EP2794875B1 - Modifizierte leichte enterokinasekette - Google Patents
Modifizierte leichte enterokinasekette Download PDFInfo
- Publication number
- EP2794875B1 EP2794875B1 EP12816050.4A EP12816050A EP2794875B1 EP 2794875 B1 EP2794875 B1 EP 2794875B1 EP 12816050 A EP12816050 A EP 12816050A EP 2794875 B1 EP2794875 B1 EP 2794875B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- light chain
- analogue
- enterokinase light
- enterokinase
- protein
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 108010013369 Enteropeptidase Proteins 0.000 title claims description 97
- 102100029727 Enteropeptidase Human genes 0.000 title claims description 91
- 101001012262 Bos taurus Enteropeptidase Proteins 0.000 claims description 109
- 108090000623 proteins and genes Proteins 0.000 claims description 50
- 150000001413 amino acids Chemical group 0.000 claims description 49
- 102000004169 proteins and genes Human genes 0.000 claims description 49
- 238000000034 method Methods 0.000 claims description 45
- 230000000694 effects Effects 0.000 claims description 42
- 210000004027 cell Anatomy 0.000 claims description 39
- 210000003000 inclusion body Anatomy 0.000 claims description 29
- 102000037865 fusion proteins Human genes 0.000 claims description 26
- 108020001507 fusion proteins Proteins 0.000 claims description 26
- 238000006467 substitution reaction Methods 0.000 claims description 25
- 238000003776 cleavage reaction Methods 0.000 claims description 23
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 23
- 230000007017 scission Effects 0.000 claims description 23
- 241000588724 Escherichia coli Species 0.000 claims description 21
- 230000008569 process Effects 0.000 claims description 19
- 238000004519 manufacturing process Methods 0.000 claims description 16
- 230000002209 hydrophobic effect Effects 0.000 claims description 13
- 230000004048 modification Effects 0.000 claims description 13
- 238000012986 modification Methods 0.000 claims description 13
- 238000004153 renaturation Methods 0.000 claims description 8
- 240000004808 Saccharomyces cerevisiae Species 0.000 claims description 7
- 108091033319 polynucleotide Proteins 0.000 claims description 7
- 102000040430 polynucleotide Human genes 0.000 claims description 7
- 239000002157 polynucleotide Substances 0.000 claims description 7
- 108010060175 trypsinogen activation peptide Proteins 0.000 claims description 7
- 230000001580 bacterial effect Effects 0.000 claims description 5
- 238000012258 culturing Methods 0.000 claims description 4
- 239000001963 growth medium Substances 0.000 claims description 3
- 239000000411 inducer Substances 0.000 claims description 3
- 230000035772 mutation Effects 0.000 claims description 3
- 230000003381 solubilizing effect Effects 0.000 claims description 3
- 241000894006 Bacteria Species 0.000 claims description 2
- 102100025101 GATA-type zinc finger protein 1 Human genes 0.000 claims 1
- 101710198884 GATA-type zinc finger protein 1 Proteins 0.000 claims 1
- DTHNMHAUYICORS-KTKZVXAJSA-N Glucagon-like peptide 1 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1N=CNC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 DTHNMHAUYICORS-KTKZVXAJSA-N 0.000 claims 1
- 235000001014 amino acid Nutrition 0.000 description 46
- 239000000872 buffer Substances 0.000 description 44
- 235000018102 proteins Nutrition 0.000 description 37
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 35
- 239000004202 carbamide Substances 0.000 description 35
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 33
- 239000007983 Tris buffer Substances 0.000 description 32
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 32
- 102000004190 Enzymes Human genes 0.000 description 21
- 108090000790 Enzymes Proteins 0.000 description 21
- 108060008226 thioredoxin Proteins 0.000 description 20
- 238000000746 purification Methods 0.000 description 17
- 229960003180 glutathione Drugs 0.000 description 16
- 108010053070 Glutathione Disulfide Proteins 0.000 description 15
- 125000003275 alpha amino acid group Chemical group 0.000 description 15
- 230000014509 gene expression Effects 0.000 description 15
- YPZRWBKMTBYPTK-BJDJZHNGSA-N glutathione disulfide Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@H](C(=O)NCC(O)=O)CSSC[C@@H](C(=O)NCC(O)=O)NC(=O)CC[C@H](N)C(O)=O YPZRWBKMTBYPTK-BJDJZHNGSA-N 0.000 description 15
- 102100036407 Thioredoxin Human genes 0.000 description 14
- 238000010790 dilution Methods 0.000 description 11
- 239000012895 dilution Substances 0.000 description 11
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 10
- 238000001994 activation Methods 0.000 description 10
- 108020004414 DNA Proteins 0.000 description 9
- 230000002255 enzymatic effect Effects 0.000 description 9
- 238000000855 fermentation Methods 0.000 description 8
- 230000004151 fermentation Effects 0.000 description 8
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 8
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 7
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 7
- 125000000539 amino acid group Chemical group 0.000 description 7
- 230000006698 induction Effects 0.000 description 7
- 150000007523 nucleic acids Chemical group 0.000 description 7
- 239000008188 pellet Substances 0.000 description 7
- 229920001223 polyethylene glycol Polymers 0.000 description 7
- 102000004196 processed proteins & peptides Human genes 0.000 description 7
- 241000283690 Bos taurus Species 0.000 description 6
- 102000002933 Thioredoxin Human genes 0.000 description 6
- 230000004913 activation Effects 0.000 description 6
- 238000005571 anion exchange chromatography Methods 0.000 description 6
- 238000002474 experimental method Methods 0.000 description 6
- BPHPUYQFMNQIOC-NXRLNHOXSA-N isopropyl beta-D-thiogalactopyranoside Chemical compound CC(C)S[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O BPHPUYQFMNQIOC-NXRLNHOXSA-N 0.000 description 6
- 108020004707 nucleic acids Proteins 0.000 description 6
- 102000039446 nucleic acids Human genes 0.000 description 6
- 229920001184 polypeptide Polymers 0.000 description 6
- 239000000758 substrate Substances 0.000 description 6
- 229940094937 thioredoxin Drugs 0.000 description 6
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 5
- 108091005804 Peptidases Proteins 0.000 description 5
- 239000004365 Protease Substances 0.000 description 5
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 5
- 238000007792 addition Methods 0.000 description 5
- 230000003197 catalytic effect Effects 0.000 description 5
- 238000005119 centrifugation Methods 0.000 description 5
- 238000006243 chemical reaction Methods 0.000 description 5
- 238000004191 hydrophobic interaction chromatography Methods 0.000 description 5
- 239000002609 medium Substances 0.000 description 5
- 239000000047 product Substances 0.000 description 5
- 239000011535 reaction buffer Substances 0.000 description 5
- 239000011780 sodium chloride Substances 0.000 description 5
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 4
- 239000002202 Polyethylene glycol Substances 0.000 description 4
- 238000004587 chromatography analysis Methods 0.000 description 4
- 238000011534 incubation Methods 0.000 description 4
- 230000001939 inductive effect Effects 0.000 description 4
- 239000012139 lysis buffer Substances 0.000 description 4
- 229920002523 polyethylene Glycol 1000 Polymers 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 239000006228 supernatant Substances 0.000 description 4
- 229920000858 Cyclodextrin Polymers 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 101001012451 Homo sapiens Enteropeptidase Proteins 0.000 description 3
- 239000004472 Lysine Substances 0.000 description 3
- 108091028043 Nucleic acid sequence Proteins 0.000 description 3
- 102000035195 Peptidases Human genes 0.000 description 3
- FKMJXALNHKIDOD-LBPRGKRZSA-N TAMe Chemical compound NC(=N)NCCC[C@@H](C(=O)OC)NS(=O)(=O)C1=CC=C(C)C=C1 FKMJXALNHKIDOD-LBPRGKRZSA-N 0.000 description 3
- 108090000631 Trypsin Proteins 0.000 description 3
- 102000004142 Trypsin Human genes 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- 230000002776 aggregation Effects 0.000 description 3
- 238000004220 aggregation Methods 0.000 description 3
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 3
- 238000010828 elution Methods 0.000 description 3
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 3
- 239000013612 plasmid Substances 0.000 description 3
- 230000002797 proteolythic effect Effects 0.000 description 3
- 239000000523 sample Substances 0.000 description 3
- 230000007928 solubilization Effects 0.000 description 3
- 238000005063 solubilization Methods 0.000 description 3
- 239000012588 trypsin Substances 0.000 description 3
- 235000014469 Bacillus subtilis Nutrition 0.000 description 2
- 108091026890 Coding region Proteins 0.000 description 2
- 108020004635 Complementary DNA Proteins 0.000 description 2
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 2
- 108010024636 Glutathione Proteins 0.000 description 2
- ZRALSGWEFCBTJO-UHFFFAOYSA-N Guanidine Chemical compound NC(N)=N ZRALSGWEFCBTJO-UHFFFAOYSA-N 0.000 description 2
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 2
- 102000018690 Trypsinogen Human genes 0.000 description 2
- 108010027252 Trypsinogen Proteins 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 238000010804 cDNA synthesis Methods 0.000 description 2
- 150000001720 carbohydrates Chemical class 0.000 description 2
- 235000014633 carbohydrates Nutrition 0.000 description 2
- 239000002299 complementary DNA Substances 0.000 description 2
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- 238000004925 denaturation Methods 0.000 description 2
- 230000036425 denaturation Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 239000003599 detergent Substances 0.000 description 2
- VHJLVAABSRFDPM-QWWZWVQMSA-N dithiothreitol Chemical compound SC[C@@H](O)[C@H](O)CS VHJLVAABSRFDPM-QWWZWVQMSA-N 0.000 description 2
- TWFOCWXYZXHJSL-ZDUSSCGKSA-N ethyl (2s)-2-(benzylamino)-5-(diaminomethylideneamino)pentanoate Chemical compound NC(N)=NCCC[C@@H](C(=O)OCC)NCC1=CC=CC=C1 TWFOCWXYZXHJSL-ZDUSSCGKSA-N 0.000 description 2
- 230000005284 excitation Effects 0.000 description 2
- 230000005714 functional activity Effects 0.000 description 2
- 230000004927 fusion Effects 0.000 description 2
- 238000001155 isoelectric focusing Methods 0.000 description 2
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 238000005457 optimization Methods 0.000 description 2
- 238000010647 peptide synthesis reaction Methods 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 239000012723 sample buffer Substances 0.000 description 2
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical compound O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 description 2
- 230000028327 secretion Effects 0.000 description 2
- 125000006850 spacer group Chemical group 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 210000005253 yeast cell Anatomy 0.000 description 2
- 125000003088 (fluoren-9-ylmethoxy)carbonyl group Chemical group 0.000 description 1
- GOJUJUVQIVIZAV-UHFFFAOYSA-N 2-amino-4,6-dichloropyrimidine-5-carbaldehyde Chemical group NC1=NC(Cl)=C(C=O)C(Cl)=N1 GOJUJUVQIVIZAV-UHFFFAOYSA-N 0.000 description 1
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 1
- 240000006439 Aspergillus oryzae Species 0.000 description 1
- LEVWYRKDKASIDU-QWWZWVQMSA-N D-cystine Chemical compound OC(=O)[C@H](N)CSSC[C@@H](N)C(O)=O LEVWYRKDKASIDU-QWWZWVQMSA-N 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 108010025076 Holoenzymes Proteins 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- CHJJGSNFBQVOTG-UHFFFAOYSA-N N-methyl-guanidine Natural products CNC(N)=N CHJJGSNFBQVOTG-UHFFFAOYSA-N 0.000 description 1
- 108010002747 Pfu DNA polymerase Proteins 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 241000589516 Pseudomonas Species 0.000 description 1
- 101000702488 Rattus norvegicus High affinity cationic amino acid transporter 1 Proteins 0.000 description 1
- 108010022999 Serine Proteases Proteins 0.000 description 1
- 102000012479 Serine Proteases Human genes 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 238000012870 ammonium sulfate precipitation Methods 0.000 description 1
- 238000005349 anion exchange Methods 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 244000052616 bacterial pathogen Species 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000011098 chromatofocusing Methods 0.000 description 1
- 238000011097 chromatography purification Methods 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229940097362 cyclodextrins Drugs 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 1
- 229960003067 cystine Drugs 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 239000003398 denaturant Substances 0.000 description 1
- 239000012470 diluted sample Substances 0.000 description 1
- SWSQBOPZIKWTGO-UHFFFAOYSA-N dimethylaminoamidine Natural products CN(C)C(N)=N SWSQBOPZIKWTGO-UHFFFAOYSA-N 0.000 description 1
- 230000002183 duodenal effect Effects 0.000 description 1
- -1 e.g. Proteins 0.000 description 1
- 238000001952 enzyme assay Methods 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 229940045883 glutathione disulfide Drugs 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 239000000833 heterodimer Substances 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 239000002198 insoluble material Substances 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 230000007154 intracellular accumulation Effects 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 238000011031 large-scale manufacturing process Methods 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 238000013507 mapping Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- UPSFMJHZUCSEHU-JYGUBCOQSA-N n-[(2s,3r,4r,5s,6r)-2-[(2r,3s,4r,5r,6s)-5-acetamido-4-hydroxy-2-(hydroxymethyl)-6-(4-methyl-2-oxochromen-7-yl)oxyoxan-3-yl]oxy-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl]acetamide Chemical compound CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1O[C@H]1[C@H](O)[C@@H](NC(C)=O)[C@H](OC=2C=C3OC(=O)C=C(C)C3=CC=2)O[C@@H]1CO UPSFMJHZUCSEHU-JYGUBCOQSA-N 0.000 description 1
- JVXXKQIRGQDWOJ-UHFFFAOYSA-N naphthalene-2-carboxamide Chemical group C1=CC=CC2=CC(C(=O)N)=CC=C21 JVXXKQIRGQDWOJ-UHFFFAOYSA-N 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- 229940113116 polyethylene glycol 1000 Drugs 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000003614 protease activity assay Methods 0.000 description 1
- 125000006239 protecting group Chemical group 0.000 description 1
- 230000004845 protein aggregation Effects 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- 230000006337 proteolytic cleavage Effects 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 125000005931 tert-butyloxycarbonyl group Chemical group [H]C([H])([H])C(OC(*)=O)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- ODLHGICHYURWBS-LKONHMLTSA-N trappsol cyclo Chemical compound CC(O)COC[C@H]([C@H]([C@@H]([C@H]1O)O)O[C@H]2O[C@@H]([C@@H](O[C@H]3O[C@H](COCC(C)O)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](COCC(C)O)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](COCC(C)O)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](COCC(C)O)[C@H]([C@@H]([C@H]3O)O)O3)[C@H](O)[C@H]2O)COCC(O)C)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H]3O[C@@H]1COCC(C)O ODLHGICHYURWBS-LKONHMLTSA-N 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/48—Hydrolases (3) acting on peptide bonds (3.4)
- C12N9/50—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25)
- C12N9/64—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue
- C12N9/6421—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue from mammals
- C12N9/6424—Serine endopeptidases (3.4.21)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P21/00—Preparation of peptides or proteins
- C12P21/06—Preparation of peptides or proteins produced by the hydrolysis of a peptide bond, e.g. hydrolysate products
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y304/00—Hydrolases acting on peptide bonds, i.e. peptidases (3.4)
- C12Y304/21—Serine endopeptidases (3.4.21)
- C12Y304/21009—Enteropeptidase (3.4.21.9), i.e. enterokinase
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/50—Fusion polypeptide containing protease site
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/62—DNA sequences coding for fusion proteins
Definitions
- the present invention is related to novel mammalian enterokinase analogues, methods of making such and the use of said mammalian enterokinase analogues for cleaving proteins having an enterokinase cleavage site.
- the serine protease enterokinase in short enterokinase or EK
- enteropeptidase is a heterodimeric glycoprotein, a mammalian enzyme catalyzing the conversion of trypsinogen into active trypsin.
- Enterokinase has preference for the substrate sequence Asp-Asp-Asp-Asp-Lys ((Asp) 4 -Lys, DDDDK), where it selectively cleaves after lysine.
- Enterokinase isolated from bovine duodenal mucosa exhibits a molecular weight (MW) of 150,000 and a carbohydrate content of 35 percent.
- the enzyme is comprised of a heavy chain (MW ⁇ 115,000) and a disulfide-linked light chain (MW ⁇ 35,000) ( Liepnieks et al., J. Biol. Chem., 254(5): 1677-1683 (1979 )).
- the function of the heavy chain is to anchor the enzyme to the mucosal membrane.
- the light chain acts as the catalytic subunit.
- Tan et al (Protein, expression and purification, academic press, San Diego, CA, vol. 56, no. 1, 3 October 2007 (2007-10-03), pages 40-47 ) discloses purification and refolding optimization of recombinant bovine enterokinase light chain overexpressed in Escherichia coli.
- CN1470634 discloses enterpeptidase light chain variant with high activity and high stability.
- E.coli In E.coli many mammalian proteins are expressed as fusion proteins, which have to be cleaved to release the mature, active protein. For that purpose a processing enzyme is needed, preferably one which cleaves directly at the junction leaving no extra amino acids on the product. Enterokinase is such an enzyme, and much effort has been made to establish a recombinant process to obtain enterokinase or enterokinase analogues in E.coli. However, the results so far have been rather poor: Available commercial products are expensive and of low specific activity, due to inefficient renaturation of precipitated EK or inefficient secretion of soluble EK.
- a process in E.coli aiming at a soluble EK product leads to a mixture of soluble and insoluble protein, requiring 2 routes of purification, expensive affinity columns and low yields altogether.
- the EK has to be produced as insoluble material in inclusion bodies. They are easy to isolate but challenging to renature in satisfactory yields, due to possible aggregation of the protein.
- An object of the invention is to obtain a mammalian enterokinase analogue with improved properties.
- the present invention is related to mammalian enterokinase analogues mutated in appropriate sites.
- One or more substitutions of an enterokinase analogue of the invention may e.g. be from hydrophobic to hydrophilic, charged amino acids relative to the amino acids in the parent (wild type) mammalian enterokinase.
- a bovine enterokinase light chain analogue which comprises an amino acid sequence set forth in SEQ ID NO: 1, wherein said analogue comprises substitutions C112A, L134K and I135K, said analogue comprises less than 10 amino acid modifications relative to SEQ ID NO: 1, and said analogue has enterokinase protease activity.
- the invention is also related to a method for obtaining improved solubility in a renaturation process of an enterokinase light chain analogue comprising the step of mutating hydrophobic amino acids of wild type bovine enterokinase light chain to hydrophilic amino acids and optionally mutating other amino acids of wild type bovine enterokinase light chain, wherein the hydrophobic amino acids subject to mutation are present on the surface of folded wild type bovine enterokinase light chain, said analogue comprises substitutions C112A, L134K and I135K, said analogue comprises less than 10 amino acid modifications relative to SEQ ID NO: 1, and said analogue has enterokinase protease activity.
- the invention provides an improved production process for obtaining mammalian enterokinase analogues. Also or alternatively, in a second aspect, the invention provides an improved production process resulting in improved production yield.
- the method for production of a bovine enterokinase light chain analogue comprises the steps:
- the invention provides a method for recombinantly producing a peptide or protein in a bacterial or yeast host cell.
- the method comprises:
- the invention may also solve further problems that will be apparent from the disclosure of the exemplary embodiments.
- the present invention is related to mammalian enterokinase analogues mutated in appropriate sites.
- One or more substitutions of an enterokinase analogue of the invention may e.g. be from hydrophobic to hydrophilic, charged amino acids relative to the amino acids in the parent (wild type) mammalian enterokinase.
- one or more substitutions of a mammalian enterokinase analogue of the invention is from hydrophobic to hydrophilic, charged amino acids relative to the amino acids in wild type bovine enterokinase.
- the hydrophobic amino acids subject to mutation are present on the surface of folded wild type mammalian enterokinase light chain such as folded wild type bovine enterokinase light chain.
- the wild type bovine enterokinase light chain generally exhibits good activity in the presence of various detergents and denaturants over a wide pH range (4.5-9.5) and temperature range (4-45 °C). Therefore, the enterokinase light chain as a powerful tool has been used in biotechnology for the in vitro cleavage of fusion proteins.
- the mammalian enterokinase analogue is a mammalian enterokinase light chain analogue such as a bovine enterokinase light chain analogue.
- the mammalian enterokinase analogue is a bovine enterokinase light chain analogue.
- the bovine light chain analogue comprises substitution(s) in position 134 and/or position 135.
- the bovine enterokinase light chain analogue comprises substitutions in positions 112, 134 and/or 135.
- the bovine enterokinase light chain analogue comprises at least two substitutions.
- the bovine enterokinase light chain analogue comprises at least three substitutions. In one aspect the bovine enterokinase light chain analogue comprises substitutions in positions 112, 134 and 135. In one aspect the bovine enterokinase light chain analogue comprises the substitutions C112A, L134K and I135K.
- Novel bovine enterokinase light chain analogues include those having the primary structural conformation (i. e., amino acid sequence) of the light chain of wild type bovine enterokinase.
- the light chain of wild type bovine enterokinase has the sequence substantially as set forth in SEQ ID NO:1.
- bovine enterokinase light chain analogues of the invention have enterokinase protease activity.
- Antibodies to such proteases are also available.
- the bovine enterokinase light chain analogue described by the present invention maintains enterokinase wild type protease activity for use as a restriction proteases to specifically cleave fusion proteins.
- bovine enterokinase as used herein means the bovine enterokinase enzyme whose structure and properties are well-known. Mammalian enterokinases are carbohydrate containing heterodimers with a heavy chain of 650-800 amino acids and a catalytic light chain of around 235 amino acids and an overall homology of 75-80% ( Liepniecks et al., J. Biol. Chem. 254 , 1677 (1979 ), Matsushima et al., J.Biol. Chem. 269 (31), 19976 (1994 ), Kitamoto et al., Biochemistry 34, 4562 (1995 ) for bovine, porcine and human enterokinase, respectively).
- bovine enterokinase light chain means the light chain of bovine enterokinase having 4 disulphide bridges.
- the bovine enterokinase light chain is e.g. described in LaVallie et al, above.
- the term "surface" in connection with amino acids present on the surface of folded wild type bovine enterokinase light chain means amino acids identified as present on the surface of the folded wild type bovine enterokinase light chain on a 3D structure as e.g. described in Mod Base P 98072.
- An enterokinase light chain according to the invention is herein to be understood as bovine enterokinase light chain or an enterokinase light chain from another species such as porcine or human enterokinase light chain.
- enterokinase light chain peptide means a peptide which is either bovine enterokinase light chain or an analog or a derivative thereof with enterokinase activity.
- enterokinase activity means the capability of cleaving peptide or protein substrates at a specific site; for protein substrates, this is generally following the sequence (Asp) 4 -Lys, or a similar sequence such as those described in Light et al., Anal. Biochem. 106: 199(1980 ); (a cluster of negatively charged amino acids followed by a positively charged amino acid).
- such activity is measured by activation of trypsinogen by cleaving the N-terminal propeptide (containing (Asp) 4 -Lys) with the enterokinase or enterokinase analogue and subsequently assaying the amount of active trypsin generated using tosyl-arginine-methylester (TAME).
- trypsinogen by cleaving the N-terminal propeptide (containing (Asp) 4 -Lys) with the enterokinase or enterokinase analogue and subsequently assaying the amount of active trypsin generated using tosyl-arginine-methylester (TAME).
- enterokinase activity can be measured directly by incubating the enzyme with the peptide substrate Gly (Asp) 4 -Lys-ss-naphthylamide and measuring the increase in fluorescence (excitation at 337 nm, emission at 420 nm) generated by cleavage and release of the ss-NA (ss-naphthylamide) moiety.
- Gly (Asp) 4 -Lys-ss-naphthylamide the peptide substrate Gly (Asp) 4 -Lys-ss-naphthylamide and measuring the increase in fluorescence (excitation at 337 nm, emission at 420 nm) generated by cleavage and release of the ss-NA (ss-naphthylamide) moiety.
- Bovine enterokinase is also active on some trypsin substrates like TAME and BAEE (benzyl-arginine
- wild type enterokinase light chain as used herein is intended to mean an enterokinase light chain before any substitutions according to the invention have been applied thereto.
- enterokinase light chain analogue or "bovine enterokinase light chain analogue” as used herein means a modified bovine enterokinase light chain wherein one or more amino acid residues of the enterokinase light chain have been substituted by other amino acid residues and/or wherein one or more amino acid residues have been deleted from the enterokinase light chain and/or wherein one or more amino acid residues have been added and/or inserted to the enterokinase light chain.
- an enterokinase light chain analogue comprises less than 10 amino acid modifications (substitutions, deletions, additions (including insertions) and any combination thereof) relative to bovine enterokinase light chain, alternatively less than 9, 8, 7, 6, 5, 4, 3or 2 modifications relative to bovine enterokinase light chain.
- an enterokinase light chain analogue comprises 5 amino acid modifications, in one aspect 4 amino acid modifications, in one aspect 3 amino acid modifications, in one aspect 2 amino acid modifications and in one aspect 1 amino acid modification relative to bovine enterokinase light chain.
- Modifications in the enterokinase molecule light chain are denoted stating the position and the one or three letter code for the amino acid residue substituting the native amino acid residue.
- terms like 134K and 135K designates that the amino acid in position 134 and 135, respectively, is K.
- the corresponding expressions are 134Lys and 135Lys, respectively.
- 112Ala, 134Lys, 135Lys bovine enterokinase light chain is an analogue of bovine enterokinase light chain where the amino acid in position 112 is substituted with alanine, the amino acid in position 134 is substituted with lysine and the amino acid in position 135 is substituted with lysine.
- amino acid residue is an amino acid from which, formally, a hydroxy group has been removed from a carboxy group and/or from which, formally, a hydrogen atom has been removed from an amino group.
- bovine enterokinase light chain analogues are such wherein Leu in position 134 is substituted with Lys or another charged amino acid, at position 135 where Ile is substituted with Lys or another charged amino acid.
- Cys in position 112 may be substituted with a number of amino acids including Ala and Ser.
- bovine enterokinase light chain analogues include, without limitation: 134Lys bovine enterokinase light chain; 135Lys bovine enterokinase light chain; 134Lys,135Lys bovine enterokinase light chain; 112Ala, 134Lys, 135Lys bovine enterokinase light chain; 112Ala, 134Lys bovine enterokinase light chain; 112Ala, 135Lys bovine enterokinase light chain and any such combinations including substitutions with other charged amino acids.
- a bovine enterokinase light chain analogue which has improved solubility in a renaturation process relative to natural bovine enterokinase light chain.
- a bovine enterokinase light chain analogue according to the invention has one or more surface oriented hydrophobic amino acids which have been mutated to hydrophilic, charged amino acids wherein improved solubility in a renaturation process relative to natural bovine enterokinase light chain is obtained.
- surface oriented hydrophobic amino acids for substitution to hydrophilic charged amino acids are selected after aligning the bovine enterokinase light chain with other serine proteases and scanning the solvent-accessable surfaces through a computational 3D model of enterokinase.
- refolding a bovine enterokinase light chain analogue according to the invention is known to the person skilled in the art.
- refolding may be carried out by denaturation in urea, followed by oxidative refolding in glutathione or another re-dox environment.
- a buffer (refolding buffer) is used during the refolding process.
- the refolding buffer comprises urea.
- the refolding buffer comprises between 0M and 2M urea.
- the refolding buffer comprises between 0.5M and 2M urea, between 0M and 1.5M urea or between 0.5M and 1.5M urea.
- the refolding buffer comprises about 1M urea.
- the initial concentration of inclusion body may affect the refolding yield.
- the concentration of inclusion body is between 1 and 4 mg/ml .
- the thioredoxin (Trx) tag is removed during refolding, i.e. during dilution and incubation under refolding conditions. It has thus been found that refolding and activation may be obtained without addition of an activation enzyme.
- the linker connecting the trx tag and the bovine enterokinase light chain analogue of the invention is removed by autocleavage. It has thus by the inventors surprisingly been found that the linker connecting the trx tag and the bovine enterokinase light chain analogue of the invention facilitates the refolding.
- a bovine enterokinase light chain analogue according to the invention has the substitutions L134K and I135K, where the bovine enterokinase light chain analogue is more soluble during the renaturation process relative to wild type EK.
- a bovine enterokinase light chain analogue according to the invention further has the substitution C112A.
- a bovine enterokinase light chain analogue of the invention has full enterokinase activity compared to wild type bovine enterokinase.
- a bovine enterokinase light chain analogue of the invention has a substantially equivalent functional or biological activity as wild type bovine enterokinase.
- a bovine enterokinase light chain analogue has substantially equivalent functional or biological activities (i.e., is a functional equivalent) of the polypeptide having the amino acid sequence set forth as SEQ ID NO: 1 (e.g., has a substantially equivalent enteropeptidase activities).
- Nucleic acid forms encoding enterokinase light chain analogues are also described herein.
- Nucleic acids include genomic DNA (gDNA), complementary DNA (cDNA), synthetic DNA prepared by chemical synthesis as well as DNA with deletions or substitutions, allelic variants and sequences that hybridize thereto under stringent conditions as long as they encode enterokinase light chain analogues.
- a nucleic acid comprising a polynucleotide sequence, and wherein said nucleic acid encodes a mammalian enterokinase light chain analogue such as a bovine enterokinase light chain analogue according to the invention.
- the nucleic acid is operably linked to an inducible promoter.
- a recombinant vector is provided which comprises the nucleic acid operably linked to the inducible promoter.
- the inducible promoter is selected from a group consisiting of AraB, T7, trp, lac, tac.
- a further embodiment of the invention provides a host cell comprising the recombinant vector comprising the polynucleotide sequence coding for the amino acid sequence of a mammalian enterokinase light chain analogue such as a bovine enterokinase light chain analogue according to the invention.
- a mammalian enterokinase light chain analogue such as a bovine enterokinase light chain analogue according to the invention.
- a further aspect of the invention provides the host cell comprising the recombinant vector comprising the polynucleotide sequence coding for the amino acid sequence encoding a mammalian enterokinase light chain analogue such as a bovine enterokinase light chain analogue according to the invention.
- the host cell is selected from a group consisting of E.coli, B.subtilis, S.saccaromyces and A.oryzae.
- enterokinase light chain The production of polypeptides, e.g., enterokinase light chain, is well known in the art.
- the bovine enterokinase light chain analogue may for instance be produced by classical peptide synthesis, e.g., solid phase peptide synthesis using t-Boc or Fmoc chemistry or other well established techniques, see, e.g., Greene and Wuts, "Protective Groups in Organic Synthesis", John Wiley & Sons, 1999 .
- the bovine enterokinase light chain analogue may also be produced by a method which comprises culturing a host cell containing a DNA sequence encoding the analogue and capable of expressing the bovine enterokinase light chain analogue in a suitable nutrient medium under conditions permitting the expression of the bovine enterokinase light chain analogue.
- a method which comprises culturing a host cell containing a DNA sequence encoding the analogue and capable of expressing the bovine enterokinase light chain analogue in a suitable nutrient medium under conditions permitting the expression of the bovine enterokinase light chain analogue.
- enterokinase in microorganisms such as, e.g., Escherichia coli and Saccharomyces cerevisiae are, e.g., disclosed in WO 94/16083 .
- the bovine enterokinase light chain analogue is produced by expressing a DNA sequence encoding the bovine enterokinase light chain analogue in question or a precursor thereof in a suitable host cell by well known technique as disclosed in e.g. WO 94/16083
- the bovine enterokinase light chain analogues of the invention may be recovered from the cell culture medium or from the cells.
- the bovine enterokinase light chain analogues of the present invention may be purified by a variety of procedures known in the art including, but not limited to, chromatography (e.g., ion exchange, affinity, hydrophobic, chromatofocusing, and size exclusion), electrophoretic procedures (e.g., preparative isoelectric focusing (IEF), differential solubility (e.g., ammonium sulfate precipitation), or extraction (see, e.g., Protein Purification, J.-C. Janson and Lars Ryden, editors, VCH Publishers, New York, 1989 ).
- chromatography e.g., ion exchange, affinity, hydrophobic, chromatofocusing, and size exclusion
- electrophoretic procedures e.g., preparative isoelectric focusing (IEF), differential solubility (e.g., ammonium s
- the bovine enterokinase light chain analogues of the present invention are purified using anion exchange chromatography.
- the anion exchange chromatography is followed by hydrophobic interaction chromatography.
- the bovine enterokinase light chain analogues of the present invention are purified using Q HP anion exchange chromatography.
- the Q HP anion exchange chromatography is followed by Phenyl FF hydrophobic interaction chromatography.
- an improved process for production of a mammalian enterokinase light chain analogue such as a bovine enterokinase light chain analogue is provided, wherein said method comprises the steps:
- the invention provides a new recombinant process for production of mammalian enterokinase light chain analogue such as a bovine enterokinase light chain analogue in E.coli in a very efficient and economic way.
- mammalian enterokinase light chain analogue such as a bovine enterokinase light chain analogue in E.coli in a very efficient and economic way.
- a bovine enterokinase light chain analogue according to the invention may e.g. be localized in the inclusion bodies of E. coli or in the secreted material of yeast. In one embodiment expression of enterokinase is localized in the inclusion bodies of E. coli.
- E. coli Various strains of E. coli are useful as host cells for the production of non-glycosylated, homogeneous enterokinase activity are also well-known in the art.
- a non-exclusive list of such strains includes E.coli B BL21 DE3, E.coli K12 W3110, MC1061, DH1, K803, HB101, JM101 and other K12 like strains.
- other bacterial species may be used, including B. subtilis, various strains of Pseudomonas, other bacilli and the like.
- yeast cells are also available as host cells for expression of the enterokinase activity of the present invention.
- Yeast cells are especially useful as a host for pre/pro fusion to mature enterokinase. When expressed using a suitable yeast vector, the fusion is secreted by virtue of a signal peptide.
- the bovine enterokinase light chain analogue of this invention When expressed in bacterial cells, it may be expressed intracellularly usually as inclusion bodies, or it may be secreted from bacterial cells in active form if a secretory signal is included. Where necessary or desired, as when reduced bioactivity is observed, the enterokinase activity may be obtained by conventional methods such as solubilization of protein in urea or guanidine HCI, followed by dilution to reduce the concentration of these reagents and treatment with oxidizing agents such as dithiothreitol or ss-mercapto ethanol to enhance refolding.
- oxidizing agents such as dithiothreitol or ss-mercapto ethanol
- the bovine enterokinase light chain analogues according to the invention are enzymatically active proteases which cleave specifically after a (Asp) 4 -Lys (DDDDK) sequence in various numbers of fused protein products between affinity tag and the mature protein.
- the bovine enterokinase light chain analogues according to the invention have retained enzymatic activity
- a process for preparing a bovine enterokinase light chain analogue in E. coli cells is obtained, wherein the E. coli cells are transformed with a plasmid carrying the bovine enterokinase light chain analogue gene and an inducible promoter by fermentation involving batch and fed batch stages and isolation and purification of the expressed protein from the cultures.
- a refolding process for a bovine enterokinase light chain analogue according to the invention is obtained, wherein the expression of the enterokinase light chain analogue is in the form of inclusion bodies in recombinant E. coli.
- denaturation followed by refolding in a redox system is used.
- enterokinase light chain analogues of the invention may be used in a method for cleaving proteins having an enterokinase cleavage site, and especially fusion proteins having such a cleavage site engineered into their sequence.
- the amounts needed are readily determined empirically by one skilled in the art.
- fusion protein as used herein is meant to refer to a protein created through genetic engineering from two or more proteins or peptides.
- a fusion protein can refer to a protein in which a Asp-Asp-Asp-Asp-Lys (D4K) sequence has been intentionally introduced for specific cleavage. Generally, cleavage of the fusion protein generates two polypeptides.
- a fusion protein according to the invention can be a recombinant fusion protein.
- a fusion protein can be generated, for example, from the addition of a vector-derived residue peptide at one terminus, for example the N-terminus, in addition to the amino acid sequence of the wild type protein of interest. In this way, for example, a recombinant fusion protein can be constructed to have Asp-Asp-Asp-Lys (D4K) cleavage site in the vector upstream joined to the protein of interest.
- operably linked denotes herein a configuration in which a control sequence is placed at an appropriate position relative to the coding sequence of the polynucleotide sequence such that the control sequence directs the expression of the coding sequence of a polypeptide.
- protease is intended to include any polypeptide/s, alone or in combination with other polypeptides, that break peptide bonds between amino acids of proteins.
- proteolytic activity is meant to refer to the cleavage activity of a substrate by an enzyme.
- the term refers to the enzymatic cleavage by enteropeptidases.
- the term is meant to refer to the specific activity of a bovine enterokinase light chain analogue of the invention for Asp-Asp-Asp-Asp-Lys cleavage sites.
- Non-specific proteolytic activity is meant to refer to cleavage activity that is not directed to a specific cleavage site.
- Specific proteolytic activity is meant to refer to cleavage activity that is directed to a specific cleavage site.
- a bovine enterokinase light chain analogue according to the invention is superior for cleavage of fusion proteins when compared to the bovine-derived two-chain form.
- the enterokinase light chain analogue of the invention is incorporated as one of the fusion protein partners to yet another protein.
- the fusion protein results in the release of additional enterokinase activity which in turn can catalyze many more proteolytic cleavages of fusion proteins.
- large amounts of enterokinase activity can be produced from a fusion protein in an autocatalytic manner.
- Another particular aspect of the invention teaches a method for cleavage of a protein containing an Asp-Asp-Asp-Asp-Lys cleavage site using any of the bovine enterokinase light chain analogues of the invention described herein, the method comprising contacting the protein with any of the bovine enterokinase light chain analogues of the invention, and wherein the contacting of the protein with the bovine enterokinase light chain analogue results in specific cleavage.
- the protein is a fusion protein.
- the fusion protein is a recombinant fusion protein.
- the protein is bacterially produced.
- the protein is a synthetic protein.
- the invention teaches a method for the preparation of recombinant protein using any of the bovine enterokinase light chain analogues according to the invention as described herein, the method comprising providing a recombinant fusion protein containing a Asp-Asp-Asp-Asp-Lys cleavage site, and contacting the fusion protein with any of the bovine enterokinase light chain analogues of the invention, wherein contacting the recombinant fusion protein with the bovine enterokinase light chain analogue results in Asp-Asp-Asp-Asp-Lys specific cleavage and preparation of recombinant protein.
- bovine enterokinase light chain analogues a production process for making bovine enterokinase light chain analogues.
- the bovine enterokinase light chain analogues were fused to thioredoxin tag expressed as inclusion bodies in E.coli. After refolding and auto-activation, the active enterokinase light chain analogue was purified by Q HP anion exchange chromatography. Moreover, it was found that triple substitutions (C112A, L134K and I135K) of bovine enterokinase light chain (EK LM ), which improved the surface hydrophilic properties, increased the refolding yield 4 fold without loosing activity.
- EK LM triple substitutions
- the yield of purified enterokinase light chain analogue was 800mg/L from a culture of 4g/L, and the specific activity was determined as 5000 ⁇ 10 EU/mg.
- our enterokinase light chain analogue production process provides a valuable tool for processing therapeutic fusion proteins and other fusion proteins.
- the DNA sequence encoding the catalytic subunit of bovine enterokinase was amplified with the following primers:
- These two primers contained Kpn I and EcoR I restriction enzyme sites, respectively.
- the target fragment was introduced into pET32a (Novagen) from Kpnl and EcoRI site. Routine PCR reaction was performed using Pfu DNA Polymerase from Stratagene. The sequence of plasmid pET32a-EK L was confirmed by sequencing. Three substitution sites, i.e. C112A, L134K, 1135K were introduced by using QuikChange® XL Site-Directed Mutagenesis Kit from Stratagene with the primers:
- Cells from a glycerol stock were inoculated on an EC1 plate grown overnight at 37°C, and washed with 0.9% sodium chloride (NaCl) to suspend the cells.
- the culture was allowed to grow in a fermentor containing fermentation defined medium (FDM) at 37°C for 16 hrs, and induced with 1.0mM IPTG at an OD600 of 150, and then grown for 6 hours at 37°C before harvesting by centrifugation.
- FDM fermentation defined medium
- Trx-linker-EK L and Trx-linker-EK LM in E.coli BL21 were expressed in fed-batch fermentation. As shown in Fig.1 , no apparent leaky expression judged by SDS-PAGE was observed before IPTG induction. An induced band just above 43kD on SDS-PAGE by IPTG appeared, and it was confirmed by LC-MS that this band represented the target protein. Moreover, the expression level of the target protein was dependent upon the induction time. 4hrs or 6hrs of induction for Trx-linker-EK L and Trx-linker-EK LM by using fermentation defined medium (FDM), respectively gave acceptable expression, and ⁇ 4g/L of the target proteins was achieved.
- FDM fermentation defined medium
- lysis buffer (1:10, w/w) containing 20mM Tris, pH 8.0, and lysed by French press.
- Inclusion bodies were sedimented at 20,000g for 1hr at 4°C, and then washed once by using lysis buffer.
- the inclusion bodies were solublized to 3.2mg/ml in buffer containing 20mM Tris, 8M urea, pH8.0, 20mM DTT and incubated at 4°C for 3hrs. After centrifugation at 20,000g for 30min, the solublized EK (i.e.
- Trx-linker-EK L and/or Trx-linker-EK LM was diluted 80 fold into refolding buffer containing 20mM Tris, 1M Urea, 1mM GSSG, 3mM GSH, pH 8.3 and incubated at 20°C for 24hrs.
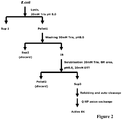
- the process scheme is shown in Fig.2 .
- the inclusion bodies were solublized in the buffer containing 5-8M urea and 10-20mM DTT. It should be noted that the inclusion body concentration affected the refolding yield. It was found that the refolding yield of 4mg/ml Trx-linker-EK LM was 2 fold higher than that of 6mg/ml Trx-linker-EK LM ( Fig.3A ).
- the refolding occurred by dilution.
- the amount of purified enzyme from a fixed volume was also dependant upon the Trx linker EK concentration in the refolding buffer, and reached a maximum when Trx-linker-EK LM concentration was 120 ⁇ g/ml.
- the auto-catalytic activation occurred concomitantly with the refolding process.
- the active EK was liberated from Trx-linker-EK by the escape active EK, which specifically cleaved Trx tag off at DDDDK recognition site just before the mature EK.
- the refolding and auto-catalytic activation process seemed optimal at48hrs ( Fig.4 ).
- Considering the inhibition of EK by urea it was found that the refolding yield was largely reduced if above 2M urea in refolding buffer. Our result showed that 1M urea in refolding buffer was optimal ( Fig.5 ).
- GSSG/GSH in the ratio 1:3 was found optimal and better than Cystine/cystein ( Fig.6 ).
- the active EK after refolding and auto-activation was purified and concentrated by one step anion exchange chromatographic purification (QHP column, Fig.7A ). It was found that Trx tag was in P1, EK LM was mainly in P2 together with the impurity of Trx tag, and P3 contained trace amount of EK LM , which is confirmed by the activity assay shown in Fig. 7C . It should be noted that high purity EK LM (>90%) was obtained by further purification of P2 using hydrophobic interaction chromatography (HIC) ( Fig.7B ). Moreover, the enzymatic activity of each fraction was also assayed ( Fig.7C ), and pooled.
- HIC hydrophobic interaction chromatography
- Trx-linker-EK L the refolding yield was rather low beyond 40 ⁇ g/ml of Trx-linker-EK L during the refolding process (4.4% at 40 ⁇ g/ml), which made this process practically difficult. In other words, a huge holding tank is required to produce large amount of EK ( ⁇ 1,000g).
- EK LM greatly improved the refolding yield, especially when EK LM concentration in refolding buffer was beyond 40 ⁇ g/ml, which is the bottle neck for the large scale production of EK L ( Fig.3A ).
- Trx-linker-EK LM As shown in Fig.3A , at 40 ⁇ g/ml of Trx-linker-EK LM concentration in the refolding buffer, the refolding yield of Trx-linker-EK LM (17%) was 4 fold higher than that of Trx-linker-EK L (4.4%). Moreover, ⁇ 16mg of active EK LM could be purified from 1L refolding tank in which the EK LM concentration is 120 ⁇ g/ml.
- EK L and EK LM The specific enzymatic activity between EK L and EK LM was compared as in Fig. 8 .
- the triple substitutions of EK LM had no apparent effect on enzyme activity, which was evidenced by the fact that EK L and EK LM have similar bands on SDS-PAGE if loaded the same activity.
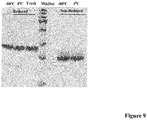
- EK LM was quite stable if stored in buffer containing 20mM Tris, 200mM NaCl at -80°C or 4°C. No apparent degradation and decrease of activity were observed up to 3 months ( Fig.9 ).
- the enzymatic activity was measured directly using a fluorogenic substrate, GDDDDK-Beta-naphthylamide.
- the reaction was started with addition of 1ul sample into each well of Fluorescent 96 well plate containing 100ul of reaction buffer. After mixing for 10 seconds, the fluorescence was measured with Fluostar OPTIMA (excitation at 340nM and emission at 420nM).
- the enzyme activity was defined by arbitrary unit (EU), which derived from slope*60/30,000, where the slope represented linear range.
- TrxEK LM cell pellet was resuspended in 100ml of lysis buffer (20mM Tris, pH 8.0), and the cells were disrupted by using a homogenizer under a pressure of 30,000psi. After the supernatant was discarded, the IBs weighed 3.53g. The isolated IBs were resuspended in 70ml of solublization buffer (20mM Tris, 8M urea, pH8.0, 20mM DTT (freshly added)) and incubated at 4°C for 4hrs. The solublized samples were clarified by centrifugation.
- 16ml of IBs solution was diluted into 500ml refolding buffer (20mM Tris, 1mM GSSG, 3mM GSH, 1M Urea, pH 8.0) and stirred at 20°C for 54hrs.
- concentration of protein during refolding is 60 ⁇ g/ml.
- the elution fractions with highest enzyme activity were combined resulting in a pool volume of 30ml and total enzyme activity of 14,100EU.
- the protein amount was 2.82mg.
- Trx-linkerEK LM cell pellet was resuspended in 1000ml of lysis buffer (20mM Tris, pH 8.0), and the cells were disrupted by using a homogenizer under a pressure of 30,000psi. After the supernatant was discarded, the IBs weighed 22g and were washed by 1000ml of 20mM Tris, pH 8.0 once. After wash, the IBs solution was divided into 6 bottles for centrifugation. After the supernatant was discarded, 41ml of solublization buffer (20mM Tris, 8M urea, pH8.0, 20mM DTT (freshly added)) was added into one bottle and incubated at 4°C for 3hrs. The solublized IBs were clarified by centrifugation and the final volume was 43ml.
- solublization buffer 20mM Tris, 8M urea, pH8.0, 20mM DTT (freshly added)
- the enzyme activity of elution fractions 18-23 is higher than the other fractions through activity test.
- the elution fractions with highest enzyme activity were combined resulting in a pool volume of 30ml and total enzyme activity of 24,900EU.
- the protein amount was 4.98mg.
- EK LM protein 2.82mg was produced from 0.5L of refolding solution when using TrxEK LM when the protein concentration was 60 ⁇ g/ml during refolding, whereas 4.98mg of EK protein was produced from Trx-linker-EK LM version under the same conditions.
- Trx-linker-EK LM version 3.82mg was produced from Trx-linker-EK LM version under the same conditions.
- Example 6 Components optimization of the refolding buffer
- the inclusion bodies were solubilized to 7.3mg/ml in the buffer containing 20mM Tris, 8M urea, pH8.0, 20mM DTT, and then the solubilized Trx-linker-EK LM was added into the optimized refolding buffer containing certain additive by 20-fold dilution. The mixture was incubated at 4°C for 20hrs and the amount of correctly refolded Trx-linker-EK LM was quantified by protease activity assay as described in Example 4.
Claims (7)
- Rinder-Enterokinase-Leichtkettennalogon, umfassend eine in SEQ ID NO: 1 dargestellte Aminosäuresequenz, wobei das Analogon die Substitutionen C112A, L134K und I135K umfasst und das Analogon weniger als 10 Aminosäuremodifikationen relativ zu SEQ ID NO: 1 aufweist und das Analogon Enterokinase-Protease-Aktivität aufweist.
- Verfahren zum Erhalten einer verbesserten Löslichkeit in einem Renaturierungsprozess eines Enterokinase-Leichtkettenanalogons, umfassend den Schritt des Mutierens hydrophober Aminosäuren von Leichtketten-Wildtyp-Rinder-Enterokinase von SEQ ID NO: 1 zu hydrophilen Aminosäuren und gegebenenfalls Mutieren anderer Aminosäuren von Leichtketten-Wildtyp-Rinder-Enterokinase, wobei die der Mutation unterworfenen hydrophoben Aminosäuren auf der Fläche von gefalteter Leichtketten-Wildtyp-Rinder-Enterokinase vorhanden sind, das Analogon die Substitutionen C112A, L134K und I135K umfasst, das Analogon relativ zu SEQ ID NO: 1 weniger als 10 Aminosäuremodifikationen umfasst und das Analogon Enterokinase-Protease-Aktivität aufweist.
- Verfahren zum Herstellen eines Rinder-Enterokinase-Leichtkettenanalogons, wobei das Rinder-Enterokinase-Leichtkettenanalogon ein Analogon nach Anspruch 1 ist, und wobei das Verfahren die Schritte umfasst:a) Kultivieren der Wirtszellen in einem Wachstumsmedium, das einen Induktor umfasst, wobei die Wirtszellen eine Polynukleotidsequenz umfassen, welche die Aminosäuresequenz des Enterokinase-Leichtkettenanalogons codiert;b) Wiedergewinnen der Zellen mit dem Enterokinase-Leichtkettenanalogon in Einschlusskörpern;c) Auflösen und Rückfalten des Enterokinase-Leichtkettenanalogons; undd) Reinigen des Enterokinase-Leichtkettenanalogons.
- Verfahren zur rekombinanten Herstellung eines Peptids oder Proteins in einer Bakterien- oder Hefewirtszelle, umfassend:a) Exprimieren eines Fusionsproteins, welches das herzustellende Peptid oder Protein umfasst, in Hefe oder Bakterien;b) Spalten des Fusionsproteins mit dem Rinder-Enterokinase-Leichtkettenanalogon nach Anspruch 1; undc) Isolieren des hergestellten Peptids oder Proteins.
- Verfahren zur rekombinanten Herstellung eines Peptids oder Proteins nach Anspruch 4, wobei das in Schritt a) exprimierte Fusionsprotein ferner eine Asp-Asp-Asp-Asp-Asp-Lys-Spaltstelle umfasst.
- Verfahren zur rekombinanten Herstellung eines Peptids oder Proteins nach einem der Ansprüche 4 bis 5, wobei die Wirtszelle E. coli ist.
- Verfahren zur rekombinanten Herstellung eines Peptids oder Proteins nach einem der Ansprüche 4 bis 6, wobei das herzustellende Peptid oder Protein ein GLP-1-Peptid ist.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2011002169 | 2011-12-23 | ||
| PCT/EP2012/076372 WO2013092855A1 (en) | 2011-12-23 | 2012-12-20 | Modified enterokinase light chain |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2794875A1 EP2794875A1 (de) | 2014-10-29 |
| EP2794875B1 true EP2794875B1 (de) | 2020-07-22 |
Family
ID=47561561
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP12816050.4A Active EP2794875B1 (de) | 2011-12-23 | 2012-12-20 | Modifizierte leichte enterokinasekette |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US9611466B2 (de) |
| EP (1) | EP2794875B1 (de) |
| JP (1) | JP6138151B2 (de) |
| ES (1) | ES2819288T3 (de) |
| WO (1) | WO2013092855A1 (de) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9982287B2 (en) * | 2013-12-17 | 2018-05-29 | Novo Nordisk A/S | Enterokinase cleavable polypeptides |
| WO2017118752A1 (en) * | 2016-01-07 | 2017-07-13 | Novo Nordisk A/S | Modified enterokinase light chain and its preparation method |
| CN109136209B (zh) * | 2018-07-18 | 2021-07-06 | 上海雅心生物技术有限公司 | 肠激酶轻链突变体及其应用 |
| CN114807101B (zh) * | 2022-06-20 | 2022-09-16 | 北京惠之衡生物科技有限公司 | 包含牛肠激酶轻链蛋白的融合蛋白及其表达载体和重组工程菌 |
| CN116064491A (zh) * | 2022-06-20 | 2023-05-05 | 北京惠之衡生物科技有限公司 | 牛肠激酶轻链蛋白突变体及其重组融合蛋白 |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE69432787D1 (de) | 1993-01-15 | 2003-07-10 | Inst Genetics Llc | Klonierung von Enterokinase und Verfahren zu deren Verwendung |
| KR19990073067A (ko) | 1999-03-13 | 1999-10-05 | 문정복 | 레일천공기 |
| KR100507980B1 (ko) | 2002-06-18 | 2005-08-17 | 프로테온 주식회사 | 엔테로키나제 경사슬의 아미노말단 또는 카르복실말단이 변형된 재조합 엔테로키나제 |
| CN1214107C (zh) | 2002-07-26 | 2005-08-10 | 刘建宁 | 一种具有高活性和高稳定性的肠激酶轻链变体 |
| CN1869236A (zh) | 2005-05-24 | 2006-11-29 | 上海新生源医药研究有限公司 | 一种重组牛肠激酶的生产方法 |
| WO2008136014A1 (en) | 2007-05-07 | 2008-11-13 | Usv Limited | Cloning and expression of enterokinase and its process for production |
| CN101613687A (zh) | 2007-05-11 | 2009-12-30 | 上海张江生物技术有限公司 | 重组牛肠激酶、其制备方法及用途 |
| CN102061302B (zh) | 2010-11-26 | 2012-07-04 | 扬子江药业集团北京海燕药业有限公司 | 肠激酶轻链基因的合成及其表达产物的制备方法 |
-
2012
- 2012-12-20 ES ES12816050T patent/ES2819288T3/es active Active
- 2012-12-20 JP JP2014548013A patent/JP6138151B2/ja active Active
- 2012-12-20 EP EP12816050.4A patent/EP2794875B1/de active Active
- 2012-12-20 US US14/364,464 patent/US9611466B2/en active Active
- 2012-12-20 WO PCT/EP2012/076372 patent/WO2013092855A1/en active Application Filing
Non-Patent Citations (1)
| Title |
|---|
| None * |
Also Published As
| Publication number | Publication date |
|---|---|
| ES2819288T3 (es) | 2021-04-15 |
| EP2794875A1 (de) | 2014-10-29 |
| JP6138151B2 (ja) | 2017-05-31 |
| US9611466B2 (en) | 2017-04-04 |
| US20150064744A1 (en) | 2015-03-05 |
| JP2015502167A (ja) | 2015-01-22 |
| WO2013092855A1 (en) | 2013-06-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Choi et al. | Recombinant enterokinase light chain with affinity tag: expression from Saccharomyces cerevisiae and its utilities in fusion protein technology | |
| EP2794875B1 (de) | Modifizierte leichte enterokinasekette | |
| JP4538501B2 (ja) | ポリペプチドのc末端修飾 | |
| CN109486800B (zh) | 一种新型赖氨酰肽链内切酶及其制备方法 | |
| US20160083713A1 (en) | Novel peptidyl alpha-hydroxyglycine alpha-amidating lyases | |
| WO2012104099A1 (en) | Process for the production of recombinant trypsin | |
| US7892787B2 (en) | Method for production of recombinant growth hormone in form of hybrid protein | |
| CN109439643B (zh) | 一种新型赖氨酸特异性内切酶及其制备方法 | |
| ES2754271T3 (es) | Polipéptidos escindibles por enteroquinasa | |
| CN103998606B (zh) | 修饰的肠激酶轻链 | |
| WO2017118752A1 (en) | Modified enterokinase light chain and its preparation method | |
| US11267863B2 (en) | N-terminal fusion partner for producing recombinant polypeptide, and method for producing recombinant polypeptide using same | |
| Liu et al. | Large scale preparation of recombinant human parathyroid hormone 1–84 from Escherichia coli | |
| CN112824527A (zh) | 人工设计的赖氨酰内切酶及编码序列和发酵方法 | |
| US20090258394A1 (en) | Method for the production of high-level soluble human recombinant interferon alpha in e. coli and vectors useful for such a production | |
| US7662935B2 (en) | Processing of peptides and proteins | |
| Yang et al. | High-level production of a candidacidal peptide lactoferrampin in Escherichia coli by fusion expression | |
| Su-Xia et al. | Cloning and expression of a new rat procarboxypeptidase B gene in Escherichia coli and purification of recombination carboxypeptidase B | |
| Song et al. | Engineered recombinant enteropeptidase catalytic subunit: effect of N-terminal modification | |
| EP1841862B1 (de) | Ubp1-proteasemutante sowie ihre codierende sequenz, deren anwendungen und heterogenes proteinexpressionssystem | |
| CN111197041B (zh) | 制备青鳉鱼肠激酶活性亚基的方法、其产物和用途 | |
| Son et al. | Effects of β-mercaptoethanol and hydrogen peroxide on enzymatic conversion of human proinsulin to insulin | |
| KR100562873B1 (ko) | 인간 인슐린 발현 플라스미드 및 이를 이용한 인간인슐린의 제조 방법 | |
| KR101002166B1 (ko) | 재조합형 gst 융합 단백질의 정제방법 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20140723 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| DAX | Request for extension of the european patent (deleted) | ||
| 17Q | First examination report despatched |
Effective date: 20160224 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| GRAJ | Information related to disapproval of communication of intention to grant by the applicant or resumption of examination proceedings by the epo deleted |
Free format text: ORIGINAL CODE: EPIDOSDIGR1 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAJ | Information related to disapproval of communication of intention to grant by the applicant or resumption of examination proceedings by the epo deleted |
Free format text: ORIGINAL CODE: EPIDOSDIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| INTG | Intention to grant announced |
Effective date: 20191121 |
|
| INTG | Intention to grant announced |
Effective date: 20191203 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTC | Intention to grant announced (deleted) | ||
| GRAJ | Information related to disapproval of communication of intention to grant by the applicant or resumption of examination proceedings by the epo deleted |
Free format text: ORIGINAL CODE: EPIDOSDIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| INTG | Intention to grant announced |
Effective date: 20200103 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| INTC | Intention to grant announced (deleted) | ||
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20200227 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602012071393 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1293419 Country of ref document: AT Kind code of ref document: T Effective date: 20200815 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1293419 Country of ref document: AT Kind code of ref document: T Effective date: 20200722 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201022 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201022 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201023 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201123 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201122 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2819288 Country of ref document: ES Kind code of ref document: T3 Effective date: 20210415 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602012071393 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 |
|
| 26N | No opposition filed |
Effective date: 20210423 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20201231 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20200722 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201220 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201220 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201231 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201231 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200722 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201231 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20221122 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20230102 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20231124 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20231122 Year of fee payment: 12 Ref country code: DE Payment date: 20231121 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20240102 Year of fee payment: 12 |