JP5667877B2 - プロテアーゼ検出 - Google Patents
プロテアーゼ検出 Download PDFInfo
- Publication number
- JP5667877B2 JP5667877B2 JP2010533653A JP2010533653A JP5667877B2 JP 5667877 B2 JP5667877 B2 JP 5667877B2 JP 2010533653 A JP2010533653 A JP 2010533653A JP 2010533653 A JP2010533653 A JP 2010533653A JP 5667877 B2 JP5667877 B2 JP 5667877B2
- Authority
- JP
- Japan
- Prior art keywords
- polypeptide
- amino acid
- chromogenic
- fragment
- terminus
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 108091005804 Peptidases Proteins 0.000 title claims description 64
- 239000004365 Protease Substances 0.000 title claims description 35
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 title claims 9
- 238000001514 detection method Methods 0.000 title description 15
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 151
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 128
- 229920001184 polypeptide Polymers 0.000 claims description 121
- 150000001413 amino acids Chemical class 0.000 claims description 120
- 239000012634 fragment Substances 0.000 claims description 28
- 238000000034 method Methods 0.000 claims description 27
- 238000012800 visualization Methods 0.000 claims description 23
- 150000001299 aldehydes Chemical class 0.000 claims description 22
- 125000003277 amino group Chemical group 0.000 claims description 16
- 239000007787 solid Substances 0.000 claims description 14
- 239000012501 chromatography medium Substances 0.000 claims description 13
- 229910052757 nitrogen Inorganic materials 0.000 claims description 8
- 125000003118 aryl group Chemical group 0.000 claims description 7
- 238000003776 cleavage reaction Methods 0.000 claims description 6
- 239000012528 membrane Substances 0.000 claims description 6
- 230000007017 scission Effects 0.000 claims description 6
- 150000003935 benzaldehydes Chemical class 0.000 claims description 4
- KJPRLNWUNMBNBZ-UHFFFAOYSA-N cinnamic aldehyde Chemical class O=CC=CC1=CC=CC=C1 KJPRLNWUNMBNBZ-UHFFFAOYSA-N 0.000 claims description 4
- 239000000463 material Substances 0.000 claims description 4
- 239000000126 substance Substances 0.000 claims description 4
- KJPRLNWUNMBNBZ-QPJJXVBHSA-N (E)-cinnamaldehyde Chemical class O=C\C=C\C1=CC=CC=C1 KJPRLNWUNMBNBZ-QPJJXVBHSA-N 0.000 claims description 3
- HUMNYLRZRPPJDN-UHFFFAOYSA-N benzenecarboxaldehyde Natural products O=CC1=CC=CC=C1 HUMNYLRZRPPJDN-UHFFFAOYSA-N 0.000 claims description 3
- 210000004899 c-terminal region Anatomy 0.000 claims description 3
- 229940117916 cinnamic aldehyde Drugs 0.000 claims description 3
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 3
- QNGNSVIICDLXHT-UHFFFAOYSA-N para-ethylbenzaldehyde Natural products CCC1=CC=C(C=O)C=C1 QNGNSVIICDLXHT-UHFFFAOYSA-N 0.000 claims description 3
- 238000012360 testing method Methods 0.000 claims description 3
- 239000011148 porous material Substances 0.000 claims description 2
- MHABMANUFPZXEB-UHFFFAOYSA-N O-demethyl-aloesaponarin I Natural products O=C1C2=CC=CC(O)=C2C(=O)C2=C1C=C(O)C(C(O)=O)=C2C MHABMANUFPZXEB-UHFFFAOYSA-N 0.000 claims 2
- 125000003275 alpha amino acid group Chemical group 0.000 claims 1
- 229940024606 amino acid Drugs 0.000 description 93
- 235000001014 amino acid Nutrition 0.000 description 93
- ALYNCZNDIQEVRV-UHFFFAOYSA-N 4-aminobenzoic acid Chemical compound NC1=CC=C(C(O)=O)C=C1 ALYNCZNDIQEVRV-UHFFFAOYSA-N 0.000 description 71
- 102000035195 Peptidases Human genes 0.000 description 55
- 239000000523 sample Substances 0.000 description 37
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 36
- WZSDWSACAGBYQU-UHFFFAOYSA-N 2-(dimethylamino)-3-phenylprop-2-enal Chemical group CN(C)C(C=O)=CC1=CC=CC=C1 WZSDWSACAGBYQU-UHFFFAOYSA-N 0.000 description 31
- 235000019419 proteases Nutrition 0.000 description 24
- 238000006243 chemical reaction Methods 0.000 description 22
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 21
- 102000016387 Pancreatic elastase Human genes 0.000 description 21
- 108010067372 Pancreatic elastase Proteins 0.000 description 21
- 239000000758 substrate Substances 0.000 description 21
- 238000003786 synthesis reaction Methods 0.000 description 19
- 239000011159 matrix material Substances 0.000 description 18
- 230000015572 biosynthetic process Effects 0.000 description 16
- 238000003556 assay Methods 0.000 description 13
- 239000011347 resin Substances 0.000 description 13
- 229920005989 resin Polymers 0.000 description 13
- 239000000047 product Substances 0.000 description 12
- 239000000203 mixture Substances 0.000 description 9
- 239000000243 solution Substances 0.000 description 9
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 8
- 239000002250 absorbent Substances 0.000 description 7
- 230000002745 absorbent Effects 0.000 description 7
- 238000005859 coupling reaction Methods 0.000 description 7
- 239000010931 gold Substances 0.000 description 7
- 229910052737 gold Inorganic materials 0.000 description 7
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 6
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 6
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 239000000020 Nitrocellulose Substances 0.000 description 6
- -1 alanine amino acid Chemical class 0.000 description 6
- 230000008878 coupling Effects 0.000 description 6
- 238000010168 coupling process Methods 0.000 description 6
- 229920001220 nitrocellulos Polymers 0.000 description 6
- 239000002245 particle Substances 0.000 description 6
- 230000008859 change Effects 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 5
- 238000001819 mass spectrum Methods 0.000 description 5
- 210000004898 n-terminal fragment Anatomy 0.000 description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 5
- 125000003088 (fluoren-9-ylmethoxy)carbonyl group Chemical group 0.000 description 4
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- 108090000526 Papain Proteins 0.000 description 4
- 239000003593 chromogenic compound Substances 0.000 description 4
- 238000010511 deprotection reaction Methods 0.000 description 4
- 238000002845 discoloration Methods 0.000 description 4
- 229940055729 papain Drugs 0.000 description 4
- 235000019834 papain Nutrition 0.000 description 4
- 239000007790 solid phase Substances 0.000 description 4
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 4
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 3
- DGPBVJWCIDNDPN-UHFFFAOYSA-N 2-(dimethylamino)benzaldehyde Chemical compound CN(C)C1=CC=CC=C1C=O DGPBVJWCIDNDPN-UHFFFAOYSA-N 0.000 description 3
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- 239000004471 Glycine Substances 0.000 description 3
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 3
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 3
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 3
- 239000004473 Threonine Substances 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 102000004142 Trypsin Human genes 0.000 description 3
- 108090000631 Trypsin Proteins 0.000 description 3
- 238000010521 absorption reaction Methods 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 229960003767 alanine Drugs 0.000 description 3
- 235000004279 alanine Nutrition 0.000 description 3
- 229960004050 aminobenzoic acid Drugs 0.000 description 3
- HOPRXXXSABQWAV-UHFFFAOYSA-N anhydrous collidine Natural products CC1=CC=NC(C)=C1C HOPRXXXSABQWAV-UHFFFAOYSA-N 0.000 description 3
- 229960002685 biotin Drugs 0.000 description 3
- 235000020958 biotin Nutrition 0.000 description 3
- 239000011616 biotin Substances 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- UTBIMNXEDGNJFE-UHFFFAOYSA-N collidine Natural products CC1=CC=C(C)C(C)=N1 UTBIMNXEDGNJFE-UHFFFAOYSA-N 0.000 description 3
- UAOMVDZJSHZZME-UHFFFAOYSA-N diisopropylamine Chemical compound CC(C)NC(C)C UAOMVDZJSHZZME-UHFFFAOYSA-N 0.000 description 3
- 229940088598 enzyme Drugs 0.000 description 3
- 229960002449 glycine Drugs 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 230000017854 proteolysis Effects 0.000 description 3
- 229960001153 serine Drugs 0.000 description 3
- 239000002002 slurry Substances 0.000 description 3
- GFYHSKONPJXCDE-UHFFFAOYSA-N sym-collidine Natural products CC1=CN=C(C)C(C)=C1 GFYHSKONPJXCDE-UHFFFAOYSA-N 0.000 description 3
- 230000002194 synthesizing effect Effects 0.000 description 3
- 229960002898 threonine Drugs 0.000 description 3
- 239000012588 trypsin Substances 0.000 description 3
- NGNBDVOYPDDBFK-UHFFFAOYSA-N 2-[2,4-di(pentan-2-yl)phenoxy]acetyl chloride Chemical compound CCCC(C)C1=CC=C(OCC(Cl)=O)C(C(C)CCC)=C1 NGNBDVOYPDDBFK-UHFFFAOYSA-N 0.000 description 2
- CXOWHCCVISNMIX-UHFFFAOYSA-N 2-aminonaphthalene-1-carboxylic acid Chemical compound C1=CC=CC2=C(C(O)=O)C(N)=CC=C21 CXOWHCCVISNMIX-UHFFFAOYSA-N 0.000 description 2
- JMTMSDXUXJISAY-UHFFFAOYSA-N 2H-benzotriazol-4-ol Chemical compound OC1=CC=CC2=C1N=NN2 JMTMSDXUXJISAY-UHFFFAOYSA-N 0.000 description 2
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 2
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 2
- 108010028275 Leukocyte Elastase Proteins 0.000 description 2
- 102000016799 Leukocyte elastase Human genes 0.000 description 2
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- 108010090804 Streptavidin Proteins 0.000 description 2
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 2
- 239000003875 Wang resin Substances 0.000 description 2
- NERFNHBZJXXFGY-UHFFFAOYSA-N [4-[(4-methylphenyl)methoxy]phenyl]methanol Chemical compound C1=CC(C)=CC=C1COC1=CC=C(CO)C=C1 NERFNHBZJXXFGY-UHFFFAOYSA-N 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 125000000539 amino acid group Chemical group 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 238000001311 chemical methods and process Methods 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 238000003745 diagnosis Methods 0.000 description 2
- 239000000539 dimer Substances 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 2
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 description 2
- NPZTUJOABDZTLV-UHFFFAOYSA-N hydroxybenzotriazole Substances O=C1C=CC=C2NNN=C12 NPZTUJOABDZTLV-UHFFFAOYSA-N 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- CVWZIGOXWLVKHP-FQEVSTJZSA-N n-[(2s)-5-(diaminomethylideneamino)-1-(naphthalen-1-ylamino)-1-oxopentan-2-yl]benzamide Chemical compound N([C@@H](CCCNC(=N)N)C(=O)NC=1C2=CC=CC=C2C=CC=1)C(=O)C1=CC=CC=C1 CVWZIGOXWLVKHP-FQEVSTJZSA-N 0.000 description 2
- 125000001624 naphthyl group Chemical group 0.000 description 2
- 238000010647 peptide synthesis reaction Methods 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 230000002797 proteolythic effect Effects 0.000 description 2
- 238000000425 proton nuclear magnetic resonance spectrum Methods 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 230000007306 turnover Effects 0.000 description 2
- 238000011144 upstream manufacturing Methods 0.000 description 2
- 229960004295 valine Drugs 0.000 description 2
- 239000004474 valine Substances 0.000 description 2
- UGNIYGNGCNXHTR-SFHVURJKSA-N (2s)-2-(9h-fluoren-9-ylmethoxycarbonylamino)-3-methylbutanoic acid Chemical compound C1=CC=C2C(COC(=O)N[C@@H](C(C)C)C(O)=O)C3=CC=CC=C3C2=C1 UGNIYGNGCNXHTR-SFHVURJKSA-N 0.000 description 1
- OSNIIMCBVLBNGS-UHFFFAOYSA-N 1-(1,3-benzodioxol-5-yl)-2-(dimethylamino)propan-1-one Chemical compound CN(C)C(C)C(=O)C1=CC=C2OCOC2=C1 OSNIIMCBVLBNGS-UHFFFAOYSA-N 0.000 description 1
- LNETULKMXZVUST-UHFFFAOYSA-N 1-naphthoic acid Chemical compound C1=CC=C2C(C(=O)O)=CC=CC2=C1 LNETULKMXZVUST-UHFFFAOYSA-N 0.000 description 1
- RUFPHBVGCFYCNW-UHFFFAOYSA-N 1-naphthylamine Chemical compound C1=CC=C2C(N)=CC=CC2=C1 RUFPHBVGCFYCNW-UHFFFAOYSA-N 0.000 description 1
- XXMFJKNOJSDQBM-UHFFFAOYSA-N 2,2,2-trifluoroacetic acid;hydrate Chemical compound [OH3+].[O-]C(=O)C(F)(F)F XXMFJKNOJSDQBM-UHFFFAOYSA-N 0.000 description 1
- ITRMROGJSNWFKO-FOCLMDBBSA-N 4,4'-azodibenzenearsonic acid Chemical compound C1=CC([As](O)(=O)O)=CC=C1\N=N\C1=CC=C([As](O)(O)=O)C=C1 ITRMROGJSNWFKO-FOCLMDBBSA-N 0.000 description 1
- TYMLOMAKGOJONV-UHFFFAOYSA-N 4-nitroaniline Chemical compound NC1=CC=C([N+]([O-])=O)C=C1 TYMLOMAKGOJONV-UHFFFAOYSA-N 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 1
- 206010007269 Carcinogenicity Diseases 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 241000579835 Merops Species 0.000 description 1
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N N-phenyl amine Natural products NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 1
- 125000001429 N-terminal alpha-amino-acid group Chemical group 0.000 description 1
- 102000015636 Oligopeptides Human genes 0.000 description 1
- 108010038807 Oligopeptides Proteins 0.000 description 1
- 229920005439 Perspex® Polymers 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- 206010070834 Sensitisation Diseases 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 125000002490 anilino group Chemical group [H]N(*)C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 229960003121 arginine Drugs 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 235000009697 arginine Nutrition 0.000 description 1
- 150000001483 arginine derivatives Chemical class 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- 229960005261 aspartic acid Drugs 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 238000002820 assay format Methods 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- 238000010256 biochemical assay Methods 0.000 description 1
- 239000012267 brine Substances 0.000 description 1
- 235000011089 carbon dioxide Nutrition 0.000 description 1
- 230000007670 carcinogenicity Effects 0.000 description 1
- 231100000260 carcinogenicity Toxicity 0.000 description 1
- LBJNMUFDOHXDFG-UHFFFAOYSA-N copper;hydrate Chemical compound O.[Cu].[Cu] LBJNMUFDOHXDFG-UHFFFAOYSA-N 0.000 description 1
- 229960002433 cysteine Drugs 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 1
- 229940043279 diisopropylamine Drugs 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 238000002330 electrospray ionisation mass spectrometry Methods 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 229960002989 glutamic acid Drugs 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 229960002743 glutamine Drugs 0.000 description 1
- 235000004554 glutamine Nutrition 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 229960002885 histidine Drugs 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 230000005661 hydrophobic surface Effects 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 230000032297 kinesis Effects 0.000 description 1
- 238000011031 large-scale manufacturing process Methods 0.000 description 1
- 229960003136 leucine Drugs 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 229960003646 lysine Drugs 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- 229960004452 methionine Drugs 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 208000028169 periodontal disease Diseases 0.000 description 1
- 229960005190 phenylalanine Drugs 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 239000004926 polymethyl methacrylate Substances 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000002953 preparative HPLC Methods 0.000 description 1
- 229960002429 proline Drugs 0.000 description 1
- 230000006337 proteolytic cleavage Effects 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 239000012488 sample solution Substances 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 230000008313 sensitization Effects 0.000 description 1
- 238000007086 side reaction Methods 0.000 description 1
- 239000000741 silica gel Substances 0.000 description 1
- 229910002027 silica gel Inorganic materials 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 150000003568 thioethers Chemical class 0.000 description 1
- 150000003573 thiols Chemical class 0.000 description 1
- 239000012780 transparent material Substances 0.000 description 1
- QXTIBZLKQPJVII-UHFFFAOYSA-N triethylsilicon Chemical compound CC[Si](CC)CC QXTIBZLKQPJVII-UHFFFAOYSA-N 0.000 description 1
- UCPYLLCMEDAXFR-UHFFFAOYSA-N triphosgene Chemical compound ClC(Cl)(Cl)OC(=O)OC(Cl)(Cl)Cl UCPYLLCMEDAXFR-UHFFFAOYSA-N 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- 229960004799 tryptophan Drugs 0.000 description 1
- 229960004441 tyrosine Drugs 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/02—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing at least one abnormal peptide link
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/34—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving hydrolase
- C12Q1/37—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving hydrolase involving peptidase or proteinase
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
- B01L3/50—Containers for the purpose of retaining a material to be analysed, e.g. test tubes
- B01L3/505—Containers for the purpose of retaining a material to be analysed, e.g. test tubes flexible containers not provided for above
- B01L3/5055—Hinged, e.g. opposable surfaces
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/04—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing only normal peptide links
- C07K5/08—Tripeptides
- C07K5/0802—Tripeptides with the first amino acid being neutral
- C07K5/0804—Tripeptides with the first amino acid being neutral and aliphatic
- C07K5/0808—Tripeptides with the first amino acid being neutral and aliphatic the side chain containing 2 to 4 carbon atoms, e.g. Val, Ile, Leu
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
- C07K7/04—Linear peptides containing only normal peptide links
- C07K7/06—Linear peptides containing only normal peptide links having 5 to 11 amino acids
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/58—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving labelled substances
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/58—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving labelled substances
- G01N33/583—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving labelled substances with non-fluorescent dye label
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/08—Geometry, shape and general structure
- B01L2300/0809—Geometry, shape and general structure rectangular shaped
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- General Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Immunology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Analytical Chemistry (AREA)
- Hematology (AREA)
- Medicinal Chemistry (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Microbiology (AREA)
- Physics & Mathematics (AREA)
- Biotechnology (AREA)
- Urology & Nephrology (AREA)
- Biomedical Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- General Physics & Mathematics (AREA)
- Cell Biology (AREA)
- Food Science & Technology (AREA)
- Pathology (AREA)
- Clinical Laboratory Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Peptides Or Proteins (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Enzymes And Modification Thereof (AREA)
Description
(i)先行請求項のいずれか一項に記載のポリペプチドに該サンプルをさらし、該プロテアーゼに該ポリペプチドを切断させて断片(該断片はそのN末端に発色性アミノ酸を示す。)を得る工程、
(ii)共役アルデヒドを、該発色性アミノ酸を含む該断片と反応させて、着色付加体を得る工程、および
(iii)該着色付加体を検出する工程であって、該着色付加体の存在が該サンプル中のプロテアーゼ酵素の存在を示す工程、
を含む方法を提供する。
i.1つの発色性アミノ酸を含むアミノ酸二量体を合成し、
ii.該ポリペプチドの合成中に、該二量体を該ポリペプチドの残部に取り込ませる工程を含んでなる、本発明の製造方法を提供する
好都合には、工程(ii)は、該二量体を新生オリゴペプチドに結合させることを含む。
本発明のポリペプチドおよび
該ポリペプチドが固定化されうる固体支持体
を含んでなる、サンプル中のプロテアーゼ酵素を検出するための製品を提供する。
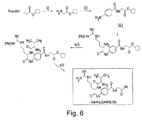
図6を参照して、Val−Arg−[pABA]−Glyの合成を以下に説明する。
サンプル溶液中のパパインの存在を検出するためのアッセイにおいて、発色性ポリペプチドVal−Arg−[pABA]−Glyを使用した。プロテアーゼ酵素であるパパインを含むサンプルを、図3に記載されている実施形態によるブックレット(図8Aを参照されたい。)の第1シートに適用した。該ブックレットの第1シートは該発色性ポリペプチドで予め含浸されていた。5分後、該ブックレットの第1および第2シートを一緒に折り畳んだ。該ブックレットの第2シートはDMACで予め含浸されており、該ブックレットの第2シート内の穴から観察可能なとおり、10分後、DMACは黄色から赤色に変化した。また、対照として、該サンプルの代わりに水を使用して、該アッセイを反復した。変色の結果を図8Bのグラフに示す。
2つのペプチド、すなわち、AAPV−[pABA]−GGCおよびAAPV−[ANA]−GGC(ここで、pABAはパラ−アミノ安息香酸であり、ANAは2−アミノナフトエ酸である。)を合成した。両方のペプチドを、好中球エラスターゼ酵素に対する基質としてのそれらの適合性に関して試験した。AAPV−[pABA]−GGCおよびAAPV−[ANA]−GGCのエレクトロスプレー質量スペクトルを、それぞれ、図10Aおよび10Bに示す。
2つのペプチド、すなわち、AAPV−[pABA]−GGCおよびAAPV−[ANA]−GGCの存在下の酵素好中球エラスターゼの活性に関して試験するために、もう1つのアッセイ形態を用いた。固体表面への結合の手段として、該ペプチドのC末端システイン基を使用した。ヨードアセチル基で官能基化された半融ポリエチレンフリットを50mM リン酸ナトリウム、5mM EDTA(pH7.4)中で10分間洗浄した。ついで該フリットを、30分間、増感のために0.5mg/ml ペプチド(PBS中)の溶液に移した。ついで該フリットを、リン酸EDTAバッファー中でもう一度、10分間洗浄した。該フリットを空カラム内にローディングし、200μlのエラスターゼを30秒の概算流動接触時間で加えた。該アッセイを展開するために、100μlの実施用DMAC溶液(0.3mg/ml DMAC、50mM HCl)を該カラムに通過させた。
2つのペプチドの間で、それらの存在下で検出されるエラスターゼ活性の点で、明らかな相違が観察される。エラスターゼ基質は、好ましくは、セリン、トレオニン(共に親水性側鎖を有する。)またはグリシンをP1’位に有する(情報源:MEROPSデータベース)。したがって、大きな疎水性表面を有する嵩高い発色性アミノ酸がこれらの好ましいアミノ酸に対して適度に立体的に合致するとは考えにくい。ナフトエ酸(ANA)はパラ−アミノ安息香酸(pABA)より嵩高く、より疎水性であり、したがって、AAPV−[ANA]−GGCは不良なエラスターゼ基質である。これらの両方の分子の構造を図9に示す。pABA発色性アミノ酸は、セリンおよびトレオニンに対して、遥かに良好に立体的に合致し、したがってAAPV−[pABA]−GGCは、エラスターゼに対する基質として、有意に、より好適であり、基質および酵素が混合されると、エラスターゼ活性をもたらす。したがってAAPV−[pABA]−GGCはエラスターゼの存在の検出において使用されうる。
発色性アミノ酸を含有するペプチドの合成を改善するために開発された新規タイプのビルディングブロックを製造した。この場合、該ビルディングブロックの合成は自動化合成に適合しうる。このビルディングブロックを合成するために用いた方法は、実施例1に記載されているBTC/コリジン法の使用を要さない。BTC/コリジン法は有効であるが、過酷な条件を要し、不溶性沈殿物を生成して、それを、自動化ペプチド合成との併用に不適当なものにする。
Claims (22)
- サンプル中のプロテアーゼ酵素の検出方法であって、
(i)該サンプルを、発色性アミノ酸を含むポリペプチドに曝し、プロテアーゼに該ポリペプチドを切断させて、そのN末端に発色性アミノ酸を示す断片を得る工程、ここで、該発色性アミノ酸は、立体的な等価性において天然アミノ酸に合致し、そのNおよびC末端において少なくとも1つのアミノ酸に隣接しており、該発色性アミノ酸のアミン基が5未満のpKaを有し、および該発色性アミノ酸が共役アルデヒドと反応可能であり、ならびに該ポリペプチドが、該発色性アミノ酸のアミノ基を含むペプチド結合を切断し得る標的プロテアーゼに対する標的配列を含む、
(ii)共役アルデヒドを、該発色性アミノ酸を含む該断片と反応させて、着色付加体を得る工程、および
(iii)該着色付加体を検出する工程であって、該着色付加体の存在が該サンプル中のプロテアーゼ酵素の存在を示す工程、
を含む方法。 - 発色性アミノ酸が、該発色性アミノ酸のアミノ基の窒素原子に直接結合した芳香環部分を含む請求項1に記載の方法。
- 標的配列が、発色性アミノ酸を含む、請求項1または2に記載の方法。
- 共役アルデヒドが、置換ベンズアルデヒド、シンナムアルデヒド、トランス,トランス フェニルペンタジエナール、DMACまたはDMABである、請求項1〜3のいずれか一項に記載の方法。
- ポリペプチドが該ポリペプチドのC末端もしくはN末端において又はその付近において固体支持体上に固定化されている、請求項1〜4のいずれか一項に記載の方法。
- ポリペプチドのC末端もしくはN末端において又はその付近において第1および第2結合部分を更に含む、請求項1〜5のいずれか一項に記載の方法。
- ポリペプチドが2〜100アミノ酸長、または3〜40アミノ酸長である、請求項1〜6のいずれか一項に記載の方法。
- (i)発色性アミノ酸を含むポリペプチド、ここで、該発色性アミノ酸は、立体的な等価性において天然アミノ酸に合致し、そのNおよびC末端において少なくとも1つのアミノ酸に隣接しており、該発色性アミノ酸のアミン基が5未満のpKaを有し、および該発色性アミノ酸が共役アルデヒドと反応可能であり、ならびに該ポリペプチドが、該発色性アミノ酸のアミノ基を含むペプチド結合を切断し得る標的プロテアーゼに対する標的配列を含む、および
(ii)該ポリペプチドが固定化され得る固体支持体
を含んでなる、サンプル中のプロテアーゼ酵素を検出するための製品。 - 発色性アミノ酸が、該発色性アミノ酸のアミノ基の窒素原子に直接結合した芳香環部分を含む請求項8に記載の製品。
- 標的配列が、発色性アミノ酸を含む、請求項8または9に記載の製品。
- 共役アルデヒドが、置換ベンズアルデヒド、シンナムアルデヒド、トランス,トランス フェニルペンタジエナール、DMACまたはDMABである、請求項8〜10のいずれか一項に記載の製品。
- ポリペプチドが該ポリペプチドのC末端もしくはN末端において又はその付近において固体支持体上に固定化されている、請求項8〜11のいずれか一項に記載の製品。
- ポリペプチドのC末端もしくはN末端において又はその付近において第1および第2結合部分を更に含む、請求項8〜12のいずれか一項に記載の製品。
- ポリペプチドが2〜100アミノ酸長、または3〜40アミノ酸長である、請求項8〜13のいずれか一項に記載の製品。
- 固体支持体が、ヒンジにより連結された第1および第2シートを含み、ポリペプチドが第1シート上に固定化されており、および共役アルデヒドが第2シート上に位置していて、それらのシートを一緒に折り畳むことにより第1シートから第2シートへの物質の移動が可能となる、請求項8〜14のいずれか一項に記載の製品。
- 製品が更に、第1シート上に固定化されたポリペプチドと、第2シート上に位置する共役アルデヒドとの間に介在し得る膜を含み、該膜が、閾サイズより大きなサイズを有する物質の、第1シートから第2シートへの通過を妨げ、該ポリペプチドが、該発色性アミノ酸を含む断片を遊離するようプロテアーゼ酵素により切断可能であり、該断片が該閾サイズより小さい、請求項15に記載の製品。
- 固体支持体がクロマトグラフィー媒体を含む、請求項8〜14のいずれか一項に記載の製品。
- クロマトグラフィー媒体が更に、プロテアーゼ酵素による切断の後にポリペプチドから遊離可能な発色性アミノ酸を含む該ポリペプチドの断片に結合し得る断片結合性分子を含み、該ポリペプチドが指示区域において該クロマトグラフィー媒体上に固定化可能であり、ならびに該断片結合性分子が可視化区域において該クロマトグラフィー媒体上に固定化可能である、請求項17に記載の製品。
- ポリペプチドが第1および第2断片へと切断可能であり、第1断片が該発色性アミノ酸を含み、ならびに製品が更に、第2断片と結合可能な検出可能な標識、および該クロマトグラフィー媒体内または上に固定化された第1および第2捕捉分子を含み、第1捕捉分子が第1断片に結合可能であり、および第2捕捉分子が第2断片または該検出可能標識に結合可能である、請求項17に記載の製品。
- 標識が、第2断片に結合可能な結合性成分を含む、請求項19に記載の製品。
- クロマトグラフィー媒体が、
a)試験ストリップ、または
b)多孔性物質のカラム
を含む、請求項17〜20のいずれか一項に記載の製品。 - 共役アルデヒドをさらに含む、請求項8〜21のいずれか一項に記載の製品。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB0722287.0 | 2007-11-13 | ||
| GB0722287A GB2454672A (en) | 2007-11-13 | 2007-11-13 | Chromogenic protease substrates |
| PCT/GB2008/003833 WO2009063208A2 (en) | 2007-11-13 | 2008-11-13 | Protease detection |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2011503160A JP2011503160A (ja) | 2011-01-27 |
| JP2011503160A5 JP2011503160A5 (ja) | 2011-12-22 |
| JP5667877B2 true JP5667877B2 (ja) | 2015-02-12 |
Family
ID=38896238
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010533653A Expired - Fee Related JP5667877B2 (ja) | 2007-11-13 | 2008-11-13 | プロテアーゼ検出 |
Country Status (11)
| Country | Link |
|---|---|
| US (2) | US8993253B2 (ja) |
| EP (1) | EP2222404B1 (ja) |
| JP (1) | JP5667877B2 (ja) |
| CN (1) | CN101896272B (ja) |
| AU (1) | AU2008322724B2 (ja) |
| CA (1) | CA2705432A1 (ja) |
| GB (1) | GB2454672A (ja) |
| IL (1) | IL205699A0 (ja) |
| NZ (1) | NZ585207A (ja) |
| WO (1) | WO2009063208A2 (ja) |
| ZA (1) | ZA201003313B (ja) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2435510A (en) * | 2006-02-23 | 2007-08-29 | Mologic Ltd | Enzyme detection product and methods |
| BRPI0918652B1 (pt) | 2008-09-17 | 2021-10-19 | Chiasma, Inc. | Composição farmacêutica compreendendo um meio hidrofóbico e uma forma sólida que compreende polipeptídeo e sal de ácido graxo de cadeia média, processo de produção da mesma e forma de dosagem oral |
| GB201116523D0 (en) | 2011-09-23 | 2011-11-09 | Systagenix Wound Man Ip Co Bv | Wound prognosis |
| US9932622B2 (en) | 2011-01-31 | 2018-04-03 | Woundchek Laboratories B.V. | Wound prognosis |
| EP2734846A4 (en) * | 2011-07-22 | 2015-03-18 | Rapid Pathogen Screening Inc | LATERAL FLOW ASSAYS BASED ON ENZYMATIC CLIP |
| GB201206976D0 (en) | 2012-04-20 | 2012-06-06 | Mologic Ltd | An enzyme detection device |
| GB201206977D0 (en) * | 2012-04-20 | 2012-06-06 | Mologic Ltd | An enzyme detection device |
| JP6510747B2 (ja) * | 2013-08-02 | 2019-05-08 | 東洋ビーネット株式会社 | プロテアーゼ活性測定法 |
| US10238709B2 (en) | 2015-02-03 | 2019-03-26 | Chiasma, Inc. | Method of treating diseases |
| GB201605110D0 (en) | 2016-03-24 | 2016-05-11 | Mologic Ltd | Detecting sepsis |
| GB201614053D0 (en) * | 2016-08-17 | 2016-09-28 | Microarray Ltd | Determining the condition of a wound |
| CN106442969B (zh) * | 2016-08-23 | 2018-08-31 | 中国人民解放军军事医学科学院微生物流行病研究所 | 一种用于检测肉毒毒素的试剂盒 |
| GB201902458D0 (en) | 2019-02-22 | 2019-04-10 | Mologic Ltd | Treatment stratification for an excerbation of inflamation |
| US11141457B1 (en) | 2020-12-28 | 2021-10-12 | Amryt Endo, Inc. | Oral octreotide therapy and contraceptive methods |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4588836A (en) * | 1982-09-01 | 1986-05-13 | Toyo Jozo Kabushiki Kaisha | Novel synthetic substrate and assay method using the same |
| JPS5988099A (ja) * | 1982-11-15 | 1984-05-21 | Toyo Jozo Co Ltd | 新規な酵素活性の測定法 |
| JPS5942350A (ja) * | 1982-09-01 | 1984-03-08 | Toyo Jozo Co Ltd | 新規な合成基質を用いるL‐ロイシンアミノペプチダーゼおよびγ‐グルタミルトランスペプチダーゼからなる群より選ばれるペプチダーゼの活性測定法 |
| DE3413311A1 (de) * | 1984-04-09 | 1985-10-17 | Behringwerke Ag, 3550 Marburg | Reagenz zur bestimmung der thromboplastinzeit |
| HU194913B (en) * | 1986-01-03 | 1988-03-28 | Innofinance Altalanos Innovaci | Process for producing novel gonadoliberin derivatives containing in the sixth position aromatic amino carboxylic acid and medical preparations containing these compounds |
| GB2435510A (en) | 2006-02-23 | 2007-08-29 | Mologic Ltd | Enzyme detection product and methods |
| GB2435511A (en) * | 2006-02-23 | 2007-08-29 | Mologic Ltd | Protease detection |
-
2007
- 2007-11-13 GB GB0722287A patent/GB2454672A/en not_active Withdrawn
-
2008
- 2008-11-13 CA CA2705432A patent/CA2705432A1/en not_active Abandoned
- 2008-11-13 WO PCT/GB2008/003833 patent/WO2009063208A2/en active Application Filing
- 2008-11-13 JP JP2010533653A patent/JP5667877B2/ja not_active Expired - Fee Related
- 2008-11-13 NZ NZ585207A patent/NZ585207A/en not_active IP Right Cessation
- 2008-11-13 EP EP08849127.9A patent/EP2222404B1/en not_active Not-in-force
- 2008-11-13 AU AU2008322724A patent/AU2008322724B2/en not_active Ceased
- 2008-11-13 CN CN2008801158369A patent/CN101896272B/zh not_active Expired - Fee Related
- 2008-11-13 US US12/742,867 patent/US8993253B2/en not_active Expired - Fee Related
-
2010
- 2010-05-11 ZA ZA2010/03313A patent/ZA201003313B/en unknown
- 2010-05-11 IL IL205699A patent/IL205699A0/en unknown
-
2014
- 2014-11-25 US US14/553,182 patent/US9376706B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| EP2222404A2 (en) | 2010-09-01 |
| CN101896272B (zh) | 2013-07-31 |
| US20110086370A1 (en) | 2011-04-14 |
| CA2705432A1 (en) | 2009-05-22 |
| GB2454672A (en) | 2009-05-20 |
| GB0722287D0 (en) | 2007-12-27 |
| EP2222404B1 (en) | 2018-08-01 |
| NZ585207A (en) | 2011-09-30 |
| CN101896272A (zh) | 2010-11-24 |
| AU2008322724A1 (en) | 2009-05-22 |
| AU2008322724B2 (en) | 2014-03-27 |
| WO2009063208A3 (en) | 2009-08-13 |
| US20150152471A1 (en) | 2015-06-04 |
| US8993253B2 (en) | 2015-03-31 |
| WO2009063208A2 (en) | 2009-05-22 |
| ZA201003313B (en) | 2011-08-31 |
| US9376706B2 (en) | 2016-06-28 |
| IL205699A0 (en) | 2010-11-30 |
| JP2011503160A (ja) | 2011-01-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5667877B2 (ja) | プロテアーゼ検出 | |
| JP2007527511A (ja) | プロテアーゼ検定 | |
| Andresen et al. | Functional peptide microarrays for specific and sensitive antibody diagnostics | |
| EP2839288B1 (en) | An enzyme detection device | |
| WO2007141030A2 (en) | A method for the detection of enzymatic reactions | |
| US20230258653A1 (en) | Multiplexed bead-based analytical assays | |
| CN101180405A (zh) | 酶分析方法 | |
| EP1509542A1 (en) | Methods for measuring kinase activity and phosphatase activity | |
| Hua et al. | Antibody developments and immunoassays for organophosphorus chemicals: a review | |
| JP5337960B2 (ja) | ヒストン脱アセチル化酵素活性の測定方法 | |
| JPWO2005071056A1 (ja) | バイオチップ及びそれを用いた試料溶液の機能性検査方法 | |
| EP2545384B1 (en) | Method for determining the concentration of a peptide | |
| EP1628998B1 (en) | A tag for purification of peptides | |
| JP4547538B2 (ja) | ダイオキシン結合材料及びダイオキシンの検出又は定量方法 | |
| JP2010122002A (ja) | 抗体の検出方法及び該方法に用いられる試薬キット | |
| Guo et al. | A novel quantitative proteomics reagent based on soluble nanopolymers | |
| EP1547999A1 (en) | Standard compound for immunoassay for dioxin and method of immunoassay for dioxin | |
| WO2007053594A2 (en) | Large dynamic range proteomic analysis methods and compositions for practicing the same | |
| JP5344450B2 (ja) | 電解発光物質を内封するリポソームを用いた迅速高感度アッセイ法 | |
| Maier et al. | Synthesis and quality control of thiol tagged compound libraries for chemical microarrays | |
| JP2004357706A (ja) | 酵素活性検出用基板及びそれを用いた酵素活性の検出方法 | |
| WO2005052584A1 (en) | Method of immobilizing a substance of interest to a solid phase | |
| US20070184510A1 (en) | Method and apparatus for monitoring enzyme mixtures | |
| JP2006166830A (ja) | プロテアーゼ活性の測定方法及びそのための試薬 | |
| JP2011037791A (ja) | 抗体−ルシフェリルペプチド複合体、これを用いた被検物質の測定方法及び被検物質の測定試薬並びに測定試薬の調製方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20111104 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20111104 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20131015 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140109 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140117 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140414 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20141118 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20141215 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5667877 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |