JP4634367B2 - ピリミジン化合物 - Google Patents
ピリミジン化合物 Download PDFInfo
- Publication number
- JP4634367B2 JP4634367B2 JP2006503537A JP2006503537A JP4634367B2 JP 4634367 B2 JP4634367 B2 JP 4634367B2 JP 2006503537 A JP2006503537 A JP 2006503537A JP 2006503537 A JP2006503537 A JP 2006503537A JP 4634367 B2 JP4634367 B2 JP 4634367B2
- Authority
- JP
- Japan
- Prior art keywords
- amino
- het
- formula
- hydrochloride
- phenyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 150000003230 pyrimidines Chemical class 0.000 title description 2
- 150000001875 compounds Chemical class 0.000 claims abstract description 289
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 25
- 125000000217 alkyl group Chemical group 0.000 claims description 87
- -1 hydroxy, mercapto Chemical class 0.000 claims description 83
- 125000003342 alkenyl group Chemical group 0.000 claims description 53
- 206010028980 Neoplasm Diseases 0.000 claims description 47
- 125000000304 alkynyl group Chemical group 0.000 claims description 43
- 125000005843 halogen group Chemical group 0.000 claims description 42
- 150000003839 salts Chemical class 0.000 claims description 42
- 239000012453 solvate Substances 0.000 claims description 36
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 34
- 229910052760 oxygen Inorganic materials 0.000 claims description 34
- 229910052799 carbon Inorganic materials 0.000 claims description 33
- 125000005842 heteroatom Chemical group 0.000 claims description 33
- 229910052717 sulfur Inorganic materials 0.000 claims description 33
- 238000011282 treatment Methods 0.000 claims description 33
- 125000000392 cycloalkenyl group Chemical group 0.000 claims description 32
- 125000001072 heteroaryl group Chemical group 0.000 claims description 30
- 125000000623 heterocyclic group Chemical group 0.000 claims description 28
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 26
- 229910052757 nitrogen Inorganic materials 0.000 claims description 24
- 241001465754 Metazoa Species 0.000 claims description 20
- 125000004122 cyclic group Chemical group 0.000 claims description 20
- 229910052739 hydrogen Inorganic materials 0.000 claims description 20
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 20
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 15
- 125000004432 carbon atom Chemical group C* 0.000 claims description 14
- 125000003118 aryl group Chemical group 0.000 claims description 13
- 125000004043 oxo group Chemical group O=* 0.000 claims description 12
- 125000004429 atom Chemical group 0.000 claims description 11
- KXDAEFPNCMNJSK-UHFFFAOYSA-N Benzamide Chemical compound NC(=O)C1=CC=CC=C1 KXDAEFPNCMNJSK-UHFFFAOYSA-N 0.000 claims description 10
- 125000002947 alkylene group Chemical group 0.000 claims description 9
- 125000006704 (C5-C6) cycloalkyl group Chemical group 0.000 claims description 7
- NYGVOZNTKCVNRH-UHFFFAOYSA-N 1-[2-[[5-bromo-2-(3,4,5-trimethoxyanilino)pyrimidin-4-yl]amino]phenyl]ethanone;hydrochloride Chemical compound Cl.COC1=C(OC)C(OC)=CC(NC=2N=C(NC=3C(=CC=CC=3)C(C)=O)C(Br)=CN=2)=C1 NYGVOZNTKCVNRH-UHFFFAOYSA-N 0.000 claims description 4
- DSWNFPUXAGOUNB-UHFFFAOYSA-N 1-[2-[[5-nitro-2-(3,4,5-trimethoxyanilino)pyrimidin-4-yl]amino]phenyl]ethanone;hydrochloride Chemical compound Cl.COC1=C(OC)C(OC)=CC(NC=2N=C(NC=3C(=CC=CC=3)C(C)=O)C(=CN=2)[N+]([O-])=O)=C1 DSWNFPUXAGOUNB-UHFFFAOYSA-N 0.000 claims description 4
- BPKHLVYIRZXGNH-UHFFFAOYSA-N 2-[[2-(4-acetamidoanilino)-5-nitropyrimidin-4-yl]amino]-n-methylbenzamide;hydrochloride Chemical compound Cl.CNC(=O)C1=CC=CC=C1NC1=NC(NC=2C=CC(NC(C)=O)=CC=2)=NC=C1[N+]([O-])=O BPKHLVYIRZXGNH-UHFFFAOYSA-N 0.000 claims description 4
- GLBLAEZTBNRZKL-UHFFFAOYSA-N 2-[[2-(4-acetamidoanilino)-5-nitropyrimidin-4-yl]amino]benzoic acid;hydrochloride Chemical compound Cl.C1=CC(NC(=O)C)=CC=C1NC1=NC=C([N+]([O-])=O)C(NC=2C(=CC=CC=2)C(O)=O)=N1 GLBLAEZTBNRZKL-UHFFFAOYSA-N 0.000 claims description 4
- LTRGJYMLSZPWOC-UHFFFAOYSA-N 2-[[5-nitro-2-(3,4,5-trimethoxyanilino)pyrimidin-4-yl]amino]benzoic acid;hydrochloride Chemical compound Cl.COC1=C(OC)C(OC)=CC(NC=2N=C(NC=3C(=CC=CC=3)C(O)=O)C(=CN=2)[N+]([O-])=O)=C1 LTRGJYMLSZPWOC-UHFFFAOYSA-N 0.000 claims description 4
- 125000005809 3,4,5-trimethoxyphenyl group Chemical group [H]C1=C(OC([H])([H])[H])C(OC([H])([H])[H])=C(OC([H])([H])[H])C([H])=C1* 0.000 claims description 4
- TZNMFMQKKIZUGH-UHFFFAOYSA-N 4-[2-(4-methylbenzoyl)anilino]-2-(3,4,5-trimethoxyanilino)pyrimidine-5-carbonitrile Chemical compound COC1=C(OC)C(OC)=CC(NC=2N=C(NC=3C(=CC=CC=3)C(=O)C=3C=CC(C)=CC=3)C(C#N)=CN=2)=C1 TZNMFMQKKIZUGH-UHFFFAOYSA-N 0.000 claims description 4
- XERNFKJNZREEBZ-UHFFFAOYSA-N 5-[[5-bromo-4-(2-methylsulfanylanilino)pyrimidin-2-yl]amino]-1,3-dihydrobenzimidazol-2-one;hydrochloride Chemical compound Cl.CSC1=CC=CC=C1NC1=NC(NC=2C=C3NC(=O)NC3=CC=2)=NC=C1Br XERNFKJNZREEBZ-UHFFFAOYSA-N 0.000 claims description 4
- LXCWMSHRUOVNGC-UHFFFAOYSA-N 8-[[5-nitro-2-(3,4,5-trimethoxyanilino)pyrimidin-4-yl]amino]-3,4-dihydro-2h-naphthalen-1-one;hydrochloride Chemical compound Cl.COC1=C(OC)C(OC)=CC(NC=2N=C(NC=3C=4C(=O)CCCC=4C=CC=3)C(=CN=2)[N+]([O-])=O)=C1 LXCWMSHRUOVNGC-UHFFFAOYSA-N 0.000 claims description 4
- UYEYYPXZHRAVBF-UHFFFAOYSA-N 8-[[5-nitro-2-(3,4,5-trimethoxyanilino)pyrimidin-4-yl]amino]naphthalen-2-ol;hydrochloride Chemical compound Cl.COC1=C(OC)C(OC)=CC(NC=2N=C(NC=3C4=CC(O)=CC=C4C=CC=3)C(=CN=2)[N+]([O-])=O)=C1 UYEYYPXZHRAVBF-UHFFFAOYSA-N 0.000 claims description 4
- OKONJKKSCHMKAJ-UHFFFAOYSA-N [5-chloro-2-[[5-nitro-2-(3,4,5-trimethoxyanilino)pyrimidin-4-yl]amino]phenyl]-(2-fluorophenyl)methanone;hydrochloride Chemical compound Cl.COC1=C(OC)C(OC)=CC(NC=2N=C(NC=3C(=CC(Cl)=CC=3)C(=O)C=3C(=CC=CC=3)F)C(=CN=2)[N+]([O-])=O)=C1 OKONJKKSCHMKAJ-UHFFFAOYSA-N 0.000 claims description 4
- 125000004450 alkenylene group Chemical group 0.000 claims description 4
- 229910052794 bromium Inorganic materials 0.000 claims description 4
- IDHZUXWTOZDXQU-UHFFFAOYSA-N n-[4-[[4-(2-acetylanilino)-5-bromopyrimidin-2-yl]amino]phenyl]acetamide;hydrochloride Chemical compound Cl.C1=CC(NC(=O)C)=CC=C1NC1=NC=C(Br)C(NC=2C(=CC=CC=2)C(C)=O)=N1 IDHZUXWTOZDXQU-UHFFFAOYSA-N 0.000 claims description 4
- FSHZMEGTFLXHEO-UHFFFAOYSA-N n-[4-[[5-bromo-4-(2-morpholin-4-ylanilino)pyrimidin-2-yl]amino]phenyl]acetamide;hydrochloride Chemical compound Cl.C1=CC(NC(=O)C)=CC=C1NC1=NC=C(Br)C(NC=2C(=CC=CC=2)N2CCOCC2)=N1 FSHZMEGTFLXHEO-UHFFFAOYSA-N 0.000 claims description 4
- DRBPJIVAWIHDAW-UHFFFAOYSA-N n-[4-[[5-bromo-4-(3,4,5-trimethoxyanilino)pyrimidin-2-yl]amino]phenyl]acetamide;hydrochloride Chemical compound Cl.COC1=C(OC)C(OC)=CC(NC=2C(=CN=C(NC=3C=CC(NC(C)=O)=CC=3)N=2)Br)=C1 DRBPJIVAWIHDAW-UHFFFAOYSA-N 0.000 claims description 4
- 125000004527 pyrimidin-4-yl group Chemical group N1=CN=C(C=C1)* 0.000 claims description 4
- ZCHBHKSZZHBKDX-UHFFFAOYSA-N 2-[[2-(4-acetamidoanilino)-5-nitropyrimidin-4-yl]amino]-6-methylbenzoic acid;hydrochloride Chemical compound Cl.C1=CC(NC(=O)C)=CC=C1NC1=NC=C([N+]([O-])=O)C(NC=2C(=C(C)C=CC=2)C(O)=O)=N1 ZCHBHKSZZHBKDX-UHFFFAOYSA-N 0.000 claims description 3
- BQDHCGJUSXMIIS-UHFFFAOYSA-N 2-[[5-nitro-2-(3,4,5-trimethoxyanilino)pyrimidin-4-yl]amino]benzamide;hydrochloride Chemical compound Cl.COC1=C(OC)C(OC)=CC(NC=2N=C(NC=3C(=CC=CC=3)C(N)=O)C(=CN=2)[N+]([O-])=O)=C1 BQDHCGJUSXMIIS-UHFFFAOYSA-N 0.000 claims description 3
- VCLQPDIBPALDEZ-UHFFFAOYSA-N 5-hydroxy-2-[[5-nitro-2-(3,4,5-trimethoxyanilino)pyrimidin-4-yl]amino]benzoic acid;hydrochloride Chemical compound Cl.COC1=C(OC)C(OC)=CC(NC=2N=C(NC=3C(=CC(O)=CC=3)C(O)=O)C(=CN=2)[N+]([O-])=O)=C1 VCLQPDIBPALDEZ-UHFFFAOYSA-N 0.000 claims description 3
- YMQJLYHOOFWQJZ-UHFFFAOYSA-N dimethyl benzene-1,4-dicarboxylate hydrochloride Chemical compound Cl.COC(C1=CC=C(C(=O)OC)C=C1)=O YMQJLYHOOFWQJZ-UHFFFAOYSA-N 0.000 claims description 3
- HVUUHKHRYNKNRO-UHFFFAOYSA-N n-[4-[[4-(2-benzoylanilino)-5-nitropyrimidin-2-yl]amino]phenyl]acetamide;hydrochloride Chemical compound Cl.C1=CC(NC(=O)C)=CC=C1NC1=NC=C([N+]([O-])=O)C(NC=2C(=CC=CC=2)C(=O)C=2C=CC=CC=2)=N1 HVUUHKHRYNKNRO-UHFFFAOYSA-N 0.000 claims description 3
- 229910052727 yttrium Inorganic materials 0.000 claims description 3
- FRKVTFVAWJNBDS-UHFFFAOYSA-N 5-bromo-4-n-(2-morpholin-4-ylphenyl)-2-n-(3,4,5-trimethoxyphenyl)pyrimidine-2,4-diamine;hydrochloride Chemical compound Cl.COC1=C(OC)C(OC)=CC(NC=2N=C(NC=3C(=CC=CC=3)N3CCOCC3)C(Br)=CN=2)=C1 FRKVTFVAWJNBDS-UHFFFAOYSA-N 0.000 claims description 2
- 238000000034 method Methods 0.000 abstract description 55
- 230000008569 process Effects 0.000 abstract description 2
- 239000008177 pharmaceutical agent Substances 0.000 abstract 1
- 238000006243 chemical reaction Methods 0.000 description 71
- 210000004027 cell Anatomy 0.000 description 60
- 239000007787 solid Substances 0.000 description 53
- 235000002639 sodium chloride Nutrition 0.000 description 46
- 239000000203 mixture Substances 0.000 description 45
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 43
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 40
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 37
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 33
- 239000002246 antineoplastic agent Substances 0.000 description 30
- 239000003112 inhibitor Substances 0.000 description 30
- 230000011278 mitosis Effects 0.000 description 29
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 27
- 125000004093 cyano group Chemical group *C#N 0.000 description 26
- 239000003054 catalyst Substances 0.000 description 24
- 239000002904 solvent Substances 0.000 description 23
- 230000002401 inhibitory effect Effects 0.000 description 22
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 21
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 21
- 102100031463 Serine/threonine-protein kinase PLK1 Human genes 0.000 description 19
- 108010056274 polo-like kinase 1 Proteins 0.000 description 19
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 18
- 238000009472 formulation Methods 0.000 description 17
- 229910052763 palladium Inorganic materials 0.000 description 17
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 17
- 201000011510 cancer Diseases 0.000 description 16
- 230000004663 cell proliferation Effects 0.000 description 16
- 238000004519 manufacturing process Methods 0.000 description 16
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 15
- 229940034982 antineoplastic agent Drugs 0.000 description 15
- 150000001721 carbon Chemical group 0.000 description 15
- 229940127089 cytotoxic agent Drugs 0.000 description 14
- 239000003814 drug Substances 0.000 description 14
- 239000011541 reaction mixture Substances 0.000 description 14
- 239000012442 inert solvent Substances 0.000 description 13
- DLFVBJFMPXGRIB-UHFFFAOYSA-N Acetamide Chemical compound CC(N)=O DLFVBJFMPXGRIB-UHFFFAOYSA-N 0.000 description 12
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 12
- 108091000080 Phosphotransferase Proteins 0.000 description 12
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 12
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 12
- 230000022131 cell cycle Effects 0.000 description 12
- 239000003795 chemical substances by application Substances 0.000 description 12
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 12
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 12
- 102000020233 phosphotransferase Human genes 0.000 description 12
- 239000000843 powder Substances 0.000 description 12
- 239000000243 solution Substances 0.000 description 12
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 12
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 11
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 11
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 11
- 230000010261 cell growth Effects 0.000 description 11
- 230000001404 mediated effect Effects 0.000 description 11
- 239000004480 active ingredient Substances 0.000 description 10
- YNHIGQDRGKUECZ-UHFFFAOYSA-L bis(triphenylphosphine)palladium(ii) dichloride Chemical compound [Cl-].[Cl-].[Pd+2].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 YNHIGQDRGKUECZ-UHFFFAOYSA-L 0.000 description 10
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 10
- NFHFRUOZVGFOOS-UHFFFAOYSA-N palladium;triphenylphosphane Chemical compound [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 10
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 10
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 9
- 206010006187 Breast cancer Diseases 0.000 description 9
- 208000026310 Breast neoplasm Diseases 0.000 description 9
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 9
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 9
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 9
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 8
- 102000009465 Growth Factor Receptors Human genes 0.000 description 8
- 108010009202 Growth Factor Receptors Proteins 0.000 description 8
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 8
- 230000004913 activation Effects 0.000 description 8
- 238000001994 activation Methods 0.000 description 8
- 238000003556 assay Methods 0.000 description 8
- 229940088597 hormone Drugs 0.000 description 8
- 239000005556 hormone Substances 0.000 description 8
- 239000000543 intermediate Substances 0.000 description 8
- 238000007254 oxidation reaction Methods 0.000 description 8
- 239000012071 phase Substances 0.000 description 8
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 8
- 108091008598 receptor tyrosine kinases Proteins 0.000 description 8
- 230000011664 signaling Effects 0.000 description 8
- 0 Cc1nc(*)c(*)c(Cl)n1 Chemical compound Cc1nc(*)c(*)c(Cl)n1 0.000 description 7
- 108020004414 DNA Proteins 0.000 description 7
- 208000035475 disorder Diseases 0.000 description 7
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 7
- 230000014509 gene expression Effects 0.000 description 7
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 7
- 102000037979 non-receptor tyrosine kinases Human genes 0.000 description 7
- 108091008046 non-receptor tyrosine kinases Proteins 0.000 description 7
- 239000007800 oxidant agent Substances 0.000 description 7
- 230000003647 oxidation Effects 0.000 description 7
- 230000002062 proliferating effect Effects 0.000 description 7
- 102000027426 receptor tyrosine kinases Human genes 0.000 description 7
- 239000000126 substance Substances 0.000 description 7
- CYPYTURSJDMMMP-WVCUSYJESA-N (1e,4e)-1,5-diphenylpenta-1,4-dien-3-one;palladium Chemical compound [Pd].[Pd].C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1 CYPYTURSJDMMMP-WVCUSYJESA-N 0.000 description 6
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 6
- 239000007995 HEPES buffer Substances 0.000 description 6
- 206010060862 Prostate cancer Diseases 0.000 description 6
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 6
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 6
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 6
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- 208000035269 cancer or benign tumor Diseases 0.000 description 6
- 238000005660 chlorination reaction Methods 0.000 description 6
- UKJLNMAFNRKWGR-UHFFFAOYSA-N cyclohexatrienamine Chemical group NC1=CC=C=C[CH]1 UKJLNMAFNRKWGR-UHFFFAOYSA-N 0.000 description 6
- UAOMVDZJSHZZME-UHFFFAOYSA-N diisopropylamine Chemical compound CC(C)NC(C)C UAOMVDZJSHZZME-UHFFFAOYSA-N 0.000 description 6
- 230000006870 function Effects 0.000 description 6
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 6
- 230000002018 overexpression Effects 0.000 description 6
- 108090000623 proteins and genes Proteins 0.000 description 6
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 6
- 125000001424 substituent group Chemical group 0.000 description 6
- 210000001519 tissue Anatomy 0.000 description 6
- 102100021569 Apoptosis regulator Bcl-2 Human genes 0.000 description 5
- 206010014733 Endometrial cancer Diseases 0.000 description 5
- 206010014759 Endometrial neoplasm Diseases 0.000 description 5
- 101000971171 Homo sapiens Apoptosis regulator Bcl-2 Proteins 0.000 description 5
- 101000691614 Homo sapiens Serine/threonine-protein kinase PLK3 Proteins 0.000 description 5
- 102100031462 Serine/threonine-protein kinase PLK2 Human genes 0.000 description 5
- 102100026209 Serine/threonine-protein kinase PLK3 Human genes 0.000 description 5
- 239000002253 acid Substances 0.000 description 5
- 125000003282 alkyl amino group Chemical group 0.000 description 5
- 125000005157 alkyl carboxy group Chemical group 0.000 description 5
- 150000005215 alkyl ethers Chemical class 0.000 description 5
- 125000004390 alkyl sulfonyl group Chemical group 0.000 description 5
- 230000027455 binding Effects 0.000 description 5
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 5
- 230000030833 cell death Effects 0.000 description 5
- GBRBMTNGQBKBQE-UHFFFAOYSA-L copper;diiodide Chemical compound I[Cu]I GBRBMTNGQBKBQE-UHFFFAOYSA-L 0.000 description 5
- 125000004663 dialkyl amino group Chemical group 0.000 description 5
- 239000003085 diluting agent Substances 0.000 description 5
- 239000003937 drug carrier Substances 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- 230000005764 inhibitory process Effects 0.000 description 5
- 239000007788 liquid Substances 0.000 description 5
- 239000000546 pharmaceutical excipient Substances 0.000 description 5
- 229910000027 potassium carbonate Inorganic materials 0.000 description 5
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 5
- 102000004169 proteins and genes Human genes 0.000 description 5
- 102000005962 receptors Human genes 0.000 description 5
- 230000019491 signal transduction Effects 0.000 description 5
- 239000011780 sodium chloride Substances 0.000 description 5
- 125000000475 sulfinyl group Chemical group [*:2]S([*:1])=O 0.000 description 5
- 239000000725 suspension Substances 0.000 description 5
- MHCRXTLQJHXSHN-UHFFFAOYSA-N 2-[(2-chloro-5-nitropyrimidin-4-yl)amino]benzamide Chemical compound NC(=O)C1=CC=CC=C1NC1=NC(Cl)=NC=C1[N+]([O-])=O MHCRXTLQJHXSHN-UHFFFAOYSA-N 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 101150012716 CDK1 gene Proteins 0.000 description 4
- 108091007914 CDKs Proteins 0.000 description 4
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 4
- 206010009944 Colon cancer Diseases 0.000 description 4
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 4
- 101100059559 Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) nimX gene Proteins 0.000 description 4
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 4
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 4
- 101001059454 Homo sapiens Serine/threonine-protein kinase MARK2 Proteins 0.000 description 4
- 101000729945 Homo sapiens Serine/threonine-protein kinase PLK2 Proteins 0.000 description 4
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 4
- 241000124008 Mammalia Species 0.000 description 4
- JRNVZBWKYDBUCA-UHFFFAOYSA-N N-chlorosuccinimide Chemical compound ClN1C(=O)CCC1=O JRNVZBWKYDBUCA-UHFFFAOYSA-N 0.000 description 4
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 4
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 4
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 4
- 102000014400 SH2 domains Human genes 0.000 description 4
- 108050003452 SH2 domains Proteins 0.000 description 4
- 102000000395 SH3 domains Human genes 0.000 description 4
- 108050008861 SH3 domains Proteins 0.000 description 4
- 102100028904 Serine/threonine-protein kinase MARK2 Human genes 0.000 description 4
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 4
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 4
- 229920002472 Starch Polymers 0.000 description 4
- 108091008605 VEGF receptors Proteins 0.000 description 4
- 102100033177 Vascular endothelial growth factor receptor 2 Human genes 0.000 description 4
- 229940122803 Vinca alkaloid Drugs 0.000 description 4
- 229940100198 alkylating agent Drugs 0.000 description 4
- 239000002168 alkylating agent Substances 0.000 description 4
- 229940044684 anti-microtubule agent Drugs 0.000 description 4
- 239000000074 antisense oligonucleotide Substances 0.000 description 4
- 238000012230 antisense oligonucleotides Methods 0.000 description 4
- 239000011230 binding agent Substances 0.000 description 4
- FJDQFPXHSGXQBY-UHFFFAOYSA-L caesium carbonate Chemical compound [Cs+].[Cs+].[O-]C([O-])=O FJDQFPXHSGXQBY-UHFFFAOYSA-L 0.000 description 4
- 229910000024 caesium carbonate Inorganic materials 0.000 description 4
- 239000002775 capsule Substances 0.000 description 4
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 4
- 239000003153 chemical reaction reagent Substances 0.000 description 4
- 238000000576 coating method Methods 0.000 description 4
- 238000007796 conventional method Methods 0.000 description 4
- 239000006071 cream Substances 0.000 description 4
- 230000001419 dependent effect Effects 0.000 description 4
- 201000004101 esophageal cancer Diseases 0.000 description 4
- 239000008187 granular material Substances 0.000 description 4
- 229910052736 halogen Inorganic materials 0.000 description 4
- 150000002367 halogens Chemical class 0.000 description 4
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 4
- 229910052744 lithium Inorganic materials 0.000 description 4
- 239000000314 lubricant Substances 0.000 description 4
- 208000020816 lung neoplasm Diseases 0.000 description 4
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 4
- CHMBIJAOCISYEW-UHFFFAOYSA-N n-(4-aminophenyl)acetamide Chemical compound CC(=O)NC1=CC=C(N)C=C1 CHMBIJAOCISYEW-UHFFFAOYSA-N 0.000 description 4
- 210000005170 neoplastic cell Anatomy 0.000 description 4
- LXNAVEXFUKBNMK-UHFFFAOYSA-N palladium(II) acetate Substances [Pd].CC(O)=O.CC(O)=O LXNAVEXFUKBNMK-UHFFFAOYSA-N 0.000 description 4
- YJVFFLUZDVXJQI-UHFFFAOYSA-L palladium(ii) acetate Chemical compound [Pd+2].CC([O-])=O.CC([O-])=O YJVFFLUZDVXJQI-UHFFFAOYSA-L 0.000 description 4
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 4
- 230000026731 phosphorylation Effects 0.000 description 4
- 238000006366 phosphorylation reaction Methods 0.000 description 4
- 229910052697 platinum Inorganic materials 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 108020003175 receptors Proteins 0.000 description 4
- 239000008107 starch Substances 0.000 description 4
- 235000019698 starch Nutrition 0.000 description 4
- VZGDMQKNWNREIO-UHFFFAOYSA-N tetrachloromethane Chemical compound ClC(Cl)(Cl)Cl VZGDMQKNWNREIO-UHFFFAOYSA-N 0.000 description 4
- 238000002560 therapeutic procedure Methods 0.000 description 4
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 4
- SUXDKWUJQKIXPP-UHFFFAOYSA-N 1-[2-[(5-bromo-2-chloropyrimidin-4-yl)amino]phenyl]ethanone Chemical compound CC(=O)C1=CC=CC=C1NC1=NC(Cl)=NC=C1Br SUXDKWUJQKIXPP-UHFFFAOYSA-N 0.000 description 3
- WEJRZRSLNWNCSA-UHFFFAOYSA-N 1-oxo-1-(2-sulfamoylhydrazinyl)ethane Chemical group CC(=O)NNS(N)(=O)=O WEJRZRSLNWNCSA-UHFFFAOYSA-N 0.000 description 3
- GIKMWFAAEIACRF-UHFFFAOYSA-N 2,4,5-trichloropyrimidine Chemical compound ClC1=NC=C(Cl)C(Cl)=N1 GIKMWFAAEIACRF-UHFFFAOYSA-N 0.000 description 3
- DPVIABCMTHHTGB-UHFFFAOYSA-N 2,4,6-trichloropyrimidine Chemical compound ClC1=CC(Cl)=NC(Cl)=N1 DPVIABCMTHHTGB-UHFFFAOYSA-N 0.000 description 3
- WLZKNGHHZJMUBW-UHFFFAOYSA-N 2-[(2-chloro-5-nitropyrimidin-4-yl)amino]benzoic acid Chemical compound OC(=O)C1=CC=CC=C1NC1=NC(Cl)=NC=C1[N+]([O-])=O WLZKNGHHZJMUBW-UHFFFAOYSA-N 0.000 description 3
- ODWSMCBPWVISAP-UHFFFAOYSA-N 2-chloro-n-(2,3-dihydro-1,4-benzodioxin-5-yl)-5-nitropyrimidin-4-amine Chemical compound [O-][N+](=O)C1=CN=C(Cl)N=C1NC1=CC=CC2=C1OCCO2 ODWSMCBPWVISAP-UHFFFAOYSA-N 0.000 description 3
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical compound N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 3
- MPCUACOCIITPDA-UHFFFAOYSA-N 8-[(2-chloro-5-nitropyrimidin-4-yl)amino]-3,4-dihydro-2h-naphthalen-1-one Chemical compound [O-][N+](=O)C1=CN=C(Cl)N=C1NC1=CC=CC2=C1C(=O)CCC2 MPCUACOCIITPDA-UHFFFAOYSA-N 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 3
- 102000016736 Cyclin Human genes 0.000 description 3
- 108050006400 Cyclin Proteins 0.000 description 3
- 108090000266 Cyclin-dependent kinases Proteins 0.000 description 3
- 102000003903 Cyclin-dependent kinases Human genes 0.000 description 3
- 230000006820 DNA synthesis Effects 0.000 description 3
- 229940124087 DNA topoisomerase II inhibitor Drugs 0.000 description 3
- 101000582926 Dictyostelium discoideum Probable serine/threonine-protein kinase PLK Proteins 0.000 description 3
- QMMFVYPAHWMCMS-UHFFFAOYSA-N Dimethyl sulfide Chemical compound CSC QMMFVYPAHWMCMS-UHFFFAOYSA-N 0.000 description 3
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 3
- 108060006698 EGF receptor Proteins 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- 108010010803 Gelatin Proteins 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 3
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 3
- 206010025323 Lymphomas Diseases 0.000 description 3
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 3
- 206010033128 Ovarian cancer Diseases 0.000 description 3
- 206010061535 Ovarian neoplasm Diseases 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 3
- RWRDLPDLKQPQOW-UHFFFAOYSA-N Pyrrolidine Chemical compound C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 description 3
- 239000000317 Topoisomerase II Inhibitor Substances 0.000 description 3
- 239000003377 acid catalyst Substances 0.000 description 3
- 230000000340 anti-metabolite Effects 0.000 description 3
- 229940100197 antimetabolite Drugs 0.000 description 3
- 239000002256 antimetabolite Substances 0.000 description 3
- 238000013459 approach Methods 0.000 description 3
- 125000002619 bicyclic group Chemical group 0.000 description 3
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 3
- 229940127093 camptothecin Drugs 0.000 description 3
- VSJKWCGYPAHWDS-FQEVSTJZSA-N camptothecin Chemical compound C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-FQEVSTJZSA-N 0.000 description 3
- 230000000973 chemotherapeutic effect Effects 0.000 description 3
- 238000002512 chemotherapy Methods 0.000 description 3
- 239000000460 chlorine Substances 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 208000029742 colonic neoplasm Diseases 0.000 description 3
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 239000007884 disintegrant Substances 0.000 description 3
- 150000004141 diterpene derivatives Chemical class 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 125000001153 fluoro group Chemical group F* 0.000 description 3
- 229920000159 gelatin Polymers 0.000 description 3
- 239000008273 gelatin Substances 0.000 description 3
- 235000019322 gelatine Nutrition 0.000 description 3
- 235000011852 gelatine desserts Nutrition 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 201000010536 head and neck cancer Diseases 0.000 description 3
- 208000014829 head and neck neoplasm Diseases 0.000 description 3
- 150000002430 hydrocarbons Chemical group 0.000 description 3
- ZMZDMBWJUHKJPS-UHFFFAOYSA-N hydrogen thiocyanate Natural products SC#N ZMZDMBWJUHKJPS-UHFFFAOYSA-N 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- CDAISMWEOUEBRE-GPIVLXJGSA-N inositol Chemical compound O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@H](O)[C@@H]1O CDAISMWEOUEBRE-GPIVLXJGSA-N 0.000 description 3
- 239000003446 ligand Substances 0.000 description 3
- 201000005202 lung cancer Diseases 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- HHKFILBTVSLJTM-UHFFFAOYSA-N methyl 2-[(2-chloro-5-nitropyrimidin-4-yl)amino]benzoate Chemical compound COC(=O)C1=CC=CC=C1NC1=NC(Cl)=NC=C1[N+]([O-])=O HHKFILBTVSLJTM-UHFFFAOYSA-N 0.000 description 3
- CXKWCBBOMKCUKX-UHFFFAOYSA-M methylene blue Chemical compound [Cl-].C1=CC(N(C)C)=CC2=[S+]C3=CC(N(C)C)=CC=C3N=C21 CXKWCBBOMKCUKX-UHFFFAOYSA-M 0.000 description 3
- 229960000907 methylthioninium chloride Drugs 0.000 description 3
- 230000000394 mitotic effect Effects 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- 230000027498 negative regulation of mitosis Effects 0.000 description 3
- 231100000252 nontoxic Toxicity 0.000 description 3
- 230000003000 nontoxic effect Effects 0.000 description 3
- 239000012038 nucleophile Substances 0.000 description 3
- 239000003921 oil Substances 0.000 description 3
- 239000002674 ointment Substances 0.000 description 3
- 239000006072 paste Substances 0.000 description 3
- 239000012286 potassium permanganate Substances 0.000 description 3
- 230000000861 pro-apoptotic effect Effects 0.000 description 3
- 230000035755 proliferation Effects 0.000 description 3
- 125000004368 propenyl group Chemical group C(=CC)* 0.000 description 3
- 235000018102 proteins Nutrition 0.000 description 3
- 108700042226 ras Genes Proteins 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 229940124530 sulfonamide Drugs 0.000 description 3
- 150000003456 sulfonamides Chemical class 0.000 description 3
- 238000003786 synthesis reaction Methods 0.000 description 3
- 239000006188 syrup Substances 0.000 description 3
- 235000020357 syrup Nutrition 0.000 description 3
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 3
- 210000004881 tumor cell Anatomy 0.000 description 3
- 239000003981 vehicle Substances 0.000 description 3
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 3
- 239000001993 wax Substances 0.000 description 3
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 description 2
- QBSFUIVGMLHKMH-UHFFFAOYSA-N 1-[2-[(2-chloro-5-nitropyrimidin-4-yl)amino]phenyl]ethanone Chemical compound CC(=O)C1=CC=CC=C1NC1=NC(Cl)=NC=C1[N+]([O-])=O QBSFUIVGMLHKMH-UHFFFAOYSA-N 0.000 description 2
- FCEHBMOGCRZNNI-UHFFFAOYSA-N 1-benzothiophene Chemical compound C1=CC=C2SC=CC2=C1 FCEHBMOGCRZNNI-UHFFFAOYSA-N 0.000 description 2
- HZNVUJQVZSTENZ-UHFFFAOYSA-N 2,3-dichloro-5,6-dicyano-1,4-benzoquinone Chemical compound ClC1=C(Cl)C(=O)C(C#N)=C(C#N)C1=O HZNVUJQVZSTENZ-UHFFFAOYSA-N 0.000 description 2
- INUSQTPGSHFGHM-UHFFFAOYSA-N 2,4-dichloro-5-nitropyrimidine Chemical compound [O-][N+](=O)C1=CN=C(Cl)N=C1Cl INUSQTPGSHFGHM-UHFFFAOYSA-N 0.000 description 2
- PXBFMLJZNCDSMP-UHFFFAOYSA-N 2-Aminobenzamide Chemical compound NC(=O)C1=CC=CC=C1N PXBFMLJZNCDSMP-UHFFFAOYSA-N 0.000 description 2
- MEOADRWUNVLPKF-UHFFFAOYSA-N 2-[(2-chloro-5-nitropyrimidin-4-yl)amino]-5-hydroxybenzoic acid Chemical compound OC(=O)C1=CC(O)=CC=C1NC1=NC(Cl)=NC=C1[N+]([O-])=O MEOADRWUNVLPKF-UHFFFAOYSA-N 0.000 description 2
- ILNUIRZRRBTPSA-UHFFFAOYSA-N 2-[(2-chloro-5-nitropyrimidin-4-yl)amino]-n-methylbenzamide Chemical compound CNC(=O)C1=CC=CC=C1NC1=NC(Cl)=NC=C1[N+]([O-])=O ILNUIRZRRBTPSA-UHFFFAOYSA-N 0.000 description 2
- VTFSOARZHNPMLF-UHFFFAOYSA-N 2-[[2-(4-acetamidoanilino)-5-nitropyrimidin-4-yl]amino]-6-methylbenzoic acid Chemical compound C1=CC(NC(=O)C)=CC=C1NC1=NC=C([N+]([O-])=O)C(NC=2C(=C(C)C=CC=2)C(O)=O)=N1 VTFSOARZHNPMLF-UHFFFAOYSA-N 0.000 description 2
- XEFRNCLPPFDWAC-UHFFFAOYSA-N 3,4,5-trimethoxyaniline Chemical compound COC1=CC(N)=CC(OC)=C1OC XEFRNCLPPFDWAC-UHFFFAOYSA-N 0.000 description 2
- NHQDETIJWKXCTC-UHFFFAOYSA-N 3-chloroperbenzoic acid Chemical compound OOC(=O)C1=CC=CC(Cl)=C1 NHQDETIJWKXCTC-UHFFFAOYSA-N 0.000 description 2
- VJUZNSSAVKQGJO-UHFFFAOYSA-N 5-bromo-2-chloro-n-(2-morpholin-4-ylphenyl)pyrimidin-4-amine Chemical compound ClC1=NC=C(Br)C(NC=2C(=CC=CC=2)N2CCOCC2)=N1 VJUZNSSAVKQGJO-UHFFFAOYSA-N 0.000 description 2
- 229920001817 Agar Polymers 0.000 description 2
- 102400000068 Angiostatin Human genes 0.000 description 2
- 108010079709 Angiostatins Proteins 0.000 description 2
- 102000051485 Bcl-2 family Human genes 0.000 description 2
- 108700038897 Bcl-2 family Proteins 0.000 description 2
- 108010006654 Bleomycin Proteins 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- KLWPJMFMVPTNCC-UHFFFAOYSA-N Camptothecin Natural products CCC1(O)C(=O)OCC2=C1C=C3C4Nc5ccccc5C=C4CN3C2=O KLWPJMFMVPTNCC-UHFFFAOYSA-N 0.000 description 2
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 2
- 108091060290 Chromatid Proteins 0.000 description 2
- 231100001074 DNA strand break Toxicity 0.000 description 2
- 229940123780 DNA topoisomerase I inhibitor Drugs 0.000 description 2
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 241000255581 Drosophila <fruit fly, genus> Species 0.000 description 2
- 108700007536 Drosophila polo Proteins 0.000 description 2
- 102000001301 EGF receptor Human genes 0.000 description 2
- 102400001047 Endostatin Human genes 0.000 description 2
- 108010079505 Endostatins Proteins 0.000 description 2
- 102000018233 Fibroblast Growth Factor Human genes 0.000 description 2
- 108050007372 Fibroblast Growth Factor Proteins 0.000 description 2
- 102000016621 Focal Adhesion Protein-Tyrosine Kinases Human genes 0.000 description 2
- 108010067715 Focal Adhesion Protein-Tyrosine Kinases Proteins 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- 101000851007 Homo sapiens Vascular endothelial growth factor receptor 2 Proteins 0.000 description 2
- SIKJAQJRHWYJAI-UHFFFAOYSA-N Indole Chemical compound C1=CC=C2NC=CC2=C1 SIKJAQJRHWYJAI-UHFFFAOYSA-N 0.000 description 2
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical class C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 2
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 2
- 240000007472 Leucaena leucocephala Species 0.000 description 2
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 2
- JLTDJTHDQAWBAV-UHFFFAOYSA-N N,N-dimethylaniline Chemical compound CN(C)C1=CC=CC=C1 JLTDJTHDQAWBAV-UHFFFAOYSA-N 0.000 description 2
- MZRVEZGGRBJDDB-UHFFFAOYSA-N N-Butyllithium Chemical compound [Li]CCCC MZRVEZGGRBJDDB-UHFFFAOYSA-N 0.000 description 2
- KIMWOULVHFLJIU-UHFFFAOYSA-N N-Methylanthranilamide Chemical compound CNC(=O)C1=CC=CC=C1N KIMWOULVHFLJIU-UHFFFAOYSA-N 0.000 description 2
- 108091034117 Oligonucleotide Proteins 0.000 description 2
- 108091008606 PDGF receptors Proteins 0.000 description 2
- 102000038030 PI3Ks Human genes 0.000 description 2
- 108091007960 PI3Ks Proteins 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 2
- OFBQJSOFQDEBGM-UHFFFAOYSA-N Pentane Chemical compound CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 2
- 108090000430 Phosphatidylinositol 3-kinases Proteins 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 2
- 102000011653 Platelet-Derived Growth Factor Receptors Human genes 0.000 description 2
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 102000001253 Protein Kinase Human genes 0.000 description 2
- 108090000315 Protein Kinase C Proteins 0.000 description 2
- 102000003923 Protein Kinase C Human genes 0.000 description 2
- KYQCOXFCLRTKLS-UHFFFAOYSA-N Pyrazine Chemical compound C1=CN=CC=N1 KYQCOXFCLRTKLS-UHFFFAOYSA-N 0.000 description 2
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 2
- KAESVJOAVNADME-UHFFFAOYSA-N Pyrrole Chemical compound C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 description 2
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 2
- 108091005682 Receptor kinases Proteins 0.000 description 2
- 230000018199 S phase Effects 0.000 description 2
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- WQDUMFSSJAZKTM-UHFFFAOYSA-N Sodium methoxide Chemical compound [Na+].[O-]C WQDUMFSSJAZKTM-UHFFFAOYSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 2
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical compound C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 2
- 239000000365 Topoisomerase I Inhibitor Substances 0.000 description 2
- 102100029823 Tyrosine-protein kinase BTK Human genes 0.000 description 2
- 102000009484 Vascular Endothelial Growth Factor Receptors Human genes 0.000 description 2
- 241001660687 Xantho Species 0.000 description 2
- QUPQDXFHLRFEPF-UHFFFAOYSA-N [2-[(2-chloro-5-nitropyrimidin-4-yl)amino]phenyl]-phenylmethanone Chemical compound [O-][N+](=O)C1=CN=C(Cl)N=C1NC1=CC=CC=C1C(=O)C1=CC=CC=C1 QUPQDXFHLRFEPF-UHFFFAOYSA-N 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 229930183665 actinomycin Natural products 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 239000008272 agar Substances 0.000 description 2
- 235000010419 agar Nutrition 0.000 description 2
- 150000001345 alkine derivatives Chemical class 0.000 description 2
- 230000029936 alkylation Effects 0.000 description 2
- 238000005804 alkylation reaction Methods 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 230000033115 angiogenesis Effects 0.000 description 2
- 239000004037 angiogenesis inhibitor Substances 0.000 description 2
- 229940121369 angiogenesis inhibitor Drugs 0.000 description 2
- 239000005557 antagonist Substances 0.000 description 2
- RWZYAGGXGHYGMB-UHFFFAOYSA-N anthranilic acid Chemical compound NC1=CC=CC=C1C(O)=O RWZYAGGXGHYGMB-UHFFFAOYSA-N 0.000 description 2
- 230000000692 anti-sense effect Effects 0.000 description 2
- 230000006907 apoptotic process Effects 0.000 description 2
- FZCSTZYAHCUGEM-UHFFFAOYSA-N aspergillomarasmine B Natural products OC(=O)CNC(C(O)=O)CNC(C(O)=O)CC(O)=O FZCSTZYAHCUGEM-UHFFFAOYSA-N 0.000 description 2
- 150000001540 azides Chemical class 0.000 description 2
- 239000000440 bentonite Substances 0.000 description 2
- 235000012216 bentonite Nutrition 0.000 description 2
- 229910000278 bentonite Inorganic materials 0.000 description 2
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- 230000003115 biocidal effect Effects 0.000 description 2
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical class N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 2
- 239000002981 blocking agent Substances 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- ZADPBFCGQRWHPN-UHFFFAOYSA-N boronic acid Chemical compound OBO ZADPBFCGQRWHPN-UHFFFAOYSA-N 0.000 description 2
- ODWXUNBKCRECNW-UHFFFAOYSA-M bromocopper(1+) Chemical compound Br[Cu+] ODWXUNBKCRECNW-UHFFFAOYSA-M 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 2
- 229910002091 carbon monoxide Inorganic materials 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 2
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 230000032823 cell division Effects 0.000 description 2
- 210000003793 centrosome Anatomy 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 210000004756 chromatid Anatomy 0.000 description 2
- 239000003086 colorant Substances 0.000 description 2
- 238000002648 combination therapy Methods 0.000 description 2
- BERDEBHAJNAUOM-UHFFFAOYSA-N copper(i) oxide Chemical compound [Cu]O[Cu] BERDEBHAJNAUOM-UHFFFAOYSA-N 0.000 description 2
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 2
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 2
- 230000002380 cytological effect Effects 0.000 description 2
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 2
- 229940043279 diisopropylamine Drugs 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- CNXMDTWQWLGCPE-UHFFFAOYSA-N ditert-butyl-(2-phenylphenyl)phosphane Chemical group CC(C)(C)P(C(C)(C)C)C1=CC=CC=C1C1=CC=CC=C1 CNXMDTWQWLGCPE-UHFFFAOYSA-N 0.000 description 2
- VSJKWCGYPAHWDS-UHFFFAOYSA-N dl-camptothecin Natural products C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)C5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-UHFFFAOYSA-N 0.000 description 2
- 238000007876 drug discovery Methods 0.000 description 2
- 238000002330 electrospray ionisation mass spectrometry Methods 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- 210000002950 fibroblast Anatomy 0.000 description 2
- 229940126864 fibroblast growth factor Drugs 0.000 description 2
- 230000003176 fibrotic effect Effects 0.000 description 2
- 238000011049 filling Methods 0.000 description 2
- 235000013355 food flavoring agent Nutrition 0.000 description 2
- 239000001530 fumaric acid Substances 0.000 description 2
- XGALLCVXEZPNRQ-UHFFFAOYSA-N gefitinib Chemical compound C=12C=C(OCCCN3CCOCC3)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 XGALLCVXEZPNRQ-UHFFFAOYSA-N 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 238000000227 grinding Methods 0.000 description 2
- 230000009036 growth inhibition Effects 0.000 description 2
- 125000001188 haloalkyl group Chemical group 0.000 description 2
- 235000015220 hamburgers Nutrition 0.000 description 2
- 239000002944 hormone and hormone analog Substances 0.000 description 2
- 239000002955 immunomodulating agent Substances 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 229960000367 inositol Drugs 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- AWJUIBRHMBBTKR-UHFFFAOYSA-N isoquinoline Chemical compound C1=NC=CC2=CC=CC=C21 AWJUIBRHMBBTKR-UHFFFAOYSA-N 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 208000032839 leukemia Diseases 0.000 description 2
- 108020001756 ligand binding domains Proteins 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- 235000019359 magnesium stearate Nutrition 0.000 description 2
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 201000001441 melanoma Diseases 0.000 description 2
- 210000003584 mesangial cell Anatomy 0.000 description 2
- 108020004999 messenger RNA Proteins 0.000 description 2
- 208000030159 metabolic disease Diseases 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- VAMXMNNIEUEQDV-UHFFFAOYSA-N methyl anthranilate Chemical compound COC(=O)C1=CC=CC=C1N VAMXMNNIEUEQDV-UHFFFAOYSA-N 0.000 description 2
- 125000002950 monocyclic group Chemical group 0.000 description 2
- 230000035772 mutation Effects 0.000 description 2
- YUSMFBGAHGKJDT-UHFFFAOYSA-N n-(2-chloro-5-nitropyrimidin-4-yl)-1h-indol-4-amine Chemical compound [O-][N+](=O)C1=CN=C(Cl)N=C1NC1=CC=CC2=C1C=CN2 YUSMFBGAHGKJDT-UHFFFAOYSA-N 0.000 description 2
- 239000007922 nasal spray Substances 0.000 description 2
- 230000035407 negative regulation of cell proliferation Effects 0.000 description 2
- 229910052759 nickel Inorganic materials 0.000 description 2
- 208000002154 non-small cell lung carcinoma Diseases 0.000 description 2
- 210000001331 nose Anatomy 0.000 description 2
- 108020004707 nucleic acids Proteins 0.000 description 2
- 102000039446 nucleic acids Human genes 0.000 description 2
- 150000007523 nucleic acids Chemical class 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 239000012074 organic phase Substances 0.000 description 2
- 239000012285 osmium tetroxide Substances 0.000 description 2
- 229910000489 osmium tetroxide Inorganic materials 0.000 description 2
- CTSLXHKWHWQRSH-UHFFFAOYSA-N oxalyl chloride Chemical compound ClC(=O)C(Cl)=O CTSLXHKWHWQRSH-UHFFFAOYSA-N 0.000 description 2
- 201000002528 pancreatic cancer Diseases 0.000 description 2
- 208000008443 pancreatic carcinoma Diseases 0.000 description 2
- 239000012188 paraffin wax Substances 0.000 description 2
- VLTRZXGMWDSKGL-UHFFFAOYSA-N perchloric acid Chemical compound OCl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-N 0.000 description 2
- 125000005010 perfluoroalkyl group Chemical group 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 230000035790 physiological processes and functions Effects 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 2
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 2
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 2
- SCVFZCLFOSHCOH-UHFFFAOYSA-M potassium acetate Chemical compound [K+].CC([O-])=O SCVFZCLFOSHCOH-UHFFFAOYSA-M 0.000 description 2
- NROKBHXJSPEDAR-UHFFFAOYSA-M potassium fluoride Chemical compound [F-].[K+] NROKBHXJSPEDAR-UHFFFAOYSA-M 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- 229960005205 prednisolone Drugs 0.000 description 2
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 108090000765 processed proteins & peptides Proteins 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 230000005855 radiation Effects 0.000 description 2
- 230000021014 regulation of cell growth Effects 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- CDAISMWEOUEBRE-UHFFFAOYSA-N scyllo-inosotol Natural products OC1C(O)C(O)C(O)C(O)C1O CDAISMWEOUEBRE-UHFFFAOYSA-N 0.000 description 2
- 238000009491 slugging Methods 0.000 description 2
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 2
- 235000017557 sodium bicarbonate Nutrition 0.000 description 2
- 229910000029 sodium carbonate Inorganic materials 0.000 description 2
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 2
- JQWHASGSAFIOCM-UHFFFAOYSA-M sodium periodate Chemical compound [Na+].[O-]I(=O)(=O)=O JQWHASGSAFIOCM-UHFFFAOYSA-M 0.000 description 2
- MFRIHAYPQRLWNB-UHFFFAOYSA-N sodium tert-butoxide Chemical compound [Na+].CC(C)(C)[O-] MFRIHAYPQRLWNB-UHFFFAOYSA-N 0.000 description 2
- 239000007921 spray Substances 0.000 description 2
- 206010041823 squamous cell carcinoma Diseases 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- 125000005017 substituted alkenyl group Chemical group 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 125000005420 sulfonamido group Chemical group S(=O)(=O)(N*)* 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 230000004083 survival effect Effects 0.000 description 2
- 239000000454 talc Substances 0.000 description 2
- 229910052623 talc Inorganic materials 0.000 description 2
- 235000012222 talc Nutrition 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 2
- 238000011200 topical administration Methods 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 150000004654 triazenes Chemical class 0.000 description 2
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 2
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 description 2
- 239000002691 unilamellar liposome Substances 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- 229940124676 vascular endothelial growth factor receptor Drugs 0.000 description 2
- RGHNJXZEOKUKBD-NRXMZTRTSA-N (2r,3r,4r,5s)-2,3,4,5,6-pentahydroxyhexanoic acid Chemical compound OC[C@H](O)[C@@H](O)[C@@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-NRXMZTRTSA-N 0.000 description 1
- CRYXXTXWUCPIMU-WNQIDUERSA-N (2s)-2-aminobutanediamide;phenol Chemical compound OC1=CC=CC=C1.NC(=O)[C@@H](N)CC(N)=O CRYXXTXWUCPIMU-WNQIDUERSA-N 0.000 description 1
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 1
- LKJPYSCBVHEWIU-KRWDZBQOSA-N (R)-bicalutamide Chemical compound C([C@@](O)(C)C(=O)NC=1C=C(C(C#N)=CC=1)C(F)(F)F)S(=O)(=O)C1=CC=C(F)C=C1 LKJPYSCBVHEWIU-KRWDZBQOSA-N 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical class OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- UWYVPFMHMJIBHE-OWOJBTEDSA-N (e)-2-hydroxybut-2-enedioic acid Chemical compound OC(=O)\C=C(\O)C(O)=O UWYVPFMHMJIBHE-OWOJBTEDSA-N 0.000 description 1
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 1
- OTPDWCMLUKMQNO-UHFFFAOYSA-N 1,2,3,4-tetrahydropyrimidine Chemical compound C1NCC=CN1 OTPDWCMLUKMQNO-UHFFFAOYSA-N 0.000 description 1
- VDFVNEFVBPFDSB-UHFFFAOYSA-N 1,3-dioxane Chemical compound C1COCOC1 VDFVNEFVBPFDSB-UHFFFAOYSA-N 0.000 description 1
- WNXJIVFYUVYPPR-UHFFFAOYSA-N 1,3-dioxolane Chemical compound C1COCO1 WNXJIVFYUVYPPR-UHFFFAOYSA-N 0.000 description 1
- OGYGFUAIIOPWQD-UHFFFAOYSA-N 1,3-thiazolidine Chemical compound C1CSCN1 OGYGFUAIIOPWQD-UHFFFAOYSA-N 0.000 description 1
- TUSDEZXZIZRFGC-UHFFFAOYSA-N 1-O-galloyl-3,6-(R)-HHDP-beta-D-glucose Chemical class OC1C(O2)COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC1C(O)C2OC(=O)C1=CC(O)=C(O)C(O)=C1 TUSDEZXZIZRFGC-UHFFFAOYSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- WNWHHMBRJJOGFJ-UHFFFAOYSA-N 16-methylheptadecan-1-ol Chemical class CC(C)CCCCCCCCCCCCCCCO WNWHHMBRJJOGFJ-UHFFFAOYSA-N 0.000 description 1
- BAXOFTOLAUCFNW-UHFFFAOYSA-N 1H-indazole Chemical compound C1=CC=C2C=NNC2=C1 BAXOFTOLAUCFNW-UHFFFAOYSA-N 0.000 description 1
- LBLYYCQCTBFVLH-UHFFFAOYSA-N 2-Methylbenzenesulfonic acid Chemical compound CC1=CC=CC=C1S(O)(=O)=O LBLYYCQCTBFVLH-UHFFFAOYSA-N 0.000 description 1
- DEKWHWQPSCWZAS-UHFFFAOYSA-N 2-[(2-chloro-5-nitropyrimidin-4-yl)amino]-6-methylbenzoic acid Chemical compound CC1=CC=CC(NC=2C(=CN=C(Cl)N=2)[N+]([O-])=O)=C1C(O)=O DEKWHWQPSCWZAS-UHFFFAOYSA-N 0.000 description 1
- GGVLVRBBVDAZMN-UHFFFAOYSA-N 2-[(2-chloro-5-nitropyrimidin-4-yl)amino]-n-cyclohexylbenzamide Chemical compound [O-][N+](=O)C1=CN=C(Cl)N=C1NC1=CC=CC=C1C(=O)NC1CCCCC1 GGVLVRBBVDAZMN-UHFFFAOYSA-N 0.000 description 1
- YHBZJCBYHUVKCM-UHFFFAOYSA-N 2-amino-n-tert-butylbenzamide Chemical compound CC(C)(C)NC(=O)C1=CC=CC=C1N YHBZJCBYHUVKCM-UHFFFAOYSA-N 0.000 description 1
- UPHOPMSGKZNELG-UHFFFAOYSA-N 2-hydroxynaphthalene-1-carboxylic acid Chemical class C1=CC=C2C(C(=O)O)=C(O)C=CC2=C1 UPHOPMSGKZNELG-UHFFFAOYSA-N 0.000 description 1
- OPJKGTJXHVPYIM-UHFFFAOYSA-N 2-methylprop-2-enamide;phenol Chemical compound CC(=C)C(N)=O.OC1=CC=CC=C1 OPJKGTJXHVPYIM-UHFFFAOYSA-N 0.000 description 1
- WLJVXDMOQOGPHL-PPJXEINESA-N 2-phenylacetic acid Chemical compound O[14C](=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-PPJXEINESA-N 0.000 description 1
- MGADZUXDNSDTHW-UHFFFAOYSA-N 2H-pyran Chemical compound C1OC=CC=C1 MGADZUXDNSDTHW-UHFFFAOYSA-N 0.000 description 1
- ZZVDXRCAGGQFAK-UHFFFAOYSA-N 2h-oxazaphosphinine Chemical class N1OC=CC=P1 ZZVDXRCAGGQFAK-UHFFFAOYSA-N 0.000 description 1
- WEVYNIUIFUYDGI-UHFFFAOYSA-N 3-[6-[4-(trifluoromethoxy)anilino]-4-pyrimidinyl]benzamide Chemical compound NC(=O)C1=CC=CC(C=2N=CN=C(NC=3C=CC(OC(F)(F)F)=CC=3)C=2)=C1 WEVYNIUIFUYDGI-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- LUNUNJFSHKSXGQ-UHFFFAOYSA-N 4-Aminoindole Chemical compound NC1=CC=CC2=C1C=CN2 LUNUNJFSHKSXGQ-UHFFFAOYSA-N 0.000 description 1
- WQGHFKPVYWGAHQ-UHFFFAOYSA-N 4-[2-(4-methylbenzoyl)anilino]-2-methylsulfonylpyrimidine-5-carbonitrile Chemical compound C1=CC(C)=CC=C1C(=O)C1=CC=CC=C1NC1=NC(S(C)(=O)=O)=NC=C1C#N WQGHFKPVYWGAHQ-UHFFFAOYSA-N 0.000 description 1
- BCXSVFBDMPSKPT-UHFFFAOYSA-N 5-amino-1,3-dihydrobenzimidazol-2-one Chemical compound NC1=CC=C2NC(=O)NC2=C1 BCXSVFBDMPSKPT-UHFFFAOYSA-N 0.000 description 1
- SIKXIUWKPGWBBF-UHFFFAOYSA-N 5-bromo-2,4-dichloropyrimidine Chemical compound ClC1=NC=C(Br)C(Cl)=N1 SIKXIUWKPGWBBF-UHFFFAOYSA-N 0.000 description 1
- ZEFUJFGFQYHXNN-UHFFFAOYSA-N 5-bromo-2-chloro-n-(3,4,5-trimethoxyphenyl)pyrimidin-4-amine Chemical compound COC1=C(OC)C(OC)=CC(NC=2C(=CN=C(Cl)N=2)Br)=C1 ZEFUJFGFQYHXNN-UHFFFAOYSA-N 0.000 description 1
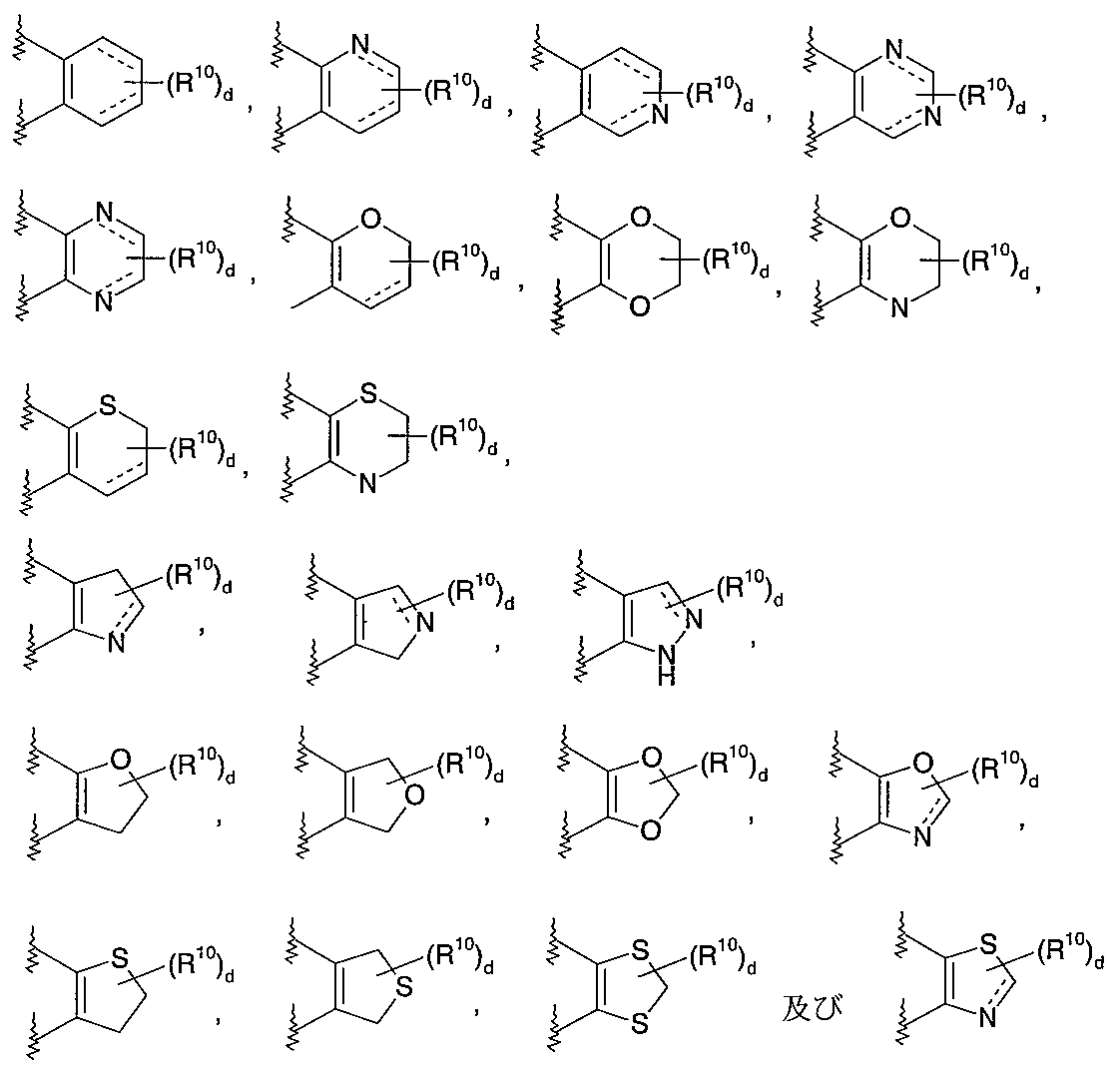
- 125000004070 6 membered heterocyclic group Chemical group 0.000 description 1
- WYWHKKSPHMUBEB-UHFFFAOYSA-N 6-Mercaptoguanine Natural products N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- NUPVJXALSSTAIQ-UHFFFAOYSA-N 8-[(2-chloro-5-nitropyrimidin-4-yl)amino]naphthalen-2-ol Chemical compound C12=CC(O)=CC=C2C=CC=C1NC1=NC(Cl)=NC=C1[N+]([O-])=O NUPVJXALSSTAIQ-UHFFFAOYSA-N 0.000 description 1
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 1
- FOFIZBDOYSOLKU-UHFFFAOYSA-N 8-amino-3,4-dihydro-2h-naphthalen-1-one Chemical compound C1CCC(=O)C2=C1C=CC=C2N FOFIZBDOYSOLKU-UHFFFAOYSA-N 0.000 description 1
- GJCOSYZMQJWQCA-UHFFFAOYSA-N 9H-xanthene Chemical compound C1=CC=C2CC3=CC=CC=C3OC2=C1 GJCOSYZMQJWQCA-UHFFFAOYSA-N 0.000 description 1
- IKHGUXGNUITLKF-UHFFFAOYSA-N Acetaldehyde Chemical compound CC=O IKHGUXGNUITLKF-UHFFFAOYSA-N 0.000 description 1
- 206010000830 Acute leukaemia Diseases 0.000 description 1
- 108010029445 Agammaglobulinaemia Tyrosine Kinase Proteins 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 102100022014 Angiopoietin-1 receptor Human genes 0.000 description 1
- 108091023037 Aptamer Proteins 0.000 description 1
- BFYIZQONLCFLEV-DAELLWKTSA-N Aromasine Chemical compound O=C1C=C[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CC(=C)C2=C1 BFYIZQONLCFLEV-DAELLWKTSA-N 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 201000001320 Atherosclerosis Diseases 0.000 description 1
- 206010004446 Benign prostatic hyperplasia Diseases 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- SESFRYSPDFLNCH-UHFFFAOYSA-N Benzyl benzoate Natural products C=1C=CC=CC=1C(=O)OCC1=CC=CC=C1 SESFRYSPDFLNCH-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-DCSYEGIMSA-N Beta-Lactose Chemical compound OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-DCSYEGIMSA-N 0.000 description 1
- 239000002028 Biomass Substances 0.000 description 1
- 208000006386 Bone Resorption Diseases 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- GRJCWULLAJEIIT-UHFFFAOYSA-N CC(C)(C)NC(c1ccccc1Nc1nc(Nc(cc2OC)cc(OC)c2OC)ncc1[N+]([O-])=O)=O Chemical compound CC(C)(C)NC(c1ccccc1Nc1nc(Nc(cc2OC)cc(OC)c2OC)ncc1[N+]([O-])=O)=O GRJCWULLAJEIIT-UHFFFAOYSA-N 0.000 description 1
- DTGURCGTPUDALE-UHFFFAOYSA-N COc(cc(cc1OC)Nc(nc2Nc3cccc(cc4)c3cc4O)ncc2[N+]([O-])=O)c1OC Chemical compound COc(cc(cc1OC)Nc(nc2Nc3cccc(cc4)c3cc4O)ncc2[N+]([O-])=O)c1OC DTGURCGTPUDALE-UHFFFAOYSA-N 0.000 description 1
- ZVAQGTCPHGPJCX-UHFFFAOYSA-N COc(cc(cc1OC)Nc(nc2Nc3cccc4c3OCCO4)ncc2[N+]([O-])=O)c1OC Chemical compound COc(cc(cc1OC)Nc(nc2Nc3cccc4c3OCCO4)ncc2[N+]([O-])=O)c1OC ZVAQGTCPHGPJCX-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 102000053642 Catalytic RNA Human genes 0.000 description 1
- 108090000994 Catalytic RNA Proteins 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 108010066551 Cholestenone 5 alpha-Reductase Proteins 0.000 description 1
- 206010063209 Chronic allograft nephropathy Diseases 0.000 description 1
- 108010060385 Cyclin B1 Proteins 0.000 description 1
- 108010024986 Cyclin-Dependent Kinase 2 Proteins 0.000 description 1
- 108010025464 Cyclin-Dependent Kinase 4 Proteins 0.000 description 1
- 108010025468 Cyclin-Dependent Kinase 6 Proteins 0.000 description 1
- 102100036239 Cyclin-dependent kinase 2 Human genes 0.000 description 1
- 102100036252 Cyclin-dependent kinase 4 Human genes 0.000 description 1
- 102100026804 Cyclin-dependent kinase 6 Human genes 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- 102000053602 DNA Human genes 0.000 description 1
- 102000003915 DNA Topoisomerases Human genes 0.000 description 1
- 108090000323 DNA Topoisomerases Proteins 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- 101100216227 Dictyostelium discoideum anapc3 gene Proteins 0.000 description 1
- 101100327311 Dictyostelium discoideum anapc6 gene Proteins 0.000 description 1
- BUDQDWGNQVEFAC-UHFFFAOYSA-N Dihydropyran Chemical compound C1COC=CC1 BUDQDWGNQVEFAC-UHFFFAOYSA-N 0.000 description 1
- 101100015729 Drosophila melanogaster drk gene Proteins 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 108010041308 Endothelial Growth Factors Proteins 0.000 description 1
- 241000792859 Enema Species 0.000 description 1
- 102100038595 Estrogen receptor Human genes 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 239000001263 FEMA 3042 Chemical class 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- 102000012673 Follicle Stimulating Hormone Human genes 0.000 description 1
- 108010079345 Follicle Stimulating Hormone Proteins 0.000 description 1
- 230000037059 G2/M phase arrest Effects 0.000 description 1
- 102100032340 G2/mitotic-specific cyclin-B1 Human genes 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- 206010018364 Glomerulonephritis Diseases 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 102000006771 Gonadotropins Human genes 0.000 description 1
- 108010086677 Gonadotropins Proteins 0.000 description 1
- 108010069236 Goserelin Proteins 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000753291 Homo sapiens Angiopoietin-1 receptor Proteins 0.000 description 1
- 101001056180 Homo sapiens Induced myeloid leukemia cell differentiation protein Mcl-1 Proteins 0.000 description 1
- 101000605743 Homo sapiens Kinesin-like protein KIF23 Proteins 0.000 description 1
- 101000904173 Homo sapiens Progonadoliberin-1 Proteins 0.000 description 1
- 101150057269 IKBKB gene Proteins 0.000 description 1
- JJKOTMDDZAJTGQ-DQSJHHFOSA-N Idoxifene Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN2CCCC2)=CC=1)/C1=CC=C(I)C=C1 JJKOTMDDZAJTGQ-DQSJHHFOSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 108700002232 Immediate-Early Genes Proteins 0.000 description 1
- 102100026539 Induced myeloid leukemia cell differentiation protein Mcl-1 Human genes 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 102100023915 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- VQTUBCCKSQIDNK-UHFFFAOYSA-N Isobutene Chemical group CC(C)=C VQTUBCCKSQIDNK-UHFFFAOYSA-N 0.000 description 1
- 238000010824 Kaplan-Meier survival analysis Methods 0.000 description 1
- 102100038406 Kinesin-like protein KIF23 Human genes 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- 239000005411 L01XE02 - Gefitinib Substances 0.000 description 1
- 108091054455 MAP kinase family Proteins 0.000 description 1
- 102000043136 MAP kinase family Human genes 0.000 description 1
- 108010058398 Macrophage Colony-Stimulating Factor Receptor Proteins 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- SPAGIJMPHSUYSE-UHFFFAOYSA-N Magnesium peroxide Chemical compound [Mg+2].[O-][O-] SPAGIJMPHSUYSE-UHFFFAOYSA-N 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 102000029749 Microtubule Human genes 0.000 description 1
- 108091022875 Microtubule Proteins 0.000 description 1
- 229920000715 Mucilage Polymers 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 1
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- LFTLOKWAGJYHHR-UHFFFAOYSA-N N-methylmorpholine N-oxide Chemical compound CN1(=O)CCOCC1 LFTLOKWAGJYHHR-UHFFFAOYSA-N 0.000 description 1
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N N-phenyl amine Natural products NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 1
- ZFVLZXXZUGHWSP-UHFFFAOYSA-L N1=CN=CC=C1.[Cr](=O)(=O)(O)Cl Chemical compound N1=CN=CC=C1.[Cr](=O)(=O)(O)Cl ZFVLZXXZUGHWSP-UHFFFAOYSA-L 0.000 description 1
- 101150111783 NTRK1 gene Proteins 0.000 description 1
- 101150117329 NTRK3 gene Proteins 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 101150056950 Ntrk2 gene Proteins 0.000 description 1
- REYJJPSVUYRZGE-UHFFFAOYSA-N Octadecylamine Chemical compound CCCCCCCCCCCCCCCCCCN REYJJPSVUYRZGE-UHFFFAOYSA-N 0.000 description 1
- 108700020796 Oncogene Proteins 0.000 description 1
- ZCQWOFVYLHDMMC-UHFFFAOYSA-N Oxazole Chemical compound C1=COC=N1 ZCQWOFVYLHDMMC-UHFFFAOYSA-N 0.000 description 1
- WYNCHZVNFNFDNH-UHFFFAOYSA-N Oxazolidine Chemical compound C1COCN1 WYNCHZVNFNFDNH-UHFFFAOYSA-N 0.000 description 1
- CBENFWSGALASAD-UHFFFAOYSA-N Ozone Chemical compound [O-][O+]=O CBENFWSGALASAD-UHFFFAOYSA-N 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- LRBQNJMCXXYXIU-PPKXGCFTSA-N Penta-digallate-beta-D-glucose Chemical class OC1=C(O)C(O)=CC(C(=O)OC=2C(=C(O)C=C(C=2)C(=O)OC[C@@H]2[C@H]([C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)O2)OC(=O)C=2C=C(OC(=O)C=3C=C(O)C(O)=C(O)C=3)C(O)=C(O)C=2)O)=C1 LRBQNJMCXXYXIU-PPKXGCFTSA-N 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- PCNDJXKNXGMECE-UHFFFAOYSA-N Phenazine Natural products C1=CC=CC2=NC3=CC=CC=C3N=C21 PCNDJXKNXGMECE-UHFFFAOYSA-N 0.000 description 1
- 102000003993 Phosphatidylinositol 3-kinases Human genes 0.000 description 1
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 1
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 241001495452 Podophyllum Species 0.000 description 1
- 101710182846 Polyhedrin Proteins 0.000 description 1
- 108010039918 Polylysine Proteins 0.000 description 1
- 229920001710 Polyorthoester Polymers 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 102100024028 Progonadoliberin-1 Human genes 0.000 description 1
- 208000004403 Prostatic Hyperplasia Diseases 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 108091008611 Protein Kinase B Proteins 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical compound C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 description 1
- 238000012228 RNA interference-mediated gene silencing Methods 0.000 description 1
- 238000010240 RT-PCR analysis Methods 0.000 description 1
- 102000003901 Ras GTPase-activating proteins Human genes 0.000 description 1
- 108090000231 Ras GTPase-activating proteins Proteins 0.000 description 1
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 1
- 108010031091 Separase Proteins 0.000 description 1
- 102000005734 Separase Human genes 0.000 description 1
- 229920002684 Sepharose Polymers 0.000 description 1
- 241000287219 Serinus canaria Species 0.000 description 1
- 229920001800 Shellac Polymers 0.000 description 1
- 206010041067 Small cell lung cancer Diseases 0.000 description 1
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- BCKXLBQYZLBQEK-KVVVOXFISA-M Sodium oleate Chemical compound [Na+].CCCCCCCC\C=C/CCCCCCCC([O-])=O BCKXLBQYZLBQEK-KVVVOXFISA-M 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 208000005718 Stomach Neoplasms Diseases 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- 239000012505 Superdex™ Substances 0.000 description 1
- 101000996723 Sus scrofa Gonadotropin-releasing hormone receptor Proteins 0.000 description 1
- 108091005735 TGF-beta receptors Proteins 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 229940123237 Taxane Drugs 0.000 description 1
- DHXVGJBLRPWPCS-UHFFFAOYSA-N Tetrahydropyran Chemical compound C1CCOCC1 DHXVGJBLRPWPCS-UHFFFAOYSA-N 0.000 description 1
- YPWFISCTZQNZAU-UHFFFAOYSA-N Thiane Chemical compound C1CCSCC1 YPWFISCTZQNZAU-UHFFFAOYSA-N 0.000 description 1
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 1
- ZMZDMBWJUHKJPS-UHFFFAOYSA-M Thiocyanate anion Chemical compound [S-]C#N ZMZDMBWJUHKJPS-UHFFFAOYSA-M 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- 102000004357 Transferases Human genes 0.000 description 1
- 108090000992 Transferases Proteins 0.000 description 1
- 102000016715 Transforming Growth Factor beta Receptors Human genes 0.000 description 1
- 206010052779 Transplant rejections Diseases 0.000 description 1
- YZCKVEUIGOORGS-NJFSPNSNSA-N Tritium Chemical compound [3H] YZCKVEUIGOORGS-NJFSPNSNSA-N 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 102000014384 Type C Phospholipases Human genes 0.000 description 1
- 108010079194 Type C Phospholipases Proteins 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 241000269370 Xenopus <genus> Species 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- IPVMBFZZDHPOPJ-UHFFFAOYSA-N [5-chloro-2-[(2-chloro-5-nitropyrimidin-4-yl)amino]phenyl]-(2-fluorophenyl)methanone Chemical compound [O-][N+](=O)C1=CN=C(Cl)N=C1NC1=CC=C(Cl)C=C1C(=O)C1=CC=CC=C1F IPVMBFZZDHPOPJ-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- AXJDEHNQPMZKOS-UHFFFAOYSA-N acetylazanium;chloride Chemical compound [Cl-].CC([NH3+])=O AXJDEHNQPMZKOS-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 102000035181 adaptor proteins Human genes 0.000 description 1
- 108091005764 adaptor proteins Proteins 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 239000003463 adsorbent Substances 0.000 description 1
- 239000000556 agonist Substances 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 150000001350 alkyl halides Chemical class 0.000 description 1
- 229940045714 alkyl sulfonate alkylating agent Drugs 0.000 description 1
- 150000008052 alkyl sulfonates Chemical class 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Chemical class OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 235000001014 amino acid Nutrition 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 229960003437 aminoglutethimide Drugs 0.000 description 1
- ROBVIMPUHSLWNV-UHFFFAOYSA-N aminoglutethimide Chemical compound C=1C=C(N)C=CC=1C1(CC)CCC(=O)NC1=O ROBVIMPUHSLWNV-UHFFFAOYSA-N 0.000 description 1
- 229920000469 amphiphilic block copolymer Polymers 0.000 description 1
- 229940035676 analgesics Drugs 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 229960002932 anastrozole Drugs 0.000 description 1
- YBBLVLTVTVSKRW-UHFFFAOYSA-N anastrozole Chemical compound N#CC(C)(C)C1=CC(C(C)(C#N)C)=CC(CN2N=CN=C2)=C1 YBBLVLTVTVSKRW-UHFFFAOYSA-N 0.000 description 1
- 239000003098 androgen Substances 0.000 description 1
- 150000001448 anilines Chemical class 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 229940045799 anthracyclines and related substance Drugs 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000002424 anti-apoptotic effect Effects 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000003474 anti-emetic effect Effects 0.000 description 1
- 229940046836 anti-estrogen Drugs 0.000 description 1
- 230000001833 anti-estrogenic effect Effects 0.000 description 1
- 230000000118 anti-neoplastic effect Effects 0.000 description 1
- 230000001028 anti-proliverative effect Effects 0.000 description 1
- 239000000051 antiandrogen Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000002111 antiemetic agent Substances 0.000 description 1
- 229940125683 antiemetic agent Drugs 0.000 description 1
- 229940045686 antimetabolites antineoplastic purine analogs Drugs 0.000 description 1
- 239000003080 antimitotic agent Substances 0.000 description 1
- 229940045719 antineoplastic alkylating agent nitrosoureas Drugs 0.000 description 1
- 229940045688 antineoplastic antimetabolites pyrimidine analogues Drugs 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- 239000003886 aromatase inhibitor Substances 0.000 description 1
- 229940046844 aromatase inhibitors Drugs 0.000 description 1
- 206010003246 arthritis Diseases 0.000 description 1
- 239000008122 artificial sweetener Substances 0.000 description 1
- 235000021311 artificial sweeteners Nutrition 0.000 description 1
- METKIMKYRPQLGS-UHFFFAOYSA-N atenolol Chemical compound CC(C)NCC(O)COC1=CC=C(CC(N)=O)C=C1 METKIMKYRPQLGS-UHFFFAOYSA-N 0.000 description 1
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 1
- 239000003899 bactericide agent Substances 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- RFRXIWQYSOIBDI-UHFFFAOYSA-N benzarone Chemical compound CCC=1OC2=CC=CC=C2C=1C(=O)C1=CC=C(O)C=C1 RFRXIWQYSOIBDI-UHFFFAOYSA-N 0.000 description 1
- JUHORIMYRDESRB-UHFFFAOYSA-N benzathine Chemical compound C=1C=CC=CC=1CNCCNCC1=CC=CC=C1 JUHORIMYRDESRB-UHFFFAOYSA-N 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 1
- 229940092714 benzenesulfonic acid Drugs 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 229960004365 benzoic acid Drugs 0.000 description 1
- KYZHGEFMXZOSJN-UHFFFAOYSA-N benzoic acid isobutyl ester Natural products CC(C)COC(=O)C1=CC=CC=C1 KYZHGEFMXZOSJN-UHFFFAOYSA-N 0.000 description 1
- SNIABFMMCKVXSY-UHFFFAOYSA-N benzoylazanium;chloride Chemical compound Cl.NC(=O)C1=CC=CC=C1 SNIABFMMCKVXSY-UHFFFAOYSA-N 0.000 description 1
- WPSOODLAKZMRBF-UHFFFAOYSA-N benzyl 2-[(2-chloro-5-nitropyrimidin-4-yl)amino]benzoate Chemical compound [O-][N+](=O)C1=CN=C(Cl)N=C1NC1=CC=CC=C1C(=O)OCC1=CC=CC=C1 WPSOODLAKZMRBF-UHFFFAOYSA-N 0.000 description 1
- 229960002903 benzyl benzoate Drugs 0.000 description 1
- 125000000051 benzyloxy group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])O* 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 229960000997 bicalutamide Drugs 0.000 description 1
- 229920002988 biodegradable polymer Polymers 0.000 description 1
- 239000004621 biodegradable polymer Substances 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000012455 biphasic mixture Substances 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 230000024279 bone resorption Effects 0.000 description 1
- BGECDVWSWDRFSP-UHFFFAOYSA-N borazine Chemical compound B1NBNBN1 BGECDVWSWDRFSP-UHFFFAOYSA-N 0.000 description 1
- 125000005620 boronic acid group Chemical class 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- HGXJOXHYPGNVNK-UHFFFAOYSA-N butane;ethenoxyethane;tin Chemical compound CCCC[Sn](CCCC)(CCCC)C(=C)OCC HGXJOXHYPGNVNK-UHFFFAOYSA-N 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 230000005907 cancer growth Effects 0.000 description 1
- 125000002837 carbocyclic group Chemical group 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 229960004562 carboplatin Drugs 0.000 description 1
- 229940105329 carboxymethylcellulose Drugs 0.000 description 1
- 238000004517 catalytic hydrocracking Methods 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000012820 cell cycle checkpoint Effects 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 150000005829 chemical entities Chemical class 0.000 description 1
- 229960004630 chlorambucil Drugs 0.000 description 1
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 125000001309 chloro group Chemical group Cl* 0.000 description 1
- NEHMKBQYUWJMIP-UHFFFAOYSA-N chloromethane Chemical compound ClC NEHMKBQYUWJMIP-UHFFFAOYSA-N 0.000 description 1
- VDANGULDQQJODZ-UHFFFAOYSA-N chloroprocaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1Cl VDANGULDQQJODZ-UHFFFAOYSA-N 0.000 description 1
- 229960002023 chloroprocaine Drugs 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 1
- 229960001231 choline Drugs 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 101150116749 chuk gene Proteins 0.000 description 1
- 230000007882 cirrhosis Effects 0.000 description 1
- 208000019425 cirrhosis of liver Diseases 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 108010045512 cohesins Proteins 0.000 description 1
- 239000008119 colloidal silica Substances 0.000 description 1
- 238000012875 competitive assay Methods 0.000 description 1
- 235000009508 confectionery Nutrition 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- 229920006037 cross link polymer Polymers 0.000 description 1
- 125000001047 cyclobutenyl group Chemical group C1(=CCC1)* 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000000596 cyclohexenyl group Chemical group C1(=CCCCC1)* 0.000 description 1
- PJKIIBRREOYEKJ-UHFFFAOYSA-N cyclohexyl 2-[(2-chloro-5-nitropyrimidin-4-yl)amino]benzoate Chemical compound [O-][N+](=O)C1=CN=C(Cl)N=C1NC1=CC=CC=C1C(=O)OC1CCCCC1 PJKIIBRREOYEKJ-UHFFFAOYSA-N 0.000 description 1
- 125000000640 cyclooctyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 125000002433 cyclopentenyl group Chemical group C1(=CCCC1)* 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 229960000978 cyproterone acetate Drugs 0.000 description 1
- UWFYSQMTEOIJJG-FDTZYFLXSA-N cyproterone acetate Chemical compound C1=C(Cl)C2=CC(=O)[C@@H]3C[C@@H]3[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)C)[C@@]1(C)CC2 UWFYSQMTEOIJJG-FDTZYFLXSA-N 0.000 description 1
- 229960000684 cytarabine Drugs 0.000 description 1
- 230000021953 cytokinesis Effects 0.000 description 1
- 239000000824 cytostatic agent Substances 0.000 description 1
- 230000001085 cytostatic effect Effects 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 231100000599 cytotoxic agent Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 229960003901 dacarbazine Drugs 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 229960000975 daunorubicin Drugs 0.000 description 1
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- 229940043237 diethanolamine Drugs 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- TZKNQZFXNYWYPZ-UHFFFAOYSA-N ditert-butyl-[2-(2-propan-2-ylnaphthalen-1-yl)phenyl]phosphane Chemical compound CC(C)C1=CC=C2C=CC=CC2=C1C1=CC=CC=C1P(C(C)(C)C)C(C)(C)C TZKNQZFXNYWYPZ-UHFFFAOYSA-N 0.000 description 1
- 229960003668 docetaxel Drugs 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 239000006196 drop Substances 0.000 description 1
- 238000002651 drug therapy Methods 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- JWJOTENAMICLJG-QWBYCMEYSA-N dutasteride Chemical compound O=C([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)N[C@@H]4CC3)C)CC[C@@]21C)NC1=CC(C(F)(F)F)=CC=C1C(F)(F)F JWJOTENAMICLJG-QWBYCMEYSA-N 0.000 description 1
- 229960004199 dutasteride Drugs 0.000 description 1
- 239000012039 electrophile Substances 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 230000002357 endometrial effect Effects 0.000 description 1
- 239000007920 enema Substances 0.000 description 1
- 229940079360 enema for constipation Drugs 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 238000006911 enzymatic reaction Methods 0.000 description 1
- 108060002566 ephrin Proteins 0.000 description 1
- 102000012803 ephrin Human genes 0.000 description 1
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 description 1
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 description 1
- 210000002615 epidermis Anatomy 0.000 description 1
- 229940011871 estrogen Drugs 0.000 description 1
- 239000000262 estrogen Substances 0.000 description 1
- 239000000328 estrogen antagonist Substances 0.000 description 1
- 108010038795 estrogen receptors Proteins 0.000 description 1
- 229940012017 ethylenediamine Drugs 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- 210000003527 eukaryotic cell Anatomy 0.000 description 1
- 229960000255 exemestane Drugs 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000003889 eye drop Substances 0.000 description 1
- 229940012356 eye drops Drugs 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- DBEPLOCGEIEOCV-WSBQPABSSA-N finasteride Chemical compound N([C@@H]1CC2)C(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H](C(=O)NC(C)(C)C)[C@@]2(C)CC1 DBEPLOCGEIEOCV-WSBQPABSSA-N 0.000 description 1
- 229960004039 finasteride Drugs 0.000 description 1
- 239000010419 fine particle Substances 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 239000005454 flavour additive Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 229960002074 flutamide Drugs 0.000 description 1
- MKXKFYHWDHIYRV-UHFFFAOYSA-N flutamide Chemical compound CC(C)C(=O)NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 MKXKFYHWDHIYRV-UHFFFAOYSA-N 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 229940028334 follicle stimulating hormone Drugs 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 210000003953 foreskin Anatomy 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 229960002598 fumaric acid Drugs 0.000 description 1
- LRBQNJMCXXYXIU-QWKBTXIPSA-N gallotannic acid Chemical class OC1=C(O)C(O)=CC(C(=O)OC=2C(=C(O)C=C(C=2)C(=O)OC[C@H]2[C@@H]([C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)O2)OC(=O)C=2C=C(OC(=O)C=3C=C(O)C(O)=C(O)C=3)C(O)=C(O)C=2)O)=C1 LRBQNJMCXXYXIU-QWKBTXIPSA-N 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 206010017758 gastric cancer Diseases 0.000 description 1
- 229960002584 gefitinib Drugs 0.000 description 1
- 238000002523 gelfiltration Methods 0.000 description 1
- 230000009368 gene silencing by RNA Effects 0.000 description 1
- 229960002518 gentamicin Drugs 0.000 description 1
- 125000002686 geranylgeranyl group Chemical group [H]C([*])([H])/C([H])=C(C([H])([H])[H])/C([H])([H])C([H])([H])/C([H])=C(C([H])([H])[H])/C([H])([H])C([H])([H])/C([H])=C(C([H])([H])[H])/C([H])([H])C([H])([H])C([H])=C(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 229960002989 glutamic acid Drugs 0.000 description 1
- XLXSAKCOAKORKW-UHFFFAOYSA-N gonadorelin Chemical compound C1CCC(C(=O)NCC(N)=O)N1C(=O)C(CCCN=C(N)N)NC(=O)C(CC(C)C)NC(=O)CNC(=O)C(NC(=O)C(CO)NC(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C(CC=1NC=NC=1)NC(=O)C1NC(=O)CC1)CC1=CC=C(O)C=C1 XLXSAKCOAKORKW-UHFFFAOYSA-N 0.000 description 1
- 239000002622 gonadotropin Substances 0.000 description 1
- 229960003690 goserelin acetate Drugs 0.000 description 1
- 238000005469 granulation Methods 0.000 description 1
- 230000003179 granulation Effects 0.000 description 1
- 101150098203 grb2 gene Proteins 0.000 description 1
- 229940022353 herceptin Drugs 0.000 description 1
- IPCSVZSSVZVIGE-UHFFFAOYSA-N hexadecanoic acid Chemical compound CCCCCCCCCCCCCCCC(O)=O IPCSVZSSVZVIGE-UHFFFAOYSA-N 0.000 description 1
- GNOIPBMMFNIUFM-UHFFFAOYSA-N hexamethylphosphoric triamide Chemical compound CN(C)P(=O)(N(C)C)N(C)C GNOIPBMMFNIUFM-UHFFFAOYSA-N 0.000 description 1
- 229940125697 hormonal agent Drugs 0.000 description 1
- 230000036571 hydration Effects 0.000 description 1
- 238000006703 hydration reaction Methods 0.000 description 1
- 239000000017 hydrogel Substances 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical class I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 238000007327 hydrogenolysis reaction Methods 0.000 description 1
- 229940071870 hydroiodic acid Drugs 0.000 description 1
- 239000008309 hydrophilic cream Substances 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 229910052738 indium Inorganic materials 0.000 description 1
- PZOUSPYUWWUPPK-UHFFFAOYSA-N indole Natural products CC1=CC=CC2=C1C=CN2 PZOUSPYUWWUPPK-UHFFFAOYSA-N 0.000 description 1
- RKJUIXBNRJVNHR-UHFFFAOYSA-N indolenine Natural products C1=CC=C2CC=NC2=C1 RKJUIXBNRJVNHR-UHFFFAOYSA-N 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 102000006495 integrins Human genes 0.000 description 1
- 108010044426 integrins Proteins 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 229940084651 iressa Drugs 0.000 description 1
- 229960004768 irinotecan Drugs 0.000 description 1
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 1
- ZLTPDFXIESTBQG-UHFFFAOYSA-N isothiazole Chemical compound C=1C=NSC=1 ZLTPDFXIESTBQG-UHFFFAOYSA-N 0.000 description 1
- CTAPFRYPJLPFDF-UHFFFAOYSA-N isoxazole Chemical compound C=1C=NOC=1 CTAPFRYPJLPFDF-UHFFFAOYSA-N 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 229960003881 letrozole Drugs 0.000 description 1
- HPJKCIUCZWXJDR-UHFFFAOYSA-N letrozole Chemical compound C1=CC(C#N)=CC=C1C(N1N=CN=C1)C1=CC=C(C#N)C=C1 HPJKCIUCZWXJDR-UHFFFAOYSA-N 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- UBJFKNSINUCEAL-UHFFFAOYSA-N lithium;2-methylpropane Chemical compound [Li+].C[C-](C)C UBJFKNSINUCEAL-UHFFFAOYSA-N 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 239000001630 malic acid Chemical class 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 229960004296 megestrol acetate Drugs 0.000 description 1
- RQZAXGRLVPAYTJ-GQFGMJRRSA-N megestrol acetate Chemical compound C1=C(C)C2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)C)[C@@]1(C)CC2 RQZAXGRLVPAYTJ-GQFGMJRRSA-N 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 239000001525 mentha piperita l. herb oil Substances 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 238000006263 metalation reaction Methods 0.000 description 1
- 208000037819 metastatic cancer Diseases 0.000 description 1
- 208000011575 metastatic malignant neoplasm Diseases 0.000 description 1
- 206010061289 metastatic neoplasm Diseases 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- WSFSSNUMVMOOMR-BJUDXGSMSA-N methanone Chemical compound O=[11CH2] WSFSSNUMVMOOMR-BJUDXGSMSA-N 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- MITGWRGLCKMXAF-UHFFFAOYSA-N methyl 2-[(2-chloro-5-nitropyrimidin-4-yl)amino]-6-methylbenzoate Chemical compound COC(=O)C1=C(C)C=CC=C1NC1=NC(Cl)=NC=C1[N+]([O-])=O MITGWRGLCKMXAF-UHFFFAOYSA-N 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 125000002816 methylsulfanyl group Chemical group [H]C([H])([H])S[*] 0.000 description 1
- 210000004688 microtubule Anatomy 0.000 description 1
- 230000008880 microtubule cytoskeleton organization Effects 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 239000003595 mist Substances 0.000 description 1
- 239000003226 mitogen Substances 0.000 description 1
- 238000002625 monoclonal antibody therapy Methods 0.000 description 1
- 125000001620 monocyclic carbocycle group Chemical group 0.000 description 1
- BLCLNMBMMGCOAS-UHFFFAOYSA-N n-[1-[[1-[[1-[[1-[[1-[[1-[[1-[2-[(carbamoylamino)carbamoyl]pyrrolidin-1-yl]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-[(2-methylpropan-2-yl)oxy]-1-oxopropan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amin Chemical compound C1CCC(C(=O)NNC(N)=O)N1C(=O)C(CCCN=C(N)N)NC(=O)C(CC(C)C)NC(=O)C(COC(C)(C)C)NC(=O)C(NC(=O)C(CO)NC(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C(CC=1NC=NC=1)NC(=O)C1NC(=O)CC1)CC1=CC=C(O)C=C1 BLCLNMBMMGCOAS-UHFFFAOYSA-N 0.000 description 1
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- HXFFNPYZSTYGCK-UHFFFAOYSA-N n-tert-butyl-2-[[5-nitro-2-(3,4,5-trimethoxyanilino)pyrimidin-4-yl]amino]benzamide;hydrochloride Chemical compound Cl.COC1=C(OC)C(OC)=CC(NC=2N=C(NC=3C(=CC=CC=3)C(=O)NC(C)(C)C)C(=CN=2)[N+]([O-])=O)=C1 HXFFNPYZSTYGCK-UHFFFAOYSA-N 0.000 description 1
- KVBGVZZKJNLNJU-UHFFFAOYSA-N naphthalene-2-sulfonic acid Chemical compound C1=CC=CC2=CC(S(=O)(=O)O)=CC=C21 KVBGVZZKJNLNJU-UHFFFAOYSA-N 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 210000003928 nasal cavity Anatomy 0.000 description 1
- 235000021096 natural sweeteners Nutrition 0.000 description 1
- 239000006199 nebulizer Substances 0.000 description 1
- 230000017095 negative regulation of cell growth Effects 0.000 description 1
- 201000009925 nephrosclerosis Diseases 0.000 description 1
- 230000004770 neurodegeneration Effects 0.000 description 1
- 208000015122 neurodegenerative disease Diseases 0.000 description 1
- 229960002653 nilutamide Drugs 0.000 description 1
- XWXYUMMDTVBTOU-UHFFFAOYSA-N nilutamide Chemical compound O=C1C(C)(C)NC(=O)N1C1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 XWXYUMMDTVBTOU-UHFFFAOYSA-N 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 230000000269 nucleophilic effect Effects 0.000 description 1
- 230000030648 nucleus localization Effects 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 239000003883 ointment base Substances 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 201000008482 osteoarthritis Diseases 0.000 description 1
- 210000002997 osteoclast Anatomy 0.000 description 1
- 230000002611 ovarian Effects 0.000 description 1
- WCPAKWJPBJAGKN-UHFFFAOYSA-N oxadiazole Chemical compound C1=CON=N1 WCPAKWJPBJAGKN-UHFFFAOYSA-N 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- AHHWIHXENZJRFG-UHFFFAOYSA-N oxetane Chemical compound C1COC1 AHHWIHXENZJRFG-UHFFFAOYSA-N 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- MUJIDPITZJWBSW-UHFFFAOYSA-N palladium(2+) Chemical compound [Pd+2] MUJIDPITZJWBSW-UHFFFAOYSA-N 0.000 description 1
- 125000001312 palmitoyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 239000011236 particulate material Substances 0.000 description 1
- 235000019477 peppermint oil Nutrition 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 150000008105 phosphatidylcholines Chemical class 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- YJGVMLPVUAXIQN-XVVDYKMHSA-N podophyllotoxin Chemical compound COC1=C(OC)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@H](O)[C@@H]3[C@@H]2C(OC3)=O)=C1 YJGVMLPVUAXIQN-XVVDYKMHSA-N 0.000 description 1
- 229920000111 poly(butyric acid) Polymers 0.000 description 1
- 229920001610 polycaprolactone Polymers 0.000 description 1
- 229920002721 polycyanoacrylate Polymers 0.000 description 1
- 229920000656 polylysine Polymers 0.000 description 1
- 229920006324 polyoxymethylene Polymers 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 235000011056 potassium acetate Nutrition 0.000 description 1
- 239000011698 potassium fluoride Substances 0.000 description 1
- 235000003270 potassium fluoride Nutrition 0.000 description 1
- 229910000160 potassium phosphate Inorganic materials 0.000 description 1
- 235000011009 potassium phosphates Nutrition 0.000 description 1
- LPNYRYFBWFDTMA-UHFFFAOYSA-N potassium tert-butoxide Chemical compound [K+].CC(C)(C)[O-] LPNYRYFBWFDTMA-UHFFFAOYSA-N 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 238000004393 prognosis Methods 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 125000002568 propynyl group Chemical group [*]C#CC([H])([H])[H] 0.000 description 1
- 239000011253 protective coating Substances 0.000 description 1
- 239000003528 protein farnesyltransferase inhibitor Substances 0.000 description 1
- 108060006633 protein kinase Proteins 0.000 description 1
- IGFXRKMLLMBKSA-UHFFFAOYSA-N purine Chemical compound N1=C[N]C2=NC=NC2=C1 IGFXRKMLLMBKSA-UHFFFAOYSA-N 0.000 description 1
- PBMFSQRYOILNGV-UHFFFAOYSA-N pyridazine Chemical compound C1=CC=NN=C1 PBMFSQRYOILNGV-UHFFFAOYSA-N 0.000 description 1
- OYRRZWATULMEPF-UHFFFAOYSA-N pyrimidin-4-amine Chemical compound NC1=CC=NC=N1 OYRRZWATULMEPF-UHFFFAOYSA-N 0.000 description 1
- 125000000714 pyrimidinyl group Chemical group 0.000 description 1
- 150000003242 quaternary ammonium salts Chemical class 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 108010077182 raf Kinases Proteins 0.000 description 1
- 102000009929 raf Kinases Human genes 0.000 description 1
- 229960004622 raloxifene Drugs 0.000 description 1
- GZUITABIAKMVPG-UHFFFAOYSA-N raloxifene Chemical compound C1=CC(O)=CC=C1C1=C(C(=O)C=2C=CC(OCCN3CCCCC3)=CC=2)C2=CC=C(O)C=C2S1 GZUITABIAKMVPG-UHFFFAOYSA-N 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 230000022983 regulation of cell cycle Effects 0.000 description 1
- 230000020273 regulation of microtubule cytoskeleton organization Effects 0.000 description 1
- 230000007363 regulatory process Effects 0.000 description 1
- 208000037803 restenosis Diseases 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 108091092562 ribozyme Proteins 0.000 description 1
- 235000019204 saccharin Nutrition 0.000 description 1
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 description 1
- 229940081974 saccharin Drugs 0.000 description 1
- 239000000901 saccharin and its Na,K and Ca salt Substances 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 108010017797 serum-inducible kinase Proteins 0.000 description 1
- 239000004208 shellac Substances 0.000 description 1
- 229940113147 shellac Drugs 0.000 description 1
- ZLGIYFNHBLSMPS-ATJNOEHPSA-N shellac Chemical compound OCCCCCC(O)C(O)CCCCCCCC(O)=O.C1C23[C@H](C(O)=O)CCC2[C@](C)(CO)[C@@H]1C(C(O)=O)=C[C@@H]3O ZLGIYFNHBLSMPS-ATJNOEHPSA-N 0.000 description 1
- 235000013874 shellac Nutrition 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 239000010802 sludge Substances 0.000 description 1
- 208000000587 small cell lung carcinoma Diseases 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 235000015424 sodium Nutrition 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- WXMKPNITSTVMEF-UHFFFAOYSA-M sodium benzoate Chemical compound [Na+].[O-]C(=O)C1=CC=CC=C1 WXMKPNITSTVMEF-UHFFFAOYSA-M 0.000 description 1
- 239000004299 sodium benzoate Substances 0.000 description 1
- 235000010234 sodium benzoate Nutrition 0.000 description 1
- 239000012312 sodium hydride Substances 0.000 description 1
- 229910000104 sodium hydride Inorganic materials 0.000 description 1
- KSAVQLQVUXSOCR-UHFFFAOYSA-M sodium lauroyl sarcosinate Chemical compound [Na+].CCCCCCCCCCCC(=O)N(C)CC([O-])=O KSAVQLQVUXSOCR-UHFFFAOYSA-M 0.000 description 1
- RYYKJJJTJZKILX-UHFFFAOYSA-M sodium octadecanoate Chemical compound [Na+].CCCCCCCCCCCCCCCCCC([O-])=O RYYKJJJTJZKILX-UHFFFAOYSA-M 0.000 description 1
- 238000005063 solubilization Methods 0.000 description 1
- 230000007928 solubilization Effects 0.000 description 1
- 230000020347 spindle assembly Effects 0.000 description 1
- 102000009076 src-Family Kinases Human genes 0.000 description 1
- 108010087686 src-Family Kinases Proteins 0.000 description 1
- 239000012192 staining solution Substances 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 150000003429 steroid acids Chemical class 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 239000012258 stirred mixture Substances 0.000 description 1
- 239000011550 stock solution Substances 0.000 description 1
- 201000011549 stomach cancer Diseases 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 125000000547 substituted alkyl group Chemical group 0.000 description 1
- 125000004426 substituted alkynyl group Chemical group 0.000 description 1
- 125000005346 substituted cycloalkyl group Chemical group 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 229960001603 tamoxifen Drugs 0.000 description 1
- 229920002258 tannic acid Chemical class 0.000 description 1
- 235000015523 tannic acid Nutrition 0.000 description 1
- 229940033123 tannic acid Drugs 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- NRUKOCRGYNPUPR-QBPJDGROSA-N teniposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 NRUKOCRGYNPUPR-QBPJDGROSA-N 0.000 description 1
- 229960001278 teniposide Drugs 0.000 description 1
- KKEYFWRCBNTPAC-UHFFFAOYSA-L terephthalate(2-) Chemical compound [O-]C(=O)C1=CC=C(C([O-])=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-L 0.000 description 1
- 125000004213 tert-butoxy group Chemical group [H]C([H])([H])C(O*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- RAOIDOHSFRTOEL-UHFFFAOYSA-N tetrahydrothiophene Chemical compound C1CCSC1 RAOIDOHSFRTOEL-UHFFFAOYSA-N 0.000 description 1
- 150000003536 tetrazoles Chemical class 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- VLLMWSRANPNYQX-UHFFFAOYSA-N thiadiazole Chemical compound C1=CSN=N1.C1=CSN=N1 VLLMWSRANPNYQX-UHFFFAOYSA-N 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- XSROQCDVUIHRSI-UHFFFAOYSA-N thietane Chemical compound C1CSC1 XSROQCDVUIHRSI-UHFFFAOYSA-N 0.000 description 1
- 125000003396 thiol group Chemical class [H]S* 0.000 description 1
- BRNULMACUQOKMR-UHFFFAOYSA-N thiomorpholine Chemical compound C1CSCCN1 BRNULMACUQOKMR-UHFFFAOYSA-N 0.000 description 1
- 229930192474 thiophene Natural products 0.000 description 1
- 230000001732 thrombotic effect Effects 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- MNRILEROXIRVNJ-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=NC=N[C]21 MNRILEROXIRVNJ-UHFFFAOYSA-N 0.000 description 1
- 229960000303 topotecan Drugs 0.000 description 1
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 description 1
- 229960005026 toremifene Drugs 0.000 description 1
- XFCLJVABOIYOMF-QPLCGJKRSA-N toremifene Chemical compound C1=CC(OCCN(C)C)=CC=C1C(\C=1C=CC=CC=1)=C(\CCCl)C1=CC=CC=C1 XFCLJVABOIYOMF-QPLCGJKRSA-N 0.000 description 1
- 230000002110 toxicologic effect Effects 0.000 description 1
- 231100000027 toxicology Toxicity 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 102000035160 transmembrane proteins Human genes 0.000 description 1
- 108091005703 transmembrane proteins Proteins 0.000 description 1
- 150000003852 triazoles Chemical class 0.000 description 1
- 125000004205 trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- 229910052722 tritium Inorganic materials 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 1
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 1
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 241000701447 unidentified baculovirus Species 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- 239000008215 water for injection Substances 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 230000029663 wound healing Effects 0.000 description 1
- 229920001285 xanthan gum Polymers 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229930195724 β-lactose Natural products 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/28—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to ring carbon atoms
- C07D239/32—One oxygen, sulfur or nitrogen atom
- C07D239/42—One nitrogen atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/28—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to ring carbon atoms
- C07D239/46—Two or more oxygen, sulphur or nitrogen atoms
- C07D239/48—Two nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
Landscapes
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Hematology (AREA)
- Oncology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Polysaccharides And Polysaccharide Derivatives (AREA)
- Catalysts (AREA)
Description
R1は、H、ハロ、アルキル、アルケニル、アルキニルからなる群から選択され;
R2は、ハロ、アルキル、アルケニル、アルキニル、シクロアルキル、シクロアルケニル、-(R7)g-C(O)R6、-(R7)g-CO2R6、-(R7)g-C(O)N(R6)2、-(R7)g-OR6、-O-(R7)g-Ay、-(R7)g-S(O)eR6、-(R7)g-N(R6)2、-(R7)g-N(R6)C(O)R6、-(R7)g-CN、-(R7)g-SCN、-NO2、-N3、Ay、並びにN、O及びSから選択される1又は2個のヘテロ原子を含む5〜9員のヘテロアリールからなる群から選択され;
各R3は、同一又は異なって、そして独立して、H又アルキルであり;
Yは、-C(O)R8、-C(S)R8、-S(O)eR9、-S(O)eN(R9)2、-N(R9)2、-N(R9)-S(O)eR9、-N(R9)-C(O)R9、-N(R9)-CO2R9及び-N(R9)-C(O)N(R9)2からなる群から選択されるか、
又は、Yは、C-2及びC-3と一緒になって、式A:
ここで、R8はアルキル、アルケニル、アルキニル、シクロアルキル、シクロアルケニル、Ay、Het、-OR6、-O-(R7)g-Ay、-O-(R7)g-Het、-N(R6)2、-N(R6)-(R7)g-Ay、-N(R6)-(R7)g-Het、-N(R6)-(R7)g-OR6、-N(R6)-(R7)g-C(O)R6、-N(R6)-(R7)g-CO2R6、-N(R6)-(R7)g-SO2R6及び-N(R6)-(R7)g-N(R6)2からなる群から選択され;
各R9は、同一又は異なって、そして独立して、H、アルキル、アルケニル、アルキニル、シクロアルキル、シクロアルケニル、Ay及びHetからなる群から選択され;
cは、0又は1であり;
Z1、Z2、Z3及びZ4は、それぞれ独立して、C、O、S及びNからなる群から選択され、ここで、cが0である場合には、Z1、Z2及びZ3の少なくとも1つはCであり、そしてcが1である場合には、Z1、Z2、Z3及びZ4の少なくとも2つはCであり;
各破線は、任意に二重結合を表し;
dは、0、1又は2であり;
各R10は、同一又は異なって、そして独立して、ハロ、アルキル、オキソ、ヒドロキシ、メルカプト及びアミノからなる群から選択され;
aは、0、1、2又は3であり;
各R4は、同一又は異なって、そして独立して、ハロ、アルキル、アルケニル、アルキニル、-(R7)g-シクロアルキル、-(R7)g-シクロアルケニル、-(R7)g-Ay、-(R7)g-Het、-(R7)g-C(O)R6、-(R7)g-C(O)Ay、-(R7)g-C(O)Het、-(R7)g-CO2R6、-(R7)g-CO2Ay、-(R7)g-CO2Het、-(R7)g-C(O)N(R6)2、-(R7)g-OR6、-(R7)g-OAy、-(R7)g-OHet、-(R7)g-OC(O)R6、-(R7)g-OC(O)Ay、-(R7)g-OC(O)Het、-(R7)g-S(O)eR6、-(R7)g-S(O)eAy、-(R7)g-S(O)eHet、-(R7)g-S(O)eN(R6)2、-(R7)g-S(O)eN(R6)Ay、-(R7)g-S(O)eN(R6)Het、-(R7)g-N(R6)2、-(R7)g-N(R6)Ay、-(R7)g-N(R6)Het、-(R7)g-N(R6)C(O)R6、-(R7)g-N(R6)C(O)Ay、-(R7)g-N(R6)C(O)Het、-(R7)g-N(R6)C(O)N(R6)2、-(R7)g-N(R6)S(O)eR6、-(R7)g-N(R6)S(O)eAy、-(R7)g-N(R6)S(O)eHet、-NO2、-CN、-SCN及び-N3からなる群から選択されるか、又は
2個の隣接するR4は、それらが結合している炭素原子と一緒になって、フェニル又はN、O及びSからなる群から選択される1又は2個のヘテロ原子を含む5若しくは6員のヘテロ環若しくはヘテロアリールを形成し;
各eは、同一又は異なって、そして独立して、0、1又は2であり;
bは、0、1、2、3、4又は5であり;
各R5は、同一又は異なって、式(R7)g-R11の基であるか、又は2個の隣接するR5基は、それぞれ同一又は異なって、そして独立して、アルキル、アルケニル、-OR6、-S(O)eR6及び-N(R6)2からなる群から選択され、そしてそれらが結合している炭素原子と一緒になって、C5-6シクロアルキル、C5-6シクロアルケニル又はN、O及びSからなる群から選択される1又は2個のヘテロ原子を含む5若しくは6員のヘテロ環若しくはヘテロアリールを形成し;
各R6は、同一又は異なって、そして独立して、H、アルキル、アルケニル、アルキニル、シクロアルキル及びシクロアルケニルからなる群から選択され;
gは、0又は1であり;
R7はアルキレン又はアルケニレンであり;
Ayは、アリールであり;
Hetは、N、O及びSからなる群から選択される1、2又は3個のヘテロ原子を含む5員又は6員のヘテロ環若しくはヘテロアリールであり;
R11は、ハロ、アルキル、アルケニル、アルキニル、シクロアルキル、シクロアルケニル、Ay、Het、-C(O)R6、-C(O)Ay、-C(O)Het、-CO2R6、-CO2Ay、-CO2Het、-C(O)-R7-OR6、-C(O)-R7-OAy、-C(O)-R7-OHet、-C(O)N(R6)2、-C(O)N(R6)Ay、-C(O)N(R6)Het、-C(O)N(R6)-(R7)g-N(R6)2、-C(O)N(R6)-(R7)g-CO2R6、-C(O)N(R6)-(R7)g-S(O)eR6、-OR6、-OC(O)R6、-O-(R7)g-Ay、-OC(O)Ay、-O-(R7)g-Het、-OC(O)Het、-O-R7-OR6、-O-R7-N(R6)2、-S(O)eR6、-S(O)e-(R7)g-Ay、-S(O)e-(R7)g-Het、-S(O)e-(R7)g-N(R6)2、-S(O)e-(R7)g-N(R6)Ay、-S(O)e-(R7)g-N(R6)Het、-S(O)eN(R6)-(R7)g-C(O)R6、-S(O)eN(R6)-(R7)g-C(O)Ay、-S(O)eN(R6)-(R7)g-C(O)Het、-N(R6)2、-N(R6)-(R7)g-Ay、-N(R6)-(R7)g-Het、-N(R6)-(R7)g-C(O)R6、-N(R6)-C(O)-(R7)g-Ay、-N(R6)-C(O)-(R7)g-Het、-N(R6)-C(O)-(R7)g-N(R6)2、-N(R6)-C(O)-(R7)g-N(R6)Ay、-N(R6)-C(O)-(R7)g-N(R6)Het、-N(R6)-C(O)-(R7)g-N(R6)-(R7-O)h-N(R6)-CO2R6、-N(R6)-(R7)g-S(O)eR6、-N(R6)-(R7)g-S(O)eAy、-N(R6)-(R7)g-S(O)eHet、-N(R6)-R7-N(R6)2、-N(R6)-R7-OR6、-CN、-SCN、-NO2及び-N3からなる群から選択され;そして
hは、1〜20であり;
ここで、R1が-CH3であり、R2がBr又はNO2であり、両方のR3がHであり、aが0であり、そしてbが0又は1であり、R5が-CO2Hである場合には、Yは-CO2Hではない。]
の化合物又はその薬学的に許容される塩、溶媒和物若しくは生理的機能性誘導体(physiologically functional derivative)が提供される。
本明細書で用いられるように、「本発明の化合物」又は「式(I)の化合物」は、式(I)の化合物又はその薬学的に許容される塩、溶媒和物若しくは生理的機能性誘導体を意味する。同様に、単離可能な中間体、例えば式(IV)の化合物などに関して、「式(番号)の化合物」というフレーズは、その式を有する化合物又はその薬学的に許容される塩、溶媒和物若しくは生理的機能性誘導体を意味する。
R1は、H、ハロ、アルキル、アルケニル、アルキニルからなる群から選択され;
R2は、ハロ、アルキル、アルケニル、アルキニル、シクロアルキル、シクロアルケニル、-(R7)g-C(O)R6、-(R7)g-CO2R6、-(R7)g-C(O)N(R6)2、-(R7)g-OR6、-O-(R7)g-Ay、-(R7)g-S(O)eR6、-(R7)g-N(R6)2、-(R7)g-N(R6)C(O)R6、-(R7)g-CN、-(R7)g-SCN、-NO2、-N3、Ay、並びにN、O及びSから選択される1又は2個のヘテロ原子を含む5〜9員のヘテロアリールからなる群から選択され;
各R3は、同一又は異なって、そして独立して、H又アルキルであり;
Yは、-C(O)R8、-C(S)R8、-S(O)eR9、-S(O)eN(R9)2、-N(R9)2、-N(R9)-S(O)eR9、-N(R9)-C(O)R9、-N(R9)-CO2R9及び-N(R9)-C(O)N(R9)2からなる群から選択されるか、
又は、Yは、C-2及びC-3と一緒になって、式A:
ここで、R8はアルキル、アルケニル、アルキニル、シクロアルキル、シクロアルケニル、Ay、Het、-OR6、-O-(R7)g-Ay、-O-(R7)g-Het、-N(R6)2、-N(R6)-(R7)g-Ay、-N(R6)-(R7)g-Het、-N(R6)-(R7)g-OR6、-N(R6)-(R7)g-C(O)R6、-N(R6)-(R7)g-CO2R6、-N(R6)-(R7)g-SO2R6及び-N(R6)-(R7)g-N(R6)2からなる群から選択され;
各R9は、同一又は異なって、そして独立して、H、アルキル、アルケニル、アルキニル、シクロアルキル、シクロアルケニル、Ay及びHetからなる群から選択され;
cは、0又は1であり;
Z1、Z2、Z3及びZ4は、それぞれ独立して、C、O、S及びNからなる群から選択され、ここで、cが0である場合には、Z1、Z2及びZ3の少なくとも1つはCであり、そしてcが1である場合には、Z1、Z2、Z3及びZ4の少なくとも2つはCであり;
各破線は、任意に二重結合を表し;
dは、0、1又は2であり;
各R10は、同一又は異なって、そして独立して、ハロ、アルキル、オキソ、ヒドロキシ、メルカプト及びアミノからなる群から選択され;
aは、0、1、2又は3であり;
各R4は、同一又は異なって、そして独立して、ハロ、アルキル、アルケニル、アルキニル、-(R7)g-シクロアルキル、-(R7)g-シクロアルケニル、-(R7)g-Ay、-(R7)g-Het、-(R7)g-C(O)R6、-(R7)g-C(O)Ay、-(R7)g-C(O)Het、-(R7)g-CO2R6、-(R7)g-CO2Ay、-(R7)g-CO2Het、-(R7)g-C(O)N(R6)2、-(R7)g-OR6、-(R7)g-OAy、-(R7)g-OHet、-(R7)g-OC(O)R6、-(R7)g-OC(O)Ay、-(R7)g-OC(O)Het、-(R7)g-S(O)eR6、-(R7)g-S(O)eAy、-(R7)g-S(O)eHet、-(R7)g-S(O)eN(R6)2、-(R7)g-S(O)eN(R6)Ay、-(R7)g-S(O)eN(R6)Het、-(R7)g-N(R6)2、-(R7)g-N(R6)Ay、-(R7)g-N(R6)Het、-(R7)g-N(R6)C(O)R6、-(R7)g-N(R6)C(O)Ay、-(R7)g-N(R6)C(O)Het、-(R7)g-N(R6)C(O)N(R6)2、-(R7)g-N(R6)S(O)eR6、-(R7)g-N(R6)S(O)eAy、-(R7)g-N(R6)S(O)eHet、-NO2、-CN、-SCN及び-N3からなる群から選択されるか、又は
2個の隣接するR4は、それらが結合している炭素原子と一緒になって、フェニル又はN、O及びSからなる群から選択される1又は2個のヘテロ原子を含む5若しくは6員のヘテロ環若しくはヘテロアリールを形成し;
各eは、同一又は異なって、そして独立して、0、1又は2であり;
bは、0、1、2、3、4又は5であり;
各R5は、同一又は異なって、式(R7)g-R11の基であるか、又は2個の隣接するR5基は、それぞれ同一又は異なって、そして独立して、アルキル、アルケニル、-OR6、-S(O)eR6及び-N(R6)2からなる群から選択され、そしてそれらが結合している炭素原子と一緒になって、C5-6シクロアルキル、C5-6シクロアルケニル又はN、O及びSからなる群から選択される1又は2個のヘテロ原子を含む5若しくは6員のヘテロ環若しくはヘテロアリールを形成し;
各R6は、同一又は異なって、そして独立して、H、アルキル、アルケニル、アルキニル、シクロアルキル及びシクロアルケニルからなる群から選択され;
gは、0又は1であり;
R7はアルキレン又はアルケニレンであり;
Ayは、アリールであり;
Hetは、N、O及びSからなる群から選択される1、2又は3個のヘテロ原子を含む5員又は6員のヘテロ環若しくはヘテロアリールであり;
R11は、ハロ、アルキル、アルケニル、アルキニル、シクロアルキル、シクロアルケニル、Ay、Het、-C(O)R6、-C(O)Ay、-C(O)Het、-CO2R6、-CO2Ay、-CO2Het、-C(O)-R7-OR6、-C(O)-R7-OAy、-C(O)-R7-OHet、-C(O)N(R6)2、-C(O)N(R6)Ay、-C(O)N(R6)Het、-C(O)N(R6)-(R7)g-N(R6)2、-C(O)N(R6)-(R7)g-CO2R6、-C(O)N(R6)-(R7)g-S(O)eR6、-OR6、-OC(O)R6、-O-(R7)g-Ay、-OC(O)Ay、-O-(R7)g-Het、-OC(O)Het、-O-R7-OR6、-O-R7-N(R6)2、-S(O)eR6、-S(O)e-(R7)g-Ay、-S(O)e-(R7)g-Het、-S(O)e-(R7)g-N(R6)2、-S(O)e-(R7)g-N(R6)Ay、-S(O)e-(R7)g-N(R6)Het、-S(O)eN(R6)-(R7)g-C(O)R6、-S(O)eN(R6)-(R7)g-C(O)Ay、-S(O)eN(R6)-(R7)g-C(O)Het、-N(R6)2、-N(R6)-(R7)g-Ay、-N(R6)-(R7)g-Het、-N(R6)-(R7)g-C(O)R6、-N(R6)-C(O)-(R7)g-Ay、-N(R6)-C(O)-(R7)g-Het、-N(R6)-C(O)-(R7)g-N(R6)2、-N(R6)-C(O)-(R7)g-N(R6)Ay、-N(R6)-C(O)-(R7)g-N(R6)Het、-N(R6)-C(O)-(R7)g-N(R6)-(R7-O)h-N(R6)-CO2R6、-N(R6)-(R7)g-S(O)eR6、-N(R6)-(R7)g-S(O)eAy、-N(R6)-(R7)g-S(O)eHet、-N(R6)-R7-N(R6)2、-N(R6)-R7-OR6、-CN、-SCN、-NO2及び-N3からなる群から選択され;そして
hは、1〜20であり;
ここで、R1が-CH3であり、R2がBr又はNO2であり、両方のR3がHであり、aが0であり、そしてbが0又は1であり、R5が-CO2Hである場合には、Yは-CO2Hではない。]
の化合物又はその薬学的に許容される塩、溶媒和物若しくは生理的機能性誘導体を提供する。
cは0又は1であり;
Z1、Z2、Z3及びZ4は、それぞれ独立して、C、O、S及びNからなる群から選択され、ここで、cが0である場合には、Z1、Z2及びZ3の少なくとも1つはC(炭素)であり、そしてcが1である場合には、Z1、Z2、Z3及びZ4の少なくとも2つはC(炭素)であり;
dは0、1又は2であり;
各R10は同一又は異なって、そして独立して、ハロ、アルキル、オキソ、ヒドロキシ、メルカプト及びアミノからなる群から選択される。
2-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)安息香酸塩酸塩;
2-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)ベンズアミド塩酸塩;
N-(tert-ブチル)-2-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)ベンズアミド塩酸塩;
N-[4-({4-[(2-ベンゾイルフェニル)アミノ]-5-ニトロピリミジン-2-イル}アミノ)フェニル]アセトアミド塩酸塩;
8-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)-3,4-ジヒドロナフタレン-1(2H)-オン塩酸塩;
1-[2-({5-ブロモ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)フェニル]エタノン塩酸塩;
2-[(2-{[4-(アセチルアミノ)フェニル]アミノ}-5-ニトロピリミジン-4-イル)アミノ]-N-メチルベンズアミド塩酸塩;
N4-(1H-インドール-4-イル)-5-ニトロ-N2-(3,4,5-トリメトキシフェニル)ピリミジン-2,4-ジアミン塩酸塩;
2-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)安息香酸メチル塩酸塩;
N4-(2,3-ジヒドロ-1,4-ベンゾジオキシン-5-イル)-5-ニトロ-N2-(3,4,5-トリメトキシフェニル)ピリミジン-2,4-ジアミン塩酸塩;
2-[(2-{[4-(アセチルアミノ)フェニル]アミノ}-5-ニトロピリミジン-4-イル)アミノ]安息香酸シクロヘキシル塩酸塩;
5-ヒドロキシ-2-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)安息香酸塩酸塩;
2-[(2-{[4-(アセチルアミノ)フェニル]アミノ}-5-ニトロピリミジン-4-イル)アミノ]安息香酸塩酸塩;
N-[4-({4-[(2-アセチルフェニル)アミノ]-5-ブロモピリミジン-2-イル}アミノ)フェニル]アセトアミド塩酸塩;
N-[4-({5-ブロモ-4-[(2-モルホリン-4-イルフェニル)アミノ]ピリミジン-2-イル}アミノ)フェニル]アセトアミド塩酸塩;
5-[(5-ブロモ-4-{[2-(メチルチオ)フェニル]アミノ}ピリミジン-2-イル)アミノ]-1,3-ジヒドロ-2H-ベンズイミダゾール-2-オン塩酸塩;
5-ブロモ-N4-(2-モルホリン-4-イルフェニル)-N2-(3,4,5-トリメトキシフェニル)ピリミジン-2,4-ジアミン塩酸塩;
N-[4-({5-ブロモ-4-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-2-イル}アミノ)フェニル]アセトアミド塩酸塩;
2-[(2-{[4-(アセチルアミノ)フェニル]アミノ}-5-ニトロピリミジン-4-イル)アミノ]テレフタル酸ジメチル塩酸塩;
2-[(2-{[4-(アセチルアミノ)フェニル]アミノ}-5-ニトロピリミジン-4-イル)アミノ]安息香酸ベンジル塩酸塩;
2-[(2-{[4-(アセチルアミノ)フェニル]アミノ}-5-ニトロピリミジン-4-イル)アミノ]-6-メチル安息香酸メチル塩酸塩;
2-[(2-{[4-(アセチルアミノ)フェニル]アミノ}-5-ニトロピリミジン-4-イル)アミノ]安息香酸イソブチル塩酸塩;
2-[(2-{[4-(アセチルアミノ)フェニル]アミノ}-5-ニトロピリミジン-4-イル)アミノ]-6-メチル安息香酸塩酸塩;
N-シクロヘキシル-2-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)ベンズアミド塩酸塩;
1-[2-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)フェニル]エタノン塩酸塩;
[5-クロロ-2-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)フェニル](2-フルオロフェニル)メタノン塩酸塩;
8-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)-2-ナフトール塩酸塩;
N-(tert-ブチル)-2-({5-メチルケトン-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)ベンズアミド;
4-{[2-(4-メチルベンゾイル)フェニル]アミノ}-2-[(3,4,5-トリメトキシフェニル)アミノ]-ピリミジン-5-カルボニトリル;
並びにその薬学的に許容される塩、溶媒和物及び生理機能性誘導体。
R1は、H、ハロ、アルキル、アルケニル、アルキニルからなる群から選択され;
R2は、ハロ、アルキル、アルケニル、アルキニル、シクロアルキル、シクロアルケニル、-(R7)g-C(O)R6、-(R7)g-CO2R6、-(R7)g-C(O)N(R6)2、-(R7)g-OR6、-O-(R7)g-Ay、-(R7)g-S(O)eR6、-(R7)g-N(R6)2、-(R7)g-N(R6)C(O)R6、-(R7)g-CN、-(R7)g-SCN、-NO2、-N3、Ay、並びにN、O及びSから選択される1又は2個のヘテロ原子を含む5〜9員のヘテロアリールからなる群から選択され;
各R3は、同一又は異なって、そして独立して、H又アルキルであり;
Yは、-C(O)R8、-C(S)R8、-S(O)eR9、-S(O)eN(R9)2、-N(R9)2、-N(R9)-S(O)eR9、-N(R9)-C(O)R9、-N(R9)-CO2R9及び-N(R9)-C(O)N(R9)2からなる群から選択されるか、
又は、Yは、C-2及びC-3と一緒になって、式A:
ここで、R8はアルキル、アルケニル、アルキニル、シクロアルキル、シクロアルケニル、Ay、Het、-OR6、-O-(R7)g-Ay、-O-(R7)g-Het、-N(R6)2、-N(R6)-(R7)g-Ay、-N(R6)-(R7)g-Het、-N(R6)-(R7)g-OR6、-N(R6)-(R7)g-C(O)R6、-N(R6)-(R7)g-CO2R6、-N(R6)-(R7)g-SO2R6及び-N(R6)-(R7)g-N(R6)2からなる群から選択され;
各R9は、同一又は異なって、そして独立して、H、アルキル、アルケニル、アルキニル、シクロアルキル、シクロアルケニル、Ay及びHetからなる群から選択され;
cは、0又は1であり;
Z1、Z2、Z3及びZ4は、それぞれ独立して、C、O、S及びNからなる群から選択され、ここで、cが0である場合には、Z1、Z2及びZ3の少なくとも1つはCであり、そしてcが1である場合には、Z1、Z2、Z3及びZ4の少なくとも2つはCであり;
各破線は、任意に二重結合を表し;
dは、0、1又は2であり;
各R10は、同一又は異なって、そして独立して、ハロ、アルキル、オキソ、ヒドロキシ、メルカプト及びアミノからなる群から選択され;
aは、0、1、2又は3であり;
各R4は、同一又は異なって、そして独立して、ハロ、アルキル、アルケニル、アルキニル、-(R7)g-シクロアルキル、-(R7)g-シクロアルケニル、-(R7)g-Ay、-(R7)g-Het、-(R7)g-C(O)R6、-(R7)g-C(O)Ay、-(R7)g-C(O)Het、-(R7)g-CO2R6、-(R7)g-CO2Ay、-(R7)g-CO2Het、-(R7)g-C(O)N(R6)2、-(R7)g-OR6、-(R7)g-OAy、-(R7)g-OHet、-(R7)g-OC(O)R6、-(R7)g-OC(O)Ay、-(R7)g-OC(O)Het、-(R7)g-S(O)eR6、-(R7)g-S(O)eAy、-(R7)g-S(O)eHet、-(R7)g-S(O)eN(R6)2、-(R7)g-S(O)eN(R6)Ay、-(R7)g-S(O)eN(R6)Het、-(R7)g-N(R6)2、-(R7)g-N(R6)Ay、-(R7)g-N(R6)Het、-(R7)g-N(R6)C(O)R6、-(R7)g-N(R6)C(O)Ay、-(R7)g-N(R6)C(O)Het、-(R7)g-N(R6)C(O)N(R6)2、-(R7)g-N(R6)S(O)eR6、-(R7)g-N(R6)S(O)eAy、-(R7)g-N(R6)S(O)eHet、-NO2、-CN、-SCN及び-N3からなる群から選択されるか、又は
2個の隣接するR4は、それらが結合している炭素原子と一緒になって、フェニル又はN、O及びSからなる群から選択される1又は2個のヘテロ原子を含む5若しくは6員のヘテロ環若しくはヘテロアリールを形成し;
各eは、同一又は異なって、そして独立して、0、1又は2であり;
bは、0、1、2、3、4又は5であり;
各R5は、同一又は異なって、式(R7)g-R11の基であるか、又は2個の隣接するR5基は、それぞれ同一又は異なって、そして独立して、アルキル、アルケニル、-OR6、-S(O)eR6及び-N(R6)2からなる群から選択され、そしてそれらが結合している炭素原子と一緒になって、C5-6シクロアルキル、C5-6シクロアルケニル又はN、O及びSからなる群から選択される1又は2個のヘテロ原子を含む5若しくは6員のヘテロ環若しくはヘテロアリールを形成し;
各R6は、同一又は異なって、そして独立して、H、アルキル、アルケニル、アルキニル、シクロアルキル及びシクロアルケニルからなる群から選択され;
gは、0又は1であり;
R7はアルキレン又はアルケニレンであり;
Ayは、アリールであり;
Hetは、N、O及びSからなる群から選択される1、2又は3個のヘテロ原子を含む5員又は6員のヘテロ環若しくはヘテロアリールであり;
R11は、ハロ、アルキル、アルケニル、アルキニル、シクロアルキル、シクロアルケニル、Ay、Het、-C(O)R6、-C(O)Ay、-C(O)Het、-CO2R6、-CO2Ay、-CO2Het、-C(O)-R7-OR6、-C(O)-R7-OAy、-C(O)-R7-OHet、-C(O)N(R6)2、-C(O)N(R6)Ay、-C(O)N(R6)Het、-C(O)N(R6)-(R7)g-N(R6)2、-C(O)N(R6)-(R7)g-CO2R6、-C(O)N(R6)-(R7)g-S(O)eR6、-OR6、-OC(O)R6、-O-(R7)g-Ay、-OC(O)Ay、-O-(R7)g-Het、-OC(O)Het、-O-R7-OR6、-O-R7-N(R6)2、-S(O)eR6、-S(O)e-(R7)g-Ay、-S(O)e-(R7)g-Het、-S(O)e-(R7)g-N(R6)2、-S(O)e-(R7)g-N(R6)Ay、-S(O)e-(R7)g-N(R6)Het、-S(O)eN(R6)-(R7)g-C(O)R6、-S(O)eN(R6)-(R7)g-C(O)Ay、-S(O)eN(R6)-(R7)g-C(O)Het、-N(R6)2、-N(R6)-(R7)g-Ay、-N(R6)-(R7)g-Het、-N(R6)-(R7)g-C(O)R6、-N(R6)-C(O)-(R7)g-Ay、-N(R6)-C(O)-(R7)g-Het、-N(R6)-C(O)-(R7)g-N(R6)2、-N(R6)-C(O)-(R7)g-N(R6)Ay、-N(R6)-C(O)-(R7)g-N(R6)Het、-N(R6)-C(O)-(R7)g-N(R6)-(R7-O)h-N(R6)-CO2R6、-N(R6)-(R7)g-S(O)eR6、-N(R6)-(R7)g-S(O)eAy、-N(R6)-(R7)g-S(O)eHet、-N(R6)-R7-N(R6)2、-N(R6)-R7-OR6、-CN、-SCN、-NO2及び-N3からなる群から選択され;
hは、1〜20であり;そして
R15は、ハロ及び-S(O)eR6からなる群から選択される。
a)式(II)の化合物を式(III)の化合物と反応させて式(IV)の化合物を製造し;そして
b)式(IV)の化合物を式(V)の化合物と反応させて式(I)の化合物を製造すること
を含む方法により製造することができる。
R16はアルケニル又はアルキニルであり;
R17は、R2からなる群から選択され、すなわち、アルキル、-C(O)R6、-CO2R6、-C(O)N(R6)2、-OR6、-S(O)eR6、-N(R6)2、-N(R6)COR6、-CN、-SCN、Ay、並びにN、O及びSから選択される1若しくは2個のヘテロ原子を含む5〜9員のヘテロアリールからなる群から選択され;
そして他の全ての基は上記で定義したとおりである。
本反応は等モル量の式(I-A)の化合物と式(XIV)のトリブチル錫化合物との反応により行れるが、本反応は過剰量の式(XIV)の化合物の存在下で行うこともできる。パラジウム触媒は式(I-A)の化合物に対して好ましくは5〜20モル%の量で存在する。好適なパラジウム触媒の例は、ジクロロビス(トリフェニルホスフィン)パラジウム(II)、及びトリフェニルホスフィンと一緒の酢酸パラジウム(II)、及びトリス(ジベンジリデンアセトン)ジパラジウム(0)を含むが、これらに限定されるものではない。パラジウム触媒は、典型的にはヨウ化銅と錯体生成したものである。臭化銅はパラジウム触媒に対して好ましくは200モル%の量で存在する。塩基は、式(I-A)の化合物と同様の、又はその200モル%より大きい割合で典型的に存在する。好適な溶剤は、ジオキサン、N,N-ジメチルホルムアミド及びテトラヒドロフランを含むが、これらに限定されるものではない。式(XIV)の化合物は、商業的供給源から入手できるか、又は当業者に公知の方法を用いて慎重に単離した化合物として製造することができる。(Kukla, M. J. Bioorg. Med. Chem. Let. 2001, 11, 2235; Yamada, K. J. Med. Chem. 2001, 44, 3355)。
実施例39: 4-{[2-(4-メチルベンゾイル)フェニル]アミノ}-2-[(3,4,5-トリメトキシフェニル)アミノ]-ピリミジン-5-カルボニトリル
I. PLK1の阻害に関するアッセイ
A. 6x N末端His標識PLKキナーゼドメインの作製
6x N末端His標識PLKキナーゼドメイン(MKKGHHHHHHDが先行するアミノ酸21〜346)は、バキュロウイルスに感染したT. ni細胞から、ポリヘドリンプロモーターで制御して作製した。全ての手順は4℃で行った。細胞を25 mM HEPES、200 mM NaCl、25 mM イミダゾール;pH8.0中で溶解した。このホモジネートを SLA-1500 ローターで14K rpm で40分間遠心分離し、上澄み液を1.2ミクロンフィルターに通して濾過した。上澄み液をニッケルキレート化 Sepharose (Amersham Pharmacia) カラム上に負荷し、25 mM HEPES、500 mM NaCl、25 mM イミダゾール;pH8.0で洗浄した。次いでカラムを16.6%Bの段階(ここで、緩衝液Bは25 mM HEPES、500 mM NaCl、300 mM イミダゾール;pH8.0である)で洗浄した。16.6%B〜100%Bの10倍カラム容量線形勾配を用いてタンパク質を溶離した。PLKを含む分画をSDS-PAGEにより決定した。10 kDa 分子量カットオフ膜を用いてPLKを濃縮し、25 mM HEPES、1 mM DTT、500 mM NaCl;pH8.0中で平衡化したSuperdex 75 ゲル濾過 (Amersham Pharmacia) カラム上に負荷した。PLKを含む分画をSDS-PAGEにより決定した。PLKをプールし、アリコートに分割し、−80℃で保存した。サンプルは質量分析により品質管理した。
化合物をプレートに加えた(100% DMSO 中1μl)。DMSO(最終2%)及びEDTA(最終55.5 mM)をコントロールとして用いた。反応混合物Aは4℃で下記のように調製する:
反応混合物A(基質混合物):
25 mM HEPES、pH7.2
15 mM MgCl2
2μM ATP
0.1μCi/ウェル 33P-γ ATP (10Ci/mMol)
2μM 基質ペプチド(ビオチン-Ahx-SFNDTLDFD)
反応混合物Bは4℃で下記のように調製する:
反応混合物B(酵素混合物):
25 mM HEPES、pH7.2
15 mM MgCl2
0.15 mg/ml BSA
2 mM DTT
2〜10 nM PLK1キナーゼドメイン
反応混合物A(20μl)をウェルごとに加える。反応混合物B(20μl)をウェルごとに加える。室温で1.5時間インキュベートする。酵素反応を175μl のSPA/EDTAビーズ混合物(29 mM EDTA、標準ダルベッコPBS(Mg2+及びCa2+を含まない)中の2.5 mg/ml ストレプトアビジン被覆SPA、60μM ATP)で停止する。プレートを1,000×gで7分間密封遠心分離(室温で1時間インキュベートした後)するか、又は一夜沈降させ、次いでプレートを Packard TopCount において30秒/ウェルでカウントする。
得られたデータを下記表1に報告する。表1において、+ = pIC50 < 5;++ = pIC 50 5〜7;+++ = pIC50 > 7である。
正常非と包皮細胞線維芽細胞(HFF)、並びにヒトの結腸癌(HCT116、RKO)、肺癌(H460)、前立腺癌(PC3)及び乳癌(MCF7)細胞株を、10%ウシ胎仔血清(FBS)を含む高グルコースDMEM (Life Technologies) 中で、加湿した10%CO2、90%空気インキュベーター中37℃で培養した。トリプシン/EDTAを用いて細胞を採取し、血球計算器を用いてカウントし、96ウェル組織培養プレート (Falcon 3075) に入れた100μlの適切な培地に下記の密度で塗布した:HFFは5,000細胞/ウェル、HCT116は3,000細胞/ウェル、RKOは2,500細胞/ウェル、H460は2,000細胞/ウェル、PC3は8,000細胞/ウェル、MCF7は4,000細胞/ウェル。翌日、化合物を、DMSO中の10 mM ストック溶液から、100μg/ml のゲンタマイシンを含むDMEM中で最終必要濃度の2倍に希釈した。100μl/ウェルのこれらの希釈物を、細胞プレート上に現在あるのと同じ培地100μlに加えた。DMEM中で希釈した化合物を全ての細胞株に加えた。全てのウェル中のDMSO最終濃度は0.3%であった。細胞を37℃、10%CO2で3日間インキュベートした。培地を吸引により除去した。細胞をウェル当たり90μl のメチレンブルー (Sigma M9140、50:50のエタノール:水中0.5%) で染色し、室温で30分間インキュベートすることにより細胞バイオマスを評価した。染色液を除去し、プレートを穏やかな水流下ですすぎ、空気乾燥した。細胞から染色を遊離させるために、100μl の可溶化溶液を加え(PBS中1% N-ラウロイルザルコシン、ナトリウム塩、Sigma L5125)、プレートを穏やかに30分間振盪した。620 nM での光学密度をマイクロプレートリーダーで測定した。細胞成長のパーセント阻害をビヒクルで処理したコントロールウェルに対して計算した。細胞成長を50%阻害する化合物の濃度(IC50)を、非線形回帰 (Levenberg-Marquardt) 及び式 y = Vmax *(1−(x/(K+x)))+ Y2を用いて補間した。ここで、式中の「K」はIC50と等しかった。得られたデータを下記表1に報告する。表1において、+ = 10 〜 > 30 uM;++ = 1〜10 uM;+++ = < 1 uM である。
Claims (3)
- 式(I):
R1は、H、ハロ、アルキル、アルケニル、アルキニルからなる群から選択され;
R2は、ハロ、アルキル、アルケニル、アルキニル、シクロアルキル、シクロアルケニル、-(R7)g-C(O)R6、-(R7)g-CO2R6、-(R7)g-C(O)N(R6)2、-(R7)g-OR6、-O-(R7)g-Ay、-(R7)g-S(O)eR6、-(R7)g-N(R6)2、-(R7)g-N(R6)C(O)R6、-(R7)g-CN、-(R7)g-SCN、-NO2、-N3、Ay、並びにN、O及びSから選択される1又は2個のヘテロ原子を含む5〜9員のヘテロアリールからなる群から選択され;
各R3は、同一又は異なって、そして独立して、H又アルキルであり;
Yは、-C(O)R8、-C(S)R8、-S(O)eR9、-S(O)eN(R9)2、-N(R9)2、-N(R9)-S(O)eR9、-N(R9)-C(O)R9、-N(R9)-CO2R9及び-N(R9)-C(O)N(R9)2からなる群から選択されるか、
又は、Yは、C-2及びC-3と一緒になって、式A:
ここで、R8はアルキル、アルケニル、アルキニル、シクロアルキル、シクロアルケニル、Ay、Het、-OR6、-O-(R7)g-Ay、-O-(R7)g-Het、-N(R6)2、-N(R6)-(R7)g-Ay、-N(R6)-(R7)g-Het、-N(R6)-(R7)g-OR6、-N(R6)-(R7)g-C(O)R6、-N(R6)-(R7)g-CO2R6、-N(R6)-(R7)g-SO2R6及び-N(R6)-(R7)g-N(R6)2からなる群から選択され;
各R9は、同一又は異なって、そして独立して、H、アルキル、アルケニル、アルキニル、シクロアルキル、シクロアルケニル、Ay及びHetからなる群から選択され;
cは、0又は1であり;
Z1、Z2、Z3及びZ4は、それぞれ独立して、C、O、S及びNからなる群から選択され、ここで、cが0である場合には、Z1、Z2及びZ3の少なくとも1つはCであり、そしてcが1である場合には、Z1、Z2、Z3及びZ4の少なくとも2つはCであり;
各破線は、任意に二重結合を表し;
dは、0、1又は2であり;
各R10は、同一又は異なって、そして独立して、ハロ、アルキル、オキソ、ヒドロキシ、メルカプト及びアミノからなる群から選択され;
aは、0、1、2又は3であり;
各R4は、同一又は異なって、そして独立して、ハロ、アルキル、アルケニル、アルキニル、-(R7)g-シクロアルキル、-(R7)g-シクロアルケニル、-(R7)g-Ay、-(R7)g-Het、-(R7)g-C(O)R6、-(R7)g-C(O)Ay、-(R7)g-C(O)Het、-(R7)g-CO2R6、-(R7)g-CO2Ay、-(R7)g-CO2Het、-(R7)g-C(O)N(R6)2、-(R7)g-OR6、-(R7)g-OAy、-(R7)g-OHet、-(R7)g-OC(O)R6、-(R7)g-OC(O)Ay、-(R7)g-OC(O)Het、-(R7)g-S(O)eR6、-(R7)g-S(O)eAy、-(R7)g-S(O)eHet、-(R7)g-S(O)eN(R6)2、-(R7)g-S(O)eN(R6)Ay、-(R7)g-S(O)eN(R6)Het、-(R7)g-N(R6)2、-(R7)g-N(R6)Ay、-(R7)g-N(R6)Het、-(R7)g-N(R6)C(O)R6、-(R7)g-N(R6)C(O)Ay、-(R7)g-N(R6)C(O)Het、-(R7)g-N(R6)C(O)N(R6)2、-(R7)g-N(R6)S(O)eR6、-(R7)g-N(R6)S(O)eAy、-(R7)g-N(R6)S(O)eHet、-NO2、-CN、-SCN及び-N3からなる群から選択されるか、又は
2個の隣接するR4は、それらが結合している炭素原子と一緒になって、フェニル又はN、O及びSからなる群から選択される1又は2個のヘテロ原子を含む5若しくは6員のヘテロ環若しくはヘテロアリールを形成し;
各eは、同一又は異なって、そして独立して、0、1又は2であり;
bは、0、1、2、3、4又は5であり;
各R5は、同一又は異なって、式(R7)g-R11の基であるか、又は2個の隣接するR5基は、それぞれ同一又は異なって、そして独立して、アルキル、アルケニル、-OR6、-S(O)eR6及び-N(R6)2からなる群から選択され、そしてそれらが結合している炭素原子と一緒になって、C5-6シクロアルキル、C5-6シクロアルケニル又はN、O及びSからなる群から選択される1又は2個のヘテロ原子を含む5若しくは6員のヘテロ環若しくはヘテロアリールを形成し;
各R6は、同一又は異なって、そして独立して、H、アルキル、アルケニル、アルキニル、シクロアルキル及びシクロアルケニルからなる群から選択され;
gは、0又は1であり;
R7はアルキレン又はアルケニレンであり;
Ayは、アリールであり;
Hetは、N、O及びSからなる群から選択される1、2又は3個のヘテロ原子を含む5員又は6員のヘテロ環若しくはヘテロアリールであり;
R11は、ハロ、アルキル、アルケニル、アルキニル、シクロアルキル、シクロアルケニル、Ay、Het、-C(O)R6、-C(O)Ay、-C(O)Het、-CO2R6、-CO2Ay、-CO2Het、-C(O)-R7-OR6、-C(O)-R7-OAy、-C(O)-R7-OHet、-C(O)N(R6)2、-C(O)N(R6)Ay、-C(O)N(R6)Het、-C(O)N(R6)-(R7)g-N(R6)2、-C(O)N(R6)-(R7)g-CO2R6、-C(O)N(R6)-(R7)g-S(O)eR6、-OR6、-OC(O)R6、-O-(R7)g-Ay、-OC(O)Ay、-O-(R7)g-Het、-OC(O)Het、-O-R7-OR6、-O-R7-N(R6)2、-S(O)eR6、-S(O)e-(R7)g-Ay、-S(O)e-(R7)g-Het、-S(O)e-(R7)g-N(R6)2、-S(O)e-(R7)g-N(R6)Ay、-S(O)e-(R7)g-N(R6)Het、-S(O)eN(R6)-(R7)g-C(O)R6、-S(O)eN(R6)-(R7)g-C(O)Ay、-S(O)eN(R6)-(R7)g-C(O)Het、-N(R6)2、-N(R6)-(R7)g-Ay、-N(R6)-(R7)g-Het、-N(R6)-(R7)g-C(O)R6、-N(R6)-C(O)-(R7)g-Ay、-N(R6)-C(O)-(R7)g-Het、-N(R6)-C(O)-(R7)g-N(R6)2、-N(R6)-C(O)-(R7)g-N(R6)Ay、-N(R6)-C(O)-(R7)g-N(R6)Het、-N(R6)-C(O)-(R7)g-N(R6)-(R7-O)h-N(R6)-CO2R6、-N(R6)-(R7)g-S(O)eR6、-N(R6)-(R7)g-S(O)eAy、-N(R6)-(R7)g-S(O)eHet、-N(R6)-R7-N(R6)2、-N(R6)-R7-OR6、-CN、-SCN、-NO2及び-N3からなる群から選択され;そして
hは、1〜20であり;
ここで、R1が-CH3であり、R2がBr又はNO2であり、両方のR3がHであり、aが0であり、そしてbが0又は1であり、R5が-CO2Hである場合には、Yは-CO2Hではない。]
の化合物又はその薬学的に許容される塩若しくは溶媒和物。 - 2-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)安息香酸塩酸塩;
2-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)ベンズアミド塩酸塩;
N-(tert-ブチル)-2-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)ベンズアミド塩酸塩;
N-[4-({4-[(2-ベンゾイルフェニル)アミノ]-5-ニトロピリミジン-2-イル}アミノ)フェニル]アセトアミド塩酸塩;
8-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)-3,4-ジヒドロナフタレン-1(2H)-オン塩酸塩;
1-[2-({5-ブロモ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)フェニル]エタノン塩酸塩;
2-[(2-{[4-(アセチルアミノ)フェニル]アミノ}-5-ニトロピリミジン-4-イル)アミノ]-N-メチルベンズアミド塩酸塩;
N4-(1H-インドール-4-イル)-5-ニトロ-N2-(3,4,5-トリメトキシフェニル)ピリミジン-2,4-ジアミン塩酸塩;
2-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)安息香酸メチル塩酸塩;
N4-(2,3-ジヒドロ-1,4-ベンゾジオキシン-5-イル)-5-ニトロ-N2-(3,4,5-トリメトキシフェニル)ピリミジン-2,4-ジアミン塩酸塩;
2-[(2-{[4-(アセチルアミノ)フェニル]アミノ}-5-ニトロピリミジン-4-イル)アミノ]安息香酸シクロヘキシル塩酸塩;
5-ヒドロキシ-2-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)安息香酸塩酸塩;
2-[(2-{[4-(アセチルアミノ)フェニル]アミノ}-5-ニトロピリミジン-4-イル)アミノ]安息香酸塩酸塩;
N-[4-({4-[(2-アセチルフェニル)アミノ]-5-ブロモピリミジン-2-イル}アミノ)フェニル]アセトアミド塩酸塩;
N-[4-({5-ブロモ-4-[(2-モルホリン-4-イルフェニル)アミノ]ピリミジン-2-イル}アミノ)フェニル]アセトアミド塩酸塩;
5-[(5-ブロモ-4-{[2-(メチルチオ)フェニル]アミノ}ピリミジン-2-イル)アミノ]-1,3-ジヒドロ-2H-ベンズイミダゾール-2-オン塩酸塩;
5-ブロモ-N4-(2-モルホリン-4-イルフェニル)-N2-(3,4,5-トリメトキシフェニル)ピリミジン-2,4-ジアミン塩酸塩;
N-[4-({5-ブロモ-4-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-2-イル}アミノ)フェニル]アセトアミド塩酸塩;
2-[(2-{[4-(アセチルアミノ)フェニル]アミノ}-5-ニトロピリミジン-4-イル)アミノ]テレフタル酸ジメチル塩酸塩;
2-[(2-{[4-(アセチルアミノ)フェニル]アミノ}-5-ニトロピリミジン-4-イル)アミノ]安息香酸ベンジル塩酸塩;
2-[(2-{[4-(アセチルアミノ)フェニル]アミノ}-5-ニトロピリミジン-4-イル)アミノ]-6-メチル安息香酸メチル塩酸塩;
2-[(2-{[4-(アセチルアミノ)フェニル]アミノ}-5-ニトロピリミジン-4-イル)アミノ]安息香酸イソブチル塩酸塩;
2-[(2-{[4-(アセチルアミノ)フェニル]アミノ}-5-ニトロピリミジン-4-イル)アミノ]-6-メチル安息香酸塩酸塩;
N-シクロヘキシル-2-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)ベンズアミド塩酸塩;
1-[2-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)フェニル]エタノン塩酸塩;
[5-クロロ-2-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)フェニル](2-フルオロフェニル)メタノン塩酸塩;
8-({5-ニトロ-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)-2-ナフトール塩酸塩;
N-(tert-ブチル)-2-({5-メチルケトン-2-[(3,4,5-トリメトキシフェニル)アミノ]ピリミジン-4-イル}アミノ)ベンズアミド;
4-{[2-(4-メチルベンゾイル)フェニル]アミノ}-2-[(3,4,5-トリメトキシフェニル)アミノ]-ピリミジン-5-カルボニトリル;並びに
その薬学的に許容される塩及び溶媒和物
からなる群から選択される化合物。 - 動物における感受性新生物の治療に使用するための、請求項1又は2記載の化合物を含有する医薬組成物。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US44879503P | 2003-02-20 | 2003-02-20 | |
| PCT/US2004/004197 WO2004074244A2 (en) | 2003-02-20 | 2004-02-11 | Pyrimidine compounds |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2006518386A JP2006518386A (ja) | 2006-08-10 |
| JP4634367B2 true JP4634367B2 (ja) | 2011-02-16 |
Family
ID=32908652
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2006503537A Expired - Lifetime JP4634367B2 (ja) | 2003-02-20 | 2004-02-11 | ピリミジン化合物 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US7514446B2 (ja) |
| EP (1) | EP1597251B1 (ja) |
| JP (1) | JP4634367B2 (ja) |
| AT (1) | ATE433447T1 (ja) |
| DE (1) | DE602004021472D1 (ja) |
| ES (1) | ES2325440T3 (ja) |
| WO (1) | WO2004074244A2 (ja) |
Families Citing this family (104)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TWI329105B (en) | 2002-02-01 | 2010-08-21 | Rigel Pharmaceuticals Inc | 2,4-pyrimidinediamine compounds and their uses |
| GB0206215D0 (en) | 2002-03-15 | 2002-05-01 | Novartis Ag | Organic compounds |
| ATE451104T1 (de) | 2002-07-29 | 2009-12-15 | Rigel Pharmaceuticals Inc | Verfahren zur behandlung oder pruvention von autoimmunkrankheiten mit 2,4-pyrimidindiamin- verbindungen |
| GB0305929D0 (en) | 2003-03-14 | 2003-04-23 | Novartis Ag | Organic compounds |
| PL1656372T3 (pl) | 2003-07-30 | 2013-08-30 | Rigel Pharmaceuticals Inc | Związki 2,4-pirymidynodiaminy do stosowania w leczeniu lub zapobieganiu chorobom autoimmunologicznym |
| US20050113398A1 (en) * | 2003-08-07 | 2005-05-26 | Ankush Argade | 2,4-pyrimidinediamine compounds and uses as anti-proliferative agents |
| CA2533320A1 (en) * | 2003-08-15 | 2006-02-24 | Novartis Ag | 2, 4-pyrimidinediamines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders |
| GB0321710D0 (en) * | 2003-09-16 | 2003-10-15 | Novartis Ag | Organic compounds |
| CN100584832C (zh) * | 2003-09-18 | 2010-01-27 | 诺瓦提斯公司 | 可用于治疗增殖性病症的2,4-二(苯基氨基)嘧啶类 |
| US7521457B2 (en) | 2004-08-20 | 2009-04-21 | Boehringer Ingelheim International Gmbh | Pyrimidines as PLK inhibitors |
| EP1815206B1 (en) | 2004-10-13 | 2016-04-06 | PTC Therapeutics, Inc. | Compounds for nonsense suppression, and methods for their use |
| US7557207B2 (en) | 2004-11-24 | 2009-07-07 | Rigel Pharmaceuticals, Inc. | Spiro 2,4-pyrimidinediamine compounds and their uses |
| ATE519759T1 (de) | 2004-12-30 | 2011-08-15 | Exelixis Inc | Pyrimidinderivate als kinasemodulatoren und anwendungsverfahren |
| BRPI0606318B8 (pt) | 2005-01-19 | 2021-05-25 | Rigel Pharmaceuticals Inc | composto, composição, e, uso de um composto |
| WO2006129100A1 (en) * | 2005-06-03 | 2006-12-07 | Glaxo Group Limited | Novel compounds |
| US20070203161A1 (en) | 2006-02-24 | 2007-08-30 | Rigel Pharmaceuticals, Inc. | Compositions and methods for inhibition of the jak pathway |
| WO2006133426A2 (en) | 2005-06-08 | 2006-12-14 | Rigel Pharmaceuticals, Inc. | Compositions and methods for inhibition of the jak pathway |
| TW200736232A (en) * | 2006-01-26 | 2007-10-01 | Astrazeneca Ab | Pyrimidine derivatives |
| US7569561B2 (en) | 2006-02-22 | 2009-08-04 | Boehringer Ingelheim International Gmbh | 2,4-diaminopyrimidines useful for treating cell proliferation diseases |
| ES2622493T3 (es) | 2006-02-24 | 2017-07-06 | Rigel Pharmaceuticals, Inc. | Composiciones y métodos para la inhibición de la ruta de JAK |
| US7504513B2 (en) | 2006-02-27 | 2009-03-17 | Hoffman-La Roche Inc. | Thiazolyl-benzimidazoles |
| CA2648170A1 (en) * | 2006-04-10 | 2007-10-18 | Boehringer Ingelheim International Gmbh | 2, 4-diaminopyrimidine derivatives and their use for the treatment of cancer |
| PE20120006A1 (es) | 2006-05-15 | 2012-02-02 | Boehringer Ingelheim Int | Compuestos derivados de pirimidina como inhibidores de la quinasa aurora |
| US8198421B2 (en) * | 2006-06-19 | 2012-06-12 | Asklepios Biopharmaceutical, Inc. | Modified factor VIII and factor IX genes and vectors for gene therapy |
| EP2043651A2 (en) * | 2006-07-05 | 2009-04-08 | Exelixis, Inc. | Methods of using igf1r and abl kinase modulators |
| US20100010025A1 (en) * | 2006-07-21 | 2010-01-14 | Norvartis Ag | Pyrimidine Derivatives |
| SI2091918T1 (sl) | 2006-12-08 | 2015-01-30 | Irm Llc | Spojine in sestavki kot inhibitorji protein-kinaze |
| PE20081636A1 (es) * | 2007-01-26 | 2009-01-10 | Smithkline Beecham Corp | Inhibidores de antranilamida para aurora quinasa |
| TW200840581A (en) * | 2007-02-28 | 2008-10-16 | Astrazeneca Ab | Novel pyrimidine derivatives |
| EP2100894A1 (en) | 2008-03-12 | 2009-09-16 | 4Sc Ag | Pyridopyrimidines used as Plk1 (polo-like kinase) inhibitors |
| WO2009127642A2 (en) * | 2008-04-15 | 2009-10-22 | Cellzome Limited | Use of lrrk2 inhibitors for neurodegenerative diseases |
| NZ589315A (en) | 2008-04-16 | 2012-11-30 | Portola Pharm Inc | 2,6-diamino-pyrimidin-5-yl-carboxamides as Spleen tryosine kinase (syk) or Janus kinase (JAK) inhibitors |
| US8138339B2 (en) | 2008-04-16 | 2012-03-20 | Portola Pharmaceuticals, Inc. | Inhibitors of protein kinases |
| US9273077B2 (en) | 2008-05-21 | 2016-03-01 | Ariad Pharmaceuticals, Inc. | Phosphorus derivatives as kinase inhibitors |
| HUE035029T2 (en) | 2008-05-21 | 2018-03-28 | Ariad Pharma Inc | Kinase inhibitor phosphorus derivatives |
| SG10201510696RA (en) | 2008-06-27 | 2016-01-28 | Celgene Avilomics Res Inc | Heteroaryl compounds and uses thereof |
| US8338439B2 (en) | 2008-06-27 | 2012-12-25 | Celgene Avilomics Research, Inc. | 2,4-disubstituted pyrimidines useful as kinase inhibitors |
| US11351168B1 (en) | 2008-06-27 | 2022-06-07 | Celgene Car Llc | 2,4-disubstituted pyrimidines useful as kinase inhibitors |
| PL2350075T3 (pl) | 2008-09-22 | 2014-07-31 | Array Biopharma Inc | Podstawione związki imidazo[1,2b]pirydazynowe jako inhibitory kinaz Trk |
| TWI491605B (zh) | 2008-11-24 | 2015-07-11 | Boehringer Ingelheim Int | 新穎化合物 |
| AR074209A1 (es) | 2008-11-24 | 2010-12-29 | Boehringer Ingelheim Int | Derivados de pirimidina utiles para el tratamiento del cancer |
| CA2745596A1 (en) | 2008-12-18 | 2010-10-28 | F. Hoffmann-La Roche Ag | Thiazolyl-benzimidazoles |
| ES2624622T3 (es) | 2008-12-30 | 2017-07-17 | Rigel Pharmaceuticals, Inc. | Inhibidores de pirimidindiamina cinasa |
| ES2659725T3 (es) | 2009-05-05 | 2018-03-19 | Dana-Farber Cancer Institute, Inc. | Inhibidores de EGFR y procedimiento de tratamiento de trastornos |
| TW201100441A (en) | 2009-06-01 | 2011-01-01 | Osi Pharm Inc | Amino pyrimidine anticancer compounds |
| WO2010142766A2 (en) * | 2009-06-10 | 2010-12-16 | Cellzome Limited | Pyrimidine derivatives as zap-70 inhibitors |
| AR077468A1 (es) | 2009-07-09 | 2011-08-31 | Array Biopharma Inc | Compuestos de pirazolo (1,5 -a) pirimidina sustituidos como inhibidores de trk- quinasa |
| KR20130031296A (ko) | 2010-05-21 | 2013-03-28 | 케밀리아 에이비 | 신규한 피리미딘 유도체 |
| CN103096716B (zh) | 2010-08-10 | 2016-03-02 | 西建阿维拉米斯研究公司 | Btk抑制剂的苯磺酸盐 |
| WO2012061303A1 (en) | 2010-11-01 | 2012-05-10 | Avila Therapeutics, Inc. | Heteroaryl compounds and uses thereof |
| BR112013010564B1 (pt) | 2010-11-01 | 2021-09-21 | Celgene Car Llc | Compostos heterocíclicos e composições compreendendo os mesmos |
| WO2012064706A1 (en) | 2010-11-10 | 2012-05-18 | Avila Therapeutics, Inc. | Mutant-selective egfr inhibitors and uses thereof |
| EP2646448B1 (en) | 2010-11-29 | 2017-08-30 | OSI Pharmaceuticals, LLC | Macrocyclic kinase inhibitors |
| DK2688883T3 (en) | 2011-03-24 | 2016-09-05 | Noviga Res Ab | pyrimidine |
| JP5999177B2 (ja) | 2011-05-04 | 2016-09-28 | アリアド・ファーマシューティカルズ・インコーポレイテッド | Egfr発動性がんの細胞増殖阻害用化合物 |
| WO2013013188A1 (en) | 2011-07-21 | 2013-01-24 | Tolero Pharmaceuticals, Inc. | Heterocyclic protein kinase inhibitors |
| TW201325593A (zh) | 2011-10-28 | 2013-07-01 | Celgene Avilomics Res Inc | 治療布魯頓(bruton’s)酪胺酸激酶疾病或病症之方法 |
| MX363551B (es) | 2011-11-23 | 2019-03-27 | Portola Pharmaceuticals Inc Star | Compuestos derivados de pirazina como inhibidores de cinasa. |
| WO2013138495A1 (en) | 2012-03-15 | 2013-09-19 | Celgene Avilomics Research, Inc. | Solid forms of an epidermal growth factor receptor kinase inhibitor |
| SG11201405692UA (en) | 2012-03-15 | 2014-10-30 | Celgene Avilomics Res Inc | Salts of an epidermal growth factor receptor kinase inhibitor |
| AU2013204563B2 (en) | 2012-05-05 | 2016-05-19 | Takeda Pharmaceutical Company Limited | Compounds for inhibiting cell proliferation in EGFR-driven cancers |
| WO2014100748A1 (en) | 2012-12-21 | 2014-06-26 | Celgene Avilomics Research, Inc. | Heteroaryl compounds and uses thereof |
| WO2014124230A2 (en) | 2013-02-08 | 2014-08-14 | Celgene Avilomics Research, Inc. | Erk inhibitors and uses thereof |
| TR201911151T4 (tr) | 2013-03-14 | 2019-08-21 | Tolero Pharmaceuticals Inc | Jak2 ve alk2 inhibitörleri ve bunların kullanım yöntemleri. |
| US9611283B1 (en) | 2013-04-10 | 2017-04-04 | Ariad Pharmaceuticals, Inc. | Methods for inhibiting cell proliferation in ALK-driven cancers |
| CN104230954A (zh) * | 2013-06-08 | 2014-12-24 | 中国科学院上海药物研究所 | 2,4-二氨基嘧啶类化合物及其医药用途 |
| US9492471B2 (en) | 2013-08-27 | 2016-11-15 | Celgene Avilomics Research, Inc. | Methods of treating a disease or disorder associated with Bruton'S Tyrosine Kinase |
| US9415049B2 (en) | 2013-12-20 | 2016-08-16 | Celgene Avilomics Research, Inc. | Heteroaryl compounds and uses thereof |
| US10005760B2 (en) | 2014-08-13 | 2018-06-26 | Celgene Car Llc | Forms and compositions of an ERK inhibitor |
| JP6684780B2 (ja) * | 2014-08-25 | 2020-04-22 | ソーク インスティテュート フォー バイオロジカル スタディーズ | 新規ulk1阻害剤およびそれを使用する方法 |
| CN105503827B (zh) * | 2014-10-11 | 2019-09-24 | 上海翰森生物医药科技有限公司 | Egfr抑制剂及其制备方法和用途 |
| CA2992586A1 (en) | 2015-07-16 | 2017-01-19 | Array Biopharma, Inc. | Substituted pyrazolo[1,5-a]pyridine compounds as ret kinase inhibitors |
| EP3368039A1 (en) | 2015-10-26 | 2018-09-05 | The Regents of The University of Colorado, A Body Corporate | Point mutations in trk inhibitor-resistant cancer and methods relating to the same |
| EP3439663B1 (en) | 2016-04-04 | 2024-07-17 | Loxo Oncology, Inc. | Methods of treating pediatric cancers |
| GEP20227339B (en) | 2016-04-04 | 2022-01-25 | Loxo Oncology Inc | Liquid formulations of (s)-n-(5-((r)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)- pyrazolo[1,5-a]pyrimidin-3-yl)-3-hydro-xypyrrolidine-1-carboxamide |
| US10045991B2 (en) | 2016-04-04 | 2018-08-14 | Loxo Oncology, Inc. | Methods of treating pediatric cancers |
| CA3024603A1 (en) | 2016-05-18 | 2017-11-23 | Charles Todd Eary | Process for the preparation of (s)-n-(5-((r)-2-(2,5-difluorophenyl)pyrrolidin-1-yl)-pyrazolo[1,5-a]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide and salts thereof |
| JOP20190077A1 (ar) | 2016-10-10 | 2019-04-09 | Array Biopharma Inc | مركبات بيرازولو [1، 5-a]بيريدين بها استبدال كمثبطات كيناز ret |
| TWI704148B (zh) | 2016-10-10 | 2020-09-11 | 美商亞雷生物製藥股份有限公司 | 作為ret激酶抑制劑之經取代吡唑并[1,5-a]吡啶化合物 |
| JOP20190092A1 (ar) | 2016-10-26 | 2019-04-25 | Array Biopharma Inc | عملية لتحضير مركبات بيرازولو[1، 5-a]بيريميدين وأملاح منها |
| WO2018136661A1 (en) | 2017-01-18 | 2018-07-26 | Andrews Steven W | SUBSTITUTED PYRAZOLO[1,5-a]PYRAZINE COMPOUNDS AS RET KINASE INHIBITORS |
| WO2018136663A1 (en) | 2017-01-18 | 2018-07-26 | Array Biopharma, Inc. | Ret inhibitors |
| JOP20190213A1 (ar) | 2017-03-16 | 2019-09-16 | Array Biopharma Inc | مركبات حلقية ضخمة كمثبطات لكيناز ros1 |
| CN107235931B (zh) * | 2017-07-11 | 2019-09-24 | 大连医科大学 | 新型嘧啶类抗肿瘤化合物及其制备方法与用途 |
| TW202410896A (zh) | 2017-10-10 | 2024-03-16 | 美商絡速藥業公司 | 6-(2-羥基-2-甲基丙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮雜雙環[3.1.1]庚-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-甲腈之調配物 |
| TWI791053B (zh) | 2017-10-10 | 2023-02-01 | 美商亞雷生物製藥股份有限公司 | 6-(2-羥基-2-甲基丙氧基)-4-(6-(6-((6-甲氧基吡啶-3-基)甲基)-3,6-二氮雜雙環[3.1.1]庚-3-基)吡啶-3-基)吡唑并[1,5-a]吡啶-3-甲腈之結晶形式及其醫藥組合物 |
| TWI802635B (zh) | 2018-01-18 | 2023-05-21 | 美商亞雷生物製藥股份有限公司 | 作為ret激酶抑制劑之經取代吡咯并[2,3-d]嘧啶化合物 |
| CA3087972C (en) | 2018-01-18 | 2023-01-10 | Array Biopharma Inc. | Substituted pyrazolyl[4,3-c]pyridinecompounds as ret kinase inhibitors |
| WO2019143991A1 (en) | 2018-01-18 | 2019-07-25 | Array Biopharma Inc. | SUBSTITUTED PYRAZOLO[3,4-d]PYRIMIDINE COMPOUNDS AS RET KINASE INHIBITORS |
| CN112533602A (zh) | 2018-04-05 | 2021-03-19 | 大日本住友制药肿瘤公司 | Axl激酶抑制剂及其用途 |
| MX2020010556A (es) | 2018-04-13 | 2021-03-02 | Sumitomo Pharma Oncology Inc | Inhibidores de cinasa de insercion proviral en linfomas murinos (pim) para el tratamiento de neoplasias mieloproliferativas y fibrosis asociadas con cancer. |
| CA3103995A1 (en) | 2018-07-26 | 2020-01-30 | Sumitomo Dainippon Pharma Oncology, Inc. | Methods for treating diseases associated with abnormal acvr1 expression and acvr1 inhibitors for use in the same |
| KR102653681B1 (ko) | 2018-07-31 | 2024-04-03 | 록쏘 온콜로지, 인코포레이티드 | (s)-5-아미노-3-(4-((5-플루오로-2-메톡시벤즈아미도)메틸)페닐)-1-(1,1,1-트리플루오로프로판-2-일)-1h-피라졸-4-카르복스아미드의분무-건조된 분산물 및 제제 |
| CA3111984A1 (en) | 2018-09-10 | 2020-03-19 | Array Biopharma Inc. | Fused heterocyclic compounds as ret kinase inhibitors |
| EP3898626A1 (en) | 2018-12-19 | 2021-10-27 | Array Biopharma, Inc. | Substituted pyrazolo[1,5-a]pyridine compounds as inhibitors of fgfr tyrosine kinases |
| WO2020131674A1 (en) | 2018-12-19 | 2020-06-25 | Array Biopharma Inc. | 7-((3,5-dimethoxyphenyl)amino)quinoxaline derivatives as fgfr inhibitors for treating cancer |
| MX2021009371A (es) | 2019-02-12 | 2021-09-10 | Sumitomo Pharma Oncology Inc | Formulaciones que comprenden inhibidores de proteina cinasa heterociclicos. |
| CA3145864A1 (en) | 2019-07-03 | 2021-01-07 | Sumitomo Dainippon Pharma Oncology, Inc. | Tyrosine kinase non-receptor 1 (tnk1) inhibitors and uses thereof |
| CN112876420B (zh) * | 2019-11-29 | 2023-01-24 | 沈阳化工研究院有限公司 | 一种硫代苯甲酰衍生物及其应用 |
| CA3161339A1 (en) | 2019-12-27 | 2021-07-01 | Schrodinger, Inc. | Cyclic compounds and methods of using same |
| WO2022055963A1 (en) | 2020-09-10 | 2022-03-17 | Schrödinger, Inc. | Heterocyclic pericondensed cdc7 kinase inhibitors for the treatment of cancer |
| US20240148732A1 (en) | 2021-01-26 | 2024-05-09 | Schrödinger, Inc. | Tricyclic compounds useful in the treatment of cancer, autoimmune and inflammatory disorders |
| TW202300150A (zh) | 2021-03-18 | 2023-01-01 | 美商薛定諤公司 | 環狀化合物及其使用方法 |
| WO2024097653A1 (en) | 2022-10-31 | 2024-05-10 | Sumitomo Pharma America, Inc. | Pim1 inhibitor for treating myeloproliferative neoplasms |
Family Cites Families (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB9523675D0 (en) * | 1995-11-20 | 1996-01-24 | Celltech Therapeutics Ltd | Chemical compounds |
| GB9622363D0 (en) | 1996-10-28 | 1997-01-08 | Celltech Therapeutics Ltd | Chemical compounds |
| KR100699514B1 (ko) | 1998-03-27 | 2007-03-26 | 얀센 파마슈티카 엔.브이. | Hiv를 억제하는 피리미딘 유도체 |
| AU5438299A (en) | 1998-08-29 | 2000-03-21 | Astrazeneca Ab | Pyrimidine compounds |
| KR100658489B1 (ko) | 1998-11-10 | 2006-12-18 | 얀센 파마슈티카 엔.브이. | Hiv 복제를 억제하는 피리미딘 |
| GB9828511D0 (en) * | 1998-12-24 | 1999-02-17 | Zeneca Ltd | Chemical compounds |
| HUP0301117A3 (en) | 2000-02-17 | 2004-01-28 | Amgen Inc Thousand Oaks | Imidazole derivatives kinase inhibitors, their use, process for their preparation and pharmaceutical compositions containing them |
| GB0004890D0 (en) | 2000-03-01 | 2000-04-19 | Astrazeneca Uk Ltd | Chemical compounds |
| GB0004887D0 (en) | 2000-03-01 | 2000-04-19 | Astrazeneca Uk Ltd | Chemical compounds |
| GB0004886D0 (en) | 2000-03-01 | 2000-04-19 | Astrazeneca Uk Ltd | Chemical compounds |
| GB0004888D0 (en) * | 2000-03-01 | 2000-04-19 | Astrazeneca Uk Ltd | Chemical compounds |
| MXPA03005696A (es) | 2000-12-21 | 2003-10-06 | Glaxo Group Ltd | Pirimidinaminas como moduladores de angiogenesis. |
| US20040171630A1 (en) | 2001-06-19 | 2004-09-02 | Yuntae Kim | Tyrosine kinase inhibitors |
| WO2003030909A1 (en) * | 2001-09-25 | 2003-04-17 | Bayer Pharmaceuticals Corporation | 2- and 4-aminopyrimidines n-substtituded by a bicyclic ring for use as kinase inhibitors in the treatment of cancer |
| DE50212771D1 (de) * | 2001-10-17 | 2008-10-23 | Boehringer Ingelheim Pharma | Pyrimidinderivate, arzneimittel enthaltend diese verbindungen, deren verwendung und verfahren zu ihrer herstellung |
| TWI329105B (en) * | 2002-02-01 | 2010-08-21 | Rigel Pharmaceuticals Inc | 2,4-pyrimidinediamine compounds and their uses |
| GB0206215D0 (en) | 2002-03-15 | 2002-05-01 | Novartis Ag | Organic compounds |
| AU2002350719A1 (en) | 2002-11-29 | 2004-06-23 | Janssen Pharmaceutica N.V. | Pharmaceutical compositions comprising a basic respectively acidic drug compound, a surfactant and a physiologically tolerable water-soluble acid respectively base |
| EP1598343A1 (de) | 2004-05-19 | 2005-11-23 | Boehringer Ingelheim International GmbH | 2-Arylaminopyrimidine als PLK Inhibitoren |
-
2004
- 2004-02-11 JP JP2006503537A patent/JP4634367B2/ja not_active Expired - Lifetime
- 2004-02-11 EP EP04710264A patent/EP1597251B1/en not_active Expired - Lifetime
- 2004-02-11 DE DE602004021472T patent/DE602004021472D1/de not_active Expired - Lifetime
- 2004-02-11 AT AT04710264T patent/ATE433447T1/de not_active IP Right Cessation
- 2004-02-11 ES ES04710264T patent/ES2325440T3/es not_active Expired - Lifetime
- 2004-02-11 WO PCT/US2004/004197 patent/WO2004074244A2/en active Application Filing
- 2004-02-11 US US10/545,352 patent/US7514446B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| US7514446B2 (en) | 2009-04-07 |
| US20070010668A1 (en) | 2007-01-11 |
| WO2004074244A3 (en) | 2004-11-11 |
| ES2325440T3 (es) | 2009-09-04 |
| JP2006518386A (ja) | 2006-08-10 |
| EP1597251B1 (en) | 2009-06-10 |
| EP1597251A2 (en) | 2005-11-23 |
| WO2004074244A2 (en) | 2004-09-02 |
| ATE433447T1 (de) | 2009-06-15 |
| DE602004021472D1 (en) | 2009-07-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4634367B2 (ja) | ピリミジン化合物 | |
| JP5103403B2 (ja) | 2−ピリミジニルピラゾロピリジンErbBキナーゼ阻害剤 | |
| JP5132319B2 (ja) | 2−ピリミジニルピラゾロピリジンErbBキナーゼ阻害剤 | |
| JP4280442B2 (ja) | イミダゾ[1,2a]ピリジンおよびピラゾロ[2,3a]ピリジン誘導体 | |
| EP1641805B1 (en) | Thiazolo-, oxazalo and imidazolo-quinazoline compounds capable of inhibiting protein kinases | |
| US7189712B2 (en) | 1,3-Oxazole compounds for the treatment of cancer | |
| EP1463730B1 (en) | Pyrazolopyridazine derivatives | |
| SK3542002A3 (en) | Pteridinones as kinase inhibitors | |
| MX2013003918A (es) | Derivados de bencimidazol como inhibidores de cinasa pi3. | |
| JP2003502280A (ja) | 置換アザ−オキシインドール誘導体 | |
| EP1937671A2 (en) | Benzimidazole thiophene compounds as plk inhibitors | |
| JP2007519720A (ja) | チアゾール化合物 | |
| EP1608656B1 (en) | Imidazotriazine compounds for the treatment of cancer diseases | |
| JP2010508301A (ja) | 5−置換及び6−置換ベンゾイミダゾールチオフェン化合物 | |
| US7812022B2 (en) | 2-pyrimidinyl pyrazolopyridine ErbB kinase inhibitors | |
| JP2004508372A (ja) | オキシインドール誘導体 | |
| US20070142437A1 (en) | Chemical compounds |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20061114 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100309 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20100603 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20100610 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100909 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20101019 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20101118 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4634367 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20131126 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20131126 Year of fee payment: 3 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20131126 Year of fee payment: 3 |
|
| R371 | Transfer withdrawn |
Free format text: JAPANESE INTERMEDIATE CODE: R371 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| S533 | Written request for registration of change of name |
Free format text: JAPANESE INTERMEDIATE CODE: R313533 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R360 | Written notification for declining of transfer of rights |
Free format text: JAPANESE INTERMEDIATE CODE: R360 |
|
| R371 | Transfer withdrawn |
Free format text: JAPANESE INTERMEDIATE CODE: R371 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20131126 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R153 | Grant of patent term extension |
Free format text: JAPANESE INTERMEDIATE CODE: R153 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R153 | Grant of patent term extension |
Free format text: JAPANESE INTERMEDIATE CODE: R153 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R153 | Grant of patent term extension |
Free format text: JAPANESE INTERMEDIATE CODE: R153 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R153 | Grant of patent term extension |
Free format text: JAPANESE INTERMEDIATE CODE: R153 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |