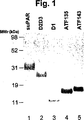
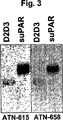
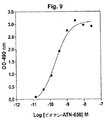
JP4890449B2 - 下流uPAR相互作用を阻害するウロキナーゼ型プラスミノーゲン活性化因子(uPA)とそのレセプター(uPAR)との複合体に結合するリガンド:診断または治療における同定および使用 - Google Patents
下流uPAR相互作用を阻害するウロキナーゼ型プラスミノーゲン活性化因子(uPA)とそのレセプター(uPAR)との複合体に結合するリガンド:診断または治療における同定および使用 Download PDFInfo
- Publication number
- JP4890449B2 JP4890449B2 JP2007515288A JP2007515288A JP4890449B2 JP 4890449 B2 JP4890449 B2 JP 4890449B2 JP 2007515288 A JP2007515288 A JP 2007515288A JP 2007515288 A JP2007515288 A JP 2007515288A JP 4890449 B2 JP4890449 B2 JP 4890449B2
- Authority
- JP
- Japan
- Prior art keywords
- antigen
- antibody
- upar
- seq
- binding fragment
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2896—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against molecules with a "CD"-designation, not provided for elsewhere
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/40—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/08—Drugs for genital or sexual disorders; Contraceptives for gonadal disorders or for enhancing fertility, e.g. inducers of ovulation or of spermatogenesis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/06—Antiglaucoma agents or miotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/24—Drugs for disorders of the endocrine system of the sex hormones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/02—Antithrombotic agents; Anticoagulants; Platelet aggregation inhibitors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/08—Vasodilators for multiple indications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Immunology (AREA)
- Molecular Biology (AREA)
- Oncology (AREA)
- Diabetes (AREA)
- Hematology (AREA)
- Endocrinology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Reproductive Health (AREA)
- Genetics & Genomics (AREA)
- Ophthalmology & Optometry (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Rheumatology (AREA)
- Physical Education & Sports Medicine (AREA)
- Pain & Pain Management (AREA)
- Communicable Diseases (AREA)
- Neurosurgery (AREA)
- Dermatology (AREA)
- Pulmonology (AREA)
- Gynecology & Obstetrics (AREA)
- Pregnancy & Childbirth (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US57389604P | 2004-05-25 | 2004-05-25 | |
| US60/573,896 | 2004-05-25 | ||
| PCT/US2005/018322 WO2005116077A2 (en) | 2004-05-25 | 2005-05-25 | LIGANDS BINDING THE COMPLEX OF UROKINASE-TYPE PLASMINOGEN ACTIVATOR (uPA) AND ITS RECEPTOR (uPAR) THAT INHIBIT DOWNSTREAM uPAR INTERACTIONS: IDENTIFICATION AND USE IN DIAGNOSIS OR THERAPY |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2008509086A JP2008509086A (ja) | 2008-03-27 |
| JP2008509086A5 JP2008509086A5 (enExample) | 2008-07-10 |
| JP4890449B2 true JP4890449B2 (ja) | 2012-03-07 |
Family
ID=35451457
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007515288A Expired - Lifetime JP4890449B2 (ja) | 2004-05-25 | 2005-05-25 | 下流uPAR相互作用を阻害するウロキナーゼ型プラスミノーゲン活性化因子(uPA)とそのレセプター(uPAR)との複合体に結合するリガンド:診断または治療における同定および使用 |
Country Status (16)
| Country | Link |
|---|---|
| US (1) | US8101726B2 (enExample) |
| EP (1) | EP1765397B1 (enExample) |
| JP (1) | JP4890449B2 (enExample) |
| KR (1) | KR20070047246A (enExample) |
| CN (1) | CN101022830A (enExample) |
| AU (1) | AU2005248400A1 (enExample) |
| CA (1) | CA2568428C (enExample) |
| DK (1) | DK1765397T3 (enExample) |
| ES (1) | ES2398221T3 (enExample) |
| IL (1) | IL179565A0 (enExample) |
| MX (1) | MXPA06013709A (enExample) |
| PL (1) | PL1765397T3 (enExample) |
| PT (1) | PT1765397E (enExample) |
| RU (1) | RU2006145905A (enExample) |
| WO (1) | WO2005116077A2 (enExample) |
| ZA (1) | ZA200610578B (enExample) |
Families Citing this family (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8343489B2 (en) * | 2006-03-21 | 2013-01-01 | Weaver David T | Methods for humanizing antibodies and humanized antibodies made thereby |
| CN1919874B (zh) * | 2006-09-18 | 2010-09-08 | 中国人民解放军军事医学科学院生物工程研究所 | 一种抗尿激酶型纤溶酶激活剂受体的抗体样分子ATF-Fc融合蛋白及其用途 |
| DK2117571T3 (en) * | 2006-12-08 | 2017-05-08 | Monopar Therapeutics Inc | Urokinase-type plasminogen activator receptor epitope |
| US8815519B2 (en) * | 2006-12-22 | 2014-08-26 | Hvidovre Hospital | Method for predicting cancer and other diseases |
| EP2050764A1 (en) | 2007-10-15 | 2009-04-22 | sanofi-aventis | Novel polyvalent bispecific antibody format and uses thereof |
| EP2730282A1 (en) | 2007-11-08 | 2014-05-14 | The General Hospital Corporation | Methods and compositions for the treatment of proteinuric diseases |
| EP2352503B1 (en) * | 2008-11-06 | 2017-07-19 | University of Miami | Role of soluble upar in the pathogenesis of proteinuric kidney disease |
| CN102597775A (zh) | 2009-09-25 | 2012-07-18 | 佐马技术有限公司 | 筛选方法 |
| CN103391787A (zh) | 2010-12-22 | 2013-11-13 | 意大利癌症研究基金会分子肿瘤学研究所(Ifom) | uPAR拮抗剂及其应用 |
| WO2012097313A2 (en) | 2011-01-14 | 2012-07-19 | The Regents Of The University Of California | Therapeutic antibodies against ror-1 protein and methods for use of same |
| US20130344074A1 (en) | 2011-03-16 | 2013-12-26 | Sanofi | Uses of a dual v region antibody-like protein |
| US9867923B2 (en) | 2011-05-09 | 2018-01-16 | University Of Miami | Reducing soluble urokinase receptor in the circulation |
| AU2012293720A1 (en) | 2011-08-05 | 2014-02-27 | Ifom Fondazione Istituto Firc Di Oncologia Molecolare | Constitutively active uPAR variants and their use for the generation and isolation of inhibitory antibodies |
| PT3929207T (pt) * | 2013-03-15 | 2024-10-21 | Univ Texas | Utilização do péptido fttftvt no tratamento de fibrose |
| CN105368859B (zh) * | 2015-11-25 | 2019-10-18 | 王任直 | 一种嵌合抗原受体hCD87-CAR及载有hCD87-CAR基因结构的慢病毒及质粒及其应用 |
| US10959062B1 (en) | 2018-04-19 | 2021-03-23 | Tango Tango, Inc. | System and method for push-to-talk over cellular integration with software defined radio |
| US10681506B1 (en) | 2018-04-19 | 2020-06-09 | Tango Tango, Inc. | System and method for push-to-talk over cellular integration with software defined radio |
| EP3849580A4 (en) | 2018-09-10 | 2022-06-15 | Lung Therapeutics, Inc. | Modified peptide fragments of cav-1 protein and the use thereof in the treatment of fibrosis |
| WO2020069498A1 (en) | 2018-09-28 | 2020-04-02 | Jochen Reiser | Supar and prediction and treatment of acute kidney injury |
| JP7774871B2 (ja) * | 2019-10-28 | 2025-11-25 | モナシュ ユニバーシティ | プラスミンとの結合のための抗体 |
| JP2023532426A (ja) * | 2020-06-15 | 2023-07-28 | モノパー セラピューティクス、インコーポレイテッド | 重症COVID-19疾患の治療のためのウロキナーゼプラスミノーゲン活性化因子受容体(uPAR)の正確な放射線免疫治療標的化 |
| IL306074A (en) | 2021-03-24 | 2023-11-01 | Alkermes Inc | UPAR antibodies and fusion proteins with them |
| CA3223227A1 (en) * | 2021-05-21 | 2022-11-24 | NorthStar Medical Technologies, LLC | Urokinase plasminogen activator receptor-targeted radiopharmaceutical |
| WO2022261398A1 (en) * | 2021-06-11 | 2022-12-15 | Memorial Sloan-Kettering Cancer Center | Antigen recognizing receptors targeting upar and uses thereof |
| US20250197838A1 (en) * | 2022-03-24 | 2025-06-19 | The United States Government As Represented By The Department Of Veterans Affairs | Urokinase-type plasminogen activator receptor binding peptides and methods of use |
| EP4610269A1 (en) | 2024-02-29 | 2025-09-03 | 3B Pharmaceuticals GmbH | Urokinase plasminogen activator surface receptor (upar) ligands for diagnostic or therapeutic use |
| WO2025207781A1 (en) | 2024-03-26 | 2025-10-02 | Monopar Therapeutics Inc. | Antibody radioisotope constructs |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5532132A (en) * | 1993-09-28 | 1996-07-02 | President And Fellows Of Harvard University | Method for regulating formation of a complex of plasminogen activator, its receptor and inhibitor |
| DE10117381A1 (de) * | 2001-04-06 | 2002-10-10 | Wilex Biotechnology Gmbh | Herstellung polyklonaler, monospezifischer Antikörper gegen die uPAR-Varianten del4, del5 und del4+5 sowie deren Verwendung für diagnostische und therapeutische Zwecke |
| US20030219837A1 (en) * | 2002-03-18 | 2003-11-27 | Cress Anne E. | Integrin ligand |
| DK2117571T3 (en) * | 2006-12-08 | 2017-05-08 | Monopar Therapeutics Inc | Urokinase-type plasminogen activator receptor epitope |
-
2005
- 2005-05-25 US US11/597,689 patent/US8101726B2/en active Active
- 2005-05-25 DK DK05754219.3T patent/DK1765397T3/da active
- 2005-05-25 PL PL05754219T patent/PL1765397T3/pl unknown
- 2005-05-25 ZA ZA200610578A patent/ZA200610578B/xx unknown
- 2005-05-25 WO PCT/US2005/018322 patent/WO2005116077A2/en not_active Ceased
- 2005-05-25 ES ES05754219T patent/ES2398221T3/es not_active Expired - Lifetime
- 2005-05-25 CN CNA2005800249140A patent/CN101022830A/zh active Pending
- 2005-05-25 JP JP2007515288A patent/JP4890449B2/ja not_active Expired - Lifetime
- 2005-05-25 RU RU2006145905/13A patent/RU2006145905A/ru not_active Application Discontinuation
- 2005-05-25 AU AU2005248400A patent/AU2005248400A1/en not_active Abandoned
- 2005-05-25 CA CA2568428A patent/CA2568428C/en not_active Expired - Lifetime
- 2005-05-25 PT PT57542193T patent/PT1765397E/pt unknown
- 2005-05-25 MX MXPA06013709A patent/MXPA06013709A/es active IP Right Grant
- 2005-05-25 KR KR1020067027198A patent/KR20070047246A/ko not_active Withdrawn
- 2005-05-25 EP EP05754219A patent/EP1765397B1/en not_active Expired - Lifetime
-
2006
- 2006-11-23 IL IL179565A patent/IL179565A0/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| EP1765397A4 (en) | 2009-05-13 |
| PL1765397T3 (pl) | 2013-04-30 |
| CA2568428C (en) | 2020-12-29 |
| MXPA06013709A (es) | 2008-01-16 |
| AU2005248400A1 (en) | 2005-12-08 |
| JP2008509086A (ja) | 2008-03-27 |
| DK1765397T3 (da) | 2013-02-04 |
| IL179565A0 (en) | 2007-05-15 |
| PT1765397E (pt) | 2013-02-04 |
| EP1765397B1 (en) | 2012-10-24 |
| ES2398221T3 (es) | 2013-03-14 |
| RU2006145905A (ru) | 2008-06-27 |
| CN101022830A (zh) | 2007-08-22 |
| US8101726B2 (en) | 2012-01-24 |
| CA2568428A1 (en) | 2005-12-08 |
| US20090180952A1 (en) | 2009-07-16 |
| WO2005116077A3 (en) | 2005-12-29 |
| KR20070047246A (ko) | 2007-05-04 |
| EP1765397A2 (en) | 2007-03-28 |
| ZA200610578B (en) | 2009-09-30 |
| WO2005116077A2 (en) | 2005-12-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4890449B2 (ja) | 下流uPAR相互作用を阻害するウロキナーゼ型プラスミノーゲン活性化因子(uPA)とそのレセプター(uPAR)との複合体に結合するリガンド:診断または治療における同定および使用 | |
| EP2117571B1 (en) | Urokinase-type plasminogen activator receptor epitope | |
| CN112135626B (zh) | 抗tigit抗体及其用途 | |
| KR101531317B1 (ko) | 항-ανβ6 항체 및 이의 용도 | |
| CN116410316A (zh) | 抗cd47抗体及其用途 | |
| US20250353915A1 (en) | Antibodies to galectin-3 and methods of use thereof | |
| EP4169947A1 (en) | Anti-claudin18.2 antibody and use thereof | |
| IL255416B1 (en) | Anti – cd71 antibodies, activatable anti – cd71 antibodies compositions comprising same and uses thereof | |
| JP2008538289A (ja) | 結腸癌および膵臓癌のための、組換え型モノクローナル抗体および対応する抗原 | |
| US20240026009A1 (en) | Antibodies to galectin-3 and methods of use thereof | |
| KR20100023869A (ko) | 페리오스틴의 Exon-17 부위에 의해 코드되는 펩티드에 대한 항체를 함유하는 암 치료제 | |
| US7951589B2 (en) | Monoclonal antibodies specific for the chemotactic epitope of the urokinase-type plasminogen activator receptor | |
| WO2007134274A2 (en) | Antibodies to urokinase- type plasminogen activator receptor(upar)bind cancer stem cells: use in diagnosis and therapy | |
| HK40084746A (en) | Coagulation factor xi (fxi) binding protein | |
| US20050232926A1 (en) | Antibodies specific for cancer associated antigen SM5-1 and uses thereof | |
| HK40042152B (zh) | 抗tigit抗体及其用途 | |
| JPWO1999060025A1 (ja) | 遺伝子組換え抗体 | |
| MXPA00009667A (en) | Humanized antibody against human tissue factor (tf) and process for constructing humanized antibody | |
| HK1011560A1 (en) | Method of diagnosing and treating epithelioma | |
| HK1011560B (en) | Method of diagnosing and treating epithelioma |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080521 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20080521 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110224 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110506 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110513 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110623 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110630 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110725 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20110725 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110822 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20111027 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20111117 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20111214 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 Ref document number: 4890449 Country of ref document: JP |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20141222 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| EXPY | Cancellation because of completion of term |