JP2005298974A - 水素化ホウ素を製造するための電解方法 - Google Patents
水素化ホウ素を製造するための電解方法 Download PDFInfo
- Publication number
- JP2005298974A JP2005298974A JP2005114236A JP2005114236A JP2005298974A JP 2005298974 A JP2005298974 A JP 2005298974A JP 2005114236 A JP2005114236 A JP 2005114236A JP 2005114236 A JP2005114236 A JP 2005114236A JP 2005298974 A JP2005298974 A JP 2005298974A
- Authority
- JP
- Japan
- Prior art keywords
- cathode
- borohydride
- solution
- electrolysis
- solvent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 238000005868 electrolysis reaction Methods 0.000 title claims abstract description 20
- 238000004519 manufacturing process Methods 0.000 title claims abstract description 9
- 229910010277 boron hydride Inorganic materials 0.000 title abstract description 7
- UORVGPXVDQYIDP-UHFFFAOYSA-N borane Chemical compound B UORVGPXVDQYIDP-UHFFFAOYSA-N 0.000 title abstract description 6
- 238000000034 method Methods 0.000 claims abstract description 18
- 239000003125 aqueous solvent Substances 0.000 claims description 10
- 239000002904 solvent Substances 0.000 claims description 7
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 claims description 4
- 150000004678 hydrides Chemical class 0.000 claims description 4
- 238000005984 hydrogenation reaction Methods 0.000 claims description 3
- 239000003054 catalyst Substances 0.000 claims description 2
- 230000000694 effects Effects 0.000 claims description 2
- 229910052751 metal Inorganic materials 0.000 claims description 2
- 239000002184 metal Substances 0.000 claims description 2
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 18
- 229910000033 sodium borohydride Inorganic materials 0.000 description 17
- 239000012279 sodium borohydride Substances 0.000 description 17
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 12
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 9
- 238000006243 chemical reaction Methods 0.000 description 9
- 238000007323 disproportionation reaction Methods 0.000 description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 9
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 8
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 8
- -1 borate ester Chemical class 0.000 description 7
- 238000005481 NMR spectroscopy Methods 0.000 description 6
- 238000002474 experimental method Methods 0.000 description 6
- 229910002804 graphite Inorganic materials 0.000 description 6
- 239000010439 graphite Substances 0.000 description 6
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 5
- 239000003513 alkali Substances 0.000 description 5
- 125000004432 carbon atom Chemical group C* 0.000 description 5
- 239000011521 glass Substances 0.000 description 5
- GNOIPBMMFNIUFM-UHFFFAOYSA-N hexamethylphosphoric triamide Chemical compound CN(C)P(=O)(N(C)C)N(C)C GNOIPBMMFNIUFM-UHFFFAOYSA-N 0.000 description 5
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 4
- ZOMNIUBKTOKEHS-UHFFFAOYSA-L dimercury dichloride Chemical class Cl[Hg][Hg]Cl ZOMNIUBKTOKEHS-UHFFFAOYSA-L 0.000 description 4
- WRECIMRULFAWHA-UHFFFAOYSA-N trimethyl borate Chemical compound COB(OC)OC WRECIMRULFAWHA-UHFFFAOYSA-N 0.000 description 4
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 3
- 125000000217 alkyl group Chemical group 0.000 description 3
- 150000001642 boronic acid derivatives Chemical class 0.000 description 3
- 239000010406 cathode material Substances 0.000 description 3
- 229910052740 iodine Inorganic materials 0.000 description 3
- 239000011630 iodine Substances 0.000 description 3
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 2
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- MWPLVEDNUUSJAV-UHFFFAOYSA-N anthracene Chemical compound C1=CC=CC2=CC3=CC=CC=C3C=C21 MWPLVEDNUUSJAV-UHFFFAOYSA-N 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- ZOXJGFHDIHLPTG-IGMARMGPSA-N boron-11 atom Chemical compound [11B] ZOXJGFHDIHLPTG-IGMARMGPSA-N 0.000 description 2
- 238000011088 calibration curve Methods 0.000 description 2
- 229940075397 calomel Drugs 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- WDECIBYCCFPHNR-UHFFFAOYSA-N chrysene Chemical compound C1=CC=CC2=CC=C3C4=CC=CC=C4C=CC3=C21 WDECIBYCCFPHNR-UHFFFAOYSA-N 0.000 description 2
- 230000005518 electrochemistry Effects 0.000 description 2
- 229910021397 glassy carbon Inorganic materials 0.000 description 2
- 150000002500 ions Chemical class 0.000 description 2
- AWJUIBRHMBBTKR-UHFFFAOYSA-N isoquinoline Chemical compound C1=NC=CC2=CC=CC=C21 AWJUIBRHMBBTKR-UHFFFAOYSA-N 0.000 description 2
- MHCFAGZWMAWTNR-UHFFFAOYSA-M lithium perchlorate Chemical compound [Li+].[O-]Cl(=O)(=O)=O MHCFAGZWMAWTNR-UHFFFAOYSA-M 0.000 description 2
- 229910001486 lithium perchlorate Inorganic materials 0.000 description 2
- 229910052759 nickel Inorganic materials 0.000 description 2
- 239000003791 organic solvent mixture Substances 0.000 description 2
- 229910052763 palladium Inorganic materials 0.000 description 2
- YNPNZTXNASCQKK-UHFFFAOYSA-N phenanthrene Chemical compound C1=CC=C2C3=CC=CC=C3C=CC2=C1 YNPNZTXNASCQKK-UHFFFAOYSA-N 0.000 description 2
- 229910052697 platinum Inorganic materials 0.000 description 2
- 239000003880 polar aprotic solvent Substances 0.000 description 2
- 239000004810 polytetrafluoroethylene Substances 0.000 description 2
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 150000003109 potassium Chemical class 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 239000011541 reaction mixture Substances 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 238000004448 titration Methods 0.000 description 2
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical class C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 125000003545 alkoxy group Chemical group 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 229910052796 boron Inorganic materials 0.000 description 1
- 239000007810 chemical reaction solvent Substances 0.000 description 1
- XTEGARKTQYYJKE-UHFFFAOYSA-M chlorate Inorganic materials [O-]Cl(=O)=O XTEGARKTQYYJKE-UHFFFAOYSA-M 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- LYCAIKOWRPUZTN-UHFFFAOYSA-N ethylene glycol Natural products OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 1
- 150000002334 glycols Chemical class 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 229910052741 iridium Inorganic materials 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 229910001496 lithium tetrafluoroborate Inorganic materials 0.000 description 1
- IOEDDFFKYCBADJ-UHFFFAOYSA-M lithium;4-methylbenzenesulfonate Chemical compound [Li+].CC1=CC=C(S([O-])(=O)=O)C=C1 IOEDDFFKYCBADJ-UHFFFAOYSA-M 0.000 description 1
- OWNSEPXOQWKTKG-UHFFFAOYSA-M lithium;methanesulfonate Chemical compound [Li+].CS([O-])(=O)=O OWNSEPXOQWKTKG-UHFFFAOYSA-M 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 229910052987 metal hydride Inorganic materials 0.000 description 1
- 150000004681 metal hydrides Chemical class 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- VLTRZXGMWDSKGL-UHFFFAOYSA-M perchlorate Inorganic materials [O-]Cl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-M 0.000 description 1
- VLTRZXGMWDSKGL-UHFFFAOYSA-N perchloric acid Chemical compound OCl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-N 0.000 description 1
- 125000005575 polycyclic aromatic hydrocarbon group Chemical group 0.000 description 1
- 239000003586 protic polar solvent Substances 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 230000000171 quenching effect Effects 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 229910052703 rhodium Inorganic materials 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 150000005621 tetraalkylammonium salts Chemical class 0.000 description 1
- 230000036962 time dependent Effects 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B1/00—Electrolytic production of inorganic compounds or non-metals
- C25B1/01—Products
- C25B1/28—Per-compounds
- C25B1/30—Peroxides
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B1/00—Electrolytic production of inorganic compounds or non-metals
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B1/00—Electrolytic production of inorganic compounds or non-metals
- C25B1/01—Products
- C25B1/14—Alkali metal compounds
Landscapes
- Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Electrolytic Production Of Non-Metals, Compounds, Apparatuses Therefor (AREA)
Abstract
【解決手段】 水素化トリアルコキシホウ素の溶液が陰極と接触している、電解セル中の陽極と陰極との間に電流を流すことによる水素化ホウ素の製造方法。
【選択図】なし
Description
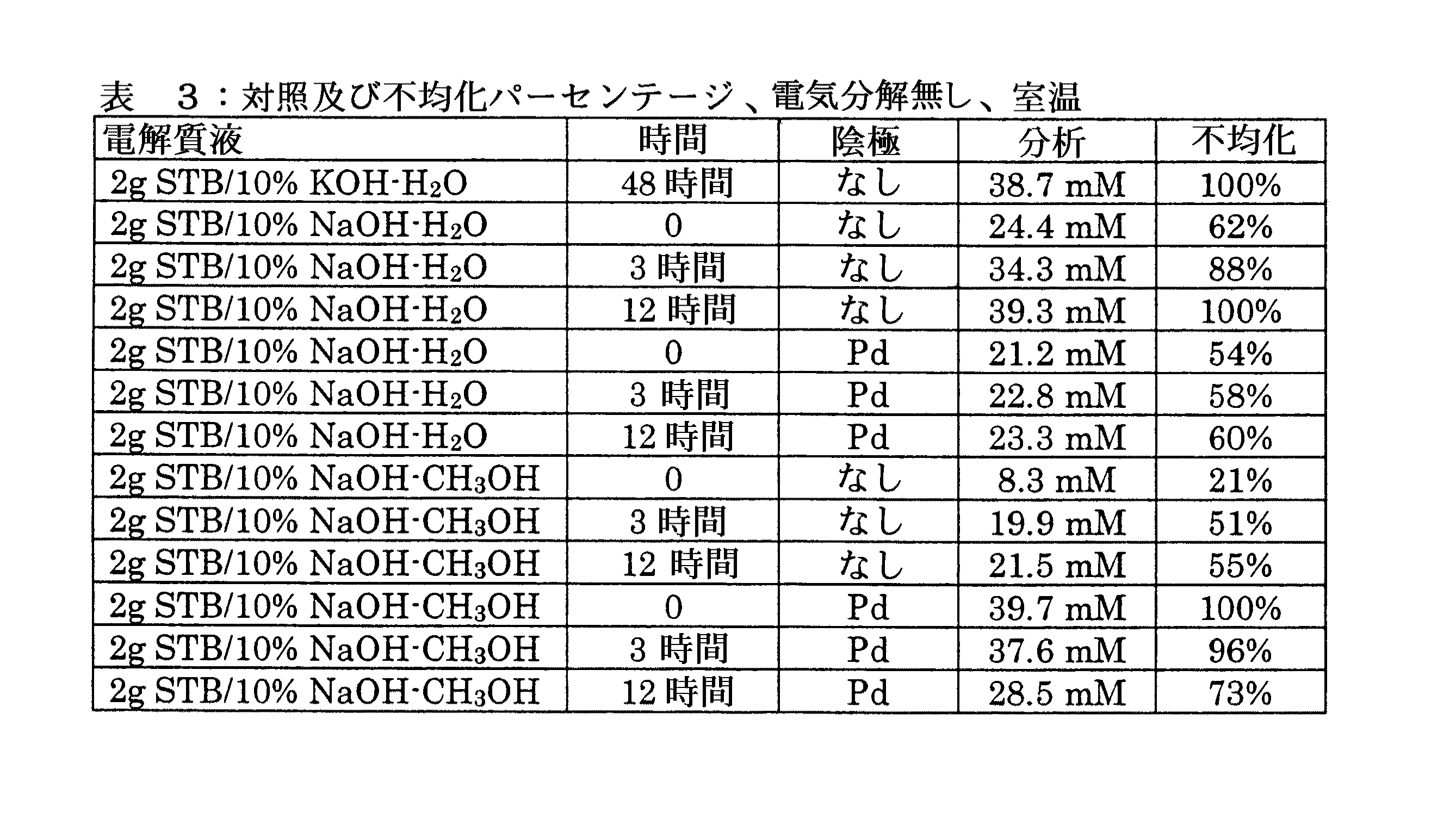
対応するガラスカバーを有する三つの区画(アノード液、カソード液および参照)からなるフリット分離ガラスH−セルに、陰極、および、グラファイトロッド陽極(5cm2電極面積;溶液に暴露される残余の電極領域はPTFEテープでマスクされた)が装着された。飽和カロメル参照電極は、参照区画中に挿入された。カソード溶液はカソード液区画に添加され、10重量%水酸化ナトリウム水溶液が陽極区画(35mL)および参照区画(10mL)に添加された。電極は、エレクトロシンセシスCo.410ポテンシオスタット、420A DC電源および640クーロメータからなるポテンシオスタット系に接続された。セルは、一定の温度を維持するため室温水浴中に垂下され、磁気撹拌器が陰極区画を良く撹拌された状態に維持するため利用された。作用電極(陰極)のための電位および初期電流が、その後セットされた。
(A)10%水酸化ナトリウム100mLおよびSTBを2gからなるカソード液を使用して、上記一般的手順に従った。陰極の電位は、カロメル参照に対して−1.5Vにセットされた。初期電流は、550mA(110mA/cm2電流密度)であった。定電位において7225クーロンの電荷(0.0750モルの電子)が通過した後、反応を停止した。水素化ホウ素ナトリウム製造についての6−電子プロセス(six−electron process)に基づいて、100%効率で12.5ミリモルまでの水素化ホウ素ナトリウムが形成され得た。反応混合物中の水素化ホウ素ナトリウムの実際の濃度を明確にするために、ホウ素−11NMRピーク強度を使用して異なる濃度の一連の水素化ホウ素カリウム試料により検量曲線が作成された。直線のキャリブレーションが4.5ミリモル/L〜13.5ミリモル/Lの濃度範囲で得られた。この曲線に基づいて、実験試料の濃度は、18.3ミリモル/Lであった。これは1.83ミリモルの全SBHに相当し、15%の電流効率を示している。
相当するガラスカバーを有する三つの区画(アノード液、カソード液および参照)からなるフリット分離ガラスH−セルに、陰極、および、グラファイトロッド陽極(5cm2電極面積;溶液に暴露される残余の電極領域はPTFEテープでマスクされた)が装着された。飽和カロメル参照電極は、参照区画中に挿入された。カソード液は、100mLのDMF中、0.5M過塩素酸リチウム、5mLのTMB(4.6g、44.3ミリモル)であった。アノード液は、0.5M過塩素酸リチウム/DMF(35mL)であった。電極は、エレクトロシンセシスCo.410ポテンシオスタット、420A DC電源および640クーロメータからなるポテンシオスタット系に接続された。セルは、一定の温度を維持するため室温水浴中に垂下され、磁気撹拌器が陰極区画を良く撹拌された状態に維持するため利用された。定電位は、−3.90Vにセットされ、初期電流は150mAであり、通過した電荷は1390クーロンであった。第二の実験において、ニッケルロッドに取り付けられたニッケルフラグ陰極(5cm2)が使用された。定電位は、−3.5Vにセットされ、初期電流は85mAであり、通過した電荷は1054クーロンであった。ホウ素NMR分析により、一種のホウ素水素化物種に対し予期された領域における約0.17ppmのダブレットの存在が示されたが、水素化ホウ素について予期された位置ではなかった。
Claims (6)
- 電解セル中の陽極と陰極との間に電流を流すことを含み、ここで水素化トリアルコキシホウ素溶液が陰極と接触している、水素化ホウ素を製造する方法。
- 陰極と接触する溶媒が非水性溶媒である、請求項1に記載の方法。
- 再生可能レドックス種が陰極付近に存在する、請求項1に記載の方法。
- 陰極が、水素化触媒としての活性を有する金属を含む、請求項1に記載の方法。
- a)電解セル中の陽極と陰極との間に電流を流し(ここでホウ酸エステル溶液が陰極と接触している)、それにより水素化トリアルコキシホウ素溶液を製造する工程;および
b)第二電解セル中の第二陽極と第二陰極との間に電流を流す(ここで水素化トリアルコキシホウ素溶液が第二陰極と接触している)工程
を含む水素化ホウ素を製造する方法。 - 陰極および第二陰極と接触している溶媒が非水性溶媒を含む、請求項5に記載の方法。
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US56160304P | 2004-04-13 | 2004-04-13 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2005298974A true JP2005298974A (ja) | 2005-10-27 |
| JP4303215B2 JP4303215B2 (ja) | 2009-07-29 |
Family
ID=34940713
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2005114236A Expired - Fee Related JP4303215B2 (ja) | 2004-04-13 | 2005-04-12 | 水素化ホウ素を製造するための電解方法 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US7638029B2 (ja) |
| EP (1) | EP1586677A1 (ja) |
| JP (1) | JP4303215B2 (ja) |
| KR (1) | KR100729987B1 (ja) |
| CN (1) | CN1690250B (ja) |
| CA (1) | CA2503297C (ja) |
| TW (1) | TWI310369B (ja) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009525939A (ja) * | 2006-02-08 | 2009-07-16 | ロス アラモス ナショナル セキュリティ,リミテッド ライアビリテイ カンパニー | ボラン類のエネルギー効率の良い合成 |
| JP2010069399A (ja) * | 2008-09-17 | 2010-04-02 | Toshiba Corp | ホウ素分離システム |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060102491A1 (en) * | 2004-11-10 | 2006-05-18 | Kelly Michael T | Processes for separating metals from metal salts |
| US8021536B2 (en) * | 2006-04-13 | 2011-09-20 | Air Products And Chemical, Inc. | Method and apparatus for achieving maximum yield in the electrolytic preparation of group IV and V hydrides |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3734842A (en) | 1971-05-05 | 1973-05-22 | H Cooper | Electrolytic process for the production of alkali metal borohydrides |
| US4808282A (en) * | 1987-01-05 | 1989-02-28 | The Dow Chemical Company | Alkaline earth metal compounds and alkali metal substances via electrochemical process |
| US4904357A (en) * | 1989-05-30 | 1990-02-27 | Southwestern Analytical | Production of quaternary ammonium and quaternary phosphonium borohydrides |
| US4931154A (en) | 1989-07-17 | 1990-06-05 | Southwestern Analytical Chemicals, Inc. | Production of metal borohydrides and organic onium borohydrides |
| US5804329A (en) * | 1995-12-28 | 1998-09-08 | National Patent Development Corporation | Electroconversion cell |
| JP2003247088A (ja) | 2002-02-22 | 2003-09-05 | Nissan Motor Co Ltd | 水素化硼素化合物の製造方法および装置 |
-
2005
- 2005-03-31 CA CA002503297A patent/CA2503297C/en not_active Expired - Fee Related
- 2005-03-31 TW TW094110369A patent/TWI310369B/zh not_active IP Right Cessation
- 2005-04-05 EP EP05252119A patent/EP1586677A1/en not_active Withdrawn
- 2005-04-12 CN CN2005100650176A patent/CN1690250B/zh not_active Expired - Fee Related
- 2005-04-12 JP JP2005114236A patent/JP4303215B2/ja not_active Expired - Fee Related
- 2005-04-12 US US11/104,121 patent/US7638029B2/en not_active Expired - Fee Related
- 2005-04-13 KR KR1020050030542A patent/KR100729987B1/ko not_active Expired - Fee Related
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009525939A (ja) * | 2006-02-08 | 2009-07-16 | ロス アラモス ナショナル セキュリティ,リミテッド ライアビリテイ カンパニー | ボラン類のエネルギー効率の良い合成 |
| JP2010069399A (ja) * | 2008-09-17 | 2010-04-02 | Toshiba Corp | ホウ素分離システム |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20060045643A (ko) | 2006-05-17 |
| CA2503297A1 (en) | 2005-10-13 |
| US7638029B2 (en) | 2009-12-29 |
| CN1690250B (zh) | 2013-09-25 |
| JP4303215B2 (ja) | 2009-07-29 |
| KR100729987B1 (ko) | 2007-06-20 |
| CN1690250A (zh) | 2005-11-02 |
| TWI310369B (en) | 2009-06-01 |
| EP1586677A1 (en) | 2005-10-19 |
| TW200538392A (en) | 2005-12-01 |
| CA2503297C (en) | 2009-10-20 |
| US20050224364A1 (en) | 2005-10-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2010529033A (ja) | 電解質としてのイオン性液体 | |
| US11359295B2 (en) | Electrohydrogenation of nitriles | |
| JP2012528825A (ja) | 非対称性ビアリールアルコールを製造する方法 | |
| US4131521A (en) | Electrochemical synthesis of organic carbonates | |
| JP4303215B2 (ja) | 水素化ホウ素を製造するための電解方法 | |
| Mohammadzadeh et al. | The electrochemical behavior of 4-nitrobenzyl bromide and its catalytic activity for reduction of CO2 in the acetonitrile solvent at the Cu/Pd/rGO/GCE surface | |
| Zhao et al. | Influences of the operative parameters and the nature of the substrate on the electrocarboxylation of benzophenones | |
| US11198669B2 (en) | Method for preparing primary diamines by Kolbe electrolysis coupling reaction | |
| CN111809195B (zh) | α-二硫醚二羧酸类化合物的电化学催化氧化偶联合成方法 | |
| Lamoureux et al. | An electrolysis cell with close consecutive flow-through porous electrodes for particular organic electrosynthesis | |
| JP6495925B2 (ja) | 電気化学的脱炭酸プロセスのための溶融カルボキシレート電解質 | |
| Perovic et al. | Efficient hydrogen production using ternary Ni–Cu–Mo ionic activator | |
| US6419814B1 (en) | Methods for electrochemical synthesis of organoiodonium salts and derivatives | |
| García-Cruz et al. | Surprising electrooxidation of propargyl alcohol to (Z)-3-(2-propynoxy)-2-propenoic acid at a NiOOH electrode in alkaline medium | |
| JP4587329B2 (ja) | 脂肪族または脂環式c−原子と結合した第1級アミノ基およびシクロプロピル単位を有する、第1級アミンの製造方法 | |
| Du et al. | Reduction of 1-(2-Chloroethyl)-2-nitrobenzene and 1-(2-Bromoethyl)-2-nitrobenzene at carbon cathodes: Electrosynthetic routes to 1-Nitro-2-vinylbenzene and 1H-indole | |
| CN119194469B (zh) | 一种电化学合成胺类化合物的方法 | |
| US9206515B2 (en) | Method of producing coupled radical products via desulfoxylation | |
| JP2632832B2 (ja) | ポリフルオロベンジルアルコールの製造方法 | |
| Shamsipur et al. | Electrochemical oxidation of catechols in the presence of ethyl‐2‐chloroacetoacetate. Synthesis and mechanistic study | |
| JPS62294191A (ja) | アルコキシ酢酸の製法 | |
| Perovic et al. | Efficient hydrogen production using ternary NieCueMo ionic activator | |
| WO2020203638A1 (ja) | 水素化物イオン含有組成物、水素化物イオン含有組成物の製造方法、及び、化合物のヒドリド還元方法 | |
| JPH0375391A (ja) | m‐シアノベンジルアルコールの製造方法 | |
| WO2014138252A1 (en) | Method of producing coupled radical products via desulfoxylation |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| RD02 | Notification of acceptance of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7422 Effective date: 20061017 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20071015 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20071112 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20080208 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080226 |
|
| RD02 | Notification of acceptance of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7422 Effective date: 20080226 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20080215 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20080312 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20080317 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080411 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20081125 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090218 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20090402 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20090423 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120501 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130501 Year of fee payment: 4 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |