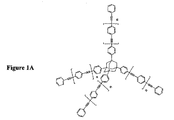
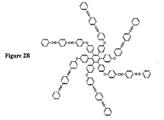
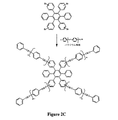
JP2004504455A - 有機組成物における熱硬化性分子の組成物および方法 - Google Patents
有機組成物における熱硬化性分子の組成物および方法 Download PDFInfo
- Publication number
- JP2004504455A JP2004504455A JP2002514210A JP2002514210A JP2004504455A JP 2004504455 A JP2004504455 A JP 2004504455A JP 2002514210 A JP2002514210 A JP 2002514210A JP 2002514210 A JP2002514210 A JP 2002514210A JP 2004504455 A JP2004504455 A JP 2004504455A
- Authority
- JP
- Japan
- Prior art keywords
- aryl
- polymer
- arylene ether
- branched
- dielectric constant
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 229920001187 thermosetting polymer Polymers 0.000 title claims abstract description 79
- 238000000034 method Methods 0.000 title claims description 34
- 239000000203 mixture Substances 0.000 title description 12
- 125000003118 aryl group Chemical group 0.000 claims abstract description 106
- 239000000178 monomer Substances 0.000 claims abstract description 86
- 229920000642 polymer Polymers 0.000 claims abstract description 73
- -1 arylene ether Chemical compound 0.000 claims abstract description 54
- 150000001875 compounds Chemical group 0.000 claims abstract description 42
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 claims abstract description 42
- ORILYTVJVMAKLC-UHFFFAOYSA-N adamantane Chemical compound C1C(C2)CC3CC1CC2C3 ORILYTVJVMAKLC-UHFFFAOYSA-N 0.000 claims abstract description 40
- 238000006243 chemical reaction Methods 0.000 claims abstract description 29
- 238000010348 incorporation Methods 0.000 claims abstract description 18
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims abstract description 16
- 229910052710 silicon Inorganic materials 0.000 claims abstract description 12
- 239000003989 dielectric material Substances 0.000 claims abstract description 11
- ZICQBHNGXDOVJF-UHFFFAOYSA-N diamantane Chemical compound C1C2C3CC(C4)CC2C2C4C3CC1C2 ZICQBHNGXDOVJF-UHFFFAOYSA-N 0.000 claims abstract description 9
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims abstract description 8
- 238000004519 manufacturing process Methods 0.000 claims abstract description 7
- 230000007423 decrease Effects 0.000 claims description 4
- 239000010703 silicon Substances 0.000 abstract description 3
- 125000000732 arylene group Chemical group 0.000 abstract 2
- 125000004429 atom Chemical group 0.000 description 14
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 12
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 12
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 9
- UEXCJVNBTNXOEH-UHFFFAOYSA-N Ethynylbenzene Chemical group C#CC1=CC=CC=C1 UEXCJVNBTNXOEH-UHFFFAOYSA-N 0.000 description 9
- 238000006352 cycloaddition reaction Methods 0.000 description 8
- 239000011159 matrix material Substances 0.000 description 8
- 0 C1C=C*=CC1 Chemical compound C1C=C*=CC1 0.000 description 7
- 238000004132 cross linking Methods 0.000 description 7
- 238000010586 diagram Methods 0.000 description 7
- NFHFRUOZVGFOOS-UHFFFAOYSA-N palladium;triphenylphosphane Chemical compound [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 7
- 239000007787 solid Substances 0.000 description 7
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 6
- 238000007792 addition Methods 0.000 description 6
- QARVLSVVCXYDNA-UHFFFAOYSA-N bromobenzene Chemical compound BrC1=CC=CC=C1 QARVLSVVCXYDNA-UHFFFAOYSA-N 0.000 description 6
- 239000011810 insulating material Substances 0.000 description 6
- 239000000463 material Substances 0.000 description 6
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 6
- 239000000047 product Substances 0.000 description 6
- XMWRBQBLMFGWIX-UHFFFAOYSA-N C60 fullerene Chemical class C12=C3C(C4=C56)=C7C8=C5C5=C9C%10=C6C6=C4C1=C1C4=C6C6=C%10C%10=C9C9=C%11C5=C8C5=C8C7=C3C3=C7C2=C1C1=C2C4=C6C4=C%10C6=C9C9=C%11C5=C5C8=C3C3=C7C1=C1C2=C4C6=C2C9=C5C3=C12 XMWRBQBLMFGWIX-UHFFFAOYSA-N 0.000 description 5
- QXJJQWWVWRCVQT-UHFFFAOYSA-K calcium;sodium;phosphate Chemical compound [Na+].[Ca+2].[O-]P([O-])([O-])=O QXJJQWWVWRCVQT-UHFFFAOYSA-K 0.000 description 5
- 229910003472 fullerene Inorganic materials 0.000 description 5
- 239000002105 nanoparticle Substances 0.000 description 5
- 239000011148 porous material Substances 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- UHOVQNZJYSORNB-UHFFFAOYSA-N benzene Substances C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 4
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 4
- 125000000524 functional group Chemical group 0.000 description 4
- 238000004128 high performance liquid chromatography Methods 0.000 description 4
- 239000010410 layer Substances 0.000 description 4
- 230000007246 mechanism Effects 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 239000007783 nanoporous material Substances 0.000 description 4
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 4
- 239000002904 solvent Substances 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- 239000011800 void material Substances 0.000 description 4
- 235000012431 wafers Nutrition 0.000 description 4
- UCCUXODGPMAHRL-UHFFFAOYSA-N 1-bromo-4-iodobenzene Chemical compound BrC1=CC=C(I)C=C1 UCCUXODGPMAHRL-UHFFFAOYSA-N 0.000 description 3
- 238000007341 Heck reaction Methods 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 238000007080 aromatic substitution reaction Methods 0.000 description 3
- 125000002619 bicyclic group Chemical group 0.000 description 3
- 125000004432 carbon atom Chemical group C* 0.000 description 3
- 239000003054 catalyst Substances 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 239000010408 film Substances 0.000 description 3
- 239000012467 final product Substances 0.000 description 3
- 125000005842 heteroatom Chemical group 0.000 description 3
- 238000007339 nucleophilic aromatic substitution reaction Methods 0.000 description 3
- 229910052763 palladium Inorganic materials 0.000 description 3
- 125000001424 substituent group Chemical group 0.000 description 3
- HIXDQWDOVZUNNA-UHFFFAOYSA-N 2-(3,4-dimethoxyphenyl)-5-hydroxy-7-methoxychromen-4-one Chemical compound C=1C(OC)=CC(O)=C(C(C=2)=O)C=1OC=2C1=CC=C(OC)C(OC)=C1 HIXDQWDOVZUNNA-UHFFFAOYSA-N 0.000 description 2
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 2
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- RDOXTESZEPMUJZ-UHFFFAOYSA-N anisole Chemical compound COC1=CC=CC=C1 RDOXTESZEPMUJZ-UHFFFAOYSA-N 0.000 description 2
- MWPLVEDNUUSJAV-UHFFFAOYSA-N anthracene Chemical compound C1=CC=CC2=CC3=CC=CC=C3C=C21 MWPLVEDNUUSJAV-UHFFFAOYSA-N 0.000 description 2
- 150000001491 aromatic compounds Chemical class 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 239000004020 conductor Substances 0.000 description 2
- VPUGDVKSAQVFFS-UHFFFAOYSA-N coronene Chemical compound C1=C(C2=C34)C=CC3=CC=C(C=C3)C4=C4C3=CC=C(C=C3)C4=C2C3=C1 VPUGDVKSAQVFFS-UHFFFAOYSA-N 0.000 description 2
- 150000001923 cyclic compounds Chemical class 0.000 description 2
- 238000009795 derivation Methods 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 229920002521 macromolecule Polymers 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 230000000269 nucleophilic effect Effects 0.000 description 2
- QJPQVXSHYBGQGM-UHFFFAOYSA-N palladium;triphenylphosphane Chemical compound [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 QJPQVXSHYBGQGM-UHFFFAOYSA-N 0.000 description 2
- YNPNZTXNASCQKK-UHFFFAOYSA-N phenanthrene Chemical compound C1=CC=C2C3=CC=CC=C3C=CC2=C1 YNPNZTXNASCQKK-UHFFFAOYSA-N 0.000 description 2
- 229910000027 potassium carbonate Inorganic materials 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 239000011541 reaction mixture Substances 0.000 description 2
- 238000010992 reflux Methods 0.000 description 2
- 238000010561 standard procedure Methods 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 230000003335 steric effect Effects 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 230000002194 synthesizing effect Effects 0.000 description 2
- 239000010409 thin film Substances 0.000 description 2
- CWMFRHBXRUITQE-UHFFFAOYSA-N trimethylsilylacetylene Chemical group C[Si](C)(C)C#C CWMFRHBXRUITQE-UHFFFAOYSA-N 0.000 description 2
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- CZUXGRWFZTZQBE-UHFFFAOYSA-N 1,3,5,7-tetrakis(4-methoxyphenyl)adamantane Chemical compound COC1=CC=C(C=C1)C12CC3(CC(CC(C1)(C3)C3=CC=C(C=C3)OC)(C2)C2=CC=C(C=C2)OC)C2=CC=C(C=C2)OC CZUXGRWFZTZQBE-UHFFFAOYSA-N 0.000 description 1
- WAUKYPQSFIENDE-UHFFFAOYSA-N 1-ethynyl-4-(2-phenylethynyl)benzene Chemical compound C1=CC(C#C)=CC=C1C#CC1=CC=CC=C1 WAUKYPQSFIENDE-UHFFFAOYSA-N 0.000 description 1
- RCOPXPNGDKPSLU-UHFFFAOYSA-N 1-fluoro-4-(2-phenylethynyl)benzene Chemical compound C1=CC(F)=CC=C1C#CC1=CC=CC=C1 RCOPXPNGDKPSLU-UHFFFAOYSA-N 0.000 description 1
- VXEGSRKPIUDPQT-UHFFFAOYSA-N 4-[4-(4-methoxyphenyl)piperazin-1-yl]aniline Chemical compound C1=CC(OC)=CC=C1N1CCN(C=2C=CC(N)=CC=2)CC1 VXEGSRKPIUDPQT-UHFFFAOYSA-N 0.000 description 1
- 125000004800 4-bromophenyl group Chemical group [H]C1=C([H])C(*)=C([H])C([H])=C1Br 0.000 description 1
- XLHCHVUFUPJPEO-UHFFFAOYSA-N Brc(cc1)ccc1C#Cc1ccccc1 Chemical compound Brc(cc1)ccc1C#Cc1ccccc1 XLHCHVUFUPJPEO-UHFFFAOYSA-N 0.000 description 1
- YXBSWVITYJEYIR-UHFFFAOYSA-N C(C(CC(C1)(C2)C3=CC=CC=C3)(CC2(C2)C3=CC=CC=C3)C3=CC=CC=C3)C12C1=CC=CC=C1.Br.Br Chemical compound C(C(CC(C1)(C2)C3=CC=CC=C3)(CC2(C2)C3=CC=CC=C3)C3=CC=CC=C3)C12C1=CC=CC=C1.Br.Br YXBSWVITYJEYIR-UHFFFAOYSA-N 0.000 description 1
- GXHZPXCJUCGLLI-UHFFFAOYSA-N C(C(CC(C1)(C2)C3=CC=CC=C3)(CC2(C2)C3=CC=CC=C3)C3=CC=CC=C3)C12C1=CC=CC=C1.Br.Br.Br Chemical compound C(C(CC(C1)(C2)C3=CC=CC=C3)(CC2(C2)C3=CC=CC=C3)C3=CC=CC=C3)C12C1=CC=CC=C1.Br.Br.Br GXHZPXCJUCGLLI-UHFFFAOYSA-N 0.000 description 1
- PVDBILXAJAJAOK-UHFFFAOYSA-N CC1=CCC([CH+]Cc2ccccc2)C=C1 Chemical compound CC1=CCC([CH+]Cc2ccccc2)C=C1 PVDBILXAJAJAOK-UHFFFAOYSA-N 0.000 description 1
- FSWCCQWDVGZMRD-UHFFFAOYSA-N CC1CC=CCC1 Chemical compound CC1CC=CCC1 FSWCCQWDVGZMRD-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 239000004971 Cross linker Substances 0.000 description 1
- 238000005698 Diels-Alder reaction Methods 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- OHZZCRCAPUAKNQ-UHFFFAOYSA-N OC1=C(C=CC=C1)C12CC3(CC(CC(C1)(C3)C3=C(C=CC=C3)O)(C2)C2=C(C=CC=C2)O)C2=C(C=CC=C2)O Chemical compound OC1=C(C=CC=C1)C12CC3(CC(CC(C1)(C3)C3=C(C=CC=C3)O)(C2)C2=C(C=CC=C2)O)C2=C(C=CC=C2)O OHZZCRCAPUAKNQ-UHFFFAOYSA-N 0.000 description 1
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 1
- JPYHHZQJCSQRJY-UHFFFAOYSA-N Phloroglucinol Natural products CCC=CCC=CCC=CCC=CCCCCC(=O)C1=C(O)C=C(O)C=C1O JPYHHZQJCSQRJY-UHFFFAOYSA-N 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- WAVPCXOQEHSDIB-UHFFFAOYSA-N [3-ethynyl-4-(2-phenylethynyl)phenyl]-trimethylsilane Chemical compound C[Si](C1=CC(=C(C=C1)C#CC1=CC=CC=C1)C#C)(C)C WAVPCXOQEHSDIB-UHFFFAOYSA-N 0.000 description 1
- 239000002390 adhesive tape Substances 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- BOQVAQFBJWXETA-UHFFFAOYSA-N bicyclo[2.2.1]heptane Chemical compound C1CC2CCC1C2.C1CC2CCC1C2 BOQVAQFBJWXETA-UHFFFAOYSA-N 0.000 description 1
- YNHIGQDRGKUECZ-UHFFFAOYSA-L bis(triphenylphosphine)palladium(ii) dichloride Chemical compound [Cl-].[Cl-].[Pd+2].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 YNHIGQDRGKUECZ-UHFFFAOYSA-L 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 229920001795 coordination polymer Polymers 0.000 description 1
- LSXDOTMGLUJQCM-UHFFFAOYSA-M copper(i) iodide Chemical compound I[Cu] LSXDOTMGLUJQCM-UHFFFAOYSA-M 0.000 description 1
- RKTYLMNFRDHKIL-UHFFFAOYSA-N copper;5,10,15,20-tetraphenylporphyrin-22,24-diide Chemical compound [Cu+2].C1=CC(C(=C2C=CC([N-]2)=C(C=2C=CC=CC=2)C=2C=CC(N=2)=C(C=2C=CC=CC=2)C2=CC=C3[N-]2)C=2C=CC=CC=2)=NC1=C3C1=CC=CC=C1 RKTYLMNFRDHKIL-UHFFFAOYSA-N 0.000 description 1
- GBRBMTNGQBKBQE-UHFFFAOYSA-L copper;diiodide Chemical compound I[Cu]I GBRBMTNGQBKBQE-UHFFFAOYSA-L 0.000 description 1
- 239000003431 cross linking reagent Substances 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 125000002897 diene group Chemical group 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- 229920006351 engineering plastic Polymers 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 230000009477 glass transition Effects 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 125000001475 halogen functional group Chemical group 0.000 description 1
- 230000026030 halogenation Effects 0.000 description 1
- 238000005658 halogenation reaction Methods 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 239000003999 initiator Substances 0.000 description 1
- 229920000592 inorganic polymer Polymers 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- UZKWTJUDCOPSNM-UHFFFAOYSA-N methoxybenzene Substances CCCCOC=C UZKWTJUDCOPSNM-UHFFFAOYSA-N 0.000 description 1
- 239000012044 organic layer Substances 0.000 description 1
- 229920000620 organic polymer Polymers 0.000 description 1
- 125000002524 organometallic group Chemical group 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- QCDYQQDYXPDABM-UHFFFAOYSA-N phloroglucinol Chemical compound OC1=CC(O)=CC(O)=C1 QCDYQQDYXPDABM-UHFFFAOYSA-N 0.000 description 1
- 229960001553 phloroglucinol Drugs 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 229920000090 poly(aryl ether) Polymers 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000000425 proton nuclear magnetic resonance spectrum Methods 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000000197 pyrolysis Methods 0.000 description 1
- 238000010526 radical polymerization reaction Methods 0.000 description 1
- 238000007348 radical reaction Methods 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 238000007363 ring formation reaction Methods 0.000 description 1
- 230000008054 signal transmission Effects 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 239000005049 silicon tetrachloride Substances 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G65/00—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule
- C08G65/02—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule from cyclic ethers by opening of the heterocyclic ring
- C08G65/30—Post-polymerisation treatment, e.g. recovery, purification, drying
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G61/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G61/12—Macromolecular compounds containing atoms other than carbon in the main chain of the macromolecule
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G61/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G61/02—Macromolecular compounds containing only carbon atoms in the main chain of the macromolecule, e.g. polyxylylenes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2650/00—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule
- C08G2650/28—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule characterised by the polymer type
- C08G2650/60—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule characterised by the polymer type containing acetylenic group
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Polyoxymethylene Polymers And Polymers With Carbon-To-Carbon Bonds (AREA)
- Other Resins Obtained By Reactions Not Involving Carbon-To-Carbon Unsaturated Bonds (AREA)
- Formation Of Insulating Films (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/618,945 US6469123B1 (en) | 2000-07-19 | 2000-07-19 | Compositions and methods for thermosetting molecules in organic compositions |
| US09/897,936 US20020022708A1 (en) | 2000-07-19 | 2001-07-05 | Compositions and methods for thermosetting molecules in organic compositions |
| PCT/US2001/022204 WO2002008308A1 (en) | 2000-07-19 | 2001-07-13 | Compositions and methods for thermosetting molecules in organic compositions |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2004504455A true JP2004504455A (ja) | 2004-02-12 |
| JP2004504455A5 JP2004504455A5 (enExample) | 2008-08-21 |
Family
ID=27088367
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2002514210A Withdrawn JP2004504455A (ja) | 2000-07-19 | 2001-07-13 | 有機組成物における熱硬化性分子の組成物および方法 |
Country Status (9)
| Country | Link |
|---|---|
| US (2) | US20020022708A1 (enExample) |
| EP (1) | EP1309639A4 (enExample) |
| JP (1) | JP2004504455A (enExample) |
| KR (1) | KR100620207B1 (enExample) |
| CN (1) | CN1458945A (enExample) |
| AU (1) | AU2001280549A1 (enExample) |
| MY (1) | MY134260A (enExample) |
| TW (1) | TWI241310B (enExample) |
| WO (1) | WO2002008308A1 (enExample) |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006233128A (ja) * | 2005-02-28 | 2006-09-07 | Fuji Photo Film Co Ltd | カゴ構造を有する重合体、それを含む膜形成用組成物、絶縁膜および電子デバイス |
| JP2007119706A (ja) * | 2005-09-28 | 2007-05-17 | Fujifilm Corp | 重合体および膜形成用組成物 |
| JP2007161780A (ja) * | 2005-12-09 | 2007-06-28 | Fujifilm Corp | 膜形成用組成物、該組成物を用いた絶縁膜及び電子デバイス |
| JP2007161788A (ja) * | 2005-12-09 | 2007-06-28 | Fujifilm Corp | 重合体および膜形成用組成物 |
| JP2008231174A (ja) * | 2007-03-19 | 2008-10-02 | Fujifilm Corp | 膜形成用組成物、絶縁膜及び電子デバイス |
| US7501185B2 (en) | 2004-06-10 | 2009-03-10 | Fujifilm Corporation | Film-forming composition, insulating material-forming composition, insulating film and electronic device |
| JP2009088028A (ja) * | 2007-09-27 | 2009-04-23 | Sumitomo Bakelite Co Ltd | 有機絶縁膜及び半導体装置 |
| WO2010067683A1 (ja) | 2008-12-10 | 2010-06-17 | 富士フイルム株式会社 | 組成物 |
| US7750102B2 (en) | 2007-02-06 | 2010-07-06 | Fujifilm Corporation | Insulating film for semiconductor integrated circuit |
| US7799843B2 (en) | 2008-02-29 | 2010-09-21 | Fujifilm Corporation | Film |
| KR20170076578A (ko) | 2015-12-24 | 2017-07-04 | 에아.워타 가부시키가이샤 | 다가 알킨 화합물, 그 제법 및 용도 |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6987147B2 (en) * | 2001-10-01 | 2006-01-17 | Honeywell International Inc. | Low dielectric constant materials with improved thermo-mechanical strength and processability |
| US20030143332A1 (en) * | 2002-01-31 | 2003-07-31 | Sumitomo Chemical Company, Limited | Coating solution for forming insulating film |
| US20080159114A1 (en) * | 2007-01-02 | 2008-07-03 | Dipietro Richard Anthony | High density data storage medium, method and device |
| US7558186B2 (en) * | 2007-01-02 | 2009-07-07 | International Business Machines Corporation | High density data storage medium, method and device |
| GB2451865A (en) | 2007-08-15 | 2009-02-18 | Univ Liverpool | Microporous polymers from alkynyl monomers |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4918158A (en) * | 1986-07-21 | 1990-04-17 | Fluorochem Inc. | 1,3-diethynyladamantane and methods of polymerization thereof |
| US5017734A (en) * | 1989-12-11 | 1991-05-21 | Kurt Baum | Ethynyl adamantane derivatives and methods of polymerization thereof |
| JPH04314394A (ja) | 1991-04-12 | 1992-11-05 | Fujitsu Ltd | ガラスセラミック回路基板とその製造方法 |
| US5347063A (en) | 1993-03-09 | 1994-09-13 | Mobil Oil Corporation | Method for direct arylation of diamondoids |
| US5576355A (en) * | 1993-06-04 | 1996-11-19 | Mobil Oil Corp. | Diamondoid derivatives for pharmaceutical use |
| US5744399A (en) | 1995-11-13 | 1998-04-28 | Lsi Logic Corporation | Process for forming low dielectric constant layers using fullerenes |
| DE69930874T2 (de) * | 1998-11-24 | 2006-11-02 | Dow Global Technologies, Inc., Midland | Eine zusammensetzung enthaltend einen vernetzbaren matrixpercursor und eine porenstruktur bildendes material und eine daraus hergestellte poröse matrix |
| US6413202B1 (en) * | 1999-01-21 | 2002-07-02 | Alliedsignal, Inc. | Solvent systems for polymeric dielectric materials |
| US6509415B1 (en) * | 2000-04-07 | 2003-01-21 | Honeywell International Inc. | Low dielectric constant organic dielectrics based on cage-like structures |
| US6444715B1 (en) * | 2000-06-06 | 2002-09-03 | Honeywell International Inc. | Low dielectric materials and methods of producing same |
| US6469123B1 (en) * | 2000-07-19 | 2002-10-22 | Honeywell International Inc. | Compositions and methods for thermosetting molecules in organic compositions |
| US6423811B1 (en) * | 2000-07-19 | 2002-07-23 | Honeywell International Inc. | Low dielectric constant materials with polymeric networks |
-
2001
- 2001-07-05 US US09/897,936 patent/US20020022708A1/en not_active Abandoned
- 2001-07-13 CN CN01815765A patent/CN1458945A/zh active Pending
- 2001-07-13 EP EP01958944A patent/EP1309639A4/en not_active Withdrawn
- 2001-07-13 KR KR1020037000737A patent/KR100620207B1/ko not_active Expired - Fee Related
- 2001-07-13 WO PCT/US2001/022204 patent/WO2002008308A1/en not_active Ceased
- 2001-07-13 JP JP2002514210A patent/JP2004504455A/ja not_active Withdrawn
- 2001-07-13 AU AU2001280549A patent/AU2001280549A1/en not_active Abandoned
- 2001-07-19 TW TW090117706A patent/TWI241310B/zh not_active IP Right Cessation
- 2001-12-13 MY MYPI20015668A patent/MY134260A/en unknown
-
2002
- 2002-10-08 US US10/267,380 patent/US6803441B2/en not_active Expired - Fee Related
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7501185B2 (en) | 2004-06-10 | 2009-03-10 | Fujifilm Corporation | Film-forming composition, insulating material-forming composition, insulating film and electronic device |
| JP2006233128A (ja) * | 2005-02-28 | 2006-09-07 | Fuji Photo Film Co Ltd | カゴ構造を有する重合体、それを含む膜形成用組成物、絶縁膜および電子デバイス |
| JP2007119706A (ja) * | 2005-09-28 | 2007-05-17 | Fujifilm Corp | 重合体および膜形成用組成物 |
| JP2007161780A (ja) * | 2005-12-09 | 2007-06-28 | Fujifilm Corp | 膜形成用組成物、該組成物を用いた絶縁膜及び電子デバイス |
| JP2007161788A (ja) * | 2005-12-09 | 2007-06-28 | Fujifilm Corp | 重合体および膜形成用組成物 |
| US7750102B2 (en) | 2007-02-06 | 2010-07-06 | Fujifilm Corporation | Insulating film for semiconductor integrated circuit |
| JP2008231174A (ja) * | 2007-03-19 | 2008-10-02 | Fujifilm Corp | 膜形成用組成物、絶縁膜及び電子デバイス |
| JP2009088028A (ja) * | 2007-09-27 | 2009-04-23 | Sumitomo Bakelite Co Ltd | 有機絶縁膜及び半導体装置 |
| US7799843B2 (en) | 2008-02-29 | 2010-09-21 | Fujifilm Corporation | Film |
| WO2010067683A1 (ja) | 2008-12-10 | 2010-06-17 | 富士フイルム株式会社 | 組成物 |
| KR20170076578A (ko) | 2015-12-24 | 2017-07-04 | 에아.워타 가부시키가이샤 | 다가 알킨 화합물, 그 제법 및 용도 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN1458945A (zh) | 2003-11-26 |
| TWI241310B (en) | 2005-10-11 |
| EP1309639A1 (en) | 2003-05-14 |
| EP1309639A4 (en) | 2004-12-08 |
| MY134260A (en) | 2007-11-30 |
| KR100620207B1 (ko) | 2006-09-13 |
| WO2002008308A1 (en) | 2002-01-31 |
| KR20030031123A (ko) | 2003-04-18 |
| AU2001280549A1 (en) | 2002-02-05 |
| US20030096938A1 (en) | 2003-05-22 |
| US20020022708A1 (en) | 2002-02-21 |
| US6803441B2 (en) | 2004-10-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2004504455A (ja) | 有機組成物における熱硬化性分子の組成物および方法 | |
| CN1227276C (zh) | 基于笼形结构的低介电常数有机电介质 | |
| KR100433722B1 (ko) | 중합체 시스템을 열에 의해 가교 결합시키기 위한 작용기 | |
| JP2007332373A (ja) | 低誘電率材料およびその調製方法 | |
| US6469123B1 (en) | Compositions and methods for thermosetting molecules in organic compositions | |
| KR20030022291A (ko) | 고분자 망상구조를 지닌 저유전율 물질 | |
| JP2004535497A (ja) | 籠状構造に基づく低誘電率有機誘電体 | |
| EP1476416B1 (en) | Multifunctional monomers and their use in making cross-linked polymers and porous films | |
| US7049386B2 (en) | Compositions and methods for thermosetting molecules in organic compositions | |
| US20040247896A1 (en) | Organic compositions | |
| WO2003057749A1 (en) | Organic compositions | |
| HK1060139A (en) | Compositions and methods for thermosetting molecules in organic compositions | |
| KR20060056712A (ko) | 포토리소그래피에 사용되는 바텀 레지스트용 폴리머 및 그제조 방법 | |
| JP4885724B2 (ja) | 結合メソゲン性ポラゲン形成性部分を含有する多官能性モノマー及びそれからのポリアリーレン組成物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| RD03 | Notification of appointment of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7423 Effective date: 20080701 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080704 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20080704 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20080704 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20080826 |