H]21 OOKAZRDERJMRCJ-KOUAFAAESA-N 0.000 description 2
- HUWSZNZAROKDRZ-RRLWZMAJSA-N (3r,4r)-3-azaniumyl-5-[[(2s,3r)-1-[(2s)-2,3-dicarboxypyrrolidin-1-yl]-3-methyl-1-oxopentan-2-yl]amino]-5-oxo-4-sulfanylpentane-1-sulfonate Chemical compound OS(=O)(=O)CC[C@@H](N)[C@@H](S)C(=O)N[C@@H]([C@H](C)CC)C(=O)N1CCC(C(O)=O)[C@H]1C(O)=O HUWSZNZAROKDRZ-RRLWZMAJSA-N 0.000 description 2
- MPDDTAJMJCESGV-CTUHWIOQSA-M (3r,5r)-7-[2-(4-fluorophenyl)-5-[methyl-[(1r)-1-phenylethyl]carbamoyl]-4-propan-2-ylpyrazol-3-yl]-3,5-dihydroxyheptanoate Chemical compound C1([C@@H](C)N(C)C(=O)C2=NN(C(CC[C@@H](O)C[C@@H](O)CC([O-])=O)=C2C(C)C)C=2C=CC(F)=CC=2)=CC=CC=C1 MPDDTAJMJCESGV-CTUHWIOQSA-M 0.000 description 2
- YQOLEILXOBUDMU-KRWDZBQOSA-N (4R)-5-[(6-bromo-3-methyl-2-pyrrolidin-1-ylquinoline-4-carbonyl)amino]-4-(2-chlorophenyl)pentanoic acid Chemical compound CC1=C(C2=C(C=CC(=C2)Br)N=C1N3CCCC3)C(=O)NC[C@H](CCC(=O)O)C4=CC=CC=C4Cl YQOLEILXOBUDMU-KRWDZBQOSA-N 0.000 description 2
- STPKWKPURVSAJF-LJEWAXOPSA-N (4r,5r)-5-[4-[[4-(1-aza-4-azoniabicyclo[2.2.2]octan-4-ylmethyl)phenyl]methoxy]phenyl]-3,3-dibutyl-7-(dimethylamino)-1,1-dioxo-4,5-dihydro-2h-1$l^{6}-benzothiepin-4-ol Chemical compound O[C@H]1C(CCCC)(CCCC)CS(=O)(=O)C2=CC=C(N(C)C)C=C2[C@H]1C(C=C1)=CC=C1OCC(C=C1)=CC=C1C[N+]1(CC2)CCN2CC1 STPKWKPURVSAJF-LJEWAXOPSA-N 0.000 description 2
- DEVSOMFAQLZNKR-RJRFIUFISA-N (z)-3-[3-[3,5-bis(trifluoromethyl)phenyl]-1,2,4-triazol-1-yl]-n'-pyrazin-2-ylprop-2-enehydrazide Chemical compound FC(F)(F)C1=CC(C(F)(F)F)=CC(C2=NN(\C=C/C(=O)NNC=3N=CC=NC=3)C=N2)=C1 DEVSOMFAQLZNKR-RJRFIUFISA-N 0.000 description 2
- WSLDOOZREJYCGB-UHFFFAOYSA-N 1,2-Dichloroethane Chemical compound ClCCCl WSLDOOZREJYCGB-UHFFFAOYSA-N 0.000 description 2
- KKHFRAFPESRGGD-UHFFFAOYSA-N 1,3-dimethyl-7-[3-(n-methylanilino)propyl]purine-2,6-dione Chemical compound C1=NC=2N(C)C(=O)N(C)C(=O)C=2N1CCCN(C)C1=CC=CC=C1 KKHFRAFPESRGGD-UHFFFAOYSA-N 0.000 description 2
- KQZLRWGGWXJPOS-NLFPWZOASA-N 1-[(1R)-1-(2,4-dichlorophenyl)ethyl]-6-[(4S,5R)-4-[(2S)-2-(hydroxymethyl)pyrrolidin-1-yl]-5-methylcyclohexen-1-yl]pyrazolo[3,4-b]pyrazine-3-carbonitrile Chemical compound ClC1=C(C=CC(=C1)Cl)[C@@H](C)N1N=C(C=2C1=NC(=CN=2)C1=CC[C@@H]([C@@H](C1)C)N1[C@@H](CCC1)CO)C#N KQZLRWGGWXJPOS-NLFPWZOASA-N 0.000 description 2
- WZZBNLYBHUDSHF-DHLKQENFSA-N 1-[(3s,4s)-4-[8-(2-chloro-4-pyrimidin-2-yloxyphenyl)-7-fluoro-2-methylimidazo[4,5-c]quinolin-1-yl]-3-fluoropiperidin-1-yl]-2-hydroxyethanone Chemical compound CC1=NC2=CN=C3C=C(F)C(C=4C(=CC(OC=5N=CC=CN=5)=CC=4)Cl)=CC3=C2N1[C@H]1CCN(C(=O)CO)C[C@@H]1F WZZBNLYBHUDSHF-DHLKQENFSA-N 0.000 description 2
- ONBQEOIKXPHGMB-VBSBHUPXSA-N 1-[2-[(2s,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-4,6-dihydroxyphenyl]-3-(4-hydroxyphenyl)propan-1-one Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1OC1=CC(O)=CC(O)=C1C(=O)CCC1=CC=C(O)C=C1 ONBQEOIKXPHGMB-VBSBHUPXSA-N 0.000 description 2
- UNILWMWFPHPYOR-KXEYIPSPSA-M 1-[6-[2-[3-[3-[3-[2-[2-[3-[[2-[2-[[(2r)-1-[[2-[[(2r)-1-[3-[2-[2-[3-[[2-(2-amino-2-oxoethoxy)acetyl]amino]propoxy]ethoxy]ethoxy]propylamino]-3-hydroxy-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-3-[(2r)-2,3-di(hexadecanoyloxy)propyl]sulfanyl-1-oxopropan-2-yl Chemical compound O=C1C(SCCC(=O)NCCCOCCOCCOCCCNC(=O)COCC(=O)N[C@@H](CSC[C@@H](COC(=O)CCCCCCCCCCCCCCC)OC(=O)CCCCCCCCCCCCCCC)C(=O)NCC(=O)N[C@H](CO)C(=O)NCCCOCCOCCOCCCNC(=O)COCC(N)=O)CC(=O)N1CCNC(=O)CCCCCN\1C2=CC=C(S([O-])(=O)=O)C=C2CC/1=C/C=C/C=C/C1=[N+](CC)C2=CC=C(S([O-])(=O)=O)C=C2C1 UNILWMWFPHPYOR-KXEYIPSPSA-M 0.000 description 2
- QXOGPTXQGKQSJT-UHFFFAOYSA-N 1-amino-4-[4-(3,4-dimethylphenyl)sulfanylanilino]-9,10-dioxoanthracene-2-sulfonic acid Chemical compound Cc1ccc(Sc2ccc(Nc3cc(c(N)c4C(=O)c5ccccc5C(=O)c34)S(O)(=O)=O)cc2)cc1C QXOGPTXQGKQSJT-UHFFFAOYSA-N 0.000 description 2
- VFFRLRQQWXGEBX-UHFFFAOYSA-N 1-aminonaphthalene-2-carboxylic acid Chemical compound C1=CC=C2C(N)=C(C(O)=O)C=CC2=C1 VFFRLRQQWXGEBX-UHFFFAOYSA-N 0.000 description 2
- AXTGDCSMTYGJND-UHFFFAOYSA-N 1-dodecylazepan-2-one Chemical compound CCCCCCCCCCCCN1CCCCCC1=O AXTGDCSMTYGJND-UHFFFAOYSA-N 0.000 description 2
- JMHURTPKAKXCGY-BBRMVZONSA-N 1-hydroxy-n-[(3s,5s)-5-(5-methyl-1,3-oxazol-2-yl)pyrrolidin-3-yl]naphthalene-2-carboxamide Chemical compound O1C(C)=CN=C1[C@H]1NC[C@@H](NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)C1 JMHURTPKAKXCGY-BBRMVZONSA-N 0.000 description 2
- IMOZBKVIYJMWFA-PXNSSMCTSA-N 1-hydroxy-n-[(3s,5s)-5-(5-phenyl-1,3-oxazol-2-yl)pyrrolidin-3-yl]naphthalene-2-carboxamide Chemical compound O1C([C@H]2NC[C@H](C2)NC(=O)C2=C(C3=CC=CC=C3C=C2)O)=NC=C1C1=CC=CC=C1 IMOZBKVIYJMWFA-PXNSSMCTSA-N 0.000 description 2
- BJQMWOSPZUZYNW-YUMQZZPRSA-N 1-o-tert-butyl 2-o-methyl (2s,4s)-4-azidopyrrolidine-1,2-dicarboxylate Chemical compound COC(=O)[C@@H]1C[C@H](N=[N+]=[N-])CN1C(=O)OC(C)(C)C BJQMWOSPZUZYNW-YUMQZZPRSA-N 0.000 description 2
- RNMVWSAJMIKMDY-IUCAKERBSA-N 1-o-tert-butyl 2-o-methyl (2s,4s)-4-hydroxypiperidine-1,2-dicarboxylate Chemical compound COC(=O)[C@@H]1C[C@@H](O)CCN1C(=O)OC(C)(C)C RNMVWSAJMIKMDY-IUCAKERBSA-N 0.000 description 2
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 2
- QKWWDTYDYOFRJL-UHFFFAOYSA-N 2,2-dimethoxyethanamine Chemical compound COC(CN)OC QKWWDTYDYOFRJL-UHFFFAOYSA-N 0.000 description 2
- HZNVUJQVZSTENZ-UHFFFAOYSA-N 2,3-dichloro-5,6-dicyano-1,4-benzoquinone Chemical compound ClC1=C(Cl)C(=O)C(C#N)=C(C#N)C1=O HZNVUJQVZSTENZ-UHFFFAOYSA-N 0.000 description 2
- WGFNXGPBPIJYLI-UHFFFAOYSA-N 2,6-difluoro-3-[(3-fluorophenyl)sulfonylamino]-n-(3-methoxy-1h-pyrazolo[3,4-b]pyridin-5-yl)benzamide Chemical compound C1=C2C(OC)=NNC2=NC=C1NC(=O)C(C=1F)=C(F)C=CC=1NS(=O)(=O)C1=CC=CC(F)=C1 WGFNXGPBPIJYLI-UHFFFAOYSA-N 0.000 description 2
- FQMZXMVHHKXGTM-UHFFFAOYSA-N 2-(1-adamantyl)-n-[2-[2-(2-hydroxyethylamino)ethylamino]quinolin-5-yl]acetamide Chemical compound C1C(C2)CC(C3)CC2CC13CC(=O)NC1=CC=CC2=NC(NCCNCCO)=CC=C21 FQMZXMVHHKXGTM-UHFFFAOYSA-N 0.000 description 2
- VCUXVXLUOHDHKK-UHFFFAOYSA-N 2-(2-aminopyrimidin-4-yl)-4-(2-chloro-4-methoxyphenyl)-1,3-thiazole-5-carboxamide Chemical compound ClC1=CC(OC)=CC=C1C1=C(C(N)=O)SC(C=2N=C(N)N=CC=2)=N1 VCUXVXLUOHDHKK-UHFFFAOYSA-N 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- PYRKKGOKRMZEIT-UHFFFAOYSA-N 2-[6-(2-cyclopropylethoxy)-9-(2-hydroxy-2-methylpropyl)-1h-phenanthro[9,10-d]imidazol-2-yl]-5-fluorobenzene-1,3-dicarbonitrile Chemical compound C1=C2C3=CC(CC(C)(O)C)=CC=C3C=3NC(C=4C(=CC(F)=CC=4C#N)C#N)=NC=3C2=CC=C1OCCC1CC1 PYRKKGOKRMZEIT-UHFFFAOYSA-N 0.000 description 2
- FMKGJQHNYMWDFJ-CVEARBPZSA-N 2-[[4-(2,2-difluoropropoxy)pyrimidin-5-yl]methylamino]-4-[[(1R,4S)-4-hydroxy-3,3-dimethylcyclohexyl]amino]pyrimidine-5-carbonitrile Chemical compound FC(COC1=NC=NC=C1CNC1=NC=C(C(=N1)N[C@H]1CC([C@H](CC1)O)(C)C)C#N)(C)F FMKGJQHNYMWDFJ-CVEARBPZSA-N 0.000 description 2
- VVCMGAUPZIKYTH-VGHSCWAPSA-N 2-acetyloxybenzoic acid;[(2s,3r)-4-(dimethylamino)-3-methyl-1,2-diphenylbutan-2-yl] propanoate;1,3,7-trimethylpurine-2,6-dione Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O.CN1C(=O)N(C)C(=O)C2=C1N=CN2C.C([C@](OC(=O)CC)([C@H](C)CN(C)C)C=1C=CC=CC=1)C1=CC=CC=C1 VVCMGAUPZIKYTH-VGHSCWAPSA-N 0.000 description 2
- YSUIQYOGTINQIN-UZFYAQMZSA-N 2-amino-9-[(1S,6R,8R,9S,10R,15R,17R,18R)-8-(6-aminopurin-9-yl)-9,18-difluoro-3,12-dihydroxy-3,12-bis(sulfanylidene)-2,4,7,11,13,16-hexaoxa-3lambda5,12lambda5-diphosphatricyclo[13.2.1.06,10]octadecan-17-yl]-1H-purin-6-one Chemical compound NC1=NC2=C(N=CN2[C@@H]2O[C@@H]3COP(S)(=O)O[C@@H]4[C@@H](COP(S)(=O)O[C@@H]2[C@@H]3F)O[C@H]([C@H]4F)N2C=NC3=C2N=CN=C3N)C(=O)N1 YSUIQYOGTINQIN-UZFYAQMZSA-N 0.000 description 2
- TVTJUIAKQFIXCE-HUKYDQBMSA-N 2-amino-9-[(2R,3S,4S,5R)-4-fluoro-3-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-7-prop-2-ynyl-1H-purine-6,8-dione Chemical compound NC=1NC(C=2N(C(N(C=2N=1)[C@@H]1O[C@@H]([C@H]([C@H]1O)F)CO)=O)CC#C)=O TVTJUIAKQFIXCE-HUKYDQBMSA-N 0.000 description 2
- LFOIDLOIBZFWDO-UHFFFAOYSA-N 2-methoxy-6-[6-methoxy-4-[(3-phenylmethoxyphenyl)methoxy]-1-benzofuran-2-yl]imidazo[2,1-b][1,3,4]thiadiazole Chemical compound N1=C2SC(OC)=NN2C=C1C(OC1=CC(OC)=C2)=CC1=C2OCC(C=1)=CC=CC=1OCC1=CC=CC=C1 LFOIDLOIBZFWDO-UHFFFAOYSA-N 0.000 description 2
- JWUJQDFVADABEY-UHFFFAOYSA-N 2-methyltetrahydrofuran Chemical compound CC1CCCO1 JWUJQDFVADABEY-UHFFFAOYSA-N 0.000 description 2
- QBWKPGNFQQJGFY-QLFBSQMISA-N 3-[(1r)-1-[(2r,6s)-2,6-dimethylmorpholin-4-yl]ethyl]-n-[6-methyl-3-(1h-pyrazol-4-yl)imidazo[1,2-a]pyrazin-8-yl]-1,2-thiazol-5-amine Chemical compound N1([C@H](C)C2=NSC(NC=3C4=NC=C(N4C=C(C)N=3)C3=CNN=C3)=C2)C[C@H](C)O[C@H](C)C1 QBWKPGNFQQJGFY-QLFBSQMISA-N 0.000 description 2
- XMIIGOLPHOKFCH-UHFFFAOYSA-N 3-phenylpropionic acid Chemical compound OC(=O)CCC1=CC=CC=C1 XMIIGOLPHOKFCH-UHFFFAOYSA-N 0.000 description 2
- WYFCZWSWFGJODV-MIANJLSGSA-N 4-[[(1s)-2-[(e)-3-[3-chloro-2-fluoro-6-(tetrazol-1-yl)phenyl]prop-2-enoyl]-5-(4-methyl-2-oxopiperazin-1-yl)-3,4-dihydro-1h-isoquinoline-1-carbonyl]amino]benzoic acid Chemical compound O=C1CN(C)CCN1C1=CC=CC2=C1CCN(C(=O)\C=C\C=1C(=CC=C(Cl)C=1F)N1N=NN=C1)[C@@H]2C(=O)NC1=CC=C(C(O)=O)C=C1 WYFCZWSWFGJODV-MIANJLSGSA-N 0.000 description 2
- XFJBGINZIMNZBW-CRAIPNDOSA-N 5-chloro-2-[4-[(1r,2s)-2-[2-(5-methylsulfonylpyridin-2-yl)oxyethyl]cyclopropyl]piperidin-1-yl]pyrimidine Chemical compound N1=CC(S(=O)(=O)C)=CC=C1OCC[C@H]1[C@@H](C2CCN(CC2)C=2N=CC(Cl)=CN=2)C1 XFJBGINZIMNZBW-CRAIPNDOSA-N 0.000 description 2
- RSIWALKZYXPAGW-NSHDSACASA-N 6-(3-fluorophenyl)-3-methyl-7-[(1s)-1-(7h-purin-6-ylamino)ethyl]-[1,3]thiazolo[3,2-a]pyrimidin-5-one Chemical compound C=1([C@@H](NC=2C=3N=CNC=3N=CN=2)C)N=C2SC=C(C)N2C(=O)C=1C1=CC=CC(F)=C1 RSIWALKZYXPAGW-NSHDSACASA-N 0.000 description 2
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- 241000416162 Astragalus gummifer Species 0.000 description 2
- 239000005711 Benzoic acid Substances 0.000 description 2
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 2
- JQUCWIWWWKZNCS-LESHARBVSA-N C(C1=CC=CC=C1)(=O)NC=1SC[C@H]2[C@@](N1)(CO[C@H](C2)C)C=2SC=C(N2)NC(=O)C2=NC=C(C=C2)OC(F)F Chemical compound C(C1=CC=CC=C1)(=O)NC=1SC[C@H]2[C@@](N1)(CO[C@H](C2)C)C=2SC=C(N2)NC(=O)C2=NC=C(C=C2)OC(F)F JQUCWIWWWKZNCS-LESHARBVSA-N 0.000 description 2
- BQXUPNKLZNSUMC-YUQWMIPFSA-N CCN(CCCCCOCC(=O)N[C@H](C(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](C)c1ccc(cc1)-c1scnc1C)C(C)(C)C)CCOc1ccc(cc1)C(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 Chemical compound CCN(CCCCCOCC(=O)N[C@H](C(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](C)c1ccc(cc1)-c1scnc1C)C(C)(C)C)CCOc1ccc(cc1)C(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 BQXUPNKLZNSUMC-YUQWMIPFSA-N 0.000 description 2
- HSWBVYNHHPZLEY-UHFFFAOYSA-N COC(=O)C1CC(CCN1)NC(=O)C2=C(C3=CC=CC=C3C=C2)O Chemical compound COC(=O)C1CC(CCN1)NC(=O)C2=C(C3=CC=CC=C3C=C2)O HSWBVYNHHPZLEY-UHFFFAOYSA-N 0.000 description 2
- FBRDVNQHUHAZKO-UHFFFAOYSA-N COC(=O)C1CC(CN1)NC(=O)C2=C(C3=CC=CC=C3C=C2)O Chemical compound COC(=O)C1CC(CN1)NC(=O)C2=C(C3=CC=CC=C3C=C2)O FBRDVNQHUHAZKO-UHFFFAOYSA-N 0.000 description 2
- PKMUHQIDVVOXHQ-HXUWFJFHSA-N C[C@H](C1=CC(C2=CC=C(CNC3CCCC3)S2)=CC=C1)NC(C1=C(C)C=CC(NC2CNC2)=C1)=O Chemical compound C[C@H](C1=CC(C2=CC=C(CNC3CCCC3)S2)=CC=C1)NC(C1=C(C)C=CC(NC2CNC2)=C1)=O PKMUHQIDVVOXHQ-HXUWFJFHSA-N 0.000 description 2
- 239000004215 Carbon black (E152) Substances 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 229940126657 Compound 17 Drugs 0.000 description 2
- 229940127007 Compound 39 Drugs 0.000 description 2
- 229910021595 Copper(I) iodide Inorganic materials 0.000 description 2
- RGHNJXZEOKUKBD-SQOUGZDYSA-N D-gluconic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 description 2
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 2
- QUSNBJAOOMFDIB-UHFFFAOYSA-N Ethylamine Chemical compound CCN QUSNBJAOOMFDIB-UHFFFAOYSA-N 0.000 description 2
- XPDWGBQVDMORPB-UHFFFAOYSA-N Fluoroform Chemical compound FC(F)F XPDWGBQVDMORPB-UHFFFAOYSA-N 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- 229940122604 HCV protease inhibitor Drugs 0.000 description 2
- 229940121759 Helicase inhibitor Drugs 0.000 description 2
- 101000600434 Homo sapiens Putative uncharacterized protein encoded by MIR7-3HG Proteins 0.000 description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 2
- 229940124257 Interferon receptor agonist Drugs 0.000 description 2
- 108010047761 Interferon-alpha Proteins 0.000 description 2
- 102000006992 Interferon-alpha Human genes 0.000 description 2
- AMIMRNSIRUDHCM-UHFFFAOYSA-N Isopropylaldehyde Chemical compound CC(C)C=O AMIMRNSIRUDHCM-UHFFFAOYSA-N 0.000 description 2
- 229930194542 Keto Natural products 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- BZLVMXJERCGZMT-UHFFFAOYSA-N Methyl tert-butyl ether Chemical compound COC(C)(C)C BZLVMXJERCGZMT-UHFFFAOYSA-N 0.000 description 2
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 2
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 2
- LVDRREOUMKACNJ-BKMJKUGQSA-N N-[(2R,3S)-2-(4-chlorophenyl)-1-(1,4-dimethyl-2-oxoquinolin-7-yl)-6-oxopiperidin-3-yl]-2-methylpropane-1-sulfonamide Chemical compound CC(C)CS(=O)(=O)N[C@H]1CCC(=O)N([C@@H]1c1ccc(Cl)cc1)c1ccc2c(C)cc(=O)n(C)c2c1 LVDRREOUMKACNJ-BKMJKUGQSA-N 0.000 description 2
- OPFJDXRVMFKJJO-ZHHKINOHSA-N N-{[3-(2-benzamido-4-methyl-1,3-thiazol-5-yl)-pyrazol-5-yl]carbonyl}-G-dR-G-dD-dD-dD-NH2 Chemical compound S1C(C=2NN=C(C=2)C(=O)NCC(=O)N[C@H](CCCN=C(N)N)C(=O)NCC(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC(O)=O)C(N)=O)=C(C)N=C1NC(=O)C1=CC=CC=C1 OPFJDXRVMFKJJO-ZHHKINOHSA-N 0.000 description 2
- QOVYHDHLFPKQQG-NDEPHWFRSA-N N[C@@H](CCC(=O)N1CCC(CC1)NC1=C2C=CC=CC2=NC(NCC2=CN(CCCNCCCNC3CCCCC3)N=N2)=N1)C(O)=O Chemical compound N[C@@H](CCC(=O)N1CCC(CC1)NC1=C2C=CC=CC2=NC(NCC2=CN(CCCNCCCNC3CCCCC3)N=N2)=N1)C(O)=O QOVYHDHLFPKQQG-NDEPHWFRSA-N 0.000 description 2
- 101800001020 Non-structural protein 4A Proteins 0.000 description 2
- XYFCBTPGUUZFHI-UHFFFAOYSA-N Phosphine Chemical compound P XYFCBTPGUUZFHI-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 229940122488 Primase inhibitor Drugs 0.000 description 2
- 102100037401 Putative uncharacterized protein encoded by MIR7-3HG Human genes 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- LCTONWCANYUPML-UHFFFAOYSA-N Pyruvic acid Chemical compound CC(=O)C(O)=O LCTONWCANYUPML-UHFFFAOYSA-N 0.000 description 2
- 108091027967 Small hairpin RNA Proteins 0.000 description 2
- 108020004459 Small interfering RNA Proteins 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 2
- 229920001615 Tragacanth Polymers 0.000 description 2
- LJOOWESTVASNOG-UFJKPHDISA-N [(1s,3r,4ar,7s,8s,8as)-3-hydroxy-8-[2-[(4r)-4-hydroxy-6-oxooxan-2-yl]ethyl]-7-methyl-1,2,3,4,4a,7,8,8a-octahydronaphthalen-1-yl] (2s)-2-methylbutanoate Chemical compound C([C@H]1[C@@H](C)C=C[C@H]2C[C@@H](O)C[C@@H]([C@H]12)OC(=O)[C@@H](C)CC)CC1C[C@@H](O)CC(=O)O1 LJOOWESTVASNOG-UFJKPHDISA-N 0.000 description 2
- SPXSEZMVRJLHQG-XMMPIXPASA-N [(2R)-1-[[4-[(3-phenylmethoxyphenoxy)methyl]phenyl]methyl]pyrrolidin-2-yl]methanol Chemical compound C(C1=CC=CC=C1)OC=1C=C(OCC2=CC=C(CN3[C@H](CCC3)CO)C=C2)C=CC=1 SPXSEZMVRJLHQG-XMMPIXPASA-N 0.000 description 2
- IOSLINNLJFQMFF-XMMPIXPASA-N [(2R)-1-[[4-[[3-[(4-fluorophenyl)methylsulfanyl]phenoxy]methyl]phenyl]methyl]pyrrolidin-2-yl]methanol Chemical compound FC1=CC=C(CSC=2C=C(OCC3=CC=C(CN4[C@H](CCC4)CO)C=C3)C=CC=2)C=C1 IOSLINNLJFQMFF-XMMPIXPASA-N 0.000 description 2
- LNUFLCYMSVYYNW-ZPJMAFJPSA-N [(2r,3r,4s,5r,6r)-2-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[[(3s,5s,8r,9s,10s,13r,14s,17r)-10,13-dimethyl-17-[(2r)-6-methylheptan-2-yl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-yl]oxy]-4,5-disulfo Chemical compound O([C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1C[C@@H]2CC[C@H]3[C@@H]4CC[C@@H]([C@]4(CC[C@@H]3[C@@]2(C)CC1)C)[C@H](C)CCCC(C)C)[C@H]1O[C@H](COS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]1OS(O)(=O)=O LNUFLCYMSVYYNW-ZPJMAFJPSA-N 0.000 description 2
- PSLUFJFHTBIXMW-WYEYVKMPSA-N [(3r,4ar,5s,6s,6as,10s,10ar,10bs)-3-ethenyl-10,10b-dihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-6-(2-pyridin-2-ylethylcarbamoyloxy)-5,6,6a,8,9,10-hexahydro-2h-benzo[f]chromen-5-yl] acetate Chemical compound O([C@@H]1[C@@H]([C@]2(O[C@](C)(CC(=O)[C@]2(O)[C@@]2(C)[C@@H](O)CCC(C)(C)[C@@H]21)C=C)C)OC(=O)C)C(=O)NCCC1=CC=CC=N1 PSLUFJFHTBIXMW-WYEYVKMPSA-N 0.000 description 2
- SMNRFWMNPDABKZ-WVALLCKVSA-N [[(2R,3S,4R,5S)-5-(2,6-dioxo-3H-pyridin-3-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [[[(2R,3S,4S,5R,6R)-4-fluoro-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl] hydrogen phosphate Chemical compound OC[C@H]1O[C@H](OP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)C2C=CC(=O)NC2=O)[C@H](O)[C@@H](F)[C@@H]1O SMNRFWMNPDABKZ-WVALLCKVSA-N 0.000 description 2
- WREOTYWODABZMH-DTZQCDIJSA-N [[(2r,3s,4r,5r)-3,4-dihydroxy-5-[2-oxo-4-(2-phenylethoxyamino)pyrimidin-1-yl]oxolan-2-yl]methoxy-hydroxyphosphoryl] phosphono hydrogen phosphate Chemical compound O[C@@H]1[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@H]1N(C=C\1)C(=O)NC/1=N\OCCC1=CC=CC=C1 WREOTYWODABZMH-DTZQCDIJSA-N 0.000 description 2
- QQIRAVWVGBTHMJ-UHFFFAOYSA-N [dimethyl-(trimethylsilylamino)silyl]methane;lithium Chemical compound [Li].C[Si](C)(C)N[Si](C)(C)C QQIRAVWVGBTHMJ-UHFFFAOYSA-N 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- YQNQNVDNTFHQSW-UHFFFAOYSA-N acetic acid [2-[[(5-nitro-2-thiazolyl)amino]-oxomethyl]phenyl] ester Chemical compound CC(=O)OC1=CC=CC=C1C(=O)NC1=NC=C([N+]([O-])=O)S1 YQNQNVDNTFHQSW-UHFFFAOYSA-N 0.000 description 2
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 125000004453 alkoxycarbonyl group Chemical group 0.000 description 2
- 125000000278 alkyl amino alkyl group Chemical group 0.000 description 2
- 125000003282 alkyl amino group Chemical group 0.000 description 2
- 125000003806 alkyl carbonyl amino group Chemical group 0.000 description 2
- 125000004448 alkyl carbonyl group Chemical group 0.000 description 2
- 125000004656 alkyl sulfonylamino group Chemical group 0.000 description 2
- 125000004414 alkyl thio group Chemical group 0.000 description 2
- 125000002947 alkylene group Chemical group 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 239000007900 aqueous suspension Substances 0.000 description 2
- 125000004658 aryl carbonyl amino group Chemical group 0.000 description 2
- 125000005129 aryl carbonyl group Chemical group 0.000 description 2
- 150000005840 aryl radicals Chemical class 0.000 description 2
- 125000004657 aryl sulfonyl amino group Chemical group 0.000 description 2
- 125000004391 aryl sulfonyl group Chemical group 0.000 description 2
- 239000012298 atmosphere Substances 0.000 description 2
- HUMNYLRZRPPJDN-UHFFFAOYSA-N benzaldehyde Chemical compound O=CC1=CC=CC=C1 HUMNYLRZRPPJDN-UHFFFAOYSA-N 0.000 description 2
- 235000010233 benzoic acid Nutrition 0.000 description 2
- IOJUPLGTWVMSFF-UHFFFAOYSA-N benzothiazole Chemical group C1=CC=C2SC=NC2=C1 IOJUPLGTWVMSFF-UHFFFAOYSA-N 0.000 description 2
- VTRGJDSQUBURDB-MJGOQNOKSA-N benzyl (2s,4r)-2-(1,3-benzoxazol-2-yl)-4-[(2-methylpropan-2-yl)oxy]pyrrolidine-1-carboxylate Chemical compound N1([C@@H](C[C@H](C1)OC(C)(C)C)C=1OC2=CC=CC=C2N=1)C(=O)OCC1=CC=CC=C1 VTRGJDSQUBURDB-MJGOQNOKSA-N 0.000 description 2
- BETCDXCQKDUZDM-XLIONFOSSA-N benzyl (2s,4r)-2-[(2-bromophenyl)carbamoyl]-4-[(2-methylpropan-2-yl)oxy]pyrrolidine-1-carboxylate Chemical compound N1([C@@H](C[C@H](C1)OC(C)(C)C)C(=O)NC=1C(=CC=CC=1)Br)C(=O)OCC1=CC=CC=C1 BETCDXCQKDUZDM-XLIONFOSSA-N 0.000 description 2
- LQHRHGJOXONZKB-REWPJTCUSA-N benzyl (2s,4s)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]-2-(5-methyl-1,3-oxazol-2-yl)pyrrolidine-1-carboxylate Chemical compound O1C(C)=CN=C1[C@H]1N(C(=O)OCC=2C=CC=CC=2)C[C@@H](NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)C1 LQHRHGJOXONZKB-REWPJTCUSA-N 0.000 description 2
- CADQCPJTJPOQAT-IGKIAQTJSA-N benzyl (2s,4s)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]-2-(5-phenyl-1,3-oxazol-2-yl)pyrrolidine-1-carboxylate Chemical compound N1([C@@H](C[C@@H](C1)NC(=O)C1=C(C2=CC=CC=C2C=C1)O)C=1OC(=CN=1)C=1C=CC=CC=1)C(=O)OCC1=CC=CC=C1 CADQCPJTJPOQAT-IGKIAQTJSA-N 0.000 description 2
- CIEOIHPZYFYLHR-GJZGRUSLSA-N benzyl (2s,4s)-4-amino-2-(5-tert-butyl-1,3-oxazol-2-yl)pyrrolidine-1-carboxylate Chemical compound O1C(C(C)(C)C)=CN=C1[C@H]1N(C(=O)OCC=2C=CC=CC=2)C[C@@H](N)C1 CIEOIHPZYFYLHR-GJZGRUSLSA-N 0.000 description 2
- WGQKYBSKWIADBV-UHFFFAOYSA-N benzylamine Chemical compound NCC1=CC=CC=C1 WGQKYBSKWIADBV-UHFFFAOYSA-N 0.000 description 2
- 239000012867 bioactive agent Substances 0.000 description 2
- LHHCSNFAOIFYRV-DOVBMPENSA-N boceprevir Chemical compound O=C([C@@H]1[C@@H]2[C@@H](C2(C)C)CN1C(=O)[C@@H](NC(=O)NC(C)(C)C)C(C)(C)C)NC(C(=O)C(N)=O)CC1CCC1 LHHCSNFAOIFYRV-DOVBMPENSA-N 0.000 description 2
- XTEOJPUYZWEXFI-UHFFFAOYSA-N butyl n-[3-[4-(imidazol-1-ylmethyl)phenyl]-5-(2-methylpropyl)thiophen-2-yl]sulfonylcarbamate Chemical compound S1C(CC(C)C)=CC(C=2C=CC(CN3C=NC=C3)=CC=2)=C1S(=O)(=O)NC(=O)OCCCC XTEOJPUYZWEXFI-UHFFFAOYSA-N 0.000 description 2
- PFKFTWBEEFSNDU-UHFFFAOYSA-N carbonyldiimidazole Chemical compound C1=CN=CN1C(=O)N1C=CN=C1 PFKFTWBEEFSNDU-UHFFFAOYSA-N 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 229940110456 cocoa butter Drugs 0.000 description 2
- 235000019868 cocoa butter Nutrition 0.000 description 2
- 229940125773 compound 10 Drugs 0.000 description 2
- 229940125797 compound 12 Drugs 0.000 description 2
- 229940126543 compound 14 Drugs 0.000 description 2
- 229940125758 compound 15 Drugs 0.000 description 2
- 229940126142 compound 16 Drugs 0.000 description 2
- 229940125782 compound 2 Drugs 0.000 description 2
- 229940125810 compound 20 Drugs 0.000 description 2
- 229940126086 compound 21 Drugs 0.000 description 2
- 229940126208 compound 22 Drugs 0.000 description 2
- 229940125961 compound 24 Drugs 0.000 description 2
- 229940125846 compound 25 Drugs 0.000 description 2
- 229940125851 compound 27 Drugs 0.000 description 2
- 229940127204 compound 29 Drugs 0.000 description 2
- 229940125877 compound 31 Drugs 0.000 description 2
- 229940125878 compound 36 Drugs 0.000 description 2
- 229940126540 compound 41 Drugs 0.000 description 2
- 229940125936 compound 42 Drugs 0.000 description 2
- 229940125844 compound 46 Drugs 0.000 description 2
- 229940127271 compound 49 Drugs 0.000 description 2
- 229940126545 compound 53 Drugs 0.000 description 2
- 229940127113 compound 57 Drugs 0.000 description 2
- 229940125900 compound 59 Drugs 0.000 description 2
- 229940126179 compound 72 Drugs 0.000 description 2
- 125000004093 cyano group Chemical group *C#N 0.000 description 2
- ZVTDLPBHTSMEJZ-JSZLBQEHSA-N danoprevir Chemical compound O=C([C@@]12C[C@H]1\C=C/CCCCC[C@@H](C(N1C[C@@H](C[C@H]1C(=O)N2)OC(=O)N1CC2=C(F)C=CC=C2C1)=O)NC(=O)OC(C)(C)C)NS(=O)(=O)C1CC1 ZVTDLPBHTSMEJZ-JSZLBQEHSA-N 0.000 description 2
- FAMRKDQNMBBFBR-BQYQJAHWSA-N diethyl azodicarboxylate Substances CCOC(=O)\N=N\C(=O)OCC FAMRKDQNMBBFBR-BQYQJAHWSA-N 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical compound C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 2
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 2
- 231100000673 dose–response relationship Toxicity 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 150000002085 enols Chemical group 0.000 description 2
- BJXYHBKEQFQVES-NWDGAFQWSA-N enpatoran Chemical compound N[C@H]1CN(C[C@H](C1)C(F)(F)F)C1=C2C=CC=NC2=C(C=C1)C#N BJXYHBKEQFQVES-NWDGAFQWSA-N 0.000 description 2
- 238000010931 ester hydrolysis Methods 0.000 description 2
- VUFKXQKHPMMQHX-IBVKSMDESA-N ethyl (2s,4r)-1-(cyclohexylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]-2-methylpyrrolidine-2-carboxylate Chemical compound C([C@@H](C[C@@]1(C)C(=O)OCC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1CCCCC1 VUFKXQKHPMMQHX-IBVKSMDESA-N 0.000 description 2
- HTWFMRWIMYKEMH-UWVGGRQHSA-N ethyl (2s,4s)-4-amino-1-(2-methylpropyl)pyrrolidine-2-carboxylate Chemical compound CCOC(=O)[C@@H]1C[C@H](N)CN1CC(C)C HTWFMRWIMYKEMH-UWVGGRQHSA-N 0.000 description 2
- WXQGAEBQJGCJLF-RYUDHWBXSA-N ethyl (2s,4s)-4-amino-1-(cyclohexanecarbonyl)pyrrolidine-2-carboxylate Chemical compound CCOC(=O)[C@@H]1C[C@H](N)CN1C(=O)C1CCCCC1 WXQGAEBQJGCJLF-RYUDHWBXSA-N 0.000 description 2
- CDORZWJWKVMOER-STQMWFEESA-N ethyl (2s,4s)-4-amino-1-(cyclohexylmethyl)pyrrolidine-2-carboxylate Chemical compound CCOC(=O)[C@@H]1C[C@H](N)CN1CC1CCCCC1 CDORZWJWKVMOER-STQMWFEESA-N 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- FAMRKDQNMBBFBR-UHFFFAOYSA-N ethyl n-ethoxycarbonyliminocarbamate Chemical compound CCOC(=O)N=NC(=O)OCC FAMRKDQNMBBFBR-UHFFFAOYSA-N 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 239000012467 final product Substances 0.000 description 2
- 229940125777 fusion inhibitor Drugs 0.000 description 2
- 239000007789 gas Substances 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 239000007903 gelatin capsule Substances 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- JAXFJECJQZDFJS-XHEPKHHKSA-N gtpl8555 Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@@H]1C(=O)N[C@H](B1O[C@@]2(C)[C@H]3C[C@H](C3(C)C)C[C@H]2O1)CCC1=CC=C(F)C=C1 JAXFJECJQZDFJS-XHEPKHHKSA-N 0.000 description 2
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 2
- 125000004404 heteroalkyl group Chemical group 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 108010010648 interferon alfacon-1 Proteins 0.000 description 2
- 239000000543 intermediate Substances 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 2
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 2
- 229960004592 isopropanol Drugs 0.000 description 2
- ZLVXBBHTMQJRSX-VMGNSXQWSA-N jdtic Chemical compound C1([C@]2(C)CCN(C[C@@H]2C)C[C@H](C(C)C)NC(=O)[C@@H]2NCC3=CC(O)=CC=C3C2)=CC=CC(O)=C1 ZLVXBBHTMQJRSX-VMGNSXQWSA-N 0.000 description 2
- 150000002576 ketones Chemical class 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- 239000000787 lecithin Substances 0.000 description 2
- 235000010445 lecithin Nutrition 0.000 description 2
- 229940067606 lecithin Drugs 0.000 description 2
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 2
- 210000004185 liver Anatomy 0.000 description 2
- RENRQMCACQEWFC-UGKGYDQZSA-N lnp023 Chemical compound C1([C@H]2N(CC=3C=4C=CNC=4C(C)=CC=3OC)CC[C@@H](C2)OCC)=CC=C(C(O)=O)C=C1 RENRQMCACQEWFC-UGKGYDQZSA-N 0.000 description 2
- 239000006210 lotion Substances 0.000 description 2
- 108010026228 mRNA guanylyltransferase Proteins 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- 230000008018 melting Effects 0.000 description 2
- IPMKGDUTQHAECL-UQBPGWFLSA-N methyl (2r,4s)-1-(cyclohexylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]-2-methylpyrrolidine-2-carboxylate Chemical compound C([C@H](C[C@]1(C)C(=O)OC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1CCCCC1 IPMKGDUTQHAECL-UQBPGWFLSA-N 0.000 description 2
- IPMKGDUTQHAECL-CLOONOSVSA-N methyl (2s,4r)-1-(cyclohexylmethyl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]-2-methylpyrrolidine-2-carboxylate Chemical compound C([C@@H](C[C@@]1(C)C(=O)OC)NC(=O)C=2C(=C3C=CC=CC3=CC=2)O)N1CC1CCCCC1 IPMKGDUTQHAECL-CLOONOSVSA-N 0.000 description 2
- DROVTANONRBMRA-RYUDHWBXSA-N methyl (2s,4s)-4-amino-1-benzylpyrrolidine-2-carboxylate Chemical compound COC(=O)[C@@H]1C[C@H](N)CN1CC1=CC=CC=C1 DROVTANONRBMRA-RYUDHWBXSA-N 0.000 description 2
- ZBELDPMWYXDLNY-UHFFFAOYSA-N methyl 9-(4-bromo-2-fluoroanilino)-[1,3]thiazolo[5,4-f]quinazoline-2-carboximidate Chemical compound C12=C3SC(C(=N)OC)=NC3=CC=C2N=CN=C1NC1=CC=C(Br)C=C1F ZBELDPMWYXDLNY-UHFFFAOYSA-N 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 235000010981 methylcellulose Nutrition 0.000 description 2
- 210000000214 mouth Anatomy 0.000 description 2
- TXXHDPDFNKHHGW-UHFFFAOYSA-N muconic acid Chemical compound OC(=O)C=CC=CC(O)=O TXXHDPDFNKHHGW-UHFFFAOYSA-N 0.000 description 2
- YCJZWBZJSYLMPB-UHFFFAOYSA-N n-(2-chloropyrimidin-4-yl)-2,5-dimethyl-1-phenylimidazole-4-carboxamide Chemical compound CC=1N(C=2C=CC=CC=2)C(C)=NC=1C(=O)NC1=CC=NC(Cl)=N1 YCJZWBZJSYLMPB-UHFFFAOYSA-N 0.000 description 2
- NLHSEBRVAHJNEU-KSSFIOAISA-N n-[(3s,5s)-5-(1,3-benzoxazol-2-yl)pyrrolidin-3-yl]-1-hydroxynaphthalene-2-carboxamide Chemical compound C1=CC=C2OC([C@H]3NC[C@H](C3)NC(=O)C3=C(C4=CC=CC=C4C=C3)O)=NC2=C1 NLHSEBRVAHJNEU-KSSFIOAISA-N 0.000 description 2
- UFWIBTONFRDIAS-UHFFFAOYSA-N naphthalene-acid Chemical group C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 2
- 150000002790 naphthalenes Chemical group 0.000 description 2
- IOMMMLWIABWRKL-WUTDNEBXSA-N nazartinib Chemical compound C1N(C(=O)/C=C/CN(C)C)CCCC[C@H]1N1C2=C(Cl)C=CC=C2N=C1NC(=O)C1=CC=NC(C)=C1 IOMMMLWIABWRKL-WUTDNEBXSA-N 0.000 description 2
- 229960002480 nitazoxanide Drugs 0.000 description 2
- 231100000252 nontoxic Toxicity 0.000 description 2
- 230000003000 nontoxic effect Effects 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 235000019198 oils Nutrition 0.000 description 2
- 239000002674 ointment Substances 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 239000003961 penetration enhancing agent Substances 0.000 description 2
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 2
- 239000008024 pharmaceutical diluent Substances 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 125000000286 phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 description 2
- 229920006316 polyvinylpyrrolidine Polymers 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- WGYKZJWCGVVSQN-UHFFFAOYSA-N propylamine Chemical compound CCCN WGYKZJWCGVVSQN-UHFFFAOYSA-N 0.000 description 2
- 125000006239 protecting group Chemical group 0.000 description 2
- 235000018102 proteins Nutrition 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- JTZZSQYMACOLNN-VDWJNHBNSA-N simeprevir Chemical compound O=C([C@@]12C[C@H]1\C=C/CCCCN(C)C(=O)[C@H]1[C@H](C(N2)=O)C[C@H](C1)OC=1C2=CC=C(C(=C2N=C(C=1)C=1SC=C(N=1)C(C)C)C)OC)NS(=O)(=O)C1CC1 JTZZSQYMACOLNN-VDWJNHBNSA-N 0.000 description 2
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 2
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 2
- JUJBNYBVVQSIOU-UHFFFAOYSA-M sodium;4-[2-(4-iodophenyl)-3-(4-nitrophenyl)tetrazol-2-ium-5-yl]benzene-1,3-disulfonate Chemical compound [Na+].C1=CC([N+](=O)[O-])=CC=C1N1[N+](C=2C=CC(I)=CC=2)=NC(C=2C(=CC(=CC=2)S([O-])(=O)=O)S([O-])(=O)=O)=N1 JUJBNYBVVQSIOU-UHFFFAOYSA-M 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 230000002459 sustained effect Effects 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 230000008685 targeting Effects 0.000 description 2
- 125000001981 tert-butyldimethylsilyl group Chemical group [H]C([H])([H])[Si]([H])(C([H])([H])[H])[*]C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- FPGGTKZVZWFYPV-UHFFFAOYSA-M tetrabutylammonium fluoride Chemical compound [F-].CCCC[N+](CCCC)(CCCC)CCCC FPGGTKZVZWFYPV-UHFFFAOYSA-M 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 238000004809 thin layer chromatography Methods 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- 238000011200 topical administration Methods 0.000 description 2
- 235000010487 tragacanth Nutrition 0.000 description 2
- 239000000196 tragacanth Substances 0.000 description 2
- 229940116362 tragacanth Drugs 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- 125000000026 trimethylsilyl group Chemical group [H]C([H])([H])[Si]([*])(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 239000008215 water for injection Substances 0.000 description 2
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- WJQQGUSRJSMVDW-MFKMUULPSA-N (2S,4R)-2-[(2-bromophenyl)carbamoyl]-4-[(2-methylpropan-2-yl)oxy]pyrrolidine-1-carboxylic acid Chemical compound BrC1=C(C=CC=C1)NC(=O)[C@H]1N(C[C@@H](C1)OC(C)(C)C)C(=O)O WJQQGUSRJSMVDW-MFKMUULPSA-N 0.000 description 1
- ZIXHOTAOUGGFJY-ZJUUUORDSA-N (2S,4R)-4-[(2-methylpropan-2-yl)oxy]-2-(2-oxopropylcarbamoyl)pyrrolidine-1-carboxylic acid Chemical compound C(C)(C)(C)O[C@@H]1C[C@H](N(C1)C(=O)O)C(NCC(C)=O)=O ZIXHOTAOUGGFJY-ZJUUUORDSA-N 0.000 description 1
- DZRQMHSNVNTFAQ-IVGJVWKCSA-N (2r,3r)-2,3-dihydroxybutanedioic acid;(2r,3s,4r,5s)-1-(6-ethoxyhexyl)-2-methylpiperidine-3,4,5-triol Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O.CCOCCCCCCN1C[C@H](O)[C@@H](O)[C@@H](O)[C@H]1C DZRQMHSNVNTFAQ-IVGJVWKCSA-N 0.000 description 1
- BENKAPCDIOILGV-RNFRBKRXSA-N (2r,4r)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid Chemical compound CC(C)(C)OC(=O)N1C[C@H](O)C[C@@H]1C(O)=O BENKAPCDIOILGV-RNFRBKRXSA-N 0.000 description 1
- SQJVUMFKCPOWJL-HQJQHLMTSA-N (2s,4r)-4-hydroxy-2-methyl-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid Chemical compound CC(C)(C)OC(=O)N1C[C@H](O)C[C@@]1(C)C(O)=O SQJVUMFKCPOWJL-HQJQHLMTSA-N 0.000 description 1
- WBGTVBYNNXLLJP-FBCQKBJTSA-N (2s,4r)-4-hydroxy-2-methylpyrrolidine-1,2-dicarboxylic acid Chemical compound OC(=O)[C@]1(C)C[C@@H](O)CN1C(O)=O WBGTVBYNNXLLJP-FBCQKBJTSA-N 0.000 description 1
- WQBOWGPMMLEVOY-IMJSIDKUSA-N (2s,4s)-4-azidopyrrolidine-1,2-dicarboxylic acid Chemical compound OC(=O)[C@@H]1C[C@H](N=[N+]=[N-])CN1C(O)=O WQBOWGPMMLEVOY-IMJSIDKUSA-N 0.000 description 1
- GCAZZUFIDGXTDA-YUMQZZPRSA-N (2s,4s)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]piperidine-2-carboxylic acid Chemical compound CC(C)(C)OC(=O)N1CC[C@H](O)C[C@H]1C(O)=O GCAZZUFIDGXTDA-YUMQZZPRSA-N 0.000 description 1
- BENKAPCDIOILGV-BQBZGAKWSA-N (2s,4s)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid Chemical compound CC(C)(C)OC(=O)N1C[C@@H](O)C[C@H]1C(O)=O BENKAPCDIOILGV-BQBZGAKWSA-N 0.000 description 1
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 1
- YQUCBFIQSJVCOR-JOCHJYFZSA-N (7r)-14-cyclohexyl-7-{[2-(dimethylamino)ethyl](methyl)amino}-7,8-dihydro-6h-indolo[1,2-e][1,5]benzoxazocine-11-carboxylic acid Chemical compound C([C@@H](CN1C2=CC(=CC=C22)C(O)=O)N(C)CCN(C)C)OC3=CC=CC=C3C1=C2C1CCCCC1 YQUCBFIQSJVCOR-JOCHJYFZSA-N 0.000 description 1
- 125000006272 (C3-C7) cycloalkyl group Chemical group 0.000 description 1
- MIOPJNTWMNEORI-GMSGAONNSA-N (S)-camphorsulfonic acid Chemical compound C1C[C@@]2(CS(O)(=O)=O)C(=O)C[C@@H]1C2(C)C MIOPJNTWMNEORI-GMSGAONNSA-N 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- WBYWAXJHAXSJNI-VOTSOKGWSA-M .beta-Phenylacrylic acid Natural products [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- ZORQXIQZAOLNGE-UHFFFAOYSA-N 1,1-difluorocyclohexane Chemical compound FC1(F)CCCCC1 ZORQXIQZAOLNGE-UHFFFAOYSA-N 0.000 description 1
- CSNIZNHTOVFARY-UHFFFAOYSA-N 1,2-benzothiazole Chemical group C1=CC=C2C=NSC2=C1 CSNIZNHTOVFARY-UHFFFAOYSA-N 0.000 description 1
- KTZQTRPPVKQPFO-UHFFFAOYSA-N 1,2-benzoxazole Chemical group C1=CC=C2C=NOC2=C1 KTZQTRPPVKQPFO-UHFFFAOYSA-N 0.000 description 1
- QFMZQPDHXULLKC-UHFFFAOYSA-N 1,2-bis(diphenylphosphino)ethane Chemical compound C=1C=CC=CC=1P(C=1C=CC=CC=1)CCP(C=1C=CC=CC=1)C1=CC=CC=C1 QFMZQPDHXULLKC-UHFFFAOYSA-N 0.000 description 1
- DDMOUSALMHHKOS-UHFFFAOYSA-N 1,2-dichloro-1,1,2,2-tetrafluoroethane Chemical compound FC(F)(Cl)C(F)(F)Cl DDMOUSALMHHKOS-UHFFFAOYSA-N 0.000 description 1
- BCMCBBGGLRIHSE-UHFFFAOYSA-N 1,3-benzoxazole Chemical group C1=CC=C2OC=NC2=C1 BCMCBBGGLRIHSE-UHFFFAOYSA-N 0.000 description 1
- SGUVLZREKBPKCE-UHFFFAOYSA-N 1,5-diazabicyclo[4.3.0]-non-5-ene Chemical compound C1CCN=C2CCCN21 SGUVLZREKBPKCE-UHFFFAOYSA-N 0.000 description 1
- XRYRJXQJSTWFDB-UHFFFAOYSA-N 1-(cyclohexylmethyl)pyrrolidin-1-ium-2-carboxylate Chemical compound OC(=O)C1CCCN1CC1CCCCC1 XRYRJXQJSTWFDB-UHFFFAOYSA-N 0.000 description 1
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide Substances CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 description 1
- VQFKFAKEUMHBLV-BYSUZVQFSA-N 1-O-(alpha-D-galactosyl)-N-hexacosanoylphytosphingosine Chemical compound CCCCCCCCCCCCCCCCCCCCCCCCCC(=O)N[C@H]([C@H](O)[C@H](O)CCCCCCCCCCCCCC)CO[C@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O VQFKFAKEUMHBLV-BYSUZVQFSA-N 0.000 description 1
- CRTRUQUJSMFWPD-UHFFFAOYSA-N 1-amino-3,3-dimethylbutan-2-one Chemical compound CC(C)(C)C(=O)CN CRTRUQUJSMFWPD-UHFFFAOYSA-N 0.000 description 1
- MNMDZSAWTQOEHX-BDAKNGLRSA-N 1-o-tert-butyl 2-o-ethyl (2s,4r)-4-hydroxypyrrolidine-1,2-dicarboxylate Chemical compound CCOC(=O)[C@@H]1C[C@@H](O)CN1C(=O)OC(C)(C)C MNMDZSAWTQOEHX-BDAKNGLRSA-N 0.000 description 1
- CTBPJCTYTFHHRY-PRHODGIISA-N 1-o-tert-butyl 2-o-methyl (2r,4r)-4-hydroxy-2-methylpyrrolidine-1,2-dicarboxylate Chemical compound COC(=O)[C@@]1(C)C[C@@H](O)CN1C(=O)OC(C)(C)C CTBPJCTYTFHHRY-PRHODGIISA-N 0.000 description 1
- MZMNEDXVUJLQAF-HTQZYQBOSA-N 1-o-tert-butyl 2-o-methyl (2r,4r)-4-hydroxypyrrolidine-1,2-dicarboxylate Chemical compound COC(=O)[C@H]1C[C@@H](O)CN1C(=O)OC(C)(C)C MZMNEDXVUJLQAF-HTQZYQBOSA-N 0.000 description 1
- UKVKNGDXVREPDE-HIFRSBDPSA-N 1-o-tert-butyl 2-o-methyl (2s,4r)-4-(4-methylphenyl)sulfonyloxypyrrolidine-1,2-dicarboxylate Chemical compound C1N(C(=O)OC(C)(C)C)[C@H](C(=O)OC)C[C@H]1OS(=O)(=O)C1=CC=C(C)C=C1 UKVKNGDXVREPDE-HIFRSBDPSA-N 0.000 description 1
- CTBPJCTYTFHHRY-PELKAZGASA-N 1-o-tert-butyl 2-o-methyl (2s,4r)-4-hydroxy-2-methylpyrrolidine-1,2-dicarboxylate Chemical compound COC(=O)[C@]1(C)C[C@@H](O)CN1C(=O)OC(C)(C)C CTBPJCTYTFHHRY-PELKAZGASA-N 0.000 description 1
- MZMNEDXVUJLQAF-YUMQZZPRSA-N 1-o-tert-butyl 2-o-methyl (2s,4s)-4-hydroxypyrrolidine-1,2-dicarboxylate Chemical compound COC(=O)[C@@H]1C[C@H](O)CN1C(=O)OC(C)(C)C MZMNEDXVUJLQAF-YUMQZZPRSA-N 0.000 description 1
- YRAJNWYBUCUFBD-UHFFFAOYSA-N 2,2,6,6-tetramethylheptane-3,5-dione Chemical compound CC(C)(C)C(=O)CC(=O)C(C)(C)C YRAJNWYBUCUFBD-UHFFFAOYSA-N 0.000 description 1
- DJWDAKFSDBOQJK-UHFFFAOYSA-N 2,5-diazabicyclo[2.2.2]octane Chemical compound C1NC2CCC1NC2 DJWDAKFSDBOQJK-UHFFFAOYSA-N 0.000 description 1
- UDSAJFSYJMHNFI-UHFFFAOYSA-N 2,6-diazaspiro[3.3]heptane Chemical compound C1NCC11CNC1 UDSAJFSYJMHNFI-UHFFFAOYSA-N 0.000 description 1
- JXZYSNWHGBGZAI-GOSISDBHSA-N 2-[(1r)-5-cyano-8-methyl-1-propyl-4,9-dihydro-3h-pyrano[3,4-b]indol-1-yl]acetic acid Chemical compound N1C2=C(C)C=CC(C#N)=C2C2=C1[C@@](CCC)(CC(O)=O)OCC2 JXZYSNWHGBGZAI-GOSISDBHSA-N 0.000 description 1
- HEQOJEGTZCTHCF-UHFFFAOYSA-N 2-amino-1-phenylethanone Chemical compound NCC(=O)C1=CC=CC=C1 HEQOJEGTZCTHCF-UHFFFAOYSA-N 0.000 description 1
- SCVJRXQHFJXZFZ-KVQBGUIXSA-N 2-amino-9-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-3h-purine-6-thione Chemical compound C1=2NC(N)=NC(=S)C=2N=CN1[C@H]1C[C@H](O)[C@@H](CO)O1 SCVJRXQHFJXZFZ-KVQBGUIXSA-N 0.000 description 1
- AOPBDRUWRLBSDB-UHFFFAOYSA-N 2-bromoaniline Chemical compound NC1=CC=CC=C1Br AOPBDRUWRLBSDB-UHFFFAOYSA-N 0.000 description 1
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 description 1
- UPHOPMSGKZNELG-UHFFFAOYSA-N 2-hydroxynaphthalene-1-carboxylic acid Chemical compound C1=CC=C2C(C(=O)O)=C(O)C=CC2=C1 UPHOPMSGKZNELG-UHFFFAOYSA-N 0.000 description 1
- YHWMFDLNZGIJSD-UHFFFAOYSA-N 2h-1,4-oxazine Chemical compound C1OC=CN=C1 YHWMFDLNZGIJSD-UHFFFAOYSA-N 0.000 description 1
- LTNUSYNQZJZUSY-UHFFFAOYSA-N 3,3-dimethylbutanal Chemical compound CC(C)(C)CC=O LTNUSYNQZJZUSY-UHFFFAOYSA-N 0.000 description 1
- MLMQPDHYNJCQAO-UHFFFAOYSA-N 3,3-dimethylbutyric acid Chemical compound CC(C)(C)CC(O)=O MLMQPDHYNJCQAO-UHFFFAOYSA-N 0.000 description 1
- LKDJYZBKCVSODK-UHFFFAOYSA-N 3,8-diazabicyclo[3.2.1]octane Chemical compound C1NCC2CCC1N2 LKDJYZBKCVSODK-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- ZRPLANDPDWYOMZ-UHFFFAOYSA-N 3-cyclopentylpropionic acid Chemical compound OC(=O)CCC1CCCC1 ZRPLANDPDWYOMZ-UHFFFAOYSA-N 0.000 description 1
- QOXOZONBQWIKDA-UHFFFAOYSA-N 3-hydroxypropyl Chemical group [CH2]CCO QOXOZONBQWIKDA-UHFFFAOYSA-N 0.000 description 1
- 125000006201 3-phenylpropyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- NYPIRLYMDJMKGW-VPCXQMTMSA-N 4-amino-1-[(2r,3r,4r,5r)-3-fluoro-4-hydroxy-5-(hydroxymethyl)-3-methyloxolan-2-yl]pyrimidin-2-one Chemical compound C[C@@]1(F)[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)N=C(N)C=C1 NYPIRLYMDJMKGW-VPCXQMTMSA-N 0.000 description 1
- RJWBTWIBUIGANW-UHFFFAOYSA-N 4-chlorobenzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=C(Cl)C=C1 RJWBTWIBUIGANW-UHFFFAOYSA-N 0.000 description 1
- GIXOPPBZUGBQBY-UHFFFAOYSA-N 4-methylbenzenesulfonic acid;naphthalene-2-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1.C1=CC=CC2=CC(S(=O)(=O)O)=CC=C21 GIXOPPBZUGBQBY-UHFFFAOYSA-N 0.000 description 1
- QJMYXHKGEGNLED-UHFFFAOYSA-N 5-(2-hydroxyethylamino)-1h-pyrimidine-2,4-dione Chemical compound OCCNC1=CNC(=O)NC1=O QJMYXHKGEGNLED-UHFFFAOYSA-N 0.000 description 1
- WPMJNLCLKAKMLA-UHFFFAOYSA-N 5-(3,3-dimethylbut-1-ynyl)-3-[(4-hydroxycyclohexyl)-[(4-methylcyclohexyl)-oxomethyl]amino]-2-thiophenecarboxylic acid Chemical compound C1CC(C)CCC1C(=O)N(C1=C(SC(=C1)C#CC(C)(C)C)C(O)=O)C1CCC(O)CC1 WPMJNLCLKAKMLA-UHFFFAOYSA-N 0.000 description 1
- TZYVRXZQAWPIAB-FCLHUMLKSA-N 5-amino-3-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-4h-[1,3]thiazolo[4,5-d]pyrimidine-2,7-dione Chemical compound O=C1SC=2C(=O)NC(N)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O TZYVRXZQAWPIAB-FCLHUMLKSA-N 0.000 description 1
- WTDWVLJJJOTABN-UHFFFAOYSA-N 5-cyclopropyl-2-(4-fluorophenyl)-6-[(2-hydroxyethyl)(methylsulfonyl)amino]-n-methyl-1-benzofuran-3-carboxamide Chemical compound C1=C2C(C(=O)NC)=C(C=3C=CC(F)=CC=3)OC2=CC(N(CCO)S(C)(=O)=O)=C1C1CC1 WTDWVLJJJOTABN-UHFFFAOYSA-N 0.000 description 1
- JYIAZVFJRYLCBH-UHFFFAOYSA-N 8-hydroxyquinoline-7-carboxylic acid Chemical compound C1=CC=NC2=C(O)C(C(=O)O)=CC=C21 JYIAZVFJRYLCBH-UHFFFAOYSA-N 0.000 description 1
- AMKGKYQBASDDJB-UHFFFAOYSA-N 9$l^{2}-borabicyclo[3.3.1]nonane Chemical compound C1CCC2CCCC1[B]2 AMKGKYQBASDDJB-UHFFFAOYSA-N 0.000 description 1
- PVRFQJIRERYGTQ-DSQUMVBZSA-N 9-[(2s,4ar,6r,7r,7ar)-7-fluoro-7-methyl-2-oxo-2-propan-2-yloxy-4,4a,6,7a-tetrahydrofuro[3,2-d][1,3,2]dioxaphosphinin-6-yl]-6-ethoxypurin-2-amine Chemical compound C([C@H]1O2)O[P@@](=O)(OC(C)C)O[C@H]1[C@](F)(C)[C@@H]2N1C(N=C(N)N=C2OCC)=C2N=C1 PVRFQJIRERYGTQ-DSQUMVBZSA-N 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 229940077274 Alpha glucosidase inhibitor Drugs 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- LBRXHOPVDMLJCW-RZSVFLSASA-N C/C(/N)=C(\C=C/C=C)/Br Chemical compound C/C(/N)=C(\C=C/C=C)/Br LBRXHOPVDMLJCW-RZSVFLSASA-N 0.000 description 1
- XUCMXXAZYCWLEN-BTFOHMIHSA-N C/C=C(/COC(N(C[C@@H](C1)OS(c2ccc(C)cc2)(=O)=O)C1c1nc(cccc2)c2[o]1)=O)\C=C/C=C Chemical compound C/C=C(/COC(N(C[C@@H](C1)OS(c2ccc(C)cc2)(=O)=O)C1c1nc(cccc2)c2[o]1)=O)\C=C/C=C XUCMXXAZYCWLEN-BTFOHMIHSA-N 0.000 description 1
- XXJSUHHTUVHWAB-UHFFFAOYSA-N C1C(CNC1C(=O)O)NC(=O)C2=C(C3=CC=CC=C3C=C2)O Chemical compound C1C(CNC1C(=O)O)NC(=O)C2=C(C3=CC=CC=C3C=C2)O XXJSUHHTUVHWAB-UHFFFAOYSA-N 0.000 description 1
- VAUZVEOCJUXLBS-QWRGUYRKSA-N C1[C@H](N)CN[C@@H]1C1=NC=C(C=2C=CC=CC=2)O1 Chemical compound C1[C@H](N)CN[C@@H]1C1=NC=C(C=2C=CC=CC=2)O1 VAUZVEOCJUXLBS-QWRGUYRKSA-N 0.000 description 1
- HAHPRFKJPFJPKI-JTQLQIEISA-N CC(C)(C)CCN(CC(C1)=[N+]=N)[C@@H]1C(OC)=O Chemical compound CC(C)(C)CCN(CC(C1)=[N+]=N)[C@@H]1C(OC)=O HAHPRFKJPFJPKI-JTQLQIEISA-N 0.000 description 1
- JNIVAMILFKQRQC-HOCLYGCPSA-N CC(C)(C)OC(N(CC[C@@H](C1)OS(c2ccc(C)cc2)(=O)=O)[C@@H]1C(OC)=O)=O Chemical compound CC(C)(C)OC(N(CC[C@@H](C1)OS(c2ccc(C)cc2)(=O)=O)[C@@H]1C(OC)=O)=O JNIVAMILFKQRQC-HOCLYGCPSA-N 0.000 description 1
- SDIKSYQJURNZIL-BDAKNGLRSA-N CC(C)(C)OC(N(CC[C@H](C1)N=[N+]=N)[C@@H]1C(OC)=O)=O Chemical compound CC(C)(C)OC(N(CC[C@H](C1)N=[N+]=N)[C@@H]1C(OC)=O)=O SDIKSYQJURNZIL-BDAKNGLRSA-N 0.000 description 1
- HAOXYRATYSKLSG-OAHLLOKOSA-N CC(C)(C)O[C@H](C1)CN(C(OCc2ccccc2)=O)C1=C Chemical compound CC(C)(C)O[C@H](C1)CN(C(OCc2ccccc2)=O)C1=C HAOXYRATYSKLSG-OAHLLOKOSA-N 0.000 description 1
- VTRGJDSQUBURDB-DUSLRRAJSA-N CC(C)(C)O[C@H](CC1c2nc(cccc3)c3[o]2)CN1C(OCc1ccccc1)=O Chemical compound CC(C)(C)O[C@H](CC1c2nc(cccc3)c3[o]2)CN1C(OCc1ccccc1)=O VTRGJDSQUBURDB-DUSLRRAJSA-N 0.000 description 1
- LDCHQLFDKUTDSP-MDWODPDHSA-N CC1(C=CC=CC1)c1cnc([C@H](C2)N(CC3CCCCC3)C[C@H]2NC(c(ccc2c3cccc2)c3O)=O)[o]1 Chemical compound CC1(C=CC=CC1)c1cnc([C@H](C2)N(CC3CCCCC3)C[C@H]2NC(c(ccc2c3cccc2)c3O)=O)[o]1 LDCHQLFDKUTDSP-MDWODPDHSA-N 0.000 description 1
- KCBAMQOKOLXLOX-BSZYMOERSA-N CC1=C(SC=N1)C2=CC=C(C=C2)[C@H](C)NC(=O)[C@@H]3C[C@H](CN3C(=O)[C@H](C(C)(C)C)NC(=O)CCCCCCCCCCNCCCONC(=O)C4=C(C(=C(C=C4)F)F)NC5=C(C=C(C=C5)I)F)O Chemical compound CC1=C(SC=N1)C2=CC=C(C=C2)[C@H](C)NC(=O)[C@@H]3C[C@H](CN3C(=O)[C@H](C(C)(C)C)NC(=O)CCCCCCCCCCNCCCONC(=O)C4=C(C(=C(C=C4)F)F)NC5=C(C=C(C=C5)I)F)O KCBAMQOKOLXLOX-BSZYMOERSA-N 0.000 description 1
- JGLMVXWAHNTPRF-CMDGGOBGSA-N CCN1N=C(C)C=C1C(=O)NC1=NC2=CC(=CC(OC)=C2N1C\C=C\CN1C(NC(=O)C2=CC(C)=NN2CC)=NC2=CC(=CC(OCCCN3CCOCC3)=C12)C(N)=O)C(N)=O Chemical compound CCN1N=C(C)C=C1C(=O)NC1=NC2=CC(=CC(OC)=C2N1C\C=C\CN1C(NC(=O)C2=CC(C)=NN2CC)=NC2=CC(=CC(OCCCN3CCOCC3)=C12)C(N)=O)C(N)=O JGLMVXWAHNTPRF-CMDGGOBGSA-N 0.000 description 1
- XICHBRZVQFJBGG-UHFFFAOYSA-N CCOC(=O)C1CCCN1CC(C)C Chemical compound CCOC(=O)C1CCCN1CC(C)C XICHBRZVQFJBGG-UHFFFAOYSA-N 0.000 description 1
- JAYRWHQRUSBHNP-QWRGUYRKSA-N COC(=O)[C@@H]1C[C@H](N)CN1CC1CCCC1 Chemical compound COC(=O)[C@@H]1C[C@H](N)CN1CC1CCCC1 JAYRWHQRUSBHNP-QWRGUYRKSA-N 0.000 description 1
- KBWOQDZAOSEOGF-ZDUSSCGKSA-N COC([C@H](C1)N(CC2CCCCC2)CCC1=[N+]=N)=O Chemical compound COC([C@H](C1)N(CC2CCCCC2)CCC1=[N+]=N)=O KBWOQDZAOSEOGF-ZDUSSCGKSA-N 0.000 description 1
- WCIFBCVKXZIWBR-JEAXJGTLSA-N COC([C@H](C1)NCC[C@H]1N=[N+]=[N-])O Chemical compound COC([C@H](C1)NCC[C@H]1N=[N+]=[N-])O WCIFBCVKXZIWBR-JEAXJGTLSA-N 0.000 description 1
- ZRZSUEGVAWYGBP-WABBHOIFSA-N C[N+](=N)=N[C@@H](C1)CN[C@@H]1C(O)OC Chemical compound C[N+](=N)=N[C@@H](C1)CN[C@@H]1C(O)OC ZRZSUEGVAWYGBP-WABBHOIFSA-N 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-N Caprylic acid Natural products CCCCCCCC(O)=O WWZKQHOCKIZLMA-UHFFFAOYSA-N 0.000 description 1
- 239000005973 Carvone Substances 0.000 description 1
- 102000053642 Catalytic RNA Human genes 0.000 description 1
- 108090000994 Catalytic RNA Proteins 0.000 description 1
- 101710163595 Chaperone protein DnaK Proteins 0.000 description 1
- WBYWAXJHAXSJNI-SREVYHEPSA-N Cinnamic acid Chemical compound OC(=O)\C=C/C1=CC=CC=C1 WBYWAXJHAXSJNI-SREVYHEPSA-N 0.000 description 1
- 229940126639 Compound 33 Drugs 0.000 description 1
- 102000001493 Cyclophilins Human genes 0.000 description 1
- 108010068682 Cyclophilins Proteins 0.000 description 1
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 1
- 108010036949 Cyclosporine Proteins 0.000 description 1
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Natural products OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 239000004338 Dichlorodifluoromethane Substances 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 108010008165 Etanercept Proteins 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- 238000005863 Friedel-Crafts acylation reaction Methods 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- CATMPQFFVNKDEY-YPMHNXCESA-N Golotimod Chemical compound C1=CC=C2C(C[C@H](NC(=O)CC[C@@H](N)C(O)=O)C(O)=O)=CNC2=C1 CATMPQFFVNKDEY-YPMHNXCESA-N 0.000 description 1
- 101710178376 Heat shock 70 kDa protein Proteins 0.000 description 1
- 101710152018 Heat shock cognate 70 kDa protein Proteins 0.000 description 1
- 101710113864 Heat shock protein 90 Proteins 0.000 description 1
- 102100034051 Heat shock protein HSP 90-alpha Human genes 0.000 description 1
- 108060003951 Immunoglobulin Proteins 0.000 description 1
- 102000000521 Immunophilins Human genes 0.000 description 1
- 108010016648 Immunophilins Proteins 0.000 description 1
- 101710200424 Inosine-5'-monophosphate dehydrogenase Proteins 0.000 description 1
- 108010015268 Integration Host Factors Proteins 0.000 description 1
- 108010078049 Interferon alpha-2 Proteins 0.000 description 1
- 102100040018 Interferon alpha-2 Human genes 0.000 description 1
- 108010086140 Interferon alpha-beta Receptor Proteins 0.000 description 1
- 102000007438 Interferon alpha-beta Receptor Human genes 0.000 description 1
- 102100026720 Interferon beta Human genes 0.000 description 1
- 102100037850 Interferon gamma Human genes 0.000 description 1
- 108010079944 Interferon-alpha2b Proteins 0.000 description 1
- 108090000467 Interferon-beta Proteins 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102000015696 Interleukins Human genes 0.000 description 1
- 108010063738 Interleukins Proteins 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- 108060004795 Methyltransferase Proteins 0.000 description 1
- 108700011259 MicroRNAs Proteins 0.000 description 1
- 229920000881 Modified starch Polymers 0.000 description 1
- TXXHDPDFNKHHGW-CCAGOZQPSA-N Muconic acid Natural products OC(=O)\C=C/C=C\C(O)=O TXXHDPDFNKHHGW-CCAGOZQPSA-N 0.000 description 1
- SJRJJKPEHAURKC-UHFFFAOYSA-N N-Methylmorpholine Chemical compound CN1CCOCC1 SJRJJKPEHAURKC-UHFFFAOYSA-N 0.000 description 1
- PCLIMKBDDGJMGD-UHFFFAOYSA-N N-bromosuccinimide Chemical compound BrN1C(=O)CCC1=O PCLIMKBDDGJMGD-UHFFFAOYSA-N 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- NMKQYVIKIBQVFX-BQBZGAKWSA-N N[C@H]1C[C@H](N(C1)C(=O)O)C=1OC(=CN=1)C Chemical compound N[C@H]1C[C@H](N(C1)C(=O)O)C=1OC(=CN=1)C NMKQYVIKIBQVFX-BQBZGAKWSA-N 0.000 description 1
- JOTPMBSPOYXURM-WDSKDSINSA-N N[C@H]1C[C@H](N(C1)C(=O)O)C=1OC=CN1 Chemical compound N[C@H]1C[C@H](N(C1)C(=O)O)C=1OC=CN1 JOTPMBSPOYXURM-WDSKDSINSA-N 0.000 description 1
- DSXMPZUQTIWCPB-QWRGUYRKSA-N N[C@H]1C[C@H](N(C1)CC1CCCCC1)C(=O)O Chemical compound N[C@H]1C[C@H](N(C1)CC1CCCCC1)C(=O)O DSXMPZUQTIWCPB-QWRGUYRKSA-N 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 101710144111 Non-structural protein 3 Proteins 0.000 description 1
- 101800001019 Non-structural protein 4B Proteins 0.000 description 1
- SJLAZODPABVGMQ-KSSFIOAISA-N O1C(=NC2=C1C=CC=C2)[C@H]1N(C[C@H](C1)NC(=O)C1=C(C2=CC=CC=C2C=C1)O)C(=O)O Chemical compound O1C(=NC2=C1C=CC=C2)[C@H]1N(C[C@H](C1)NC(=O)C1=C(C2=CC=CC=C2C=C1)O)C(=O)O SJLAZODPABVGMQ-KSSFIOAISA-N 0.000 description 1
- OQZKLJQSYZUGTE-BQBZGAKWSA-N O1C(C)=CN=C1[C@H]1NC[C@@H](N)C1 Chemical compound O1C(C)=CN=C1[C@H]1NC[C@@H](N)C1 OQZKLJQSYZUGTE-BQBZGAKWSA-N 0.000 description 1
- OHWIHFWQMRBQMV-UHFFFAOYSA-N O=CNc(cccc1)c1Br Chemical compound O=CNc(cccc1)c1Br OHWIHFWQMRBQMV-UHFFFAOYSA-N 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 101800000212 P1A protein Proteins 0.000 description 1
- 101800000862 Protease cofactor Proteins 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 101800001554 RNA-directed RNA polymerase Proteins 0.000 description 1
- PNUZDKCDAWUEGK-CYZMBNFOSA-N Sitafloxacin Chemical compound C([C@H]1N)N(C=2C(=C3C(C(C(C(O)=O)=CN3[C@H]3[C@H](C3)F)=O)=CC=2F)Cl)CC11CC1 PNUZDKCDAWUEGK-CYZMBNFOSA-N 0.000 description 1
- MHFMTUBUVQZIRE-WINRQGAFSA-N Sovaprevir Chemical compound C([C@H](C(=O)N1[C@@H](C[C@H](C1)OC=1C2=CC=C(C=C2N=C(C=1)C=1C=CC=CC=1)OC)C(=O)N[C@]1([C@@H](C1)C=C)C(=O)NS(=O)(=O)C1CC1)C(C)(C)C)C(=O)N1CCCCC1 MHFMTUBUVQZIRE-WINRQGAFSA-N 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- 230000005867 T cell response Effects 0.000 description 1
- 239000012317 TBTU Substances 0.000 description 1
- QYTDEUPAUMOIOP-UHFFFAOYSA-N TEMPO Chemical group CC1(C)CCCC(C)(C)N1[O] QYTDEUPAUMOIOP-UHFFFAOYSA-N 0.000 description 1
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 1
- 108010078233 Thymalfasin Proteins 0.000 description 1
- 102400000800 Thymosin alpha-1 Human genes 0.000 description 1
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 1
- OKJPEAGHQZHRQV-UHFFFAOYSA-N Triiodomethane Natural products IC(I)I OKJPEAGHQZHRQV-UHFFFAOYSA-N 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- 238000005644 Wolff-Kishner reduction reaction Methods 0.000 description 1
- HTJGLYIJVSDQAE-VWNXEWBOSA-N [(1s,6s,7s,8r,8ar)-1,7,8-trihydroxy-1,2,3,5,6,7,8,8a-octahydroindolizin-6-yl] butanoate Chemical compound O[C@H]1[C@H](O)[C@@H](OC(=O)CCC)CN2CC[C@H](O)[C@@H]21 HTJGLYIJVSDQAE-VWNXEWBOSA-N 0.000 description 1
- ZWELIJXAKMASLK-UGKPPGOTSA-N [(2r,3r,4r,5r)-4-acetyloxy-5-(5-amino-2-oxo-[1,3]thiazolo[4,5-d]pyrimidin-3-yl)-2-(hydroxymethyl)oxolan-3-yl] acetate Chemical compound CC(=O)O[C@@H]1[C@H](OC(=O)C)[C@@H](CO)O[C@H]1N1C(=O)SC2=CN=C(N)N=C21 ZWELIJXAKMASLK-UGKPPGOTSA-N 0.000 description 1
- HOOMGTNENMZAFP-NYNCVSEMSA-N [(2r,3r,5s)-2-(5-amino-2-oxo-[1,3]thiazolo[4,5-d]pyrimidin-3-yl)-5-(hydroxymethyl)oxolan-3-yl] acetate Chemical compound CC(=O)O[C@@H]1C[C@@H](CO)O[C@H]1N1C(=O)SC2=CN=C(N)N=C21 HOOMGTNENMZAFP-NYNCVSEMSA-N 0.000 description 1
- DGEZNRSVGBDHLK-UHFFFAOYSA-N [1,10]phenanthroline Chemical compound C1=CN=C2C3=NC=CC=C3C=CC2=C1 DGEZNRSVGBDHLK-UHFFFAOYSA-N 0.000 description 1
- CIUQDSCDWFSTQR-UHFFFAOYSA-N [C]1=CC=CC=C1 Chemical compound [C]1=CC=CC=C1 CIUQDSCDWFSTQR-UHFFFAOYSA-N 0.000 description 1
- CLZISMQKJZCZDN-UHFFFAOYSA-N [benzotriazol-1-yloxy(dimethylamino)methylidene]-dimethylazanium Chemical compound C1=CC=C2N(OC(N(C)C)=[N+](C)C)N=NC2=C1 CLZISMQKJZCZDN-UHFFFAOYSA-N 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 108010080374 albuferon Proteins 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 229910001413 alkali metal ion Inorganic materials 0.000 description 1
- 229910001420 alkaline earth metal ion Inorganic materials 0.000 description 1
- 125000004183 alkoxy alkyl group Chemical group 0.000 description 1
- 125000004457 alkyl amino carbonyl group Chemical group 0.000 description 1
- 125000004471 alkyl aminosulfonyl group Chemical group 0.000 description 1
- 125000005115 alkyl carbamoyl group Chemical group 0.000 description 1
- 125000005466 alkylenyl group Chemical group 0.000 description 1
- 239000003888 alpha glucosidase inhibitor Substances 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 150000001409 amidines Chemical class 0.000 description 1
- 235000001014 amino acid Nutrition 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 125000004103 aminoalkyl group Chemical group 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 239000000908 ammonium hydroxide Substances 0.000 description 1
- 229940124599 anti-inflammatory drug Drugs 0.000 description 1
- 230000000842 anti-protozoal effect Effects 0.000 description 1
- 239000003904 antiprotozoal agent Substances 0.000 description 1
- 239000000074 antisense oligonucleotide Substances 0.000 description 1
- 238000012230 antisense oligonucleotides Methods 0.000 description 1
- 239000008365 aqueous carrier Substances 0.000 description 1
- 239000008135 aqueous vehicle Substances 0.000 description 1
- 239000012300 argon atmosphere Substances 0.000 description 1
- 235000021311 artificial sweeteners Nutrition 0.000 description 1
- 125000005100 aryl amino carbonyl group Chemical group 0.000 description 1
- 125000005116 aryl carbamoyl group Chemical group 0.000 description 1
- 229960002118 asunaprevir Drugs 0.000 description 1
- HONIICLYMWZJFZ-UHFFFAOYSA-N azetidine Chemical compound C1CNC1 HONIICLYMWZJFZ-UHFFFAOYSA-N 0.000 description 1
- 125000002393 azetidinyl group Chemical group 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 1
- 229940092714 benzenesulfonic acid Drugs 0.000 description 1
- 125000004618 benzofuryl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000003236 benzoyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C(*)=O 0.000 description 1
- VHANWEHPHUEIKW-OFVILXPXSA-N benzyl (2s,4s)-2-(1,3-benzoxazol-2-yl)-4-[(1-hydroxynaphthalene-2-carbonyl)amino]pyrrolidine-1-carboxylate Chemical compound N1([C@@H](C[C@@H](C1)NC(=O)C1=C(C2=CC=CC=C2C=C1)O)C=1OC2=CC=CC=C2N=1)C(=O)OCC1=CC=CC=C1 VHANWEHPHUEIKW-OFVILXPXSA-N 0.000 description 1
- VALPXRNQZYGXPS-UHFFFAOYSA-N benzyl pyrrolidine-1-carboxylate Chemical compound C1CCCN1C(=O)OCC1=CC=CC=C1 VALPXRNQZYGXPS-UHFFFAOYSA-N 0.000 description 1
- GONOPSZTUGRENK-UHFFFAOYSA-N benzyl(trichloro)silane Chemical compound Cl[Si](Cl)(Cl)CC1=CC=CC=C1 GONOPSZTUGRENK-UHFFFAOYSA-N 0.000 description 1
- 125000001584 benzyloxycarbonyl group Chemical group C(=O)(OCC1=CC=CC=C1)* 0.000 description 1
- MUALRAIOVNYAIW-UHFFFAOYSA-N binap Chemical group C1=CC=CC=C1P(C=1C(=C2C=CC=CC2=CC=1)C=1C2=CC=CC=C2C=CC=1P(C=1C=CC=CC=1)C=1C=CC=CC=1)C1=CC=CC=C1 MUALRAIOVNYAIW-UHFFFAOYSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 229920002988 biodegradable polymer Polymers 0.000 description 1
- 239000004621 biodegradable polymer Substances 0.000 description 1
- 239000004305 biphenyl Substances 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- AZWXAPCAJCYGIA-UHFFFAOYSA-N bis(2-methylpropyl)alumane Chemical compound CC(C)C[AlH]CC(C)C AZWXAPCAJCYGIA-UHFFFAOYSA-N 0.000 description 1
- SIPUZPBQZHNSDW-UHFFFAOYSA-N bis(2-methylpropyl)aluminum Chemical compound CC(C)C[Al]CC(C)C SIPUZPBQZHNSDW-UHFFFAOYSA-N 0.000 description 1
- 229960000517 boceprevir Drugs 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- 150000001722 carbon compounds Chemical class 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 229960004424 carbon dioxide Drugs 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 229950003414 celgosivir Drugs 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 238000001516 cell proliferation assay Methods 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- KYKAJFCTULSVSH-UHFFFAOYSA-N chloro(fluoro)methane Chemical compound F[C]Cl KYKAJFCTULSVSH-UHFFFAOYSA-N 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 229960001265 ciclosporin Drugs 0.000 description 1
- PJZPDFUUXKKDNB-KNINVFKUSA-N ciluprevir Chemical compound N([C@@H]1C(=O)N2[C@H](C(N[C@@]3(C[C@H]3\C=C/CCCCC1)C(O)=O)=O)C[C@H](C2)OC=1C2=CC=C(C=C2N=C(C=1)C=1N=C(NC(C)C)SC=1)OC)C(=O)OC1CCCC1 PJZPDFUUXKKDNB-KNINVFKUSA-N 0.000 description 1
- 229950006631 ciluprevir Drugs 0.000 description 1
- 235000013985 cinnamic acid Nutrition 0.000 description 1
- 229930016911 cinnamic acid Natural products 0.000 description 1
- 230000007882 cirrhosis Effects 0.000 description 1
- 208000019425 cirrhosis of liver Diseases 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 229940125904 compound 1 Drugs 0.000 description 1
- 229940125833 compound 23 Drugs 0.000 description 1
- 229940127573 compound 38 Drugs 0.000 description 1
- 239000013068 control sample Substances 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- LSXDOTMGLUJQCM-UHFFFAOYSA-M copper(i) iodide Chemical compound I[Cu] LSXDOTMGLUJQCM-UHFFFAOYSA-M 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- INVYSLWXPIEDIQ-UHFFFAOYSA-N cyclobutanecarbaldehyde Chemical compound O=CC1CCC1 INVYSLWXPIEDIQ-UHFFFAOYSA-N 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- RVOJTCZRIKWHDX-UHFFFAOYSA-N cyclohexanecarbonyl chloride Chemical compound ClC(=O)C1CCCCC1 RVOJTCZRIKWHDX-UHFFFAOYSA-N 0.000 description 1
- 125000000640 cyclooctyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- VELDYOPRLMJFIK-UHFFFAOYSA-N cyclopentanecarbaldehyde Chemical compound O=CC1CCCC1 VELDYOPRLMJFIK-UHFFFAOYSA-N 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 229930182912 cyclosporin Natural products 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- NBRBXGKOEOGLOI-UHFFFAOYSA-N dasabuvir Chemical compound C1=C(C(C)(C)C)C(OC)=C(C=2C=C3C=CC(NS(C)(=O)=O)=CC3=CC=2)C=C1N1C=CC(=O)NC1=O NBRBXGKOEOGLOI-UHFFFAOYSA-N 0.000 description 1
- 229960001418 dasabuvir Drugs 0.000 description 1
- BMAIGAHXAJEULY-UKTHLTGXSA-N deleobuvir Chemical compound C12=CC=C(C(=O)NC3(CCC3)C=3N(C4=CC(\C=C\C(O)=O)=CC=C4N=3)C)C=C2N(C)C(C=2N=CC(Br)=CN=2)=C1C1CCCC1 BMAIGAHXAJEULY-UKTHLTGXSA-N 0.000 description 1
- 230000000368 destabilizing effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 125000004985 dialkyl amino alkyl group Chemical group 0.000 description 1
- 125000004663 dialkyl amino group Chemical group 0.000 description 1
- 125000005117 dialkylcarbamoyl group Chemical group 0.000 description 1
- 125000000664 diazo group Chemical group [N-]=[N+]=[*] 0.000 description 1
- 238000006193 diazotization reaction Methods 0.000 description 1
- WMKGGPCROCCUDY-PHEQNACWSA-N dibenzylideneacetone Chemical compound C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1 WMKGGPCROCCUDY-PHEQNACWSA-N 0.000 description 1
- PXBRQCKWGAHEHS-UHFFFAOYSA-N dichlorodifluoromethane Chemical compound FC(F)(Cl)Cl PXBRQCKWGAHEHS-UHFFFAOYSA-N 0.000 description 1
- 235000019404 dichlorodifluoromethane Nutrition 0.000 description 1
- 229940042935 dichlorodifluoromethane Drugs 0.000 description 1
- 229940087091 dichlorotetrafluoroethane Drugs 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- BOBUBHOXRCYKLI-UHFFFAOYSA-N ditert-butyl(cyclopenta-2,4-dien-1-yl)phosphane;iron(2+);(2,3,4,5-tetraphenylcyclopenta-1,4-dien-1-yl)benzene Chemical compound [Fe+2].CC(C)(C)P(C(C)(C)C)C1=CC=C[CH-]1.C1=CC=CC=C1C1=C(C=2C=CC=CC=2)[C-](C=2C=CC=CC=2)C(C=2C=CC=CC=2)=C1C1=CC=CC=C1 BOBUBHOXRCYKLI-UHFFFAOYSA-N 0.000 description 1
- MOTZDAYCYVMXPC-UHFFFAOYSA-N dodecyl hydrogen sulfate Chemical compound CCCCCCCCCCCCOS(O)(=O)=O MOTZDAYCYVMXPC-UHFFFAOYSA-N 0.000 description 1
- 239000008298 dragée Substances 0.000 description 1
- 239000000890 drug combination Substances 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 229940073621 enbrel Drugs 0.000 description 1
- 239000002702 enteric coating Substances 0.000 description 1
- 238000009505 enteric coating Methods 0.000 description 1
- 210000002615 epidermis Anatomy 0.000 description 1
- AFAXGSQYZLGZPG-UHFFFAOYSA-N ethanedisulfonic acid Chemical compound OS(=O)(=O)CCS(O)(=O)=O AFAXGSQYZLGZPG-UHFFFAOYSA-N 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- XZNLICJJUUMHOD-RYUDHWBXSA-N ethyl (2s,4s)-4-amino-1-(cyclopentylmethyl)pyrrolidine-2-carboxylate Chemical compound CCOC(=O)[C@@H]1C[C@H](N)CN1CC1CCCC1 XZNLICJJUUMHOD-RYUDHWBXSA-N 0.000 description 1
- VFRSADQPWYCXDG-LEUCUCNGSA-N ethyl (2s,5s)-5-methylpyrrolidine-2-carboxylate;2,2,2-trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F.CCOC(=O)[C@@H]1CC[C@H](C)N1 VFRSADQPWYCXDG-LEUCUCNGSA-N 0.000 description 1
- YSHYBOANKZNAAU-UHFFFAOYSA-N ethyl 1-(cyclohexanecarbonyl)pyrrolidine-2-carboxylate Chemical compound CCOC(=O)C1CCCN1C(=O)C1CCCCC1 YSHYBOANKZNAAU-UHFFFAOYSA-N 0.000 description 1
- IXNFXSRMSVKKNP-UHFFFAOYSA-N ethyl 1-(cyclopentylmethyl)pyrrolidine-2-carboxylate Chemical compound CCOC(=O)C1CCCN1CC1CCCC1 IXNFXSRMSVKKNP-UHFFFAOYSA-N 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 239000003349 gelling agent Substances 0.000 description 1
- 239000000174 gluconic acid Substances 0.000 description 1
- 235000012208 gluconic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 150000002334 glycols Chemical class 0.000 description 1
- 108010049353 golotimod Proteins 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 230000005802 health problem Effects 0.000 description 1
- 210000002443 helper t lymphocyte Anatomy 0.000 description 1
- 208000010710 hepatitis C virus infection Diseases 0.000 description 1
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 1
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000004634 hexahydroazepinyl group Chemical group N1(CCCCCC1)* 0.000 description 1
- FFUAGWLWBBFQJT-UHFFFAOYSA-N hexamethyldisilazane Chemical compound C[Si](C)(C)N[Si](C)(C)C FFUAGWLWBBFQJT-UHFFFAOYSA-N 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 229960004931 histamine dihydrochloride Drugs 0.000 description 1
- PPZMYIBUHIPZOS-UHFFFAOYSA-N histamine dihydrochloride Chemical compound Cl.Cl.NCCC1=CN=CN1 PPZMYIBUHIPZOS-UHFFFAOYSA-N 0.000 description 1
- 239000008240 homogeneous mixture Substances 0.000 description 1
- 102000011749 human hepatitis C immune globulin Human genes 0.000 description 1
- 108010062138 human hepatitis C immune globulin Proteins 0.000 description 1
- 229940048921 humira Drugs 0.000 description 1
- 150000004678 hydrides Chemical class 0.000 description 1
- RCBVKBFIWMOMHF-UHFFFAOYSA-L hydroxy-(hydroxy(dioxo)chromio)oxy-dioxochromium;pyridine Chemical compound C1=CC=NC=C1.C1=CC=NC=C1.O[Cr](=O)(=O)O[Cr](O)(=O)=O RCBVKBFIWMOMHF-UHFFFAOYSA-L 0.000 description 1
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 1
- 125000002636 imidazolinyl group Chemical group 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 102000018358 immunoglobulin Human genes 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 239000002955 immunomodulating agent Substances 0.000 description 1
- 229940121354 immunomodulator Drugs 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 229940090438 infergen Drugs 0.000 description 1
- 239000003978 infusion fluid Substances 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 229940047122 interleukins Drugs 0.000 description 1
- 239000013067 intermediate product Substances 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 229940065638 intron a Drugs 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- JJWLVOIRVHMVIS-UHFFFAOYSA-N isopropylamine Chemical compound CC(C)N JJWLVOIRVHMVIS-UHFFFAOYSA-N 0.000 description 1
- 125000002183 isoquinolinyl group Chemical group C1(=NC=CC2=CC=CC=C12)* 0.000 description 1
- ZLTPDFXIESTBQG-UHFFFAOYSA-N isothiazole Chemical compound C=1C=NSC=1 ZLTPDFXIESTBQG-UHFFFAOYSA-N 0.000 description 1
- CTAPFRYPJLPFDF-UHFFFAOYSA-N isoxazole Chemical compound C=1C=NOC=1 CTAPFRYPJLPFDF-UHFFFAOYSA-N 0.000 description 1
- 125000003965 isoxazolidinyl group Chemical group 0.000 description 1
- 125000000468 ketone group Chemical group 0.000 description 1
- 229940043355 kinase inhibitor Drugs 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 208000019423 liver disease Diseases 0.000 description 1
- 108010046177 locteron Proteins 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 238000003670 luciferase enzyme activity assay Methods 0.000 description 1
- 238000003468 luciferase reporter gene assay Methods 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- 238000001819 mass spectrum Methods 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 238000002483 medication Methods 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- QJWIOUAZOWOZDS-WHFBIAKZSA-N methyl (2s,4s)-4-azidopyrrolidine-2-carboxylate Chemical compound COC(=O)[C@@H]1C[C@H](N=[N+]=[N-])CN1 QJWIOUAZOWOZDS-WHFBIAKZSA-N 0.000 description 1
- NGJACAHAGMHHQT-UHFFFAOYSA-N methyl 1-benzylpyrrolidine-2-carboxylate Chemical compound COC(=O)C1CCCN1CC1=CC=CC=C1 NGJACAHAGMHHQT-UHFFFAOYSA-N 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- WBYWAXJHAXSJNI-UHFFFAOYSA-N methyl p-hydroxycinnamate Natural products OC(=O)C=CC1=CC=CC=C1 WBYWAXJHAXSJNI-UHFFFAOYSA-N 0.000 description 1
- GDOPTJXRTPNYNR-UHFFFAOYSA-N methyl-cyclopentane Natural products CC1CCCC1 GDOPTJXRTPNYNR-UHFFFAOYSA-N 0.000 description 1
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 description 1
- 125000004170 methylsulfonyl group Chemical group [H]C([H])([H])S(*)(=O)=O 0.000 description 1
- 239000002679 microRNA Substances 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 235000019426 modified starch Nutrition 0.000 description 1
- VOWOEBADKMXUBU-UHFFFAOYSA-J molecular oxygen;tetrachlorite;hydrate Chemical compound O.O=O.[O-]Cl=O.[O-]Cl=O.[O-]Cl=O.[O-]Cl=O VOWOEBADKMXUBU-UHFFFAOYSA-J 0.000 description 1
- 150000004682 monohydrates Chemical class 0.000 description 1
- 125000002757 morpholinyl group Chemical group 0.000 description 1
- 239000002324 mouth wash Substances 0.000 description 1
- 229940051866 mouthwash Drugs 0.000 description 1
- OKDQKPLMQBXTNH-UHFFFAOYSA-N n,n-dimethyl-2h-pyridin-1-amine Chemical compound CN(C)N1CC=CC=C1 OKDQKPLMQBXTNH-UHFFFAOYSA-N 0.000 description 1
- XMZSTQYSBYEENY-RMKNXTFCSA-N n-[4-[(e)-2-[3-tert-butyl-5-(2,4-dioxopyrimidin-1-yl)-2-methoxyphenyl]ethenyl]phenyl]methanesulfonamide Chemical compound C1=C(N2C(NC(=O)C=C2)=O)C=C(C(C)(C)C)C(OC)=C1\C=C\C1=CC=C(NS(C)(=O)=O)C=C1 XMZSTQYSBYEENY-RMKNXTFCSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N n-hexanoic acid Natural products CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- OHDXDNUPVVYWOV-UHFFFAOYSA-N n-methyl-1-(2-naphthalen-1-ylsulfanylphenyl)methanamine Chemical compound CNCC1=CC=CC=C1SC1=CC=CC2=CC=CC=C12 OHDXDNUPVVYWOV-UHFFFAOYSA-N 0.000 description 1
- 125000004593 naphthyridinyl group Chemical group N1=C(C=CC2=CC=CN=C12)* 0.000 description 1
- RICZEKWVNZFTNZ-LFGITCQGSA-N narlaprevir Chemical compound N([C@H](C(=O)N1C[C@H]2[C@H](C2(C)C)[C@H]1C(=O)N[C@@H](CCCC)C(=O)C(=O)NC1CC1)C(C)(C)C)C(=O)NC1(CS(=O)(=O)C(C)(C)C)CCCCC1 RICZEKWVNZFTNZ-LFGITCQGSA-N 0.000 description 1
- 229950003504 narlaprevir Drugs 0.000 description 1
- 235000021096 natural sweeteners Nutrition 0.000 description 1
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 238000006396 nitration reaction Methods 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 125000003835 nucleoside group Chemical group 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- PIDFDZJZLOTZTM-KHVQSSSXSA-N ombitasvir Chemical compound COC(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)NC1=CC=C([C@H]2N([C@@H](CC2)C=2C=CC(NC(=O)[C@H]3N(CCC3)C(=O)[C@@H](NC(=O)OC)C(C)C)=CC=2)C=2C=CC(=CC=2)C(C)(C)C)C=C1 PIDFDZJZLOTZTM-KHVQSSSXSA-N 0.000 description 1
- 239000006186 oral dosage form Substances 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 150000002895 organic esters Chemical class 0.000 description 1
- 238000006053 organic reaction Methods 0.000 description 1
- AICOOMRHRUFYCM-ZRRPKQBOSA-N oxazine, 1 Chemical compound C([C@@H]1[C@H](C(C[C@]2(C)[C@@H]([C@H](C)N(C)C)[C@H](O)C[C@]21C)=O)CC1=CC2)C[C@H]1[C@@]1(C)[C@H]2N=C(C(C)C)OC1 AICOOMRHRUFYCM-ZRRPKQBOSA-N 0.000 description 1
- 125000000160 oxazolidinyl group Chemical group 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 125000003566 oxetanyl group Chemical group 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- 125000004043 oxo group Chemical group O=* 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- MUJIDPITZJWBSW-UHFFFAOYSA-N palladium(2+) Chemical compound [Pd+2] MUJIDPITZJWBSW-UHFFFAOYSA-N 0.000 description 1
- LXNAVEXFUKBNMK-UHFFFAOYSA-N palladium(II) acetate Substances [Pd].CC(O)=O.CC(O)=O LXNAVEXFUKBNMK-UHFFFAOYSA-N 0.000 description 1
- YJVFFLUZDVXJQI-UHFFFAOYSA-L palladium(ii) acetate Chemical compound [Pd+2].CC([O-])=O.CC([O-])=O YJVFFLUZDVXJQI-UHFFFAOYSA-L 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- QNGNSVIICDLXHT-UHFFFAOYSA-N para-ethylbenzaldehyde Natural products CCC1=CC=C(C=O)C=C1 QNGNSVIICDLXHT-UHFFFAOYSA-N 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- UAUIUKWPKRJZJV-MDJGTQRPSA-N paritaprevir Chemical compound C1=NC(C)=CN=C1C(=O)N[C@@H]1C(=O)N2C[C@H](OC=3C4=CC=CC=C4C4=CC=CC=C4N=3)C[C@H]2C(=O)N[C@]2(C(=O)NS(=O)(=O)C3CC3)C[C@@H]2\C=C/CCCCC1 UAUIUKWPKRJZJV-MDJGTQRPSA-N 0.000 description 1
- 229960002754 paritaprevir Drugs 0.000 description 1
- 239000006072 paste Substances 0.000 description 1
- 235000014594 pastries Nutrition 0.000 description 1
- 239000001814 pectin Substances 0.000 description 1
- 235000010987 pectin Nutrition 0.000 description 1
- 229920001277 pectin Polymers 0.000 description 1
- 229940002988 pegasys Drugs 0.000 description 1
- 108010092853 peginterferon alfa-2a Proteins 0.000 description 1
- 108010092851 peginterferon alfa-2b Proteins 0.000 description 1
- 229940106366 pegintron Drugs 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 230000003285 pharmacodynamic effect Effects 0.000 description 1
- 229960003742 phenol Drugs 0.000 description 1
- 150000002989 phenols Chemical class 0.000 description 1
- UYWQUFXKFGHYNT-UHFFFAOYSA-N phenylmethyl ester of formic acid Natural products O=COCC1=CC=CC=C1 UYWQUFXKFGHYNT-UHFFFAOYSA-N 0.000 description 1
- 229910000073 phosphorus hydride Inorganic materials 0.000 description 1
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 125000004193 piperazinyl group Chemical group 0.000 description 1
- 125000003386 piperidinyl group Chemical group 0.000 description 1
- IUGYQRQAERSCNH-UHFFFAOYSA-N pivalic acid Chemical compound CC(C)(C)C(O)=O IUGYQRQAERSCNH-UHFFFAOYSA-N 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 239000004626 polylactic acid Substances 0.000 description 1
- 230000008092 positive effect Effects 0.000 description 1
- WSHYKIAQCMIPTB-UHFFFAOYSA-M potassium;2-oxo-3-(3-oxo-1-phenylbutyl)chromen-4-olate Chemical compound [K+].[O-]C=1C2=CC=CC=C2OC(=O)C=1C(CC(=O)C)C1=CC=CC=C1 WSHYKIAQCMIPTB-UHFFFAOYSA-M 0.000 description 1
- RYXIBQLRUHDYEE-UHFFFAOYSA-M potassium;5-(cyclohexen-1-yl)-3-[(4-methoxycyclohexyl)-(4-methylcyclohexanecarbonyl)amino]thiophene-2-carboxylate Chemical compound [K+].C1CC(OC)CCC1N(C1=C(SC(=C1)C=1CCCCC=1)C([O-])=O)C(=O)C1CCC(C)CC1 RYXIBQLRUHDYEE-UHFFFAOYSA-M 0.000 description 1
- 238000002953 preparative HPLC Methods 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 150000003138 primary alcohols Chemical class 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- XEABSBMNTNXEJM-UHFFFAOYSA-N propagermanium Chemical compound OC(=O)CC[Ge](=O)O[Ge](=O)CCC(O)=O XEABSBMNTNXEJM-UHFFFAOYSA-N 0.000 description 1
- 229950002828 propagermanium Drugs 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 229940021993 prophylactic vaccine Drugs 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000003373 pyrazinyl group Chemical group 0.000 description 1
- 125000003226 pyrazolyl group Chemical group 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 125000004076 pyridyl group Chemical group 0.000 description 1
- 125000000714 pyrimidinyl group Chemical group 0.000 description 1
- 125000000719 pyrrolidinyl group Chemical group 0.000 description 1
- 125000000168 pyrrolyl group Chemical group 0.000 description 1
- 229940107700 pyruvic acid Drugs 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 1
- 125000004621 quinuclidinyl group Chemical group N12C(CC(CC1)CC2)* 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 229940044601 receptor agonist Drugs 0.000 description 1
- 239000000018 receptor agonist Substances 0.000 description 1
- 229940116176 remicade Drugs 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 229950010550 resiquimod Drugs 0.000 description 1
- BXNMTOQRYBFHNZ-UHFFFAOYSA-N resiquimod Chemical compound C1=CC=CC2=C(N(C(COCC)=N3)CC(C)(C)O)C3=C(N)N=C21 BXNMTOQRYBFHNZ-UHFFFAOYSA-N 0.000 description 1
- 210000002345 respiratory system Anatomy 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000008593 response to virus Effects 0.000 description 1
- 210000003705 ribosome Anatomy 0.000 description 1
- 108091092562 ribozyme Proteins 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 150000003333 secondary alcohols Chemical class 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000011450 sequencing therapy Methods 0.000 description 1
- DEKOYVOWOVJMPM-RLHIPHHXSA-N setrobuvir Chemical compound N1([C@H]2[C@@H]3CC[C@@H](C3)[C@H]2C(O)=C(C1=O)C=1NC2=CC=C(C=C2S(=O)(=O)N=1)NS(=O)(=O)C)CC1=CC=C(F)C=C1 DEKOYVOWOVJMPM-RLHIPHHXSA-N 0.000 description 1
- 230000035939 shock Effects 0.000 description 1
- 238000010898 silica gel chromatography Methods 0.000 description 1
- 229920002379 silicone rubber Polymers 0.000 description 1
- 239000004945 silicone rubber Substances 0.000 description 1
- 229960002091 simeprevir Drugs 0.000 description 1
- 238000009097 single-agent therapy Methods 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- SSERCMQZZYTNBY-UHFFFAOYSA-M sodium;3-[(4-hydroxycyclohexyl)-(4-methylcyclohexanecarbonyl)amino]-5-phenylthiophene-2-carboxylate Chemical compound [Na+].C1CC(C)CCC1C(=O)N(C1=C(SC(=C1)C=1C=CC=CC=1)C([O-])=O)C1CCC(O)CC1 SSERCMQZZYTNBY-UHFFFAOYSA-M 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 239000001593 sorbitan monooleate Substances 0.000 description 1
- 235000011069 sorbitan monooleate Nutrition 0.000 description 1
- 229940035049 sorbitan monooleate Drugs 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- LBJQKYPPYSCCBH-UHFFFAOYSA-N spiro[3.3]heptane Chemical compound C1CCC21CCC2 LBJQKYPPYSCCBH-UHFFFAOYSA-N 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 238000011272 standard treatment Methods 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- PXQLVRUNWNTZOS-UHFFFAOYSA-N sulfanyl Chemical class [SH] PXQLVRUNWNTZOS-UHFFFAOYSA-N 0.000 description 1
- 230000000153 supplemental effect Effects 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 238000003419 tautomerization reaction Methods 0.000 description 1
- BBAWEDCPNXPBQM-GDEBMMAJSA-N telaprevir Chemical compound N([C@H](C(=O)N[C@H](C(=O)N1C[C@@H]2CCC[C@@H]2[C@H]1C(=O)N[C@@H](CCC)C(=O)C(=O)NC1CC1)C(C)(C)C)C1CCCCC1)C(=O)C1=CN=CC=N1 BBAWEDCPNXPBQM-GDEBMMAJSA-N 0.000 description 1
- PGJHKGDUVQTASP-UHFFFAOYSA-N tert-butyl ethanimidate Chemical compound CC(=N)OC(C)(C)C PGJHKGDUVQTASP-UHFFFAOYSA-N 0.000 description 1
- 125000005931 tert-butyloxycarbonyl group Chemical group [H]C([H])([H])C(OC(*)=O)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- MLGCXEBRWGEOQX-UHFFFAOYSA-N tetradifon Chemical compound C1=CC(Cl)=CC=C1S(=O)(=O)C1=CC(Cl)=C(Cl)C=C1Cl MLGCXEBRWGEOQX-UHFFFAOYSA-N 0.000 description 1
- 125000003718 tetrahydrofuranyl group Chemical group 0.000 description 1
- 125000001412 tetrahydropyranyl group Chemical group 0.000 description 1
- 125000003507 tetrahydrothiofenyl group Chemical group 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 229940021747 therapeutic vaccine Drugs 0.000 description 1
- VLLMWSRANPNYQX-UHFFFAOYSA-N thiadiazole Chemical compound C1=CSN=N1.C1=CSN=N1 VLLMWSRANPNYQX-UHFFFAOYSA-N 0.000 description 1
- 125000001984 thiazolidinyl group Chemical group 0.000 description 1
- 125000004568 thiomorpholinyl group Chemical group 0.000 description 1
- NZVYCXVTEHPMHE-ZSUJOUNUSA-N thymalfasin Chemical compound CC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O NZVYCXVTEHPMHE-ZSUJOUNUSA-N 0.000 description 1
- 229960004231 thymalfasin Drugs 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 239000012049 topical pharmaceutical composition Substances 0.000 description 1
- 125000002088 tosyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1C([H])([H])[H])S(*)(=O)=O 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 230000037317 transdermal delivery Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- CYRMSUTZVYGINF-UHFFFAOYSA-N trichlorofluoromethane Chemical compound FC(Cl)(Cl)Cl CYRMSUTZVYGINF-UHFFFAOYSA-N 0.000 description 1
- 229940029284 trichlorofluoromethane Drugs 0.000 description 1
- IMNIMPAHZVJRPE-UHFFFAOYSA-N triethylenediamine Chemical compound C1CN2CCN1CC2 IMNIMPAHZVJRPE-UHFFFAOYSA-N 0.000 description 1
- ITMCEJHCFYSIIV-UHFFFAOYSA-M triflate Chemical compound [O-]S(=O)(=O)C(F)(F)F ITMCEJHCFYSIIV-UHFFFAOYSA-M 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 229960000281 trometamol Drugs 0.000 description 1
- 108010077753 type II interferon receptor Proteins 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 230000007502 viral entry Effects 0.000 description 1
- 239000011345 viscous material Substances 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/04—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/04—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D207/10—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D207/16—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D211/00—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings
- C07D211/04—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D211/06—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D211/36—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D211/60—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/06—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
Landscapes
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Virology (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Oncology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Communicable Diseases (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Molecular Biology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Hydrogenated Pyridines (AREA)
- Plural Heterocyclic Compounds (AREA)
- Pyrrole Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Description
nは、1または2であり;
Qは、1個以上のQ’で置換されたフェニルまたはナフタレンであり;
Q’は、ヒドロキシル、低級アルキル、またはハロであり;
R1は、低級アルキル、シクロアルキル、フェニル、またはヘテロシクロアルキルであり;
R2は、−C(=O)OR2’、−C(=O)R2’、−C(=O)ON(R2’)2、任意に1個以上のR2’で置換されていてもよい単環式または二環式ヘテロアリールであり;
R2’はそれぞれ、独立してH、低級アルキルまたはヘテロシクロアルキルであり;
R3は、Hまたは低級アルキルであり;
Xは、CH2またはC(=O)である]
またはその医薬的に許容できる塩を提供する。
本出願は、式の化合物および医薬的に許容できる賦形剤を含む医薬組成物を提供する。
本明細書中で用いる句“a”または“an”の物は、1以上のその物を表わす;たとえば、a compoundは、one or more compounds(1種類以上の化合物)またはat least one compound(少なくとも1種類の化合物)を表わす。したがって、“a” (または“an”)、“one or more(1以上)”、および“at least one(少なくとも1)”は本明細書中で互換性をもって使用できる。
本明細書中で用いる用語“任意の(optional)”または“任意に(optionally)”は、それに続いて記載する事象または状況が出現してもよいが、その必要はないこと、およびその記載はその事象または状況が出現する場合とそれが出現しない場合とを含むことを意味する。たとえば、“任意に置換されていてもよい”は、その任意に置換されていてもよい部分が水素原子または置換基を含むことができることを意味する。
本明細書中で用いる用語“アルキル”は、1〜10個の炭素原子を含む非分枝鎖または分枝鎖、飽和、一価の炭化水素残基を表わす。用語“低級アルキル”は、1〜6個の炭素原子を含む直鎖または分枝鎖の炭化水素残基を表わす。本明細書中で用いる“C1−10アルキル”は、1〜10個の炭素から構成されるアルキルを表わす。アルキル基の例には低級アルキルが含まれるが、これらに限定されず、メチル、エチル、プロピル、i−プロピル、n−ブチル、i−ブチル、t−ブチルまたはペンチル、イソペンチル、ネオペンチル、ヘキシル、ヘプチル、およびオクチルが含まれる。
用語“ハロアルコキシ”または“ハロ−低級アルコキシ”または“低級ハロアルコキシ”は、低級アルコキシ基において1個以上の炭素原子が1個以上のハロゲン原子で置換されたものを表わす。
本明細書中で用いる用語“ヘテロアリール”または“ヘテロ芳香族”は、少なくとも1つの芳香環または部分不飽和環をもつ、環当たり4〜8個の原子を含む環原子5〜12個の単環式または二環式ラジカルであって、1個以上のN、OまたはSヘテロ原子を含み、残りの環原子が炭素であるものを意味する;ヘテロアリールラジカルの結合点は芳香環または部分不飽和環上にあると理解される。当業者に周知のように、ヘテロアリール環はそれらの全炭素カウンターパートより低い芳香族性をもつ。したがって、本発明の目的にとって、ヘテロアリール基はある程度の芳香族性をもつだけでよい。ヘテロアリール部分の例には5〜6個の環原子および1〜3個のヘテロ原子をもつ単環式芳香族複素環が含まれ、これには下記のものが含まれるが、これらに限定されない:ピリジニル、ピリミジニル、ピラジニル、オキサジニル、ピロリル、ピラゾリル、イミダゾリル、オキサゾリル、4,5−ジヒドロ−オキサゾリル、5,6−ジヒドロ−4H−[1,3]オキサゾリル、イソオキサゾール、チアゾール、イソチアゾール、トリアゾリン、チアジアゾールおよびオキサジアキソリン(oxadiaxoline);これらは任意に、下記のものから選択される1個以上、好ましくは1または2個の置換基で置換されていてもよい:ヒドロキシ、シアノ、アルキル、アルコキシ、チオ、低級ハロアルコキシ、アルキルチオ、ハロ、低級ハロアルキル、アルキルスルフィニル、アルキルスルホニル、ハロゲン、アミノ、アルキルアミノ、ジアルキルアミノ、アミノアルキル、アルキルアミノアルキル、およびジアルキルアミノアルキル、ニトロ、アルコキシカルボニルおよびカルバモイル、アルキルカルバモイル、ジアルキルカルバモイル、アリールカルバモイル、アルキルカルボニルアミノおよびアリールカルボニルアミノ。二環式部分の例には下記のものが含まれるが、これらに限定されない:キノリニル、イソキノリニル、ベンゾフリル、ベンゾチオフェニル、ベンゾオキサゾール、ベンゾイソオキサゾール、ベンゾチアゾール、ナフチリジニル、5,6,7,8−テトラヒドロ−[1,6]ナフチリジニル、およびベンゾイソチアゾール。二環式部分は任意にいずれの環において置換されていてもよいが、結合点はヘテロ原子を含む環上にある。
本出願は、式Iの化合物
nは、1または2であり;
Qは、1個以上のQ’で置換されたフェニルまたはナフタレンであり;
Q’は、ヒドロキシル、低級アルキル、またはハロであり;
R1は、低級アルキル、シクロアルキル、フェニル、またはヘテロシクロアルキルであり;
R2は、−C(=O)OR2’、−C(=O)R2’、−C(=O)ON(R2’)2、任意に1個以上のR2’で置換されていてもよい単環式または二環式ヘテロアリールであり;
R2’はそれぞれ、独立してH、低級アルキルまたはヘテロシクロアルキルであり;
R3は、Hまたは低級アルキルであり;
Xは、CH2またはC(=O)である]
またはその医薬的に許容できる塩を提供する。
本出願は、R3がHである、式Iの化合物を提供する。
本出願は、R3がHであり、かつnが1である、式Iの化合物を提供する。
本出願は、XがCH2であり、R3がHである、式Iの化合物を提供する。
本出願は、XがCH2であり、かつnが1である、式Iの化合物を提供する。
本出願は、R2が−C(=O)N(R2’)2である、式Iの化合物を提供する。
本出願は、R2が−C(=O)N(R2’)2であり、かつR3がHである、式Iの化合物を提供する。
本出願は、R2が−C(=O)N(R2’)2であり、XがCH2であり、R3がHであり、かつnが1である、式Iの化合物を提供する。
本出願は、R2が−C(=O)OR2’である、式Iの化合物を提供する。
本出願は、R2が−C(=O)OR2’であり、かつR2’が低級アルキルである、式Iの化合物を提供する。
本出願は、R2’が低級アルキルであり、XがCH2であり、R3がHであり、かつnが1である、式Iの化合物を提供する。
本出願は、R1が低級アルキルまたはシクロアルキルである、式Iの化合物を提供する。
本出願は、R1が低級アルキルまたはシクロアルキルであり、R2が−C(=O)OR2’であり、R2’が低級アルキルであり、XがCH2であり、R3がHであり、かつnが1である、式Iの化合物を提供する。
本出願は、R3がHである、式Iの化合物を提供する。
本出願は、R3がHであり、かつnが1である、式Iの化合物を提供する。
本出願は、XがCH2であり、かつR3がHである、式Iの化合物を提供する。
本出願は、XがCH2であり、かつnが1である、式Iの化合物を提供する。
本出願は、R2が−C(=O)N(R2’)2である、式Iの化合物を提供する。
本出願は、R2が−C(=O)N(R2’)2であり、かつR3がHである、式Iの化合物を提供する。
本出願は、R2が−C(=O)N(R2’)2であり、XがCH2であり、R3がHであり、かつnが1である、式Iの化合物を提供する。
本出願は、R2が−C(=O)OR2’である、式Iの化合物を提供する。
本出願は、R2が−C(=O)OR2’であり、かつR2’が低級アルキルである、式Iの化合物を提供する。
本出願は、R2’が低級アルキルであり、XがCH2であり、R3がHであり、かつnが1である、式Iの化合物を提供する。
本出願は、R1が低級アルキルまたはシクロアルキルである、式Iの化合物を提供する。
本出願は、R1が低級アルキルまたはシクロアルキルであり、R2が−C(=O)OR2’であり、R2’が低級アルキルであり、XがCH2であり、R3がHであり、かつnが1である、式Iの化合物を提供する。
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 tert−ブチルエステル;
(2S,4S)−1−(3,3−ジメチル−ブチル)−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピペリジン−2−カルボン酸 エチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
1−ヒドロキシ−ナフタレン−2−カルボン酸 ((3S,5S)−5−ベンゾオキサゾール−2−イル−1−シクロヘキシルメチル−ピロリジン−3−イル)−アミド;
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピペリジン−2−カルボン酸 メチルエステル;
1−ヒドロキシ−ナフタレン−2−カルボン酸 [(3S,5S)−1−シクロヘキシルメチル−5−(5−メチル−オキサゾール−2−イル)−ピロリジン−3−イル]−アミド;
1−ヒドロキシ−ナフタレン−2−カルボン酸 [(3S,5S)−1−シクロヘキシルメチル−5−(5−フェニル−オキサゾール−2−イル)−ピロリジン−3−イル]−アミド;
1−ヒドロキシ−ナフタレン−2−カルボン酸 ((3S,5S)−1−シクロヘキシルメチル−5−オキサゾール−2−イル−ピロリジン−3−イル)−アミド;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4S)−1−ベンジル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4S)−1−シクロペンチルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 ベンジルアミド;
(2S,4S)−1−シクロヘキサンカルボニル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 イソプロピルアミド;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 プロピルアミド;
(2S,4S)−1−シクロブチルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルアミド;
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピペリジン−2−カルボン酸 エチルアミド;
(2R,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−2−メチル−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4S)−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−1−イソブチル−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4S)−4−[(1−アミノ−ナフタレン−2−カルボニル)−アミノ]−1−シクロヘキシルメチル−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(8−ヒドロキシ−キノリン−7−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸アミド;
1−ヒドロキシ−ナフタレン−2−カルボン酸 [(3S,5S)−1−シクロヘキシルメチル−5−(ピロリジン−1−カルボニル)−ピロリジン−3−イル]−アミド;
(2S,4R)−1−ベンジル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル;および
1−ヒドロキシ−ナフタレン−2−カルボン酸 [(3S,5S)−5−(アゼチジン−1−カルボニル)−1−シクロヘキシルメチル−ピロリジン−3−イル]−アミド。
本出願は、さらに、免疫系調節薬もしくはHCVの複製を阻害する抗ウイルス薬またはその組合わせを投与することを含む、上記方法を提供する。
本出願は、抗ウイルス薬がHCVプロテアーゼ阻害薬、HCVポリメラーゼ阻害薬、HCVヘリカーゼ阻害薬、HCVプライマーゼ阻害薬、HCV融合阻害薬、およびその組合わせからなる群から選択される、上記方法を提供する。
本出願は、式Iの化合物および医薬的に許容できる賦形剤を含む組成物を提供する。
本出願は、本明細書に記載する化合物、組成物、または方法を提供する。
化合物
本発明により包含される、本発明の範囲に含まれる代表的化合物の例を下記の表に提示する。これらの例およびそれに続く製造法は、当業者が本発明をより明瞭に理解し、実施することができるように提供される。それらは本発明の限定とみなすべきではなく、本発明の説明および代表例にすぎない。
一般的スキーム
合成−一般的反応スキーム
幾つかの経路で投与するための本発明化合物の医薬組成物をこの例に記載するように調製した。
本発明の化合物を多様な経口投与剤形およびキャリヤー中に配合することができる。経口投与は、錠剤、コーティング錠、糖衣丸、硬および軟ゼラチンカプセル剤、液剤、乳剤、シロップ剤、または懸濁液剤の形態で行なうことができる。本発明の化合物は、他の投与経路のうちでは特に、連続(静脈内点滴) 局所 非経口、筋肉内、静脈内、皮下、経皮(これは透過促進剤を含有することができる)、口腔、鼻腔、吸入および坐剤投与を含めた他の投与経路で投与した場合に有効である。好ましい投与様式は、一般に、好都合な各日投与計画を用いる経口様式であり、これは疾患の程度および有効成分に対する患者の応答に従って調整できる。
適応症
本発明の化合物およびそれらの異性体形態ならびにその医薬的に許容できる塩類は、HCV感染症を治療および予防するのに有用である。
本出願は、式Iの化合物を投与することを含む、細胞においてHCVの複製を阻害するための方法を提供する。
本発明の化合物およびそれらの異性体形態ならびにその医薬的に許容できる塩類は、単独で、またはHCVライフサイクルに関与するウイルスもしくは細胞のエレメントもしくは機能をターゲティングする他の化合物と併用した際に、HCV感染症を治療および予防するのに有用である。本発明に有用なクラスの化合物には、限定ではなく、すべてのクラスのHCV抗ウイルス薬が含まれる。
本出願は、さらに、免疫系調節薬もしくはHCVの複製を阻害する抗ウイルス薬またはその組合わせを投与することを含む、上記方法を提供する。
本出願は、抗ウイルス薬がHCVプロテアーゼ阻害薬、HCVポリメラーゼ阻害薬、HCVヘリカーゼ阻害薬、HCVプライマーゼ阻害薬、HCV融合阻害薬、およびその組合わせからなる群から選択される、上記方法を提供する。
一般的に用いられる略号には下記のものが含まれる:アセチル(Ac)、アゾ−ビス−イソブチロニトリル(AIBN)、大気(Atm)、9−ボラビシクロ[3.3.1]ノナン(9−BBNまたはBBN)、2,2’−ビス(ジフェニルホスフィノ)−1,1’−ビナフチル(BINAP)、tert−ブトキシカルボニル(Boc)、ピロ炭酸ジ−tert−ブチルまたはbocアンヒドリド(BOC2O)、ベンジル(Bn)、ブチル(Bu)、ケミカルアブストラクツ登録番号(CASRN)、ベンジルオキシカルボニル(CBZまたはZ)、カルボニルジイミダゾール(CDI)、1,4−ジアザビシクロ[2.2.2]オクタン(DABCO)、三フッ化ジエチルアミノ硫黄(DAST)、ジベンジリデンアセトン(dba)、1,5−ジアザビシクロ[4.3.0]ノナ−5−エン(DBN)、1,8−ジアザビシクロ[5.4.0]ウンデカ−7−エン(DBU)、N,N’−ジシクロヘキシルカルボジイミド(DCC)、1,2−ジクロロエタン(DCE)、ジクロロメタン(DCM)、2,3−ジクロロ−5,6−ジシアノ−1,4−ベンゾキノン(DDQ)、アゾジカルボン酸ジエチル(DEAD)、アゾジカルボン酸 ジ−イソ−プロピル(DIAD)、水素化ジ−イソ−ブチルアルミニウム(DIBALまたはDIBAL−H)、ジ−イソ−プロピルエチルアミン(DIPEA)、N,N−ジメチルアセトアミド(DMA)、4−N,N−ジメチルアミノピリジン(DMAP)、N,N−ジメチルホルムアミド(DMF)、ジメチルスルホキシド(DMSO)、1,1’−ビス−(ジフェニルホスフィノ)エタン(dppe)、1,1’−ビス−(ジフェニルホスフィノ)フェロセン(dppf)、1−(3−ジメチルアミノプロピル)−3−エチルカルボジイミド塩酸塩(EDCI)、2−エトキシ−1−エトキシカルボニル−1,2−ジヒドロキノリン(EEDQ)、エチル(Et)、酢酸エチル(EtOAc)、エタノール(EtOH)、2−エトキシ−2H−キノリン−1−カルボン酸 エチルエステル(EEDQ)、ジエチルエーテル(Et2O)、エチルイソプロピルエーテル(EtOiPr)、O−(7−アザベンゾトリアゾール−1−イル)−N、N,N’N’−テトラメチルウロニウムヘキサフルオロホスフェート酢酸(HATU)、酢酸(HOAc)、1−N−ヒドロキシベンゾトリアゾール(HOBt)、高速液体クロマトグラフィー(HPLC)、イソ−プロパノール(IPA)、イソプロピルマグネシウムクロリド(iPrMgCl)、ヘキサメチルジシラザン(HMDS)、液体クロマトグラフィー質量分析(LCMS)、リチウムヘキサメチルジシラザン(LiHMDS)、メタ−クロロペルオキシ安息香酸(m−CPBA)、メタノール(MeOH)、融点(mp)、MeSO2−(メシルまたはMs)、メチル(Me)、アセトニトリル(MeCN)、m−クロロ過安息香酸(MCPBA)、質量スペクトル(ms)、メチル t−ブチルエーテル(MTBE)、メチルテトラヒドロフラン(MeTHF)、N−ブロモスクシンイミド(NBS)、n−ブチルリチウム(nBuLi)、N−カルボキシアンヒドリド(NCA)、N−クロロスクシンイミド(NCS)、N−メチルモルホリン(NMM)、N−メチルピロリドン(NMP)、クロロクロム酸ピリジニウム(PCC)、ジクロロ−((ビス−ジフェニルホスフィノ)フェロセニル)パラジウム(II)(Pd(dppf)Cl2)、酢酸パラジウム(II)(Pd(OAc)2)、トリス(ジベンジリデンアセトン)二パラジウム(0)(Pd2(dba)3)、二クロム酸ピリジニウム(PDC)、フェニル(Ph)、プロピル(Pr)、イソ−プロピル(i−Pr)、ポンド/平方インチ(psi)、ピリジン(pyr)、1,2,3,4,5−ペンタフェニル−1’−(ジ−tert−ブチルホスフィノ)フェロセン(Q−Phos)、室温(周囲温度、rtまたはRT)、sec−ブチルリチウム(sBuLi)、tert−ブチルジメチルシリルまたはt−BuMe2Si(TBDMS)、フッ化テトラ−n−ブチルアンモニウム(TBAF)、トリエチルアミン(TEAまたはEt3N)、2,2,6,6−テトラメチルピペリジン 1−オキシル(TEMPO)、トリフレートまたはCF3SO2−(Tf)、トリフルオロ酢酸(TFA)、1,1’−ビス−2,2,6,6−テトラメチルヘプタン−2,6−ジオン(TMHD)、O−ベンゾトリアゾール−1−イル−N,N,N’,N’−テトラメチルウロニウムテトラフルオロボレート(TBTU)、薄層クロマトグラフィー(TLC)、テトラヒドロフラン(THF)、トリメチルシリルまたはMe3Si(TMS)、p−トルエンスルホン酸1水和物(TsOHまたはpTsOH)、4−Me−C6H4SO2−またはトシル(Ts)、およびN−ウレタン−N−カルボキシアンヒドリド(UNCA)。接頭辞ノルマル(n)、イソ(i−)、第二級(sec−)、第三級(tert−)およびneoを含む一般的な名称は、アルキル部分と共に用いられる場合、それらの慣用される意味をもつ。(J. Rigaudy and D. P. Klesney, Nomenclature in Organic Chemistry, IUPAC 1979 Pergamon Press, Oxford.)。
本発明の化合物は、以下の具体例のセクションに記載される具体的な合成反応に示す多様な方法により製造できる。
(2S,4S)−4−アミノ−1−シクロヘキシルメチル−ピロリジン−2−カルボン酸 メチルエステルの製造
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル
(2S,4S)−1−ベンジル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル
(2S,4S)−1−(3,3−ジメチル−ブチル)−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル
(2S,4S)−1−シクロペンチルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル
(2S,4S)−1−シクロブチルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル
(2S,4S)−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−1−イソブチル−ピロリジン−2−カルボン酸 エチルエステル
(2S,4S)−1−シクロヘキサンカルボニル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 tert−ブチルエステル
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルアミド
1−ヒドロキシ−ナフタレン−2−カルボン酸 [(3S,5S)−5−(アゼチジン−1−カルボニル)−1−シクロヘキシルメチル−ピロリジン−3−イル]−アミド
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 プロピルアミド
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 イソプロピルアミド
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 ベンジルアミド
1−ヒドロキシ−ナフタレン−2−カルボン酸 [(3S,5S)−1−シクロヘキシルメチル−5−(ピロリジン−1−カルボニル)−ピロリジン−3−イル]−アミド
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸アミド
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−2−メチル−ピロリジン−2−カルボン酸 メチルエステル
(2R,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−2−メチル−ピロリジン−2−カルボン酸 メチルエステル
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−2−メチル−ピロリジン−2−カルボン酸 メチルエステル
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−2−メチル−ピロリジン−2−カルボン酸 エチルエステル
(2S,4S)−4−[(1−アミノ−ナフタレン−2−カルボニル)−アミノ]−1−シクロヘキシルメチル−ピロリジン−2−カルボン酸 メチルエステル
(2S,4S)−1−シクロヘキシルメチル−4−[(8−ヒドロキシ−キノリン−7−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピペリジン−2−カルボン酸 メチルエステル
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピペリジン−2−カルボン酸 エチルエステル
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピペリジン−2−カルボン酸 エチルアミド
1−ヒドロキシ−ナフタレン−2−カルボン酸 ((3S,5S)−1−シクロヘキシルメチル−5−オキサゾール−2−イル−ピロリジン−3−イル)−アミド
1−ヒドロキシ−ナフタレン−2−カルボン酸 [(3S,5S)−1−シクロヘキシルメチル−5−(5−メチル−オキサゾール−2−イル)−ピロリジン−3−イル]−アミド
1−ヒドロキシ−ナフタレン−2−カルボン酸 [(3S,5S)−1−シクロヘキシルメチル−5−(5−フェニル−オキサゾール−2−イル)−ピロリジン−3−イル]−アミド
1−ヒドロキシ−ナフタレン−2−カルボン酸 ((3S,5S)−5−ベンゾオキサゾール−2−イル−1−シクロヘキシルメチル−ピロリジン−3−イル)−アミド
レプリコンルシフェラーゼレポーターアッセイを用いる、化合物のHCV GT1b阻害レプリコン活性の測定
2209−23細胞系はRocheにおいて、先に記載されたようにヘパトーマ細胞系Huh−7をGT−1b Con1サブゲノム バイシストロニックレプリコン(bicistronic replicon)で安定トランスフェクションすることにより開発された。サブゲノムレプリコン細胞系を、R. Bartenschlager (J Virol. 2003 Mar; 77 (5):3007-19)から得られたキュアした(cured)Huh7細胞において樹立した。GT−1a H77サブゲノムレプリコンベクターpRLuc H77 1b 75 S/Iは、GT−1b Con1サブゲノムレプリコンの非構造領域を、GT−1b Con1株に由来するNS3タンパク質の最初の75アミノ酸以外において、H77株のひとつで置き換えることにより作製された(J Virol. 2001 77:5352-59)。GT−1a pRLuc H77 1b 75 S/Iサブゲノムレプリコン細胞系を、R. Bartenschlager. (J Virol. 2003 Mar; 77 (5):3007-19)から得られたキュアしたHuh7細胞において樹立した。
細胞生存性試験のために、2209−23細胞をウェル当たり5000個の細胞密度で透明平底96ウェルプレート(Becton Dickinson,カタログ# 35 3075)にプレーティングした。WST−1細胞増殖アッセイ(Roche Diagnostic,カタログ# 11644807001)を用いて細胞生存性を測定した。アッセイプレートをレプリコンアッセイの場合と同じ方式で設定した。製造業者の指示に従って、化合物インキュベーションの3日後に10μlのWST−1試薬を各ウェルに37℃および5% CO2で2時間添加した。450nm(基準フィルター650nm)における吸収の読みを、MRX Revelationマイクロタイタープレートリーダー(Lab System)により測定した。CC50値は化合物が存在しない非処理対照と比較して細胞生存性を50%低下させるのに要した化合物濃度と定義され、化合物用量応答データの非線形フィッティングにより決定された。代表的なアッセイデータを下記の表IIにみることができる。
本明細書は以下の発明の開示を包含する:
[1]式Iの化合物
Aは、CHまたはNであり;
nは、1または2であり;
R 1 は、低級アルキル、シクロアルキル、フェニル、またはヘテロシクロアルキルであり;
R 2 は、−C(=O)OR 2 ’、−C(=O)N(R 2 ’) 2 、任意に1個以上のR 2 ’で置換されていてもよい単環式または二環式ヘテロアリールであり;
R 2 ’はそれぞれ、独立してH、低級アルキルまたはヘテロシクロアルキルであり;
R 3 は、Hまたは低級アルキルであり;
R 4 は、ヒドロキシルまたはアミノであり;
Xは、CH 2 またはC(=O)である]
またはその医薬的に許容できる塩。
[2]R 3 がHであり、かつXがCH 2 である、[2]に記載の化合物。
[3]nが2である、[2]に記載の化合物。
[4]nが1である、[2]に記載の化合物。
[5]R 2 が−C(=O)N(R 2 ’) 2 である、[4]に記載の化合物。
[6]R 2 が、任意に1個以上のR 2 ’で置換されていてもよい単環式または二環式ヘテロアリールである、[4]に記載の化合物。
[7]R 2 が−C(=O)OR 2 ’である、[4]に記載の化合物。
[8]R 2 ’が低級アルキルである、[7]に記載の化合物。
[9]R 1 が低級アルキルまたはシクロアルキルである、[8]に記載の化合物。
[10]R 1 がシクロヘキシルである、[9]に記載の化合物。
[11]下記の群から選択される化合物:
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 tert−ブチルエステル;
(2S,4S)−1−(3,3−ジメチル−ブチル)−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピペリジン−2−カルボン酸 エチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
1−ヒドロキシ−ナフタレン−2−カルボン酸 ((3S,5S)−5−ベンゾオキサゾール−2−イル−1−シクロヘキシルメチル−ピロリジン−3−イル)−アミド;
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピペリジン−2−カルボン酸 メチルエステル;
1−ヒドロキシ−ナフタレン−2−カルボン酸 [(3S,5S)−1−シクロヘキシルメチル−5−(5−メチル−オキサゾール−2−イル)−ピロリジン−3−イル]−アミド;
1−ヒドロキシ−ナフタレン−2−カルボン酸 [(3S,5S)−1−シクロヘキシルメチル−5−(5−フェニル−オキサゾール−2−イル)−ピロリジン−3−イル]−アミド;
1−ヒドロキシ−ナフタレン−2−カルボン酸 ((3S,5S)−1−シクロヘキシルメチル−5−オキサゾール−2−イル−ピロリジン−3−イル)−アミド;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4S)−1−ベンジル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4S)−1−シクロペンチルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 ベンジルアミド;
(2S,4S)−1−シクロヘキサンカルボニル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 イソプロピルアミド;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 プロピルアミド;
(2S,4S)−1−シクロブチルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルアミド;
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピペリジン−2−カルボン酸 エチルアミド;
(2R,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−2−メチル−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4S)−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−1−イソブチル−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4S)−4−[(1−アミノ−ナフタレン−2−カルボニル)−アミノ]−1−シクロヘキシルメチル−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(8−ヒドロキシ−キノリン−7−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸アミド;
1−ヒドロキシ−ナフタレン−2−カルボン酸 [(3S,5S)−1−シクロヘキシルメチル−5−(ピロリジン−1−カルボニル)−ピロリジン−3−イル]−アミド;
(2S,4R)−1−ベンジル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル;および
1−ヒドロキシ−ナフタレン−2−カルボン酸 [(3S,5S)−5−(アゼチジン−1−カルボニル)−1−シクロヘキシルメチル−ピロリジン−3−イル]−アミド。
[12]療法有効物質として使用するための、[1]〜[11]のいずれかに記載の化合物。
[13]C型肝炎ウイルス(HCV)感染症の治療または予防のための、[1]〜[11]のいずれかに記載の化合物の使用。
[14]C型肝炎ウイルス(HCV)感染症の治療または予防のための医薬を調製するための、[1]〜[11]のいずれかに記載の化合物の使用。
[15]C型肝炎ウイルス(HCV)感染症の治療または予防のための、[1]〜[11]のいずれかに記載の化合物。
[16]その必要がある患者に療法有効量の[1]〜[11]のいずれかに記載の化合物を投与することを含む、C型肝炎ウイルス(HCV)感染症を処置するための方法。
[17][1]〜[11]のいずれかに記載の化合物および医薬的に許容できる賦形剤を含む医薬組成物。
[18]以上に記載した発明。
Claims (17)
- R3がHであり、かつXがCH2である、請求項1に記載の化合物。
- nが2である、請求項2に記載の化合物。
- nが1である、請求項2に記載の化合物。
- R2が−C(=O)N(R2’)2である、請求項4に記載の化合物。
- R2が、任意に1個以上のR2’で置換されていてもよい単環式または二環式ヘテロアリールである、請求項4に記載の化合物。
- R2が−C(=O)OR2’である、請求項4に記載の化合物。
- R2’が低級アルキルである、請求項7に記載の化合物。
- R1が低級アルキルまたはシクロアルキルである、請求項8に記載の化合物。
- R1がシクロヘキシルである、請求項9に記載の化合物。
- 下記の群から選択される化合物:
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 tert−ブチルエステル;
(2S,4S)−1−(3,3−ジメチル−ブチル)−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピペリジン−2−カルボン酸 エチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
1−ヒドロキシ−ナフタレン−2−カルボン酸 ((3S,5S)−5−ベンゾオキサゾール−2−イル−1−シクロヘキシルメチル−ピロリジン−3−イル)−アミド;
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピペリジン−2−カルボン酸 メチルエステル;
1−ヒドロキシ−ナフタレン−2−カルボン酸 [(3S,5S)−1−シクロヘキシルメチル−5−(5−メチル−オキサゾール−2−イル)−ピロリジン−3−イル]−アミド;
1−ヒドロキシ−ナフタレン−2−カルボン酸 [(3S,5S)−1−シクロヘキシルメチル−5−(5−フェニル−オキサゾール−2−イル)−ピロリジン−3−イル]−アミド;
1−ヒドロキシ−ナフタレン−2−カルボン酸 ((3S,5S)−1−シクロヘキシルメチル−5−オキサゾール−2−イル−ピロリジン−3−イル)−アミド;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4S)−1−ベンジル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4S)−1−シクロペンチルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 ベンジルアミド;
(2S,4S)−1−シクロヘキサンカルボニル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 イソプロピルアミド;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 プロピルアミド;
(2S,4S)−1−シクロブチルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルアミド;
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピペリジン−2−カルボン酸 エチルアミド;
(2R,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−2−メチル−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4S)−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−1−イソブチル−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4S)−4−[(1−アミノ−ナフタレン−2−カルボニル)−アミノ]−1−シクロヘキシルメチル−ピロリジン−2−カルボン酸 メチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(8−ヒドロキシ−キノリン−7−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4S)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸アミド;
1−ヒドロキシ−ナフタレン−2−カルボン酸 [(3S,5S)−1−シクロヘキシルメチル−5−(ピロリジン−1−カルボニル)−ピロリジン−3−イル]−アミド;
(2S,4R)−1−ベンジル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 エチルエステル;
(2S,4R)−1−シクロヘキシルメチル−4−[(1−ヒドロキシ−ナフタレン−2−カルボニル)−アミノ]−ピロリジン−2−カルボン酸 メチルエステル;および
1−ヒドロキシ−ナフタレン−2−カルボン酸 [(3S,5S)−5−(アゼチジン−1−カルボニル)−1−シクロヘキシルメチル−ピロリジン−3−イル]−アミド。 - 療法有効物質として使用するための、請求項1〜11のいずれか1項に記載の化合物。
- 請求項1〜11のいずれか1項に記載の化合物を含む、C型肝炎ウイルス(HCV)感染症の治療または予防のための医薬組成物。
- C型肝炎ウイルス(HCV)感染症の治療または予防のための医薬を調製するための、請求項1〜11のいずれか1項に記載の化合物の使用。
- C型肝炎ウイルス(HCV)感染症の治療または予防のための、請求項1〜11のいずれか1項に記載の化合物。
- 請求項1〜11のいずれか1項に記載の化合物を含む、C型肝炎ウイルス(HCV)感染症を治療するための医薬組成物。
- 請求項1〜11のいずれか1項に記載の化合物および医薬的に許容できる賦形剤を含む医薬組成物。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261602687P | 2012-02-24 | 2012-02-24 | |
| US61/602,687 | 2012-02-24 | ||
| PCT/EP2013/053409 WO2013124335A1 (en) | 2012-02-24 | 2013-02-21 | Antiviral compounds |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2015508088A JP2015508088A (ja) | 2015-03-16 |
| JP6092261B2 true JP6092261B2 (ja) | 2017-03-08 |
Family
ID=47740975
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014558092A Expired - Fee Related JP6092261B2 (ja) | 2012-02-24 | 2013-02-21 | 抗ウイルス化合物 |
Country Status (10)
| Country | Link |
|---|---|
| US (2) | US9090559B2 (ja) |
| EP (1) | EP2817291A1 (ja) |
| JP (1) | JP6092261B2 (ja) |
| KR (1) | KR20140130449A (ja) |
| CN (1) | CN104185624B (ja) |
| BR (1) | BR112014015582A8 (ja) |
| CA (1) | CA2857262A1 (ja) |
| MX (1) | MX2014009799A (ja) |
| RU (1) | RU2014137052A (ja) |
| WO (1) | WO2013124335A1 (ja) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013124335A1 (en) * | 2012-02-24 | 2013-08-29 | F. Hoffmann-La Roche Ag | Antiviral compounds |
| ES2989988T3 (es) | 2016-05-10 | 2024-11-28 | C4 Therapeutics Inc | Degronímeros heterorocíclicos para la degradación de proteínas diana |
| CN109641874A (zh) | 2016-05-10 | 2019-04-16 | C4医药公司 | 用于靶蛋白降解的c3-碳连接的戊二酰亚胺降解决定子体 |
| CN109790143A (zh) | 2016-05-10 | 2019-05-21 | C4医药公司 | 用于靶蛋白降解的胺连接的c3-戊二酰亚胺降解决定子体 |
| WO2017197036A1 (en) | 2016-05-10 | 2017-11-16 | C4 Therapeutics, Inc. | Spirocyclic degronimers for target protein degradation |
| CN118440096A (zh) | 2017-06-20 | 2024-08-06 | C4医药公司 | 用于蛋白降解的n/o-连接的降解决定子和降解决定子体 |
| WO2019028362A1 (en) * | 2017-08-04 | 2019-02-07 | Dyax Corp. | INHIBITORS OF PLASMATIC KALLIKREIN AND USES THEREOF |
| US11837363B2 (en) | 2020-11-04 | 2023-12-05 | Hill-Rom Services, Inc. | Remote management of patient environment |
Family Cites Families (42)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4213765A (en) | 1979-01-02 | 1980-07-22 | Union Carbide Corporation | Oxidative coal desulfurization using lime to regenerate alkali metal hydroxide from reaction product |
| US5807876A (en) | 1996-04-23 | 1998-09-15 | Vertex Pharmaceuticals Incorporated | Inhibitors of IMPDH enzyme |
| US6054472A (en) | 1996-04-23 | 2000-04-25 | Vertex Pharmaceuticals, Incorporated | Inhibitors of IMPDH enzyme |
| TR199802136T2 (xx) | 1996-04-23 | 2001-06-21 | Vertex Pharmaceuticals Incorporated | �MPDH enzimi inhibit�rleri olarak �re t�revleri. |
| DK0932617T3 (da) * | 1996-10-18 | 2002-04-22 | Vertex Pharma | Inhibitorer af serinproteaser, især af hepatitis C-virus-NS3-protease |
| GB9623908D0 (en) | 1996-11-18 | 1997-01-08 | Hoffmann La Roche | Amino acid derivatives |
| ATE244717T1 (de) | 1997-03-14 | 2003-07-15 | Vertex Pharma | Inhibitoren des impdh-enzyms |
| US6010848A (en) | 1997-07-02 | 2000-01-04 | Smithkline Beecham Corporation | Screening methods using an atpase protein from hepatitis C virus |
| PT1012180E (pt) | 1997-08-11 | 2005-04-29 | Boehringer Ingelheim Ca Ltd | Analogos de peptidos inibidores da hepatite c |
| ATE298317T1 (de) | 1998-07-27 | 2005-07-15 | Angeletti P Ist Richerche Bio | Diketosäure-derivate als hemmstoffe von polymerasen |
| US6323180B1 (en) | 1998-08-10 | 2001-11-27 | Boehringer Ingelheim (Canada) Ltd | Hepatitis C inhibitor tri-peptides |
| JP2002523371A (ja) | 1998-08-21 | 2002-07-30 | バイロファーマ・インコーポレイテッド | ウイルス感染および関連疾患を治療または予防するための化合物、組成物および方法 |
| MXPA01002253A (es) | 1998-09-04 | 2003-06-04 | Viropharma Inc | Metodo para tratar o prevenir infecciones virales y enfermedades asociadas. |
| US6316492B1 (en) | 1998-09-25 | 2001-11-13 | Viropharma Incorporated | Methods for treating or preventing viral infections and associated diseases |
| ES2405316T3 (es) | 1999-03-19 | 2013-05-30 | Vertex Pharmaceuticals Incorporated | Inhibidores de la enzima IMPDH |
| CA2389745C (en) | 1999-11-04 | 2010-03-23 | Shire Biochem Inc. | Method for the treatment or prevention of flaviviridae viral infection using nucleoside analogues |
| JP2004509066A (ja) | 2000-05-10 | 2004-03-25 | スミスクライン・ビーチャム・コーポレイション | 新規抗感染症薬 |
| US6448281B1 (en) | 2000-07-06 | 2002-09-10 | Boehringer Ingelheim (Canada) Ltd. | Viral polymerase inhibitors |
| SV2003000617A (es) | 2000-08-31 | 2003-01-13 | Lilly Co Eli | Inhibidores de la proteasa peptidomimetica ref. x-14912m |
| EP2206709A3 (en) | 2001-06-11 | 2011-06-29 | Virochem Pharma Inc. | Thiophene derivatives as antiviral agents for flavivirus infection |
| US6887877B2 (en) | 2001-06-11 | 2005-05-03 | Virochem Pharma Inc. | Compounds and methods for the treatment or prevention of Flavivirus infections |
| AR035543A1 (es) | 2001-06-26 | 2004-06-16 | Japan Tobacco Inc | Agente terapeutico para la hepatitis c que comprende un compuesto de anillo condensado, compuesto de anillo condensado, composicion farmaceutica que lo comprende, compuestos de benzimidazol, tiazol y bifenilo utiles como intermediarios para producir dichos compuestos, uso del compuesto de anillo con |
| EP1411928A1 (en) | 2001-07-20 | 2004-04-28 | Boehringer Ingelheim (Canada) Ltd. | Viral polymerase inhibitors |
| EP2335700A1 (en) | 2001-07-25 | 2011-06-22 | Boehringer Ingelheim (Canada) Ltd. | Hepatitis C virus polymerase inhibitors with a heterobicylic structure |
| DE60221875T2 (de) | 2001-11-02 | 2007-12-20 | Glaxo Group Ltd., Greenford | 4-(6-gliedrige)-heteroaryl-acyl-pyrrolidinderivate als hcv-inhibitoren |
| WO2003037893A1 (en) | 2001-11-02 | 2003-05-08 | Glaxo Group Limited | Acyl dihydro pyrrole derivatives as hcv inhibitors |
| US20050043545A1 (en) | 2001-11-02 | 2005-02-24 | Gianpalol Bravi | 4-(5-Membered)-heteroaryl acyl pyrrolidine derivatives as hcv inhibitors |
| WO2004113353A1 (en) | 2003-06-19 | 2004-12-29 | Amedis Pharmaceuticals Ltd. | Silicon-comprising aminothiazole derivatives as cdk inhibitors |
| CA2552319C (en) | 2004-01-30 | 2012-08-21 | Medivir Ab | Hcv ns-3 serine protease inhibitors |
| US20070167426A1 (en) | 2004-06-02 | 2007-07-19 | Schering Corporation | Compounds for the treatment of inflammatory disorders and microbial diseases |
| US7601686B2 (en) * | 2005-07-11 | 2009-10-13 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| GB0526614D0 (en) | 2005-12-30 | 2006-02-08 | Novartis Ag | Organic compounds |
| BRPI0708524A2 (pt) | 2006-03-03 | 2011-05-31 | Novartis Ag | compostos de n-formil hidroxilamina |
| US8329159B2 (en) | 2006-08-11 | 2012-12-11 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| DE102006048924A1 (de) | 2006-10-17 | 2008-04-24 | Bayer Healthcare Ag | Acylaminopyrazole |
| DE602008003221D1 (de) | 2007-06-07 | 2010-12-09 | Hoffmann La Roche | Prolinamidderivate als nk3-antagonisten |
| WO2010009196A1 (en) | 2008-07-15 | 2010-01-21 | Temple University Of The Commonwealth System Of Higher Education | Synthesis of bis-peptides oligomers comprising at least one n-substituted diketopiperazine as structural moiety |
| US20110195929A1 (en) * | 2008-08-05 | 2011-08-11 | Summit Corporation Plc | Compounds for the treatment of flaviviral infections |
| WO2011031934A1 (en) * | 2009-09-11 | 2011-03-17 | Enanta Pharmaceuticals, Inc. | Hepatitis c virus inhibitors |
| WO2011049988A2 (en) * | 2009-10-20 | 2011-04-28 | Eiger Biopharmaceuticals, Inc. | Indazoles to treat flaviviridae virus infection |
| EP2345643A1 (en) * | 2009-12-29 | 2011-07-20 | Polichem S.A. | New tertiary 8-hydroxyquinoline-7-carboxamide derivatives and uses thereof |
| WO2013124335A1 (en) * | 2012-02-24 | 2013-08-29 | F. Hoffmann-La Roche Ag | Antiviral compounds |
-
2013
- 2013-02-21 WO PCT/EP2013/053409 patent/WO2013124335A1/en not_active Ceased
- 2013-02-21 US US14/376,336 patent/US9090559B2/en not_active Expired - Fee Related
- 2013-02-21 KR KR1020147023576A patent/KR20140130449A/ko not_active Withdrawn
- 2013-02-21 CA CA2857262A patent/CA2857262A1/en not_active Abandoned
- 2013-02-21 BR BR112014015582A patent/BR112014015582A8/pt not_active IP Right Cessation
- 2013-02-21 CN CN201380010537.XA patent/CN104185624B/zh not_active Expired - Fee Related
- 2013-02-21 MX MX2014009799A patent/MX2014009799A/es unknown
- 2013-02-21 JP JP2014558092A patent/JP6092261B2/ja not_active Expired - Fee Related
- 2013-02-21 RU RU2014137052A patent/RU2014137052A/ru not_active Application Discontinuation
- 2013-02-21 EP EP13705187.6A patent/EP2817291A1/en not_active Withdrawn
-
2015
- 2015-06-09 US US14/734,176 patent/US9403805B2/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| HK1204618A1 (en) | 2015-11-27 |
| CN104185624B (zh) | 2016-10-12 |
| KR20140130449A (ko) | 2014-11-10 |
| US20150274711A1 (en) | 2015-10-01 |
| BR112014015582A2 (pt) | 2017-06-13 |
| CN104185624A (zh) | 2014-12-03 |
| US9403805B2 (en) | 2016-08-02 |
| BR112014015582A8 (pt) | 2017-07-04 |
| RU2014137052A (ru) | 2016-04-10 |
| EP2817291A1 (en) | 2014-12-31 |
| CA2857262A1 (en) | 2013-08-29 |
| MX2014009799A (es) | 2014-09-08 |
| JP2015508088A (ja) | 2015-03-16 |
| US20150005283A1 (en) | 2015-01-01 |
| US9090559B2 (en) | 2015-07-28 |
| WO2013124335A1 (en) | 2013-08-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6092261B2 (ja) | 抗ウイルス化合物 | |
| JP5808496B2 (ja) | 抗ウイルス化合物 | |
| JP5923181B2 (ja) | Hcvns5aの阻害剤 | |
| JP6018715B2 (ja) | 抗ウイルス化合物 | |
| US20120328565A1 (en) | Antiviral compounds | |
| JP2016510050A (ja) | 抗ウイルス性化合物 | |
| JP6122514B2 (ja) | 抗ウイルス化合物 | |
| HK1204618B (en) | Antiviral compounds | |
| HK1200847B (en) | Inhibitors of hcv ns5a |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20160219 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20160907 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20160908 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20161129 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20170110 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20170208 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6092261 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| LAPS | Cancellation because of no payment of annual fees |