EP2912151B1 - Use of viscosity modifier in high viscosity index lubricating oil base stock combinations - Google Patents
Use of viscosity modifier in high viscosity index lubricating oil base stock combinations Download PDFInfo
- Publication number
- EP2912151B1 EP2912151B1 EP13773574.2A EP13773574A EP2912151B1 EP 2912151 B1 EP2912151 B1 EP 2912151B1 EP 13773574 A EP13773574 A EP 13773574A EP 2912151 B1 EP2912151 B1 EP 2912151B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- viscosity
- group
- copolymer
- acid
- oil
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- UAEPNZWRGJTJPN-UHFFFAOYSA-N CC1CCCCC1 Chemical compound CC1CCCCC1 UAEPNZWRGJTJPN-UHFFFAOYSA-N 0.000 description 1
- ALHGTRBFOGTTAW-UHFFFAOYSA-O [IH]=CCC1[NH2+]C1 Chemical compound [IH]=CCC1[NH2+]C1 ALHGTRBFOGTTAW-UHFFFAOYSA-O 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M149/00—Lubricating compositions characterised by the additive being a macromolecular compound containing nitrogen
- C10M149/02—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- C10M149/06—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds containing monomers having an unsaturated radical bound to an amido or imido group
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M145/00—Lubricating compositions characterised by the additive being a macromolecular compound containing oxygen
- C10M145/02—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- C10M145/10—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds containing monomers having an unsaturated radical bound to a carboxyl radical, e.g. acrylate
- C10M145/12—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds containing monomers having an unsaturated radical bound to a carboxyl radical, e.g. acrylate monocarboxylic
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M145/00—Lubricating compositions characterised by the additive being a macromolecular compound containing oxygen
- C10M145/02—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- C10M145/10—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds containing monomers having an unsaturated radical bound to a carboxyl radical, e.g. acrylate
- C10M145/16—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds containing monomers having an unsaturated radical bound to a carboxyl radical, e.g. acrylate polycarboxylic
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M149/00—Lubricating compositions characterised by the additive being a macromolecular compound containing nitrogen
- C10M149/02—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- C10M149/04—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds containing monomers having an unsaturated radical bound to an amino group
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M169/00—Lubricating compositions characterised by containing as components a mixture of at least two types of ingredient selected from base-materials, thickeners or additives, covered by the preceding groups, each of these compounds being essential
- C10M169/04—Mixtures of base-materials and additives
- C10M169/041—Mixtures of base-materials and additives the additives being macromolecular compounds only
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2205/00—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions
- C10M2205/02—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions containing acyclic monomers
- C10M2205/028—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions containing acyclic monomers containing aliphatic monomers having more than four carbon atoms
- C10M2205/0285—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions containing acyclic monomers containing aliphatic monomers having more than four carbon atoms used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2209/00—Organic macromolecular compounds containing oxygen as ingredients in lubricant compositions
- C10M2209/02—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- C10M2209/08—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds containing monomers having an unsaturated radical bound to a carboxyl radical, e.g. acrylate type
- C10M2209/084—Acrylate; Methacrylate
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2217/00—Organic macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2217/06—Macromolecular compounds obtained by functionalisation op polymers with a nitrogen containing compound
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2020/00—Specified physical or chemical properties or characteristics, i.e. function, of component of lubricating compositions
- C10N2020/01—Physico-chemical properties
- C10N2020/019—Shear stability
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2030/00—Specified physical or chemical properties which is improved by the additive characterising the lubricating composition, e.g. multifunctional additives
- C10N2030/02—Pour-point; Viscosity index
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2030/00—Specified physical or chemical properties which is improved by the additive characterising the lubricating composition, e.g. multifunctional additives
- C10N2030/04—Detergent property or dispersant property
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2030/00—Specified physical or chemical properties which is improved by the additive characterising the lubricating composition, e.g. multifunctional additives
- C10N2030/06—Oiliness; Film-strength; Anti-wear; Resistance to extreme pressure
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2030/00—Specified physical or chemical properties which is improved by the additive characterising the lubricating composition, e.g. multifunctional additives
- C10N2030/08—Resistance to extreme temperature
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2030/00—Specified physical or chemical properties which is improved by the additive characterising the lubricating composition, e.g. multifunctional additives
- C10N2030/10—Inhibition of oxidation, e.g. anti-oxidants
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2030/00—Specified physical or chemical properties which is improved by the additive characterising the lubricating composition, e.g. multifunctional additives
- C10N2030/68—Shear stability
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/04—Oil-bath; Gear-boxes; Automatic transmissions; Traction drives
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/04—Oil-bath; Gear-boxes; Automatic transmissions; Traction drives
- C10N2040/044—Oil-bath; Gear-boxes; Automatic transmissions; Traction drives for manual transmissions
Definitions
- This disclosure relates to the use of high viscosity index lubricating oil base stock (metallocene catalyzed polyalphaolefin and polyalphaolefin fluid) and viscosity modifier (e.g., ester-containing copolymer) combinations, and of lubricating oils derived therefrom.
- This disclosure relates to lubricating driveline devices, e.g., gears and transmissions, using the lubricating oils to improve fuel efficiency without sacrificing driveline device durability.
- Lubricants in commercial use today are prepared from a variety of natural and synthetic base stocks admixed with various additive packages and solvents depending upon their intended application.
- the base stocks typically include mineral oils, polyalphaolefins (PAO), gas-to-liquid base oils (GTL), silicone oils, phosphate esters, diesters, polyol esters, and the like.
- WO2007/145924 discloses lubricating oils comprising metallocene catalyzed PAO.
- Viscosity index improvers are known to be added to lubricating oil compositions to reduce the change in viscosity of the lubricant as a function of temperature.
- the most conventional types of viscosity index improvers used in axle and transmission oil applications include polyisobutylene and polymers of methacrylates.
- More recent viscosity index improver technologies consist of olefins (such as copolymers of alpha-olefins and maleic anhydride and esterified derivatives thereof). These viscosity index improvers tend to incorporate ester functional groups in pendant/grafted/branched groups.
- the ester functional groups may be derived from linear alkyl alcohols with 1 to 40 carbon atoms.
- Lubricants capable of performing at lower viscosity typically provide increased fuel economy (thus improving corporate average fuel efficiency (CAFE), NEDC (European Driving Cycle), or FTP-75 (Federal Test Procedure), or Japanese test cycle (JC-08)). Conversely, higher viscosity fluids contribute to elevated gear and transmission operating temperatures, which are believed to reduce fuel economy and diminish durability.
- CAFE corporate average fuel efficiency
- NEDC European Driving Cycle
- FTP-75 Federal Test Procedure
- JC-08 Japanese test cycle
- Driveline power transmitting devices such as axles and transmissions-present highly complex technological challenges for axle and manual transmission lubricants.
- These lubricants are required to ensure hardware durability in the form wear protection and high load-carrying capacity, while delivering enhanced fuel efficiency benefits over extended periods.
- transmissions typically require specific frictional characteristics of fluids that are compatible with synchronizer material or design.
- One of the important parameters influencing performance is lubricant viscosity.
- Lubricants capable of performing at lower viscosity typically provide increased fuel economy. However viscosity that is too low to maintain sufficient and stable oil film between surface asperities results in elevated gear and transmission operating temperatures, which are believed to reduce fuel economy due to higher friction in contact zones. Therefore, increasing lubricant viscosity is conventionally believed to provide better wear protection and durability to gears and transmissions.
- a viscosity modifier comprising a copolymer having units derived from monomers of (i) an ⁇ -olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with an alcohol in a driveline device lubricated with a lubricating composition comprising:
- the above lubricating composition can be produced by a, process comprising:
- a method of lubricating a mechanical device comprises supplying to the device the above lubricating composition.
- the mechanical device comprises a driveline device, e.g., gears or transmissions.
- fuel efficiency can be improved, while maintaining or improving wear control, load carrying capacity and/or traction reduction in a driveline device, e.g., gears or transmissions, lubricated with a lubricating composition.
- a driveline device e.g., gears or transmissions
- higher viscosity fluids can result in lower fuel efficiency due to churning losses.
- the internal friction of the fluid measured by its traction properties is an indicator of its efficiency benefits in high pressure contact areas within axles.
- the method of blending of this disclosure delivers lower traction and lower viscosity fluids.
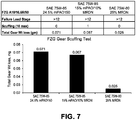
- the lubricating compositions used in this disclosure exhibit improved wear control (as determined by ASTM D4172), load carrying capacity (as determined by ASTM D2783) and/or traction reduction (as determined by Mini-Traction Machine (MTM) Ball-on-Disc apparatus) with said lubricating composition as compared to wear control, load carrying capacity and traction reduction achieved, at an equal or lower kinematic viscosity (Kv@100°C), with a lubricating composition containing a viscosity modifier other than a viscosity modifier comprising a copolymer having units derived from monomers of (i) an ⁇ -olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with an alcohol.
- a polymer is referred to as comprising homopolymers and copolymers, where copolymers include any polymer having two or more chemically distinct monomers.
- polyalphaolefin or “PAO” includes homopolymers and copolymers of C 3 or greater alphaolefin monomers.
- the lubricating compositions used according to this disclosure exhibit improved wear control (as determined by ASTM D4172), improved load carrying capacity (as determined by ASTM D2783) and/or improved traction reduction (as determined by Mini-Traction Machine (MTM) Ball-on-Disc apparatus) as compared to wear control, load carrying capacity and traction reduction achieved, at an equal or lower kinematic viscosity (Kv@100°C), with a lubricating composition containing a viscosity modifier other than a viscosity modifier comprising a copolymer having units derived from monomers of (i) an ⁇ -olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with an alcohol.
- the lubricating compositions used according to this disclosure are also capable of providing at least one of improved oxidative stability, reduced mechanical device operating temperatures, increased mechanical device durability, improved shear stability, improved viscosity index, improved low temperature viscometrics and improved high temperature viscometrics.
- this disclosure relates to the use of a combination of a high viscosity synthetic base stock and an ester-containing viscosity modifier that enables improvement in wear control, load carrying capacity and traction and provides improved efficiency at equal or lower kinematic viscosity (Kv @100°C).
- Current high performance commercial axle fluids are blended with low viscosity synthetic base stocks (such as ⁇ 10 cSt PAO) in combination with conventional viscosity modifiers.
- higher viscosity fluids can result in lower fuel efficiency due to churning losses.
- the internal friction of the fluid measured by its traction properties provides an indicator of its efficiency benefits in high pressure contact areas within axles.
- the method of blending a high viscosity synthetic base stock and an ester-containing viscosity modifier in accordance with this disclosure provides lower traction and lower viscosity fluids.
- This disclosure relates to lubricating driveline devices, e.g., gears and transmissions, using the lubricating oils to improve fuel efficiency without sacrificing driveline device durability.
- Lubricating oils that are useful in the present disclosure are both natural oils and synthetic oils. Natural and synthetic oils (or mixtures thereof) can be used unrefined, refined, or rerefined (the latter is also known as reclaimed or reprocessed oil). Unrefined oils are those obtained directly from a natural or synthetic source and used without added purification. These include shale oil obtained directly from retorting operations, petroleum oil obtained directly from primary distillation, and ester oil obtained directly from an esterification process. Refined oils are similar to the oils discussed for unrefined oils except refined oils are subjected to one or more purification steps to improve the at least one lubricating oil property.
- Groups I, II, III, IV, V and VI are broad categories of base oil stocks developed and defined by the American Petroleum Institute (API Publication 1509; www.API.org) to create guidelines for lubricant base oils.
- Group I base stocks generally have a viscosity index of between 80 to 120 and contain greater than 0.03% sulfur and less than 90% saturates.
- Group II base stocks generally have a viscosity index of between 80 to 120, and contain less than or equal to 0.03% sulfur and greater than or equal to 90% saturates.
- Group III stock generally has a viscosity index greater than 120 and contains less than or equal to 0.03% sulfur and greater than 90% saturates.
- Group IV includes polyalphaolefins (PAO).
- Group V base stocks include base stocks not included in Groups I-IV. The table below summarizes properties of each of these six groups.
- Group VI are polyinternal olefins ("PIO").
- Polyinternal olefins are long-chain hydrocarbons, typically a linear backbone with some branching randomly attached; they are obtained by oligomerization of internal n-olefins.
- the catalyst is usually a BF3 complex with a proton source that leads to a cationic polymerization, or promoted BF3 or AlCl3 catalyst system.
- the process to produce polyinternal olefins (PIO) consists of four steps: reaction, neutralization/washing, hydrogenation and distillation. These steps are somewhat similar to PAO process.
- PIO are typically available in low viscosity grades, 4 cSt, 6 cSt and 8 cSt.
- n-olefins used as starting material are n-C 12 -C 18 internal olefins, more preferably, n-C 14 -C 16 olefins are used.
- PIO can be made with VI and pour points very similar to PAO, only slightly inferior. They can be used in engine and industrial lubricant formulations. For more detailed discussion, see Chapter 2, Polyintemalolefins in the book, "Synthetics, Mineral Oils, and Bio-Based Lubricants-Chemistry and Technology" Edited by Leslie R. Rudnick, p.
- PIO was classified by itself as Group VI fluid in API base stock classification
- Base Stock Properties Saturates Sulfur Viscosity Index Group I ⁇ 90 and/or >0.03% and ⁇ 80 and ⁇ 120
- Group IV Includes polyalphaolefins (PAO) Group V All other base oil stocks not included in Groups I, II, III or IV Group VI Polyinternal olefins (PIO)
- Natural oils include animal oils, vegetable oils (castor oil and lard oil, for example), and mineral oils. Animal and vegetable oils possessing favorable thermal oxidative stability can be used. Of the natural oils, mineral oils are preferred. Mineral oils vary widely as to their crude source, for example, as to whether they are paraffinic, naphthenic, or mixed paraffinic-naphthenic. Oils derived from coal or shale are also useful in the present disclosure. Natural oils vary also as to the method used for their production and purification, for example, their distillation range and whether they are straight run or cracked, hydrorefined, or solvent extracted.
- Group II and/or Group III hydroprocessed or hydrocracked base stocks as well as synthetic oils such as polyalphaolefins, alkyl aromatics and synthetic esters, i.e., Group IV and Group V oils are also well known base stock oils.
- Synthetic oils include hydrocarbon oil such as polymerized and interpolymerized olefins (polybutylenes, polypropylenes, propylene isobutylene copolymers, ethylene-olefin copolymers, and ethylene-alphaolefin copolymers, for example).
- Polyalphaolefin (PAO) oil base stocks the Group IV API base stocks, are a commonly used synthetic hydrocarbon oil.
- PAOs derived from C 8 , C 10 , C 12 , C 14 olefins or mixtures thereof may be utilized. See U.S. Patent Nos. 4,956,122 ; 4,827,064 ; and 4,827,073 .
- Group IV oils, that is, the PAO base stocks have viscosity indices preferably greater than 130, more preferably greater than 135, still more preferably greater than 140.
- Esters in a minor amount may be useful in the lubricating oils of this disclosure. Additive solvency and seal compatibility characteristics may be secured by the use of esters such as the esters of dibasic acids with monoalkanols and the polyol esters of monocarboxylic acids.
- Esters of the former type include, for example, the esters of dicarboxylic acids such as phthalic acid, succinic acid, sebacic acid, fumaric acid, adipic acid, linoleic acid dimer, malonic acid, alkyl malonic acid, alkenyl malonic acid, etc., with a variety of alcohols such as butyl alcohol, hexyl alcohol, dodecyl alcohol, 2-ethylhexyl alcohol, etc.
- dicarboxylic acids such as phthalic acid, succinic acid, sebacic acid, fumaric acid, adipic acid, linoleic acid dimer, malonic acid, alkyl malonic acid, alkenyl malonic acid, etc.
- alcohols such as butyl alcohol, hexyl alcohol, dodecyl alcohol, 2-ethylhexyl alcohol, etc.
- esters include dibutyl adipate, di(2-ethylhexyl) sebacate, di-n-hexyl fumarate, dioctyl sebacate, diisooctyl azelate, diisodecyl azelate, dioctyl phthalate, didecyl phthalate, dieicosyl sebacate, etc.
- Particularly useful synthetic esters are those which are obtained by reacting one or more polyhydric alcohols, preferably the hindered polyols such as the neopentyl polyols; e.g., neopentyl glycol, trimethylol ethane, 2-methyl-2-propyl-1,3-propanediol, trimethylol propane, pentaerythritol and dipentaerythritol with alkanoic acids containing at least 4 carbon atoms, preferably C 5 to C 30 acids such as saturated straight chain fatty acids including caprylic acid, capric acids, lauric acid, myristic acid, palmitic acid, stearic acid, arachic acid, and behenic acid, or the corresponding branched chain fatty acids or unsaturated fatty acids such as oleic acid, or mixtures of any of these materials.
- the hindered polyols such as the neopentyl polyols
- Esters should be used in an amount such that the improved wear and corrosion resistance provided by the lubricating oils of this disclosure are not adversely affected.
- the esters preferably have a D5293 viscosity of less than 10,000 cP at -35°C.
- Non-conventional or unconventional base stocks and/or base oils include one or a mixture of base stock(s) and/or base oil(s) derived from: (1) one or more Gas-to-Liquids (GTL) materials, as well as (2) hydrodewaxed, or hydroisomerized/cat (and/or solvent) dewaxed base stock(s) and/or base oils derived from synthetic wax, natural wax or waxy feeds, mineral and/or non-mineral oil waxy feed stocks such as gas oils, slack waxes (derived from the solvent dewaxing of natural oils, mineral oils or synthetic oils; e.g., Fischer-Tropsch feed stocks), natural waxes, and waxy stocks such as gas oils, waxy fuels hydrocracker bottoms, waxy raffinate, hydrocrackate, thermal crackates, foots oil or other mineral, mineral oil, or even non-petroleum oil derived waxy materials such as waxy materials recovered from coal liquefaction or shale oil, linear or
- GTL materials are materials that are derived via one or more synthesis, combination, transformation, rearrangement, and/or degradation/deconstructive processes from gaseous carbon-containing compounds, hydrogen-containing compounds and/or elements as feed stocks such as hydrogen, carbon dioxide, carbon monoxide, water, methane, ethane, ethylene, acetylene, propane, propylene, propyne, butane, butylenes, and butynes.
- GTL base stocks and/or base oils are GTL materials of lubricating viscosity that are generally derived from hydrocarbons; for example, waxy synthesized hydrocarbons, that are themselves derived from simpler gaseous carbon-containing compounds, hydrogen-containing compounds and/or elements as feed stocks.
- GTL base stock(s) and/or base oil(s) include oils boiling in the lube oil boiling range (1) separated/fractionated from synthesized GTL materials such as, for example, by distillation and subsequently subjected to a final wax processing step which involves either or both of a catalytic dewaxing process, or a solvent dewaxing process, to produce lube oils of reduced/low pour point; (2) synthesized wax isomerates, comprising, for example, hydrodewaxed or hydroisomerized cat and/or solvent dewaxed synthesized wax or waxy hydrocarbons; (3) hydrodewaxed or hydroisomerized cat and/or solvent dewaxed Fischer-Tropsch (F-T) material (i.e., hydrocarbons, waxy hydrocarbons, waxes and possible analogous oxygenates); preferably hydrodewaxed or hydroisomerized/followed by cat and/or solvent dewaxing dewaxed F-T waxy hydrocarbons, or hydrodewaxed
- GTL base stock(s) and/or base oil(s) derived from GTL materials are characterized typically as having kinematic viscosities at 100°C of from 2 mm 2 /s to 50 mm 2 /s (ASTM D445). They are further characterized typically as having pour points of -5°C to -40°C or lower (ASTM D97). They are also characterized typically as having viscosity indices of 80 to 140 or greater (ASTM D2270).
- GTL base stock(s) and/or base oil(s) are typically highly paraffinic (>90% saturates), and may contain mixtures of monocycloparaffins and multicycloparaffins in combination with non-cyclic isoparaffins.
- the ratio of the naphthenic (i.e., cycloparaffin) content in such combinations varies with the catalyst and temperature used.
- GTL base stock(s) and/or base oil(s) typically have very low sulfur and nitrogen content, generally containing less than 10 ppm, and more typically less than 5 ppm of each of these elements.
- the sulfur and nitrogen content of GTL base stock(s) and/or base oil(s) obtained from F-T material, especially F-T wax, is essentially nil.
- the absence of phosphorous and aromatics make this materially especially suitable for the formulation of low SAP products.
- GTL base stock and/or base oil and/or wax isomerate base stock and/or base oil is to be understood as embracing individual fractions of such materials of wide viscosity range as recovered in the production process, mixtures of two or more of such fractions, as well as mixtures of one or two or more low viscosity fractions with one, two or more higher viscosity fractions to produce a blend wherein the blend exhibits a target kinematic viscosity.
- the GTL material, from which the GTL base stock(s) and/or base oil(s) is/are derived is preferably an F-T material (i.e., hydrocarbons, waxy hydrocarbons, wax).
- Base oils for use in the formulated lubricating oils useful in the present disclosure are any of the variety of oils corresponding to API Group I, Group II, Group III, Group IV, Group V and Group VI oils and mixtures thereof, preferably API Group II, Group III, Group IV, Group V and Group VI oils and mixtures thereof, more preferably the Group III to Group VI base oils due to their exceptional volatility, stability, viscometric and cleanliness features.
- Minor quantities of Group I stock such as the amount used to dilute additives for blending into formulated lube oil products, can be tolerated but should be kept to a minimum, i.e. amounts only associated with their use as diluent/carrier oil for additives used on an "as-received" basis.
- Even in regard to the Group II stocks it is preferred that the Group II stock be in the higher quality range associated with that stock, i.e. a Group II stock having a viscosity index in the range 100 ⁇ VI ⁇ 120.
- GTL base stock(s) and/or base oil(s) are typically highly paraffinic (>90% saturates), and may contain mixtures of monocycloparaffins and multicycloparaffins in combination with non-cyclic isoparaffins.
- the ratio of the naphthenic (i.e., cycloparaffin) content in such combinations varies with the catalyst and temperature used.
- GTL base stock(s) and/or base oil(s) and hydrodewaxed, or hydroisomerized/cat (and/or solvent) dewaxed base stock(s) and/or base oil(s) typically have very low sulfur and nitrogen content, generally containing less than 10 ppm, and more typically less than 5 ppm of each of these elements.
- the sulfur and nitrogen content of GTL base stock(s) and/or base oil(s) obtained from F-T material, especially F-T wax, is essentially nil.
- the absence of phosphorous and aromatics make this material especially suitable for the formulation of low sulfur, sulfated ash, and phosphorus (low SAP) products.
- Polyalphaolefins are preferred lubricating oil basestocks of this disclosure.
- the PAOs can preferably comprise one or more C 8 to C 12 monomers.
- the PAOs have a viscosity (Kv 100 ) from 2 to 700 cSt at 100°C, preferably from 3 to 155 cSt at 100°C, and more preferably from 4 to 150 cSt at 100°C; and a viscosity index (VI) from 130 to 207, preferably from 140 to 200, and more preferably from 150 to 197.
- viscosity (Kv 100 ) is determined by ASTM D 445-01
- viscosity index (VI) is determined by ASTM D 2270-93 (1998).
- the PAOs useful in this disclosure can have a pour point (PP) less than -25°C; a molecular weight distribution (Mw/Mn) less than 2.0; and a glass transition temperature T g less than -60°C.
- any PAO described herein may have a kinematic viscosity (Kv) at 100°C in any of the following ranges: from 65 to 1,000 cSt, from 100 to 950 cSt, from 250 cSt to 900 cSt, from 400 cSt to 800 cSt, wherein all values are measured by ASTM D445-01.
- Kv kinematic viscosity
- the PAOs useful in this disclosure have a high viscosity index and a Kv 100 of 2 cSt or more, alternatively 3 cSt or more, alternatively 4 cSt or more, with a VI of 130 or more, alternatively 140 or more, alternatively 150 or more.
- base stock VI is a function of fluid viscosity. Usually, the higher the VI, the better it is for lube application. Base stock VI also depends on feed composition. Fluids made from single 1-octene, 1-nonene, 1-decene, or 1-dodecene have excellent VI and good low pour point.
- Fluids made from two or more olefins selected from C 8 to C 12 alphaolefins generally have excellent high VI and superior low pour points if the average carbon chain length of feed LAOs is kept within 8 to 12 carbons.
- a relatively much lower average chain length in the feed (much below 6 carbons) of the mixed LAO would result in lower VI. Too high of a average chain length in the feed (much above 12 carbons) of the mixed LAO would result in very high pour point, around room temperature.
- Viscosity Index is an empirical, unitless number which indicates the rate of change in the viscosity of an oil within a given temperature range and is related to kinematic viscosities measured at 40°C and 100°C (typically using ASTM Method D 445). Fluids exhibiting a relatively large change in viscosity with temperature are said to have a low viscosity index.
- the low VI oil for example, will thin out at elevated temperatures faster than the high VI oil.
- the high VI oil is more desirable because it has higher viscosity at higher temperature, which translates into better or thicker lubrication film and better protection of the contacting machine elements.
- the viscosity of the high VI oil will not increase as much as the viscosity of low VI oil. This is advantageous because the excessive high viscosity of the low VI oil will decrease the efficiency of the operating machine.
- high VI oil has performance advantages in both high and low temperature operation.
- the PAOs useful in this disclosure have low pour points (PP) less than -25°C, preferably less than -30°C, and more preferably less than -35°C. As used herein, pour point is determined by ASTM D97.
- any PAO described herein may have a pour point of less than -25°C (as measured by ASTM D97), preferably less than -35°C, preferably less than -45°C, preferably less than -55°C, preferably less than -65°C, and preferably between -25°C and -75°C.
- the PAOs useful in this disclosure have a narrow molecular weight distribution (Mw/Mn) less than 2.0, preferably less than 1.95, and more preferably less than 1.9 as synthesized.
- Mw/Mn molecular weight distribution
- Mw/Mn is determined by GPC using a column for medium to low molecular weight polymers, tetrahydrofuran as solvent and polystyrene as calibration standard.
- the PAOs useful in this disclosure have a Mw of 100,000 g/mol or less, or between 2000 and 80,000 g/mol, or between 2500 and 60,000 g/mol, or between 2800 and 50,000 g/mol, or between 3360 and 40,000 g/mol.
- Preferred Mw's include those from 840 to 55,100 g/mol, or from 900 to 45,000 g/mol, or 1000 to 40,000 g/mol, or 2,000 to 37,500 g/mol.
- preferred Mw's include 2240 to 67900 g/mol and 2240 to 37200 g/mol.
- the PAOs useful in this disclosure preferably have an Mn of 50,000 g/mol or less, or 40,000 g/mol or less, or between 2000 and 40,000 g/mol, or between 2500 and 30,000 g/mol, preferably between 5000 and 20,000 g/mol.
- Preferred Mn ranges include those from 2800 to 10,000 g/mol or from 2800 to 8,000 g/mol.
- preferred Mn ranges are from 2000 to 20,900 g/mol, or 2800 to 20,000 g/mol, or 2000 to 17000 g/mol, or 2000 to 12000 g/mol, or 2800 to 29000 g/mol, or 2800 to 17000 g/mol, or 2000 to 5000 g/mol.
- the Mw and Mn are measured by GPC using a column for medium to low molecular weight polymers, tetrahydrofuran as solvent and polystyrene as calibration standard, correlated with the fluid viscosity according to a power equation.
- the PAOs described herein have a narrow molecular weight distribution of greater than 1 and less than 2, alternatively less than 1.95, alternatively less than 1.90, alternatively less than 1.85.
- the Mn and Mw are measured by gel permeation chromatography (GPC) using a column for medium to low molecular weight polymers, tetrahydrofuran as solvent and narrow molecular weight distribution polystyrene as calibration standard, correlated with the fluid viscosity according to a power equation.
- the MWD of PAO is a function of fluid viscosity.
- any of the polyalphaolefins described herein preferably have an Mw/Mn of between 1 and 2.0, alternatively between 1 and 1.95, depending on fluid viscosity.
- the PAOs useful in this disclosure have low glass transition temperature T g less than -60°C, preferably less than -70°C, and more preferably less than -80°C.
- glass transition temperature T g is determined by differential scanning calorimetry (DSC).
- DSC differential scanning calorimetry
- the polyolefin products produced in accordance with the process of this disclosure have no crystallization peak as measured by differential scanning calorimetry and high thermal stability.
- the PAOs useful in this disclosure can comprise a single alphaolefin monomer type, or may comprise two or more different alphaolefin monomers.
- this disclosure relates to PAOs comprising a molar amount of C 8 to C 12 alphaolefin monomers selected from the group consisting of 55 mol% or more, 60 mol% or more, 65 mol% or more, 70 mol% or more, 75 mol% or more, 80 mol% or more, 85 mol% or more, 90 mol% or more, 95 mol% or more, 100 mol%, all based on the total moles of monomers present in the polyalphaolefin, as measured by 13 CNMR.
- the PAOs comprise polymers of one or more alphaolefins (also known as 1-olefins) with carbon numbers of C 8 to C 12 .
- at least one of the alphaolefins is a linear alphaolefin (LAO); more preferably, all the alphaolefins are LAOS.
- LAOs include 1-octene, 1-nonene, 1-decene, 1-undecene, and 1-dodecene, and blends thereof.
- the PAO comprises polymers of two or more C 8 to C 12 LAOs to make 'copolymer' or 'terpolymer' or higher-order copolymer combinations.
- Other embodiments involve polymerization of a mixture of LAOs selected from C 8 to C 12 LAOs with even carbon numbers, preferably a mixture of two or three LAOs selected from 1-octene, 1-decene, and 1-dodecene.
- the PAO comprises polymers of a single alphaolefin species having a total carbon count of 8 to 12. In other embodiments, the PAO comprises polymers of mixed (i.e., two or more) alphaolefin species, wherein each alphaolefin species has a carbon number of 8 to 12. In other embodiments, the PAO comprises polymers of mixed alphaolefin species wherein the molar-average carbon number (“C LAO ") is 8 to 12 or 9 to 11.
- C LAO molar-average carbon number
- any PAO described herein may have a density of 0.75 to 0.96 g/cm 3 , preferably 0.80 to 0.94 g/cm 3 , alternatively from 0.76 to 0.855 g/cm 3 .
- the high viscosity PAOs useful in this disclosure are desirable for use as lubricating oil base stocks and also blend stocks with API Groups I to V or gas-to-liquid (GTL) derived lube base stocks for use in industrial and automotive engine or gear oil, especially certain high Kv 100 grades of 65 to 155 cSt which are especially desirable for use as lubricating oil base stocks or blend stocks with Groups I to V or GTL-derived lube base stocks for use in industrial and automotive engine or gear oil.
- GTL gas-to-liquid
- PAOs can be used as lubricating oil base stocks and also superior blend stocks. They can be blend stocks with any of the API Group I to V and GTL fluids to give the optimum viscometrics, solvency, high and low temperature lubricity, etc.
- the PAOs can be further blended with proper additives, including antioxidants, antiwear additives, friction modifiers, dispersants, detergents, corrosion inhibitors, defoamants, extreme pressure additives, seal swell additives, and optionally viscosity modifiers, etc. Description of typical additives can be found in the book " Lubricant Additives: Chemistry and Applications," L.R. Rudnick, ed. Marcel Dekker Inc., New York, 2001 .
- the PAOs can be produced by conventional methods known in the art.
- the high viscosity PAOs used in this disclosure is prepared from different feed olefins using metallocene catalysts.
- the metallocene catalyst system, products, process and feeds are described, for example, in U.S. Application Publication No. 2011/0136714 .
- the basestock component of the present lubricating oils will typically be from 80 to 99 weight percent of the total composition (all proportions and percentages set out in this specification are by weight unless the contrary is stated) and more usually in the range of 90 to 99 weight percent.
- the viscosity modifiers useful in the lubricating compositions of the disclosure include substantially linear polymers with a weight average molecular weight of 45,000 or less, or 35,000 or less, or 25,000 or less, or 8000 to 25,000, or 12,000 to 20,000.
- the substantially linear polymers are copolymers comprising units derived from monomers (i) an ⁇ -olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with an alcohol.
- the substantially linear polymer may be a copolymer comprising units derived from monomers (i) one or more alpha olefins and (ii) one or more alkyl (meth) acrylate esters.
- the ethylenically unsaturated carboxylic acid may be esterified with alcohol before or after polymerization with the ⁇ -olefin.
- the ethylenically unsaturated carboxylic acid may be esterified with alcohol before polymerization with the ⁇ -olefin. In one embodiment the ethylenically unsaturated carboxylic acid may be esterified with alcohol after polymerization with the ⁇ -olefin.
- a commercially available copolymer prepared by esterification before polymerization is available from Akzo Nobel sold under the tradename Ketjenlube®3700.
- the alcohol may have 1 to 40, or 1 to 30, or 4 to 20, or 6 to 16 carbon atoms.
- Examples of a suitable alcohol include 2-ethylhexanol, 2- butyloctanol, 2-hexyldecanol, 2-octyldodecanol, 2-decyltetradecanol, butanol, pentanol, hexanol, heptanol, octanol, nonanol, decanol, undecanol, dodecanol, tridecanol, tetradecanol, pentadecanol, hexadecanol, heptadecanol, octadecanol, eicosanol, or mixtures thereof.
- a copolymer of this type is described in more detail in U.S. Patent Nos. 4,526,950 , 6,419,714 , 6,573,224 , or 6,174,843 .
- the ethylenically unsaturated carboxylic acid may be esterified with alcohol after polymerization with the ⁇ -olefin.
- a copolymer of this type may be a substantially linear polymer that may in one embodiment be (a) a copolymer comprising units derived from monomers (i) an ⁇ -olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with a primary alcohol branched at the ⁇ - or higher position, wherein the copolymer typically has a reduced specific viscosity of up to 0.2, (b) a poly(meth)acrylate, or mixtures thereof.
- the substantially linear polymer may be present in the lubricating compositions described herein at 0.1 wt% to 50 wt%, or 2 wt% to 40 wt%, or 5 wt% to 30 wt%, or 8 wt% to 20 wt% of the lubricating composition.
- the lubricating composition contains 65 to 99 wt % of synthetic base stock and 1 to 35 wt % of substantially linear polymer.
- the lubricating composition contains 75 to 98 wt% of synthetic base stock and 2 to 25 wt% of substantially linear polymer.
- the substantially linear polymer may be a copolymer comprising units derived from monomers (i) one or more alpha olefins and (ii) one or more alkyl (meth)acrylate esters.
- the substantially linear polymer includes a mixtures of (a) a copolymer comprising units derived from monomers of (i) an ⁇ -olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with a primary alcohol, and (b) a poly(meth)acrylate.
- the poly(meth)acrylate (typically a polymethacrylate) can have units derived from a mixture of alkyl (meth)acrylate ester monomers containing (a) 8 to 24, or 12 to 18, or to 15 carbon atoms in the alcohol-derived portion of the ester group and (b) 6 to 11, or 8 to 11, or 8 carbon atoms in the alcohol-derived portion of the ester group, and which have 2-(C 1-4 alkyl)-substituents, and optionally, at least one monomer selected from the group consisting of (meth)acrylic acid esters containing 1 to 7 carbon atoms in the alcohol-derived portion of the ester group and which are different from (meth)acrylic acid esters (a) and (b), vinyl aromatic compounds (or vinyl aromatic monomers); and nitrogen-containing vinyl monomers; provided that no more than 60% by weight, or no more than 50% by weight, or no more than 35% by weight of the esters contain not more than 10 carbon atoms in the alcohol-derived portion of the ester group
- the linear polymer of this type is described in more detail in US 6,124,249 or EP 0 937 769 A1 .
- the linear polymer may further contain a third monomer.
- the third monomer may be styrene, or mixtures thereof.
- the third monomer may be present in an amount 0% to 25% of the polymer composition, or from 1% to 15% of the composition, 2% to 10% of the composition, or even from 1% to 3% of the composition.
- the mole ratio of esters (a) to esters (b) in the copolymer ranges from 95:5 to 35:65, or 90:10 to 60:40, or 80:20 to 50:50.
- the esters are usually aliphatic esters, typically alkyl esters.
- the ester of (a) may be a C 12-15 alkyl methacrylate and the ester of (b) may be 2-ethylhexyl methacrylate.
- ester groups in ester (a) contain branched alkyl groups.
- the ester groups may contain 2 to 65%, or 5 to 60% or greater of the ester groups having branched alkyl groups.
- the C 1-4 alkyl substituents may be methyl, ethyl, and any isomers of propyl and butyl.
- the weight average molecular weight of the poly(meth)acrylate may be 45,000 or less, or 35,000 or less, or 25,000 or less, or 8000 to 25,000, or 12,000 to 20,000.
- the substantially linear polymer includes a copolymer comprising units derived from monomers (i) an ⁇ -olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with a primary alcohol branched at the ⁇ - or higher position, wherein the copolymer typically has a reduced specific viscosity of up to 0.2, or up to 0.15, or up to 0.10, or up to 0.08.
- the reduced specific viscosity may be up to 0.08 (or 0.02 to 0.08 (or 0.02 to 0.07, 0.03 to 0.07 or 0.04 to 0.06).
- a measurement correlating with molecular weight of the copolymer (or interpolymer such as an alternating copolymer) may be expressed in terms of the "reduced specific viscosity" of the copolymer which is a recognized means of expressing the molecular size of a polymeric substance.
- the copolymer may be derived from monomers (i) an ⁇ -olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof, wherein 0.1 to 99.89% of the carboxylic acid units are esterified with a primary alcohol branched at the ⁇ - or higher position, wherein 0.1 to 99.89% of the carboxylic acid units are esterified with a linear alcohol or an alpha-branched alcohol (e.g., a secondary alcohol), wherein 0.01 to 10% of the carboxylic acid units has at least one of an amino-, amido- and/or imido- group, and wherein the copolymer has a reduced specific viscosity (prior to esterification) of up to 0.08.
- monomers i) an ⁇ -olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof, wherein 0.1 to 99.89% of the carboxylic acid units are esterified with
- the copolymer may be derived from monomers (i) an ⁇ -olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof, wherein 0.1 to 99.89% of the carboxylic acid units are esterified with a primary alcohol branched at the ⁇ - or higher position, wherein 0.1 to 99.9% of the carboxylic acid units are esterified with a linear alcohol or an alpha-branched alcohol, wherein 0 to 10% of the carboxylic acid units has at least one of an amino-, amido- and/or imido- group, and wherein the copolymer has a reduced specific viscosity of up to 0.08.
- a linear alcohol may include methanol, ethanol, propanol, butanol, pentanol, hexanol, heptanol, octanol, nonanol, decanol, undecanol, dodecanol, tridecanol, tetradecanol, pentadecanol, hexadecanol, heptadecanol, octadecanol, nonadecanol, eicosanol, or mixtures thereof.
- the linear alcohol contains 6 to 30, or 8 to 20, or 8 to 15 carbon atoms (typically 8 to 15 carbon atoms).
- the linear alcohol may include commercially available materials such as Oxo Alcohol® 7911, Oxo Alcohol® 7900 and Oxo Alcohol® 1 100 of Monsanto; Alphanol® 79 of ICI; Nafol® 1620, Alfol® 610 and Alfol® 810 of Condea (now Sasol); Epal® 610 and Epal® 810 of Ethyl Corporation (now Afton); Linevol® 79, Linevol® 911 and Dobanol® 25 L of Shell AG; Lial® 125 of Condea Augusta, Milan; Dehydad® and Lorol® of Henkel KGaA (now Cognis) as well as Linopol® 7-11 and Acropol® 91 of Ugine Kuhlmann.
- Commercially available materials such as Oxo Alcohol® 7911, Oxo Alcohol® 7900 and Oxo Alcohol® 1 100 of Monsanto; Alphanol® 79 of ICI; Nafol® 1620, Alfol® 610 and Alfo
- the copolymer may be derived from monomers of (i) an ⁇ -olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof, wherein 5 to 15% of the carboxylic acid units are esterified with a primary alcohol branched at the ⁇ - or higher position, wherein 0.1 to 95% of the carboxylic acid units are esterified with a linear alcohol or an alpha-branched alcohol, wherein 0 to less than 2% of the carboxylic acid units has at least one of an amino-, amido- and/or imido- group, and wherein the copolymer has a reduced specific viscosity of up to 0.08.
- the copolymer comprises units derived from monomers (i) an ⁇ -olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with a primary alcohol branched at the ⁇ - or higher position.
- the copolymer may be represented by the formula below. Ester or other groups with the primary alcohol-derived moiety branched at the ⁇ - or higher position may be represented within the ( ) w shown in the formula: wherein
- the copolymer with pendant groups may contain 0.10% to 100%, or 0.5% to 20%, or 0.75% to 10%, branched hydrocarbyl groups represented by a group within ( ) y of the formula (I) above, expressed as a percentage of the total number of pendant groups .
- the pendant groups of formula (1) may also be used to define the ester groups as defined above by the phrase "esterified with a primary alcohol branched at the ⁇ - or higher position".
- X may be derived from an ethylenically unsaturated carboxylic acid or derivatives thereof.
- a suitable carboxylic acid or derivatives thereof typically include maleic anhydride, maleic acid, (meth)acrylic acid, itaconic anhydride or itaconic acid.
- the ethylenically unsaturated carboxylic acid or derivatives thereof may be at least one of maleic anhydride or maleic acid.
- X is other than an alkylene group, connecting the copolymer backbone and the branched hydrocarbyl groups.
- the pendant groups may be esterified, amidated or imidated functional groups.
- the pendant groups may be derived from esterified and/or amidated functional groups.
- the copolymer includes esterified pendant groups.
- the pendant groups may be derived from Guerbet alcohols.
- the Guerbet alcohols may contain 10 to 60, or 12 to 60, or 16 to 40 carbon atoms.
- the primary alcohol branched at the ⁇ - or higher position described herein may be a Guerbet alcohol. Methods to prepare Guerbet alcohols are disclosed in U.S. Patent No. 4,767,815 .
- the pendant groups may contain a total combined number of carbon atoms on R' and R"in the range of 12 to 60, or 14 to 50, or 16 to 40, or 18 to 40, or 20 to 36.
- Each of R' and R" may individually contain 5 to 25, or 8 to 32, or 10 to 18 methylene carbon atoms. In one embodiment the number of carbon atoms on each R' and R"group maybe 10 to 24.
- Suitable primary alcohol branched at the ⁇ - or higher position examples include 2-ethylhexanol, 2-propyl heptanol, 2-butyloctanol, 2-hexyldecanol, 2-octyldodecanol, 2-decyltetradecanol, or mixtures thereof.
- the ethylenically unsaturated carboxylic acid or derivatives thereof may be an acid or anhydride or derivatives thereof that may be wholly esterified, partially esterified or mixtures thereof.
- other functional groups include acids, salts or mixtures thereof.
- Suitable salts include alkali metals, alkaline earth metals or mixtures thereof.
- the salts include lithium, sodium, potassium, magnesium, calcium or mixtures thereof.
- the unsaturated carboxylic acid or derivatives thereof includes acrylic acid, methyl acrylate, methacrylic acid, maleic acid or anhydride, fumaric acid, itaconic acid or anhydride or mixtures thereof, or substituted equivalents thereof.
- Suitable examples of the ethylenically unsaturated carboxylic acid or derivatives thereof include itaconic anhydride, maleic anhydride, methyl maleic anhydride, ethyl maleic anhydride, dimethyl maleic anhydride or mixtures thereof.
- the ethylenically unsaturated carboxylic acid or derivatives thereof includes maleic anhydride or derivatives thereof.
- Examples of an alpha-olefin include 1-decene, 1-undecene, 1-dodecene, 1-tridecene, 1-tetradecene, 1-pentadecene, 1-hexadecene, 1-hepta-decene, 1-octadecene, or mixtures thereof.
- An example of a useful alpha-olefm is 1-dodecene.
- the alpha-olefin may be a branched alpha-olefin, or mixtures thereof. If the ⁇ -olefin is branched, the number of carbon atoms of the ⁇ -olefin may range from 4 to 32, or 6 to 20, or 8 to 16.
- the copolymer of the disclosure further includes a nitrogen containing group such as those disclosed above.
- the nitrogen containing group may be derived from a nitrogen containing compound capable of being incorporated during copolymerization.
- the copolymer of the disclosure further includes a nitrogen containing group that may be capable of reacting with the functionalized copolymer backbone, typically for capping the copolymer backbone. The capping may result in the copolymer having ester, amide, imide or amine groups.
- the nitrogen group is described in more detail in PCT Patent Application No. PCT/US09/052028 .
- the copolymer comprises units derived from monomers (i) an ⁇ -olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof may be further reacted with an amine to additionally provide oxidation control.
- the copolymer with oxidation control contains an incorporated residue of an amine-containing compound such as morpholines, pyrrolidinones, imidazolidinones, acetamides, ⁇ -alanine alkyl esters, or mixtures thereof.
- nitrogen-containing compounds examples include 3-morpholin-4-yl-propylamine, 3-morpholin-4-yl-ethylamine, [beta]-alanine alkyl esters (typically alkyl esters have 1 to 30, or 6 to 20 carbon atoms), or mixtures thereof.
- the compounds based on imidazolidinones, cyclic carbamates or pyrrolidinones may be derived from a compound of general structure: wherein:
- the imidazolidinone includes 1-(2-amino-ethyl)-imidazolidin-2-one (may also be called aminoethylethyleneurea), 1-(3-amino-propyl)-imidazolidin-2-one, 1-(2-hydroxy-ethyl)-imidazolidin-2-one, 1-(3-amino-propyl)-pyrrolidin-2-one, 1-(3-amino-ethyl)-pyrrolidin-2-one, or mixtures thereof.
- 1-(2-amino-ethyl)-imidazolidin-2-one may also be called aminoethylethyleneurea
- 1-(3-amino-propyl)-imidazolidin-2-one 1-(2-hydroxy-ethyl)-imidazolidin-2-one
- 1-(3-amino-propyl)-pyrrolidin-2-one 1-(3-amino-ethyl)-pyrrolidin-2-one
- the copolymer may be reacted with an amine-containing compound selected from morpholines, imidazolidinones, and mixtures thereof.
- the formulated lubricating oil useful in the present disclosure may additionally contain one or more of the other commonly used lubricating oil performance additives including but not limited to dispersants, other detergents, corrosion inhibitors, rust inhibitors, metal deactivators, other antiwear agents and/or extreme pressure additives, anti-seizure agents, wax modifiers, viscosity index improvers, fluid-loss additives, seal compatibility agents, other friction modifiers, lubricity agents, antistaining agents, chromophoric agents, defoamants, demulsifiers, emulsifiers, densifiers, wetting agents, gelling agents, tackiness agents, colorants, and others.
- dispersants including but not limited to dispersants, other detergents, corrosion inhibitors, rust inhibitors, metal deactivators, other antiwear agents and/or extreme pressure additives, anti-seizure agents, wax modifiers, viscosity index improvers, fluid-loss additives, seal compatibility agents, other friction
- Typical antioxidant include phenolic antioxidants, aminic antioxidants and oil-soluble copper complexes.
- the phenolic antioxidants include sulfurized and non-sulfurized phenolic antioxidants.
- the terms "phenolic type” or “phenolic antioxidant” used herein includes compounds having one or more than one hydroxyl group bound to an aromatic ring which may itself be mononuclear, e.g., benzyl, or poly-nuclear, e.g., naphthyl and spiro aromatic compounds.
- phenol type includes phenol per se, catechol, resorcinol, hydroquinone, naphthol, etc., as well as alkyl or alkenyl and sulfurized alkyl or alkenyl derivatives thereof, and bisphenol type compounds including such bi-phenol compounds linked by alkylene bridges sulfuric bridges or oxygen bridges.
- Alkyl phenols include mono- and poly-alkyl or alkenyl phenols, the alkyl or alkenyl group containing from 3-100 carbons, preferably 4 to 50 carbons and sulfurized derivatives thereof, the number of alkyl or alkenyl groups present in the aromatic ring ranging from 1 to up to the available unsatisfied valences of the aromatic ring remaining after counting the number of hydroxyl groups bound to the aromatic ring.
- the phenolic anti-oxidant may be represented by the general formula: (R) x -Ar-(OH) y where Ar is selected from the group consisting of: wherein R is a C 3 -C 100 alkyl or alkenyl group, a sulfur substituted alkyl or alkenyl group, preferably a C 4 -C 50 alkyl or alkenyl group or sulfur substituted alkyl or alkenyl group, more preferably C 3 -C 100 alkyl or sulfur substituted alkyl group, most preferably a C 4 -C 50 alkyl group, R g is a C 1 -C 100 alkylene or sulfur substituted alkylene group, preferably a C 2 -C 50 alkylene or sulfur substituted alkylene group, more preferably a C 2 -C 2 alkylene or sulfur substituted alkylene group, y is at least 1 to up to the available valences of Ar, x ranges from 0 to up to the available valences of Ar
- Preferred phenolic antioxidant compounds are the hindered phenolics and phenolic esters which contain a sterically hindered hydroxyl group, and these include those derivatives of dihydroxy aryl compounds in which the hydroxyl groups are in the o- or p-position to each other.
- Typical phenolic anti-oxidants include the hindered phenols substituted with C 1 + alkyl groups and the alkylene coupled derivatives of these hindered phenols.
- phenolic materials of this type 2-t-butyl-4-heptyl phenol; 2-t-butyl-4-octyl phenol; 2-t-butyl-4-dodecyl phenol; 2,6-di-t-butyl-4-heptyl phenol; 2,6-di-t-butyl-4-dodecyl phenol; 2-methyl-6-t-butyl-4-heptyl phenol; 2-methyl-6-t-butyl-4-dodecyl phenol; 2,6-di-t-butyl-4 methyl phenol; 2,6-di-t-butyl-4-ethyl phenol; and 2,6-di-t-butyl 4 alkoxy phenol; and
- Phenolic type antioxidants are well known in the lubricating industry and commercial examples such as Ethanox® 4710, Irganox® 1076, Irganox® L1035, Irganox® 1010, Irganox® L109, Irganox® L118, Irganox® L135 and the like are familiar to those skilled in the art. The above is presented only by way of exemplification, not limitation on the type of phenolic anti-oxidants which can be used.
- the phenolic antioxidant can be employed in an amount in the range of 0.1 to 3 wt%, preferably 0.25 to 2.5 wt%, more preferably 0.5 to 2 wt% on an active ingredient basis.
- Aromatic amine antioxidants include phenyl- ⁇ -naphthyl amine which is described by the following molecular structure: wherein R z is hydrogen or a C 1 to C 14 linear or C 3 to C 14 branched alkyl group, preferably C 1 to C 10 linear or C 3 to C 10 branched alkyl group, more preferably linear or branched C 6 to C 8 and n is an integer ranging from 1 to 5 preferably 1.
- R z is hydrogen or a C 1 to C 14 linear or C 3 to C 14 branched alkyl group, preferably C 1 to C 10 linear or C 3 to C 10 branched alkyl group, more preferably linear or branched C 6 to C 8 and n is an integer ranging from 1 to 5 preferably 1.
- a particular example is Irganox L06.
- aromatic amine antioxidants include other alkylated and non-alkylated aromatic amines such as aromatic monoamines of the formula R 8 R 9 R 10 N where R 8 is an aliphatic, aromatic or substituted aromatic group, R 9 is an aromatic or a substituted aromatic group, and R 10 is H, alkyl, aryl or R 11 S(O) x R 12 where R 11 is an alkylene, alkenylene, or aralkylene group, R 12 is a higher alkyl group, or an alkenyl, aryl, or alkaryl group, and x is 0, 1 or 2.
- the aliphatic group R 8 may contain from 1 to 20 carbon atoms, and preferably contains from 6 to 12 carbon atoms.
- the aliphatic group is a saturated aliphatic group.
- both R 8 and R 9 are aromatic or substituted aromatic groups, and the aromatic group may be a fused ring aromatic group such as naphthyl.
- Aromatic groups R 8 and R 9 may be joined together with other groups such as S.
- Typical aromatic amines anti-oxidants have alkyl substituent groups of at least 6 carbon atoms.
- Examples of aliphatic groups include hexyl, heptyl, octyl, nonyl, and decyl. Generally, the aliphatic groups will not contain more than 14 carbon atoms.
- the general types of such other additional amine antioxidants which may be present include diphenylamines, phenothiazines, imidodibenzyls and diphenyl phenylene diamines. Mixtures of two or more of such other additional aromatic amines may also be present. Polymeric amine antioxidants can also be used.
- oil-soluble copper compounds are oil-soluble copper compounds. Any oil-soluble suitable copper compound may be blended into the lubricating oil.
- suitable copper antioxidants include copper dihydrocarbyl thio- or dithio-phosphates and copper salts of carboxylic acid (naturally occurring or synthetic).
- suitable copper salts include copper dithiacarbamates, sulphonates, phenates, and acetylacetonates.
- Basic, neutral, or acidic copper Cu(I) and or Cu(II) salts derived from alkenyl succinic acids or anhydrides are known to be particularly useful.
- antioxidants may be used individually or as mixtures of one or more types of antioxidants, the total amount employed being an amount of 0.50 to 5 wt%, preferably 0.75 to 3 wt% (on an as-received basis).
- alkali or alkaline earth metal salicylate detergent which is an optional component in the present disclosure
- other detergents may also be present. While such other detergents can be present, it is preferred that the amount employed be such as to not interfere with the synergistic effect attributable to the presence of the salicylate. Therefore, most preferably such other detergents are not employed.
- additional detergents can include alkali and alkaline earth metal phenates, sulfonates, carboxylates, phosphonates and mixtures thereof.
- These supplemental detergents can have total base number (TBN) ranging from neutral to highly overbased, i.e. TBN of 0 to over 500, preferably 2 to 400, more preferably 5 to 300, and they can be present either individually or in combination with each other in an amount in the range of from 0 to 10 wt%, preferably 0.5 to 5 wt% (active ingredient) based on the total weight of the formulated lubricating oil.
- TBN total base number
- Such additional other detergents include by way of example and not limitation calcium phenates, calcium sulfonates, magnesium phenates, magnesium sulfonates and other related components (including borated detergents).
- Dispersants help keep these byproducts in solution, thus diminishing their deposition on metal surfaces.
- Dispersants may be ashless or ash-forming in nature.
- the dispersant is ashless.
- So called ashless dispersants are organic materials that form substantially no ash upon combustion.
- non-metal-containing or borated metal-free dispersants are considered ashless.
- metal-containing detergents discussed above form ash upon combustion.
- Suitable dispersants typically contain a polar group attached to a relatively high molecular weight hydrocarbon chain.
- the polar group typically contains at least one element of nitrogen, oxygen, or phosphorus.
- Typical hydrocarbon chains contain 50 to 400 carbon atoms.
- a particularly useful class of dispersants are the alkenylsuccinic derivatives, typically produced by the reaction of a long chain substituted alkenyl succinic compound, usually a substituted succinic anhydride, with a polyhydroxy or polyamino compound.
- the long chain group constituting the oleophilic portion of the molecule which confers solubility in the oil, is normally a polyisobutylene group.
- Hydrocarbyl-substituted succinic acid compounds are popular dispersants.
- succinimide, succinate esters, or succinate ester amides prepared by the reaction of a hydrocarbon-substituted succinic acid compound preferably having at least 50 carbon atoms in the hydrocarbon substituent, with at least one equivalent of an alkylene amine are particularly useful.
- Succinimides are formed by the condensation reaction between alkenyl succinic anhydrides and amines. Molar ratios can vary depending on the amine or polyamine. For example, the molar ratio of alkenyl succinic anhydride to TEPA can vary from 1:1 to 5:1.
- Succinate esters are formed by the condensation reaction between alkenyl succinic anhydrides and alcohols or polyols. Molar ratios can vary depending on the alcohol or polyol used. For example, the condensation product of an alkenyl succinic anhydride and pentaerythritol is a useful dispersant.
- Succinate ester amides are formed by condensation reaction between alkenyl succinic anhydrides and alkanol amines.
- suitable alkanol amines include ethoxylated polyalkylpolyamines, propoxylated polyalkylpolyamines and polyalkenylpolyamines such as polyethylene polyamines.
- propoxylated hexamethylenediamine is propoxylated hexamethylenediamine.
- the molecular weight of the alkenyl succinic anhydrides will typically range between 800 and 2,500.
- the above products can be post-reacted with various reagents such as sulfur, oxygen, formaldehyde, carboxylic acids such as oleic acid, and boron compounds such as borate esters or highly borated dispersants.
- the dispersants can be borated with from 0.1 to 5 moles of boron per mole of dispersant reaction product.
- Mannich base dispersants are made from the reaction of alkylphenols, formaldehyde, and amines. Process aids and catalysts, such as oleic acid and sulfonic acids, can also be part of the reaction mixture. Molecular weights of the alkylphenols range from 800 to 2,500 or more.
- Typical high molecular weight aliphatic acid modified Mannich condensation products can be prepared from high molecular weight alkyl-substituted hydroxyaromatics or HN(R) 2 group-containing reactants.
- high molecular weight alkyl-substituted hydroxyaromatic compounds are polypropylphenol, polybutylphenol, and other polyalkylphenols. These polyalkylphenols can be obtained by the alkylation, in the presence of an alkylating catalyst, such as BF 3 , of phenol with high molecular weight polypropylene, polybutylene, and other polyalkylene compounds to give alkyl substituents on the benzene ring of phenol having an average 600-100,000 molecular weight.
- an alkylating catalyst such as BF 3
- HN(R) 2 group-containing reactants are alkylene polyamines, principally polyethylene polyamines.
- Other representative organic compounds containing at least one HN(R) 2 group suitable for use in the preparation of Mannich condensation products are well known and include the mono- and di-amino alkanes and their substituted analogs, e.g., ethylamine and diethanol amine; aromatic diamines, e.g., phenylene diamine, diamino naphthalenes; heterocyclic amines, e.g., morpholine, pyrrole, pyrrolidine, imidazole, imidazolidine, and piperidine; melamine and their substituted analogs.
- alkylene polyamine reactants include ethylenediamine, diethylene triamine, triethylene tetraamine, tetraethylene pentaamine, pentaethylene hexamine, hexaethylene heptaamine, heptaethylene octaamine, octaethylene nonaamine, nonaethylene decamine, and decaethylene undecamine and mixture of such amines having nitrogen contents corresponding to the alkylene polyamines, in the formula H 2 N-(Z-NH-) n H, mentioned before, Z is a divalent ethylene and n is 1 to 10 of the foregoing formula.
- propylene polyamines such as propylene diamine and di-, tri-, tetra-, pentapropylene tri-, tetra-, penta- and hexaamines are also suitable reactants.
- the alkylene polyamines are usually obtained by the reaction of ammonia and dihalo alkanes, such as dichloro alkanes.
- the alkylene polyamines obtained from the reaction of 2 to 11 moles of ammonia with 1 to 10 moles of dichloroalkanes having 2 to 6 carbon atoms and the chlorines on different carbons are suitable alkylene polyamine reactants.
- Aldehyde reactants useful in the preparation of the high molecular products useful in this disclosure include the aliphatic aldehydes such as formaldehyde (also as paraformaldehyde and formalin), acetaldehyde and aldol ( ⁇ -hydroxybutyraldehyde). Formaldehyde or a formaldehyde-yielding reactant is preferred.
- Preferred dispersants include borated and non-borated succinimides, including those derivatives from mono-succinimides, bis-succinimides, and/or mixtures of mono- and bis-succinimides, wherein the hydrocarbyl succinimide is derived from a hydrocarbylene group such as polyisobutylene having a Mn of from 500 to 5000 or more or a mixture of such hydrocarbylene groups.
- Other preferred dispersants include succinic acid-esters and amides, alkylphenol-polyamine-coupled Mannich adducts, their capped derivatives, and other related components.
- Such additives may be used in an amount of 0.1 to 20 wt%, preferably 0.1 to 8 wt%, more preferably 1 to 6 wt% (on an as-received basis) based on the weight of the total lubricant.
- pour point depressants also known as lube oil flow improvers
- Pour point depressant may be added to lower the minimum temperature at which the fluid will flow or can be poured.
- suitable pour point depressants include alkylated naphthalenes polymethacrylates, polyacrylates, polyarylamides, condensation products of haloparaffin waxes and aromatic compounds, vinyl carboxylate polymers, and terpolymers of dialkylfumarates, vinyl esters of fatty acids and allyl vinyl ethers.
- Such additives may be used in amount of 0.0 to 0.5 wt%, preferably 0 to 0.3 wt%, more preferably 0.001 to 0.1 wt% on an as-received basis.
- Corrosion inhibitors are used to reduce the degradation of metallic parts that are in contact with the lubricating oil composition.
- Suitable corrosion inhibitors include aryl thiazines, alkyl substituted dimercapto thiodiazoles thiadiazoles and mixtures thereof.
- Such additives may be used in an amount of 0.01 to 5 wt%, preferably 0.01 to 1.5 wt%, more preferably 0.01 to 0.2 wt%, still more preferably 0.01 to 0.1 wt% (on an as-received basis) based on the total weight of the lubricating oil composition.
- Seal compatibility agents help to swell elastomeric seals by causing a chemical reaction in the fluid or physical change in the elastomer.
- Suitable seal compatibility agents for lubricating oils include organic phosphates, aromatic esters, aromatic hydrocarbons, esters (butylbenzyl phthalate, for example), and polybutenyl succinic anhydride and sulfolane-type seal swell agents such as Lubrizol 730-type seal swell additives. Such additives may be used in an amount of 0.01 to 3 wt%, preferably 0.01 to 2 wt% on an as-received basis.
- Anti-foam agents may advantageously be added to lubricant compositions. These agents retard the formation of stable foams. Silicones and organic polymers are typical anti-foam agents. For example, polysiloxanes, such as silicon oil or polydimethyl siloxane, provide antifoam properties. Anti-foam agents are commercially available and may be used in conventional minor amounts along with other additives such as demulsifiers; usually the amount of these additives combined is less than 1 percent, preferably 0.001 to 0.5 wt%, more preferably 0.001 to 0.2 wt%, still more preferably 0.0001 to 0.15 wt% (on an as-received basis) based on the total weight of the lubricating oil composition.
- Antirust additives are additives that protect lubricated metal surfaces against chemical attack by water or other contaminants.
- One type of antirust additive is a polar compound that wets the metal surface preferentially, protecting it with a film of oil.
- Another type of antirust additive absorbs water by incorporating it in a water-in-oil emulsion so that only the oil touches the surface.
- Yet another type of antirust additive chemically adheres to the metal to produce a nonreactive surface.
- suitable additives include zinc dithiophosphates, metal phenolates, basic metal sulfonates, fatty acids and amines. Such additives may be used in an amount of 0.01 to 5 wt%, preferably 0.01 to 1.5 wt% on an as-received basis.
- organo molybdenum-nitrogen complexes embraces the organo molybdenum-nitrogen complexes described in U.S. Patent 4,889,647 .
- the complexes are reaction products of a fatty oil, dithanolamine and a molybdenum source. Specific chemical structures have not been assigned to the complexes.
- U.S. Patent 4,889,647 reports an infrared spectrum for a typical reaction product of that disclosure; the spectrum identifies an ester carbonyl band at 1740 cm -1 and an amide carbonyl band at 1620 cm -1 .
- the fatty oils are glyceryl esters of higher fatty acids containing at least 12 carbon atoms up to 22 carbon atoms or more.
- the molybdenum source is an oxygen-containing compound such as ammonium molybdates, molybdenum oxides and mixtures.
- organo molybdenum complexes which can be used in the present disclosure are tri-nuclear molybdenum-sulfur compounds described in EP 1 040 115 and WO 99/31113 and the molybdenum complexes described in U.S. Patent No. 4,978,464 .
- the lubricating composition may optionally further contain other known viscosity modifiers.
- the viscosity modifiers may be hydrogenated styrene-butadiene rubbers, ethylenepropylene copolymers, hydrogenated styrene-isoprene polymers, hydrogenated diene polymers, polyalkyl styrenes, polyolefins, esters of maleic anhydride-styrene copolymers, or mixtures thereof.
- the lubricating compositions can include at least one antiwear agent.
- suitable antiwear agents include oil soluble amine salts of phosphorus compounds, sulphurised olefins, metal dihydrocarbyldithio-phosphates (such as zinc dialkyldithiophosphates), thiocarbamate-containing compounds, such as thiocarbamate esters, thiocarbamate amides, thiocarbamic ethers, alkylene-coupled thiocarbamates, and bis(S-alkyldithiocarbamyl) disulphides.
- the oil soluble phosphorus amine salt antiwear agent includes an amine salt of a phosphorus acid ester or mixtures thereof.
- the amine salt of a phosphorus acid ester includes phosphoric acid esters and amine salts thereof; dialkyldithiophosphoric acid esters and amine salts thereof; amine salts of phosphites; and amine salts of phosphorus-containing carboxylic esters, ethers, and amides; and mixtures thereof.
- the amine salt of a phosphorus acid ester may be used alone or in combination.
- the oil soluble phosphorus amine salt includes partial amine salt-partial metal salt compounds or mixtures thereof.
- the phosphorus compound further includes a sulphur atom in the molecule.
- the amine salt of the phosphorus compound may be ashless, i.e., metal-free (prior to being mixed with other components).
- the amines which may be suitable for use as the amine salt include primary amines, secondary amines, tertiary amines, and mixtures thereof.
- the amines include those with at least one hydrocarbyl group, or, in certain embodiments, two or three hydrocarbyl groups.
- the hydrocarbyl groups may contain 2 to 30 carbon atoms, or in other embodiments 8 to 26, or 10 to 20, or 13 to 19 carbon atoms.
- Primary amines include ethylamine, propylamine, butylamine, 2-ethylhexylamine, octylamine, and dodecylamine, as well as such fatty amines as n-octylamine, n-decylamine, n-dodecylamine, n-tetradecylamine, n-hexadecylamine, n-octadecylamine and oleyamine.
- fatty amines include commercially available fatty amines such as "Armeen®” amines (products available from Akzo Chemicals, Chicago, Illinois), such as Armeen C, Armeen O, Armeen OL, Armeen T, Armeen HT, Armeen S and Armeen SD, wherein the letter designation relates to the fatty group, such as coco, oleyl, tallow, or stearyl groups.
- suitable secondary amines include dimethylamine, diethylamine, dipropylamine, dibutylamine, diamylamine, dihexylamine, diheptylamine, methylethylamine, ethylbutylamine and ethylamylamine.
- the secondary amines may be cyclic amines such as piperidine, piperazine and morpholine.
- the amine may also be a tertiary-aliphatic primary amine.
- the aliphatic group in this case may be an alkyl group containing 2 to 30, or 6 to 26, or 8 to 24 carbon atoms.
- Tertiary alkyl amines include monoamines such as tert-butylamine, tert-hexylamine, 1-methyl-1-amino-cyclohexane, tert-octylamine, tert-decylamine, tertdodecylamine, tert-tetradecylamine, tert-hexadecylamine, tert-octadecylamine, tert-tetracosanylamine, and tert-octacosanylamine.
- the phosphorus acid amine salt includes an amine with C 11 to C 14 tertiary alkyl primary groups or mixtures thereof. In one embodiment the phosphorus acid amine salt includes an amine with C 14 to C 18 tertiary alkyl primary amines or mixtures thereof. In one embodiment the phosphorus acid amine salt includes an amine with C 18 to C 22 tertiary alkyl primary amines or mixtures thereof.
- amines may also be used in the disclosure.
- a useful mixture of amines is "Primene® 81R” and “Primene® JMT.”
- Primene® 81R and Primene® JMT are mixtures of C 11 to C 14 tertiary alkyl primary amines and C 18 to C 22 tertiary alkyl primary amines, respectively.
- oil soluble amine salts of phosphorus compounds include a sulphur-free amine salt of a phosphorus-containing compound may be obtained/obtainable by a process comprising: reacting an amine with either (i) a hydroxy-substituted di-ester of phosphoric acid, or (ii) a phosphorylated hydroxy-substituted di- or tri-ester of phosphoric acid.
- a process comprising: reacting an amine with either (i) a hydroxy-substituted di-ester of phosphoric acid, or (ii) a phosphorylated hydroxy-substituted di- or tri-ester of phosphoric acid.
- the hydrocarbyl amine salt of an alkylphosphoric acid ester is the reaction product of a C 14 to C 18 alkylated phosphoric acid with Primene 81RTM (produced and sold by Rohm & Haas) which is a mixture of C 11 to C 14 tertiary alkyl primary amines.
- hydrocarbyl amine salts of dialkyldithiophosphoric acid esters include the reaction product(s) of isopropyl, methyl-amyl (4-methyl-2-pentyl or mixtures thereof), 2-ethylhexyl, heptyl, octyl or nonyl dithiophosphoric acids with ethylene diamine, morpholine, or Primene 81RTM, and mixtures thereof.
- the dithiophosphoric acid may be reacted with an epoxide or a glycol. This reaction product is further reacted with a phosphorus acid, anhydride, or lower ester.
- the epoxide includes an aliphatic epoxide or a styrene oxide. Examples of useful epoxides include ethylene oxide, propylene oxide, butene oxide, octene oxide, dodecene oxide, and styrene oxide. In one embodiment the epoxide may be propylene oxide.
- the glycols may be aliphatic glycols having from 1 to 12, or from 2 to 6, or 2 to 3 carbon atoms.
- dithiophosphoric acids glycols, epoxides, inorganic phosphorus reagents and methods of reacting the same are described in U.S. Patent Nos. 3,197,405 and 3,544,465 .
- the resulting acids may then be salted with amines.
- An example of suitable dithiophosphoric acid is prepared by adding phosphorus pentoxide (64 grams) at 58°C over a period of 45 minutes to 514 grams of hydroxypropyl 0,0-di(4-methyl-2-pentyl)phosphorodithioate (prepared by reacting di(4-methyl-2-pentyl)-phosphorodithioic acid with 1.3 moles of propylene oxide at 25°C).
- the mixture may be heated at 75°C for 2.5 hours, mixed with a diatomaceous earth and filtered at 70°C.
- the filtrate contains 11.8% by weight phosphorus, 15.2% by weight sulphur, and an acid number of 87 (bromophenol blue).
- the dithiocarbamate-containing compounds may be prepared by reacting a dithiocarbamate acid or salt with an unsaturated compound.
- the dithiocarbamate containing compounds may also be prepared by simultaneously reacting an amine, carbon disulphide and an unsaturated compound. Generally, the reaction occurs at a temperature from 25°C to 125°C.
- Suitable olefins that may be sulphurised to form an the sulphurised olefin include propylene, butylene, isobutylene, pentene, hexane, heptene, octane, nonene, decene, undecene, dodecene, undecyl, tridecene, tetradecene, pentadecene, hexadecene, heptadecene, octadecene, octadecenene, nonodecene, eicosene or mixtures thereof.
- hexadecene, heptadecene, octadecene, octadecenene, nonodecene, eicosene or mixtures thereof and their dimers, trimers and tetramers are especially useful olefins.
- the olefin may be a Diels-Alder adduct of a diene such as 1,3-butadiene and an unsaturated ester, such as, butylacrylate.
- sulphurised olefin includes fatty acids and their esters.
- the fatty acids are often obtained from vegetable oil or animal oil; and typically contain 4 to 22 carbon atoms.
- suitable fatty acids and their esters include triglycerides, oleic acid, linoleic acid, palmitoleic acid or mixtures thereof.
- the fatty acids are obtained from lard oil, tall oil, peanut oil, soybean oil, cottonseed oil, sunflower seed oil or mixtures thereof.
- fatty acids and/or ester are mixed with olefins.
- the ashless antiwear agent may be a monoester of a polyol and an aliphatic carboxylic acid, often an acid containing 12 to 24 carbon atoms.
- the monoester of a polyol and an aliphatic carboxylic acid is in the form of a mixture with a sunflower oil or the like, which may be present in the friction modifier mixture from 5 to 95, in several embodiments from 10 to 90, or from 20 to 85, or 20 to 80 weight percent of said mixture.
- the aliphatic carboxylic acids (especially a monocarboxylic acid) which form the esters are those acids typically containing 12 to 24, or from 14 to 20 carbon atoms. Examples of carboxylic acids include dodecanoic acid, stearic acid, lauric acid, behenic acid, and oleic acid.
- Polyols include diols, triols, and alcohols with higher numbers of alcoholic OH groups.
- Polyhydric alcohols include ethylene glycols, including di-, tri- and tetraethylene glycols; propylene glycols, including di-, tri- and tetrapropylene glycols; glycerol; butane diol; hexane diol; sorbitol; arabitol; mannitol; sucrose; fructose; glucose; cyclohexane diol; erythritol; and pentaerythritols, including di- and tripentaerythritol.
- the polyol is diethylene glycol, triethylene glycol, glycerol, sorbitol, penta erythritol or dipentaerythritol.
- glycerol monooleate The commercially available monoester known as "glycerol monooleate” is believed to include 60 + 5 percent by weight of the chemical species glycerol monooleate, along with 35 + 5 percent glycerol dioleate, and less than 5 percent trioleate and oleic acid.
- the amounts of the monoesters, described above, are calculated based on the actual, corrected, amount of polyol monoester present in any such mixture.
- EP agents that are soluble in the oil include sulphur- and chlorosulphur-containing EP agents, chlorinated hydrocarbon EP agents and phosphorus EP agents.
- EP agents include chlorinated wax; sulphurised olefins (such as sulphurised isobutylene), organic sulphides and polysulphides such as dibenzyldisulphide, bis-(chlorobenzyl) disulphide, dibutyl tetrasulphide, sulphurised methyl ester of oleic acid, sulphurised alkylphenol, sulphurised dipentene, sulphurised terpene, and sulphurised Diels-Alder adducts; phosphosulphurised hydrocarbons such as the reaction product of phosphorus sulphide with turpentine or methyl oleate; phosphorus esters such as the dihydrocarbon and trihydrocarbon phosphites, e.g
- the method and lubricating compositions of this disclosure may be suitable for greases, gear oils, axle oils, drive shaft oils, traction oils, manual transmission oils, automatic transmission oils, metal working fluids, hydraulic oils, or internal combustion engine oils.
- the method and lubricating composition of the disclosure may be suitable for at least one of gear oils, axle oils, drive shaft oils, traction oils, manual transmission oils or automatic transmission oils. In one embodiment the disclosure provides a method of lubricating a manual transmission.
- An automatic transmission includes continuously variable transmissions (CVT), infinitely variable transmissions (IVT), toroidal transmissions, continuously slipping torque converter clutches (CSTCC), stepped automatic transmissions or dual clutch transmissions (DCT).
- CVT continuously variable transmissions
- IVT infinitely variable transmissions
- CSTCC continuously slipping torque converter clutches
- DCT dual clutch transmissions
- the internal combustion engines may be 2-stroke or 4-stroke engines.
- Suitable internal combustion engines include marine diesel engines, aviation piston engines, low-load diesel engines, and automobile and truck engines.
- (meth) acrylic and related terms includes both acrylic and methacrylic groups.
- a primary alcohol branched at the ⁇ - or higher position relates to an alcohol with branching at the 2-position or a higher position (e.g., 3-, or 4-, or 5-, or 6-, or 7-position, etc.).
- the number of carbon atoms present in the ester groups of the polymers of the disclosure is counted to include only those carbon atoms of the alcohol-derived portion of the ester group. Specifically, the number of carbon atoms excludes the carbonyl carbon of the ester.
- hydrocarbyl substituent or “hydrocarbyl group” is used in its ordinary sense, which is well-known to those skilled in the art. Specifically, it refers to a group having a carbon atom directly attached to the remainder of the molecule and having predominantly hydrocarbon character.
- hydrocarbyl groups include: hydrocarbon substituents, including aliphatic, alicyclic, and aromatic substituents; substituted hydrocarbon substituents, that is, substituents containing non-hydrocarbon groups which, in the context of this disclosure, do not alter the predominantly hydrocarbon nature of the substituent; and hetero substituents, that is, substituents which similarly have a predominantly hydrocarbon character but contain other than carbon in a ring or chain.
- Lubricant compositions were prepared by blending a polyalphaolefin base stock (e.g., mPAO 700, mPAO 450, mPAO 300 , mPAO 150, mPAO 65, mPAO 14, PAO 70, PAO 4, and PAO 6), a hybrid olefin ester polymer (HOEP) viscosity modifier (MeridianTM), and an axle oil additive package.
- HOEP hybrid olefin ester polymer
- the MeridianTM viscosity modifier is available from Lubrizol Corporation.
- the axle oil additive package used in Examples 1 and 2 is available from Lubrizol Corporation as AnglamolTM 6043M.
- the axle oil additive package used in Examples 3 and 4 is available from Afton Chemicals.
- FIG. 1 shows 4-Ball Wear Scar (ASTM D4172) is reduced when using the MeridianTM viscosity modifier alone or in combination with mPAO 150 or mPAO 65.
- a surprising aspect of this disclosure is that base oil and viscosity modifiers are not expected to have significant impact on wear or load-carrying properties of the fluid.
- Fig. 10 shows the results of 4-Ball Wear Scar (ASTM D4172) for the designated lubricant compositions (i.e., blends).
- Fig. 4 shows on average 25-30% lower traction coefficients for lubricating compositions containing mPAO 150, mPAO 65, and/or MeridianTM compared to a conventional viscosity modifier. Lower traction indicates potential axle efficiency enhancement and therefore improved fuel economy.
- Fig. 11 shows the results of 4-Ball EP (ASTM D2783) load wear index for the designated lubricant compositions (i.e., blends).
- Fig. 12 shows the results of High Frequency Reciprocating Rig (HFRR) friction coefficient for the designated lubricant compositions (i.e., blends).
- HFRR High Frequency Reciprocating Rig
- Fig. 7 indicates a potential antiwear/antiscuffing advantage for a MeridianTM viscosity modifier in the FZG scuffing test even in a lower SAE 75W-80 viscosity formulation containing all MeridianTM as the viscosity modifier.
- a trend reflecting lower Total Gear weight loss with increased concentration of MeridianTM was observed in the FZG test. All formulations showed excellent performance by delivering "> Stage 12" passing results, a requirement for premium high performance gear oils.
- Fig. 8 shows surprisingly excellent sludge and varnish results for fluids evaluated in 100-hr L60-1 Thermal and Oxidative Stability test, which is double the standard duration (i.e., 50 hours) required for SAE J2360 high performance gear oil specification.
- the results clearly demonstrate that incorporation of the MeridianTM viscosity modifier in the lubricating compositions of this disclosure provides improved stability and cleanliness even for already high performing technology. This further substantiates the potential to formulation even higher performing lower viscosity finished products by incorporating new synthetic base fluids and viscosity modifiers.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Organic Chemistry (AREA)
- Lubricants (AREA)
Description
- This disclosure relates to the use of high viscosity index lubricating oil base stock (metallocene catalyzed polyalphaolefin and polyalphaolefin fluid) and viscosity modifier (e.g., ester-containing copolymer) combinations, and of lubricating oils derived therefrom. This disclosure relates to lubricating driveline devices, e.g., gears and transmissions, using the lubricating oils to improve fuel efficiency without sacrificing driveline device durability.
- Environmental regulations are driving vehicle fuel economy standards and there is increased emphasis on and market demand for higher efficiency driveline fluids, particularly axle oils that can deliver fuel economy and maintain hardware protection capability. A proven approach to enhancing lubricant derived fuel efficiency is lower fluid viscosity. Since equipment durability cannot be compromised, equal or lower viscosity lubricants must deliver improved efficiency while retaining the same level of protection against various types of hardware damage (e.g., wear, micropitting, scuffing, scoring, and the like). Improved durability and reduced component wear increases equipment operating life and reduces maintenance costs and downtime. Improved shear stability is likewise desirable to provide enduring performance (i.e., oil film stability) throughout the useful life of the lubricant. Additionally, different transmission applications have specific friction requirements, some of which benefit from higher friction.
- Lubricants in commercial use today are prepared from a variety of natural and synthetic base stocks admixed with various additive packages and solvents depending upon their intended application. The base stocks typically include mineral oils, polyalphaolefins (PAO), gas-to-liquid base oils (GTL), silicone oils, phosphate esters, diesters, polyol esters, and the like.
WO2007/145924 discloses lubricating oils comprising metallocene catalyzed PAO. - Viscosity index improvers are known to be added to lubricating oil compositions to reduce the change in viscosity of the lubricant as a function of temperature. The most conventional types of viscosity index improvers used in axle and transmission oil applications include polyisobutylene and polymers of methacrylates. More recent viscosity index improver technologies consist of olefins (such as copolymers of alpha-olefins and maleic anhydride and esterified derivatives thereof). These viscosity index improvers tend to incorporate ester functional groups in pendant/grafted/branched groups. The ester functional groups may be derived from linear alkyl alcohols with 1 to 40 carbon atoms. Recent attempts have been made to produce viscosity index improvers from copolymers of alpha-olefins. However, such viscosity index improvers remain susceptible to viscosity loss when subjected to high shear conditions. Low temperature flow, oxidation stability, deposit control, thickening efficiency, and shear stability are performance attributes that can be controlled by design. Different core chemical structures and evolving process technologies enable design of molecules that deliver varying degrees of performance in these areas.
WO2010/014655 ,EP0471266 ,EP0566048 ,US 3,856,685 andEP1975222 disclose copolymer viscosity modifiers. - Lubricants capable of performing at lower viscosity (in, for instance, driveline devices) typically provide increased fuel economy (thus improving corporate average fuel efficiency (CAFE), NEDC (European Driving Cycle), or FTP-75 (Federal Test Procedure), or Japanese test cycle (JC-08)). Conversely, higher viscosity fluids contribute to elevated gear and transmission operating temperatures, which are believed to reduce fuel economy and diminish durability.
- Driveline power transmitting devices such as axles and transmissions-present highly complex technological challenges for axle and manual transmission lubricants. These lubricants are required to ensure hardware durability in the form wear protection and high load-carrying capacity, while delivering enhanced fuel efficiency benefits over extended periods. Additionally, transmissions typically require specific frictional characteristics of fluids that are compatible with synchronizer material or design. One of the important parameters influencing performance is lubricant viscosity. Lubricants capable of performing at lower viscosity typically provide increased fuel economy. However viscosity that is too low to maintain sufficient and stable oil film between surface asperities results in elevated gear and transmission operating temperatures, which are believed to reduce fuel economy due to higher friction in contact zones. Therefore, increasing lubricant viscosity is conventionally believed to provide better wear protection and durability to gears and transmissions.
- Consequently, it would be desirable to provide a correctly balanced lubricant composition to meet the needs of mechanical devices such as gears and transmissions, especially axle fluids and manual transmission fluids (MTFs). The discovery of new ways to control or adjust frictional properties of a lube formulation would be very beneficial.
- The present disclosure provides solutions and advantages, which shall become apparent as described below.
- This disclosure relates to the use of a viscosity modifier comprising a copolymer having units derived from monomers of (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with an alcohol in a driveline device lubricated with a lubricating composition comprising:
- a first base stock component comprising one or more metallocene catalyzed polyalphaolefins (mPAOs), each mPAO having a kinematic viscosity (Kv100) from 40 cSt to 155 cSt and a viscosity index (VI) from 150 to 207; and
- a second base stock comprising one or more polyalphaolefins (PAOs), each PAO having a kinematic viscosity (Kv100) less than 10 cSt and a VI from 130 to 145;
- for improving at least one of wear control (as determined by ASTM D4172), load carrying capacity (as determined by ASTM D2783) and traction reduction (as determined by Mini-Traction Machine (MTM) Ball-on-Disc apparatus) as compared to at least one of wear control, load carrying capacity and traction reduction achieved with a lubricating composition containing a viscosity modifier other than said viscosity modifier copolymer, at an equal or lower kinematic viscosity (Kv@100°C).
- The above lubricating composition can be produced by a, process comprising:
- providing a first base stock component comprising one or more metallocene catalyzed polyalphaolefins (mPAOs), each mPAO having a kinematic viscosity (Kv100) from 40 cSt to 155 cSt and a viscosity index (VI) from 150 to 207;
- providing a second base stock comprising one or more polyalphaolefins (PAOs), each PAO having a kinematic viscosity (Kv100) less than 10 cSt and a VI from 130 to 145;
- providing a viscosity modifier comprising a copolymer having units derived from monomers of (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with an alcohol; and
- blending the first base stock component, second base stock component and viscosity modifier in amounts sufficient to produce the lubricating composition.
- A method of lubricating a mechanical device comprises supplying to the device the above lubricating composition. The mechanical device comprises a driveline device, e.g., gears or transmissions.
- By using this disclosure fuel efficiency can be improved, while maintaining or improving wear control, load carrying capacity and/or traction reduction in a driveline device, e.g., gears or transmissions, lubricated with a lubricating composition. In driveline devices such as axles, higher viscosity fluids can result in lower fuel efficiency due to churning losses. The internal friction of the fluid measured by its traction properties is an indicator of its efficiency benefits in high pressure contact areas within axles. The method of blending of this disclosure delivers lower traction and lower viscosity fluids.
- It has been surprisingly found that the lubricating compositions used in this disclosure exhibit improved wear control (as determined by ASTM D4172), load carrying capacity (as determined by ASTM D2783) and/or traction reduction (as determined by Mini-Traction Machine (MTM) Ball-on-Disc apparatus) with said lubricating composition as compared to wear control, load carrying capacity and traction reduction achieved, at an equal or lower kinematic viscosity (Kv@100°C), with a lubricating composition containing a viscosity modifier other than a viscosity modifier comprising a copolymer having units derived from monomers of (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with an alcohol.
- Further objects, features and advantages of the present disclosure will be understood by reference to the following drawings and detailed description.
-
-
Fig. 1 depicts Four-Ball Wear Scar (ASTM D4172) results for lubricating compositions in Example 1. Consistently lower wear is achieved by incorporating a viscosity modifier comprising a copolymer having units derived from monomers of (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with an alcohol. -
Fig. 2 depicts LWI results which are a measure of the relative ability of a lubricant to prevent wear under applied loads. LWI is calculated from data obtained from the Four Ball EP Method (ASTM D2783) in Example 2. Across a broad range of finished lubricant kinematic viscosity at 100°C (Kv100), the combination of viscosity modifier and mPAO 65 showed surprisingly higher LWI than either the Meridian VM or the mPAO 65 alone. -
Fig. 3 depicts average friction coefficient measurements for lubricating compositions in Example 2. The combination of mPAO 65 and Meridian show surprisingly higher friction than other base oil and/or VM combinations indicating the possibility to control friction of lubricants designed for automotive transmission applications such at continuously variable transmissions (CVT) or infinitely variable transmissions (IVT). -
Fig. 4 depicts average traction coefficient for lubricating compositions in Example 1. Improved traction coefficients are observed over a range of finished lubricant kinematic viscosity at 100°C for these compositions compared to conventional PIB-based viscosity modifier. -
Fig. 5 is a tabular listing of tapered roller bearing 40-hr shear stability test results for lubricating compositions in Example 3. -
Fig. 6 depicts 40-hr shear kinematic viscosity (Kv100) loss (%) for lubricating compositions in Example 3. The reduction in viscosity loss observed with the combination of Meridian andmPAO 150 compared to either high viscosity component by itself is unexpected. -
Fig. 7 depicts FZG scuffing testing results for lubricating compositions in Example 4. Results indicate unexpected directional improvement in wear protection with higher concentration of Meridian viscosity modifier even at lower finished fluid viscosity. -
Fig. 8 depicts thermal oxidation results for lubricating compositions in Example 4. Oxidation and deposit control in severe L60-1 test improves with incorporation of Meridian viscosity modifier. -
Fig. 9 depicts L-42 high speed shock testing results for lubricating compositions in Example 4. -
Fig. 10 shows the results of 4-Ball Wear Scar (ASTM D4172) for the designated lubricant compositions (i.e., blends) of Example 1. -
Fig. 11 shows the results of 4-Ball EP (ASTM D2783) load wear index for the designated lubricant compositions (i.e., blends) of Example 2. -
Fig. 12 shows the results of High Frequency Reciprocating Rig (HFRR) friction coefficient for the designated lubricant compositions (i.e., blends) of Example 2. - All numerical values within the detailed description take into account experimental error and variations that would be expected by a person having ordinary skill in the art.
- For purposes of this disclosure and the claims thereto, a polymer is referred to as comprising homopolymers and copolymers, where copolymers include any polymer having two or more chemically distinct monomers.
- For the purposes of this disclosure and the claims thereto the term "polyalphaolefin" or "PAO" includes homopolymers and copolymers of C3 or greater alphaolefin monomers.
- The lubricating compositions used according to this disclosure exhibit improved wear control (as determined by ASTM D4172), improved load carrying capacity (as determined by ASTM D2783) and/or improved traction reduction (as determined by Mini-Traction Machine (MTM) Ball-on-Disc apparatus) as compared to wear control, load carrying capacity and traction reduction achieved, at an equal or lower kinematic viscosity (Kv@100°C), with a lubricating composition containing a viscosity modifier other than a viscosity modifier comprising a copolymer having units derived from monomers of (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with an alcohol.
- The lubricating compositions used according to this disclosure are also capable of providing at least one of improved oxidative stability, reduced mechanical device operating temperatures, increased mechanical device durability, improved shear stability, improved viscosity index, improved low temperature viscometrics and improved high temperature viscometrics.
- In an embodiment, this disclosure relates to the use of a combination of a high viscosity synthetic base stock and an ester-containing viscosity modifier that enables improvement in wear control, load carrying capacity and traction and provides improved efficiency at equal or lower kinematic viscosity (Kv @100°C). Current high performance commercial axle fluids are blended with low viscosity synthetic base stocks (such as <10 cSt PAO) in combination with conventional viscosity modifiers. In axles, higher viscosity fluids can result in lower fuel efficiency due to churning losses. The internal friction of the fluid measured by its traction properties provides an indicator of its efficiency benefits in high pressure contact areas within axles. The method of blending a high viscosity synthetic base stock and an ester-containing viscosity modifier in accordance with this disclosure provides lower traction and lower viscosity fluids.
- This disclosure relates to lubricating driveline devices, e.g., gears and transmissions, using the lubricating oils to improve fuel efficiency without sacrificing driveline device durability.
- A wide range of lubricating oils is known in the art. Lubricating oils that are useful in the present disclosure are both natural oils and synthetic oils. Natural and synthetic oils (or mixtures thereof) can be used unrefined, refined, or rerefined (the latter is also known as reclaimed or reprocessed oil). Unrefined oils are those obtained directly from a natural or synthetic source and used without added purification. These include shale oil obtained directly from retorting operations, petroleum oil obtained directly from primary distillation, and ester oil obtained directly from an esterification process. Refined oils are similar to the oils discussed for unrefined oils except refined oils are subjected to one or more purification steps to improve the at least one lubricating oil property. One skilled in the art is familiar with many purification processes. These processes include solvent extraction, secondary distillation, acid extraction, base extraction, filtration, and percolation. Rerefined oils are obtained by processes analogous to refined oils but using an oil that has been previously used as a feed stock.
- Groups I, II, III, IV, V and VI are broad categories of base oil stocks developed and defined by the American Petroleum Institute (API Publication 1509; www.API.org) to create guidelines for lubricant base oils. Group I base stocks generally have a viscosity index of between 80 to 120 and contain greater than 0.03% sulfur and less than 90% saturates. Group II base stocks generally have a viscosity index of between 80 to 120, and contain less than or equal to 0.03% sulfur and greater than or equal to 90% saturates. Group III stock generally has a viscosity index greater than 120 and contains less than or equal to 0.03% sulfur and greater than 90% saturates. Group IV includes polyalphaolefins (PAO). Group V base stocks include base stocks not included in Groups I-IV. The table below summarizes properties of each of these six groups.
- Group VI are polyinternal olefins ("PIO"). Polyinternal olefins are long-chain hydrocarbons, typically a linear backbone with some branching randomly attached; they are obtained by oligomerization of internal n-olefins. The catalyst is usually a BF3 complex with a proton source that leads to a cationic polymerization, or promoted BF3 or AlCl3 catalyst system. The process to produce polyinternal olefins (PIO) consists of four steps: reaction, neutralization/washing, hydrogenation and distillation. These steps are somewhat similar to PAO process. PIO are typically available in low viscosity grades, 4 cSt, 6 cSt and 8 cSt. If necessary, low viscosity, 1.5 to 3.9 cSt can also be made conveniently by the BF3 process or other cationic processes. Typically, the n-olefins used as starting material are n-C12-C18 internal olefins, more preferably, n-C14-C16 olefins are used. PIO can be made with VI and pour points very similar to PAO, only slightly inferior. They can be used in engine and industrial lubricant formulations. For more detailed discussion, see
Chapter 2, Polyintemalolefins in the book, "Synthetics, Mineral Oils, and Bio-Based Lubricants-Chemistry and Technology" Edited by Leslie R. Rudnick, p. 37-46, published by CRC Press, Taylor & Francis Group, 2006Base Stock Properties Saturates Sulfur Viscosity Index Group I <90 and/or >0.03% and ≥80 and <120 Group II ≥90 and ≤0.03% and ≥80 and <120 Group III ≥90 and ≤0.03% and ≥120 Group IV Includes polyalphaolefins (PAO) Group V All other base oil stocks not included in Groups I, II, III or IV Group VI Polyinternal olefins (PIO) - Natural oils include animal oils, vegetable oils (castor oil and lard oil, for example), and mineral oils. Animal and vegetable oils possessing favorable thermal oxidative stability can be used. Of the natural oils, mineral oils are preferred. Mineral oils vary widely as to their crude source, for example, as to whether they are paraffinic, naphthenic, or mixed paraffinic-naphthenic. Oils derived from coal or shale are also useful in the present disclosure. Natural oils vary also as to the method used for their production and purification, for example, their distillation range and whether they are straight run or cracked, hydrorefined, or solvent extracted.
- Group II and/or Group III hydroprocessed or hydrocracked base stocks, as well as synthetic oils such as polyalphaolefins, alkyl aromatics and synthetic esters, i.e., Group IV and Group V oils are also well known base stock oils.
- Synthetic oils include hydrocarbon oil such as polymerized and interpolymerized olefins (polybutylenes, polypropylenes, propylene isobutylene copolymers, ethylene-olefin copolymers, and ethylene-alphaolefin copolymers, for example). Polyalphaolefin (PAO) oil base stocks, the Group IV API base stocks, are a commonly used synthetic hydrocarbon oil. By way of example, PAOs derived from C8, C10, C12, C14 olefins or mixtures thereof may be utilized. See
U.S. Patent Nos. 4,956,122 ;4,827,064 ; and4,827,073 . Group IV oils, that is, the PAO base stocks have viscosity indices preferably greater than 130, more preferably greater than 135, still more preferably greater than 140. - Esters in a minor amount may be useful in the lubricating oils of this disclosure. Additive solvency and seal compatibility characteristics may be secured by the use of esters such as the esters of dibasic acids with monoalkanols and the polyol esters of monocarboxylic acids. Esters of the former type include, for example, the esters of dicarboxylic acids such as phthalic acid, succinic acid, sebacic acid, fumaric acid, adipic acid, linoleic acid dimer, malonic acid, alkyl malonic acid, alkenyl malonic acid, etc., with a variety of alcohols such as butyl alcohol, hexyl alcohol, dodecyl alcohol, 2-ethylhexyl alcohol, etc. Specific examples of these types of esters include dibutyl adipate, di(2-ethylhexyl) sebacate, di-n-hexyl fumarate, dioctyl sebacate, diisooctyl azelate, diisodecyl azelate, dioctyl phthalate, didecyl phthalate, dieicosyl sebacate, etc.
- Particularly useful synthetic esters are those which are obtained by reacting one or more polyhydric alcohols, preferably the hindered polyols such as the neopentyl polyols; e.g., neopentyl glycol, trimethylol ethane, 2-methyl-2-propyl-1,3-propanediol, trimethylol propane, pentaerythritol and dipentaerythritol with alkanoic acids containing at least 4 carbon atoms, preferably C5 to C30 acids such as saturated straight chain fatty acids including caprylic acid, capric acids, lauric acid, myristic acid, palmitic acid, stearic acid, arachic acid, and behenic acid, or the corresponding branched chain fatty acids or unsaturated fatty acids such as oleic acid, or mixtures of any of these materials.
- Esters should be used in an amount such that the improved wear and corrosion resistance provided by the lubricating oils of this disclosure are not adversely affected. The esters preferably have a D5293 viscosity of less than 10,000 cP at -35°C.
- Non-conventional or unconventional base stocks and/or base oils include one or a mixture of base stock(s) and/or base oil(s) derived from: (1) one or more Gas-to-Liquids (GTL) materials, as well as (2) hydrodewaxed, or hydroisomerized/cat (and/or solvent) dewaxed base stock(s) and/or base oils derived from synthetic wax, natural wax or waxy feeds, mineral and/or non-mineral oil waxy feed stocks such as gas oils, slack waxes (derived from the solvent dewaxing of natural oils, mineral oils or synthetic oils; e.g., Fischer-Tropsch feed stocks), natural waxes, and waxy stocks such as gas oils, waxy fuels hydrocracker bottoms, waxy raffinate, hydrocrackate, thermal crackates, foots oil or other mineral, mineral oil, or even non-petroleum oil derived waxy materials such as waxy materials recovered from coal liquefaction or shale oil, linear or branched hydrocarbyl compounds with carbon number of 20 or greater, preferably 30 or greater and mixtures of such base stocks and/or base oils.
- GTL materials are materials that are derived via one or more synthesis, combination, transformation, rearrangement, and/or degradation/deconstructive processes from gaseous carbon-containing compounds, hydrogen-containing compounds and/or elements as feed stocks such as hydrogen, carbon dioxide, carbon monoxide, water, methane, ethane, ethylene, acetylene, propane, propylene, propyne, butane, butylenes, and butynes. GTL base stocks and/or base oils are GTL materials of lubricating viscosity that are generally derived from hydrocarbons; for example, waxy synthesized hydrocarbons, that are themselves derived from simpler gaseous carbon-containing compounds, hydrogen-containing compounds and/or elements as feed stocks. GTL base stock(s) and/or base oil(s) include oils boiling in the lube oil boiling range (1) separated/fractionated from synthesized GTL materials such as, for example, by distillation and subsequently subjected to a final wax processing step which involves either or both of a catalytic dewaxing process, or a solvent dewaxing process, to produce lube oils of reduced/low pour point; (2) synthesized wax isomerates, comprising, for example, hydrodewaxed or hydroisomerized cat and/or solvent dewaxed synthesized wax or waxy hydrocarbons; (3) hydrodewaxed or hydroisomerized cat and/or solvent dewaxed Fischer-Tropsch (F-T) material (i.e., hydrocarbons, waxy hydrocarbons, waxes and possible analogous oxygenates); preferably hydrodewaxed or hydroisomerized/followed by cat and/or solvent dewaxing dewaxed F-T waxy hydrocarbons, or hydrodewaxed or hydroisomerized/followed by cat (or solvent) dewaxing dewaxed, F-T waxes, or mixtures thereof.
- GTL base stock(s) and/or base oil(s) derived from GTL materials, especially, hydrodewaxed or hydroisomerized/followed by cat and/or solvent dewaxed wax or waxy feed, preferably F-T material derived base stock(s) and/or base oil(s), are characterized typically as having kinematic viscosities at 100°C of from 2 mm2/s to 50 mm2/s (ASTM D445). They are further characterized typically as having pour points of -5°C to -40°C or lower (ASTM D97). They are also characterized typically as having viscosity indices of 80 to 140 or greater (ASTM D2270).
- In addition, the GTL base stock(s) and/or base oil(s) are typically highly paraffinic (>90% saturates), and may contain mixtures of monocycloparaffins and multicycloparaffins in combination with non-cyclic isoparaffins. The ratio of the naphthenic (i.e., cycloparaffin) content in such combinations varies with the catalyst and temperature used. Further, GTL base stock(s) and/or base oil(s) typically have very low sulfur and nitrogen content, generally containing less than 10 ppm, and more typically less than 5 ppm of each of these elements. The sulfur and nitrogen content of GTL base stock(s) and/or base oil(s) obtained from F-T material, especially F-T wax, is essentially nil. In addition, the absence of phosphorous and aromatics make this materially especially suitable for the formulation of low SAP products.
- The term GTL base stock and/or base oil and/or wax isomerate base stock and/or base oil is to be understood as embracing individual fractions of such materials of wide viscosity range as recovered in the production process, mixtures of two or more of such fractions, as well as mixtures of one or two or more low viscosity fractions with one, two or more higher viscosity fractions to produce a blend wherein the blend exhibits a target kinematic viscosity.
- The GTL material, from which the GTL base stock(s) and/or base oil(s) is/are derived is preferably an F-T material (i.e., hydrocarbons, waxy hydrocarbons, wax).
- Base oils for use in the formulated lubricating oils useful in the present disclosure are any of the variety of oils corresponding to API Group I, Group II, Group III, Group IV, Group V and Group VI oils and mixtures thereof, preferably API Group II, Group III, Group IV, Group V and Group VI oils and mixtures thereof, more preferably the Group III to Group VI base oils due to their exceptional volatility, stability, viscometric and cleanliness features. Minor quantities of Group I stock, such as the amount used to dilute additives for blending into formulated lube oil products, can be tolerated but should be kept to a minimum, i.e. amounts only associated with their use as diluent/carrier oil for additives used on an "as-received" basis. Even in regard to the Group II stocks, it is preferred that the Group II stock be in the higher quality range associated with that stock, i.e. a Group II stock having a viscosity index in the
range 100 < VI < 120. - In addition, the GTL base stock(s) and/or base oil(s) are typically highly paraffinic (>90% saturates), and may contain mixtures of monocycloparaffins and multicycloparaffins in combination with non-cyclic isoparaffins. The ratio of the naphthenic (i.e., cycloparaffin) content in such combinations varies with the catalyst and temperature used. Further, GTL base stock(s) and/or base oil(s) and hydrodewaxed, or hydroisomerized/cat (and/or solvent) dewaxed base stock(s) and/or base oil(s) typically have very low sulfur and nitrogen content, generally containing less than 10 ppm, and more typically less than 5 ppm of each of these elements. The sulfur and nitrogen content of GTL base stock(s) and/or base oil(s) obtained from F-T material, especially F-T wax, is essentially nil. In addition, the absence of phosphorous and aromatics make this material especially suitable for the formulation of low sulfur, sulfated ash, and phosphorus (low SAP) products.
- Polyalphaolefins (PAOs) are preferred lubricating oil basestocks of this disclosure. The PAOs can preferably comprise one or more C8 to C12 monomers. The PAOs have a viscosity (Kv100) from 2 to 700 cSt at 100°C, preferably from 3 to 155 cSt at 100°C, and more preferably from 4 to 150 cSt at 100°C; and a viscosity index (VI) from 130 to 207, preferably from 140 to 200, and more preferably from 150 to 197. As used herein, viscosity (Kv100) is determined by ASTM D 445-01, and viscosity index (VI) is determined by ASTM D 2270-93 (1998).
- The PAOs useful in this disclosure can have a pour point (PP) less than -25°C; a molecular weight distribution (Mw/Mn) less than 2.0; and a glass transition temperature Tg less than -60°C.
- In another embodiment according to the present disclosure, any PAO described herein may have a kinematic viscosity (Kv) at 100°C in any of the following ranges: from 65 to 1,000 cSt, from 100 to 950 cSt, from 250 cSt to 900 cSt, from 400 cSt to 800 cSt, wherein all values are measured by ASTM D445-01.
- The PAOs useful in this disclosure have a high viscosity index and a Kv100 of 2 cSt or more, alternatively 3 cSt or more, alternatively 4 cSt or more, with a VI of 130 or more, alternatively 140 or more, alternatively 150 or more. Usually base stock VI is a function of fluid viscosity. Usually, the higher the VI, the better it is for lube application. Base stock VI also depends on feed composition. Fluids made from single 1-octene, 1-nonene, 1-decene, or 1-dodecene have excellent VI and good low pour point. Fluids made from two or more olefins selected from C8 to C12 alphaolefins generally have excellent high VI and superior low pour points if the average carbon chain length of feed LAOs is kept within 8 to 12 carbons. A relatively much lower average chain length in the feed (much below 6 carbons) of the mixed LAO would result in lower VI. Too high of a average chain length in the feed (much above 12 carbons) of the mixed LAO would result in very high pour point, around room temperature.
- The viscosity-temperature relationship of lubricating oil is one of the critical criteria which must be considered when selecting a lubricant for a particular application. Viscosity Index (VI) is an empirical, unitless number which indicates the rate of change in the viscosity of an oil within a given temperature range and is related to kinematic viscosities measured at 40°C and 100°C (typically using ASTM Method D 445). Fluids exhibiting a relatively large change in viscosity with temperature are said to have a low viscosity index. The low VI oil, for example, will thin out at elevated temperatures faster than the high VI oil. Usually, the high VI oil is more desirable because it has higher viscosity at higher temperature, which translates into better or thicker lubrication film and better protection of the contacting machine elements. As the oil operating temperature decreases, the viscosity of the high VI oil will not increase as much as the viscosity of low VI oil. This is advantageous because the excessive high viscosity of the low VI oil will decrease the efficiency of the operating machine. Thus high VI oil has performance advantages in both high and low temperature operation.
- The PAOs useful in this disclosure have low pour points (PP) less than -25°C, preferably less than -30°C, and more preferably less than -35°C. As used herein, pour point is determined by ASTM D97.
- In an embodiment of this disclosure, any PAO described herein may have a pour point of less than -25°C (as measured by ASTM D97), preferably less than -35°C, preferably less than -45°C, preferably less than -55°C, preferably less than -65°C, and preferably between -25°C and -75°C.
- The PAOs useful in this disclosure have a narrow molecular weight distribution (Mw/Mn) less than 2.0, preferably less than 1.95, and more preferably less than 1.9 as synthesized. As used herein, molecular weight distribution (Mw/Mn) is determined by GPC using a column for medium to low molecular weight polymers, tetrahydrofuran as solvent and polystyrene as calibration standard.
- The PAOs useful in this disclosure have a Mw of 100,000 g/mol or less, or between 2000 and 80,000 g/mol, or between 2500 and 60,000 g/mol, or between 2800 and 50,000 g/mol, or between 3360 and 40,000 g/mol. Preferred Mw's include those from 840 to 55,100 g/mol, or from 900 to 45,000 g/mol, or 1000 to 40,000 g/mol, or 2,000 to 37,500 g/mol. Alternatively preferred Mw's include 2240 to 67900 g/mol and 2240 to 37200 g/mol.
- The PAOs useful in this disclosure preferably have an Mn of 50,000 g/mol or less, or 40,000 g/mol or less, or between 2000 and 40,000 g/mol, or between 2500 and 30,000 g/mol, preferably between 5000 and 20,000 g/mol. Preferred Mn ranges include those from 2800 to 10,000 g/mol or from 2800 to 8,000 g/mol. Alternatively preferred Mn ranges are from 2000 to 20,900 g/mol, or 2800 to 20,000 g/mol, or 2000 to 17000 g/mol, or 2000 to 12000 g/mol, or 2800 to 29000 g/mol, or 2800 to 17000 g/mol, or 2000 to 5000 g/mol.
- The Mw and Mn are measured by GPC using a column for medium to low molecular weight polymers, tetrahydrofuran as solvent and polystyrene as calibration standard, correlated with the fluid viscosity according to a power equation.
- In another embodiment, the PAOs described herein have a narrow molecular weight distribution of greater than 1 and less than 2, alternatively less than 1.95, alternatively less than 1.90, alternatively less than 1.85. The Mn and Mw are measured by gel permeation chromatography (GPC) using a column for medium to low molecular weight polymers, tetrahydrofuran as solvent and narrow molecular weight distribution polystyrene as calibration standard, correlated with the fluid viscosity according to a power equation. The MWD of PAO is a function of fluid viscosity. Alternatively any of the polyalphaolefins described herein preferably have an Mw/Mn of between 1 and 2.0, alternatively between 1 and 1.95, depending on fluid viscosity.
- The PAOs useful in this disclosure have low glass transition temperature Tg less than -60°C, preferably less than -70°C, and more preferably less than -80°C. As used herein, glass transition temperature Tg is determined by differential scanning calorimetry (DSC). The polyolefin products produced in accordance with the process of this disclosure have no crystallization peak as measured by differential scanning calorimetry and high thermal stability.
- The PAOs useful in this disclosure can comprise a single alphaolefin monomer type, or may comprise two or more different alphaolefin monomers. In one embodiment, this disclosure relates to PAOs comprising a molar amount of C8 to C12 alphaolefin monomers selected from the group consisting of 55 mol% or more, 60 mol% or more, 65 mol% or more, 70 mol% or more, 75 mol% or more, 80 mol% or more, 85 mol% or more, 90 mol% or more, 95 mol% or more, 100 mol%, all based on the total moles of monomers present in the polyalphaolefin, as measured by 13CNMR. When two or more alphaolefin monomers are present, it is sometimes desirable to add propylene, or butene (typically 1-butene) olefins into the feed. Use of these smaller olefins in the feed offers the advantage of lower feed cost and/or more abundant feed source. When adding C3 or 1-C4 olefins as one of the feed components, it is important to maintain the total average carbon chain length of the feed LAO (linear alphaolefin) between 8 to 12 carbons.
- In one or more embodiments, the PAOs comprise polymers of one or more alphaolefins (also known as 1-olefins) with carbon numbers of C8 to C12. Preferably, at least one of the alphaolefins is a linear alphaolefin (LAO); more preferably, all the alphaolefins are LAOS. Suitable LAOs include 1-octene, 1-nonene, 1-decene, 1-undecene, and 1-dodecene, and blends thereof.
- In one or more embodiments, the PAO comprises polymers of two or more C8 to C12 LAOs to make 'copolymer' or 'terpolymer' or higher-order copolymer combinations. Other embodiments involve polymerization of a mixture of LAOs selected from C8 to C12 LAOs with even carbon numbers, preferably a mixture of two or three LAOs selected from 1-octene, 1-decene, and 1-dodecene.
- In one or more embodiments, the PAO comprises polymers of a single alphaolefin species having a total carbon count of 8 to 12. In other embodiments, the PAO comprises polymers of mixed (i.e., two or more) alphaolefin species, wherein each alphaolefin species has a carbon number of 8 to 12. In other embodiments, the PAO comprises polymers of mixed alphaolefin species wherein the molar-average carbon number ("CLAO") is 8 to 12 or 9 to 11.
- In another embodiment according to the present disclosure, any PAO described herein may have a density of 0.75 to 0.96 g/cm3, preferably 0.80 to 0.94 g/cm3, alternatively from 0.76 to 0.855 g/cm3.
- The high viscosity PAOs useful in this disclosure are desirable for use as lubricating oil base stocks and also blend stocks with API Groups I to V or gas-to-liquid (GTL) derived lube base stocks for use in industrial and automotive engine or gear oil, especially certain high Kv100 grades of 65 to 155 cSt which are especially desirable for use as lubricating oil base stocks or blend stocks with Groups I to V or GTL-derived lube base stocks for use in industrial and automotive engine or gear oil.
- These higher viscosity PAOs can be used as lubricating oil base stocks and also superior blend stocks. They can be blend stocks with any of the API Group I to V and GTL fluids to give the optimum viscometrics, solvency, high and low temperature lubricity, etc. The PAOs can be further blended with proper additives, including antioxidants, antiwear additives, friction modifiers, dispersants, detergents, corrosion inhibitors, defoamants, extreme pressure additives, seal swell additives, and optionally viscosity modifiers, etc. Description of typical additives can be found in the book "Lubricant Additives: Chemistry and Applications," L.R. Rudnick, ed. Marcel Dekker Inc., New York, 2001.
- The PAOs can be produced by conventional methods known in the art. The high viscosity PAOs used in this disclosure is prepared from different feed olefins using metallocene catalysts. The metallocene catalyst system, products, process and feeds are described, for example, in
U.S. Application Publication No. 2011/0136714 . - The basestock component of the present lubricating oils will typically be from 80 to 99 weight percent of the total composition (all proportions and percentages set out in this specification are by weight unless the contrary is stated) and more usually in the range of 90 to 99 weight percent.
- The viscosity modifiers useful in the lubricating compositions of the disclosure include substantially linear polymers with a weight average molecular weight of 45,000 or less, or 35,000 or less, or 25,000 or less, or 8000 to 25,000, or 12,000 to 20,000.
- The substantially linear polymers are copolymers comprising units derived from monomers (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with an alcohol. In one embodiment, the substantially linear polymer may be a copolymer comprising units derived from monomers (i) one or more alpha olefins and (ii) one or more alkyl (meth) acrylate esters. The ethylenically unsaturated carboxylic acid may be esterified with alcohol before or after polymerization with the α-olefin. In one embodiment the ethylenically unsaturated carboxylic acid may be esterified with alcohol before polymerization with the α-olefin. In one embodiment the ethylenically unsaturated carboxylic acid may be esterified with alcohol after polymerization with the α-olefin.
- A commercially available copolymer prepared by esterification before polymerization is available from Akzo Nobel sold under the tradename Ketjenlube®3700. The alcohol may have 1 to 40, or 1 to 30, or 4 to 20, or 6 to 16 carbon atoms. Examples of a suitable alcohol include 2-ethylhexanol, 2- butyloctanol, 2-hexyldecanol, 2-octyldodecanol, 2-decyltetradecanol, butanol, pentanol, hexanol, heptanol, octanol, nonanol, decanol, undecanol, dodecanol, tridecanol, tetradecanol, pentadecanol, hexadecanol, heptadecanol, octadecanol, eicosanol, or mixtures thereof. A copolymer of this type is described in more detail in
U.S. Patent Nos. 4,526,950 ,6,419,714 ,6,573,224 , or6,174,843 . - The ethylenically unsaturated carboxylic acid may be esterified with alcohol after polymerization with the α-olefin. A copolymer of this type may be a substantially linear polymer that may in one embodiment be (a) a copolymer comprising units derived from monomers (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with a primary alcohol branched at the β - or higher position, wherein the copolymer typically has a reduced specific viscosity of up to 0.2, (b) a poly(meth)acrylate, or mixtures thereof.
- The substantially linear polymer may be present in the lubricating compositions described herein at 0.1 wt% to 50 wt%, or 2 wt% to 40 wt%, or 5 wt% to 30 wt%, or 8 wt% to 20 wt% of the lubricating composition. In certain embodiments the lubricating composition contains 65 to 99 wt % of synthetic base stock and 1 to 35 wt % of substantially linear polymer. In other embodiments, the lubricating composition contains 75 to 98 wt% of synthetic base stock and 2 to 25 wt% of substantially linear polymer.
- In one embodiment, the substantially linear polymer may be a copolymer comprising units derived from monomers (i) one or more alpha olefins and (ii) one or more alkyl (meth)acrylate esters. In another embodiment the substantially linear polymer includes a mixtures of (a) a copolymer comprising units derived from monomers of (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with a primary alcohol, and (b) a poly(meth)acrylate.
- The poly(meth)acrylate (typically a polymethacrylate) can have units derived from a mixture of alkyl (meth)acrylate ester monomers containing (a) 8 to 24, or 12 to 18, or to 15 carbon atoms in the alcohol-derived portion of the ester group and (b) 6 to 11, or 8 to 11, or 8 carbon atoms in the alcohol-derived portion of the ester group, and which have 2-(C1-4 alkyl)-substituents, and optionally, at least one monomer selected from the group consisting of (meth)acrylic acid esters containing 1 to 7 carbon atoms in the alcohol-derived portion of the ester group and which are different from (meth)acrylic acid esters (a) and (b), vinyl aromatic compounds (or vinyl aromatic monomers); and nitrogen-containing vinyl monomers; provided that no more than 60% by weight, or no more than 50% by weight, or no more than 35% by weight of the esters contain not more than 10 carbon atoms in the alcohol-derived portion of the ester group. The linear polymer of this type is described in more detail in
US 6,124,249 orEP 0 937 769 A1amount 0% to 25% of the polymer composition, or from 1% to 15% of the composition, 2% to 10% of the composition, or even from 1% to 3% of the composition. - Typically, the mole ratio of esters (a) to esters (b) in the copolymer ranges from 95:5 to 35:65, or 90:10 to 60:40, or 80:20 to 50:50.
- The esters are usually aliphatic esters, typically alkyl esters. In one embodiment the ester of (a) may be a C12-15 alkyl methacrylate and the ester of (b) may be 2-ethylhexyl methacrylate.
- In one embodiment, the ester groups in ester (a) contain branched alkyl groups. The ester groups may contain 2 to 65%, or 5 to 60% or greater of the ester groups having branched alkyl groups.
- The C1-4 alkyl substituents may be methyl, ethyl, and any isomers of propyl and butyl.
- The weight average molecular weight of the poly(meth)acrylate may be 45,000 or less, or 35,000 or less, or 25,000 or less, or 8000 to 25,000, or 12,000 to 20,000.
- In one embodiment the substantially linear polymer includes a copolymer comprising units derived from monomers (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with a primary alcohol branched at the β- or higher position, wherein the copolymer typically has a reduced specific viscosity of up to 0.2, or up to 0.15, or up to 0.10, or up to 0.08. In one embodiment the reduced specific viscosity may be up to 0.08 (or 0.02 to 0.08 (or 0.02 to 0.07, 0.03 to 0.07 or 0.04 to 0.06).
- A measurement correlating with molecular weight of the copolymer (or interpolymer such as an alternating copolymer) may be expressed in terms of the "reduced specific viscosity" of the copolymer which is a recognized means of expressing the molecular size of a polymeric substance. As used herein, the reduced specific viscosity (abbreviated as RSV) is the value typically obtained in accordance with the formula RSV = (Relative Viscosity - 1)/Concentration, wherein the relative viscosity is determined by measuring, by means of a dilution viscometer, the viscosity of a solution of 1.6 g of the polymer in 100 cm3 of acetone and the viscosity of acetone at 30°C. For purpose of computation by the above formula, the concentration is adjusted to 1.6 g of the copolymer per 100 cm3 of acetone. A more detailed discussion of the reduced specific viscosity, also known as the specific viscosity, as well as its relationship to the average molecular weight of a copolymer, appears in Paul J. Flory, Principles of Polymer Chemistry, (1953 Edition) pages 308 et seq.
- In one embodiment the copolymer may be derived from monomers (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof,
wherein 0.1 to 99.89% of the carboxylic acid units are esterified with a primary alcohol branched at the β- or higher position,
wherein 0.1 to 99.89% of the carboxylic acid units are esterified with a linear alcohol or an alpha-branched alcohol (e.g., a secondary alcohol),
wherein 0.01 to 10% of the carboxylic acid units has at least one of an amino-, amido- and/or imido- group, and
wherein the copolymer has a reduced specific viscosity (prior to esterification) of up to 0.08. - In one embodiment the copolymer may be derived from monomers (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof,
wherein 0.1 to 99.89% of the carboxylic acid units are esterified with a primary alcohol branched at the β- or higher position,
wherein 0.1 to 99.9% of the carboxylic acid units are esterified with a linear alcohol or an alpha-branched alcohol,
wherein 0 to 10% of the carboxylic acid units has at least one of an amino-, amido- and/or imido- group, and
wherein the copolymer has a reduced specific viscosity of up to 0.08. - A linear alcohol may include methanol, ethanol, propanol, butanol, pentanol, hexanol, heptanol, octanol, nonanol, decanol, undecanol, dodecanol, tridecanol, tetradecanol, pentadecanol, hexadecanol, heptadecanol, octadecanol, nonadecanol, eicosanol, or mixtures thereof. In one embodiment the linear alcohol contains 6 to 30, or 8 to 20, or 8 to 15 carbon atoms (typically 8 to 15 carbon atoms).
- The linear alcohol may include commercially available materials such as Oxo Alcohol® 7911, Oxo Alcohol® 7900 and
Oxo Alcohol® 1 100 of Monsanto; Alphanol® 79 of ICI; Nafol® 1620, Alfol® 610 and Alfol® 810 of Condea (now Sasol); Epal® 610 and Epal® 810 of Ethyl Corporation (now Afton); Linevol® 79, Linevol® 911 and Dobanol® 25 L of Shell AG; Lial® 125 of Condea Augusta, Milan; Dehydad® and Lorol® of Henkel KGaA (now Cognis) as well as Linopol® 7-11 and Acropol® 91 of Ugine Kuhlmann. - In one embodiment the copolymer may be derived from monomers of (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof,
wherein 5 to 15% of the carboxylic acid units are esterified with a primary alcohol branched at the β- or higher position,
wherein 0.1 to 95% of the carboxylic acid units are esterified with a linear alcohol or an alpha-branched alcohol,
wherein 0 to less than 2% of the carboxylic acid units has at least one of an amino-, amido- and/or imido- group, and
wherein the copolymer has a reduced specific viscosity of up to 0.08. - In one embodiment the copolymer comprises units derived from monomers (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with a primary alcohol branched at the β- or higher position. In certain embodiments the copolymer may be represented by the formula below. Ester or other groups with the primary alcohol-derived moiety branched at the β- or higher position may be represented within the ( )w shown in the formula:
- Formula (I) may comprise a copolymer backbone (BB), and one or more pendant groups as shown, wherein BB may be derived from a copolymer of (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof (typically fumaric acid, maleic anhydride, maleic acid, (meth)acrylic acid, itaconic anhydride or itaconic acid);
- X may be a functional group which either (i) contains a carbon and at least one oxygen or nitrogen atom (such as an ester or amide, or imide linkage), or (ii) is an alkylene group with 1 to 5 carbon atoms (typically -CH2-), connecting the copolymer backbone and a branched hydrocarbyl group contained within ( )y, (typically X may be a functional group which either (i) contains a carbon and at least one oxygen or nitrogen atom);
- w may be the number of pendant groups attached to the copolymer backbone, which may be in the range of 2 to 2000, or 2 to 500, or 5 to 250;
- y may be 0, 1, 2 or 3, provided that in at least 1 mol% of the pendant groups, y is not zero; and with the proviso that when y is 0, X is bonded to a terminal group in a manner sufficient to satisfy the valence of X, wherein the terminal group is selected from hydrogen, alkyl, aryl, a metal (typically introduced during neutralization of ester reactions; suitable metals include calcium, magnesium, barium, zinc, sodium, potassium or lithium) or ammonium cation, and mixtures thereof;
- p may be an integer in the range of 1 to 15 (or 1 to 8, or 1 to 4); and
- R' and R" may independently be linear or branched hydrocarbyl groups, and the combined total number of carbon atoms present in R' and R" may be at least 12 (or at least 16, or at least 18 or at least 20).
- In different embodiments the copolymer with pendant groups may contain 0.10% to 100%, or 0.5% to 20%, or 0.75% to 10%, branched hydrocarbyl groups represented by a group within ( )y of the formula (I) above, expressed as a percentage of the total number of pendant groups . The pendant groups of formula (1) may also be used to define the ester groups as defined above by the phrase "esterified with a primary alcohol branched at the β- or higher position".
- In different embodiments the functional groups defined by X on the formula above, may comprise at least one of -CO2-, -C(O)N= or -(CH2)V-, wherein v is an integer in the range of 1 to 20, or 1 to 10, or 1 to 2.
- In one embodiment X may be derived from an ethylenically unsaturated carboxylic acid or derivatives thereof. Examples of a suitable carboxylic acid or derivatives thereof typically include maleic anhydride, maleic acid, (meth)acrylic acid, itaconic anhydride or itaconic acid. In one embodiment the ethylenically unsaturated carboxylic acid or derivatives thereof may be at least one of maleic anhydride or maleic acid.
- In one embodiment X is other than an alkylene group, connecting the copolymer backbone and the branched hydrocarbyl groups.
- In different embodiments the pendant groups may be esterified, amidated or imidated functional groups.
- In one embodiment the pendant groups may be derived from esterified and/or amidated functional groups.
- In one embodiment the copolymer includes esterified pendant groups. The pendant groups may be derived from Guerbet alcohols. The Guerbet alcohols may contain 10 to 60, or 12 to 60, or 16 to 40 carbon atoms. In one embodiment the primary alcohol branched at the β- or higher position described herein may be a Guerbet alcohol. Methods to prepare Guerbet alcohols are disclosed in
U.S. Patent No. 4,767,815 . - Examples of suitable groups for R' and R"on the formula defined above include the following:
- 1) alkyl groups containing C15-16 polymethylene groups, such as 2-C1-15 alkyl-hexadecyl groups (e.g. 2-octylhexadecyl) and 2-alkyl-octadecyl groups (e.g., 2-ethyloctadecyl, 2-tetradecyl-octadecyl and 2-hexadecyloctadecyl);
- 2) alkyl groups containing C13-14 polymethylene groups, such as 1-C1-15 alkyl-tetradecyl groups (e.g., 2-hexyltetradecyl, 2-decyltetradecyl and 2-undecyltridecyl) and 2-C1-15 alkyl-hexadecyl groups (e.g., 2-ethyl-hexadecyl and 2-dodecylhexadecyl);
- 3) alkyl groups containing C10-12 polymethylene groups, such as 2-C1-15 alkyl-dodecyl groups (e.g., 2-octyldodecyl) and 2-C1-15 alkyl-dodecyl groups (2-hexyldodecyl and 2-octyldodecyl), 2-C1-15 alkyl-tetradecyl groups (e.g., 2-hexyltetradecyl and 2-decyltetradecyl);
- 4) alkyl groups containing C6-9 polymethylene groups, such as 2-C1-15 alkyl-decyl groups (e.g., 2-octyldecyl) and 2,4-di-C1-15 alkyl-decyl groups (e.g., 2-ethyl-4-butyl-decyl group);
- 5) alkyl groups containing C1-5 polymethylene groups, such as 2-(3-methylhexyl)-7-methyl-decyl and 2-(1,4-dimethylbutyl)-5,7,7-trimethyl-octyl groups; and
- 6) and mixtures of two or more branched alkyl groups, such as alkyl residues of oxoalcohols corresponding to propylene oligomers (from hexamer to undecamer), ethylene/propylene (molar ratio 16:1-1:11) oligomers, iso-butene oligomers (from pentamer to octamer), C5-17 α-olefin oligomers (from dimer to hexamer).
- The pendant groups may contain a total combined number of carbon atoms on R' and R"in the range of 12 to 60, or 14 to 50, or 16 to 40, or 18 to 40, or 20 to 36.
- Each of R' and R"may individually contain 5 to 25, or 8 to 32, or 10 to 18 methylene carbon atoms. In one embodiment the number of carbon atoms on each R' and R"group maybe 10 to 24.
- Examples of suitable primary alcohol branched at the β- or higher position include 2-ethylhexanol, 2-propyl heptanol, 2-butyloctanol, 2-hexyldecanol, 2-octyldodecanol, 2-decyltetradecanol, or mixtures thereof.
- The ethylenically unsaturated carboxylic acid or derivatives thereof may be an acid or anhydride or derivatives thereof that may be wholly esterified, partially esterified or mixtures thereof. When partially esterified, other functional groups include acids, salts or mixtures thereof. Suitable salts include alkali metals, alkaline earth metals or mixtures thereof. The salts include lithium, sodium, potassium, magnesium, calcium or mixtures thereof. The unsaturated carboxylic acid or derivatives thereof includes acrylic acid, methyl acrylate, methacrylic acid, maleic acid or anhydride, fumaric acid, itaconic acid or anhydride or mixtures thereof, or substituted equivalents thereof.
- Suitable examples of the ethylenically unsaturated carboxylic acid or derivatives thereof include itaconic anhydride, maleic anhydride, methyl maleic anhydride, ethyl maleic anhydride, dimethyl maleic anhydride or mixtures thereof.
- In one embodiment the ethylenically unsaturated carboxylic acid or derivatives thereof includes maleic anhydride or derivatives thereof.
- Examples of an alpha-olefin include 1-decene, 1-undecene, 1-dodecene, 1-tridecene, 1-tetradecene, 1-pentadecene, 1-hexadecene, 1-hepta-decene, 1-octadecene, or mixtures thereof. An example of a useful alpha-olefm is 1-dodecene. The alpha-olefin may be a branched alpha-olefin, or mixtures thereof. If the α-olefin is branched, the number of carbon atoms of the α-olefin may range from 4 to 32, or 6 to 20, or 8 to 16.
- In one embodiment the copolymer of the disclosure further includes a nitrogen containing group such as those disclosed above. The nitrogen containing group may be derived from a nitrogen containing compound capable of being incorporated during copolymerization. In one embodiment the copolymer of the disclosure further includes a nitrogen containing group that may be capable of reacting with the functionalized copolymer backbone, typically for capping the copolymer backbone. The capping may result in the copolymer having ester, amide, imide or amine groups. The nitrogen group is described in more detail in PCT Patent Application No.
PCT/US09/052028 - In one embodiment the copolymer comprises units derived from monomers (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof may be further reacted with an amine to additionally provide oxidation control. Typically, the copolymer with oxidation control contains an incorporated residue of an amine-containing compound such as morpholines, pyrrolidinones, imidazolidinones, acetamides, β-alanine alkyl esters, or mixtures thereof. Examples of suitable nitrogen-containing compounds include 3-morpholin-4-yl-propylamine, 3-morpholin-4-yl-ethylamine, [beta]-alanine alkyl esters (typically alkyl esters have 1 to 30, or 6 to 20 carbon atoms), or mixtures thereof.
-
- X = -OH or NH2;
- Hy" may be hydrogen, or a hydrocarbyl group (typically alkyl, or C1-4-, or C2-- alkyl); Hy may be a hydrocarbylene group (typically alkylene, or C1-4-, or C2- alkylene); Q = >NH, >NR, >CH2, >CHR, >CR2, or -O- (typically >NH, or >NR) and R may be C1-4 alkyl.
- In one embodiment the imidazolidinone includes 1-(2-amino-ethyl)-imidazolidin-2-one (may also be called aminoethylethyleneurea), 1-(3-amino-propyl)-imidazolidin-2-one, 1-(2-hydroxy-ethyl)-imidazolidin-2-one, 1-(3-amino-propyl)-pyrrolidin-2-one, 1-(3-amino-ethyl)-pyrrolidin-2-one, or mixtures thereof.
- In one embodiment the copolymer may be reacted with an amine-containing compound selected from morpholines, imidazolidinones, and mixtures thereof.
- Other illustrative copolymers and interpolymers useful as viscosity modifiers of this disclosure are described, for example, in
U.S. Patent Application Publication Nos. 2010/0144566 and2011/0190182 , and alsoWO 2011/066242 . - The formulated lubricating oil useful in the present disclosure may additionally contain one or more of the other commonly used lubricating oil performance additives including but not limited to dispersants, other detergents, corrosion inhibitors, rust inhibitors, metal deactivators, other antiwear agents and/or extreme pressure additives, anti-seizure agents, wax modifiers, viscosity index improvers, fluid-loss additives, seal compatibility agents, other friction modifiers, lubricity agents, antistaining agents, chromophoric agents, defoamants, demulsifiers, emulsifiers, densifiers, wetting agents, gelling agents, tackiness agents, colorants, and others. For a review of many commonly used additives, see Klamann in Lubricants and Related Products, Verlag Chemie, Deerfield Beach, FL; ISBN 0-89573-177-0. Reference is also made to "Lubricant Additives" by M. W. Ranney, published by Noyes Data Corporation of Parkridge, NJ (1973).
- The types and quantities of performance additives used in combination with the instant disclosure in lubricant compositions are not limited by the examples shown herein as illustrations.
- Typical antioxidant include phenolic antioxidants, aminic antioxidants and oil-soluble copper complexes.
- The phenolic antioxidants include sulfurized and non-sulfurized phenolic antioxidants. The terms "phenolic type" or "phenolic antioxidant" used herein includes compounds having one or more than one hydroxyl group bound to an aromatic ring which may itself be mononuclear, e.g., benzyl, or poly-nuclear, e.g., naphthyl and spiro aromatic compounds. Thus "phenol type" includes phenol per se, catechol, resorcinol, hydroquinone, naphthol, etc., as well as alkyl or alkenyl and sulfurized alkyl or alkenyl derivatives thereof, and bisphenol type compounds including such bi-phenol compounds linked by alkylene bridges sulfuric bridges or oxygen bridges. Alkyl phenols include mono- and poly-alkyl or alkenyl phenols, the alkyl or alkenyl group containing from 3-100 carbons, preferably 4 to 50 carbons and sulfurized derivatives thereof, the number of alkyl or alkenyl groups present in the aromatic ring ranging from 1 to up to the available unsatisfied valences of the aromatic ring remaining after counting the number of hydroxyl groups bound to the aromatic ring.
- Generally, therefore, the phenolic anti-oxidant may be represented by the general formula:
(R)x-Ar-(OH)y
where Ar is selected from the group consisting of: - Preferred phenolic antioxidant compounds are the hindered phenolics and phenolic esters which contain a sterically hindered hydroxyl group, and these include those derivatives of dihydroxy aryl compounds in which the hydroxyl groups are in the o- or p-position to each other. Typical phenolic anti-oxidants include the hindered phenols substituted with C1+ alkyl groups and the alkylene coupled derivatives of these hindered phenols. Examples of phenolic materials of this type 2-t-butyl-4-heptyl phenol; 2-t-butyl-4-octyl phenol; 2-t-butyl-4-dodecyl phenol; 2,6-di-t-butyl-4-heptyl phenol; 2,6-di-t-butyl-4-dodecyl phenol; 2-methyl-6-t-butyl-4-heptyl phenol; 2-methyl-6-t-butyl-4-dodecyl phenol; 2,6-di-t-butyl-4 methyl phenol; 2,6-di-t-butyl-4-ethyl phenol; and 2,6-di-t-
butyl 4 alkoxy phenol; and - Phenolic type antioxidants are well known in the lubricating industry and commercial examples such as Ethanox® 4710, Irganox® 1076, Irganox® L1035, Irganox® 1010, Irganox® L109, Irganox® L118, Irganox® L135 and the like are familiar to those skilled in the art. The above is presented only by way of exemplification, not limitation on the type of phenolic anti-oxidants which can be used.
- The phenolic antioxidant can be employed in an amount in the range of 0.1 to 3 wt%, preferably 0.25 to 2.5 wt%, more preferably 0.5 to 2 wt% on an active ingredient basis.
- Aromatic amine antioxidants include phenyl-α-naphthyl amine which is described by the following molecular structure:
- Other aromatic amine antioxidants include other alkylated and non-alkylated aromatic amines such as aromatic monoamines of the formula R8R9R10N where R8 is an aliphatic, aromatic or substituted aromatic group, R9 is an aromatic or a substituted aromatic group, and R10 is H, alkyl, aryl or R11S(O)xR12 where R11 is an alkylene, alkenylene, or aralkylene group, R12 is a higher alkyl group, or an alkenyl, aryl, or alkaryl group, and x is 0, 1 or 2. The aliphatic group R8 may contain from 1 to 20 carbon atoms, and preferably contains from 6 to 12 carbon atoms. The aliphatic group is a saturated aliphatic group. Preferably, both R8 and R9 are aromatic or substituted aromatic groups, and the aromatic group may be a fused ring aromatic group such as naphthyl. Aromatic groups R8 and R9 may be joined together with other groups such as S.
- Typical aromatic amines anti-oxidants have alkyl substituent groups of at least 6 carbon atoms. Examples of aliphatic groups include hexyl, heptyl, octyl, nonyl, and decyl. Generally, the aliphatic groups will not contain more than 14 carbon atoms. The general types of such other additional amine antioxidants which may be present include diphenylamines, phenothiazines, imidodibenzyls and diphenyl phenylene diamines. Mixtures of two or more of such other additional aromatic amines may also be present. Polymeric amine antioxidants can also be used.
- Another class of antioxidant used in lubricating oil compositions and which may also be present are oil-soluble copper compounds. Any oil-soluble suitable copper compound may be blended into the lubricating oil. Examples of suitable copper antioxidants include copper dihydrocarbyl thio- or dithio-phosphates and copper salts of carboxylic acid (naturally occurring or synthetic). Other suitable copper salts include copper dithiacarbamates, sulphonates, phenates, and acetylacetonates. Basic, neutral, or acidic copper Cu(I) and or Cu(II) salts derived from alkenyl succinic acids or anhydrides are known to be particularly useful.
- Such antioxidants may be used individually or as mixtures of one or more types of antioxidants, the total amount employed being an amount of 0.50 to 5 wt%, preferably 0.75 to 3 wt% (on an as-received basis).
- In addition to the alkali or alkaline earth metal salicylate detergent which is an optional component in the present disclosure, other detergents may also be present. While such other detergents can be present, it is preferred that the amount employed be such as to not interfere with the synergistic effect attributable to the presence of the salicylate. Therefore, most preferably such other detergents are not employed.
- If such additional detergents are present, they can include alkali and alkaline earth metal phenates, sulfonates, carboxylates, phosphonates and mixtures thereof. These supplemental detergents can have total base number (TBN) ranging from neutral to highly overbased, i.e. TBN of 0 to over 500, preferably 2 to 400, more preferably 5 to 300, and they can be present either individually or in combination with each other in an amount in the range of from 0 to 10 wt%, preferably 0.5 to 5 wt% (active ingredient) based on the total weight of the formulated lubricating oil. As previously stated, however, it is preferred that such other detergent not be present in the formulation.
- Such additional other detergents include by way of example and not limitation calcium phenates, calcium sulfonates, magnesium phenates, magnesium sulfonates and other related components (including borated detergents).
- During engine operation, oil-insoluble oxidation byproducts are produced. Dispersants help keep these byproducts in solution, thus diminishing their deposition on metal surfaces. Dispersants may be ashless or ash-forming in nature. Preferably, the dispersant is ashless. So called ashless dispersants are organic materials that form substantially no ash upon combustion. For example, non-metal-containing or borated metal-free dispersants are considered ashless. In contrast, metal-containing detergents discussed above form ash upon combustion.
- Suitable dispersants typically contain a polar group attached to a relatively high molecular weight hydrocarbon chain. The polar group typically contains at least one element of nitrogen, oxygen, or phosphorus. Typical hydrocarbon chains contain 50 to 400 carbon atoms.
- A particularly useful class of dispersants are the alkenylsuccinic derivatives, typically produced by the reaction of a long chain substituted alkenyl succinic compound, usually a substituted succinic anhydride, with a polyhydroxy or polyamino compound. The long chain group constituting the oleophilic portion of the molecule which confers solubility in the oil, is normally a polyisobutylene group. Many examples of this type of dispersant are well known commercially and in the literature. Exemplary U.S. patents describing such dispersants are
3,172,892 ;3,215,707 ;3,219,666 ;3,316,177 ;3,341,542 ;3,444,170 ;3,454,607 ;3,541,012 ;3,630,904 ;3,632,511 ;3,787,374 and4,234,435 . Other types of dispersant are described inU.S. Pat. Nos. 3,036,003 ;3,200,107 ;3,254,025 ;3,275,554 ;3,438,757 ;3,454,555 ;3,565,804 ;3,413,347 ;3,697,574 ;3,725,277 ;3,725,480 ;3,726,882 ;4,454,059 ;3,329,658 ;3,449,250 ;3,519,565 ;3,666,730 ;3,687,849 ;3,702,300 ;4,100,082 ;5,705,458 . A further description of dispersants may be found, for example, in European Patent Application No.471 071 - Hydrocarbyl-substituted succinic acid compounds are popular dispersants. In particular, succinimide, succinate esters, or succinate ester amides prepared by the reaction of a hydrocarbon-substituted succinic acid compound preferably having at least 50 carbon atoms in the hydrocarbon substituent, with at least one equivalent of an alkylene amine are particularly useful.
- Succinimides are formed by the condensation reaction between alkenyl succinic anhydrides and amines. Molar ratios can vary depending on the amine or polyamine. For example, the molar ratio of alkenyl succinic anhydride to TEPA can vary from 1:1 to 5:1.
- Succinate esters are formed by the condensation reaction between alkenyl succinic anhydrides and alcohols or polyols. Molar ratios can vary depending on the alcohol or polyol used. For example, the condensation product of an alkenyl succinic anhydride and pentaerythritol is a useful dispersant.
- Succinate ester amides are formed by condensation reaction between alkenyl succinic anhydrides and alkanol amines. For example, suitable alkanol amines include ethoxylated polyalkylpolyamines, propoxylated polyalkylpolyamines and polyalkenylpolyamines such as polyethylene polyamines. One example is propoxylated hexamethylenediamine.
- The molecular weight of the alkenyl succinic anhydrides will typically range between 800 and 2,500. The above products can be post-reacted with various reagents such as sulfur, oxygen, formaldehyde, carboxylic acids such as oleic acid, and boron compounds such as borate esters or highly borated dispersants. The dispersants can be borated with from 0.1 to 5 moles of boron per mole of dispersant reaction product.
- Mannich base dispersants are made from the reaction of alkylphenols, formaldehyde, and amines. Process aids and catalysts, such as oleic acid and sulfonic acids, can also be part of the reaction mixture. Molecular weights of the alkylphenols range from 800 to 2,500 or more.
- Typical high molecular weight aliphatic acid modified Mannich condensation products can be prepared from high molecular weight alkyl-substituted hydroxyaromatics or HN(R)2 group-containing reactants.
- Examples of high molecular weight alkyl-substituted hydroxyaromatic compounds are polypropylphenol, polybutylphenol, and other polyalkylphenols. These polyalkylphenols can be obtained by the alkylation, in the presence of an alkylating catalyst, such as BF3, of phenol with high molecular weight polypropylene, polybutylene, and other polyalkylene compounds to give alkyl substituents on the benzene ring of phenol having an average 600-100,000 molecular weight.
- Examples of HN(R)2 group-containing reactants are alkylene polyamines, principally polyethylene polyamines. Other representative organic compounds containing at least one HN(R)2 group suitable for use in the preparation of Mannich condensation products are well known and include the mono- and di-amino alkanes and their substituted analogs, e.g., ethylamine and diethanol amine; aromatic diamines, e.g., phenylene diamine, diamino naphthalenes; heterocyclic amines, e.g., morpholine, pyrrole, pyrrolidine, imidazole, imidazolidine, and piperidine; melamine and their substituted analogs.
- Examples of alkylene polyamine reactants include ethylenediamine, diethylene triamine, triethylene tetraamine, tetraethylene pentaamine, pentaethylene hexamine, hexaethylene heptaamine, heptaethylene octaamine, octaethylene nonaamine, nonaethylene decamine, and decaethylene undecamine and mixture of such amines having nitrogen contents corresponding to the alkylene polyamines, in the formula H2N-(Z-NH-)nH, mentioned before, Z is a divalent ethylene and n is 1 to 10 of the foregoing formula. Corresponding propylene polyamines such as propylene diamine and di-, tri-, tetra-, pentapropylene tri-, tetra-, penta- and hexaamines are also suitable reactants. The alkylene polyamines are usually obtained by the reaction of ammonia and dihalo alkanes, such as dichloro alkanes. Thus the alkylene polyamines obtained from the reaction of 2 to 11 moles of ammonia with 1 to 10 moles of dichloroalkanes having 2 to 6 carbon atoms and the chlorines on different carbons are suitable alkylene polyamine reactants.
- Aldehyde reactants useful in the preparation of the high molecular products useful in this disclosure include the aliphatic aldehydes such as formaldehyde (also as paraformaldehyde and formalin), acetaldehyde and aldol (β-hydroxybutyraldehyde). Formaldehyde or a formaldehyde-yielding reactant is preferred.
- Preferred dispersants include borated and non-borated succinimides, including those derivatives from mono-succinimides, bis-succinimides, and/or mixtures of mono- and bis-succinimides, wherein the hydrocarbyl succinimide is derived from a hydrocarbylene group such as polyisobutylene having a Mn of from 500 to 5000 or more or a mixture of such hydrocarbylene groups. Other preferred dispersants include succinic acid-esters and amides, alkylphenol-polyamine-coupled Mannich adducts, their capped derivatives, and other related components. Such additives may be used in an amount of 0.1 to 20 wt%, preferably 0.1 to 8 wt%, more preferably 1 to 6 wt% (on an as-received basis) based on the weight of the total lubricant.
- Conventional pour point depressants (also known as lube oil flow improvers) may also be present. Pour point depressant may be added to lower the minimum temperature at which the fluid will flow or can be poured. Examples of suitable pour point depressants include alkylated naphthalenes polymethacrylates, polyacrylates, polyarylamides, condensation products of haloparaffin waxes and aromatic compounds, vinyl carboxylate polymers, and terpolymers of dialkylfumarates, vinyl esters of fatty acids and allyl vinyl ethers. Such additives may be used in amount of 0.0 to 0.5 wt%, preferably 0 to 0.3 wt%, more preferably 0.001 to 0.1 wt% on an as-received basis.
- Corrosion inhibitors are used to reduce the degradation of metallic parts that are in contact with the lubricating oil composition. Suitable corrosion inhibitors include aryl thiazines, alkyl substituted dimercapto thiodiazoles thiadiazoles and mixtures thereof. Such additives may be used in an amount of 0.01 to 5 wt%, preferably 0.01 to 1.5 wt%, more preferably 0.01 to 0.2 wt%, still more preferably 0.01 to 0.1 wt% (on an as-received basis) based on the total weight of the lubricating oil composition.
- Seal compatibility agents help to swell elastomeric seals by causing a chemical reaction in the fluid or physical change in the elastomer. Suitable seal compatibility agents for lubricating oils include organic phosphates, aromatic esters, aromatic hydrocarbons, esters (butylbenzyl phthalate, for example), and polybutenyl succinic anhydride and sulfolane-type seal swell agents such as Lubrizol 730-type seal swell additives. Such additives may be used in an amount of 0.01 to 3 wt%, preferably 0.01 to 2 wt% on an as-received basis.
- Anti-foam agents may advantageously be added to lubricant compositions. These agents retard the formation of stable foams. Silicones and organic polymers are typical anti-foam agents. For example, polysiloxanes, such as silicon oil or polydimethyl siloxane, provide antifoam properties. Anti-foam agents are commercially available and may be used in conventional minor amounts along with other additives such as demulsifiers; usually the amount of these additives combined is less than 1 percent, preferably 0.001 to 0.5 wt%, more preferably 0.001 to 0.2 wt%, still more preferably 0.0001 to 0.15 wt% (on an as-received basis) based on the total weight of the lubricating oil composition.
- Antirust additives (or corrosion inhibitors) are additives that protect lubricated metal surfaces against chemical attack by water or other contaminants. One type of antirust additive is a polar compound that wets the metal surface preferentially, protecting it with a film of oil. Another type of antirust additive absorbs water by incorporating it in a water-in-oil emulsion so that only the oil touches the surface. Yet another type of antirust additive chemically adheres to the metal to produce a nonreactive surface. Examples of suitable additives include zinc dithiophosphates, metal phenolates, basic metal sulfonates, fatty acids and amines. Such additives may be used in an amount of 0.01 to 5 wt%, preferably 0.01 to 1.5 wt% on an as-received basis.
- The term "organo molybdenum-nitrogen complexes" embraces the organo molybdenum-nitrogen complexes described in
U.S. Patent 4,889,647 . The complexes are reaction products of a fatty oil, dithanolamine and a molybdenum source. Specific chemical structures have not been assigned to the complexes.U.S. Patent 4,889,647 reports an infrared spectrum for a typical reaction product of that disclosure; the spectrum identifies an ester carbonyl band at 1740 cm-1 and an amide carbonyl band at 1620 cm-1. The fatty oils are glyceryl esters of higher fatty acids containing at least 12 carbon atoms up to 22 carbon atoms or more. The molybdenum source is an oxygen-containing compound such as ammonium molybdates, molybdenum oxides and mixtures. - Other organo molybdenum complexes which can be used in the present disclosure are tri-nuclear molybdenum-sulfur compounds described in
EP 1 040 115WO 99/31113 U.S. Patent No. 4,978,464 . - In addition to the copolymers described herein as part of the disclosure the lubricating composition may optionally further contain other known viscosity modifiers. The viscosity modifiers may be hydrogenated styrene-butadiene rubbers, ethylenepropylene copolymers, hydrogenated styrene-isoprene polymers, hydrogenated diene polymers, polyalkyl styrenes, polyolefins, esters of maleic anhydride-styrene copolymers, or mixtures thereof.
- The lubricating compositions can include at least one antiwear agent. Examples of suitable antiwear agents include oil soluble amine salts of phosphorus compounds, sulphurised olefins, metal dihydrocarbyldithio-phosphates (such as zinc dialkyldithiophosphates), thiocarbamate-containing compounds, such as thiocarbamate esters, thiocarbamate amides, thiocarbamic ethers, alkylene-coupled thiocarbamates, and bis(S-alkyldithiocarbamyl) disulphides.
- In one embodiment the oil soluble phosphorus amine salt antiwear agent includes an amine salt of a phosphorus acid ester or mixtures thereof. The amine salt of a phosphorus acid ester includes phosphoric acid esters and amine salts thereof; dialkyldithiophosphoric acid esters and amine salts thereof; amine salts of phosphites; and amine salts of phosphorus-containing carboxylic esters, ethers, and amides; and mixtures thereof. The amine salt of a phosphorus acid ester may be used alone or in combination.
- In one embodiment the oil soluble phosphorus amine salt includes partial amine salt-partial metal salt compounds or mixtures thereof. In one embodiment the phosphorus compound further includes a sulphur atom in the molecule. In one embodiment the amine salt of the phosphorus compound may be ashless, i.e., metal-free (prior to being mixed with other components).
- The amines which may be suitable for use as the amine salt include primary amines, secondary amines, tertiary amines, and mixtures thereof. The amines include those with at least one hydrocarbyl group, or, in certain embodiments, two or three hydrocarbyl groups. The hydrocarbyl groups may contain 2 to 30 carbon atoms, or in
other embodiments 8 to 26, or 10 to 20, or 13 to 19 carbon atoms. - Primary amines include ethylamine, propylamine, butylamine, 2-ethylhexylamine, octylamine, and dodecylamine, as well as such fatty amines as n-octylamine, n-decylamine, n-dodecylamine, n-tetradecylamine, n-hexadecylamine, n-octadecylamine and oleyamine. Other useful fatty amines include commercially available fatty amines such as "Armeen®" amines (products available from Akzo Chemicals, Chicago, Illinois), such as Armeen C, Armeen O, Armeen OL, Armeen T, Armeen HT, Armeen S and Armeen SD, wherein the letter designation relates to the fatty group, such as coco, oleyl, tallow, or stearyl groups.
- Examples of suitable secondary amines include dimethylamine, diethylamine, dipropylamine, dibutylamine, diamylamine, dihexylamine, diheptylamine, methylethylamine, ethylbutylamine and ethylamylamine. The secondary amines may be cyclic amines such as piperidine, piperazine and morpholine.
- The amine may also be a tertiary-aliphatic primary amine. The aliphatic group in this case may be an alkyl group containing 2 to 30, or 6 to 26, or 8 to 24 carbon atoms. Tertiary alkyl amines include monoamines such as tert-butylamine, tert-hexylamine, 1-methyl-1-amino-cyclohexane, tert-octylamine, tert-decylamine, tertdodecylamine, tert-tetradecylamine, tert-hexadecylamine, tert-octadecylamine, tert-tetracosanylamine, and tert-octacosanylamine.
- In one embodiment the phosphorus acid amine salt includes an amine with C11 to C14 tertiary alkyl primary groups or mixtures thereof. In one embodiment the phosphorus acid amine salt includes an amine with C14 to C18 tertiary alkyl primary amines or mixtures thereof. In one embodiment the phosphorus acid amine salt includes an amine with C18 to C22 tertiary alkyl primary amines or mixtures thereof.
- Mixtures of amines may also be used in the disclosure. In one embodiment a useful mixture of amines is "Primene® 81R" and "Primene® JMT." Primene® 81R and Primene® JMT (both produced and sold by Rohm & Haas) are mixtures of C11 to C14 tertiary alkyl primary amines and C18 to C22 tertiary alkyl primary amines, respectively.
- In one embodiment oil soluble amine salts of phosphorus compounds include a sulphur-free amine salt of a phosphorus-containing compound may be obtained/obtainable by a process comprising: reacting an amine with either (i) a hydroxy-substituted di-ester of phosphoric acid, or (ii) a phosphorylated hydroxy-substituted di- or tri-ester of phosphoric acid. A more detailed description of compounds of this type is disclosed in International Application
PCT/US08/051126 . - In one embodiment the hydrocarbyl amine salt of an alkylphosphoric acid ester is the reaction product of a C14 to C18 alkylated phosphoric acid with Primene 81R™ (produced and sold by Rohm & Haas) which is a mixture of C11 to C14 tertiary alkyl primary amines.
- Examples of hydrocarbyl amine salts of dialkyldithiophosphoric acid esters include the reaction product(s) of isopropyl, methyl-amyl (4-methyl-2-pentyl or mixtures thereof), 2-ethylhexyl, heptyl, octyl or nonyl dithiophosphoric acids with ethylene diamine, morpholine, or Primene 81R™, and mixtures thereof.
- In one embodiment the dithiophosphoric acid may be reacted with an epoxide or a glycol. This reaction product is further reacted with a phosphorus acid, anhydride, or lower ester. The epoxide includes an aliphatic epoxide or a styrene oxide. Examples of useful epoxides include ethylene oxide, propylene oxide, butene oxide, octene oxide, dodecene oxide, and styrene oxide. In one embodiment the epoxide may be propylene oxide. The glycols may be aliphatic glycols having from 1 to 12, or from 2 to 6, or 2 to 3 carbon atoms. The dithiophosphoric acids, glycols, epoxides, inorganic phosphorus reagents and methods of reacting the same are described in
U.S. Patent Nos. 3,197,405 and3,544,465 . The resulting acids may then be salted with amines. An example of suitable dithiophosphoric acid is prepared by adding phosphorus pentoxide (64 grams) at 58°C over a period of 45 minutes to 514 grams ofhydroxypropyl 0,0-di(4-methyl-2-pentyl)phosphorodithioate (prepared by reacting di(4-methyl-2-pentyl)-phosphorodithioic acid with 1.3 moles of propylene oxide at 25°C). The mixture may be heated at 75°C for 2.5 hours, mixed with a diatomaceous earth and filtered at 70°C. The filtrate contains 11.8% by weight phosphorus, 15.2% by weight sulphur, and an acid number of 87 (bromophenol blue). - The dithiocarbamate-containing compounds may be prepared by reacting a dithiocarbamate acid or salt with an unsaturated compound. The dithiocarbamate containing compounds may also be prepared by simultaneously reacting an amine, carbon disulphide and an unsaturated compound. Generally, the reaction occurs at a temperature from 25°C to 125°C.
- Examples of suitable olefins that may be sulphurised to form an the sulphurised olefin include propylene, butylene, isobutylene, pentene, hexane, heptene, octane, nonene, decene, undecene, dodecene, undecyl, tridecene, tetradecene, pentadecene, hexadecene, heptadecene, octadecene, octadecenene, nonodecene, eicosene or mixtures thereof. In one embodiment, hexadecene, heptadecene, octadecene, octadecenene, nonodecene, eicosene or mixtures thereof and their dimers, trimers and tetramers are especially useful olefins. Alternatively, the olefin may be a Diels-Alder adduct of a diene such as 1,3-butadiene and an unsaturated ester, such as, butylacrylate.
- Another class of sulphurised olefin includes fatty acids and their esters. The fatty acids are often obtained from vegetable oil or animal oil; and typically contain 4 to 22 carbon atoms. Examples of suitable fatty acids and their esters include triglycerides, oleic acid, linoleic acid, palmitoleic acid or mixtures thereof. Often, the fatty acids are obtained from lard oil, tall oil, peanut oil, soybean oil, cottonseed oil, sunflower seed oil or mixtures thereof. In one embodiment fatty acids and/or ester are mixed with olefins.
- In an alternative embodiment, the ashless antiwear agent may be a monoester of a polyol and an aliphatic carboxylic acid, often an acid containing 12 to 24 carbon atoms. Often the monoester of a polyol and an aliphatic carboxylic acid is in the form of a mixture with a sunflower oil or the like, which may be present in the friction modifier mixture from 5 to 95, in several embodiments from 10 to 90, or from 20 to 85, or 20 to 80 weight percent of said mixture. The aliphatic carboxylic acids (especially a monocarboxylic acid) which form the esters are those acids typically containing 12 to 24, or from 14 to 20 carbon atoms. Examples of carboxylic acids include dodecanoic acid, stearic acid, lauric acid, behenic acid, and oleic acid.
- Polyols include diols, triols, and alcohols with higher numbers of alcoholic OH groups. Polyhydric alcohols include ethylene glycols, including di-, tri- and tetraethylene glycols; propylene glycols, including di-, tri- and tetrapropylene glycols; glycerol; butane diol; hexane diol; sorbitol; arabitol; mannitol; sucrose; fructose; glucose; cyclohexane diol; erythritol; and pentaerythritols, including di- and tripentaerythritol. Often the polyol is diethylene glycol, triethylene glycol, glycerol, sorbitol, penta erythritol or dipentaerythritol.
- The commercially available monoester known as "glycerol monooleate" is believed to include 60 + 5 percent by weight of the chemical species glycerol monooleate, along with 35 + 5 percent glycerol dioleate, and less than 5 percent trioleate and oleic acid. The amounts of the monoesters, described above, are calculated based on the actual, corrected, amount of polyol monoester present in any such mixture.
- Extreme Pressure (EP) agents that are soluble in the oil include sulphur- and chlorosulphur-containing EP agents, chlorinated hydrocarbon EP agents and phosphorus EP agents. Examples of such EP agents include chlorinated wax; sulphurised olefins (such as sulphurised isobutylene), organic sulphides and polysulphides such as dibenzyldisulphide, bis-(chlorobenzyl) disulphide, dibutyl tetrasulphide, sulphurised methyl ester of oleic acid, sulphurised alkylphenol, sulphurised dipentene, sulphurised terpene, and sulphurised Diels-Alder adducts; phosphosulphurised hydrocarbons such as the reaction product of phosphorus sulphide with turpentine or methyl oleate; phosphorus esters such as the dihydrocarbon and trihydrocarbon phosphites, e.g., dibutyl phosphite, diheptyl phosphite, dicyclohexyl phosphite, pentylphenyl phosphite; dipentylphenyl phosphite, tridecyl phosphite, distearyl phosphite and polypropylene substituted phenol phosphite; metal thiocarbamates such as zinc dioctyldithio carbamate and barium heptylphenol diacid; amine salts of alkyl and dialkylphosphoric acids or derivatives; and mixtures thereof (as described in
U.S. Patent No. 3,197,405 ). - The method and lubricating compositions of this disclosure may be suitable for greases, gear oils, axle oils, drive shaft oils, traction oils, manual transmission oils, automatic transmission oils, metal working fluids, hydraulic oils, or internal combustion engine oils.
- In one embodiment the method and lubricating composition of the disclosure may be suitable for at least one of gear oils, axle oils, drive shaft oils, traction oils, manual transmission oils or automatic transmission oils. In one embodiment the disclosure provides a method of lubricating a manual transmission.
- An automatic transmission includes continuously variable transmissions (CVT), infinitely variable transmissions (IVT), toroidal transmissions, continuously slipping torque converter clutches (CSTCC), stepped automatic transmissions or dual clutch transmissions (DCT).
- The internal combustion engines may be 2-stroke or 4-stroke engines. Suitable internal combustion engines include marine diesel engines, aviation piston engines, low-load diesel engines, and automobile and truck engines.
- As used herein, the term "(meth) acrylic" and related terms includes both acrylic and methacrylic groups.
- As used herein, the term "a primary alcohol branched at the β- or higher position" relates to an alcohol with branching at the 2-position or a higher position (e.g., 3-, or 4-, or 5-, or 6-, or 7-position, etc.).
- As used herein the number of carbon atoms present in the ester groups of the polymers of the disclosure is counted to include only those carbon atoms of the alcohol-derived portion of the ester group. Specifically, the number of carbon atoms excludes the carbonyl carbon of the ester.
- As used herein, the term "hydrocarbyl substituent" or "hydrocarbyl group" is used in its ordinary sense, which is well-known to those skilled in the art. Specifically, it refers to a group having a carbon atom directly attached to the remainder of the molecule and having predominantly hydrocarbon character. Examples of hydrocarbyl groups include: hydrocarbon substituents, including aliphatic, alicyclic, and aromatic substituents; substituted hydrocarbon substituents, that is, substituents containing non-hydrocarbon groups which, in the context of this disclosure, do not alter the predominantly hydrocarbon nature of the substituent; and hetero substituents, that is, substituents which similarly have a predominantly hydrocarbon character but contain other than carbon in a ring or chain.
- The following examples are for purposes of illustration only and are non-limiting examples.
- Lubricant compositions (i.e., axle oil blends) were prepared by blending a polyalphaolefin base stock (e.g., mPAO 700, mPAO 450, mPAO 300 ,
mPAO 150,mPAO 65,mPAO 14,PAO 70,PAO 4, and PAO 6), a hybrid olefin ester polymer (HOEP) viscosity modifier (Meridian™), and an axle oil additive package. The Meridian™ viscosity modifier is available from Lubrizol Corporation. The axle oil additive package used in Examples 1 and 2 is available from Lubrizol Corporation asAnglamol™ 6043M. The axle oil additive package used in Examples 3 and 4 is available from Afton Chemicals. - Wear control and load carrying capacity were determined for lubricant compositions prepared as described hereinabove. The results in
Fig. 1 show 4-Ball Wear Scar (ASTM D4172) is reduced when using the Meridian™ viscosity modifier alone or in combination withmPAO 150 ormPAO 65. A surprising aspect of this disclosure is that base oil and viscosity modifiers are not expected to have significant impact on wear or load-carrying properties of the fluid.Fig. 10 shows the results of 4-Ball Wear Scar (ASTM D4172) for the designated lubricant compositions (i.e., blends). - Over a broad viscosity range, a synergy was observed where mixtures of
mPAO 65 and Meridian™ gave higher Load Wear Index (LWI) than either high viscosity component alone (Fig. 2 ). The average friction coefficient measurements for multiple blends over a broad viscosity range (Fig. 3 ) show lower friction as the relative concentration of high viscosity components increase. Surprisingly, however, the combination ofmPAO 65 and Meridian™ showed higher friction than either component alone. This synergy implies the possibility of adjusting friction in applications such as transmissions, which require a specific balance of frictional characteristics, i.e., higher friction in some cases.Fig. 4 shows on average 25-30% lower traction coefficients for lubricatingcompositions containing mPAO 150,mPAO 65, and/or Meridian™ compared to a conventional viscosity modifier. Lower traction indicates potential axle efficiency enhancement and therefore improved fuel economy.Fig. 11 shows the results of 4-Ball EP (ASTM D2783) load wear index for the designated lubricant compositions (i.e., blends).Fig. 12 shows the results of High Frequency Reciprocating Rig (HFRR) friction coefficient for the designated lubricant compositions (i.e., blends). - Similar to Example 1 using a lubricating
composition containing mPAO 150 and Meridian™ (except for a different axle oil additive package), an unexpected synergy was observed as shown inFigs. 5 and6 . Keeping to total concentration of high viscosity material constant at 25 wt%, less viscosity loss was observed for combinations of mPAO and Meridian™ than for either component alone. This synergy was obvious in terms of absolute viscosity loss and percent loss and shown inFig. 5 . Results are illustrated graphically inFig. 6 . Durability of oil film due to enhanced shear stability results in consistent performance and protection of hardware over an extended period time compared to less shear stable fluids. For high dispersant treated systems such as the lubricating composition of this Example 3, this disclosure provides a method for improving shear stability of axle fluids. - Similar to Examples 1 and 3 using a lubricating
compositions containing mPAO 150 and Meridian™ (except for different axle oil additive packages),Fig. 7 indicates a potential antiwear/antiscuffing advantage for a Meridian™ viscosity modifier in the FZG scuffing test even in alower SAE 75W-80 viscosity formulation containing all Meridian™ as the viscosity modifier. A trend reflecting lower Total Gear weight loss with increased concentration of Meridian™ was observed in the FZG test. All formulations showed excellent performance by delivering ">Stage 12" passing results, a requirement for premium high performance gear oils. - Additional performance testing of these lower viscosity axle fluid formulations were conducted to assess the impact of the high VI synthetic components in areas such as oxidation stability and deposit control.
Fig. 8 shows surprisingly excellent sludge and varnish results for fluids evaluated in 100-hr L60-1 Thermal and Oxidative Stability test, which is double the standard duration (i.e., 50 hours) required for SAE J2360 high performance gear oil specification. The results clearly demonstrate that incorporation of the Meridian™ viscosity modifier in the lubricating compositions of this disclosure provides improved stability and cleanliness even for already high performing technology. This further substantiates the potential to formulation even higher performing lower viscosity finished products by incorporating new synthetic base fluids and viscosity modifiers. - Referring to
Fig. 9 , additional durability testing in a high speed shock test for axle/gear oils used to assess anti-scoring performance of fluids showed that even at lower kinematic viscosity @100°C, these oils still retained passing performance under severe operating conditions. In the L-42 test procedure, which is part of the SAE J2360 gear oil specification, the ring and pinion gears are evaluated for scoring after a series of high speed accelerations and rapid decelerations at temperatures up to 280°F. In order to pass, the candidate lubricant must show less scoring than the established passing reference oil. - When numerical lower limits and numerical upper limits are listed herein, ranges from any lower limit to any upper limit are contemplated. While the illustrative embodiments of the disclosure have been described with particularity, it will be understood that various other modifications will be apparent to and can be readily made by those skilled in the art without departing from the scope of the disclosure.
Claims (9)
- Use of a viscosity modifier comprising a copolymer having units derived from monomers of (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof esterified with an alcohol for improving at least one of wear control as determined by ASTM D4172, load carrying capacity as determined by ASTM D2783 and traction reduction as determined by Mini-Traction Machine (MTM, Ball-on-Disc apparatus) in a driveline device lubricated with a lubricating composition as compared to at least one of wear control, load carrying capacity and traction reduction achieved with a lubricating composition containing a viscosity modifier other than said viscosity modifier copolymer, at an equal or lower kinematic viscosity (Kv@100°C), said lubricating composition comprising comprising:a first base stock component comprising one or more metallocene catalyzed polyalphaolefins (mPAOs), each mPAO having a kinematic viscosity (Kv100) from 40 cSt to 155 cSt and a viscosity index (VI) from 150 to 207; anda second base stock comprising one or more polyalphaolefins (PAOs), each PAO having a kinematic viscosity (Kv100) less than 10 cSt and a VI from 130 to 145.
- The use of claim 1, wherein the viscosity modifier is represented by the formula:
- The use of claim 1 or 2, wherein the α-olefin is selected from the group consisting of 1-decene, 1-undecene, 1-dodecene, 1-tridecene, 1-tetradecene, 1-pentadecene, 1-hexadecene, 1-hepta-decene, 1-octadecene, and mixtures thereof; the ethylenically unsaturated carboxylic acid or derivative thereof is selected from the group consisting of acrylic acid, methyl acrylate, methacrylic acid, maleic acid or anhydride, fumaric acid, itaconic acid or anhydride or mixtures thereof, and substituted equivalents thereof; and the alcohol is selected from the group consisting of 2-ethylhexanol, 2- butyloctanol, 2-hexyldecanol, 2-octyldodecanol, 2-decyltetradecanol, butanol, pentanol, hexanol, heptanol, octanol, nonanol, decanol, undecanol, dodecanol, tridecanol, tetradecanol, pentadecanol, hexadecanol, heptadecanol, octadecanol, eicosanol, and mixtures thereof.
- The use of claims 1-3, wherein the viscosity modifier is a copolymer derived from monomers of (i) an α-olefin and (ii) an ethylenically unsaturated carboxylic acid or derivatives thereof,
wherein 0.1 to 99.89 percent of the carboxylic acid units are esterified with a primary alcohol branched at the β- or higher position,
wherein 0.1 to 99.89 percent of the carboxylic acid units are esterified with a linear alcohol or an alpha-branched alcohol, and
wherein 0.01 to 10 percent of the carboxylic acid units has at least one of an amino-, amido- and/or imido- group. - The use of claims 1-3, wherein the viscosity modifier copolymer has a weight average molecular weight of 45,000 or less.
- The use of claims 1-4, wherein the lubricating oil base stock is present in an amount from 60 weight percent to 98 weight percent, and the viscosity modifier is present in an amount from 2 weight percent to 40 weight percent, based on the total weight of the lubricating composition; preferably wherein the lubricating composition further comprises one or more of an antioxidant, detergent, dispersant, pour point depressant, corrosion inhibitor, metal deactivator, seal compatibility additive, anti-foam agent, inhibitor, antiwear, extreme pressure agent, friction modifier, and anti-rust additive.
- The use of claims 1-6, wherein the lubricating composition is an axle fluid or a manual transmission fluid (MTF).
- The use of claims 1-7, further comprising improving fuel efficiency or shear stability.
- The use of claims 1-8, wherein the driveline device comprises gears or transmissions.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261717947P | 2012-10-24 | 2012-10-24 | |
| PCT/US2013/062147 WO2014065984A1 (en) | 2012-10-24 | 2013-09-27 | High viscosity index lubricating oil base stock viscosity modifier combinations, and lubricating oils derived therefrom |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2912151A1 EP2912151A1 (en) | 2015-09-02 |
| EP2912151B1 true EP2912151B1 (en) | 2019-08-07 |
Family
ID=49304430
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP13773574.2A Active EP2912151B1 (en) | 2012-10-24 | 2013-09-27 | Use of viscosity modifier in high viscosity index lubricating oil base stock combinations |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US20140113847A1 (en) |
| EP (1) | EP2912151B1 (en) |
| SG (2) | SG10201703150VA (en) |
| WO (1) | WO2014065984A1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107955680A (en) * | 2017-12-05 | 2018-04-24 | 广州市联诺化工科技有限公司 | A kind of import cutter special environment protection antirust oil and preparation method thereof |
Families Citing this family (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007011462A1 (en) | 2005-07-19 | 2007-01-25 | Exxonmobil Chemical Patents Inc. | Lubricants from mixed alpha-olefin feeds |
| SG11201503993WA (en) * | 2012-12-03 | 2015-06-29 | Lubrizol Corp | Industrial gear oils imparting reduced gearbox operating temperatures |
| US20140187457A1 (en) * | 2013-01-03 | 2014-07-03 | Exxonmobil Research And Engineering Company | Lubricating compositions having improved shear stability |
| JPWO2014142157A1 (en) * | 2013-03-14 | 2017-02-16 | 出光興産株式会社 | High temperature lubricating oil composition |
| US10227544B2 (en) * | 2013-08-15 | 2019-03-12 | Infineum International Limited | Automotive transmission fluid compositions for improved energy efficiency |
| US20160168504A1 (en) * | 2014-12-10 | 2016-06-16 | Hyundai Motor Company | Low viscosity gear oil composition providing enhanced fuel efficiency |
| JP6500271B2 (en) * | 2015-03-30 | 2019-04-17 | 出光興産株式会社 | Lubricating oil composition |
| JP6789615B2 (en) * | 2015-03-31 | 2020-11-25 | 出光興産株式会社 | Lubricating oil composition for transmission |
| EP3322780B1 (en) * | 2015-07-16 | 2019-09-18 | Basf Se | Corrosion inhibitors for fuels |
| EP3337880A1 (en) | 2015-08-21 | 2018-06-27 | ExxonMobil Chemical Patents Inc. | Lubricant base stock blends |
| US10059898B2 (en) | 2015-08-21 | 2018-08-28 | Exxonmobil Chemical Patents Inc. | High-viscosity metallocene polyalpha-olefins with high electrohydrodynamic performance |
| US10611980B2 (en) | 2015-10-15 | 2020-04-07 | Exxonmobil Chemical Patents Inc. | Lubricant containing high-viscosity metallocene polyalpha-olefins |
| JP6775605B2 (en) | 2016-05-18 | 2020-10-28 | エボニック オペレーションズ ゲーエムベーハー | Abrasion resistant copolymer and lubricant composition |
| KR102251044B1 (en) * | 2016-05-18 | 2021-05-14 | 에보니크 오퍼레이션즈 게엠베하 | Anti-wear copolymer and lubricant composition |
| EP3481920B1 (en) | 2016-07-05 | 2021-08-11 | Basf Se | Use of corrosion inhibitors for fuels and lubricants |
| US10144894B2 (en) * | 2016-07-20 | 2018-12-04 | Exxonmobil Chemical Patents Inc. | Shear-stable oil compositions and processes for making the same |
| US10351488B2 (en) | 2016-08-02 | 2019-07-16 | Exxonmobil Chemical Patents Inc. | Unsaturated polyalpha-olefin materials |
| JP2018039943A (en) * | 2016-09-09 | 2018-03-15 | 昭和シェル石油株式会社 | Lubricating oil composition for automatic transmission |
| US10160926B2 (en) * | 2016-11-25 | 2018-12-25 | Hyundai Motor Company | Axle oil composition having enhanced fuel efficiency and low viscosity |
| FR3060604B1 (en) * | 2016-12-15 | 2021-05-28 | Skf Ab | GREASE COMPOSITIONS AND THEIR MANUFACTURING PROCESS |
| FR3060605B1 (en) | 2016-12-15 | 2021-05-28 | Skf Ab | GREASE COMPOSITIONS AND THEIR MANUFACTURING PROCESS |
| US20190136147A1 (en) * | 2017-11-03 | 2019-05-09 | Exxonmobil Research And Engineering Company | Lubricant compositions with improved performance and methods of preparing and using the same |
| WO2019160630A1 (en) | 2018-02-19 | 2019-08-22 | Exxonmobil Chemical Patents Inc. | Functional fluids comprising low-viscosity polyalpha-olefin base stock |
| CA3093399C (en) | 2018-03-06 | 2022-03-22 | Valvoline Licensing And Intellectual Property Llc | Traction fluid composition comprising a hydrogenated alpha dimethyl styrene dimer base oil, a polyisobutene viscosity modifier, and a comb-polymethacrylate viscosity modifier |
| WO2019208373A1 (en) * | 2018-04-26 | 2019-10-31 | トヨタ自動車株式会社 | Lubricant composition |
| KR102654520B1 (en) * | 2018-10-18 | 2024-04-03 | 현대자동차주식회사 | Automatic transmission oil composition |
| WO2020131515A2 (en) * | 2018-12-19 | 2020-06-25 | Exxonmobil Research And Engineering Company | Lubricant compositions with improved wear control |
| US11597273B2 (en) * | 2019-01-08 | 2023-03-07 | Ford Global Technologies, Llc | Vehicular gear system friction reduction |
| JP7437897B2 (en) * | 2019-09-04 | 2024-02-26 | 住友化学株式会社 | Method for manufacturing resin container, storage bag, and lubricating oil |
| KR102842991B1 (en) * | 2019-11-26 | 2025-08-06 | 현대자동차주식회사 | composition of continuously variable transmission oil for fuel efficiency and durance performance |
| WO2023133247A1 (en) * | 2022-01-06 | 2023-07-13 | Tai Chih Cheng | Methods of viscosity modification of mineral oils for immersion cooling system |
| CN116445204A (en) * | 2023-04-27 | 2023-07-18 | 亚培烯科技(上海)有限公司 | Lubricating oil and preparation method and application thereof |
Family Cites Families (56)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3036003A (en) | 1957-08-07 | 1962-05-22 | Sinclair Research Inc | Lubricating oil composition |
| US3444170A (en) | 1959-03-30 | 1969-05-13 | Lubrizol Corp | Process which comprises reacting a carboxylic intermediate with an amine |
| DE1248643B (en) | 1959-03-30 | 1967-08-31 | The Lubrizol Corporation, Cleveland, Ohio (V. St. A.) | Process for the preparation of oil-soluble aylated amines |
| US3215707A (en) | 1960-06-07 | 1965-11-02 | Lubrizol Corp | Lubricant |
| US3200107A (en) | 1961-06-12 | 1965-08-10 | Lubrizol Corp | Process for preparing acylated amine-cs2 compositions and products |
| US3087936A (en) | 1961-08-18 | 1963-04-30 | Lubrizol Corp | Reaction product of an aliphatic olefinpolymer-succinic acid producing compound with an amine and reacting the resulting product with a boron compound |
| US3449250A (en) | 1962-05-14 | 1969-06-10 | Monsanto Co | Dispersency oil additives |
| US3329658A (en) | 1962-05-14 | 1967-07-04 | Monsanto Co | Dispersency oil additives |
| US3197405A (en) | 1962-07-09 | 1965-07-27 | Lubrizol Corp | Phosphorus-and nitrogen-containing compositions and process for preparing the same |
| NL137371C (en) | 1963-08-02 | |||
| US3316177A (en) | 1964-12-07 | 1967-04-25 | Lubrizol Corp | Functional fluid containing a sludge inhibiting detergent comprising the polyamine salt of the reaction product of maleic anhydride and an oxidized interpolymer of propylene and ethylene |
| NL145565B (en) | 1965-01-28 | 1975-04-15 | Shell Int Research | PROCESS FOR PREPARING A LUBRICANT COMPOSITION. |
| US3574576A (en) | 1965-08-23 | 1971-04-13 | Chevron Res | Distillate fuel compositions having a hydrocarbon substituted alkylene polyamine |
| US3697574A (en) | 1965-10-22 | 1972-10-10 | Standard Oil Co | Boron derivatives of high molecular weight mannich condensation products |
| US3413347A (en) | 1966-01-26 | 1968-11-26 | Ethyl Corp | Mannich reaction products of high molecular weight alkyl phenols, aldehydes and polyaminopolyalkyleneamines |
| US3519565A (en) | 1967-09-19 | 1970-07-07 | Lubrizol Corp | Oil-soluble interpolymers of n-vinylthiopyrrolidones |
| US3541012A (en) | 1968-04-15 | 1970-11-17 | Lubrizol Corp | Lubricants and fuels containing improved acylated nitrogen additives |
| US3544465A (en) | 1968-06-03 | 1970-12-01 | Mobil Oil Corp | Esters of phosphorodithioates |
| GB1244435A (en) | 1968-06-18 | 1971-09-02 | Lubrizol Corp | Oil-soluble graft polymers derived from degraded ethylene-propylene interpolymers |
| GB1282887A (en) | 1968-07-03 | 1972-07-26 | Lubrizol Corp | Acylation of nitrogen-containing products |
| US3725480A (en) | 1968-11-08 | 1973-04-03 | Standard Oil Co | Ashless oil additives |
| US3726882A (en) | 1968-11-08 | 1973-04-10 | Standard Oil Co | Ashless oil additives |
| US3702300A (en) | 1968-12-20 | 1972-11-07 | Lubrizol Corp | Lubricant containing nitrogen-containing ester |
| US3454607A (en) | 1969-02-10 | 1969-07-08 | Lubrizol Corp | High molecular weight carboxylic compositions |
| US3632511A (en) | 1969-11-10 | 1972-01-04 | Lubrizol Corp | Acylated nitrogen-containing compositions processes for their preparationand lubricants and fuels containing the same |
| US3787374A (en) | 1971-09-07 | 1974-01-22 | Lubrizol Corp | Process for preparing high molecular weight carboxylic compositions |
| JPS515645B2 (en) * | 1971-12-02 | 1976-02-21 | ||
| US4100082A (en) | 1976-01-28 | 1978-07-11 | The Lubrizol Corporation | Lubricants containing amino phenol-detergent/dispersant combinations |
| US4454059A (en) | 1976-11-12 | 1984-06-12 | The Lubrizol Corporation | Nitrogenous dispersants, lubricants and concentrates containing said nitrogenous dispersants |
| US4234435A (en) | 1979-02-23 | 1980-11-18 | The Lubrizol Corporation | Novel carboxylic acid acylating agents, derivatives thereof, concentrate and lubricant compositions containing the same, and processes for their preparation |
| US4956122A (en) | 1982-03-10 | 1990-09-11 | Uniroyal Chemical Company, Inc. | Lubricating composition |
| US4526950A (en) | 1982-04-20 | 1985-07-02 | The Lubrizol Corporation | Method for preparing interpolymers |
| US4889647A (en) | 1985-11-14 | 1989-12-26 | R. T. Vanderbilt Company, Inc. | Organic molybdenum complexes |
| US4827064A (en) | 1986-12-24 | 1989-05-02 | Mobil Oil Corporation | High viscosity index synthetic lubricant compositions |
| US4767815A (en) | 1987-11-09 | 1988-08-30 | Gaf Corporation | Guerbet alcohol esters |
| US4827073A (en) | 1988-01-22 | 1989-05-02 | Mobil Oil Corporation | Process for manufacturing olefinic oligomers having lubricating properties |
| US4978464A (en) | 1989-09-07 | 1990-12-18 | Exxon Research And Engineering Company | Multi-function additive for lubricating oils |
| US5366648A (en) | 1990-02-23 | 1994-11-22 | The Lubrizol Corporation | Functional fluids useful at high temperatures |
| DE4025494A1 (en) * | 1990-08-11 | 1992-02-13 | Roehm Gmbh | SYNTHESIC OILS, WHOLE OR PARTLY FROM OLIGOMERS OR CONSIST OF COOLIGOMERS OF (METH) ACRYLIC ACID ESTERS AND 1-ALKENES |
| US6174843B1 (en) | 1990-08-13 | 2001-01-16 | Nalco Chemical Company | Composition and method for lubricant wax dispersant and pour point improver |
| ES2051608T3 (en) * | 1991-01-11 | 1994-06-16 | Mobil Oil Corp | LUBRICATING COMPOSITIONS. |
| DE4212569A1 (en) * | 1992-04-15 | 1993-10-21 | Roehm Gmbh | Synthetic oils containing cooligomers, consisting of 1-alkenes and (meth) acrylic acid esters |
| AU719520B2 (en) | 1995-09-19 | 2000-05-11 | Lubrizol Corporation, The | Additive compositions for lubricants and functional fluids |
| US6573224B2 (en) | 1997-01-03 | 2003-06-03 | Bardahl Manufacturing Corporation | Two-cycle engine lubricant composition comprising an ester copolymer and a diester |
| WO1999031113A1 (en) | 1997-12-12 | 1999-06-24 | Infineum Usa L.P. | Method for the preparation of trinuclear molybdenum-sulfur compounds and their use as lubricant additives |
| US6419714B2 (en) | 1999-07-07 | 2002-07-16 | The Lubrizol Corporation | Emulsifier for an acqueous hydrocarbon fuel |
| WO2007145924A1 (en) * | 2006-06-06 | 2007-12-21 | Exxonmobil Research And Engineering Company | High viscosity metallocene catalyst pao novel base stock lubricant blends |
| US8501675B2 (en) * | 2006-06-06 | 2013-08-06 | Exxonmobil Research And Engineering Company | High viscosity novel base stock lubricant viscosity blends |
| US8535514B2 (en) * | 2006-06-06 | 2013-09-17 | Exxonmobil Research And Engineering Company | High viscosity metallocene catalyst PAO novel base stock lubricant blends |
| WO2008094807A2 (en) | 2007-01-29 | 2008-08-07 | The Lubrizol Corporation | Lubricant compositions |
| US7888298B2 (en) * | 2007-03-20 | 2011-02-15 | Exxonmobil Research And Engineering Company | Lubricant compositions with improved properties |
| CN102171258B (en) * | 2008-07-31 | 2014-10-15 | 卢布里佐尔公司 | Copolymer and lubricating composition thereof |
| ES2665459T3 (en) * | 2009-11-06 | 2018-04-25 | Cognis Ip Management Gmbh | Lubricating compositions |
| AU2010324872B2 (en) | 2009-11-24 | 2016-12-15 | The Lubrizol Corporation | Lubricating composition containing viscosity modifier combination |
| JP2013249461A (en) * | 2012-06-04 | 2013-12-12 | Showa Shell Sekiyu Kk | Lubricating oil composition |
| US20140187457A1 (en) * | 2013-01-03 | 2014-07-03 | Exxonmobil Research And Engineering Company | Lubricating compositions having improved shear stability |
-
2013
- 2013-09-24 US US14/034,864 patent/US20140113847A1/en not_active Abandoned
- 2013-09-27 SG SG10201703150VA patent/SG10201703150VA/en unknown
- 2013-09-27 WO PCT/US2013/062147 patent/WO2014065984A1/en not_active Ceased
- 2013-09-27 EP EP13773574.2A patent/EP2912151B1/en active Active
- 2013-09-27 SG SG11201501766XA patent/SG11201501766XA/en unknown
Non-Patent Citations (1)
| Title |
|---|
| None * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107955680A (en) * | 2017-12-05 | 2018-04-24 | 广州市联诺化工科技有限公司 | A kind of import cutter special environment protection antirust oil and preparation method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2912151A1 (en) | 2015-09-02 |
| US20140113847A1 (en) | 2014-04-24 |
| SG11201501766XA (en) | 2015-04-29 |
| SG10201703150VA (en) | 2017-06-29 |
| WO2014065984A1 (en) | 2014-05-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2912151B1 (en) | Use of viscosity modifier in high viscosity index lubricating oil base stock combinations | |
| US11629308B2 (en) | Low viscosity gear oil compositions for electric and hybrid vehicles | |
| US20140187457A1 (en) | Lubricating compositions having improved shear stability | |
| US10208269B2 (en) | Low viscosity ester lubricant and method for using | |
| EP2941476B1 (en) | Use for improving high temperature performance in an engine | |
| WO2018067906A1 (en) | High conductivity lubricating oils for electric and hybrid vehicles | |
| WO2018067903A1 (en) | Method for controlling electrical conductivity of lubricating oils in electric vehicle powertrains | |
| US20180100120A1 (en) | Method for preventing or minimizing electrostatic discharge and dielectric breakdown in electric vehicle powertrains | |
| WO2020123440A1 (en) | Method for improving oxidation and deposit resistance of lubricating oils | |
| WO2020068439A1 (en) | Low viscosity lubricating oils with improved oxidative stability and traction performance | |
| EP2726582A1 (en) | Lubricating compositions containing polyalkylene glycol mono ethers | |
| US20140274848A1 (en) | Low traction energy conserving fluids containing base stock blends | |
| US20140274849A1 (en) | Lubricating composition providing high wear resistance | |
| US10611980B2 (en) | Lubricant containing high-viscosity metallocene polyalpha-olefins | |
| WO2020131310A1 (en) | Method for improving high temperature antifoaming performance of a lubricating oil | |
| US20130023455A1 (en) | Lubricating Compositions Containing Polyetheramines | |
| US20210189282A1 (en) | Lubricating oil compositions and methods of use | |
| WO2020131515A2 (en) | Lubricant compositions with improved wear control | |
| US20130165354A1 (en) | Method for improving engine fuel efficiency | |
| WO2020264534A2 (en) | Method for reducing solubilized copper levels in wind turbine gear oils | |
| US20200190425A1 (en) | Lubricating oil compositions having functionalized quercetin antioxidants |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20150522 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAX | Request for extension of the european patent (deleted) | ||
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20180810 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20190305 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP Ref country code: AT Ref legal event code: REF Ref document number: 1163873 Country of ref document: AT Kind code of ref document: T Effective date: 20190815 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602013058837 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20190807 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20191209 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20191107 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20191107 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1163873 Country of ref document: AT Kind code of ref document: T Effective date: 20190807 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20191207 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20191108 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200224 Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602013058837 Country of ref document: DE |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG2D | Information on lapse in contracting state deleted |
Ref country code: IS |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190927 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190930 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190930 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190927 |
|
| 26N | No opposition filed |
Effective date: 20200603 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20190930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190930 Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20191007 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20130927 Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190807 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230518 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20250926 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20250923 Year of fee payment: 13 |