EP2365101B1 - Process for producing sintered ore - Google Patents
Process for producing sintered ore Download PDFInfo
- Publication number
- EP2365101B1 EP2365101B1 EP09830482.7A EP09830482A EP2365101B1 EP 2365101 B1 EP2365101 B1 EP 2365101B1 EP 09830482 A EP09830482 A EP 09830482A EP 2365101 B1 EP2365101 B1 EP 2365101B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- sintering
- gaseous fuel
- carbonaceous material
- bed
- sintered ore
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000000034 method Methods 0.000 title description 34
- 230000008569 process Effects 0.000 title description 9
- 238000005245 sintering Methods 0.000 claims description 206
- 239000000446 fuel Substances 0.000 claims description 130
- 238000002485 combustion reaction Methods 0.000 claims description 81
- 239000003575 carbonaceous material Substances 0.000 claims description 66
- 239000002994 raw material Substances 0.000 claims description 44
- 238000004519 manufacturing process Methods 0.000 claims description 20
- 239000007789 gas Substances 0.000 description 66
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 41
- 239000000571 coke Substances 0.000 description 28
- 238000002347 injection Methods 0.000 description 17
- 239000007924 injection Substances 0.000 description 17
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 15
- 230000007423 decrease Effects 0.000 description 13
- 230000009467 reduction Effects 0.000 description 12
- 238000009826 distribution Methods 0.000 description 11
- 230000000717 retained effect Effects 0.000 description 11
- 239000010410 layer Substances 0.000 description 10
- 238000012360 testing method Methods 0.000 description 10
- 238000010586 diagram Methods 0.000 description 9
- 239000001569 carbon dioxide Substances 0.000 description 8
- 229910002092 carbon dioxide Inorganic materials 0.000 description 8
- 230000000694 effects Effects 0.000 description 8
- 238000002844 melting Methods 0.000 description 8
- WETINTNJFLGREW-UHFFFAOYSA-N calcium;iron;tetrahydrate Chemical compound O.O.O.O.[Ca].[Fe].[Fe] WETINTNJFLGREW-UHFFFAOYSA-N 0.000 description 7
- 230000008018 melting Effects 0.000 description 7
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 6
- 230000015556 catabolic process Effects 0.000 description 6
- 230000003247 decreasing effect Effects 0.000 description 6
- 238000006731 degradation reaction Methods 0.000 description 6
- 238000002474 experimental method Methods 0.000 description 6
- 229910052595 hematite Inorganic materials 0.000 description 6
- 239000011019 hematite Substances 0.000 description 6
- LIKBJVNGSGBSGK-UHFFFAOYSA-N iron(3+);oxygen(2-) Chemical group [O-2].[O-2].[O-2].[Fe+3].[Fe+3] LIKBJVNGSGBSGK-UHFFFAOYSA-N 0.000 description 6
- 239000000047 product Substances 0.000 description 6
- 239000000523 sample Substances 0.000 description 6
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 5
- 238000006243 chemical reaction Methods 0.000 description 5
- 238000007599 discharging Methods 0.000 description 5
- 230000001965 increasing effect Effects 0.000 description 5
- 229910052500 inorganic mineral Inorganic materials 0.000 description 5
- 239000011707 mineral Substances 0.000 description 5
- 235000010755 mineral Nutrition 0.000 description 5
- 239000001301 oxygen Substances 0.000 description 5
- 229910052760 oxygen Inorganic materials 0.000 description 5
- 239000002245 particle Substances 0.000 description 5
- ODINCKMPIJJUCX-UHFFFAOYSA-N Calcium oxide Chemical compound [Ca]=O ODINCKMPIJJUCX-UHFFFAOYSA-N 0.000 description 4
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 4
- 239000000378 calcium silicate Substances 0.000 description 4
- 229910052918 calcium silicate Inorganic materials 0.000 description 4
- OYACROKNLOSFPA-UHFFFAOYSA-N calcium;dioxido(oxo)silane Chemical compound [Ca+2].[O-][Si]([O-])=O OYACROKNLOSFPA-UHFFFAOYSA-N 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 230000000052 comparative effect Effects 0.000 description 4
- 239000001257 hydrogen Substances 0.000 description 4
- 229910052739 hydrogen Inorganic materials 0.000 description 4
- 230000006872 improvement Effects 0.000 description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 3
- 150000002431 hydrogen Chemical class 0.000 description 3
- 229910052742 iron Inorganic materials 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- 238000010587 phase diagram Methods 0.000 description 3
- 239000002344 surface layer Substances 0.000 description 3
- OTMSDBZUPAUEDD-UHFFFAOYSA-N Ethane Chemical compound CC OTMSDBZUPAUEDD-UHFFFAOYSA-N 0.000 description 2
- 229910000831 Steel Inorganic materials 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 238000007664 blowing Methods 0.000 description 2
- 239000000292 calcium oxide Substances 0.000 description 2
- 235000012255 calcium oxide Nutrition 0.000 description 2
- 239000000428 dust Substances 0.000 description 2
- 239000003345 natural gas Substances 0.000 description 2
- 238000005453 pelletization Methods 0.000 description 2
- 230000035699 permeability Effects 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 239000001294 propane Substances 0.000 description 2
- 239000010959 steel Substances 0.000 description 2
- 238000004441 surface measurement Methods 0.000 description 2
- 206010017740 Gas poisoning Diseases 0.000 description 1
- 235000019738 Limestone Nutrition 0.000 description 1
- BPQQTUXANYXVAA-UHFFFAOYSA-N Orthosilicate Chemical compound [O-][Si]([O-])([O-])[O-] BPQQTUXANYXVAA-UHFFFAOYSA-N 0.000 description 1
- 208000005374 Poisoning Diseases 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- RHZUVFJBSILHOK-UHFFFAOYSA-N anthracen-1-ylmethanolate Chemical compound C1=CC=C2C=C3C(C[O-])=CC=CC3=CC2=C1 RHZUVFJBSILHOK-UHFFFAOYSA-N 0.000 description 1
- 239000003830 anthracite Substances 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 239000001273 butane Substances 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 239000010459 dolomite Substances 0.000 description 1
- 229910000514 dolomite Inorganic materials 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000004880 explosion Methods 0.000 description 1
- 239000002360 explosive Substances 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 230000004907 flux Effects 0.000 description 1
- 239000002737 fuel gas Substances 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- SZVJSHCCFOBDDC-UHFFFAOYSA-N iron(II,III) oxide Inorganic materials O=[Fe]O[Fe]O[Fe]=O SZVJSHCCFOBDDC-UHFFFAOYSA-N 0.000 description 1
- 239000006028 limestone Substances 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 1
- OFBQJSOFQDEBGM-UHFFFAOYSA-N n-pentane Natural products CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 1
- 231100000572 poisoning Toxicity 0.000 description 1
- 230000000607 poisoning effect Effects 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B1/00—Preliminary treatment of ores or scrap
- C22B1/14—Agglomerating; Briquetting; Binding; Granulating
- C22B1/16—Sintering; Agglomerating
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B1/00—Preliminary treatment of ores or scrap
- C22B1/14—Agglomerating; Briquetting; Binding; Granulating
- C22B1/16—Sintering; Agglomerating
- C22B1/20—Sintering; Agglomerating in sintering machines with movable grates
- C22B1/205—Sintering; Agglomerating in sintering machines with movable grates regulation of the sintering process
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B1/00—Preliminary treatment of ores or scrap
- C22B1/14—Agglomerating; Briquetting; Binding; Granulating
- C22B1/16—Sintering; Agglomerating
- C22B1/20—Sintering; Agglomerating in sintering machines with movable grates
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B1/00—Preliminary treatment of ores or scrap
- C22B1/14—Agglomerating; Briquetting; Binding; Granulating
- C22B1/24—Binding; Briquetting ; Granulating
- C22B1/242—Binding; Briquetting ; Granulating with binders
- C22B1/244—Binding; Briquetting ; Granulating with binders organic
- C22B1/245—Binding; Briquetting ; Granulating with binders organic with carbonaceous material for the production of coked agglomerates
Definitions
- the present invention relates to a method for producing sintered ore using a downward-suction-type sintering machine, in which high-strength, high-quality sintered ore can be produced at high yield and low cost, and moreover, carbon dioxide (CO 2 ) emission can be reduced, thus being environmentally friendly.
- Sintered ore which is a main raw material in the blast furnace iron making process, is generally produced by the process shown in Fig. 1 .
- raw materials for sintered ore include fine iron ore; under-sieve fine of sintered ore; recovered fine in iron works; CaO-containing auxiliary raw materials, such as limestone and dolomite; a pelletizing aid, such as burnt lime; coke breeze; and anthracite.
- These raw materials are fed from respective hoppers 1 at predetermined rates onto a conveyor. An adequate amount of water is added to the fed raw materials by drum mixers 2 and 3, and the like, and mixing and pelletizing are performed to thereby obtain a sintering raw material composed of quasi-particles having a average particle size of 3 to 6 mm.
- the sintering raw material is then charged from surge hopper 5, which are provided on a sintering machine, through a drum feeder 6 and a charge chute 7 onto a sintering machine pallet 8 of an endless moving type to form a charged bed 9 which is also referred to as a sintering bed.
- the thickness (height) of the sintering bed is usually about 400 to 800 mm.
- the carbonaceous material in the surface layer of the sintering bed is ignited by an ignition furnace 10 disposed above the sintering bed 9, and air is sucked downward with a wind box 11 disposed beneath the pallet 8 so that the carbonaceous material in the sintering bed is gradually burned.
- the sintering raw material is melted by the combustion heat generated in this step, and thereby a sintered cake is obtained.
- the sintered cake thus obtained is crushed and subjected to particle size regulation, and agglomerates with a size of about 5 mm or more are collected as product sintered ore.
- combustion zone a combustion/melting zone
- the combustion zone moves from the upper portion to the lower portion of the sintering bed as the pallet 8 moves toward the downstream side.
- a sintered cake layer (hereinafter, may be simply referred to as a "sinter layer”) is formed after the combustion zone has passed.
- the moisture contained in the sintering raw material is evaporated by means of the combustion heat of the carbonaceous material and concentrated in the sintering raw material located in the lower layer, the temperature of which has not yet been increased, to form a wet zone.
- the moisture concentration increases to a certain extent or higher, the space between particles of the sintering raw material, which serves as the flow path for suction gas, is filled with moisture, resulting in an increase in airflow resistance.
- the melted portion occurring in the combustion zone required for the sintering reaction also becomes a factor for increasing airflow resistance.
- Fig. 2 shows the distribution of pressure loss and temperature in the sintering bed when the combustion zone moving in the sintering bed with a thickness of 600 mm is located at the position about 400 mm above the pallet in the sintering bed (200 mm down from the surface of the sintering bed).
- the pressure loss distribution at this stage about 60% is in the wet zone, and about 40% is in the combustion zone.
- the production amount (t/hr) of a sintering machine is generally determined by productivity of sinter (t/hr ⁇ m 2 ) ⁇ area of sintering machine (m 2 ). That is, the production amount of a sintering machine varies depending on the machine width and machine length of the sintering machine, the thickness of the layer of raw material deposited (thickness of the sintering bed), the bulk density of the sintering raw material, sintering (combustion) time, yield, and the like.
- Fig. 3 shows the change in temperature with time at a certain point in the sintering bed when sintered ore productivity is high and low, i.e., when the moving speed of the pallet of the sintering machine is high and low.
- the time in which the temperature is retained at 1,200°C, at which particles of sintering raw material start to melt, or higher (hereinafter, referred to as the "high-temperature holding time") is denoted by t 1 in the case of low productivity and denoted by t 2 in the case of high productivity.
- the productivity is high, since the moving speed of the pallet is high, the high-temperature holding time t 2 is shorter than t 1 in the case of low productivity.
- the high-temperature holding time decreases, insufficient sintering easily occurs, resulting in a decrease in the cold strength of sintered ore and a decrease in the yield. Consequently, in order to produce high-strength sintered ore in a short period of time, at high yield, and with high productivity, it is necessary to extend the "high-temperature holding time" by taking any measures so as to enhance the strength of the sintered cake, i.e., the cold strength of sintered ore.
- indices showing the cold strength of sintered ore generally, SI (shatter index) and TI (tumbler index) are used.
- Fig. 4 is a diagram schematically showing a process in which a carbonaceous material in the surface layer of the sintering bed ignited with an ignition furnace continues to burn by means of air being sucked to thereby form a combustion zone, and the combustion zone gradually moves from the upper portion to the lower portion of the sintering bed, resulting in formation of a sintered cake.
- Fig. 5(a) schematically shows the temperature distribution when the combustion zone is present in each of the upper portion, the middle portion, and the lower portion of the sintering bed in the section within the thick frame shown in Fig. 4 .
- the strength of sintered ore is influenced by the time in which the temperature is retained at 1,200°C or higher, more accurately, by the product of temperature and time in which the temperature is retained at 1,200°C or higher. As this value increases, the strength of sintered ore increases.
- the middle portion and the lower portion of the sintering bed are preheated because the combustion heat generated by combustion of the carbonaceous material in the upper portion of the sintering bed is transmitted thereto together with air being sucked. Therefore, the middle portion and the lower portion of the sintering bed are maintained at high temperatures for a long period of time.
- Japanese Unexamined Patent Application Publication No. 48-18102 proposes a technique in which, after a sintering bed is ignited, a gaseous fuel is injected over the sintering bed.
- Japanese Examined Patent Application Publication No. 46-27126 proposes a technique in which, after a sintering bed is ignited, a combustible gas is added to air to be sucked into the sintering bed.
- 55-18585 proposes a technique in which, in order to increase the temperature of a sintering bed of a sintering raw material to high temperatures, a hood is provided on the sintering bed, and air or a mixed gas including coke-oven gas is blown from the hood at the position just behind an ignition furnace. Furthermore, Japanese Unexamined Patent Application Publication No. 5-311257 proposes a technique in which a low melting flux, a carbonaceous material, and a combustible gas are blown in at the same time at the position just behind an ignition furnace.
- WO 2007/052776 discloses a method for producing a sintered ore by feeding a gaseous fuel from the upper part of a charge layer of a sintering raw material deposited on a pallet in a sintering machine, said gaseous fuel being diluted to have a concentration of a lower flammable limit or less, wherein the feed position, the highest attainable temperature in the charge layer, and/or holding time in a high temperature range is regulated during the sintering process.
- a gaseous fuel diluted to a predetermined concentration is introduced into the sintering bed in a downward-suction-type sintering machine, and combustion can be caused in a targeted position in the sintering bed. Therefore, by appropriately controlling the highest achieving temperature and the high-temperature holding time during combustion of the sintering raw material, it is possible to increase the strength of sintered ore in the upper portion of the sintering bed in which the cold strength of sintered ore tends to be decreased because of the shortage of the amount of heat, or it is possible to further increase the strength of sintered ore in the middle and lower portions of the sintering bed.
- a method for producing sintered ore includes a charging step of charging a sintering raw material including fine ore and a carbonaceous material onto a cyclically moving pallet to form a sintering bed; an ignition step of igniting the carbonaceous material in the surface of the sintering bed with an ignition furnace; a gaseous fuel feeding step of feeding a gaseous fuel into the air above the sintering bed so that the gaseous fuel is fed as a diluted gaseous fuel, the concentration of which is a lower flammable limit concentration or less; and a sintering step of sucking the diluted gaseous fuel and air with a wind box disposed under the pallet, into the sintering bed, combusting the carbonaceous material in the sintering bed and combusting the diluted gaseous fuel in the sintering bed in which the carbonaceous material has been combusted, to thereby perform sintering, wherein the amount of carbonace
- the replacement ratio is in a range of 2 to 6.
- the temperature of the combustion/melting zone in the sintering step can be set such that the highest achieving temperature does not exceed 1,380°C and can be retained in the range of 1,200°C to 1,380°C for a long period of time. Therefore, high-strength sintered ore can be produced at high yield stably while ensuring high productivity.
- the carbonaceous material can be reduced in an amount equivalent to or more than the combustion heat of the gaseous fuel fed, the carbonaceous material cost can be reduced, and emission of carbon dioxide generated in the sintering step can be greatly reduced.
- a method for producing sintered ore according to the present invention includes a charging step, an ignition step, a gaseous fuel feeding step, and a sintering step.
- a charging step a sintering raw material including fine ore and a carbonaceous material is charged onto a cyclically moving pallet to form a sintering bed.
- the ignition step the carbonaceous material in the surface layer of the sintering bed is ignited with an ignition furnace.
- the gaseous fuel feeding step on the downstream side in the pallet travel direction of the ignition furnace, by discharging a high-concentration gaseous fuel at high speed from a gaseous fuel feed device into the air above the sintering bed, the gaseous fuel is instantaneously mixed with air to produce a diluted gaseous fuel having a predetermined concentration equal to or less than the lower flammable limit concentration, and the diluted gaseous fuel together with air is sucked, with a wind box disposed under the pallet, into the sintering bed.
- the carbonaceous material in the sintering bed is burned by means of the air sucked into the sintering bed to melt and sinter the sintering raw material by the resulting combustion heat, and by burning the diluted gaseous fuel at a predetermined position in the sintering bed through which the combustion zone has passed to further promote melting and sintering to form a sintered cake.
- the gaseous fuel is instantaneously mixed with the surrounding air to dilute the gaseous fuel to a concentration equal to or less than the lower flammable limit concentration of the gaseous fuel, and then the diluted gaseous fuel is introduced into the sintering bed.
- the reason for dilution before introduction into the sintering bed is described below.
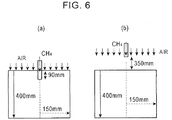
- An experiment device was fabricated by filling a sintering pot of 300 mm ⁇ in inner diameter ⁇ 400 mm in height with a sintered cake, in which it was possible to suck air through the sintered cake from below the sintered cake.
- a nozzle was embedded at the position 90 mm deep from the surface in the center of the sintered cake, and a 100%-concentration methane gas was injected into the sintered cake in an amount of 1% by volume relative to air being sucked.
- the methane gas concentration distribution was measured in the circumferential direction and the depth direction in the sintered cake, and the results thereof are shown in Table 1. Furthermore, as shown in Fig.
- examples of the method of feeding a diluted gaseous fuel with the concentration described above into the sintering bed include a method of injecting from directly above in which a gaseous fuel, such as city gas, LNG, or C gas, with a high concentration is discharged into the air and diluted with the surrounding air to a predetermined concentration, and then the diluted gaseous fuel is introduced into the sintering bed; and a premix injection method (so-called premix type) in which a gaseous fuel is mixed with the air and diluted to a predetermined concentration in advance, and the premix is fed from above the sintering bed.
- Table 3 evaluates the advantages and disadvantages of each method.
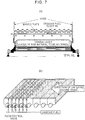
- a device having a gaseous fuel feed means in which a plurality of gaseous fuel feed pipes are arranged along the direction of pallet travel, and slits or openings for discharging the gaseous fuel are provided on the pipes or nozzles are fixed to the pipes as shown in Fig. 8 may be preferably used.
- Table 4 shows the lower flammable limit concentration, the feed concentration, and the like of gaseous fuels (city gas, coke-oven gas (C gas), and blast furnace gas (B gas)) used in the steel industry.
- concentration of the gaseous fuel when fed into the sintering raw material from the standpoint of preventing explosion and a fire (ignition), safety increases as the lower flammable limit concentration decreases.
- city gas uses natural gas (LNG) containing methane as a main component, and although city gas has a lower flammable limit concentration close to that of C gas, city gas has a higher calorific value than C gas. Therefore, the feed concentration of C gas can be decreased.
- LNG natural gas
- Table 5 shows the burning components (hydrogen, CO, and methane) contained in the gaseous fuel, and the lower and upper flammable limit concentrations, combustion speed at laminar flow and at turbulent flow, and the like of the burning components.
- backfire prevention is necessary. This is considered to be achieved by discharging the gaseous fuel at a speed at least equal to or higher than the laminar flow combustion speed, preferably, at a high speed equal to or higher than the turbulent flow combustion speed. For example, in the case of city gas containing methane as a main component, if discharge is performed at a speed exceeding 3.7 m/s, there is no possibility of backfiring.
- the gaseous fuel that can be fed into the sintering bed in addition to the city gas (LNG) described above, any of B gas, C gas, CO gas, ethane gas, propane gas, butane gas, and a mixed gas of these can be used. Note that, in the case where B gas or C gas is used, it is necessary to increase the gas discharge rate and to separately take measures against CO.
- the concentration of the combustible gas (burning component) contained therein is preferably diluted to 3/4 (75%) or less of the lower flammable limit concentration in the air at normal temperature.
- the lower limit concentration of the diluted gaseous fuel is preferably 1% or more of the lower flammable limit concentration.
- the reason for this is that at less than 1% of the lower flammable limit concentration, the amount of heat generated by combustion is insufficient, and it is not possible to enhance the strength of sintered ore and to obtain the effect of improving the yield.
- the concentration of the diluted gaseous fuel to be fed into the sintering bed in the present invention is preferably in the range of 1% to 75% of the lower flammable limit concentration.
- the suitable concentration of the diluted gaseous fuel is in the range of 0.05% to 3.6% by volume.
- the formation of calcium ferrite depends on the time in which the temperature is retained at 1,200°C or higher, more accurately, by the product of temperature and time in which the temperature is retained in the range of 1,200°C to 1,380°C. Consequently, in order to obtain sintered ore having high strength, good reducibility, and a low RDI, it is a problem to achieve a heat pattern in which the temperature in the sintering bed during sintering is retained in the range of 1,200°C to 1,380°C for a long period of time.
- a sintering method in which, in addition to the carbonaceous material, a diluted gaseous fuel is further fed into the sintering bed.
- Fig. 11(b) shows the relationship between temperature and time at the position denoted by ⁇ in Fig. 11(a) in the sintering bed and compares the case where a diluted gaseous fuel is fed with the case where a diluted gaseous fuel is not fed in a sintering test using a test pot made of transparent quartz.
- the broken line in the graph indicates the example in which 5% by mass of coke is blended as a carbonaceous material into a sintering raw material, and a gaseous fuel is not fed.
- the temperature in the sintering bed at the time in which the combustion/melting zone is passing the point is retained at a temperature of 1,200°C or higher, which is effective for sintering, for about two minutes.
- the solid line in the graph indicates the example in which LNG in an amount equivalent to 0.4% by mass of coke in terms of calorific value is fed into the sintering bed, and the amount of carbonaceous material (coke) in the sintering raw material is reduced by that amount to 4.6% by mass, the total calorific value being fixed.
- the diluted gaseous fuel fed into the sintering bed is burned at the upper side of the combustion position (combustion zone) of coke, i.e., in the region through which the combustion zone has already passed and in which the temperature has started to decrease, and since the region is reheated, the time in which the temperature is retained at 1,200°C or higher, which is effective for sintering, is greatly extended. Moreover, in spite of the fact that the amount of coke is reduced with the feeding of the gaseous fuel, the extension is achieved without increasing the highest achieving temperature in the sintering bed to a temperature exceeding 1,380°C, which decreases the strength of coke, when the gas combustion/melting zone passes.
- Fig. 12 shows the results of a sintering experiment conducted in which the amount of coke in the sintering bed, the concentration of a gaseous fuel (LNG) fed, and the feeding position are changed to four levels in the sintering experiment shown in Fig. 11 .
- Fig. 12(a) shows the time-positions at which coke in the sintering bed and gaseous fuels are burned.
- Fig. 12(b) shows the change of temperature with time at the position denoted by ⁇ in Fig. 12(a) .
- 12(b) indicates the change in temperature in the example in which 5% by mass of coke is contained as a carbonaceous material in a sintering raw material and no gaseous fuel is fed. Furthermore, the curve (thin dotted line) of level B indicates the example in which LNG diluted to 0.1% by volume is fed and the amount of coke is reduced to 4.6% by mass. In this example, the dilute concentration of LNG is low at 0.1% by volume, and the calorific value is low. As a result, it is not possible to fully cover the shortage in the amount of heat due to the reduction in the amount of coke, and the effect of feeding the gaseous fuel is insufficient.
- the curve (thick broken line) of level D indicates the example in which LNG diluted to 4.0% by volume is fed and coke is reduced to 4.6% by mass.
- the combustion temperature of the diluted gaseous fuel depends on the temperature, and as the concentration increases, the combustion temperature decreases. Consequently, 4.0% by volume LNG is burned in the upper portion of the sintering bed which is largely deviated from the combustion position of coke and in which sintering has been completed and the temperature has decreased. Therefore, although the temperature in the sintering bed has two peaks, the extension of the temperature of 1,200°C or higher, which is effective for sintering, is not achieved.
- the curve (thick solid line) of level C indicates the case where LNG diluted to 0.4% by volume is fed and coke is reduced to 4.6% by mass.
- the combustion temperature of the diluted gaseous fuel shifts to the high temperature side, both effects of coke burning and LNG burning are overlapped, and the holding time at a temperature of 1,200°C or higher is largely extended compared with the cases of levels A, B, and C.
- the amount of carbonaceous material contained in the sintering raw material was set at 5.0% by mass when the gaseous fuel was not blown into the raw material, and varied in the range of 4.8% to 4.0% by mass when the gaseous fuel was blown into the raw material.
- the relationships between the combustion heat of the gaseous fuel fed, the combustion heat equivalent to the amount of carbonaceous material reduced, and quality of sintered ore and productivity were investigated. Table 7 No.
- Coke breeze ratio (%) Combustion heat of coke breeze Reduction in amount of coke breeze (kcal) B LNG Injection conditions Combustion heat of LNG B/A Sintering time (min) Quality characteristics of sintered ore Productivity (t/hr ⁇ m 2 ) (kcal/m 2 /min) (kcal) LNG concentration (%) LNG amount (Nm 3 /min) Injection time (min) (kcal/m 2 /min) (kcal) A Shatter strength (%) Product yield (%) Reducibility (%) 1 5.0 10027 11676 0 0.0 0 0 0 0 - 16.4 89.6 70.9 60.4 1.15 2 4.8 9397 11209 -467 0.6 0.0084 4 1159 329 1.42 16.8 92.0 74.5 63.6 1.18 3 4.6 9059 10742 -934 0.6 0.0084 4 1159 329 2.84 16.7 92.0 75.8 65.9 1.21 4 4.4 8878 10275 -1401 0.6 0.0084 4 1159 329 4.
- the results of the pot test are shown in Table 7. Furthermore, the relationship between a replacement ratio B/A and each of quality (shatter strength and reducibility) of sintered ore, product yield, and production rate is shown in Fig. 13 , the replacement ratio being defined as a ratio of B to A (B/A), where A is the combustion heat of the gaseous fuel fed, and B is the combustion heat equivalent to the amount of carbonaceous material reduced.
- the shatter strength was measured in accordance with JIS M8711, and the reducibility was measured in accordance with JIS M8713.
- the reason for the improvement of quality characteristics of sintered ore and productivity even if the carbonaceous material is reduced in the amount equivalent to or more than the combustion heat of the diluted gaseous fuel fed, consequently, even if the total combustion heat of the gaseous fuel and the carbonaceous material is reduced, is considered to be as follows.
- the diluted gaseous fuel fed into the sintering bed is burned at the upper side of the combustion position (combustion zone) of coke, i.e., in the region through which the combustion zone has already passed and in which the temperature has started to decrease, and the region is reheated.
- the temperature in the sintering bed at the time when the gas combustion/melting zone passes does not increase to a temperature exceeding 1,380°C, which decreases the strength of coke, and the time in which the temperature is retained at 1,200°C or higher, which is effective for sintering, is greatly extended.
- the amount of carbonaceous material is excessively reduced, i.e., if the replacement ratio B/A is excessively increased, the total combustion heat of the gaseous fuel and the carbonaceous material excessively decreases, resulting in degradation of quality characteristics of sintered ore and productivity. Furthermore, the effect of feeding the gaseous fuel is recognized even at a replacement ratio B/A of 10 or more, and the upper limit thereof is about 15, as described later in Examples. Consequently, the amount of carbonaceous material in the sintering raw material is reduced in accordance with the gaseous fuel to be fed such that the replacement ratio B/A is in the range of 1.5 to 10, and preferably in the range of 2 to 6.
- the carbonaceous material can be reduced in an amount equivalent to or more than the combustion heat of the gaseous fuel fed, quality improvement of sintered ore and productivity improvement can be achieved at low cost, and in addition, the amount of carbon dioxide generated by combustion of the carbonaceous material can be greatly reduced. Consequently, the present invention is considered to be an earth-conscious, environmentally friendly technique.
- a sintering experiment was carried out, in which, using an actual sintering machine provided with gaseous fuel feed equipment as shown in Fig. 14 , under the conditions shown in Table 8, the gaseous fuel was blown in and at the same time, the amount of carbonaceous material in the sintering raw material was reduced, and the influence on the quality (tumbler strength and reducibility) of sintered ore was confirmed.
- the tumbler strength is most widely used as an index of strength of sintered ore obtained by an actual sintering machine, and is strongly correlated to shatter strength.
- the tumbler strength TI was determined, in accordance with JIS M8712, by a method in which the sample was tumbled in a rotary drum and screened with a 6.3-mm sieve, and the ratio between the mass of the sample tested to the mass of +6.3 mm sample after testing was calculated.
- reducibility was determined, in accordance with JIS M8713, by a method in which 500 g of sintered ore sample screened to 19.0 to 22.4 mm was reduced, at 900°C, using a reducing gas containing 30% by volume of CO and 70% by volume of N 2 for 180 minutes, and the percentage of the amount of reduced oxygen relative to the amount of reducible oxygen before reduction was calculated.
Landscapes
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Manufacturing & Machinery (AREA)
- Geochemistry & Mineralogy (AREA)
- Geology (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Environmental & Geological Engineering (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Manufacture And Refinement Of Metals (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2008305842A JP4911163B2 (ja) | 2008-12-01 | 2008-12-01 | 焼結鉱の製造方法 |
| PCT/JP2009/070440 WO2010064717A1 (ja) | 2008-12-01 | 2009-11-30 | 焼結鉱の製造方法 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP2365101A1 EP2365101A1 (en) | 2011-09-14 |
| EP2365101A4 EP2365101A4 (en) | 2016-04-06 |
| EP2365101B1 true EP2365101B1 (en) | 2018-10-03 |
Family
ID=42233363
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP09830482.7A Active EP2365101B1 (en) | 2008-12-01 | 2009-11-30 | Process for producing sintered ore |
Country Status (9)
| Country | Link |
|---|---|
| EP (1) | EP2365101B1 (pt) |
| JP (1) | JP4911163B2 (pt) |
| KR (1) | KR101353113B1 (pt) |
| CN (1) | CN102232120B (pt) |
| AU (1) | AU2009323269B2 (pt) |
| BR (1) | BRPI0923299B1 (pt) |
| TR (1) | TR201816067T4 (pt) |
| TW (1) | TWI460278B (pt) |
| WO (1) | WO2010064717A1 (pt) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5439981B2 (ja) * | 2008-12-03 | 2014-03-12 | Jfeスチール株式会社 | 焼結鉱の製造方法 |
| KR20140145629A (ko) | 2012-06-13 | 2014-12-23 | 제이에프이 스틸 가부시키가이샤 | 소결광의 제조 방법 |
| CN103512351B (zh) * | 2012-06-20 | 2015-10-07 | 鞍钢股份有限公司 | 一种金属化烧结矿的烧结装置及其生产方法 |
| KR20150036547A (ko) | 2012-07-18 | 2015-04-07 | 제이에프이 스틸 가부시키가이샤 | 소결기의 기체 연료 공급 장치 |
| JP5561443B2 (ja) * | 2012-07-18 | 2014-07-30 | Jfeスチール株式会社 | 焼結鉱の製造方法 |
| KR20150059784A (ko) * | 2012-11-20 | 2015-06-02 | 제이에프이 스틸 가부시키가이샤 | 소결기의 산소-기체 연료 공급 장치 |
| CN104342550A (zh) * | 2013-07-26 | 2015-02-11 | 上海梅山钢铁股份有限公司 | 焦炉煤气辅助烧结的方法 |
| JP6213734B2 (ja) * | 2014-02-24 | 2017-10-18 | Jfeスチール株式会社 | 焼結鉱の製造方法 |
| JP6295791B2 (ja) * | 2014-04-04 | 2018-03-20 | 新日鐵住金株式会社 | 焼結鉱製造方法、及び、石炭チャー、又は無煙炭若しくは半無煙炭の評価方法 |
| CN110319694A (zh) * | 2018-03-31 | 2019-10-11 | 高彦 | 烧结装置及控制方法 |
| JP7554979B2 (ja) | 2020-12-07 | 2024-09-24 | 日本製鉄株式会社 | 焼結鉱の製造方法 |
| CN114058840A (zh) * | 2021-10-20 | 2022-02-18 | 包头钢铁(集团)有限责任公司 | 一种使用可燃气体改善烧结矿质量的方法 |
| CN115218669B (zh) * | 2021-11-22 | 2024-06-11 | 中冶长天国际工程有限责任公司 | 一种燃气间歇吸入辅助烧结的装置及其方法 |
| CN115218670B (zh) * | 2021-11-22 | 2024-06-07 | 中冶长天国际工程有限责任公司 | 一种燃气水蒸气间隔喷吹辅助烧结的方法 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS4627126B1 (pt) * | 1967-05-17 | 1971-08-06 | ||
| JPS4818102B1 (pt) | 1968-11-12 | 1973-06-04 | ||
| JPS5518585A (en) | 1978-07-27 | 1980-02-08 | Sumitomo Metal Ind Ltd | Manufacture of sintered ore |
| JPH05311257A (ja) | 1992-05-11 | 1993-11-22 | Nippon Steel Corp | 焼結鉱の製造方法 |
| KR100515042B1 (ko) * | 2000-08-19 | 2005-09-15 | 주식회사 포스코 | 액화천연가스를 이용한 배가스 순환식 철광석 소결장치 및그 방법 |
| EP1956101B1 (en) * | 2005-10-31 | 2012-08-15 | JFE Steel Corporation | Process for producing sintered ore |
-
2008
- 2008-12-01 JP JP2008305842A patent/JP4911163B2/ja active Active
-
2009
- 2009-11-30 CN CN2009801483459A patent/CN102232120B/zh active Active
- 2009-11-30 BR BRPI0923299-0A patent/BRPI0923299B1/pt active IP Right Grant
- 2009-11-30 KR KR1020117013500A patent/KR101353113B1/ko active IP Right Grant
- 2009-11-30 WO PCT/JP2009/070440 patent/WO2010064717A1/ja active Application Filing
- 2009-11-30 EP EP09830482.7A patent/EP2365101B1/en active Active
- 2009-11-30 TR TR2018/16067T patent/TR201816067T4/tr unknown
- 2009-11-30 AU AU2009323269A patent/AU2009323269B2/en active Active
- 2009-12-01 TW TW098140952A patent/TWI460278B/zh active
Also Published As
| Publication number | Publication date |
|---|---|
| BRPI0923299B1 (pt) | 2017-10-31 |
| EP2365101A1 (en) | 2011-09-14 |
| EP2365101A4 (en) | 2016-04-06 |
| KR101353113B1 (ko) | 2014-01-17 |
| BRPI0923299A8 (pt) | 2016-09-13 |
| WO2010064717A1 (ja) | 2010-06-10 |
| AU2009323269B2 (en) | 2013-10-31 |
| JP2010126802A (ja) | 2010-06-10 |
| JP4911163B2 (ja) | 2012-04-04 |
| TWI460278B (zh) | 2014-11-11 |
| TR201816067T4 (tr) | 2018-11-21 |
| CN102232120A (zh) | 2011-11-02 |
| BRPI0923299A2 (pt) | 2016-01-05 |
| CN102232120B (zh) | 2013-08-07 |
| AU2009323269A1 (en) | 2011-07-07 |
| KR20110084321A (ko) | 2011-07-21 |
| TW201033373A (en) | 2010-09-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2365101B1 (en) | Process for producing sintered ore | |
| EP1956101B1 (en) | Process for producing sintered ore | |
| KR101387811B1 (ko) | 소결광의 제조방법 및 소결기 | |
| KR101475130B1 (ko) | 소결광의 제조 방법 | |
| JP5544784B2 (ja) | 焼結機 | |
| AU2012395098B2 (en) | Oxygen-gas fuel supply apparatus for sintering machine | |
| JP2008291362A (ja) | 焼結機への希釈気体燃料吹込み操業時の操業解析プログラムおよび焼結機への希釈気体燃料吹込み時の操業解析・制御装置 | |
| JP2015157980A (ja) | 焼結鉱の製造方法 | |
| JP5439981B2 (ja) | 焼結鉱の製造方法 | |
| JP2011169570A (ja) | 焼結機 | |
| JP5428195B2 (ja) | 焼結機 | |
| EP2876175B1 (en) | Method for producing sinter | |
| JP5682099B2 (ja) | 焼結鉱の製造方法 | |
| JP2010106341A (ja) | 焼結鉱の製造方法 | |
| EP2862949B1 (en) | Method for manufacturing sintered ore | |
| JP5439982B2 (ja) | 焼結鉱の製造方法 | |
| JP5428194B2 (ja) | 焼結機 | |
| JP2013057423A (ja) | 焼結機の酸素−気体燃料供給装置 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20110511 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK SM TR |
|
| DAX | Request for extension of the european patent (deleted) | ||
| RA4 | Supplementary search report drawn up and despatched (corrected) |
Effective date: 20160309 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: C22B 1/16 20060101ALI20160303BHEP Ipc: C22B 1/20 20060101AFI20160303BHEP |
|
| 17Q | First examination report despatched |
Effective date: 20170407 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| INTG | Intention to grant announced |
Effective date: 20180531 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP Ref country code: AT Ref legal event code: REF Ref document number: 1048674 Country of ref document: AT Kind code of ref document: T Effective date: 20181015 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602009054903 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20181003 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1048674 Country of ref document: AT Kind code of ref document: T Effective date: 20181003 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190103 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190103 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190203 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190203 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190104 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602009054903 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181130 Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20181130 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181130 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181130 |
|
| 26N | No opposition filed |
Effective date: 20190704 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181130 Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181130 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181130 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181003 Ref country code: MK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181003 Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20091130 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20230929 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20231012 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: TR Payment date: 20231128 Year of fee payment: 15 Ref country code: DE Payment date: 20231003 Year of fee payment: 15 |