EP3502131A1 - Reconstituted hdl formulation - Google Patents
Reconstituted hdl formulation Download PDFInfo
- Publication number
- EP3502131A1 EP3502131A1 EP19151328.2A EP19151328A EP3502131A1 EP 3502131 A1 EP3502131 A1 EP 3502131A1 EP 19151328 A EP19151328 A EP 19151328A EP 3502131 A1 EP3502131 A1 EP 3502131A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- formulation according
- apolipoprotein
- rhdl formulation
- rhdl
- sucrose
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 179
- 238000009472 formulation Methods 0.000 title claims abstract description 153
- 238000004108 freeze drying Methods 0.000 claims abstract description 65
- 239000003381 stabilizer Substances 0.000 claims abstract description 64
- 102000007592 Apolipoproteins Human genes 0.000 claims abstract description 58
- 108010071619 Apolipoproteins Proteins 0.000 claims abstract description 58
- 150000002632 lipids Chemical class 0.000 claims abstract description 42
- 108010010234 HDL Lipoproteins Proteins 0.000 claims abstract description 31
- 102000015779 HDL Lipoproteins Human genes 0.000 claims abstract description 31
- 229930006000 Sucrose Natural products 0.000 claims description 81
- 239000005720 sucrose Substances 0.000 claims description 81
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 claims description 77
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 claims description 29
- 229960002429 proline Drugs 0.000 claims description 29
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 claims description 25
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 25
- 108010059886 Apolipoprotein A-I Proteins 0.000 claims description 23
- 102000005666 Apolipoprotein A-I Human genes 0.000 claims description 23
- 150000003904 phospholipids Chemical class 0.000 claims description 22
- 229940024606 amino acid Drugs 0.000 claims description 21
- 235000001014 amino acid Nutrition 0.000 claims description 21
- 150000001413 amino acids Chemical class 0.000 claims description 21
- 235000000346 sugar Nutrition 0.000 claims description 20
- 150000005846 sugar alcohols Chemical class 0.000 claims description 20
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 claims description 15
- 229930195725 Mannitol Natural products 0.000 claims description 15
- 239000003599 detergent Substances 0.000 claims description 15
- 239000000594 mannitol Substances 0.000 claims description 15
- 235000010355 mannitol Nutrition 0.000 claims description 15
- 201000010099 disease Diseases 0.000 claims description 13
- 238000000034 method Methods 0.000 claims description 13
- 208000035475 disorder Diseases 0.000 claims description 12
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 claims description 12
- ATBOMIWRCZXYSZ-XZBBILGWSA-N [1-[2,3-dihydroxypropoxy(hydroxy)phosphoryl]oxy-3-hexadecanoyloxypropan-2-yl] (9e,12e)-octadeca-9,12-dienoate Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCC\C=C\C\C=C\CCCCC ATBOMIWRCZXYSZ-XZBBILGWSA-N 0.000 claims description 10
- AWUCVROLDVIAJX-UHFFFAOYSA-N alpha-glycerophosphate Natural products OCC(O)COP(O)(O)=O AWUCVROLDVIAJX-UHFFFAOYSA-N 0.000 claims description 10
- 208000004476 Acute Coronary Syndrome Diseases 0.000 claims description 9
- NRHMKIHPTBHXPF-TUJRSCDTSA-M sodium cholate Chemical compound [Na+].C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC([O-])=O)C)[C@@]2(C)[C@@H](O)C1 NRHMKIHPTBHXPF-TUJRSCDTSA-M 0.000 claims description 9
- 150000002016 disaccharides Chemical class 0.000 claims description 7
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 claims description 6
- 208000024172 Cardiovascular disease Diseases 0.000 claims description 4
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 claims description 4
- 239000012634 fragment Substances 0.000 claims description 4
- BTCSSZJGUNDROE-UHFFFAOYSA-N gamma-aminobutyric acid Chemical compound NCCCC(O)=O BTCSSZJGUNDROE-UHFFFAOYSA-N 0.000 claims description 4
- FSYKKLYZXJSNPZ-UHFFFAOYSA-N sarcosine Chemical compound C[NH2+]CC([O-])=O FSYKKLYZXJSNPZ-UHFFFAOYSA-N 0.000 claims description 4
- 229960001153 serine Drugs 0.000 claims description 4
- 125000000185 sucrose group Chemical group 0.000 claims description 4
- 206010002383 Angina Pectoris Diseases 0.000 claims description 3
- 201000001320 Atherosclerosis Diseases 0.000 claims description 3
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 claims description 3
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 claims description 3
- 208000035150 Hypercholesterolemia Diseases 0.000 claims description 3
- 206010020961 Hypocholesterolaemia Diseases 0.000 claims description 3
- 208000010125 myocardial infarction Diseases 0.000 claims description 3
- 239000000600 sorbitol Substances 0.000 claims description 3
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 claims description 2
- OGNSCSPNOLGXSM-UHFFFAOYSA-N (+/-)-DABA Natural products NCCC(N)C(O)=O OGNSCSPNOLGXSM-UHFFFAOYSA-N 0.000 claims description 2
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 claims description 2
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 claims description 2
- 229930091371 Fructose Natural products 0.000 claims description 2
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 claims description 2
- 239000005715 Fructose Substances 0.000 claims description 2
- 239000004471 Glycine Substances 0.000 claims description 2
- SQUHHTBVTRBESD-UHFFFAOYSA-N Hexa-Ac-myo-Inositol Natural products CC(=O)OC1C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C1OC(C)=O SQUHHTBVTRBESD-UHFFFAOYSA-N 0.000 claims description 2
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 claims description 2
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 claims description 2
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 claims description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 claims description 2
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 claims description 2
- 239000004472 Lysine Substances 0.000 claims description 2
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 claims description 2
- 108010077895 Sarcosine Proteins 0.000 claims description 2
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 claims description 2
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 claims description 2
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 claims description 2
- 235000004279 alanine Nutrition 0.000 claims description 2
- 229960003767 alanine Drugs 0.000 claims description 2
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 claims description 2
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 claims description 2
- PXEDJBXQKAGXNJ-QTNFYWBSSA-L disodium L-glutamate Chemical compound [Na+].[Na+].[O-]C(=O)[C@@H](N)CCC([O-])=O PXEDJBXQKAGXNJ-QTNFYWBSSA-L 0.000 claims description 2
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 claims description 2
- FBPFZTCFMRRESA-GUCUJZIJSA-N galactitol Chemical compound OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-GUCUJZIJSA-N 0.000 claims description 2
- 229960003692 gamma aminobutyric acid Drugs 0.000 claims description 2
- 229960002591 hydroxyproline Drugs 0.000 claims description 2
- CDAISMWEOUEBRE-GPIVLXJGSA-N inositol Chemical compound O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@H](O)[C@@H]1O CDAISMWEOUEBRE-GPIVLXJGSA-N 0.000 claims description 2
- 229960000367 inositol Drugs 0.000 claims description 2
- 239000008101 lactose Substances 0.000 claims description 2
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 claims description 2
- 150000002772 monosaccharides Chemical group 0.000 claims description 2
- 235000013923 monosodium glutamate Nutrition 0.000 claims description 2
- 229940043230 sarcosine Drugs 0.000 claims description 2
- CDAISMWEOUEBRE-UHFFFAOYSA-N scyllo-inosotol Natural products OC1C(O)C(O)C(O)C(O)C1O CDAISMWEOUEBRE-UHFFFAOYSA-N 0.000 claims description 2
- 229940073490 sodium glutamate Drugs 0.000 claims description 2
- 150000004043 trisaccharides Chemical class 0.000 claims description 2
- 239000000811 xylitol Substances 0.000 claims description 2
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 claims description 2
- 235000010447 xylitol Nutrition 0.000 claims description 2
- 229960002675 xylitol Drugs 0.000 claims description 2
- BVHLGVCQOALMSV-JEDNCBNOSA-N L-lysine hydrochloride Chemical compound Cl.NCCCC[C@H](N)C(O)=O BVHLGVCQOALMSV-JEDNCBNOSA-N 0.000 claims 1
- 229960002449 glycine Drugs 0.000 claims 1
- 229960003646 lysine Drugs 0.000 claims 1
- 229960005337 lysine hydrochloride Drugs 0.000 claims 1
- 229960001855 mannitol Drugs 0.000 claims 1
- 231100000417 nephrotoxicity Toxicity 0.000 abstract description 6
- 230000007774 longterm Effects 0.000 abstract description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 48
- 239000002245 particle Substances 0.000 description 26
- 235000012000 cholesterol Nutrition 0.000 description 23
- 235000018102 proteins Nutrition 0.000 description 22
- 102000004169 proteins and genes Human genes 0.000 description 22
- 108090000623 proteins and genes Proteins 0.000 description 22
- 102100031538 Phosphatidylcholine-sterol acyltransferase Human genes 0.000 description 20
- 108010011964 Phosphatidylcholine-sterol O-acyltransferase Proteins 0.000 description 19
- 210000004027 cell Anatomy 0.000 description 17
- 239000000243 solution Substances 0.000 description 16
- 230000000694 effects Effects 0.000 description 12
- 238000009826 distribution Methods 0.000 description 9
- 210000002381 plasma Anatomy 0.000 description 9
- 238000003860 storage Methods 0.000 description 8
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 7
- BHQCQFFYRZLCQQ-OELDTZBJSA-N cholic acid Chemical compound C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)[C@@H](O)C1 BHQCQFFYRZLCQQ-OELDTZBJSA-N 0.000 description 7
- 229940099352 cholate Drugs 0.000 description 6
- -1 proline Chemical class 0.000 description 6
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 5
- 102000004895 Lipoproteins Human genes 0.000 description 5
- 108090001030 Lipoproteins Proteins 0.000 description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 5
- 239000003613 bile acid Substances 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- MWRBNPKJOOWZPW-CLFAGFIQSA-N dioleoyl phosphatidylethanolamine Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(COP(O)(=O)OCCN)OC(=O)CCCCCCC\C=C/CCCCCCCC MWRBNPKJOOWZPW-CLFAGFIQSA-N 0.000 description 4
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 4
- 238000005755 formation reaction Methods 0.000 description 4
- PEDCQBHIVMGVHV-UHFFFAOYSA-N glycerol Substances OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 4
- 238000001802 infusion Methods 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 239000002953 phosphate buffered saline Substances 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 239000000523 sample Substances 0.000 description 4
- 239000011780 sodium chloride Substances 0.000 description 4
- 239000008215 water for injection Substances 0.000 description 4
- PORPENFLTBBHSG-MGBGTMOVSA-N 1,2-dihexadecanoyl-sn-glycerol-3-phosphate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(O)=O)OC(=O)CCCCCCCCCCCCCCC PORPENFLTBBHSG-MGBGTMOVSA-N 0.000 description 3
- TZCPCKNHXULUIY-RGULYWFUSA-N 1,2-distearoyl-sn-glycero-3-phosphoserine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCC TZCPCKNHXULUIY-RGULYWFUSA-N 0.000 description 3
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 3
- ZWZWYGMENQVNFU-UHFFFAOYSA-N Glycerophosphorylserin Natural products OC(=O)C(N)COP(O)(=O)OCC(O)CO ZWZWYGMENQVNFU-UHFFFAOYSA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- JLPULHDHAOZNQI-JLOPVYAASA-N [(2r)-3-hexadecanoyloxy-2-[(9e,12e)-octadeca-9,12-dienoyl]oxypropyl] 2-(trimethylazaniumyl)ethyl phosphate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCC\C=C\C\C=C\CCCCC JLPULHDHAOZNQI-JLOPVYAASA-N 0.000 description 3
- 229940098773 bovine serum albumin Drugs 0.000 description 3
- BIABMEZBCHDPBV-UHFFFAOYSA-N dipalmitoyl phosphatidylglycerol Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCCCCCCCCCC BIABMEZBCHDPBV-UHFFFAOYSA-N 0.000 description 3
- 239000003814 drug Substances 0.000 description 3
- 238000000605 extraction Methods 0.000 description 3
- 239000013020 final formulation Substances 0.000 description 3
- 238000007710 freezing Methods 0.000 description 3
- 230000008014 freezing Effects 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- SDEURMLKLAEUAY-JFSPZUDSSA-N (2-{[(2r)-2,3-bis[(13z)-docos-13-enoyloxy]propyl phosphonato]oxy}ethyl)trimethylazanium Chemical compound CCCCCCCC\C=C/CCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCC\C=C/CCCCCCCC SDEURMLKLAEUAY-JFSPZUDSSA-N 0.000 description 2
- RUDATBOHQWOJDD-UHFFFAOYSA-N (3beta,5beta,7alpha)-3,7-Dihydroxycholan-24-oic acid Natural products OC1CC2CC(O)CCC2(C)C2C1C1CCC(C(CCC(O)=O)C)C1(C)CC2 RUDATBOHQWOJDD-UHFFFAOYSA-N 0.000 description 2
- CITHEXJVPOWHKC-UUWRZZSWSA-N 1,2-di-O-myristoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCC CITHEXJVPOWHKC-UUWRZZSWSA-N 0.000 description 2
- MLKLDGSYMHFAOC-AREMUKBSSA-N 1,2-dicapryl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCC MLKLDGSYMHFAOC-AREMUKBSSA-N 0.000 description 2
- KILNVBDSWZSGLL-KXQOOQHDSA-N 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCC KILNVBDSWZSGLL-KXQOOQHDSA-N 0.000 description 2
- YFWHNAWEOZTIPI-DIPNUNPCSA-N 1,2-dioctadecanoyl-sn-glycerol-3-phosphate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(O)=O)OC(=O)CCCCCCCCCCCCCCCCC YFWHNAWEOZTIPI-DIPNUNPCSA-N 0.000 description 2
- WTBFLCSPLLEDEM-JIDRGYQWSA-N 1,2-dioleoyl-sn-glycero-3-phospho-L-serine Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCC\C=C/CCCCCCCC WTBFLCSPLLEDEM-JIDRGYQWSA-N 0.000 description 2
- LVNGJLRDBYCPGB-UHFFFAOYSA-N 1,2-distearoylphosphatidylethanolamine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(COP([O-])(=O)OCC[NH3+])OC(=O)CCCCCCCCCCCCCCCCC LVNGJLRDBYCPGB-UHFFFAOYSA-N 0.000 description 2
- PAZGBAOHGQRCBP-ZCXUNETKSA-N 1-Palmitoyl-2-oleoylglycero-3-phosphoglycerol Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCC\C=C/CCCCCCCC PAZGBAOHGQRCBP-ZCXUNETKSA-N 0.000 description 2
- CFWRDBDJAOHXSH-SECBINFHSA-N 2-azaniumylethyl [(2r)-2,3-diacetyloxypropyl] phosphate Chemical compound CC(=O)OC[C@@H](OC(C)=O)COP(O)(=O)OCCN CFWRDBDJAOHXSH-SECBINFHSA-N 0.000 description 2
- UMCMPZBLKLEWAF-BCTGSCMUSA-N 3-[(3-cholamidopropyl)dimethylammonio]propane-1-sulfonate Chemical compound C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCCC[N+](C)(C)CCCS([O-])(=O)=O)C)[C@@]2(C)[C@@H](O)C1 UMCMPZBLKLEWAF-BCTGSCMUSA-N 0.000 description 2
- GUQQBLRVXOUDTN-XOHPMCGNSA-N 3-[dimethyl-[3-[[(4r)-4-[(3r,5s,7r,8r,9s,10s,12s,13r,14s,17r)-3,7,12-trihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]pentanoyl]amino]propyl]azaniumyl]-2-hydroxypropane-1-sulfonate Chemical compound C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCCC[N+](C)(C)CC(O)CS([O-])(=O)=O)C)[C@@]2(C)[C@@H](O)C1 GUQQBLRVXOUDTN-XOHPMCGNSA-N 0.000 description 2
- 108010087614 Apolipoprotein A-II Proteins 0.000 description 2
- 102000009081 Apolipoprotein A-II Human genes 0.000 description 2
- KLFKZIQAIPDJCW-HTIIIDOHSA-N Dipalmitoylphosphatidylserine Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCC KLFKZIQAIPDJCW-HTIIIDOHSA-N 0.000 description 2
- 235000010469 Glycine max Nutrition 0.000 description 2
- 244000068988 Glycine max Species 0.000 description 2
- FVJZSBGHRPJMMA-IOLBBIBUSA-N PG(18:0/18:0) Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@@H](O)CO)OC(=O)CCCCCCCCCCCCCCCCC FVJZSBGHRPJMMA-IOLBBIBUSA-N 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- DSNRWDQKZIEDDB-GCMPNPAFSA-N [(2r)-3-[2,3-dihydroxypropoxy(hydroxy)phosphoryl]oxy-2-[(z)-octadec-9-enoyl]oxypropyl] (z)-octadec-9-enoate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@H](COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCC\C=C/CCCCCCCC DSNRWDQKZIEDDB-GCMPNPAFSA-N 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- RUDATBOHQWOJDD-BSWAIDMHSA-N chenodeoxycholic acid Chemical compound C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)CC1 RUDATBOHQWOJDD-BSWAIDMHSA-N 0.000 description 2
- 238000007398 colorimetric assay Methods 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- KXGVEGMKQFWNSR-LLQZFEROSA-N deoxycholic acid Chemical compound C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)[C@@H](O)C1 KXGVEGMKQFWNSR-LLQZFEROSA-N 0.000 description 2
- KXGVEGMKQFWNSR-UHFFFAOYSA-N deoxycholic acid Natural products C1CC2CC(O)CCC2(C)C2C1C1CCC(C(CCC(O)=O)C)C1(C)C(O)C2 KXGVEGMKQFWNSR-UHFFFAOYSA-N 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- LHCZDUCPSRJDJT-UHFFFAOYSA-N dilauroyl phosphatidylglycerol Chemical compound CCCCCCCCCCCC(=O)OCC(COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCCCCCC LHCZDUCPSRJDJT-UHFFFAOYSA-N 0.000 description 2
- 229960003724 dimyristoylphosphatidylcholine Drugs 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 230000032050 esterification Effects 0.000 description 2
- 238000005886 esterification reaction Methods 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 231100000304 hepatotoxicity Toxicity 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 210000004185 liver Anatomy 0.000 description 2
- 230000007056 liver toxicity Effects 0.000 description 2
- 230000014759 maintenance of location Effects 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 150000002482 oligosaccharides Chemical class 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 150000003905 phosphatidylinositols Chemical class 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 230000000069 prophylactic effect Effects 0.000 description 2
- 235000004252 protein component Nutrition 0.000 description 2
- 239000013074 reference sample Substances 0.000 description 2
- 230000004141 reverse cholesterol transport Effects 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 150000003626 triacylglycerols Chemical class 0.000 description 2
- RUDATBOHQWOJDD-UZVSRGJWSA-N ursodeoxycholic acid Chemical compound C([C@H]1C[C@@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)CC1 RUDATBOHQWOJDD-UZVSRGJWSA-N 0.000 description 2
- HSINOMROUCMIEA-FGVHQWLLSA-N (2s,4r)-4-[(3r,5s,6r,7r,8s,9s,10s,13r,14s,17r)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]-2-methylpentanoic acid Chemical compound C([C@@]12C)C[C@@H](O)C[C@H]1[C@@H](CC)[C@@H](O)[C@@H]1[C@@H]2CC[C@]2(C)[C@@H]([C@H](C)C[C@H](C)C(O)=O)CC[C@H]21 HSINOMROUCMIEA-FGVHQWLLSA-N 0.000 description 1
- HVYWMOMLDIMFJA-AAUXEULTSA-N (3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol Chemical compound CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4[14CH2][C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O HVYWMOMLDIMFJA-AAUXEULTSA-N 0.000 description 1
- BHQCQFFYRZLCQQ-UHFFFAOYSA-N (3alpha,5alpha,7alpha,12alpha)-3,7,12-trihydroxy-cholan-24-oic acid Natural products OC1CC2CC(O)CCC2(C)C2C1C1CCC(C(CCC(O)=O)C)C1(C)C(O)C2 BHQCQFFYRZLCQQ-UHFFFAOYSA-N 0.000 description 1
- HVYWMOMLDIMFJA-OZXVBKCJSA-N (3r,8s,9s,10r,13r,14s,17r)-10,13-dimethyl-17-[(2r)-6-methylheptan-2-yl]-1,2-ditritio-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-3-ol Chemical compound C([C@@H]12)C[C@]3(C)[C@@H]([C@H](C)CCCC(C)C)CC[C@H]3[C@@H]1CC=C1[C@]2(C)C([3H])C([3H])[C@H](O)C1 HVYWMOMLDIMFJA-OZXVBKCJSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- DBTMGCOVALSLOR-UHFFFAOYSA-N 32-alpha-galactosyl-3-alpha-galactosyl-galactose Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(OC2C(C(CO)OC(O)C2O)O)OC(CO)C1O DBTMGCOVALSLOR-UHFFFAOYSA-N 0.000 description 1
- BNPGUGDYJRQSOU-UHFFFAOYSA-N 4,4,6,6-tetramethyl-3-phenylheptane-3-sulfonic acid Chemical compound CC(C)(C)CC(C)(C)C(S(O)(=O)=O)(CC)C1=CC=CC=C1 BNPGUGDYJRQSOU-UHFFFAOYSA-N 0.000 description 1
- DVKQVRZMKBDMDH-UUOKFMHZSA-N 8-Br-cAMP Chemical compound C([C@H]1O2)OP(O)(=O)O[C@H]1[C@@H](O)[C@@H]2N1C(N=CN=C2N)=C2N=C1Br DVKQVRZMKBDMDH-UUOKFMHZSA-N 0.000 description 1
- 108010006533 ATP-Binding Cassette Transporters Proteins 0.000 description 1
- 102000005416 ATP-Binding Cassette Transporters Human genes 0.000 description 1
- 108010061118 Apolipoprotein A-V Proteins 0.000 description 1
- 102000011936 Apolipoprotein A-V Human genes 0.000 description 1
- 102000006996 Aryldialkylphosphatase Human genes 0.000 description 1
- 108010008184 Aryldialkylphosphatase Proteins 0.000 description 1
- 108010024976 Asparaginase Proteins 0.000 description 1
- LZZYPRNAOMGNLH-UHFFFAOYSA-M Cetrimonium bromide Chemical compound [Br-].CCCCCCCCCCCCCCCC[N+](C)(C)C LZZYPRNAOMGNLH-UHFFFAOYSA-M 0.000 description 1
- 239000004380 Cholic acid Substances 0.000 description 1
- RXVWSYJTUUKTEA-UHFFFAOYSA-N D-maltotriose Natural products OC1C(O)C(OC(C(O)CO)C(O)C(O)C=O)OC(CO)C1OC1C(O)C(O)C(O)C(CO)O1 RXVWSYJTUUKTEA-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-QTVWNMPRSA-N D-mannopyranose Chemical compound OC[C@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-QTVWNMPRSA-N 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- JZNWSCPGTDBMEW-UHFFFAOYSA-N Glycerophosphorylethanolamin Natural products NCCOP(O)(=O)OCC(O)CO JZNWSCPGTDBMEW-UHFFFAOYSA-N 0.000 description 1
- 238000008214 LDL Cholesterol Methods 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- BACYUWVYYTXETD-UHFFFAOYSA-N N-Lauroylsarcosine Chemical compound CCCCCCCCCCCC(=O)N(C)CC(O)=O BACYUWVYYTXETD-UHFFFAOYSA-N 0.000 description 1
- RWKUXQNLWDTSLO-GWQJGLRPSA-N N-hexadecanoylsphingosine-1-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)N[C@@H](COP([O-])(=O)OCC[N+](C)(C)C)[C@H](O)\C=C\CCCCCCCCCCCCC RWKUXQNLWDTSLO-GWQJGLRPSA-N 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 108010080283 Pre-beta High-Density Lipoproteins Proteins 0.000 description 1
- MUPFEKGTMRGPLJ-RMMQSMQOSA-N Raffinose Natural products O(C[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O[C@@]2(CO)[C@H](O)[C@@H](O)[C@@H](CO)O2)O1)[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 MUPFEKGTMRGPLJ-RMMQSMQOSA-N 0.000 description 1
- 101000650578 Salmonella phage P22 Regulatory protein C3 Proteins 0.000 description 1
- 208000001163 Tangier disease Diseases 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 101001040920 Triticum aestivum Alpha-amylase inhibitor 0.28 Proteins 0.000 description 1
- 229920004890 Triton X-100 Polymers 0.000 description 1
- 239000013504 Triton X-100 Substances 0.000 description 1
- FTNIPWXXIGNQQF-UHFFFAOYSA-N UNPD130147 Natural products OC1C(O)C(O)C(CO)OC1OC1C(CO)OC(OC2C(OC(OC3C(OC(OC4C(OC(O)C(O)C4O)CO)C(O)C3O)CO)C(O)C2O)CO)C(O)C1O FTNIPWXXIGNQQF-UHFFFAOYSA-N 0.000 description 1
- MUPFEKGTMRGPLJ-UHFFFAOYSA-N UNPD196149 Natural products OC1C(O)C(CO)OC1(CO)OC1C(O)C(O)C(O)C(COC2C(C(O)C(O)C(CO)O2)O)O1 MUPFEKGTMRGPLJ-UHFFFAOYSA-N 0.000 description 1
- LEBBDRXHHNYZIA-LDUWYPJVSA-N [(2s,3r,4s,5r,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] n-[(z)-1,3-dihydroxyoctadec-4-en-2-yl]carbamate Chemical compound CCCCCCCCCCCCC\C=C/C(O)C(CO)NC(=O)O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O LEBBDRXHHNYZIA-LDUWYPJVSA-N 0.000 description 1
- 239000000370 acceptor Substances 0.000 description 1
- 102000005421 acetyltransferase Human genes 0.000 description 1
- 108020002494 acetyltransferase Proteins 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 description 1
- BNABBHGYYMZMOA-AHIHXIOASA-N alpha-maltoheptaose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)O[C@H](O[C@@H]2[C@H](O[C@H](O[C@@H]3[C@H](O[C@H](O[C@@H]4[C@H](O[C@H](O[C@@H]5[C@H](O[C@H](O[C@@H]6[C@H](O[C@H](O)[C@H](O)[C@H]6O)CO)[C@H](O)[C@H]5O)CO)[C@H](O)[C@H]4O)CO)[C@H](O)[C@H]3O)CO)[C@H](O)[C@H]2O)CO)[C@H](O)[C@H]1O BNABBHGYYMZMOA-AHIHXIOASA-N 0.000 description 1
- OCIBBXPLUVYKCH-QXVNYKTNSA-N alpha-maltohexaose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)O[C@H](O[C@@H]2[C@H](O[C@H](O[C@@H]3[C@H](O[C@H](O[C@@H]4[C@H](O[C@H](O[C@@H]5[C@H](O[C@H](O)[C@H](O)[C@H]5O)CO)[C@H](O)[C@H]4O)CO)[C@H](O)[C@H]3O)CO)[C@H](O)[C@H]2O)CO)[C@H](O)[C@H]1O OCIBBXPLUVYKCH-QXVNYKTNSA-N 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 108010040033 apolipoprotein A-I Milano Proteins 0.000 description 1
- 108010073614 apolipoprotein A-IV Proteins 0.000 description 1
- 238000000149 argon plasma sintering Methods 0.000 description 1
- 210000001367 artery Anatomy 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 239000003833 bile salt Substances 0.000 description 1
- 238000010364 biochemical engineering Methods 0.000 description 1
- 229960000074 biopharmaceutical Drugs 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 244000309466 calf Species 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 229930183167 cerebroside Natural products 0.000 description 1
- 150000001784 cerebrosides Chemical class 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 229940009025 chenodeoxycholate Drugs 0.000 description 1
- 229960001091 chenodeoxycholic acid Drugs 0.000 description 1
- 150000001840 cholesterol esters Chemical class 0.000 description 1
- 229960002471 cholic acid Drugs 0.000 description 1
- 235000019416 cholic acid Nutrition 0.000 description 1
- 239000002872 contrast media Substances 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 229940009976 deoxycholate Drugs 0.000 description 1
- 229960003964 deoxycholic acid Drugs 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 239000012538 diafiltration buffer Substances 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- ZGSPNIOCEDOHGS-UHFFFAOYSA-L disodium [3-[2,3-di(octadeca-9,12-dienoyloxy)propoxy-oxidophosphoryl]oxy-2-hydroxypropyl] 2,3-di(octadeca-9,12-dienoyloxy)propyl phosphate Chemical compound [Na+].[Na+].CCCCCC=CCC=CCCCCCCCC(=O)OCC(OC(=O)CCCCCCCC=CCC=CCCCCC)COP([O-])(=O)OCC(O)COP([O-])(=O)OCC(OC(=O)CCCCCCCC=CCC=CCCCCC)COC(=O)CCCCCCCC=CCC=CCCCCC ZGSPNIOCEDOHGS-UHFFFAOYSA-L 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 238000002825 functional assay Methods 0.000 description 1
- 229930182830 galactose Natural products 0.000 description 1
- 150000002270 gangliosides Chemical class 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 239000000644 isotonic solution Substances 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 238000011031 large-scale manufacturing process Methods 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 238000005567 liquid scintillation counting Methods 0.000 description 1
- 239000012931 lyophilized formulation Substances 0.000 description 1
- 239000008176 lyophilized powder Substances 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- DJMVHSOAUQHPSN-UHFFFAOYSA-N malto-hexaose Natural products OC1C(O)C(OC(C(O)CO)C(O)C(O)C=O)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(OC4C(C(O)C(O)C(CO)O4)O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 DJMVHSOAUQHPSN-UHFFFAOYSA-N 0.000 description 1
- FJCUPROCOFFUSR-UHFFFAOYSA-N malto-pentaose Natural products OC1C(O)C(OC(C(O)CO)C(O)C(O)C=O)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 FJCUPROCOFFUSR-UHFFFAOYSA-N 0.000 description 1
- FJCUPROCOFFUSR-GMMZZHHDSA-N maltopentaose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O[C@H]([C@H](O)CO)[C@H](O)[C@@H](O)C=O)O[C@H](CO)[C@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O[C@@H]2[C@@H]([C@@H](O)[C@H](O[C@@H]3[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O3)O)[C@@H](CO)O2)O)[C@@H](CO)O1 FJCUPROCOFFUSR-GMMZZHHDSA-N 0.000 description 1
- FYGDTMLNYKFZSV-UHFFFAOYSA-N mannotriose Natural products OC1C(O)C(O)C(CO)OC1OC1C(CO)OC(OC2C(OC(O)C(O)C2O)CO)C(O)C1O FYGDTMLNYKFZSV-UHFFFAOYSA-N 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 238000002414 normal-phase solid-phase extraction Methods 0.000 description 1
- 229920001542 oligosaccharide Polymers 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 150000008104 phosphatidylethanolamines Chemical class 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 229940068965 polysorbates Drugs 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- MUPFEKGTMRGPLJ-ZQSKZDJDSA-N raffinose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[C@@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O2)O)O1 MUPFEKGTMRGPLJ-ZQSKZDJDSA-N 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 108700004121 sarkosyl Proteins 0.000 description 1
- 238000003345 scintillation counting Methods 0.000 description 1
- 238000001542 size-exclusion chromatography Methods 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 150000003408 sphingolipids Chemical class 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- 229960001661 ursodiol Drugs 0.000 description 1
- FYGDTMLNYKFZSV-BYLHFPJWSA-N β-1,4-galactotrioside Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@H](CO)O[C@@H](O[C@@H]2[C@@H](O[C@@H](O)[C@H](O)[C@H]2O)CO)[C@H](O)[C@H]1O FYGDTMLNYKFZSV-BYLHFPJWSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/1703—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- A61K38/1709—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/10—Alcohols; Phenols; Salts thereof, e.g. glycerol; Polyethylene glycols [PEG]; Poloxamers; PEG/POE alkyl ethers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/24—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing atoms other than carbon, hydrogen, oxygen, halogen, nitrogen or sulfur, e.g. cyclomethicone or phospholipids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/26—Carbohydrates, e.g. sugar alcohols, amino sugars, nucleic acids, mono-, di- or oligo-saccharides; Derivatives thereof, e.g. polysorbates, sorbitan fatty acid esters or glycyrrhizin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/19—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles lyophilised, i.e. freeze-dried, solutions or dispersions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/02—Nutrients, e.g. vitamins, minerals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/775—Apolipopeptides
Definitions
- the present invention relates to reconstituted high density lipoprotein formulations, and in particular to formulations with suitable stability and biological properties for pharmaceutical use.
- High-density lipoproteins form a range of lipoprotein particles found in normal serum.
- Mature HDL particles are present in the form of a globular structure containing proteins and lipids. Within the outer layer of these particles are the more polar lipids, phospholipids and free cholesterol, all having charged groups orientated outwards, towards the aqueous environment. The more hydrophobic lipids, such as esterified cholesterol and triglycerides, reside in the core of the particle. Newly formed or nascent HDL particles lack the lipid and are discoidal in shape. Protein components are embedded in the outer layer.
- the main protein component is apolipoprotein A-I (Apo A-I) with smaller amounts of Apo A-II, Apo A-IV, Apo CIII, Apo D, Apo E and Apo J.
- Various other proteins reside on the HDL particle, such as lecithin-cholesterol acetyl transferase, PAF acetylhydrolase and paraoxonase.
- rHDL reconstituted HDL
- the artificial particles contain components of the natural particles, in particular Apo A-I and lipids.
- rHDL reconstituted HDL
- lipids e.g. 1,2-dipalmitoyl- sn -glycero-3-[phospho- rac -(I-glycerol)] (DPPG)).
- rHDL formulations It is convenient for rHDL formulations to be lyophilized (freeze-dried) before use. Lyophilization is a commonly used method for preparing solid protein pharmaceuticals. However, this process generates a variety of freezing and drying stresses, such as concentration of the solubilized protein, formation of ice crystals, pH changes, etc. All of these stresses can denature proteins to various degrees. Thus, stabilizers are often required in a protein formulation to protect protein stability both during freezing and drying processes. In order to maintain the stability of rHDL formulations during lyophilization, stabilizers like sugars and sugar alcohols have been used.
- US 5,089,602 discloses plasma-derived lipoproteins that are stabilized with 10% sucrose or a mixture of 10% sucrose and 5% mannitol.
- WO 2012/000048 discloses sugar and sugar alcohol stabilizers used at a concentration from about 65 to 85 g/L of rHDL formulation (equivalent to about 6.5 to 8.5% w/w).
- WO 2012/109162 discloses sucrose and mannitol as stabilizers, used in a mixture at 4% w/w and 2% w/w respectively.
- An investigation into the manufacturing and shelf stability of rHDL was carried out in Kim et al, Biotechnology and Bioprocess Engineering 16, 785-792 (2011 ).
- rHDL with an Apo A-I:soybean PC ratio of 1:150 could not be sufficiently stabilized with 1 or 5% sucrose, whereas 10% sucrose was described as optimal.
- rHDL formulations of these documents are intended for infusion therapy, but high sugar concentrations in infusion products may cause or exacerbate renal problems. This is a particular problem in the target patient population for rHDL, because these patients are often renally impaired.
- an object of the present invention was to provide alternative or improved rHDL formulations compared to these previous formulations.
- the inventors sought stable rHDL formulations with reduced renal toxicity.
- the rHDL formulation of claim 1 shows good long-term stability. By containing less lyophilization stabilizer than previous formulations, the formulation also presents less risk of renal toxicity.
- the low lyophilization stabilizer concentration may also allow the rHDL to perform better in functional assays of rHDL function.
- amino acids, particularly proline are useful lyophilization stabilizers for rHDL formulations.
- the invention provides an rHDL formulation comprising an apolipoprotein, a lipid and a lyophilization stabilizer, wherein the ratio between the apolipoprotein and the lipid is from about 1:20 to about 1:120 (mol:mol).
- the lyophilization stabilizer is present in a concentration from about 1.0% to about 6.0% (w/w of rHDL formulation), e.g. from 1.0, 1.1, 1.2 or 1.3 to 5.5, 5.6, 5.7, 5.8, 5.9, or 6.0.
- This low amount of lyophilization stabilizer may reduce the risk of renal toxicity. It is also particularly suitable for patients receiving contrast agents during acute coronary syndrome therapy (ACS), since these agents may compete with lyophilization stabilizer for clearance in the kidneys.
- the lyophilization stabilizer is present in a concentration from about 1.0% to less than 6.0% e.g. from about 1.0% to 5.9%.
- the lyophilization stabilizer is present in a concentration from about 3.0% to less than 6.0%, e.g. from about 3.0% to 5.9%. More preferably, the lyophilization stabilizer is present in a concentration from about 4.0% to 5.5%, particularly 4.3% to 5.3%, more particularly 4.3% to 5.0%, and most preferably 4.6% to 4.8% (w/w).
- Such formulations show good stability and low renal toxicity.
- the ratio between the apolipoprotein and the lyophilization stabilizer is from about 1:1 to about 1:3 (w:w).
- the ratio between the apolipoprotein and the lyophilization stabilizer is from about 1:1 to about 1:2.4 (w:w), e.g. less than 1:2 (w:w).
- the ratio between the apolipoprotein and the lyophilization stabilizer may be less than this, e.g. from about 1:1 to about 1:7, and in particular from about 1:1 to about 1:5 (w:w).
- the invention also provides an rHDL formulation comprising an apolipoprotein, a lipid and a lyophilization stabilizer, wherein the lyophilization stabilizer comprises an amino acid.
- the amino acid is proline.
- the inventors have found that amino acids are good lyophilization stabilizers for rHDL formulations, particularly when in a mixture with low amounts of other stabilizers.
- the invention also provides the aforementioned rHDL formulation for preventing or treating a disease, disorder or condition in a human.
- the disease, disorder or condition is responsive to prophylactic or therapeutic administration of the rHDL formulation.
- reconstituted HDL (rHDL) formulation means any artificially-produced lipoprotein formulation or composition that is functionally similar to, analogous to, corresponds to, or mimics, high density lipoprotein (HDL), typically present in blood plasma.
- rHDL formulations include within their scope “HDL mimetics” and “synthetic HDL particles”.
- lyophilization stabilizer means a substance that stabilizes protein during lyophilization. Such lyophilization stabilizers are well known in the art and are reviewed in, for example, Wang (2000) International Journal of Pharmaceuticals 203:1-60 .
- a preferred lyophilization stabilizer for use in the invention comprises a sugar, a sugar alcohol, an amino acid, or a mixture thereof.
- disaccharides such as sucrose are particularly suitable sugars for use as the lyophilization stabilizer.
- Other disaccharides that may be used include fructose, trehalose, maltose and lactose.
- trisaccharides like raffinose and maltotriose may be used.
- Larger oligosaccharides may also be suitable, e.g. maltopentaose, maltohexaose and maltoheptaose.
- monosaccharides like glucose, mannose and galactose may be used. These mono-, di-, tri- and larger oligo-saccharides may be used either alone or in combination with each other.
- lyophilization stabilizers that are sugar alcohols may also be used. These sugar alcohols may also be used either alone or in combination.
- a particular sugar alcohol for use in the invention is mannitol.
- sugar alcohols that may be used include inositol, xylitol, galactitol, and sorbitol.
- Other polyols like glycerol may also be suitable.
- Amino acids that may be used as lyophilization stabilizers include proline, glycine, serine, alanine, and lysine. Modified amino acids may also be used, for example 4-hydroxyproline, L-serine, sodium glutamate, sarcosine, and ⁇ -aminobutyric acid. The inventors have found that proline is a particularly suitable amino acid for use as a lyophilization stabilizer.
- the lyophilization stabilizer comprises a mixture of a sugar and a sugar alcohol.
- a mixture of sucrose and mannitol may be used.
- the sugar and the sugar alcohol may be mixed in any suitable ratio, e.g. from about 1:1 (w:w) to about 3:1 (w:w), and in particular about 2:1 (w:w).
- Ratios less than 2:1 are particularly envisaged, e.g. less than 3:2.
- the ratio is greater than 1:5, e.g. greater than 1:2 (w:w).
- the formulation comprises less than 4% sucrose and 2% mannitol (w/w of rHDL formulation), for example 3% sucrose and 2% mannitol.
- the formulation comprises 4% sucrose and less than 2% mannitol. In some embodiments the formulation comprises less than 4% sucrose and less than 2% mannitol e.g. about 1.0% to 3.9% sucrose and about 1.0% to 1.9% (w/w) mannitol.
- the lyophilization stabilizer comprises a mixture of a sugar and an amino acid.
- a mixture of sucrose and proline may be used.
- the sugar and the amino acid may be mixed in any suitable ratio, e.g. from about 1:1 to about 3:1 (w:w), and in particular about 2:1 (w:w). Ratios less than 2:1 are particularly envisaged, e.g. less than 3:2 (w:w). Typically, the ratio is greater than 1:5, e.g. greater than 1:2 (w:w).
- the amino acid is present in a concentration of from about 1.0 to about 2.5% e.g.
- the formulation comprises 1.0% sucrose and 2.2% proline, or 3.0% sucrose and 1.5% proline, or 4% sucrose and 1.2% proline.
- the amino acid may be added to the sugar to maintain an isotonic solution. Solutions with an osmolality of greater than 350 mosmol/kg are typically hypertonic, while those of less than 250 mosmol/kg are typically hypotonic. Solutions with an osmolality of from 250 mosmol/kg to 350 mosmol/kg are typically isotonic.
- the lyophilization stabilizer comprises a mixture of a sugar alcohol and an amino acid.
- the lyophilization stabilizer may comprise a mixture of a sugar, a sugar alcohol, and an amino acid.
- the apolipoprotein may be any apolipoprotein which is a functional, biologically active component of naturally-occurring HDL or of a reconstituted high density lipoprotein/rHDL.
- the apolipoprotein is either a plasma-derived or recombinant apolipoprotein such as Apo A-I, Apo A-II, Apo A-V, pro-Apo A-I or a variant such as Apo A-I Milano.
- the apolipoprotein is Apo A-I. More preferably the Apo A-I is either recombinantly derived comprising a wild type sequence or the Milano sequence or alternatively it is purified from human plasma.
- the apolipoprotein may be in the form of a biologically-active fragment of apolipoprotein. Such fragments may be naturally-occurring, chemically synthetized or recombinant.
- a biologically-active fragment of Apo A-I preferably has at least 50%, 60%, 70%, 80%, 90% or 95% to 100% or even greater than 100% of the lecithin-cholesterol acyltransferase (LCAT) stimulatory activity of Apo A-I.
- LCAT lecithin-cholesterol acyltransferase
- the molar ratio of apolipoprotein:lipid is typically from about 1:20 to about 1:120, and preferably from about 1:20 to about 1:100, more preferably from about 1:20 to about 1:75 (mol:mol), and in particular from 1:45 to 1:65.
- This range includes molar ratios such as about 1:25, 1:30, 1:35, 1:40, 1:45, 1:50, 1:55, 1:60, 1:65, 1:70, 1:75, 1:80, 1:85, 1:90, 1:95 and 1:100.
- a particularly advantageous ratio of apolipoprotein:lipid is from 1:40 to 1:65 (mol:mol). This ensures that the rHDL formulation according to the present invention comprises a lipid at a level which does not cause liver toxicity.
- the molar ratio of apolipoprotein:lipid may be in a range from about 1:80 to about 1:120.
- the ratio may be from 1:100 to 1:115, or from 1:105 to 1:110.
- the molar ratio may be for example from 1:80 to 1:90, from 1:90 to 1:100, or from 1:100 to 1:110.
- the rHDL formulation according to the present invention comprises additionally a detergent in order to further stabilize the rHDL particles.
- the detergent may be any ionic (e.g. cationic, anionic, zwitterionic) detergent or nonionic detergent, inclusive of bile acids and salts thereof, suitable for use in rHDL formulations.
- Ionic detergents may include bile acids and salts thereof, polysorbates (e.g. PS80), 3-[(3-Cholamidopropyl)dimethylammonio]-1-propane-sulfonate(CHAPS), 3-[(3-Cholamidopropyl)-dimethylammonio]-2-hydroxy-1-propanesulfonate (CHAPSO), cetyl trimethyl-ammonium bromide, lauroylsarcosine, tert-octyl phenyl propanesulfonic acid and 4'-amino-7-benzamido-taurocholic acid.
- polysorbates e.g. PS80
- CHPS 3-[(3-Cholamidopropyl)dimethylammonio]-1-propane-sulfonate
- CHAPSO 3-[(3-Cholamidopropyl)-dimethylammonio]-2-hydroxy-1-propanesulfonate
- Bile acids are typically dihydroxylated or trihydroxylated steroids with 24 carbons, including cholic acid, deoxycholic acid, chenodeoxycholic acid or ursodeoxycholic acid.
- the detergent is a bile salt such as a cholate, deoxycholate, chenodeoxycholate or ursodeoxycholate salt.
- a particularly preferred detergent is sodium cholate.
- the concentration of the detergent, in particular of sodium cholate is preferably 0.3 to 1.5 mg/mL.
- the bile acid concentration can be determined using various methods including colorimetric assay (for example, see Lerch et. al., 1996, Vox Sang. 71:155-164 ; Sharma, 2012, Int. J. Pharm Biomed.
- the rHDL formulation comprises cholate levels of 0.5 mg/mL to 1.5 mg/mL as determined by colorimetric assay and a lyophilization stabilizer in a concentration from about 4.0% to 5.5%, particularly 4.3% to 5.3%, more particularly 4.3% to 5.0%, and most preferably 4.6% to 4.8% (w/w).
- the lyophilization stabilizer is sucrose.
- the ratio between the apolipoprotein and the lyophilization stabilizer is usually adjusted so this ratio is from about 1:1 to about 1:7 (w:w). More preferably, the ratio is from about 1:1 to about 1:3, in particular about 1:1.1 to about 1:2. In specific embodiments the rHDL formulations thus have ratios of 1:1.1, 1:1.2, 1:1.3, 1:1.4, 1:1.5, 1:1.6, 1:1.7, 1:1.8, 1:1.9 or 1:2 (w:w). It is however contemplated that for particular embodiments where there are low amounts of protein (e.g. ⁇ 20mg/mL) that the ratio between the apolipoprotein and the lyophilization stabilizer can be extended to as much as about 1:7 (w:w), e.g. about 1:4.5 (w:w).
- the apolipoprotein is at a concentration from about 5 to about 50 mg/ml. This includes 5, 8, 10, 15, 20, 25, 30, 35, 40, 45 and 50 mg/ml and any ranges between these amounts.
- the apolipoprotein is, preferably, at a concentration from about 25 to 45 mg/ml. In other embodiments, the apolipoprotein may be at a concentration of from about 5 to 20 mg/ml, e.g. about 8 to 12 mg/ml.
- the lipid may be any lipid which is a functional, biologically active component of naturally occurring HDL or of reconstituted high density lipoprotein (rHDL).
- lipids include phospholipids, cholesterol, cholesterol-esters, fatty acids and/or triglycerides.
- the lipid is at least one charged or non-charged phospholipid or a mixture thereof.
- the rHDL formulation according to the present invention comprises a combination of a detergent and a non-charged phospholipid.
- the rHDL formulation comprises a charged phospholipid but no detergent at all.
- the rHDL formulation comprises charged and non-charged lipids as well as a detergent.
- non-charged phospholipids also called neutral phospholipids
- neutral phospholipids are phospholipids that have a net charge of about zero at physiological pH.
- Non-charged phospholipids may be zwitterions, although other types of net neutral phospholipids are known and may be used.
- Charge phospholipids are phospholipids that have a net charge at physiological pH.
- the charged phospholipid may comprise a single type of charged phospholipid, or a mixture of two or more different, typically like-charged phospholipids. In some examples, the charged phospholipids are negatively charged glycophospholipids.
- the formulation according to the present invention may also comprise a mixture of different lipids, such as a mixture of several non-charged lipids or of a non-charged lipid and a charged lipid.
- phospholipids include phosphatidylcholine (lecithin), phosphatidic acid, phosphatidylethanolamine (cephalin), phosphatidylglycerol (PG), phosphatidylserine (PS), phosphatidylinositol (PI) and sphinogomyelin (SM) or natural or synthetic derivatives thereof.
- Natural derivatives include egg phosphatidylcholine, egg phosphatidylglycerol, soy bean phosphatidylcholine, hydrogenated soy bean phosphatidylcholine, soy bean phosphatidylglycerol, brain phosphatidylserine, sphingolipids, brain sphingomyelin, egg sphingomyelin, galactocerebroside, gangliosides, cerebrosides, cephalin, cardiolipin and dicetylphospate.
- Synthetic derivatives include dipalmitoylphosphatidylcholine (DPPC), didecanoylphosphatidylcholine (DDPC), dierucoylphosphatidylcholine (DEPC), dimyristoylphosphatidylcholine (DLPC), palmitoyloleoylphosphatidylcholine (PMPC), palmitoylstearoylphosphatidylcholine (PSPC), dioleoylphosphatidylethanolamine (DOPE), dilauroylphosphatidylglycerol (DLPG), distearoylphosphatidylglycerol (DSPG), dioleoylphosphatidylglycerol (DOPG), palmitoyloleoylphosphatidylglycerol (POPG), dimyrstolyphosphatidic acid (DMPA), dipalmitoylphosphatidic acid (DPPA), distearoylphosphatidic acid (DSPA), dipalmitoy
- the phospholipid can also be a derivative or analogue of any of the above phospholipids. Best results could be obtained with phosphatidylcholine.
- the lipids in the formulation according to the present invention are sphingomyelin and a negatively charged phospholipid, such as phosphatidylglycerol (e.g. DPPG).
- DPPG phosphatidylglycerol
- a mixture of sphingomyelin and phosphatidylglycerol (particularly DPPG) is specifically envisaged for use in the invention.
- the sphingomyelin and the phosphatidylglycerol may be present in any suitable ratio, e.g. from 90:10 to 99:1 (w:w), typically 95:5 to 98:2 and most typically 97:3.
- the formulation according to the present invention typically has a lyophilization stabilizer concentration from about 1.0% to about 6.0% e.g. from 1.0, 1.1, 1.2 or 1.3% to 5.5, 5.6, 5.7, 5.8, 5.9, or 6.0%, preferably from about 1.0% to less than 6.0%, e.g. from about 1.0% to 5.9% (w/w of rHDL formulation). Preferably from about 3.0% to less than 6.0%, e.g.
- the lyophilization stabilizer is preferably a sugar (e.g. sucrose), optionally in combination with a sugar alcohol such as mannitol or sorbitol, or an amino acid such as proline.
- the rHDL formulation according to the present invention has a pH in the range of 6 to 8, preferably within the range of 7 to 8. Even more preferably the pH is in the range of 7.3 to 7.7.
- the formulation is lyophilized. Due to the presence of lyophilization stabilizer, preferably of sucrose, sucrose and mannitol, or sucrose and proline, in combination with the apolipoprotein:lipid ratio, the lyophilisation yields in a stable powder having a long shelf life. This powder may be stored, used directly or after storage as a powder or used after rehydration to form the reconstituted high density lipoprotein formulation.
- lyophilization stabilizer preferably of sucrose, sucrose and mannitol, or sucrose and proline
- the invention may be used for large scale production of reconstituted high density lipoprotein.
- the lyophilized product may be prepared for bulk preparations, or alternatively, the mixed protein/lipid solution may be apportioned in smaller containers (for example, single dose units) prior to lyophilization, and such smaller units may be used as sterile unit dosage forms.
- the lyophilized formulation can be reconstituted in order to obtain a solution or suspension of the protein-lipid complex, that is the reconstituted high density lipoprotein.
- the lyophilized powder is rehydrated with an aqueous solution to a suitable volume.
- Preferred aqueous solutions are water for injection (WFI), phosphate-buffer saline or a physiological saline solution.
- the mixture can be agitated to facilitate rehydration.
- the reconstitution step is conducted at room temperature.
- the invention provides a method of producing an rHDL formulation including the step of adding the lyophilization stabilizer to the solution comprising the lipid, and the apolipoprotein until a concentration of from about 1.0% to about 6.0% (w/w of rHDL formulation) is reached, e.g. from 1.0, 1.1, 1.2 or 1.3 to 5.5, 5.6, 5.7, 5.8, 5.9, or 6.0.
- the lyophilization stabilizer is added until a concentration from about 1.0% to less than 6.0% e.g. from about 1.0% to 5.9% is reached.
- lyophilization stabilizer is added until a concentration from about 3.0% to less than 6.0%, e.g.
- the lyophilization stabilizer is added until a concentration from about 4.0% to 5.5%, particularly 4.3% to 5.3%, more particularly 4.3% to 5.0%, and most preferably 4.6% to 4.8% (w/w) is reached.
- the solution may already contain stabilizer.
- the solution additionally includes a detergent such as sodium cholate.
- a detergent such as sodium cholate.
- the rHDL formulation is manufactured by combining Apo A-I purified from plasma, with phosphatidylcholine (PC) in the presence of sodium cholate and sucrose at a concentration from about 1.0% to about 6.0%, preferably from about 1.0% to less than 6.0% w/w to produce disc shaped, non-covalently associated particles (MW approximately 144 kDa).
- the rHDL formulation is comprised of an Apo A-I (recombinant or purified from plasma) and phoshatidylcholine stabilized by cholate and sucrose at a concentration from about 1.0% to about 6.0% w/w, preferably from about 1.0% to less than 6.0%.
- the cholate levels are from about 0.5 to about 1.5 mg/mL.
- the recombinant Apo A-I comprises either a wild type sequence or the Milano sequence (which when expressed forms dimers).
- the lyophilized rHDL formulation of the present invention may be formed using any method of lyophilization known in the art, including, but not limited to, freeze drying, i.e. the apolipoprotein/lipid-containing solution is subjected to freezing followed by reduced pressure evaporation.
- the lyophilized rHDL formulations that are provided can retain substantially their original stability characteristics for at least 2, 4, 6, 8, 10, 12, 18, 24, 36 or more months.
- lyophilized rHDL formulations stored at 2-8 °C or 25 °C can typically retain substantially the same molecular size distribution as measured by HPLC-SEC when stored for 6 months or longer.
- Particular embodiments of the rHDL formulation can be stable and suitable for commercial pharmaceutical use for at least 6 months, 12 months, 18 months, 24 months, 36 months or even longer when stored at 2-8 °C and/or room temperature.
- the rHDL formulation according to the present invention may be used in preventing or treating a disease, disorder or condition in a human.
- the disease, disorder or condition is responsive to prophylactic or therapeutic administration of the rHDL formulation according to the present invention.
- diseases, disorders or conditions include atherosclerosis; cardiovascular disease (e.g. acute coronary syndrome (ACS) such as angina pectoris and myocardial infarction); or diseases, disorders or conditions such as diabetes that predispose to ACS; hypercholesterolaemia (e.g. elevated serum cholesterol or elevated LDL cholesterol) and hypocholesterolaemia resulting from reduced levels of high-density lipoprotein (HDL), such as being symptomatic of Tangier disease.
- ACS acute coronary syndrome
- HDL high-density lipoprotein
- rHDL formulations according to the present invention may be administered by any route of administration known in the art.
- rHDL formulations are administered parenterally, such as by intravenous (IV) infusion or injection.
- the rHDL formulation comprises Apo A-I (recombinant or purified from plasma) which has been reconstituted to form particles suitable for IV infusion.
- the administered dosage of the rHDL formulation may be in the range of from about 1 to about 120 mg/kg body weight.
- the dosage is in the range of from about 5 mg/kg to about 80 mg/kg inclusive of 8 mg/kg, 10 mg/kg, 12 mg/kg, 20 mg/kg, 30 mg/kg, 40 mg/kg, 50 mg/kg, 60 mg/kg, and 70 mg/kg dosages.
- delivery can be achieved by fixed dosages of rHDL, that is, in an amount independent of patient body weight.
- Preferred fixed dosages include 0.1-15 g, 0.5-12 g, 1-10 g, 2-9 g, 3-8 g, 4-7 g or 5-6 g of apolipoprotein.
- Particularly preferred fixed dosages include 1-2 g, 3-4 g, 5-6 g or 6-7g of apolipoprotein.
- Non-limiting examples of specific fixed dosages include 0.25 g, 0.5 g, 1.0 g, 1.7 g, 2.0 g, 3.4 g, 4.0 g, 5.1 g, 6.0 g, 6.8 g and 8.0 g of apolipoprotein.
- a vial preferably comprises the lyophilized rHDL formulation with a protein content of 0.25 g, 0.5 g, 1 g, 2 g, 2.5 g, 3 g, 3.5 g, 4 g, 4.5 g, 5 g, 5.5 g, 6 g, 6.5 g, 7 g, 8 g or 10 g per vial. More preferably the protein content is either 0.5 g, 1 g, 2 g, 4 g, 6 g, 8 g or 10 g per vial.
- the invention also provides an apolipoprotein kit comprising one or more unit doses of the apolipoprotein formulation disclosed herein and one or more other kit components.
- the kit is for prophylactically or therapeutically treating a disease, disorder or condition in a human, as hereinbefore described.
- Non-limiting examples of one or more other kit components include instructions for use; vials, containers or other storage vessels containing each of the unit doses; delivery devices such as needles, catheters, syringes, tubing and the like; and/or packaging suitable for safely and conveniently storing and/or transporting the kit.
- the instructions for use are a label or package insert, wherein the label or package insert indicates that the apolipoprotein formulation may be used to treat a disease or condition such as cardiovascular disease by administering a fixed dose amount to a human subject in need thereof.
- a 'package insert' refers to instructions included in commercial packages of the apolipoprotein formulations, that contains information about the indications, usage, dosage, administration, contraindications and/or warnings concerning the use of such apolipoprotein formations.
- a 'vial' refers to a container which holds an apolipoprotein formulation.
- the vial may be sealed by a stopper pierceable by a syringe.
- the vial is formed from a glass material.
- the apolipoprotein formulation in the vial can be in various states including liquid, lyophilized, frozen etc.
- the fixed dosage apolipoprotein formulation is preferably stable as turbidity is a preferred measure. A turbidity level of below about 5, 10, 15, 20, or 30 NTU can generally be considered a stable dosage apolipoprotein formulation.
- Turbidity measurements can be taken by incubating the apolipoprotein formulations over time periods such as 0 hr, 2 hr, 4 hr, 6 hr, 12 hr, 18 hr, 24 hr, 36 hr, 72 hr, 7 days and 14 days at storage temperatures such as room temperature or 2 to 8 °C.
- the apolipoprotein formulation is considered to be stable as a liquid when it is stored for 14 days at room temperature and exhibits a turbidity of less than about 15 NTU.
- the kit may facilitate administration of the apolipoprotein formulation by a health professional or self-administration by a patient or caregiver.
- the term “comprising” encompasses “including” as well as “consisting” e.g. a formulation or a component of a formulation that is described as “comprising” X may consist exclusively of X or may include something additional e . g . X + Y.
- the invention can also provide a process involving less than the total number of steps.
- the different steps can be performed at very different times by different people in different places ( e . g . in different countries).
- a process comprising a step of mixing two or more components does not require any specific order of mixing.
- components can be mixed in any order. Where there are three components then two components can be combined with each other, and then the combination may be combined with the third component, etc.
- sodium cholate (New Zealand Pharmaceuticals) was dissolved in buffer (10 mM NaCl, 1 mM EDTA, 10 mM TRIS, pH 8.0) and stirred until clear. Soybean phosphatidylcholine (Phospholipid GmbH) was added to an appropriate volume of the cholate and stirred for 16 h at room temperature.
- the Apo A-I solution was diluted to a protein concentration of 9.0 mg/mL (determined by OD280) with 10 mM NaCl and mixed with an appropriate volume of the lipid solution to obtain protein to lipid ratio in the range of 1:45 to 1:65. The mixture was stirred at 2-8 °C for 30 min to 16 h.
- the HDL mimetics were prepared by cholate dialysis using 1% sucrose as a diafiltration buffer. The eluate was concentrated to a protein concentration of 33 to 38 g protein /L. Sucrose was added to obtain the desired concentration (1%, 2%, 3%, 4%, 5%, 6.5%, 7%, 10% w/w). The pH of the solution was adjusted, with 0.2 M NaOH to pH 7.50 ⁇ 0.1 after which WFI (water for injection) was added to obtain a protein concentration of 30 mg/mL. The final formulations were then sterile filtered through a 0.2 + 0.1 ⁇ m filter and filled into 100 mL glass vials at 1.7 g protein per vial and lyophilized.
- proline was added to the desired concentration. Proline maintains an isotonic formulation.
- HPLC-SEC Size exclusion chromatography
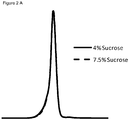
- Figure 1 shows a complete chromatogram of (1) internal control, 2: 5% w/w sucrose, 3: 6.5% w/w sucrose, 4: 7.5% w/w sucrose and 5: 10% w/w sucrose.
- Figures 2B and 2C show the results for sucrose concentrations 1, 2, 3, and 4% (w/w) and formulations comprising sucrose and proline.
- HDL particles are capable of sequestering cholesterol from plaques formed along artery walls or cells by interaction with the ATP-binding cassette transporter A1 (ABCA1).
- ABCA1 ATP-binding cassette transporter A1
- LCAT Lecithin-cholesterol acyltransferase
- a plasma enzyme converts the free cholesterol into cholesteryl ester (a more hydrophobic form of cholesterol), which is then sequestered into the core of the HDL particle before being transported to the liver to be metabolized. If the sucrose content in the final formulation affected the efficacy of the rHDL particle, LCAT activity would decrease.
- LCAT lecithin-cholesterol acyltransferase
- the cholesterol and cholesteryl ester is extraction by liquid extraction with n-hexane.
- the cholesteryl ester was separated from unesterified cholesterol using a solid phase extraction column (SampliQ Amino, Agilent) and measured by scintillation counting.
- the count rate of the sample stored at 4 °C is subtracted from the count rate of the sample stored at 37 °C.
- the same procedure is also performed with a reference sample.
- the LCAT activity is expressed as % of the Reference sample.
- Figures 3A and 3B show LCAT activity for 4-10% w/w sucrose formulations.
- Figure 3C shows LCAT activity for 1-4% w/w sucrose formulations. Very little difference is seen in LCAT activity when the sucrose ranges from 5-10% w/w in the final formulation ( Figure 3A ), however a slight decreasing trend is evident when the sucrose is further reduced to 4% w/w ( Figure 3B ).
- Figure 3D shows LCAT activity for formulations comprising sucrose and proline. No apparent trend in LCAT activity is observed for formulations containing sucrose and proline. Thus the efficacy of the HDL particle in sucrose/proline formulations is maintained.
- Reverse cholesterol transport is a pathway by which accumulated cholesterol is transported from the vessel wall to the liver for excretion.
- Cells efflux free cholesterol to lipid-poor Apo A-I via the ABCA1 pathway.
- the cholesterol efflux assay measures the capacity of HDL to accept cholesterol released from cells. It is anticipated that if sucrose content affected particle formation and/or integrity, differences would affect cholesterol efflux.
- RAW264.7 cells were obtained from the American Type Culture Collection (ATCC). Cells were cultured in DMEM (Dulbecco's modified Eagle's medium, Gibco) supplemented with 10% (v/v) foetal calf serum (FCS, Gibco), 2 mM glutamine, 100 units/mL penicillin and 100 ⁇ g/mL streptomycin in a humidified CO 2 incubator at 37 °C.
- ATCC American Type Culture Collection
- cells were seeded into 24-well plates at a density of 0.35 x 10 6 cells per well. The following day, cells were labeled with [1,2- 3 H]cholesterol (1 ⁇ Ci/mL, GE) in DMEM supplemented with 5% (v/v) FCS.
- Radioactivity in cell supernatants was measured by liquid scintillation counting.
- Total cell-associated [ 3 H]cholesterol was determined after extraction of cells in control wells for at least 30 minutes with 0.1 M Triton X-100. Cholesterol efflux was expressed as the percentage of the radioactivity released from cells into the medium relative to the total radioactivity in cells and medium. The difference in efflux between control and 8Br-cAMP-stimulated cells was taken as a measure of ABCA1-dependent efflux.
- Figures 4A and 4B show that as sucrose concentration decreases from 7.5% w/w to 4% w/w the cholesterol efflux increased. No apparent difference in cholesterol efflux was observed between the proline containing formulations and the 7.5% sucrose formulation ( Figure 4C ).
- turbidity is used to describe the cloudiness or haze in a solution. Strictly, turbidity arises from the multiple scattering events of visible light by elements present in the solution. Since turbidity arises from the net scattered light, it depends on the sample path length, protein concentration and size of the protein/aggregates/particles. Given that all reduced sucrose formulations contained the same protein concentration upon reconstitution and were measured with the same path length, differences in turbidity can be attributed to differences in the size and/or number of protein/aggregates/particles resulting from the various sucrose formulations.
- Turbidity was determined with a LED nephelometer (Hach 2100AN Turbiditimeter, Loveland, CO.) using formacin as a standard. Results are given as relative light scattering (NTU).
- Formulations containing 4-10% w/w sucrose produced similar turbid solutions upon reconstitution ( Figures 5A & 5B ).
- Sucrose concentrations of less than 4% showed increased turbidity ( Figures 5E and 5G ). Based on turbidity, sucrose concentrations of 4% (w/w) and above are optimum.
- lyophilized rHDL formulations prepared as per Example 1 was examined before and after storage (protected from light) at 40 °C for 12 weeks. Parameters tested included pH, turbidity, LCAT activation, HPLC-SEC (aggregate content, % lipoprotein in single peak and its relative retention time) and cholesterol efflux (C-efflux) (Tables 1 & 2). The results indicate that the formulations remain stable over the storage period.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Molecular Biology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Diabetes (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Zoology (AREA)
- Gastroenterology & Hepatology (AREA)
- Biophysics (AREA)
- Hematology (AREA)
- Obesity (AREA)
- Biochemistry (AREA)
- Immunology (AREA)
- Marine Sciences & Fisheries (AREA)
- Genetics & Genomics (AREA)
- Toxicology (AREA)
- Dermatology (AREA)
- Heart & Thoracic Surgery (AREA)
- Cardiology (AREA)
- Emergency Medicine (AREA)
- Endocrinology (AREA)
- Nutrition Science (AREA)
- Urology & Nephrology (AREA)
- Vascular Medicine (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261721771P | 2012-11-02 | 2012-11-02 | |
| EP13153903 | 2013-02-04 | ||
| US13/803,863 US9125943B2 (en) | 2012-11-02 | 2013-03-14 | Reconstituted HDL formulation |
| AU2013205684A AU2013205684B2 (en) | 2012-11-02 | 2013-04-10 | Reconstituted hdl formulation |
| EP13851610.9A EP2916857B1 (en) | 2012-11-02 | 2013-10-31 | Reconstituted hdl formulation |
| PCT/AU2013/001260 WO2014066943A1 (en) | 2012-11-02 | 2013-10-31 | Reconstituted hdl formulation |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP13851610.9A Division EP2916857B1 (en) | 2012-11-02 | 2013-10-31 | Reconstituted hdl formulation |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| EP3502131A1 true EP3502131A1 (en) | 2019-06-26 |
Family
ID=47665977
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP19151328.2A Pending EP3502131A1 (en) | 2012-11-02 | 2013-10-31 | Reconstituted hdl formulation |
| EP13851610.9A Active EP2916857B1 (en) | 2012-11-02 | 2013-10-31 | Reconstituted hdl formulation |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP13851610.9A Active EP2916857B1 (en) | 2012-11-02 | 2013-10-31 | Reconstituted hdl formulation |
Country Status (15)
| Country | Link |
|---|---|
| US (6) | US9125943B2 (enExample) |
| EP (2) | EP3502131A1 (enExample) |
| JP (1) | JP6340372B2 (enExample) |
| KR (1) | KR102263810B1 (enExample) |
| CN (2) | CN108057023B (enExample) |
| AU (1) | AU2013205684B2 (enExample) |
| BR (1) | BR112015009748B1 (enExample) |
| CA (1) | CA2889785C (enExample) |
| IL (1) | IL238504B (enExample) |
| MX (1) | MX364587B (enExample) |
| NZ (1) | NZ631131A (enExample) |
| PL (1) | PL2916857T3 (enExample) |
| RU (1) | RU2669568C2 (enExample) |
| SG (1) | SG11201503083UA (enExample) |
| WO (1) | WO2014066943A1 (enExample) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2617977T3 (es) | 2010-06-30 | 2017-06-20 | Csl Limited | Una formulación de lipoproteína de alta densidad reconstituida y método de producción de la misma |
| US9125943B2 (en) * | 2012-11-02 | 2015-09-08 | Csl Limited | Reconstituted HDL formulation |
| NZ631116A (en) * | 2013-06-05 | 2018-07-27 | Csl Ltd | Process for preparing apolipoprotein a-i (apo a-i) |
| CA2920391C (en) | 2013-08-08 | 2023-03-28 | Csl Limited | Contaminant removal method |
| BR112019001459A2 (pt) | 2016-07-27 | 2019-05-07 | Hartis-Pharma Sa | combinação, composição farmacêutica, e, método de prevenção e/ou de tratamento de uma doença ou de um distúrbio. |
| KR20240044543A (ko) * | 2016-11-10 | 2024-04-04 | 시에스엘 리미티드 | 심근경색의 재구성된 고밀도 지질단백질 치료 |
| CN112546201A (zh) * | 2019-09-26 | 2021-03-26 | 中国科学院生物物理研究所 | 人工脂蛋白颗粒apoA-Ⅳ脂肪体在治疗和/或预防糖尿病中的应用 |
| WO2025185021A1 (zh) * | 2024-03-05 | 2025-09-12 | 维康平生(北京)生物科技有限公司 | 一种脂肪体的冷冻干燥方法 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5089602A (en) | 1988-02-08 | 1992-02-18 | Rotkreuzstiftung Zentrallaboratorium Blutspendedienst Srk | Process for the manufacture of apolipoproteins from human blood plasma or serum |
| EP0663407A1 (de) * | 1993-12-31 | 1995-07-19 | Rotkreuzstiftung Zentrallaboratorium Blutspendedienst Srk | Verfahren zur Herstellung von rekonstituiertem Lipoprotein |
| WO2012000048A1 (en) | 2010-06-30 | 2012-01-05 | Csl Limited | A reconstituted high density lipoprotein formulation and production method thereof |
| WO2012109162A1 (en) | 2011-02-07 | 2012-08-16 | Cerenis Therapeutics Holding S.A. | Lipoprotein complexes and manufacturing and uses thereof |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| PL372925A1 (en) | 2001-09-28 | 2005-08-08 | Esperion Therapeutics Inc. | Prevention and treatment of restenosis by local administration of drug |
| MXPA04011312A (es) | 2002-05-17 | 2005-02-14 | Esperion Therapeutics Inc | Metodo para el tratamiento de desordenes dislipidemicos. |
| BR0310100A (pt) | 2002-05-17 | 2007-04-27 | Esperion Therapeutics Inc | métodos para tratar, prevenir ou reduzir a lesão de reperfusão isquêmica em um tecido ou órgão, e para prevenir ou tratar de uma condição associada com a privação de oxigênio, seguida por suprimento de oxigênio aumentado a um tecido ou órgão em necessidade disto |
| PE20050438A1 (es) | 2003-10-20 | 2005-06-14 | Esperion Therapeutics Inc | Formulas farmaceuticas, metodos y regimenes de dosificacion para el tratamiento y la prevencion de sindromes coronarios agudos |
| US20070254832A1 (en) * | 2006-02-17 | 2007-11-01 | Pressler Milton L | Methods for the treatment of macular degeneration and related eye conditions |
| KR100992488B1 (ko) | 2006-12-29 | 2010-11-05 | 주식회사 녹십자 | ApoA-I의 정제방법 및 정제된ApoA-I을 이용한재구축 HDL의 생산 방법 |
| NZ582170A (en) | 2007-08-17 | 2012-02-24 | Csl Behring Gmbh | Methods for purification of alpha-1-antitrypsin and apolipoprotein a-i |
| US8734853B2 (en) | 2008-11-17 | 2014-05-27 | University Of North Texas Health Science Center At Fort Worth | HDL particles for delivery of nucleic acids |
| US9125943B2 (en) * | 2012-11-02 | 2015-09-08 | Csl Limited | Reconstituted HDL formulation |
-
2013
- 2013-03-14 US US13/803,863 patent/US9125943B2/en active Active
- 2013-04-10 AU AU2013205684A patent/AU2013205684B2/en active Active
- 2013-10-31 RU RU2015120808A patent/RU2669568C2/ru active
- 2013-10-31 CA CA2889785A patent/CA2889785C/en active Active
- 2013-10-31 EP EP19151328.2A patent/EP3502131A1/en active Pending
- 2013-10-31 CN CN201711141045.0A patent/CN108057023B/zh active Active
- 2013-10-31 US US14/439,094 patent/US9925236B2/en active Active
- 2013-10-31 MX MX2015004546A patent/MX364587B/es active IP Right Grant
- 2013-10-31 WO PCT/AU2013/001260 patent/WO2014066943A1/en not_active Ceased
- 2013-10-31 NZ NZ631131A patent/NZ631131A/en unknown
- 2013-10-31 PL PL13851610T patent/PL2916857T3/pl unknown
- 2013-10-31 CN CN201380057007.0A patent/CN104755096B/zh active Active
- 2013-10-31 SG SG11201503083UA patent/SG11201503083UA/en unknown
- 2013-10-31 JP JP2015539998A patent/JP6340372B2/ja active Active
- 2013-10-31 BR BR112015009748-0A patent/BR112015009748B1/pt active IP Right Grant
- 2013-10-31 EP EP13851610.9A patent/EP2916857B1/en active Active
- 2013-10-31 KR KR1020157014530A patent/KR102263810B1/ko active Active
-
2015
- 2015-04-28 IL IL238504A patent/IL238504B/en active IP Right Grant
-
2018
- 2018-02-12 US US15/894,397 patent/US10603355B2/en active Active
-
2020
- 2020-03-23 US US16/826,585 patent/US11464828B2/en active Active
-
2022
- 2022-09-02 US US17/902,580 patent/US11957731B2/en active Active
-
2024
- 2024-04-12 US US18/634,576 patent/US20250099537A1/en active Pending
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5089602A (en) | 1988-02-08 | 1992-02-18 | Rotkreuzstiftung Zentrallaboratorium Blutspendedienst Srk | Process for the manufacture of apolipoproteins from human blood plasma or serum |
| EP0663407A1 (de) * | 1993-12-31 | 1995-07-19 | Rotkreuzstiftung Zentrallaboratorium Blutspendedienst Srk | Verfahren zur Herstellung von rekonstituiertem Lipoprotein |
| US5652339A (en) * | 1993-12-31 | 1997-07-29 | Rotkreuzstiftung Zentrallaboratorium | Method of producing reconstituted lipoproteins |
| WO2012000048A1 (en) | 2010-06-30 | 2012-01-05 | Csl Limited | A reconstituted high density lipoprotein formulation and production method thereof |
| WO2012109162A1 (en) | 2011-02-07 | 2012-08-16 | Cerenis Therapeutics Holding S.A. | Lipoprotein complexes and manufacturing and uses thereof |
Non-Patent Citations (6)
| Title |
|---|
| BORTNICK, J BIOL CHEM., vol. 275, no. 37, 2000, pages 28634 - 40 |
| KIM ET AL., BIOTECHNOLOGY AND BIOPROCESS ENGINEERING, vol. 16, 2011, pages 785 - 792 |
| LERCH, VOX SANG, vol. 71, 1996, pages 155 - 164 |
| SHARMA, INT. J. PHARM BIOMED., vol. 3, no. 2, 2012, pages 28 - 34 |
| STOKKE; NORUM, SCAND J CLIN LAB INVEST, vol. 27, no. 1, 1971, pages 21 - 7 |
| WANG, INTERNATIONAL JOURNAL OF PHARMACEUTICALS, vol. 203, 2000, pages 1 - 60 |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11957731B2 (en) | Reconstituted HDL formulation | |
| US20240216467A1 (en) | Dosage regime for apolipoprotein formulations | |
| HK40010383A (en) | Reconstituted hdl formulation | |
| DK2916857T3 (en) | RECONSTITUTED HDL FORMULATION | |
| HK1212599B (en) | Reconstituted hdl formulation | |
| RU2776110C2 (ru) | Схема дозирования для составов аполипопротеина | |
| HK1199813B (en) | Dosage regime for apolipoprotein formulations | |
| NZ624558B2 (en) | Dosage regime for apolipoprotein formulations |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION HAS BEEN PUBLISHED |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 2916857 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20191219 |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 40010383 Country of ref document: HK |
|
| RAP3 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: CSL LIMITED |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20240704 |