JP5510911B2 - Composite interior coating material for buildings - Google Patents
Composite interior coating material for buildings Download PDFInfo
- Publication number
- JP5510911B2 JP5510911B2 JP2008083902A JP2008083902A JP5510911B2 JP 5510911 B2 JP5510911 B2 JP 5510911B2 JP 2008083902 A JP2008083902 A JP 2008083902A JP 2008083902 A JP2008083902 A JP 2008083902A JP 5510911 B2 JP5510911 B2 JP 5510911B2
- Authority
- JP
- Japan
- Prior art keywords
- coating material
- layer
- antibacterial
- binder
- inorganic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000000463 material Substances 0.000 title claims description 126
- 239000011248 coating agent Substances 0.000 title claims description 116
- 238000000576 coating method Methods 0.000 title claims description 111
- 239000002131 composite material Substances 0.000 title claims description 23
- 239000011148 porous material Substances 0.000 claims description 54
- 239000000126 substance Substances 0.000 claims description 45
- 230000000844 anti-bacterial effect Effects 0.000 claims description 42
- 239000011941 photocatalyst Substances 0.000 claims description 37
- 239000011230 binding agent Substances 0.000 claims description 33
- 239000010457 zeolite Substances 0.000 claims description 31
- 229910021536 Zeolite Inorganic materials 0.000 claims description 28
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 claims description 28
- 239000000383 hazardous chemical Substances 0.000 claims description 25
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 claims description 23
- 238000001179 sorption measurement Methods 0.000 claims description 21
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 claims description 19
- 238000002156 mixing Methods 0.000 claims description 17
- 239000000835 fiber Substances 0.000 claims description 9
- 239000000203 mixture Substances 0.000 claims description 9
- 239000010419 fine particle Substances 0.000 claims description 8
- 239000011362 coarse particle Substances 0.000 claims description 7
- 238000005342 ion exchange Methods 0.000 claims description 7
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 claims description 6
- 239000003795 chemical substances by application Substances 0.000 claims description 5
- 229910052500 inorganic mineral Inorganic materials 0.000 claims description 4
- 238000010521 absorption reaction Methods 0.000 claims description 3
- 239000005995 Aluminium silicate Substances 0.000 claims description 2
- 235000012211 aluminium silicate Nutrition 0.000 claims description 2
- 230000003197 catalytic effect Effects 0.000 claims description 2
- 239000010410 layer Substances 0.000 description 101
- 235000019645 odor Nutrition 0.000 description 30
- 239000012855 volatile organic compound Substances 0.000 description 30
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 26
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 26
- 239000007789 gas Substances 0.000 description 19
- 239000002994 raw material Substances 0.000 description 15
- 239000000843 powder Substances 0.000 description 13
- 239000005909 Kieselgur Substances 0.000 description 9
- 239000004566 building material Substances 0.000 description 8
- 239000011505 plaster Substances 0.000 description 8
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 7
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical class [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 7
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 description 7
- 239000000920 calcium hydroxide Substances 0.000 description 7
- 229910001861 calcium hydroxide Inorganic materials 0.000 description 7
- 235000011116 calcium hydroxide Nutrition 0.000 description 7
- 239000004568 cement Substances 0.000 description 7
- 238000000354 decomposition reaction Methods 0.000 description 7
- 229910052680 mordenite Inorganic materials 0.000 description 7
- 238000010276 construction Methods 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- MWUXSHHQAYIFBG-UHFFFAOYSA-N Nitric oxide Chemical compound O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 description 5
- 239000004372 Polyvinyl alcohol Substances 0.000 description 5
- 239000004113 Sepiolite Substances 0.000 description 5
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 5
- 239000003610 charcoal Substances 0.000 description 5
- 238000003795 desorption Methods 0.000 description 5
- 239000002245 particle Substances 0.000 description 5
- 229920002451 polyvinyl alcohol Polymers 0.000 description 5
- 229920002620 polyvinyl fluoride Polymers 0.000 description 5
- 229910052624 sepiolite Inorganic materials 0.000 description 5
- 235000019355 sepiolite Nutrition 0.000 description 5
- 239000000741 silica gel Substances 0.000 description 5
- 229910002027 silica gel Inorganic materials 0.000 description 5
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 4
- 229910001583 allophane Inorganic materials 0.000 description 4
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 238000005336 cracking Methods 0.000 description 4
- 239000010440 gypsum Substances 0.000 description 4
- 229910052602 gypsum Inorganic materials 0.000 description 4
- 230000006872 improvement Effects 0.000 description 4
- 238000000034 method Methods 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 239000000377 silicon dioxide Substances 0.000 description 4
- 238000002336 sorption--desorption measurement Methods 0.000 description 4
- 239000002023 wood Substances 0.000 description 4
- 229920000049 Carbon (fiber) Polymers 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- 239000000853 adhesive Substances 0.000 description 3
- 230000001070 adhesive effect Effects 0.000 description 3
- 239000003242 anti bacterial agent Substances 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 238000002485 combustion reaction Methods 0.000 description 3
- 238000001035 drying Methods 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 238000010438 heat treatment Methods 0.000 description 3
- 239000012784 inorganic fiber Substances 0.000 description 3
- 238000009413 insulation Methods 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 238000007254 oxidation reaction Methods 0.000 description 3
- 239000011120 plywood Substances 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 239000004576 sand Substances 0.000 description 3
- -1 shirasu Substances 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- 239000004677 Nylon Substances 0.000 description 2
- 229920002978 Vinylon Polymers 0.000 description 2
- 229940009868 aluminum magnesium silicate Drugs 0.000 description 2
- JYIBXUUINYLWLR-UHFFFAOYSA-N aluminum;calcium;potassium;silicon;sodium;trihydrate Chemical compound O.O.O.[Na].[Al].[Si].[K].[Ca] JYIBXUUINYLWLR-UHFFFAOYSA-N 0.000 description 2
- WMGSQTMJHBYJMQ-UHFFFAOYSA-N aluminum;magnesium;silicate Chemical compound [Mg+2].[Al+3].[O-][Si]([O-])([O-])[O-] WMGSQTMJHBYJMQ-UHFFFAOYSA-N 0.000 description 2
- 229960000892 attapulgite Drugs 0.000 description 2
- 229910000019 calcium carbonate Inorganic materials 0.000 description 2
- 239000000378 calcium silicate Substances 0.000 description 2
- 229910052918 calcium silicate Inorganic materials 0.000 description 2
- OYACROKNLOSFPA-UHFFFAOYSA-N calcium;dioxido(oxo)silane Chemical compound [Ca+2].[O-][Si]([O-])=O OYACROKNLOSFPA-UHFFFAOYSA-N 0.000 description 2
- 239000004917 carbon fiber Substances 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 2
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 2
- 239000000679 carrageenan Substances 0.000 description 2
- 235000010418 carrageenan Nutrition 0.000 description 2
- 229920001525 carrageenan Polymers 0.000 description 2
- 229940113118 carrageenan Drugs 0.000 description 2
- 229910052619 chlorite group Inorganic materials 0.000 description 2
- QBWCMBCROVPCKQ-UHFFFAOYSA-N chlorous acid Chemical group OCl=O QBWCMBCROVPCKQ-UHFFFAOYSA-N 0.000 description 2
- 238000004140 cleaning Methods 0.000 description 2
- 229910001603 clinoptilolite Inorganic materials 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- GUJOJGAPFQRJSV-UHFFFAOYSA-N dialuminum;dioxosilane;oxygen(2-);hydrate Chemical compound O.[O-2].[O-2].[O-2].[Al+3].[Al+3].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O GUJOJGAPFQRJSV-UHFFFAOYSA-N 0.000 description 2
- 230000007613 environmental effect Effects 0.000 description 2
- 239000002657 fibrous material Substances 0.000 description 2
- 239000003365 glass fiber Substances 0.000 description 2
- 231100001261 hazardous Toxicity 0.000 description 2
- 150000002484 inorganic compounds Chemical class 0.000 description 2
- 229910010272 inorganic material Inorganic materials 0.000 description 2
- 229910052622 kaolinite Inorganic materials 0.000 description 2
- 230000004298 light response Effects 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- WSFSSNUMVMOOMR-NJFSPNSNSA-N methanone Chemical compound O=[14CH2] WSFSSNUMVMOOMR-NJFSPNSNSA-N 0.000 description 2
- 229910052618 mica group Inorganic materials 0.000 description 2
- 239000011707 mineral Substances 0.000 description 2
- 235000010755 mineral Nutrition 0.000 description 2
- 229910052901 montmorillonite Inorganic materials 0.000 description 2
- 239000004570 mortar (masonry) Substances 0.000 description 2
- 229920001778 nylon Polymers 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 150000002894 organic compounds Chemical class 0.000 description 2
- 229910052625 palygorskite Inorganic materials 0.000 description 2
- 230000035699 permeability Effects 0.000 description 2
- 239000003208 petroleum Substances 0.000 description 2
- 230000001699 photocatalysis Effects 0.000 description 2
- 229940068984 polyvinyl alcohol Drugs 0.000 description 2
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 2
- 238000010298 pulverizing process Methods 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- 238000009738 saturating Methods 0.000 description 2
- 208000008842 sick building syndrome Diseases 0.000 description 2
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 2
- 239000002356 single layer Substances 0.000 description 2
- 239000002002 slurry Substances 0.000 description 2
- 229910000269 smectite group Inorganic materials 0.000 description 2
- 239000000661 sodium alginate Substances 0.000 description 2
- 235000010413 sodium alginate Nutrition 0.000 description 2
- 229940005550 sodium alginate Drugs 0.000 description 2
- 230000001954 sterilising effect Effects 0.000 description 2
- 238000004659 sterilization and disinfection Methods 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 239000000454 talc Substances 0.000 description 2
- 229910052623 talc Inorganic materials 0.000 description 2
- 239000004408 titanium dioxide Substances 0.000 description 2
- 238000009423 ventilation Methods 0.000 description 2
- 239000010455 vermiculite Substances 0.000 description 2
- 229910052902 vermiculite Inorganic materials 0.000 description 2
- 235000019354 vermiculite Nutrition 0.000 description 2
- UHVMMEOXYDMDKI-JKYCWFKZSA-L zinc;1-(5-cyanopyridin-2-yl)-3-[(1s,2s)-2-(6-fluoro-2-hydroxy-3-propanoylphenyl)cyclopropyl]urea;diacetate Chemical compound [Zn+2].CC([O-])=O.CC([O-])=O.CCC(=O)C1=CC=C(F)C([C@H]2[C@H](C2)NC(=O)NC=2N=CC(=CC=2)C#N)=C1O UHVMMEOXYDMDKI-JKYCWFKZSA-L 0.000 description 2
- 239000004925 Acrylic resin Substances 0.000 description 1
- 229920000178 Acrylic resin Polymers 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- JPVYNHNXODAKFH-UHFFFAOYSA-N Cu2+ Chemical compound [Cu+2] JPVYNHNXODAKFH-UHFFFAOYSA-N 0.000 description 1
- RWSOTUBLDIXVET-UHFFFAOYSA-N Dihydrogen sulfide Chemical compound S RWSOTUBLDIXVET-UHFFFAOYSA-N 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- 241000257303 Hymenoptera Species 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- FOIXSVOLVBLSDH-UHFFFAOYSA-N Silver ion Chemical compound [Ag+] FOIXSVOLVBLSDH-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 241000607479 Yersinia pestis Species 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- PTFCDOFLOPIGGS-UHFFFAOYSA-N Zinc dication Chemical compound [Zn+2] PTFCDOFLOPIGGS-UHFFFAOYSA-N 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 230000003373 anti-fouling effect Effects 0.000 description 1
- 230000000843 anti-fungal effect Effects 0.000 description 1
- 230000002421 anti-septic effect Effects 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 208000006673 asthma Diseases 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 238000005341 cation exchange Methods 0.000 description 1
- 238000004040 coloring Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 239000000306 component Substances 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 229910001431 copper ion Inorganic materials 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 239000002781 deodorant agent Substances 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 230000008034 disappearance Effects 0.000 description 1
- TXKMVPPZCYKFAC-UHFFFAOYSA-N disulfur monoxide Inorganic materials O=S=S TXKMVPPZCYKFAC-UHFFFAOYSA-N 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 238000004817 gas chromatography Methods 0.000 description 1
- 229910000037 hydrogen sulfide Inorganic materials 0.000 description 1
- 238000005286 illumination Methods 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 239000001023 inorganic pigment Substances 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 230000001678 irradiating effect Effects 0.000 description 1
- 238000004898 kneading Methods 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 239000002808 molecular sieve Substances 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 239000003973 paint Substances 0.000 description 1
- 239000010451 perlite Substances 0.000 description 1
- 235000019362 perlite Nutrition 0.000 description 1
- 238000007539 photo-oxidation reaction Methods 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 238000007873 sieving Methods 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- XTQHKBHJIVJGKJ-UHFFFAOYSA-N sulfur monoxide Chemical compound S=O XTQHKBHJIVJGKJ-UHFFFAOYSA-N 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 239000002344 surface layer Substances 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 230000036962 time dependent Effects 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
Images
Description
本発明は、住空間の環境保全と改善を目的とする建築用材であり、詳しくは建造物の内装仕上げに使用される複合内装塗材に関するものである。 The present invention is a building materials for the purpose of improving the environmental conservation living space, but details about the composite interior coating materials used for interior finish of buildings.
建造物の内装仕上げ構造としては、石膏ボード、木材、合板などからなる下地材の表面に珪藻土、活性炭、活性炭素繊維、分子ふるい炭素、シリカゲル、活性アルミナ、ゼオライト等の無機多孔質物質を配合した塗材やシラスなどの微粒子を吹き付けて多孔質の壁面を形成する技術が知られている。無機多孔質物質はその細孔内に種々の分子を物理吸着することが知られており、室内の湿度調整、臭気成分、揮発性有機化合物(以下、VOCと称する)を吸着する機能を有する内壁等の製品が市販されている。一方、臭気成分やVOC除去を目的に、光触媒を塗布した建造物の内壁等が開発されており、内壁、天井、壁、床、浴槽、洗面スペース、蛇口などを酸化チタンでコーティングして抗菌性を付与した製品が市販されている。代表的な光触媒活性物質としては酸化チタンが知られており、近紫外線を照射すると酸化反応を触媒することが知られている。また、少量の不純物を添加(ドープ)することにより400〜600nmの可視光で励起する可視光応答型の光触媒も開発されている。 As the interior finish structure of the building, inorganic porous materials such as diatomaceous earth, activated carbon, activated carbon fiber, molecular sieve carbon, silica gel, activated alumina, zeolite, etc. are blended on the surface of the base material made of gypsum board, wood, plywood, etc. A technique for forming a porous wall surface by spraying fine particles such as a coating material or shirasu is known. It is known that an inorganic porous material physically adsorbs various molecules in its pores, and has an inner wall functioning to adjust indoor humidity, adsorb odor components, and volatile organic compounds (hereinafter referred to as VOC). Such products are commercially available. On the other hand, the inner walls of buildings with photocatalyst applied have been developed for the purpose of removing odor components and VOCs, and the inner walls, ceiling, walls, floors, bathtubs, wash spaces, faucets, etc. are coated with titanium oxide for antibacterial properties. The product which gave is marketed. Titanium oxide is known as a typical photocatalytic active substance, and it is known to catalyze an oxidation reaction when irradiated with near ultraviolet rays. In addition, a visible light responsive photocatalyst that is excited by visible light of 400 to 600 nm by adding a small amount of impurities (doping) has been developed.
例えば、特許文献1には、基材の塗装をした表面に、光触媒を担持させた粉末を散布し、塗装した塗膜に粉体を固着させてなることを特徴とする建築材が開示されており、前記の光触媒が酸化チタンであり、光触媒を担持する粉体がシリカ、ケイ砂、ゼオライト、シリカゲル、珪藻土、アロフェンから選ばれるものであることが記載されている。これにより基材の材質が制限されることがなく、光触媒の機能を十分に発揮させることができるとの記載がある。
For example,
また、特許文献2には粉末状の炭と、消石灰又は漆喰と、ポリビニルアルコールと、水とを有することを特徴とする防蟻塗装剤が開示されており、二酸化チタン、ゼオライトを添加したことを特徴とする防蟻塗装剤についての記載がある。これにより一般住宅等の木造建造物に用いられる木材を白蟻等の害虫から長期間にわたって保守し、調湿機能、防臭機能、抗菌機能、防カビ機能、防汚機能、防腐機能、害虫駆除機能及び紫外線除去機能を付加し、さらに、作業性が良好で、環境に優しく、なおかつ低コストを実現する防蟻塗装剤及びそれを塗装した建材用ボードを提供できるとの記載がある。
Further,
本発明は無機多孔質物質への臭気成分、VOC等の分子の吸着・脱着反応と抗菌・有害物分解性物質による該分子の分解反応を協調せしめ、一度、無機多孔質物質に吸着した臭気成分、VOC等の分子が室内に再度放出されることのない建造物用複合内装塗材及び建造物用複合内装塗装構造を提供すること、さらには、第1層の抗菌・有害物分解性物質により無機多孔質物質の細孔に吸着した分子を分解することにより、細孔を飽和させることなくセルフクリーニングできる能力を有する建造物用複合内装塗材を提供することを目的とする。 The present invention coordinates an odor component to an inorganic porous material, an adsorption / desorption reaction of a molecule such as VOC, and a decomposition reaction of the molecule by an antibacterial / hazardous substance decomposable substance, and once the odor component adsorbed to the inorganic porous material Providing a composite interior coating material for buildings and a composite interior coating structure for buildings in which molecules such as VOC are not released into the room again, and further, by the antibacterial / hazardous substance decomposable substance in the first layer An object of the present invention is to provide a composite interior coating material for buildings having the capability of self-cleaning without saturating the pores by decomposing molecules adsorbed on the pores of the inorganic porous material.
従来、無機多孔質物質を単独で使用した場合は、細孔内に吸着する分子の絶対的量には上限があり、分子の吸着量が増えるにつれて細孔が飽和状態に近づきやがては飽和し、分子を吸着することができなくなるおそれがあった。また、物理吸着は共有結合等の相互作用と比較して格段に弱い相互作用であるため、吸着した分子は温度の上昇、減圧によって容易に脱着されることが知られている。従って、室温上昇などの室内環境の変化に応じて一度、吸着された臭気成分、VOCが室内に再放出され、居住者が知らないうちに臭気成分、VOCに暴露されるという問題があった。特に冬季には暖房器具の使用により無機多孔質物質が暖められ、一度、無機多孔質物質に吸着されたVOC等の分子が放出されやすい環境になり易かった。それに加えて、開放型燃焼器具を使用する場合には、暖房器具そのものから、二酸化炭素のみならず、一酸化窒素、窒素酸化物、硫黄酸化物、ホルムアルデヒド、VOCが発生する事が報告されており、冬季に石油ファンヒーター、対流式石油ストーブなどの開放型燃焼器具を使用する世帯では、居住者が高濃度の臭気成分、VOCに曝されやすく上記の問題点がより顕著に現れやすかった。昔の日本家屋では、室内の換気量が自然と大きいために、建材に用いられる接着剤、開放型燃焼器具等から発生する臭気成分、VOCは大きな問題とならなかったが、近年の高気密化が為された住宅環境においては建築物の気密化による換気量の減少により室内の臭気成分、VOCは大きな問題となり、それはシックハウス症候群等の問題からも明らかである。 Conventionally, when an inorganic porous material is used alone, there is an upper limit to the absolute amount of molecules adsorbed in the pores, and as the amount of adsorbed molecules increases, the pores approach saturation and eventually become saturated. There was a risk that the molecules could not be adsorbed. In addition, since physical adsorption is a much weaker interaction than an interaction such as a covalent bond, it is known that the adsorbed molecule is easily desorbed by a rise in temperature or a reduced pressure. Therefore, once adsorbed odor component and VOC are re-released indoors in response to changes in the room environment such as a rise in room temperature, there is a problem that the odor component and VOC are exposed without the resident knowing. In particular, in the winter season, the inorganic porous material is warmed by the use of a heating appliance, and it is easy to be in an environment where molecules such as VOC once adsorbed to the inorganic porous material are easily released. In addition, when using an open-type combustion appliance, it is reported that not only carbon dioxide but also nitrogen monoxide, nitrogen oxide, sulfur oxide, formaldehyde, and VOC are generated from the heating appliance itself. In households that use open-type combustion appliances such as oil fan heaters and convection type oil stoves in the winter, the above problems are more likely to appear more prominently because residents are more likely to be exposed to high-concentration odor components and VOCs. In old Japanese houses, indoor ventilation is naturally large, so odor components and VOCs generated from adhesives used in building materials, open-type combustion appliances, etc. have not been a major problem, but in recent years airtightness has increased. In a residential environment where the air quality has been reduced, indoor odor components and VOCs become a major problem due to a decrease in ventilation due to the airtightness of buildings, which is apparent from problems such as sick house syndrome.
特許文献1には基材の塗装を施した表面に、光触媒を担持させた粉体を散布し、塗装した塗膜に粉体を固着させてなることを特徴とする建築材であって、前記光触媒が酸化チタンであり、光触媒を担持する前記粉体がシリカ、ケイ砂、ゼオライト、シリカゲル、珪藻土、アロフェン等である建築材が開示されているが、無機多孔質物質(粉体)への臭気成分、VOC等の分子の吸着・脱着反応と抗菌・有害物分解性物質による該分子の分解反応を協調させる手段及び無機多孔質物質(粉体)の細孔の飽和を防ぐ手段については検討が為されておらず、建築材が暖房等の外的要因で暖められた際には前記粉体に吸着した臭気成分、VOCが光触媒の作用により分解されずに室内に漏れ出すおそれがある。それに加えて、光触媒を担持させた粉体を塗膜表面に固着させる構成とした場合、摩擦により固着させた前記粉体が脱落し、ガス吸着能、分解能を長期にわたって維持することは難しいという問題がある。
一方、特許文献2には粉末状の炭と、消石灰又は漆喰と、ポリビニルアルコールと、水とを有することを特徴とする防蟻塗装剤及びそれを塗布した建材用ボードが開示されており、二酸化チタン、ゼオライトを添加する旨の記載が請求項3及び請求項4に見られる。ここで提案されている防蟻塗装剤は二酸化チタン、ゼオライト、粉末状の炭、消石灰又は漆喰、ポリビニルアルコール及び水を混合した1層からなるものであり、このような単一層からなる塗装構造では抗菌・有害物分解性物質(光触媒)と無機多孔質物質(ゼオライト)の役割が明確でなく、特許文献1と同様に抗菌・有害物分解性物質と無機多孔質物質の機能の協調は図り難い。そこで本発明は上記のような室内空間への臭気成分、VOCの再放出及び無機多孔質物質の細孔の飽和現象を防ぐ手段を開発すべく検討を行った。
On the other hand,
本発明では表面塗材としての抗菌・有害物分解性物質を含有する第1層塗材と、内面塗材としての吸着吸放湿能を有する無機多孔質物質を含有する第2層塗材と、からなる建造物用複合内装塗材であって、第1層塗材は、抗菌・有害物質分解性物質として酸化チタン光触媒と、バインダーとを含有し、第2層塗材は、無機多孔質物質とバインダーを含有し、無機多孔質物質は物理吸着能及びイオン交換能を有する細粒と粗粒の天然ゼオライトの混合物を主成分とするものであり、前記の細粒と粗粒は天然ゼオライトを破砕した後で風力分級したものであり、細粒に対して粗粒を重量比で2/3倍量から1.5倍量配合して細粒と粗粒の天然ゼオライトの混合物とし、該細粒と粗粒の天然ゼオライトの混合物を第2層塗材の主成分として配合することにより表面を凹凸状にした建造物用複合内装塗材により上記の課題を解決する。すなわち、抗菌機能・有害物分解機能を担う層と臭気成分、VOC、水分子を吸着する層の2層構造とすることにより、第2層による分子の吸着作用と第1層による分解作用を協調せしめ、第2層で吸着・捕捉した臭気成分、VOCを第1層で室内側に漏らすことなく分解することができるようになるのである。また、第2層塗材中の無機多孔質物質を細粒と粗粒の天然ゼオライトの混合物としているので、第2層塗材の表面を凹凸状として抗菌・有害物分解性物質の摩耗等による消失を防ぐことができる。
In the present invention, a first layer coating material containing an antibacterial / hazardous substance decomposable substance as a surface coating material, and a second layer coating material containing an inorganic porous material having adsorption / desorption / release characteristics as an inner surface coating material, The first layer coating material contains a titanium oxide photocatalyst as an antibacterial / hazardous substance decomposable substance and a binder, and the second layer coating material is an inorganic porous material. The inorganic porous material contains a mixture of fine and coarse natural zeolite having physical adsorption ability and ion exchange ability, and the fine and coarse particles are natural zeolite. After pulverizing, the coarse particles are blended in a ratio of 2/3 to 1.5 times by weight with respect to the fine particles to obtain a mixture of fine and coarse natural zeolite, A mixture of fine and coarse natural zeolite is blended as the main component of the second layer coating material. Thus, the above-described problems are solved by the composite interior coating material for buildings having an uneven surface. In other words, by adopting a two-layer structure consisting of a layer responsible for antibacterial and harmful substance decomposition functions and a layer that adsorbs odor components, VOCs, and water molecules, the adsorption of molecules by the second layer and the decomposition by the first layer are coordinated. In other words, the odor component and VOC adsorbed and trapped in the second layer can be decomposed without leaking to the indoor side in the first layer. In addition, since the inorganic porous material in the second layer coating material is a mixture of fine and coarse natural zeolite, the surface of the second layer coating material is made uneven so that it can be worn by antibacterial / hazardous substance decomposable substances. Disappearance can be prevented.
より具体的には、前記第2層塗材を、物理吸着能に加えイオン交換能を有する無機多孔質物質と親水性バインダーからなる塗材とし、その上に第1層を形成することで、上記の課題を解決することができる。すなわち、光触媒を含有する第1層と無機多孔質物質を含有する第2層を別個独立に設けることにより、吸着した分子を分解する第1層と分子を吸着する役割を担う第2層という具合に役割分担が明確になり、第2層に含まれる無機多孔質物質に一度、吸着した臭気成分、ホルムアルデヒドなどのVOCが室内に漏れ出すことのないよう、第1層に含有させる抗菌・有害物分解性物質の密度を調節することができるようになる。また、ここで、第2層の主原料となる無機多孔質物質としてイオン交換能を有する物質を選択すればイオン交換により極性物質が保持されるため、温度の上昇等の外的要因により、第2層中の無機多孔質物質から一度吸着されたVOC分子が再放出される際に、炭などの比較的弱い相互作用と言われる分子間引力に起因する吸着(物理吸着)に比して、第2層からの分子の脱着速度を低下させることができると期待される。本発明では、イオン交換能を有する無機多孔質物質として沸石属に属する天然鉱物(以下、天然ゼオライトと称する)を主成分として使用する。天然ゼオライトを主成分とする第2層塗材には天然ゼオライトに加えてセピオライト、シラス、珪藻土、ガラスバルーンなどの軽量骨材を加えてもよい。 More specifically, the second layer coating material is a coating material composed of an inorganic porous material having an ion exchange capacity in addition to a physical adsorption capacity and a hydrophilic binder, and the first layer is formed thereon, The above problems can be solved. That is, by providing the first layer containing the photocatalyst and the second layer containing the inorganic porous material separately, the first layer that decomposes the adsorbed molecules and the second layer that plays the role of adsorbing the molecules. Antibacterial and hazardous substances contained in the first layer so that the odor components adsorbed once on the inorganic porous material contained in the second layer and VOCs such as formaldehyde do not leak into the room The density of the degradable substance can be adjusted. Here, if a substance having ion exchange ability is selected as the inorganic porous material that is the main raw material of the second layer, the polar substance is retained by ion exchange. Therefore, due to external factors such as temperature rise, When VOC molecules once adsorbed from the inorganic porous material in the two layers are re-released, compared to adsorption (physical adsorption) caused by intermolecular attraction, which is said to be a relatively weak interaction such as charcoal, It is expected that the desorption rate of molecules from the second layer can be reduced. In the present invention, natural mineral belonging to the zeolite genus as the inorganic porous pledge membrane having an ion exchange capacity (hereinafter, referred to as natural zeolite) used as a main component. The second layer coating material to the natural zeolite as the main component in addition to the natural zeolite sepiolite, shirasu, diatomaceous earth, may be added to lightweight aggregates such as glass balloons.
第2層塗材中には無機多孔質物質を含有させているため、元来ある程度の断熱性を有するが、必要に応じて、セピオライト、シラス、珪藻土、ガラスバルーンなどの軽量骨材を第2層塗材に加え第2層に空隙がより多く含まれるように構成すれば、塗材の昇温を抑制し、温度上昇に伴う第2層の無機多孔質物質に吸着した分子の脱着速度をさらに低下させることが可能となる。ここで軽量骨材の最大粒径は、第2層塗材における塗厚の範囲内であるものとする。 Since the second layer coating material contains an inorganic porous material, it originally has a certain degree of heat insulation, but if necessary, lightweight aggregates such as sepiolite, shirasu, diatomaceous earth, and glass balloons can be used as the second layer. If the second layer is configured to include more voids in addition to the layer coating material, the temperature rise of the coating material is suppressed, and the desorption rate of molecules adsorbed on the inorganic porous material of the second layer accompanying the temperature increase is increased. Further reduction is possible. Here, the maximum particle size of the lightweight aggregate is assumed to be within the range of the coating thickness in the second layer coating material.
第1層塗材の抗菌・有害物分解性物質としては抗菌活性及び有害物分解能を有するものであればよいが、好ましいものの一例としては酸化チタン光触媒が挙げられる。酸化チタン光触媒は紫外線照射等の特殊な処理を施さずとも励起することが好ましく、室内照明、すなわち一般的な蛍光灯及びLEDが発する可視光(400〜800nm)で励起され、触媒機能を発揮する可視光応答型酸化光触媒を用いると、北側に面する部屋等であっても日当たりが悪いために光触媒活性が低く発明の効果が得られないといったことがなく好ましい。この抗菌・有害物分解性物質とバインダーとを混合することにより第1層を形成するが、バインダーとしてはシリカゾル、アルミナゾル、アルミナセメントのような無機質バインダーを用いる。また、前記の第1層で使用するバインダーに代えて少量の第2層塗材を用いてもよく、その場合は第2層の上に薄く塗布して形成するとよい。この方法によると、既に調整した第2層塗材の一部をそのまま第1層塗材のバインダーに流用できるので、新たに第1層塗材用にバインダーを調整する必要がなく、作業工程の簡略化、低コスト化を図ることができる。 The antibacterial / hazardous substance decomposable substance of the first layer coating material may be any substance having antibacterial activity and harmful substance resolution, and a preferable example is a titanium oxide photocatalyst. The titanium oxide photocatalyst is preferably excited without being subjected to a special treatment such as ultraviolet irradiation, and is excited by visible light (400 to 800 nm) emitted from indoor lighting, that is, a general fluorescent lamp and LED, and exhibits a catalytic function. Use of a visible light responsive oxidation photocatalyst is preferred because even in a room facing the north side, the sunlight is poor and the photocatalytic activity is low and the effects of the invention cannot be obtained. The first layer is formed by mixing this antibacterial / hazardous substance decomposable substance and a binder. As the binder, an inorganic binder such as silica sol, alumina sol, or alumina cement is used. In addition, a small amount of the second layer coating material may be used instead of the binder used in the first layer, and in that case, it may be formed by thinly coating on the second layer. According to this method, since a part of the already adjusted second layer coating material can be used as it is for the binder of the first layer coating material, there is no need to newly adjust the binder for the first layer coating material. Simplification and cost reduction can be achieved.
第2層塗材において用いられる無機多孔質物質は物理吸着により気体分子を吸着するものであればよく、そのようなものの一例としては発泡セメント、珪藻土、シリカゲル、アロフェン、活性炭、天然ゼオライトが挙げられる。そのなかでも天然ゼオライトの日本国内での生産量は月間数十万トンにものぼり、比較的安価で手に入ることに加えて、陽イオン交換能、分子ふるい作用を有し、塊状で産出し、破砕により必要とする粒度を得るのに加工しやすいため、本発明では特に天然ゼオライトを主成分とする。特に上述のようにイオン交換によって物理吸着よりも強く極性分子を吸着すると考えられるため、物理吸着に比して室温上昇による分子の脱着速度を低下させることができる点で好ましい。天然ゼオライトの結晶構造の大半は解析されており、4、5、6、7及び12員の4面体環のつくる輪が互いに連結してトンネルとカゴを形成していることを特徴とする。トンネルの最小の幅はそれを通すことのできる1分子の最大直径の近似的な尺度となっているため、吸着させたい目的の分子のサイズ及び分子を吸着できる最大容量に応じて適宜、天然沸石族に属する鉱物を選択すればよい。特に好ましいものとしてはクリノプチロライト、モルデナイトが挙げられる。 The inorganic porous material used in the second layer coating material only needs to adsorb gas molecules by physical adsorption , and examples of such materials include foamed cement, diatomaceous earth, silica gel, allophane, activated carbon, and natural zeolite. . Among them, the production of natural zeolite in Japan is several hundred thousand tons per month. In addition to being available at a relatively low cost, it has a cation exchange capacity and molecular sieving action, and is produced in bulk. , workable other Me to obtain a particle size that required by crushing, and especially composed mainly of natural zeolite in the present invention. In particular, it is considered that polar molecules are adsorbed more strongly than physical adsorption by ion exchange as described above, which is preferable in that the desorption rate of molecules due to an increase in room temperature can be reduced as compared with physical adsorption. Most of the crystal structure of natural zeolite has been analyzed and is characterized by the fact that the rings formed by 4, 5, 6, 7 and 12-membered tetrahedral rings are connected to each other to form a tunnel and a cage. The minimum width of the tunnel is an approximate measure of the maximum diameter of a molecule that can pass through it, so that natural zeolites are appropriately selected according to the size of the target molecule to be adsorbed and the maximum capacity to adsorb the molecule. Select a mineral belonging to the group. Particularly preferred are clinoptilolite and mordenite.
また、光酸化触媒活性が低下する暗所での抗菌機能を補う目的で第1層塗材中に抗菌性物質としての無機質抗菌材を含有する複合内装塗材及び複合内装塗材構造としてもよい。無機質抗菌剤としては例えば銀、銅、亜鉛等が挙げられる。これらの無機質抗菌剤を使用する場合には、上述の無機多孔質物質に担持させて使用する。 Moreover, it is good also as a composite interior coating material and the composite interior coating material structure which contain the inorganic antibacterial material as an antibacterial substance in the 1st layer coating material in order to supplement the antibacterial function in the dark where photooxidation catalyst activity falls. . Examples of inorganic antibacterial agents include silver, copper, and zinc. When these inorganic antibacterial agents are used, they are used by being supported on the above-mentioned inorganic porous material.
第2層には必要に応じて施工時の作業性を考慮して、可塑性を上げてひび割れ防止し、塗材の磨耗強度を向上させるためのバインダーを第2層塗材中に含有させてもよい。バインダーとして好ましいものとしては、カオリン族(カオリナイト、ハロサイト等)、スメクタイト族(モンモリロナイト等)、バーミキュライト族、タルク族、雲母族、緑泥石族、アタパルジャイト、セピオライト、珪酸アルミニウムマグネシウムなどの無機化合物からなるバインダー若しくはカルボキシメチルセルロース、アルギン酸ナトリウム、ポリビニルアルコール、カラギーナンなど親水性有機化合物若しくは炭素繊維、ガラス繊維、金属繊維等の無機質繊維又は石油系繊維(ナイロン、ビニロン等)、天然繊維等の有機質繊維材料が挙げられる。これらのバインダーを適宜、第2層中に配合することでひび割れの防止、塗材の磨耗強度の向上が図られ、また塗材は粘性のあるスラリー状となり施工性を向上することができる。また、塗材が硬化し難い場合はセメント、硫酸カルシウム、水酸化カルシウム、炭酸カルシウム、珪酸カルシウム等の親水性硬化剤を添加してもよい。 In consideration of workability during construction, the second layer may contain a binder for increasing the plasticity to prevent cracking and improving the wear strength of the coating material. Good. Preferred binders include inorganic compounds such as kaolin group (kaolinite, halosite, etc.), smectite group (montmorillonite, etc.), vermiculite group, talc group, mica group, chlorite group, attapulgite, sepiolite, and aluminum magnesium silicate. Binder or carboxymethyl cellulose, hydrophilic organic compounds such as sodium alginate, polyvinyl alcohol, and carrageenan, or inorganic fibers such as carbon fibers, glass fibers, and metal fibers, or petroleum fibers (nylon, vinylon, etc.), and organic fiber materials such as natural fibers. Can be mentioned. By appropriately blending these binders in the second layer, cracking can be prevented and the wear strength of the coating material can be improved, and the coating material can be made into a viscous slurry to improve workability. If the coating material is difficult to cure, a hydrophilic curing agent such as cement, calcium sulfate, calcium hydroxide, calcium carbonate, calcium silicate may be added.
前記の第1層塗材及び第2層塗材に用いて、まず下地材に対して内面塗材としての吸着吸放湿能を有する無機多孔質物質を含有する第2層塗材を塗装し、次いで表面塗材としての抗菌・有害物分解性物質を含有する第1層塗材を塗布して仕上げることで建造物用複合内装塗材を提供することができる。 Using the first layer coating material and the second layer coating material, first, a second layer coating material containing an inorganic porous material having an adsorption / desorption capacity as an inner surface coating material is applied to the base material. Then, a composite interior coating material for buildings can be provided by applying and finishing a first layer coating material containing an antibacterial / hazardous substance decomposable substance as a surface coating material.
第1層に含まれる抗菌・有害物分解性物質による酸化反応速度が第2層からの臭気成分、VOCの放出速度に比して小さいために、室内に未分解の臭気成分、VOCが拡散してしまい、かえって室内の臭気成分、VOCの濃度が高くなってしまうこと防ぐことができ、室内側に臭気成分、VOCを再放出することのない建造物用複合内装塗材を提供することができる。 Since the oxidation reaction rate by the antibacterial / hazardous substance decomposable substance contained in the first layer is smaller than the odor component and VOC release rate from the second layer, undegraded odor component and VOC diffuse in the room. Therefore, it is possible to prevent the indoor odor component and VOC from becoming high in concentration, and to provide a composite interior coating material for buildings that does not re-release the odor component and VOC to the indoor side. .
第1層に含まれる抗菌・有害物分解性物質の作用により第2層に含まれる無機多孔質物質に吸着・捕捉された分子が分解される。これにより、無機多孔質物質の細孔が吸着した分子で飽和して一定量以上の分子を吸着できなくなることを防ぐことができる。 Molecules adsorbed and trapped in the inorganic porous substance contained in the second layer are decomposed by the action of the antibacterial / hazardous substance-degrading substance contained in the first layer. Thereby, it can prevent that the pore of an inorganic porous material is saturated with the molecule | numerator which adsorb | sucked, and it becomes impossible to adsorb | suck a molecule | numerator more than a fixed amount.
光及び外気と接触する第1層として殺菌・有害物分解層を形成することにより、内面構造中に無駄に抗菌材、光触媒を使用することが無くなるので製造コストの低減を図ることができる。また、光触媒を表層のみに配置することにより光触媒の機能を十分発揮させることができる。 By forming the sterilization / hazardous substance decomposition layer as the first layer in contact with light and outside air, the use of antibacterial materials and photocatalysts in the inner surface structure is eliminated, so that the manufacturing cost can be reduced. Moreover, the function of a photocatalyst can be sufficiently exhibited by disposing the photocatalyst only on the surface layer.
第2層の室内側の表面は、後述のように粗粒の無機多孔性物質を配合することにより凹凸状になる。凹凸状の第2層に被覆される第1層は摩擦により経時的にある程度消失してしまうが、その凹部に塗着させた殺菌・有害物分解層は磨耗等で失われることはない。それにより半永久的に吸放湿材に吸着された臭い成分、VOCなどを分解すると共に、壁表面の汚れ防止やカビ類の発生防止の機能を果たすことができる。 The indoor-side surface of the second layer becomes uneven when a coarse inorganic porous material is blended as will be described later. The first layer covered with the uneven second layer disappears to some extent due to friction, but the sterilization / hazardous substance decomposition layer applied to the recess is not lost due to wear or the like. Thereby, the odor component adsorbed by the moisture absorbing / releasing material, VOC and the like can be decomposed, and the function of preventing the contamination of the wall surface and the generation of molds can be achieved.
本発明に係る建造物用複合内装塗材は、単にそれぞれの塗材を混合して一層にして用いた場合に比較して、湿度の調整や臭気の緩和、有害物質の分解などより複合的な機能をより効率的に発揮することができる。これらの湿度調整や臭気や有害物質の除去・抗菌性の機能は上述のとおり持続性があり、特に化学物質過敏症者のいる世帯や感染症が生じやすい高齢者施設や医療施設向けに最適である。また、結露によるカビの発生や、過乾燥状態によるアレルギー症状及び喘息症状の悪化の対策として有効であり、住環境改善に大いに貢献するものである。 The composite interior coating material for buildings according to the present invention is more complex than adjusting the humidity, reducing odor, decomposing harmful substances, etc. Functions can be exhibited more efficiently. These humidity adjustment, removal of odors and harmful substances, and antibacterial functions are sustainable as described above, and are especially suitable for households with chemical-sensitive people and elderly facilities and medical facilities that are susceptible to infectious diseases. is there. In addition, it is effective as a countermeasure against the occurrence of mold due to condensation, and worsening of allergic symptoms and asthma symptoms due to overdried conditions, and greatly contributes to the improvement of the living environment.
以下、本発明の実施例につき、具体的に説明する。図1は本発明の建造物用複合内装塗材の断面を模式化して示した図である。 Examples of the present invention will be specifically described below. FIG. 1 is a diagram schematically showing a cross section of a composite interior coating material for buildings of the present invention.
木材(構造用合板、ベニヤ板等)、モルタル、石膏ボード等の下地材1に骨材、有機系又は無機系バインダーからなる下地接着材を厚さ5mm以下程度にまんべんなく鏝、エアブラシ、刷毛などで塗りつけ第3層2とする。下地材1に接する第3層2はシリコン樹脂、アクリル樹脂などの有機系若しくはセメント、石膏などの無機系の硬化接着機能を持つバインダーを主成分とし、第2層3を下地材1に接着させる役割を果たす。さらに、できるだけ透湿性、ガス透過性の小さい材料を用いることにより、下地材1から第2層3への湿気、臭気・揮発性の有害化学物質の移動を遮断することができる。このとき、もし下地材1に第2層3が必要とされる強度で接着可能な場合、また下地材1より揮発性の有害物質飛散のおそれがない場合、その飛散量が極小である場合、下地材1より第2層3へ木材アク等の溶出によるシミが生じず外観を損ねるおそれがない場合等には第3層2は省いても構わない。そのような下地材の材料としては石膏ボード等が挙げられる。
Apply base adhesive material made of aggregate, organic or inorganic binder to
下地材1の上に直接、又は上述の手順で下地材の上に積層した第3層2の上に室内のVOC、臭気成分等を吸着し、かつ吸湿・放湿する能力(吸着吸放湿能)を有する第2層塗材を鏝、刷毛等にて塗布することにより第2層3を形成する。ここで第2層3の主原料としては1種類以上の無機多孔質物質5を用いる。無機多孔質物質5として好ましいものの一例としては発泡セメント、珪藻土(ダイアトマイト)、シリカゲル、アロフェン、活性炭、合成沸石、天然ゼオライト等が挙げられる。天然ゼオライトの中でも、モルデン沸石及びクリノプチロライトは塊状で多く産出し、破砕等で加工が容易であり本発明で使用する無機多孔質物質として好ましい。これら無機多孔質物質5から、天然ゼオライトが主成分となるように、少なくとも1種類以上を選択し破砕機、分級機等により粗粒(200μm以上)と微粒(200μm以下)に加工した後、必要に応じて施工時の作業性を考慮して、可塑性を上げてひび割れ防止し、塗材の磨耗強度を向上させるためのバインダーを配合して第2層塗材とし、第3層2の表面若しくは下地材1に厚さ1mm〜20mmになるように鏝、刷毛、エアブラシ等を用いて該塗材を塗り、吸着吸放湿能を有する第2層3を形成する。ここで上記の親水性硬化剤、有機系バインダー、無機系バインダー、無機質繊維又は有機質繊維の全てについて必ずしも添加する必要はないが、塗布性の向上、養生後のクラック防止、下地への接着性の向上、塗材の耐磨耗性の向上等の目的に応じて、それぞれを適宜選択して配合することで、第2層塗材の品質をコントロールすることができる。バインダーとして好ましいものとしては、カオリン族(カオリナイト、ハロサイト等)、スメクタイト族(モンモリロナイト等)、バーミキュライト族、タルク族、雲母族、緑泥石族、アタパルジャイト、セピオライト、珪酸アルミニウムマグネシウムなどの無機化合物からなるバインダー若しくはカルボキシメチルセルロース、アルギン酸ナトリウム、ポリビニルアルコール、カラギーナンなど親水性有機化合物若しくは炭素繊維、ガラス繊維、金属繊維等の無機質繊維又は石油系繊維(ナイロン、ビニロン等)、天然繊維等の有機質繊維材料が挙げられる。これらのバインダーを適宜、第2層3中に配合することでひび割れの防止、塗材の磨耗強度の向上が図られ、また塗材は粘性のあるスラリー状となり施工性を向上することができる。また、塗材が硬化し難い場合はセメント、硫酸カルシウム、水酸化カルシウム、炭酸カルシウム、珪酸カルシウム等の親水性硬化剤を添加してもよい。また、無機多孔質物質5の粗粒と微粉末の粒度配合比を適宜調整することにより、第2層3の断面を凸凹状に形成することができるし、後述の抗菌・有害物分解性物質6を含む第1層4を塗り重ねる基礎となる第2層3の表面は、抗菌・有害物分解性物質6を含む第1層塗材の塗着を高めるために粗粒の無機多孔質物質5又は適当な粒径の川砂など骨材となるものを配合することにより凹凸状としてもよい。また、塗材の着色を目的として黄土や炭などの天然材料、弁柄などの無機顔料を用いてもよい。このようにして形成した第2層3の主原料である無機多孔質物質5は、室内の湿度調整並びに室内の湿気、臭気成分及び有害化学物質を吸着する役割を果たす。また、第2層塗材中には無機多孔質物質5を含有させているため、元来ある程度の断熱性を有するが、必要に応じて、セピオライト、シラス、珪藻土、ガラスバルーンなどの軽量骨材7を第2層塗材に加え第2層3に空隙がより多く含まれるように構成すれば、塗材の昇温を抑制し、温度上昇に伴う第2層3の無機多孔質物質5に吸着した分子の脱着速度をさらに低下させることが可能となる。ここで軽量骨材7の最大粒径は、第2層塗材における塗厚の範囲内であるものとする。
Ability to adsorb indoor VOCs, odorous components, etc., and absorb and release moisture directly on the
続いて、第2層3の室内側表面に抗菌・有害物分解性物質6としての酸化チタン光触媒とバインダーとからなる第1層塗材を塗布して、抗菌・有害物分解能を有する第1層4を形成する。ここで酸化チタン光触媒の添加量については0.01〜20g/m2を目安として、酸化チタン光触媒の濃度を増減させて室内に臭気成分やVOCが漏れ出さないようにその濃度を適宜、調節すればよいが、より高い抗菌・有害物分解能が求められる場合、後述のように第2層塗材を無機質バインダーに代えて添加する場合等においては上記の目安を超えて酸化チタン光触媒を添加してもよい。また、暗所での抗菌機能が望まれる場合にはさらに銀イオン、銅イオン、亜鉛イオンなどの無機質抗菌剤を抗菌・有害物分解性物質6として第1層塗材に添加するとよい。このとき、バインダーとしてシリカゾル、アルミナゾル、アルミナセメントのような無機質バインダーを用いるが、抗菌・有害物分解性物質6を施工現場に入る前にあらかじめバインダーと混合して塗布してもよいし、施工現場にて抗菌・有害物分解性物質6をバインダーに加えても良い。また、第2層塗材を上記の無機質バインダーに代えて添加してもよい。第1層4中の酸化チタン光触媒は明所にて抗菌性を示すほか、第2層3が吸着した室内の臭気成分やVOCを分解する機能を有する。これにより、本発明の複合内装塗材は細孔の飽和により室内の臭気成分やVOCの吸着性能が落ちることなく、継続的にその機能を発揮することができる。また、第1層4中に無機質抗菌材を添加した場合は、暗所でも抗菌性能を発揮し、常に衛生的な室内環境を提供できる。
Subsequently, a first layer coating material composed of a titanium oxide photocatalyst as an antibacterial / hazardous substance
以下、実施例により更に具体的に説明する。 Hereinafter, the present invention will be described more specifically with reference to examples.
実施例1
合板あるいはモルタル表面あるいは仕上げの塗装膜の上に直接、表1に示される配合比(グラム)でそれぞれの原料を混合した後、同量の水を加水し、鏝で塗布して第2層を、厚さ3.0mmで施工した。結果、サンプル1〜8のいずれにおいても混練および鏝による施工が可能であった。なかでもサンプル1〜6については、いずれも鏝で施工が容易であり、さらにサンプル2〜4については、最も施工性に優れていた。さらに、サンプル2〜8については養生後(養生温度は室温)もクラックは発生せず、表面の耐磨耗性も問題はなかった。サンプル1については、微粒が多いため加水量が足りず、乾燥後クラックが発生したが、表面の粉落ちは問題なかった。なお、ここで無機多孔質物質としてはモルデナイトを主原料として選択し、クラッシャー粉砕・風力分級により得られた200μm以上の粗粒と200μm以下の微粒について前記塗材に配合した。またバインダーとしてカオリン属無機鉱物および天然繊維を使用し、親水性硬化剤としては水酸化カルシウムを使用した。なお、本願発明はこの実施例に限定して理解されるべきではない。
Example 1
After mixing each raw material directly on the plywood or mortar surface or the finished coating film with the blending ratio (grams) shown in Table 1, add the same amount of water, apply with boil and apply the second layer. The construction was performed with a thickness of 3.0 mm. As a result, in any of
実施例2
表1のサンプル2と同じ原料配合比で、厚さ12.5mm、面積9cm2のプラスターボード下地に塗材を鏝で塗り付け、塗布厚3mmのサンプルを作成した。その表面に、酸化
チタン重量が1m2当たり10gになるよう可視光応答型光触媒を塗布したものを作成し「サンプル+光触媒」(Run.4)とした。比較対象のためにブランクとしてテドラーバッグに何も入れないもの(Run.1)、プラスターボード下地のみ(Run.2)、光触媒を塗布しないサンプル(Run.3)を用意した。それぞれを容積5リットルのテドラーバッグに入れ、アセトンを2μリットル注入後直ちにアセトンガスの濃度をガスクロマトグラフにより測定した。その後、適宜1μリットルずつアセトンを追加しながら温度20℃、照度1200ルクスの条件で蛍光灯照射を行いながらサンプルを静置し、アセトンガス濃度の経時的変化をガスクロマトグラフにより測定した。結果は表2及び図2にまとめたとおりであるが、比較対照のためのRun.1及びRun.2では、アセトンガスの吸着が起こらず、アセトン注入量に比例しアセトン濃度が上昇していることが確認された。Run.3およびRun.4では、当初塗材によるアセトンガスの吸着がありガス濃度はRun.1およびRun.2に比べ低く、同程度の濃度で推移しているが、光照射400時間を境に光触媒を塗布したもの(Run.4)が塗布していないもの(Run.3)に比べてガス濃度が低減したことを確認できた。
Example 2
A coating material was applied to a plaster board base having a thickness of 12.5 mm and an area of 9 cm 2 at the same raw material mixing ratio as that of
実施例3
表1のサンプル2と同じ原料配合比で、Run.1〜2までの塗材原料を準備した。そして、酸化チタン重量が1m2当たり1gになるよう可視光応答型光触媒を調整し、塗材原料塗布後、表面に光触媒を塗布したもの(Run.1)、光触媒を予め原料塗材中に混ぜ込んで塗布したもの(Run.2)について、それぞれ厚さ9.5mm、面積100cm2のプラスターボード下地に塗材を鏝で塗り付け、塗布厚3mmのサンプルを作成した。それぞれを養生乾燥したのち、容積5リットルのテドラーバッグに入れ、アセトンを50μリットル注入後暗所に5日間静置し、アセトンガスの濃度をガスクロマトグラフにより測定した。その後、温度20℃、照度1200ルクスの条件で45時間蛍光灯照射を行い、アセトンガスの濃度変化をガスクロマトグラフにより測定した。結果は表3にまとめたとおりであるが、光触媒を表面に塗布したRun.1は、塗材に光触媒を全量練りこんだRun.2に比べ、アセトン濃度が大幅に低下していることが確認できた。なお、アセトンガス残存率(%)=照射後/照射前×100にて算出した。
Example 3
In the same raw material blending ratio as
参考例1
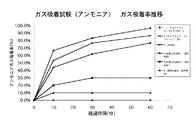
表1のサンプル2と基本的に同じ配合比で、そのうちモルデナイト量について表4に示される配合比(グラム)に置き換えたサンプルをRun.1、2として作成した。また、消石灰および珪藻土を主成分にした既存商品(Run.4〜6)を、それぞれの原料を混合した後、厚さ9.5mm、面積9cm2のプラスターボード下地に塗材を鏝で塗り付け、塗布厚3mmのサンプルを作成した。それぞれを養生乾燥したのち、容積5リットルのテドラーバッグに入れ、濃度15〜20ppmのアンモニアガスを封入し、直ちにアンモニアガスの濃度を検知管により測定した。このとき、比較としてテドラーバックに何も入れないもの(Run.4)も作成した。その後、初期濃度からのアンモニアガス吸着率の経時的変化を検知管により測定した。結果は表4及び図3にまとめたとおりであるが、沸石属であるモルデナイトを塗材の主原料とした場合(Run.1)、最もアンモニアガスの吸着率が高く、モルデナイトを他の無機多孔質物質であるパーライトに置き換えた場合(Run.2)においては、吸着率がやや低下した。また、漆喰系および珪藻土系既存品(Run.4〜6)については、総じて沸石(モルデナイト)を使用したときよりアンモニアガスの吸着率が低下した。
Reference example 1
A sample in which the blending ratio was basically the same as that of
参考例2
表5に示される配合比(グラム)でそれぞれの原料を混合した後、厚さ12.5mmのプラスターボード下地に塗材を鏝で塗り付け、塗布厚3mmのサンプルを作成した。結果は表5にて示しているが、養生後目視にて確認を行ったところ、天然繊維を含まないRun.2については乾燥後クラックが発生した。また、各サンプルについての付着強度を試験した結果、無機バインダーを添加したRun.1について、最もよい結果が得られた。なお、付着強度の試験はJIS A 6909(建築用仕上塗材)の付着強度の試験方法に準じて行った。
Reference example 2
After mixing each raw material with the compounding ratio (gram) shown in Table 5, a coating material was applied with a scissors on a 12.5 mm thick plaster board substrate to prepare a sample having a coating thickness of 3 mm. The results are shown in Table 5, but when visually confirmed after curing, Run. For No. 2, cracks occurred after drying. In addition, as a result of testing the adhesion strength for each sample, Run. For 1 the best result was obtained. The adhesion strength test was performed in accordance with the adhesion strength test method of JIS A 6909 (finishing coating material for construction).
表1のサンプル2と同じ原料配合比で、Run.1の塗材原料を準備した。そして、厚さ9.5mm、面積9cm2のプラスターボード下地に塗材を鏝で塗り付け、塗布厚3mmのサンプルを作成した。あわせて比較対照として、表6のRun.3〜5に示すサンプルを準備した。それぞれを養生乾燥したのち、第2層の下地に面する側(サンプル下部)をホットプレートにて62.5℃に加熱し、第1層の室内側の表面(サンプル上部)の温度変化を継続的に測定した。結果は表6にまとめたとおりであるが、塗材を塗布しなかったRun.2に比べ、Run.1はサンプル上部の温度上昇が抑えられている。またRun.3〜5といった既存の内装材に比べても、塗材の温度上昇抑制効果は大きく、高い断熱性を有することが明らかとなった。
In the same raw material blending ratio as
参考例3
表1のサンプル3と同じ原料配合比で、面積9cm2のプラスチック板に塗材を鏝で塗り付け塗布厚5mmのサンプルを作成した。そして、同サンプルを容積10リットルのテドラーバッグに入れたもの、また対照として何も入れなかったもの(Blank)について、それぞれ対象ガスを初期濃度10〜12ppmになるように封入した後、検知管によりガス吸着試験を経時的に行った。結果を表7に記す。
Reference example 3
A sample having a coating thickness of 5 mm was prepared by applying the coating material on a plastic plate having an area of 9 cm 2 with the same raw material blending ratio as
表7によると、代表的な臭気物質であるアンモニアと硫化水素、またシックハウス症候群の原因物質といわれるホルムアルデヒドのいずれについても、速やかにガス濃度の低下が確認された。 According to Table 7, a decrease in gas concentration was quickly confirmed for both ammonia and hydrogen sulfide, which are representative odor substances, and formaldehyde, which is a causative substance of sick house syndrome.
このように本発明を適用すれば、無機多孔質物質への臭気成分、VOC等の分子の吸着・脱着反応と抗菌・有害物分解性物質による該分子の分解反応を協調せしめ、一度、無機多孔質物質に吸着した臭気成分、VOC等の分子が室内に再度放出されることのない建造物用複合内装塗材及び建造物用複合内装塗装構造を提供することができ、さらには、第1層の抗菌・有害物分解性物質により無機多孔質物質の細孔が吸着した分子を分解することにより、細孔を飽和させることなくセルフクリーニングできる能力を有する建造物用複合内装塗材及び建造物用複合内装塗装構造を提供することができる。 As described above, by applying the present invention, the adsorption / desorption reaction of molecules such as odor components and VOCs on the inorganic porous material and the decomposition reaction of the molecule by the antibacterial / hazardous substance decomposable substance are coordinated. It is possible to provide a composite interior coating material for a building and a composite interior coating structure for a building in which molecules such as odor components and VOCs adsorbed on the material are not released again into the room, and further, the first layer For composite interior coating materials for buildings and structures that have the capability of self-cleaning without saturating the pores by decomposing the molecules adsorbed by the pores of the inorganic porous material with the antibacterial and harmful substance-degrading substance A composite interior paint structure can be provided.
1 下地材
2 第3層
3 第2層
4 第1層
5 無機多孔質物質
6 抗菌・有害物分解性物質
7 軽量骨材
DESCRIPTION OF
Claims (4)
第1層塗材は、抗菌・有害物質分解性物質として酸化チタン光触媒と、バインダーとを含有し、
第2層塗材は、無機多孔質物質とバインダーを含有し、無機多孔質物質は物理吸着能及びイオン交換能を有する細粒と粗粒の天然ゼオライトの混合物を主成分とするものであり、
前記の細粒と粗粒は天然ゼオライトを破砕した後で風力分級したものであり、細粒に対して粗粒を重量比で2/3倍量から1.5倍量配合して細粒と粗粒の天然ゼオライトの混合物とし、
該細粒と粗粒の天然ゼオライトの混合物を第2層塗材の主成分として配合することにより表面を凹凸状にした建造物用複合内装塗材。 It consists of a first layer coating material containing an antibacterial / hazardous substance decomposable substance as a surface coating material, and a second layer coating material containing an inorganic porous material having adsorption / absorption / release properties as an inner surface coating material. A composite interior coating material for buildings,
The first layer coating material contains a titanium oxide photocatalyst as an antibacterial / hazardous substance decomposable substance and a binder,
The second layer coating material contains an inorganic porous material and a binder, and the inorganic porous material is mainly composed of a mixture of fine and coarse natural zeolite having physical adsorption capacity and ion exchange capacity,
The fine particles and coarse particles are those obtained by crushing natural zeolite and then air-classified, and the fine particles are mixed with 2 to 1.5 times the amount of coarse particles by weight. A mixture of coarse natural zeolite,
Building composite interior coating material in which the surface uneven by blending a mixture of natural zeolites of the fine and coarse grains as a main component of the second Sonurizai.
第2層塗材中のバインダーは、カオリン系無機鉱物、天然繊維及び親水性硬化剤の混合物である請求項1記載の建造物用複合内装塗材。 The binder in the first layer coating material is an inorganic binder,
The binder in the second Sonuri material, kaolin inorganic mineral mixture der Ru請 Motomeko 1 building composite interior coating material according natural fibers and hydrophilic curing agent.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2008083902A JP5510911B2 (en) | 2007-08-17 | 2008-03-27 | Composite interior coating material for buildings |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2007212774 | 2007-08-17 | ||
| JP2007212774 | 2007-08-17 | ||
| JP2008083902A JP5510911B2 (en) | 2007-08-17 | 2008-03-27 | Composite interior coating material for buildings |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2009068324A JP2009068324A (en) | 2009-04-02 |
| JP2009068324A5 JP2009068324A5 (en) | 2011-05-12 |
| JP5510911B2 true JP5510911B2 (en) | 2014-06-04 |
Family
ID=40604869
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008083902A Active JP5510911B2 (en) | 2007-08-17 | 2008-03-27 | Composite interior coating material for buildings |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP5510911B2 (en) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4766180B2 (en) * | 2009-05-13 | 2011-09-07 | 日立化成工業株式会社 | Adhesive composition |
| KR101194992B1 (en) | 2010-07-01 | 2012-11-05 | 박영웅 | The Methods and the Baking Paints to Make Clean Coatings by using the Porous Particles Containing Water as Additives |
| CN105113730B (en) * | 2015-07-21 | 2018-03-20 | 平江县长城建筑工程有限公司 | Anticracking protects the water-based dry powder building colour-wash external wall construction method of the accumulation of salt in the surface soil of color Diamond Search zero |
| JP7084693B2 (en) * | 2016-03-08 | 2022-06-15 | 日鉄鋼板株式会社 | Light utilization space system |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH07188487A (en) * | 1993-12-27 | 1995-07-25 | Kyowa Leather Cloth Co Ltd | Vinyl chloride resin composition, sheet and decorative wall material |
| JPH09231821A (en) * | 1995-12-22 | 1997-09-05 | Toto Ltd | Luminaire and method for maintaining illuminance |
| JP2000096799A (en) * | 1998-09-17 | 2000-04-04 | Osaka Gas Co Ltd | Wall material and its execution |
| JP2002138376A (en) * | 2000-10-26 | 2002-05-14 | Toto Ltd | Interior material |
| JP2003033903A (en) * | 2001-07-25 | 2003-02-04 | Toppan Printing Co Ltd | Decorative material |
| JP4575759B2 (en) * | 2003-12-04 | 2010-11-04 | エスケー化研株式会社 | Laminated body |
| JP2007085118A (en) * | 2005-09-26 | 2007-04-05 | Matsushita Electric Works Ltd | Interior finish member |
| JP2007204966A (en) * | 2006-01-31 | 2007-08-16 | Dainippon Printing Co Ltd | Decorative sheet and decorative plate using the same |
| JP2008038365A (en) * | 2006-08-02 | 2008-02-21 | Mitsui Mining Co Ltd | Interior finishing wall of building, and finishing coating material therefor |
-
2008
- 2008-03-27 JP JP2008083902A patent/JP5510911B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| JP2009068324A (en) | 2009-04-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5510911B2 (en) | Composite interior coating material for buildings | |
| JP3334767B2 (en) | Building materials with moisture absorption and release functions | |
| JP2015519408A (en) | Environmentally-friendly water-based paint composition for interior finishing materials of buildings | |
| JP2009068324A5 (en) | ||
| JP2008038365A (en) | Interior finishing wall of building, and finishing coating material therefor | |
| KR20110096652A (en) | Radon reduction functional inorganic paint composition | |
| JP6584115B2 (en) | Complex for adsorbing and absorbing chemical substances and method for producing the same | |
| JP4575759B2 (en) | Laminated body | |
| KR101044239B1 (en) | Adhesive composition contained charcoal for construction materials | |
| KR101082012B1 (en) | Furniture having antimicrobial and air-puritying functions panel | |
| JP5136872B2 (en) | Interior finish with humidity control and paintability, as well as formaldehyde reduction | |
| JP3848605B2 (en) | Building material having environmental improvement function and method for manufacturing the same | |
| KR20120043459A (en) | Composition for inner and outer building materials | |
| JP2002321303A (en) | Deodorizing humidity conditioning sheet material | |
| JP5051985B2 (en) | Manufacturing method of building material having adsorption function and photocatalytic function | |
| JP2009002145A (en) | Plate-like ventilation and lamination material | |
| JP3970012B2 (en) | Raw material composition for coating materials | |
| JP2004285716A (en) | Functional building material | |
| KR101683100B1 (en) | Manufacturing method for furniture member for furniture containing ocher and vermiculite, and furniture member manufactured by the same | |
| KR20060056201A (en) | Material for reducing the sick house syndrome | |
| JP2005105010A (en) | Inorganic coating material and voc-adsorbing functional material using the same | |
| JP2007153627A (en) | Production method of zeolite building material | |
| KR101138230B1 (en) | Furniture having antimicrobal and air-purifying functions and furniture manufacturing method thereof | |
| KR101625903B1 (en) | A green painting composite of structure for air purifying | |
| KR101394694B1 (en) | Construction panel for purifiyng indoor air and manufacturing method thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110325 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20110325 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20120711 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120724 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120924 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20120925 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130625 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130826 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20130828 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20140225 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20140318 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5510911 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |