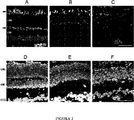
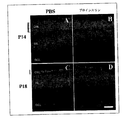
JP4727748B2 - 神経保護医薬組成物の製造のためのプロインスリンの使用、それを含む治療組成物、およびそれらの応用 - Google Patents
神経保護医薬組成物の製造のためのプロインスリンの使用、それを含む治療組成物、およびそれらの応用 Download PDFInfo
- Publication number
- JP4727748B2 JP4727748B2 JP2009511534A JP2009511534A JP4727748B2 JP 4727748 B2 JP4727748 B2 JP 4727748B2 JP 2009511534 A JP2009511534 A JP 2009511534A JP 2009511534 A JP2009511534 A JP 2009511534A JP 4727748 B2 JP4727748 B2 JP 4727748B2
- Authority
- JP
- Japan
- Prior art keywords
- proinsulin
- nucleotide sequence
- compound
- human
- seq
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 108010076181 Proinsulin Proteins 0.000 title claims description 194
- 239000008194 pharmaceutical composition Substances 0.000 title claims description 56
- 230000000324 neuroprotective effect Effects 0.000 title claims description 21
- 238000004519 manufacturing process Methods 0.000 title claims description 11
- 239000000203 mixture Substances 0.000 title description 9
- 230000001225 therapeutic effect Effects 0.000 title description 9
- 239000002773 nucleotide Substances 0.000 claims description 75
- 125000003729 nucleotide group Chemical group 0.000 claims description 75
- 150000001875 compounds Chemical class 0.000 claims description 62
- 108090000623 proteins and genes Proteins 0.000 claims description 55
- 230000000694 effects Effects 0.000 claims description 49
- 230000004770 neurodegeneration Effects 0.000 claims description 32
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 claims description 29
- 102000004169 proteins and genes Human genes 0.000 claims description 27
- 238000011282 treatment Methods 0.000 claims description 27
- 208000015122 neurodegenerative disease Diseases 0.000 claims description 26
- 239000013604 expression vector Substances 0.000 claims description 23
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 23
- 210000001519 tissue Anatomy 0.000 claims description 19
- 230000001939 inductive effect Effects 0.000 claims description 18
- 108091028043 Nucleic acid sequence Proteins 0.000 claims description 17
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 17
- 102000004877 Insulin Human genes 0.000 claims description 16
- 108090001061 Insulin Proteins 0.000 claims description 16
- 210000003205 muscle Anatomy 0.000 claims description 16
- 229940125396 insulin Drugs 0.000 claims description 15
- 239000012634 fragment Substances 0.000 claims description 14
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 13
- 208000007014 Retinitis pigmentosa Diseases 0.000 claims description 12
- 201000010099 disease Diseases 0.000 claims description 10
- 238000003782 apoptosis assay Methods 0.000 claims description 9
- 239000003814 drug Substances 0.000 claims description 9
- 230000005522 programmed cell death Effects 0.000 claims description 9
- 230000000626 neurodegenerative effect Effects 0.000 claims description 8
- 230000004112 neuroprotection Effects 0.000 claims description 8
- 230000002265 prevention Effects 0.000 claims description 8
- 208000035475 disorder Diseases 0.000 claims description 7
- 210000005260 human cell Anatomy 0.000 claims description 7
- 230000001684 chronic effect Effects 0.000 claims description 6
- 210000003527 eukaryotic cell Anatomy 0.000 claims description 6
- 230000002503 metabolic effect Effects 0.000 claims description 5
- 239000002671 adjuvant Substances 0.000 claims description 4
- 239000000969 carrier Substances 0.000 claims description 4
- 230000009885 systemic effect Effects 0.000 claims description 4
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 3
- 125000003275 alpha amino acid group Chemical group 0.000 claims 10
- 241000699670 Mus sp. Species 0.000 description 97
- 210000004027 cell Anatomy 0.000 description 46
- 230000002207 retinal effect Effects 0.000 description 46
- 238000000034 method Methods 0.000 description 33
- 150000001413 amino acids Chemical group 0.000 description 32
- 230000004044 response Effects 0.000 description 28
- 210000001525 retina Anatomy 0.000 description 27
- 230000007850 degeneration Effects 0.000 description 22
- 230000014509 gene expression Effects 0.000 description 19
- 108091008695 photoreceptors Proteins 0.000 description 17
- 241000699666 Mus <mouse, genus> Species 0.000 description 15
- 210000002966 serum Anatomy 0.000 description 15
- 239000000243 solution Substances 0.000 description 15
- 230000009261 transgenic effect Effects 0.000 description 15
- 230000034994 death Effects 0.000 description 14
- 108020004414 DNA Proteins 0.000 description 12
- 230000000946 synaptic effect Effects 0.000 description 12
- 201000007737 Retinal degeneration Diseases 0.000 description 11
- 230000030833 cell death Effects 0.000 description 11
- 210000003169 central nervous system Anatomy 0.000 description 11
- 230000035772 mutation Effects 0.000 description 11
- 230000004258 retinal degeneration Effects 0.000 description 11
- 238000011830 transgenic mouse model Methods 0.000 description 11
- 108010005939 Ciliary Neurotrophic Factor Proteins 0.000 description 10
- 102100031614 Ciliary neurotrophic factor Human genes 0.000 description 10
- 241000699660 Mus musculus Species 0.000 description 10
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 10
- 210000002569 neuron Anatomy 0.000 description 10
- 239000013598 vector Substances 0.000 description 10
- 241001465754 Metazoa Species 0.000 description 9
- 239000000284 extract Substances 0.000 description 9
- 230000008569 process Effects 0.000 description 9
- 230000004382 visual function Effects 0.000 description 9
- 102000004190 Enzymes Human genes 0.000 description 8
- 108090000790 Enzymes Proteins 0.000 description 8
- 102000016349 Myosin Light Chains Human genes 0.000 description 8
- 108010067385 Myosin Light Chains Proteins 0.000 description 8
- 238000011161 development Methods 0.000 description 8
- 230000018109 developmental process Effects 0.000 description 8
- 229940088598 enzyme Drugs 0.000 description 8
- 238000001415 gene therapy Methods 0.000 description 8
- 238000007920 subcutaneous administration Methods 0.000 description 8
- 230000004083 survival effect Effects 0.000 description 8
- 241000700159 Rattus Species 0.000 description 7
- 239000002299 complementary DNA Substances 0.000 description 7
- 210000005036 nerve Anatomy 0.000 description 7
- 239000013612 plasmid Substances 0.000 description 7
- ZOOGRGPOEVQQDX-UUOKFMHZSA-N 3',5'-cyclic GMP Chemical compound C([C@H]1O2)OP(O)(=O)O[C@H]1[C@@H](O)[C@@H]2N1C(N=C(NC2=O)N)=C2N=C1 ZOOGRGPOEVQQDX-UUOKFMHZSA-N 0.000 description 6
- 238000006243 chemical reaction Methods 0.000 description 6
- ZOOGRGPOEVQQDX-UHFFFAOYSA-N cyclic GMP Natural products O1C2COP(O)(=O)OC2C(O)C1N1C=NC2=C1NC(N)=NC2=O ZOOGRGPOEVQQDX-UHFFFAOYSA-N 0.000 description 6
- 230000002068 genetic effect Effects 0.000 description 6
- 210000004940 nucleus Anatomy 0.000 description 6
- 239000000523 sample Substances 0.000 description 6
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 5
- 229930040373 Paraformaldehyde Natural products 0.000 description 5
- 108090001050 Phosphoric Diester Hydrolases Proteins 0.000 description 5
- 210000004369 blood Anatomy 0.000 description 5
- 239000008280 blood Substances 0.000 description 5
- 230000006378 damage Effects 0.000 description 5
- 230000003111 delayed effect Effects 0.000 description 5
- 238000004925 denaturation Methods 0.000 description 5
- 230000036425 denaturation Effects 0.000 description 5
- 239000008103 glucose Substances 0.000 description 5
- 239000008187 granular material Substances 0.000 description 5
- 238000002372 labelling Methods 0.000 description 5
- 230000003902 lesion Effects 0.000 description 5
- 210000004498 neuroglial cell Anatomy 0.000 description 5
- 229920002866 paraformaldehyde Polymers 0.000 description 5
- 239000000047 product Substances 0.000 description 5
- 239000011780 sodium chloride Substances 0.000 description 5
- 238000010186 staining Methods 0.000 description 5
- 238000002560 therapeutic procedure Methods 0.000 description 5
- FWBHETKCLVMNFS-UHFFFAOYSA-N 4',6-Diamino-2-phenylindol Chemical compound C1=CC(C(=N)N)=CC=C1C1=CC2=CC=C(C(N)=N)C=C2N1 FWBHETKCLVMNFS-UHFFFAOYSA-N 0.000 description 4
- 208000003098 Ganglion Cysts Diseases 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- 208000005400 Synovial Cyst Diseases 0.000 description 4
- 229920004890 Triton X-100 Polymers 0.000 description 4
- 239000013504 Triton X-100 Substances 0.000 description 4
- 230000006907 apoptotic process Effects 0.000 description 4
- 230000004300 dark adaptation Effects 0.000 description 4
- 239000002552 dosage form Substances 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- 239000003102 growth factor Substances 0.000 description 4
- 208000017532 inherited retinal dystrophy Diseases 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 239000012528 membrane Substances 0.000 description 4
- 108020004999 messenger RNA Proteins 0.000 description 4
- 238000010172 mouse model Methods 0.000 description 4
- 210000000653 nervous system Anatomy 0.000 description 4
- 210000001428 peripheral nervous system Anatomy 0.000 description 4
- 210000000608 photoreceptor cell Anatomy 0.000 description 4
- 238000011002 quantification Methods 0.000 description 4
- 238000010254 subcutaneous injection Methods 0.000 description 4
- 239000007929 subcutaneous injection Substances 0.000 description 4
- 238000013518 transcription Methods 0.000 description 4
- 230000035897 transcription Effects 0.000 description 4
- 230000000007 visual effect Effects 0.000 description 4
- NCYCYZXNIZJOKI-IOUUIBBYSA-N 11-cis-retinal Chemical compound O=C/C=C(\C)/C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C NCYCYZXNIZJOKI-IOUUIBBYSA-N 0.000 description 3
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 3
- 101710186708 Agglutinin Proteins 0.000 description 3
- 102000004219 Brain-derived neurotrophic factor Human genes 0.000 description 3
- 108090000715 Brain-derived neurotrophic factor Proteins 0.000 description 3
- 102100033215 DNA nucleotidylexotransferase Human genes 0.000 description 3
- 238000002965 ELISA Methods 0.000 description 3
- 108010067770 Endopeptidase K Proteins 0.000 description 3
- 102000003974 Fibroblast growth factor 2 Human genes 0.000 description 3
- 108090000379 Fibroblast growth factor 2 Proteins 0.000 description 3
- 201000011240 Frontotemporal dementia Diseases 0.000 description 3
- 101710146024 Horcolin Proteins 0.000 description 3
- 101710189395 Lectin Proteins 0.000 description 3
- 101710179758 Mannose-specific lectin Proteins 0.000 description 3
- 101710150763 Mannose-specific lectin 1 Proteins 0.000 description 3
- 101710150745 Mannose-specific lectin 2 Proteins 0.000 description 3
- 102000004330 Rhodopsin Human genes 0.000 description 3
- 108090000820 Rhodopsin Proteins 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 230000009692 acute damage Effects 0.000 description 3
- 239000000910 agglutinin Substances 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 230000001640 apoptogenic effect Effects 0.000 description 3
- 230000003412 degenerative effect Effects 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 230000004438 eyesight Effects 0.000 description 3
- 238000003205 genotyping method Methods 0.000 description 3
- 238000009396 hybridization Methods 0.000 description 3
- 230000001976 improved effect Effects 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 239000012139 lysis buffer Substances 0.000 description 3
- 210000001161 mammalian embryo Anatomy 0.000 description 3
- 238000010369 molecular cloning Methods 0.000 description 3
- 230000001537 neural effect Effects 0.000 description 3
- 239000008363 phosphate buffer Substances 0.000 description 3
- 239000002243 precursor Substances 0.000 description 3
- 230000000750 progressive effect Effects 0.000 description 3
- 230000000717 retained effect Effects 0.000 description 3
- 210000003699 striated muscle Anatomy 0.000 description 3
- 238000013519 translation Methods 0.000 description 3
- 230000000472 traumatic effect Effects 0.000 description 3
- 241000701161 unidentified adenovirus Species 0.000 description 3
- 239000013603 viral vector Substances 0.000 description 3
- 208000024827 Alzheimer disease Diseases 0.000 description 2
- 208000031277 Amaurotic familial idiocy Diseases 0.000 description 2
- 201000004569 Blindness Diseases 0.000 description 2
- 102000029816 Collagenase Human genes 0.000 description 2
- 108060005980 Collagenase Proteins 0.000 description 2
- AHCYMLUZIRLXAA-SHYZEUOFSA-N Deoxyuridine 5'-triphosphate Chemical compound O1[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)C[C@@H]1N1C(=O)NC(=O)C=C1 AHCYMLUZIRLXAA-SHYZEUOFSA-N 0.000 description 2
- 241000282326 Felis catus Species 0.000 description 2
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 2
- 241000287828 Gallus gallus Species 0.000 description 2
- 206010064571 Gene mutation Diseases 0.000 description 2
- 208000010412 Glaucoma Diseases 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 208000023105 Huntington disease Diseases 0.000 description 2
- 102000003746 Insulin Receptor Human genes 0.000 description 2
- 108010001127 Insulin Receptor Proteins 0.000 description 2
- 108090000723 Insulin-Like Growth Factor I Proteins 0.000 description 2
- 201000002832 Lewy body dementia Diseases 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- 239000000020 Nitrocellulose Substances 0.000 description 2
- 108700026244 Open Reading Frames Proteins 0.000 description 2
- 208000018737 Parkinson disease Diseases 0.000 description 2
- 208000037273 Pathologic Processes Diseases 0.000 description 2
- 102000004590 Peripherins Human genes 0.000 description 2
- 108010003081 Peripherins Proteins 0.000 description 2
- 208000000609 Pick Disease of the Brain Diseases 0.000 description 2
- 108010076504 Protein Sorting Signals Proteins 0.000 description 2
- 208000009415 Spinocerebellar Ataxias Diseases 0.000 description 2
- BGDKAVGWHJFAGW-UHFFFAOYSA-N Tropicamide Chemical compound C=1C=CC=CC=1C(CO)C(=O)N(CC)CC1=CC=NC=C1 BGDKAVGWHJFAGW-UHFFFAOYSA-N 0.000 description 2
- 201000004810 Vascular dementia Diseases 0.000 description 2
- 239000011543 agarose gel Substances 0.000 description 2
- 206010002026 amyotrophic lateral sclerosis Diseases 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 210000003050 axon Anatomy 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- 229960002424 collagenase Drugs 0.000 description 2
- RGWHQCVHVJXOKC-SHYZEUOFSA-J dCTP(4-) Chemical compound O=C1N=C(N)C=CN1[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O)[C@@H](O)C1 RGWHQCVHVJXOKC-SHYZEUOFSA-J 0.000 description 2
- 230000001934 delay Effects 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 231100000673 dose–response relationship Toxicity 0.000 description 2
- 239000007850 fluorescent dye Substances 0.000 description 2
- 238000013467 fragmentation Methods 0.000 description 2
- 238000006062 fragmentation reaction Methods 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 210000001153 interneuron Anatomy 0.000 description 2
- 208000002780 macular degeneration Diseases 0.000 description 2
- 230000014759 maintenance of location Effects 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 201000006417 multiple sclerosis Diseases 0.000 description 2
- 229920001220 nitrocellulos Polymers 0.000 description 2
- 238000012758 nuclear staining Methods 0.000 description 2
- 210000001328 optic nerve Anatomy 0.000 description 2
- 230000009054 pathological process Effects 0.000 description 2
- 210000005047 peripherin Anatomy 0.000 description 2
- 230000008823 permeabilization Effects 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 108010066381 preproinsulin Proteins 0.000 description 2
- 230000004224 protection Effects 0.000 description 2
- 230000001681 protective effect Effects 0.000 description 2
- 238000000751 protein extraction Methods 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 230000008439 repair process Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 210000003994 retinal ganglion cell Anatomy 0.000 description 2
- 210000001116 retinal neuron Anatomy 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- 208000020431 spinal cord injury Diseases 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 230000002459 sustained effect Effects 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 210000000225 synapse Anatomy 0.000 description 2
- 210000002504 synaptic vesicle Anatomy 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- GPRLSGONYQIRFK-MNYXATJNSA-N triton Chemical compound [3H+] GPRLSGONYQIRFK-MNYXATJNSA-N 0.000 description 2
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 1
- 108020005345 3' Untranslated Regions Proteins 0.000 description 1
- 108020003589 5' Untranslated Regions Proteins 0.000 description 1
- 239000013607 AAV vector Substances 0.000 description 1
- 208000036443 AIPL1-related retinopathy Diseases 0.000 description 1
- 108700028369 Alleles Proteins 0.000 description 1
- 108091093088 Amplicon Proteins 0.000 description 1
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 1
- 241000972773 Aulopiformes Species 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 244000025254 Cannabis sativa Species 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 1
- 208000017667 Chronic Disease Diseases 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 238000007400 DNA extraction Methods 0.000 description 1
- 108010008286 DNA nucleotidylexotransferase Proteins 0.000 description 1
- 206010012289 Dementia Diseases 0.000 description 1
- 206010067889 Dementia with Lewy bodies Diseases 0.000 description 1
- 241000255581 Drosophila <fruit fly, genus> Species 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 238000012286 ELISA Assay Methods 0.000 description 1
- 238000008157 ELISA kit Methods 0.000 description 1
- 108700024394 Exon Proteins 0.000 description 1
- 102000003971 Fibroblast Growth Factor 1 Human genes 0.000 description 1
- 108090000386 Fibroblast Growth Factor 1 Proteins 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 206010019899 Hereditary retinal dystrophy Diseases 0.000 description 1
- 101000976075 Homo sapiens Insulin Proteins 0.000 description 1
- 206010060378 Hyperinsulinaemia Diseases 0.000 description 1
- 102000004218 Insulin-Like Growth Factor I Human genes 0.000 description 1
- 108090001117 Insulin-Like Growth Factor II Proteins 0.000 description 1
- 102000048143 Insulin-Like Growth Factor II Human genes 0.000 description 1
- 241000581650 Ivesia Species 0.000 description 1
- YQEZLKZALYSWHR-UHFFFAOYSA-N Ketamine Chemical compound C=1C=CC=C(Cl)C=1C1(NC)CCCCC1=O YQEZLKZALYSWHR-UHFFFAOYSA-N 0.000 description 1
- 241000713666 Lentivirus Species 0.000 description 1
- 208000009829 Lewy Body Disease Diseases 0.000 description 1
- 101710135898 Myc proto-oncogene protein Proteins 0.000 description 1
- 102100038895 Myc proto-oncogene protein Human genes 0.000 description 1
- 102000003505 Myosin Human genes 0.000 description 1
- 108060008487 Myosin Proteins 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 108010025020 Nerve Growth Factor Proteins 0.000 description 1
- 102000007072 Nerve Growth Factors Human genes 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 208000022873 Ocular disease Diseases 0.000 description 1
- 102000004861 Phosphoric Diester Hydrolases Human genes 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 108020005091 Replication Origin Proteins 0.000 description 1
- 208000017442 Retinal disease Diseases 0.000 description 1
- 102000013275 Somatomedins Human genes 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 101710150448 Transcriptional regulator Myc Proteins 0.000 description 1
- 102000010989 Type 6 Cyclic Nucleotide Phosphodiesterases Human genes 0.000 description 1
- 108010037638 Type 6 Cyclic Nucleotide Phosphodiesterases Proteins 0.000 description 1
- 230000004308 accommodation Effects 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 230000002424 anti-apoptotic effect Effects 0.000 description 1
- 239000000074 antisense oligonucleotide Substances 0.000 description 1
- 238000012230 antisense oligonucleotides Methods 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- 210000004507 artificial chromosome Anatomy 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 230000008827 biological function Effects 0.000 description 1
- 229960000074 biopharmaceutical Drugs 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 210000004155 blood-retinal barrier Anatomy 0.000 description 1
- 230000004378 blood-retinal barrier Effects 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 229940077737 brain-derived neurotrophic factor Drugs 0.000 description 1
- 235000011089 carbon dioxide Nutrition 0.000 description 1
- 210000003986 cell retinal photoreceptor Anatomy 0.000 description 1
- 230000007248 cellular mechanism Effects 0.000 description 1
- 230000033077 cellular process Effects 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 210000003837 chick embryo Anatomy 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 230000006726 chronic neurodegeneration Effects 0.000 description 1
- 239000013065 commercial product Substances 0.000 description 1
- 201000006754 cone-rod dystrophy Diseases 0.000 description 1
- 238000004624 confocal microscopy Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 231100000858 damage to nervous tissue Toxicity 0.000 description 1
- 230000000254 damaging effect Effects 0.000 description 1
- 230000027832 depurination Effects 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 210000002257 embryonic structure Anatomy 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 230000000763 evoking effect Effects 0.000 description 1
- 239000013613 expression plasmid Substances 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- 238000007710 freezing Methods 0.000 description 1
- 230000008014 freezing Effects 0.000 description 1
- 230000005714 functional activity Effects 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 230000002518 glial effect Effects 0.000 description 1
- 230000004153 glucose metabolism Effects 0.000 description 1
- 210000003128 head Anatomy 0.000 description 1
- 210000002287 horizontal cell Anatomy 0.000 description 1
- 230000003451 hyperinsulinaemic effect Effects 0.000 description 1
- 201000008980 hyperinsulinism Diseases 0.000 description 1
- 230000002055 immunohistochemical effect Effects 0.000 description 1
- 238000012744 immunostaining Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- PBGKTOXHQIOBKM-FHFVDXKLSA-N insulin (human) Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@H]1CSSC[C@H]2C(=O)N[C@H](C(=O)N[C@@H](CO)C(=O)N[C@H](C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3C=CC(O)=CC=3)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3NC=NC=3)NC(=O)[C@H](CO)NC(=O)CNC1=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O)=O)CSSC[C@@H](C(N2)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)CN)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C=CC=CC=1)C(C)C)C1=CN=CN1 PBGKTOXHQIOBKM-FHFVDXKLSA-N 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 229960003299 ketamine Drugs 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 210000003141 lower extremity Anatomy 0.000 description 1
- 208000038015 macular disease Diseases 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 230000007102 metabolic function Effects 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 238000000520 microinjection Methods 0.000 description 1
- 230000009456 molecular mechanism Effects 0.000 description 1
- 239000003068 molecular probe Substances 0.000 description 1
- 210000000327 mueller cell Anatomy 0.000 description 1
- 210000001178 neural stem cell Anatomy 0.000 description 1
- 239000004090 neuroprotective agent Substances 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 230000004297 night vision Effects 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- LRMQCJCMKQSEJD-UHFFFAOYSA-N oligo b Polymers O1C(N2C3=NC=NC(N)=C3N=C2)C(OC)C(OC(=O)C=2C=C3C4(OC(=O)C3=CC=2)C2=CC=C(O)C=C2OC2=CC(O)=CC=C24)C1COP(O)(=O)OC1C(C(O2)N3C(N=C(N)C(C)=C3)=O)OCC12COP(O)(=O)OC(C1OC)C(COP(O)(=O)OC2C3(COP(O)(=O)OC4C(C(OC4COP(O)(=O)OC4C(C(OC4COP(O)(=O)OC4C(C(OC4COP(O)(=O)OC4C5(COP(O)(=O)OC6C(C(OC6COP(O)(=O)OC6C7(COP(O)(=O)OC8C(C(OC8COP(O)(=O)OC8C9(CO)COC8C(O9)N8C(N=C(N)C(C)=C8)=O)N8C(NC(=O)C=C8)=O)OC)COC6C(O7)N6C(N=C(N)C(C)=C6)=O)N6C(N=C(N)C=C6)=O)OC)COC4C(O5)N4C(N=C(N)C(C)=C4)=O)N4C5=NC=NC(N)=C5N=C4)OC)N4C5=C(C(NC(N)=N5)=O)N=C4)OC)N4C5=C(C(NC(N)=N5)=O)N=C4)OC)COC2C(O3)N2C(N=C(N)C(C)=C2)=O)OC1N1C=CC(=O)NC1=O LRMQCJCMKQSEJD-UHFFFAOYSA-N 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 230000004792 oxidative damage Effects 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 229940127557 pharmaceutical product Drugs 0.000 description 1
- 230000008832 photodamage Effects 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920000729 poly(L-lysine) polymer Polymers 0.000 description 1
- 230000008488 polyadenylation Effects 0.000 description 1
- 229920002704 polyhistidine Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 108010005636 polypeptide C Proteins 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 238000004321 preservation Methods 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 208000037821 progressive disease Diseases 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 230000009993 protective function Effects 0.000 description 1
- 210000001747 pupil Anatomy 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000020129 regulation of cell death Effects 0.000 description 1
- 230000022532 regulation of transcription, DNA-dependent Effects 0.000 description 1
- 230000004043 responsiveness Effects 0.000 description 1
- 230000004243 retinal function Effects 0.000 description 1
- 230000004500 retinal neurogenesis Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 235000019515 salmon Nutrition 0.000 description 1
- 238000005185 salting out Methods 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- 230000003584 silencer Effects 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 230000008093 supporting effect Effects 0.000 description 1
- 230000005026 transcription initiation Effects 0.000 description 1
- 230000005030 transcription termination Effects 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 229960004791 tropicamide Drugs 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 230000004304 visual acuity Effects 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 239000003643 water by type Substances 0.000 description 1
- BPICBUSOMSTKRF-UHFFFAOYSA-N xylazine Chemical compound CC1=CC=CC(C)=C1NC1=NCCCS1 BPICBUSOMSTKRF-UHFFFAOYSA-N 0.000 description 1
- 229960001600 xylazine Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/22—Hormones
- A61K38/28—Insulins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/06—Antiglaucoma agents or miotics
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Organic Chemistry (AREA)
- Neurology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Immunology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Diabetes (AREA)
- Endocrinology (AREA)
- Zoology (AREA)
- Gastroenterology & Hepatology (AREA)
- Epidemiology (AREA)
- Ophthalmology & Optometry (AREA)
- Psychology (AREA)
- Hospice & Palliative Care (AREA)
- Psychiatry (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ESP200601314 | 2006-05-22 | ||
| ES200601314A ES2331342B1 (es) | 2006-05-22 | 2006-05-22 | Uso de la proinsulina para la elaboracion de una composicion farmaceutica neuroprotectora, composicion terapeutica que la contiene y sus aplicaciones. |
| PCT/ES2007/070097 WO2007135220A1 (es) | 2006-05-22 | 2007-05-21 | Uso de la proinsulina para la elaboración de una composición farmacéutica neuroprotectora, composición terapéutica que la contiene y sus aplicaciones |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2009537612A JP2009537612A (ja) | 2009-10-29 |
| JP2009537612A5 JP2009537612A5 (enExample) | 2010-02-12 |
| JP4727748B2 true JP4727748B2 (ja) | 2011-07-20 |
Family
ID=38722988
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009511534A Expired - Fee Related JP4727748B2 (ja) | 2006-05-22 | 2007-05-21 | 神経保護医薬組成物の製造のためのプロインスリンの使用、それを含む治療組成物、およびそれらの応用 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20100330042A1 (enExample) |
| EP (1) | EP2042189B1 (enExample) |
| JP (1) | JP4727748B2 (enExample) |
| AU (1) | AU2007253212B2 (enExample) |
| CA (1) | CA2652983C (enExample) |
| ES (2) | ES2331342B1 (enExample) |
| WO (1) | WO2007135220A1 (enExample) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20110021974A1 (en) * | 2010-10-05 | 2011-01-27 | Shantha Totada R | Retinitis pigmentosa treatment and prophalaxis |
| EP2872183B1 (en) | 2012-07-11 | 2018-09-26 | The Trustees Of The University Of Pennsylvania | Aav-mediated gene therapy for rpgr x-linked retinal degeneration |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA1176160A (en) * | 1981-08-27 | 1984-10-16 | Ronald E. Chance | Human proinsulin pharmaceutical formulations |
| US5652214A (en) * | 1989-06-05 | 1997-07-29 | Cephalon, Inc. | Treating disorders by application of insulin-like growth factors and analogs |
| US5667968A (en) * | 1989-08-30 | 1997-09-16 | Regeneron Pharmaceuticals, Inc. | Prevention of retinal injury and degeneration by specific factors |
| EP0788512B1 (en) * | 1994-07-08 | 1999-03-24 | The Trustees Of Dartmouth College | Proinsulin peptide compounds for detecting and treating type i diabetes |
| JP2003526321A (ja) * | 1998-07-15 | 2003-09-09 | ウイスコンシン アラムナイ リサーチ フオンデーシヨン | 合成ベータ細胞による糖尿病の治療 |
| DE10055857A1 (de) * | 2000-11-10 | 2002-08-22 | Creative Peptides Sweden Ab Dj | Neue pharmazeutische Depotformulierung |
| US7312192B2 (en) * | 2001-09-07 | 2007-12-25 | Biocon Limited | Insulin polypeptide-oligomer conjugates, proinsulin polypeptide-oligomer conjugates and methods of synthesizing same |
| JP2005534650A (ja) * | 2002-06-11 | 2005-11-17 | ザ バーナム インスティテュート | エリスロポイエチンおよびインスリン様増殖因子の神経保護的相乗作用 |
-
2006
- 2006-05-22 ES ES200601314A patent/ES2331342B1/es not_active Expired - Fee Related
-
2007
- 2007-05-21 US US12/227,554 patent/US20100330042A1/en not_active Abandoned
- 2007-05-21 CA CA2652983A patent/CA2652983C/en not_active Expired - Fee Related
- 2007-05-21 AU AU2007253212A patent/AU2007253212B2/en not_active Ceased
- 2007-05-21 EP EP07765882.1A patent/EP2042189B1/en not_active Not-in-force
- 2007-05-21 JP JP2009511534A patent/JP4727748B2/ja not_active Expired - Fee Related
- 2007-05-21 WO PCT/ES2007/070097 patent/WO2007135220A1/es not_active Ceased
- 2007-05-21 ES ES07765882T patent/ES2433389T3/es active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CA2652983C (en) | 2016-07-19 |
| ES2331342A1 (es) | 2009-12-29 |
| EP2042189B1 (en) | 2013-06-26 |
| WO2007135220A1 (es) | 2007-11-29 |
| CA2652983A1 (en) | 2007-11-29 |
| AU2007253212B2 (en) | 2012-09-27 |
| US20100330042A1 (en) | 2010-12-30 |
| EP2042189A1 (en) | 2009-04-01 |
| AU2007253212A1 (en) | 2007-11-29 |
| JP2009537612A (ja) | 2009-10-29 |
| ES2331342B1 (es) | 2010-10-13 |
| ES2433389T3 (es) | 2013-12-10 |
| EP2042189A4 (en) | 2010-02-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Petters et al. | Genetically engineered large animal model for studying cone photoreceptor survival and degeneration in retinitis pigmentosa | |
| Kobayashi et al. | HRG4 (UNC119) mutation found in cone–rod dystrophy causes retinal degeneration in a transgenic model | |
| Bessant et al. | Molecular genetics and prospects for therapy of the inherited retinal dystrophies | |
| JP7615238B2 (ja) | 網膜ジストロフィーを治療するrdh12コード領域を含むウイルスベクターおよび方法 | |
| Pang et al. | AAV-mediated gene therapy in mouse models of recessive retinal degeneration | |
| US11351225B2 (en) | Methods for modulating development and function of photoreceptor cells | |
| JP4727748B2 (ja) | 神経保護医薬組成物の製造のためのプロインスリンの使用、それを含む治療組成物、およびそれらの応用 | |
| JP2018500906A (ja) | 網膜変性処置のためのotx2過剰発現トランスジェニック網膜色素上皮細胞 | |
| US20040055023A1 (en) | Joint utilization of the insulin gene and the glucokinase gene in the development of therapeutic approaches for diabetes mellitus | |
| KR102168332B1 (ko) | 망막색소변성증의 진단에 이용 가능한 15개의 ush2a 유전자 돌연변이체 및 이외 용도 | |
| Wei | Neural stem cell-based GDNF and CNTF for the treatment of retinal degeneration in a mouse model of CLN7 disease | |
| Aguirre et al. | The use of canine models to develop translational gene therapies for the treatment of six forms of inheritedretinal degenerationsb (Work conducted at the University of Pennsylvania-USA) | |
| CA3082586C (en) | Viral vectors comprising rdh12 coding regions and methods of treating retinal dystrophies | |
| Sharma | Retinal Transplants: Growth Differentiation Integration Organization and Survival | |
| HK40106588A (en) | Viral vectors comprising rdh12 coding regions and methods of treating retinal dystrophies | |
| WO2005023311A2 (en) | Novel targets for the treatment of retina diseases | |
| RAKÓCZY et al. | Eye diseases | |
| HK40037202A (en) | Viral vectors comprising rdh12 coding regions and methods of treating retinal dystrophies | |
| HK40037202B (en) | Viral vectors comprising rdh12 coding regions and methods of treating retinal dystrophies | |
| Ramon et al. | Molecular biology of retinitis pigmentosa: therapeutic implications | |
| Lopes | Characterization of human stem cells and therapeutic strategies involving IGF-1 and shRNA in Huntington's disease | |
| EA049649B1 (ru) | Вирусные векторы, содержащие области, кодирующие rdh12, и способы лечения дистрофий сетчатки | |
| Beltran | Cellular and molecular studies of ciliary neurotrophic factor receptor alpha expression and ciliary neurotrophic factor mediated neuroprotection in the canine retina |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20091214 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20091214 |
|
| A871 | Explanation of circumstances concerning accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A871 Effective date: 20091214 |
|
| A975 | Report on accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A971005 Effective date: 20100120 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100226 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20100526 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20100602 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100628 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100921 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20101221 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110104 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110120 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20110318 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20110413 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140422 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |