JP4684655B2 - 4−ヒドロキシタモキシフェンによる乳房密度低下 - Google Patents
4−ヒドロキシタモキシフェンによる乳房密度低下 Download PDFInfo
- Publication number
- JP4684655B2 JP4684655B2 JP2004560488A JP2004560488A JP4684655B2 JP 4684655 B2 JP4684655 B2 JP 4684655B2 JP 2004560488 A JP2004560488 A JP 2004560488A JP 2004560488 A JP2004560488 A JP 2004560488A JP 4684655 B2 JP4684655 B2 JP 4684655B2
- Authority
- JP
- Japan
- Prior art keywords
- hydroxy tamoxifen
- breast
- tamoxifen
- tissue
- administration
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/13—Amines
- A61K31/135—Amines having aromatic rings, e.g. ketamine, nortriptyline
- A61K31/138—Aryloxyalkylamines, e.g. propranolol, tamoxifen, phenoxybenzamine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
- A61K47/38—Cellulose; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0014—Skin, i.e. galenical aspects of topical compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/06—Ointments; Bases therefor; Other semi-solid forms, e.g. creams, sticks, gels
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/24—Drugs for disorders of the endocrine system of the sex hormones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/24—Drugs for disorders of the endocrine system of the sex hormones
- A61P5/30—Oestrogens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/24—Drugs for disorders of the endocrine system of the sex hormones
- A61P5/32—Antioestrogens
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- General Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Diabetes (AREA)
- Endocrinology (AREA)
- Dermatology (AREA)
- Inorganic Chemistry (AREA)
- Oncology (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicinal Preparation (AREA)
Description
et al., 1993)。
et al., 1997; Boyd et al., 1992, 1995)。乳房密度と乳癌リスクとの間の関連は、高密度乳房組織での間質および上皮細胞増殖の増加が原因となっているように思われる。
et al., 1988; Kolb et al., 2002)。さらに、高密度マンモグラフィーパターンは、X線技師の自信が低下して、擬陽性診断も多くなる。その擬陽性診断は、患者を細針吸引および生検などの不必要な侵襲的処置に曝すことで、苦痛と追加的保健コストの両方を引き起こすことになる。
1996)がそのような場合を報告している。
et al., 1999)。サンら(Son et al.)は、乳癌手術後に20mg/日のタモキシフェン治療を受けた女性の59.8%で乳房柔組織の減少を認めている。閉経前女性では、サン(Son)らは、87%の低下を認めており、それに対してタモキシフェン投与を受けなかった患者では36%に過ぎず、健常対照被験者ではわずか10%であった。
et al., 1982; Kuiper et al., 1997)。トランス4−ヒドロキシタモキシフェンは、トランス−タモキシフェンと比較して、正常ヒト上皮乳房細胞の培養での増殖を100倍阻害する(Malet
et al., 1988)。
1982)を見よ。それらの各化合物は、各種細胞で多様かつ予測できない生理活性を示し、その一部は各化合物のエストロゲン受容体配座に対する個別的効果によって測定される。すなわち、各化合物のエストロゲン受容体結合により、特有の受容体−リガンド配座が生じ、それが各種補因子を召集することで、異なる化合物では薬理特性が変動することになる(Wijayaratne
et al., 1999; Giambiagi et al., 1988)。
et al., 2001)。対照的に、4−ヒドロキシタモキシフェンは哺乳動物癌細胞系でエストロンスルファターゼ活性に対するかなりの阻害効果を示すが、それに関してタモキシフェンはほとんど効果がない(Chetrite
et al., 1993)。
1982)に由来する合成が記載されている。その合成は、下記の数段階で行われる。
段階2 段階1とは別に、1,2−ジフェニル−1−ブタノンのヒドロキシル化による1−(4−ヒドロキシフェニル)−2−フェニル−1−ブタノンの形成;
段階3−段階1の生成物と段階2の生成物との間の反応による1−(4−ジメチルアミノエトキシフェニル)−1−[p−2−テトラヒドロピラニルオキシ)フェニル]−2−フェニルブタン−1−オールの形成;
段階4 メタノール/塩酸による脱水によるシスおよびトランス異性体の混合物としての1−[p−(β−ジメチルアミノエトキシ)フェニル]−トランス−1−(p−ヒドロキシフェニル)−2−フェニル−1−ブト−1−エン =4−OH−タモキシフェンの生成;
段階5 クロマトグラフィーおよび結晶化によるシスおよびトランス異性体の分離による一定の比活性の実現。
et al., supra)。従って本発明に関して、4−ヒドロキシタモキシフェンはいずれの皮膚表面にも投与可能であるが、好ましくは片方または両方の乳房に投与する。
(Lippincott Williams & Wilkins, 2000), pp.836-58; PERCUTANEOUS ABSORPTION:
DRUGS COSMETICS MECHANISMS METHODOLOGY, Bronaugh and Maibach (Marcel Dekker,
1999))。これらの刊行物が明らかにしているように、医薬分野での当業者は、各種の要素および方法を駆使して、効果的な経皮送達を得ることができる。
乳癌患者4名に、12時間〜7日間の所定の間隔で乳房に直接投与することでアルコール溶液での[3H]−4−ヒドロキシタモキシフェンを投与してから、患部組織の摘出手術を行った。手術後、摘出組織と腫瘍周囲の正常乳房組織の両方に放射能が含まれていた(Kuttennetal.,1985)。
この試験では、タモキシフェンの経口投与後の4−ヒドロキシタモキシフェンの組織濃度および血漿濃度と、水性アルコールゲルでの経皮投与後の4−ヒドロキシタモキシフェンの組織濃度と血漿濃度とを比較した(Pujol et al.)。
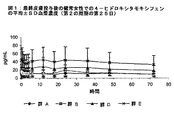
乳癌手術の予定がある患者31名を5群中の1群に無作為に割り当てた。その患者に、表3に示したように経口タモキシフェンまたは経皮4−ヒドロキシタモキシフェンのいずれかを投与した。投与は1日1回行い、3〜4週間続けてから手術を行った。この試験では、3つの異なる用量の4−ヒドロキシタモキシフェン(0.5、1または2mg/日)および2種類の投与面積(両方の乳房あるいは両腕、両前腕および両肩などの大面積の皮膚のいずれかに)を評価した。1群の患者には、20mg/日(10mgを1日2回)の経口タモキシフェンの投与を行った(ノルバルデックス(Nolvaldex;登録商標))。
本試験は、年齢18〜45歳の健常閉経前女性における局所投与4−ヒドロキシタモキシフェンゲルの耐容性および薬物動態を示すものである。各参加者には、2月経周期の期間にわたり、1日1回のゲル投与を行った。
本試験の主目的は、経皮投与した場合に、4−ヒドロキシタモキシフェンが乳房組織のマンモグラフィー密度を効果的に低下させることを示すことにある。
Claims (3)
- 被験者のマンモグラフィー感度を上げるための医薬であって、4−ヒドロキシタモキシフェンを含み、経皮投与される医薬。
- マンモグラフィーを実施する前に被験者に経皮投与される、請求項1記載の医薬。
- 前記4−ヒドロキシタモキシフェンが水性アルコールゲルに製剤されている請求項1〜2のいずれかに記載の医薬。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US43395802P | 2002-12-18 | 2002-12-18 | |
| PCT/EP2003/015030 WO2004054558A2 (en) | 2002-12-18 | 2003-12-15 | Reduction of breast density with 4-hydroxy tamoxifen |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010279107A Division JP2011052020A (ja) | 2002-12-18 | 2010-12-15 | 4−ヒドロキシタモキシフェンによる乳房密度低下 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2006514645A JP2006514645A (ja) | 2006-05-11 |
| JP2006514645A5 JP2006514645A5 (ja) | 2007-02-08 |
| JP4684655B2 true JP4684655B2 (ja) | 2011-05-18 |
Family
ID=32595254
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2004560488A Expired - Lifetime JP4684655B2 (ja) | 2002-12-18 | 2003-12-15 | 4−ヒドロキシタモキシフェンによる乳房密度低下 |
| JP2010279107A Pending JP2011052020A (ja) | 2002-12-18 | 2010-12-15 | 4−ヒドロキシタモキシフェンによる乳房密度低下 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010279107A Pending JP2011052020A (ja) | 2002-12-18 | 2010-12-15 | 4−ヒドロキシタモキシフェンによる乳房密度低下 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US7485623B2 (ja) |
| EP (3) | EP1572171B1 (ja) |
| JP (2) | JP4684655B2 (ja) |
| AU (1) | AU2003296757A1 (ja) |
| DE (1) | DE60327363D1 (ja) |
| WO (1) | WO2004054558A2 (ja) |
Families Citing this family (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2002366800B2 (en) * | 2001-12-20 | 2006-08-03 | Femmepharma Holding Company, Inc. | Vaginal delivery of drugs |
| AU2003303033B2 (en) * | 2002-12-18 | 2009-06-04 | Besins Healthcare Luxembourg Sarl | Treatment of mastalgia with 4-hydroxy tamoxifen |
| WO2004060322A2 (en) * | 2003-01-02 | 2004-07-22 | Femmepharma Holding Company, Inc. | Pharmaceutical preparations for treatments of diseases and disorders of the breast |
| US9173836B2 (en) | 2003-01-02 | 2015-11-03 | FemmeParma Holding Company, Inc. | Pharmaceutical preparations for treatments of diseases and disorders of the breast |
| US7704516B2 (en) * | 2003-04-01 | 2010-04-27 | Laboratories Besins International Sa | Percutaneous composition comprising 4-hydroxy tamoxifen |
| AU2004246812B8 (en) | 2003-06-09 | 2010-01-14 | Besins Healthcare Luxembourg Sarl | Treatment and prevention of excessive scarring with 4-hydroxy tamoxifen |
| US7968532B2 (en) * | 2003-12-15 | 2011-06-28 | Besins Healthcare Luxembourg | Treatment of gynecomastia with 4-hydroxy tamoxifen |
| EP1748770B1 (en) * | 2004-03-22 | 2008-04-09 | Laboratoires Besins International | Treatment and prevention of benign breast disease with 4-hydroxy tamoxifen |
| EP1579857A1 (en) * | 2004-03-22 | 2005-09-28 | Laboratoires Besins International | Chemically stable compositions of 4-hydroxy tamoxifen |
| US7507769B2 (en) * | 2004-03-22 | 2009-03-24 | Laboratoires Besins International | Treatment and prevention of benign breast disease with 4-hydroxy tamoxifen |
| EP1579856A1 (en) * | 2004-03-22 | 2005-09-28 | Laboratoires Besins International | Treatment and prevention of benign breast disease with 4-hydroxy tamoxifen |
| DE602005011314D1 (de) * | 2004-10-14 | 2009-01-08 | Besins Int Lab | 4-hydroxytamoxifengel-formulierungen |
| EP1647271A1 (en) * | 2004-10-14 | 2006-04-19 | Laboratoires Besins International | 4-Hydroxy tamoxifen gel formulations |
| GB0602739D0 (en) * | 2006-02-10 | 2006-03-22 | Ccbr As | Breast tissue density measure |
| CA2674078C (en) * | 2006-12-26 | 2012-03-20 | Femmepharma Holding Company, Inc. | Topical administration of danazol |
| WO2009065918A1 (en) * | 2007-11-22 | 2009-05-28 | Novo Nordisk Health Care Ag | Stabilisation of liquid-formulated factor vii(a) polypeptides by aldehyde-containing compounds |
| US20110003000A1 (en) * | 2009-07-06 | 2011-01-06 | Femmepharma Holding Company, Inc. | Transvaginal Delivery of Drugs |
| BR112016026459B1 (pt) * | 2014-05-12 | 2022-09-27 | Quest Diagnostics Investments Incorporated | Métodos para determinar a quantidade de norendoxifeno e para determinar a quantidade de tamoxifeno e metabólitos do mesmo em uma amostra humana por espectrometria de massas |
| CA3032153A1 (en) | 2016-08-19 | 2018-02-22 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Selective estrogen-receptor modulators (serms) confer protection against photoreceptor degeneration |
| SG11202002105WA (en) | 2017-09-11 | 2020-04-29 | Atossa Therapeutics Inc | Methods for making and using endoxifen |
| JP7662204B2 (ja) | 2019-07-03 | 2025-04-15 | アトッサ・セラピューティクス・インコーポレイテッド | エンドキシフェンの徐放性組成物 |
| WO2023026139A1 (en) * | 2021-08-23 | 2023-03-02 | Singh Divya Dhananjay | Pharmaceutical composition comprising 4- hydroxytamoxifen for the treatment of mastalgia |
Family Cites Families (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2558373B1 (fr) * | 1984-01-20 | 1987-07-03 | Mauvais Jarvis Pierre | Medicament antioestrogene a base de 4-hydroxytamoxifene pour administration percutanee |
| DE3781546D1 (de) | 1987-04-21 | 1992-10-08 | Heumann Pharma Gmbh & Co | Stabile loesungsmitteladdukte von z-1-(p-beta-dimethylamino-ethoxyphenyl)-1-(p-hydroxyphenyl)-2-phenylbut-1-en. |
| US5045553A (en) * | 1987-06-24 | 1991-09-03 | Fujisawa Pharmaceutical Company, Ltd. | Pharmaceutical composition for percutaneous drug absorption and percutaneous drug absorption promoter |
| US5002938A (en) * | 1988-03-21 | 1991-03-26 | Bristol-Myers Squibb Company | Antifungal gel formulations |
| DE3836862A1 (de) | 1988-10-27 | 1990-05-03 | Schering Ag | Mittel zur transdermalen applikation von steroidhormonen |
| TW218849B (ja) | 1991-05-17 | 1994-01-11 | Bristol Myers Squibb Co | |
| JPH0679002A (ja) * | 1993-12-14 | 1994-03-22 | Hisamitsu Pharmaceut Co Inc | 経皮投与用パッチシステム |
| US5613958A (en) | 1993-05-12 | 1997-03-25 | Pp Holdings Inc. | Transdermal delivery systems for the modulated administration of drugs |
| DE4407742C1 (de) * | 1994-03-08 | 1995-06-22 | Hexal Pharma Gmbh | Transdermales System in Form eines Pflasters mit einem Tamoxifen-Derivat |
| US5720963A (en) | 1994-08-26 | 1998-02-24 | Mary Kay Inc. | Barrier disruption treatments for structurally deteriorated skin |
| DE4434165A1 (de) * | 1994-09-24 | 1996-03-28 | Cassella Ag | Haarfärbemittel |
| US6083996A (en) * | 1997-11-05 | 2000-07-04 | Nexmed Holdings, Inc. | Topical compositions for NSAI drug delivery |
| US6013270A (en) | 1998-04-20 | 2000-01-11 | The Procter & Gamble Company | Skin care kit |
| DE10033853A1 (de) | 2000-07-12 | 2002-01-31 | Hexal Ag | Transdermales therapeutisches System mit hochdispersem Siliziumdioxid |
| US6503894B1 (en) | 2000-08-30 | 2003-01-07 | Unimed Pharmaceuticals, Inc. | Pharmaceutical composition and method for treating hypogonadism |
| PT1317921E (pt) | 2001-12-07 | 2009-11-06 | Besins Mfg Belgium | Composição farmacêutica sob a forma de gel ou de solução à base de di-hidrotestosterona, seu processo de preparação e suas utilizações |
| AU2003303033B2 (en) | 2002-12-18 | 2009-06-04 | Besins Healthcare Luxembourg Sarl | Treatment of mastalgia with 4-hydroxy tamoxifen |
| US7704516B2 (en) | 2003-04-01 | 2010-04-27 | Laboratories Besins International Sa | Percutaneous composition comprising 4-hydroxy tamoxifen |
| AU2004246812B8 (en) | 2003-06-09 | 2010-01-14 | Besins Healthcare Luxembourg Sarl | Treatment and prevention of excessive scarring with 4-hydroxy tamoxifen |
| US7968532B2 (en) | 2003-12-15 | 2011-06-28 | Besins Healthcare Luxembourg | Treatment of gynecomastia with 4-hydroxy tamoxifen |
| US7507769B2 (en) | 2004-03-22 | 2009-03-24 | Laboratoires Besins International | Treatment and prevention of benign breast disease with 4-hydroxy tamoxifen |
| EP1579857A1 (en) | 2004-03-22 | 2005-09-28 | Laboratoires Besins International | Chemically stable compositions of 4-hydroxy tamoxifen |
| EP1647271A1 (en) | 2004-10-14 | 2006-04-19 | Laboratoires Besins International | 4-Hydroxy tamoxifen gel formulations |
-
2003
- 2003-12-15 EP EP03813147A patent/EP1572171B1/en not_active Expired - Lifetime
- 2003-12-15 DE DE60327363T patent/DE60327363D1/de not_active Expired - Lifetime
- 2003-12-15 WO PCT/EP2003/015030 patent/WO2004054558A2/en active Application Filing
- 2003-12-15 AU AU2003296757A patent/AU2003296757A1/en not_active Abandoned
- 2003-12-15 JP JP2004560488A patent/JP4684655B2/ja not_active Expired - Lifetime
- 2003-12-15 US US10/734,644 patent/US7485623B2/en not_active Expired - Lifetime
- 2003-12-15 EP EP08103001.7A patent/EP1952809B1/en not_active Expired - Lifetime
- 2003-12-15 EP EP09000839.2A patent/EP2050443B1/en not_active Expired - Lifetime
-
2010
- 2010-12-15 JP JP2010279107A patent/JP2011052020A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| EP1952809A3 (en) | 2012-06-20 |
| AU2003296757A8 (en) | 2004-07-09 |
| WO2004054558A3 (en) | 2004-10-28 |
| EP1952809A2 (en) | 2008-08-06 |
| AU2003296757A1 (en) | 2004-07-09 |
| EP1572171B1 (en) | 2009-04-22 |
| WO2004054558A2 (en) | 2004-07-01 |
| EP1952809B1 (en) | 2017-08-02 |
| JP2011052020A (ja) | 2011-03-17 |
| EP2050443B1 (en) | 2017-05-24 |
| JP2006514645A (ja) | 2006-05-11 |
| US20040138314A1 (en) | 2004-07-15 |
| EP2050443A1 (en) | 2009-04-22 |
| US7485623B2 (en) | 2009-02-03 |
| DE60327363D1 (de) | 2009-06-04 |
| EP1572171A2 (en) | 2005-09-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4684655B2 (ja) | 4−ヒドロキシタモキシフェンによる乳房密度低下 | |
| JP5490346B2 (ja) | 4−ヒドロキシタモキシフェンによる乳癌の予防および治療 | |
| JP4938237B2 (ja) | 4−ヒドロキシタモキシフェンによる乳房痛の治療 | |
| JP4682129B2 (ja) | 4−ヒドロキシタモキシフェンによる過剰瘢痕化の治療及び予防 | |
| JP5489407B2 (ja) | 4−ヒドロキシタモキシフェンの化学的に安定な組成物 | |
| EP1579856A1 (en) | Treatment and prevention of benign breast disease with 4-hydroxy tamoxifen | |
| JP5072588B2 (ja) | 4−ヒドロキシタモキシフェンを用いた良性乳房疾患の治療および予防 | |
| JP2007529420A (ja) | 女性化乳房治療用の薬剤を製造する際の4−ヒドロキシタモキシフェンの使用 | |
| HK1077512A1 (en) | Reduction of breast density with 4-hydroxy tamoxifen | |
| HK1077512B (en) | Reduction of breast density with 4-hydroxy tamoxifen | |
| HK1130435B (en) | Reduction of breast density with 4-hydroxy tamoxifen | |
| MXPA05013435A (en) | Treatment and prevention of excessive scarring with 4-hydroxy tamoxifen |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20061214 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20061214 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100526 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100825 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100915 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20101215 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20110126 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20110209 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140218 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4684655 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| EXPY | Cancellation because of completion of term |