JP5343092B2 - 細胞分離を行う精密濾過の方法及び装置 - Google Patents
細胞分離を行う精密濾過の方法及び装置 Download PDFInfo
- Publication number
- JP5343092B2 JP5343092B2 JP2010545073A JP2010545073A JP5343092B2 JP 5343092 B2 JP5343092 B2 JP 5343092B2 JP 2010545073 A JP2010545073 A JP 2010545073A JP 2010545073 A JP2010545073 A JP 2010545073A JP 5343092 B2 JP5343092 B2 JP 5343092B2
- Authority
- JP
- Japan
- Prior art keywords
- microfiltration
- porous
- membrane
- pores
- patch
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000001471 micro-filtration Methods 0.000 title claims abstract description 92
- 238000000926 separation method Methods 0.000 title claims description 26
- 238000000034 method Methods 0.000 title abstract description 61
- 210000004027 cell Anatomy 0.000 claims abstract description 204
- 239000011148 porous material Substances 0.000 claims abstract description 167
- 239000012528 membrane Substances 0.000 claims abstract description 142
- 208000005443 Circulating Neoplastic Cells Diseases 0.000 claims abstract description 46
- 229920000052 poly(p-xylylene) Polymers 0.000 claims abstract description 42
- 238000001914 filtration Methods 0.000 claims description 53
- 239000012530 fluid Substances 0.000 claims description 21
- -1 polysiloxane Polymers 0.000 claims description 20
- 238000003491 array Methods 0.000 claims description 18
- 239000000463 material Substances 0.000 claims description 18
- 239000002245 particle Substances 0.000 claims description 16
- 229910052710 silicon Inorganic materials 0.000 claims description 12
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims description 11
- 239000010703 silicon Substances 0.000 claims description 11
- 210000000601 blood cell Anatomy 0.000 claims description 7
- 229920000515 polycarbonate Polymers 0.000 claims description 5
- 239000004417 polycarbonate Substances 0.000 claims description 5
- 239000004642 Polyimide Substances 0.000 claims description 3
- 239000004809 Teflon Substances 0.000 claims description 3
- 229920006362 Teflon® Polymers 0.000 claims description 3
- 229920002678 cellulose Polymers 0.000 claims description 3
- 239000001913 cellulose Substances 0.000 claims description 3
- 229920000058 polyacrylate Polymers 0.000 claims description 3
- 229920000728 polyester Polymers 0.000 claims description 3
- 229920001721 polyimide Polymers 0.000 claims description 3
- 229920001296 polysiloxane Polymers 0.000 claims description 3
- 230000002093 peripheral effect Effects 0.000 claims 5
- 230000000903 blocking effect Effects 0.000 claims 1
- 239000010410 layer Substances 0.000 description 119
- 239000010408 film Substances 0.000 description 48
- 239000000523 sample Substances 0.000 description 33
- 210000004369 blood Anatomy 0.000 description 29
- 239000008280 blood Substances 0.000 description 29
- 210000004881 tumor cell Anatomy 0.000 description 25
- 210000000170 cell membrane Anatomy 0.000 description 23
- 230000008569 process Effects 0.000 description 22
- 238000004519 manufacturing process Methods 0.000 description 21
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 18
- 238000010166 immunofluorescence Methods 0.000 description 18
- 239000002953 phosphate buffered saline Substances 0.000 description 18
- 210000005266 circulating tumour cell Anatomy 0.000 description 17
- 238000001514 detection method Methods 0.000 description 15
- 229920002120 photoresistant polymer Polymers 0.000 description 14
- 238000010186 staining Methods 0.000 description 12
- 238000003556 assay Methods 0.000 description 11
- 239000000126 substance Substances 0.000 description 10
- 239000000758 substrate Substances 0.000 description 9
- VRBFTYUMFJWSJY-UHFFFAOYSA-N 28804-46-8 Chemical compound ClC1CC(C=C2)=CC=C2C(Cl)CC2=CC=C1C=C2 VRBFTYUMFJWSJY-UHFFFAOYSA-N 0.000 description 8
- 102000011782 Keratins Human genes 0.000 description 8
- 108010076876 Keratins Proteins 0.000 description 8
- 238000000151 deposition Methods 0.000 description 7
- 125000004404 heteroalkyl group Chemical group 0.000 description 7
- 238000002372 labelling Methods 0.000 description 7
- 239000000203 mixture Substances 0.000 description 7
- 229910052760 oxygen Inorganic materials 0.000 description 7
- 230000003068 static effect Effects 0.000 description 7
- 238000012360 testing method Methods 0.000 description 7
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- 206010028980 Neoplasm Diseases 0.000 description 6
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 6
- 125000000217 alkyl group Chemical group 0.000 description 6
- 238000004458 analytical method Methods 0.000 description 6
- 230000006378 damage Effects 0.000 description 6
- 125000005842 heteroatom Chemical group 0.000 description 6
- 239000002609 medium Substances 0.000 description 6
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical compound [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 6
- UXDBPOWEWOXJCE-DIPNUNPCSA-N 1,2-dihexadecyl-sn-glycero-3-phosphoethanolamine Chemical compound CCCCCCCCCCCCCCCCOC[C@H](COP(O)(=O)OCCN)OCCCCCCCCCCCCCCCC UXDBPOWEWOXJCE-DIPNUNPCSA-N 0.000 description 5
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 5
- 238000013459 approach Methods 0.000 description 5
- 239000000090 biomarker Substances 0.000 description 5
- 201000011510 cancer Diseases 0.000 description 5
- 230000003833 cell viability Effects 0.000 description 5
- 239000003153 chemical reaction reagent Substances 0.000 description 5
- 230000006870 function Effects 0.000 description 5
- 230000007246 mechanism Effects 0.000 description 5
- 229920000642 polymer Polymers 0.000 description 5
- 102000004169 proteins and genes Human genes 0.000 description 5
- 108090000623 proteins and genes Proteins 0.000 description 5
- 230000002829 reductive effect Effects 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 102000018651 Epithelial Cell Adhesion Molecule Human genes 0.000 description 4
- 108010066687 Epithelial Cell Adhesion Molecule Proteins 0.000 description 4
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 4
- 229930006000 Sucrose Natural products 0.000 description 4
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 4
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 4
- 125000004432 carbon atom Chemical group C* 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 230000008021 deposition Effects 0.000 description 4
- 239000004205 dimethyl polysiloxane Substances 0.000 description 4
- 239000000706 filtrate Substances 0.000 description 4
- 125000000524 functional group Chemical group 0.000 description 4
- 229910052736 halogen Inorganic materials 0.000 description 4
- 150000002367 halogens Chemical class 0.000 description 4
- 229910052739 hydrogen Inorganic materials 0.000 description 4
- 239000001257 hydrogen Substances 0.000 description 4
- 238000002955 isolation Methods 0.000 description 4
- 238000005374 membrane filtration Methods 0.000 description 4
- 208000037819 metastatic cancer Diseases 0.000 description 4
- 208000011575 metastatic malignant neoplasm Diseases 0.000 description 4
- 244000005700 microbiome Species 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- 239000001301 oxygen Substances 0.000 description 4
- 238000000059 patterning Methods 0.000 description 4
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 description 4
- 238000004088 simulation Methods 0.000 description 4
- 239000005720 sucrose Substances 0.000 description 4
- WGTYBPLFGIVFAS-UHFFFAOYSA-M tetramethylammonium hydroxide Chemical compound [OH-].C[N+](C)(C)C WGTYBPLFGIVFAS-UHFFFAOYSA-M 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 3
- 241001494479 Pecora Species 0.000 description 3
- 206010060862 Prostate cancer Diseases 0.000 description 3
- 102000007066 Prostate-Specific Antigen Human genes 0.000 description 3
- 108010072866 Prostate-Specific Antigen Proteins 0.000 description 3
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 3
- DPKHZNPWBDQZCN-UHFFFAOYSA-N acridine orange free base Chemical compound C1=CC(N(C)C)=CC2=NC3=CC(N(C)C)=CC=C3C=C21 DPKHZNPWBDQZCN-UHFFFAOYSA-N 0.000 description 3
- 125000003118 aryl group Chemical group 0.000 description 3
- DZBUGLKDJFMEHC-UHFFFAOYSA-N benzoquinolinylidene Natural products C1=CC=CC2=CC3=CC=CC=C3N=C21 DZBUGLKDJFMEHC-UHFFFAOYSA-N 0.000 description 3
- 238000004113 cell culture Methods 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 239000000975 dye Substances 0.000 description 3
- 229940088598 enzyme Drugs 0.000 description 3
- 238000005530 etching Methods 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 238000011049 filling Methods 0.000 description 3
- 239000007850 fluorescent dye Substances 0.000 description 3
- 229910052731 fluorine Inorganic materials 0.000 description 3
- 230000000670 limiting effect Effects 0.000 description 3
- 150000002632 lipids Chemical class 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- 210000005087 mononuclear cell Anatomy 0.000 description 3
- 210000004940 nucleus Anatomy 0.000 description 3
- 210000003463 organelle Anatomy 0.000 description 3
- 238000000206 photolithography Methods 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 238000011084 recovery Methods 0.000 description 3
- 239000000377 silicon dioxide Substances 0.000 description 3
- 235000012239 silicon dioxide Nutrition 0.000 description 3
- 239000002356 single layer Substances 0.000 description 3
- IHQKEDIOMGYHEB-UHFFFAOYSA-M sodium dimethylarsinate Chemical compound [Na+].C[As](C)([O-])=O IHQKEDIOMGYHEB-UHFFFAOYSA-M 0.000 description 3
- 125000001424 substituent group Chemical group 0.000 description 3
- 125000000547 substituted alkyl group Chemical group 0.000 description 3
- 229910052717 sulfur Inorganic materials 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- 239000012103 Alexa Fluor 488 Substances 0.000 description 2
- NOWKCMXCCJGMRR-UHFFFAOYSA-N Aziridine Chemical compound C1CN1 NOWKCMXCCJGMRR-UHFFFAOYSA-N 0.000 description 2
- 206010006187 Breast cancer Diseases 0.000 description 2
- 208000026310 Breast neoplasm Diseases 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 206010027476 Metastases Diseases 0.000 description 2
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 2
- 125000003282 alkyl amino group Chemical group 0.000 description 2
- 125000004414 alkyl thio group Chemical group 0.000 description 2
- 239000012491 analyte Substances 0.000 description 2
- 238000000137 annealing Methods 0.000 description 2
- 239000000427 antigen Substances 0.000 description 2
- 102000036639 antigens Human genes 0.000 description 2
- 108091007433 antigens Proteins 0.000 description 2
- 125000005239 aroylamino group Chemical group 0.000 description 2
- 125000004104 aryloxy group Chemical group 0.000 description 2
- 210000001124 body fluid Anatomy 0.000 description 2
- 239000010839 body fluid Substances 0.000 description 2
- 229910052794 bromium Inorganic materials 0.000 description 2
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 description 2
- 125000001951 carbamoylamino group Chemical group C(N)(=O)N* 0.000 description 2
- 239000006143 cell culture medium Substances 0.000 description 2
- 230000006037 cell lysis Effects 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 229910052801 chlorine Inorganic materials 0.000 description 2
- 230000000295 complement effect Effects 0.000 description 2
- 210000000805 cytoplasm Anatomy 0.000 description 2
- 238000000432 density-gradient centrifugation Methods 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000006911 enzymatic reaction Methods 0.000 description 2
- 210000002919 epithelial cell Anatomy 0.000 description 2
- 239000000834 fixative Substances 0.000 description 2
- 238000002825 functional assay Methods 0.000 description 2
- 125000005843 halogen group Chemical group 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 125000001072 heteroaryl group Chemical group 0.000 description 2
- 238000005286 illumination Methods 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 208000014674 injury Diseases 0.000 description 2
- 230000014759 maintenance of location Effects 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 230000009401 metastasis Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 125000004433 nitrogen atom Chemical group N* 0.000 description 2
- 108091033319 polynucleotide Proteins 0.000 description 2
- 102000040430 polynucleotide Human genes 0.000 description 2
- 239000002157 polynucleotide Substances 0.000 description 2
- 239000013641 positive control Substances 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 108090000765 processed proteins & peptides Proteins 0.000 description 2
- 239000011541 reaction mixture Substances 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 238000001878 scanning electron micrograph Methods 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 125000004434 sulfur atom Chemical group 0.000 description 2
- 230000004083 survival effect Effects 0.000 description 2
- 230000008733 trauma Effects 0.000 description 2
- 239000000439 tumor marker Substances 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 description 1
- BZTDTCNHAFUJOG-UHFFFAOYSA-N 6-carboxyfluorescein Chemical compound C12=CC=C(O)C=C2OC2=CC(O)=CC=C2C11OC(=O)C2=CC=C(C(=O)O)C=C21 BZTDTCNHAFUJOG-UHFFFAOYSA-N 0.000 description 1
- 239000012109 Alexa Fluor 568 Substances 0.000 description 1
- 241000203069 Archaea Species 0.000 description 1
- 238000012935 Averaging Methods 0.000 description 1
- 108090001008 Avidin Proteins 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 206010055113 Breast cancer metastatic Diseases 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 241000195493 Cryptophyta Species 0.000 description 1
- XZMCDFZZKTWFGF-UHFFFAOYSA-N Cyanamide Chemical compound NC#N XZMCDFZZKTWFGF-UHFFFAOYSA-N 0.000 description 1
- 206010061819 Disease recurrence Diseases 0.000 description 1
- 206010013496 Disturbance in attention Diseases 0.000 description 1
- 239000012591 Dulbecco’s Phosphate Buffered Saline Substances 0.000 description 1
- 241000206602 Eukaryota Species 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- CBENFWSGALASAD-UHFFFAOYSA-N Ozone Chemical compound [O-][O+]=O CBENFWSGALASAD-UHFFFAOYSA-N 0.000 description 1
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 description 1
- 206010036790 Productive cough Diseases 0.000 description 1
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 description 1
- PLXBWHJQWKZRKG-UHFFFAOYSA-N Resazurin Chemical compound C1=CC(=O)C=C2OC3=CC(O)=CC=C3[N+]([O-])=C21 PLXBWHJQWKZRKG-UHFFFAOYSA-N 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- 108090000190 Thrombin Proteins 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- OCBFFGCSTGGPSQ-UHFFFAOYSA-N [CH2]CC Chemical group [CH2]CC OCBFFGCSTGGPSQ-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 125000002252 acyl group Chemical group 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 230000001464 adherent effect Effects 0.000 description 1
- 125000005236 alkanoylamino group Chemical group 0.000 description 1
- 125000003342 alkenyl group Chemical group 0.000 description 1
- 125000003545 alkoxy group Chemical group 0.000 description 1
- 125000000033 alkoxyamino group Chemical group 0.000 description 1
- 125000004453 alkoxycarbonyl group Chemical group 0.000 description 1
- 125000005194 alkoxycarbonyloxy group Chemical group 0.000 description 1
- 125000004656 alkyl sulfonylamino group Chemical group 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 125000004103 aminoalkyl group Chemical group 0.000 description 1
- 125000004397 aminosulfonyl group Chemical group NS(=O)(=O)* 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 125000001769 aryl amino group Chemical group 0.000 description 1
- 125000005161 aryl oxy carbonyl group Chemical group 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 125000001584 benzyloxycarbonyl group Chemical group C(=O)(OCC1=CC=CC=C1)* 0.000 description 1
- 229920000249 biocompatible polymer Polymers 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 125000003739 carbamimidoyl group Chemical group C(N)(=N)* 0.000 description 1
- 125000001589 carboacyl group Chemical group 0.000 description 1
- 150000007942 carboxylates Chemical class 0.000 description 1
- 230000021164 cell adhesion Effects 0.000 description 1
- 230000022131 cell cycle Effects 0.000 description 1
- 230000005779 cell damage Effects 0.000 description 1
- 208000037887 cell injury Diseases 0.000 description 1
- 230000006727 cell loss Effects 0.000 description 1
- 210000003855 cell nucleus Anatomy 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 238000013043 cell viability test Methods 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 1
- 125000001309 chloro group Chemical group Cl* 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 238000000942 confocal micrograph Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 210000004748 cultured cell Anatomy 0.000 description 1
- 210000000448 cultured tumor cell Anatomy 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 125000004093 cyano group Chemical group *C#N 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 238000012137 double-staining Methods 0.000 description 1
- 238000007877 drug screening Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 239000002532 enzyme inhibitor Substances 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 150000004820 halides Chemical class 0.000 description 1
- 125000001188 haloalkyl group Chemical group 0.000 description 1
- 208000019622 heart disease Diseases 0.000 description 1
- 208000013210 hematogenous Diseases 0.000 description 1
- FFUAGWLWBBFQJT-UHFFFAOYSA-N hexamethyldisilazane Chemical compound C[Si](C)(C)N[Si](C)(C)C FFUAGWLWBBFQJT-UHFFFAOYSA-N 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229920002674 hyaluronan Polymers 0.000 description 1
- 229960003160 hyaluronic acid Drugs 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- ORTFAQDWJHRMNX-UHFFFAOYSA-N hydroxidooxidocarbon(.) Chemical group O[C]=O ORTFAQDWJHRMNX-UHFFFAOYSA-N 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 125000002768 hydroxyalkyl group Chemical group 0.000 description 1
- 238000010185 immunofluorescence analysis Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 230000002934 lysing effect Effects 0.000 description 1
- 108010082117 matrigel Proteins 0.000 description 1
- 238000009285 membrane fouling Methods 0.000 description 1
- 125000005358 mercaptoalkyl group Chemical group 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 206010061289 metastatic neoplasm Diseases 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 125000001181 organosilyl group Chemical group [SiH3]* 0.000 description 1
- 229910052762 osmium Inorganic materials 0.000 description 1
- SYQBFIAQOQZEGI-UHFFFAOYSA-N osmium atom Chemical compound [Os] SYQBFIAQOQZEGI-UHFFFAOYSA-N 0.000 description 1
- 229910000489 osmium tetroxide Inorganic materials 0.000 description 1
- 239000012285 osmium tetroxide Substances 0.000 description 1
- 125000004043 oxo group Chemical group O=* 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 239000013610 patient sample Substances 0.000 description 1
- 125000006678 phenoxycarbonyl group Chemical group 0.000 description 1
- PSBAIJVSCTZDDB-UHFFFAOYSA-N phenyl acetylsalicylate Chemical compound CC(=O)OC1=CC=CC=C1C(=O)OC1=CC=CC=C1 PSBAIJVSCTZDDB-UHFFFAOYSA-N 0.000 description 1
- UYWQUFXKFGHYNT-UHFFFAOYSA-N phenylmethyl ester of formic acid Natural products O=COCC1=CC=CC=C1 UYWQUFXKFGHYNT-UHFFFAOYSA-N 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 229920001993 poloxamer 188 Polymers 0.000 description 1
- 229920002959 polymer blend Polymers 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 238000012805 post-processing Methods 0.000 description 1
- 230000001376 precipitating effect Effects 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 238000004393 prognosis Methods 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 210000001938 protoplast Anatomy 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 238000005956 quaternization reaction Methods 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000007261 regionalization Effects 0.000 description 1
- 230000003252 repetitive effect Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 210000000582 semen Anatomy 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 210000003802 sputum Anatomy 0.000 description 1
- 208000024794 sputum Diseases 0.000 description 1
- 239000011550 stock solution Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 125000000446 sulfanediyl group Chemical group *S* 0.000 description 1
- 210000004243 sweat Anatomy 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 239000010409 thin film Substances 0.000 description 1
- 229960004072 thrombin Drugs 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- ZMANZCXQSJIPKH-UHFFFAOYSA-O triethylammonium ion Chemical compound CC[NH+](CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-O 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 238000012795 verification Methods 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 239000011534 wash buffer Substances 0.000 description 1
- 238000001039 wet etching Methods 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D69/00—Semi-permeable membranes for separation processes or apparatus characterised by their form, structure or properties; Manufacturing processes specially adapted therefor
- B01D69/12—Composite membranes; Ultra-thin membranes
- B01D69/1216—Three or more layers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D61/00—Processes of separation using semi-permeable membranes, e.g. dialysis, osmosis or ultrafiltration; Apparatus, accessories or auxiliary operations specially adapted therefor
- B01D61/14—Ultrafiltration; Microfiltration
- B01D61/18—Apparatus therefor
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D67/00—Processes specially adapted for manufacturing semi-permeable membranes for separation processes or apparatus
- B01D67/0002—Organic membrane manufacture
- B01D67/0023—Organic membrane manufacture by inducing porosity into non porous precursor membranes
- B01D67/0032—Organic membrane manufacture by inducing porosity into non porous precursor membranes by elimination of segments of the precursor, e.g. nucleation-track membranes, lithography or laser methods
- B01D67/0034—Organic membrane manufacture by inducing porosity into non porous precursor membranes by elimination of segments of the precursor, e.g. nucleation-track membranes, lithography or laser methods by micromachining techniques, e.g. using masking and etching steps, photolithography
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2325/00—Details relating to properties of membranes
- B01D2325/02—Details relating to pores or porosity of the membranes
- B01D2325/021—Pore shapes
- B01D2325/0212—Symmetric or isoporous membranes
Landscapes
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Water Supply & Treatment (AREA)
- Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Manufacturing & Machinery (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Description
本発明は、契約第CA123027の下、アメリカ国立衛生研究所により支援された研究の間に行われた。政府は本発明においてある種の権利を有している。
Claims (19)
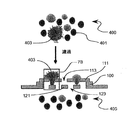
- 精密濾過パッチであり、前記精密濾過パッチは:
多孔質の上層膜を含み、前記多孔質の上層膜が、前記多孔質の上層膜を通じて、前記多孔質の上層膜の上部表面から前記多孔質の上層膜の下部表面へ垂直に伸びる上層膜のポアを有し;
多孔質の下層膜を含み、前記多孔質の下層膜が、前記多孔質の下層膜を通じて、前記多孔質の下層膜の上部表面から前記多孔質の下層膜の下部表面へ垂直に伸びる下層膜のポアを有し、前記下層膜のポアのいくつかが前記下層膜内に、前記上層多孔質膜内に分布される前記上層膜のポアのいくつからか水平にずらされるように配置され;
前記多孔質の上層膜の前記下部表面と前記多孔質の下層膜の前記上部表面との間の平面空間で定められる単一のギャップを含み、
前記ギャップが前記多孔質の上層膜の前記下部表面と前記多孔質の下層膜の前記上部表面との間の平面空間及び複数の下層膜のポアを含み、
前記多孔質の上層膜の前記下部表面と前記多孔質の下層膜の前記上部表面のギャップ距離が前記ギャップで定められる領域にわたり実質的に等しく、
前記ギャップ距離が、前記精密濾過パッチにより補足されないように粒子よりも大きく、かつ前記上層膜のポアから前記下層膜のポアへの粒子の流れを阻害する構造が前記ギャップには存在せず;及び
前記ギャップを囲む周辺構造を含み、前記周辺構造が前記多孔質の上層膜を前記多孔質の下層膜に接続する、精密濾過パッチ。 - 請求項1に記載のいくつかの精密濾過パッチを含む精密濾過アレイであって、各精密濾過パッチが、第一の六角形のアレイに配置される上層膜のポア、及び、第二の六角形のアレイに配置される下層膜のポアを含み、前記いくつかの精密濾過パッチは、精密濾過パッチのアレイに配置され、前記いくつかの精密濾過パッチは、前記上層膜が前記下層膜に接触する領域によって互いから隔てられる、精密濾過アレイ。
- 請求項1に記載のいくつかの精密濾過パッチを含む精密濾過アレイであって、前記下層膜のポアは、前記上層膜のポアの位置によって画定される中心となる領域の周囲で六角形のアレイに分布され、前記いくつかの精密濾過パッチは、精密濾過パッチのアレイに配置され、前記いくつかの精密濾過パッチは、前記多孔質の上層膜が前記多孔質の下層膜に接触する領域によって互いから隔てられる、精密濾過アレイ。
- 請求項2に記載の1又は複数の精密濾過アレイを含む精密濾過システムであって、前記いくつかの精密濾過パッチが、シリコンウエハーの少なくとも一部内で形成され、当該精密濾過システムは:
前記精密濾過パッチの前記多孔質の上層膜の上に位置づけられる上方領域、及び、
前記精密濾過パッチの前記多孔質の下層膜の下に位置づけられる下方領域、
をさらに含み、
前記上方領域は加圧可能であり、
前記下方領域は流体の受容を可能にするよう構成される、精密濾過システム。 - 請求項3に記載の1又は複数の精密濾過アレイを含む精密濾過システムであって、前記いくつかの精密濾過パッチが2つのパリレン層を含み、当該精密濾過システムが:
前記精密濾過パッチの前記多孔質の上層膜の上に位置づけられる上方領域、及び、
前記精密濾過パッチの前記多孔質の下層膜の下に位置づけられる下方領域、
をさらに含み、
前記上方領域は加圧可能であり、
前記下方領域は流体の受容を可能にするよう構成される、精密濾過システム。 - 請求項1、2又は4のうちいずれか1項に記載の精密濾過パッチであって、いくつかの上層膜のポアの直径が、選択された粒子の直径を有する粒子を捕獲するような大きさにされ、前記ギャップの距離が、前記上層膜のポアのうち少なくとも1つの中に捕獲された粒子に背圧を提供するよう前記多孔質の下層膜の位置を定めるように選択される、精密濾過パッチ。
- 請求項6に記載の精密濾過パッチであって、前記上層膜のポア及び下層膜のポアが、血液細胞の直径よりも大きく且つ循環性腫瘍細胞の直径よりも小さい直径を有し、前記ギャップの距離が前記循環性腫瘍細胞の直径よりも小さい、精密濾過パッチ。
- 請求項1、3又は5のうちいずれか1項に記載の精密濾過パッチであって、いくつかの上層膜のポアの直径が、捕獲されることになる粒子の粒径よりも大きい大きさにされ、前記下層膜のポアの直径が、捕獲されることになる粒子の粒径よりも小さい大きさにされ、前記ギャップの距離が、捕獲されることになる粒子の粒径よりも小さい、精密濾過パッチ。
- 請求項1乃至8のうちいずれか1項に記載の精密濾過パッチであって、前記下層膜のポアが、血液細胞の直径よりも大きく且つ循環性腫瘍細胞の直径よりも小さい直径を有し、前記ギャップの距離が、前記循環性腫瘍細胞の直径よりも小さい、精密濾過パッチ。
- 前記多孔質の上層膜及び/又は多孔質の下層膜がパリレンを含む、請求項1乃至9のうちいずれか一項に記載の精密濾過パッチ。
- 請求項1、2、4、7又は9のうちいずれか1項に記載の精密濾過パッチであって、前記上層膜のポアが約9マイクロメートルの直径を有し、前記下層膜のポアが約8マイクロメートルの直径を有し、前記ギャップの距離が約6.5マイクロメートルである、精密濾過パッチ。
- 請求項1、3、5、8又は9のうちいずれか1項に記載の精密濾過パッチであって、前記上層膜のポアが約40マイクロメートルの直径を有し、前記下層膜のポアが約8マイクロメートルの直径を有し、前記ギャップの距離が約5.5マイクロメートルである、精密濾過パッチ。
- 請求項1乃至12のうちいずれか1項に記載の精密濾過パッチであり、前記上層の多孔質膜、下層の多孔質膜又はそれらの両方が、ポリイミド、ポリシロキサン、ポリエステル、ポリアクリレート、セルロース、テフロン(TM)及びポリカーボネートを含む群から選択される1つの材料を含む、精密濾過パッチ。
- 請求項1乃至12のうちいずれか1項に記載の精密濾過パッチであり、前記上層の多孔質膜が、下層の多孔質膜と、前記上層の多孔質膜が下層の多孔質膜から分離可能に接続される、精密濾過パッチ。
- 細胞分離装置であり、前記構造が:
複数の上層膜のポアを含む多孔質の上層膜;
複数の下層膜のポアを含む多孔質の下層膜を含み、
それぞれの上層膜のポアが、それぞれの上層膜のポアの下及び回りに分布されるいくつかの下層膜のポアを含み、及び前記いくつかの下層膜のポアがそれぞれの上層膜のポアから水平にずらされ、それぞれの下層膜ポアが少なくとも1つの上層膜ポアよりも小さい直径を有し;
前記上層の多孔質膜及び下層の多孔質膜を分離する単一のギャップを含み、
前記上層の多孔質膜及び下層の多孔質膜のギャップ距離が、少なくとも1つの下層膜のポアの直径よりも小さく、かつ前記ギャップ内には、上層膜ポアから下層膜ポアへの粒子の流れを阻止する構造が存在せず;及び
複数の上層膜ポア及び下層膜ポアを囲み、かつ前記単一ギャップを囲む周辺壁を含み、
前記周辺壁が前記上層の多孔質膜及び下層の多孔質膜を接続する、細胞分離装置。 - 請求項15に記載の細胞分離装置であり、前記上層の多孔質膜、下層の多孔質膜またはそれらの両方が、ポリイミド、ポリシロキサン、ポリエステル、ポリアクリレート、セルロース、テフロン(TM)及びポリカーボネートを含む群から選択される1つの材料を含む、細胞分離装置。
- 請求項15又は16のうちいずれか1項に記載の細胞分離装置であり、前記上層の多孔質膜が循環腫瘍細胞の直径よりも大きく、前記ギャップ距離が循環腫瘍細胞の直径よりも小さい、細胞分離装置。
- 請求項15乃至17のうちいずれか1項に記載の細胞分離装置であり、前記上層の多孔質膜が、それぞれの上層膜ポアの回りに六角型状に設けられる、細胞分離装置。
- 請求項15乃至18のうちいずれか1項に記載の細胞分離装置であり、前記周辺壁が、細胞が前記細胞分離構造により捕獲された後で、前記下層の多孔質膜から前記上層の多孔質膜を分離することが可能に形成される、細胞分離装置。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US6281408P | 2008-01-29 | 2008-01-29 | |
| US61/062,814 | 2008-01-29 | ||
| PCT/US2009/032054 WO2009097247A1 (en) | 2008-01-29 | 2009-01-26 | Method and apparatus for microfiltration to perform cell separation |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2011510656A JP2011510656A (ja) | 2011-04-07 |
| JP2011510656A5 JP2011510656A5 (ja) | 2012-03-01 |
| JP5343092B2 true JP5343092B2 (ja) | 2013-11-13 |
Family
ID=40898143
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010545073A Active JP5343092B2 (ja) | 2008-01-29 | 2009-01-26 | 細胞分離を行う精密濾過の方法及び装置 |
Country Status (4)
| Country | Link |
|---|---|
| US (2) | US8114289B2 (ja) |
| EP (1) | EP2238232B1 (ja) |
| JP (1) | JP5343092B2 (ja) |
| WO (1) | WO2009097247A1 (ja) |
Families Citing this family (52)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8980568B2 (en) | 2001-10-11 | 2015-03-17 | Aviva Biosciences Corporation | Methods and compositions for detecting non-hematopoietic cells from a blood sample |
| US8986944B2 (en) | 2001-10-11 | 2015-03-24 | Aviva Biosciences Corporation | Methods and compositions for separating rare cells from fluid samples |
| EP2041299A4 (en) * | 2006-07-14 | 2010-01-13 | Aviva Biosciences Corp | METHOD AND COMPOSITIONS FOR DETECTION OF RARE CELLS FROM A BIOLOGICAL SAMPLE |
| JP5343092B2 (ja) | 2008-01-29 | 2013-11-13 | カリフォルニア インスティチュート オブ テクノロジー | 細胞分離を行う精密濾過の方法及び装置 |
| WO2010135603A2 (en) | 2009-05-20 | 2010-11-25 | California Institute Of Technology | Method for cancer detection, diagnosis and prognosis |
| JP5704590B2 (ja) * | 2010-02-05 | 2015-04-22 | 国立大学法人東京農工大学 | サイズ選択マイクロキャビティアレイを用いた循環腫瘍細胞の検出 |
| EP2566598B1 (en) * | 2010-05-03 | 2020-06-03 | Creatv Microtech, Inc. | Polymer microfilters |
| US20110294206A1 (en) | 2010-05-28 | 2011-12-01 | California Institute Of Technology | Methods and design of membrane filters |
| US9733235B2 (en) | 2010-05-28 | 2017-08-15 | California Instute Of Technology | Methods and design of membrane filters |
| WO2012045016A2 (en) * | 2010-09-30 | 2012-04-05 | California Institute Of Technology | Particulate nanosorting stack |
| EP2634244A4 (en) | 2010-10-25 | 2017-03-22 | Cytogen Co. Ltd. | Cell collection apparatus |
| KR101254675B1 (ko) | 2010-10-25 | 2013-04-15 | 주식회사 싸이토젠 | 세포 채집 장치 |
| CN103608682B (zh) * | 2011-05-05 | 2017-01-18 | 安派科生物医学科技有限公司 | 肿瘤细胞检测仪 |
| US8961627B2 (en) | 2011-07-07 | 2015-02-24 | David J Edlund | Hydrogen generation assemblies and hydrogen purification devices |
| US9327217B2 (en) * | 2011-09-21 | 2016-05-03 | Nanyang Technological University | Multilayer filter |
| KR101933618B1 (ko) | 2011-11-29 | 2018-12-31 | 삼성전자주식회사 | 표적 물질 검출 및 분리 장치, 및 이를 이용한 표적 물질 검출 및 분리 방법 |
| WO2013109749A1 (en) * | 2012-01-17 | 2013-07-25 | The Penn State Research Foundation | Flexible filter device for capturing of particles or cells in a fluid |
| WO2013138522A2 (en) | 2012-03-16 | 2013-09-19 | Genelux Corporation | Methods for assessing effectiveness and monitoring oncolytic virus treatment |
| TWI498273B (zh) * | 2012-04-02 | 2015-09-01 | Nat Applied Res Laboratories | 微型篩網裝置及其製造方法 |
| KR101211862B1 (ko) * | 2012-04-30 | 2012-12-12 | 한국기계연구원 | 자기장을 이용하는 자가 세포 추출장치 및 이를 이용하는 방법 |
| TWI463129B (zh) | 2012-05-07 | 2014-12-01 | Nat Applied Res Laboratories | 微粒偵測用微型篩網裝置 |
| FR2992227B1 (fr) * | 2012-06-20 | 2022-04-01 | Commissariat Energie Atomique | Ensemble de filtration pour filtrer des nanoparticules comportant un filtre et un support de filtre, appareil et procede de montage de l'ensemble associes et procede de collecte et d'analyse de nanoparticules associe. |
| US10717040B2 (en) | 2012-08-30 | 2020-07-21 | Element 1 Corp. | Hydrogen purification devices |
| US9187324B2 (en) | 2012-08-30 | 2015-11-17 | Element 1 Corp. | Hydrogen generation assemblies and hydrogen purification devices |
| US11738305B2 (en) | 2012-08-30 | 2023-08-29 | Element 1 Corp | Hydrogen purification devices |
| KR101388155B1 (ko) | 2013-03-05 | 2014-04-23 | 한국과학기술원 | 마이크로비드의 선택적 수집을 위한 마이크로비드 포획 어레이 기판 및 이의 제조 방법 |
| WO2014142754A1 (en) * | 2013-03-13 | 2014-09-18 | Cellsievo Pte Ltd | Microsieve |
| CN104520420B (zh) * | 2013-07-24 | 2016-09-07 | 株式会社奥普特尼克斯精密 | 末梢循环肿瘤细胞或稀少细胞分离用装置及末梢循环肿瘤细胞或稀少细胞分离方法 |
| CA2946468C (en) | 2014-05-01 | 2022-10-04 | King Abdullah University Of Science And Technology | A microfluidic device that separates cells |
| WO2015184321A2 (en) * | 2014-05-29 | 2015-12-03 | Siemens Healthcare Diagnostics Inc. | Rare molecule detection |
| WO2016117486A1 (ja) * | 2015-01-19 | 2016-07-28 | 株式会社村田製作所 | 多孔質構造体およびその製造方法 |
| JPWO2016136273A1 (ja) | 2015-02-27 | 2017-12-07 | 凸版印刷株式会社 | 細胞を分離する方法、およびそのためのデバイス |
| WO2016140183A1 (ja) * | 2015-03-05 | 2016-09-09 | 株式会社村田製作所 | 多孔体および濾過装置 |
| KR101766450B1 (ko) | 2015-04-30 | 2017-08-08 | 주식회사 싸이토젠 | 표적 세포 회수 방법 |
| WO2017022891A1 (ko) * | 2015-08-05 | 2017-02-09 | 주식회사 싸이토젠 | 세포 포집 필터 및 이를 갖는 세포 포집 장치 |
| WO2017119850A1 (en) * | 2016-01-08 | 2017-07-13 | Singapore University Of Technology And Design | A method of forming a thin film through-hole membrane |
| SG11201807509PA (en) * | 2016-03-03 | 2018-09-27 | Yasuiki Umezu | Method for detecting or separating/obtaining circulating tumor cell employing cell proliferation method |
| US11096612B2 (en) * | 2016-04-27 | 2021-08-24 | The Hong Kong University Of Science And Technology | Methods of personalized microfiltration to detect cells from blood |
| CA3027616A1 (en) | 2016-06-15 | 2017-12-21 | Mimetas B.V. | Cell culture device and methods |
| US10053660B2 (en) | 2016-07-12 | 2018-08-21 | California Institute Of Technology | Substrates for high-density cell growth and metabolite exchange |
| RU175245U1 (ru) * | 2017-01-13 | 2017-11-28 | Сергей Викторович Минаев | Сепарационное устройство для определения биохимических показателей и маркеров заболеваний в плазме крови |
| CN111343919B (zh) * | 2017-11-21 | 2023-10-10 | Bbb有限公司 | 生物传感器 |
| TWI700246B (zh) * | 2018-01-04 | 2020-08-01 | 美商1號組件公司 | 氫氣淨化裝置 |
| EP4234077B1 (en) | 2018-04-11 | 2024-07-10 | W. L. Gore & Associates, Inc. | Metal supported powder catalyst matrix and processes for multiphase chemical reactions |
| CN109874316B (zh) * | 2018-05-25 | 2022-10-11 | 江苏汇先医药技术有限公司 | 用于从样本中富集筛选目标物例如细胞、细菌或生物分子的装置 |
| CN112423869B (zh) * | 2018-07-19 | 2023-03-17 | W.L.戈尔及同仁股份有限公司 | 包括多孔聚对二甲苯膜或多孔聚对二甲苯/聚四氟乙烯复合膜的高流量液体过滤装置 |
| CN109554285B (zh) * | 2018-12-29 | 2024-06-28 | 北京大学 | 一种柔性微孔膜及应用其制备的细胞分离装置 |
| US20210101117A1 (en) * | 2019-10-02 | 2021-04-08 | General Biologicals Corporation | Microfilter, manufacturing method and microfiltration unit |
| KR20220121889A (ko) * | 2020-01-17 | 2022-09-01 | 더블유.엘. 고어 앤드 어소시에이트스, 인코포레이티드 | 유기 용매 나노여과를 위한 나노선택성 표면을 가지는 복합 막 |
| US11896934B2 (en) * | 2020-12-08 | 2024-02-13 | Global Life Sciences Solutions Operations Uk Limited | Porous polymeric membrane with tear prevention ring |
| CN114018835B (zh) * | 2021-09-22 | 2023-03-17 | 浙江大学 | 微量全血预处理和血浆自动定量分配装置及分析方法 |
| US20230139619A1 (en) * | 2021-10-28 | 2023-05-04 | Southwest Research Institute | Devices for cell separation |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5480552A (en) * | 1992-01-10 | 1996-01-02 | Baxter International Inc. | Method for concentrating a solute with an oscillating filtration device |
| US5985164A (en) * | 1994-03-07 | 1999-11-16 | Regents Of The University Of California | Method for forming a filter |
| AU1389799A (en) * | 1997-11-07 | 1999-05-31 | California Institute Of Technology | Micromachined membrane particle filter using parylene reinforcement |
| AU8315701A (en) * | 2000-08-07 | 2002-02-18 | Cuno Inc | Unsupported multizone microporous membrane |
| US6736971B2 (en) * | 2000-08-07 | 2004-05-18 | Cuno Incorporated | Pre-metered, unsupported multilayer microporous membrane |
| NL1016030C1 (nl) * | 2000-08-28 | 2002-03-01 | Aquamarijn Holding B V | Sproei inrichting met een nozzleplaat, een nozzleplaat, alsmede werkwijzen ter vervaardiging en voor toepassing van een dergelijke nozzleplaat. |
| KR20060004958A (ko) * | 2003-04-25 | 2006-01-16 | 제이에스알 가부시끼가이샤 | 바이오칩 및 바이오칩 키트 및 그의 제조 방법 및 사용방법 |
| US7226540B2 (en) * | 2004-02-24 | 2007-06-05 | Becton, Dickinson And Company | MEMS filter module |
| JP5079506B2 (ja) * | 2004-07-30 | 2012-11-21 | プルリセレクト ゲーエムベーハー | 動物についての、バイオテクノロジー(生物学的研究を含む)および医学的診断での適用のための、細胞、生体粒子および/または分子を液体から単離するための装置ならびに方法 |
| JP4653995B2 (ja) * | 2004-10-08 | 2011-03-16 | Jsr株式会社 | バイオセパレーション用フィルターの製造方法並びにバイオセパレーション用フィルターおよびそれを用いたバイオセパレーション用キット |
| US7846393B2 (en) * | 2005-04-21 | 2010-12-07 | California Institute Of Technology | Membrane filter for capturing circulating tumor cells |
| US7846743B2 (en) * | 2005-04-21 | 2010-12-07 | California Institute Of Technology | Uses of parylene membrane filters |
| WO2006116327A1 (en) * | 2005-04-21 | 2006-11-02 | California Institute Of Technology | Uses of parylene membrane filters |
| FR2902799B1 (fr) * | 2006-06-27 | 2012-10-26 | Millipore Corp | Procede et unite de preparation d'un echantillon pour l'analyse microbiologique d'un liquide |
| JP5343092B2 (ja) | 2008-01-29 | 2013-11-13 | カリフォルニア インスティチュート オブ テクノロジー | 細胞分離を行う精密濾過の方法及び装置 |
-
2009
- 2009-01-26 JP JP2010545073A patent/JP5343092B2/ja active Active
- 2009-01-26 US US12/360,067 patent/US8114289B2/en active Active
- 2009-01-26 EP EP09706188.1A patent/EP2238232B1/en active Active
- 2009-01-26 WO PCT/US2009/032054 patent/WO2009097247A1/en active Application Filing
-
2011
- 2011-12-29 US US13/340,516 patent/US8815092B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| EP2238232B1 (en) | 2019-08-14 |
| US20090188864A1 (en) | 2009-07-30 |
| US8815092B2 (en) | 2014-08-26 |
| US20120097610A1 (en) | 2012-04-26 |
| EP2238232A1 (en) | 2010-10-13 |
| EP2238232A4 (en) | 2013-11-27 |
| JP2011510656A (ja) | 2011-04-07 |
| WO2009097247A1 (en) | 2009-08-06 |
| US8114289B2 (en) | 2012-02-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5343092B2 (ja) | 細胞分離を行う精密濾過の方法及び装置 | |
| Zheng et al. | 3D microfilter device for viable circulating tumor cell (CTC) enrichment from blood | |
| JP6982327B2 (ja) | マイクロ流体アッセイのための方法、組成物およびシステム | |
| JP5086241B2 (ja) | パリレンメンブレンフィルターの使用 | |
| JP7219089B2 (ja) | 磁気浮上を用いた生物学的および非生物学的な部分の選別 | |
| US20130122539A1 (en) | Microsieve for cells and particles filtration | |
| US20130255361A1 (en) | Methods and Devices for Multi-Dimensional Separation, Isolation and Characterization of Circulating Tumour Cells | |
| US20150118728A1 (en) | Apparatus and method for separating a biological entity from a sample volume | |
| US20140315295A1 (en) | Polymer microfilters, devices comprising the same, methods of manufacturing the same, and uses thereof | |
| Chen et al. | Surface‐micromachined microfiltration membranes for efficient isolation and functional immunophenotyping of subpopulations of immune cells | |
| WO2013126774A2 (en) | Microfluidic devices for capture of target species | |
| Casadei et al. | Cross‐flow microfiltration for isolation, selective capture and release of liposarcoma extracellular vesicles | |
| Cheng et al. | 3D spiral channels combined with flexible micro-sieve for high-throughput rare tumor cell enrichment and assay from clinical pleural effusion samples | |
| JP2014534436A (ja) | 血液または他の媒質からの粒子の濾過 | |
| Hao et al. | Separable Bilayer Microfiltration Device for Label-Free Enrichment of Viable Circulating Tumor Cells | |
| KR102722301B1 (ko) | 자기 부상을 이용한 생물학적 및 비-생물학적 모이어티의 분류 | |
| Castro | Micron–and Submicron-Scale High Porosity Polymer Membranes and Their Use for Cell Isolation | |
| Zheng et al. | A novel 3D micro membrane filtration device for capture viable rare circulating tumor cells from whole blood | |
| Varhue | Microfluidic Device Design for Selective Enrichment of Biocolloids Based on their Deformability and Polarization | |
| CN117448275A (zh) | 一种CTCs释放制剂及CTCs的分离方法 | |
| Hur | High-throughput Rare Cell Detection and Separation Using Inertial Microfluidics | |
| WO2008008248A2 (en) | Biosurfaces for receiving, retaining & displaying biological moieties |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120112 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20120112 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20121115 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20121204 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130301 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130308 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130327 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130403 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130425 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20130716 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20130812 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5343092 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |