JP5779594B2 - Hmbを含有するプラスチック包装栄養液剤 - Google Patents
Hmbを含有するプラスチック包装栄養液剤 Download PDFInfo
- Publication number
- JP5779594B2 JP5779594B2 JP2012551326A JP2012551326A JP5779594B2 JP 5779594 B2 JP5779594 B2 JP 5779594B2 JP 2012551326 A JP2012551326 A JP 2012551326A JP 2012551326 A JP2012551326 A JP 2012551326A JP 5779594 B2 JP5779594 B2 JP 5779594B2
- Authority
- JP
- Japan
- Prior art keywords
- soluble
- nutrient solution
- protein
- calcium
- hmb
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65B—MACHINES, APPARATUS OR DEVICES FOR, OR METHODS OF, PACKAGING ARTICLES OR MATERIALS; UNPACKING
- B65B55/00—Preserving, protecting or purifying packages or package contents in association with packaging
- B65B55/02—Sterilising, e.g. of complete packages
- B65B55/12—Sterilising contents prior to, or during, packaging
- B65B55/14—Sterilising contents prior to, or during, packaging by heat
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/40—Complete food formulations for specific consumer groups or specific purposes, e.g. infant formula
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2/00—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor
- A61L2/02—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor using physical phenomena
- A61L2/04—Heat
- A61L2/06—Hot gas
- A61L2/07—Steam
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65B—MACHINES, APPARATUS OR DEVICES FOR, OR METHODS OF, PACKAGING ARTICLES OR MATERIALS; UNPACKING
- B65B55/00—Preserving, protecting or purifying packages or package contents in association with packaging
- B65B55/02—Sterilising, e.g. of complete packages
- B65B55/04—Sterilising wrappers or receptacles prior to, or during, packaging
- B65B55/10—Sterilising wrappers or receptacles prior to, or during, packaging by liquids or gases
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65B—MACHINES, APPARATUS OR DEVICES FOR, OR METHODS OF, PACKAGING ARTICLES OR MATERIALS; UNPACKING
- B65B55/00—Preserving, protecting or purifying packages or package contents in association with packaging
- B65B55/02—Sterilising, e.g. of complete packages
- B65B55/12—Sterilising contents prior to, or during, packaging
- B65B55/18—Sterilising contents prior to, or during, packaging by liquids or gases
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2002/00—Food compositions, function of food ingredients or processes for food or foodstuffs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2/00—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor
- A61L2/16—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor using chemical substances
- A61L2/18—Liquid substances or solutions comprising solids or dissolved gases
- A61L2/186—Peroxide solutions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2/00—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor
- A61L2/16—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor using chemical substances
- A61L2/22—Phase substances, e.g. smokes, aerosols or sprayed or atomised substances
-
- A61L2103/23—
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Mechanical Engineering (AREA)
- Polymers & Plastics (AREA)
- Mycology (AREA)
- Food Science & Technology (AREA)
- Nutrition Science (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Pediatric Medicine (AREA)
- Coloring Foods And Improving Nutritive Qualities (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicinal Preparation (AREA)
Description
本発明の栄養液剤は脂質、蛋白質及び糖質の少なくとも1種を含有しており、ヒト経口投与に適しており、想定投与温度で飲用可能な粘度をもつ。これらの組成物は最も典型的には水中油型、油中水型又は複合型水性乳剤等の乳剤として製剤化され、更に典型的には連続水相と不連続油相をもつ水中油型乳剤として製剤化される。栄養液剤は保存安定性とすることができる。
栄養液剤はHMBを含有しており、あるいは経口栄養製品用として適切であると共に他の点で栄養液剤の必須成分又は特徴に適合可能な任意HMB源を含有する。
栄養液剤はHMB以外に、脂質、蛋白質及び糖質の少なくとも1種を含有する。一般に、本願に定義する栄養液剤の必須成分に適合可能であるならば、栄養製品で使用するのに適切な公知又は非公知の任意脂質源、蛋白質源及び糖質源も本願で使用するのに適切であると思われる。
本発明の栄養液剤は製品安定性を改善し、経時的な苦い風味と後味の発生を最小限にするために、選択された量の可溶性蛋白質を含有することができる。
本発明の栄養組成物は製品安定性を改善すると共に苦い風味と後味の経時的発生を最小限にするために、総可溶性カルシウムに対して選択された重量比の可溶性カルシウム結合能(SCBC)を含有する乳剤態様を含むことができる。
比=SCBC/[可溶性カルシウム]
SCBC=(0.32×[可溶性クエン酸塩]+0.63[可溶性リン酸塩]+0.013×[可溶性蛋白質])
に従って求められる。
本発明の栄養液剤は対象個体における健康な筋肉の成長又は維持用に望ましい場合には更にカルシウムを含有することができる。カルシウムHMB又はカルシウムHMB・1水和物をHMB源として使用する場合には、カルシウムの一部又は全部を提供することができる。しかし、栄養液剤の必須成分と適合可能であるならば、任意の他のカルシウム源を使用することができる。
本発明の栄養組成物は対象使用者に健康な筋肉を維持し易くするために更にビタミンDを含有することができる。ビタミンD形態としては、ビタミンD2(エルゴカルシフェロール)及びビタミンD3(コレカルシフェロール)又は栄養製品で使用するのに適した他の形態が挙げられる。
栄養液剤は更に、製剤の物理的、化学的、快楽的又は加工特性を改変することや、対象集団で使用時に医薬成分又は付加栄養成分として機能することが可能な他の非必須原料を含有することができる。多数のこのような非必須原料が公知であり、又は公知ではないが、他の栄養製品で使用するのに適しており、このような非必須材料も経口投与に安全且つ有効であると共に選択された製品形態の必須原料及び他の原料と適合可能であるならば、本願に記載する栄養液剤で使用することができる。
本発明の栄養液剤は栄養製品又は食品用に適したプラスチックパッケージの内側に収容される。プラスチックパッケージは米国食品医薬品局又は他の適切な監督機関により承認された食品グレードプラスチックから製造すべきである。
本願に記載する栄養液剤は補助、主要もしくは単独栄養源を提供するため、及び/又は本願に記載するような1種以上の効果を個体に提供するために有用である。このような方法によると、必要に応じて所望レベルの栄養を提供するように液剤を経口投与することができ、最も典型的には1日1〜2包を1日1回又は2回以上に分けて投与し、例えば1包量は典型的には約100〜約300ml、例えば約150〜約250ml、例えば約190ml〜約240mlとし、1包当たり約0.4〜約3.0g、例えば約0.75〜約2.0g、例えば約1.5gのカルシウムHMBを含有する。
栄養液剤は栄養乳剤又は他の栄養液剤の製造、最も典型的には栄養水性乳剤又は牛乳ベースの乳剤の製造に適した任意の公知又は非公知方法により製造することができる。
本実施例は再構成後のPediaSure(登録商標)粉末(栄養乳剤)におけるHMBの緩衝作用を例証する。PediaSure(登録商標)粉末(Abbott Laboratories,Columbus Ohio)の対照再構成サンプル(HMB非含有)と、再構成後の粉末1キログラム当たり5.17グラムのHMBを添加して強化したPediaSure(登録商標)粉末の再構成サンプルに室温で既知量の希塩酸を加える。HMB含有サンプルを強化するために使用するHMBはカルシウムHMB・1水和物からカチオン交換によりカルシウムを除去することにより製造する。遊離HMBをサンプルに加える前に、そのpHを水酸化ナトリウムで6.7に調整する。等モル量のナトリウムを塩化ナトリウムとして対照サンプルに加える。連続撹拌下で、塩酸添加から1分後に各サンプルのpHを測定する。pH読み値から水素イオン濃度(H+)を計算する。結果を下表に示す。
本実施例は再構成後のPediaSure(登録商標)粉末(栄養乳剤)におけるHMBの緩衝作用を例証する。PediaSure(登録商標)粉末の対照再構成サンプル(HMB非含有)と、再構成後の粉末1キログラム当たり5.17グラムのHMBを添加して強化したPediaSure(登録商標)粉末の再構成サンプルに既知量の過酸化水素(再構成後の粉末1kg当たり1.32mg)を加える。HMBを含有するサンプルを強化するために使用するHMBはカルシウムHMB・1水和物からカチオン交換によりカルシウムを除去することにより製造する。遊離HMBをサンプルに加える前に、そのpHを水酸化ナトリウムで6.7に調整する。等モル量のナトリウムを塩化ナトリウムとして対照サンプルに加える。連続撹拌下に、室温で1時間後に各サンプルのpHを測定し、pH値から[H+]濃度を計算する。結果を下表に示す。
本実施例は栄養乳剤としての即時飲用可能な液剤におけるHMBの緩衝作用を例証する。市販品であるEnsure(登録商標)Plus(サンプル#1)(Abbott Laboratories,Columbus,Ohio)とサンプル#2(Ensure(登録商標)Plusに乳剤1キログラム当たり6.5グラムのカルシウムHMBと、乳剤1kg当たり2.380グラム(g)のリン酸塩を加えた液体栄養乳剤)の緩衝能を塩酸滴定と水酸化ナトリウム滴定により比較する。結果を下表に示す。
以下の実施例は本願に記載する製造方法により製造することができる本発明の保存安定性栄養液剤のいくつかの例を例証し、例示する各組成物は特に指定しない限り、無菌処理態様とレトルト包装態様を含む。製剤はプラスチック容器に包装され、レトルト殺菌法又は無菌殺菌法により殺菌された保存安定性栄養液剤である。組成物は経時的に苦い風味又は後味を殆ど又は全く生じず、1〜25℃の保存温度で12〜18カ月間の保存期間中にpH安定及び物理的安定に維持される。
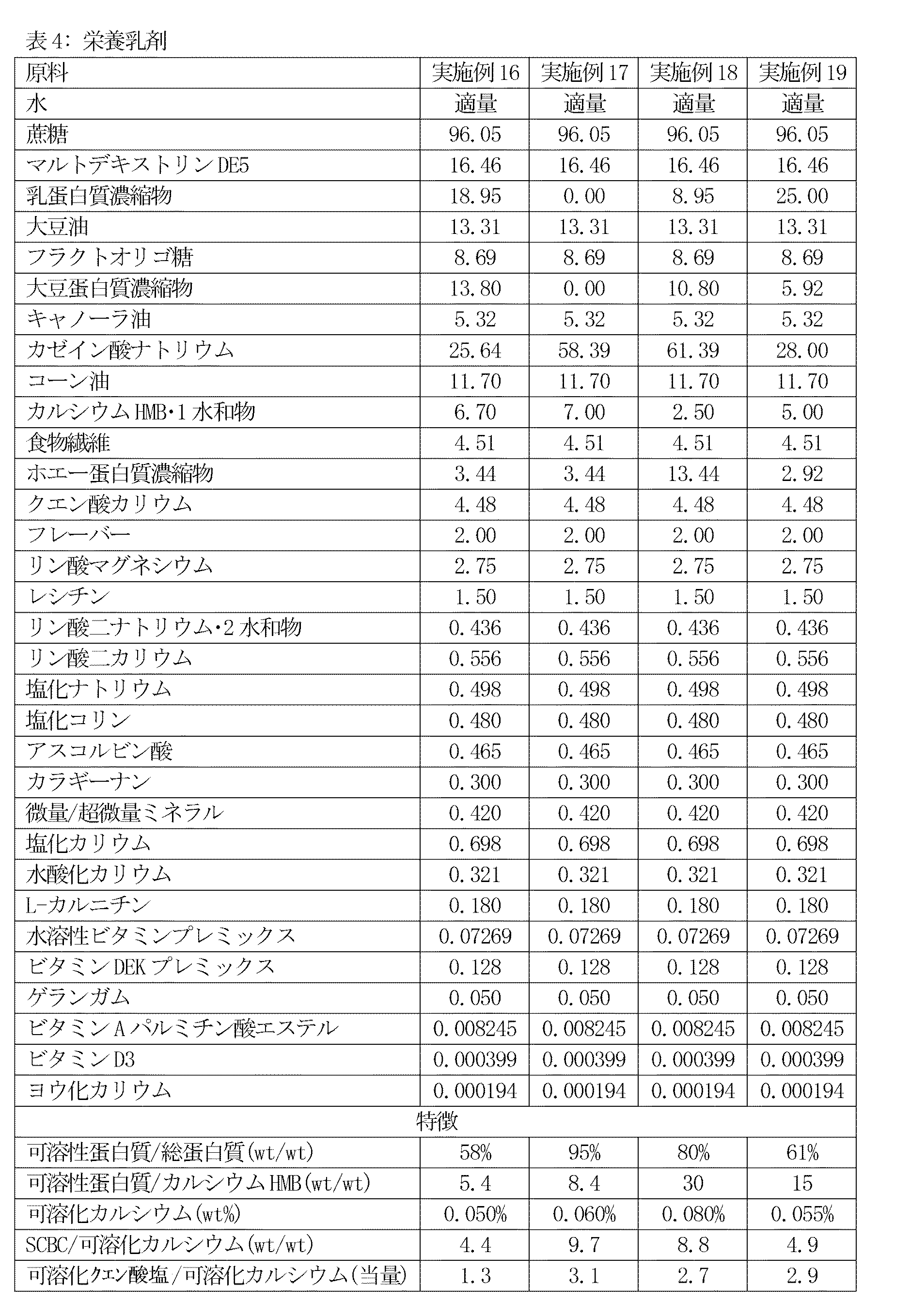
実施例4〜7は下表に原料を示す本発明の栄養乳剤を例証する。原料の全数量値は特に指定しない限り、1000キログラム製品バッチ当たりのキログラムとして表す。
これらの実施例は下表に原料を示す本発明の栄養乳剤を例証する。原料の全数量値は特に指定しない限り、1000キログラム製品バッチ当たりのキログラムとして表す。
これらの実施例は下表に原料を示す本発明の栄養乳剤を例証する。原料の全数量値は特に指定しない限り、1000キログラム製品バッチ当たりのキログラムとして表す。
これらの実施例は下表に原料を示す本発明の栄養乳剤を例証する。原料の全数量値は特に指定しない限り、1000キログラム製品バッチ当たりのキログラムとして表す。
これらの実施例は下表に原料を示す本発明の栄養乳剤を例証する。原料の全数量値は特に指定しない限り、1000キログラム製品バッチ当たりのキログラムとして表す。
これらの実施例は下表に原料を示す本発明の透明非乳剤型液剤を例証する。原料の全数量値は特に指定しない限り、1000キログラム製品バッチ当たりのキログラムとして表す。液剤はpHを4.5〜7.2に調整する。
Claims (15)
- プラスチックパッケージと前記パッケージに収容された栄養液剤とを含む包装された組成物であって、前記栄養液剤がβ−ヒドロキシ−β−メチルブチレート、可溶性カルシウム、可溶性クエン酸塩、可溶性リン酸塩、可溶性蛋白質、並びに蛋白質、糖質、及び脂質の少なくとも1種を含み、
a)前記栄養液剤は、
0.32×[可溶性クエン酸塩]+0.63[可溶性リン酸塩]+0.013×[可溶性蛋白質]
に等しい可溶性カルシウム結合能を有し、
b)前記栄養液剤は、可溶性カルシウムに対する可溶性カルシウム結合能の重量比を2.3:1から12.0:1で有する、
前記組成物。 - 栄養液剤がレトルト殺菌されている、請求項1に記載の包装組成物。
- 栄養液剤が無菌包装されている、請求項1に記載の包装組成物。
- 栄養液剤が、栄養液剤重量当り0.1%〜8%のβ−ヒドロキシ−β−メチルブチレートを含む、請求項1に記載の包装組成物。
- 栄養液剤が蛋白質、糖質、脂質およびβ−ヒドロキシ−β−メチルブチレートを含んでおり、前記蛋白質が35〜100重量%の可溶性蛋白質を含み、ホスホセリン含有蛋白質1キログラム当り少なくとも100mmolのホスホセリンを有するホスホセリン含有蛋白質を含む、請求項1に記載の包装組成物。
- プラスチックパッケージと前記パッケージに収容されたレトルト殺菌栄養液剤とを含む包装された組成物であって、前記栄養液剤が栄養液剤1キログラム当り少なくとも4.5グラムのβ−ヒドロキシ−β−メチルブチレート、可溶性カルシウム、可溶性クエン酸塩、可溶性リン酸塩、可溶性蛋白質、蛋白質、糖質、及び脂質を含んでおり、
a)前記蛋白質が35%〜100%の可溶性蛋白質を含み、
b)前記栄養液剤は、
0.32×[可溶性クエン酸塩]+0.63[可溶性リン酸塩]+0.013×[可溶性蛋白質]
に等しい可溶性カルシウム結合能を有し、
c)前記栄養液剤は、可溶性カルシウムに対する可溶性カルシウム結合能の重量比を2.3:1から12.0:1で有する、
前記組成物。 - 可溶性蛋白質が、ホスホセリン含有蛋白質1キログラム当り少なくとも100mmolのホスホセリンを有するホスホセリン含有蛋白質を含む、請求項6に記載の包装組成物。
- プラスチックパッケージが13立方センチメートル未満のヘッドスペースを含む、請求項1または請求項6に記載の包装組成物。
- プラスチックパッケージと前記パッケージに収容された無菌殺菌栄養液剤とを含む包装された組成物であって、前記栄養液剤が栄養液剤1キログラム当り少なくとも4.5グラムのβ−ヒドロキシ−β−メチルブチレート、可溶性カルシウム、可溶性クエン酸塩、可溶性リン酸塩、可溶性蛋白質、蛋白質、糖質、及び脂質を含んでおり、
a)前記蛋白質が35%〜100%の可溶性蛋白質を含み、
b)前記栄養液剤は、
0.32×[可溶性クエン酸塩]+0.63[可溶性リン酸塩]+0.013×[可溶性蛋白質]
に等しい可溶性カルシウム結合能を有し、
c)前記栄養液剤は、可溶性カルシウムに対する可溶性カルシウム結合能の重量比を2.3:1から12.0:1で有する、
前記組成物。 - 可溶性蛋白質が、ホスホセリン含有蛋白質1キログラム当り少なくとも100mmolのホスホセリンを有するホスホセリン含有蛋白質を含む、請求項9に記載の包装組成物。
- プラスチックパッケージが再封可能である、請求項1、請求項6または請求項9に記載の包装組成物。
- 可溶性蛋白質がカゼイン酸ナトリウム、ホエー蛋白質濃縮物およびその組合せからなる群から選択される、請求項5、請求項6または請求項9に記載の包装組成物。
- 栄養液剤がカルシウムβ−ヒドロキシ−β−メチルブチレートに対して5:1〜12:1の重量比の可溶性蛋白質を含む、請求項5、請求項6または請求項9に記載の包装組成物。
- プラスチックパッケージ入りpH安定性栄養液剤の製造方法であって、
a)β−ヒドロキシ−β−メチルブチレート、可溶性カルシウム、可溶性クエン酸塩、可溶性リン酸塩、可溶性蛋白質、蛋白質、糖質、及び脂質を配合し、栄養液剤を形成する工程であって、
i)前記蛋白質が35%〜100%の可溶性蛋白質を含み、
ii)前記栄養液剤は、
0.32×[可溶性クエン酸塩]+0.63[可溶性リン酸塩]+0.013×[可溶性蛋白質]
に等しい可溶性カルシウム結合能を有し、
iii)前記栄養液剤は、可溶性カルシウムに対する可溶性カルシウム結合能の重量比を2.3:1から12.0:1で有する、前記工程と;
b)栄養液剤をプラスチックパッケージに導入する工程と;
c)プラスチックパッケージ内の栄養液剤をレトルト殺菌する工程と
を含む、前記方法。 - プラスチックパッケージ入りpH安定性栄養液剤の製造方法であって、
a)β−ヒドロキシ−β−メチルブチレート、可溶性カルシウム、可溶性クエン酸塩、可溶性リン酸塩、可溶性蛋白質、蛋白質、糖質、及び脂質を配合し、栄養液剤を形成する工程であって、
i)前記蛋白質が35%〜100%の可溶性蛋白質を含み、
ii)前記栄養液剤は、
0.32×[可溶性クエン酸塩]+0.63[可溶性リン酸塩]+0.013×[可溶性蛋白質]
に等しい可溶性カルシウム結合能を有し、
iii)前記栄養液剤は、可溶性カルシウムに対する可溶性カルシウム結合能の重量比を2.3:1から12.0:1で有する、前記工程と;
b)栄養液剤を殺菌する工程と;
c)プラスチックパッケージを殺菌する工程と;
d)殺菌した栄養液剤を殺菌したプラスチックパッケージに導入する工程と
を含む、前記方法。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US29971810P | 2010-01-29 | 2010-01-29 | |
| US61/299,718 | 2010-01-29 | ||
| PCT/US2011/022938 WO2011094551A1 (en) | 2010-01-29 | 2011-01-28 | Plastic packaged nutritonal liquids comprising hmb |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2013517807A JP2013517807A (ja) | 2013-05-20 |
| JP2013517807A5 JP2013517807A5 (ja) | 2015-03-26 |
| JP5779594B2 true JP5779594B2 (ja) | 2015-09-16 |
Family
ID=43928426
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012551326A Expired - Fee Related JP5779594B2 (ja) | 2010-01-29 | 2011-01-28 | Hmbを含有するプラスチック包装栄養液剤 |
Country Status (18)
| Country | Link |
|---|---|
| US (1) | US20110250322A1 (ja) |
| EP (1) | EP2528457B1 (ja) |
| JP (1) | JP5779594B2 (ja) |
| CN (2) | CN102762112A (ja) |
| AU (1) | AU2011210685A1 (ja) |
| BR (1) | BR112012018857A2 (ja) |
| CA (1) | CA2785526C (ja) |
| CO (1) | CO6592062A2 (ja) |
| EC (1) | ECSP12012055A (ja) |
| ES (1) | ES2533357T3 (ja) |
| IL (1) | IL220659A0 (ja) |
| MX (1) | MX2012008783A (ja) |
| PE (1) | PE20121727A1 (ja) |
| PH (1) | PH12012501495A1 (ja) |
| RU (1) | RU2012125880A (ja) |
| SG (1) | SG182807A1 (ja) |
| TW (1) | TWI535388B (ja) |
| WO (1) | WO2011094551A1 (ja) |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2011210691A1 (en) | 2010-01-29 | 2012-07-12 | Abbott Laboratories | Nutritional emulsions comprising calcium HMB |
| US9693577B2 (en) | 2010-01-29 | 2017-07-04 | Abbott Laboratories | Method of preparing a nutritional powder comprising spray dried HMB |
| JP6076741B2 (ja) | 2010-01-29 | 2017-02-08 | アボット・ラボラトリーズAbbott Laboratories | 無菌包装されているhmbを含む栄養液 |
| TWI526161B (zh) | 2010-06-10 | 2016-03-21 | 亞培公司 | 包含鈣hmb及可溶性蛋白質之實質上透明營養液 |
| WO2013056048A2 (en) * | 2011-10-13 | 2013-04-18 | Abbott Laboratories | Nutritional products comprising beta-alanine |
| JP6346896B2 (ja) * | 2012-09-21 | 2018-06-20 | アボット・ラボラトリーズAbbott Laboratories | カルシウムβ−ヒドロキシ−β−メチルブチレート、タンパク質および低レベルの電解質を含む栄養組成物 |
| SG10201706315RA (en) * | 2012-10-24 | 2017-09-28 | Abbott Lab | High protein, low viscosity liquid nutritional product with hmb |
| CN105188417B (zh) * | 2013-03-15 | 2017-08-08 | 雅培制药有限公司 | 包括β‑羟基‑β‑甲基丁酸钙、酪蛋白磷酸肽和蛋白质的营养组合物 |
| WO2015037720A1 (ja) * | 2013-09-12 | 2015-03-19 | 株式会社明治 | 筋肉合成促進剤 |
| WO2015095725A1 (en) * | 2013-12-19 | 2015-06-25 | Abbott Laboratories | Methods and compositions for attenuating muscle protein degradation and preserving lean body mass |
| BE1027258B1 (nl) * | 2019-12-10 | 2020-12-01 | Culinor Nv | Werkwijze voor het verwerken van voeding |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2674102B1 (fr) * | 1991-03-18 | 1994-05-20 | Meiji Seika Kaisha Ltd | Aliment pour porcs et methode d'elevage des porcs utilisant cet aliment. |
| US5447732A (en) * | 1992-11-25 | 1995-09-05 | Ajinomoto Co., Inc. | High-absorption mineral-containing composition and foods |
| JP3381202B2 (ja) * | 1993-10-06 | 2003-02-24 | キユーピー株式会社 | 可撓性容器入り滅菌液状乳化食品 |
| JP3393946B2 (ja) * | 1995-01-19 | 2003-04-07 | テルモ株式会社 | 液状栄養食および高カロリー栄養剤 |
| EP0865735A1 (en) * | 1997-03-19 | 1998-09-23 | Societe Des Produits Nestle S.A. | Process for sterilising beverages |
| US20060062827A1 (en) * | 2002-12-20 | 2006-03-23 | Gel Dynamics, Llc | Nutritional supplement composition and method |
| US20050215640A1 (en) * | 2004-03-26 | 2005-09-29 | Baxter Jeffrey H | HMB compositions and uses thereof |
| DE102004036047A1 (de) * | 2004-07-24 | 2006-02-23 | Bioghurt Biogarde Gmbh & Co. Kg | Physiologisch aktive Zusammensetzung |
| JP4262710B2 (ja) * | 2004-10-15 | 2009-05-13 | 株式会社大塚製薬工場 | 流動食の製造方法 |
| AU2006331950A1 (en) * | 2005-12-19 | 2007-07-05 | Abbott Laboratories | Use of beta-hydroxy-beta-methylbutyrate to modulate the imbalance of type 1 and type 2 cytokine production |
| JP2009034018A (ja) * | 2007-07-31 | 2009-02-19 | Ajinomoto Co Inc | トマト風味栄養組成物 |
| CN104944346B (zh) * | 2009-02-06 | 2017-08-25 | 大日本印刷株式会社 | 饮料灌装方法及饮料灌装装置 |
-
2011
- 2011-01-28 CN CN201180007418XA patent/CN102762112A/zh active Pending
- 2011-01-28 WO PCT/US2011/022938 patent/WO2011094551A1/en not_active Ceased
- 2011-01-28 CN CN201611216419.6A patent/CN107080236A/zh active Pending
- 2011-01-28 PH PH1/2012/501495A patent/PH12012501495A1/en unknown
- 2011-01-28 CA CA2785526A patent/CA2785526C/en active Active
- 2011-01-28 BR BRBR112012018857-7A patent/BR112012018857A2/pt not_active IP Right Cessation
- 2011-01-28 SG SG2012056560A patent/SG182807A1/en unknown
- 2011-01-28 JP JP2012551326A patent/JP5779594B2/ja not_active Expired - Fee Related
- 2011-01-28 EP EP11705725.7A patent/EP2528457B1/en not_active Not-in-force
- 2011-01-28 ES ES11705725.7T patent/ES2533357T3/es active Active
- 2011-01-28 PE PE2012001075A patent/PE20121727A1/es not_active Application Discontinuation
- 2011-01-28 US US13/016,059 patent/US20110250322A1/en not_active Abandoned
- 2011-01-28 TW TW100103535A patent/TWI535388B/zh not_active IP Right Cessation
- 2011-01-28 MX MX2012008783A patent/MX2012008783A/es active IP Right Grant
- 2011-01-28 RU RU2012125880/13A patent/RU2012125880A/ru not_active Application Discontinuation
- 2011-01-28 AU AU2011210685A patent/AU2011210685A1/en not_active Abandoned
-
2012
- 2012-06-26 IL IL220659A patent/IL220659A0/en unknown
- 2012-07-19 CO CO12122194A patent/CO6592062A2/es not_active Application Discontinuation
- 2012-07-23 EC ECSP12012055 patent/ECSP12012055A/es unknown
Also Published As
| Publication number | Publication date |
|---|---|
| CA2785526C (en) | 2015-11-24 |
| EP2528457B1 (en) | 2015-01-14 |
| RU2012125880A (ru) | 2014-03-10 |
| US20110250322A1 (en) | 2011-10-13 |
| AU2011210685A1 (en) | 2012-07-05 |
| IL220659A0 (en) | 2012-08-30 |
| HK1178027A1 (en) | 2013-09-06 |
| EP2528457A1 (en) | 2012-12-05 |
| PE20121727A1 (es) | 2013-01-13 |
| SG182807A1 (en) | 2012-09-27 |
| CA2785526A1 (en) | 2011-08-04 |
| BR112012018857A2 (pt) | 2015-09-01 |
| WO2011094551A1 (en) | 2011-08-04 |
| CN102762112A (zh) | 2012-10-31 |
| ES2533357T3 (es) | 2015-04-09 |
| JP2013517807A (ja) | 2013-05-20 |
| MX2012008783A (es) | 2012-08-17 |
| CN107080236A (zh) | 2017-08-22 |
| TWI535388B (zh) | 2016-06-01 |
| TW201200036A (en) | 2012-01-01 |
| CO6592062A2 (es) | 2013-01-02 |
| PH12012501495A1 (en) | 2012-10-22 |
| ECSP12012055A (es) | 2012-08-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5779594B2 (ja) | Hmbを含有するプラスチック包装栄養液剤 | |
| JP6076741B2 (ja) | 無菌包装されているhmbを含む栄養液 | |
| TWI526161B (zh) | 包含鈣hmb及可溶性蛋白質之實質上透明營養液 | |
| CN102711525B (zh) | 包含hmb钙和可溶蛋白的营养乳液 | |
| CN102711524B (zh) | 包含hmb钙的营养乳剂 | |
| HK1178027B (en) | Plastic packaged nutritonal liquids comprising hmb | |
| HK1171342B (en) | Aseptically packaged nutritional liquids comprising hmb |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140127 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20140127 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20140805 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140902 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20141126 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20141203 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20141222 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150129 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20150129 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20150616 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20150713 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5779594 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |