EP2919932B1 - Method of preparing a cast aluminium composite material - Google Patents
Method of preparing a cast aluminium composite material Download PDFInfo
- Publication number
- EP2919932B1 EP2919932B1 EP13854766.6A EP13854766A EP2919932B1 EP 2919932 B1 EP2919932 B1 EP 2919932B1 EP 13854766 A EP13854766 A EP 13854766A EP 2919932 B1 EP2919932 B1 EP 2919932B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- composite material
- additive
- molten
- particles
- concentration
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C32/00—Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of oxides, carbides, borides, nitrides, silicides or other metal compounds, e.g. oxynitrides, sulfides, whether added as such or formed in situ
- C22C32/0047—Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of oxides, carbides, borides, nitrides, silicides or other metal compounds, e.g. oxynitrides, sulfides, whether added as such or formed in situ with carbides, nitrides, borides or silicides as the main non-metallic constituents
- C22C32/0052—Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of oxides, carbides, borides, nitrides, silicides or other metal compounds, e.g. oxynitrides, sulfides, whether added as such or formed in situ with carbides, nitrides, borides or silicides as the main non-metallic constituents only carbides
- C22C32/0057—Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of oxides, carbides, borides, nitrides, silicides or other metal compounds, e.g. oxynitrides, sulfides, whether added as such or formed in situ with carbides, nitrides, borides or silicides as the main non-metallic constituents only carbides based on B4C
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22C—FOUNDRY MOULDING
- B22C9/00—Moulds or cores; Moulding processes
- B22C9/22—Moulds for peculiarly-shaped castings
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22D—CASTING OF METALS; CASTING OF OTHER SUBSTANCES BY THE SAME PROCESSES OR DEVICES
- B22D19/00—Casting in, on, or around objects which form part of the product
- B22D19/14—Casting in, on, or around objects which form part of the product the objects being filamentary or particulate in form
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22D—CASTING OF METALS; CASTING OF OTHER SUBSTANCES BY THE SAME PROCESSES OR DEVICES
- B22D2/00—Arrangement of indicating or measuring devices, e.g. for temperature or viscosity of the fused mass
- B22D2/008—Arrangement of indicating or measuring devices, e.g. for temperature or viscosity of the fused mass for the viscosity of the molten metal
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22D—CASTING OF METALS; CASTING OF OTHER SUBSTANCES BY THE SAME PROCESSES OR DEVICES
- B22D21/00—Casting non-ferrous metals or metallic compounds so far as their metallurgical properties are of importance for the casting procedure; Selection of compositions therefor
- B22D21/002—Castings of light metals
- B22D21/007—Castings of light metals with low melting point, e.g. Al 659 degrees C, Mg 650 degrees C
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22D—CASTING OF METALS; CASTING OF OTHER SUBSTANCES BY THE SAME PROCESSES OR DEVICES
- B22D25/00—Special casting characterised by the nature of the product
- B22D25/06—Special casting characterised by the nature of the product by its physical properties
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C1/00—Making non-ferrous alloys
- C22C1/02—Making non-ferrous alloys by melting
- C22C1/026—Alloys based on aluminium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C1/00—Making non-ferrous alloys
- C22C1/10—Alloys containing non-metals
- C22C1/1036—Alloys containing non-metals starting from a melt
- C22C1/1068—Making hard metals based on borides, carbides, nitrides, oxides or silicides
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C21/00—Alloys based on aluminium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C49/00—Alloys containing metallic or non-metallic fibres or filaments
- C22C49/02—Alloys containing metallic or non-metallic fibres or filaments characterised by the matrix material
- C22C49/04—Light metals
- C22C49/06—Aluminium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C49/00—Alloys containing metallic or non-metallic fibres or filaments
- C22C49/14—Alloys containing metallic or non-metallic fibres or filaments characterised by the fibres or filaments
Definitions
- the present invention relates to cast aluminum/boron carbide composite metal matrix material having products obtained from a peritectic reaction to increase their fluidity prior to casting.
- the reaction products are obtained by using additives capable of undergoing a perictectic reaction with the boron of the boron carbide.
- US2003175543 discloses a hybrid composite reinforced metal matrix in which the metal is aluminum, aluminum alloy, or a magnesium alloy containing a relatively high percentage of aluminum.
- the metal matrix also includes a hardening agent which is at least one intermetallic compound of aluminum with at least one second metal chosen from iron, nickel, titanium, zirconium, cobalt and niobium.
- CA2491429 discloses a cast article from an aluminum alloy having the following composition in weight percent: Silicon 6.0 - 25.0, Copper 5.0 - 8.0, Iron 0.05 - 1.2, Magnesium 0.5 - 1.5, Nickel 0.05 - 0.9, Manganese 0.05 -1.2, Titanium 0.05 - 1.2, Zirconium 0.05- 1.2, Vanadium 0.05 - 1.2, Zinc 0.05 - 0.9, Strontium 0.001 - 0.1, Phosphorus 0.001 - 0.1, and the balance is Aluminum, wherein the silicon-to-magnesium ratio is 10 - 25, and the copper-to-magnesium ratio is 4 - 15.
- US2009263277 discloses a L12 aluminum alloy having magnesium or nickel; at least one of scandium, erbium, thulium, ytterbium, and lutetium; at least one of gadolinium, yttrium, zirconium, titanium, hafnium, and niobium; and at least one ceramic reinforcement.
- Aluminum oxide, silicon carbide, aluminum nitride, titanium boride, titanium diboride and titanium carbide are suitable ceramic reinforcement particles.
- titanium can be added.
- reaction products are formed near the interface of the B 4 C particles and the aluminum matrix which "poison" the B 4 C particles.
- the reaction products are taught to shield the B 4 C particles from the aluminum.
- the means and methods would preferably provide/maintain a fluidity amenable to shaping and/or casting in industrial settings.
- the present disclosure provides a method of preparing a cast composite material comprising aluminum, products of a peritectic reaction between an additive and boron as well as dispersed boron carbide particles.
- the presence of the products of the peritectic reaction maintains the fluidity of the molten composite material, prior to casting, and facilitate castability and shaping of the composite material.
- the present disclosure provides a cast composite material comprising (i) aluminum, (ii) products of a peritectic reaction between an additive and boron, (iii) dispersed boron carbide particles and (iv) optionally titanium.
- the additive is selected from the group consisting of chromium, molybdenum, vanadium, niobium, zirconium, strontium, scandium, and any combination thereof.
- a sample of the composite material has a fluidity, after having been heated, prior to casting, to a temperature of about 700°C for about 120 minutes, corresponding to a cast length of at least 100 mm when measured using a mold having a groove for containing the sample, the groove having a width of about 33 mm, a height of between about 6.5 mm and about 4.0 mm and being downwardly inclined, from an horizontal axis, of about 10°.
- the cast length of the sample is at least 190 mm.
- the cast composite material is submitted to holding during a holding time and to casting during a casting time and wherein the holding time and the casting time amounts to 120 minutes.
- the products of the peritectic reaction are provided by combining a molten aluminum or a molten aluminum alloy with the additive capable of undergoing the peritectic reaction (prior to the incorporation of boron carbide particles).
- the additive is selected from the group consisting of zirconium, strontium, scandium and any combination thereof.
- the additive is scandium.
- the additive is strontium.
- the additive is zirconium.
- the concentration (v/v) of the dispersed boron carbide particles is between 4% and 40% with respect to the total volume of the cast composite material.
- the concentration (w/w) of the additive can be between 0.47% and 8.00% with respect to the total weight of the cast composite material and optionally, the composite material can further comprise titanium at a concentration (w/w) between 0.50% and 4.00% with respect to the total weight of the cast composite material.
- the concentration (v/v) of the dispersed boron carbide particles is between 4.5% and 18.9% with respect to the total volume of the cast composite material.
- the concentration (w/w) of the additive can be between 0.38% and 4.00% with respect to the total weight of the cast composite material and, optionally, the cast composite material further comprises titanium at a concentration (w/w) between 0.40% and 2.00% with respect to the total weight of the cast composite material.
- the concentration (v/v) of the dispersed boron carbide particles is between 19.0% and 28.0% with respect to the total volume of the cast composite material.
- the concentration (w/w) of the additive can be between 1.68% and 6.00% with respect to the total weight of the cast composite material and, optionally, the cast composite material can further comprise titanium at a concentration (w/w) between 1.80% and 3.00% with respect to the total weight of the cast composite material.
- the concentration (v/v) of the dispersed boron carbide particles is between 25.0% and 28.0% or between 28.0% and 33.0% with respect to the total volume of the cast composite material.
- the concentration (w/w) of the additive can be between 0.94% and 4.00% with respect to the total weight of the cast composite material and, optionally, the cast composite material can further comprise titanium at a concentration (w/w) between 1.00% and 2.00% with respect to the total weight of the cast composite material.
- the present disclosure provides a method of preparing a cast composite material.
- the method comprises: (a) combining (i) a molten aluminum alloy comprising an additive capable of undergoing a peritectic reaction with boron (ii) between 4 and 40 v/v % of a source of boron carbide particles based on a total volume of the cast composite material so as to provide a molten composite material comprising products of the peritectic reaction between the additive and boron and dispersed boron carbide particles provided the molten aluminum alloy is free of titanium, wherein: the additive is selected from the group consisting of chromium, molybdenum, vanadium, niobium, zirconium, strontium, scandium, and any combination thereof, the concentration of the additive being between 1.10% w/w and 8.00% w/w% with respect to the total weight of the cast composite material; a sample of the composite material has a fluidity, after having been heated, prior to casting
- the cast length is at least 190 mm.
- the method further comprises, prior to step (b), holding the molten composite material during a holding time and casting the molten composite during a casting time, wherein the holding time and the casting time amounts to 120 minutes.
- the method further comprises, prior to step (a), providing the molten aluminum alloy by combining a molten aluminum or a molten aluminum alloy with the additive capable of undergoing the peritectic reaction.
- the present disclosure provides a method of improving the casting and/or shaping properties of a molten composite material comprising aluminum, products of a peritectic reaction between an additive and boron, and dispersed boron carbide particles.
- the method comprises combining (i) a molten aluminum alloy comprising the additive capable of undergoing a peritectic reaction with boron with (ii) a source of boron carbide particles so as to provide a molten composite material.
- the additive is selected from the group consisting of chromium, molybdenum, vanadium, niobium, zirconium, strontium, scandium, and any combination thereof.
- a sample of the composite material has a fluidity, after having been heated, prior to casting, to a temperature of about 700°C for about 120 minutes, corresponding to a cast length of at least 100 mm when measured using a mold having a groove for containing the sample, the groove having a width of about 33 mm, a height of between about 6.5 mm and about 4.0 mm and being downwardly inclined, from an horizontal axis, of about 10°.
- the present disclosure provides a method of facilitating shaping of a molten composite material of a molten composite material comprising aluminum, products of a peritectic reaction between an additive and boron, and dispersed boron carbide particles.
- the method comprises combining (i) a molten aluminum alloy comprising the additive capable of undergoing a peritectic reaction with boron with (ii) a source of boron carbide particles so as to provide a molten composite material.
- the additive is selected from the group consisting of chromium, molybdenum, vanadium, niobium, zirconium, strontium, scandium, and any combination thereof.
- B 4 C powder of exceptional quality.
- High quality B 4 C powders have a good granulometric distribution and have a minimum of fine powder-like particles. Generally, only such B 4 C powder may be incorporated in high amounts into the metal matrix.
- B 4 C powder is not of such good quality, significant losses of fluidity during the holding time of the molten metal, i.e. prior to casting, can be observed.
- increasing the holding temperature of the molten metal does not compensate for this loss of fluidity because this can favor the reaction between the aluminum and the B 4 C powder thereby further increasing the viscosity (loss of fluidity). In such circumstances, the molten metal behaves as a thixotropic material.
- Figure 1 shows the loss of fluidity during the holding time of a molten composite, prior to casting.
- a certain fluidity is required for a certain amount of time for allowing the shaping/casting of the Al-B 4 C mixture.
- the curves shown in Figure 1 show that the B 4 C powder used in the preparation of the composite material increased the viscosity of the material and failed to meet the fluidity required in industrial settings for subsequent steps (shaping for example), even in the presence of titanium.
- a cast Al-B 4 C composite material comprising aluminum, products of a peritectic reaction and dispersed boron carbide particles.
- the composite material is obtained by first combining aluminum (or an aluminum alloy) with an additive (or a combination of additives) capable of undergoing a peritectic reaction with the boron of the boron carbide particles and ultimately provide products of such peritectic reaction in the aluminum or the aluminum alloy. Once the additive has been included in the aluminum (or in the aluminum alloy), boron carbide particles are combined with the aluminum (or the aluminum alloy), thereby causing the peritectic reaction between the additive and the boron.
- the use of the additive in the aluminum/aluminum alloy (and, ultimately the presence of peritectic reaction products in the composite material) has been shown useful for maintaining the fluidity of the molten composite and as such imparts good castability to a molten composite material.
- the use of the additive inhibits or slows down the formation of reaction products occurring during holding of the molten composite (such as, for example, the reaction products occurring between Al and B 4 C).
- the additives can be used to limit the use of titanium in such composite materials without altering substantially their fluidity.
- This maintenance in fluidity of the molten composite material can allow for the lengthening of the holding time of the molten mixture in the furnace, for using a lower grade of B 4 C source as well as for facilitating shaping and/or casting of the resulting metal matrix composite(s).
- the composite material Prior to being casted, the composite material is in a molten state and has a fluidity.
- the molten composite material has, prior to casting, a fluidity which permits casting in an industrial setting.
- Such mold currently used and known in the art, measures the length of a sample of the composite material before it solidifies. The length measured with a K-mold is referred to as a cast length.
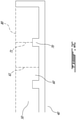
- a K-mold that can be used to determine the fluidity of a sample of a molten composite material is shown in Figure 2 .
- a K-mold is usually composed of two engageable portions, a lower inclined portion 10 (as shown in Figure 2A ) and a groove-containing portion 40 (as shown in Figures 2B and 2C ).
- the inclined portion 10 is engaged with the groove-containing portion 40.
- the sample is allowed to cast along the inclined portion 10 and within the groove 50 until it sets.
- the length covered by the sample is a measure of fluidity and refers to a cast length.
- the lower inclined portion is usually monolithic and comprises a plane 15 having a smooth surface and being downwardly inclined from an horizontal axis 20 by an angle 30 of about 10°.
- the plane 15 is for contacting directly the external sides 55 of the groove-containing portion 40 (shown on Figures 2B and 2C ) and for providing an angle to the groove of about 10°.
- the groove-containing portion 40 is a partially hollowed structure defining an enclosable groove 50 for containing the sample of the molten composite ( Figure 2B ). As shown on Figure 2B , the groove-containing portion has external sides 55 for contacting directly the plane 15 of the inclined portion 10. In the embodiment shown in Figure 2B , the groove 50 contains two different sections: sections 60 and sections 70 (defining a protuberance). In some embodiments, the K-mold comprises at least four sections 70 (e.g., four protuberances) located at a distance of 93 mm, 130 mm, 168 mm and 205 mm from the start of the mold (e.g., the position at which the sample starts contacting the inclined plane 15).
- Figure 2C shows an enlarged of the enclosable groove 50.
- the sections 60 have a similar height 61 of about 6.5 mm.
- the height 61 is constant between the length defined by the external walls 55.
- the height 61 is measured with respect to the axis 80 defined by the inclined plane 15 (when the groove-containing portion 40 is engaged with the inclined portion 10).
- the sections 70 also have a similar height 71 of about 4 mm.
- the height 71 is constant between the length defined by the external walls 55.
- the height is measured with respect to the axis 80 defined by the inclined plane 15 (when the groove-containing portion 40 is engaged on the inclined portion 10).
- the cast composite material has a fluidity, preferably prior to casting, corresponding to a cast sample length of at least 100 mm, at least 120 mm, at least 140 mm, at least 160 mm, at least 180 mm, at least 190 mm or at least 200 mm.
- the sample used for determining the fluidity of the composite material can be heated at a temperature of about 700°C and for about 120 min to reproduce the industrial casting settings.
- the present disclosure also provides a method of manufacturing a cast composite material.
- a molten aluminum alloy (also referred to as an aluminum-base matrix alloy) comprising the additive (or a combination of additives) capable of undergoing the peritectic reaction is combined with a source of boron carbide to provide a molten composite.
- the fluidity of the molten composite can be maintained at acceptable industrial levels for a longer period of time when compared to a similar molten composite which lacks the additive.
- the aluminum or the aluminum alloy used is provided in a molten form.
- the aluminum or the aluminum alloy is preferably heated to its melting temperature prior to its combination with the B 4 C particles.
- the aluminum alloy comprises (in embodiments consists essentially of and, in further embodiments, consists of) an additive capable of undergoing the peritectic reaction, the remainder being essentially aluminum or an aluminum alloy.
- Unavoidable or inevitable impurities can also be present in the alloy (for a total of impurities of at most 0.15% w/w).
- Exemplary aluminum alloys include, but are not limited to, alloys from the 11xx series and from the 6xxx series.
- Ti can be included in the aluminum or the aluminum alloy. In an alternative embodiment, if Ti is present in the aluminum or the molten aluminum alloy, it is considered to be a trace element (e.g. its concentration does not exceed the concentration of inevitable impurities).
- the composite material comprises between 4% and 40% (v/v) of B 4 C particles and the molarity of the additive (or the combination of additives) in the composite material is between 0.01044 and 0.08351.
- the combined molarity of the additive (or the combination of additives) and Ti is between 0.01044 and 0.08351.
- the concentration of the additive in the composite material can be between 0.47% to 15.32%, 0.47% to 8.00%, 0.90% to 8.00%, 0.95% to 8.00%, 1.00% to 8.00% or 1.10% to 8.00% with respect to the total weight of the composite material (comprising the B 4 C particles).
- the combined concentration of the additive and Ti in the composite can be between 0.47% to 15.32%, 0.47% to 8.00%, 0.90% to 8.00%, 0.95% to 8.00%, 1.00% to 8.00% or 1.10% to 8.00% with respect to the total weight of the composite material (comprising the B 4 C particles).
- the composite material comprises between 4.5% and 18.9% (v/v) of B 4 C particles and the molarity of the additive (or the combination of additives) in the composite material is between 0.00835 and 0.04175.
- the combined molarity of the additive (or the combination of additives) and Ti is between 0.00835 and 0.04175.
- the concentration of the additive in the composite material can be between 0.38% to 7.68%, 0.38% to 4.00%, 0.90% to 4.00%, 0.95% to 4.00%, 1.00% to 4.00% or 1.10% to 4.00% with respect to the total weight of the composite material (comprising the B 4 C particles).
- the combined concentration of the additive and Ti in the composite can be between 0.38% to 7.68%, 0.38% to 4.00%, 0.90% to 4.00%, 0.90% to 4.00%, 1.00% to 4.00% or 1.10% to 4.00% with respect to the total weight of the composite material (comprising the B 4 C particles).
- the composite material comprises between 19% and 28% (v/v) of B 4 C particles and the molarity of the additive (or the combination of additives) in the composite material is between 0.03758 and 0.06263.
- the combined molarity of the additive (or the combination of additives) and Ti is between 0.03758 and 0.06263.
- the concentration of the additive in the composite material can be between 1.69% to 11.51% or 1.69% to 6.00% with respect to the total weight of the composite material (comprising the B 4 C particles).
- the combined concentration of the additive and Ti in the composite can be between 1.69% to 11.51% or 1.69% to 6.00% with respect to the total weight of the composite material (comprising the B 4 C particles).
- the composite material comprises between 25% and 28% (v/v) or between 28% and 33% (v/v) of B 4 C particles and the molarity of the additive (or the combination of additives) in the composite material is between 0.02088 and 0.04175.
- the combined molarity of the additive (or the combination of additives) and Ti is between 0.02088 and 0.04175.
- the concentration of the additive in the composite material can be between 0.94% to 7.68%, 0.94% to 4.00%, 0.95% to 4.00%, 1.00% to 4.00% or 1.10% to 4.00% with respect to the total weight of the composite material (comprising the B 4 C particles).
- the combined concentration of the additive and Ti in the composite can be between 0.94% to 7.68%, 0.94% to 4.00%, 0.95% to 4.00%, 1.00% to 4.00% or 1.10% to 4.00% with respect to the total weight of the composite material (comprising the B 4 C particles).
- the concentration of the additive is to be understood to include all forms of the additives (including soluble additive, excess additive that comes out of solution as intermetallics or refractory compounds, as well additive included in a B-containing peritectic reaction product).
- the additive capable of causing the formation of a peritectic reaction's product can be added in any convenient form, including master alloy (for example an AI-10% additive master alloy) or as additive-containing granules or powders.
- the additive as a form of a powder to wrought alloys (including AA1xxx, AA2xxx, AA3xxx, AA4xxx or AA6xxx) or casting alloys (including AA2xx or AA3xx).
- the titanium concentration or molarity given in the foregoing description represent titanium in all forms (including soluble Ti, excess Ti coming out of solution as intermetallics or refractory compounds, as well Ti-B compounds).
- the titanium can be added in any convenient form, including master alloy (for example an AI-10% Ti master alloy) or as titanium containing granules or powders.
- a titanium as an aluminum alloy such as, for example, wrought alloys (including AA2xxx, AA3xxx, AA4xxx or AA6xxx), or casting alloys (including AA2xx or AA3xx).
- the additive capable of causing the formation of a peritectic reaction's product can be zirconium and the aluminum alloy can comprises or contains zirconium.
- the composite material does not comprise Ti (if present, Ti is considered to be a trace element).
- Ti can be present in the composite material.
- zirconium can be provided at a concentration of between about 0.95 to about 7.61, between about 1.00 to about 7.61 or between about 1.10 to about 7.61 weight percentage with respect to the composite material's (comprising the B 4 C particles) total weight.
- Ti when present, it can be provided at a concentration of between about 0.50 to about 4.00, between about 0.90 to about 4.00, between about 0.95 to about 4.00, between about 1.00 to about 4.00 or between about 1.10 to about 4.00 weight percentage with respect to the composite material's (comprising the B 4 C particles) total weight.
- zirconium in a composite material comprising between 4.5% and 18.9% (v/v) of B 4 C particles, zirconium can be provided at a concentration of between about 0.76 to about 3.81, between about 0.90 to about 3.81, between about 0.95 to about 3.81, between about 1.00 to about 3.81 or between about 1.10 to about 3.81 weight percentage with respect to the composite material's (comprising the B 4 C particles) total weight.
- Ti when it is present, it can be provided at a concentration of between about 0.40 to about 2.00, between about 0.90 to about 2.00, between about 0.95 to about 2.00, between about 1.00 to about 2.00 or between about 1.10 to about 2.00 weight percentage with respect to the composite material's (comprising the B 4 C particles) total weigh.
- zirconium in a composite material comprising between 19% and 28% (v/v) of B 4 C particles, zirconium can be provided at a concentration of between about 3.43 to about 5.71 weight percentage with respect to the composite material's (comprising the B 4 C particles) total weight.
- Ti when it is present, it can be provided at a concentration of between about 1.80 to about 3.00 weight percentage with respect of the composite material's (comprising the B 4 C particles) total weight.
- zirconium in a composite material comprising between 25% and 28% (v/v) or 28% and 33% of B 4 C particles, zirconium can be provided at a concentration of between about 1.90 to about 3.81 weight percentage with respect to the composite material's (comprising the B 4 C particles) total weight.
- Ti when Ti is present, it can be provided at whereas titanium can be provided at a concentration between about 1.00 to about 2.00 or between about 1.10 to about 2.00 weight percentage with respect of the composite material's (comprising the B 4 C particles) total weight.
- zirconium concentrations given in the foregoing description whether with reference to the aluminum alloy or the total composite material, represent zirconium in all forms (including soluble Zr, excess Zr coming out of solution as intermetallics or refractory compounds, as well Zr-B compounds).
- the zirconium can be added in any convenient form, including master alloy (for example an AI-10% Zr master alloy) or as zirconium containing granules or powders.
- an AA1xxx alloy containing zirconium in the aluminum alloy it may be advisable to use an AA1xxx alloy containing zirconium in the aluminum alloy.
- a zirconium as an aluminum alloy such as, for example, wrought alloys (including AA2xxx, AA3xxx, AA4xxx or AA6xxx), or casting alloys (including AA2xx or AA3xx).
- the additive capable of causing the formation of a peritectic reaction's product can be strontium and, in some aspects not covered by the claims, the aluminum alloy can comprises or contains strontium in combination with titanium.
- strontium can be provided at a concentration of between about 0.91 to about 7.32, between about 0.95 to about 7.32, between about 1.00 to about 7.32 or between about 1.10 to about 7.32 weight percentage with respect to the composite material's (comprising the B 4 C particles) total weight
- titanium can be provided at a concentration of between about 0.50 to about 4.00, between about 0.90 to about 4.00, between about 0.95 to about 4.00, between about 1.00 to about 4.00 or between about 1.10 to about 4.00 weight percentage with respect to the composite material's (comprising the B 4 C particles) total weight.
- strontium can be provided at a concentration of between about 0.73 to about 3.66, between about 0.90 to about 3.66, between about 0.95 to about 3.66, between about 1.00 to about 3.66 or between about 1.10 to about 3.66 weight percentage with respect to the composite material's (comprising the B 4 C particles) total weight
- titanium can be provided at a concentration of between about 0.40 to about 2.00, between about 0.90 to about 2.00, between about 0.95 to about 2.00, between about 1.00 to about 2.00 or between about 1.10 to about 2.00 weight percentage with respect to the composite material's (comprising the B 4 C particles) total weight.
- strontium can be provided at a concentration of between about 3.29 to about 5.49 weight percentage with respect to the composite material (comprising the B 4 C particles) total weight, whereas in some aspects not covered by the claims, titanium can be provided at a concentration between about 1.80 to about 3.00 weight percentage with respect of the composite material's (comprising the B 4 C particles) total weight.
- strontium can be provided at a concentration of between about 1.83 to about 3.66 weight percentage with respect to the composite material (comprising the B 4 C particles) total weight
- titanium can be provided at a concentration between about 1.00 to about 2.00 or between about 1.10 to about 2.00 weight percentage with respect of the composite material's (comprising the B 4 C particles) total weight.
- strontium concentrations given in the foregoing description represent strontium in all forms (including soluble Sr, excess Sr coming out of solution as intermetallics or refractory compounds, as well Sr-B compounds).
- the strontium can be added in any convenient form, including master alloy (for example an Al-10% Sr master alloy) or as strontium containing granules or powders. In some embodiments, it may be advisable to use an AA1xxx alloy containing strontium in the aluminum alloy.
- a strontium as an aluminum alloy such as, for example, wrought alloys (including AA2xxx, AA3xxx, AA4xxx or AA6xxx), or casting alloys (including AA2xx or AA3xx).
- the additive capable of causing the formation of a peritectic reaction's product can be scandium and the aluminum alloy can comprises or contains scandium in combination with titanium.
- scandium can be provided at a concentration of between about 0.47 to about 3.75, between about 0.90 to about 3.75, between about 1.00 to about 3.75 or between about 1.10 to about 3.75 weight percentage with respect to the composite material's (comprising the B 4 C particles) total weight
- titanium can be provided at a concentration between about 0.50 to about 4.00, between about 0.90 to about 4.00, between about 0.95 to about 4.00, between about 1.00 to about 4.00 or between about 1.10 to about 4.00 with respect to the composite material's (comprising the B 4 C particles) total weight.
- scandium can be provided at a concentration of between about 0.38 to about 1.88, between about 0.90 to about 1.88, between about 1.00 to about 1.88 or between about 1.10 to about 1.88 weight percentage with respect to the composite material's (comprising the B 4 C particles) total weight
- titanium can be provided at a concentration of between about 0.40 to about 2.00, between about 0.90 to about 2.00, between about 0.95 to about 2.00, between about 1.00 to about 2.00 or between about 1.10 to about 2.00 weight percentage with respect to the composite material's (comprising the B 4 C particles) total weight.
- scandium can be provided at a concentration of between about 1.69 to about 2.82 weight percentage with respect to the composite material (comprising the B 4 C particles) total weight
- titanium can be provided at a concentration between about 1.80 to about 3.00 weight percentage with respect of the composite material's (comprising the B 4 C particles) total weight.
- scandium can be provided at a concentration of between about 0.94 to about 1.88, between about 1.00 to about 1.88 or between about 1.10 to about 3.88 weight percentage with respect to the composite material (comprising the B 4 C particles) total weight
- titanium can be provided at a concentration between about 1.00 to about 2.00 or between about 1.10 to about 2.00 weight percentage with respect of the composite material's (comprising the B 4 C particles) total weight.
- the scandium concentrations given in the foregoing description represent scandium in all forms (including soluble Sc, excess Sc coming out of solution as intermetallics or refractory compounds, as well Sc-B compounds).
- the scandium can be added in any convenient form, including master alloy (for example an AI-10% Sc master alloy) or as scandium containing granules or powders. In some embodiments, it may be advisable to use an AA1xxx alloy containing scandium in the aluminum alloy.
- a scandium as an aluminum alloy such as, for example, wrought alloys (including AA2xxx, AA3xxx, AA4xxx or AA6xxx), or casting alloys (including AA2xx or AA3xx).
- the additive can be used in the methods described herein to form reaction products with B having an enthalpy of formation which is more negative than the one associated to AlB 2 .
- the reaction product could be a compound such as (Ti,V)B 2 .
- the formation of such reaction products would stop or reduce the reaction between the aluminum melt containing titanium and the B 4 C particles.
- the present disclosure thus provides for the addition of additive capable of causing the formation of a peritectic reaction's products as inhibitors of the reaction between the aluminum melt and the B 4 C particles to maintain the fluidity of the composite until the composite is shaped, preferably cast.
- the additive includes, but is not limited to chromium (Cr), molybdenum (Mo), vanadium (V), niobium (Nb), hafnium (Hf), zirconium (Zr), strontium (Sr), scandium (Sc), tantalum (Ta), tungsten (W) as well as any combination thereof.
- the additive includes, but is not limited to (Mo), vanadium (V), niobium (Nb), hafnium (Hf), zirconium (Zr), strontium (Sr), scandium (Sc) as well as any combination thereof.
- the additive includes, but is not limited to zirconium (Zr), strontium (Sr), scandium (Sc) as well as any combination thereof.
- the additive comprises or consists of Cr.
- the additive comprises or consists of Mo.
- the additive comprises or consists of V.
- the additive comprises or consists of Nb.
- the additive comprises or consists of Ta.
- the additive comprises or consists of W.
- the additive comprises or consists of Hf.
- the additive comprises or consists of Zr.
- the additive comprises or consists of Sr.
- the additive comprises or consists of Sc. In one embodiment, the additive comprises or consists of a combination of Zr and Sc. In another embodiment, the additive comprises or consists of a combination of Zr and Sr. In yet another embodiment, the additive comprises or consists of a combination of Sr and Sc. In yet another embodiment, the additive comprises or consists of a combination of Zr, Sr and Sc.
- the additive(s), and in some aspects not covered by the claims optionally titanium is (are) added to molten aluminum or to a molten aluminum or a molten aluminum alloy.
- an aluminum alloy containing titanium can be used.
- the titanium is first added to the molten aluminum/alloy and then the additive(s) is (are) added.
- the additive(s) is (are) first added to the molten aluminum/alloy and then the titanium is added.
- the titanium and the additive(s) are added simultaneously to the molten aluminum/alloy.
- the molten aluminum alloy is combined with a source of boron carbide (a boron carbide powder (such as a free-flowing powder) for example) to provide a molten composite material comprising dispersed boron carbide particles.
- a source of boron carbide a boron carbide powder (such as a free-flowing powder) for example
- the aluminum alloy supplied with the additive and optionally titanium
- the boron carbide particles are in a solid form and are at least partially in association with the products of the peritectic reaction.
- the mixing/stirring be carried out in a manner that allows the appropriate wetting of the B 4 C particles in the composite material.
- the molten composite material due to the presence of the products of the peritectic reaction products, has a fluidity amenable for casting in an industrial setting.
- Fluidity can be determined by various ways as known by those skilled in the art. In one example, fluidity is measured with a viscometer. In another example, fluidity is assessed by measuring the length of a cast sample in a mold. In order to do so, it is possible to add a quantity of B 4 C powder inside a reactor (capacity of about 35 kg for example) that contains liquid aluminum-based mixture at a specific temperature (about 700°C for example) to which a vacuum is applied.
- Samples of the mixture of molten metal and B 4 C powder can be obtained at a fixed interval (for example, every 20 minutes) using a step-mold and having a predetermined length.
- a K-mold step-mold can be used. Fluidity is quantified as the distance achieved/covered by the resulting mixture before its solidification.
- the K-mold can be a graphite-coated stainless steel step-mould having a sample-receiving chamber or groove having a width of 33 mm (and, in some embodiments, a maximal length of 315 mm) and being inclined at an angle of about 10°.
- a molten composite material achieving a distance of 100 mm after 120 minutes of holding time in the K-mold described above is considered as having a fair fluidity for direct chilled casting.
- a molten composite material achieving a distance of 190 mm after 120 minutes of holding time in the K-mold described above is considered as having an excellent fluidity for direct chilled casting.
- the fluidity of the composite material is 190 mm or more after 120 min.
- the fluidity of the composite material is of 200 mm or more after 120 minutes.
- the fluidity of the molten composite is at least 100 mm when measured at a temperature of about 700°C after a holding time of 120 min.
- the fluidity of the composite material is at least 105 mm, 110 mm, 115 mm, 120 mm, 125 mm, 130 mm, 135 mm, 140 mm, 145 mm, 150 mm, 155 mm, 160 mm, 165 mm, 170 mm, 175 mm, 180 mm, 181 mm, 182 mm, 183 mm, 184 mm, 185 mm, 186 mm, 187 mm, 188 mm, 189 mm, 190 mm, 191 mm, 192 mm, 193 mm, 194 mm, 195 mm, 196 mm, 197 mm, 198 mm, 199 mm or 200 mm when measured at a temperature of about 700°C after a holding time of 120 min.
- the fluidity of the molten composite can vary upon holding time and holding temperature.
- the fluidity of the composite material is not measured upon the mixture of the aluminum alloy and the boron carbide particles, but after the peritectic reaction products have been formed or even molten composite material is held at a specific temperature (e.g., holding temperature) and for a specific amount of time (e.g., holding time).
- the composite material is held at a specific temperature for allowing the material to remain in a molten state.
- the composite material is held at a minimal holding temperature of equal to or higher than about 660°C, 670°C, 680°C, 690°C, 700°C, 710°C, 720°C, 730°C, 740°C, 750°C, 760°C, 770°C, 780°C, 790°C or 800°C.
- the composite material is held at a maximal holding temperature equal to or lower than about 800°C, 790°C, 780°C, 770°C, 760°C, 750°C, 740°C, 730°C, 720°C, 710°C, 700°C, 690°C, 680°C, 670°C or 660°C.
- the composite material is held at a temperature ranging between the minimal holding temperature as defined above and the maximal holding temperature as defined above.
- the composite material is held for a minimal holding time of equal to or higher than about 20 min, 30 min, 40, min, 50 min, 60 min, 70 min, 80 min, 90 min, 100 min ,110 min, 120 min, 130 min, 140 min, 150 min, 160 min, 170 min, 180 min, 190 min or 200 min.
- the composite material is held for a maximal holding time equal to or lower than about 200 min, 190 min, 180 min, 170 min, 160 min, 150 min, 140 min, 130 min, 120 min, 110 min, 100 min, 90 min, 80 min, 70 min, 60 min, 50 min, 40 min 30 min or 20 min.
- the specific holding time ranges between the minimal holding time as defined above and the maximal holding time as defined above.
- the composite material is casted for a minimal casting time of equal to or higher than about 20 min, 30 min, 40, min, 50 min, 60 min, 70 min, 80 min, 90 min, 100 min ,110 min, 120 min, 130 min, 140 min, 150 min, 160 min, 170 min, 180 min, 190 min or 200 min.
- the composite material is casted for a maximal casting time equal to or lower than about 200 min, 190 min, 180 min, 170 min, 160 min, 150 min, 140 min, 130 min, 120 min, 110 min, 100 min, 90 min, 80 min, 70 min, 60 min, 50 min, 40 min 30 min or 20 min.
- the specific casting time ranges between the minimal casting time as defined above and the maximal casting time as defined above.
- the molten composite is amenable any form of casting (including DC casting of billets or slabs), casting of ingots for future remelting and casting as well as casting into shapes using any convenient form of shape casting.
- the cast composite can be further processed and is well adapted for further operations such as (a) remelting and casting a shape, (b) extrusion and (c) rolling or (d) forging.
- the method described herein may be used for the preparation of any shaped aluminum boron carbide composite material, particularly those containing high levels of B 4 C.
- a lower-grade B 4 C powder can be used without altering significantly the fluidity of the molten Al-Ti-B 4 C composite.
- a primary aluminum metal alloy (AA1100) was melted in a reactor at a temperature of 765°C. Ti was added and then Sr was added. Thereafter, B 4 C particles were injected into the melt. The final concentrations of Ti and Sr were both 1.65 wt%. The final concentration of the B 4 C particles was 28 vol %.
- a primary aluminum metal alloy (AA1100) was melted in a reactor at a temperature of 765°C. Zr was added. Thereafter, B 4 C particles were injected into the melt. The final concentration of Zr was 3.8 wt%. The final concentration of the B 4 C particles was 19 vol %.
- a primary aluminum metal alloy (AA1100) was melted in a reactor at a temperature of 765°C. Zr was added. Thereafter, B 4 C particles were injected into the melt. The final concentration of Zr was 3.8 wt%. The final concentration of the B 4 C particles was 19 vol %.
Landscapes
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Chemical & Material Sciences (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Manufacture Of Alloys Or Alloy Compounds (AREA)
- Powder Metallurgy (AREA)
- Mold Materials And Core Materials (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261727949P | 2012-11-19 | 2012-11-19 | |
| PCT/CA2013/050881 WO2014075194A1 (en) | 2012-11-19 | 2013-11-19 | Additives for improving the castability of aluminum-boron carbide composite material |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP2919932A1 EP2919932A1 (en) | 2015-09-23 |
| EP2919932A4 EP2919932A4 (en) | 2016-06-15 |
| EP2919932B1 true EP2919932B1 (en) | 2022-03-23 |
Family
ID=50730443
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP13854766.6A Active EP2919932B1 (en) | 2012-11-19 | 2013-11-19 | Method of preparing a cast aluminium composite material |
Country Status (8)
| Country | Link |
|---|---|
| US (2) | US20150275338A1 (enExample) |
| EP (1) | EP2919932B1 (enExample) |
| JP (1) | JP6245267B2 (enExample) |
| KR (1) | KR102014330B1 (enExample) |
| CN (2) | CN104755194A (enExample) |
| AU (1) | AU2013344742C1 (enExample) |
| CA (1) | CA2890771A1 (enExample) |
| WO (1) | WO2014075194A1 (enExample) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2983948C (en) | 2015-05-01 | 2023-02-21 | Universite Du Quebec A Chicoutimi | Composite material having improved mechanical properties at elevated temperatures |
| KR102865965B1 (ko) * | 2024-09-05 | 2025-09-29 | 주식회사 코나솔 | 중성자흡수체용 알루미늄-탄화붕소 복합재료의 제조 방법 |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3178807A (en) * | 1961-10-05 | 1965-04-20 | Du Pont | Cermet of aluminum with boron carbide or silicon carbide |
| IL86947A (en) * | 1987-07-15 | 1992-08-18 | Lanxide Technology Co Ltd | Process for preparing self-supporting bodies and products made thereby |
| US5700962A (en) * | 1996-07-01 | 1997-12-23 | Alyn Corporation | Metal matrix compositions for neutron shielding applications |
| JP2002178130A (ja) * | 2000-09-12 | 2002-06-25 | Jason Sin Hin Lo | ハイブリッド金属マトリクス組成物及びその製造方法 |
| US6918970B2 (en) * | 2002-04-10 | 2005-07-19 | The United States Of America As Represented By The Administrator Of The National Aeronautics And Space Administration | High strength aluminum alloy for high temperature applications |
| DE60323525D1 (de) * | 2002-10-25 | 2008-10-23 | Alcan Int Ltd | Verbesserter aluminiumlegierung-borkarbid-verbundwerkstoff |
| ES2347450T3 (es) * | 2004-04-22 | 2010-10-29 | Alcan International Limited | Metodo mejorado para reciclar un material compuesto al-b, c. |
| DE102005046766B3 (de) * | 2005-09-29 | 2007-04-05 | Airbus Deutschland Gmbh | Leitungshalterung in einem Flugzeug |
| CN200948493Y (zh) * | 2006-09-04 | 2007-09-19 | 包头铝业股份有限公司 | 铝板锭铸造设备 |
| US8017072B2 (en) * | 2008-04-18 | 2011-09-13 | United Technologies Corporation | Dispersion strengthened L12 aluminum alloys |
| US8758529B2 (en) * | 2010-06-30 | 2014-06-24 | GM Global Technology Operations LLC | Cast aluminum alloys |
-
2013
- 2013-11-19 AU AU2013344742A patent/AU2013344742C1/en active Active
- 2013-11-19 CN CN201380055557.9A patent/CN104755194A/zh active Pending
- 2013-11-19 US US14/443,964 patent/US20150275338A1/en not_active Abandoned
- 2013-11-19 CN CN202010441074.4A patent/CN111575522A/zh active Pending
- 2013-11-19 CA CA2890771A patent/CA2890771A1/en not_active Abandoned
- 2013-11-19 KR KR1020157010756A patent/KR102014330B1/ko active Active
- 2013-11-19 EP EP13854766.6A patent/EP2919932B1/en active Active
- 2013-11-19 WO PCT/CA2013/050881 patent/WO2014075194A1/en not_active Ceased
- 2013-11-19 JP JP2015542126A patent/JP6245267B2/ja active Active
-
2019
- 2019-06-28 US US16/456,793 patent/US20200002792A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| KR102014330B1 (ko) | 2019-08-26 |
| AU2013344742B2 (en) | 2017-03-30 |
| WO2014075194A1 (en) | 2014-05-22 |
| EP2919932A4 (en) | 2016-06-15 |
| AU2013344742C1 (en) | 2017-10-19 |
| CN111575522A (zh) | 2020-08-25 |
| US20200002792A1 (en) | 2020-01-02 |
| CA2890771A1 (en) | 2014-05-22 |
| AU2013344742A1 (en) | 2015-05-14 |
| JP6245267B2 (ja) | 2017-12-13 |
| US20150275338A1 (en) | 2015-10-01 |
| CN104755194A (zh) | 2015-07-01 |
| AU2013344742A8 (en) | 2015-05-21 |
| JP2016502604A (ja) | 2016-01-28 |
| EP2919932A1 (en) | 2015-09-23 |
| KR20150101443A (ko) | 2015-09-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2556247C2 (ru) | Алюминий-медный сплав для литья | |
| EP2885437B1 (en) | Al-nb-b master alloy for grain refining | |
| EP2634278A1 (en) | Magnesium alloy having excellent ignition resistance and mechanical properties, and method for manufacturing same | |
| JP7152977B2 (ja) | アルミニウム合金 | |
| JP6880203B2 (ja) | 付加製造技術用のアルミニウム合金 | |
| RU2673270C2 (ru) | Композиция алюминиевого сплава с улучшенными механическими свойствами при повышенной температуре | |
| CN105821260B (zh) | 一种铝合金用的铝钪锆中间合金及其生产方法 | |
| JP2021507088A5 (enExample) | ||
| KR102567776B1 (ko) | 상승된 온도에서 개선된 기계적 특성을 갖는 복합 재료 | |
| CN102433475A (zh) | 一种高强高硬铝合金及其制备方法 | |
| EP2919932B1 (en) | Method of preparing a cast aluminium composite material | |
| EP3732309B1 (en) | Aluminium alloy | |
| CN102433471A (zh) | 一种高韧性的铝合金及其制备方法 | |
| KR102353612B1 (ko) | 마그네슘 합금, 이를 이용한 마그네슘 합금 판재 및 이의 제조방법 | |
| CN102418008A (zh) | 一种用HfC去除夹杂的高强度铝合金及其制备方法 | |
| CN102433470B (zh) | 一种与TiAlN和CaH3粉末共溶的铝合金及熔炼方法 | |
| CN102505084A (zh) | 添加TaC的高强度铝合金及其制备方法 | |
| CN102560199A (zh) | 采用 TiSiN和EuH2粉末处理的铝合金及其制备方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20150603 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAX | Request for extension of the european patent (deleted) | ||
| RA4 | Supplementary search report drawn up and despatched (corrected) |
Effective date: 20160512 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: B22D 21/00 20060101ALI20160506BHEP Ipc: B22C 9/22 20060101ALI20160506BHEP Ipc: C22C 1/10 20060101ALI20160506BHEP Ipc: B22D 25/06 20060101ALI20160506BHEP Ipc: G01N 33/20 20060101ALI20160506BHEP Ipc: B22D 19/14 20060101AFI20160506BHEP Ipc: B22D 2/00 20060101ALI20160506BHEP Ipc: C22C 32/00 20060101ALI20160506BHEP |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20180430 |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: ANDRADE, NEIVI Inventor name: LANGLAIS, JOSEPH Inventor name: LAURIN, JEAN-ALAIN |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R079 Ref document number: 602013081212 Country of ref document: DE Free format text: PREVIOUS MAIN CLASS: B22D0019140000 Ipc: B22D0025060000 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: B22D 25/06 20060101AFI20210720BHEP Ipc: B22D 21/00 20060101ALI20210720BHEP Ipc: B22C 9/22 20060101ALI20210720BHEP Ipc: B22D 2/00 20060101ALI20210720BHEP Ipc: B22D 19/14 20060101ALI20210720BHEP Ipc: C22C 1/10 20060101ALI20210720BHEP Ipc: C22C 32/00 20060101ALI20210720BHEP |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| GRAJ | Information related to disapproval of communication of intention to grant by the applicant or resumption of examination proceedings by the epo deleted |
Free format text: ORIGINAL CODE: EPIDOSDIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| INTG | Intention to grant announced |
Effective date: 20210914 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTC | Intention to grant announced (deleted) | ||
| INTG | Intention to grant announced |
Effective date: 20211109 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602013081212 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1477096 Country of ref document: AT Kind code of ref document: T Effective date: 20220415 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG9D |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20220323 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220623 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220623 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220624 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220725 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220723 Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602013081212 Country of ref document: DE |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 |
|
| 26N | No opposition filed |
Effective date: 20230102 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230526 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20221119 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20221130 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20221130 Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20221130 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: UEP Ref document number: 1477096 Country of ref document: AT Kind code of ref document: T Effective date: 20220323 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20221119 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20221119 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20221119 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20221130 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20131119 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20220323 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20241015 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20241021 Year of fee payment: 12 Ref country code: AT Payment date: 20241025 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CZ Payment date: 20241030 Year of fee payment: 12 |