JP2010520184A - ブプレノルフィンとナルメフェンを含む改良医薬組成物 - Google Patents
ブプレノルフィンとナルメフェンを含む改良医薬組成物 Download PDFInfo
- Publication number
- JP2010520184A JP2010520184A JP2009551254A JP2009551254A JP2010520184A JP 2010520184 A JP2010520184 A JP 2010520184A JP 2009551254 A JP2009551254 A JP 2009551254A JP 2009551254 A JP2009551254 A JP 2009551254A JP 2010520184 A JP2010520184 A JP 2010520184A
- Authority
- JP
- Japan
- Prior art keywords
- buprenorphine
- nalmefene
- composition
- patient
- drug
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/485—Morphinan derivatives, e.g. morphine, codeine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/30—Drugs for disorders of the nervous system for treating abuse or dependence
- A61P25/36—Opioid-abuse
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Epidemiology (AREA)
- Emergency Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Addiction (AREA)
- Pain & Pain Management (AREA)
- Psychiatry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
侵害受容試験
コールドプレッサー(cold pressor:CP)試験を用いて、ブプレノルフィン、ブプレノルフィンとナルメフェンとの組み合わせ、すなわちナルメフェン組み合わせの抗侵害受容を評価した。化合物の形態は、ブプレノルフィンHCl及びナルメフェンHCl二水和物であった。CP試験では、2つのプラスチック製の円筒形容器を用い、一方を温水で満たし、もう一方を水と砕いた氷とを組み合わせたもので満たして“雪解け状態(slushy)”にした。被験者は、利き手ではないほうの前腕と手を温水にちょうど2分間浸した。1分45秒の時点で、浸した腕に装着した血圧測定器のカフを、拡張期血圧から20mmHg下の圧力にまで膨らませた。血圧測定器のカフにより、低温に対する反応を調べるにあたって、血流の役割が最小限に抑えられた。ちょうど2分の時点で、前腕を温水から冷水に移動させた。全手順を通して被験者の両眼を覆うことにより、被験者が注意散漫となったり時間の見当をつけてしまうことを最小限に抑えた。前腕を冷水に浸した際、被験者には、最初に痛みを感じた時点で知らせるようにと依頼し(痛覚閾値、CPTHR)、次に、痛みにそれ以上耐えられなくなるまで腕を浸したままにするよう依頼した(疼痛耐性、CPTOL)。疼痛閾値及び耐容時間を、冷水に浸してから秒単位で記録した。被験者には非通知の180秒のカットオフ時間で冷水浴を打ち切った。180秒を越えると、しびれ感から疼痛耐性がもはや正確には評価できないからである。疼痛耐性(CPTOL)は、現在の調査において報告される疼痛応答パラメータである。
試験前、被験者は、既往症及び薬物乱用等の要因に基づいた選択基準及び除外基準に従ってスクリーニングされた。
スクリーニングされた適切な被験者を、以下の手順に従って試験した。試験当日、到着時に被験者は尿サンプルを提出し、尿サンプルを乱用薬物(オピオイド、カンナビノイド、ベンゾジアゼピン、交感神経刺激アミン)について試験し、女性被験者については妊娠について試験した。22ゲージの留置静脈カテーテルを各腕で最も利用しやすい前腕静脈に挿入した(利き手ではない腕のCP試験における浸漬水位より上)。雄型のルアーロックアダプタインジェクションサイトを、各カテーテルに取り付けた。1つのカテーテルは試験日を通じて血液の採取に使用し、もう一方は注入に用いた。次に参加者をモニタに接続し、モニタを、試験セッションの間、生理学的パラメータを連続的に監視するように設定した。
ベースライン値が条件によって異なっていたことから、CPTOLデータをベースラインからの%変化として表すことによって、異なる薬剤組み合わせの効果を比較した。各条件における各時点での各参加者の応答を、以下に挙げる方程式に従って、ベースラインとなる応答からの%変化として表した。データを、各条件についての各薬剤投与後試験セッションにおける値の平均(±SEM)として表す。
(薬剤投与後潜時(post−drug latency)−ベースライン潜時(baseline latency)/ベースライン潜時)x100
これにより、CPTOLの%変化についての値が得られる。
19〜27歳(平均±SEM、21.7±1.8歳)の4人の健康な白人の参加者(男性2人、女性2人)が研究に参加した。平均体重は77.5kg(±9.1、範囲63〜104kg)であり、スクリーニング時の平均CPTOLは38.7秒(±5.6秒、範囲25〜52秒)であった。年齢(p=0.277)又はスクリーニング時のCPTOL(p=0.974)において男女間で大きな違いはなかった。
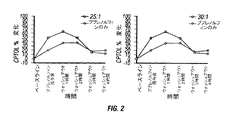
比較例として、実施例1と同じ被験者に、別の日に、静脈内注入によりブプレノルフィンと生理食塩水とを(以下、「BUPのみ」と記載する)を投与した。体重1kgあたり0.5μgの用量でブプレノルフィンを再度投与した。CPTOLの結果を図5に示す。
実施例1と同じ被験者に、別の日に、静脈内注入によりブプレノルフィンとナルメフェンとを30:1の比で投与した。ブプレノルフィンは体重1kgあたり0.5μgの用量で投与された。CPTOLの結果を図1に示す。問題となるような有害作用は見られなかった。
CPTOLのベースラインからの%変化を、実施例1、2、3について計算し、結果を図2に示す。ブプレノルフィン単独と比較するとブプレノルフィンとナルメフェンとの組み合わせのほうが際立って有益であることが見てとれる。
Claims (7)
- 非経口単位剤形又は粘膜若しくは真皮を経由した送達に適した単位剤形の鎮痛組成物であって、ブプレノルフィンと、患者の血漿に送達される又は到達するブプレノルフィン対ナルメフェンの質量比が22.6:1〜40:1の範囲となるような量のナルメフェンとを含むことを特徴とする組成物。
- 前記単位剤形におけるブプレノルフィンの量が10μg〜8mgである、請求項1に記載の組成物。
- 前記組成物が、ブプレノルフィンとナルメフェンとを23:36、好ましくは24:32、好ましくは25:30の比で含有する、請求項1又は2に記載の組成物。
- 患者の痛みを治療するための方法であって、患者の血漿に送達される又は到達するブプレノルフィン対ナルメフェンの質量比が、ブプレノルフィン:ナルメフェンで22.6:1〜40:1の範囲となるように、ブプレノルフィンとナルメフェンとを患者に非経口又は経皮又は経粘膜投与することを含むことを特徴とする方法。
- 痛みを治療するための薬物の製造におけるブプレノルフィン及びナルメフェンの使用であって、質量比22.6:1〜40:1の範囲で薬物が患者に送達されるような又は患者の血漿に到達するような量でブプレノルフィン及びナルメフェンを使用することを特徴とする使用。
- ブプレノルフィンの投与が、24時間あたり体重1kgにつき0.25〜640μgの範囲でなされる、請求項5又は6に記載の使用方法。
- 実質的に本発明に従って前述したような組成物又は方法又は使用。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB0703933.2 | 2007-03-01 | ||
| GB0703933A GB2447013A (en) | 2007-03-01 | 2007-03-01 | Analgesic composition containing buprenorphone and nalmefene |
| PCT/GB2008/000522 WO2008104736A1 (en) | 2007-03-01 | 2008-02-15 | Improved medicinal compositions comprising buprenorphine and nalmefene |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2010520184A true JP2010520184A (ja) | 2010-06-10 |
| JP2010520184A5 JP2010520184A5 (ja) | 2011-04-07 |
| JP5577102B2 JP5577102B2 (ja) | 2014-08-20 |
Family
ID=37965707
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009551254A Expired - Fee Related JP5577102B2 (ja) | 2007-03-01 | 2008-02-15 | ブプレノルフィンとナルメフェンを含む改良医薬組成物 |
Country Status (21)
| Country | Link |
|---|---|
| US (1) | US8497280B2 (ja) |
| EP (1) | EP2114452B1 (ja) |
| JP (1) | JP5577102B2 (ja) |
| KR (1) | KR101437461B1 (ja) |
| CN (1) | CN101622012B (ja) |
| AR (1) | AR065582A1 (ja) |
| AU (1) | AU2008220572B2 (ja) |
| BR (1) | BRPI0807903A2 (ja) |
| CA (1) | CA2678568C (ja) |
| CL (1) | CL2008000607A1 (ja) |
| DK (1) | DK2114452T3 (ja) |
| ES (1) | ES2476865T3 (ja) |
| GB (1) | GB2447013A (ja) |
| HR (1) | HRP20140591T1 (ja) |
| MX (1) | MX2009009134A (ja) |
| PE (2) | PE20090625A1 (ja) |
| PL (1) | PL2114452T3 (ja) |
| PT (1) | PT2114452E (ja) |
| TW (1) | TWI468163B (ja) |
| WO (1) | WO2008104736A1 (ja) |
| ZA (1) | ZA200905692B (ja) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021515034A (ja) * | 2018-02-22 | 2021-06-17 | アビオール、インコーポレイテッド | 経粘膜フィルム組成物ならびにそれを作製及び使用する方法 |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| UA102128C2 (en) | 2008-12-05 | 2013-06-10 | Х. Луннбек А/С | Nalmefene hydrochloride dihydrate |
| PL2435439T3 (pl) * | 2009-05-25 | 2016-05-31 | H Lundbeck As | Wytwarzanie chlorowodorku nalmefenu z naltreksonu |
| DE112013002074T5 (de) | 2012-04-17 | 2015-01-29 | Purdue Pharma L.P. | Systeme und Methoden zur Behandlung einer Opioid-induzierten unerwünschten pharmakodynamischen Reaktion |
| CN103637983B (zh) * | 2013-12-16 | 2017-08-04 | 科贝源(北京)生物医药科技有限公司 | 一种含有盐酸纳美芬的药物组合物及其制备方法 |
| US9849124B2 (en) | 2014-10-17 | 2017-12-26 | Purdue Pharma L.P. | Systems and methods for treating an opioid-induced adverse pharmacodynamic response |
| CN104922061B (zh) * | 2015-05-26 | 2017-09-22 | 成都天台山制药有限公司 | 盐酸纳美芬注射液药物组合物和制法 |
| JP2020526500A (ja) | 2017-06-30 | 2020-08-31 | パーデュー、ファーマ、リミテッド、パートナーシップ | 治療方法及びその剤形 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0319243A1 (en) * | 1987-12-03 | 1989-06-07 | Reckitt And Colman Products Limited | Pharmaceutical compositions |
| US5272149A (en) * | 1992-05-05 | 1993-12-21 | Stalling Reginald W | Symptom controlled receptor substitution for addiction withdrawl |
| JP2001526229A (ja) * | 1997-12-22 | 2001-12-18 | ユーロ−セルティーク,エス.エイ. | オピオイド投薬剤形の乱用を防止する方法 |
| JP2003514013A (ja) * | 1999-11-19 | 2003-04-15 | レキット ベンキサー ヘルスケア (ユーケイ) リミテッド | 医薬組成物 |
| US20050191340A1 (en) * | 2002-08-09 | 2005-09-01 | Gruenenthal Gmbh | Opioid-receptor antagonists in transdermal systems having buprenorphine |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5149538A (en) * | 1991-06-14 | 1992-09-22 | Warner-Lambert Company | Misuse-resistive transdermal opioid dosage form |
| US6096756A (en) * | 1992-09-21 | 2000-08-01 | Albert Einstein College Of Medicine Of Yeshiva University | Method of simultaneously enhancing analgesic potency and attenuating dependence liability caused by morphine and other bimodally-acting opioid agonists |
| WO2007145704A2 (en) * | 2006-04-24 | 2007-12-21 | Gloucester Pharmaceuticals | Gemcitabine combination therapy |
| WO2007146730A2 (en) * | 2006-06-08 | 2007-12-21 | Gloucester Pharmaceuticals | Deacetylase inhibitor therapy |
| US20110015168A1 (en) * | 2007-01-19 | 2011-01-20 | Mitchell Keegan | Methods for increasing levels of human fetal hemoglobin |
-
2007
- 2007-03-01 GB GB0703933A patent/GB2447013A/en not_active Withdrawn
-
2008
- 2008-02-15 WO PCT/GB2008/000522 patent/WO2008104736A1/en not_active Ceased
- 2008-02-15 CN CN2008800067678A patent/CN101622012B/zh not_active Expired - Fee Related
- 2008-02-15 HR HRP20140591AT patent/HRP20140591T1/hr unknown
- 2008-02-15 DK DK08709413.2T patent/DK2114452T3/da active
- 2008-02-15 JP JP2009551254A patent/JP5577102B2/ja not_active Expired - Fee Related
- 2008-02-15 PT PT87094132T patent/PT2114452E/pt unknown
- 2008-02-15 PL PL08709413T patent/PL2114452T3/pl unknown
- 2008-02-15 CA CA2678568A patent/CA2678568C/en not_active Expired - Fee Related
- 2008-02-15 EP EP08709413.2A patent/EP2114452B1/en not_active Not-in-force
- 2008-02-15 BR BRPI0807903-0A2A patent/BRPI0807903A2/pt not_active IP Right Cessation
- 2008-02-15 KR KR1020097018310A patent/KR101437461B1/ko not_active Expired - Fee Related
- 2008-02-15 AU AU2008220572A patent/AU2008220572B2/en not_active Ceased
- 2008-02-15 ES ES08709413.2T patent/ES2476865T3/es active Active
- 2008-02-15 US US12/529,314 patent/US8497280B2/en not_active Expired - Fee Related
- 2008-02-15 MX MX2009009134A patent/MX2009009134A/es active IP Right Grant
- 2008-02-27 TW TW97106776A patent/TWI468163B/zh not_active IP Right Cessation
- 2008-02-28 CL CL200800607A patent/CL2008000607A1/es unknown
- 2008-02-29 AR ARP080100883A patent/AR065582A1/es unknown
- 2008-02-29 PE PE2008000411A patent/PE20090625A1/es not_active Application Discontinuation
- 2008-02-29 PE PE2012000471A patent/PE20120956A1/es not_active Application Discontinuation
-
2009
- 2009-08-17 ZA ZA200905692A patent/ZA200905692B/xx unknown
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0319243A1 (en) * | 1987-12-03 | 1989-06-07 | Reckitt And Colman Products Limited | Pharmaceutical compositions |
| US5272149A (en) * | 1992-05-05 | 1993-12-21 | Stalling Reginald W | Symptom controlled receptor substitution for addiction withdrawl |
| JP2001526229A (ja) * | 1997-12-22 | 2001-12-18 | ユーロ−セルティーク,エス.エイ. | オピオイド投薬剤形の乱用を防止する方法 |
| JP2003514013A (ja) * | 1999-11-19 | 2003-04-15 | レキット ベンキサー ヘルスケア (ユーケイ) リミテッド | 医薬組成物 |
| US20050191340A1 (en) * | 2002-08-09 | 2005-09-01 | Gruenenthal Gmbh | Opioid-receptor antagonists in transdermal systems having buprenorphine |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021515034A (ja) * | 2018-02-22 | 2021-06-17 | アビオール、インコーポレイテッド | 経粘膜フィルム組成物ならびにそれを作製及び使用する方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2678568C (en) | 2015-06-16 |
| EP2114452B1 (en) | 2014-04-09 |
| EP2114452A1 (en) | 2009-11-11 |
| MX2009009134A (es) | 2009-09-03 |
| CA2678568A1 (en) | 2008-09-04 |
| HK1139868A1 (en) | 2010-09-30 |
| JP5577102B2 (ja) | 2014-08-20 |
| GB2447013A (en) | 2008-09-03 |
| AU2008220572B2 (en) | 2014-03-27 |
| GB0703933D0 (en) | 2007-04-11 |
| ES2476865T3 (es) | 2014-07-15 |
| CN101622012A (zh) | 2010-01-06 |
| BRPI0807903A2 (pt) | 2014-06-17 |
| WO2008104736A1 (en) | 2008-09-04 |
| US8497280B2 (en) | 2013-07-30 |
| AU2008220572A1 (en) | 2008-09-04 |
| PE20120956A1 (es) | 2012-08-01 |
| DK2114452T3 (da) | 2014-06-30 |
| CN101622012B (zh) | 2012-05-30 |
| KR20090115862A (ko) | 2009-11-09 |
| PT2114452E (pt) | 2014-07-15 |
| CL2008000607A1 (es) | 2008-10-03 |
| PL2114452T3 (pl) | 2014-09-30 |
| PE20090625A1 (es) | 2009-06-04 |
| US20100152222A1 (en) | 2010-06-17 |
| TW200900067A (en) | 2009-01-01 |
| KR101437461B1 (ko) | 2014-09-05 |
| HRP20140591T1 (hr) | 2014-09-12 |
| AR065582A1 (es) | 2009-06-17 |
| ZA200905692B (en) | 2010-10-27 |
| TWI468163B (zh) | 2015-01-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5577101B2 (ja) | ブプレノルフィンとナルトレキソンを含む改良医薬組成物 | |
| TWI451868B (zh) | 醫藥組成物之改良及相關之改良 | |
| JP5577102B2 (ja) | ブプレノルフィンとナルメフェンを含む改良医薬組成物 | |
| AU2014201782A1 (en) | Improved medicinal compositions comprising buprenorphine and naltrexone | |
| AU2014201777A1 (en) | Improvements in and relating to medicinal compositions | |
| HK1139868B (en) | Improved medicinal compositions comprising buprenorphine and nalmefene | |
| HK1139867A (en) | Improved medicinal compositions comprising buprenorphine and naltrexone | |
| HK1139871B (en) | Improvements in and relating to medicinal compositions |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110214 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20110214 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20121207 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130321 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130620 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130627 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130712 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130722 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130924 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20131125 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140220 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140227 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140523 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20140616 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20140707 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5577102 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| S533 | Written request for registration of change of name |
Free format text: JAPANESE INTERMEDIATE CODE: R313533 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| LAPS | Cancellation because of no payment of annual fees |