EP2323973B1 - Verfahren zur herstellung von aromatischen isocyanaten - Google Patents
Verfahren zur herstellung von aromatischen isocyanaten Download PDFInfo
- Publication number
- EP2323973B1 EP2323973B1 EP09781542A EP09781542A EP2323973B1 EP 2323973 B1 EP2323973 B1 EP 2323973B1 EP 09781542 A EP09781542 A EP 09781542A EP 09781542 A EP09781542 A EP 09781542A EP 2323973 B1 EP2323973 B1 EP 2323973B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- mixing chamber
- phosgene
- amine
- diameter
- axis
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C263/00—Preparation of derivatives of isocyanic acid
- C07C263/10—Preparation of derivatives of isocyanic acid by reaction of amines with carbonyl halides, e.g. with phosgene
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01F—MIXING, e.g. DISSOLVING, EMULSIFYING OR DISPERSING
- B01F23/00—Mixing according to the phases to be mixed, e.g. dispersing or emulsifying
- B01F23/40—Mixing liquids with liquids; Emulsifying
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J19/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J19/26—Nozzle-type reactors, i.e. the distribution of the initial reactants within the reactor is effected by their introduction or injection through nozzles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C263/00—Preparation of derivatives of isocyanic acid
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C265/00—Derivatives of isocyanic acid
- C07C265/12—Derivatives of isocyanic acid having isocyanate groups bound to carbon atoms of six-membered aromatic rings
Definitions
- the production of isocyanates by phosgenation of the corresponding amines can be carried out in principle by a liquid-phase or a gas-phase phosgenation.
- the liquid phase phosgenation is characterized in that the reaction can be carried out at lower temperatures than the gas phase phosgenation and evaporation of the reactants is not required.
- Axis of the mixing chamber arranged levels is added, for example DD-A 300 168 known.
- the object of the present invention is to provide a process for the preparation of isocyanates by reacting the corresponding amines with phosgene in the liquid phase, in which a lower secondary component formation can be achieved in comparison with the processes known from the prior art.
- the object is achieved by a process for the preparation of isocyanates by reacting the corresponding amines with phosgene in the liquid phase, optionally in the presence of at least one inert medium, in which first the amine and the phosgene are mixed in a mixing chamber to a reaction mixture and the reaction mixture a reactor is supplied.
- the amine is added through an aperture coaxial with the mixing chamber and the phosgene is added through feed ports in at least two planes perpendicular to the axis of the mixing chamber. In this case, at least one plane in the main flow direction of the reaction mixture upstream and a plane downstream of the opening of the addition of the amine is arranged.
- the average residence time of the reaction mixture in the mixing chamber is according to the invention a maximum of 18.5 ms.
- V is the volume of the mixing chamber
- V * is the total volume flow of the educt streams.
- the volume of the mixing chamber is the volume up to the end of the constriction, that is, until it enters the zone of constant cross section which adjoins the mixing chamber.
- the volume of the central nozzle which projects into the mixing chamber is not part of the volume of the mixing chamber.
- the amine used to prepare isocyanates is, for example, a monoamine, a diamine, a triamine or higher amine. However, preference is given to using monoamines or diamines. Depending on the amine used, the corresponding monoisocyanates, diisocyanates, triisocyanates or higher isocyanates result. Monoisocyanates or diisocyanates are preferably prepared by the process according to the invention.
- the amines and isocyanates may be aliphatic, cycloaliphatic or aromatic.
- Cycloaliphatic isocyanates are those which contain at least one cycloaliphatic ring system.
- Aromatic isocyanates are those which have at least one isocyanate group bonded to at least one aromatic ring system.
- (Cyclo) aliphatic isocyanates are in the context of this application briefly for cycloaliphatic and / or aliphatic isocyanates.
- aromatic diisocyanates are monomeric 2,4'- or 4,4'-methylene-di (phenyl isocyanate) (MDI) and its higher oligomers (PMDI) or mixtures thereof, 2,4- and / or 2,6-tolylene diisocyanate (TID) and 1,5- or 1,8-naphthyl diisocyanate (NDI).
- MDI 2,4'- or 4,4'-methylene-di
- PMDI oligomers
- TID 2,4- and / or 2,6-tolylene diisocyanate
- NDI 1,5- or 1,8-naphthyl diisocyanate
- Preferred (cyclo) aliphatic diisocyanates are those having 4 to 20 C atoms.
- Examples of common aliphatic diisocyanates are 1,4-tetramethylene diisocyanate, hexamethylene diisocyanate (1,6-diisocyanatohexane), 1,8-octamethylene diisocyanate, 1,10-decamethylene diisocyanate, 1,12-dodecamethylene diisocyanate, 1,14-tetradecamethylene diisocyanate, derivatives of lysine diisocyanate , Tetramethylxylylene diisocyanate (TMXDI), trimethylhexane diisocyanate or tetramethylhexane diisocyanate, and also 3 (or 4) -, 8 (or 9) -bis (isocyanatomethyl) -tricyclo [5.2.1.0 2,6 ] decane isomer mixtures, and cycloaliphatic diisocyanates, such as 1 , 4-, 1,3- or 1,2-diisocyanatocyclohexane,
- MDI / PMDI isomer and oligomer mixtures are particularly preferred.
- Aliphatic, cycloaliphatic or aromatic amines may also be used to prepare monoisocyanates.
- Aniline is particularly preferred as the aromatic amine.
- the phosgene may be dissolved in an inert solvent prior to addition to the mixing chamber.
- Suitable inert solvents in which the phosgene is dissolved are suitable for example, chlorinated aromatic hydrocarbons, for example monochlorobenzene or dichlorobenzene, or toluene.
- the ratio of phosgene to inert solvent is preferably in the range from 1: 0 to 1: 2, in particular in the range from 1: 0 to 1: 1.
- the phosgene is added via at least two feed openings in the at least two planes arranged perpendicular to the axis of the mixing chamber.
- the feed openings through which the phosgene is added are preferably arranged so that the main directions of the feed openings meet in the axis of the mixing chamber.
- the phosgene jets added via the feed openings strike directly the amine which is added through the opening arranged coaxially with the mixing chamber.
- phosgene jets leaving the feed ports also meet at the axis of the mixing chamber. This results in a uniform phosgene distribution in the flow direction of the amine.
- the supply openings of the first level are arranged rotated to the supply openings of the second level about the axis of the mixing chamber. It is particularly preferred if the supply openings are arranged rotated by 90 degrees to each other at every two supply openings per level.
- the mixing chamber in which the amine is mixed with the phosgene preferably has a ratio of length to diameter (UD ratio) which is in the range of 1 to 2 and in particular in the range of 1 to 1.5.
- the coaxial with the axis of the mixing chamber arranged opening through which the amine is added preferably projects into the mixing chamber.
- the opening through which the amine is added for example, formed as a nozzle.
- the opening through which the amine is added is then the exit orifice of the nozzle.
- the ratio of the diameter of the opening over which the amine is added based on the diameter of the mixing chamber is preferably in the range of 0.05 to 0.5, more preferably in the range of 0.1 to 0.4, and more preferably in the range of 0 , 15 to 0.35.
- the ratio of the plane to downstream distance is Opening, through which the amine is added, is arranged, to the opening through which the amine is added, based on the diameter of the mixing chamber in the range of 0 to 1, more preferably in the range of 0.01 to 0.5 and in particular in the range of 0.05 to 0.2.
- the spacing of the feed ports of the plane is that of the orifice , via which the amine is added, is closest, corresponding to the distance of the plane of the feed openings, when only one plane is arranged downstream of the opening over which the amine is added.
- the spacing of the feed ports is the plane that of the orifice , via which the amine is added, is closest, corresponding to the distance of the plane of the feed openings, when only one level upstream of the opening over which the amine is added is located.
- the phosgene is preferably added through feed openings in a maximum of five planes arranged perpendicular to the axis of the mixing chamber. It is more preferred if the phosgene is added through feed openings in a maximum of three planes arranged perpendicular to the axis of the mixing chamber, and particularly preferred if the phosgene is added through feed openings in two planes arranged perpendicular to the axis of the mixing chamber.
- the number of supply openings in the individual levels is preferably at most five, more preferably at most four and in particular two.
- the number of feed openings in the individual levels results in a good distribution of the phosgene in the mixing chamber.
- the feed openings of the individual planes are preferably rotated uniformly relative to one another.
- the diameter of the feed openings through which the phosgene is added is preferably smaller than the spacing of the planes in which the feed openings are arranged.
- the diameter of the feed openings is preferably in the range from 0.01 to 0.5, more preferably in the range from 0.02 to 0.3, and in particular in the range from 0.03 to 0.25, based on the diameter of the mixing chamber.
- the feed openings can open into the mixing chamber at any angle.
- the axes of the feed openings intersect with the axis of the mixing chamber, more preferably open the feed openings at an angle of 90 ° in the mixing chamber.
- the feed ports through which the phosgene is added are preferably nozzle orifices. This means that the phosgene is supplied to the mixing chamber via lines and at the end of the lines a cross-sectional constriction is formed in the form of a nozzle. The phosgene then enters the mixing chamber from the nozzle.
- the feed opening of the phosgene is preferably flush with the wall of the mixing chamber.
- the nozzles may have both circular and also deviating from the circular shape openings.
- the mixing chamber in which the amine is mixed with the phosgene is preferably rotationally symmetric. If the mixing chamber does not have a circular cross-section, the diameter of the mixing chamber is always the hydraulic diameter.
- the mixing chamber preferably has a diameter constriction, through which backmixing of the reaction mixture takes place.
- the backmixing results from the deflection of the flow due to the diameter constriction.
- the diameter restriction at the downstream end of the mixing chamber is preferably made at an angle in the range of 10 to 80 ° to the axis of the mixing chamber. More preferably, the diameter constriction is made at the downstream end at an angle of 15 to 60 degrees and more preferably at an angle of 18 to 40 degrees to the axis of the mixing chamber.
- the diameter restriction at the downstream end of the mixing chamber is preferably a conical constriction.
- the Ratio of the diameter of the diameter restriction, to which the cross section is reduced, based on the diameter of the mixing chamber in the range of 0.2 to 0.7, more preferably in the range of 0.25 to 0.65 and in particular in the range of 0, 3 to 0.6. In addition to backmixing, the diameter narrowing thus also accelerates the reaction mixture.
- the diameter constriction is preferably followed by a zone with a constant diameter in which only little backmixing takes place.
- the residence time of the reaction mixture in the constant diameter zone is preferably at most 50 ms, in particular at most 30 ms.
- the length of the constant diameter zone based on the diameter of this zone is preferably in the range of 1 to 10, more preferably in the range of 1.5 to 9, and especially in the range of 2 to 8.
- the zone of constant diameter is followed by a zone with a cross-sectional widening, the cross-sectional widening having an opening angle with respect to the axis of the zone at which no separation of the flow takes place.
- This means that the cross-sectional widening is formed in the form of a diffuser.
- the cross-sectional widening expands the diameter until the diameter of the reactor, which is preferably designed as a tubular reactor, is reached. In this case, it is possible for the diameter to be expanded stepwise, with an area of constant diameter being arranged in each case between the individual stages in which the diameter is widened.
- the opening angle of the cross-sectional widening to the axis of the zone is preferably less than 15 °, more preferably less than 10 ° and particularly preferably less than 8 °.
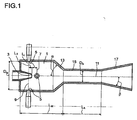
- the sole figure shows a device for mixing amine and phosgene in the liquid phase.
- An apparatus for mixing amine and phosgene comprises a mixing chamber 1 into which phosgene and amine are fed. Through an opening 3, which is arranged coaxially to the mixing chamber 1, the amine is preferably added. Alternatively, however, it is also possible for the phosgene to be fed through the opening 3 arranged coaxially with the mixing chamber. However, it is preferable to add the amine through the orifice 3 coaxial with the mixing chamber.
- the orifice 3, which is coaxial with the mixing chamber 1, is formed in the form of a nozzle projecting into the mixing chamber 1 as shown.
- the supply openings 5 are also preferably formed as nozzles.
- the feed openings 5 are arranged in at least two planes 7, 9, which are arranged perpendicular to the axis of the mixing chamber.
- the levels 7, 9 are shown here by dashed lines.
- the supply openings 5 are arranged in two planes 7, 9.
- a first level 7 is arranged downstream of the coaxially arranged opening 3 and a second level 9 upstream.
- the supply openings 5 are arranged in more than two planes.
- the supply openings 5 are arranged in more than two levels 7, 9, in each case at least one level upstream and at least one level downstream of the coaxially arranged opening 3 is arranged.
- two feed openings 5 are arranged in each plane 7, 9, wherein the feed openings 5 are diametrically opposed in each case. Due to the arrangement in which the supply openings 5 are diametrically opposed, the main directions of the supply openings 5 meet in the axis 11 of the mixing chamber 1.
- the ratio of the distance L 1 of the first plane 7 to the coaxially arranged to the mixing chamber opening 3 based on the diameter D M of the mixing chamber 1 is preferably in the range of 0 to 1, more preferably in the range of 0.01 to 0.5 and in particular Range from 0.05 to 0.2.
- supply ports 5 are disposed in more than one plane downstream of the coaxial with the mixing chamber arranged opening 3, this is the distance of the plane which is the coaxial with the mixing chamber arranged opening 3 closest.
- the ratio of the distance L 2 of the second plane 9, which is arranged upstream of the coaxial with the mixing chamber 1 arranged opening 3, based on the diameter D M of the mixing chamber 1 is also preferably in the range of 0 to 1, more preferably in the range of 0.01 to 0.5 and in particular in the range of 0.05 to 0.2. If supply openings 5 are arranged in more than two levels upstream of the opening 3 coaxial with the mixing chamber 1, this corresponds to the distance of the plane closest to the opening 3.
- the mixing chamber 1 has a diameter constriction 13.
- the diameter constriction 13 is preferably conical and designed with an angle ⁇ in the range of 10 to 80 °, preferably at an angle in the range of 15 to 60 ° and particularly preferably at an angle of 18 to 40 ° to the axis 11 of the mixing chamber 1.
- the diameter constriction 13 is followed by a zone of constant diameter 15.
- the zone 15 of constant diameter has a diameter D A , wherein the ratio of the diameter D A of the zone 15 of constant diameter to the diameter D M of the mixing chamber 1 as already described above in the range of 0.2 to 0.7, more preferably in the range from 0.25 to 0.65 and especially in the range of 0.3 to 0.6.
- the diameter constriction 13 the diameter decreases from the diameter D M of the mixing chamber 1 to the diameter D A of the zone 15 of constant diameter.
- the zone 15 of constant diameter is followed by a cross-sectional widening 17.
- the cross-sectional widening 17 is preferably designed in the form of a diffuser.
- the cross-sectional widening 17 has an opening angle ⁇ , which is selected so that in the cross-sectional widening 17 no separation of the flow occurs.
- ⁇ opening angle
- the diameter in the cross-sectional widening 17 is gradually expanded. In this case, between the individual stages, in which the diameter is widened, in each case a region of constant diameter is arranged. Alternatively, it is also possible that in each case an area is formed between the individual stages, in which the diameter widens conically.
- the cross-sectional enlargement 17 is particularly preferably conical and the opening angle ⁇ of the cross-sectional widening 17 is preferably ⁇ 15 °, more preferably ⁇ 10 ° and particularly preferably ⁇ 8 °.
- the length of the cross-sectional widening 17 is chosen such that the diameter widens to the diameter of the reactor adjoining the apparatus for mixing the amine and phosgene, which is not shown here.
- the ratio of the length L M of the mixing chamber 1 with respect to the diameter D M is preferably in the range between 1 and 2 and in particular in the range from 1 to 1.5.
- the ratio of the length L A of the zone 15 of constant diameter with respect to the diameter D A of the zone of constant diameter is preferably in the range of 1 to 10, more preferably in the range of 1.5 to 9 and in particular in the range of 2 to 8.
- a device which comprises a mixing chamber with a diameter of 40 mm and a length of 66 mm.
- an amine supply means which projects 26 mm into the mixing chamber, opens with a diameter of 20 mm and a nozzle diameter of 5.5 mm.
- two feed openings are arranged diametrically 6 mm above the outlet cross section of the central nozzle and two feed openings diametrically 6 mm below the outlet cross section of the central nozzle.
- the diameter of the feed openings above the exit cross section of the central nozzle is 5.1 mm with a diameter of the feeder of 15 mm and the nozzle diameter of the feed openings below, i. downstream of the opening of the central nozzle is 6.9 mm with a diameter of the feeder of 20 mm.
- the mixing chamber has a conical constriction at an angle of 25 °, the diameter of the mixing chamber diameter of 40 mm to the outlet diameter of 25 mm decreases.
- the total length of the mixing chamber comprising the cylindrical part and the conical part is 66 mm.
- the mixing chamber is followed by a zone of constant diameter with a length of 180 mm.
- the zone of constant diameter is followed by an extension with an opening angle of 6 °. With the extension of the diameter increases to the diameter of the subsequent tubular reactor.
- amine-containing material stream 3.75 m 3 / h of an amine-containing material stream are fed via the central nozzle and 11.2 m 3 / h of a phosgene-containing stream via the feed openings.
- the amine-containing stream contains from 34 to 36% by weight of MDA / PMDA in an amount of from 50.4 to 51.1% by weight of MDA and from 54 to 56% by weight of monochlorobenzene, and the phosgene-containing stream contains from 66 to 70% by weight. % Phosgene and 30 to 34% by weight monochlorobenzene.
- the residence time in the mixing zone is 17 ms.
- the residence time in the constant diameter zone is approximately 21 ms.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP09781542A EP2323973B1 (de) | 2008-08-07 | 2009-08-06 | Verfahren zur herstellung von aromatischen isocyanaten |
| PL09781542T PL2323973T3 (pl) | 2008-08-07 | 2009-08-06 | Sposób wytwarzania aromatycznych izocyjanianów |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP08161976 | 2008-08-07 | ||
| PCT/EP2009/060184 WO2010015667A1 (de) | 2008-08-07 | 2009-08-06 | Verfahren zur herstellung von aromatischen isocyanaten |
| EP09781542A EP2323973B1 (de) | 2008-08-07 | 2009-08-06 | Verfahren zur herstellung von aromatischen isocyanaten |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2323973A1 EP2323973A1 (de) | 2011-05-25 |
| EP2323973B1 true EP2323973B1 (de) | 2012-03-21 |
Family
ID=41353335
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP09781542A Active EP2323973B1 (de) | 2008-08-07 | 2009-08-06 | Verfahren zur herstellung von aromatischen isocyanaten |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US8829232B2 (pl) |
| EP (1) | EP2323973B1 (pl) |
| JP (1) | JP5443487B2 (pl) |
| KR (1) | KR101639087B1 (pl) |
| CN (1) | CN102119145B (pl) |
| AT (1) | ATE550317T1 (pl) |
| PL (1) | PL2323973T3 (pl) |
| PT (1) | PT2323973E (pl) |
| WO (1) | WO2010015667A1 (pl) |
Families Citing this family (58)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5416830B2 (ja) | 2009-04-24 | 2014-02-12 | ビーエーエスエフ ソシエタス・ヨーロピア | 色の安定なmdaとmdiの製造方法 |
| EP2446247B1 (de) | 2009-06-24 | 2013-05-01 | Basf Se | Verfahren zur erfassung von wassereintritten in phosgenführenden anlagen |
| WO2010149544A2 (en) | 2009-06-26 | 2010-12-29 | Basf Se | Process for the production of isocyanates, preferably diisocyanates and polyisocyanates with solvent recirculation |
| JP2012532909A (ja) | 2009-07-14 | 2012-12-20 | ビーエーエスエフ ソシエタス・ヨーロピア | 明色ジフェニルメタンイソシアネート類の製造方法 |
| KR20120050444A (ko) | 2009-07-16 | 2012-05-18 | 바스프 에스이 | 디페닐메탄 디이소시아네이트 계열의 명색 이소시아네이트의 제조 방법 |
| KR20120041257A (ko) | 2009-08-11 | 2012-04-30 | 바스프 에스이 | 기상 포스겐화에 의한 디이소시아네이트의 제조 방법 |
| JP2013505280A (ja) | 2009-09-22 | 2013-02-14 | ビーエーエスエフ ソシエタス・ヨーロピア | イソシアナートの製造方法 |
| WO2011044514A2 (en) | 2009-10-09 | 2011-04-14 | Dow Global Technologies, Inc | Isothermal multitube reactors and processes incorporating the same |
| BR112012007921A2 (pt) | 2009-10-09 | 2019-09-24 | Dow Global Technologies Llc | processo contínuo, em fase gasosa, via radicais livres para a produção de propeno e alcenos superiores clorados ou fluorados, processo para preparar um produto a jusante, e processo para preparar 2,3,3,3-tetraflúor-prop-1-eno (hfo-1234yf) ou 1,3,3,3-tetraflúor-1-eno (hfo-1234ze) |
| WO2011051314A1 (de) | 2009-10-27 | 2011-05-05 | Basf Se | Verfahren zur koppelproduktion von di- und/oder polyisocyanaten und glykolen |
| WO2011067369A1 (de) | 2009-12-04 | 2011-06-09 | Basf Se | Verfahren zur herstellung von isocyanaten |
| US8609899B2 (en) | 2010-05-17 | 2013-12-17 | Basf Se | Process for preparing toluenediamine by hydrogenation of dinitrotoluene |
| WO2011159409A1 (en) | 2010-06-14 | 2011-12-22 | Dow Global Technologies Llc | Static reactive jet mixer, and methods of mixing during an amine - phosgene mixing process |
| PT3009185T (pt) | 2010-09-28 | 2017-11-14 | Dow Global Technologies Llc | Misturador estático de fluxo reativo com obstruções de fluxo cruzado |
| US9321720B2 (en) | 2010-10-14 | 2016-04-26 | Basf Se | Process for preparing isocyanates |
| PT2627629T (pt) | 2010-10-14 | 2022-03-10 | Basf Se | Método para a preparação de isocianatos |
| PL393216A1 (pl) * | 2010-12-10 | 2012-06-18 | Zakłady Chemiczne Zachem Spółka Akcyjna | Sposób otrzymywania toluilenodiizocyjanianu (TDI) poprzez prowadzenia reakcji fosgenowania toluilenodiaminy (TDA) w fazie gazowej oraz urządzenie do otrzymywania toluilenodiizocyjanianu (TDI) poprzez prowadzenie reakcji fosgenowania toluilenodiaminy (TDA) w fazie gazowej |
| WO2012166394A1 (en) | 2011-05-31 | 2012-12-06 | Dow Global Technologies, Llc | Process for the production of chlorinated propenes |
| CA2836493A1 (en) | 2011-05-31 | 2012-12-06 | Max Markus Tirtowidjojo | Process for the production of chlorinated propenes |
| WO2012170239A1 (en) | 2011-06-08 | 2012-12-13 | Dow Agrosciences, Llc | Process for the production of chlorinated and/or fluorinated propenes |
| US9475739B2 (en) | 2011-08-07 | 2016-10-25 | Blue Cube Ip Llc | Process for the production of chlorinated propenes |
| CN103717557A (zh) | 2011-08-07 | 2014-04-09 | 陶氏环球技术有限责任公司 | 生产氯化的丙烯的方法 |
| US8816126B2 (en) | 2011-09-02 | 2014-08-26 | Basf Se | Process for preparing isocyanates |
| BR112014004622A8 (pt) | 2011-09-02 | 2017-06-20 | Basf Se | processo para a preparação de isocianatos |
| US9067855B2 (en) | 2011-11-21 | 2015-06-30 | Dow Global Technologies Llc | Process for the production of chlorinated alkanes |
| US9284239B2 (en) | 2011-12-02 | 2016-03-15 | Blue Cube Ip Llc | Process for the production of chlorinated alkanes |
| US9199899B2 (en) | 2011-12-02 | 2015-12-01 | Blue Cube Ip Llc | Process for the production of chlorinated alkanes |
| US9334205B2 (en) | 2011-12-13 | 2016-05-10 | Blue Cube Ip Llc | Process for the production of chlorinated propanes and propenes |
| EP2794528B1 (en) | 2011-12-22 | 2020-02-26 | Blue Cube IP LLC | Process for the production of tetrachloromethane |
| US9512049B2 (en) | 2011-12-23 | 2016-12-06 | Dow Global Technologies Llc | Process for the production of alkenes and/or aromatic compounds |
| WO2014046977A1 (en) | 2012-09-20 | 2014-03-27 | Dow Global Technologies, Llc | Process for the production of chlorinated propenes |
| WO2014046970A1 (en) | 2012-09-20 | 2014-03-27 | Dow Global Technologies, Llc | Process for the production of chlorinated propenes |
| CN104718020A (zh) | 2012-09-30 | 2015-06-17 | 陶氏环球技术有限公司 | 堰式骤冷器和并入所述堰式骤冷器的工艺 |
| JP6363610B2 (ja) | 2012-10-26 | 2018-07-25 | ブルー キューブ アイピー エルエルシー | 混合器およびそれを組み込んだプロセス |
| PL402054A1 (pl) * | 2012-12-14 | 2014-06-23 | Zakłady Chemiczne Zachem Spółka Akcyjna | Sposób fosgenowania toluilenodiaminy (TDA) w fazie gazowej w specjalnej konstrukcji reaktorze |
| EP2935165A1 (en) | 2012-12-18 | 2015-10-28 | Blue Cube IP LLC | Process for the production of chlorinated propenes |
| US9475740B2 (en) | 2012-12-19 | 2016-10-25 | Blue Cube Ip Llc | Process for the production of chlorinated propenes |
| WO2014134233A2 (en) | 2013-02-27 | 2014-09-04 | Dow Global Technologies Llc | Process for the production of chlorinated propenes |
| CN105026348A (zh) | 2013-03-09 | 2015-11-04 | 蓝立方知识产权有限责任公司 | 用于生产氯化烷烃的方法 |
| KR101416760B1 (ko) * | 2014-03-25 | 2014-07-09 | 금호석유화학 주식회사 | 고속 분사를 이용한 이소시아네이트 제조용 혼합 반응기 |
| JP5815087B2 (ja) * | 2013-12-10 | 2015-11-17 | コリア クムホ ペトロケミカル カンパニー., リミテッド | 高速噴射を利用した異種流体の混合反応器 |
| KR20160138410A (ko) * | 2014-03-27 | 2016-12-05 | 코베스트로 도이칠란트 아게 | 기체 상 포스겐화 플랜트의 가동 방법 |
| CN104874335A (zh) * | 2015-05-14 | 2015-09-02 | 万华化学集团股份有限公司 | 一种制备异氰酸酯的反应器及其用于制备异氰酸酯的方法 |
| CN108137488B (zh) | 2015-09-30 | 2021-02-26 | 科思创德国股份有限公司 | 制备异氰酸酯的方法 |
| CN109790104B (zh) | 2016-10-10 | 2022-09-09 | 巴斯夫欧洲公司 | 氢化甲苯二胺(tda)焦油的方法 |
| CN107597028B (zh) * | 2017-09-21 | 2020-05-08 | 万华化学(宁波)有限公司 | 一种制备异氰酸酯的反应器及方法 |
| EP4122597A3 (en) * | 2017-10-05 | 2023-02-15 | Novomer, Inc. | A process for producing a diisocyanate product, a process for producing an aromatic isocyanate product and a process for producing a polyurethane product |
| KR20190061837A (ko) * | 2017-11-28 | 2019-06-05 | 한화케미칼 주식회사 | 반응기 |
| US12064733B2 (en) | 2018-07-30 | 2024-08-20 | Dow Global Technologies Llc | Static mixing device and method for mixing phosgene and an organic amine |
| CN111589380B (zh) * | 2020-06-18 | 2024-11-19 | 靖江神驹容器制造有限公司 | 气相反应器 |
| CN116034102B (zh) | 2020-09-01 | 2025-11-14 | 巴斯夫欧洲公司 | 制备异氰酸酯的方法 |
| KR102728114B1 (ko) * | 2020-09-22 | 2024-11-07 | 주식회사 엘지화학 | 올리고머 제조 장치 |
| KR20220074330A (ko) * | 2020-11-27 | 2022-06-03 | 한화솔루션 주식회사 | 반응기 |
| CN114230489A (zh) * | 2021-12-31 | 2022-03-25 | 浙江丽水有邦新材料有限公司 | 一种间甲苯基异氰酸酯的制备、提纯方法及提纯装置 |
| CN114409572A (zh) * | 2021-12-31 | 2022-04-29 | 浙江丽水有邦新材料有限公司 | 一种十二烷基异氰酸酯的制备、提纯方法及提纯装置 |
| CN115253969B (zh) * | 2022-07-27 | 2023-08-04 | 宁夏瑞泰科技股份有限公司 | 制备异氰酸酯的反应器系统及使用其制备异氰酸酯的方法 |
| EP4378576A1 (en) | 2022-11-29 | 2024-06-05 | Basf Se | Apparatus and process for preparing isocyanates |
| KR20250106479A (ko) * | 2024-01-03 | 2025-07-10 | 이근형 | 기체 용해 장치 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3744001C1 (de) * | 1987-12-24 | 1989-06-08 | Bayer Ag | Verfahren zur kontinuierlichen Herstellung von Mono- oder Polyisocyanaten |
| DD300168A7 (de) * | 1988-12-21 | 1992-05-27 | Schwarzheide Synthesewerk Veb | Verfahren und Vorrichtung zur kontinuierlichen Umsetzung von Diaminodiphenylmethan/Polyamin-Gemischen mit Phosgen zu Polyisocyanaten |
| DE10032269A1 (de) * | 2000-07-03 | 2002-01-31 | Basf Ag | Verfahren und Vorrichtung zur Verringerung von Nebenprodukten bei der Vermischung von Eduktströmen |
| DE10161384A1 (de) * | 2001-12-14 | 2003-06-18 | Bayer Ag | Verbessertes Verfahren für die Herstellung von (/Poly)-isocyanaten in der Gasphase |
| DE10222023A1 (de) * | 2002-05-17 | 2003-11-27 | Bayer Ag | Verfahren zur Herstellung von Isocyanaten in der Gasphase |
| DE10349504A1 (de) * | 2003-10-23 | 2005-05-25 | Bayer Technology Services Gmbh | Verfahren zur Herstellung von Isocyanaten in der Gasphase |
| DE102004030164A1 (de) * | 2004-06-22 | 2006-01-19 | Basf Ag | Verfahren zur Herstellung von Isocyanaten |
| DE102005036870A1 (de) | 2005-08-02 | 2007-02-08 | Bayer Materialscience Ag | Verfahren zur Gasphasenphosgenierung |
| CN101153015B (zh) * | 2006-09-28 | 2010-06-16 | 宁波万华聚氨酯有限公司 | 一种孔射流式反应器及利用该反应器制备异氰酸酯的方法 |
| US8173833B2 (en) * | 2006-11-07 | 2012-05-08 | Basf Aktiengesellschaft | Method for the production of isocyanates |
-
2009
- 2009-08-06 CN CN200980131058.7A patent/CN102119145B/zh active Active
- 2009-08-06 PT PT09781542T patent/PT2323973E/pt unknown
- 2009-08-06 WO PCT/EP2009/060184 patent/WO2010015667A1/de not_active Ceased
- 2009-08-06 KR KR1020117003595A patent/KR101639087B1/ko active Active
- 2009-08-06 PL PL09781542T patent/PL2323973T3/pl unknown
- 2009-08-06 AT AT09781542T patent/ATE550317T1/de active
- 2009-08-06 EP EP09781542A patent/EP2323973B1/de active Active
- 2009-08-06 JP JP2011521576A patent/JP5443487B2/ja active Active
- 2009-08-06 US US13/057,869 patent/US8829232B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| US8829232B2 (en) | 2014-09-09 |
| US20110251425A1 (en) | 2011-10-13 |
| KR101639087B1 (ko) | 2016-07-13 |
| JP2011529947A (ja) | 2011-12-15 |
| PL2323973T3 (pl) | 2012-08-31 |
| WO2010015667A1 (de) | 2010-02-11 |
| KR20110084152A (ko) | 2011-07-21 |
| PT2323973E (pt) | 2012-05-07 |
| ATE550317T1 (de) | 2012-04-15 |
| CN102119145A (zh) | 2011-07-06 |
| EP2323973A1 (de) | 2011-05-25 |
| JP5443487B2 (ja) | 2014-03-19 |
| CN102119145B (zh) | 2014-06-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2323973B1 (de) | Verfahren zur herstellung von aromatischen isocyanaten | |
| EP1924552B1 (de) | Verfahren und vorrichtung zur herstellung von isocyanaten | |
| EP2091912B1 (de) | Verfahren zur herstellung von isocyanaten | |
| EP2079684B1 (de) | Verfahren zur herstellung von isocyanaten | |
| EP0065727B1 (de) | Verfahren zur kontinuierlichen Herstellung von organischen Mono- oder Polyisocyanaten | |
| EP2307356B1 (de) | Verfahren zur herstellung von isocyanaten | |
| EP0928785B1 (de) | Verfahren zur Phosgenierung von Aminen in der Gasphase unter Einsatz von Mikrostrukturmischern | |
| DE69526010T2 (de) | Verfahren zur Herstellung von aromatischen Polyisocyanatverbindungen in der Gasphase | |
| EP0749958B1 (de) | Verfahren zur Herstellung von Triisocyanaten | |
| EP1275640B2 (de) | Verfahren zur Herstellung von (cyclo)aliphatischen Isocyanaten | |
| EP1761483B1 (de) | Verfahren zur herstellung von isocyanaten | |
| EP1555258B1 (de) | Verfahren zur Herstellung von Diisocyanaten und/oder Triisocyanaten | |
| WO2009027232A1 (de) | Verfahren zur herstellung von isocyanaten | |
| DE10222023A1 (de) | Verfahren zur Herstellung von Isocyanaten in der Gasphase | |
| EP2188248A1 (de) | Verfahren zur herstellung von isocyanaten | |
| EP2079685A1 (de) | Verfahren zur herstellung von isocyanaten | |
| EP1319655A2 (de) | Verbessertes Verfahren für die Herstellung von (Poly-)isocyanaten in der Gasphase | |
| EP2077150A1 (de) | Verfahren und Mischaggregat zur Herstellung von Isocyanaten durch Phosgenierung primärer Amine | |
| DE4217019A1 (de) | Verfahren zur Herstellung von aromatischen Diisocyanaten | |
| DE2624285A1 (de) | Verfahren zur kontinuierlichen herstellung von organischen isocyanaten | |
| EP1401802A1 (de) | Verfahren zur herstellung von isocyanaten | |
| DD300168A7 (de) | Verfahren und Vorrichtung zur kontinuierlichen Umsetzung von Diaminodiphenylmethan/Polyamin-Gemischen mit Phosgen zu Polyisocyanaten |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20110307 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: AL BA RS |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| DAX | Request for extension of the european patent (deleted) | ||
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: GERMAN |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 550317 Country of ref document: AT Kind code of ref document: T Effective date: 20120415 |
|
| REG | Reference to a national code |
Ref country code: PT Ref legal event code: SC4A Free format text: AVAILABILITY OF NATIONAL TRANSLATION Effective date: 20120419 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 502009003107 Country of ref document: DE Effective date: 20120516 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: T3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120621 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 |
|
| LTIE | Lt: invalidation of european patent or patent extension |
Effective date: 20120321 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120622 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 |
|
| REG | Reference to a national code |
Ref country code: PL Ref legal event code: T3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120721 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 |
|
| REG | Reference to a national code |
Ref country code: HU Ref legal event code: AG4A Ref document number: E014287 Country of ref document: HU |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 |
|
| 26N | No opposition filed |
Effective date: 20130102 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120831 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 502009003107 Country of ref document: DE Effective date: 20130102 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120702 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120806 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120621 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PL Payment date: 20130722 Year of fee payment: 5 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20130806 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130831 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130831 Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120806 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130806 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120321 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 7 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MM01 Ref document number: 550317 Country of ref document: AT Kind code of ref document: T Effective date: 20140806 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20140806 Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20140806 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20150831 Year of fee payment: 7 |
|
| REG | Reference to a national code |
Ref country code: PL Ref legal event code: LAPE |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20170428 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160831 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20250825 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: HU Payment date: 20250725 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PT Payment date: 20250716 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20250827 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20250825 Year of fee payment: 17 |