JP2008500113A - 健康条件を判別するための方法および装置 - Google Patents
健康条件を判別するための方法および装置 Download PDFInfo
- Publication number
- JP2008500113A JP2008500113A JP2007515315A JP2007515315A JP2008500113A JP 2008500113 A JP2008500113 A JP 2008500113A JP 2007515315 A JP2007515315 A JP 2007515315A JP 2007515315 A JP2007515315 A JP 2007515315A JP 2008500113 A JP2008500113 A JP 2008500113A
- Authority
- JP
- Japan
- Prior art keywords
- subject
- vascular
- thermal energy
- temperature
- energy sensor
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000000034 method Methods 0.000 title claims abstract description 275
- 230000036541 health Effects 0.000 title claims abstract description 180
- 230000002792 vascular Effects 0.000 claims description 478
- 230000008859 change Effects 0.000 claims description 115
- 230000004044 response Effects 0.000 claims description 87
- 238000012360 testing method Methods 0.000 claims description 86
- 230000000638 stimulation Effects 0.000 claims description 56
- 230000008878 coupling Effects 0.000 claims description 47
- 238000010168 coupling process Methods 0.000 claims description 47
- 238000005859 coupling reaction Methods 0.000 claims description 47
- 238000012631 diagnostic technique Methods 0.000 claims description 41
- 238000005259 measurement Methods 0.000 claims description 31
- 239000003814 drug Substances 0.000 claims description 28
- 229940079593 drug Drugs 0.000 claims description 27
- 235000016709 nutrition Nutrition 0.000 claims description 23
- 230000035764 nutrition Effects 0.000 claims description 21
- 238000002560 therapeutic procedure Methods 0.000 claims description 19
- 230000006870 function Effects 0.000 claims description 16
- 230000017531 blood circulation Effects 0.000 claims description 14
- 230000008753 endothelial function Effects 0.000 claims description 14
- 239000000523 sample Substances 0.000 claims description 14
- 239000000021 stimulant Substances 0.000 claims description 14
- 210000001367 artery Anatomy 0.000 claims description 13
- 238000012544 monitoring process Methods 0.000 claims description 13
- 208000002815 pulmonary hypertension Diseases 0.000 claims description 10
- 210000000329 smooth muscle myocyte Anatomy 0.000 claims description 10
- 230000036772 blood pressure Effects 0.000 claims description 9
- 208000024172 Cardiovascular disease Diseases 0.000 claims description 8
- 208000019116 sleep disease Diseases 0.000 claims description 8
- 201000001320 Atherosclerosis Diseases 0.000 claims description 7
- 208000008589 Obesity Diseases 0.000 claims description 7
- 206010012601 diabetes mellitus Diseases 0.000 claims description 7
- 235000020824 obesity Nutrition 0.000 claims description 7
- 238000012216 screening Methods 0.000 claims description 7
- 208000012322 Raynaud phenomenon Diseases 0.000 claims description 6
- 230000004064 dysfunction Effects 0.000 claims description 6
- 208000019622 heart disease Diseases 0.000 claims description 6
- 230000009257 reactivity Effects 0.000 claims description 6
- 230000005586 smoking cessation Effects 0.000 claims description 6
- 230000002889 sympathetic effect Effects 0.000 claims description 5
- 210000002808 connective tissue Anatomy 0.000 claims description 4
- 208000018631 connective tissue disease Diseases 0.000 claims description 4
- 230000002526 effect on cardiovascular system Effects 0.000 claims description 4
- 239000005457 ice water Substances 0.000 claims description 4
- 230000000451 tissue damage Effects 0.000 claims description 4
- 231100000827 tissue damage Toxicity 0.000 claims description 4
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 claims description 3
- 239000011575 calcium Substances 0.000 claims description 3
- 229910052791 calcium Inorganic materials 0.000 claims description 3
- 230000006835 compression Effects 0.000 claims description 3
- 238000007906 compression Methods 0.000 claims description 3
- 230000004907 flux Effects 0.000 claims description 3
- 208000032873 genetic essential hypertension Diseases 0.000 claims description 3
- 239000011521 glass Substances 0.000 claims description 3
- 239000004973 liquid crystal related substance Substances 0.000 claims description 3
- 208000034189 Sclerosis Diseases 0.000 claims description 2
- 230000003906 autonomic nervous system functioning Effects 0.000 claims description 2
- 238000009432 framing Methods 0.000 claims description 2
- 239000011810 insulating material Substances 0.000 claims description 2
- 238000012806 monitoring device Methods 0.000 claims description 2
- 210000005036 nerve Anatomy 0.000 claims 1
- 230000008719 thickening Effects 0.000 claims 1
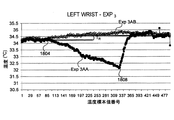
- 238000009529 body temperature measurement Methods 0.000 description 49
- 230000009471 action Effects 0.000 description 47
- 239000002085 irritant Substances 0.000 description 47
- 231100000021 irritant Toxicity 0.000 description 47
- 230000009849 deactivation Effects 0.000 description 40
- 239000000126 substance Substances 0.000 description 37
- 210000000707 wrist Anatomy 0.000 description 26
- 210000003371 toe Anatomy 0.000 description 24
- 210000000245 forearm Anatomy 0.000 description 22
- 239000000853 adhesive Substances 0.000 description 18
- 230000001070 adhesive effect Effects 0.000 description 18
- 238000013461 design Methods 0.000 description 14
- SNIOPGDIGTZGOP-UHFFFAOYSA-N Nitroglycerin Chemical compound [O-][N+](=O)OCC(O[N+]([O-])=O)CO[N+]([O-])=O SNIOPGDIGTZGOP-UHFFFAOYSA-N 0.000 description 13
- 210000002302 brachial artery Anatomy 0.000 description 13
- 229960003711 glyceryl trinitrate Drugs 0.000 description 13
- 239000000006 Nitroglycerin Substances 0.000 description 11
- 238000004458 analytical method Methods 0.000 description 11
- 238000002360 preparation method Methods 0.000 description 11
- 206010020772 Hypertension Diseases 0.000 description 10
- RYYVLZVUVIJVGH-UHFFFAOYSA-N caffeine Chemical compound CN1C(=O)N(C)C(=O)C2=C1N=CN2C RYYVLZVUVIJVGH-UHFFFAOYSA-N 0.000 description 10
- 230000003278 mimic effect Effects 0.000 description 10
- 230000035488 systolic blood pressure Effects 0.000 description 10
- 230000000694 effects Effects 0.000 description 9
- 210000002414 leg Anatomy 0.000 description 9
- 210000003038 endothelium Anatomy 0.000 description 8
- 239000012530 fluid Substances 0.000 description 8
- 210000001519 tissue Anatomy 0.000 description 8
- 210000004204 blood vessel Anatomy 0.000 description 7
- 239000000463 material Substances 0.000 description 7
- 239000008280 blood Substances 0.000 description 6
- 210000004369 blood Anatomy 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 5
- LPHGQDQBBGAPDZ-UHFFFAOYSA-N Isocaffeine Natural products CN1C(=O)N(C)C(=O)C2=C1N(C)C=N2 LPHGQDQBBGAPDZ-UHFFFAOYSA-N 0.000 description 5
- 208000025747 Rheumatic disease Diseases 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- 229960001948 caffeine Drugs 0.000 description 5
- VJEONQKOZGKCAK-UHFFFAOYSA-N caffeine Natural products CN1C(=O)N(C)C(=O)C2=C1C=CN2C VJEONQKOZGKCAK-UHFFFAOYSA-N 0.000 description 5
- 230000004087 circulation Effects 0.000 description 5
- 210000000624 ear auricle Anatomy 0.000 description 5
- 210000001331 nose Anatomy 0.000 description 5
- MWUXSHHQAYIFBG-UHFFFAOYSA-N Nitric oxide Chemical compound O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 description 4
- 208000005434 White Coat Hypertension Diseases 0.000 description 4
- 238000001816 cooling Methods 0.000 description 4
- 238000009413 insulation Methods 0.000 description 4
- 230000002452 interceptive effect Effects 0.000 description 4
- 210000000664 rectum Anatomy 0.000 description 4
- 230000000391 smoking effect Effects 0.000 description 4
- 230000004936 stimulating effect Effects 0.000 description 4
- 238000003860 storage Methods 0.000 description 4
- 229940124549 vasodilator Drugs 0.000 description 4
- 239000003071 vasodilator agent Substances 0.000 description 4
- OIPILFWXSMYKGL-UHFFFAOYSA-N acetylcholine Chemical compound CC(=O)OCC[N+](C)(C)C OIPILFWXSMYKGL-UHFFFAOYSA-N 0.000 description 3
- 229960004373 acetylcholine Drugs 0.000 description 3
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 238000004891 communication Methods 0.000 description 3
- 238000002405 diagnostic procedure Methods 0.000 description 3
- 238000010586 diagram Methods 0.000 description 3
- 235000005911 diet Nutrition 0.000 description 3
- 210000002683 foot Anatomy 0.000 description 3
- 238000010438 heat treatment Methods 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- 239000001301 oxygen Substances 0.000 description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- 101000599852 Homo sapiens Intercellular adhesion molecule 1 Proteins 0.000 description 2
- 102100037877 Intercellular adhesion molecule 1 Human genes 0.000 description 2
- 208000030831 Peripheral arterial occlusive disease Diseases 0.000 description 2
- YEESUBCSWGVPCE-UHFFFAOYSA-N azanylidyneoxidanium iron(2+) pentacyanide Chemical compound [Fe++].[C-]#N.[C-]#N.[C-]#N.[C-]#N.[C-]#N.N#[O+] YEESUBCSWGVPCE-UHFFFAOYSA-N 0.000 description 2
- 239000006071 cream Substances 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 238000003745 diagnosis Methods 0.000 description 2
- 230000000378 dietary effect Effects 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 230000002708 enhancing effect Effects 0.000 description 2
- -1 for example Substances 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 230000001631 hypertensive effect Effects 0.000 description 2
- 210000003205 muscle Anatomy 0.000 description 2
- 229960002460 nitroprusside Drugs 0.000 description 2
- 238000011275 oncology therapy Methods 0.000 description 2
- 238000000554 physical therapy Methods 0.000 description 2
- 230000035935 pregnancy Effects 0.000 description 2
- 201000002859 sleep apnea Diseases 0.000 description 2
- 230000006641 stabilisation Effects 0.000 description 2
- 238000011105 stabilization Methods 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 230000035900 sweating Effects 0.000 description 2
- 208000019553 vascular disease Diseases 0.000 description 2
- 230000004218 vascular function Effects 0.000 description 2
- 239000005526 vasoconstrictor agent Substances 0.000 description 2
- 102000016752 1-Alkyl-2-acetylglycerophosphocholine Esterase Human genes 0.000 description 1
- 239000005541 ACE inhibitor Substances 0.000 description 1
- 108010024976 Asparaginase Proteins 0.000 description 1
- 108010074051 C-Reactive Protein Proteins 0.000 description 1
- 102100032752 C-reactive protein Human genes 0.000 description 1
- 208000008960 Diabetic foot Diseases 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 229940123457 Free radical scavenger Drugs 0.000 description 1
- 102000001554 Hemoglobins Human genes 0.000 description 1
- 108010054147 Hemoglobins Proteins 0.000 description 1
- 101000622304 Homo sapiens Vascular cell adhesion protein 1 Proteins 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-N L-arginine Chemical compound OC(=O)[C@@H](N)CCCN=C(N)N ODKSFYDXXFIFQN-BYPYZUCNSA-N 0.000 description 1
- 229930064664 L-arginine Natural products 0.000 description 1
- 235000014852 L-arginine Nutrition 0.000 description 1
- KCWZGJVSDFYRIX-YFKPBYRVSA-N N(gamma)-nitro-L-arginine methyl ester Chemical compound COC(=O)[C@@H](N)CCCN=C(N)N[N+]([O-])=O KCWZGJVSDFYRIX-YFKPBYRVSA-N 0.000 description 1
- 102000003800 Selectins Human genes 0.000 description 1
- 108090000184 Selectins Proteins 0.000 description 1
- OUUQCZGPVNCOIJ-UHFFFAOYSA-M Superoxide Chemical compound [O-][O] OUUQCZGPVNCOIJ-UHFFFAOYSA-M 0.000 description 1
- 239000000150 Sympathomimetic Substances 0.000 description 1
- 102100023543 Vascular cell adhesion protein 1 Human genes 0.000 description 1
- 210000001015 abdomen Anatomy 0.000 description 1
- JUGOREOARAHOCO-UHFFFAOYSA-M acetylcholine chloride Chemical compound [Cl-].CC(=O)OCC[N+](C)(C)C JUGOREOARAHOCO-UHFFFAOYSA-M 0.000 description 1
- 229960004266 acetylcholine chloride Drugs 0.000 description 1
- 210000000577 adipose tissue Anatomy 0.000 description 1
- 239000002333 angiotensin II receptor antagonist Substances 0.000 description 1
- 229940044094 angiotensin-converting-enzyme inhibitor Drugs 0.000 description 1
- 230000003143 atherosclerotic effect Effects 0.000 description 1
- 210000004027 cell Anatomy 0.000 description 1
- 238000004590 computer program Methods 0.000 description 1
- 230000001054 cortical effect Effects 0.000 description 1
- 230000001120 cytoprotective effect Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- XEYBHCRIKKKOSS-UHFFFAOYSA-N disodium;azanylidyneoxidanium;iron(2+);pentacyanide Chemical compound [Na+].[Na+].[Fe+2].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].[O+]#N XEYBHCRIKKKOSS-UHFFFAOYSA-N 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 230000003511 endothelial effect Effects 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 210000004904 fingernail bed Anatomy 0.000 description 1
- 230000004217 heart function Effects 0.000 description 1
- 230000020169 heat generation Effects 0.000 description 1
- 230000000393 hypersympathetic effect Effects 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 230000003340 mental effect Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 230000037323 metabolic rate Effects 0.000 description 1
- 239000011859 microparticle Substances 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- 239000000734 parasympathomimetic agent Substances 0.000 description 1
- 230000001499 parasympathomimetic effect Effects 0.000 description 1
- 229940005542 parasympathomimetics Drugs 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 230000037081 physical activity Effects 0.000 description 1
- 230000035479 physiological effects, processes and functions Effects 0.000 description 1
- 201000011461 pre-eclampsia Diseases 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- AAEVYOVXGOFMJO-UHFFFAOYSA-N prometryn Chemical compound CSC1=NC(NC(C)C)=NC(NC(C)C)=N1 AAEVYOVXGOFMJO-UHFFFAOYSA-N 0.000 description 1
- 239000002516 radical scavenger Substances 0.000 description 1
- 208000020685 sleep-wake disease Diseases 0.000 description 1
- 210000002460 smooth muscle Anatomy 0.000 description 1
- 229940083618 sodium nitroprusside Drugs 0.000 description 1
- 210000004003 subcutaneous fat Anatomy 0.000 description 1
- 230000001975 sympathomimetic effect Effects 0.000 description 1
- 229940064707 sympathomimetics Drugs 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000006438 vascular health Effects 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 230000004580 weight loss Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/43—Detecting, measuring or recording for evaluating the reproductive systems
- A61B5/4375—Detecting, measuring or recording for evaluating the reproductive systems for evaluating the male reproductive system
- A61B5/4393—Sexual arousal or erectile dysfunction evaluation, e.g. tumescence evaluation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/01—Measuring temperature of body parts ; Diagnostic temperature sensing, e.g. for malignant or inflamed tissue
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/40—Detecting, measuring or recording for evaluating the nervous system
- A61B5/4029—Detecting, measuring or recording for evaluating the nervous system for evaluating the peripheral nervous systems
- A61B5/4035—Evaluating the autonomic nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/68—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient
- A61B5/6801—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient specially adapted to be attached to or worn on the body surface
- A61B5/6802—Sensor mounted on worn items
- A61B5/681—Wristwatch-type devices
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/68—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient
- A61B5/6801—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient specially adapted to be attached to or worn on the body surface
- A61B5/6813—Specially adapted to be attached to a specific body part
- A61B5/6824—Arm or wrist
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Veterinary Medicine (AREA)
- Biophysics (AREA)
- Pathology (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Public Health (AREA)
- Physics & Mathematics (AREA)
- Surgery (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Neurology (AREA)
- Gynecology & Obstetrics (AREA)
- Reproductive Health (AREA)
- Neurosurgery (AREA)
- Physiology (AREA)
- Measuring And Recording Apparatus For Diagnosis (AREA)
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US57425504P | 2004-05-26 | 2004-05-26 | |
| US58577304P | 2004-07-06 | 2004-07-06 | |
| US62414604P | 2004-11-02 | 2004-11-02 | |
| US62600604P | 2004-11-08 | 2004-11-08 | |
| US62817304P | 2004-11-15 | 2004-11-15 | |
| PCT/US2005/018437 WO2005118516A2 (en) | 2004-05-26 | 2005-05-25 | Method and apparatus for determining health condition |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2008500113A true JP2008500113A (ja) | 2008-01-10 |
| JP2008500113A5 JP2008500113A5 (enExample) | 2008-07-10 |
Family
ID=35463417
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007515315A Pending JP2008500113A (ja) | 2004-05-26 | 2005-05-25 | 健康条件を判別するための方法および装置 |
Country Status (3)
| Country | Link |
|---|---|
| EP (1) | EP1758841A2 (enExample) |
| JP (1) | JP2008500113A (enExample) |
| WO (1) | WO2005118516A2 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013538610A (ja) * | 2010-08-30 | 2013-10-17 | ビック・バイオレクス・エス・エー | シェービングカートリッジ用の保護カバー、シェービングアセンブリ、レザー、そうしたレザーを用いたシェービング方法、ならびに保護カバーの製造方法 |
| JP2015528371A (ja) * | 2012-09-17 | 2015-09-28 | エイ. ローズ、ドナルド, | 最適治療パラメータ判定法 |
| JP2017176876A (ja) * | 2011-12-27 | 2017-10-05 | コーニンクレッカ フィリップス エヌ ヴェKoninklijke Philips N.V. | 磁気共鳴サーモグラフィー:熱的異常についての高解像度画像化 |
| WO2021182936A1 (ko) * | 2020-03-11 | 2021-09-16 | 고려대학교 산학협력단 | 진단 결과 데이터 결정 방법 및 장치 |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20070225614A1 (en) | 2004-05-26 | 2007-09-27 | Endothelix, Inc. | Method and apparatus for determining vascular health conditions |
| GB0603006D0 (en) * | 2006-02-15 | 2006-03-29 | Dialog Devices Ltd | Assessing blood supply to a peripheral portion of an animal |
| IL178673A0 (en) * | 2006-04-03 | 2007-02-11 | Ilan Menashe | Improved digital thermometer |
| US8986342B2 (en) | 2007-11-25 | 2015-03-24 | Ic Therapeutics | Methods and apparatus for repeated ischemic conditioning treatment of hypertension and other medical conditions |
| PL226889B1 (pl) | 2011-05-31 | 2017-09-29 | Politechnika Łódzka | Sposób okreslania parametrów dooceny funkcji sródbłonka naczyniowego |
| US8868157B1 (en) | 2011-11-09 | 2014-10-21 | VisionQuest Biomedical LLC | Thermal optical imager system and method for detection of peripheral neuropathy |
| US12089925B2 (en) | 2014-09-15 | 2024-09-17 | Attenti Electronic Monitoring Ltd. | Impairment detection |
| ES2986414T3 (es) | 2014-09-15 | 2024-11-11 | Attenti Electronic Monitoring Ltd | Detección de deficiencias con consideraciones ambientales |
| WO2016044199A1 (en) | 2014-09-15 | 2016-03-24 | 3M Innovative Properties Company | Impairment detection with biological considerations |
| CN109770864B (zh) * | 2017-11-13 | 2021-11-30 | 深圳市倍泰健康测量分析技术有限公司 | 一种血管内皮功能检测装置、方法及存储介质 |
| CN112535464A (zh) * | 2020-12-21 | 2021-03-23 | 安徽华米智能科技有限公司 | 基于pai的状态评估方法、装置、设备和存储介质 |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH1085223A (ja) * | 1996-09-19 | 1998-04-07 | Matsushita Electric Ind Co Ltd | 体温計 |
| JP2001004453A (ja) * | 1999-06-21 | 2001-01-12 | Atsuo Morikawa | 自動検温システム |
| JP2001238967A (ja) * | 2000-02-28 | 2001-09-04 | Japan Science & Technology Corp | 皮膚状態帰還式褥創予防治療システム |
| JP2001513677A (ja) * | 1997-02-28 | 2001-09-04 | キューアールエス ダイアグノスティック リミテッド ライアビリティ カンパニー | 実時間生物学データ収集用パーソナルコンピュータカード |
| JP2003503693A (ja) * | 1999-06-23 | 2003-01-28 | エリアフ ルビンスタイン、 | 熱警報システム |
| JP2004130124A (ja) * | 2002-09-18 | 2004-04-30 | Hidetoshi Wakamatsu | 生理状態管理システムおよび生理状態管理方法 |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3463854A (en) * | 1968-05-08 | 1969-08-26 | Tibor L Kopjas | Method of treatment for effecting vasodilation of the small arteries in human beings |
| SE425331B (sv) * | 1979-01-17 | 1982-09-20 | Erling Nilsson | Anordning for detektering av cirkulationsrubbningar i en patients extremiter pa grundval av extremiteternas hudtemperatur |
| US4428382A (en) * | 1980-09-03 | 1984-01-31 | Gst Laboratories, Inc. | Method for identifying the presence of abnormal tissue |
| US5050612A (en) * | 1989-09-12 | 1991-09-24 | Matsumura Kenneth N | Device for computer-assisted monitoring of the body |
| US5634468A (en) * | 1992-04-03 | 1997-06-03 | Micromedical Industries Limited | Sensor patch and system for physiological monitoring |
| US6240306B1 (en) * | 1995-08-09 | 2001-05-29 | Rio Grande Medical Technologies, Inc. | Method and apparatus for non-invasive blood analyte measurement with fluid compartment equilibration |
| US6090050A (en) * | 1998-07-16 | 2000-07-18 | Salix Medical, Inc. | Thermometric apparatus and method |
| US6077228A (en) * | 1998-11-04 | 2000-06-20 | Schonberger; Milton | Breast temperature scanner |
| US6447460B1 (en) * | 1998-12-09 | 2002-09-10 | Kci Licensing, Inc. | Method for automated exclusion of deep venous thrombosis |
| US7277744B2 (en) * | 1999-03-22 | 2007-10-02 | Schaefer Allan L | Early detection of inflammation and infection using infrared thermography |
| US6332867B1 (en) * | 1999-06-09 | 2001-12-25 | Vsm Technology Inc. | Method and apparatus for measuring values of physiological parameters |
| WO2004017905A2 (en) * | 2002-08-23 | 2004-03-04 | The Board Of Regents Of University Of Texas System | Method and apparatus for noninvasively evaluating endothelial function |
-
2005
- 2005-05-25 WO PCT/US2005/018437 patent/WO2005118516A2/en not_active Ceased
- 2005-05-25 EP EP05753422A patent/EP1758841A2/en not_active Withdrawn
- 2005-05-25 JP JP2007515315A patent/JP2008500113A/ja active Pending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH1085223A (ja) * | 1996-09-19 | 1998-04-07 | Matsushita Electric Ind Co Ltd | 体温計 |
| JP2001513677A (ja) * | 1997-02-28 | 2001-09-04 | キューアールエス ダイアグノスティック リミテッド ライアビリティ カンパニー | 実時間生物学データ収集用パーソナルコンピュータカード |
| JP2001004453A (ja) * | 1999-06-21 | 2001-01-12 | Atsuo Morikawa | 自動検温システム |
| JP2003503693A (ja) * | 1999-06-23 | 2003-01-28 | エリアフ ルビンスタイン、 | 熱警報システム |
| JP2001238967A (ja) * | 2000-02-28 | 2001-09-04 | Japan Science & Technology Corp | 皮膚状態帰還式褥創予防治療システム |
| JP2004130124A (ja) * | 2002-09-18 | 2004-04-30 | Hidetoshi Wakamatsu | 生理状態管理システムおよび生理状態管理方法 |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013538610A (ja) * | 2010-08-30 | 2013-10-17 | ビック・バイオレクス・エス・エー | シェービングカートリッジ用の保護カバー、シェービングアセンブリ、レザー、そうしたレザーを用いたシェービング方法、ならびに保護カバーの製造方法 |
| JP2017176876A (ja) * | 2011-12-27 | 2017-10-05 | コーニンクレッカ フィリップス エヌ ヴェKoninklijke Philips N.V. | 磁気共鳴サーモグラフィー:熱的異常についての高解像度画像化 |
| JP2015528371A (ja) * | 2012-09-17 | 2015-09-28 | エイ. ローズ、ドナルド, | 最適治療パラメータ判定法 |
| WO2021182936A1 (ko) * | 2020-03-11 | 2021-09-16 | 고려대학교 산학협력단 | 진단 결과 데이터 결정 방법 및 장치 |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2005118516A3 (en) | 2009-04-09 |
| WO2005118516A2 (en) | 2005-12-15 |
| EP1758841A2 (en) | 2007-03-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8551008B2 (en) | Method and apparatus for determining vascular health conditions | |
| US10517487B2 (en) | Methods and apparatus for assessing vascular health | |
| ES2951015T3 (es) | Monitorización del flujo sanguíneo por ultrasonidos | |
| ES2336137T3 (es) | Monitor de perfusion cerebral. | |
| US20070118045A1 (en) | Iontophoresis challenge for monitoring cardiovascular status | |
| US20080027330A1 (en) | Risk assessment method for acute cardiovascular events | |
| US20080081963A1 (en) | Methods and Apparatus for Profiling Cardiovascular Vulnerability to Mental Stress | |
| JP2008500113A (ja) | 健康条件を判別するための方法および装置 | |
| EP1951110B1 (en) | Apparatus for measuring biologic parameters | |
| Hales et al. | Observations on a new non‐invasive monitor of skin blood flow | |
| US20070225606A1 (en) | Method and apparatus for comprehensive assessment of vascular health | |
| US20070173727A1 (en) | Method and apparatus for isolating the vascular component in digital temerature monitoring | |
| US20100081941A1 (en) | Cardiovascular health station methods and apparatus | |
| Zalewski et al. | Whole-body cryostimulation increases parasympathetic outflow and decreases core body temperature | |
| Wieling et al. | The assessment of cardiovascular reflex activity: standardization is needed | |
| Kelechi et al. | A descriptive study of skin temperature, tissue perfusion, and tissue oxygen in patients with chronic venous disease | |
| Kamshilin et al. | Imaging photoplethysmography and its applications | |
| RU2677590C1 (ru) | Способ оценки микроциркуляторных нарушений у больных с нарушениями углеводного обмена | |
| Zvan et al. | Influence of the cold pressor test on the middle cerebral artery circulation | |
| US20200187789A1 (en) | Methods and apparatus for assessing vascular health | |
| Costa et al. | Core body temperature evaluation: suitability of measurement procedures | |
| Mayrovitz | Assessment of human microvascular function | |
| RU2582764C1 (ru) | Способ диагностики склонности к ангиоспазму периферического сосудистого русла | |
| Murray | Laboratory assessment of Raynaud’s phenomenon | |
| Brierley-bowers et al. | Measures of autonomic nervous system regulation |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080526 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20080526 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100914 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20110308 |