JP5196992B2 - 腹膜透析装置および腹膜透析装置において実施される方法 - Google Patents
腹膜透析装置および腹膜透析装置において実施される方法 Download PDFInfo
- Publication number
- JP5196992B2 JP5196992B2 JP2007336517A JP2007336517A JP5196992B2 JP 5196992 B2 JP5196992 B2 JP 5196992B2 JP 2007336517 A JP2007336517 A JP 2007336517A JP 2007336517 A JP2007336517 A JP 2007336517A JP 5196992 B2 JP5196992 B2 JP 5196992B2
- Authority
- JP
- Japan
- Prior art keywords
- volume
- value
- fill
- total
- parameter
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000000034 method Methods 0.000 title claims description 50
- 238000000502 dialysis Methods 0.000 title claims description 28
- 239000000385 dialysis solution Substances 0.000 claims description 28
- 238000002560 therapeutic procedure Methods 0.000 claims description 27
- 238000004364 calculation method Methods 0.000 claims description 12
- 230000008859 change Effects 0.000 claims description 7
- 230000008569 process Effects 0.000 claims description 6
- 230000000284 resting effect Effects 0.000 claims 8
- 210000000683 abdominal cavity Anatomy 0.000 claims 6
- 230000001225 therapeutic effect Effects 0.000 claims 2
- 238000003860 storage Methods 0.000 description 32
- 238000010200 validation analysis Methods 0.000 description 12
- 230000004622 sleep time Effects 0.000 description 9
- 239000000243 solution Substances 0.000 description 6
- 230000006870 function Effects 0.000 description 3
- 230000014759 maintenance of location Effects 0.000 description 3
- 210000004303 peritoneum Anatomy 0.000 description 3
- 238000011340 continuous therapy Methods 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 239000002699 waste material Substances 0.000 description 2
- 208000001647 Renal Insufficiency Diseases 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 201000006370 kidney failure Diseases 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 210000003200 peritoneal cavity Anatomy 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/14—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis
- A61M1/28—Peritoneal dialysis ; Other peritoneal treatment, e.g. oxygenation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/14—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis
- A61M1/15—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis with a cassette forming partially or totally the flow circuit for the treating fluid, e.g. the dialysate fluid circuit or the treating gas circuit
- A61M1/159—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis with a cassette forming partially or totally the flow circuit for the treating fluid, e.g. the dialysate fluid circuit or the treating gas circuit specially adapted for peritoneal dialysis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/14—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis
- A61M1/28—Peritoneal dialysis ; Other peritoneal treatment, e.g. oxygenation
- A61M1/282—Operational modes
-
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H20/00—ICT specially adapted for therapies or health-improving plans, e.g. for handling prescriptions, for steering therapy or for monitoring patient compliance
- G16H20/40—ICT specially adapted for therapies or health-improving plans, e.g. for handling prescriptions, for steering therapy or for monitoring patient compliance relating to mechanical, radiation or invasive therapies, e.g. surgery, laser therapy, dialysis or acupuncture
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/12—General characteristics of the apparatus with interchangeable cassettes forming partially or totally the fluid circuit
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/50—General characteristics of the apparatus with microprocessors or computers
- A61M2205/502—User interfaces, e.g. screens or keyboards
- A61M2205/505—Touch-screens; Virtual keyboard or keypads; Virtual buttons; Soft keys; Mouse touches
Landscapes
- Health & Medical Sciences (AREA)
- Urology & Nephrology (AREA)
- Heart & Thoracic Surgery (AREA)
- Emergency Medicine (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Hematology (AREA)
- Vascular Medicine (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Primary Health Care (AREA)
- Medical Informatics (AREA)
- Epidemiology (AREA)
- Surgery (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- External Artificial Organs (AREA)
Description
Claims (20)
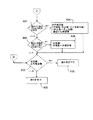
- 制御コンピュータを備える腹膜透析装置において実施される方法であって、前記制御コンピュータが、
腹膜透析装置の作動に用いられる治療パラメータの値を新しい値に変更するための命令を受け取る工程、
新しい値が治療パラメータに対する所定の最大値を超えるか否かを判定する工程、
新しい値が所定の最大値を超えている場合、新しい値を拒否する工程、
新しい値が所定の最大値未満である場合、新しい値が治療パラメータに対する所定の最小値未満であるか否かを判定する工程、
新しい値が所定の最小値未満である場合、その新しい値を拒否する工程、および
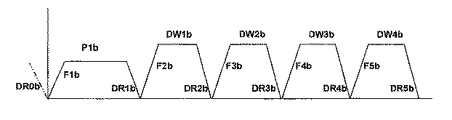
新しい値が所定の最小値を超える場合、少なくとも部分的に新しい値に基づいて、腹腔内に充填される透析溶液の全充填容積パラメータのための更新された値を計算する工程であって、前記計算は最後の充填容積および休止数が共にゼロであるか、あるいは最後の充填容積および休止数のいずれか一方または両方がゼロより大きいかを判定することを含む工程を実行することからなり、
前記治療パラメータは、腹腔内に充填される透析溶液の充填容積、充填数、最初の充填容積、最後の充填容積、休止数、休止のために腹腔内に充填される透析溶液の容積(すなわち休止容積)、タイダル療法における透析溶液の充填容積(すなわちタイダル充填容積)、タイダル療法における透析溶液の排液容積(すなわちタイダル排液容積)のうちの1つ以上からなる方法。 - 最後の充填容積のみがゼロより大きい場合、全充填容積パラメータのための更新された値は、少なくとも部分的に、充填数、充填容積、および最後の充填容積に基づいて計算される請求項1に記載の方法。
- 休止数のみがゼロより大きい場合、全充填容積パラメータのための更新された値は、少なくとも部分的に、充填数、充填容積、休止数、および休止容積に基づいて計算される請求項1または2に記載の方法。
- 最後の充填容積および休止数が共にゼロより大きい場合、全充填容積パラメータのための更新された値は、少なくとも部分的に、充填数、充填容積、休止数、休止容積、および最後の充填容積に基づいて計算される請求項1〜3のいずれか一項に記載の方法。
- 最後の充填容積および休止数が共にゼロである場合、全充填容積パラメータのための更新された値は、少なくとも部分的に、充填数および充填容積に基づいて計算される請求項1〜4のいずれか一項に記載の方法。
- 前記制御コンピュータが全充填容積パラメータの更新された値を透析溶液の全容積の値と比較する工程を更に備える請求項1〜5のいずれか一項に記載の方法。
- 透析溶液の全容積の値がユーザ入力として受け取られる請求項6に記載の方法。
- 全充填容積パラメータの更新された値が透析溶液の全容積の値よりも大きい場合、新しい値が拒否される請求項6または7に記載の方法。
- 全充填容積パラメータの更新された値が透析溶液の全容積の値よりも小さい場合、治療パラメータの値が新しい値に変更される請求項6〜8のいずれか一項に記載の方法。
- 治療パラメータが、充填容積、充填数、最後の充填容積、休止数、休止容積、タイダル充填容積、およびタイダル排液容積のうちの1つ以上からなる請求項1〜9のいずれか一項に記載の方法。
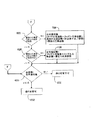
- 制御コンピュータを備える腹膜透析装置であって、前記制御コンピュータは下記の工程、すなわち、
腹膜透析装置の作動に用いられる治療パラメータの値を新しい値に変更するための命令を受け取る工程、
新しい値が治療パラメータに対する所定の最大値を超えるか否かを判定する工程、
新しい値が所定の最大値を超えている場合、新しい値を拒否する工程、
新しい値が所定の最大値未満である場合、新しい値が治療パラメータに対する所定の最小値未満であるか否かを判定する工程、
新しい値が所定の最小値未満である場合、その新しい値を拒否する工程、および
新しい値が所定の最小値を超える場合、少なくとも部分的に新しい値に基づいて、腹腔内に充填される透析溶液の全充填容積パラメータのための更新された値を計算する工程であって、前記計算は最後の充填容積および休止数が共にゼロであるか、あるいは最後の充填容積および休止数のいずれか一方または両方がゼロより大きいかを判定することを含む工程を実行するように構成されており、
前記治療パラメータは、腹腔内に充填される透析溶液の充填容積、充填数、最初の充填容積、最後の充填容積、休止数、休止のために腹腔内に充填される透析溶液の容積(すなわち休止容積)、タイダル療法における透析溶液の充填容積(すなわちタイダル充填容積)、タイダル療法における透析溶液の排液容積(すなわちタイダル排液容積)のうちの1つ以上からなる装置。 - 最後の充填容積のみがゼロより大きい場合、全充填容積パラメータのための更新された値は、少なくとも部分的に、充填数、充填容積、および最後の充填容積に基づいて計算される請求項11に記載の装置。
- 休止数のみがゼロより大きい場合、全充填容積パラメータのための更新された値は、少なくとも部分的に、充填数、充填容積、休止数、および休止容積に基づいて計算される請求項11または12に記載の装置。
- 最後の充填容積および休止数が共にゼロより大きい場合、全充填容積パラメータのための更新された値は、少なくとも部分的に、充填数、充填容積、休止数、休止容積、および最後の充填容積に基づいて計算される請求項11〜13のいずれか一項に記載の装置。
- 最後の充填容積および休止数が共にゼロである場合、全充填容積パラメータのための更新された値は、少なくとも部分的に、充填数および充填容積に基づいて計算される請求項11〜14のいずれか一項に記載の装置。
- 制御コンピュータは、更に全充填容積パラメータの更新された値を透析溶液の全容積の値と比較するように構成される請求項11〜15のいずれか一項に記載の装置。
- 透析溶液の全容積の値がユーザ入力として受け取られる請求項16に記載の装置。
- 全充填容積パラメータの更新された値が透析溶液の全容積の値よりも大きい場合、新しい値が拒否される請求項16または17に記載の装置。
- 全充填容積パラメータの更新された値が透析溶液の全容積の値よりも小さい場合、治療パラメータの値が新しい値に変更される請求項16〜18のいずれか一項に記載の装置。
- 治療パラメータが、充填容積、充填数、最後の充填容積、休止数、休止容積、タイダル充填容積、およびタイダル排液容積のうちの1つ以上からなる請求項11〜19のいずれか一項に記載の装置。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/648,154 | 2006-12-29 | ||
| US11/648,154 US20080161751A1 (en) | 2006-12-29 | 2006-12-29 | Peritoneal dialysis therapy validation |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2008173469A JP2008173469A (ja) | 2008-07-31 |
| JP2008173469A5 JP2008173469A5 (ja) | 2011-07-14 |
| JP5196992B2 true JP5196992B2 (ja) | 2013-05-15 |
Family
ID=39301523
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007336517A Active JP5196992B2 (ja) | 2006-12-29 | 2007-12-27 | 腹膜透析装置および腹膜透析装置において実施される方法 |
Country Status (4)
| Country | Link |
|---|---|
| US (2) | US20080161751A1 (ja) |
| EP (2) | EP2517742B1 (ja) |
| JP (1) | JP5196992B2 (ja) |
| CN (1) | CN101269247A (ja) |
Families Citing this family (90)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7153286B2 (en) | 2002-05-24 | 2006-12-26 | Baxter International Inc. | Automated dialysis system |
| US8029454B2 (en) | 2003-11-05 | 2011-10-04 | Baxter International Inc. | High convection home hemodialysis/hemofiltration and sorbent system |
| US10537671B2 (en) | 2006-04-14 | 2020-01-21 | Deka Products Limited Partnership | Automated control mechanisms in a hemodialysis apparatus |
| US20080058697A1 (en) | 2006-04-14 | 2008-03-06 | Deka Products Limited Partnership | Heat exchange systems, devices and methods |
| US8366316B2 (en) | 2006-04-14 | 2013-02-05 | Deka Products Limited Partnership | Sensor apparatus systems, devices and methods |
| US8042563B2 (en) | 2007-02-27 | 2011-10-25 | Deka Products Limited Partnership | Cassette system integrated apparatus |
| US8393690B2 (en) | 2007-02-27 | 2013-03-12 | Deka Products Limited Partnership | Enclosure for a portable hemodialysis system |
| US9028691B2 (en) | 2007-02-27 | 2015-05-12 | Deka Products Limited Partnership | Blood circuit assembly for a hemodialysis system |
| US20090107335A1 (en) | 2007-02-27 | 2009-04-30 | Deka Products Limited Partnership | Air trap for a medical infusion device |
| US8491184B2 (en) | 2007-02-27 | 2013-07-23 | Deka Products Limited Partnership | Sensor apparatus systems, devices and methods |
| US8425471B2 (en) | 2007-02-27 | 2013-04-23 | Deka Products Limited Partnership | Reagent supply for a hemodialysis system |
| US8317492B2 (en) | 2007-02-27 | 2012-11-27 | Deka Products Limited Partnership | Pumping cassette |
| US8357298B2 (en) | 2007-02-27 | 2013-01-22 | Deka Products Limited Partnership | Hemodialysis systems and methods |
| CA2681912C (en) | 2007-02-27 | 2015-09-29 | Deka Products Limited Partnership | Hemodialysis systems and methods |
| US8409441B2 (en) | 2007-02-27 | 2013-04-02 | Deka Products Limited Partnership | Blood treatment systems and methods |
| US8562834B2 (en) | 2007-02-27 | 2013-10-22 | Deka Products Limited Partnership | Modular assembly for a portable hemodialysis system |
| US8105487B2 (en) | 2007-09-25 | 2012-01-31 | Fresenius Medical Care Holdings, Inc. | Manifolds for use in conducting dialysis |
| US9308307B2 (en) | 2007-09-13 | 2016-04-12 | Fresenius Medical Care Holdings, Inc. | Manifold diaphragms |
| US9358331B2 (en) | 2007-09-13 | 2016-06-07 | Fresenius Medical Care Holdings, Inc. | Portable dialysis machine with improved reservoir heating system |
| US8597505B2 (en) | 2007-09-13 | 2013-12-03 | Fresenius Medical Care Holdings, Inc. | Portable dialysis machine |
| US8240636B2 (en) | 2009-01-12 | 2012-08-14 | Fresenius Medical Care Holdings, Inc. | Valve system |
| US8771508B2 (en) | 2008-08-27 | 2014-07-08 | Deka Products Limited Partnership | Dialyzer cartridge mounting arrangement for a hemodialysis system |
| EP2246080B1 (en) * | 2007-10-12 | 2016-02-10 | DEKA Products Limited Partnership | An extracorporeal blood flow system |
| US20090113335A1 (en) * | 2007-10-30 | 2009-04-30 | Baxter International Inc. | Dialysis system user interface |
| CA2960103C (en) | 2007-11-29 | 2020-03-10 | Fredenius Medical Care Holdings, Inc. | System and method for conducting hemodialysis and hemofiltration |
| CA3017406C (en) | 2008-01-23 | 2023-08-22 | Deka Products Limited Partnership | Fluid handling cassette for use with a peritoneal dialysis system |
| US10195330B2 (en) | 2008-01-23 | 2019-02-05 | Deka Products Limited Partnership | Medical treatment system and methods using a plurality of fluid lines |
| US11975128B2 (en) | 2008-01-23 | 2024-05-07 | Deka Products Limited Partnership | Medical treatment system and methods using a plurality of fluid lines |
| US9078971B2 (en) | 2008-01-23 | 2015-07-14 | Deka Products Limited Partnership | Medical treatment system and methods using a plurality of fluid lines |
| US8708950B2 (en) | 2010-07-07 | 2014-04-29 | Deka Products Limited Partnership | Medical treatment system and methods using a plurality of fluid lines |
| US10201647B2 (en) | 2008-01-23 | 2019-02-12 | Deka Products Limited Partnership | Medical treatment system and methods using a plurality of fluid lines |
| US11833281B2 (en) | 2008-01-23 | 2023-12-05 | Deka Products Limited Partnership | Pump cassette and methods for use in medical treatment system using a plurality of fluid lines |
| US8882700B2 (en) * | 2008-05-02 | 2014-11-11 | Baxter International Inc. | Smart patient transfer set for peritoneal dialysis |
| US8057679B2 (en) * | 2008-07-09 | 2011-11-15 | Baxter International Inc. | Dialysis system having trending and alert generation |
| US9147045B2 (en) | 2008-07-09 | 2015-09-29 | Baxter International Inc. | Peritoneal equilibration test and dialysis system using same |
| US8062513B2 (en) | 2008-07-09 | 2011-11-22 | Baxter International Inc. | Dialysis system and machine having therapy prescription recall |
| US8168063B2 (en) * | 2008-07-09 | 2012-05-01 | Baxter International Inc. | Dialysis system having filtering method for determining therapy prescriptions |
| US7981281B2 (en) * | 2008-07-09 | 2011-07-19 | Baxter International, Inc. | Dialysis system having regimen generation methodology |
| US9514283B2 (en) * | 2008-07-09 | 2016-12-06 | Baxter International Inc. | Dialysis system having inventory management including online dextrose mixing |
| AU2009302327C1 (en) | 2008-10-07 | 2015-09-10 | Fresenius Medical Care Holdings, Inc. | Priming system and method for dialysis systems |
| MX347636B (es) | 2008-10-30 | 2017-04-03 | Fresenius Medical Care Holdings Inc | Sistema de dialisis modular portatil. |
| US8142649B2 (en) * | 2009-01-29 | 2012-03-27 | Baxter International Inc. | Method for optimizing tidal therapies employing ultrafiltrate trending |
| US8182673B2 (en) | 2009-01-29 | 2012-05-22 | Baxter International Inc. | Drain and fill logic for automated peritoneal dialysis |
| US8521482B2 (en) * | 2009-02-20 | 2013-08-27 | Baxter International Inc. | Simulation of patient drain phase in peritoneal dialysis |
| WO2010114932A1 (en) | 2009-03-31 | 2010-10-07 | Xcorporeal, Inc. | Modular reservoir assembly for a hemodialysis and hemofiltration system |
| US20100279250A1 (en) * | 2009-04-29 | 2010-11-04 | Inter-Med, Inc. | Programmable dental device |
| US8282829B2 (en) | 2009-05-20 | 2012-10-09 | Baxter International Inc. | System and method for automated data collection of twenty-four hour ultrafiltration and other patient parameters using wired or wireless technology |
| US8926551B2 (en) * | 2009-07-07 | 2015-01-06 | Baxter Healthcare Inc. | Peritoneal dialysis therapy with large dialysis solution volumes |
| US9020827B2 (en) | 2009-10-16 | 2015-04-28 | Baxter International Inc. | Peritoneal dialysis optimized using a patient hand-held scanning device |
| MX2012005088A (es) | 2009-10-30 | 2012-10-03 | Deka Products Lp | Aparato y metodo para detectar la desconexion de un dispositivo de acceso intravascular. |
| DE102009054395A1 (de) | 2009-11-24 | 2011-06-01 | Fresenius Medical Care Deutschland Gmbh | Verfahren zum Anpassen von Grenzwertfenstern, Steuervorrichtung, medizinische Behandlungsvorrichtung und medizinische Überwachungsvorrichtung |
| KR101466459B1 (ko) * | 2010-07-14 | 2014-11-28 | 아사히 가세이 메디컬 가부시키가이샤 | 혈액 투석 장치 |
| US8936720B2 (en) * | 2010-09-17 | 2015-01-20 | Baxter International Inc. | Drain and fill logic for automated peritoneal dialysis |
| US9069886B2 (en) * | 2010-09-29 | 2015-06-30 | Terumo Kabushiki Kaisha | Home medical apparatus |
| AU2012230767A1 (en) | 2011-03-23 | 2013-10-31 | Nxstage Medical, Inc. | Peritoneal dialysis systems, devices, and methods |
| US9861733B2 (en) | 2012-03-23 | 2018-01-09 | Nxstage Medical Inc. | Peritoneal dialysis systems, devices, and methods |
| CA3166031A1 (en) | 2011-05-24 | 2012-11-29 | Deka Products Limited Partnership | Hemodialysis system |
| SG10201604167XA (en) | 2011-05-24 | 2016-07-28 | Deka Products Lp | Blood treatment systems and methods |
| JP5433634B2 (ja) * | 2011-06-10 | 2014-03-05 | 日機装株式会社 | 血液浄化装置 |
| DE102011105916B4 (de) * | 2011-06-29 | 2013-03-28 | Fresenius Medical Care Deutschland Gmbh | Dialysemaschine |
| EP3165245B1 (en) | 2011-08-02 | 2019-02-20 | Medtronic, Inc. | Hemodialysis system having a flow path with a controlled compliant volume |
| EP3002989B1 (en) | 2011-11-04 | 2017-04-19 | DEKA Products Limited Partnership | Medical treatment system and methods using a plurality of fluid lines |
| DE102011121668A1 (de) * | 2011-12-20 | 2013-06-20 | Fresenius Medical Care Deutschland Gmbh | Verfahren und Vorrichtung zur Vorbereitung von medizinischen Behandlungsvorrichtungen |
| JP5867200B2 (ja) * | 2012-03-15 | 2016-02-24 | 富士通株式会社 | プログラム、および情報処理装置 |
| US9201036B2 (en) | 2012-12-21 | 2015-12-01 | Fresenius Medical Care Holdings, Inc. | Method and system of monitoring electrolyte levels and composition using capacitance or induction |
| US9157786B2 (en) | 2012-12-24 | 2015-10-13 | Fresenius Medical Care Holdings, Inc. | Load suspension and weighing system for a dialysis machine reservoir |
| US10065003B2 (en) | 2012-12-28 | 2018-09-04 | Gambro Lundia Ab | Syringe pump engagement detection apparatus and methods |
| CA2895179C (en) | 2012-12-31 | 2021-09-07 | Gambro Lundia Ab | Treatment profiles |
| US9713666B2 (en) | 2013-01-09 | 2017-07-25 | Medtronic, Inc. | Recirculating dialysate fluid circuit for blood measurement |
| US9579443B2 (en) | 2013-01-10 | 2017-02-28 | Fresenius Medical Care Holdings, Inc. | Peritoneal dialysis systems and related devices and methods |
| US10850016B2 (en) | 2013-02-01 | 2020-12-01 | Medtronic, Inc. | Modular fluid therapy system having jumpered flow paths and systems and methods for cleaning and disinfection |
| US9623164B2 (en) | 2013-02-01 | 2017-04-18 | Medtronic, Inc. | Systems and methods for multifunctional volumetric fluid control |
| US10010663B2 (en) | 2013-02-01 | 2018-07-03 | Medtronic, Inc. | Fluid circuit for delivery of renal replacement therapies |
| JP6310467B2 (ja) * | 2013-09-20 | 2018-04-11 | テルモ株式会社 | 表示装置及び表示方法 |
| US9354640B2 (en) | 2013-11-11 | 2016-05-31 | Fresenius Medical Care Holdings, Inc. | Smart actuator for valve |
| US10537875B2 (en) | 2013-11-26 | 2020-01-21 | Medtronic, Inc. | Precision recharging of sorbent materials using patient and session data |
| US9884145B2 (en) | 2013-11-26 | 2018-02-06 | Medtronic, Inc. | Parallel modules for in-line recharging of sorbents using alternate duty cycles |
| SE538124C2 (sv) * | 2014-02-28 | 2016-03-08 | Triomed Ab | Apparat för peritoneal ultrafiltration innefattande kontrollav glukoskoncentrationen i peritonealhålan |
| CN105013032B (zh) | 2014-03-31 | 2018-06-22 | 甘布罗伦迪亚股份公司 | 体外血液处理系统及用于该系统的方法 |
| US12026271B2 (en) | 2014-05-27 | 2024-07-02 | Deka Products Limited Partnership | Control systems and methods for blood or fluid handling medical devices |
| WO2015199766A1 (en) | 2014-06-24 | 2015-12-30 | Medtronic, Inc. | Modular dialysate regeneration assembly |
| WO2015199768A1 (en) | 2014-06-24 | 2015-12-30 | Medtronic, Inc. | Stacked sorbent assembly |
| WO2016087897A1 (en) * | 2014-12-04 | 2016-06-09 | Debiotech S.A. | User interface for dialysis treatment |
| WO2017180980A1 (en) * | 2016-04-14 | 2017-10-19 | Mylan Inc. | Systems, devices and methods for assessing inhalation therapy |
| US10981148B2 (en) | 2016-11-29 | 2021-04-20 | Medtronic, Inc. | Zirconium oxide module conditioning |
| CN108255381A (zh) * | 2016-12-29 | 2018-07-06 | 苏州普源精电科技有限公司 | 电子产品的区域参数修改方法及装置 |
| US10960381B2 (en) | 2017-06-15 | 2021-03-30 | Medtronic, Inc. | Zirconium phosphate disinfection recharging and conditioning |
| WO2018237375A1 (en) | 2017-06-24 | 2018-12-27 | Nxstage Medical, Inc. | METHODS AND SYSTEMS FOR PREPARING AND / OR PROCESSING PERITONEAL DIALYSIS FLUID |
| US11872337B2 (en) | 2018-02-28 | 2024-01-16 | Nxstage Medical, Inc. | Fluid preparation and treatment devices methods and systems |
| US11213616B2 (en) | 2018-08-24 | 2022-01-04 | Medtronic, Inc. | Recharge solution for zirconium phosphate |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994020154A1 (en) * | 1993-03-03 | 1994-09-15 | Deka Products Limited Partnership | Peritoneal dialysis systems and methods employing a liquid distribution and pump cassette with self-contained air isolation and removal |
| US5324422A (en) * | 1993-03-03 | 1994-06-28 | Baxter International Inc. | User interface for automated peritoneal dialysis systems |
| US5788851A (en) * | 1995-02-13 | 1998-08-04 | Aksys, Ltd. | User interface and method for control of medical instruments, such as dialysis machines |
| DE19742637C5 (de) * | 1997-09-26 | 2005-06-02 | Fresenius Medical Care Deutschland Gmbh | Vorrichtung und Verfahren zur Bedienung medizintechnischer Geräte |
| ATE414549T1 (de) * | 2002-07-19 | 2008-12-15 | Terumo Corp | Gerät zur peritonealdialyse |
| KR101112117B1 (ko) * | 2003-10-13 | 2012-02-22 | 프레제니우스 메디칼 케어 도이칠란드 게엠베하 | 복막 투석 치료를 행하기 위한 장치 |
-
2006
- 2006-12-29 US US11/648,154 patent/US20080161751A1/en not_active Abandoned
-
2007
- 2007-12-24 EP EP12168941.8A patent/EP2517742B1/en active Active
- 2007-12-24 EP EP07025089A patent/EP1938849B1/en active Active
- 2007-12-27 JP JP2007336517A patent/JP5196992B2/ja active Active
- 2007-12-29 CN CNA2007101997949A patent/CN101269247A/zh active Pending
-
2010
- 2010-12-03 US US12/960,020 patent/US20110077586A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| JP2008173469A (ja) | 2008-07-31 |
| EP2517742A2 (en) | 2012-10-31 |
| EP2517742B1 (en) | 2016-05-18 |
| CN101269247A (zh) | 2008-09-24 |
| EP1938849A2 (en) | 2008-07-02 |
| US20110077586A1 (en) | 2011-03-31 |
| EP1938849B1 (en) | 2013-03-20 |
| US20080161751A1 (en) | 2008-07-03 |
| EP1938849A3 (en) | 2009-11-18 |
| EP2517742A3 (en) | 2012-11-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5196992B2 (ja) | 腹膜透析装置および腹膜透析装置において実施される方法 | |
| EP1969507B1 (en) | Medical apparatus with improved user interface | |
| KR102014689B1 (ko) | 치료 처방 그리고 추적, 서비스, 및 재고 조사를 위한 가정용 의료 장치 시스템 및 방법 | |
| EP0548336B1 (en) | Method of monitoring a dialysis unit | |
| US8900172B2 (en) | Method for controlling a blood treatment apparatus, control device, dispensing device and blood treatment apparatus | |
| US20080307353A1 (en) | Medical Apparatus and User Interface for a Medical Apparatus | |
| US20130317420A1 (en) | Device and method for entering user information into medical devices | |
| JPH11262522A (ja) | 専門の医療機器を操作するための装置及び方法 | |
| US9878082B2 (en) | Method and apparatus for optimizing an extracorporeal blood treatment | |
| US20110066693A1 (en) | Medical machine for fluid treatment | |
| US20160199562A1 (en) | Medical fluid treatment machines and related systems and methods | |
| EP3227812B1 (en) | User interface for dialysis treatment | |
| WO2020165108A1 (en) | System for sharing dialysis services | |
| US11577012B2 (en) | Auto adjustment of blood treatment parameters based on patient comfort | |
| JP2020089493A (ja) | 透析システムの管理装置およびプログラム | |
| JP2023119258A (ja) | 血液浄化装置および血液浄化装置の制御方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20101227 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110601 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20120220 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120925 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20121019 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20130122 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20130205 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20160215 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5196992 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |