JP4409935B2 - アミノキノリンおよびアミノピリジン誘導体およびそのアデノシンa3リガンドとしての使用 - Google Patents
アミノキノリンおよびアミノピリジン誘導体およびそのアデノシンa3リガンドとしての使用 Download PDFInfo
- Publication number
- JP4409935B2 JP4409935B2 JP2003500059A JP2003500059A JP4409935B2 JP 4409935 B2 JP4409935 B2 JP 4409935B2 JP 2003500059 A JP2003500059 A JP 2003500059A JP 2003500059 A JP2003500059 A JP 2003500059A JP 4409935 B2 JP4409935 B2 JP 4409935B2
- Authority
- JP
- Japan
- Prior art keywords
- group
- branched
- linear
- atom
- cyano
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 title description 42
- 239000002126 C01EB10 - Adenosine Substances 0.000 title description 24
- 229960005305 adenosine Drugs 0.000 title description 24
- 239000003446 ligand Substances 0.000 title description 4
- 150000003927 aminopyridines Chemical class 0.000 title 1
- 150000005010 aminoquinolines Chemical class 0.000 title 1
- 150000001875 compounds Chemical class 0.000 claims description 86
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims description 45
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 44
- -1 methylenedioxy group Chemical group 0.000 claims description 40
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 26
- 150000003839 salts Chemical class 0.000 claims description 26
- 125000003545 alkoxy group Chemical group 0.000 claims description 25
- 238000000034 method Methods 0.000 claims description 25
- 239000012453 solvate Substances 0.000 claims description 25
- 125000005843 halogen group Chemical group 0.000 claims description 24
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 20
- 229910052757 nitrogen Inorganic materials 0.000 claims description 17
- 125000004430 oxygen atom Chemical group O* 0.000 claims description 15
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 15
- 230000007062 hydrolysis Effects 0.000 claims description 13
- 238000006460 hydrolysis reaction Methods 0.000 claims description 13
- 238000002360 preparation method Methods 0.000 claims description 13
- 229910052717 sulfur Inorganic materials 0.000 claims description 13
- 125000004434 sulfur atom Chemical group 0.000 claims description 13
- 125000002541 furyl group Chemical group 0.000 claims description 10
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 10
- 125000001544 thienyl group Chemical group 0.000 claims description 10
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 6
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 claims description 5
- 125000001541 3-thienyl group Chemical group S1C([H])=C([*])C([H])=C1[H] 0.000 claims description 4
- 125000004172 4-methoxyphenyl group Chemical group [H]C1=C([H])C(OC([H])([H])[H])=C([H])C([H])=C1* 0.000 claims description 4
- 125000004453 alkoxycarbonyl group Chemical group 0.000 claims description 4
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 claims description 4
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 claims description 4
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 4
- SPDQSSIORISFDN-UHFFFAOYSA-N n-[4-(benzylamino)-3-cyanoquinolin-2-yl]-3-methylbenzamide Chemical compound CC1=CC=CC(C(=O)NC=2C(=C(NCC=3C=CC=CC=3)C3=CC=CC=C3N=2)C#N)=C1 SPDQSSIORISFDN-UHFFFAOYSA-N 0.000 claims description 4
- 125000000020 sulfo group Chemical group O=S(=O)([*])O[H] 0.000 claims description 4
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 claims description 4
- 125000000040 m-tolyl group Chemical group [H]C1=C([H])C(*)=C([H])C(=C1[H])C([H])([H])[H] 0.000 claims description 3
- XSWSGVCWMTYDHC-UHFFFAOYSA-N n-[3-cyano-4-(furan-2-ylmethylamino)quinolin-2-yl]furan-2-carboxamide Chemical compound C=1C=COC=1C(=O)NC(C=1C#N)=NC2=CC=CC=C2C=1NCC1=CC=CO1 XSWSGVCWMTYDHC-UHFFFAOYSA-N 0.000 claims description 3
- JAPZLARAKVKDGB-UHFFFAOYSA-N n-[3-cyano-4-(furan-2-ylmethylamino)quinolin-2-yl]thiophene-3-carboxamide Chemical compound C1=CSC=C1C(=O)NC(C=1C#N)=NC2=CC=CC=C2C=1NCC1=CC=CO1 JAPZLARAKVKDGB-UHFFFAOYSA-N 0.000 claims description 3
- RBVFCHLFDTUSCP-UHFFFAOYSA-N n-[3-cyano-4-(thiophen-2-ylmethylamino)quinolin-2-yl]thiophene-3-carboxamide Chemical compound C1=CSC=C1C(=O)NC(C=1C#N)=NC2=CC=CC=C2C=1NCC1=CC=CS1 RBVFCHLFDTUSCP-UHFFFAOYSA-N 0.000 claims description 3
- 125000003682 3-furyl group Chemical group O1C([H])=C([*])C([H])=C1[H] 0.000 claims description 2
- 230000001476 alcoholic effect Effects 0.000 claims description 2
- 230000008569 process Effects 0.000 claims description 2
- 229910001854 alkali hydroxide Inorganic materials 0.000 claims 1
- 150000008044 alkali metal hydroxides Chemical class 0.000 claims 1
- FTQWRYSLUYAIRQ-UHFFFAOYSA-N n-[(octadecanoylamino)methyl]octadecanamide Chemical compound CCCCCCCCCCCCCCCCCC(=O)NCNC(=O)CCCCCCCCCCCCCCCCC FTQWRYSLUYAIRQ-UHFFFAOYSA-N 0.000 claims 1
- JMMDWIFCQSCQSV-UHFFFAOYSA-N n-[4-(benzylamino)-3-cyanoquinolin-2-yl]thiophene-3-carboxamide Chemical compound C1=CSC=C1C(=O)NC(C=1C#N)=NC2=CC=CC=C2C=1NCC1=CC=CC=C1 JMMDWIFCQSCQSV-UHFFFAOYSA-N 0.000 claims 1
- 102000005962 receptors Human genes 0.000 description 27
- 108020003175 receptors Proteins 0.000 description 27
- 230000000694 effects Effects 0.000 description 22
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 12
- 230000027455 binding Effects 0.000 description 12
- 102000009346 Adenosine receptors Human genes 0.000 description 11
- 108050000203 Adenosine receptors Proteins 0.000 description 11
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 10
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 9
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 9
- 238000002844 melting Methods 0.000 description 9
- 230000008018 melting Effects 0.000 description 9
- 239000000243 solution Substances 0.000 description 9
- 238000012360 testing method Methods 0.000 description 9
- 239000000872 buffer Substances 0.000 description 8
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 7
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical group C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 7
- 239000007983 Tris buffer Substances 0.000 description 7
- 210000004027 cell Anatomy 0.000 description 7
- 239000012528 membrane Substances 0.000 description 7
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 6
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 6
- 230000005764 inhibitory process Effects 0.000 description 6
- 239000000543 intermediate Substances 0.000 description 6
- 239000000203 mixture Substances 0.000 description 6
- 239000002244 precipitate Substances 0.000 description 6
- 239000011541 reaction mixture Substances 0.000 description 6
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 6
- XMNBZTMWILHWPS-UHFFFAOYSA-N 2-amino-4-(benzylamino)quinoline-3-carbonitrile Chemical compound N#CC=1C(N)=NC2=CC=CC=C2C=1NCC1=CC=CC=C1 XMNBZTMWILHWPS-UHFFFAOYSA-N 0.000 description 5
- 238000006243 chemical reaction Methods 0.000 description 5
- 201000010099 disease Diseases 0.000 description 5
- LELCQSVSHYQGMI-UHFFFAOYSA-N 2-amino-4-(thiophen-2-ylmethylamino)quinoline-3-carbonitrile Chemical compound N#CC=1C(N)=NC2=CC=CC=C2C=1NCC1=CC=CS1 LELCQSVSHYQGMI-UHFFFAOYSA-N 0.000 description 4
- RRHVNLMYGHTZJC-UHFFFAOYSA-N 2-amino-4-chloroquinoline-3-carbonitrile Chemical compound C1=CC=C2C(Cl)=C(C#N)C(N)=NC2=C1 RRHVNLMYGHTZJC-UHFFFAOYSA-N 0.000 description 4
- 125000000175 2-thienyl group Chemical group S1C([*])=C([H])C([H])=C1[H] 0.000 description 4
- 101710169336 5'-deoxyadenosine deaminase Proteins 0.000 description 4
- 102000055025 Adenosine deaminases Human genes 0.000 description 4
- 229920002873 Polyethylenimine Polymers 0.000 description 4
- 239000005557 antagonist Substances 0.000 description 4
- WGQKYBSKWIADBV-UHFFFAOYSA-N benzylamine Chemical compound NCC1=CC=CC=C1 WGQKYBSKWIADBV-UHFFFAOYSA-N 0.000 description 4
- 210000003169 central nervous system Anatomy 0.000 description 4
- 239000002178 crystalline material Substances 0.000 description 4
- 238000001514 detection method Methods 0.000 description 4
- 239000003365 glass fiber Substances 0.000 description 4
- 230000009871 nonspecific binding Effects 0.000 description 4
- 239000008194 pharmaceutical composition Substances 0.000 description 4
- 230000000704 physical effect Effects 0.000 description 4
- 125000001424 substituent group Chemical group 0.000 description 4
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 3
- MCLFCZKVPRPRDS-UHFFFAOYSA-N 2-amino-4-(furan-2-ylmethylamino)quinoline-3-carbonitrile Chemical compound N#CC=1C(N)=NC2=CC=CC=C2C=1NCC1=CC=CO1 MCLFCZKVPRPRDS-UHFFFAOYSA-N 0.000 description 3
- 125000002941 2-furyl group Chemical group O1C([*])=C([H])C([H])=C1[H] 0.000 description 3
- 206010001052 Acute respiratory distress syndrome Diseases 0.000 description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical group CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 239000004480 active ingredient Substances 0.000 description 3
- 108060000200 adenylate cyclase Proteins 0.000 description 3
- 102000030621 adenylate cyclase Human genes 0.000 description 3
- 125000006615 aromatic heterocyclic group Chemical group 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 210000003630 histaminocyte Anatomy 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 238000002560 therapeutic procedure Methods 0.000 description 3
- 238000011282 treatment Methods 0.000 description 3
- RIRGCFBBHQEQQH-SSFGXONLSA-N (-)-n6-(2-phenylisopropyl)adenosine Chemical compound C([C@@H](C)NC=1C=2N=CN(C=2N=CN=1)[C@H]1[C@@H]([C@H](O)[C@@H](CO)O1)O)C1=CC=CC=C1 RIRGCFBBHQEQQH-SSFGXONLSA-N 0.000 description 2
- LOGOEBMHHXYBID-WBKNRDRNSA-N (2s,3s,4r,5r)-5-[6-[(4-amino-3-iodanylphenyl)methylamino]purin-9-yl]-3,4-dihydroxy-n-methyloxolane-2-carboxamide Chemical compound O[C@@H]1[C@H](O)[C@@H](C(=O)NC)O[C@H]1N1C2=NC=NC(NCC=3C=C([125I])C(N)=CC=3)=C2N=C1 LOGOEBMHHXYBID-WBKNRDRNSA-N 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- RUQIUASLAXJZIE-UHFFFAOYSA-N 3-methoxybenzoyl chloride Chemical compound COC1=CC=CC(C(Cl)=O)=C1 RUQIUASLAXJZIE-UHFFFAOYSA-N 0.000 description 2
- 125000004207 3-methoxyphenyl group Chemical group [H]C1=C([H])C(*)=C([H])C(OC([H])([H])[H])=C1[H] 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 2
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 2
- FFBDFADSZUINTG-UHFFFAOYSA-N DPCPX Chemical compound N1C=2C(=O)N(CCC)C(=O)N(CCC)C=2N=C1C1CCCC1 FFBDFADSZUINTG-UHFFFAOYSA-N 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- 208000010412 Glaucoma Diseases 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 206010020751 Hypersensitivity Diseases 0.000 description 2
- KYQCOXFCLRTKLS-UHFFFAOYSA-N Pyrazine Chemical compound C1=CN=CC=N1 KYQCOXFCLRTKLS-UHFFFAOYSA-N 0.000 description 2
- KAESVJOAVNADME-UHFFFAOYSA-N Pyrrole Chemical compound C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 230000010933 acylation Effects 0.000 description 2
- 238000005917 acylation reaction Methods 0.000 description 2
- MXMOTZIXVICDSD-UHFFFAOYSA-N anisoyl chloride Chemical compound COC1=CC=C(C(Cl)=O)C=C1 MXMOTZIXVICDSD-UHFFFAOYSA-N 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 230000033228 biological regulation Effects 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- DDRPCXLAQZKBJP-UHFFFAOYSA-N furfurylamine Chemical compound NCC1=CC=CO1 DDRPCXLAQZKBJP-UHFFFAOYSA-N 0.000 description 2
- 210000002216 heart Anatomy 0.000 description 2
- 239000005457 ice water Substances 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 108020004999 messenger RNA Proteins 0.000 description 2
- ZFBYATAXTNWUKQ-UHFFFAOYSA-N n-[4-(benzylamino)-3-cyanoquinolin-2-yl]thiophene-2-carboxamide Chemical compound C=1C=CSC=1C(=O)NC(C=1C#N)=NC2=CC=CC=C2C=1NCC1=CC=CC=C1 ZFBYATAXTNWUKQ-UHFFFAOYSA-N 0.000 description 2
- SQMWSBKSHWARHU-SDBHATRESA-N n6-cyclopentyladenosine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C2=NC=NC(NC3CCCC3)=C2N=C1 SQMWSBKSHWARHU-SDBHATRESA-N 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 2
- 230000035790 physiological processes and functions Effects 0.000 description 2
- 230000001681 protective effect Effects 0.000 description 2
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 230000000946 synaptic effect Effects 0.000 description 2
- 210000001550 testis Anatomy 0.000 description 2
- QTWBEVAYYDZLQL-UHFFFAOYSA-N thiophene-3-carbonyl chloride Chemical compound ClC(=O)C=1C=CSC=1 QTWBEVAYYDZLQL-UHFFFAOYSA-N 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- GCMNJUJAKQGROZ-UHFFFAOYSA-N 1,2-Dihydroquinolin-2-imine Chemical group C1=CC=CC2=NC(N)=CC=C21 GCMNJUJAKQGROZ-UHFFFAOYSA-N 0.000 description 1
- ZRSGZIMDIHBXIN-UHFFFAOYSA-N 1,3-benzodioxole-5-carbonyl chloride Chemical compound ClC(=O)C1=CC=C2OCOC2=C1 ZRSGZIMDIHBXIN-UHFFFAOYSA-N 0.000 description 1
- YNGDWRXWKFWCJY-UHFFFAOYSA-N 1,4-Dihydropyridine Chemical class C1C=CNC=C1 YNGDWRXWKFWCJY-UHFFFAOYSA-N 0.000 description 1
- QWENRTYMTSOGBR-UHFFFAOYSA-N 1H-1,2,3-Triazole Chemical compound C=1C=NNN=1 QWENRTYMTSOGBR-UHFFFAOYSA-N 0.000 description 1
- WOXFMYVTSLAQMO-UHFFFAOYSA-N 2-Pyridinemethanamine Chemical compound NCC1=CC=CC=N1 WOXFMYVTSLAQMO-UHFFFAOYSA-N 0.000 description 1
- USKBIWBLJHCVOP-UHFFFAOYSA-N 2-amino-4-oxo-1h-quinoline-3-carbonitrile Chemical compound C1=CC=C2C(O)=C(C#N)C(N)=NC2=C1 USKBIWBLJHCVOP-UHFFFAOYSA-N 0.000 description 1
- OFTKFKYVSBNYEC-UHFFFAOYSA-N 2-furoyl chloride Chemical compound ClC(=O)C1=CC=CO1 OFTKFKYVSBNYEC-UHFFFAOYSA-N 0.000 description 1
- YHOYYHYBFSYOSQ-UHFFFAOYSA-N 3-methylbenzoyl chloride Chemical compound CC1=CC=CC(C(Cl)=O)=C1 YHOYYHYBFSYOSQ-UHFFFAOYSA-N 0.000 description 1
- 125000001999 4-Methoxybenzoyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1OC([H])([H])[H])C(*)=O 0.000 description 1
- NSPMIYGKQJPBQR-UHFFFAOYSA-N 4H-1,2,4-triazole Chemical compound C=1N=CNN=1 NSPMIYGKQJPBQR-UHFFFAOYSA-N 0.000 description 1
- 208000026872 Addison Disease Diseases 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- 206010006458 Bronchitis chronic Diseases 0.000 description 1
- 206010006482 Bronchospasm Diseases 0.000 description 1
- PAOANWZGLPPROA-RQXXJAGISA-N CGS-21680 Chemical compound O[C@@H]1[C@H](O)[C@@H](C(=O)NCC)O[C@H]1N1C2=NC(NCCC=3C=CC(CCC(O)=O)=CC=3)=NC(N)=C2N=C1 PAOANWZGLPPROA-RQXXJAGISA-N 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 102000015554 Dopamine receptor Human genes 0.000 description 1
- 108050004812 Dopamine receptor Proteins 0.000 description 1
- 208000000059 Dyspnea Diseases 0.000 description 1
- 206010013975 Dyspnoeas Diseases 0.000 description 1
- 206010014561 Emphysema Diseases 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N Furan Chemical compound C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- 206010021143 Hypoxia Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 208000007201 Myocardial reperfusion injury Diseases 0.000 description 1
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- ZCQWOFVYLHDMMC-UHFFFAOYSA-N Oxazole Chemical compound C1=COC=N1 ZCQWOFVYLHDMMC-UHFFFAOYSA-N 0.000 description 1
- PCNDJXKNXGMECE-UHFFFAOYSA-N Phenazine Natural products C1=CC=CC2=NC3=CC=CC=C3N=C21 PCNDJXKNXGMECE-UHFFFAOYSA-N 0.000 description 1
- 102000011420 Phospholipase D Human genes 0.000 description 1
- 108090000553 Phospholipase D Proteins 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical compound C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 description 1
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 1
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical group N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 1
- 208000013616 Respiratory Distress Syndrome Diseases 0.000 description 1
- 206010039710 Scleroderma Diseases 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- 102000014384 Type C Phospholipases Human genes 0.000 description 1
- 108010079194 Type C Phospholipases Proteins 0.000 description 1
- 206010045240 Type I hypersensitivity Diseases 0.000 description 1
- FXTRYBYAYXLBQD-UHFFFAOYSA-N [1,3]thiazolo[5,4-b][1,8]naphthyridine Chemical compound C1=CN=C2N=C(SC=N3)C3=CC2=C1 FXTRYBYAYXLBQD-UHFFFAOYSA-N 0.000 description 1
- RJDMBYPRCCOTKM-MCDZGGTQSA-N [C@@H]1([C@H](O)[C@H](O)[C@@H](CO)O1)N1C=NC=2C(N)=NC=NC12.NC1=CC=C(CNC(=O)C)C=C1 Chemical compound [C@@H]1([C@H](O)[C@H](O)[C@@H](CO)O1)N1C=NC=2C(N)=NC=NC12.NC1=CC=C(CNC(=O)C)C=C1 RJDMBYPRCCOTKM-MCDZGGTQSA-N 0.000 description 1
- 229960000583 acetic acid Drugs 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 150000001263 acyl chlorides Chemical class 0.000 description 1
- 239000002580 adenosine A3 receptor antagonist Substances 0.000 description 1
- 230000007815 allergy Effects 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 230000008485 antagonism Effects 0.000 description 1
- 230000003288 anthiarrhythmic effect Effects 0.000 description 1
- 230000001088 anti-asthma Effects 0.000 description 1
- 230000001430 anti-depressive effect Effects 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 230000002253 anti-ischaemic effect Effects 0.000 description 1
- 230000000648 anti-parkinson Effects 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 239000003416 antiarrhythmic agent Substances 0.000 description 1
- 239000000924 antiasthmatic agent Substances 0.000 description 1
- 239000000935 antidepressant agent Substances 0.000 description 1
- 229940005513 antidepressants Drugs 0.000 description 1
- 239000000939 antiparkinson agent Substances 0.000 description 1
- 206010003119 arrhythmia Diseases 0.000 description 1
- 230000006793 arrhythmia Effects 0.000 description 1
- 206010003246 arthritis Diseases 0.000 description 1
- 208000006673 asthma Diseases 0.000 description 1
- PXXJHWLDUBFPOL-UHFFFAOYSA-N benzamidine Chemical compound NC(=N)C1=CC=CC=C1 PXXJHWLDUBFPOL-UHFFFAOYSA-N 0.000 description 1
- 230000000975 bioactive effect Effects 0.000 description 1
- 238000010170 biological method Methods 0.000 description 1
- 206010006451 bronchitis Diseases 0.000 description 1
- 230000007885 bronchoconstriction Effects 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 1
- 210000000748 cardiovascular system Anatomy 0.000 description 1
- 230000012292 cell migration Effects 0.000 description 1
- 208000007451 chronic bronchitis Diseases 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 208000025302 chronic primary adrenal insufficiency Diseases 0.000 description 1
- 230000002057 chronotropic effect Effects 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 229960000913 crospovidone Drugs 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 230000016396 cytokine production Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 229940088679 drug related substance Drugs 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 210000003979 eosinophil Anatomy 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-N ethanesulfonic acid Chemical class CCS(O)(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-N 0.000 description 1
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 1
- 229930003935 flavonoid Natural products 0.000 description 1
- 150000002215 flavonoids Chemical class 0.000 description 1
- 235000017173 flavonoids Nutrition 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- 239000012362 glacial acetic acid Substances 0.000 description 1
- 229940093915 gynecological organic acid Drugs 0.000 description 1
- 230000004217 heart function Effects 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 description 1
- 230000007954 hypoxia Effects 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 230000000297 inotrophic effect Effects 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 230000031891 intestinal absorption Effects 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 230000004410 intraocular pressure Effects 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 208000028867 ischemia Diseases 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- ZLTPDFXIESTBQG-UHFFFAOYSA-N isothiazole Chemical group C=1C=NSC=1 ZLTPDFXIESTBQG-UHFFFAOYSA-N 0.000 description 1
- CTAPFRYPJLPFDF-UHFFFAOYSA-N isoxazole Chemical compound C=1C=NOC=1 CTAPFRYPJLPFDF-UHFFFAOYSA-N 0.000 description 1
- 208000017169 kidney disease Diseases 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 238000002386 leaching Methods 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- XWFWXDCLZBTWKP-UHFFFAOYSA-N n-[4-(benzylamino)-3-cyanoquinolin-2-yl]-3-methyl-n-(3-methylbenzoyl)benzamide Chemical compound CC1=CC=CC(C(=O)N(C(=O)C=2C=C(C)C=CC=2)C=2C(=C(NCC=3C=CC=CC=3)C3=CC=CC=C3N=2)C#N)=C1 XWFWXDCLZBTWKP-UHFFFAOYSA-N 0.000 description 1
- 210000001577 neostriatum Anatomy 0.000 description 1
- 230000009251 neurologic dysfunction Effects 0.000 description 1
- 208000015015 neurological dysfunction Diseases 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 230000001777 nootropic effect Effects 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- 125000003395 phenylethylamino group Chemical group [H]N(*)C([H])([H])C([H])([H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 235000013809 polyvinylpolypyrrolidone Nutrition 0.000 description 1
- 229920000523 polyvinylpolypyrrolidone Polymers 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- PBMFSQRYOILNGV-UHFFFAOYSA-N pyridazine Chemical compound C1=CC=NN=C1 PBMFSQRYOILNGV-UHFFFAOYSA-N 0.000 description 1
- HDOUGSFASVGDCS-UHFFFAOYSA-N pyridin-3-ylmethanamine Chemical compound NCC1=CC=CN=C1 HDOUGSFASVGDCS-UHFFFAOYSA-N 0.000 description 1
- TXQWFIVRZNOPCK-UHFFFAOYSA-N pyridin-4-ylmethanamine Chemical compound NCC1=CC=NC=C1 TXQWFIVRZNOPCK-UHFFFAOYSA-N 0.000 description 1
- ATBIAJXSKNPHEI-UHFFFAOYSA-N pyridine-3-carbonyl chloride Chemical compound ClC(=O)C1=CC=CN=C1 ATBIAJXSKNPHEI-UHFFFAOYSA-N 0.000 description 1
- JWVCLYRUEFBMGU-UHFFFAOYSA-N quinazoline Chemical compound N1=CN=CC2=CC=CC=C21 JWVCLYRUEFBMGU-UHFFFAOYSA-N 0.000 description 1
- 239000002287 radioligand Substances 0.000 description 1
- 206010037844 rash Diseases 0.000 description 1
- 230000009711 regulatory function Effects 0.000 description 1
- 210000002345 respiratory system Anatomy 0.000 description 1
- 206010039083 rhinitis Diseases 0.000 description 1
- 238000013391 scatchard analysis Methods 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 210000000329 smooth muscle myocyte Anatomy 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000008247 solid mixture Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 231100001274 therapeutic index Toxicity 0.000 description 1
- 150000008634 thiazolopyrimidines Chemical class 0.000 description 1
- FKKJJPMGAWGYPN-UHFFFAOYSA-N thiophen-2-ylmethanamine Chemical compound NCC1=CC=CS1 FKKJJPMGAWGYPN-UHFFFAOYSA-N 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 231100000048 toxicity data Toxicity 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 108700012359 toxins Proteins 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- 230000002463 transducing effect Effects 0.000 description 1
- FFSJPOPLSWBGQY-UHFFFAOYSA-N triazol-4-one Chemical compound O=C1C=NN=N1 FFSJPOPLSWBGQY-UHFFFAOYSA-N 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 230000006442 vascular tone Effects 0.000 description 1
- 230000024883 vasodilation Effects 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D215/00—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems
- C07D215/02—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom
- C07D215/16—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D215/48—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen
- C07D215/54—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen attached in position 3
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/08—Bronchodilators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/06—Antiglaucoma agents or miotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/14—Decongestants or antiallergics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/78—Carbon atoms having three bonds to hetero atoms, with at the most one bond to halogen, e.g. ester or nitrile radicals
- C07D213/84—Nitriles
- C07D213/85—Nitriles in position 3
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D215/00—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems
- C07D215/02—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom
- C07D215/16—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D215/38—Nitrogen atoms
- C07D215/42—Nitrogen atoms attached in position 4
- C07D215/46—Nitrogen atoms attached in position 4 with hydrocarbon radicals, substituted by nitrogen atoms, attached to said nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D215/00—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems
- C07D215/02—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom
- C07D215/16—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D215/48—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing three or more hetero rings
Landscapes
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Pulmonology (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Neurology (AREA)
- Heart & Thoracic Surgery (AREA)
- Ophthalmology & Optometry (AREA)
- Diabetes (AREA)
- Cardiology (AREA)
- Urology & Nephrology (AREA)
- Pain & Pain Management (AREA)
- Immunology (AREA)
- Emergency Medicine (AREA)
- Rheumatology (AREA)
- Endocrinology (AREA)
- Psychiatry (AREA)
- Hematology (AREA)
- Vascular Medicine (AREA)
- Obesity (AREA)
- Psychology (AREA)
- Dermatology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Plural Heterocyclic Compounds (AREA)
- Quinoline Compounds (AREA)
Description
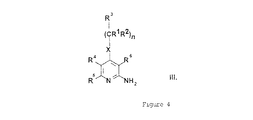
(式中、
R1は、水素原子または直鎖状もしくは分枝状C1−4アルキル基を表し、
R2は、水素原子または直鎖状もしくは分枝状C1−4アルキル基を表し、
R3は、水素原子または直鎖状もしくは分枝状C1−4アルキル基、あるいは1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、またはハロゲン原子により場合によっては置換されたフェニル基、チエニル基、またはフリル基を表し、あるいは1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、またはハロゲン原子により場合によっては置換された、1、2もしくは3個の窒素原子または1個の窒素原子と1個の酸素原子または1個の窒素原子と1個の硫黄原子を含む芳香族複素5員環または6員環を表し、
R4およびR5は、一緒になってメチレンジオキシ基、1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、ヒドロキシ基またはハロゲン原子により場合によっては置換された1,3−ブタジエニル基を形成し、
R6は、水素原子またはシアノ基、アミノカルボニル基、C1−4アルコキシカルボニル基、またはカルボキシ基を表し、
R7は、水素原子または直鎖状もしくは分枝状C1−4アルキル基、あるいはメチレンジオキシ基、1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、ヒドロキシ基、トリフルオロメチル基、シアノ基またはハロゲン原子により場合によっては置換されたフェニル基、ベンジル基、チエニル基またはフリル基を表し、あるいは1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、またはハロゲン原子により場合によっては置換された、1、2もしくは3個の窒素原子または1個の窒素原子と1個の酸素原子または1個の窒素原子と1個の硫黄原子を含む芳香族複素5員環または6員環を表し、
Xは、−CH2−基、−NH−基、−NR8−基(式中、R8は、直鎖状もしくは分枝状C1−4アルキル基またはC3−6シクロアルキル基を表す)、または硫黄原子または酸素原子またはスルホ基またはスルホキシ基を表し、
nは、0、1または2を表す。)およびその塩、溶媒和物、ならびに異性体およびその塩、溶媒和物が上記の基準を満たすことを発見した。
R1は、水素原子、または直鎖状もしくは分枝状C1−4アルキル基を表し、
R2は、水素原子、または直鎖状もしくは分枝状C1−4アルキル基を表し、
R3は、水素原子、または直鎖状もしくは分枝状C1−4アルキル基、あるいは1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、またはハロゲン原子により場合によっては置換されたフェニル基、チエニル基、またはフリル基を表し、あるいは1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、またはハロゲン原子により場合によっては置換された、1、2もしくは3個の窒素原子または1個の窒素原子と1個の酸素原子または1個の窒素原子と1個の硫黄原子を含む芳香族複素5員環または6員環を表し、
R9、R10、R11、およびR12はそれぞれ独立に、水素原子、または直鎖状もしくは分枝状C1−4アルキル基、または直鎖状もしくは分枝状C1−4アルコキシ基、またはヒドロキシ基またはハロゲン原子を意味し、あるいは
R9およびR12は水素原子を表し、R10およびR11は一緒になってメチレンジオキシ基を形成し、
R6は、水素原子、またはシアノ基、アミノカルボニル基、C1−4アルコキシカルボニル基、またはカルボキシ基を表し、
R7は、水素原子、または直鎖状もしくは分枝状C1−4アルキル基、あるいはメチレンジオキシ基、1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、ヒドロキシ基、トリフルオロメチル基、シアノ基またはハロゲン原子により場合によっては置換されたフェニル基、ベンジル基、チエニル基またはフリル基を表し、あるいは1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、またはハロゲン原子により場合によっては置換された、1、2もしくは3個の窒素原子または1個の窒素原子と1個の酸素原子または1個の窒素原子と1個の硫黄原子を含む芳香族複素5員環または6員環を表し、
Xは、−CH2−基、−NH−基、−NR8−基(式中、R8は、直鎖状もしくは分枝状C1−4アルキル基またはC3−6シクロアルキル基を表す)、または硫黄原子または酸素原子またはスルホ基またはスルホキシ基を表し、
nは、0、1または2を表す、
一般式(IA)の化合物、ならびにその塩、溶媒和物、光学活性な異性体ならびにその塩、溶媒和物によって形成される。
R1は、水素原子、またはメチル基を表し、
R2は、水素原子、またはメチル基を表し、
R3は、フェニルまたはチエニルまたはフリル基を表し、
R9、R10、R11、およびR12はそれぞれ独立に、水素原子、または直鎖状もしくは分枝状C1−4アルキル基、または直鎖状もしくは分枝状C1−4アルコキシ基、またはヒドロキシ基またはハロゲン原子を意味し、あるいは
R9およびR12は水素原子を表し、かつR10およびR11は一緒になってメチレンジオキシ基を形成し、
R6は、水素原子、またはシアノ基を表し、
R7は、4−メトキシフェニル、3−メチルフェニル、3−メトキシフェニル、3−チエニル、または3−フリル基を表し、
Xは、−NH−基または酸素原子を表し、また
nは1を表す
化合物、ならびにその塩、溶媒和物、異性体ならびにその塩、溶媒和物によって形成される。
3−メチル−N−(4−ベンジルアミノ−3−シアノキノリン−2−イル)ベンズアミド、
4−メトキシ−N−(4−ベンジルアミノ−3−シアノキノリン−2−イル)ベンズアミド、
3−メトキシ−N−(4−ベンジルアミノ−3−シアノキノリン−2−イル)ベンズアミド、
3,4−メチレンジオキシ−N−(4−ベンジルアミノ−3−シアノキノリン−2−イル)ベンズアミド、
N−(4−ベンジルアミノ−3−シアノキノリン−2−イル)チオフェン−2−カルボキサミド、
N−(4−[2−チエニルメチルアミノ]−3−シアノキノリン−2−イル)チオフェン−3−カルボキサミド、
4−メトキシ−N−(4−[2−チエニルメチルアミノ]−3−シアノキノリン−2−イル)ベンズアミド、
3,4−メチレンジオキシ−N−(4−[2−チエニルメチルアミノ]−3−シアノキノリン−2−イル)ベンズアミド、
N−(4−[2−フリルメチルアミノ]−3−シアノキノリン−2−イル)フラン−2−カルボキサミド、
N−(4−[2−フリルメチルアミノ]−3−シアノキノリン−2−イル)チオフェン−3−カルボキサミド、
ならびにその塩、溶媒和物、異性体ならびにその塩、溶媒和物が特に有利である。
3−メチル−N−(4−ベンジルアミノ−3−シアノキノリン−2−イル)ベンズアミド
一般式(I)において、R1およびR2は水素原子、R3はフェニル基を表し、R4およびR5は一緒になって1,3−ブタジエニル基を形成し、R6はシアノ基、R7は3−メチルフェニル基を表し、Xは−NH−基を意味し、nは1である。
2−アミノ−3−シアノ−4−ヒドロキシキノリン10gと塩化ホスホリル15mlとの混合物を攪拌しながら110℃で加熱した。この反応混合物を冷却し、氷水100mlに注ぎ、10%水酸化ナトリウム溶液60mlで中和した。得られた黄色の沈殿物をろ過し、水50mlで洗浄した。乾燥後に標題化合物7.5gが得られた。mp.:210℃。
2−アミノ−3−シアノ−4−クロロキノリン5gとベンジルアミン11mlを攪拌しながら130℃で加熱した。この反応混合物を水50mlに注ぎ、得られた沈殿物をろ過し、水50mlで洗浄した。黄白色の沈殿物をジメチルホルムアミドから再結晶させて、標題化合物5.2gを得た。
2−アミノ−3−シアノ−4−ベンジルアミノキノリン5gのピリジン30ml中溶液に、攪拌しながら0℃で3−メチルベンゾイルクロリド6mlを滴下した。この反応混合物を80℃で8時間攪拌し、次いで氷水150mlに注いだ。この沈殿物をろ過し、水40mlで2回洗浄した。得られた白色の結晶物質をエタノール200mlから再結晶させて、標題化合物9.2gを得た。mp.:234℃。
3−メチル−N−(3−メチルベンゾイル)−N−(4−ベンジルアミノ−3−シアノキノリン−2−イル)ベンズアミド5gのアセトニトリル80ml中溶液に1Nメタノール性水酸化カリウム溶液20mlを加えた。この反応混合物を3分間還流させ、次いで氷酢酸3mlを加え、次いで1M炭酸水素ナトリウム溶液50mlで中和し、得られた結晶をろ過した。白色の結晶物質をアセトニトリル130mlから再結晶させて、一般式(I)の標題化合物3.1gを得た。Mp.:230℃。
4−メトキシ−N−(4−ベンジルアミノ−3−シアノキノリン−2−イル)ベンズアミド
一般式(I)において、R1およびR2は水素原子、R3はフェニル基を意味し、R4およびR5は一緒になって1,3−ブタジエニル基を意味し、R6はシアノ基を意味し、R7は4−メトキシフェニル基を意味し、Xは−NH−基を意味し、nは1である。
3−メトキシ−N−(4−ベンジルアミノ−3−シアノキノリン−2−イル)ベンズアミド
一般式(I)において、R1およびR2は水素原子、R3はフェニル基を意味し、R4およびR5は一緒になって1,3−ブタジエニル基を意味し、R6はシアノ基を意味し、R7は3−メトキシフェニル基を意味し、Xは−NH−基を意味し、nは1である。
3,4−メチレンジオキシ−N−(4−ベンジルアミノ−3−シアノキノリン−2−イル)ベンズアミド
一般式(I)において、R1およびR2は水素原子、R3はフェニル基を意味し、R4およびR5は一緒になって1,3−ブタジエニル基を意味し、R6はシアノ基を意味し、R7は3,4−メチレンジオキシフェニル基を意味し、Xは−NH−基を意味し、nは1である。
N−(4−ベンジルアミノ−3−シアノキノリン−2−イル)チオフェン−2−カルボキサミド
一般式(I)において、R1およびR2は水素原子、R3はフェニル基を意味し、R4およびR5は一緒になって1,3−ブタジエニル基を意味し、R6はシアノ基を意味し、R7は2−チエニル基を意味し、Xは−NH−基を意味し、nは1である。
N−(4−[2−チエニルメチルアミノ]−3−シアノキノリン−2−イル)チオフェン−3−カルボキサミド
一般式(I)において、R1およびR2は水素原子、R3は2−チエニル基を意味し、R4およびR5は一緒になって1,3−ブタジエニル基を意味し、R6はシアノ基を意味し、R7は3−チエニル基を意味し、Xは−NH−基を意味し、nは1である。
実施例1に記載した通りに調製した2−アミノ−3−シアノ−4−クロロキノリン5gを、2−チエニルメチルアミン11mlと130℃で3時間攪拌した。この反応混合物を水50mlに注ぎ、得られた沈殿物をろ過し、水50mlで洗浄した。この黄白色の物質をエタノール25mlから再結晶させて、標題の化合物5.2gを得た。mp.:208℃。
4−メトキシ−N−(4−[2−チエニルメチルアミノ]−3−シアノキノリン−2−イル)ベンズアミド
一般式(I)において、R1およびR2は水素原子、R3は2−チエニル基を意味し、R4およびR5は一緒になって1,3−ブタジエニル基を意味し、R6はシアノ基を意味し、R7は4−メトキシフェニル基を意味し、Xは−NH−基を意味し、nは1である。
3,4−メチレンジオキシ−N−(4−[2−チエニルメチルアミノ]−3−シアノキノリン−2−イル)ベンズアミド
一般式(I)において、R1およびR2は水素原子、R3は2−チエニル基を意味し、R4およびR5は一緒になって1,3−ブタジエニル基を意味し、R6はシアノ基を意味し、R7は3,4−メチレンジオキシフェニル基を意味し、Xは−NH−基を意味し、nは1である。
N−(4−[2−フリルメチルアミノ]−3−シアノキノリン−2−イル)フラン−2−カルボキサミド
一般式(I)において、R1およびR2は水素原子、R3は2−フリル基を意味し、R4およびR5は一緒になって1,3−ブタジエニル基を意味し、R6はシアノ基を意味し、R7は2−フリル基を意味し、Xは−NH−基を意味し、nは1である。
実施例1に記載した通りに調製した2−アミノ−3−シアノ−4−クロロキノリン5gを、2−フリルメチルアミン(フルフリルアミン)1mlと130℃で3時間攪拌した。この反応混合物を水50mlに注ぎ、得られた沈殿物をろ過し、水50mlで洗浄した。この黄白色の物質をエタノール20mlから再結晶させて、標題の化合物4.8gを得た。mp.:208℃。
N−(4−[2−フリルメチルアミノ]−3−シアノキノリン−2−イル)チオフェン−3−カルボキサミド
一般式(I)において、R1およびR2は水素原子、R3は2−フリル基を意味し、R4およびR5は一緒になって1,3−ブタジエニル基を意味し、R6はシアノ基を意味し、R7は3−チエニル基を意味し、XはNH基を意味し、nは1である。
製薬業界で使用されている知られている方法によって、以下の組成物の錠剤を製造した。
ラクトース 50mg
アビセル 21mg
クロスポビドン 3mg
ステアリン酸マグネシウム 1mg
生物学
方法
ヒトアデノシンA3受容体との結合
膜懸濁液の調製:氷冷PBSで3回洗浄することによって、hA3受容体を発現するCHO細胞を回収し、1000xgで10分間遠心分離し、緩衝液(50mMトリス、10mM MgCl2、1mM EDTA、pH8.0)中で15秒間ホモジナイズし、43.000xgで10分間遠心分離し(Sigma 3K30)、膜調製物を上記の緩衝液中に懸濁させ、そのアリコートを−80℃で保管した。
ヒトアデノシンA1受容体との結合
膜懸濁液の調製:氷冷PBSで3回洗浄することによって、hA1受容体を発現するCHO細胞を回収し、1000xgで10分間遠心分離し、緩衝液(50mMトリス、pH7.4)中で15秒間ホモジナイズし、43.000xgで10分間遠心分離し(Sigma 3K30)、膜調製物を上記の緩衝液中に懸濁させ、そのアリコートを−80℃で保管した。
ヒトアデノシンA2a受容体との結合
結合プロトコル:非特異的な結合または全容積が100μLの試験化合物を特徴付けるために、膜(ヒトA2aアデノシン受容体を形質移入したHEK−293細胞、発売元:Receptor Biology、Inc.)7μg、緩衝液(50mMトリス−HCl、10mM MgCl2、1mM EDTA、2U/mLアデノシンデアミナーゼ、pH7.4)、20nM[3H]CGS−21680(2−[p−(2−カルボニルエチル)フェニルエチルアミノ]−5’−N−エチルカルボキサミド−アデノシン)(200.000dpm)および50μM NECA(5’−N−エチルカルボキサミド−アデノシン)を室温で90分間培養した。Whatman GF/Bガラス繊維フィルター(あらかじめ0.5%ポリエチレンイミンに浸漬しておく)でろ過し、96ウェルBrandel Cell Harvester上で氷冷50mMトリス、10mM MgCl2、1mM EDTA、0.9%NaCl(pH7.4)1mLで4回洗浄した。活性の検出:ベータカウンター(1450 Microbeta、Wallac)内の96ウェルプレート中でHiSafe−3カクテル存在下で行った。阻害[%]=100−((試験化合物が存在する場合の活性−非特異的な活性)/(全活性−非特異的な活性))×100
ヒトアデノシンA2b受容体との結合
結合プロトコル:非特異的な結合または全容積が100μLの試験化合物を特徴付けるために、膜(ヒトA2bアデノシン受容体を形質移入したHEK−293細胞、発売元:Receptor Biology、Inc.)20.8μg、緩衝液(50mMトリス−HCl、10mM MgCl2、1mM EDTA、0.1mMベンズアミジン、2U/mLアデノシンデアミナーゼ、pH6.5)、32.4nM[3H]DPCPX(8−シクロペンチル−1,3−ジプロピルキサンチン)(800.000dpm)および100μM NECA(5’−N−エチルカルボキサミド−アデノシン)を室温で30分間培養した。Whatman GF/Cガラス繊維フィルター(あらかじめ0.5%ポリエチレンイミンに浸漬しておく)でろ過し、96ウェルBrandel Cell Harvester上で氷50mMトリス−HCl(pH6.5)1mLで4回洗浄した。活性の検出:ベータカウンター(1450 Microbeta、Wallac)内の96ウェルプレート中でHiSafe−3カクテル存在下で行った。阻害[%]=100−((試験化合物が存在する場合の活性−非特異的な活性)/(全活性−非特異的な活性))×100
結果
本発明者らの実験条件において、1μMで80%より高い活性で放射リガンドとヒトアデノシンA3受容体との結合を阻害する場合、本発明者らは、この化合物を生物活性なものとして見なす。
Claims (7)
- 一般式(I)の化合物
R1は、水素原子、または直鎖状もしくは分枝状C1−4アルキル基を表し、
R2は、水素原子、または直鎖状もしくは分枝状C1−4アルキル基を表し、
R3は、水素原子、または直鎖状もしくは分枝状C1−4アルキル基、あるいは1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、またはハロゲン原子により場合によっては置換されたフェニル基、チエニル基、またはフリル基を表し、あるいは1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、またはハロゲン原子により場合によっては置換された、1、2もしくは3個の窒素原子または1個の窒素原子と1個の酸素原子または1個の窒素原子と1個の硫黄原子を含む芳香族複素5員環または6員環を表し、
R4およびR5は、一緒になってメチレンジオキシ基、1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、ヒドロキシ基またはハロゲン原子により場合によっては置換された1,3−ブタジエニル基を形成し、
R6は、水素原子、またはシアノ基、アミノカルボニル基、C1−4アルコキシカルボニル基、またはカルボキシ基を表し、
R7は、水素原子、または直鎖状もしくは分枝状C1−4アルキル基、あるいはメチレンジオキシ基、1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、ヒドロキシ基、トリフルオロメチル基、シアノ基またはハロゲン原子により場合によっては置換されたフェニル基、ベンジル基、チエニル基またはフリル基を表し、あるいは1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、またはハロゲン原子により場合によっては置換された、1、2もしくは3個の窒素原子または1個の窒素原子と1個の酸素原子または1個の窒素原子と1個の硫黄原子を含む芳香族複素5員環または6員環を表し、
Xは、−CH2−基、−NH−基、−NR8−基(式中、R8は、直鎖状もしくは分枝状C1−4アルキル基またはC3−6シクロアルキル基を表す)、または硫黄原子または酸素原子またはスルホ基またはスルホキシ基を表し、
nは、0、1または2を表す。)、
またはその塩もしくは溶媒和物。 -
R2は、水素原子、または直鎖状もしくは分枝状C1−4アルキル基を表し、
R3は、水素原子、または直鎖状もしくは分枝状C1−4アルキル基、あるいは1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、またはハロゲン原子により場合によっては置換されたフェニル基、チエニル基、またはフリル基を表し、あるいは1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、またはハロゲン原子により場合によっては置換された、1、2もしくは3個の窒素原子または1個の窒素原子と1個の酸素原子または1個の窒素原子と1個の硫黄原子を含む芳香族複素5員環または6員環を表し、
R9、R10、R11またはR12は互いにそれぞれ独立に、水素原子、または直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、ヒドロキシ基またはハロゲン原子を表し、あるいは
R9およびR12は水素原子を表し、R10およびR11は一緒になってメチレンジオキシ基を形成し、
R6は、水素原子、またはシアノ基、アミノカルボニル基、C1−4アルコキシカルボニル基、またはカルボキシ基を表し、
R7は、水素原子、または直鎖状もしくは分枝状C1−4アルキル基、あるいはメチレンジオキシ基、1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、ヒドロキシ基、トリフルオロメチル基、シアノ基またはハロゲン原子により場合によっては置換されたフェニル基、ベンジル基、チエニル基またはフリル基を表し、あるいは1種または複数の直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、またはハロゲン原子により場合によっては置換された、1、2もしくは3個の窒素原子または1個の窒素原子と1個の酸素原子または1個の窒素原子と1個の硫黄原子を含む芳香族複素5員環または6員環を表し、
Xは、−CH2−基、−NH−基、−NR8−基(式中、R8は、直鎖状もしくは分枝状C1−4アルキル基またはC3−6シクロアルキル基を表す)、または硫黄原子または酸素原子またはスルホ基またはスルホキシ基を表し、
nは、0、1または2を表す、
一般式(IA)の、請求項1に記載の化合物またはその塩もしくは溶媒和物。 - R1は、水素原子、またはメチル基を表し、
R2は、水素原子、またはメチル基を表し、
R3は、フェニル基、チエニル基またはフリル基を表し、
R9、R10、R11またはR12は互いにそれぞれ独立に、水素原子、または直鎖状もしくは分枝状C1−4アルキル基、直鎖状もしくは分枝状C1−4アルコキシ基、ヒドロキシ基またはハロゲン原子を表し、あるいは
R9およびR12は水素原子を表し、R10およびR11は一緒になってメチレンジオキシ基を形成し、
R6は、水素原子またはシアノ基を表し、
R7は、4−メトキシフェニル基、3−メチルフェニル基、3−チエニル基または3−フリル基を表し、
Xは、−NH−基または酸素原子を表し、また
nは1を表す、
一般式(IA)の、請求項2に記載の化合物またはその塩もしくは溶媒和物。 - 3−メチル−N−(4−ベンジルアミノ−3−シアノ−キノリン−2−イル)ベンズアミド、
4−メトキシ−N−(4−ベンジルアミノ−3−シアノ−キノリン−2−イル)ベンズアミド、
3−メトキシ−N−(4−ベンジルアミノ−3−シアノ−キノリン−2−イル)ベンズアミド、
3,4−メチレンジオキシ−N−(4−ベンジルアミノ−3−シアノ−キノリン−2−イル)ベンズアミド、
N−(4−ベンジルアミノ−3−シアノ−キノリン−2−イル)チオフェン−3−カルボキサミド、
N−(4−[2−チエニルメチルアミノ]−3−シアノ−キノリン−2−イル)チオフェン−3−カルボキサミド、
4−メトキシ−N−(4−[2−チエニルメチルアミノ]−3−シアノ−キノリン−2−イル)ベンズアミド、
3,4−メチレンジオキシ−N−(4−[2−チエニルメチルアミノ]−3−シアノ−キノリン−2−イル)ベンズアミド、
N−(4−[2−フリルメチルアミノ]−3−シアノ−キノリン−2−イル)フラン−2−カルボキサミド、
N−(4−[2−フリルメチルアミノ]−3−シアノ−キノリン−2−イル)チオフェン−3−カルボキサミド、
である請求項1から3のいずれかに記載の下記の化合物またはその塩もしくは溶媒和物。 - 一般式(II)のビス酸アミド
- アルコール性媒体中で、水酸化アルカリの存在下で選択的加水分解を行うことを特徴とする請求項5に記載の方法。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| HU0102279A HUP0102279A3 (en) | 2001-05-31 | 2001-05-31 | A3 antagonist amino-quinoline-derivatives, process for their preparation, pharmaceutical compositions containing them and their use |
| HU0200774A HUP0200774A2 (hu) | 2002-03-01 | 2002-03-01 | Amino-piridin és amino-kinolin származékok, eljárás előállításukra, ezeket tartalmazó gyógyszerkészítmények és intermedierjeik |
| PCT/HU2002/000048 WO2002096879A1 (en) | 2001-05-31 | 2002-05-29 | Aminoquinoline and aminopyridine derivatives and their use as adenosine a3 ligands |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2004536074A JP2004536074A (ja) | 2004-12-02 |
| JP2004536074A5 JP2004536074A5 (ja) | 2005-12-22 |
| JP4409935B2 true JP4409935B2 (ja) | 2010-02-03 |
Family
ID=47711452
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2003500059A Expired - Fee Related JP4409935B2 (ja) | 2001-05-31 | 2002-05-29 | アミノキノリンおよびアミノピリジン誘導体およびそのアデノシンa3リガンドとしての使用 |
Country Status (27)
| Country | Link |
|---|---|
| US (1) | US6969723B2 (ja) |
| EP (1) | EP1390349B1 (ja) |
| JP (1) | JP4409935B2 (ja) |
| KR (1) | KR100745307B1 (ja) |
| CN (1) | CN1257160C (ja) |
| AR (1) | AR036074A1 (ja) |
| AU (1) | AU2002304358B2 (ja) |
| BG (1) | BG108477A (ja) |
| BR (1) | BR0209719A (ja) |
| CA (1) | CA2448561C (ja) |
| CO (1) | CO5550457A2 (ja) |
| CZ (1) | CZ20033510A3 (ja) |
| EA (1) | EA006854B1 (ja) |
| EE (1) | EE05304B1 (ja) |
| HR (1) | HRP20031091B1 (ja) |
| IL (1) | IL158854A0 (ja) |
| IS (1) | IS2773B (ja) |
| MA (1) | MA26236A1 (ja) |
| MX (1) | MXPA03010870A (ja) |
| NO (1) | NO327710B1 (ja) |
| NZ (1) | NZ529966A (ja) |
| PL (1) | PL366516A1 (ja) |
| RS (1) | RS95003A (ja) |
| RU (1) | RU2278112C2 (ja) |
| SK (1) | SK14142003A3 (ja) |
| TN (1) | TNSN03117A1 (ja) |
| WO (1) | WO2002096879A1 (ja) |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| HUP0203976A3 (en) * | 2002-11-15 | 2004-08-30 | Sanofi Aventis | Adenozine a3 receptors, process for their preparation and pharmaceutical compositions containing them |
| EP1567497B1 (en) | 2002-12-06 | 2009-09-23 | Purdue Research Foundation | Pyridines for treating injured mammalian nerve tissue |
| US8729107B2 (en) * | 2002-12-06 | 2014-05-20 | Purdue Research Foundation | Pyridines for treating injured mammalian nerve tissue |
| KR20060065662A (ko) * | 2003-07-31 | 2006-06-14 | 사노피-아벤티스 | 아미노퀴놀린 유도체 및 아데노신 a3 리간드로서의 이의용도 |
| HUP0400812A2 (en) * | 2004-04-19 | 2006-02-28 | Sanofi Aventis | Crystalline forms of 2-amino-3-cyano-quinoline derivatives, process for their preparation and pharmaceutical compositions containing them |
| WO2006040645A1 (en) * | 2004-10-11 | 2006-04-20 | Ranbaxy Laboratories Limited | N-(3,5-dichloropyridin-4-yl)-2,4,5-alkoxy and 2,3,4-alkoxy benzamide derivatives as pde-iv (phophodiesterase type-iv) inhibitors for the treatment of inflammatory diseases such as asthma |
| HUP0402371A2 (en) * | 2004-11-15 | 2006-09-28 | Sanofi Aventis | Novel 125-i-labeled amino-quinoline derivatives |
| KR100788161B1 (ko) * | 2006-01-06 | 2007-12-21 | (주)아모레퍼시픽 | 벤즈이미다졸 아민 유도체 또는 아미노퀴놀린 유도체를 함유하는 피부 미백용 조성물 |
| HUP0700395A2 (en) * | 2007-06-07 | 2009-03-02 | Sanofi Aventis | Substituted [1,2,4] triazolo [1,5-a] quinolines, process for their preparation, pharmaceutical compositions thereof, and intermediates |
| KR101106050B1 (ko) * | 2009-03-25 | 2012-01-18 | 한국과학기술연구원 | 아미노퀴놀린 화합물, 이의 제조 방법 및 이를 함유하는 의약 조성물 |
| EP2611502A1 (en) * | 2010-09-01 | 2013-07-10 | Ambit Biosciences Corporation | Adenosine a3 receptor modulating compounds and methods of use thereof |
| CA2754237A1 (en) | 2011-05-27 | 2012-11-27 | The Regents Of The University Of California | Cyanoquinoline compounds having activity in correcting mutant-cftr processing and increasing ion transport and uses thereof |
| WO2012166654A1 (en) * | 2011-05-27 | 2012-12-06 | The Regents Of The University Of California | Cyanoquinoline compounds having activity in correcting mutant-cftr processing and increasing ion transport and uses thereof |
| ES2578363B1 (es) | 2015-01-22 | 2017-01-31 | Palobiofarma, S.L. | Moduladores de los receptores A3 de adenosina |
| UY36838A (es) | 2015-08-03 | 2017-01-31 | Bristol Myers Squibb Company Una Corporación Del Estado De Delaware | Compuestos heterocíclicos moduladores de la señalización de tnf alfa y composiciones farmacéuticas que los contienen |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE265143C (ja) | ||||
| JPH01180518A (ja) * | 1988-01-12 | 1989-07-18 | Nissan Chem Ind Ltd | 配向処理剤 |
| IL111266A (en) * | 1993-10-22 | 2002-03-10 | Zeneca Ltd | 2-HETEROARYL OR 2-ARYLPYRIDAZINO [4,5-b] QUINOLINE - 1, 10 - DIONES, THEIR PREPARATION AND PHARMACEUTICAL COMPOSITIONS CONTAINING THEM |
| AU765473B2 (en) * | 1999-04-23 | 2003-09-18 | Takeda Pharmaceutical Company Limited | 5-pyridyl-1,3-azole compounds, process for producing the same and use thereof |
-
2002
- 2002-05-29 RS YUP-950/03A patent/RS95003A/sr unknown
- 2002-05-29 MX MXPA03010870A patent/MXPA03010870A/es active IP Right Grant
- 2002-05-29 WO PCT/HU2002/000048 patent/WO2002096879A1/en active Application Filing
- 2002-05-29 EA EA200301317A patent/EA006854B1/ru not_active IP Right Cessation
- 2002-05-29 US US10/478,721 patent/US6969723B2/en not_active Expired - Fee Related
- 2002-05-29 IL IL15885402A patent/IL158854A0/xx not_active IP Right Cessation
- 2002-05-29 EE EEP200300574A patent/EE05304B1/xx not_active IP Right Cessation
- 2002-05-29 BR BR0209719-2A patent/BR0209719A/pt not_active IP Right Cessation
- 2002-05-29 CZ CZ20033510A patent/CZ20033510A3/cs unknown
- 2002-05-29 JP JP2003500059A patent/JP4409935B2/ja not_active Expired - Fee Related
- 2002-05-29 AU AU2002304358A patent/AU2002304358B2/en not_active Ceased
- 2002-05-29 PL PL02366516A patent/PL366516A1/xx not_active Application Discontinuation
- 2002-05-29 KR KR1020037015646A patent/KR100745307B1/ko not_active IP Right Cessation
- 2002-05-29 NZ NZ529966A patent/NZ529966A/en not_active IP Right Cessation
- 2002-05-29 RU RU2003137836/04A patent/RU2278112C2/ru not_active IP Right Cessation
- 2002-05-29 CA CA2448561A patent/CA2448561C/en not_active Expired - Fee Related
- 2002-05-29 EP EP02732985A patent/EP1390349B1/en not_active Expired - Lifetime
- 2002-05-29 CN CNB02810840XA patent/CN1257160C/zh not_active Expired - Fee Related
- 2002-05-29 SK SK1414-2003A patent/SK14142003A3/sk unknown
- 2002-05-31 AR ARP020102036A patent/AR036074A1/es unknown
-
2003
- 2003-07-08 TN TNPCT/HU2002/000048A patent/TNSN03117A1/en unknown
- 2003-11-10 MA MA27388A patent/MA26236A1/fr unknown
- 2003-11-26 IS IS7052A patent/IS2773B/is unknown
- 2003-11-28 NO NO20035300A patent/NO327710B1/no not_active IP Right Cessation
- 2003-12-05 CO CO03107462A patent/CO5550457A2/es not_active Application Discontinuation
- 2003-12-19 BG BG108477A patent/BG108477A/bg unknown
- 2003-12-29 HR HRP20031091AA patent/HRP20031091B1/xx not_active IP Right Cessation
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2011042653A (ja) | アデノシン受容体リガンドとして有用なトリアゾロ−キノリン誘導体 | |
| US7709489B2 (en) | Imidazoquinoline derivatives as adenosine A3 receptor ligands | |
| JP4409935B2 (ja) | アミノキノリンおよびアミノピリジン誘導体およびそのアデノシンa3リガンドとしての使用 | |
| JP5487100B2 (ja) | アデノシンa3受容体リガンドとしてのトリアゾロ[1,5−a]キノリン | |
| JP2007500689A (ja) | アミノキノリン誘導体およびアデノシンa3リガンドとしてのそれらの使用 | |
| AU2002304358A1 (en) | Aminoquinoline and aminopyridine derivatives and their use as adenosine A3 ligands | |
| JP4530663B2 (ja) | イミダゾキノリン誘導体 | |
| KR20070020335A (ko) | 아미노퀴놀린 유도체 및 아데노신 a3 리간드로서의 이의용도 | |
| TWI328581B (en) | New compounds | |
| ZA200308865B (en) | Aminoquinoline derivatives and their use as adenosine A3 ligands. |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20050111 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20050111 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20080812 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20081110 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20081117 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20081222 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20090127 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090408 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20090408 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20090514 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20090630 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090917 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20091027 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20091112 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20121120 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20121120 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20131120 Year of fee payment: 4 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |