JP5601727B2 - 高極性を有するワックス及び塩素含有熱可塑性プラスチックのための滑剤としてのその使用 - Google Patents
高極性を有するワックス及び塩素含有熱可塑性プラスチックのための滑剤としてのその使用 Download PDFInfo
- Publication number
- JP5601727B2 JP5601727B2 JP2011548611A JP2011548611A JP5601727B2 JP 5601727 B2 JP5601727 B2 JP 5601727B2 JP 2011548611 A JP2011548611 A JP 2011548611A JP 2011548611 A JP2011548611 A JP 2011548611A JP 5601727 B2 JP5601727 B2 JP 5601727B2
- Authority
- JP
- Japan
- Prior art keywords
- olefin
- copolymer wax
- reaction
- anhydride
- weight
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000000314 lubricant Substances 0.000 title description 21
- 239000001993 wax Substances 0.000 title description 18
- 229920001169 thermoplastic Polymers 0.000 title description 5
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 title description 4
- 239000000460 chlorine Substances 0.000 title description 4
- 229910052801 chlorine Inorganic materials 0.000 title description 4
- 239000004416 thermosoftening plastic Substances 0.000 title description 4
- 229920001577 copolymer Polymers 0.000 claims description 31
- 239000004711 α-olefin Substances 0.000 claims description 21
- 239000002253 acid Substances 0.000 claims description 17
- 238000006243 chemical reaction Methods 0.000 claims description 16
- 150000008064 anhydrides Chemical class 0.000 claims description 14
- 150000001336 alkenes Chemical class 0.000 claims description 12
- 125000004432 carbon atom Chemical group C* 0.000 claims description 11
- FPYJFEHAWHCUMM-UHFFFAOYSA-N maleic anhydride Chemical compound O=C1OC(=O)C=C1 FPYJFEHAWHCUMM-UHFFFAOYSA-N 0.000 claims description 11
- 238000000034 method Methods 0.000 claims description 11
- -1 maleate diesters Chemical class 0.000 claims description 10
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 claims description 10
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 claims description 8
- 229910052740 iodine Inorganic materials 0.000 claims description 8
- 239000011630 iodine Substances 0.000 claims description 8
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 claims description 8
- 150000007513 acids Chemical class 0.000 claims description 6
- 239000003999 initiator Substances 0.000 claims description 6
- 238000004519 manufacturing process Methods 0.000 claims description 6
- 229930195733 hydrocarbon Natural products 0.000 claims description 5
- 150000002430 hydrocarbons Chemical class 0.000 claims description 5
- 239000004215 Carbon black (E152) Substances 0.000 claims description 4
- 239000006057 Non-nutritive feed additive Substances 0.000 claims description 4
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 claims description 4
- 230000035515 penetration Effects 0.000 claims description 4
- OEPOKWHJYJXUGD-UHFFFAOYSA-N 2-(3-phenylmethoxyphenyl)-1,3-thiazole-4-carbaldehyde Chemical compound O=CC1=CSC(C=2C=C(OCC=3C=CC=CC=3)C=CC=2)=N1 OEPOKWHJYJXUGD-UHFFFAOYSA-N 0.000 claims description 3
- NLHHRLWOUZZQLW-UHFFFAOYSA-N Acrylonitrile Chemical compound C=CC#N NLHHRLWOUZZQLW-UHFFFAOYSA-N 0.000 claims description 3
- XTXRWKRVRITETP-UHFFFAOYSA-N Vinyl acetate Chemical compound CC(=O)OC=C XTXRWKRVRITETP-UHFFFAOYSA-N 0.000 claims description 3
- BZHJMEDXRYGGRV-UHFFFAOYSA-N Vinyl chloride Chemical compound ClC=C BZHJMEDXRYGGRV-UHFFFAOYSA-N 0.000 claims description 3
- QYKIQEUNHZKYBP-UHFFFAOYSA-N Vinyl ether Chemical compound C=COC=C QYKIQEUNHZKYBP-UHFFFAOYSA-N 0.000 claims description 3
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 claims description 2
- 239000005977 Ethylene Substances 0.000 claims description 2
- 238000006384 oligomerization reaction Methods 0.000 claims description 2
- 229920001328 Polyvinylidene chloride Polymers 0.000 claims 2
- 239000005033 polyvinylidene chloride Substances 0.000 claims 2
- 150000008065 acid anhydrides Chemical class 0.000 claims 1
- 150000001252 acrylic acid derivatives Chemical class 0.000 claims 1
- 239000004800 polyvinyl chloride Substances 0.000 description 26
- 229920000915 polyvinyl chloride Polymers 0.000 description 25
- 239000000203 mixture Substances 0.000 description 12
- 238000012545 processing Methods 0.000 description 8
- 239000000047 product Substances 0.000 description 8
- 150000002148 esters Chemical class 0.000 description 7
- 229920000642 polymer Polymers 0.000 description 7
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 6
- 239000000654 additive Substances 0.000 description 6
- 230000001050 lubricating effect Effects 0.000 description 6
- 230000000996 additive effect Effects 0.000 description 4
- 125000001931 aliphatic group Chemical group 0.000 description 4
- 235000014113 dietary fatty acids Nutrition 0.000 description 4
- 239000000194 fatty acid Substances 0.000 description 4
- 229930195729 fatty acid Natural products 0.000 description 4
- 150000004665 fatty acids Chemical class 0.000 description 4
- 150000002978 peroxides Chemical class 0.000 description 4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 238000004821 distillation Methods 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 238000000227 grinding Methods 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 239000000155 melt Substances 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 229920003023 plastic Polymers 0.000 description 3
- 239000004033 plastic Substances 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 238000002411 thermogravimetry Methods 0.000 description 3
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- BAPJBEWLBFYGME-UHFFFAOYSA-N Methyl acrylate Chemical compound COC(=O)C=C BAPJBEWLBFYGME-UHFFFAOYSA-N 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 238000012662 bulk polymerization Methods 0.000 description 2
- 239000007795 chemical reaction product Substances 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- LSXWFXONGKSEMY-UHFFFAOYSA-N di-tert-butyl peroxide Chemical compound CC(C)(C)OOC(C)(C)C LSXWFXONGKSEMY-UHFFFAOYSA-N 0.000 description 2
- 238000007720 emulsion polymerization reaction Methods 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 239000006082 mold release agent Substances 0.000 description 2
- 239000012778 molding material Substances 0.000 description 2
- 150000002763 monocarboxylic acids Chemical class 0.000 description 2
- 238000000465 moulding Methods 0.000 description 2
- 238000011056 performance test Methods 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 238000005096 rolling process Methods 0.000 description 2
- 238000007127 saponification reaction Methods 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 238000010557 suspension polymerization reaction Methods 0.000 description 2
- 239000001124 (E)-prop-1-ene-1,2,3-tricarboxylic acid Substances 0.000 description 1
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 1
- JAHNSTQSQJOJLO-UHFFFAOYSA-N 2-(3-fluorophenyl)-1h-imidazole Chemical compound FC1=CC=CC(C=2NC=CN=2)=C1 JAHNSTQSQJOJLO-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 239000004605 External Lubricant Substances 0.000 description 1
- 239000004609 Impact Modifier Substances 0.000 description 1
- 239000004610 Internal Lubricant Substances 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 239000004164 Wax ester Substances 0.000 description 1
- 229940091181 aconitic acid Drugs 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000002216 antistatic agent Substances 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 125000000751 azo group Chemical group [*]N=N[*] 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- GTZCVFVGUGFEME-IWQZZHSRSA-N cis-aconitic acid Chemical compound OC(=O)C\C(C(O)=O)=C\C(O)=O GTZCVFVGUGFEME-IWQZZHSRSA-N 0.000 description 1
- HNEGQIOMVPPMNR-IHWYPQMZSA-N citraconic acid Chemical compound OC(=O)C(/C)=C\C(O)=O HNEGQIOMVPPMNR-IHWYPQMZSA-N 0.000 description 1
- 229940018557 citraconic acid Drugs 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 238000007334 copolymerization reaction Methods 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 230000032050 esterification Effects 0.000 description 1
- 238000005886 esterification reaction Methods 0.000 description 1
- 125000002573 ethenylidene group Chemical group [*]=C=C([H])[H] 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 150000002191 fatty alcohols Chemical class 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- 239000004088 foaming agent Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 229920000578 graft copolymer Polymers 0.000 description 1
- 239000012760 heat stabilizer Substances 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 230000003301 hydrolyzing effect Effects 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 239000003077 lignite Substances 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- HNEGQIOMVPPMNR-NSCUHMNNSA-N mesaconic acid Chemical compound OC(=O)C(/C)=C/C(O)=O HNEGQIOMVPPMNR-NSCUHMNNSA-N 0.000 description 1
- LVHBHZANLOWSRM-UHFFFAOYSA-N methylenebutanedioic acid Natural products OC(=O)CC(=C)C(O)=O LVHBHZANLOWSRM-UHFFFAOYSA-N 0.000 description 1
- HNEGQIOMVPPMNR-UHFFFAOYSA-N methylfumaric acid Natural products OC(=O)C(C)=CC(O)=O HNEGQIOMVPPMNR-UHFFFAOYSA-N 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 239000012170 montan wax Substances 0.000 description 1
- 238000011017 operating method Methods 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 150000002976 peresters Chemical class 0.000 description 1
- 230000003711 photoprotective effect Effects 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 239000012744 reinforcing agent Substances 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 239000000344 soap Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- GTZCVFVGUGFEME-UHFFFAOYSA-N trans-aconitic acid Natural products OC(=O)CC(C(O)=O)=CC(O)=O GTZCVFVGUGFEME-UHFFFAOYSA-N 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 235000019386 wax ester Nutrition 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F10/00—Homopolymers and copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond
- C08F10/14—Monomers containing five or more carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F210/00—Copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond
- C08F210/14—Monomers containing five or more carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/04—Oxygen-containing compounds
- C08K5/09—Carboxylic acids; Metal salts thereof; Anhydrides thereof
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Lubricants (AREA)
Description
<15、好ましくは<13gヨウ素/100gのヨウ素価、
200〜4000mPa*sの100℃における粘度
60〜90℃の滴点、
200〜600バールのプランジャー貫通硬度(Stempelpenetrationshaerte)、
60〜100mgKOH/gの酸価、並びに
<10のヨウ素色数。
溶融粘度は、DIN 53019−2に従い、回転粘度計を用いて測定し、滴点はDIN ISO 2176に従い測定した。酸価の測定は、無水物基が加水分解的に解裂するのを避けるべく、使用する溶剤のキシレン及びエタノールが水を含まない形態で使用されたことを除いてDIN 53402に従って行った。ケン化価は、DIN規格53401に従って測定した。ヨウ素価の測定は、DIN規格14111に従って行い、ヨウ素色数の測定はDIN 6162に従って行った。TGA測定は、Mettler−Geraet SD TA 551eを用いて行った(温度プログラム:室温から300℃までの昇温、加熱速度5°/分、窒素流50ml/分)。硬度の尺度としてのプランジャー貫通度は、DGF−法 M−III 9eに従って測定した(Fiebig, Braun, Fett/Lipid 98, 1996, Nr. 2, 86(非特許文献1)を参照)。
α−オレフィンC30+(本質的に>=30個のC原子の鎖長を有するオレフィン系炭化水素からなる混合物;ChevronPhillips社の市場商品)2500gを、撹拌装置、内部温度計及び蒸留橋が装着されたガラス装置中で、窒素ブランケット下(Stickstoffueberlagerung)で溶融する。引き続き、表1に示した量の無水マレイン酸を6回分に均等に分けて、それぞれを30分間隔で計量添加した。同じ期間内で、ジ−tert.−ブチルパーオキシドを滴下漏斗から連続的に加えた。その後、1時間、後反応させた。これに続いて、揮発性画分を真空中(約30ミリバール)で留去した。約30分後、窒素を導入することにより標準圧にした。結果として得られたコポリマーワックスのデータを表1に一覧表記する。
C30+オレフィン及び無水マレイン酸からなる文献公知のコポリマーを、ドイツ国特許出願公開公報第3003797号明細書(特許文献5)、例1、ドイツ国特許出願公開公報第3510233号明細書(特許文献6)、例1、並びに欧州特許出願公開第1693047号明細書(特許文献7)、例A(コポリマーワックス1)に述べられた指示に従って製造した。測定データを表2に挙げる。
1) C30+オレフィン、アクリル酸メチル及びアクリル酸からなるコポリマーワックスを欧州特許出願公開第0545306号(特許文献8)、例1と同様に製造した。上記の例とは相違して、C24−C60−オレフィンの代わりにC30+オレフィンが使用された。それ以外の成分、並びに量比率、反応条件及び操作方法は変更しなかった。
酸価: 12mgKOH/g
ケン化価: 163mgKOH/g
滴点: 73℃
90℃における粘度:: 610mPa*s
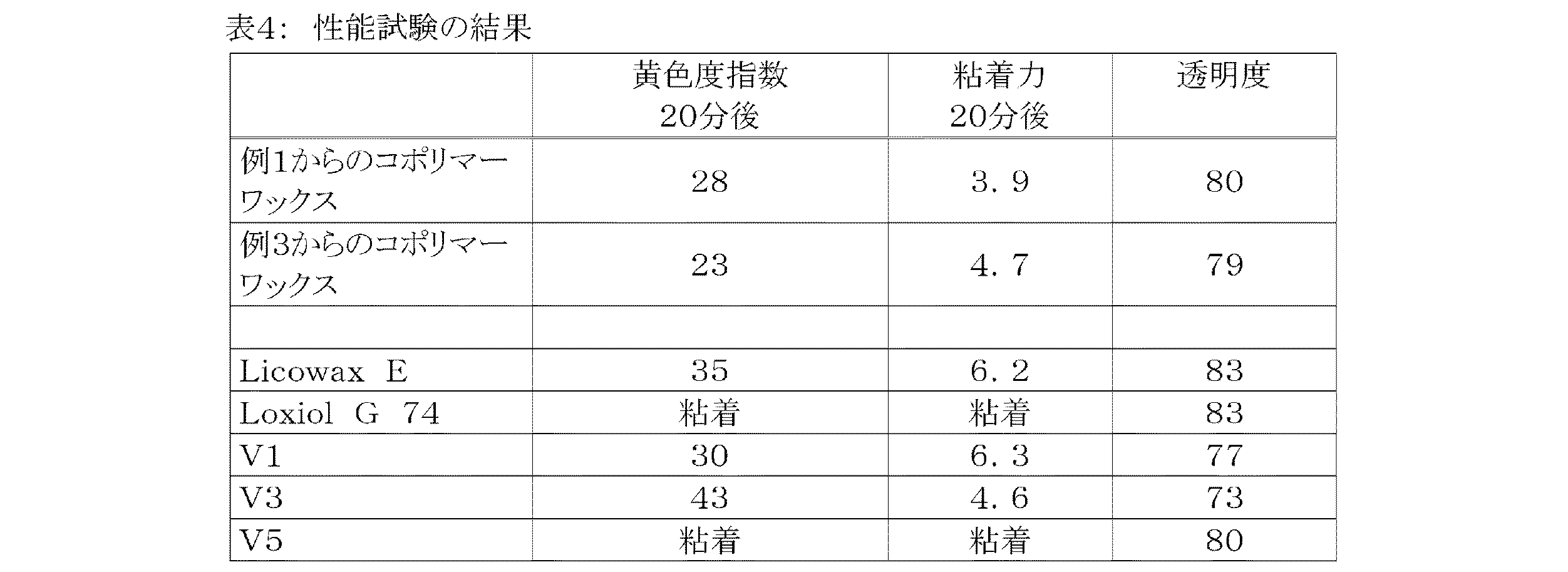
表3に示した組成に従うPVC処方を実験混合機中で前混合後に、タイプ150MのCollin計測用圧延機(Dr. Collin GmbH)で圧延シートに加工した。その際に、いずれも添加剤を含む各PVC混合物をそれぞれ220g使用し、その加工温度は195℃であった。20分の運転時間後、粘着力及び黄色度指数(Yellowness−Index(YI))を測定した。各試験処方の混合物は、2mmの層厚を有するプレートに更にプレスした(Collin Labor−Plattenpress 200P、プレス温度180℃、プレス圧200バール)。これらプレートの透明度を測定した。
Claims (9)
- <15gヨウ素/100gのヨウ素価
200〜4000mPa・sの100℃での粘度
60〜90℃の滴点、
200〜600バールのプランジャー貫通硬度、
60〜100mgKOH/gの酸価、並びに
<10のヨウ素色数、
を特徴とする、コポリマーワックスであって、
その際、該コポリマーワックスが、使用されるオレフィン系炭化水素に基づいて、少なくとも2.0重量%のラジカル開始剤の存在下で、28個以上のC原子の鎖長を有するα−オレフィンと、不飽和ポリカルボン酸又はそれらの無水物との反応によって製造され、
その際、α−オレフィンと不飽和ポリカルボン酸又は無水物が、5:1〜8:1の比率で互いに反応させ、
その際、該コポリマーワックスは、PVCもしくはポリ塩化ビニリデンのための、又は酢酸ビニル、塩化ビニリデン、ビニルエーテル、アクリルニトリル、アクリル酸エステル、マレイン酸モノエステル、マレイン酸ジエステル又はオレフィンからなる群から選択される30重量%までのコモノマーを有する塩化ビニルのコポリマーのための加工助剤として使用されることを特徴とする、上記のコポリマーワックス。 - 28個以上のC原子の鎖長を有するα−オレフィンと、不飽和ポリカルボン酸又はそれらの無水物との反応によって製造されることを特徴とする、請求項1に記載のコポリマーワックス。
- 前記α−オレフィンが、28個〜60個のC原子の鎖長を有することを特徴とする、請求項2に記載のコポリマーワックス。
- 使用されるオレフィン系炭化水素に基づいて、少なくとも2.0重量%のラジカル開始剤の存在下で、28個以上のC原子の鎖長を有するα−オレフィンと、不飽和ポリカルボン酸又はそれらの無水物との反応による、請求項1に記載のコポリマーワックスの製造方法。
- 使用されるα−オレフィンが、エチレンのオリゴマー化によって得られたものであることを特徴とする、請求項4に記載のコポリマーワックスの製造方法。
- ポリカルボン酸無水物として無水マレイン酸が使用されることを特徴とする、請求項4に記載のコポリマーワックスの製造方法。
- 前記反応が、100〜200℃の反応温度で遂行されることを特徴とする、請求項4に記載のコポリマーワックスの製造方法。
- α−オレフィン及び無水マレイン酸を、5:1〜8:1の重量比で相互に反応させることを特徴とする、請求項7に記載のコポリマーワックスの製造方法。
- PVCもしくはポリ塩化ビニリデンのための、又は酢酸ビニル、塩化ビニリデン、ビニルエーテル、アクリルニトリル、アクリル酸エステル、マレイン酸モノエステル、マレイン酸ジエステル又はオレフィンからなる群から選択される30重量%までのコモノマーを有する塩化ビニルのコポリマーのための加工助剤としての、請求項1に記載のコポリマーワックスの使用。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE102009008257A DE102009008257A1 (de) | 2009-02-10 | 2009-02-10 | Wachse mit hoher Polarität und ihre Verwendung als Gleitmittel für chlorhaltige Thermoplaste |
| DE102009008257.3 | 2009-02-10 | ||
| PCT/EP2010/000720 WO2010091825A1 (de) | 2009-02-10 | 2010-02-05 | Wachse mit hoher polarität und ihre verwendung als gleitmittel für chlorhaltige thermoplaste |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2012517488A JP2012517488A (ja) | 2012-08-02 |
| JP5601727B2 true JP5601727B2 (ja) | 2014-10-08 |
Family
ID=42105935
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011548611A Expired - Fee Related JP5601727B2 (ja) | 2009-02-10 | 2010-02-05 | 高極性を有するワックス及び塩素含有熱可塑性プラスチックのための滑剤としてのその使用 |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US20110275837A1 (ja) |
| EP (1) | EP2396352B1 (ja) |
| JP (1) | JP5601727B2 (ja) |
| DE (1) | DE102009008257A1 (ja) |
| ES (1) | ES2735513T3 (ja) |
| PL (1) | PL2396352T3 (ja) |
| PT (1) | PT2396352T (ja) |
| TR (1) | TR201908666T4 (ja) |
| WO (1) | WO2010091825A1 (ja) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2444450A1 (de) | 2010-10-19 | 2012-04-25 | Hinterwaldner Consulting & Partner (Gbr) | Zusammensetzungen zur Herstellung abhäsiver Beschichtungen |
| EP2543704A1 (en) * | 2011-07-07 | 2013-01-09 | Clariant International Ltd. | Use of waxlike products of plastics processing |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE1693047A1 (de) | 1968-03-11 | 1971-07-08 | Lentia Gmbh | Verfahren zur Herstellung von hydroxylierten Aminosteroiden |
| GB1245879A (en) * | 1968-08-29 | 1971-09-08 | Mobil Oil Corp | Fluidity improvers |
| NL162682C (nl) | 1969-02-15 | 1980-06-16 | Neynaber Chemie Gmbh | Werkwijze ter bereiding van thermoplastische kunst- stoffen die een glijmiddel bevatten. |
| JPS5222988B2 (ja) * | 1971-08-20 | 1977-06-21 | ||
| DE2306744C2 (de) | 1973-02-12 | 1982-07-08 | Neynaber Chemie Gmbh, 2854 Loxstedt | Verwendung von Mischestern als Gleitmittel-Zusatz für die formgebende Verarbeitung thermoplastischer Massen |
| JPS5223386B2 (ja) * | 1973-02-20 | 1977-06-23 | ||
| DE2748367A1 (de) | 1977-10-28 | 1979-05-03 | Akzo Gmbh | Olefin-maleinsaeureanhydrid-copolymerisate |
| DE3003797C2 (de) | 1980-02-02 | 1983-05-11 | Akzo Gmbh, 5600 Wuppertal | Verformung von Gleitmittel enthaltenden Kunststoffen |
| US4358573A (en) * | 1981-05-29 | 1982-11-09 | S. C. Johnson & Son, Inc. | Waxy maleic anhydride alpha olefin terpolymers |
| US4358564A (en) * | 1981-05-29 | 1982-11-09 | Eastman Kodak Company | Process for controlling the viscosity during the preparation of grafted polyethylene and ethylene/alpha olefin waxes |
| GB2156823B (en) | 1984-03-22 | 1987-11-25 | Mitsubishi Chem Ind | Wax and ink composition for thermal ink transfer abstract of the disclosure |
| DE4139601C2 (de) | 1991-11-30 | 1994-09-08 | Hoechst Ag | Copolymerisate und ihre Verwendung als Gleit- und Trennmittel für die Verarbeitung thermoplastischer Kunststoffe |
| DE102005007980A1 (de) | 2005-02-22 | 2006-02-23 | Clariant Gmbh | Kosmetische, pharmazeutische oder dermatologische Zubereitungen enthaltend Copolymerwachse |
-
2009
- 2009-02-10 DE DE102009008257A patent/DE102009008257A1/de not_active Withdrawn
-
2010
- 2010-02-05 EP EP10703419.1A patent/EP2396352B1/de active Active
- 2010-02-05 TR TR2019/08666T patent/TR201908666T4/tr unknown
- 2010-02-05 ES ES10703419T patent/ES2735513T3/es active Active
- 2010-02-05 US US13/144,787 patent/US20110275837A1/en not_active Abandoned
- 2010-02-05 JP JP2011548611A patent/JP5601727B2/ja not_active Expired - Fee Related
- 2010-02-05 WO PCT/EP2010/000720 patent/WO2010091825A1/de not_active Ceased
- 2010-02-05 PT PT10703419T patent/PT2396352T/pt unknown
- 2010-02-05 PL PL10703419T patent/PL2396352T3/pl unknown
Also Published As
| Publication number | Publication date |
|---|---|
| EP2396352B1 (de) | 2019-05-22 |
| PL2396352T3 (pl) | 2019-11-29 |
| PT2396352T (pt) | 2019-07-16 |
| WO2010091825A1 (de) | 2010-08-19 |
| ES2735513T3 (es) | 2019-12-19 |
| TR201908666T4 (tr) | 2019-07-22 |
| EP2396352A1 (de) | 2011-12-21 |
| JP2012517488A (ja) | 2012-08-02 |
| DE102009008257A1 (de) | 2010-08-12 |
| US20110275837A1 (en) | 2011-11-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2022029502A1 (en) | Green polyethylene wax for hot melt adhesives, coatings, and cosmetics | |
| US5306437A (en) | Copolymers and their use as lubricants and release agents for processing thermoplastics | |
| US10676618B2 (en) | Polar-modified rice husk wax | |
| JP5601727B2 (ja) | 高極性を有するワックス及び塩素含有熱可塑性プラスチックのための滑剤としてのその使用 | |
| JP5107483B1 (ja) | メタクリル系樹脂組成物および成形体 | |
| JP2012520908A (ja) | 塗料のための添加剤としてのコポリマーの使用 | |
| JP2009127053A (ja) | ワックス様アイオノマー | |
| AU2012280689B2 (en) | Use of waxlike products for plastics processing | |
| JP2006501337A (ja) | 多価アルコールのエステル及び部分エステル | |
| US10711127B2 (en) | Chlorine-containing polymer composition comprising a chlorine-containing polymer and a wax comprising a fraction consisting of oxidized hydrocarbons and a fraction consisting of non-oxidized hydrocarbons, method of processing the polymer composition and the use of the wax as external lubricant during the polymer processing | |
| JPS6241088B2 (ja) | ||
| JP6030359B2 (ja) | 塩化ビニル樹脂組成物用加工助剤および該加工助剤を含有する塩化ビニル樹脂組成物並びにその成形品 | |
| JP2001505947A (ja) | 共役ジエンおよびジエノフィル成分からなるポリマー | |
| JPH04328108A (ja) | 酸化ポリエチレンワックスのエステルおよび可塑性成形材料において滑剤としてそれを使用する方法 | |
| CN106188616A (zh) | 烯烃扩链酸润滑剂、其制备及含该润滑剂的热塑性塑料 | |
| JPH07216170A (ja) | 塩化ビニルポリマー成形材料 | |
| JPH08143732A (ja) | 塩素含有樹脂組成物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20130204 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20131211 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140114 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140411 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140418 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140513 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140520 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140612 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140619 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140710 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20140813 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20140818 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5601727 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| R371 | Transfer withdrawn |
Free format text: JAPANESE INTERMEDIATE CODE: R371 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R360 | Written notification for declining of transfer of rights |
Free format text: JAPANESE INTERMEDIATE CODE: R360 |
|
| R360 | Written notification for declining of transfer of rights |
Free format text: JAPANESE INTERMEDIATE CODE: R360 |
|
| R371 | Transfer withdrawn |
Free format text: JAPANESE INTERMEDIATE CODE: R371 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| RD02 | Notification of acceptance of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: R3D02 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |