EP2037271B1 - Procédés d'utilisation de O6-alkylguanine-ADN alkyltransférases - Google Patents
Procédés d'utilisation de O6-alkylguanine-ADN alkyltransférases Download PDFInfo
- Publication number
- EP2037271B1 EP2037271B1 EP08168182A EP08168182A EP2037271B1 EP 2037271 B1 EP2037271 B1 EP 2037271B1 EP 08168182 A EP08168182 A EP 08168182A EP 08168182 A EP08168182 A EP 08168182A EP 2037271 B1 EP2037271 B1 EP 2037271B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- label
- agt
- fusion protein
- protein
- molecule
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/531—Production of immunochemical test materials
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/20—Fusion polypeptide containing a tag with affinity for a non-protein ligand
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/60—Fusion polypeptide containing spectroscopic/fluorescent detection, e.g. green fluorescent protein [GFP]
Definitions
- the present invention relates to methods of transferring a label from a substrate to a fusion protein comprising a protein of interest and an O 6 -alkylguanine-DNA alkyltransferase (AGT), and in particular to methods which further comprise detecting and/or manipulating the labelled fusion protein.
- AGT O 6 -alkylguanine-DNA alkyltransferase
- German Patent Application No: 199 03 895 A (Kai Johnsson ) describes an ELISA assay for the detection of O 6 -alkylguanine-DNA alkyltransferase (AGT).
- ABT O 6 -alkylguanine-DNA alkyltransferase
- AGT transfers the alkyl group in a S N 2 reaction to one of its own cysteines, resulting in an irreversibly alkylated enzyme.
- overexpression of AGT in tumour cells enables them to acquire drug resistance, particularly to alkylating drugs such as procarbazine, dacarbazine, temozolomide and bis-2-chloroethyl-N-nitrosourea
- inhibitors of AGT have been proposed for use as sensitisers in chemotherapy [Pegg et al., 1995].
- DE 199 03 895 A discloses an assay for measuring levels of AGT which relies on the reaction between biotinylated O 6 -alkylguanine-derivatives and AGT which leads to biotinylation of the AGT.
- the assay is suggested for monitoring the level of AGT in tumour tissue, adjusting treatment using AGT inhibitors as sensitisers in chemotherapy and for use in screening for AGT inhibitors.
- the present invention provides a method for detecting a protein of interest in vitro or in vivo , in vivo meaning in bacterial or eukaryotic cells, in cell culture or in cell extracts, in a cell in all the compartments of a cell, on the surface of a cell or AGT fusion proteins pointing to the extracellular space, which comprises contacting an expressed fusion protein comprising the protein of interest and an O 6 - alkylguanine-DNA alkyltransferase (AGT) with a substrate having a label so that the AGT transfers the label from the substrate to become covalently bonded to the expressed fusion protein to permit detection thereof, wherein the substrate is a labelled benzyl guanine substrate represented by the general formula: wherein
- AGT O 6 -alkylguanine-DNA alkyltransferase
- a method which comprises contacting a fusion protein comprising protein of interest and an O 6 -alkylguanine-DNA alkyltransferase (AGT) and a substrate having a particular label so that the AGT transfers the label so that it is covalently bonded to the fusion protein, wherein the label is an affinity tag, a first member of a specific binding pair which is capable of specifically binding to the second member of the specific binding pair, a molecule which is attachable to a solid phase, a molecule which is capable of generating reactive radicals, a candidate compound or library of candidate compounds, a molecule which is capable of crosslinking to other biomolecules, a nucleic acid or a derivative thereof capable of undergoing base-pairing with its complementary strand, a lipid, or a hydrophobic molecule with membrane-inserting properties.
- the method may additionally involve the further step of detecting and/or manipulating the labelled
- AGT O 6 -alkylguanine-DNA alkyltransferase
- the method comprises one or more further steps, for example detecting and/or manipulating the labelled fusion protein.
- a method of detecting a fusion protein comprising protein of interest and an O 6 -alkylguanine-DNA alkyltransferase comprising contacting the fusion protein with a substrate having a particular label as defined hereinbefore so that the AGT transfers the label so that it is covalently bonded to the fusion protein and detecting the protein construct using the label.
- AGT O 6 -alkylguanine-DNA alkyltransferase
- a method of manipulating a fusion protein comprising protein of interest and an O 6 -alkylguanine-DNA alkyltransferase (AGT), the method comprising contacting the fusion protein with a substrate having a particular label as defined hereinbefore so that the AGT transfers the label so that it is covalently bonded to the fusion protein and manipulating the fusion protein using a physical and/or chemical property introduced by the label to the fusion protein.
- AGT O 6 -alkylguanine-DNA alkyltransferase
- the method may comprise detecting the protein construct using the label.
- a method of immobilising a fusion protein comprising protein of interest and an alkylguanine-DNA alkyltransferase (AGT) on a solid support comprising contacting the fusion protein with a substrate having a label which is attachable to a solid support, wherein the AGT transfers the label so that it is covalently bonded to the fusion protein which thereby can be subsequently attached to the solid support.
- the method may involve the further step of contacting the labelled fusion protein with the solid support so that it becomes immobilised on the solid support.
- the label may be covalently attached to the solid support in a subsequent reaction, or may be one member of a specific binding pair, the other member of which is attached or attachable to the solid support, either covalently or by any other means (e.g. using the specific binding pair of biotin and avidin or streptavidin).
- AGT fusion proteins both in vivo as well as in vitro .
- the term in vivo labelling of a AGT fusion protein means labelling in all compartments of a cell as well as of AGT fusion proteins pointing to the extracellular space. If the labelling of the AGT fusion protein is done in vivo and the protein fused to the AGT is a plasma membrane protein, the AGT part of the fusion protein can be either attached to the cytoplasmic or the extracellular side of the plasma membrane. If the labelling is done in vitro , the labelling of the fusion protein can be either performed in cell extracts or with purified or enriched forms of the AGT fusion protein.
- the protein of interest fused to the AGT comprises a DNA binding domain of a transcription factor or an activation domain of a transcription factor, a target substance or library of target substances is linked to the other of the DNA binding domain or the activation domain of the transcription factor, and the label is a candidate compound or library of candidate compounds suspected of interacting with the target substance(s).
- the method may further comprise transferring the candidate compound or library of candidate compounds to the AGT protein fusion and contacting the AGT fusion protein(s) labelled with the candidate compounds and the target substance(s) so that the interaction of a candidate compound joined to the AGT fusion protein and a target substance activates the transcription factor.
- the activated transcription factor can then drive the expression of a reporter which, if the method is carried out in cells, can be detected if the expression of the reporter confers a selective advantage on the cells.
- the method may involve one or more further steps such as detecting, isolating, identifying or characterising the candidate compound(s) or target substance(s).
- the O 6 -alkylguanine-DNA alkyltransferase or 'AGT' has the property of transferring a label present on a substrate to one of the cysteine residues of the AGT forming part of a fusion protein.
- the AGT is an O 6 -alkylguanine-DNA alkyltransferase, for example human O 6 -alkylguanine-DNA alkyltransferase which is described in Pegg et al. 1995 and references therein.
- other alkylguanine-DNA alkyltransferases are known, e.g.
- O 6 -alkylguanine-DNA alkyltransferase also includes variants of a wild-type AGT which may differ by virtue of one or more amino acid substitutions, deletions or additions, but which still retain the property of transferring a label present on a substrate to the AGT or the protein or peptide with which it forms a fusion.
- variants of AGTs may be chemically modified using techniques well known to those skilled in the art.
- AGT variants may be produced using protein engineering techniques known to the skilled person and/or using molecular evolution to generate and select new O 6 -alkylguanine-DNA alkyltransferases.
- the reference to the protein part of the fusion protein with the AGT is intended to include proteins, polypeptides and peptides of any length and both with and without secondary, tertiary or quaternary structure. Examples of applications of the present invention are provided below.
- the labelled substrate is a labelled benzylguanine substrate, and preferably is an O 6 -benzylguanine derivative.
- An example of such a derivative is an O 6 -benzylguanine derivative that is derivatised at the 4-position of the benzyl ring with the following general formula: wherein:
- the label part of the substrate can be chosen by those skilled in the art dependent on the application for which the fusion protein is intended.
- Examples of labels include:
- the following applications of the present invention are provided by way of examples and not limitation.
- the method disclosed herein is generally applicable to a range of applications and is capable of specifically and covalently labelling fusion proteins with (1) labels which are capable of sensing and inducing changes in the environment of the labelled fusion protein and/or (2) labels which aid in manipulating the fusion protein by the physical and/or chemical properties specifically introduced by the label to the fusion protein.
- the method disclosed herein can be used to label AGT fusion proteins both in vivo and in vitro .
- the present invention is based on the realisation that specific attachment of a label to a desired protein could be carried out by constructing a fusion protein between that protein of interest and taking advantage of the mechanism of an O 6 -alkylguanine-DNA alkyltransferase such as human DNA repair enzyme O 6 -alkylguanine-DNA alkyltransferase (hAGT).
- O 6 -alkylguanine-DNA alkyltransferase hAGT
- This enzyme irreversibly transfers the alkyl group from its substrate, O 6 -alkylguanine-DNA, to one of its cysteine residues ( Figure 1 ).
- a substrate analogue that rapidly reacts with hAGT is O 6 -benzylguanine, the second order rate constant being approximately 10 3 sec -1 M -1 ( Figure 1 ).
- the labelling of the endogenous AGT of the host is advantageously taken into account. If the endogenous AGT of the host does not accept O 6 -benzylguanine derivatives or related compounds as a substrate, the labelling of the fusion protein is specific. In mammalian cells (human, murine, rat), labelling of endogenous AGT is possible. In those experiments where the simultaneous labelling of the endogenous AGT as well as of the AGT fusion problem poses a problem, previously described AGT-deficient cell lines can be used [Kaina et al., 1991].
- the present invention can be employed in all applications of the technique were the covalent and specific attachment of a label to a AGT fusion protein is used to monitor or influence the behaviour of the AGT fusion protein or is used to manipulate the AGT fusion protein by virtue of the introduced label. Examples of applications for the use of this technology follow.
- AGT substrates such as O 6 -benzylguanine derivatives
- the substrate carries a detectable label which can be transferred to the AGT
- the present invention to be used to specifically and covalently attach the detectable label to the AGT fusion protein, either in a cell, on the surface of a cell ( in vivo ) or in vitro .
- This allows the detection and characterization of the AGT fusion protein in vivo or in vitro.
- the term in vivo includes labelling in all compartments of a cell as well as of AGT fusion proteins pointing to the extracellular space.
- AGT substrates such as O 6 -benzylguanine derivatives
- an affinity tag such as biotin
- the AGT fusion protein can be released from the affinity tag after its isolation.
- AGT substrates such as O 6 -benzylguanine derivatives
- AGT-protein fusions which are capable of generating reactive radicals, such as hydroxyl radicals, upon exposure to an external stimuli.
- the generated radicals can then inactivate the AGT fusion proteins as well as those proteins that are in close proximity of the AGT fusion protein, allowing the study the role of these proteins.
- labels are tethered metal-chelate complexes that produce hydroxyl radicals upon exposure to H 2 O 2 and ascorbate, and chromophores such as malachite green that produce hydroxyl radicals upon laser irradiation.
- CALI chromophore assisted laser induced inactivation
- the chromophore is brought in the spatial neighbourhood of the protein of interest by microinjecting chromophore-labelled antibodies specific to the protein of interest.
- labelling AGT fusion proteins with chromophores such as malachite green and subsequent laser irradiation would allow to inactivate the AGT fusion protein as well as those proteins that interact with the AGT fusion protein in a time-controlled and spatially-resolved manner.
- the method can be applied both in vivo or in vitro .
- AGT fusion proteins can be labelled with tethered metal-chelates and the AGT fusion protein and those proteins that interact with the AGT fusion protein can be inactivated in a specific manner upon exposure to H 2 O 2 and ascorbate.
- the method can not only be used to study the function of an AGT fusion protein or those that are in close proximity of the AGT fusion protein, but also to identify those proteins that are in close proximity of a AGT fusion protein.
- proteins which are in close proximity of the AGT fusion protein can be identified as such by either detecting fragments of that protein by a specific antibody, by the disappearance of those proteins on a high-resolution 2D-electrophoresis gels or by identification of the cleaved protein fragments via separation and sequencing techniques such as mass spectrometry or protein sequencing by N-terminal degradation.
- AGT substrates such as O 6 -benzylguanine derivatives
- labelled AGT substrates such as O 6 -benzylguanine derivatives
- binding partners of the ligand such as proteins
- the label is a ligand which is capable of binding to a binding partner
- contacting such a substrate with a AGT fusion protein will lead to specific attachment of the ligand to the fusion protein.
- the ligand binds to another protein Y and the dimerization of the protein Y with the labelled AGT fusion protein leads to a biological function or a measurable signal, the biological function or the measured signal depends on the addition of the AGT substrate carrying the label.
- a specific example would be the use of AGT substrates and AGT fusion protein in the so-called three-hybrid system described by Ho et al., 1996, to regulate gene expression with small molecules.
- AGT is fused to the DNA-binding domain of a transcription factor.
- a protein Y such as FKBP that binds a ligand, such as FK506, is fused to the activation domain of a transcription factor. Supplying the cells with the AGT substrate that carries the ligand, in this example FK506, will lead to the formation of a functional transcription factor and gene expression.
- O 6 -benzylguanine derivatives or related AGT substrates that carry a label and where the label is a drug or a biological active small molecule that binds to an yet unidentified protein Y.
- the goal would be to identify the target protein Y of the biological active molecule.
- AGT is fused to the DNA-binding domain of a transcription factor.
- a cDNA library of the organism which expresses the unknown target protein Y is fused to the activation domain of a transcription factor.
- O 6 -benzylguanine derivatives or related AGT substrate that carry a label and where the label is the drug or the biological active small molecule will lead to the formation of a functional transcription factor and gene expression only in the case where this molecule binds to its target protein Y present in the cDNA library and fused to the activation domain. If gene expression is coupled to a selective advantage, the corresponding host carrying the plasmid with the target gene Y of the drug or bioactive molecule can be identified.
- O 6 -benzylguanine or related AGT substrates that carry a label and where the label is a library of chemical molecules the goal would be to identify small molecules that bind to a protein Y under in vivo conditions, which might be a potential drug target.
- AGT is fused to the DNA-binding domain of a transcription factor.
- the target protein Y is fused to the activation domain of a transcription factor.
- Adding a library of small molecules attached as label to a O 6 -benzylguanine derivative will lead to the formation of a functional transcription factor and gene expression only in the case where the label (i.e. the small molecule) binds to its target protein Y fused to the activation domain. If gene expression is coupled to a selective advantage, those molecules of the library leading to the growth of the host can be identified.
- the label is biotin and the molecule attached to the surface is streptavidin or avidin.
- a carrier would be either a glass side, a microtiter plate or any functionalized polymer.
- the immobilization of the AGT substrate via its label allows the subsequent immobilization of a AGT fusion protein on the carrier by the transfer of the label to the fusion protein.
- Spotting (different) AGT fusion proteins in a spatially resolved manner on the carrier allows the creation of protein arrays.
- O 6 -benzylguanine derivatives or related AGT substrates which carry a label and where the label is a molecule that can cross-link to other proteins.
- cross-linkers are molecules containing functional groups such as maleimides, active esters or azides and others known to those proficient in the art and described in Nadeau et al., 2002.
- AGT substrates with AGT fusion proteins that interact with other proteins ( in vivo or in vitro ) can lead to the covalent cross-linking of the AGT fusion protein with its interacting protein via the label. This allows the identification of the protein interacting with the AGT fusion protein.
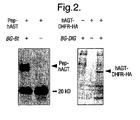
- Pep-hAGT were grown to an optical density OD 600nm of 0.6. Expression of Pep-hAGT was induced by adding IPTG to a final concentration of 1 mM. At the same time BG-Bt was added to a final concentration of 10 ⁇ M and the bacteria were incubated for 2 h at 37°C. Cells were harvested by centrifugation and the pellet washed twice to remove access BG-Bt. A resuspended aliquot of cells was analysed by Western Blotting. Biotinylated proteins were detected using a streptavidin-peroxidase conjugate ( NEN ) and a chemiluminescent peroxidase substrate ( Renaissance reagent plus, NEN ) ( Figure 2 ).
- NEN streptavidin-peroxidase conjugate
- chemiluminescent peroxidase substrate Renaissance reagent plus, NEN
- hAGT-DHFR-HA fusion protein is biotinylation in yeast using BG-Bt.
- the fusion protein is constructed on the DNA level using standard molecular biology procedures.
- the stop codon of hAGT is replaced by codons for the amino acids RSGI, which are then followed by the codon for the first amino acid of DHFR from mouse, a Met [Nunberg et al., 1980].
- the codons for the linker between hAGT and DHFR also encode for a Bgl II site, its DNA sequence being AGATCT.
- DHFR a codon for the first amino acid of the HA-tag [Kolodziej, 1991].
- the HA-tag is followed by a stop codon.
- Expression of hAGT-DHFR-HA was induced by adding CuSO 4 to a concentration of 100 ⁇ M and BG-Bt was simultaneously added to a concentration of 10 ⁇ M. Aliquots were taken after 2.5 h and 5 h and cells harvested by centrifugation.
- the pellet was washed twice to remove remaining BG-Bt. After lysis of the yeast cells by freeze/thaw cycling the cell extract was analysed for the presence of biotinylated hAGT-DHFR-HA fusion protein using an ELISA.
- the biotinylated hAGT-DHFR-HA was immobilized in streptavidin-coated microtiter wells and detected by using an antiHA-antibody ( Babco ) as a primary and an antimouse-HRP conjugate ( Sigma ) as a secondary antibody ( Figure 1 ) [Kolodziej, 1991].
- the ELISA was developed using the peroxidase substrate ABTS and the signal (absorbance) measured at OD 405nm .
- the signal for the in vivo biotinylated hAGT-DHFR-HA fusion protein was at least fivefold above background.
- the background signal was defined as the OD 405nm of cell lysates obtained from cells treated exactly as above but omitting the addition of BG-Bt.
- the following example demonstrates the feasibility of covalently labelling hAGT fusion proteins in yeast.
- the hAGT-DHFR-HA fusion protein is labelled with digoxigenin in yeast using BG-DIG.
- the construction of the hAGT-DHFR-HA fusion is described in example B.
- a culture of L40 yeast cells containing the expression vector p314AK1 in which the gene of a hAGT-DHFR fusion protein is under control of the p cup1 promoter was grown to an OD 600nm of 1.2.
- Expression of the hAGT-DHFR fusion protein was induced by adding CuSO 4 to a concentration of 100 ⁇ M and BG-DIG was simultaneously added to a concentration of 20 ⁇ M.
- HEK 293 endogenous hAGT in human cells
- BG-AcFc fluoresceine
- this example describes the use of a spectroscopic probe, it can be likewise performed with a particular label of the invention.
- HEK 293 cells were incubated with 5 ⁇ M BG-AcFc in PBS for 5min.
- the acetylated fluoresceine derivative BG-AcFc is cell-permeable and non-fluorescent but can be expected to be hydrolysed rapidly within the cell to yield the fluorescent BG-Fc.
- the cells were then washed by changing the PBS to remove any access substrate BG-AcFc and incubated in PBS for 20 min. Images were then taken with a confocal fluorescence microscope (Ext. 492 nm; Em. 510 nm).
- a confocal fluorescence microscope (Ext. 492 nm; Em. 510 nm).
- the HEK 293 cells were treated as above but incubated prior to addition of BG-AcFc overnight with O 6 -benzylguanine (1 ⁇ M). This should inactivate the endogenous hAGT and therefore prevent the accumulation of the fluorescence in the nucleus. As expected, no accumulation of fluorescence in the nucleus was observed when the cells were preincubated with O 6 -benzylguanine.
- recombinant Pep-hAGT (10 ⁇ M, as described in example A was incubated with 100 ⁇ M BG-Fc at 25 °C in 50 mM Tris-Cl, 10 mM DTT, 1 mM EDTA, 200 ⁇ g/ml BSA, 10% Glycerol, pH 7.4 for 10 minutes, followed by addition of 900 ⁇ l PBS (phosphate buffered saline: 137 mM NaCl, 2.7 mM KCI, 10 mM Na 2 HPO 4 , 1.8 mM KH 2 PO 4 , pH 7.4).
- PBS phosphate buffered saline: 137 mM NaCl, 2.7 mM KCI, 10 mM Na 2 HPO 4 , 1.8 mM KH 2 PO 4 , pH 7.4
- BG-Fc Separation of excess substrate BG-Fc was achieved by gel filtration on a NAP TM -10 Column (Pharmacia) according to the supplier's instruction.
- the Pep-hAGT was then characterized in a standard fluorescence spectrophotometer. The sample was excitated at 222, 238 and 490 nm, respectively and showed maximal emission at a wavelength of 523 nm, verifying that the protein was labelled with fluoresceine.
- a solution of 20 nM BG-Fc in PBS was measured as reference.
- the substrate's emission wavelength is 519 nm (excitation at 237, 323 and 490 nm respectively).
- fusion proteins with AGT can be directly labelled and manipulated (here immobilized) in cell extracts the following N- and C-terminal fusion proteins with hAGT proteins were constructed via standard molecular cloning procedures and cloned into a yeast expression vector:
- L40 yeast cells containing an expression vector encoding for one of the fusion protein were grown to an OD of 0.6 and expression of the fusion protein was induced by adding CuSO 4 to a concentration of 100 ⁇ M. Aliquots (2 ml) were taken after 5 h and cells harvested by centrifugation. After lysis of the cells by freeze/thaw cycling the yeast extract was incubated with BG-Bt-oligo (10 pmol) for 20 min at room temperature, leading to biotinylation of the fusion protein. Subsequently, the suspension was transferred into a streptavidin-coated microtiter plates ( Roche molecular biochemicals ) and incubated for 1 h.
- the immobilized fusion protein was detected using either an anti-HA-antibody ( Babco ) or an anti-hAGT-antibody (in the case of the SSN6-hAGT fusion protein) as a primary and an antimouse-peroxidase conjugate ( Sigma, #A4416 ) as a secondary antibody and subsequent incubation with the peroxidase substrate ABTS using standard biochemical procedures.
- an anti-HA-antibody Babco
- an anti-hAGT-antibody in the case of the SSN6-hAGT fusion protein
- an antimouse-peroxidase conjugate Sigma, #A4416
- the references are in alphabetic order.
Claims (18)
- Procédé de détection d'une protéine d'intérêt in vitro ou in vivo, in vivo signifiant dans des cellules bactériennes ou eucaryotes, dans une culture cellulaire ou dans des extraits de cellules, dans une cellule dans tous les compartiments d'une celle, sur la surface d'une cellule ou des protéines de fusion AGT pointant vers l'espace extracellulaire, qui comprend la mise en contact d'une protéine de fusion exprimée comprenant la protéine d'intérêt et une O6-alkylguanine-ADN alkyltransférase (AGT) avec un substrat ayant une étiquette de sorte que l'AGT transfère l'étiquette du substrat pour devenir liée par covalence à la protéine de fusion exprimée pour permettre sa détection, dans lequel le substrat est un substrat de benzylguanine étiqueté représenté par la formule générale :
etétiquette représente un groupe à utiliser dans la détection et/ou la manipulation de la protéine de fusion ;ou par la formule générale :
etétiquette est tel que ci-dessus ;et dans lequel l'étiquette est un marqueur d'affinité, un premier élément d'une paire de liaison spécifique qui est capable de se lier spécifiquement au second élément de la paire de liaison spécifique, une molécule qui peut être attachée à une phase solide, une molécule qui est capable de générer des radicaux réactifs, un composé candidat ou une bibliothèque de composés candidats, une molécule qui est capable de réticulation avec d'autres biomolécules, un acide nucléique ou un dérivé de celui-ci capable de subir un appariement de base avec sa chaîne complémentaire, un lipide, ou une molécule hydrophobe avec des propriétés d'insertion dans la membrane. - Procédé selon la revendication 1, dans lequel l'étiquette est un marqueur d'affinité, un premier élément d'une paire de liaison spécifique qui est capable de se lier spécifiquement au second élément de la paire de liaison spécifique, ou une molécule qui peut être attachée à une phase solide.
- Procédé selon la revendication 1 ou 2, qui comprend la détection de la protéine de fusion étiquetée dans un système in vitro.
- Procédé selon la revendication 1 ou 2, qui comprend la détection de la protéine de fusion étiquetée dans un système de culture cellulaire.
- Procédé selon la revendication 4, comprenant en outre l'étape initiale consistant à transformer les cellules avec un vecteur d'expression comprenant un acide nucléique codant la protéine de fusion liée à des séquences de commande pour diriger son expression.
- Procédé selon l'une quelconque des revendications précédentes, comprenant en outre la manipulation de la protéine de fusion étiquetée à l'aide d'une propriété introduite par l'étiquette dans la protéine de fusion.
- Procédé selon l'une quelconque des revendications précédentes, dans lequel le substrat étiqueté est incorporé dans une molécule d'acide nucléique, et la molécule d'acide nucléique est un oligonucléotide d'une longueur comprise entre 2 et 99 nucléotides.
- Procédé selon la revendication 1, dans lequel dans la seconde formule R1 est une chaîne alkyle substituée ou non substituée, un groupe cycloalkyle substitué ou non substitué avec une taille de cycle comprise entre trois et dix atomes de carbone, un hétérocycle substitué ou non substitué ayant une taille de cycle comprise entre trois et dix atomes de carbone, ou un hétérocycle aromatique substitué ou non substitué avec une taille de cycle comprise entre trois et dix atomes de carbone.
- Procédé selon l'une quelconque des revendications 1 à 8, dans lequel le groupe de liaison R2 est une chaîne alkyle substituée ou non substituée ou un poly(éthylène glycol).
- Procédé selon l'une quelconque des revendications 1 à 9, dans lequel l'étiquette est un marqueur d'affinité.
- Procédé selon l'une quelconque des revendications 1 à 9, dans lequel l'étiquette est un premier élément d'une paire de liaison spécifique qui est capable de se lier spécifiquement au second élément de la paire de liaison spécifique.
- Procédé selon l'une quelconque des revendications 1 à 11, dans lequel l'étiquette est une molécule qui peut être attachée à une phase solide.
- Procédé selon la revendication 10, 11 ou 12, dans lequel l'étiquette est la biotine, l'avidine ou la streptavidine.
- Procédé selon la revendication 10, 11 ou 12, dans lequel l'étiquette est liée à la protéine de fusion via un lieur clivable de sorte que la protéine de fusion peut être libérée par l'étiquette.
- Procédé selon la revendication 10, 11 ou 12, dans lequel l'étiquette est liée à la protéine de fusion via un lieur photoclivable de sorte que la protéine de fusion peut être libérée par l'étiquette.
- Procédé selon la revendication 11, comprenant en outre la mise en contact de la protéine de fusion avec une molécule comprenant le second élément de la paire de liaison spécifique.
- Procédé selon la revendication 11 ou 16, dans lequel les premier et second éléments de la paire de liaison spécifique sont des première et secondes protéines, un anticorps et un antigène, une enzyme et un substrat ou un ligand et un récepteur.
- Procédé selon la revendication 12, dans lequel le procédé comprend l'étape supplémentaire consistant à mettre en contact la protéine de fusion étiquetée avec le support solide de sorte qu'elle devient immobilisée sur le support solide.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP10075132.0A EP2211177B1 (fr) | 2001-04-10 | 2002-04-05 | Procédés d'utilisation de O6-alkylguanine-ADN alkyltransférases |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US28276601P | 2001-04-10 | 2001-04-10 | |
| EP06111733A EP1696234B1 (fr) | 2001-04-10 | 2002-04-05 | Procédés d'utlisation de O6-alkylguanine-ADN alkyltransférases |
| EP02720187A EP1410023B1 (fr) | 2001-04-10 | 2002-04-05 | Procedes d'utilisation de o6-alkylguanine-dna alkyltransferase |
Related Parent Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP02720187.0 Division | 2002-04-05 | ||
| EP06111733A Division EP1696234B1 (fr) | 2001-04-10 | 2002-04-05 | Procédés d'utlisation de O6-alkylguanine-ADN alkyltransférases |
| EP06111733.9 Division | 2006-03-27 |
Related Child Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP10075132.0A Division EP2211177B1 (fr) | 2001-04-10 | 2002-04-05 | Procédés d'utilisation de O6-alkylguanine-ADN alkyltransférases |
| EP10075095.9 Division-Into | 2010-03-03 | ||
| EP10075132.0 Division-Into | 2010-03-25 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2037271A1 EP2037271A1 (fr) | 2009-03-18 |
| EP2037271B1 true EP2037271B1 (fr) | 2010-09-22 |
Family
ID=23083030
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP08168182A Expired - Lifetime EP2037271B1 (fr) | 2001-04-10 | 2002-04-05 | Procédés d'utilisation de O6-alkylguanine-ADN alkyltransférases |
| EP06111733A Expired - Lifetime EP1696234B1 (fr) | 2001-04-10 | 2002-04-05 | Procédés d'utlisation de O6-alkylguanine-ADN alkyltransférases |
| EP10075132.0A Expired - Lifetime EP2211177B1 (fr) | 2001-04-10 | 2002-04-05 | Procédés d'utilisation de O6-alkylguanine-ADN alkyltransférases |
| EP02720187A Expired - Lifetime EP1410023B1 (fr) | 2001-04-10 | 2002-04-05 | Procedes d'utilisation de o6-alkylguanine-dna alkyltransferase |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP06111733A Expired - Lifetime EP1696234B1 (fr) | 2001-04-10 | 2002-04-05 | Procédés d'utlisation de O6-alkylguanine-ADN alkyltransférases |
| EP10075132.0A Expired - Lifetime EP2211177B1 (fr) | 2001-04-10 | 2002-04-05 | Procédés d'utilisation de O6-alkylguanine-ADN alkyltransférases |
| EP02720187A Expired - Lifetime EP1410023B1 (fr) | 2001-04-10 | 2002-04-05 | Procedes d'utilisation de o6-alkylguanine-dna alkyltransferase |
Country Status (15)
| Country | Link |
|---|---|
| US (2) | US7939284B2 (fr) |
| EP (4) | EP2037271B1 (fr) |
| JP (2) | JP4195815B2 (fr) |
| KR (1) | KR20040039194A (fr) |
| CN (2) | CN1975422B (fr) |
| AT (3) | ATE331220T1 (fr) |
| AU (1) | AU2002251257B2 (fr) |
| CA (1) | CA2443570A1 (fr) |
| DE (3) | DE60237792D1 (fr) |
| DK (1) | DK1410023T3 (fr) |
| HK (1) | HK1068406A1 (fr) |
| IL (1) | IL157880A0 (fr) |
| NZ (3) | NZ540410A (fr) |
| WO (1) | WO2002083937A2 (fr) |
| ZA (1) | ZA200307442B (fr) |
Families Citing this family (44)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7799524B2 (en) | 2002-10-03 | 2010-09-21 | Ecole Polytechnique Ferdeale de Lausanne | Substrates for O6-alkylguanina-DNA alkyltransferase |
| AU2003267423A1 (en) * | 2002-10-03 | 2004-04-23 | Ecole Polytechnique Federale De Lausanne (Epfl) | Protein labelling with 06-alkylguanine-DNA alkyltransferase |
| CA2514564A1 (fr) | 2003-01-31 | 2005-07-26 | Promega Corporation | Fixation covalente de groupes fonctionnels a des proteines |
| US7429472B2 (en) | 2003-01-31 | 2008-09-30 | Promega Corporation | Method of immobilizing a protein or molecule via a mutant dehalogenase that is bound to an immobilized dehalogenase substrate and linked directly or indirectly to the protein or molecule |
| EP1627226A1 (fr) * | 2003-05-23 | 2006-02-22 | Ecole Polytechnique Federale De Lausanne | Methodes pour marquage de proteines a base de proteine porteuse d'acyle. |
| US7888090B2 (en) | 2004-03-02 | 2011-02-15 | Ecole Polytechnique Federale De Lausanne | Mutants of O6-alkylguanine-DNA alkyltransferase |
| EP1730298B1 (fr) * | 2004-03-02 | 2015-11-11 | EPFL Ecole Polytechnique Fédérale de Lausanne | Substrats de o6-alkylguanine-adn-alkyltransferase |
| US7425436B2 (en) | 2004-07-30 | 2008-09-16 | Promega Corporation | Covalent tethering of functional groups to proteins and substrates therefor |
| US7825096B2 (en) | 2004-09-08 | 2010-11-02 | The United States Of America As Represented By The Department Of Health And Human Services | O6-alkylguanine-DNA alkyltransferase inactivators and beta-glucuronidase cleavable prodrugs |
| US7273714B2 (en) * | 2005-03-11 | 2007-09-25 | Vion Pharmaceuticals, Inc. | Alkylguanyltransferase assays |
| US8178314B2 (en) | 2005-04-27 | 2012-05-15 | Covalys Biosciences Ag | Pyrimidines reacting with O6-alkylguanine-DNA alkyltransferase fusion protein and method for detecting protein |
| FR2890174B1 (fr) | 2005-08-30 | 2009-04-24 | Cis Bio Internat Sa | Procede pour la mise en evidence d'un processus biologique par mesure d'un fret |
| FR2890446B1 (fr) | 2005-09-05 | 2008-04-18 | Cis Bio Internat Sa | Methode de detection d'interaction intracellulaire entre bio-molecules |
| JP5415264B2 (ja) | 2006-06-30 | 2014-02-12 | ドイスコベルク コーポレーション | 検出可能な核酸タグ |
| WO2008054821A2 (fr) | 2006-10-30 | 2008-05-08 | Promega Corporation | Protéines hydrolase mutantes à cinétique et expression fonctionnelle améliorées |
| US7897737B2 (en) | 2006-12-05 | 2011-03-01 | Lasergen, Inc. | 3′-OH unblocked, nucleotides and nucleosides, base modified with photocleavable, terminating groups and methods for their use in DNA sequencing |
| BRPI0719923B1 (pt) * | 2006-12-05 | 2021-08-17 | Agilent Technologies Inc | Métodos para sequenciamento de um ácido nucléico alvo, para conversão de um componente de ocorrência não natural em uma molécula de ácido nucléico em um componente de ocorrência natural, para terminar uma síntese de ácido nucléico, e para realizar o sequenciamento de sanger ou tipo sanger, e compostos |
| EP1978093A1 (fr) * | 2007-04-04 | 2008-10-08 | Ecole Polytechnique Fédérale de Lausanne (EPFL) | Procédé pour la réticulation de deux objets d'intérêt |
| GB0711560D0 (en) * | 2007-06-14 | 2007-07-25 | Innova Biosciences Ltd | Improvements in or relating to production of conjugates |
| US20100209933A1 (en) * | 2007-10-29 | 2010-08-19 | Mcreynolds Larry A | Methods and Compositions for Detection and Enrichment of Target Small RNAs |
| EP2093564A1 (fr) | 2008-02-25 | 2009-08-26 | Technische Universität Dresden Medizinische Fakultät Carl Gustav Carus | Procédé de distinction de granules de sécrétion d'âges différents |
| WO2009152353A2 (fr) | 2008-06-11 | 2009-12-17 | Lasergen, Inc. | Nucléotides et nucléosides et procédés pour leur utilisation dans le séquençage de l'adn |
| FR2936245B1 (fr) | 2008-09-23 | 2012-07-06 | Cis Bio Int | Nouveaux substrats d'o6-alkylguanine-adn alkyltransferase et ses mutants. |
| GB2489187B (en) | 2010-01-20 | 2015-02-11 | New England Biolabs Inc | Compositions, methods and related uses for cleaving modified DNA |
| US20130183696A1 (en) | 2010-09-15 | 2013-07-18 | Constance Neely Wilson | Methods of use and kit for measurement of lipopolysaccharide with a time resolved fluorescence based assay |
| US8889860B2 (en) | 2011-09-13 | 2014-11-18 | Lasergen, Inc. | 3′-OH unblocked, fast photocleavable terminating nucleotides and methods for nucleic acid sequencing |
| AU2012350229B2 (en) | 2011-12-09 | 2018-03-22 | Institut Pasteur | Multiplex immuno screening assay |
| US9267117B2 (en) | 2012-03-15 | 2016-02-23 | New England Biolabs, Inc. | Mapping cytosine modifications |
| WO2014153408A1 (fr) | 2013-03-19 | 2014-09-25 | Directed Genomics, Llc | Enrichissement en séquences cibles |
| JP5781589B2 (ja) * | 2013-12-20 | 2015-09-24 | レーザーゲン インコーポレイテッド | 光開裂性標識ヌクレオチドとヌクレオシドおよび標識ヌクレオチドとヌクレオシドならびにdna配列決定におけるそれらの使用法 |
| WO2016003795A1 (fr) | 2014-07-02 | 2016-01-07 | New England Biolabs, Inc. | Réactif universel de liaison aux n-glycanes |
| WO2016093838A1 (fr) | 2014-12-11 | 2016-06-16 | New England Biolabs, Inc. | Enrichissement en séquences cibles |
| CN104844710B (zh) * | 2015-04-13 | 2018-02-02 | 西北大学 | 定向固定化pega复合型树酯的制备方法及应用 |
| CN113444047B (zh) | 2016-07-20 | 2023-06-16 | 纳莹(上海)生物科技有限公司 | 一种荧光探针及其制备方法和用途 |
| US10934533B1 (en) | 2018-10-10 | 2021-03-02 | New England Biolabs, Inc. | Variant DNA polymerases having improved properties and method for improved isothermal amplification of a target DNA |
| CN111253417B (zh) * | 2019-09-04 | 2022-03-25 | 大连民族大学 | 一种嘌呤衍生物的合成及应用 |
| WO2021163559A1 (fr) | 2020-02-13 | 2021-08-19 | New England Biolabs, Inc. | Variants d'adn polymérases de la famille d |
| WO2021163561A1 (fr) | 2020-02-13 | 2021-08-19 | New England Biolabs, Inc. | Variants de polymérases d'adn de la famille d |
| EP4118233A1 (fr) | 2020-03-12 | 2023-01-18 | New England Biolabs, Inc. | Test de diagnostic rapide pour technique lampe |
| CN112010983A (zh) * | 2020-08-11 | 2020-12-01 | 西北大学 | 止咳平喘中药活性成分靶向筛选方法及试剂盒 |
| WO2022040443A2 (fr) | 2020-08-21 | 2022-02-24 | New England Biolabs, Inc. | Test de diagnostic rapide de lamp |
| WO2022250676A1 (fr) | 2021-05-27 | 2022-12-01 | New England Biolabs, Inc. | Variants de dnase i, compositions, procédés et kits |
| WO2023097226A2 (fr) | 2021-11-24 | 2023-06-01 | New England Biolabs, Inc. | Désaminases d'adn double brin |
| WO2023197015A1 (fr) | 2022-04-08 | 2023-10-12 | New England Biolabs, Inc. | Compositions et analyse d'oligoribonucléotides déphosphorylés |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB9224888D0 (en) * | 1992-11-27 | 1993-01-13 | Univ Singapore | Monoclonal antibody |
| AU697977B2 (en) * | 1993-06-08 | 1998-10-22 | Cancer Research Technology Limited | O6-substituted guanine derivatives, a process for their preparation and their use in treating tumour cells |
| DE19903895A1 (de) * | 1999-02-01 | 2000-08-03 | Kai Johnsson | Selektive Immobilisierung und Nachweis der O·6·-Alkylguanin-DNA-Alkyltransferase in der aktiven Form |
-
2002
- 2002-04-05 CN CN2006101603317A patent/CN1975422B/zh not_active Expired - Lifetime
- 2002-04-05 JP JP2002582274A patent/JP4195815B2/ja not_active Expired - Lifetime
- 2002-04-05 WO PCT/GB2002/001636 patent/WO2002083937A2/fr active IP Right Grant
- 2002-04-05 EP EP08168182A patent/EP2037271B1/fr not_active Expired - Lifetime
- 2002-04-05 NZ NZ540410A patent/NZ540410A/en unknown
- 2002-04-05 AU AU2002251257A patent/AU2002251257B2/en not_active Ceased
- 2002-04-05 EP EP06111733A patent/EP1696234B1/fr not_active Expired - Lifetime
- 2002-04-05 IL IL15788002A patent/IL157880A0/xx not_active IP Right Cessation
- 2002-04-05 KR KR10-2003-7012899A patent/KR20040039194A/ko active IP Right Grant
- 2002-04-05 EP EP10075132.0A patent/EP2211177B1/fr not_active Expired - Lifetime
- 2002-04-05 DE DE60237792T patent/DE60237792D1/de not_active Expired - Lifetime
- 2002-04-05 DK DK02720187T patent/DK1410023T3/da active
- 2002-04-05 NZ NZ528594A patent/NZ528594A/en unknown
- 2002-04-05 DE DE60212642T patent/DE60212642T2/de not_active Expired - Lifetime
- 2002-04-05 CN CNB028081137A patent/CN1295510C/zh not_active Expired - Lifetime
- 2002-04-05 DE DE60230689T patent/DE60230689D1/de not_active Expired - Lifetime
- 2002-04-05 EP EP02720187A patent/EP1410023B1/fr not_active Expired - Lifetime
- 2002-04-05 AT AT02720187T patent/ATE331220T1/de not_active IP Right Cessation
- 2002-04-05 US US10/474,796 patent/US7939284B2/en active Active
- 2002-04-05 AT AT08168182T patent/ATE482395T1/de not_active IP Right Cessation
- 2002-04-05 AT AT06111733T patent/ATE419378T1/de not_active IP Right Cessation
- 2002-04-05 NZ NZ537939A patent/NZ537939A/en unknown
- 2002-04-05 CA CA002443570A patent/CA2443570A1/fr not_active Abandoned
-
2003
- 2003-09-25 ZA ZA200307442A patent/ZA200307442B/xx unknown
-
2005
- 2005-01-18 HK HK05100472A patent/HK1068406A1/xx not_active IP Right Cessation
-
2007
- 2007-12-20 JP JP2007328757A patent/JP4226053B2/ja not_active Expired - Fee Related
-
2011
- 2011-01-24 US US13/012,234 patent/US8367361B2/en not_active Expired - Lifetime
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2037271B1 (fr) | Procédés d'utilisation de O6-alkylguanine-ADN alkyltransférases | |
| AU2002251257A1 (en) | Methods of Using O6-alkylguanine-DNA Alkyltransferases | |
| US7666612B2 (en) | Methods for protein labeling based on acyl carrier protein | |
| JP2004532028A5 (fr) | ||
| Tanaka et al. | Site‐specific protein modification on living cells catalyzed by sortase | |
| ZA200502211B (en) | Protein labelling with 06-alkylguanine-dna alkyltransferase | |
| Chung et al. | Open flower fluoroimmunoassay: a general method to make fluorescent protein-based immunosensor probes |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20081103 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 1696234 Country of ref document: EP Kind code of ref document: P Ref document number: 1410023 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE TR |
|
| 17Q | First examination report despatched |
Effective date: 20090429 |
|
| AKX | Designation fees paid |
Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE TR |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 1410023 Country of ref document: EP Kind code of ref document: P Ref document number: 1696234 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP Ref country code: CH Ref legal event code: NV Representative=s name: KIRKER & CIE S.A. |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REF | Corresponds to: |
Ref document number: 60237792 Country of ref document: DE Date of ref document: 20101104 Kind code of ref document: P |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100922 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100922 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: VDEP Effective date: 20100922 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100922 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20101223 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100922 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110124 Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100922 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100922 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110102 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20110623 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100922 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 60237792 Country of ref document: DE Effective date: 20110623 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110430 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110405 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110405 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100922 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100922 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 15 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 16 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 17 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180430 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180430 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20210315 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20210428 Year of fee payment: 20 Ref country code: FR Payment date: 20210428 Year of fee payment: 20 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 60237792 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 Expiry date: 20220404 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20220404 |