JP5723386B2 - 陰圧創傷治療法のための装置 - Google Patents
陰圧創傷治療法のための装置 Download PDFInfo
- Publication number
- JP5723386B2 JP5723386B2 JP2012543519A JP2012543519A JP5723386B2 JP 5723386 B2 JP5723386 B2 JP 5723386B2 JP 2012543519 A JP2012543519 A JP 2012543519A JP 2012543519 A JP2012543519 A JP 2012543519A JP 5723386 B2 JP5723386 B2 JP 5723386B2
- Authority
- JP
- Japan
- Prior art keywords
- negative pressure
- wound
- foam
- wound therapy
- pressure wound
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000009581 negative-pressure wound therapy Methods 0.000 title claims description 58
- 206010052428 Wound Diseases 0.000 claims description 141
- 208000027418 Wounds and injury Diseases 0.000 claims description 141
- 239000006260 foam Substances 0.000 claims description 88
- 239000000463 material Substances 0.000 claims description 75
- 239000000203 mixture Substances 0.000 claims description 25
- 238000009826 distribution Methods 0.000 claims description 22
- 238000007789 sealing Methods 0.000 claims description 21
- 238000011282 treatment Methods 0.000 claims description 16
- 125000000882 C2-C6 alkenyl group Chemical group 0.000 claims description 15
- 239000012530 fluid Substances 0.000 claims description 12
- 230000035699 permeability Effects 0.000 claims description 11
- 239000003054 catalyst Substances 0.000 claims description 10
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 claims description 10
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims description 8
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 7
- 239000004604 Blowing Agent Substances 0.000 claims description 6
- 125000003118 aryl group Chemical group 0.000 claims description 6
- 229910052739 hydrogen Inorganic materials 0.000 claims description 6
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 6
- 125000002524 organometallic group Chemical group 0.000 claims description 6
- 229910052710 silicon Inorganic materials 0.000 claims description 6
- 239000001257 hydrogen Substances 0.000 claims description 5
- 125000004435 hydrogen atom Chemical class [H]* 0.000 claims description 5
- 125000000217 alkyl group Chemical group 0.000 claims description 4
- 239000002994 raw material Substances 0.000 claims description 3
- 230000029663 wound healing Effects 0.000 claims description 3
- 239000010703 silicon Substances 0.000 claims description 2
- 125000001188 haloalkyl group Chemical group 0.000 claims 2
- 230000037431 insertion Effects 0.000 claims 2
- 238000003780 insertion Methods 0.000 claims 2
- 125000003342 alkenyl group Chemical group 0.000 claims 1
- 210000004027 cell Anatomy 0.000 description 54
- 239000000853 adhesive Substances 0.000 description 20
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 19
- 230000001070 adhesive effect Effects 0.000 description 16
- -1 polypropylene Polymers 0.000 description 15
- 238000000034 method Methods 0.000 description 14
- 239000011248 coating agent Substances 0.000 description 12
- 229920001296 polysiloxane Polymers 0.000 description 12
- 238000000576 coating method Methods 0.000 description 11
- 239000004372 Polyvinyl alcohol Substances 0.000 description 9
- 230000008901 benefit Effects 0.000 description 9
- 210000000416 exudates and transudate Anatomy 0.000 description 9
- 229920002451 polyvinyl alcohol Polymers 0.000 description 9
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 8
- 239000011888 foil Substances 0.000 description 8
- 229920000642 polymer Polymers 0.000 description 8
- 210000002421 cell wall Anatomy 0.000 description 6
- 229910052709 silver Inorganic materials 0.000 description 6
- 239000004332 silver Substances 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 5
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 5
- 239000003795 chemical substances by application Substances 0.000 description 5
- 229910052697 platinum Inorganic materials 0.000 description 5
- 229920003176 water-insoluble polymer Polymers 0.000 description 5
- AMQJEAYHLZJPGS-UHFFFAOYSA-N N-Pentanol Chemical compound CCCCCO AMQJEAYHLZJPGS-UHFFFAOYSA-N 0.000 description 4
- 239000007789 gas Substances 0.000 description 4
- ZSIAUFGUXNUGDI-UHFFFAOYSA-N hexan-1-ol Chemical compound CCCCCCO ZSIAUFGUXNUGDI-UHFFFAOYSA-N 0.000 description 4
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 4
- 230000036961 partial effect Effects 0.000 description 4
- 239000004814 polyurethane Substances 0.000 description 4
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 239000002390 adhesive tape Substances 0.000 description 3
- 230000001580 bacterial effect Effects 0.000 description 3
- BTANRVKWQNVYAZ-UHFFFAOYSA-N butan-2-ol Chemical compound CCC(C)O BTANRVKWQNVYAZ-UHFFFAOYSA-N 0.000 description 3
- 125000004432 carbon atom Chemical group C* 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 238000004132 cross linking Methods 0.000 description 3
- 239000002657 fibrous material Substances 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- 230000000704 physical effect Effects 0.000 description 3
- 229920000728 polyester Polymers 0.000 description 3
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 239000012780 transparent material Substances 0.000 description 3
- 125000000171 (C1-C6) haloalkyl group Chemical group 0.000 description 2
- KBPLFHHGFOOTCA-UHFFFAOYSA-N 1-Octanol Chemical compound CCCCCCCCO KBPLFHHGFOOTCA-UHFFFAOYSA-N 0.000 description 2
- BBMCTIGTTCKYKF-UHFFFAOYSA-N 1-heptanol Chemical compound CCCCCCCO BBMCTIGTTCKYKF-UHFFFAOYSA-N 0.000 description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical group [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical group [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- DKGAVHZHDRPRBM-UHFFFAOYSA-N Tert-Butanol Chemical compound CC(C)(C)O DKGAVHZHDRPRBM-UHFFFAOYSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 150000001298 alcohols Chemical class 0.000 description 2
- 125000004429 atom Chemical group 0.000 description 2
- WERYXYBDKMZEQL-UHFFFAOYSA-N butane-1,4-diol Chemical compound OCCCCO WERYXYBDKMZEQL-UHFFFAOYSA-N 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 239000007795 chemical reaction product Substances 0.000 description 2
- BITPLIXHRASDQB-UHFFFAOYSA-N ethenyl-[ethenyl(dimethyl)silyl]oxy-dimethylsilane Chemical compound C=C[Si](C)(C)O[Si](C)(C)C=C BITPLIXHRASDQB-UHFFFAOYSA-N 0.000 description 2
- 239000004744 fabric Substances 0.000 description 2
- 239000010408 film Substances 0.000 description 2
- QNVRIHYSUZMSGM-UHFFFAOYSA-N hexan-2-ol Chemical compound CCCCC(C)O QNVRIHYSUZMSGM-UHFFFAOYSA-N 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 125000004430 oxygen atom Chemical group O* 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 239000003566 sealing material Substances 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 230000003068 static effect Effects 0.000 description 2
- 230000001954 sterilising effect Effects 0.000 description 2
- 238000004659 sterilization and disinfection Methods 0.000 description 2
- 229920000247 superabsorbent polymer Polymers 0.000 description 2
- 229920002554 vinyl polymer Polymers 0.000 description 2
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 1
- QNVRIHYSUZMSGM-LURJTMIESA-N 2-Hexanol Natural products CCCC[C@H](C)O QNVRIHYSUZMSGM-LURJTMIESA-N 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical group [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 206010011985 Decubitus ulcer Diseases 0.000 description 1
- 241000021559 Dicerandra Species 0.000 description 1
- 235000010654 Melissa officinalis Nutrition 0.000 description 1
- 208000004221 Multiple Trauma Diseases 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 229920005830 Polyurethane Foam Polymers 0.000 description 1
- 208000004210 Pressure Ulcer Diseases 0.000 description 1
- 208000003251 Pruritus Diseases 0.000 description 1
- 229920001247 Reticulated foam Polymers 0.000 description 1
- 239000012891 Ringer solution Substances 0.000 description 1
- 229910008051 Si-OH Inorganic materials 0.000 description 1
- 229910006358 Si—OH Inorganic materials 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000002313 adhesive film Substances 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical group [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 238000003869 coulometry Methods 0.000 description 1
- 239000013039 cover film Substances 0.000 description 1
- HPXRVTGHNJAIIH-UHFFFAOYSA-N cyclohexanol Chemical compound OC1CCCCC1 HPXRVTGHNJAIIH-UHFFFAOYSA-N 0.000 description 1
- XCIXKGXIYUWCLL-UHFFFAOYSA-N cyclopentanol Chemical compound OC1CCCC1 XCIXKGXIYUWCLL-UHFFFAOYSA-N 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 239000000645 desinfectant Substances 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000016097 disease of metabolism Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000005187 foaming Methods 0.000 description 1
- 230000035876 healing Effects 0.000 description 1
- 125000006038 hexenyl group Chemical group 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 239000000865 liniment Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- 208000030159 metabolic disease Diseases 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- QQZOPKMRPOGIEB-UHFFFAOYSA-N n-butyl methyl ketone Natural products CCCCC(C)=O QQZOPKMRPOGIEB-UHFFFAOYSA-N 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 229920006264 polyurethane film Polymers 0.000 description 1
- 239000011496 polyurethane foam Substances 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- 239000004800 polyvinyl chloride Substances 0.000 description 1
- 229910000028 potassium bicarbonate Inorganic materials 0.000 description 1
- 235000015497 potassium bicarbonate Nutrition 0.000 description 1
- 239000011736 potassium bicarbonate Substances 0.000 description 1
- TYJJADVDDVDEDZ-UHFFFAOYSA-M potassium hydrogencarbonate Chemical compound [K+].OC([O-])=O TYJJADVDDVDEDZ-UHFFFAOYSA-M 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 150000003333 secondary alcohols Chemical class 0.000 description 1
- 125000006850 spacer group Chemical group 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 238000009827 uniform distribution Methods 0.000 description 1
- 239000011800 void material Substances 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/22—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing macromolecular materials
- A61L15/26—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/425—Porous materials, e.g. foams or sponges
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/90—Negative pressure wound therapy devices, i.e. devices for applying suction to a wound to promote healing, e.g. including a vacuum dressing
- A61M1/91—Suction aspects of the dressing
- A61M1/915—Constructional details of the pressure distribution manifold
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J9/00—Working-up of macromolecular substances to porous or cellular articles or materials; After-treatment thereof
- C08J9/02—Working-up of macromolecular substances to porous or cellular articles or materials; After-treatment thereof using blowing gases generated by the reacting monomers or modifying agents during the preparation or modification of macromolecules
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/90—Negative pressure wound therapy devices, i.e. devices for applying suction to a wound to promote healing, e.g. including a vacuum dressing
- A61M1/98—Containers specifically adapted for negative pressure wound therapy
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2201/00—Foams characterised by the foaming process
- C08J2201/02—Foams characterised by the foaming process characterised by mechanical pre- or post-treatments
- C08J2201/024—Preparation or use of a blowing agent concentrate, i.e. masterbatch in a foamable composition
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2205/00—Foams characterised by their properties
- C08J2205/04—Foams characterised by their properties characterised by the foam pores
- C08J2205/05—Open cells, i.e. more than 50% of the pores are open
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2207/00—Foams characterised by their intended use
- C08J2207/10—Medical applications, e.g. biocompatible scaffolds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2383/00—Characterised by the use of macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon with or without sulfur, nitrogen, oxygen, or carbon only; Derivatives of such polymers
- C08J2383/04—Polysiloxanes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L83/00—Compositions of macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon with or without sulfur, nitrogen, oxygen or carbon only; Compositions of derivatives of such polymers
- C08L83/04—Polysiloxanes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L83/00—Compositions of macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon with or without sulfur, nitrogen, oxygen or carbon only; Compositions of derivatives of such polymers
- C08L83/14—Compositions of macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon with or without sulfur, nitrogen, oxygen or carbon only; Compositions of derivatives of such polymers in which at least two but not all the silicon atoms are connected by linkages other than oxygen atoms
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Hematology (AREA)
- Materials Engineering (AREA)
- Heart & Thoracic Surgery (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Vascular Medicine (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Dispersion Chemistry (AREA)
- Organic Chemistry (AREA)
- Polymers & Plastics (AREA)
- Medicinal Chemistry (AREA)
- Materials For Medical Uses (AREA)
- Medicinal Preparation (AREA)
- Manufacture Of Porous Articles, And Recovery And Treatment Of Waste Products (AREA)
Description
(a) 前記創傷及び創傷の周辺を密閉するためのカバー材料、
(b) 前記創傷領域内に陰圧の生成のための手段、好ましくは前記創傷領域を、前記カバー材料の外部の陰圧源と機能的に接続して陰圧が前記創傷領域で生成され、流体が吸引により前記創傷領域から取り除かれるようにするための手段、
(c) 創傷皮膚材としてクロスリンクポリオルガノシロキサン系のオープンセルフォーム、を含む。
(a) 前記創傷及び創傷周辺を密閉するためのカバー材料、
(b) 前記創傷領域内に陰圧の生成のための手段、好ましくは前記創傷領域を、前記カバー材料の外部の陰圧源と機能的に接続して陰圧が前記創傷領域で生成され、流体が吸引により前記創傷領域ら取り除かれるようにするための手段、
(c) 創傷皮膚材としてクロスリンクポリオルガノシロキサン系のオープンセルフォームを含み、それにより前記フォームが被覆材として適切であり、即使用状態で提供される。
つの実施態様では、前記カバー材料の外側で前記創傷領域と陰圧源との機能的接続は少なくとも1つの接続ラインを含む。前記少なくとも1つの接続ラインは前記カバー材料を通じて配設され得る。
(i) C2〜C6アルケニル基、好ましくは1以上のビニル基を含むポリオルガノシロキサン、
(ii) 1以上のSi−H基を含むポリオルガノシロキサン、
(iii) 1以上のOH基を含む発泡剤、及び
(iv) 有機金属触媒、である。前記硬化可能な混合物の成分(i)から(iv)は、以下詳細に説明される。
ここで、R1は、独立して、C1〜C6アルキル、C1〜C6ハロアルキル、アリール、又はC2〜C6アルケニル基であり、ただし前記分子が少なくともC2〜C6アルケニル基、好ましくは少なくとも2つのC2〜C6アルケニル基又は少なくとも3つのC2〜C6アルケニル基を含む。式(1)の前記化合物は従って、好ましくは直鎖ポリシロキサンであり、M及びD、単位を含む。
前記直鎖成分(i−M/D)と共に、前記硬化可能な混合物の成分(i)(即ち、1以上のC2〜C6アルケニル基を含むポリオルガノシロキサン)はまた、分岐ポリオルガノシロキサン成分であって、M及びD単位と共にまたT及び/又はQ単位も含む分岐ポリオルガノシロキサン成分を含み得る。これらの分岐成分は、成分(i−M/D/T/Q)と参照される。C2〜C6アルケニル基のタイプ及び量に関する前記説明はまた、前記成分(i−M/D/T/Q)にも適用される。特に、成分(i−M/D/T/Q)は、少なくとも2つのC2〜C6アルケニル基、好ましくはビニル基を含む。
1つの好ましい実施態様では、成分(ii)は、式(2)のポリオルガノシロキサンを含み、R2は独立して、C1〜C6アルキル、C1〜C6ハロアルキル、アリール又は水素である。ただし好ましくは、式(2)の1分子が少なくとも2つの、好ましくは3つのシリコン原子に結合する水素を含み、それにより前記水素原子が異なるシリコン原子に結合される。前記式(2)においては、ポリオルガノシロキサンは、M及びD単位を持つ場合が好ましい。又は前記ポリオルガノシロキサンはまた、T及び/又はQ適用単位を含む。一般には、成分(ii)のシリコン原子の0.01から10%がSi−H結合を持つ。
(a) 請求項1から12のいずれか1項に記載の本発明による装置又は請求項13に記載のセットを適用するステップ、
(b) 前記創傷に陰圧被覆を適用するステップ、
(c) 最大500mmHg、好ましくは最大250mmHgの陰圧を、少なくとも30分及び最大7日間まで、好ましくは少なくとも1日及び最大6日間までの間前記創傷領域で生成させるステップを含む。
部分混合物A:
60部の粘度6000mPasのビニル基含有M/Dポリシロキサン(i)
30部の粘度5500mPasのビニル基含有M/D/Qポリシロキサン(i)
3部のn−ブタノール(iii)
0.1部のPd触媒(カルステット)
部分混合物B:
30部の粘度6000mPasのビニル基含有M/Dポリシロキサン(i)
10部の粘度5500mPasのビニル基含有M/D/Qポリシロキサン(i)
30部の粘度4500nPasのSi−H基含有M/Dポリシロキサン(iii)
部分混合物A及びBをスタティックミキサー中で22℃で混合し反応させた。20分後得られたフォームを取り出し望ましいサイズに切断した。得られたフォームは、次のパラメータで特徴付けられた:密度が0.22g/cm3、引張強度>150kPa、延性>200%、空気透過性4000l/m2秒)。
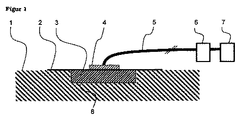
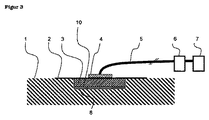
2 密閉材料(a)
3 クロスリンクポリオルガノシロキサン系オープンセルフォーム(c)
4 陰圧接続部(ポート)
5 陰圧接続ライン
6 収容器
7 陰圧ユニット
8 創傷
9 圧力分布層
10 創傷接触層
Claims (15)
- 陰圧創傷治療法のための装置であり、前記装置が:
(a) 前記創傷及び創傷周囲を密閉するためのカバー材料;
(b) 前記創傷の領域に陰圧を発生させるのに適した手段;及び
(c) 創傷被覆材としてのクロスリンクポリオルガノシロキサン系オープンセルフォーム;
を含み、
前記フォーム(c)が、DIN EN ISO9237による測定で空気透過性が1000から8000 l/(m 2 秒)である、装置。 - 請求項1に記載の陰圧創傷治療法のための装置であり、前記フォーム(c)が、次の成分;
(i) C2〜C6アルケニル基を持つ1以上の基を含むポリオルガノシロキサン、
(ii) 1以上のSi−H基を含むポリオルガノシロキサン、
(iii) 1以上のOH基を含む発泡剤、及び
(iv) 有機金属触媒、
を含む硬化可能混合物の反応により得られる、陰圧創傷治療法のための装置。 - 請求項1に記載の陰圧創傷治療法のための装置であり、前記フォーム(c)が、次の成分;
(i) 1以上のビニル基を持つポリオルガノシロキサン、
(ii) 1以上のSi−H基を含むポリオルガノシロキサン、
(iii) 1以上のOH基を含む発泡剤、及び
(iv) 有機金属触媒、
を含む硬化可能混合物の反応により得られる、陰圧創傷治療法のための装置。 - 請求項2乃至5のいずれか1項に記載の陰圧創傷治療法のための装置であり、成分(iii)としてC1〜6アルカノールが使用される、陰圧創傷治療法のための装置。
- 請求項1乃至6のいずれか1項に記載の陰圧創傷治療法のための装置であり、前記フォームが、引張強度がDIN53571による測定で100kPaから10MPaであり及び/又は延性がDIN53571による測定で100%から350%である、陰圧創傷治療法のための装置。
- 請求項1乃至7のいずれか1項に記載の陰圧創傷治療法のための装置であり、前記フォーム(c)が粘弾性を有する、陰圧創傷治療法のための装置。
- 請求項1乃至8のいずれか1項に記載の陰圧創傷治療法のための装置であり、前記フォーム(c)が、DIN EN ISO845による測定で原料密度が0.12と0.30g/cm3との間である、陰圧創傷治療法のための装置。
- 請求項1乃至9のいずれか1項に記載の陰圧創傷治療法のための装置であり、前記カバー材料(a)が、100から2500g/m2x24時間の水蒸気透過性を有する、陰圧創傷治療法のための装置。
- 請求項1乃至10のいずれか1項に記載の陰圧創傷治療法のための装置であり、さらに、前記創傷被覆材(c)及び前記カバー材料(a)との間に挿入するための少なくとも1つの追加の圧力分布層を含む、陰圧創傷治療法のための装置。
- 請求項1乃至11のいずれか1項に記載の陰圧創傷治療法のための装置であり、さらに、前記創傷の表面と前記創傷被覆材(c)との間に挿入するための創傷接触層を含む、陰圧創傷治療法のための装置。
- 陰圧創傷治療法のための即使用セットであって、前記セットが:
(a) 前記創傷及び創傷周辺を密閉するためのカバー材料、
(b) 前記創傷の領域内に陰圧を生成するのに適切な手段であり、陰圧を前記創傷の領域で生成しかつ流体を前記創傷の領域から吸引により除くことができる、手段、
(c) クロスリンクポリオルガノシロキサン系オープンセルフォーム、
を含み、前記フォームが創傷被覆材として適しており、即使用パック内に準備されており、
前記フォーム(c)が、DIN EN ISO9237による測定で空気透過性が1000から8000l/(m 2 秒)である、陰圧創傷治療法のための即使用セット。 - 陰圧創傷治療法のための即使用セットであって、前記セットが:
(a) 前記創傷及び創傷周辺を密閉するためのカバー材料、
(b) 前記創傷の領域を前記カバー材料の外側の陰圧源と機能的に接続する手段であり、陰圧を前記創傷の領域で生成しかつ流体を前記創傷の領域から吸引により除くことができる、手段、
(c) クロスリンクポリオルガノシロキサン系オープンセルフォーム、
を含み、前記フォームが創傷被覆材として適しており、即使用パック内に準備されており、
前記フォーム(c)が、DIN EN ISO9237による測定で空気透過性が1000から8000l/(m 2 秒)である、陰圧創傷治療法のための即使用セット。 - 請求項1乃至12のいずれか1項に記載の陰圧創傷治療法のための装置において使用するための、クロスリンクポリオルガノシロキサン系オープンセルフォーム。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP09015561.5 | 2009-12-16 | ||
| EP09015561.5A EP2335747B1 (de) | 2009-12-16 | 2009-12-16 | Vorrichtung zur Unterdrucktherapie von Wunden |
| PCT/EP2010/007620 WO2011072840A1 (de) | 2009-12-16 | 2010-12-14 | Vorrichtung zur unterdrucktherapie von wunden |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2013514094A JP2013514094A (ja) | 2013-04-25 |
| JP2013514094A5 JP2013514094A5 (ja) | 2013-10-31 |
| JP5723386B2 true JP5723386B2 (ja) | 2015-05-27 |
Family
ID=42122859
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012543519A Expired - Fee Related JP5723386B2 (ja) | 2009-12-16 | 2010-12-14 | 陰圧創傷治療法のための装置 |
Country Status (5)
| Country | Link |
|---|---|
| EP (2) | EP2335747B1 (ja) |
| JP (1) | JP5723386B2 (ja) |
| CN (1) | CN102740901B (ja) |
| RU (1) | RU2530054C2 (ja) |
| WO (1) | WO2011072840A1 (ja) |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB0224986D0 (en) | 2002-10-28 | 2002-12-04 | Smith & Nephew | Apparatus |
| GB201011173D0 (en) | 2010-07-02 | 2010-08-18 | Smith & Nephew | Provision of wound filler |
| RU2597393C2 (ru) | 2010-11-25 | 2016-09-10 | СМИТ ЭНД НЕФЬЮ ПиЭлСи | Композиция i-ii, содержащие ее изделия и ее применения |
| GB201020005D0 (en) * | 2010-11-25 | 2011-01-12 | Smith & Nephew | Composition 1-1 |
| US20150159066A1 (en) | 2011-11-25 | 2015-06-11 | Smith & Nephew Plc | Composition, apparatus, kit and method and uses thereof |
| US20160120706A1 (en) | 2013-03-15 | 2016-05-05 | Smith & Nephew Plc | Wound dressing sealant and use thereof |
| DE102013008964A1 (de) | 2013-05-22 | 2014-11-27 | Gmbu E.V., Fachsektion Dresden | Vorrichtung zur Behandlung von Problemwunden |
| DE102014106518A1 (de) | 2014-05-09 | 2015-11-12 | Paul Hartmann Ag | Schaumwundauflage für die Unterdrucktherapie |
| DE102014116912A1 (de) | 2014-11-19 | 2016-05-19 | Paul Hartmann Ag | Flüssigkeitsindikator für Unterdrucktherapie-Vorrichtung |
| DE102014116910A1 (de) | 2014-11-19 | 2016-05-19 | Paul Hartmann Ag | Elektronischer Flüssigkeitssensor für Unterdrucktherapie-Vorrichtung |
| DE102015007622A1 (de) | 2015-06-16 | 2016-12-22 | Paul Hartmann Ag | Vorrichtung zur Unterdrucktherapie von Wunden enthaltend siliconhaltigen Polyurethanschaumstoff |
| DE102016114786A1 (de) | 2016-08-10 | 2018-02-15 | Paul Hartmann Ag | Fassschlaufe für Saugkörper zur endoluminalen Unterdrucktherapie |
| DE102016114819A1 (de) | 2016-08-10 | 2018-02-15 | Paul Hartmann Ag | Saugkörper zur endoluminalen Unterdrucktherapie |
| DE102016114817A1 (de) | 2016-08-10 | 2018-02-15 | Paul Hartmann Ag | Medizinisches Kit zur Verwendung bei der Unterdrucktherapie von endoluminalen Wundstellen im Bereich des Gastrointestinaltraktes |
| GB201904402D0 (en) | 2019-03-29 | 2019-05-15 | Trio Healthcare Ltd | Foamed skin compatible silicone composition |
Family Cites Families (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3775452A (en) | 1971-04-28 | 1973-11-27 | Gen Electric | Platinum complexes of unsaturated siloxanes and platinum containing organopolysiloxanes |
| US4808634A (en) * | 1988-03-24 | 1989-02-28 | General Electric Company | Low density silicon foam |
| US5645081A (en) | 1991-11-14 | 1997-07-08 | Wake Forest University | Method of treating tissue damage and apparatus for same |
| GB9411429D0 (en) * | 1994-06-08 | 1994-07-27 | Seton Healthcare Group Plc | Wound dressings |
| JP3500992B2 (ja) * | 1997-11-19 | 2004-02-23 | 信越化学工業株式会社 | オルガノポリシロキサン組成物 |
| WO2001089431A1 (en) | 2000-05-22 | 2001-11-29 | Coffey Arthur C | Combination sis and vacuum bandage and method |
| JP3647389B2 (ja) * | 2000-08-01 | 2005-05-11 | ジーイー東芝シリコーン株式会社 | ポリオルガノシロキサン発泡材、発泡体およびその製造方法 |
| RU22749U1 (ru) * | 2001-02-06 | 2002-04-27 | Закрытое акционерное общество "МедСил" | Резиновая медицинская силиконовая трубка |
| US7700819B2 (en) * | 2001-02-16 | 2010-04-20 | Kci Licensing, Inc. | Biocompatible wound dressing |
| GB2415382A (en) | 2004-06-21 | 2005-12-28 | Johnson & Johnson Medical Ltd | Wound dressings for vacuum therapy |
| GB2415908A (en) | 2004-07-09 | 2006-01-11 | Ethicon Inc | Vacuum wound dressings |
| DE202004017052U1 (de) | 2004-11-02 | 2005-06-09 | Riesinger, Birgit | Vorrichtung zur Wundbehandlung unter Einsatz von Unterdruck |
| JP5154228B2 (ja) | 2004-11-05 | 2013-02-27 | コンバテック・テクノロジーズ・インコーポレイテッド | 真空創傷包帯 |
| FR2902107A1 (fr) * | 2006-06-07 | 2007-12-14 | Rhodia Recherches & Tech | Composition organopolysiloxane pour mousse elastomere |
| US7842848B2 (en) * | 2006-11-13 | 2010-11-30 | Ossur Hf | Absorbent structure in an absorbent article |
| JP2008150447A (ja) * | 2006-12-15 | 2008-07-03 | Shin Etsu Chem Co Ltd | 発泡性オルガノポリシロキサン組成物およびシリコーンゴムスポンジ |
| US8377016B2 (en) * | 2007-01-10 | 2013-02-19 | Wake Forest University Health Sciences | Apparatus and method for wound treatment employing periodic sub-atmospheric pressure |
| JP2008214440A (ja) * | 2007-03-01 | 2008-09-18 | Shin Etsu Chem Co Ltd | シリコーンゴムスポンジ組成物 |
| KR101608548B1 (ko) * | 2008-03-05 | 2016-04-01 | 케이씨아이 라이센싱 인코포레이티드 | 조직 부위에 감압을 가하고,조직 부위로부터 유체를 수집 및 저장하는 드레싱 및 방법 |
-
2009
- 2009-12-16 EP EP09015561.5A patent/EP2335747B1/de active Active
-
2010
- 2010-12-14 JP JP2012543519A patent/JP5723386B2/ja not_active Expired - Fee Related
- 2010-12-14 WO PCT/EP2010/007620 patent/WO2011072840A1/de active Application Filing
- 2010-12-14 RU RU2012129974/05A patent/RU2530054C2/ru active
- 2010-12-14 CN CN201080057146.XA patent/CN102740901B/zh active Active
- 2010-12-14 EP EP10795947A patent/EP2512544A1/de not_active Withdrawn
Also Published As
| Publication number | Publication date |
|---|---|
| EP2512544A1 (de) | 2012-10-24 |
| RU2530054C2 (ru) | 2014-10-10 |
| RU2012129974A (ru) | 2014-01-27 |
| JP2013514094A (ja) | 2013-04-25 |
| WO2011072840A1 (de) | 2011-06-23 |
| CN102740901A (zh) | 2012-10-17 |
| EP2335747A1 (de) | 2011-06-22 |
| CN102740901B (zh) | 2015-11-25 |
| EP2335747B1 (de) | 2016-12-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5723386B2 (ja) | 陰圧創傷治療法のための装置 | |
| US9452247B2 (en) | Device for negative pressure wound therapy | |
| US11931226B2 (en) | Wound dressing sealant and use thereof | |
| US11730876B2 (en) | Composition I-II and products and uses thereof | |
| AU2014229779B2 (en) | Wound dressing sealant and use thereof | |
| CN1115170C (zh) | 裹伤巾及其制造方法 | |
| EP3135304A1 (en) | Foamed silicone in wound care | |
| JP5283636B2 (ja) | 銀含有発泡体 | |
| JP2013540459A (ja) | 負圧治療のためのフォームと軟膏基材をふくむ創傷包帯 | |
| CN104203172A (zh) | 组合物、装置、套件及其方法与用途 | |
| JP2015536795A (ja) | 接着性周縁部を有する創傷被覆材 | |
| WO2012104584A1 (en) | Silicone wound dressing laminate and method for making the same | |
| MX2013014551A (es) | Dispositivos medicos y no medicos hechos de materiales de hule hidrofilico. | |
| JP2015501346A (ja) | 高粘度シリコーン接着剤 | |
| US20120046589A1 (en) | Wound dressing comprising foam and ointment base for negative pressure therapy | |
| ES2720584T3 (es) | Dispositivo para la terapia de vacío de heridas que contiene espuma de poliuretano que contiene silicona | |
| US20240139035A1 (en) | Wound dressing sealant and use thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130909 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20130909 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20140530 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140610 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140909 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20150317 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20150327 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5723386 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| LAPS | Cancellation because of no payment of annual fees |