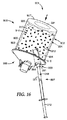
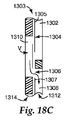
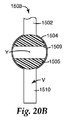
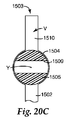
JP2011502544A - 試料の調製容器及び方法 - Google Patents
試料の調製容器及び方法 Download PDFInfo
- Publication number
- JP2011502544A JP2011502544A JP2010535042A JP2010535042A JP2011502544A JP 2011502544 A JP2011502544 A JP 2011502544A JP 2010535042 A JP2010535042 A JP 2010535042A JP 2010535042 A JP2010535042 A JP 2010535042A JP 2011502544 A JP2011502544 A JP 2011502544A
- Authority
- JP
- Japan
- Prior art keywords
- sample
- sample preparation
- valve
- self
- lid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000002360 preparation method Methods 0.000 title claims abstract description 276
- 238000000034 method Methods 0.000 title claims abstract description 74
- 239000012491 analyte Substances 0.000 claims abstract description 151
- 239000007788 liquid Substances 0.000 claims abstract description 147
- 239000000203 mixture Substances 0.000 claims abstract description 120
- 239000003085 diluting agent Substances 0.000 claims abstract description 78
- 239000012530 fluid Substances 0.000 claims abstract description 46
- 238000004891 communication Methods 0.000 claims abstract description 36
- 238000012360 testing method Methods 0.000 claims abstract description 14
- 238000003825 pressing Methods 0.000 claims abstract description 12
- 239000000523 sample Substances 0.000 claims description 482
- 239000000706 filtrate Substances 0.000 claims description 85
- 230000008878 coupling Effects 0.000 claims description 32
- 238000010168 coupling process Methods 0.000 claims description 32
- 238000005859 coupling reaction Methods 0.000 claims description 32
- 235000013305 food Nutrition 0.000 claims description 26
- 230000004888 barrier function Effects 0.000 claims description 25
- 244000005700 microbiome Species 0.000 claims description 22
- 239000000126 substance Substances 0.000 claims description 22
- 238000001914 filtration Methods 0.000 claims description 15
- 238000004458 analytical method Methods 0.000 claims description 14
- 238000003756 stirring Methods 0.000 claims description 13
- 238000001514 detection method Methods 0.000 claims description 12
- 238000003556 assay Methods 0.000 claims description 11
- 235000015097 nutrients Nutrition 0.000 claims description 11
- 244000045947 parasite Species 0.000 claims description 11
- 229910021645 metal ion Inorganic materials 0.000 claims description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 9
- 238000005336 cracking Methods 0.000 claims description 8
- 239000003053 toxin Substances 0.000 claims description 7
- 231100000765 toxin Toxicity 0.000 claims description 7
- 239000013566 allergen Substances 0.000 claims description 6
- ZKHQWZAMYRWXGA-KQYNXXCUSA-J ATP(4-) Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O)[C@@H](O)[C@H]1O ZKHQWZAMYRWXGA-KQYNXXCUSA-J 0.000 claims description 5
- ZKHQWZAMYRWXGA-UHFFFAOYSA-N Adenosine triphosphate Natural products C1=NC=2C(N)=NC=NC=2N1C1OC(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)C(O)C1O ZKHQWZAMYRWXGA-UHFFFAOYSA-N 0.000 claims description 5
- 241000193163 Clostridioides difficile Species 0.000 claims description 5
- 239000000654 additive Substances 0.000 claims description 5
- 230000000845 anti-microbial effect Effects 0.000 claims description 5
- 239000003966 growth inhibitor Substances 0.000 claims description 5
- 239000003960 organic solvent Substances 0.000 claims description 5
- 241000193738 Bacillus anthracis Species 0.000 claims description 4
- 241000194033 Enterococcus Species 0.000 claims description 4
- 108090000790 Enzymes Proteins 0.000 claims description 4
- 102000004190 Enzymes Human genes 0.000 claims description 4
- 241000191967 Staphylococcus aureus Species 0.000 claims description 4
- 239000004599 antimicrobial Substances 0.000 claims description 4
- 229940065181 bacillus anthracis Drugs 0.000 claims description 4
- 230000009977 dual effect Effects 0.000 claims description 4
- 239000006174 pH buffer Substances 0.000 claims description 4
- 239000008223 sterile water Substances 0.000 claims description 4
- 239000004094 surface-active agent Substances 0.000 claims description 4
- 241000589291 Acinetobacter Species 0.000 claims description 3
- 241000193830 Bacillus <bacterium> Species 0.000 claims description 3
- 241000193755 Bacillus cereus Species 0.000 claims description 3
- 241000589875 Campylobacter jejuni Species 0.000 claims description 3
- 241000588724 Escherichia coli Species 0.000 claims description 3
- 241000186779 Listeria monocytogenes Species 0.000 claims description 3
- 241001263478 Norovirus Species 0.000 claims description 3
- 241000714209 Norwalk virus Species 0.000 claims description 3
- 241000589517 Pseudomonas aeruginosa Species 0.000 claims description 3
- 241000702670 Rotavirus Species 0.000 claims description 3
- 241000607142 Salmonella Species 0.000 claims description 3
- 108010059993 Vancomycin Proteins 0.000 claims description 3
- 241000607598 Vibrio Species 0.000 claims description 3
- 238000004587 chromatography analysis Methods 0.000 claims description 3
- 238000004611 spectroscopical analysis Methods 0.000 claims description 3
- 241000701161 unidentified adenovirus Species 0.000 claims description 3
- 229960003165 vancomycin Drugs 0.000 claims description 3
- MYPYJXKWCTUITO-LYRMYLQWSA-N vancomycin Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=C2C=C3C=C1OC1=CC=C(C=C1Cl)[C@@H](O)[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@H]3C(=O)N[C@H]1C(=O)N[C@H](C(N[C@@H](C3=CC(O)=CC(O)=C3C=3C(O)=CC=C1C=3)C(O)=O)=O)[C@H](O)C1=CC=C(C(=C1)Cl)O2)=O)NC(=O)[C@@H](CC(C)C)NC)[C@H]1C[C@](C)(N)[C@H](O)[C@H](C)O1 MYPYJXKWCTUITO-LYRMYLQWSA-N 0.000 claims description 3
- MYPYJXKWCTUITO-UHFFFAOYSA-N vancomycin Natural products O1C(C(=C2)Cl)=CC=C2C(O)C(C(NC(C2=CC(O)=CC(O)=C2C=2C(O)=CC=C3C=2)C(O)=O)=O)NC(=O)C3NC(=O)C2NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(CC(C)C)NC)C(O)C(C=C3Cl)=CC=C3OC3=CC2=CC1=C3OC1OC(CO)C(O)C(O)C1OC1CC(C)(N)C(O)C(C)O1 MYPYJXKWCTUITO-UHFFFAOYSA-N 0.000 claims description 3
- 229930195730 Aflatoxin Natural products 0.000 claims description 2
- XWIYFDMXXLINPU-UHFFFAOYSA-N Aflatoxin G Chemical compound O=C1OCCC2=C1C(=O)OC1=C2C(OC)=CC2=C1C1C=COC1O2 XWIYFDMXXLINPU-UHFFFAOYSA-N 0.000 claims description 2
- 235000017060 Arachis glabrata Nutrition 0.000 claims description 2
- 235000010777 Arachis hypogaea Nutrition 0.000 claims description 2
- 235000018262 Arachis monticola Nutrition 0.000 claims description 2
- 206010012735 Diarrhoea Diseases 0.000 claims description 2
- 239000005409 aflatoxin Substances 0.000 claims description 2
- 238000010256 biochemical assay Methods 0.000 claims description 2
- 239000013068 control sample Substances 0.000 claims description 2
- 238000012136 culture method Methods 0.000 claims description 2
- 230000000120 cytopathologic effect Effects 0.000 claims description 2
- 238000002784 cytotoxicity assay Methods 0.000 claims description 2
- 231100000263 cytotoxicity test Toxicity 0.000 claims description 2
- 231100000655 enterotoxin Toxicity 0.000 claims description 2
- 238000010353 genetic engineering Methods 0.000 claims description 2
- 238000010324 immunological assay Methods 0.000 claims description 2
- 230000002906 microbiologic effect Effects 0.000 claims description 2
- 238000000386 microscopy Methods 0.000 claims description 2
- 235000020232 peanut Nutrition 0.000 claims description 2
- 238000002798 spectrophotometry method Methods 0.000 claims description 2
- 238000002076 thermal analysis method Methods 0.000 claims description 2
- 238000004448 titration Methods 0.000 claims description 2
- 230000000996 additive effect Effects 0.000 claims 3
- 238000000518 rheometry Methods 0.000 claims 3
- 241000193468 Clostridium perfringens Species 0.000 claims 2
- 241001553178 Arachis glabrata Species 0.000 claims 1
- 238000012211 viral plaque assay Methods 0.000 claims 1
- 239000000463 material Substances 0.000 description 44
- 239000002198 insoluble material Substances 0.000 description 23
- -1 Mercury ions Chemical class 0.000 description 21
- 239000007789 gas Substances 0.000 description 19
- 230000006870 function Effects 0.000 description 18
- 230000002093 peripheral effect Effects 0.000 description 18
- 230000008569 process Effects 0.000 description 18
- 238000013019 agitation Methods 0.000 description 11
- 239000002609 medium Substances 0.000 description 11
- 241000894006 Bacteria Species 0.000 description 10
- 239000004743 Polypropylene Substances 0.000 description 10
- 229920001155 polypropylene Polymers 0.000 description 10
- 239000002904 solvent Substances 0.000 description 10
- 229920001778 nylon Polymers 0.000 description 8
- 108020004414 DNA Proteins 0.000 description 7
- 244000000013 helminth Species 0.000 description 7
- 238000002156 mixing Methods 0.000 description 7
- 239000011148 porous material Substances 0.000 description 7
- 238000007789 sealing Methods 0.000 description 7
- 239000007787 solid Substances 0.000 description 7
- 239000002195 soluble material Substances 0.000 description 7
- 238000003466 welding Methods 0.000 description 7
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 6
- 229920001684 low density polyethylene Polymers 0.000 description 6
- 239000004702 low-density polyethylene Substances 0.000 description 6
- 239000012528 membrane Substances 0.000 description 6
- 229910052751 metal Inorganic materials 0.000 description 6
- 239000002184 metal Substances 0.000 description 6
- 229920000515 polycarbonate Polymers 0.000 description 6
- 239000004417 polycarbonate Substances 0.000 description 6
- 235000018102 proteins Nutrition 0.000 description 6
- 108090000623 proteins and genes Proteins 0.000 description 6
- 102000004169 proteins and genes Human genes 0.000 description 6
- 239000004677 Nylon Substances 0.000 description 5
- 239000004698 Polyethylene Substances 0.000 description 5
- 239000000919 ceramic Substances 0.000 description 5
- 239000003795 chemical substances by application Substances 0.000 description 5
- 238000002716 delivery method Methods 0.000 description 5
- 208000015181 infectious disease Diseases 0.000 description 5
- 239000002245 particle Substances 0.000 description 5
- 229920000573 polyethylene Polymers 0.000 description 5
- 229920000098 polyolefin Polymers 0.000 description 5
- 230000002829 reductive effect Effects 0.000 description 5
- 238000005464 sample preparation method Methods 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- 108700012359 toxins Proteins 0.000 description 5
- 238000011144 upstream manufacturing Methods 0.000 description 5
- 241000224489 Amoeba Species 0.000 description 4
- 241000233866 Fungi Species 0.000 description 4
- 241000244206 Nematoda Species 0.000 description 4
- 210000004027 cell Anatomy 0.000 description 4
- 230000005484 gravity Effects 0.000 description 4
- 239000001963 growth medium Substances 0.000 description 4
- 229920001903 high density polyethylene Polymers 0.000 description 4
- 239000004700 high-density polyethylene Substances 0.000 description 4
- 239000002207 metabolite Substances 0.000 description 4
- 238000000465 moulding Methods 0.000 description 4
- 244000052769 pathogen Species 0.000 description 4
- 229920000728 polyester Polymers 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 238000001556 precipitation Methods 0.000 description 4
- 239000004952 Polyamide Substances 0.000 description 3
- 241000700605 Viruses Species 0.000 description 3
- 239000000853 adhesive Substances 0.000 description 3
- 230000001070 adhesive effect Effects 0.000 description 3
- 229910052782 aluminium Inorganic materials 0.000 description 3
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 238000011109 contamination Methods 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 150000002739 metals Chemical class 0.000 description 3
- 229920002647 polyamide Polymers 0.000 description 3
- 229920000306 polymethylpentene Polymers 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- 238000010998 test method Methods 0.000 description 3
- 210000002700 urine Anatomy 0.000 description 3
- 229920000178 Acrylic resin Polymers 0.000 description 2
- 239000004925 Acrylic resin Substances 0.000 description 2
- 241000244203 Caenorhabditis elegans Species 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- KZBUYRJDOAKODT-UHFFFAOYSA-N Chlorine Chemical compound ClCl KZBUYRJDOAKODT-UHFFFAOYSA-N 0.000 description 2
- 241000193403 Clostridium Species 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- 241000725303 Human immunodeficiency virus Species 0.000 description 2
- 241000701806 Human papillomavirus Species 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- 241000186781 Listeria Species 0.000 description 2
- 229920001410 Microfiber Polymers 0.000 description 2
- KFSLWBXXFJQRDL-UHFFFAOYSA-N Peracetic acid Chemical compound CC(=O)OO KFSLWBXXFJQRDL-UHFFFAOYSA-N 0.000 description 2
- 241000405070 Percophidae Species 0.000 description 2
- 239000004793 Polystyrene Substances 0.000 description 2
- 241001354013 Salmonella enterica subsp. enterica serovar Enteritidis Species 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 241001148470 aerobic bacillus Species 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 238000003490 calendering Methods 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 235000013601 eggs Nutrition 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 239000004744 fabric Substances 0.000 description 2
- 239000006260 foam Substances 0.000 description 2
- 239000003365 glass fiber Substances 0.000 description 2
- 239000003673 groundwater Substances 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- 239000005556 hormone Substances 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 230000002458 infectious effect Effects 0.000 description 2
- 238000005304 joining Methods 0.000 description 2
- KWGKDLIKAYFUFQ-UHFFFAOYSA-M lithium chloride Chemical compound [Li+].[Cl-] KWGKDLIKAYFUFQ-UHFFFAOYSA-M 0.000 description 2
- 239000003658 microfiber Substances 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 239000002991 molded plastic Substances 0.000 description 2
- 230000003472 neutralizing effect Effects 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 230000001717 pathogenic effect Effects 0.000 description 2
- 238000003752 polymerase chain reaction Methods 0.000 description 2
- 229920002223 polystyrene Polymers 0.000 description 2
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 2
- 239000004810 polytetrafluoroethylene Substances 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 230000035755 proliferation Effects 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 238000010079 rubber tapping Methods 0.000 description 2
- VWDWKYIASSYTQR-UHFFFAOYSA-N sodium nitrate Chemical compound [Na+].[O-][N+]([O-])=O VWDWKYIASSYTQR-UHFFFAOYSA-N 0.000 description 2
- DAEPDZWVDSPTHF-UHFFFAOYSA-M sodium pyruvate Chemical compound [Na+].CC(=O)C([O-])=O DAEPDZWVDSPTHF-UHFFFAOYSA-M 0.000 description 2
- AKHNMLFCWUSKQB-UHFFFAOYSA-L sodium thiosulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=S AKHNMLFCWUSKQB-UHFFFAOYSA-L 0.000 description 2
- 235000019345 sodium thiosulphate Nutrition 0.000 description 2
- 230000003381 solubilizing effect Effects 0.000 description 2
- 235000013599 spices Nutrition 0.000 description 2
- 210000004215 spore Anatomy 0.000 description 2
- 229910001220 stainless steel Inorganic materials 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- 230000001954 sterilising effect Effects 0.000 description 2
- 238000004659 sterilization and disinfection Methods 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 239000001974 tryptic soy broth Substances 0.000 description 2
- 108010050327 trypticase-soy broth Proteins 0.000 description 2
- 239000013598 vector Substances 0.000 description 2
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- KKAJSJJFBSOMGS-UHFFFAOYSA-N 3,6-diamino-10-methylacridinium chloride Chemical compound [Cl-].C1=C(N)C=C2[N+](C)=C(C=C(N)C=C3)C3=CC2=C1 KKAJSJJFBSOMGS-UHFFFAOYSA-N 0.000 description 1
- 241000251468 Actinopterygii Species 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- WZPBZJONDBGPKJ-UHFFFAOYSA-N Antibiotic SQ 26917 Natural products O=C1N(S(O)(=O)=O)C(C)C1NC(=O)C(=NOC(C)(C)C(O)=O)C1=CSC(N)=N1 WZPBZJONDBGPKJ-UHFFFAOYSA-N 0.000 description 1
- 244000105624 Arachis hypogaea Species 0.000 description 1
- 241000726107 Blastocystis hominis Species 0.000 description 1
- 0 C1*(C2)C1C1C2C*=CC1 Chemical compound C1*(C2)C1C1C2C*=CC1 0.000 description 1
- 241000589876 Campylobacter Species 0.000 description 1
- 241000193155 Clostridium botulinum Species 0.000 description 1
- 241000186216 Corynebacterium Species 0.000 description 1
- 241001135265 Cronobacter sakazakii Species 0.000 description 1
- 241000195493 Cryptophyta Species 0.000 description 1
- 241000223935 Cryptosporidium Species 0.000 description 1
- 241000223936 Cryptosporidium parvum Species 0.000 description 1
- 241000016605 Cyclospora cayetanensis Species 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 241000588914 Enterobacter Species 0.000 description 1
- 241000588921 Enterobacteriaceae Species 0.000 description 1
- 241000498255 Enterobius vermicularis Species 0.000 description 1
- 241000588722 Escherichia Species 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 1
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 1
- 241000287828 Gallus gallus Species 0.000 description 1
- 241000224466 Giardia Species 0.000 description 1
- 108010034145 Helminth Proteins Proteins 0.000 description 1
- 238000004566 IR spectroscopy Methods 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 241000589248 Legionella Species 0.000 description 1
- 208000007764 Legionnaires' Disease Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- RJQXTJLFIWVMTO-TYNCELHUSA-N Methicillin Chemical compound COC1=CC=CC(OC)=C1C(=O)N[C@@H]1C(=O)N2[C@@H](C(O)=O)C(C)(C)S[C@@H]21 RJQXTJLFIWVMTO-TYNCELHUSA-N 0.000 description 1
- 241001430197 Mollicutes Species 0.000 description 1
- 241000186359 Mycobacterium Species 0.000 description 1
- 238000005481 NMR spectroscopy Methods 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- YJQPYGGHQPGBLI-UHFFFAOYSA-N Novobiocin Natural products O1C(C)(C)C(OC)C(OC(N)=O)C(O)C1OC1=CC=C(C(O)=C(NC(=O)C=2C=C(CC=C(C)C)C(O)=CC=2)C(=O)O2)C2=C1C YJQPYGGHQPGBLI-UHFFFAOYSA-N 0.000 description 1
- 244000131316 Panax pseudoginseng Species 0.000 description 1
- 235000005035 Panax pseudoginseng ssp. pseudoginseng Nutrition 0.000 description 1
- 235000003140 Panax quinquefolius Nutrition 0.000 description 1
- 239000001888 Peptone Substances 0.000 description 1
- 108010080698 Peptones Proteins 0.000 description 1
- 108020005120 Plant DNA Proteins 0.000 description 1
- 108010093965 Polymyxin B Proteins 0.000 description 1
- 102000029797 Prion Human genes 0.000 description 1
- 108091000054 Prion Proteins 0.000 description 1
- 241000589516 Pseudomonas Species 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 238000001069 Raman spectroscopy Methods 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 241000293869 Salmonella enterica subsp. enterica serovar Typhimurium Species 0.000 description 1
- 241000607768 Shigella Species 0.000 description 1
- 241000191940 Staphylococcus Species 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- 241000194017 Streptococcus Species 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- 241001489145 Trichuris trichiura Species 0.000 description 1
- 241000607265 Vibrio vulnificus Species 0.000 description 1
- 238000000441 X-ray spectroscopy Methods 0.000 description 1
- 241000607734 Yersinia <bacteria> Species 0.000 description 1
- 241000607447 Yersinia enterocolitica Species 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 229940023020 acriflavine Drugs 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 238000001042 affinity chromatography Methods 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 229960004821 amikacin Drugs 0.000 description 1
- LKCWBDHBTVXHDL-RMDFUYIESA-N amikacin Chemical compound O([C@@H]1[C@@H](N)C[C@H]([C@@H]([C@H]1O)O[C@@H]1[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O1)O)NC(=O)[C@@H](O)CCN)[C@H]1O[C@H](CN)[C@@H](O)[C@H](O)[C@H]1O LKCWBDHBTVXHDL-RMDFUYIESA-N 0.000 description 1
- 235000001014 amino acid Nutrition 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 238000005102 attenuated total reflection Methods 0.000 description 1
- 229960003644 aztreonam Drugs 0.000 description 1
- WZPBZJONDBGPKJ-VEHQQRBSSA-N aztreonam Chemical compound O=C1N(S([O-])(=O)=O)[C@@H](C)[C@@H]1NC(=O)C(=N/OC(C)(C)C(O)=O)\C1=CSC([NH3+])=N1 WZPBZJONDBGPKJ-VEHQQRBSSA-N 0.000 description 1
- 210000004666 bacterial spore Anatomy 0.000 description 1
- 239000007640 basal medium Substances 0.000 description 1
- 235000013361 beverage Nutrition 0.000 description 1
- 239000003833 bile salt Substances 0.000 description 1
- 229940093761 bile salts Drugs 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 229940011597 blastocystis hominis Drugs 0.000 description 1
- 239000007844 bleaching agent Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 235000008429 bread Nutrition 0.000 description 1
- 229960001506 brilliant green Drugs 0.000 description 1
- HXCILVUBKWANLN-UHFFFAOYSA-N brilliant green cation Chemical compound C1=CC(N(CC)CC)=CC=C1C(C=1C=CC=CC=1)=C1C=CC(=[N+](CC)CC)C=C1 HXCILVUBKWANLN-UHFFFAOYSA-N 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 239000013592 cell lysate Substances 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 235000013339 cereals Nutrition 0.000 description 1
- 235000013351 cheese Nutrition 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 235000013330 chicken meat Nutrition 0.000 description 1
- 229960005091 chloramphenicol Drugs 0.000 description 1
- WIIZWVCIJKGZOK-RKDXNWHRSA-N chloramphenicol Chemical compound ClC(Cl)C(=O)N[C@H](CO)[C@H](O)C1=CC=C([N+]([O-])=O)C=C1 WIIZWVCIJKGZOK-RKDXNWHRSA-N 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- CYDMQBQPVICBEU-UHFFFAOYSA-N chlorotetracycline Natural products C1=CC(Cl)=C2C(O)(C)C3CC4C(N(C)C)C(O)=C(C(N)=O)C(=O)C4(O)C(O)=C3C(=O)C2=C1O CYDMQBQPVICBEU-UHFFFAOYSA-N 0.000 description 1
- CYDMQBQPVICBEU-XRNKAMNCSA-N chlortetracycline Chemical compound C1=CC(Cl)=C2[C@](O)(C)[C@H]3C[C@H]4[C@H](N(C)C)C(O)=C(C(N)=O)C(=O)[C@@]4(O)C(O)=C3C(=O)C2=C1O CYDMQBQPVICBEU-XRNKAMNCSA-N 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 235000015142 cultured sour cream Nutrition 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 238000010908 decantation Methods 0.000 description 1
- 229960003964 deoxycholic acid Drugs 0.000 description 1
- 238000009795 derivation Methods 0.000 description 1
- 239000000645 desinfectant Substances 0.000 description 1
- 235000021185 dessert Nutrition 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 235000015872 dietary supplement Nutrition 0.000 description 1
- 235000021186 dishes Nutrition 0.000 description 1
- BVTBRVFYZUCAKH-UHFFFAOYSA-L disodium selenite Chemical compound [Na+].[Na+].[O-][Se]([O-])=O BVTBRVFYZUCAKH-UHFFFAOYSA-L 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000012154 double-distilled water Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 241001493065 dsRNA viruses Species 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- 208000001848 dysentery Diseases 0.000 description 1
- 235000013399 edible fruits Nutrition 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 239000013536 elastomeric material Substances 0.000 description 1
- 238000000840 electrochemical analysis Methods 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 244000079386 endoparasite Species 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 238000005530 etching Methods 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 235000019197 fats Nutrition 0.000 description 1
- 230000002550 fecal effect Effects 0.000 description 1
- 210000003608 fece Anatomy 0.000 description 1
- 238000009950 felting Methods 0.000 description 1
- 235000019688 fish Nutrition 0.000 description 1
- 235000013312 flour Nutrition 0.000 description 1
- 238000000799 fluorescence microscopy Methods 0.000 description 1
- 229920002313 fluoropolymer Polymers 0.000 description 1
- 235000012041 food component Nutrition 0.000 description 1
- 239000005417 food ingredient Substances 0.000 description 1
- 238000009615 fourier-transform spectroscopy Methods 0.000 description 1
- 238000001730 gamma-ray spectroscopy Methods 0.000 description 1
- 238000004817 gas chromatography Methods 0.000 description 1
- 235000008434 ginseng Nutrition 0.000 description 1
- 239000011491 glass wool Substances 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 235000011868 grain product Nutrition 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 229910001385 heavy metal Inorganic materials 0.000 description 1
- 238000009396 hybridization Methods 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 238000010820 immunofluorescence microscopy Methods 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 239000002917 insecticide Substances 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 238000004898 kneading Methods 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 210000002429 large intestine Anatomy 0.000 description 1
- 238000003698 laser cutting Methods 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 238000004811 liquid chromatography Methods 0.000 description 1
- 229940073577 lithium chloride Drugs 0.000 description 1
- 238000004020 luminiscence type Methods 0.000 description 1
- 229940002712 malachite green oxalate Drugs 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 230000013011 mating Effects 0.000 description 1
- 235000013372 meat Nutrition 0.000 description 1
- 238000010907 mechanical stirring Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 239000007769 metal material Substances 0.000 description 1
- 229960003085 meticillin Drugs 0.000 description 1
- 244000000010 microbial pathogen Species 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 230000004899 motility Effects 0.000 description 1
- MHWLWQUZZRMNGJ-UHFFFAOYSA-N nalidixic acid Chemical compound C1=C(C)N=C2N(CC)C=C(C(O)=O)C(=O)C2=C1 MHWLWQUZZRMNGJ-UHFFFAOYSA-N 0.000 description 1
- 229960000210 nalidixic acid Drugs 0.000 description 1
- 229920003052 natural elastomer Polymers 0.000 description 1
- 229920001194 natural rubber Polymers 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 239000004745 nonwoven fabric Substances 0.000 description 1
- YJQPYGGHQPGBLI-KGSXXDOSSA-N novobiocin Chemical compound O1C(C)(C)[C@H](OC)[C@@H](OC(N)=O)[C@@H](O)[C@@H]1OC1=CC=C(C(O)=C(NC(=O)C=2C=C(CC=C(C)C)C(O)=CC=2)C(=O)O2)C2=C1C YJQPYGGHQPGBLI-KGSXXDOSSA-N 0.000 description 1
- 229960002950 novobiocin Drugs 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 235000016709 nutrition Nutrition 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 238000000399 optical microscopy Methods 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 239000013110 organic ligand Substances 0.000 description 1
- 235000016046 other dairy product Nutrition 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 1
- 235000015927 pasta Nutrition 0.000 description 1
- 235000019319 peptone Nutrition 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 231100000614 poison Toxicity 0.000 description 1
- 229920005594 polymer fiber Polymers 0.000 description 1
- 229920000024 polymyxin B Polymers 0.000 description 1
- 229960005266 polymyxin b Drugs 0.000 description 1
- 108091033319 polynucleotide Proteins 0.000 description 1
- 102000040430 polynucleotide Human genes 0.000 description 1
- 239000002157 polynucleotide Substances 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- BFPJYWDBBLZXOM-UHFFFAOYSA-L potassium tellurite Chemical compound [K+].[K+].[O-][Te]([O-])=O BFPJYWDBBLZXOM-UHFFFAOYSA-L 0.000 description 1
- 230000001376 precipitating effect Effects 0.000 description 1
- 238000011045 prefiltration Methods 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 235000013324 preserved food Nutrition 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 238000005057 refrigeration Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 239000006254 rheological additive Substances 0.000 description 1
- 229940081623 rose bengal Drugs 0.000 description 1
- 229930187593 rose bengal Natural products 0.000 description 1
- AZJPTIGZZTZIDR-UHFFFAOYSA-L rose bengal Chemical compound [K+].[K+].[O-]C(=O)C1=C(Cl)C(Cl)=C(Cl)C(Cl)=C1C1=C2C=C(I)C(=O)C(I)=C2OC2=C(I)C([O-])=C(I)C=C21 AZJPTIGZZTZIDR-UHFFFAOYSA-L 0.000 description 1
- STRXNPAVPKGJQR-UHFFFAOYSA-N rose bengal A Natural products O1C(=O)C(C(=CC=C2Cl)Cl)=C2C21C1=CC(I)=C(O)C(I)=C1OC1=C(I)C(O)=C(I)C=C21 STRXNPAVPKGJQR-UHFFFAOYSA-N 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 235000015067 sauces Nutrition 0.000 description 1
- 238000004626 scanning electron microscopy Methods 0.000 description 1
- 235000014102 seafood Nutrition 0.000 description 1
- 210000002374 sebum Anatomy 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 239000013049 sediment Substances 0.000 description 1
- 239000006152 selective media Substances 0.000 description 1
- 229940082569 selenite Drugs 0.000 description 1
- MCAHWIHFGHIESP-UHFFFAOYSA-L selenite(2-) Chemical compound [O-][Se]([O-])=O MCAHWIHFGHIESP-UHFFFAOYSA-L 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- FHHPUSMSKHSNKW-SMOYURAASA-M sodium deoxycholate Chemical compound [Na+].C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC([O-])=O)C)[C@@]2(C)[C@@H](O)C1 FHHPUSMSKHSNKW-SMOYURAASA-M 0.000 description 1
- 239000004317 sodium nitrate Substances 0.000 description 1
- 235000010344 sodium nitrate Nutrition 0.000 description 1
- 229940001516 sodium nitrate Drugs 0.000 description 1
- 229940054269 sodium pyruvate Drugs 0.000 description 1
- 229960001471 sodium selenite Drugs 0.000 description 1
- 239000011781 sodium selenite Substances 0.000 description 1
- 235000015921 sodium selenite Nutrition 0.000 description 1
- HAEPBEMBOAIUPN-UHFFFAOYSA-L sodium tetrathionate Chemical compound O.O.[Na+].[Na+].[O-]S(=O)(=O)SSS([O-])(=O)=O HAEPBEMBOAIUPN-UHFFFAOYSA-L 0.000 description 1
- 229940001474 sodium thiosulfate Drugs 0.000 description 1
- 239000002689 soil Substances 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 235000014347 soups Nutrition 0.000 description 1
- 230000004763 spore germination Effects 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 229960000551 sulfacetamide sodium Drugs 0.000 description 1
- IHCDKJZZFOUARO-UHFFFAOYSA-M sulfacetamide sodium Chemical compound O.[Na+].CC(=O)[N-]S(=O)(=O)C1=CC=C(N)C=C1 IHCDKJZZFOUARO-UHFFFAOYSA-M 0.000 description 1
- SEEPANYCNGTZFQ-UHFFFAOYSA-N sulfadiazine Chemical compound C1=CC(N)=CC=C1S(=O)(=O)NC1=NC=CC=N1 SEEPANYCNGTZFQ-UHFFFAOYSA-N 0.000 description 1
- 229960004306 sulfadiazine Drugs 0.000 description 1
- 229960002135 sulfadimidine Drugs 0.000 description 1
- ASWVTGNCAZCNNR-UHFFFAOYSA-N sulfamethazine Chemical compound CC1=CC(C)=NC(NS(=O)(=O)C=2C=CC(N)=CC=2)=N1 ASWVTGNCAZCNNR-UHFFFAOYSA-N 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 210000004243 sweat Anatomy 0.000 description 1
- 229920003051 synthetic elastomer Polymers 0.000 description 1
- HPQYKCJIWQFJMS-UHFFFAOYSA-L tetrathionate(2-) Chemical compound [O-]S(=O)(=O)SSS([O-])(=O)=O HPQYKCJIWQFJMS-UHFFFAOYSA-L 0.000 description 1
- 238000003856 thermoforming Methods 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 239000003440 toxic substance Substances 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 238000004627 transmission electron microscopy Methods 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 241001148471 unidentified anaerobic bacterium Species 0.000 description 1
- 241001515965 unidentified phage Species 0.000 description 1
- 150000003673 urethanes Chemical class 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 238000003260 vortexing Methods 0.000 description 1
- 239000002023 wood Substances 0.000 description 1
- 239000002759 woven fabric Substances 0.000 description 1
- 210000000707 wrist Anatomy 0.000 description 1
- 229940098232 yersinia enterocolitica Drugs 0.000 description 1
- 235000013618 yogurt Nutrition 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N1/00—Sampling; Preparing specimens for investigation
- G01N1/28—Preparing specimens for investigation including physical details of (bio-)chemical methods covered elsewhere, e.g. G01N33/50, C12Q
- G01N1/38—Diluting, dispersing or mixing samples
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T436/00—Chemistry: analytical and immunological testing
- Y10T436/25—Chemistry: analytical and immunological testing including sample preparation
Landscapes
- General Health & Medical Sciences (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- Physics & Mathematics (AREA)
- General Physics & Mathematics (AREA)
- Immunology (AREA)
- Pathology (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Sampling And Sample Adjustment (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US98917507P | 2007-11-20 | 2007-11-20 | |
| PCT/US2008/084035 WO2009067513A1 (en) | 2007-11-20 | 2008-11-19 | Sample preparation container and method |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2011502544A true JP2011502544A (ja) | 2011-01-27 |
| JP2011502544A5 JP2011502544A5 (enExample) | 2011-12-22 |
Family
ID=40328661
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010535042A Withdrawn JP2011502544A (ja) | 2007-11-20 | 2008-11-19 | 試料の調製容器及び方法 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US8569072B2 (enExample) |
| EP (1) | EP2214829B1 (enExample) |
| JP (1) | JP2011502544A (enExample) |
| CN (1) | CN101909755B (enExample) |
| WO (1) | WO2009067513A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017221665A1 (ja) * | 2016-06-21 | 2017-12-28 | 株式会社サンプラテック | 細胞・生体組織輸送用容器 |
Families Citing this family (38)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2024742B1 (en) | 2006-05-22 | 2020-03-04 | 3M Innovative Properties Company | System and method for preparing samples |
| EP2231850B1 (en) | 2007-11-20 | 2019-05-15 | 3M Innovative Properties Company | Environmental sampling articles and methods |
| WO2009082667A1 (en) | 2007-12-21 | 2009-07-02 | 3M Innovative Properties Company | Microbiological systems and methods of fluid sample analysis |
| JP6062630B2 (ja) | 2008-06-26 | 2017-01-18 | スリーエム イノベイティブ プロパティズ カンパニー | グラフト化鎖を伴う固体支持体 |
| CN102325596B (zh) | 2008-12-31 | 2015-04-29 | 3M创新有限公司 | 使用微粒进行的活生物负载检测 |
| WO2010078399A2 (en) | 2008-12-31 | 2010-07-08 | 3M Innovative Properties Company | Sampling devices and methods for concentrating microorganisms |
| US9284593B2 (en) | 2009-12-30 | 2016-03-15 | 3M Innovative Properties Company | Live bioload detection using microparticles |
| US8753834B2 (en) | 2009-12-30 | 2014-06-17 | 3M Innovative Properties Company | Microbial detection article |
| DE102010046868A1 (de) * | 2010-09-29 | 2012-03-29 | Multibind Biotec Gmbh | Verfahren zur Aufarbeitung von Proben für direkte messtechnische Analysen |
| EP2741676A1 (en) | 2011-08-09 | 2014-06-18 | Cook General Biotechnology LLC | Vial useable in tissue extraction procedures |
| WO2013059526A1 (en) * | 2011-10-18 | 2013-04-25 | The Trustees Of Columbia University In The City Of New York | Medical apparatus and method for collecting biological samples |
| CN102634449B (zh) * | 2012-03-30 | 2013-11-06 | 中国检验检疫科学研究院 | 病毒性气溶胶采集富集仪 |
| ITBO20120186A1 (it) * | 2012-04-06 | 2013-10-07 | Iclinico | Dispositivo di raccolta di materiale biologico per analisi |
| US10166009B2 (en) | 2012-11-20 | 2019-01-01 | The Trustees Of Columbia University In The City Of New York | Medical apparatus and method for collecting biological samples |
| US9863199B1 (en) * | 2013-10-07 | 2018-01-09 | Kejr, Inc. | Soil sampler lay flat sheet liner system |
| WO2015084617A1 (en) * | 2013-12-05 | 2015-06-11 | 3M Innovative Properties Company | Container for a spraying device |
| WO2016131859A1 (en) * | 2015-02-20 | 2016-08-25 | Ventana Medical Systems, Inc. | Assembly for storing and transporting tissue samples immersed in a fluid |
| US10281343B2 (en) * | 2015-10-28 | 2019-05-07 | U.S. Department Of Energy | Method and apparatus for measuring a peak load |
| CN105754851B (zh) * | 2015-12-22 | 2018-06-12 | 波顿(上海)生物技术有限公司 | 一种生物香气产生和香气富集装置 |
| CN105482995B (zh) * | 2015-12-22 | 2017-09-29 | 波顿(上海)生物技术有限公司 | 一种香气产生和富集装置 |
| CN106556534A (zh) * | 2016-12-03 | 2017-04-05 | 百奥森(江苏)食品安全科技有限公司 | 一种自动化便携式拉曼检测重金属的富集装置 |
| GB201703383D0 (en) | 2017-03-02 | 2017-04-19 | Gargle Tech Ltd | Testing for particulates |
| US10330573B2 (en) | 2017-07-10 | 2019-06-25 | Cem Corporation | Rapid sample preparation for analytical analysis using dispersive energized extraction |
| US10241014B2 (en) | 2017-07-10 | 2019-03-26 | Cem Corporation | Instrument for analytical sample preparation |
| US10295447B2 (en) | 2017-07-10 | 2019-05-21 | Cem Corporation | Rapid energized dispersive solid phase extraction (SPE) for analytical analysis |
| EP3714252B1 (en) * | 2017-11-22 | 2023-05-31 | Beckman Coulter, Inc. | Diluent preparation module und method for supplying such diluent |
| CN108982169B (zh) * | 2018-05-24 | 2023-09-15 | 中国地质大学(北京) | 气体取样瓶及其使用方法 |
| EP3801851A4 (en) | 2018-06-01 | 2022-03-23 | 3M Innovative Properties Company | POROUS MEMBRANES WITH TRIBLOCK COPOLYMERS |
| WO2020012302A1 (en) | 2018-07-13 | 2020-01-16 | 3M Innovative Properties Company | Isoporous membranes including crosslinked multiblock copolymers |
| EP4205851A3 (en) | 2018-09-05 | 2023-10-04 | Hero Scientific Ltd | Testing for particulates |
| US11850320B2 (en) | 2018-12-20 | 2023-12-26 | Asp Global Manufacturing Gmbh | Liquid-chemical sterilization system with biological indicator |
| WO2020139932A1 (en) * | 2018-12-26 | 2020-07-02 | University Of Cincinnati | Device, system, and method for rapid fixation and embedding of biological specimens |
| GB2582156B (en) * | 2019-03-12 | 2022-09-28 | Quantumdx Group Ltd | Specimen container for specimen pre-processing |
| WO2021181339A1 (en) | 2020-03-11 | 2021-09-16 | Hero Scientific Ltd. | Testing devices |
| CN112147173A (zh) * | 2020-10-22 | 2020-12-29 | 张志春 | 一种高寒草地生态系统淋溶性土壤有机碳监测装置 |
| CA3202405A1 (en) | 2021-01-06 | 2022-07-14 | Zvi Feldman | Filtration sampling devices |
| CN117168936B (zh) * | 2023-11-03 | 2024-03-15 | 榆林市榆阳区市场抽检快检服务中心 | 一种食品检测样品搅拌消解装置 |
| CN120427337A (zh) * | 2025-05-06 | 2025-08-05 | 广州海洋地质调查局 | 一种沉积不溶有机质的富集设备及系统 |
Family Cites Families (96)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3163160A (en) | 1962-11-15 | 1964-12-29 | Milton J Cohen | Disposable swab and culture medium device |
| US3449081A (en) | 1965-03-29 | 1969-06-10 | Electronic Instr Co | Test kit |
| US3367191A (en) | 1965-10-22 | 1968-02-06 | Joseph D Richard | Water sampling apparatus |
| US3601317A (en) | 1970-03-09 | 1971-08-24 | George X Batlas | Atomizers spraying in all directions |
| US3748905A (en) | 1971-12-30 | 1973-07-31 | J Fletcher | Vacuum probe surface sampler |
| US3784039A (en) | 1972-01-10 | 1974-01-08 | Illinois Tool Works | Nursing bottle construction and assembly |
| US3819158A (en) | 1972-08-17 | 1974-06-25 | Lever Brothers Ltd | Devices for blending materials |
| BE849898A (fr) * | 1976-12-28 | 1977-06-28 | Dispositif de dilution et de filtration | |
| US4121306A (en) | 1977-04-15 | 1978-10-24 | Bringman Bernard B | Urinal |
| US4427406A (en) | 1982-03-22 | 1984-01-24 | Beckman Instruments, Inc. | Sectional shaped liner for a centrifuge rotor |
| US4984715A (en) | 1983-11-10 | 1991-01-15 | Green Richard D | Twin compartment squeeze bottle |
| JPS6173054A (ja) | 1984-09-17 | 1986-04-15 | メデイカル テクノロジ− コ−ポレ−シヨン | 試料調整用小瓶 |
| US4937194A (en) | 1986-05-12 | 1990-06-26 | Baxter International Inc. | Method for metering nutrient media to cell culture containers |
| US5429803A (en) | 1991-04-18 | 1995-07-04 | Lamina, Inc. | Liquid specimen container and attachable testing modules |
| US5100801A (en) | 1989-01-26 | 1992-03-31 | Biocontrol Systems, Inc. | Device for sequential microbial enrichment in a single apparatus |
| US5186897A (en) | 1989-04-11 | 1993-02-16 | Ares-Serono Research & Development Limited Partnership | Multianalyte test vehicle |
| US5230865A (en) | 1989-09-08 | 1993-07-27 | Cem Corporation | Ventable rupture diaphragm-protected container for heating contained materials by microwave radiation |
| US5252496A (en) | 1989-12-18 | 1993-10-12 | Princeton Biomeditech Corporation | Carbon black immunochemical label |
| US5403745A (en) | 1990-04-27 | 1995-04-04 | Genzyme Corporation | Determination of analytes in biological fluids in the presence of substances interfering with assays therefor |
| EP0471947A1 (en) | 1990-06-29 | 1992-02-26 | Sekisui Chemical Co., Ltd. | Culture bag |
| US5119830A (en) | 1991-04-03 | 1992-06-09 | Code Blue Medical Corporation | Analytical specimen cup with testing means |
| US5291779A (en) | 1992-07-02 | 1994-03-08 | The United States Of America As Represented By The Secretary Of The Army | High-wind snow collector |
| US5341693A (en) | 1993-01-14 | 1994-08-30 | Ocean Test Equipment, Inc. | Double wall sampler |
| US5350080A (en) | 1993-02-10 | 1994-09-27 | Hyclone Laboratories | Multi-access port for use in a cell culture media system |
| EP0697849A4 (en) | 1993-05-11 | 1997-04-02 | Munchking Bottling Inc | DISPOSABLE BOTTLE BAGS FOR USE IN INFANT CARE |
| US5403551A (en) | 1993-09-16 | 1995-04-04 | Roche Diagnostic Systems, Inc. | Assaying device and container for in field analysis of a specimen and later shipment of the unadulterated specimen |
| US5617972A (en) | 1994-03-25 | 1997-04-08 | Playtex Products Inc. | Nurser liner |
| GB2298272A (en) | 1995-02-21 | 1996-08-28 | Mini Agriculture & Fisheries | Preparing food samles for assay for microorganisms |
| US5728542A (en) | 1995-05-19 | 1998-03-17 | Charm Sciences, Inc. | Disposable test kit apparatus and method for bacteria |
| US5569225A (en) | 1995-06-29 | 1996-10-29 | Gkr Industries, Inc. | Bodily fluid test kit and method of testing bodily fluids |
| US5543115A (en) * | 1995-07-17 | 1996-08-06 | Mizuho Usa, Inc. | Specimen handling device |
| US5833860A (en) | 1995-08-28 | 1998-11-10 | Millipore Investment Holdings Limited | Centrifugal adsorptive sample preparation device and method |
| DE69704618T2 (de) | 1996-05-13 | 2002-03-07 | Filtaflex Ltd., Almonte | Mit schlaggerät versehendes beutelklopfendes suspendiergerät für mikroben |
| US7225690B1 (en) | 2002-08-02 | 2007-06-05 | A+ Manufacturing, Llc | Multi-cavity sample cylinder with integrated valving |
| US6820824B1 (en) | 1998-01-14 | 2004-11-23 | 3M Innovative Properties Company | Apparatus for spraying liquids, disposable containers and liners suitable for use therewith |
| GB9701548D0 (en) * | 1997-01-25 | 1997-03-12 | Elopak Systems | A method of handling substances and a container for such substances |
| US6146875A (en) | 1997-05-02 | 2000-11-14 | Ward; N. Robert | Method for culturing microorganisms in prefilled flexible containers |
| US6021681A (en) | 1997-06-27 | 2000-02-08 | The United States Of America As Represented By The United States Department Of Energy | Sampling device with a capped body and detachable handle |
| US6107085A (en) | 1997-07-11 | 2000-08-22 | Corning Incorporated | Self contained cell growth system |
| US6854875B2 (en) | 1997-10-29 | 2005-02-15 | Mcgill Technology Limited | Food blending apparatus |
| US6338569B1 (en) | 1998-10-27 | 2002-01-15 | Mcgill Shane R. | Food blending apparatus |
| US6168758B1 (en) | 1997-11-19 | 2001-01-02 | Starplex Scientific | Liquid sample assay device |
| US6221655B1 (en) | 1998-08-01 | 2001-04-24 | Cytosignal | Spin filter assembly for isolation and analysis |
| AU6021699A (en) | 1998-09-01 | 2000-03-21 | Penn State Research Foundation | Method and apparatus for aseptic growth or processing of biomass |
| US6516953B1 (en) | 1998-12-05 | 2003-02-11 | Becton, Dickinson And Company | Device for separating components of a fluid sample |
| US6497325B1 (en) | 1998-12-05 | 2002-12-24 | Becton Dickinson And Company | Device for separating components of a fluid sample |
| US7108662B2 (en) | 1999-04-21 | 2006-09-19 | Quest Diagnostics Incorporated | Device and method for sample collection |
| GB2350421B (en) * | 1999-05-18 | 2003-12-17 | Krysium Advisors Ltd | Apparatus and method of testing a biological fluid |
| US7223364B1 (en) | 1999-07-07 | 2007-05-29 | 3M Innovative Properties Company | Detection article having fluid control film |
| US6536687B1 (en) | 1999-08-16 | 2003-03-25 | 3M Innovative Properties Company | Mixing cup adapting assembly |
| US6682939B2 (en) * | 1999-08-25 | 2004-01-27 | The Goodyear Tire & Rubber Company | Chemical sampler system and container |
| US6180335B1 (en) | 1999-09-30 | 2001-01-30 | University Of New Mexico | Apparatus for detecting contamination in food products |
| US6136549A (en) | 1999-10-15 | 2000-10-24 | Feistel; Christopher C. | systems and methods for performing magnetic chromatography assays |
| US6471069B2 (en) | 1999-12-03 | 2002-10-29 | Becton Dickinson And Company | Device for separating components of a fluid sample |
| US6595680B2 (en) | 2000-02-24 | 2003-07-22 | Kubota Corporation | Food mixing apparatus |
| US6187209B1 (en) * | 2000-03-23 | 2001-02-13 | Bechtel Bwxt Idaho, Llc | Lined sampling vessel including a filter to separate solids from liquids on exit |
| US6541262B1 (en) * | 2000-04-28 | 2003-04-01 | Medtronic, Inc. | Method and device for testing a sample of fresh whole blood |
| JP4404445B2 (ja) | 2000-05-17 | 2010-01-27 | テルモ株式会社 | 血液フィルターおよび血液フィルターの製造方法 |
| JP4642188B2 (ja) | 2000-06-19 | 2011-03-02 | 株式会社エルメックス | フィルタ付きホモジナイズ袋 |
| US6458067B1 (en) * | 2000-06-30 | 2002-10-01 | Beckman Coulter, Inc. | Removable conformal liners for centrifuge containers |
| JP2002023902A (ja) | 2000-07-11 | 2002-01-25 | Mitsubishi Electric Corp | 半導体装置 |
| US6576193B1 (en) * | 2000-10-27 | 2003-06-10 | Shujie Cui | Device and method for collecting and testing fluid specimens |
| US6866826B2 (en) * | 2000-12-30 | 2005-03-15 | Beckman Coulter, Inc. | Large mouth centrifuge labware |
| EP1395646B1 (en) | 2001-02-28 | 2010-07-14 | BioMerieux, Inc. | Integrated filtration and detection device |
| US7188785B2 (en) | 2001-04-24 | 2007-03-13 | 3M Innovative Properties Company | Reservoir with refill inlet for hand-held spray guns |
| US6461853B1 (en) | 2001-05-17 | 2002-10-08 | Hong Zhu | Method for surface culture of microorganisms and cells in flexible culture bags |
| US6588681B2 (en) | 2001-07-09 | 2003-07-08 | 3M Innovative Properties Company | Liquid supply assembly |
| US6669908B2 (en) | 2001-07-25 | 2003-12-30 | Applied Biotech, Inc. | Urine test device |
| GB0122286D0 (en) * | 2001-09-14 | 2001-11-07 | Scient Generics Ltd | Optical coatings for high-throughput laboratory consumables |
| US7022289B1 (en) * | 2001-10-10 | 2006-04-04 | The United States Of America As Represented By The Secretary Of The Army | Chemical and biological sampling device and kit and method of use thereof |
| US7100461B2 (en) | 2002-02-27 | 2006-09-05 | Microbial-Vac Systems, Inc. | Portable contaminant sampling system |
| JP3848201B2 (ja) | 2002-03-18 | 2006-11-22 | 株式会社エルメックス | 拭き取り検査キット |
| US6955099B2 (en) | 2002-07-19 | 2005-10-18 | Goodin John W | Spill proof liquid sample cup |
| DE10232907A1 (de) | 2002-07-19 | 2004-02-05 | Agere Systems, Inc. | Visuelle graphische Anzeige der Anzahl restlicher Zeichen in einem Editierungsfeld eines elektronischen Gerätes. |
| US7517694B2 (en) * | 2002-07-26 | 2009-04-14 | Ortho-Clinical Diagnostics, Inc. | Metering tip with internal features to control fluid meniscus and oscillation |
| US6789945B2 (en) | 2002-08-02 | 2004-09-14 | Hassia Verpackungsmaschinen Gmbh | Sealed three-sided pouch with two chambers |
| US7211225B2 (en) | 2002-08-26 | 2007-05-01 | Perceptronix Medical Inc. | Filter devices for depositing material and density gradients of material from sample suspension |
| GB0222855D0 (en) * | 2002-10-03 | 2002-11-06 | Secr Defence | Sampling apparatus |
| GB0224698D0 (en) | 2002-10-24 | 2002-12-04 | 3M Innovative Properties Co | Easy clean spray gun |
| US7682824B2 (en) * | 2002-12-04 | 2010-03-23 | Smiths Detection Inc. | Holder for PCR sample collection and preparation |
| US7114403B2 (en) * | 2003-05-30 | 2006-10-03 | Oakville Hong Kong Co., Ltd | Fluid collection and application device and methods of use of same |
| US7337907B2 (en) | 2003-08-01 | 2008-03-04 | Polyzen, Inc. | Press-flat centrifuge tube and specimen collection assembly comprising same |
| WO2005050169A2 (en) | 2003-11-14 | 2005-06-02 | Oakville Hong Kong Co., Limited | Sample collection cup with integrated activatable sample analysis system |
| EP1694814A1 (en) * | 2003-12-08 | 2006-08-30 | Covaris, Inc. | Apparatus and methods for sample preparation |
| US7547526B2 (en) | 2004-03-15 | 2009-06-16 | Purdue Research Foundation | Cell concentration and pathogen recovery |
| ATE468284T1 (de) | 2004-05-28 | 2010-06-15 | Ajinomoto Kk | Abgedichteter lagerungsbeutel mit mehrfachkammerstruktur |
| US7866907B2 (en) | 2004-08-09 | 2011-01-11 | Tyco Healthcare Group Lp | Medical skin applicator apparatus |
| AT500882A3 (de) | 2004-10-06 | 2009-12-15 | Greiner Bio One Gmbh | In-vitro diagnostikum zur speichelvolumsbestimmung |
| US20060102550A1 (en) | 2004-11-18 | 2006-05-18 | Joseph Stephen C P | Liquid supply and filter assembly |
| USD532253S1 (en) | 2004-12-09 | 2006-11-21 | Mcgill Technology Limited | Milkshake container lid |
| CA2603697A1 (en) | 2005-04-04 | 2006-10-12 | John Carl Steichen | Flexible culture medium bag containing nutrient concentrate |
| US20060275798A1 (en) | 2005-04-04 | 2006-12-07 | Steichen John C | Detection of organisms using a media sachet and primer directed nucleic acid amplification |
| TW200712487A (en) * | 2005-08-02 | 2007-04-01 | 3M Innovative Properties Co | Apparatus and method for detecting an analyte |
| EP2024742B1 (en) | 2006-05-22 | 2020-03-04 | 3M Innovative Properties Company | System and method for preparing samples |
| US20070269341A1 (en) | 2006-05-22 | 2007-11-22 | 3M Innovative Properties Company | Sampling assembly and method of preparing samples |
| US7437805B2 (en) | 2006-06-23 | 2008-10-21 | Edward Alan Berich | Reclosable storage bag closure with internal valving |
-
2008
- 2008-11-19 CN CN2008801246162A patent/CN101909755B/zh not_active Expired - Fee Related
- 2008-11-19 JP JP2010535042A patent/JP2011502544A/ja not_active Withdrawn
- 2008-11-19 EP EP08851800A patent/EP2214829B1/en not_active Not-in-force
- 2008-11-19 US US12/743,449 patent/US8569072B2/en not_active Expired - Fee Related
- 2008-11-19 WO PCT/US2008/084035 patent/WO2009067513A1/en not_active Ceased
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017221665A1 (ja) * | 2016-06-21 | 2017-12-28 | 株式会社サンプラテック | 細胞・生体組織輸送用容器 |
| JPWO2017221665A1 (ja) * | 2016-06-21 | 2019-04-11 | 株式会社サンプラテック | 細胞・生体組織輸送用容器 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN101909755B (zh) | 2013-09-18 |
| WO2009067513A1 (en) | 2009-05-28 |
| EP2214829B1 (en) | 2012-12-26 |
| CN101909755A (zh) | 2010-12-08 |
| EP2214829A1 (en) | 2010-08-11 |
| US8569072B2 (en) | 2013-10-29 |
| US20100248215A1 (en) | 2010-09-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2214829B1 (en) | Sample preparation container and method | |
| US8685746B2 (en) | Sample preparation container and method | |
| US8647574B2 (en) | Sample preparation container and method | |
| CN101909757B (zh) | 环境取样的样品制备 | |
| EP2024742B1 (en) | System and method for preparing samples | |
| US9388448B2 (en) | System and method for processing samples | |
| WO2010080223A1 (en) | System and method for preparing samples |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20111101 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20111101 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20120618 |