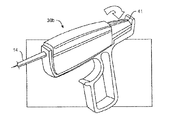
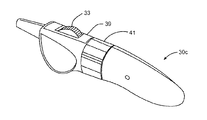
JP4271889B2 - 卵管内避妊のための配備作動システム - Google Patents
卵管内避妊のための配備作動システム Download PDFInfo
- Publication number
- JP4271889B2 JP4271889B2 JP2001517975A JP2001517975A JP4271889B2 JP 4271889 B2 JP4271889 B2 JP 4271889B2 JP 2001517975 A JP2001517975 A JP 2001517975A JP 2001517975 A JP2001517975 A JP 2001517975A JP 4271889 B2 JP4271889 B2 JP 4271889B2
- Authority
- JP
- Japan
- Prior art keywords
- contraceptive device
- sheath
- handle
- core shaft
- contraceptive
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000003433 contraceptive agent Substances 0.000 claims abstract description 254
- 230000002254 contraceptive effect Effects 0.000 claims abstract description 254
- 210000003101 oviduct Anatomy 0.000 claims abstract description 75
- 230000033001 locomotion Effects 0.000 claims description 39
- 241000973497 Siphonognathus argyrophanes Species 0.000 claims 1
- 238000000034 method Methods 0.000 abstract description 64
- 230000036541 health Effects 0.000 abstract description 7
- 210000001519 tissue Anatomy 0.000 description 25
- 210000003811 finger Anatomy 0.000 description 22
- 210000004291 uterus Anatomy 0.000 description 13
- 230000007246 mechanism Effects 0.000 description 10
- 229920000642 polymer Polymers 0.000 description 9
- 230000004044 response Effects 0.000 description 9
- 239000000835 fiber Substances 0.000 description 8
- 239000003550 marker Substances 0.000 description 8
- 210000003484 anatomy Anatomy 0.000 description 6
- 238000000576 coating method Methods 0.000 description 6
- 230000008878 coupling Effects 0.000 description 6
- 238000010168 coupling process Methods 0.000 description 6
- 238000005859 coupling reaction Methods 0.000 description 6
- 238000003384 imaging method Methods 0.000 description 6
- 229910052751 metal Inorganic materials 0.000 description 6
- 239000002184 metal Substances 0.000 description 6
- 238000002604 ultrasonography Methods 0.000 description 6
- 238000004873 anchoring Methods 0.000 description 5
- 238000013459 approach Methods 0.000 description 5
- 230000000994 depressogenic effect Effects 0.000 description 5
- 230000007774 longterm Effects 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 230000003287 optical effect Effects 0.000 description 5
- 230000035935 pregnancy Effects 0.000 description 5
- 229910001220 stainless steel Inorganic materials 0.000 description 5
- 210000003813 thumb Anatomy 0.000 description 5
- 238000012800 visualization Methods 0.000 description 5
- 239000000853 adhesive Substances 0.000 description 4
- 230000001070 adhesive effect Effects 0.000 description 4
- 239000011248 coating agent Substances 0.000 description 4
- 238000002594 fluoroscopy Methods 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- 230000001965 increasing effect Effects 0.000 description 4
- 230000036512 infertility Effects 0.000 description 4
- 208000000509 infertility Diseases 0.000 description 4
- 231100000535 infertility Toxicity 0.000 description 4
- 238000003780 insertion Methods 0.000 description 4
- 230000037431 insertion Effects 0.000 description 4
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 4
- 229920000728 polyester Polymers 0.000 description 4
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 4
- 239000004810 polytetrafluoroethylene Substances 0.000 description 4
- 239000010935 stainless steel Substances 0.000 description 4
- 208000002193 Pain Diseases 0.000 description 3
- 239000004642 Polyimide Substances 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 238000012790 confirmation Methods 0.000 description 3
- 230000004720 fertilization Effects 0.000 description 3
- 208000015181 infectious disease Diseases 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 229920001721 polyimide Polymers 0.000 description 3
- 229910001285 shape-memory alloy Inorganic materials 0.000 description 3
- 229910000679 solder Inorganic materials 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- DSUFPYCILZXJFF-UHFFFAOYSA-N 4-[[4-[[4-(pentoxycarbonylamino)cyclohexyl]methyl]cyclohexyl]carbamoyloxy]butyl n-[4-[[4-(butoxycarbonylamino)cyclohexyl]methyl]cyclohexyl]carbamate Chemical compound C1CC(NC(=O)OCCCCC)CCC1CC1CCC(NC(=O)OCCCCOC(=O)NC2CCC(CC3CCC(CC3)NC(=O)OCCCC)CC2)CC1 DSUFPYCILZXJFF-UHFFFAOYSA-N 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 2
- 206010016654 Fibrosis Diseases 0.000 description 2
- 229920006362 Teflon® Polymers 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000000740 bleeding effect Effects 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 230000000881 depressing effect Effects 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 230000009977 dual effect Effects 0.000 description 2
- 230000004761 fibrosis Effects 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 230000002458 infectious effect Effects 0.000 description 2
- 210000000754 myometrium Anatomy 0.000 description 2
- 210000002445 nipple Anatomy 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 210000003200 peritoneal cavity Anatomy 0.000 description 2
- 229910052697 platinum Inorganic materials 0.000 description 2
- -1 silk Polymers 0.000 description 2
- SQGYOTSLMSWVJD-UHFFFAOYSA-N silver(1+) nitrate Chemical compound [Ag+].[O-]N(=O)=O SQGYOTSLMSWVJD-UHFFFAOYSA-N 0.000 description 2
- 238000004804 winding Methods 0.000 description 2
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- 229920001651 Cyanoacrylate Polymers 0.000 description 1
- 229920004934 Dacron® Polymers 0.000 description 1
- 102000002322 Egg Proteins Human genes 0.000 description 1
- 108010000912 Egg Proteins Proteins 0.000 description 1
- 206010024769 Local reaction Diseases 0.000 description 1
- MWCLLHOVUTZFKS-UHFFFAOYSA-N Methyl cyanoacrylate Chemical compound COC(=O)C(=C)C#N MWCLLHOVUTZFKS-UHFFFAOYSA-N 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 208000000450 Pelvic Pain Diseases 0.000 description 1
- 229920002614 Polyether block amide Polymers 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000004809 Teflon Substances 0.000 description 1
- 239000012867 bioactive agent Substances 0.000 description 1
- 230000000975 bioactive effect Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 239000003518 caustics Substances 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 210000003679 cervix uteri Anatomy 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- 230000001427 coherent effect Effects 0.000 description 1
- 239000004020 conductor Substances 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000002059 diagnostic imaging Methods 0.000 description 1
- 230000003292 diminished effect Effects 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 239000013013 elastic material Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 230000003628 erosive effect Effects 0.000 description 1
- 230000035558 fertility Effects 0.000 description 1
- 230000005057 finger movement Effects 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 238000001794 hormone therapy Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 230000002262 irrigation Effects 0.000 description 1
- 238000003973 irrigation Methods 0.000 description 1
- 230000009191 jumping Effects 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 238000002595 magnetic resonance imaging Methods 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 230000013011 mating Effects 0.000 description 1
- 239000012567 medical material Substances 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 229910001000 nickel titanium Inorganic materials 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 239000013307 optical fiber Substances 0.000 description 1
- 210000004681 ovum Anatomy 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 239000005020 polyethylene terephthalate Substances 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 230000003014 reinforcing effect Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 231100000241 scar Toxicity 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 229910001961 silver nitrate Inorganic materials 0.000 description 1
- 210000000329 smooth muscle myocyte Anatomy 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 238000009810 tubal ligation Methods 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 238000007879 vasectomy Methods 0.000 description 1
- 238000009423 ventilation Methods 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 238000007794 visualization technique Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F6/00—Contraceptive devices; Pessaries; Applicators therefor
- A61F6/06—Contraceptive devices; Pessaries; Applicators therefor for use by females
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B1/00—Instruments for performing medical examinations of the interior of cavities or tubes of the body by visual or photographical inspection, e.g. endoscopes; Illuminating arrangements therefor
- A61B1/307—Instruments for performing medical examinations of the interior of cavities or tubes of the body by visual or photographical inspection, e.g. endoscopes; Illuminating arrangements therefor for the urinary organs, e.g. urethroscopes, cystoscopes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F6/00—Contraceptive devices; Pessaries; Applicators therefor
- A61F6/06—Contraceptive devices; Pessaries; Applicators therefor for use by females
- A61F6/14—Contraceptive devices; Pessaries; Applicators therefor for use by females intra-uterine type
- A61F6/18—Inserters or removers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F6/00—Contraceptive devices; Pessaries; Applicators therefor
- A61F6/20—Vas deferens occluders; Fallopian occluders
- A61F6/22—Vas deferens occluders; Fallopian occluders implantable in tubes
- A61F6/225—Vas deferens occluders; Fallopian occluders implantable in tubes transcervical
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/28—Surgical forceps
- A61B17/29—Forceps for use in minimally invasive surgery
- A61B17/2909—Handles
- A61B2017/2912—Handles transmission of forces to actuating rod or piston
- A61B2017/2923—Toothed members, e.g. rack and pinion
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/28—Surgical forceps
- A61B17/29—Forceps for use in minimally invasive surgery
- A61B2017/2926—Details of heads or jaws
- A61B2017/2927—Details of heads or jaws the angular position of the head being adjustable with respect to the shaft
- A61B2017/2929—Details of heads or jaws the angular position of the head being adjustable with respect to the shaft with a head rotatable about the longitudinal axis of the shaft
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/28—Surgical forceps
- A61B17/29—Forceps for use in minimally invasive surgery
- A61B2017/2926—Details of heads or jaws
- A61B2017/2932—Transmission of forces to jaw members
- A61B2017/2943—Toothed members, e.g. rack and pinion
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Reproductive Health (AREA)
- Vascular Medicine (AREA)
- Surgery (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medical Informatics (AREA)
- Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Pathology (AREA)
- Radiology & Medical Imaging (AREA)
- Urology & Nephrology (AREA)
- Biophysics (AREA)
- Molecular Biology (AREA)
- Orthopedics, Nursing, And Contraception (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Surgical Instruments (AREA)
- Error Detection And Correction (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Tents Or Canopies (AREA)
- Scissors And Nippers (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15023899P | 1999-08-23 | 1999-08-23 | |
| US60/150,238 | 1999-08-23 | ||
| PCT/US2000/023013 WO2001013833A1 (en) | 1999-08-23 | 2000-08-22 | Deployment actuation system for intrafallopian contraception |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008291545A Division JP2009056328A (ja) | 1999-08-23 | 2008-11-13 | 卵管内避妊のための配備作動システム |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2003507126A JP2003507126A (ja) | 2003-02-25 |
| JP2003507126A5 JP2003507126A5 (enExample) | 2007-10-11 |
| JP4271889B2 true JP4271889B2 (ja) | 2009-06-03 |
Family
ID=22533642
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2001517975A Expired - Fee Related JP4271889B2 (ja) | 1999-08-23 | 2000-08-22 | 卵管内避妊のための配備作動システム |
| JP2008291545A Pending JP2009056328A (ja) | 1999-08-23 | 2008-11-13 | 卵管内避妊のための配備作動システム |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008291545A Pending JP2009056328A (ja) | 1999-08-23 | 2008-11-13 | 卵管内避妊のための配備作動システム |
Country Status (9)
| Country | Link |
|---|---|
| EP (3) | EP1554999B1 (enExample) |
| JP (2) | JP4271889B2 (enExample) |
| CN (1) | CN100391421C (enExample) |
| AT (3) | ATE485020T1 (enExample) |
| AU (2) | AU781739B2 (enExample) |
| CA (3) | CA2381912C (enExample) |
| DE (3) | DE60045141D1 (enExample) |
| ES (3) | ES2355051T3 (enExample) |
| WO (2) | WO2001013833A1 (enExample) |
Families Citing this family (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7073504B2 (en) | 1996-12-18 | 2006-07-11 | Ams Research Corporation | Contraceptive system and method of use |
| ES2238759T3 (es) | 1997-06-05 | 2005-09-01 | Adiana, Inc. | Aparato para la oclusion de las trompas uterinas. |
| US8702727B1 (en) * | 1999-02-01 | 2014-04-22 | Hologic, Inc. | Delivery catheter with implant ejection mechanism |
| US6309384B1 (en) | 1999-02-01 | 2001-10-30 | Adiana, Inc. | Method and apparatus for tubal occlusion |
| ATE492251T1 (de) | 1999-08-23 | 2011-01-15 | Conceptus Inc | Einbringungs- und entfaltungskathetersystem für eileiterkontrazeptionsmittel |
| EP1499235B1 (en) * | 2002-04-17 | 2016-08-17 | Covidien LP | Endoscope structures and techniques for navigating to a target in branched structure |
| US7632291B2 (en) | 2003-06-13 | 2009-12-15 | Trivascular2, Inc. | Inflatable implant |
| US6905057B2 (en) * | 2003-09-29 | 2005-06-14 | Ethicon Endo-Surgery, Inc. | Surgical stapling instrument incorporating a firing mechanism having a linked rack transmission |
| AU2005209844B2 (en) | 2004-02-02 | 2010-07-08 | Bayer Essure, Inc. | Contraceptive with permeable and impermeable components |
| EP2559388B8 (en) * | 2004-04-28 | 2014-03-12 | Bayer Essure Inc. | Endoscopic delivery of medical devices |
| US8562628B2 (en) | 2006-04-03 | 2013-10-22 | Conceptus, Inc. | Linear motion delivery system for female sterilization device |
| PL2124831T3 (pl) | 2007-03-15 | 2017-03-31 | Ortho-Space Ltd. | Urządzenia protetyczne |
| US8231619B2 (en) | 2010-01-22 | 2012-07-31 | Cytyc Corporation | Sterilization device and method |
| US8550086B2 (en) | 2010-05-04 | 2013-10-08 | Hologic, Inc. | Radiopaque implant |
| AU2011329443B2 (en) * | 2010-11-17 | 2016-02-25 | Boston Scientific Scimed, Inc. | Stent delivery system |
| US9289307B2 (en) | 2011-10-18 | 2016-03-22 | Ortho-Space Ltd. | Prosthetic devices and methods for using same |
| US20150090271A1 (en) * | 2013-10-01 | 2015-04-02 | Julian Cruzada | Impact force dampening of spring release |
| US10959761B2 (en) | 2015-09-18 | 2021-03-30 | Ortho-Space Ltd. | Intramedullary fixated subacromial spacers |
| DE102015119639A1 (de) | 2015-11-13 | 2017-05-18 | Andrea Brohm-Schmitz-Rode | Uterotubar-Implantat-Vorrichtung |
| US11045981B2 (en) | 2017-01-30 | 2021-06-29 | Ortho-Space Ltd. | Processing machine and methods for processing dip-molded articles |
| CN114246725B (zh) * | 2021-12-16 | 2024-01-12 | 湖州市妇幼保健院 | 一种具有防黏连材料的宫内节育器 |
| CN116672139B (zh) * | 2023-07-07 | 2025-07-11 | 上海畅德医疗科技有限公司 | 输送装置 |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3042030A (en) * | 1958-11-25 | 1962-07-03 | Read Thane | Spherical type insert plug for body passageway and tool therefor |
| USRE29345E (en) * | 1973-02-26 | 1977-08-09 | The Franklin Institute | Method and apparatus for non-surgical, reversible sterilization of females |
| US3805767A (en) * | 1973-02-26 | 1974-04-23 | Erb Rene | Method and apparatus for non-surgical, reversible sterilization of females |
| US3858571A (en) | 1973-07-02 | 1975-01-07 | Arthur I Rudolph | Cornual plug |
| FR2409041A1 (fr) * | 1977-11-18 | 1979-06-15 | Wolf Gmbh Richard | Pince flexible pour saisir et mettre en place des obturateurs de trompes |
| US4246896A (en) * | 1978-10-30 | 1981-01-27 | Dynatech Corp. | Intracervical cuff (ICC) for contraception and prevention of venereal disease and applicator therefor |
| ES239677Y (es) * | 1978-11-23 | 1979-06-16 | Sopena Quesada Angel | Espermicida intrauterino |
| FR2641692A1 (fr) * | 1989-01-17 | 1990-07-20 | Nippon Zeon Co | Bouchon de fermeture d'une breche pour application medicale et dispositif pour bouchon de fermeture l'utilisant |
| US4932421A (en) * | 1989-01-23 | 1990-06-12 | Steven Kaali | Electrified intrauterine device |
| US5108366A (en) * | 1990-09-28 | 1992-04-28 | Ovamed Corporation | Delivery catheter |
| US5389100A (en) * | 1991-11-06 | 1995-02-14 | Imagyn Medical, Inc. | Controller for manipulation of instruments within a catheter |
| US5303719A (en) * | 1992-08-14 | 1994-04-19 | Wilk Peter J | Surgical method and associated instrument assembly |
| PT702944E (pt) * | 1994-09-26 | 2000-06-30 | Hugo Cimber Dr Med | Pressario oclusivo |
| US5630797A (en) * | 1995-01-17 | 1997-05-20 | Imagyn Medical, Inc. | Everting catheter system and method of utilizing the same |
| US6705323B1 (en) * | 1995-06-07 | 2004-03-16 | Conceptus, Inc. | Contraceptive transcervical fallopian tube occlusion devices and methods |
| US6176240B1 (en) * | 1995-06-07 | 2001-01-23 | Conceptus, Inc. | Contraceptive transcervical fallopian tube occlusion devices and their delivery |
| ES2348746T3 (es) * | 1995-06-07 | 2010-12-13 | Conceptus, Inc. | Dispositivos anticonceptivos transcervicales expandibles de oclusion de la trompa de falopio que tiene fijacion mecanica a la trompa de falopio y sistema de suministro. |
| AU3583397A (en) * | 1996-06-27 | 1998-01-14 | Hank H. Chen | Transcervical electroocclusive sterilization device |
| US5935137A (en) * | 1997-07-18 | 1999-08-10 | Gynecare, Inc. | Tubular fallopian sterilization device |
| CN2336770Y (zh) * | 1998-06-22 | 1999-09-08 | 王金声 | 输卵管节育器放置器 |
-
2000
- 2000-08-22 AT AT08100783T patent/ATE485020T1/de not_active IP Right Cessation
- 2000-08-22 CA CA2381912A patent/CA2381912C/en not_active Expired - Fee Related
- 2000-08-22 EP EP05006090A patent/EP1554999B1/en not_active Expired - Lifetime
- 2000-08-22 DE DE60045141T patent/DE60045141D1/de not_active Expired - Lifetime
- 2000-08-22 ES ES08100783T patent/ES2355051T3/es not_active Expired - Lifetime
- 2000-08-22 WO PCT/US2000/023013 patent/WO2001013833A1/en not_active Ceased
- 2000-08-22 EP EP00957665A patent/EP1212019B1/en not_active Expired - Lifetime
- 2000-08-22 ES ES00957665T patent/ES2241643T3/es not_active Expired - Lifetime
- 2000-08-22 JP JP2001517975A patent/JP4271889B2/ja not_active Expired - Fee Related
- 2000-08-22 WO PCT/US2000/023038 patent/WO2001013834A1/en not_active Ceased
- 2000-08-22 EP EP08100783A patent/EP1920741B1/en not_active Expired - Lifetime
- 2000-08-22 DE DE60037917T patent/DE60037917T2/de not_active Expired - Lifetime
- 2000-08-22 AU AU69251/00A patent/AU781739B2/en not_active Ceased
- 2000-08-22 CA CA2689688A patent/CA2689688C/en not_active Expired - Fee Related
- 2000-08-22 AT AT05006090T patent/ATE384508T1/de not_active IP Right Cessation
- 2000-08-22 AU AU70653/00A patent/AU7065300A/en not_active Withdrawn
- 2000-08-22 CN CNB008119678A patent/CN100391421C/zh not_active Expired - Fee Related
- 2000-08-22 AT AT00957665T patent/ATE297172T1/de not_active IP Right Cessation
- 2000-08-22 DE DE60020719T patent/DE60020719T2/de not_active Expired - Lifetime
- 2000-08-22 CA CA2772757A patent/CA2772757C/en not_active Expired - Fee Related
- 2000-08-22 ES ES05006090T patent/ES2300891T3/es not_active Expired - Lifetime
-
2008
- 2008-11-13 JP JP2008291545A patent/JP2009056328A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| EP1920741A1 (en) | 2008-05-14 |
| WO2001013834A1 (en) | 2001-03-01 |
| EP1920741B1 (en) | 2010-10-20 |
| AU781739B2 (en) | 2005-06-09 |
| CA2381912A1 (en) | 2001-03-01 |
| AU7065300A (en) | 2001-03-19 |
| ATE485020T1 (de) | 2010-11-15 |
| EP1212019B1 (en) | 2005-06-08 |
| CA2772757A1 (en) | 2001-03-01 |
| CN100391421C (zh) | 2008-06-04 |
| ATE297172T1 (de) | 2005-06-15 |
| ES2241643T3 (es) | 2005-11-01 |
| DE60020719T2 (de) | 2006-03-23 |
| DE60045141D1 (de) | 2010-12-02 |
| ES2300891T3 (es) | 2008-06-16 |
| EP1554999B1 (en) | 2008-01-23 |
| EP1212019A1 (en) | 2002-06-12 |
| WO2001013833A1 (en) | 2001-03-01 |
| DE60037917T2 (de) | 2009-01-08 |
| EP1554999A1 (en) | 2005-07-20 |
| DE60037917D1 (de) | 2008-03-13 |
| CA2772757C (en) | 2014-10-14 |
| JP2009056328A (ja) | 2009-03-19 |
| HK1081094A1 (en) | 2006-05-12 |
| JP2003507126A (ja) | 2003-02-25 |
| CA2689688C (en) | 2012-05-22 |
| CN1371264A (zh) | 2002-09-25 |
| EP1212019A4 (en) | 2003-08-27 |
| ES2355051T3 (es) | 2011-03-22 |
| ATE384508T1 (de) | 2008-02-15 |
| AU6925100A (en) | 2001-03-19 |
| CA2689688A1 (en) | 2001-03-01 |
| CA2381912C (en) | 2010-03-30 |
| DE60020719D1 (de) | 2005-07-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4271889B2 (ja) | 卵管内避妊のための配備作動システム | |
| US6709667B1 (en) | Deployment actuation system for intrafallopian contraception | |
| JP4683804B2 (ja) | 卵管内避妊のための挿入及び配備カテーテルシステム | |
| HK1081094B (en) | Deployment actuation system for intrafallopian contraception |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20070822 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20070822 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20071210 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20080310 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20080317 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080610 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20080714 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20081113 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20081225 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20090113 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20090209 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20090226 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120306 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4271889 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120306 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130306 Year of fee payment: 4 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130306 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140306 Year of fee payment: 5 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| S533 | Written request for registration of change of name |
Free format text: JAPANESE INTERMEDIATE CODE: R313533 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |