JP2012502900A - 抗癌剤並びにそれに関する転移性悪性黒色腫及び他の癌についての使用 - Google Patents
抗癌剤並びにそれに関する転移性悪性黒色腫及び他の癌についての使用 Download PDFInfo
- Publication number
- JP2012502900A JP2012502900A JP2011526634A JP2011526634A JP2012502900A JP 2012502900 A JP2012502900 A JP 2012502900A JP 2011526634 A JP2011526634 A JP 2011526634A JP 2011526634 A JP2011526634 A JP 2011526634A JP 2012502900 A JP2012502900 A JP 2012502900A
- Authority
- JP
- Japan
- Prior art keywords
- compound
- diazenyl
- dimethylamino
- formula
- solution
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 206010028980 Neoplasm Diseases 0.000 title claims abstract description 39
- 206010027480 Metastatic malignant melanoma Diseases 0.000 title claims abstract description 12
- 208000021039 metastatic melanoma Diseases 0.000 title claims abstract description 12
- 239000002246 antineoplastic agent Substances 0.000 title claims description 14
- 239000000203 mixture Substances 0.000 claims abstract description 91
- 238000000034 method Methods 0.000 claims abstract description 37
- 150000003839 salts Chemical class 0.000 claims abstract description 21
- 206010039491 Sarcoma Diseases 0.000 claims abstract description 6
- 201000009030 Carcinoma Diseases 0.000 claims abstract description 4
- 206010018338 Glioma Diseases 0.000 claims abstract description 4
- 206010025323 Lymphomas Diseases 0.000 claims abstract description 4
- 150000001875 compounds Chemical class 0.000 claims description 165
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical group CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 claims description 90
- 239000007787 solid Substances 0.000 claims description 57
- 241000282414 Homo sapiens Species 0.000 claims description 33
- 201000011510 cancer Diseases 0.000 claims description 25
- -1 CF 3 Inorganic materials 0.000 claims description 23
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Divinylene sulfide Natural products C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 claims description 19
- 229960003901 dacarbazine Drugs 0.000 claims description 18
- BRKSHTFZKOTZGD-UHFFFAOYSA-N 3-(dimethylaminodiazenyl)thiophene-2-carboxylic acid Chemical compound CN(C)N=NC=1C=CSC=1C(O)=O BRKSHTFZKOTZGD-UHFFFAOYSA-N 0.000 claims description 17
- 239000008194 pharmaceutical composition Substances 0.000 claims description 16
- 230000002401 inhibitory effect Effects 0.000 claims description 15
- BPEGJWRSRHCHSN-UHFFFAOYSA-N Temozolomide Chemical compound O=C1N(C)N=NC2=C(C(N)=O)N=CN21 BPEGJWRSRHCHSN-UHFFFAOYSA-N 0.000 claims description 14
- 229960004964 temozolomide Drugs 0.000 claims description 14
- 229910052799 carbon Inorganic materials 0.000 claims description 13
- 229940127089 cytotoxic agent Drugs 0.000 claims description 13
- 125000000896 monocarboxylic acid group Chemical group 0.000 claims description 12
- YLQBMQCUIZJEEH-UHFFFAOYSA-N Furan Chemical class C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 claims description 11
- 239000003085 diluting agent Substances 0.000 claims description 11
- 229910052739 hydrogen Inorganic materials 0.000 claims description 11
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 claims description 10
- 125000002147 dimethylamino group Chemical group [H]C([H])([H])N(*)C([H])([H])[H] 0.000 claims description 10
- 229910052757 nitrogen Inorganic materials 0.000 claims description 9
- 229930192474 thiophene Natural products 0.000 claims description 9
- XCNVKXHFBYRXOX-UHFFFAOYSA-N 3-(dimethylaminodiazenyl)-4-methoxythiophene-2,5-dicarboxamide Chemical compound COC1=C(C(N)=O)SC(C(N)=O)=C1N=NN(C)C XCNVKXHFBYRXOX-UHFFFAOYSA-N 0.000 claims description 8
- 230000010261 cell growth Effects 0.000 claims description 7
- 229910052717 sulfur Inorganic materials 0.000 claims description 7
- IHXVULHYZAGNOQ-UHFFFAOYSA-N 3-(dimethylaminodiazenyl)-5-nitrothiophene-2-carboxylic acid Chemical compound CN(C)N=NC=1C=C([N+]([O-])=O)SC=1C(O)=O IHXVULHYZAGNOQ-UHFFFAOYSA-N 0.000 claims description 6
- FFIINGQLYOWBQU-UHFFFAOYSA-N 3-(dimethylaminodiazenyl)-5-phenylthiophene-2-carboxylic acid Chemical compound S1C(C(O)=O)=C(N=NN(C)C)C=C1C1=CC=CC=C1 FFIINGQLYOWBQU-UHFFFAOYSA-N 0.000 claims description 6
- 229930012538 Paclitaxel Natural products 0.000 claims description 6
- 230000008512 biological response Effects 0.000 claims description 6
- 210000003169 central nervous system Anatomy 0.000 claims description 6
- 239000003795 chemical substances by application Substances 0.000 claims description 6
- 210000004185 liver Anatomy 0.000 claims description 6
- 229960001592 paclitaxel Drugs 0.000 claims description 6
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 claims description 6
- 150000003577 thiophenes Chemical class 0.000 claims description 6
- SRONACWOWHDIFG-UHFFFAOYSA-N 3-(dimethylaminodiazenyl)-n-(2-hydroxyethyl)thiophene-2-carboxamide Chemical compound CN(C)N=NC=1C=CSC=1C(=O)NCCO SRONACWOWHDIFG-UHFFFAOYSA-N 0.000 claims description 5
- JEKTZIPLXIBRFH-UHFFFAOYSA-N 3-(dimethylaminodiazenyl)thiophene-2-carboxamide Chemical compound CN(C)N=NC=1C=CSC=1C(N)=O JEKTZIPLXIBRFH-UHFFFAOYSA-N 0.000 claims description 5
- MPZVKKVKDAMAKY-UHFFFAOYSA-N 3-methylthieno[2,3-d]triazin-4-one Chemical compound O=C1N(C)N=NC2=C1C=CS2 MPZVKKVKDAMAKY-UHFFFAOYSA-N 0.000 claims description 5
- LFITZROYUAUXCO-UHFFFAOYSA-N 4-(dimethylaminodiazenyl)thiophene-3-carboxamide Chemical compound CN(C)N=NC1=CSC=C1C(N)=O LFITZROYUAUXCO-UHFFFAOYSA-N 0.000 claims description 5
- 206010006187 Breast cancer Diseases 0.000 claims description 5
- 208000026310 Breast neoplasm Diseases 0.000 claims description 5
- 208000006168 Ewing Sarcoma Diseases 0.000 claims description 5
- 206010027476 Metastases Diseases 0.000 claims description 5
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 5
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical class C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 claims description 5
- 125000003342 alkenyl group Chemical group 0.000 claims description 5
- 150000003973 alkyl amines Chemical class 0.000 claims description 5
- 125000000217 alkyl group Chemical group 0.000 claims description 5
- 229910052794 bromium Inorganic materials 0.000 claims description 5
- 230000022534 cell killing Effects 0.000 claims description 5
- 238000007918 intramuscular administration Methods 0.000 claims description 5
- 238000007912 intraperitoneal administration Methods 0.000 claims description 5
- 238000001990 intravenous administration Methods 0.000 claims description 5
- ZLTPDFXIESTBQG-UHFFFAOYSA-N isothiazole Chemical class C=1C=NSC=1 ZLTPDFXIESTBQG-UHFFFAOYSA-N 0.000 claims description 5
- 239000003607 modifier Substances 0.000 claims description 5
- 229910052760 oxygen Inorganic materials 0.000 claims description 5
- DENPQNAWGQXKCU-UHFFFAOYSA-N thiophene-2-carboxamide Chemical compound NC(=O)C1=CC=CS1 DENPQNAWGQXKCU-UHFFFAOYSA-N 0.000 claims description 5
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 claims description 4
- BGWLAAMXLGLLOX-UHFFFAOYSA-N 3-(dimethylaminodiazenyl)-5-nitrothiophene-2-carboxamide Chemical compound CN(C)N=NC=1C=C([N+]([O-])=O)SC=1C(N)=O BGWLAAMXLGLLOX-UHFFFAOYSA-N 0.000 claims description 4
- JODOHQOKFYCSLT-UHFFFAOYSA-N 3-(dimethylaminodiazenyl)-5-phenylthiophene-2-carboxamide Chemical compound S1C(C(N)=O)=C(N=NN(C)C)C=C1C1=CC=CC=C1 JODOHQOKFYCSLT-UHFFFAOYSA-N 0.000 claims description 4
- GBBZWFPJGBPQDG-UHFFFAOYSA-N 3-methyl-6-nitrothieno[2,3-d]triazin-4-one Chemical compound O=C1N(C)N=NC2=C1C=C([N+]([O-])=O)S2 GBBZWFPJGBPQDG-UHFFFAOYSA-N 0.000 claims description 4
- LBSBADCUEOPGFV-UHFFFAOYSA-N 3-methyl-6-phenylthieno[3,2-d]triazin-4-one Chemical compound S1C=2C(=O)N(C)N=NC=2C=C1C1=CC=CC=C1 LBSBADCUEOPGFV-UHFFFAOYSA-N 0.000 claims description 4
- JIXFBKMQZSOWLN-UHFFFAOYSA-N 4-bromo-3-(dimethylaminodiazenyl)thiophene-2-carboxamide Chemical compound CN(C)N=NC=1C(Br)=CSC=1C(N)=O JIXFBKMQZSOWLN-UHFFFAOYSA-N 0.000 claims description 4
- 208000003174 Brain Neoplasms Diseases 0.000 claims description 4
- 101100294115 Caenorhabditis elegans nhr-4 gene Proteins 0.000 claims description 4
- 102000014150 Interferons Human genes 0.000 claims description 4
- 108010050904 Interferons Proteins 0.000 claims description 4
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 claims description 4
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 claims description 4
- 108060008682 Tumor Necrosis Factor Proteins 0.000 claims description 4
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 claims description 4
- 125000006615 aromatic heterocyclic group Chemical group 0.000 claims description 4
- 229960003668 docetaxel Drugs 0.000 claims description 4
- 239000000839 emulsion Substances 0.000 claims description 4
- 229960004768 irinotecan Drugs 0.000 claims description 4
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 claims description 4
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 claims description 4
- 201000002528 pancreatic cancer Diseases 0.000 claims description 4
- XAEFZNCEHLXOMS-UHFFFAOYSA-M potassium benzoate Chemical compound [K+].[O-]C(=O)C1=CC=CC=C1 XAEFZNCEHLXOMS-UHFFFAOYSA-M 0.000 claims description 4
- AFBXRCSQERBLCD-UHFFFAOYSA-N 3-(dimethylaminodiazenyl)-n-methylthiophene-2-carboxamide Chemical compound CNC(=O)C=1SC=CC=1N=NN(C)C AFBXRCSQERBLCD-UHFFFAOYSA-N 0.000 claims description 3
- KMFWOYBEAVLDNW-UHFFFAOYSA-N 3-methylthieno[3,2-d]triazin-4-one Chemical compound O=C1N(C)N=NC2=C1SC=C2 KMFWOYBEAVLDNW-UHFFFAOYSA-N 0.000 claims description 3
- VNDSXZWEYBFGHD-UHFFFAOYSA-N 6-amino-3-methylthieno[2,3-d]triazin-4-one Chemical compound O=C1N(C)N=NC2=C1C=C(N)S2 VNDSXZWEYBFGHD-UHFFFAOYSA-N 0.000 claims description 3
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 claims description 3
- 108010063738 Interleukins Proteins 0.000 claims description 3
- 102000015696 Interleukins Human genes 0.000 claims description 3
- 210000004556 brain Anatomy 0.000 claims description 3
- 229960004679 doxorubicin Drugs 0.000 claims description 3
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 claims description 3
- 229960005277 gemcitabine Drugs 0.000 claims description 3
- 229940079322 interferon Drugs 0.000 claims description 3
- 210000004072 lung Anatomy 0.000 claims description 3
- FRBXIGJRDSLZSX-UHFFFAOYSA-N n-(2-aminoethyl)-3-(dimethylaminodiazenyl)thiophene-2-carboxamide Chemical compound CN(C)N=NC=1C=CSC=1C(=O)NCCN FRBXIGJRDSLZSX-UHFFFAOYSA-N 0.000 claims description 3
- 210000000056 organ Anatomy 0.000 claims description 3
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 claims description 2
- 108010006654 Bleomycin Proteins 0.000 claims description 2
- GAGWJHPBXLXJQN-UORFTKCHSA-N Capecitabine Chemical compound C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](C)O1 GAGWJHPBXLXJQN-UORFTKCHSA-N 0.000 claims description 2
- GAGWJHPBXLXJQN-UHFFFAOYSA-N Capecitabine Natural products C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1C1C(O)C(O)C(C)O1 GAGWJHPBXLXJQN-UHFFFAOYSA-N 0.000 claims description 2
- 206010009944 Colon cancer Diseases 0.000 claims description 2
- 102000007644 Colony-Stimulating Factors Human genes 0.000 claims description 2
- 108010071942 Colony-Stimulating Factors Proteins 0.000 claims description 2
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 claims description 2
- 208000032612 Glial tumor Diseases 0.000 claims description 2
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 claims description 2
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 claims description 2
- 102000004457 Granulocyte-Macrophage Colony-Stimulating Factor Human genes 0.000 claims description 2
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 claims description 2
- 206010062767 Hypophysitis Diseases 0.000 claims description 2
- 102000008070 Interferon-gamma Human genes 0.000 claims description 2
- 108010074328 Interferon-gamma Proteins 0.000 claims description 2
- 108090000177 Interleukin-11 Proteins 0.000 claims description 2
- 108010065805 Interleukin-12 Proteins 0.000 claims description 2
- 108010002350 Interleukin-2 Proteins 0.000 claims description 2
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 claims description 2
- 239000005411 L01XE02 - Gefitinib Substances 0.000 claims description 2
- 206010058467 Lung neoplasm malignant Diseases 0.000 claims description 2
- 206010031112 Oropharyngeal squamous cell carcinoma Diseases 0.000 claims description 2
- 206010060862 Prostate cancer Diseases 0.000 claims description 2
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 2
- 208000000102 Squamous Cell Carcinoma of Head and Neck Diseases 0.000 claims description 2
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 claims description 2
- 210000004100 adrenal gland Anatomy 0.000 claims description 2
- 229940009456 adriamycin Drugs 0.000 claims description 2
- 229960000548 alemtuzumab Drugs 0.000 claims description 2
- XCPGHVQEEXUHNC-UHFFFAOYSA-N amsacrine Chemical compound COC1=CC(NS(C)(=O)=O)=CC=C1NC1=C(C=CC=C2)C2=NC2=CC=CC=C12 XCPGHVQEEXUHNC-UHFFFAOYSA-N 0.000 claims description 2
- 229960001220 amsacrine Drugs 0.000 claims description 2
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 claims description 2
- 229960001561 bleomycin Drugs 0.000 claims description 2
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 claims description 2
- 210000000481 breast Anatomy 0.000 claims description 2
- 229960004117 capecitabine Drugs 0.000 claims description 2
- 125000006297 carbonyl amino group Chemical group [H]N([*:2])C([*:1])=O 0.000 claims description 2
- 229960004562 carboplatin Drugs 0.000 claims description 2
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 claims description 2
- 229960004316 cisplatin Drugs 0.000 claims description 2
- 208000029742 colonic neoplasm Diseases 0.000 claims description 2
- 229940047120 colony stimulating factors Drugs 0.000 claims description 2
- 125000004122 cyclic group Chemical group 0.000 claims description 2
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 claims description 2
- 229960000975 daunorubicin Drugs 0.000 claims description 2
- 210000003238 esophagus Anatomy 0.000 claims description 2
- 229960005420 etoposide Drugs 0.000 claims description 2
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 claims description 2
- 229960002949 fluorouracil Drugs 0.000 claims description 2
- 210000001035 gastrointestinal tract Anatomy 0.000 claims description 2
- XGALLCVXEZPNRQ-UHFFFAOYSA-N gefitinib Chemical compound C=12C=C(OCCCN3CCOCC3)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 XGALLCVXEZPNRQ-UHFFFAOYSA-N 0.000 claims description 2
- 229960002584 gefitinib Drugs 0.000 claims description 2
- 201000010536 head and neck cancer Diseases 0.000 claims description 2
- 208000014829 head and neck neoplasm Diseases 0.000 claims description 2
- 229940022353 herceptin Drugs 0.000 claims description 2
- 229960003130 interferon gamma Drugs 0.000 claims description 2
- 210000003734 kidney Anatomy 0.000 claims description 2
- 201000005202 lung cancer Diseases 0.000 claims description 2
- 208000020816 lung neoplasm Diseases 0.000 claims description 2
- 230000001926 lymphatic effect Effects 0.000 claims description 2
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 2
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 claims description 2
- 229960001924 melphalan Drugs 0.000 claims description 2
- 229960001428 mercaptopurine Drugs 0.000 claims description 2
- 230000009401 metastasis Effects 0.000 claims description 2
- 229960000485 methotrexate Drugs 0.000 claims description 2
- 229960004857 mitomycin Drugs 0.000 claims description 2
- 239000002105 nanoparticle Substances 0.000 claims description 2
- 208000022698 oropharynx squamous cell carcinoma Diseases 0.000 claims description 2
- 210000001672 ovary Anatomy 0.000 claims description 2
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 claims description 2
- 229960001756 oxaliplatin Drugs 0.000 claims description 2
- 210000000496 pancreas Anatomy 0.000 claims description 2
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 2
- 210000003635 pituitary gland Anatomy 0.000 claims description 2
- 210000002307 prostate Anatomy 0.000 claims description 2
- 239000003087 receptor blocking agent Substances 0.000 claims description 2
- 208000011581 secondary neoplasm Diseases 0.000 claims description 2
- 210000000952 spleen Anatomy 0.000 claims description 2
- 229960001052 streptozocin Drugs 0.000 claims description 2
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 claims description 2
- 210000001550 testis Anatomy 0.000 claims description 2
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 claims description 2
- 229960000303 topotecan Drugs 0.000 claims description 2
- 229960003048 vinblastine Drugs 0.000 claims description 2
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 claims description 2
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 claims description 2
- 229960004528 vincristine Drugs 0.000 claims description 2
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 claims description 2
- 125000006575 electron-withdrawing group Chemical group 0.000 claims 2
- 108010002352 Interleukin-1 Proteins 0.000 claims 1
- 108010002335 Interleukin-9 Proteins 0.000 claims 1
- 125000002619 bicyclic group Chemical group 0.000 claims 1
- 210000003679 cervix uteri Anatomy 0.000 claims 1
- 238000010790 dilution Methods 0.000 claims 1
- 239000012895 dilution Substances 0.000 claims 1
- 229960004641 rituximab Drugs 0.000 claims 1
- 150000004654 triazenes Chemical class 0.000 abstract description 42
- 239000012453 solvate Substances 0.000 abstract description 3
- 150000004677 hydrates Chemical class 0.000 abstract description 2
- 239000000243 solution Substances 0.000 description 144
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 110
- 210000004027 cell Anatomy 0.000 description 99
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 66
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 60
- 239000000047 product Substances 0.000 description 51
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 48
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 46
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 42
- 239000002904 solvent Substances 0.000 description 41
- 201000001441 melanoma Diseases 0.000 description 37
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 33
- 230000015572 biosynthetic process Effects 0.000 description 33
- 229910052938 sodium sulfate Inorganic materials 0.000 description 33
- 235000011152 sodium sulphate Nutrition 0.000 description 33
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 32
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical group C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 32
- 239000012267 brine Substances 0.000 description 32
- 239000003480 eluent Substances 0.000 description 32
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 32
- 239000000741 silica gel Substances 0.000 description 31
- 229910002027 silica gel Inorganic materials 0.000 description 31
- XUMBMVFBXHLACL-UHFFFAOYSA-N Melanin Chemical compound O=C1C(=O)C(C2=CNC3=C(C(C(=O)C4=C32)=O)C)=C2C4=CNC2=C1C XUMBMVFBXHLACL-UHFFFAOYSA-N 0.000 description 30
- 239000011541 reaction mixture Substances 0.000 description 29
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 28
- 239000010410 layer Substances 0.000 description 28
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 27
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 24
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 24
- 238000004458 analytical method Methods 0.000 description 22
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 22
- 238000003786 synthesis reaction Methods 0.000 description 22
- OAYLNYINCPYISS-UHFFFAOYSA-N ethyl acetate;hexane Chemical compound CCCCCC.CCOC(C)=O OAYLNYINCPYISS-UHFFFAOYSA-N 0.000 description 19
- 150000002500 ions Chemical class 0.000 description 19
- 238000012360 testing method Methods 0.000 description 18
- 238000011282 treatment Methods 0.000 description 18
- 235000019439 ethyl acetate Nutrition 0.000 description 17
- LPXPTNMVRIOKMN-UHFFFAOYSA-M sodium nitrite Chemical compound [Na+].[O-]N=O LPXPTNMVRIOKMN-UHFFFAOYSA-M 0.000 description 16
- 102000003855 L-lactate dehydrogenase Human genes 0.000 description 15
- 108700023483 L-lactate dehydrogenases Proteins 0.000 description 15
- 239000003981 vehicle Substances 0.000 description 15
- WORJEOGGNQDSOE-UHFFFAOYSA-N chloroform;methanol Chemical compound OC.ClC(Cl)Cl WORJEOGGNQDSOE-UHFFFAOYSA-N 0.000 description 14
- 230000000694 effects Effects 0.000 description 14
- 238000003756 stirring Methods 0.000 description 14
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 13
- 239000003814 drug Substances 0.000 description 13
- 239000007788 liquid Substances 0.000 description 13
- QAOWNCQODCNURD-UHFFFAOYSA-N sulfuric acid Substances OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 13
- DVNYTAVYBRSTGK-UHFFFAOYSA-N 5-aminoimidazole-4-carboxamide Chemical compound NC(=O)C=1N=CNC=1N DVNYTAVYBRSTGK-UHFFFAOYSA-N 0.000 description 12
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 12
- 229940079593 drug Drugs 0.000 description 12
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 12
- 210000004881 tumor cell Anatomy 0.000 description 12
- 238000001727 in vivo Methods 0.000 description 11
- 230000005764 inhibitory process Effects 0.000 description 11
- JRIKQFXKSGJDBO-UHFFFAOYSA-N methyl 3-(dimethylaminodiazenyl)thiophene-2-carboxylate Chemical compound COC(=O)C=1SC=CC=1N=NN(C)C JRIKQFXKSGJDBO-UHFFFAOYSA-N 0.000 description 11
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 10
- 241000699666 Mus <mouse, genus> Species 0.000 description 10
- WQDUMFSSJAZKTM-UHFFFAOYSA-N Sodium methoxide Chemical compound [Na+].[O-]C WQDUMFSSJAZKTM-UHFFFAOYSA-N 0.000 description 10
- 238000000338 in vitro Methods 0.000 description 10
- 239000012044 organic layer Substances 0.000 description 10
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 9
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 9
- 235000011114 ammonium hydroxide Nutrition 0.000 description 9
- 239000012528 membrane Substances 0.000 description 9
- 230000002829 reductive effect Effects 0.000 description 9
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 8
- 101710179684 Poly [ADP-ribose] polymerase Proteins 0.000 description 8
- 102100023712 Poly [ADP-ribose] polymerase 1 Human genes 0.000 description 8
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 8
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 8
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 8
- 239000000908 ammonium hydroxide Substances 0.000 description 8
- 239000003153 chemical reaction reagent Substances 0.000 description 8
- 229940125904 compound 1 Drugs 0.000 description 8
- 230000014509 gene expression Effects 0.000 description 8
- 210000004379 membrane Anatomy 0.000 description 8
- 239000011734 sodium Substances 0.000 description 8
- 235000010288 sodium nitrite Nutrition 0.000 description 8
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 7
- 241001465754 Metazoa Species 0.000 description 7
- 229920000776 Poly(Adenosine diphosphate-ribose) polymerase Polymers 0.000 description 7
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 7
- 239000000443 aerosol Substances 0.000 description 7
- 150000001412 amines Chemical class 0.000 description 7
- OQNGCCWBHLEQFN-UHFFFAOYSA-N chloroform;hexane Chemical compound ClC(Cl)Cl.CCCCCC OQNGCCWBHLEQFN-UHFFFAOYSA-N 0.000 description 7
- 230000012010 growth Effects 0.000 description 7
- NLKNQRATVPKPDG-UHFFFAOYSA-M potassium iodide Substances [K+].[I-] NLKNQRATVPKPDG-UHFFFAOYSA-M 0.000 description 7
- 239000000725 suspension Substances 0.000 description 7
- CDTOLFKPXAIBGJ-UHFFFAOYSA-N (aminodiazenyl)methane Chemical class CNN=N CDTOLFKPXAIBGJ-UHFFFAOYSA-N 0.000 description 6
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 6
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 6
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 6
- 0 COC(c1c(*)c(*=C)c(*)[s]1)=O Chemical compound COC(c1c(*)c(*=C)c(*)[s]1)=O 0.000 description 6
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 6
- WTDHULULXKLSOZ-UHFFFAOYSA-N Hydroxylamine hydrochloride Chemical compound Cl.ON WTDHULULXKLSOZ-UHFFFAOYSA-N 0.000 description 6
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 6
- 241000699670 Mus sp. Species 0.000 description 6
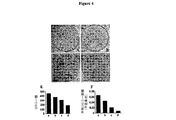
- 230000000259 anti-tumor effect Effects 0.000 description 6
- 230000004663 cell proliferation Effects 0.000 description 6
- 231100000135 cytotoxicity Toxicity 0.000 description 6
- 230000003013 cytotoxicity Effects 0.000 description 6
- 150000002148 esters Chemical class 0.000 description 6
- 230000004048 modification Effects 0.000 description 6
- 238000012986 modification Methods 0.000 description 6
- 239000002953 phosphate buffered saline Substances 0.000 description 6
- 229910000027 potassium carbonate Inorganic materials 0.000 description 6
- WBZUSDQVWKYLAC-UHFFFAOYSA-N 2-[(z)-aminodiazenyl]-3-methylimidazole-4-carboxamide Chemical compound CN1C(\N=N\N)=NC=C1C(N)=O WBZUSDQVWKYLAC-UHFFFAOYSA-N 0.000 description 5
- RRSNDVCODIMOFX-MPKOGUQCSA-N Fc1c(Cl)cccc1[C@H]1[C@@H](NC2(CCCCC2)[C@@]11C(=O)Nc2cc(Cl)ccc12)C(=O)Nc1ccc(cc1)C(=O)NCCCCCc1cccc2C(=O)N(Cc12)C1CCC(=O)NC1=O Chemical compound Fc1c(Cl)cccc1[C@H]1[C@@H](NC2(CCCCC2)[C@@]11C(=O)Nc2cc(Cl)ccc12)C(=O)Nc1ccc(cc1)C(=O)NCCCCCc1cccc2C(=O)N(Cc12)C1CCC(=O)NC1=O RRSNDVCODIMOFX-MPKOGUQCSA-N 0.000 description 5
- 229910021529 ammonia Inorganic materials 0.000 description 5
- 230000022131 cell cycle Effects 0.000 description 5
- 230000003833 cell viability Effects 0.000 description 5
- 231100000050 cytotoxic potential Toxicity 0.000 description 5
- 210000002889 endothelial cell Anatomy 0.000 description 5
- 238000002474 experimental method Methods 0.000 description 5
- 239000012091 fetal bovine serum Substances 0.000 description 5
- TWEQNZZOOFKOER-UHFFFAOYSA-N methyl 3-aminothiophene-2-carboxylate Chemical compound COC(=O)C=1SC=CC=1N TWEQNZZOOFKOER-UHFFFAOYSA-N 0.000 description 5
- HCRQNZWIOUMCOW-UHFFFAOYSA-N methyl 4-aminothiophene-2-carboxylate Chemical compound COC(=O)C1=CC(N)=CS1 HCRQNZWIOUMCOW-UHFFFAOYSA-N 0.000 description 5
- BUFZZXCVOFBHLS-UHFFFAOYSA-N methyl 4-aminothiophene-3-carboxylate Chemical compound COC(=O)C1=CSC=C1N BUFZZXCVOFBHLS-UHFFFAOYSA-N 0.000 description 5
- 239000003921 oil Substances 0.000 description 5
- 235000019198 oils Nutrition 0.000 description 5
- 210000003491 skin Anatomy 0.000 description 5
- 230000001225 therapeutic effect Effects 0.000 description 5
- WHZIZZOTISTHCT-UHFFFAOYSA-N 2-aminothiophene-3-carboxamide Chemical compound NC(=O)C=1C=CSC=1N WHZIZZOTISTHCT-UHFFFAOYSA-N 0.000 description 4
- AUMYBOLSUBPAEF-UHFFFAOYSA-N 3h-thieno[2,3-d]triazin-4-one Chemical compound O=C1NN=NC2=C1C=CS2 AUMYBOLSUBPAEF-UHFFFAOYSA-N 0.000 description 4
- 108020004414 DNA Proteins 0.000 description 4
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 4
- BAPJBEWLBFYGME-UHFFFAOYSA-N Methyl acrylate Chemical compound COC(=O)C=C BAPJBEWLBFYGME-UHFFFAOYSA-N 0.000 description 4
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 4
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 4
- 208000000453 Skin Neoplasms Diseases 0.000 description 4
- OKJPEAGHQZHRQV-UHFFFAOYSA-N Triiodomethane Natural products IC(I)I OKJPEAGHQZHRQV-UHFFFAOYSA-N 0.000 description 4
- 239000002253 acid Substances 0.000 description 4
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 4
- 238000004113 cell culture Methods 0.000 description 4
- 238000003776 cleavage reaction Methods 0.000 description 4
- 239000012043 crude product Substances 0.000 description 4
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 description 4
- GIBGVHMTXFZCBN-UHFFFAOYSA-N dimethyl 3-hydroxythiophene-2,5-dicarboxylate Chemical compound COC(=O)C1=CC(O)=C(C(=O)OC)S1 GIBGVHMTXFZCBN-UHFFFAOYSA-N 0.000 description 4
- 239000002552 dosage form Substances 0.000 description 4
- 231100000673 dose–response relationship Toxicity 0.000 description 4
- 239000003937 drug carrier Substances 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 4
- 230000010534 mechanism of action Effects 0.000 description 4
- 239000002609 medium Substances 0.000 description 4
- 150000007522 mineralic acids Chemical class 0.000 description 4
- 238000006396 nitration reaction Methods 0.000 description 4
- 229910017604 nitric acid Inorganic materials 0.000 description 4
- 150000007524 organic acids Chemical class 0.000 description 4
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 230000007017 scission Effects 0.000 description 4
- 201000000849 skin cancer Diseases 0.000 description 4
- 239000011780 sodium chloride Substances 0.000 description 4
- 230000004614 tumor growth Effects 0.000 description 4
- 238000001262 western blot Methods 0.000 description 4
- DYRYDYQWWQHRQE-UHFFFAOYSA-N 3-amino-5-phenylthiophene-2-carboxamide Chemical compound NC1=C(C(=O)N)SC(C=2C=CC=CC=2)=C1 DYRYDYQWWQHRQE-UHFFFAOYSA-N 0.000 description 3
- GVKYEBRJHLHHOE-UHFFFAOYSA-N 3-chloro-3-phenylprop-2-enenitrile Chemical compound N#CC=C(Cl)C1=CC=CC=C1 GVKYEBRJHLHHOE-UHFFFAOYSA-N 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 3
- 102000002004 Cytochrome P-450 Enzyme System Human genes 0.000 description 3
- 108010015742 Cytochrome P-450 Enzyme System Proteins 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 3
- PGBFYLVIMDQYMS-UHFFFAOYSA-N Methyl thiophene-2-carboxylate Chemical compound COC(=O)C1=CC=CS1 PGBFYLVIMDQYMS-UHFFFAOYSA-N 0.000 description 3
- 102100027467 Pro-opiomelanocortin Human genes 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical class [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- 239000004480 active ingredient Substances 0.000 description 3
- 239000002671 adjuvant Substances 0.000 description 3
- 229940100198 alkylating agent Drugs 0.000 description 3
- 239000002168 alkylating agent Substances 0.000 description 3
- 235000019270 ammonium chloride Nutrition 0.000 description 3
- 230000006907 apoptotic process Effects 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- 239000003054 catalyst Substances 0.000 description 3
- 238000002512 chemotherapy Methods 0.000 description 3
- 229940126214 compound 3 Drugs 0.000 description 3
- 239000012228 culture supernatant Substances 0.000 description 3
- 231100000433 cytotoxic Toxicity 0.000 description 3
- 230000001472 cytotoxic effect Effects 0.000 description 3
- JRBCYFRHGHSCAI-UHFFFAOYSA-N dimethyl 3-amino-4-methoxythiophene-2,5-dicarboxylate Chemical compound COC(=O)C=1SC(C(=O)OC)=C(OC)C=1N JRBCYFRHGHSCAI-UHFFFAOYSA-N 0.000 description 3
- DTHGQYUSTKUIEO-UHFFFAOYSA-N dimethyl 3-hydroxy-4-nitrothiophene-2,5-dicarboxylate Chemical compound COC(=O)C=1SC(C(=O)OC)=C([N+]([O-])=O)C=1O DTHGQYUSTKUIEO-UHFFFAOYSA-N 0.000 description 3
- JBRYAXHITCFFMI-UHFFFAOYSA-N dimethyl 3-methoxy-4-nitrothiophene-2,5-dicarboxylate Chemical compound COC(=O)C=1SC(C(=O)OC)=C([N+]([O-])=O)C=1OC JBRYAXHITCFFMI-UHFFFAOYSA-N 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 230000010595 endothelial cell migration Effects 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 239000000499 gel Substances 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 230000007062 hydrolysis Effects 0.000 description 3
- 238000006460 hydrolysis reaction Methods 0.000 description 3
- 238000003119 immunoblot Methods 0.000 description 3
- 230000008595 infiltration Effects 0.000 description 3
- 238000001764 infiltration Methods 0.000 description 3
- 108010082117 matrigel Proteins 0.000 description 3
- MKIJJIMOAABWGF-UHFFFAOYSA-N methyl 2-sulfanylacetate Chemical compound COC(=O)CS MKIJJIMOAABWGF-UHFFFAOYSA-N 0.000 description 3
- LWSWLXPUUCBUPI-UHFFFAOYSA-N methyl 3-amino-4-bromothiophene-2-carboxylate Chemical compound COC(=O)C=1SC=C(Br)C=1N LWSWLXPUUCBUPI-UHFFFAOYSA-N 0.000 description 3
- QESSCNMSOLRYBO-UHFFFAOYSA-N methyl 3-amino-5-phenylthiophene-2-carboxylate Chemical compound NC1=C(C(=O)OC)SC(C=2C=CC=CC=2)=C1 QESSCNMSOLRYBO-UHFFFAOYSA-N 0.000 description 3
- JDWYDYUXQFKWLT-UHFFFAOYSA-N methyl 4-(dimethylaminodiazenyl)thiophene-2-carboxylate Chemical compound COC(=O)C1=CC(N=NN(C)C)=CS1 JDWYDYUXQFKWLT-UHFFFAOYSA-N 0.000 description 3
- NELTUYZACMMVIC-UHFFFAOYSA-N methyl 4-(dimethylaminodiazenyl)thiophene-3-carboxylate Chemical compound COC(=O)C1=CSC=C1N=NN(C)C NELTUYZACMMVIC-UHFFFAOYSA-N 0.000 description 3
- LEAKUJFYXNILRB-UHFFFAOYSA-N methyl 4-oxothiolane-3-carboxylate Chemical compound COC(=O)C1CSCC1=O LEAKUJFYXNILRB-UHFFFAOYSA-N 0.000 description 3
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 3
- 230000005012 migration Effects 0.000 description 3
- 238000013508 migration Methods 0.000 description 3
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 3
- 230000017095 negative regulation of cell growth Effects 0.000 description 3
- 239000011148 porous material Substances 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 239000002243 precursor Substances 0.000 description 3
- 208000023958 prostate neoplasm Diseases 0.000 description 3
- BOLDJAUMGUJJKM-LSDHHAIUSA-N renifolin D Natural products CC(=C)[C@@H]1Cc2c(O)c(O)ccc2[C@H]1CC(=O)c3ccc(O)cc3O BOLDJAUMGUJJKM-LSDHHAIUSA-N 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 239000004094 surface-active agent Substances 0.000 description 3
- 230000004083 survival effect Effects 0.000 description 3
- 239000003826 tablet Substances 0.000 description 3
- 231100001274 therapeutic index Toxicity 0.000 description 3
- QERYCTSHXKAMIS-UHFFFAOYSA-N thiophene-2-carboxylic acid Chemical compound OC(=O)C1=CC=CS1 QERYCTSHXKAMIS-UHFFFAOYSA-N 0.000 description 3
- 230000001988 toxicity Effects 0.000 description 3
- 231100000419 toxicity Toxicity 0.000 description 3
- 210000003606 umbilical vein Anatomy 0.000 description 3
- WHNFPRLDDSXQCL-UAZQEYIDSA-N α-msh Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(N)=O)NC(=O)[C@H](CO)NC(C)=O)C1=CC=C(O)C=C1 WHNFPRLDDSXQCL-UAZQEYIDSA-N 0.000 description 3
- AYJOWWJCBULTIM-UHFFFAOYSA-N 3-amino-5-phenylthiophene-2-carbonitrile Chemical compound S1C(C#N)=C(N)C=C1C1=CC=CC=C1 AYJOWWJCBULTIM-UHFFFAOYSA-N 0.000 description 2
- SZHKHACZQDMGGI-UHFFFAOYSA-N 4-nitro-2-thiophenecarboxylic acid Chemical compound OC(=O)C1=CC([N+]([O-])=O)=CS1 SZHKHACZQDMGGI-UHFFFAOYSA-N 0.000 description 2
- 125000002373 5 membered heterocyclic group Chemical group 0.000 description 2
- UZSFPZHJEPYLHO-UHFFFAOYSA-N 6-phenyl-1h-thieno[3,2-d]triazin-4-one Chemical compound S1C=2C(=O)NN=NC=2C=C1C1=CC=CC=C1 UZSFPZHJEPYLHO-UHFFFAOYSA-N 0.000 description 2
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical compound N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 2
- KWOLFJPFCHCOCG-UHFFFAOYSA-N Acetophenone Chemical compound CC(=O)C1=CC=CC=C1 KWOLFJPFCHCOCG-UHFFFAOYSA-N 0.000 description 2
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 2
- 102000007469 Actins Human genes 0.000 description 2
- 108010085238 Actins Proteins 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- 102100025064 Cellular tumor antigen p53 Human genes 0.000 description 2
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 2
- 229920000742 Cotton Polymers 0.000 description 2
- 230000006820 DNA synthesis Effects 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- QUSNBJAOOMFDIB-UHFFFAOYSA-N Ethylamine Chemical compound CCN QUSNBJAOOMFDIB-UHFFFAOYSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 239000004166 Lanolin Substances 0.000 description 2
- 102400000740 Melanocyte-stimulating hormone alpha Human genes 0.000 description 2
- 101710200814 Melanotropin alpha Proteins 0.000 description 2
- GAWIPFJXHCDBHK-UHFFFAOYSA-N NN=N.C=1C=CSC=1 Chemical class NN=N.C=1C=CSC=1 GAWIPFJXHCDBHK-UHFFFAOYSA-N 0.000 description 2
- 235000019502 Orange oil Nutrition 0.000 description 2
- CBENFWSGALASAD-UHFFFAOYSA-N Ozone Chemical compound [O-][O+]=O CBENFWSGALASAD-UHFFFAOYSA-N 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 108010069820 Pro-Opiomelanocortin Proteins 0.000 description 2
- 239000000683 Pro-Opiomelanocortin Substances 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 2
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 150000001299 aldehydes Chemical class 0.000 description 2
- 230000029936 alkylation Effects 0.000 description 2
- 238000005804 alkylation reaction Methods 0.000 description 2
- 229940121369 angiogenesis inhibitor Drugs 0.000 description 2
- 239000004037 angiogenesis inhibitor Substances 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 230000003197 catalytic effect Effects 0.000 description 2
- 230000004709 cell invasion Effects 0.000 description 2
- 239000013592 cell lysate Substances 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 230000005757 colony formation Effects 0.000 description 2
- 239000003086 colorant Substances 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical compound NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- VAYGXNSJCAHWJZ-UHFFFAOYSA-N dimethyl sulfate Chemical compound COS(=O)(=O)OC VAYGXNSJCAHWJZ-UHFFFAOYSA-N 0.000 description 2
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 230000003828 downregulation Effects 0.000 description 2
- 239000000975 dye Substances 0.000 description 2
- 230000003511 endothelial effect Effects 0.000 description 2
- 238000000684 flow cytometry Methods 0.000 description 2
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 2
- 235000003599 food sweetener Nutrition 0.000 description 2
- 239000007903 gelatin capsule Substances 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- UYTPUPDQBNUYGX-UHFFFAOYSA-N guanine Chemical group O=C1NC(N)=NC2=C1N=CN2 UYTPUPDQBNUYGX-UHFFFAOYSA-N 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 210000002510 keratinocyte Anatomy 0.000 description 2
- 229940039717 lanolin Drugs 0.000 description 2
- 235000019388 lanolin Nutrition 0.000 description 2
- 238000011068 loading method Methods 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 231100000682 maximum tolerated dose Toxicity 0.000 description 2
- 210000002752 melanocyte Anatomy 0.000 description 2
- 239000002207 metabolite Substances 0.000 description 2
- 229910000000 metal hydroxide Inorganic materials 0.000 description 2
- 150000004692 metal hydroxides Chemical class 0.000 description 2
- 229910001960 metal nitrate Inorganic materials 0.000 description 2
- XNDKLLFGXIEGKL-UHFFFAOYSA-N methyl 3-(2-methoxy-2-oxoethyl)sulfanylpropanoate Chemical compound COC(=O)CCSCC(=O)OC XNDKLLFGXIEGKL-UHFFFAOYSA-N 0.000 description 2
- UOSXYPCNJPVISS-UHFFFAOYSA-N methyl 3-(dimethylaminodiazenyl)-5-nitrothiophene-2-carboxylate Chemical compound COC(=O)C=1SC([N+]([O-])=O)=CC=1N=NN(C)C UOSXYPCNJPVISS-UHFFFAOYSA-N 0.000 description 2
- PTZDYNXKMWPJIZ-UHFFFAOYSA-N methyl 3-(dimethylaminodiazenyl)-5-phenylthiophene-2-carboxylate Chemical compound CN(C)N=NC1=C(C(=O)OC)SC(C=2C=CC=CC=2)=C1 PTZDYNXKMWPJIZ-UHFFFAOYSA-N 0.000 description 2
- OSWPMRLSEDHDFF-UHFFFAOYSA-N methyl salicylate Chemical compound COC(=O)C1=CC=CC=C1O OSWPMRLSEDHDFF-UHFFFAOYSA-N 0.000 description 2
- 230000003228 microsomal effect Effects 0.000 description 2
- VMGAPWLDMVPYIA-HIDZBRGKSA-N n'-amino-n-iminomethanimidamide Chemical compound N\N=C\N=N VMGAPWLDMVPYIA-HIDZBRGKSA-N 0.000 description 2
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 2
- 230000035764 nutrition Effects 0.000 description 2
- 235000016709 nutrition Nutrition 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 239000010502 orange oil Substances 0.000 description 2
- 230000001590 oxidative effect Effects 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 2
- 239000000049 pigment Substances 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 102000004196 processed proteins & peptides Human genes 0.000 description 2
- 108090000765 processed proteins & peptides Proteins 0.000 description 2
- XJMOSONTPMZWPB-UHFFFAOYSA-M propidium iodide Chemical compound [I-].[I-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CCC[N+](C)(CC)CC)=C1C1=CC=CC=C1 XJMOSONTPMZWPB-UHFFFAOYSA-M 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 150000003212 purines Chemical class 0.000 description 2
- 230000005855 radiation Effects 0.000 description 2
- 239000002534 radiation-sensitizing agent Substances 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 238000010992 reflux Methods 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 230000028327 secretion Effects 0.000 description 2
- 238000010898 silica gel chromatography Methods 0.000 description 2
- 239000008247 solid mixture Substances 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 239000011593 sulfur Substances 0.000 description 2
- 239000003765 sweetening agent Substances 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 2
- 238000011200 topical administration Methods 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 210000002700 urine Anatomy 0.000 description 2
- AOSZTAHDEDLTLQ-AZKQZHLXSA-N (1S,2S,4R,8S,9S,11S,12R,13S,19S)-6-[(3-chlorophenyl)methyl]-12,19-difluoro-11-hydroxy-8-(2-hydroxyacetyl)-9,13-dimethyl-6-azapentacyclo[10.8.0.02,9.04,8.013,18]icosa-14,17-dien-16-one Chemical compound C([C@@H]1C[C@H]2[C@H]3[C@]([C@]4(C=CC(=O)C=C4[C@@H](F)C3)C)(F)[C@@H](O)C[C@@]2([C@@]1(C1)C(=O)CO)C)N1CC1=CC=CC(Cl)=C1 AOSZTAHDEDLTLQ-AZKQZHLXSA-N 0.000 description 1
- GLGNXYJARSMNGJ-VKTIVEEGSA-N (1s,2s,3r,4r)-3-[[5-chloro-2-[(1-ethyl-6-methoxy-2-oxo-4,5-dihydro-3h-1-benzazepin-7-yl)amino]pyrimidin-4-yl]amino]bicyclo[2.2.1]hept-5-ene-2-carboxamide Chemical compound CCN1C(=O)CCCC2=C(OC)C(NC=3N=C(C(=CN=3)Cl)N[C@H]3[C@H]([C@@]4([H])C[C@@]3(C=C4)[H])C(N)=O)=CC=C21 GLGNXYJARSMNGJ-VKTIVEEGSA-N 0.000 description 1
- SZUVGFMDDVSKSI-WIFOCOSTSA-N (1s,2s,3s,5r)-1-(carboxymethyl)-3,5-bis[(4-phenoxyphenyl)methyl-propylcarbamoyl]cyclopentane-1,2-dicarboxylic acid Chemical compound O=C([C@@H]1[C@@H]([C@](CC(O)=O)([C@H](C(=O)N(CCC)CC=2C=CC(OC=3C=CC=CC=3)=CC=2)C1)C(O)=O)C(O)=O)N(CCC)CC(C=C1)=CC=C1OC1=CC=CC=C1 SZUVGFMDDVSKSI-WIFOCOSTSA-N 0.000 description 1
- 239000001677 (2R,5R)-1,4-dithiane-2,5-diol Substances 0.000 description 1
- GHYOCDFICYLMRF-UTIIJYGPSA-N (2S,3R)-N-[(2S)-3-(cyclopenten-1-yl)-1-[(2R)-2-methyloxiran-2-yl]-1-oxopropan-2-yl]-3-hydroxy-3-(4-methoxyphenyl)-2-[[(2S)-2-[(2-morpholin-4-ylacetyl)amino]propanoyl]amino]propanamide Chemical compound C1(=CCCC1)C[C@@H](C(=O)[C@@]1(OC1)C)NC([C@H]([C@@H](C1=CC=C(C=C1)OC)O)NC([C@H](C)NC(CN1CCOCC1)=O)=O)=O GHYOCDFICYLMRF-UTIIJYGPSA-N 0.000 description 1
- QFLWZFQWSBQYPS-AWRAUJHKSA-N (3S)-3-[[(2S)-2-[[(2S)-2-[5-[(3aS,6aR)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-[1-bis(4-chlorophenoxy)phosphorylbutylamino]-4-oxobutanoic acid Chemical compound CCCC(NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)C(C)C)P(=O)(Oc1ccc(Cl)cc1)Oc1ccc(Cl)cc1 QFLWZFQWSBQYPS-AWRAUJHKSA-N 0.000 description 1
- VEEGZPWAAPPXRB-BJMVGYQFSA-N (3e)-3-(1h-imidazol-5-ylmethylidene)-1h-indol-2-one Chemical compound O=C1NC2=CC=CC=C2\C1=C/C1=CN=CN1 VEEGZPWAAPPXRB-BJMVGYQFSA-N 0.000 description 1
- JGIRDDQLZLXRKQ-UHFFFAOYSA-N (4-methoxycarbonylthiophen-3-yl)azanium;chloride Chemical compound Cl.COC(=O)C1=CSC=C1N JGIRDDQLZLXRKQ-UHFFFAOYSA-N 0.000 description 1
- SEPPVOUBHWNCAW-FNORWQNLSA-N (E)-4-oxonon-2-enal Chemical compound CCCCCC(=O)\C=C\C=O SEPPVOUBHWNCAW-FNORWQNLSA-N 0.000 description 1
- YUIOPHXTILULQC-UHFFFAOYSA-N 1,4-Dithiane-2,5-diol Chemical compound OC1CSC(O)CS1 YUIOPHXTILULQC-UHFFFAOYSA-N 0.000 description 1
- ONBQEOIKXPHGMB-VBSBHUPXSA-N 1-[2-[(2s,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-4,6-dihydroxyphenyl]-3-(4-hydroxyphenyl)propan-1-one Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1OC1=CC(O)=CC(O)=C1C(=O)CCC1=CC=C(O)C=C1 ONBQEOIKXPHGMB-VBSBHUPXSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- PBXYRYVEDSIQEF-UHFFFAOYSA-N 2-[(e)-aminodiazenyl]thiophene Chemical class NN=NC1=CC=CS1 PBXYRYVEDSIQEF-UHFFFAOYSA-N 0.000 description 1
- AZKSAVLVSZKNRD-UHFFFAOYSA-M 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide Chemical compound [Br-].S1C(C)=C(C)N=C1[N+]1=NC(C=2C=CC=CC=2)=NN1C1=CC=CC=C1 AZKSAVLVSZKNRD-UHFFFAOYSA-M 0.000 description 1
- BKDZTJNNXCNSCK-UHFFFAOYSA-N 3-aminothiophene-2-carboxamide Chemical compound NC(=O)C=1SC=CC=1N BKDZTJNNXCNSCK-UHFFFAOYSA-N 0.000 description 1
- LTFGKKLLQUAPGE-UHFFFAOYSA-N 3-nitrothiophene-2-carboxylic acid Chemical compound OC(=O)C=1SC=CC=1[N+]([O-])=O LTFGKKLLQUAPGE-UHFFFAOYSA-N 0.000 description 1
- LLBZPESJRQGYMB-UHFFFAOYSA-N 4-one Natural products O1C(C(=O)CC)CC(C)C11C2(C)CCC(C3(C)C(C(C)(CO)C(OC4C(C(O)C(O)C(COC5C(C(O)C(O)CO5)OC5C(C(OC6C(C(O)C(O)C(CO)O6)O)C(O)C(CO)O5)OC5C(C(O)C(O)C(C)O5)O)O4)O)CC3)CC3)=C3C2(C)CC1 LLBZPESJRQGYMB-UHFFFAOYSA-N 0.000 description 1
- BXJHWYVXLGLDMZ-UHFFFAOYSA-N 6-O-methylguanine Chemical compound COC1=NC(N)=NC2=C1NC=N2 BXJHWYVXLGLDMZ-UHFFFAOYSA-N 0.000 description 1
- UNEPVPOHGXLUIR-UHFFFAOYSA-N 6317-37-9 Chemical compound OC(=O)C1=CC=C([N+]([O-])=O)S1 UNEPVPOHGXLUIR-UHFFFAOYSA-N 0.000 description 1
- NALREUIWICQLPS-UHFFFAOYSA-N 7-imino-n,n-dimethylphenothiazin-3-amine;hydrochloride Chemical compound [Cl-].C1=C(N)C=C2SC3=CC(=[N+](C)C)C=CC3=NC2=C1 NALREUIWICQLPS-UHFFFAOYSA-N 0.000 description 1
- LYHRBIAPWZFXBG-UHFFFAOYSA-N 7h-imidazo[4,5-e]tetrazine Chemical compound N1=NNC2=NC=NC2=N1 LYHRBIAPWZFXBG-UHFFFAOYSA-N 0.000 description 1
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 1
- 108010017384 Blood Proteins Proteins 0.000 description 1
- 102000004506 Blood Proteins Human genes 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- FGUUSXIOTUKUDN-IBGZPJMESA-N C1(=CC=CC=C1)N1C2=C(NC([C@H](C1)NC=1OC(=NN=1)C1=CC=CC=C1)=O)C=CC=C2 Chemical compound C1(=CC=CC=C1)N1C2=C(NC([C@H](C1)NC=1OC(=NN=1)C1=CC=CC=C1)=O)C=CC=C2 FGUUSXIOTUKUDN-IBGZPJMESA-N 0.000 description 1
- KJEHEMKQYMNJQB-UHFFFAOYSA-N CC(c1cc(C(N(C)N=N2)=O)c2[s]1)=C Chemical compound CC(c1cc(C(N(C)N=N2)=O)c2[s]1)=C KJEHEMKQYMNJQB-UHFFFAOYSA-N 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 108090000397 Caspase 3 Proteins 0.000 description 1
- 102100029855 Caspase-3 Human genes 0.000 description 1
- 108010076667 Caspases Proteins 0.000 description 1
- 102000011727 Caspases Human genes 0.000 description 1
- 208000002177 Cataract Diseases 0.000 description 1
- RENMDAKOXSCIGH-UHFFFAOYSA-N Chloroacetonitrile Chemical compound ClCC#N RENMDAKOXSCIGH-UHFFFAOYSA-N 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 229940126657 Compound 17 Drugs 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 229920000858 Cyclodextrin Polymers 0.000 description 1
- 102000053602 DNA Human genes 0.000 description 1
- 230000005778 DNA damage Effects 0.000 description 1
- 231100000277 DNA damage Toxicity 0.000 description 1
- 230000004543 DNA replication Effects 0.000 description 1
- 208000008743 Desmoplastic Small Round Cell Tumor Diseases 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 239000001856 Ethyl cellulose Substances 0.000 description 1
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 1
- 208000001382 Experimental Melanoma Diseases 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 1
- 230000010337 G2 phase Effects 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- AVXURJPOCDRRFD-UHFFFAOYSA-N Hydroxylamine Chemical compound ON AVXURJPOCDRRFD-UHFFFAOYSA-N 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 101800001751 Melanocyte-stimulating hormone alpha Proteins 0.000 description 1
- 206010027145 Melanocytic naevus Diseases 0.000 description 1
- 244000246386 Mentha pulegium Species 0.000 description 1
- 235000016257 Mentha pulegium Nutrition 0.000 description 1
- 235000004357 Mentha x piperita Nutrition 0.000 description 1
- 206010059282 Metastases to central nervous system Diseases 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- HWOAYKOYQJLDSC-UHFFFAOYSA-N NC(c1cc(N=N)c[s]1)=O Chemical compound NC(c1cc(N=N)c[s]1)=O HWOAYKOYQJLDSC-UHFFFAOYSA-N 0.000 description 1
- 206010028813 Nausea Diseases 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- 101150093308 POMC gene Proteins 0.000 description 1
- 239000004264 Petrolatum Substances 0.000 description 1
- 208000009077 Pigmented Nevus Diseases 0.000 description 1
- 229920002565 Polyethylene Glycol 400 Polymers 0.000 description 1
- 102000006382 Ribonucleases Human genes 0.000 description 1
- 108010083644 Ribonucleases Proteins 0.000 description 1
- 229920001800 Shellac Polymers 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 206010042496 Sunburn Diseases 0.000 description 1
- 108010078814 Tumor Suppressor Protein p53 Proteins 0.000 description 1
- AZFNGPAYDKGCRB-XCPIVNJJSA-M [(1s,2s)-2-amino-1,2-diphenylethyl]-(4-methylphenyl)sulfonylazanide;chlororuthenium(1+);1-methyl-4-propan-2-ylbenzene Chemical compound [Ru+]Cl.CC(C)C1=CC=C(C)C=C1.C1=CC(C)=CC=C1S(=O)(=O)[N-][C@@H](C=1C=CC=CC=1)[C@@H](N)C1=CC=CC=C1 AZFNGPAYDKGCRB-XCPIVNJJSA-M 0.000 description 1
- LNUFLCYMSVYYNW-ZPJMAFJPSA-N [(2r,3r,4s,5r,6r)-2-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[[(3s,5s,8r,9s,10s,13r,14s,17r)-10,13-dimethyl-17-[(2r)-6-methylheptan-2-yl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-yl]oxy]-4,5-disulfo Chemical compound O([C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1C[C@@H]2CC[C@H]3[C@@H]4CC[C@@H]([C@]4(CC[C@@H]3[C@@]2(C)CC1)C)[C@H](C)CCCC(C)C)[C@H]1O[C@H](COS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]1OS(O)(=O)=O LNUFLCYMSVYYNW-ZPJMAFJPSA-N 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 230000002152 alkylating effect Effects 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 230000002491 angiogenic effect Effects 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000003127 anti-melanomic effect Effects 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 210000000436 anus Anatomy 0.000 description 1
- 230000001640 apoptogenic effect Effects 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- 229940120638 avastin Drugs 0.000 description 1
- 239000003899 bactericide agent Substances 0.000 description 1
- 210000002469 basement membrane Anatomy 0.000 description 1
- 235000013871 bee wax Nutrition 0.000 description 1
- 239000012166 beeswax Substances 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 235000019445 benzyl alcohol Nutrition 0.000 description 1
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000000975 bioactive effect Effects 0.000 description 1
- 230000002051 biphasic effect Effects 0.000 description 1
- GXJABQQUPOEUTA-RDJZCZTQSA-N bortezomib Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)B(O)O)NC(=O)C=1N=CC=NC=1)C1=CC=CC=C1 GXJABQQUPOEUTA-RDJZCZTQSA-N 0.000 description 1
- 230000031709 bromination Effects 0.000 description 1
- 238000005893 bromination reaction Methods 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 150000003857 carboxamides Chemical class 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 230000025084 cell cycle arrest Effects 0.000 description 1
- 230000005779 cell damage Effects 0.000 description 1
- 208000037887 cell injury Diseases 0.000 description 1
- 230000012292 cell migration Effects 0.000 description 1
- 238000002737 cell proliferation kit Methods 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 229960005395 cetuximab Drugs 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 229940112822 chewing gum Drugs 0.000 description 1
- 235000015218 chewing gum Nutrition 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 229940110456 cocoa butter Drugs 0.000 description 1
- 235000019868 cocoa butter Nutrition 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 229940075614 colloidal silicon dioxide Drugs 0.000 description 1
- 229940125773 compound 10 Drugs 0.000 description 1
- 229940125797 compound 12 Drugs 0.000 description 1
- 229940126543 compound 14 Drugs 0.000 description 1
- 229940125758 compound 15 Drugs 0.000 description 1
- 229940126142 compound 16 Drugs 0.000 description 1
- 229940125782 compound 2 Drugs 0.000 description 1
- 229940125898 compound 5 Drugs 0.000 description 1
- 239000007891 compressed tablet Substances 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 230000001186 cumulative effect Effects 0.000 description 1
- DGJMPUGMZIKDRO-UHFFFAOYSA-N cyanoacetamide Chemical compound NC(=O)CC#N DGJMPUGMZIKDRO-UHFFFAOYSA-N 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 229940104302 cytosine Drugs 0.000 description 1
- 230000001086 cytosolic effect Effects 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 239000007857 degradation product Substances 0.000 description 1
- 230000002939 deleterious effect Effects 0.000 description 1
- 238000000326 densiometry Methods 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 238000006193 diazotization reaction Methods 0.000 description 1
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 1
- 230000008034 disappearance Effects 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 210000003038 endothelium Anatomy 0.000 description 1
- 239000002702 enteric coating Substances 0.000 description 1
- 238000009505 enteric coating Methods 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 230000002327 eosinophilic effect Effects 0.000 description 1
- 210000004920 epithelial cell of skin Anatomy 0.000 description 1
- 235000019325 ethyl cellulose Nutrition 0.000 description 1
- 229920001249 ethyl cellulose Polymers 0.000 description 1
- 229940093476 ethylene glycol Drugs 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 239000010685 fatty oil Substances 0.000 description 1
- 239000012894 fetal calf serum Substances 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 235000011194 food seasoning agent Nutrition 0.000 description 1
- 239000012737 fresh medium Substances 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 231100000118 genetic alteration Toxicity 0.000 description 1
- 230000004077 genetic alteration Effects 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 210000004392 genitalia Anatomy 0.000 description 1
- 230000024924 glomerular filtration Effects 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 230000009036 growth inhibition Effects 0.000 description 1
- 229940093915 gynecological organic acid Drugs 0.000 description 1
- 125000001072 heteroaryl group Chemical group 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 235000001050 hortel pimenta Nutrition 0.000 description 1
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 239000000367 immunologic factor Substances 0.000 description 1
- 230000001506 immunosuppresive effect Effects 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 239000003701 inert diluent Substances 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 238000011396 initial chemotherapy Methods 0.000 description 1
- 229940047124 interferons Drugs 0.000 description 1
- 229940047122 interleukins Drugs 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 230000009545 invasion Effects 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- ZLVXBBHTMQJRSX-VMGNSXQWSA-N jdtic Chemical compound C1([C@]2(C)CCN(C[C@@H]2C)C[C@H](C(C)C)NC(=O)[C@@H]2NCC3=CC(O)=CC=C3C2)=CC=CC(O)=C1 ZLVXBBHTMQJRSX-VMGNSXQWSA-N 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- XMGQYMWWDOXHJM-UHFFFAOYSA-N limonene Chemical compound CC(=C)C1CCC(C)=CC1 XMGQYMWWDOXHJM-UHFFFAOYSA-N 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 208000019423 liver disease Diseases 0.000 description 1
- 230000005976 liver dysfunction Effects 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 210000001165 lymph node Anatomy 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-M mandelate Chemical compound [O-]C(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-M 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000008099 melanin synthesis Effects 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- DDLFZCCIZTVFBQ-UHFFFAOYSA-N methyl 3-nitrothiophene-2-carboxylate Chemical compound COC(=O)C=1SC=CC=1[N+]([O-])=O DDLFZCCIZTVFBQ-UHFFFAOYSA-N 0.000 description 1
- AFAFEIMOITYVED-UHFFFAOYSA-N methyl 4-bromo-3-(dimethylaminodiazenyl)thiophene-2-carboxylate Chemical compound COC(=O)C=1SC=C(Br)C=1N=NN(C)C AFAFEIMOITYVED-UHFFFAOYSA-N 0.000 description 1
- ABWPLDHHOYVTPB-UHFFFAOYSA-N methyl 4-nitrothiophene-2-carboxylate Chemical compound COC(=O)C1=CC([N+]([O-])=O)=CS1 ABWPLDHHOYVTPB-UHFFFAOYSA-N 0.000 description 1
- NNQTUMGJWXJMIR-UHFFFAOYSA-N methyl 5-aminothiophene-2-carboxylate Chemical compound COC(=O)C1=CC=C(N)S1 NNQTUMGJWXJMIR-UHFFFAOYSA-N 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- 229960001047 methyl salicylate Drugs 0.000 description 1
- 230000011987 methylation Effects 0.000 description 1
- 238000007069 methylation reaction Methods 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 238000001000 micrograph Methods 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 230000000394 mitotic effect Effects 0.000 description 1
- HDZGCSFEDULWCS-UHFFFAOYSA-N monomethylhydrazine Chemical compound CNN HDZGCSFEDULWCS-UHFFFAOYSA-N 0.000 description 1
- 210000000214 mouth Anatomy 0.000 description 1
- 210000002200 mouth mucosa Anatomy 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 239000007908 nanoemulsion Substances 0.000 description 1
- 230000008693 nausea Effects 0.000 description 1
- 210000003739 neck Anatomy 0.000 description 1
- 150000002828 nitro derivatives Chemical class 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 231100000344 non-irritating Toxicity 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 239000000346 nonvolatile oil Substances 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 230000000269 nucleophilic effect Effects 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 239000007968 orange flavor Substances 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 150000003891 oxalate salts Chemical class 0.000 description 1
- 238000007067 oxidative demethylation reaction Methods 0.000 description 1
- 239000006201 parenteral dosage form Substances 0.000 description 1
- 239000003182 parenteral nutrition solution Substances 0.000 description 1
- 229940066842 petrolatum Drugs 0.000 description 1
- 235000019271 petrolatum Nutrition 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 230000008092 positive effect Effects 0.000 description 1
- 235000010289 potassium nitrite Nutrition 0.000 description 1
- 239000004304 potassium nitrite Substances 0.000 description 1
- 150000003141 primary amines Chemical group 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 230000006337 proteolytic cleavage Effects 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 230000000637 radiosensitizating effect Effects 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 210000000664 rectum Anatomy 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- 230000008085 renal dysfunction Effects 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 description 1
- 229940081974 saccharin Drugs 0.000 description 1
- 235000019204 saccharin Nutrition 0.000 description 1
- 239000000901 saccharin and its Na,K and Ca salt Substances 0.000 description 1
- 239000012266 salt solution Substances 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical compound O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 description 1
- 238000009094 second-line therapy Methods 0.000 description 1
- 150000003335 secondary amines Chemical class 0.000 description 1
- 239000004208 shellac Substances 0.000 description 1
- 229940113147 shellac Drugs 0.000 description 1
- ZLGIYFNHBLSMPS-ATJNOEHPSA-N shellac Chemical compound OCCCCCC(O)C(O)CCCCCCCC(O)=O.C1C23[C@H](C(O)=O)CCC2[C@](C)(CO)[C@@H]1C(C(O)=O)=C[C@@H]3O ZLGIYFNHBLSMPS-ATJNOEHPSA-N 0.000 description 1
- 235000013874 shellac Nutrition 0.000 description 1
- 210000004927 skin cell Anatomy 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- WBHQBSYUUJJSRZ-UHFFFAOYSA-M sodium bisulfate Chemical compound [Na+].OS([O-])(=O)=O WBHQBSYUUJJSRZ-UHFFFAOYSA-M 0.000 description 1
- 229910000342 sodium bisulfate Inorganic materials 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- 229910052979 sodium sulfide Inorganic materials 0.000 description 1
- GRVFOGOEDUUMBP-UHFFFAOYSA-N sodium sulfide (anhydrous) Chemical compound [Na+].[Na+].[S-2] GRVFOGOEDUUMBP-UHFFFAOYSA-N 0.000 description 1
- 230000003381 solubilizing effect Effects 0.000 description 1
- 125000006850 spacer group Chemical group 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 235000000891 standard diet Nutrition 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 230000000475 sunscreen effect Effects 0.000 description 1
- 239000000516 sunscreening agent Substances 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 1
- 239000012049 topical pharmaceutical composition Substances 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 230000037317 transdermal delivery Effects 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- AYNNSCRYTDRFCP-UHFFFAOYSA-N triazene Chemical compound NN=N AYNNSCRYTDRFCP-UHFFFAOYSA-N 0.000 description 1
- 210000005239 tubule Anatomy 0.000 description 1
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 1
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 1
- 229940099039 velcade Drugs 0.000 description 1
- 239000008215 water for injection Substances 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/26—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D333/38—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/26—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D333/38—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
- C07D333/40—Thiophene-2-carboxylic acid
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D495/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms
- C07D495/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms in which the condensed system contains two hetero rings
- C07D495/04—Ortho-condensed systems
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Oncology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Heterocyclic Compounds Containing Sulfur Atoms (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Oxygen Or Sulfur (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN2230/CHE/08 | 2008-09-15 | ||
| IN2230CH2008 | 2008-09-15 | ||
| PCT/IN2009/000504 WO2010029577A2 (en) | 2008-09-15 | 2009-09-14 | Anti-cancer drugs and uses relating thereto for metastatic malignant melanoma and other cancers |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2012502900A true JP2012502900A (ja) | 2012-02-02 |
| JP2012502900A5 JP2012502900A5 (enExample) | 2012-10-25 |
Family
ID=41565903
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011526634A Pending JP2012502900A (ja) | 2008-09-15 | 2009-09-14 | 抗癌剤並びにそれに関する転移性悪性黒色腫及び他の癌についての使用 |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US8258119B2 (enExample) |
| EP (1) | EP2324006B1 (enExample) |
| JP (1) | JP2012502900A (enExample) |
| KR (1) | KR20110063537A (enExample) |
| CN (1) | CN102149703B (enExample) |
| AU (1) | AU2009290365B2 (enExample) |
| BR (1) | BRPI0913485A2 (enExample) |
| CA (1) | CA2736732A1 (enExample) |
| IL (1) | IL211366A0 (enExample) |
| NZ (1) | NZ591394A (enExample) |
| WO (1) | WO2010029577A2 (enExample) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| BRPI1006651A2 (pt) * | 2009-04-27 | 2015-08-25 | Kasina Laila Innova Pharmaceuticals Private Ltd | Medicamentos anticancer e usos relacionados para melanome maligno e outros canceres |
| NO2686520T3 (enExample) | 2011-06-06 | 2018-03-17 | ||
| CA2837560C (en) * | 2011-06-06 | 2017-02-14 | Akebia Therapeutics Inc. | Compounds and compositions for stabilizing hypoxia inducible factor-2 alpha as a method for treating cancer |
| PE20160194A1 (es) | 2013-06-13 | 2016-04-20 | Akebia Therapeutics Inc | Composiciones y metodos para tratar anemia |
| US9593095B2 (en) | 2013-06-14 | 2017-03-14 | Duke University | Methods for the treatment of bacterial infections |
| DE112014006766T5 (de) | 2014-06-27 | 2017-04-27 | Ecole Polytechnique Federale De Lausanne (Epfl) Epfl-Tto | Herstellung und medizinische Verwendung von Triazenen |
| AU2016209126A1 (en) | 2015-01-23 | 2017-08-10 | Akebia Therapeutics, Inc. | Solid forms of 2-(5-(3-fluorophenyl)-3-hydroxypicolinamido)acetic acid, compositions, and uses thereof |
| SG11201707994UA (en) | 2015-04-01 | 2017-10-30 | Akebia Therapeutics Inc | Compositions and methods for treating anemia |
| WO2016187191A1 (en) | 2015-05-18 | 2016-11-24 | Medoc Pharmaceutical Co., Ltd. | A pharmaceutical co-crystal and use thereof |
| CA2988989A1 (en) | 2015-06-19 | 2016-12-22 | Syn-Nat Products Enterprise LLC | Composition containing carboplatin and use |
| WO2016205785A1 (en) | 2015-06-19 | 2016-12-22 | Syn-Nat Products Enterprise LLC | Pharmaceutical composition of carboplatin based co-crystals and use thereof |
| JP6929234B2 (ja) | 2015-06-25 | 2021-09-01 | シン−ナット プロダクツ エンタープライズ エルエルシー | 医薬共結晶組成物及びその用途 |
| AU2019265629B2 (en) | 2018-05-09 | 2024-09-12 | Akebia Therapeutics, Inc. | Process for preparing 2-((5-(3-chlorophenyl)-3-hydroxypyridine-2-carbonyl)amino)acetic acid |
| KR102648781B1 (ko) | 2021-11-09 | 2024-03-19 | 국립공주대학교 산학협력단 | 항암 활성을 갖는 인데노피라졸론 유도체 화합물, 그의 제조방법 및 그의 용도 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS4936234B1 (enExample) * | 1968-09-09 | 1974-09-28 |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE1020641B (de) | 1955-10-15 | 1957-12-12 | Basf Ag | Verfahren zur Herstellung von Derivaten der 3-Oxythiophen-2-carbonsaeure |
-
2009
- 2009-09-14 WO PCT/IN2009/000504 patent/WO2010029577A2/en not_active Ceased
- 2009-09-14 EP EP09760623.0A patent/EP2324006B1/en not_active Not-in-force
- 2009-09-14 AU AU2009290365A patent/AU2009290365B2/en not_active Ceased
- 2009-09-14 JP JP2011526634A patent/JP2012502900A/ja active Pending
- 2009-09-14 CN CN200980135652.3A patent/CN102149703B/zh not_active Expired - Fee Related
- 2009-09-14 CA CA2736732A patent/CA2736732A1/en not_active Abandoned
- 2009-09-14 BR BRPI0913485A patent/BRPI0913485A2/pt not_active IP Right Cessation
- 2009-09-14 KR KR1020117008490A patent/KR20110063537A/ko not_active Ceased
- 2009-09-14 NZ NZ591394A patent/NZ591394A/xx not_active IP Right Cessation
- 2009-09-15 US US12/559,811 patent/US8258119B2/en not_active Expired - Fee Related
-
2011
- 2011-02-23 IL IL211366A patent/IL211366A0/en unknown
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS4936234B1 (enExample) * | 1968-09-09 | 1974-09-28 |
Non-Patent Citations (5)
| Title |
|---|
| JPN6014005693; Journal of the Chemical Society, Perkin Transactions 2; Physical Organic Chemistry No.11, 1998, p.2361-2363 * |
| JPN6014005695; Journal of Medicinal Chemistry Vol.35, No.18, 1992, p.3377-3382 * |
| JPN6014005697; Journal of Medicinal Chemistry Vol.21, No.6, 1978, p.563-574 * |
| JPN6014005699; Journal of Pharmaceutical Science Vol.60, No.4, 1971, p.554-560 * |
| JPN6014005700; Anticancer Research Vol.19, No.3A, 1999, p.2127-2131 * |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2009290365B2 (en) | 2014-08-14 |
| CN102149703B (zh) | 2014-09-10 |
| AU2009290365A1 (en) | 2010-03-18 |
| WO2010029577A3 (en) | 2010-05-06 |
| KR20110063537A (ko) | 2011-06-10 |
| CN102149703A (zh) | 2011-08-10 |
| CA2736732A1 (en) | 2010-03-18 |
| NZ591394A (en) | 2013-03-28 |
| US20100068178A1 (en) | 2010-03-18 |
| EP2324006B1 (en) | 2014-08-13 |
| US8258119B2 (en) | 2012-09-04 |
| EP2324006A2 (en) | 2011-05-25 |
| IL211366A0 (en) | 2011-04-28 |
| WO2010029577A2 (en) | 2010-03-18 |
| BRPI0913485A2 (pt) | 2016-06-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2324006B1 (en) | Anti-cancer drugs and uses relating thereto for metastatic malignant melanoma and other cancers | |
| JP5571168B2 (ja) | 抗癌剤ならびに悪性黒色腫および他の癌に関連する使用 | |
| JP6219882B2 (ja) | IRE−1αインヒビター | |
| US10315990B2 (en) | Isothiocyanate compounds and isothiocyanate XPO1 protein inhibitor drugs thereof | |
| JP2021503013A (ja) | Acss2阻害剤およびその使用方法 | |
| JP2005506334A (ja) | NF−κB阻害剤 | |
| JP2004523476A (ja) | NF−κB阻害剤 | |
| JP2021504457A (ja) | チエノ環系化合物およびその合成方法と応用 | |
| WO2003029241A1 (en) | Chk1 kinase inhibitors | |
| ES2782357T3 (es) | Inhibidores de IRE 1 alfa | |
| JP2004532797A (ja) | NF−κB阻害剤 | |
| CN114080389A (zh) | Nrf2活性化化合物 | |
| ES2285125T3 (es) | Inhibidores de nf-kb. | |
| CN106535894A (zh) | 用于癌症治疗的mcl‑1调节化合物 | |
| US9278958B2 (en) | Anti-cancer compounds | |
| CN102600164B (zh) | 嘧啶醚类化合物作为制备抗肿瘤药物的应用 | |
| HK1154863A (en) | Anti-cancer drugs and uses relating thereto for metastatic malignant melanoma and other cancers | |
| CN117986202B (zh) | 具有ptpn2抑制活性的1,2,4-噻二唑烷-3,5-二酮化合物及其制备方法和应用 | |
| KR20140072309A (ko) | 신규한 이치환 아다만틸 유도체 또는 이의 약학적으로 허용가능한 염, 이의 제조방법 및 이를 유효성분으로 함유하는 암 전이 억제용 약학적 조성물 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120907 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20120907 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140210 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140509 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20140708 |