JP5154932B2 - ゲル化速度の早い一次コーティング材を含んだコーティングされた光ファイバ及び光ファイバコーティングシステム - Google Patents
ゲル化速度の早い一次コーティング材を含んだコーティングされた光ファイバ及び光ファイバコーティングシステム Download PDFInfo
- Publication number
- JP5154932B2 JP5154932B2 JP2007530482A JP2007530482A JP5154932B2 JP 5154932 B2 JP5154932 B2 JP 5154932B2 JP 2007530482 A JP2007530482 A JP 2007530482A JP 2007530482 A JP2007530482 A JP 2007530482A JP 5154932 B2 JP5154932 B2 JP 5154932B2
- Authority
- JP
- Japan
- Prior art keywords
- optical fiber
- primary
- coating material
- acrylate
- composition
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000000576 coating method Methods 0.000 title claims description 111
- 239000011248 coating agent Substances 0.000 title claims description 107
- 239000000463 material Substances 0.000 title claims description 96
- 239000013307 optical fiber Substances 0.000 title claims description 95
- 238000001879 gelation Methods 0.000 title claims description 11
- 239000000203 mixture Substances 0.000 claims description 136
- 239000000178 monomer Substances 0.000 claims description 48
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 claims description 20
- 150000001252 acrylic acid derivatives Chemical class 0.000 claims description 11
- 238000005253 cladding Methods 0.000 claims description 6
- UHESRSKEBRADOO-UHFFFAOYSA-N ethyl carbamate;prop-2-enoic acid Chemical compound OC(=O)C=C.CCOC(N)=O UHESRSKEBRADOO-UHFFFAOYSA-N 0.000 claims description 6
- KCTAWXVAICEBSD-UHFFFAOYSA-N prop-2-enoyloxy prop-2-eneperoxoate Chemical compound C=CC(=O)OOOC(=O)C=C KCTAWXVAICEBSD-UHFFFAOYSA-N 0.000 claims description 6
- 239000004721 Polyphenylene oxide Substances 0.000 claims description 5
- 125000001931 aliphatic group Chemical group 0.000 claims description 5
- 239000007795 chemical reaction product Substances 0.000 claims description 5
- UYMKPFRHYYNDTL-UHFFFAOYSA-N ethenamine Chemical compound NC=C UYMKPFRHYYNDTL-UHFFFAOYSA-N 0.000 claims description 5
- 229920000570 polyether Polymers 0.000 claims description 5
- 238000005033 Fourier transform infrared spectroscopy Methods 0.000 claims description 4
- BBILJUBMQKCJMS-UHFFFAOYSA-N 2-methyloxirane;prop-2-enoic acid Chemical compound CC1CO1.OC(=O)C=C BBILJUBMQKCJMS-UHFFFAOYSA-N 0.000 claims description 2
- 125000004805 propylene group Chemical group [H]C([H])([H])C([H])([*:1])C([H])([H])[*:2] 0.000 claims description 2
- 238000001723 curing Methods 0.000 description 39
- 239000000654 additive Substances 0.000 description 19
- 239000000126 substance Substances 0.000 description 17
- 238000000034 method Methods 0.000 description 16
- 239000002318 adhesion promoter Substances 0.000 description 12
- 239000003365 glass fiber Substances 0.000 description 11
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 11
- 230000003287 optical effect Effects 0.000 description 11
- 239000000835 fiber Substances 0.000 description 10
- 230000009477 glass transition Effects 0.000 description 10
- 238000006243 chemical reaction Methods 0.000 description 9
- 238000005259 measurement Methods 0.000 description 9
- 238000012360 testing method Methods 0.000 description 8
- 230000000052 comparative effect Effects 0.000 description 7
- 229920000642 polymer Polymers 0.000 description 6
- 239000000047 product Substances 0.000 description 6
- 230000000996 additive effect Effects 0.000 description 5
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 239000003963 antioxidant agent Substances 0.000 description 4
- 239000003999 initiator Substances 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 238000006116 polymerization reaction Methods 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- UUEWCQRISZBELL-UHFFFAOYSA-N 3-trimethoxysilylpropane-1-thiol Chemical compound CO[Si](OC)(OC)CCCS UUEWCQRISZBELL-UHFFFAOYSA-N 0.000 description 3
- 239000012958 Amine synergist Substances 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- GWQHGNJGONBJPS-UHFFFAOYSA-N [2-[[3-but-3-enoyloxy-2,2-bis(but-3-enoyloxymethyl)propoxy]methyl]-2-(but-3-enoyloxymethyl)-3-hydroxypropyl] but-3-enoate Chemical compound C=CCC(=O)OCC(COC(=O)CC=C)(CO)COCC(COC(=O)CC=C)(COC(=O)CC=C)COC(=O)CC=C GWQHGNJGONBJPS-UHFFFAOYSA-N 0.000 description 3
- -1 alkylene glycol Chemical compound 0.000 description 3
- 230000003078 antioxidant effect Effects 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 238000010438 heat treatment Methods 0.000 description 3
- PBOSTUDLECTMNL-UHFFFAOYSA-N lauryl acrylate Chemical compound CCCCCCCCCCCCOC(=O)C=C PBOSTUDLECTMNL-UHFFFAOYSA-N 0.000 description 3
- 239000010410 layer Substances 0.000 description 3
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 3
- 230000007935 neutral effect Effects 0.000 description 3
- 229920000728 polyester Polymers 0.000 description 3
- 239000002861 polymer material Substances 0.000 description 3
- 239000003505 polymerization initiator Substances 0.000 description 3
- 239000010453 quartz Substances 0.000 description 3
- 230000005855 radiation Effects 0.000 description 3
- QNODIIQQMGDSEF-UHFFFAOYSA-N (1-hydroxycyclohexyl)-phenylmethanone Chemical compound C=1C=CC=CC=1C(=O)C1(O)CCCCC1 QNODIIQQMGDSEF-UHFFFAOYSA-N 0.000 description 2
- PJAKWOZHTFWTNF-UHFFFAOYSA-N (2-nonylphenyl) prop-2-enoate Chemical class CCCCCCCCCC1=CC=CC=C1OC(=O)C=C PJAKWOZHTFWTNF-UHFFFAOYSA-N 0.000 description 2
- QVCUKHQDEZNNOC-UHFFFAOYSA-N 1,2-diazabicyclo[2.2.2]octane Chemical compound C1CC2CCN1NC2 QVCUKHQDEZNNOC-UHFFFAOYSA-N 0.000 description 2
- JWYVGKFDLWWQJX-UHFFFAOYSA-N 1-ethenylazepan-2-one Chemical compound C=CN1CCCCCC1=O JWYVGKFDLWWQJX-UHFFFAOYSA-N 0.000 description 2
- KWVGIHKZDCUPEU-UHFFFAOYSA-N 2,2-dimethoxy-2-phenylacetophenone Chemical compound C=1C=CC=CC=1C(OC)(OC)C(=O)C1=CC=CC=C1 KWVGIHKZDCUPEU-UHFFFAOYSA-N 0.000 description 2
- VFBJXXJYHWLXRM-UHFFFAOYSA-N 2-[2-[3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoyloxy]ethylsulfanyl]ethyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate Chemical compound CC(C)(C)C1=C(O)C(C(C)(C)C)=CC(CCC(=O)OCCSCCOC(=O)CCC=2C=C(C(O)=C(C=2)C(C)(C)C)C(C)(C)C)=C1 VFBJXXJYHWLXRM-UHFFFAOYSA-N 0.000 description 2
- KBQVDAIIQCXKPI-UHFFFAOYSA-N 3-trimethoxysilylpropyl prop-2-enoate Chemical compound CO[Si](OC)(OC)CCCOC(=O)C=C KBQVDAIIQCXKPI-UHFFFAOYSA-N 0.000 description 2
- LVGFPWDANALGOY-UHFFFAOYSA-N 8-methylnonyl prop-2-enoate Chemical compound CC(C)CCCCCCCOC(=O)C=C LVGFPWDANALGOY-UHFFFAOYSA-N 0.000 description 2
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 2
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 2
- INXWLSDYDXPENO-UHFFFAOYSA-N [2-(hydroxymethyl)-3-prop-2-enoyloxy-2-[[3-prop-2-enoyloxy-2,2-bis(prop-2-enoyloxymethyl)propoxy]methyl]propyl] prop-2-enoate Chemical compound C=CC(=O)OCC(COC(=O)C=C)(CO)COCC(COC(=O)C=C)(COC(=O)C=C)COC(=O)C=C INXWLSDYDXPENO-UHFFFAOYSA-N 0.000 description 2
- QOODKTDFNWDBNR-UHFFFAOYSA-N [dodecoxy(oxiran-2-yl)methyl] prop-2-enoate Chemical compound CCCCCCCCCCCCOC(OC(=O)C=C)C1CO1 QOODKTDFNWDBNR-UHFFFAOYSA-N 0.000 description 2
- 230000005540 biological transmission Effects 0.000 description 2
- WERYXYBDKMZEQL-UHFFFAOYSA-N butane-1,4-diol Chemical compound OCCCCO WERYXYBDKMZEQL-UHFFFAOYSA-N 0.000 description 2
- 239000008199 coating composition Substances 0.000 description 2
- 239000011247 coating layer Substances 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- 238000005520 cutting process Methods 0.000 description 2
- 230000007547 defect Effects 0.000 description 2
- VFHVQBAGLAREND-UHFFFAOYSA-N diphenylphosphoryl-(2,4,6-trimethylphenyl)methanone Chemical class CC1=CC(C)=CC(C)=C1C(=O)P(=O)(C=1C=CC=CC=1)C1=CC=CC=C1 VFHVQBAGLAREND-UHFFFAOYSA-N 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012544 monitoring process Methods 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- RZFODFPMOHAYIR-UHFFFAOYSA-N oxepan-2-one;prop-2-enoic acid Chemical compound OC(=O)C=C.O=C1CCCCCO1 RZFODFPMOHAYIR-UHFFFAOYSA-N 0.000 description 2
- 229920000058 polyacrylate Polymers 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 238000000518 rheometry Methods 0.000 description 2
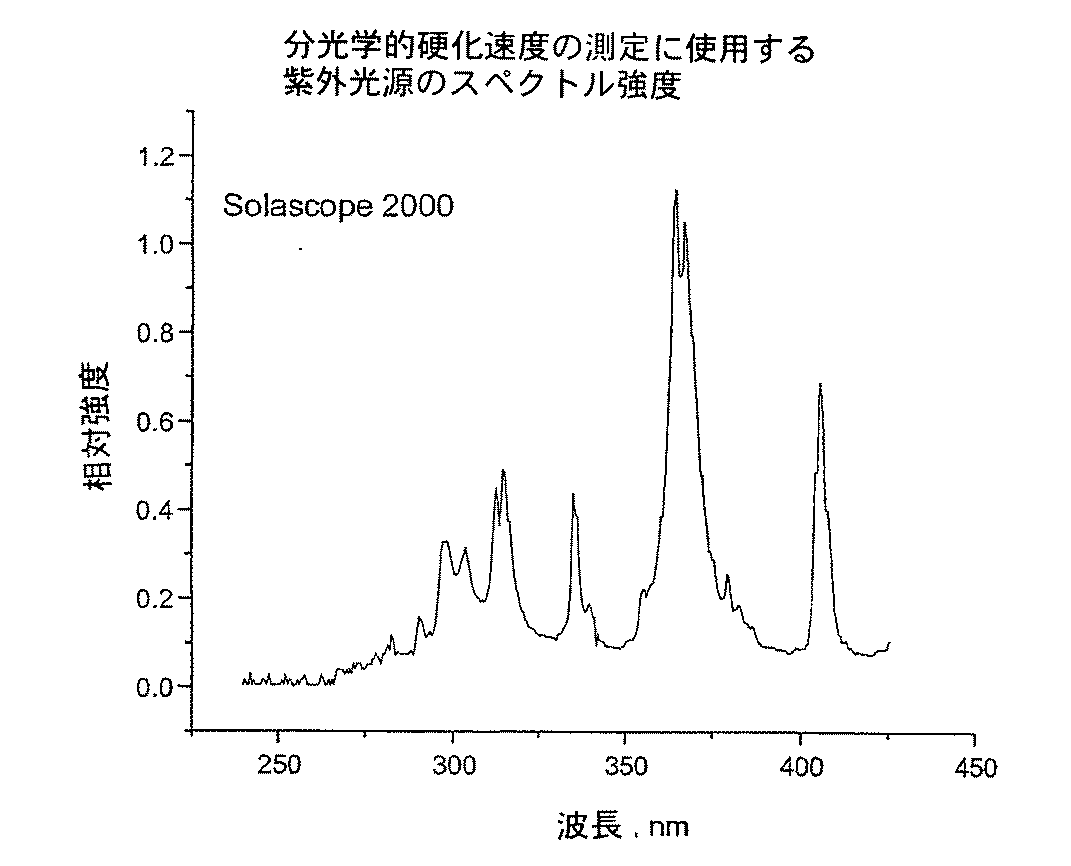
- 230000003595 spectral effect Effects 0.000 description 2
- 238000001228 spectrum Methods 0.000 description 2
- MAFQBSQRZKWGGE-UHFFFAOYSA-N trimethoxy-[2-[4-(2-trimethoxysilylethyl)phenyl]ethyl]silane Chemical compound CO[Si](OC)(OC)CCC1=CC=C(CC[Si](OC)(OC)OC)C=C1 MAFQBSQRZKWGGE-UHFFFAOYSA-N 0.000 description 2
- PSGCQDPCAWOCSH-UHFFFAOYSA-N (4,7,7-trimethyl-3-bicyclo[2.2.1]heptanyl) prop-2-enoate Chemical compound C1CC2(C)C(OC(=O)C=C)CC1C2(C)C PSGCQDPCAWOCSH-UHFFFAOYSA-N 0.000 description 1
- MYWOJODOMFBVCB-UHFFFAOYSA-N 1,2,6-trimethylphenanthrene Chemical compound CC1=CC=C2C3=CC(C)=CC=C3C=CC2=C1C MYWOJODOMFBVCB-UHFFFAOYSA-N 0.000 description 1
- ZMLPKJYZRQZLDA-UHFFFAOYSA-N 1-(2-phenylethenyl)-4-[4-(2-phenylethenyl)phenyl]benzene Chemical group C=1C=CC=CC=1C=CC(C=C1)=CC=C1C(C=C1)=CC=C1C=CC1=CC=CC=C1 ZMLPKJYZRQZLDA-UHFFFAOYSA-N 0.000 description 1
- 239000012956 1-hydroxycyclohexylphenyl-ketone Substances 0.000 description 1
- FTALTLPZDVFJSS-UHFFFAOYSA-N 2-(2-ethoxyethoxy)ethyl prop-2-enoate Chemical compound CCOCCOCCOC(=O)C=C FTALTLPZDVFJSS-UHFFFAOYSA-N 0.000 description 1
- WMYINDVYGQKYMI-UHFFFAOYSA-N 2-[2,2-bis(hydroxymethyl)butoxymethyl]-2-ethylpropane-1,3-diol Chemical compound CCC(CO)(CO)COCC(CC)(CO)CO WMYINDVYGQKYMI-UHFFFAOYSA-N 0.000 description 1
- FDSUVTROAWLVJA-UHFFFAOYSA-N 2-[[3-hydroxy-2,2-bis(hydroxymethyl)propoxy]methyl]-2-(hydroxymethyl)propane-1,3-diol;prop-2-enoic acid Chemical compound OC(=O)C=C.OC(=O)C=C.OC(=O)C=C.OC(=O)C=C.OC(=O)C=C.OCC(CO)(CO)COCC(CO)(CO)CO FDSUVTROAWLVJA-UHFFFAOYSA-N 0.000 description 1
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 description 1
- RZVINYQDSSQUKO-UHFFFAOYSA-N 2-phenoxyethyl prop-2-enoate Chemical compound C=CC(=O)OCCOC1=CC=CC=C1 RZVINYQDSSQUKO-UHFFFAOYSA-N 0.000 description 1
- WPMYUUITDBHVQZ-UHFFFAOYSA-N 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoic acid Chemical compound CC(C)(C)C1=CC(CCC(O)=O)=CC(C(C)(C)C)=C1O WPMYUUITDBHVQZ-UHFFFAOYSA-N 0.000 description 1
- QOXOZONBQWIKDA-UHFFFAOYSA-N 3-hydroxypropyl Chemical group [CH2]CCO QOXOZONBQWIKDA-UHFFFAOYSA-N 0.000 description 1
- SXIFAEWFOJETOA-UHFFFAOYSA-N 4-hydroxy-butyl Chemical group [CH2]CCCO SXIFAEWFOJETOA-UHFFFAOYSA-N 0.000 description 1
- SAZHWFFOFMSQPA-UHFFFAOYSA-N 4-phenylcoumarin Chemical compound C12=CC=CC=C2OC(=O)C=C1C1=CC=CC=C1 SAZHWFFOFMSQPA-UHFFFAOYSA-N 0.000 description 1
- JTHZUSWLNCPZLX-UHFFFAOYSA-N 6-fluoro-3-methyl-2h-indazole Chemical compound FC1=CC=C2C(C)=NNC2=C1 JTHZUSWLNCPZLX-UHFFFAOYSA-N 0.000 description 1
- DXPPIEDUBFUSEZ-UHFFFAOYSA-N 6-methylheptyl prop-2-enoate Chemical compound CC(C)CCCCCOC(=O)C=C DXPPIEDUBFUSEZ-UHFFFAOYSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 206010012442 Dermatitis contact Diseases 0.000 description 1
- 239000004593 Epoxy Substances 0.000 description 1
- 239000004386 Erythritol Substances 0.000 description 1
- UNXHWFMMPAWVPI-UHFFFAOYSA-N Erythritol Natural products OCC(O)C(O)CO UNXHWFMMPAWVPI-UHFFFAOYSA-N 0.000 description 1
- 229920003266 Leaf® Polymers 0.000 description 1
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 1
- 244000000231 Sesamum indicum Species 0.000 description 1
- 235000003434 Sesamum indicum Nutrition 0.000 description 1
- DAKWPKUUDNSNPN-UHFFFAOYSA-N Trimethylolpropane triacrylate Chemical compound C=CC(=O)OCC(CC)(COC(=O)C=C)COC(=O)C=C DAKWPKUUDNSNPN-UHFFFAOYSA-N 0.000 description 1
- 238000003848 UV Light-Curing Methods 0.000 description 1
- QYKIQEUNHZKYBP-UHFFFAOYSA-N Vinyl ether Chemical class C=COC=C QYKIQEUNHZKYBP-UHFFFAOYSA-N 0.000 description 1
- JOBBTVPTPXRUBP-UHFFFAOYSA-N [3-(3-sulfanylpropanoyloxy)-2,2-bis(3-sulfanylpropanoyloxymethyl)propyl] 3-sulfanylpropanoate Chemical compound SCCC(=O)OCC(COC(=O)CCS)(COC(=O)CCS)COC(=O)CCS JOBBTVPTPXRUBP-UHFFFAOYSA-N 0.000 description 1
- KNSXNCFKSZZHEA-UHFFFAOYSA-N [3-prop-2-enoyloxy-2,2-bis(prop-2-enoyloxymethyl)propyl] prop-2-enoate Chemical class C=CC(=O)OCC(COC(=O)C=C)(COC(=O)C=C)COC(=O)C=C KNSXNCFKSZZHEA-UHFFFAOYSA-N 0.000 description 1
- WOSYMBHLRSSRGN-UHFFFAOYSA-N [oxiran-2-yl(phenoxy)methyl] prop-2-enoate Chemical compound C1OC1C(OC(=O)C=C)OC1=CC=CC=C1 WOSYMBHLRSSRGN-UHFFFAOYSA-N 0.000 description 1
- GUCYFKSBFREPBC-UHFFFAOYSA-N [phenyl-(2,4,6-trimethylbenzoyl)phosphoryl]-(2,4,6-trimethylphenyl)methanone Chemical compound CC1=CC(C)=CC(C)=C1C(=O)P(=O)(C=1C=CC=CC=1)C(=O)C1=C(C)C=C(C)C=C1C GUCYFKSBFREPBC-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 150000007824 aliphatic compounds Chemical class 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 150000001491 aromatic compounds Chemical class 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- MQDJYUACMFCOFT-UHFFFAOYSA-N bis[2-(1-hydroxycyclohexyl)phenyl]methanone Chemical compound C=1C=CC=C(C(=O)C=2C(=CC=CC=2)C2(O)CCCCC2)C=1C1(O)CCCCC1 MQDJYUACMFCOFT-UHFFFAOYSA-N 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 238000012691 depolymerization reaction Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 229910003460 diamond Inorganic materials 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 230000008034 disappearance Effects 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- WNAHIZMDSQCWRP-UHFFFAOYSA-N dodecane-1-thiol Chemical compound CCCCCCCCCCCCS WNAHIZMDSQCWRP-UHFFFAOYSA-N 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000010894 electron beam technology Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 125000003700 epoxy group Chemical group 0.000 description 1
- UNXHWFMMPAWVPI-ZXZARUISSA-N erythritol Chemical compound OC[C@H](O)[C@H](O)CO UNXHWFMMPAWVPI-ZXZARUISSA-N 0.000 description 1
- 229940009714 erythritol Drugs 0.000 description 1
- 235000019414 erythritol Nutrition 0.000 description 1
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 1
- 230000006203 ethylation Effects 0.000 description 1
- 238000006200 ethylation reaction Methods 0.000 description 1
- LYCAIKOWRPUZTN-UHFFFAOYSA-N ethylene glycol Natural products OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 1
- 125000000816 ethylene group Chemical group [H]C([H])([*:1])C([H])([H])[*:2] 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000012632 extractable Substances 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 125000003976 glyceryl group Chemical group [H]C([*])([H])C(O[H])([H])C(O[H])([H])[H] 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 230000003301 hydrolyzing effect Effects 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 238000002329 infrared spectrum Methods 0.000 description 1
- 239000010687 lubricating oil Substances 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- NJGIAKIPSDCYAC-LURJTMIESA-N methyl (2r)-2-[(2-methylpropan-2-yl)oxycarbonylamino]-3-sulfanylpropanoate Chemical compound COC(=O)[C@H](CS)NC(=O)OC(C)(C)C NJGIAKIPSDCYAC-LURJTMIESA-N 0.000 description 1
- CRVGTESFCCXCTH-UHFFFAOYSA-N methyl diethanolamine Chemical compound OCCN(C)CCO CRVGTESFCCXCTH-UHFFFAOYSA-N 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 238000010525 oxidative degradation reaction Methods 0.000 description 1
- WKGDNXBDNLZSKC-UHFFFAOYSA-N oxido(phenyl)phosphanium Chemical compound O=[PH2]c1ccccc1 WKGDNXBDNLZSKC-UHFFFAOYSA-N 0.000 description 1
- AUONHKJOIZSQGR-UHFFFAOYSA-N oxophosphane Chemical compound P=O AUONHKJOIZSQGR-UHFFFAOYSA-N 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 230000010287 polarization Effects 0.000 description 1
- 229920000647 polyepoxide Polymers 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229920001451 polypropylene glycol Polymers 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 125000002572 propoxy group Chemical group [*]OC([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 238000010926 purge Methods 0.000 description 1
- 238000003847 radiation curing Methods 0.000 description 1
- SBIBMFFZSBJNJF-UHFFFAOYSA-N selenium;zinc Chemical compound [Se]=[Zn] SBIBMFFZSBJNJF-UHFFFAOYSA-N 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 239000012748 slip agent Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- MUTNCGKQJGXKEM-UHFFFAOYSA-N tamibarotene Chemical compound C=1C=C2C(C)(C)CCC(C)(C)C2=CC=1NC(=O)C1=CC=C(C(O)=O)C=C1 MUTNCGKQJGXKEM-UHFFFAOYSA-N 0.000 description 1
- 238000009864 tensile test Methods 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- XOALFFJGWSCQEO-UHFFFAOYSA-N tridecyl prop-2-enoate Chemical compound CCCCCCCCCCCCCOC(=O)C=C XOALFFJGWSCQEO-UHFFFAOYSA-N 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- UIYCHXAGWOYNNA-UHFFFAOYSA-N vinyl sulfide Chemical group C=CSC=C UIYCHXAGWOYNNA-UHFFFAOYSA-N 0.000 description 1
- 239000001993 wax Substances 0.000 description 1
- 238000004804 winding Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C25/00—Surface treatment of fibres or filaments made from glass, minerals or slags
- C03C25/10—Coating
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C25/00—Surface treatment of fibres or filaments made from glass, minerals or slags
- C03C25/10—Coating
- C03C25/104—Coating to obtain optical fibres
- C03C25/106—Single coatings
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C25/00—Surface treatment of fibres or filaments made from glass, minerals or slags
- C03C25/10—Coating
- C03C25/104—Coating to obtain optical fibres
- C03C25/1065—Multiple coatings
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C25/00—Surface treatment of fibres or filaments made from glass, minerals or slags
- C03C25/10—Coating
- C03C25/24—Coatings containing organic materials
- C03C25/26—Macromolecular compounds or prepolymers
- C03C25/28—Macromolecular compounds or prepolymers obtained by reactions involving only carbon-to-carbon unsaturated bonds
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D133/00—Coating compositions based on homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides, or nitriles thereof; Coating compositions based on derivatives of such polymers
- C09D133/04—Homopolymers or copolymers of esters
- C09D133/14—Homopolymers or copolymers of esters of esters containing halogen, nitrogen, sulfur or oxygen atoms in addition to the carboxy oxygen
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B6/00—Light guides; Structural details of arrangements comprising light guides and other optical elements, e.g. couplings
- G02B6/02—Optical fibres with cladding with or without a coating
- G02B6/02395—Glass optical fibre with a protective coating, e.g. two layer polymer coating deposited directly on a silica cladding surface during fibre manufacture
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Materials Engineering (AREA)
- Engineering & Computer Science (AREA)
- General Chemical & Material Sciences (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Geochemistry & Mineralogy (AREA)
- Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- General Physics & Mathematics (AREA)
- Wood Science & Technology (AREA)
- Surface Treatment Of Glass Fibres Or Filaments (AREA)
- Optical Fibers, Optical Fiber Cores, And Optical Fiber Bundles (AREA)
- Macromonomer-Based Addition Polymer (AREA)
- Paints Or Removers (AREA)
Description
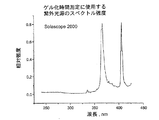
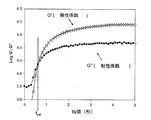
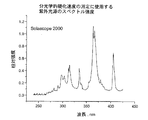
完全硬化バンド比は最後の10秒の露光の後のバンド比である。硬化速度は10%変換から40%変換までのリニア領域の%変換の変化率として計算される。
実験例
本発明が更に以下の限定されない例によって説明される。
実験例1
一次硬化組成物1乃至8、及び比較の一次硬化組成物C1が加熱バンド又は加熱マントルによって70℃に加熱された適切な容器内で高速ミキサを用いて作成された。各々のケースにおいて、秤を用いて成分の重さが量られて容器に入れられ、固体成分が完全に溶けるまで混合されて混合物は均一となる。硬化性組成物はオリゴマ、 モノマ、及び光開始剤の量が合計100重量%となるように構成され、他の添加剤はpphの単位で全混合物に添加された。BR3731、BR3741及びBR 582はボマール・スペシャリティー社によって市販されているオリゴマである。PHOTOMER 4003はコグニス社によって市販されているエトキシル化されたノニルフェノールアクリレートモノマである。N-ビニルピロリジノン及びN-ビニルカプロラクタムはアルドリッチ社によって市販されている。CN130はサートマー社によって市販されているラウリルオキシグリシジル・アクリレートモノマである。IRGACURE819及びJRGACURE 1850はチバ・スペシャリティー・ケミカル社によって市販されている光開始剤である。IRGANOX 1035はチバ・スペシャリティー・ケミカル社によって市販されている抗酸化物質である。ビス(トリメトキシシリルエチル)ベンゼン及び3-アクリルオキシプロピルトリメトキシシランはゲレスト社によって市販されている接着促進剤である。PHOTOMER 4399はコグニス社によって市販されているジペンタエリスリトール・モノヒドロキシ・ペンタアクリレートモノマである。UVITEX OBはチバ・スペシャリティー・ケミカル社によって市販されている蛍光増白剤である。トリエタノールアミンはフィッシャー・サイエンティフィック社(Fisher Scientific)によって市販されている。オリゴマ及び1以上のモノマが70℃で少なくとも1時間混合された。1以上の光開始剤及び添加剤がその後添加され、1時間混合が継続された。最後に、室温まで冷却した後、接着促進剤が添加され、30分間混合が継続された。一次硬化組成物1乃至8及び比較の一次硬化組成物C1を構成する成分が以下の表1に詳細に示されている。
Claims (4)
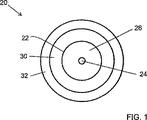
- コーティングされた光ファイバであって、コア及びクラッドを有する光ファイバと、前記光ファイバを包含する一次コーティング材と、からなり、
前記一次コーティング材は約5MPa以下のヤング率を有し、
前記一次コーティング材は紫外線強度3.4mW/cm 2 においてゲル化時間が約1.4秒より短い一次硬化組成物の硬化反応品であり、
前記一次硬化組成物は、
3000ダルトンから15000ダルトンの間の分子量を有するポリエーテル・ウレタン・アクリレート・オリゴマと、
エトキシル化されたアクリレート、エトキシル化されたアルキルフェノール・モノアクリレート、プロピレンオキサイドアクリレート、n−プロピレンオキサイドアクリレート、iso−プロピレンオキサイドアクリレート、単官能基のアクリレート、多官能のアクリレート、及びそれらの組合せから選択されたモノマと、
光開始剤と、
5乃至40重量%の濃度の単官能基の脂肪族エポキシ・アクリレート・モノマ及び2乃至40重量%の濃度のN−ビニルアミドモノマの少なくとも一方と、
を含むことを特徴とする光ファイバ。 - 前記一次硬化組成物は紫外線強度3.4mW/cm2においてゲル化時間が約1.2秒より短いことを特徴とする請求項1に記載のコーティングされた光ファイバ。
- 前記一次コーティング材は約1.5MPaより低いヤング率を有することを特徴とする請求項1に記載のコーティングされた光ファイバ。
- 前記一次硬化組成物は少なくとも約150%/秒のFTIRによって測定される分光学的硬化速度を有していることを特徴とする請求項1に記載のコーティングされた光ファイバ。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/937,069 | 2004-09-08 | ||
| US10/937,069 US7010206B1 (en) | 2004-09-08 | 2004-09-08 | Coated optical fiber and optical fiber coating system including a fast-gelling primary coating |
| PCT/US2005/031854 WO2006029202A1 (en) | 2004-09-08 | 2005-09-08 | Coated optical fiber and optical fiber coating system including a fast-gelling primary coating |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2008512334A JP2008512334A (ja) | 2008-04-24 |
| JP5154932B2 true JP5154932B2 (ja) | 2013-02-27 |
Family
ID=35503706
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007530482A Expired - Fee Related JP5154932B2 (ja) | 2004-09-08 | 2005-09-08 | ゲル化速度の早い一次コーティング材を含んだコーティングされた光ファイバ及び光ファイバコーティングシステム |
Country Status (6)
| Country | Link |
|---|---|
| US (2) | US7010206B1 (ja) |
| EP (1) | EP1799621A1 (ja) |
| JP (1) | JP5154932B2 (ja) |
| KR (1) | KR101203823B1 (ja) |
| CN (1) | CN101010265B (ja) |
| WO (1) | WO2006029202A1 (ja) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12036766B2 (en) | 2019-09-06 | 2024-07-16 | Dow Global Technologies Llc | Multilayer panel member |
| US12172349B2 (en) | 2019-09-06 | 2024-12-24 | Dow Global Technologies Llc | Flexible film fluid-dispensing device |
Families Citing this family (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7010206B1 (en) * | 2004-09-08 | 2006-03-07 | Corning Incorporated | Coated optical fiber and optical fiber coating system including a fast-gelling primary coating |
| US10578812B2 (en) | 2005-06-08 | 2020-03-03 | Commscope, Inc. Of North Carolina | Methods for forming connectorized fiber optic cabling |
| US8992098B2 (en) | 2005-06-08 | 2015-03-31 | Commscope, Inc. Of North Carolina | Methods for forming connectorized fiber optic cabling |
| US7537393B2 (en) | 2005-06-08 | 2009-05-26 | Commscope, Inc. Of North Carolina | Connectorized fiber optic cabling and methods for forming the same |
| US7742667B2 (en) * | 2005-06-08 | 2010-06-22 | Commscope, Inc. Of North Carolina | Fiber optic cables and methods for forming the same |
| CN101228468B (zh) * | 2006-08-10 | 2011-06-08 | 古河电气工业株式会社 | 光纤 |
| KR101108450B1 (ko) * | 2007-11-20 | 2012-02-20 | 아사히 가세이 겐자이 가부시키가이샤 | 열경화성 수지 발포판의 제조 방법 |
| RU2487839C2 (ru) * | 2007-11-29 | 2013-07-20 | Корнинг Инкорпорейтед | Отверждение волокна протяженными излучателями |
| WO2010036684A2 (en) | 2008-09-26 | 2010-04-01 | Corning Incorporated | High numerical aperture multimode optical fiber |
| SG176293A1 (en) * | 2009-06-04 | 2012-01-30 | Research In Motion Ltd | Methods and apparatus for use in facilitating the communication of neighboring network information to a mobile terminal with use of a radius compatible protocol |
| US8406596B2 (en) | 2009-08-12 | 2013-03-26 | Corning Incorporated | Optical fiber containing multi-layered coating system |
| US8385703B2 (en) | 2010-03-02 | 2013-02-26 | Corning Incorporated | High numerical aperture multimode optical fiber |
| US20110300367A1 (en) | 2010-06-07 | 2011-12-08 | Ching-Kee Chien | Optical Fiber With Photoacid Coating |
| US10221503B2 (en) * | 2011-04-07 | 2019-03-05 | Board Of Regents Of The University Of Texas System | Photopolymerizable compositions for solventless fiber spinning |
| RU2014115209A (ru) | 2011-09-16 | 2015-10-27 | Корнинг Инкорпорейтед | Маломодовые оптические волокна для мультиплексирования с модовым разделением |
| US8848285B2 (en) | 2012-01-12 | 2014-09-30 | Corning Incorporated | Few mode optical fibers for Er doped amplifiers, and amplifiers using such |
| US8971682B2 (en) | 2012-03-01 | 2015-03-03 | Corning Incorporated | Few mode optical fibers |
| DE102012204605B4 (de) | 2012-03-22 | 2021-09-09 | Automotive Lighting Reutlingen Gmbh | Verfahren zum Herstellen eines Lichtleitkörpers und Lichtleitkörper |
| US9197030B2 (en) | 2012-07-31 | 2015-11-24 | Corning Incorporated | Few mode rare earth doped optical fibers for optical amplifiers, and amplifiers using such fibers |
| WO2014159702A2 (en) * | 2013-03-14 | 2014-10-02 | Vascular Imaging Corporation | Optical fiber ribbon imaging guidewire and methods |
| US9383511B2 (en) * | 2013-05-02 | 2016-07-05 | Corning Incorporated | Optical fiber with large mode field diameter and low microbending losses |
| US9810838B2 (en) | 2013-09-12 | 2017-11-07 | Corning Incorporated | Fiber coatings with low young's modulus and high tear strength |
| US9244221B1 (en) | 2013-12-10 | 2016-01-26 | Corning Incorporated | Low modulus primary coatings for optical fibers |
| US9708491B2 (en) | 2014-06-04 | 2017-07-18 | Corning Incorporated | Optical fiber coating and composition |
| US10359563B2 (en) | 2015-03-20 | 2019-07-23 | Corning Incorporated | Few-mode optical fiber |
| US9874686B2 (en) | 2015-05-29 | 2018-01-23 | Corning Incorporated | Optical fiber with macrobend loss mitigating layer |
| US10604659B2 (en) | 2015-06-08 | 2020-03-31 | Dsm Ip Assets B.V. | Liquid, hybrid UV/VIS radiation curable resin compositions for additive fabrication |
| JP6880416B2 (ja) | 2015-10-01 | 2021-06-02 | ディーエスエム アイピー アセッツ ビー.ブイ.Dsm Ip Assets B.V. | 付加造形用液状ハイブリッドUV/vis線硬化性樹脂組成物 |
| JP7111432B2 (ja) * | 2017-06-02 | 2022-08-02 | コベストロ (ネザーランズ) ビー.ブイ. | 光ファイバー用耐熱放射線硬化性コーティング |
| EP3820827A1 (en) | 2018-08-30 | 2021-05-19 | DSM IP Assets B.V. | Radiation curable compositions for coating optical fiber |
| CN110045457B (zh) * | 2019-04-11 | 2020-06-26 | 电子科技大学 | 一种基于包层软化和多包层结构的声波增敏光纤 |
| CN110304821A (zh) * | 2019-07-16 | 2019-10-08 | 成都中住光纤有限公司 | 一种低衰减变化的小直径光纤及其制造方法 |
| US11822117B2 (en) * | 2019-10-08 | 2023-11-21 | Corning Incorporated | Primary coating compositions with improved microbending performance |
| CN112680020A (zh) * | 2019-10-17 | 2021-04-20 | 上海飞凯光电材料股份有限公司 | 油墨、制备方法及其应用 |
| CN112341184B (zh) * | 2020-11-09 | 2021-11-23 | 新沂市锡沂高新材料产业技术研究院有限公司 | 一种基于Isobam凝胶态浸涂技术的波导结构激光透明陶瓷光纤的制备方法 |
| CA3256585A1 (en) * | 2022-05-06 | 2023-11-09 | Igm Group B. V. | PHOTO-INITIATOR SYSTEM COMPRISING PHOSPHINE OXIDE-BASED PHOTO-INITIATORS, OXAZOLE-BASED SENSITIZING AGENTS, AND AMINE-TYPE ADDITIVES |
Family Cites Families (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5712035A (en) * | 1992-04-20 | 1998-01-27 | Dsm N.V. | Liquid curable resin composition |
| WO1996028396A1 (en) * | 1995-03-13 | 1996-09-19 | Dsm N.V. | Radiation curable optical fiber coating composition |
| CN1175036C (zh) * | 1997-04-22 | 2004-11-10 | Dsm有限公司 | 液态可固化树脂组合物 |
| US6187835B1 (en) * | 1997-06-18 | 2001-02-13 | Dsm N.V. | Radiation-curable optical fiber coatings having reduced yellowing and fast cure speed |
| CN1206280C (zh) | 1998-04-15 | 2005-06-15 | Dsmip财产有限公司 | 可辐射固化的树脂组合物 |
| US6744954B1 (en) * | 1998-11-20 | 2004-06-01 | Sumitomo Electric Industries, Ltd. | Submarine optical cable, optical fiber unit employed in the submarine optical cable, and method of making optical fiber unit |
| US6650821B1 (en) * | 1999-01-06 | 2003-11-18 | Sumitomo Electric Industries, Ltd. | Optical device and a making method thereof |
| US6316516B1 (en) * | 1999-04-29 | 2001-11-13 | Corning Incorporated | Coating composition for optical fibers |
| AU3789401A (en) * | 1999-12-30 | 2001-07-16 | Corning Incorporated | Fast curing primary optical fiber coating |
| US6489376B1 (en) * | 2000-07-31 | 2002-12-03 | Alcatel | Formulation of UV-curable coatings for optical fiber for a fast cure |
| EP1205450A1 (en) * | 2000-11-07 | 2002-05-15 | Alcatel | Coating for optical fibers and method of manufacturing thereof |
| US6916855B2 (en) * | 2000-11-22 | 2005-07-12 | Dsm Ip Assets B.V. | Radiation curable compositions |
| US6602601B2 (en) * | 2000-12-22 | 2003-08-05 | Corning Incorporated | Optical fiber coating compositions |
| WO2002066390A1 (fr) * | 2001-02-20 | 2002-08-29 | Sumitomo Electric Industries, Ltd. | Fibre optique revetue, bande centrale de fibre optique utilisant cette derniere et ensemble de fibre optique |
| JP4782294B2 (ja) | 2001-03-02 | 2011-09-28 | ディーエスエム アイピー アセッツ ビー.ブイ. | 多層被覆 |
| US6470128B1 (en) * | 2001-03-30 | 2002-10-22 | Alcatel | UV-curable coating composition for optical fiber for a fast cure and with improved adhesion to glass |
| US7276543B2 (en) * | 2001-10-09 | 2007-10-02 | Dsm Ip Assets B.V. | Radiation curable resin composition |
| US20040022511A1 (en) * | 2002-04-24 | 2004-02-05 | Eekelen Jan Van | Coated optical fibers |
| US7010206B1 (en) * | 2004-09-08 | 2006-03-07 | Corning Incorporated | Coated optical fiber and optical fiber coating system including a fast-gelling primary coating |
-
2004
- 2004-09-08 US US10/937,069 patent/US7010206B1/en not_active Expired - Lifetime
-
2005
- 2005-09-08 WO PCT/US2005/031854 patent/WO2006029202A1/en not_active Ceased
- 2005-09-08 KR KR1020077007924A patent/KR101203823B1/ko not_active Expired - Fee Related
- 2005-09-08 CN CN2005800297892A patent/CN101010265B/zh not_active Expired - Fee Related
- 2005-09-08 JP JP2007530482A patent/JP5154932B2/ja not_active Expired - Fee Related
- 2005-09-08 EP EP05803966A patent/EP1799621A1/en not_active Withdrawn
-
2006
- 2006-03-02 US US11/368,205 patent/US7221842B2/en not_active Expired - Lifetime
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12036766B2 (en) | 2019-09-06 | 2024-07-16 | Dow Global Technologies Llc | Multilayer panel member |
| US12172349B2 (en) | 2019-09-06 | 2024-12-24 | Dow Global Technologies Llc | Flexible film fluid-dispensing device |
Also Published As
| Publication number | Publication date |
|---|---|
| US7010206B1 (en) | 2006-03-07 |
| EP1799621A1 (en) | 2007-06-27 |
| WO2006029202A1 (en) | 2006-03-16 |
| CN101010265A (zh) | 2007-08-01 |
| US7221842B2 (en) | 2007-05-22 |
| US20060153513A1 (en) | 2006-07-13 |
| JP2008512334A (ja) | 2008-04-24 |
| KR101203823B1 (ko) | 2012-11-23 |
| KR20070100876A (ko) | 2007-10-12 |
| CN101010265B (zh) | 2013-02-27 |
| US20060051040A1 (en) | 2006-03-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5154932B2 (ja) | ゲル化速度の早い一次コーティング材を含んだコーティングされた光ファイバ及び光ファイバコーティングシステム | |
| US9488774B2 (en) | Primary optical fiber coating composition containing non-radiation curable component | |
| JP4931582B2 (ja) | 被覆光ファイバおよび光ファイバを被覆するのに適した硬化性組成物 | |
| CN100510822C (zh) | 光纤涂层体系以及经涂覆的光纤 | |
| US6014488A (en) | Coated optical fibers having strippable primary coatings and processes for making and using same | |
| US9678247B2 (en) | Primary optical fiber coating composition containing non-radiation curable component | |
| CN110167983A (zh) | 具有低模量和高临界应力的光纤涂层 | |
| EP3911980A1 (en) | Optical fiber cable with high fiber count | |
| CN112074493A (zh) | 具有低拔出力的光纤涂层 | |
| JP2008527420A (ja) | 被覆光ファイバおよび光ファイバの被覆に好適な硬化性組成物 | |
| JP2005504698A (ja) | 接着促進剤を用いた被覆光ファイバと、その製造方法及び使用方法 | |
| US9563013B2 (en) | Primary coatings for optical fibers having short gel times | |
| US7010205B2 (en) | Coated optical fiber and optical fiber coating system including a hydrophilic primary coating | |
| WO2019144005A1 (en) | Synthesis of oligomer for optical fiber coating | |
| WO2020117513A2 (en) | Fiber coating compositions with acylgermane photoinitiators | |
| CN114845967A (zh) | 具有低拔出力的光纤涂层 | |
| WO2021091527A1 (en) | Optical fibers with thin coatings | |
| CN111201206A (zh) | 具有快速固化速度的光纤涂层 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20080715 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20111108 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120208 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20121204 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20121206 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20151214 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5154932 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |