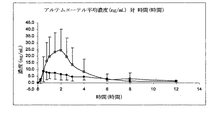
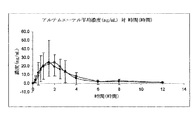
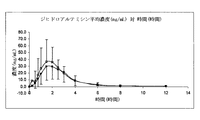
JP2012524771A - 中性油を含む舌下用の医薬組成物 - Google Patents
中性油を含む舌下用の医薬組成物 Download PDFInfo
- Publication number
- JP2012524771A JP2012524771A JP2012506583A JP2012506583A JP2012524771A JP 2012524771 A JP2012524771 A JP 2012524771A JP 2012506583 A JP2012506583 A JP 2012506583A JP 2012506583 A JP2012506583 A JP 2012506583A JP 2012524771 A JP2012524771 A JP 2012524771A
- Authority
- JP
- Japan
- Prior art keywords
- oil
- eur
- drug
- miglyol
- composition according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 230000007935 neutral effect Effects 0.000 title claims abstract description 15
- 239000008194 pharmaceutical composition Substances 0.000 title claims abstract description 14
- 239000000203 mixture Substances 0.000 claims abstract description 76
- 229940079593 drug Drugs 0.000 claims abstract description 63
- 239000003814 drug Substances 0.000 claims abstract description 63
- SNIOPGDIGTZGOP-UHFFFAOYSA-N Nitroglycerin Chemical compound [O-][N+](=O)OCC(O[N+]([O-])=O)CO[N+]([O-])=O SNIOPGDIGTZGOP-UHFFFAOYSA-N 0.000 claims abstract description 3
- 239000000006 Nitroglycerin Substances 0.000 claims abstract description 3
- 229960003711 glyceryl trinitrate Drugs 0.000 claims abstract description 3
- 229960000981 artemether Drugs 0.000 claims description 62
- SXYIRMFQILZOAM-HVNFFKDJSA-N dihydroartemisinin methyl ether Chemical compound C1C[C@H]2[C@H](C)CC[C@H]3[C@@H](C)[C@@H](OC)O[C@H]4[C@]32OO[C@@]1(C)O4 SXYIRMFQILZOAM-HVNFFKDJSA-N 0.000 claims description 62
- 239000007921 spray Substances 0.000 claims description 44
- 239000003921 oil Substances 0.000 claims description 34
- 235000019198 oils Nutrition 0.000 claims description 34
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 18
- 239000002253 acid Substances 0.000 claims description 18
- 150000003626 triacylglycerols Chemical class 0.000 claims description 14
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 10
- 239000000341 volatile oil Substances 0.000 claims description 10
- 229960003511 macrogol Drugs 0.000 claims description 8
- 239000004094 surface-active agent Substances 0.000 claims description 8
- 229960004191 artemisinin Drugs 0.000 claims description 7
- BLUAFEHZUWYNDE-NNWCWBAJSA-N artemisinin Chemical compound C([C@](OO1)(C)O2)C[C@H]3[C@H](C)CC[C@@H]4[C@@]31[C@@H]2OC(=O)[C@@H]4C BLUAFEHZUWYNDE-NNWCWBAJSA-N 0.000 claims description 7
- 229930101531 artemisinin Natural products 0.000 claims description 7
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 claims description 6
- 229940057917 medium chain triglycerides Drugs 0.000 claims description 6
- 239000003240 coconut oil Substances 0.000 claims description 5
- 235000019864 coconut oil Nutrition 0.000 claims description 5
- 229960002428 fentanyl Drugs 0.000 claims description 5
- 235000021323 fish oil Nutrition 0.000 claims description 5
- 125000005456 glyceride group Chemical group 0.000 claims description 5
- 239000003961 penetration enhancing agent Substances 0.000 claims description 5
- RZRNAYUHWVFMIP-KTKRTIGZSA-N 1-oleoylglycerol Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(O)CO RZRNAYUHWVFMIP-KTKRTIGZSA-N 0.000 claims description 4
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 claims description 4
- 239000003963 antioxidant agent Substances 0.000 claims description 4
- 230000003078 antioxidant effect Effects 0.000 claims description 4
- OGBUMNBNEWYMNJ-UHFFFAOYSA-N batilol Chemical class CCCCCCCCCCCCCCCCCCOCC(O)CO OGBUMNBNEWYMNJ-UHFFFAOYSA-N 0.000 claims description 4
- 235000012716 cod liver oil Nutrition 0.000 claims description 4
- 239000003026 cod liver oil Substances 0.000 claims description 4
- 239000006184 cosolvent Substances 0.000 claims description 4
- GPLRAVKSCUXZTP-UHFFFAOYSA-N diglycerol Chemical compound OCC(O)COCC(O)CO GPLRAVKSCUXZTP-UHFFFAOYSA-N 0.000 claims description 4
- DTMGIJFHGGCSLO-FIAQIACWSA-N ethyl (4z,7z,10z,13z,16z,19z)-docosa-4,7,10,13,16,19-hexaenoate;ethyl (5z,8z,11z,14z,17z)-icosa-5,8,11,14,17-pentaenoate Chemical class CCOC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CC.CCOC(=O)CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CC DTMGIJFHGGCSLO-FIAQIACWSA-N 0.000 claims description 4
- 239000010685 fatty oil Substances 0.000 claims description 4
- 239000000796 flavoring agent Substances 0.000 claims description 4
- RZRNAYUHWVFMIP-HXUWFJFHSA-N glycerol monolinoleate Natural products CCCCCCCCC=CCCCCCCCC(=O)OC[C@H](O)CO RZRNAYUHWVFMIP-HXUWFJFHSA-N 0.000 claims description 4
- 239000010514 hydrogenated cottonseed oil Substances 0.000 claims description 4
- 239000003346 palm kernel oil Substances 0.000 claims description 4
- 235000019865 palm kernel oil Nutrition 0.000 claims description 4
- 150000003839 salts Chemical class 0.000 claims description 4
- UFTFJSFQGQCHQW-UHFFFAOYSA-N triformin Chemical compound O=COCC(OC=O)COC=O UFTFJSFQGQCHQW-UHFFFAOYSA-N 0.000 claims description 4
- 235000013311 vegetables Nutrition 0.000 claims description 4
- 229960002970 artemotil Drugs 0.000 claims description 3
- NLYNIRQVMRLPIQ-XQLAAWPRSA-N artemotil Chemical compound C1C[C@H]2[C@H](C)CC[C@H]3[C@@H](C)[C@@H](OCC)O[C@H]4[C@]32OO[C@@]1(C)O4 NLYNIRQVMRLPIQ-XQLAAWPRSA-N 0.000 claims description 3
- 235000013355 food flavoring agent Nutrition 0.000 claims description 3
- GGCSSNBKKAUURC-UHFFFAOYSA-N sufentanil Chemical compound C1CN(CCC=2SC=CC=2)CCC1(COC)N(C(=O)CC)C1=CC=CC=C1 GGCSSNBKKAUURC-UHFFFAOYSA-N 0.000 claims description 3
- 229960004739 sufentanil Drugs 0.000 claims description 3
- KRWBMXNBYQFMLZ-HOWIICQTSA-N (6e,10e,14e,18e,22e)-3,7,11,15,19,23,27-heptamethyloctacosa-6,10,14,18,22,26-hexaen-1-ol Chemical compound OCCC(C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CCC=C(C)C KRWBMXNBYQFMLZ-HOWIICQTSA-N 0.000 claims description 2
- 239000002202 Polyethylene glycol Substances 0.000 claims description 2
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 claims description 2
- FIHJKUPKCHIPAT-AHIGJZGOSA-N artesunate Chemical compound C([C@](OO1)(C)O2)C[C@H]3[C@H](C)CC[C@@H]4[C@@]31[C@@H]2O[C@@H](OC(=O)CCC(O)=O)[C@@H]4C FIHJKUPKCHIPAT-AHIGJZGOSA-N 0.000 claims description 2
- 229960004991 artesunate Drugs 0.000 claims description 2
- YDSDEBIZUNNPOB-UHFFFAOYSA-N carfentanil Chemical compound C1CN(CCC=2C=CC=CC=2)CCC1(C(=O)OC)N(C(=O)CC)C1=CC=CC=C1 YDSDEBIZUNNPOB-UHFFFAOYSA-N 0.000 claims description 2
- 229950004689 carfentanil Drugs 0.000 claims description 2
- 239000004359 castor oil Substances 0.000 claims description 2
- 235000019438 castor oil Nutrition 0.000 claims description 2
- 239000002285 corn oil Substances 0.000 claims description 2
- 235000005687 corn oil Nutrition 0.000 claims description 2
- PWEOPMBMTXREGV-UHFFFAOYSA-N decanoic acid;octanoic acid;propane-1,2-diol Chemical compound CC(O)CO.CCCCCCCC(O)=O.CCCCCCCC(O)=O.CCCCCCCCCC(O)=O.CCCCCCCCCC(O)=O PWEOPMBMTXREGV-UHFFFAOYSA-N 0.000 claims description 2
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 claims description 2
- 230000036961 partial effect Effects 0.000 claims description 2
- 229920001223 polyethylene glycol Polymers 0.000 claims description 2
- 239000003755 preservative agent Substances 0.000 claims description 2
- 230000002335 preservative effect Effects 0.000 claims description 2
- FYPMFJGVHOHGLL-UHFFFAOYSA-N probucol Chemical compound C=1C(C(C)(C)C)=C(O)C(C(C)(C)C)=CC=1SC(C)(C)SC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 FYPMFJGVHOHGLL-UHFFFAOYSA-N 0.000 claims description 2
- 229960003912 probucol Drugs 0.000 claims description 2
- 239000010464 refined olive oil Substances 0.000 claims description 2
- 229960001967 tacrolimus Drugs 0.000 claims description 2
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 claims description 2
- PJMPHNIQZUBGLI-UHFFFAOYSA-N fentanyl Chemical compound C=1C=CC=CC=1N(C(=O)CC)C(CC1)CCN1CCC1=CC=CC=C1 PJMPHNIQZUBGLI-UHFFFAOYSA-N 0.000 claims 2
- IMYHGORQCPYVBZ-NLFFAJNJSA-N lofentanil Chemical compound CCC(=O)N([C@@]1([C@@H](CN(CCC=2C=CC=CC=2)CC1)C)C(=O)OC)C1=CC=CC=C1 IMYHGORQCPYVBZ-NLFFAJNJSA-N 0.000 claims 1
- 229950010274 lofentanil Drugs 0.000 claims 1
- 238000012377 drug delivery Methods 0.000 abstract description 13
- 238000009472 formulation Methods 0.000 description 19
- 239000003826 tablet Substances 0.000 description 19
- 238000012360 testing method Methods 0.000 description 19
- 239000000725 suspension Substances 0.000 description 16
- NOOLISFMXDJSKH-UTLUCORTSA-N (+)-Neomenthol Chemical compound CC(C)[C@@H]1CC[C@@H](C)C[C@@H]1O NOOLISFMXDJSKH-UTLUCORTSA-N 0.000 description 11
- NOOLISFMXDJSKH-UHFFFAOYSA-N DL-menthol Natural products CC(C)C1CCC(C)CC1O NOOLISFMXDJSKH-UHFFFAOYSA-N 0.000 description 11
- 238000010521 absorption reaction Methods 0.000 description 11
- MBMBGCFOFBJSGT-KUBAVDMBSA-N all-cis-docosa-4,7,10,13,16,19-hexaenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCC(O)=O MBMBGCFOFBJSGT-KUBAVDMBSA-N 0.000 description 11
- 238000010438 heat treatment Methods 0.000 description 11
- 229940041616 menthol Drugs 0.000 description 11
- 239000000243 solution Substances 0.000 description 11
- 150000007513 acids Chemical class 0.000 description 10
- VOVIALXJUBGFJZ-KWVAZRHASA-N Budesonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O VOVIALXJUBGFJZ-KWVAZRHASA-N 0.000 description 9
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 9
- 229960004436 budesonide Drugs 0.000 description 9
- 235000020673 eicosapentaenoic acid Nutrition 0.000 description 8
- 238000003760 magnetic stirring Methods 0.000 description 8
- 235000020669 docosahexaenoic acid Nutrition 0.000 description 7
- DKYWVDODHFEZIM-UHFFFAOYSA-N ketoprofen Chemical compound OC(=O)C(C)C1=CC=CC(C(=O)C=2C=CC=CC=2)=C1 DKYWVDODHFEZIM-UHFFFAOYSA-N 0.000 description 7
- 229960000991 ketoprofen Drugs 0.000 description 7
- 235000020660 omega-3 fatty acid Nutrition 0.000 description 7
- 230000036470 plasma concentration Effects 0.000 description 7
- 238000003756 stirring Methods 0.000 description 7
- DQCKKXVULJGBQN-UWFFTQNDSA-N (4r,4as,12bs)-3-(cyclopropylmethyl)-4a,9-dihydroxy-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinoline-7-one Chemical compound C([C@@]12[C@@]3(O)CCC(=O)C1OC=1C(O)=CC=C(C2=1)C[C@]31[H])CN1CC1CC1 DQCKKXVULJGBQN-UWFFTQNDSA-N 0.000 description 6
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 6
- 230000008901 benefit Effects 0.000 description 6
- 230000000694 effects Effects 0.000 description 6
- 210000001035 gastrointestinal tract Anatomy 0.000 description 6
- 229960003088 loratadine Drugs 0.000 description 6
- JCCNYMKQOSZNPW-UHFFFAOYSA-N loratadine Chemical compound C1CN(C(=O)OCC)CCC1=C1C2=NC=CC=C2CCC2=CC(Cl)=CC=C21 JCCNYMKQOSZNPW-UHFFFAOYSA-N 0.000 description 6
- 210000004877 mucosa Anatomy 0.000 description 6
- MVFGUOIZUNYYSO-UHFFFAOYSA-N prilocaine Chemical compound CCCNC(C)C(=O)NC1=CC=CC=C1C MVFGUOIZUNYYSO-UHFFFAOYSA-N 0.000 description 6
- BNRNXUUZRGQAQC-UHFFFAOYSA-N sildenafil Chemical compound CCCC1=NN(C)C(C(N2)=O)=C1N=C2C(C(=CC=1)OCC)=CC=1S(=O)(=O)N1CCN(C)CC1 BNRNXUUZRGQAQC-UHFFFAOYSA-N 0.000 description 6
- TVYLLZQTGLZFBW-ZBFHGGJFSA-N (R,R)-tramadol Chemical compound COC1=CC=CC([C@]2(O)[C@H](CCCC2)CN(C)C)=C1 TVYLLZQTGLZFBW-ZBFHGGJFSA-N 0.000 description 5
- LSQZJLSUYDQPKJ-NJBDSQKTSA-N amoxicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=C(O)C=C1 LSQZJLSUYDQPKJ-NJBDSQKTSA-N 0.000 description 5
- 229960003022 amoxicillin Drugs 0.000 description 5
- 229960002521 artenimol Drugs 0.000 description 5
- BJDCWCLMFKKGEE-ISOSDAIHSA-N artenimol Chemical compound C([C@](OO1)(C)O2)C[C@H]3[C@H](C)CC[C@@H]4[C@@]31[C@@H]2O[C@H](O)[C@@H]4C BJDCWCLMFKKGEE-ISOSDAIHSA-N 0.000 description 5
- 229930016266 dihydroartemisinin Natural products 0.000 description 5
- IVLVTNPOHDFFCJ-UHFFFAOYSA-N fentanyl citrate Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O.C=1C=CC=CC=1N(C(=O)CC)C(CC1)CCN1CCC1=CC=CC=C1 IVLVTNPOHDFFCJ-UHFFFAOYSA-N 0.000 description 5
- 238000010579 first pass effect Methods 0.000 description 5
- 210000004185 liver Anatomy 0.000 description 5
- LSQZJLSUYDQPKJ-UHFFFAOYSA-N p-Hydroxyampicillin Natural products O=C1N2C(C(O)=O)C(C)(C)SC2C1NC(=O)C(N)C1=CC=C(O)C=C1 LSQZJLSUYDQPKJ-UHFFFAOYSA-N 0.000 description 5
- 229960004380 tramadol Drugs 0.000 description 5
- SPCKHVPPRJWQRZ-UHFFFAOYSA-N 2-benzhydryloxy-n,n-dimethylethanamine;2-hydroxypropane-1,2,3-tricarboxylic acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O.C=1C=CC=CC=1C(OCCN(C)C)C1=CC=CC=C1 SPCKHVPPRJWQRZ-UHFFFAOYSA-N 0.000 description 4
- 230000009471 action Effects 0.000 description 4
- JAZBEHYOTPTENJ-JLNKQSITSA-N all-cis-5,8,11,14,17-icosapentaenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCCC(O)=O JAZBEHYOTPTENJ-JLNKQSITSA-N 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 238000006731 degradation reaction Methods 0.000 description 4
- 229960000520 diphenhydramine Drugs 0.000 description 4
- 229940090949 docosahexaenoic acid Drugs 0.000 description 4
- 229960005135 eicosapentaenoic acid Drugs 0.000 description 4
- NETZHAKZCGBWSS-CEDHKZHLSA-N nalbuphine Chemical compound C([C@]12[C@H]3OC=4C(O)=CC=C(C2=4)C[C@@H]2[C@]1(O)CC[C@@H]3O)CN2CC1CCC1 NETZHAKZCGBWSS-CEDHKZHLSA-N 0.000 description 4
- 229960000805 nalbuphine Drugs 0.000 description 4
- WWZKQHOCKIZLMA-UHFFFAOYSA-N octanoic acid Chemical compound CCCCCCCC(O)=O WWZKQHOCKIZLMA-UHFFFAOYSA-N 0.000 description 4
- 238000011282 treatment Methods 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- YJPIGAIKUZMOQA-UHFFFAOYSA-N Melatonin Natural products COC1=CC=C2N(C(C)=O)C=C(CCN)C2=C1 YJPIGAIKUZMOQA-UHFFFAOYSA-N 0.000 description 3
- FELGMEQIXOGIFQ-UHFFFAOYSA-N Ondansetron Chemical compound CC1=NC=CN1CC1C(=O)C(C=2C(=CC=CC=2)N2C)=C2CC1 FELGMEQIXOGIFQ-UHFFFAOYSA-N 0.000 description 3
- BNPSSFBOAGDEEL-UHFFFAOYSA-N albuterol sulfate Chemical compound OS(O)(=O)=O.CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1.CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1 BNPSSFBOAGDEEL-UHFFFAOYSA-N 0.000 description 3
- 150000001298 alcohols Chemical class 0.000 description 3
- DTOSIQBPPRVQHS-PDBXOOCHSA-N alpha-linolenic acid Chemical compound CC\C=C/C\C=C/C\C=C/CCCCCCCC(O)=O DTOSIQBPPRVQHS-PDBXOOCHSA-N 0.000 description 3
- 235000006708 antioxidants Nutrition 0.000 description 3
- RMRJXGBAOAMLHD-IHFGGWKQSA-N buprenorphine Chemical compound C([C@]12[C@H]3OC=4C(O)=CC=C(C2=4)C[C@@H]2[C@]11CC[C@]3([C@H](C1)[C@](C)(O)C(C)(C)C)OC)CN2CC1CC1 RMRJXGBAOAMLHD-IHFGGWKQSA-N 0.000 description 3
- 229960001736 buprenorphine Drugs 0.000 description 3
- 230000015556 catabolic process Effects 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- GHVNFZFCNZKVNT-UHFFFAOYSA-N decanoic acid Chemical compound CCCCCCCCCC(O)=O GHVNFZFCNZKVNT-UHFFFAOYSA-N 0.000 description 3
- 238000004090 dissolution Methods 0.000 description 3
- 230000000968 intestinal effect Effects 0.000 description 3
- OZWKMVRBQXNZKK-UHFFFAOYSA-N ketorolac Chemical compound OC(=O)C1CCN2C1=CC=C2C(=O)C1=CC=CC=C1 OZWKMVRBQXNZKK-UHFFFAOYSA-N 0.000 description 3
- 229960004752 ketorolac Drugs 0.000 description 3
- JTEGQNOMFQHVDC-NKWVEPMBSA-N lamivudine Chemical compound O=C1N=C(N)C=CN1[C@H]1O[C@@H](CO)SC1 JTEGQNOMFQHVDC-NKWVEPMBSA-N 0.000 description 3
- 229960001627 lamivudine Drugs 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- DRLFMBDRBRZALE-UHFFFAOYSA-N melatonin Chemical compound COC1=CC=C2NC=C(CCNC(C)=O)C2=C1 DRLFMBDRBRZALE-UHFFFAOYSA-N 0.000 description 3
- 229960003987 melatonin Drugs 0.000 description 3
- UZHSEJADLWPNLE-GRGSLBFTSA-N naloxone Chemical compound O=C([C@@H]1O2)CC[C@@]3(O)[C@H]4CC5=CC=C(O)C2=C5[C@@]13CCN4CC=C UZHSEJADLWPNLE-GRGSLBFTSA-N 0.000 description 3
- 229960004127 naloxone Drugs 0.000 description 3
- 229960005343 ondansetron Drugs 0.000 description 3
- 229940005483 opioid analgesics Drugs 0.000 description 3
- 239000007935 oral tablet Substances 0.000 description 3
- 229940096978 oral tablet Drugs 0.000 description 3
- 229960003310 sildenafil Drugs 0.000 description 3
- 229960002639 sildenafil citrate Drugs 0.000 description 3
- DEIYFTQMQPDXOT-UHFFFAOYSA-N sildenafil citrate Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O.CCCC1=NN(C)C(C(N2)=O)=C1N=C2C(C(=CC=1)OCC)=CC=1S(=O)(=O)N1CCN(C)CC1 DEIYFTQMQPDXOT-UHFFFAOYSA-N 0.000 description 3
- KFVSLSTULZVNPG-UHFFFAOYSA-N terbutaline sulfate Chemical compound [O-]S([O-])(=O)=O.CC(C)(C)[NH2+]CC(O)C1=CC(O)=CC(O)=C1.CC(C)(C)[NH2+]CC(O)C1=CC(O)=CC(O)=C1 KFVSLSTULZVNPG-UHFFFAOYSA-N 0.000 description 3
- 229960005105 terbutaline sulfate Drugs 0.000 description 3
- HBOMLICNUCNMMY-XLPZGREQSA-N zidovudine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](N=[N+]=[N-])C1 HBOMLICNUCNMMY-XLPZGREQSA-N 0.000 description 3
- 229960002555 zidovudine Drugs 0.000 description 3
- GVJHHUAWPYXKBD-IEOSBIPESA-N α-tocopherol Chemical compound OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-IEOSBIPESA-N 0.000 description 3
- OQOCQFSPEWCSDO-JLNKQSITSA-N 6Z,9Z,12Z,15Z,18Z-Heneicosapentaenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCCCC(O)=O OQOCQFSPEWCSDO-JLNKQSITSA-N 0.000 description 2
- 239000004322 Butylated hydroxytoluene Substances 0.000 description 2
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 description 2
- QFOHBWFCKVYLES-UHFFFAOYSA-N Butylparaben Chemical compound CCCCOC(=O)C1=CC=C(O)C=C1 QFOHBWFCKVYLES-UHFFFAOYSA-N 0.000 description 2
- 241001454694 Clupeiformes Species 0.000 description 2
- 244000060011 Cocos nucifera Species 0.000 description 2
- 235000013162 Cocos nucifera Nutrition 0.000 description 2
- 235000019501 Lemon oil Nutrition 0.000 description 2
- 206010028116 Mucosal inflammation Diseases 0.000 description 2
- 201000010927 Mucositis Diseases 0.000 description 2
- 235000019502 Orange oil Nutrition 0.000 description 2
- HXWJFEZDFPRLBG-UHFFFAOYSA-N Timnodonic acid Natural products CCCC=CC=CCC=CCC=CCC=CCCCC(O)=O HXWJFEZDFPRLBG-UHFFFAOYSA-N 0.000 description 2
- 208000005946 Xerostomia Diseases 0.000 description 2
- HQPCSDADVLFHHO-LTKCOYKYSA-N all-cis-8,11,14,17-icosatetraenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/CCCCCCC(O)=O HQPCSDADVLFHHO-LTKCOYKYSA-N 0.000 description 2
- 235000019513 anchovy Nutrition 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 2
- 229940095259 butylated hydroxytoluene Drugs 0.000 description 2
- OROGSEYTTFOCAN-DNJOTXNNSA-N codeine Chemical compound C([C@H]1[C@H](N(CC[C@@]112)C)C3)=C[C@H](O)[C@@H]1OC1=C2C3=CC=C1OC OROGSEYTTFOCAN-DNJOTXNNSA-N 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 238000001647 drug administration Methods 0.000 description 2
- 206010013781 dry mouth Diseases 0.000 description 2
- JAZBEHYOTPTENJ-UHFFFAOYSA-N eicosapentaenoic acid Natural products CCC=CCC=CCC=CCC=CCC=CCCCC(O)=O JAZBEHYOTPTENJ-UHFFFAOYSA-N 0.000 description 2
- 230000002440 hepatic effect Effects 0.000 description 2
- OROGSEYTTFOCAN-UHFFFAOYSA-N hydrocodone Natural products C1C(N(CCC234)C)C2C=CC(O)C3OC2=C4C1=CC=C2OC OROGSEYTTFOCAN-UHFFFAOYSA-N 0.000 description 2
- 238000010253 intravenous injection Methods 0.000 description 2
- 239000010501 lemon oil Substances 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- 238000000034 method Methods 0.000 description 2
- BQJCRHHNABKAKU-KBQPJGBKSA-N morphine Chemical compound O([C@H]1[C@H](C=C[C@H]23)O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O BQJCRHHNABKAKU-KBQPJGBKSA-N 0.000 description 2
- 229960002446 octanoic acid Drugs 0.000 description 2
- 229940126701 oral medication Drugs 0.000 description 2
- 239000010502 orange oil Substances 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 230000002035 prolonged effect Effects 0.000 description 2
- 239000003380 propellant Substances 0.000 description 2
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 150000004671 saturated fatty acids Chemical class 0.000 description 2
- 235000003441 saturated fatty acids Nutrition 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 230000001839 systemic circulation Effects 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- TVYLLZQTGLZFBW-GOEBONIOSA-N tramadol Natural products COC1=CC=CC([C@@]2(O)[C@@H](CCCC2)CN(C)C)=C1 TVYLLZQTGLZFBW-GOEBONIOSA-N 0.000 description 2
- 150000005691 triesters Chemical class 0.000 description 2
- SNICXCGAKADSCV-JTQLQIEISA-N (-)-Nicotine Chemical compound CN1CCC[C@H]1C1=CC=CN=C1 SNICXCGAKADSCV-JTQLQIEISA-N 0.000 description 1
- GYSCBCSGKXNZRH-UHFFFAOYSA-N 1-benzothiophene-2-carboxamide Chemical compound C1=CC=C2SC(C(=O)N)=CC2=C1 GYSCBCSGKXNZRH-UHFFFAOYSA-N 0.000 description 1
- SVUOLADPCWQTTE-UHFFFAOYSA-N 1h-1,2-benzodiazepine Chemical compound N1N=CC=CC2=CC=CC=C12 SVUOLADPCWQTTE-UHFFFAOYSA-N 0.000 description 1
- UETXPGADPCBQFT-UHFFFAOYSA-N 2,2-diphenyl-4-piperidin-1-ylbutanamide Chemical compound C=1C=CC=CC=1C(C=1C=CC=CC=1)(C(=O)N)CCN1CCCCC1 UETXPGADPCBQFT-UHFFFAOYSA-N 0.000 description 1
- USSIQXCVUWKGNF-UHFFFAOYSA-N 6-(dimethylamino)-4,4-diphenylheptan-3-one Chemical compound C=1C=CC=CC=1C(CC(C)N(C)C)(C(=O)CC)C1=CC=CC=C1 USSIQXCVUWKGNF-UHFFFAOYSA-N 0.000 description 1
- 239000004255 Butylated hydroxyanisole Substances 0.000 description 1
- 239000005632 Capric acid (CAS 334-48-5) Substances 0.000 description 1
- 239000005635 Caprylic acid (CAS 124-07-2) Substances 0.000 description 1
- 241000252203 Clupea harengus Species 0.000 description 1
- 241000270722 Crocodylidae Species 0.000 description 1
- 241000219104 Cucurbitaceae Species 0.000 description 1
- 240000003133 Elaeis guineensis Species 0.000 description 1
- 235000001950 Elaeis guineensis Nutrition 0.000 description 1
- JAQUASYNZVUNQP-USXIJHARSA-N Levorphanol Chemical compound C1C2=CC=C(O)C=C2[C@]23CCN(C)[C@H]1[C@@H]2CCCC3 JAQUASYNZVUNQP-USXIJHARSA-N 0.000 description 1
- 206010025476 Malabsorption Diseases 0.000 description 1
- 208000004155 Malabsorption Syndromes Diseases 0.000 description 1
- 244000246386 Mentha pulegium Species 0.000 description 1
- 235000016257 Mentha pulegium Nutrition 0.000 description 1
- 235000004357 Mentha x piperita Nutrition 0.000 description 1
- XADCESSVHJOZHK-UHFFFAOYSA-N Meperidine Chemical compound C=1C=CC=CC=1C1(C(=O)OCC)CCN(C)CC1 XADCESSVHJOZHK-UHFFFAOYSA-N 0.000 description 1
- BRUQQQPBMZOVGD-XFKAJCMBSA-N Oxycodone Chemical compound O=C([C@@H]1O2)CC[C@@]3(O)[C@H]4CC5=CC=C(OC)C2=C5[C@@]13CCN4C BRUQQQPBMZOVGD-XFKAJCMBSA-N 0.000 description 1
- UQCNKQCJZOAFTQ-ISWURRPUSA-N Oxymorphone Chemical compound O([C@H]1C(CC[C@]23O)=O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O UQCNKQCJZOAFTQ-ISWURRPUSA-N 0.000 description 1
- 240000004760 Pimpinella anisum Species 0.000 description 1
- 235000012550 Pimpinella anisum Nutrition 0.000 description 1
- 241000269821 Scombridae Species 0.000 description 1
- 244000290333 Vanilla fragrans Species 0.000 description 1
- 235000009499 Vanilla fragrans Nutrition 0.000 description 1
- 235000012036 Vanilla tahitensis Nutrition 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 230000001476 alcoholic effect Effects 0.000 description 1
- JIWBIWFOSCKQMA-LTKCOYKYSA-N all-cis-octadeca-6,9,12,15-tetraenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/CCCCC(O)=O JIWBIWFOSCKQMA-LTKCOYKYSA-N 0.000 description 1
- 229940087168 alpha tocopherol Drugs 0.000 description 1
- OBETXYAYXDNJHR-UHFFFAOYSA-N alpha-ethylcaproic acid Natural products CCCCC(CC)C(O)=O OBETXYAYXDNJHR-UHFFFAOYSA-N 0.000 description 1
- 235000020661 alpha-linolenic acid Nutrition 0.000 description 1
- 230000002421 anti-septic effect Effects 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 229940049706 benzodiazepine Drugs 0.000 description 1
- 239000003833 bile salt Substances 0.000 description 1
- 229940093761 bile salts Drugs 0.000 description 1
- 230000000975 bioactive effect Effects 0.000 description 1
- 235000021152 breakfast Nutrition 0.000 description 1
- IFKLAQQSCNILHL-QHAWAJNXSA-N butorphanol Chemical compound N1([C@@H]2CC3=CC=C(C=C3[C@@]3([C@]2(CCCC3)O)CC1)O)CC1CCC1 IFKLAQQSCNILHL-QHAWAJNXSA-N 0.000 description 1
- 229960001113 butorphanol Drugs 0.000 description 1
- 235000019282 butylated hydroxyanisole Nutrition 0.000 description 1
- CZBZUDVBLSSABA-UHFFFAOYSA-N butylated hydroxyanisole Chemical compound COC1=CC=C(O)C(C(C)(C)C)=C1.COC1=CC=C(O)C=C1C(C)(C)C CZBZUDVBLSSABA-UHFFFAOYSA-N 0.000 description 1
- 229940043253 butylated hydroxyanisole Drugs 0.000 description 1
- 229940067596 butylparaben Drugs 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 230000004087 circulation Effects 0.000 description 1
- 239000010634 clove oil Substances 0.000 description 1
- 229960004126 codeine Drugs 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- -1 containing triesters Chemical compound 0.000 description 1
- GVJHHUAWPYXKBD-UHFFFAOYSA-N d-alpha-tocopherol Natural products OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 229960004193 dextropropoxyphene Drugs 0.000 description 1
- XLMALTXPSGQGBX-GCJKJVERSA-N dextropropoxyphene Chemical compound C([C@](OC(=O)CC)([C@H](C)CN(C)C)C=1C=CC=CC=1)C1=CC=CC=C1 XLMALTXPSGQGBX-GCJKJVERSA-N 0.000 description 1
- 150000005690 diesters Chemical class 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 150000001226 dihydroartemisinin methyl ether derivatives Chemical class 0.000 description 1
- XYYVYLMBEZUESM-UHFFFAOYSA-N dihydrocodeine Natural products C1C(N(CCC234)C)C2C=CC(=O)C3OC2=C4C1=CC=C2OC XYYVYLMBEZUESM-UHFFFAOYSA-N 0.000 description 1
- 239000013583 drug formulation Substances 0.000 description 1
- 230000036267 drug metabolism Effects 0.000 description 1
- 239000002359 drug metabolite Substances 0.000 description 1
- 235000013399 edible fruits Nutrition 0.000 description 1
- 230000032050 esterification Effects 0.000 description 1
- 238000005886 esterification reaction Methods 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 235000020988 fatty fish Nutrition 0.000 description 1
- 229950010801 fenpipramide Drugs 0.000 description 1
- 229940013317 fish oils Drugs 0.000 description 1
- 235000019634 flavors Nutrition 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 235000019514 herring Nutrition 0.000 description 1
- 235000001050 hortel pimenta Nutrition 0.000 description 1
- LLPOLZWFYMWNKH-CMKMFDCUSA-N hydrocodone Chemical compound C([C@H]1[C@H](N(CC[C@@]112)C)C3)CC(=O)[C@@H]1OC1=C2C3=CC=C1OC LLPOLZWFYMWNKH-CMKMFDCUSA-N 0.000 description 1
- 229960000240 hydrocodone Drugs 0.000 description 1
- WVLOADHCBXTIJK-YNHQPCIGSA-N hydromorphone Chemical compound O([C@H]1C(CC[C@H]23)=O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O WVLOADHCBXTIJK-YNHQPCIGSA-N 0.000 description 1
- 229960001410 hydromorphone Drugs 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 230000031891 intestinal absorption Effects 0.000 description 1
- 229960003406 levorphanol Drugs 0.000 description 1
- 229960004194 lidocaine Drugs 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 229960004488 linolenic acid Drugs 0.000 description 1
- IMYHGORQCPYVBZ-UHFFFAOYSA-N lofentanyl Chemical group C1CN(CCC=2C=CC=CC=2)CC(C)C1(C(=O)OC)N(C(=O)CC)C1=CC=CC=C1 IMYHGORQCPYVBZ-UHFFFAOYSA-N 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 235000020640 mackerel Nutrition 0.000 description 1
- 230000005389 magnetism Effects 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 239000001525 mentha piperita l. herb oil Substances 0.000 description 1
- 239000001683 mentha spicata herb oil Substances 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 229960001797 methadone Drugs 0.000 description 1
- 229960005181 morphine Drugs 0.000 description 1
- DQCKKXVULJGBQN-XFWGSAIBSA-N naltrexone Chemical compound N1([C@@H]2CC3=CC=C(C=4O[C@@H]5[C@](C3=4)([C@]2(CCC5=O)O)CC1)O)CC1CC1 DQCKKXVULJGBQN-XFWGSAIBSA-N 0.000 description 1
- 229960003086 naltrexone Drugs 0.000 description 1
- 229960002715 nicotine Drugs 0.000 description 1
- SNICXCGAKADSCV-UHFFFAOYSA-N nicotine Natural products CN1CCCC1C1=CC=CN=C1 SNICXCGAKADSCV-UHFFFAOYSA-N 0.000 description 1
- 229960002085 oxycodone Drugs 0.000 description 1
- 229960005118 oxymorphone Drugs 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 239000013618 particulate matter Substances 0.000 description 1
- VOKSWYLNZZRQPF-GDIGMMSISA-N pentazocine Chemical compound C1C2=CC=C(O)C=C2[C@@]2(C)[C@@H](C)[C@@H]1N(CC=C(C)C)CC2 VOKSWYLNZZRQPF-GDIGMMSISA-N 0.000 description 1
- 229960005301 pentazocine Drugs 0.000 description 1
- 235000019477 peppermint oil Nutrition 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 229960000482 pethidine Drugs 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 210000004258 portal system Anatomy 0.000 description 1
- 229960001807 prilocaine Drugs 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 1
- 229960003415 propylparaben Drugs 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 230000001953 sensory effect Effects 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 235000019721 spearmint oil Nutrition 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 230000036962 time dependent Effects 0.000 description 1
- 229960000984 tocofersolan Drugs 0.000 description 1
- 235000010384 tocopherol Nutrition 0.000 description 1
- 229930003799 tocopherol Natural products 0.000 description 1
- 239000011732 tocopherol Substances 0.000 description 1
- 229960001295 tocopherol Drugs 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- LLPOLZWFYMWNKH-UHFFFAOYSA-N trans-dihydrocodeinone Natural products C1C(N(CCC234)C)C2CCC(=O)C3OC2=C4C1=CC=C2OC LLPOLZWFYMWNKH-UHFFFAOYSA-N 0.000 description 1
- 238000005809 transesterification reaction Methods 0.000 description 1
- 239000002076 α-tocopherol Substances 0.000 description 1
- 235000004835 α-tocopherol Nutrition 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
- A61K9/006—Oral mucosa, e.g. mucoadhesive forms, sublingual droplets; Buccal patches or films; Buccal sprays
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/12—Aerosols; Foams
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Epidemiology (AREA)
- Dispersion Chemistry (AREA)
- Nutrition Science (AREA)
- Physiology (AREA)
- Neurosurgery (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Pain & Pain Management (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/GB2009/050416 WO2010122276A1 (en) | 2009-04-23 | 2009-04-23 | Drug delivery |
| GB0906977.4 | 2009-04-23 | ||
| GB0906977A GB2469792A (en) | 2009-04-23 | 2009-04-23 | Oil-based pharmaceutical formulation for sublingual delivery |
| GBPCT/GB2009/050416 | 2009-04-23 | ||
| PCT/GB2010/050671 WO2010122355A1 (en) | 2009-04-23 | 2010-04-23 | Sublingual pharmaceutical composition comprising a neutral oil |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2012524771A true JP2012524771A (ja) | 2012-10-18 |
| JP2012524771A5 JP2012524771A5 (enExample) | 2013-05-09 |
Family
ID=42236683
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012506583A Pending JP2012524771A (ja) | 2009-04-23 | 2010-04-23 | 中性油を含む舌下用の医薬組成物 |
Country Status (15)
| Country | Link |
|---|---|
| US (1) | US20120058158A1 (enExample) |
| EP (1) | EP2421503A1 (enExample) |
| JP (1) | JP2012524771A (enExample) |
| CN (1) | CN102458358A (enExample) |
| AU (1) | AU2010240653A1 (enExample) |
| BR (1) | BRPI1013539A2 (enExample) |
| CA (1) | CA2756879A1 (enExample) |
| IL (1) | IL215454A (enExample) |
| MX (1) | MX2011010835A (enExample) |
| MY (1) | MY167918A (enExample) |
| NZ (1) | NZ595467A (enExample) |
| RU (1) | RU2011139638A (enExample) |
| SG (1) | SG175160A1 (enExample) |
| WO (1) | WO2010122355A1 (enExample) |
| ZA (1) | ZA201107089B (enExample) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2019519487A (ja) * | 2016-05-05 | 2019-07-11 | アクエスティブ セラピューティクス インコーポレイテッド | 送達増強エピネフリン組成物 |
| JP2020535162A (ja) * | 2017-09-27 | 2020-12-03 | アクエスティブ セラピューティクス インコーポレイテッド | 増強された送達のエピネフリン及びプロドラッグ組成物 |
| US11273131B2 (en) | 2016-05-05 | 2022-03-15 | Aquestive Therapeutics, Inc. | Pharmaceutical compositions with enhanced permeation |
| US12427121B2 (en) | 2016-05-05 | 2025-09-30 | Aquestive Therapeutics, Inc. | Enhanced delivery epinephrine compositions |
| US12433850B2 (en) | 2016-05-05 | 2025-10-07 | Aquestive Therapeutics, Inc. | Enhanced delivery epinephrine and prodrug compositions |
| US12465564B2 (en) | 2021-10-25 | 2025-11-11 | Aquestive Therapeutics, Inc. | Oral and nasal compositions and methods of treatment |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8852638B2 (en) | 2005-09-30 | 2014-10-07 | Durect Corporation | Sustained release small molecule drug formulation |
| US10512644B2 (en) | 2007-03-12 | 2019-12-24 | Inheris Pharmaceuticals, Inc. | Oligomer-opioid agonist conjugates |
| US8173666B2 (en) | 2007-03-12 | 2012-05-08 | Nektar Therapeutics | Oligomer-opioid agonist conjugates |
| EP2907524A1 (en) | 2007-05-25 | 2015-08-19 | RB Pharmaceuticals Limited | Sustained delivery formulations of risperidone compounds |
| GB0720967D0 (en) * | 2007-10-25 | 2007-12-05 | Protophama Ltd | Anti-material pharmaceutical composition |
| GB2513060B (en) | 2010-06-08 | 2015-01-07 | Rb Pharmaceuticals Ltd | Microparticle buprenorphine suspension |
| US9272044B2 (en) | 2010-06-08 | 2016-03-01 | Indivior Uk Limited | Injectable flowable composition buprenorphine |
| US20130254139A1 (en) * | 2012-03-21 | 2013-09-26 | Xiaoguang Lei | Systems and methods for building a universal intelligent assistant with learning capabilities |
| GB201404139D0 (en) | 2014-03-10 | 2014-04-23 | Rb Pharmaceuticals Ltd | Sustained release buprenorphine solution formulations |
| US10376586B2 (en) * | 2016-02-16 | 2019-08-13 | Entourage Bioscience, LLC | Method and compositions for solubilizing non-polar constituents |
| KR102442753B1 (ko) * | 2016-12-26 | 2022-09-16 | 셀릭스 바이오 프라이빗 리미티드 | 만성 통증 치료를 위한 화합물 |
| WO2020136620A1 (en) | 2018-12-29 | 2020-07-02 | 3M Innovative Properties Company | Oral articles and methods of use |
| PL4125843T3 (pl) | 2020-03-31 | 2025-09-22 | Remicine IP B.V. | Leczenie ciężkiego zespołu zapalnego |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63503304A (ja) * | 1986-03-10 | 1988-12-02 | ブルグハルト,クルト | 医薬製剤およびその製造方法 |
| JP2005512943A (ja) * | 2001-02-14 | 2005-05-12 | ジーダブリュー ファーマ リミテッド | 医薬製剤 |
| WO2009020666A1 (en) * | 2007-08-06 | 2009-02-12 | Insys Therapeutics Inc. | Oral cannabinoid liquid formulations and methods of treatment |
| JP2012524772A (ja) * | 2009-04-23 | 2012-10-18 | ロンドンファーマ リミテッド | ジヒドロアルテミシニンを含む舌下スプレー用製剤 |
| JP5438018B2 (ja) * | 2007-10-25 | 2014-03-12 | プロトファーマ リミテッド | 抗マラリア薬剤組成物 |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5955098A (en) * | 1996-04-12 | 1999-09-21 | Flemington Pharmaceutical Corp. | Buccal non polar spray or capsule |
| US20050281752A1 (en) * | 1997-10-01 | 2005-12-22 | Dugger Harry A Iii | Buccal, polar and non-polar spray or capsule containing drugs for treating disorders of the central nervous system |
| US8486972B2 (en) * | 2006-01-25 | 2013-07-16 | Insys Therapeutics, Inc. | Sublingual fentanyl spray |
-
2010
- 2010-04-23 EP EP10715336A patent/EP2421503A1/en not_active Withdrawn
- 2010-04-23 MX MX2011010835A patent/MX2011010835A/es active IP Right Grant
- 2010-04-23 SG SG2011073939A patent/SG175160A1/en unknown
- 2010-04-23 CA CA2756879A patent/CA2756879A1/en not_active Abandoned
- 2010-04-23 JP JP2012506583A patent/JP2012524771A/ja active Pending
- 2010-04-23 RU RU2011139638/15A patent/RU2011139638A/ru unknown
- 2010-04-23 NZ NZ595467A patent/NZ595467A/xx not_active IP Right Cessation
- 2010-04-23 CN CN2010800179717A patent/CN102458358A/zh active Pending
- 2010-04-23 US US13/265,825 patent/US20120058158A1/en not_active Abandoned
- 2010-04-23 MY MYPI2011005079A patent/MY167918A/en unknown
- 2010-04-23 BR BRPI1013539A patent/BRPI1013539A2/pt not_active Application Discontinuation
- 2010-04-23 WO PCT/GB2010/050671 patent/WO2010122355A1/en not_active Ceased
- 2010-04-23 AU AU2010240653A patent/AU2010240653A1/en not_active Abandoned
-
2011
- 2011-09-28 ZA ZA2011/07089A patent/ZA201107089B/en unknown
- 2011-10-02 IL IL215454A patent/IL215454A/en active IP Right Grant
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63503304A (ja) * | 1986-03-10 | 1988-12-02 | ブルグハルト,クルト | 医薬製剤およびその製造方法 |
| JP2005512943A (ja) * | 2001-02-14 | 2005-05-12 | ジーダブリュー ファーマ リミテッド | 医薬製剤 |
| WO2009020666A1 (en) * | 2007-08-06 | 2009-02-12 | Insys Therapeutics Inc. | Oral cannabinoid liquid formulations and methods of treatment |
| JP5438018B2 (ja) * | 2007-10-25 | 2014-03-12 | プロトファーマ リミテッド | 抗マラリア薬剤組成物 |
| JP2012524772A (ja) * | 2009-04-23 | 2012-10-18 | ロンドンファーマ リミテッド | ジヒドロアルテミシニンを含む舌下スプレー用製剤 |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2019519487A (ja) * | 2016-05-05 | 2019-07-11 | アクエスティブ セラピューティクス インコーポレイテッド | 送達増強エピネフリン組成物 |
| US11191737B2 (en) | 2016-05-05 | 2021-12-07 | Aquestive Therapeutics, Inc. | Enhanced delivery epinephrine compositions |
| US11273131B2 (en) | 2016-05-05 | 2022-03-15 | Aquestive Therapeutics, Inc. | Pharmaceutical compositions with enhanced permeation |
| JP2023052143A (ja) * | 2016-05-05 | 2023-04-11 | アクエスティブ セラピューティクス インコーポレイテッド | 送達増強エピネフリン組成物 |
| US12023309B2 (en) | 2016-05-05 | 2024-07-02 | Aquestive Therapeutics, Inc. | Enhanced delivery epinephrine compositions |
| JP7653218B2 (ja) | 2016-05-05 | 2025-03-28 | アクエスティブ セラピューティクス インコーポレイテッド | 送達増強エピネフリン組成物 |
| US12427121B2 (en) | 2016-05-05 | 2025-09-30 | Aquestive Therapeutics, Inc. | Enhanced delivery epinephrine compositions |
| US12433850B2 (en) | 2016-05-05 | 2025-10-07 | Aquestive Therapeutics, Inc. | Enhanced delivery epinephrine and prodrug compositions |
| JP2020535162A (ja) * | 2017-09-27 | 2020-12-03 | アクエスティブ セラピューティクス インコーポレイテッド | 増強された送達のエピネフリン及びプロドラッグ組成物 |
| US12465564B2 (en) | 2021-10-25 | 2025-11-11 | Aquestive Therapeutics, Inc. | Oral and nasal compositions and methods of treatment |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2421503A1 (en) | 2012-02-29 |
| IL215454A0 (en) | 2011-12-29 |
| CA2756879A1 (en) | 2010-10-28 |
| CN102458358A (zh) | 2012-05-16 |
| IL215454A (en) | 2014-11-30 |
| MX2011010835A (es) | 2012-05-08 |
| MY167918A (en) | 2018-09-27 |
| AU2010240653A1 (en) | 2011-10-20 |
| ZA201107089B (en) | 2012-12-27 |
| WO2010122355A1 (en) | 2010-10-28 |
| US20120058158A1 (en) | 2012-03-08 |
| BRPI1013539A2 (pt) | 2016-04-12 |
| RU2011139638A (ru) | 2013-05-27 |
| NZ595467A (en) | 2013-08-30 |
| SG175160A1 (en) | 2011-11-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2012524771A (ja) | 中性油を含む舌下用の医薬組成物 | |
| JP7617927B2 (ja) | 口内溶解性フィルムおよび口内溶解性フィルムを製造し、使用する方法 | |
| CN103622950B (zh) | 抗疟疾药物组合物 | |
| AU2012367017B2 (en) | Medicament delivery technology | |
| GB2469754A (en) | Sub-lingual drug delivery system using a neutral oil | |
| US20190142737A1 (en) | Sublingual spray formulation comprising dihydroartemesinin | |
| WO2010122276A1 (en) | Drug delivery | |
| HK1169939A (en) | Sublingual pharmaceutical composition comprising a neutral oil | |
| WO2010122275A1 (en) | Pharmaceutical preparation | |
| HK1190645B (en) | Anti-malarial pharmaceutical composition | |
| GB2469791A (en) | Lipophilic compositions comprising an artemisinin derivative and their therapeutic uses |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20130318 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20130318 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140225 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20140228 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20140916 |