EP1814974B1 - Wäschebehandlungsmittel - Google Patents
Wäschebehandlungsmittel Download PDFInfo
- Publication number
- EP1814974B1 EP1814974B1 EP05790693A EP05790693A EP1814974B1 EP 1814974 B1 EP1814974 B1 EP 1814974B1 EP 05790693 A EP05790693 A EP 05790693A EP 05790693 A EP05790693 A EP 05790693A EP 1814974 B1 EP1814974 B1 EP 1814974B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- dye
- group
- substituted
- acid
- granule
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Revoked
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 50
- 239000000975 dye Substances 0.000 claims abstract description 120
- 239000008187 granular material Substances 0.000 claims abstract description 89
- 239000002736 nonionic surfactant Substances 0.000 claims abstract description 31
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 16
- 229910021536 Zeolite Inorganic materials 0.000 claims description 15
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical group O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 claims description 15
- 230000002209 hydrophobic effect Effects 0.000 claims description 15
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 15
- 239000000985 reactive dye Substances 0.000 claims description 15
- 239000010457 zeolite Substances 0.000 claims description 15
- 239000000980 acid dye Substances 0.000 claims description 14
- -1 bis-azo direct violet Chemical compound 0.000 claims description 14
- 239000003599 detergent Substances 0.000 claims description 14
- 239000004744 fabric Substances 0.000 claims description 13
- 125000001273 sulfonato group Chemical group [O-]S(*)(=O)=O 0.000 claims description 12
- 239000000440 bentonite Substances 0.000 claims description 11
- 229910000278 bentonite Inorganic materials 0.000 claims description 11
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical group O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 claims description 11
- 239000000982 direct dye Substances 0.000 claims description 10
- 125000001624 naphthyl group Chemical group 0.000 claims description 10
- 125000003118 aryl group Chemical group 0.000 claims description 9
- 239000011230 binding agent Substances 0.000 claims description 9
- 239000004094 surface-active agent Substances 0.000 claims description 9
- AOMZHDJXSYHPKS-DROYEMJCSA-L Amido Black 10B Chemical compound [Na+].[Na+].[O-]S(=O)(=O)C1=CC2=CC(S([O-])(=O)=O)=C(\N=N\C=3C=CC=CC=3)C(O)=C2C(N)=C1\N=N\C1=CC=C(N(=O)=O)C=C1 AOMZHDJXSYHPKS-DROYEMJCSA-L 0.000 claims description 8
- 239000004927 clay Substances 0.000 claims description 8
- 239000007787 solid Substances 0.000 claims description 8
- SJEYSFABYSGQBG-UHFFFAOYSA-M Patent blue Chemical compound [Na+].C1=CC(N(CC)CC)=CC=C1C(C=1C(=CC(=CC=1)S([O-])(=O)=O)S([O-])(=O)=O)=C1C=CC(=[N+](CC)CC)C=C1 SJEYSFABYSGQBG-UHFFFAOYSA-M 0.000 claims description 7
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 7
- 238000010521 absorption reaction Methods 0.000 claims description 6
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 6
- 230000000007 visual effect Effects 0.000 claims description 6
- LJFWQNJLLOFIJK-UHFFFAOYSA-N solvent violet 13 Chemical compound C1=CC(C)=CC=C1NC1=CC=C(O)C2=C1C(=O)C1=CC=CC=C1C2=O LJFWQNJLLOFIJK-UHFFFAOYSA-N 0.000 claims description 5
- 239000004753 textile Substances 0.000 claims description 5
- 125000003545 alkoxy group Chemical group 0.000 claims description 4
- 125000000217 alkyl group Chemical group 0.000 claims description 4
- 125000004104 aryloxy group Chemical group 0.000 claims description 4
- 239000007844 bleaching agent Substances 0.000 claims description 4
- 150000007942 carboxylates Chemical group 0.000 claims description 4
- 239000000986 disperse dye Substances 0.000 claims description 4
- 238000000034 method Methods 0.000 claims description 4
- 239000000992 solvent dye Substances 0.000 claims description 4
- VRVDFJOCCWSFLI-UHFFFAOYSA-K trisodium 3-[[4-[(6-anilino-1-hydroxy-3-sulfonatonaphthalen-2-yl)diazenyl]-5-methoxy-2-methylphenyl]diazenyl]naphthalene-1,5-disulfonate Chemical compound [Na+].[Na+].[Na+].COc1cc(N=Nc2cc(c3cccc(c3c2)S([O-])(=O)=O)S([O-])(=O)=O)c(C)cc1N=Nc1c(O)c2ccc(Nc3ccccc3)cc2cc1S([O-])(=O)=O VRVDFJOCCWSFLI-UHFFFAOYSA-K 0.000 claims description 4
- 239000001045 blue dye Substances 0.000 claims description 3
- LHRXTFDXJQAGAV-UHFFFAOYSA-L disodium 3-hydroxy-4-(naphthalen-1-yldiazenyl)naphthalene-2,7-disulfonate Chemical compound [Na+].[Na+].Oc1c(cc2cc(ccc2c1N=Nc1cccc2ccccc12)S([O-])(=O)=O)S([O-])(=O)=O LHRXTFDXJQAGAV-UHFFFAOYSA-L 0.000 claims description 3
- IINNWAYUJNWZRM-UHFFFAOYSA-L erythrosin B Chemical compound [Na+].[Na+].[O-]C(=O)C1=CC=CC=C1C1=C2C=C(I)C(=O)C(I)=C2OC2=C(I)C([O-])=C(I)C=C21 IINNWAYUJNWZRM-UHFFFAOYSA-L 0.000 claims description 3
- LGZQSRCLLIPAEE-UHFFFAOYSA-M sodium 1-[(4-sulfonaphthalen-1-yl)diazenyl]naphthalen-2-olate Chemical compound [Na+].C1=CC=C2C(N=NC3=C4C=CC=CC4=CC=C3O)=CC=C(S([O-])(=O)=O)C2=C1 LGZQSRCLLIPAEE-UHFFFAOYSA-M 0.000 claims description 3
- PEAGNRWWSMMRPZ-UHFFFAOYSA-L woodstain scarlet Chemical compound [Na+].[Na+].OC1=CC=C2C=C(S([O-])(=O)=O)C=C(S([O-])(=O)=O)C2=C1N=NC(C=C1)=CC=C1N=NC1=CC=CC=C1 PEAGNRWWSMMRPZ-UHFFFAOYSA-L 0.000 claims description 3
- JSRUDOBCTLPTFO-UHFFFAOYSA-N 2-[5-acetamido-n-(2-acetyloxyethyl)-4-[(2-bromo-4,6-dinitrophenyl)diazenyl]-2-methoxyanilino]ethyl acetate Chemical compound C1=C(N(CCOC(C)=O)CCOC(C)=O)C(OC)=CC(N=NC=2C(=CC(=CC=2Br)[N+]([O-])=O)[N+]([O-])=O)=C1NC(C)=O JSRUDOBCTLPTFO-UHFFFAOYSA-N 0.000 claims description 2
- 239000002202 Polyethylene glycol Substances 0.000 claims description 2
- 239000002253 acid Substances 0.000 claims description 2
- QCWPZYSLMIXIHM-UHFFFAOYSA-L disodium 4-amino-5-hydroxy-3-[(3-nitrophenyl)diazenyl]-6-phenyldiazenylnaphthalene-2,7-disulfonate Chemical compound [Na+].[Na+].Nc1c(N=Nc2cccc(c2)[N+]([O-])=O)c(cc2cc(c(N=Nc3ccccc3)c(O)c12)S([O-])(=O)=O)S([O-])(=O)=O QCWPZYSLMIXIHM-UHFFFAOYSA-L 0.000 claims description 2
- XPRMZBUQQMPKCR-UHFFFAOYSA-L disodium;8-anilino-5-[[4-[(3-sulfonatophenyl)diazenyl]naphthalen-1-yl]diazenyl]naphthalene-1-sulfonate Chemical compound [Na+].[Na+].[O-]S(=O)(=O)C1=CC=CC(N=NC=2C3=CC=CC=C3C(N=NC=3C4=CC=CC(=C4C(NC=4C=CC=CC=4)=CC=3)S([O-])(=O)=O)=CC=2)=C1 XPRMZBUQQMPKCR-UHFFFAOYSA-L 0.000 claims description 2
- SEACYXSIPDVVMV-UHFFFAOYSA-L eosin Y Chemical compound [Na+].[Na+].[O-]C(=O)C1=CC=CC=C1C1=C2C=C(Br)C(=O)C(Br)=C2OC2=C(Br)C([O-])=C(Br)C=C21 SEACYXSIPDVVMV-UHFFFAOYSA-L 0.000 claims description 2
- QGAYMQGSQUXCQO-UHFFFAOYSA-L eosin b Chemical compound [Na+].[Na+].O1C(=O)C2=CC=CC=C2C21C1=CC([N+]([O-])=O)=C([O-])C(Br)=C1OC1=C2C=C([N+]([O-])=O)C([O-])=C1Br QGAYMQGSQUXCQO-UHFFFAOYSA-L 0.000 claims description 2
- 238000005469 granulation Methods 0.000 claims description 2
- 230000003179 granulation Effects 0.000 claims description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 2
- 229910052500 inorganic mineral Inorganic materials 0.000 claims description 2
- 239000011707 mineral Substances 0.000 claims description 2
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 2
- 229920001223 polyethylene glycol Polymers 0.000 claims description 2
- 239000011369 resultant mixture Substances 0.000 claims description 2
- 229960003138 rose bengal sodium Drugs 0.000 claims description 2
- FJBHGWADYLMEJG-UHFFFAOYSA-M sodium;3-[[4-[[4-(diethylamino)phenyl]-[4-[ethyl-[(3-sulfonatophenyl)methyl]azaniumylidene]cyclohexa-2,5-dien-1-ylidene]methyl]-n-ethylanilino]methyl]benzenesulfonate Chemical compound [Na+].C1=CC(N(CC)CC)=CC=C1C(C=1C=CC(=CC=1)N(CC)CC=1C=C(C=CC=1)S([O-])(=O)=O)=C(C=C1)C=CC1=[N+](CC)CC1=CC=CC(S([O-])(=O)=O)=C1 FJBHGWADYLMEJG-UHFFFAOYSA-M 0.000 claims description 2
- 229920000058 polyacrylate Polymers 0.000 claims 2
- OCQDPIXQTSYZJL-UHFFFAOYSA-N 1,4-bis(butylamino)anthracene-9,10-dione Chemical compound O=C1C2=CC=CC=C2C(=O)C2=C1C(NCCCC)=CC=C2NCCCC OCQDPIXQTSYZJL-UHFFFAOYSA-N 0.000 claims 1
- JUUJTYPMICHIEM-UHFFFAOYSA-N 1,4-bis(ethylamino)anthracene-9,10-dione Chemical compound O=C1C2=CC=CC=C2C(=O)C2=C1C(NCC)=CC=C2NCC JUUJTYPMICHIEM-UHFFFAOYSA-N 0.000 claims 1
- NLXFWUZKOOWWFD-UHFFFAOYSA-N 1-(2-hydroxyethylamino)-4-(methylamino)anthracene-9,10-dione Chemical compound O=C1C2=CC=CC=C2C(=O)C2=C1C(NCCO)=CC=C2NC NLXFWUZKOOWWFD-UHFFFAOYSA-N 0.000 claims 1
- UIHYHADQHHUIOF-UHFFFAOYSA-N 2-[n-ethyl-3-methyl-4-[(5-nitro-1,3-thiazol-2-yl)diazenyl]anilino]ethanol Chemical compound CC1=CC(N(CCO)CC)=CC=C1N=NC1=NC=C([N+]([O-])=O)S1 UIHYHADQHHUIOF-UHFFFAOYSA-N 0.000 claims 1
- FOQABOMYTOFLPZ-ISLYRVAYSA-N Disperse Red 1 Chemical compound C1=CC(N(CCO)CC)=CC=C1\N=N\C1=CC=C([N+]([O-])=O)C=C1 FOQABOMYTOFLPZ-ISLYRVAYSA-N 0.000 claims 1
- YCUVUDODLRLVIC-UHFFFAOYSA-N Sudan black B Chemical compound C1=CC(=C23)NC(C)(C)NC2=CC=CC3=C1N=NC(C1=CC=CC=C11)=CC=C1N=NC1=CC=CC=C1 YCUVUDODLRLVIC-UHFFFAOYSA-N 0.000 claims 1
- 229910052570 clay Inorganic materials 0.000 claims 1
- 229920001577 copolymer Polymers 0.000 claims 1
- OOYIOIOOWUGAHD-UHFFFAOYSA-L disodium;2',4',5',7'-tetrabromo-4,5,6,7-tetrachloro-3-oxospiro[2-benzofuran-1,9'-xanthene]-3',6'-diolate Chemical compound [Na+].[Na+].O1C(=O)C(C(=C(Cl)C(Cl)=C2Cl)Cl)=C2C21C1=CC(Br)=C([O-])C(Br)=C1OC1=C(Br)C([O-])=C(Br)C=C21 OOYIOIOOWUGAHD-UHFFFAOYSA-L 0.000 claims 1
- 229940117964 disperse blue 106 Drugs 0.000 claims 1
- AZJPTIGZZTZIDR-UHFFFAOYSA-L rose bengal Chemical compound [K+].[K+].[O-]C(=O)C1=C(Cl)C(Cl)=C(Cl)C(Cl)=C1C1=C2C=C(I)C(=O)C(I)=C2OC2=C(I)C([O-])=C(I)C=C21 AZJPTIGZZTZIDR-UHFFFAOYSA-L 0.000 claims 1
- RCTGMCJBQGBLKT-PAMTUDGESA-N scarlet red Chemical compound CC1=CC=CC=C1\N=N\C(C=C1C)=CC=C1\N=N\C1=C(O)C=CC2=CC=CC=C12 RCTGMCJBQGBLKT-PAMTUDGESA-N 0.000 claims 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 claims 1
- 239000000843 powder Substances 0.000 description 29
- 239000000243 solution Substances 0.000 description 13
- 229920000742 Cotton Polymers 0.000 description 12
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 8
- 229920000728 polyester Polymers 0.000 description 8
- 239000003795 chemical substances by application Substances 0.000 description 7
- 235000002639 sodium chloride Nutrition 0.000 description 6
- 238000005406 washing Methods 0.000 description 6
- RZVAJINKPMORJF-UHFFFAOYSA-N Acetaminophen Chemical compound CC(=O)NC1=CC=C(O)C=C1 RZVAJINKPMORJF-UHFFFAOYSA-N 0.000 description 5
- 238000002474 experimental method Methods 0.000 description 5
- 102000004190 Enzymes Human genes 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical group C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 4
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 150000001298 alcohols Chemical class 0.000 description 4
- 238000004873 anchoring Methods 0.000 description 4
- PYKYMHQGRFAEBM-UHFFFAOYSA-N anthraquinone Natural products CCC(=O)c1c(O)c2C(=O)C3C(C=CC=C3O)C(=O)c2cc1CC(=O)OC PYKYMHQGRFAEBM-UHFFFAOYSA-N 0.000 description 4
- 150000004056 anthraquinones Chemical class 0.000 description 4
- 239000002585 base Substances 0.000 description 4
- 239000000969 carrier Substances 0.000 description 4
- 235000019441 ethanol Nutrition 0.000 description 4
- 239000000835 fiber Substances 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 235000017550 sodium carbonate Nutrition 0.000 description 4
- 229910000029 sodium carbonate Inorganic materials 0.000 description 4
- QXNVGIXVLWOKEQ-UHFFFAOYSA-N Disodium Chemical compound [Na][Na] QXNVGIXVLWOKEQ-UHFFFAOYSA-N 0.000 description 3
- 206010070834 Sensitisation Diseases 0.000 description 3
- 238000005054 agglomeration Methods 0.000 description 3
- 230000002776 aggregation Effects 0.000 description 3
- 230000008021 deposition Effects 0.000 description 3
- 239000007850 fluorescent dye Substances 0.000 description 3
- 230000007794 irritation Effects 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- FBMQNRKSAWNXBT-UHFFFAOYSA-N 1,4-diaminoanthracene-9,10-dione Chemical compound O=C1C2=CC=CC=C2C(=O)C2=C1C(N)=CC=C2N FBMQNRKSAWNXBT-UHFFFAOYSA-N 0.000 description 2
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 2
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 2
- MJVAVZPDRWSRRC-UHFFFAOYSA-N Menadione Chemical compound C1=CC=C2C(=O)C(C)=CC(=O)C2=C1 MJVAVZPDRWSRRC-UHFFFAOYSA-N 0.000 description 2
- BPQQTUXANYXVAA-UHFFFAOYSA-N Orthosilicate Chemical compound [O-][Si]([O-])([O-])[O-] BPQQTUXANYXVAA-UHFFFAOYSA-N 0.000 description 2
- 229920002125 Sokalan® Polymers 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 2
- LIKZXCROQGHXTI-UHFFFAOYSA-M acid blue 25 Chemical compound [Na+].C1=2C(=O)C3=CC=CC=C3C(=O)C=2C(N)=C(S([O-])(=O)=O)C=C1NC1=CC=CC=C1 LIKZXCROQGHXTI-UHFFFAOYSA-M 0.000 description 2
- 239000002518 antifoaming agent Substances 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000008033 biological extinction Effects 0.000 description 2
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical compound C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 2
- 125000001072 heteroaryl group Chemical group 0.000 description 2
- 239000012535 impurity Substances 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- IEQIEDJGQAUEQZ-UHFFFAOYSA-N phthalocyanine Chemical compound N1C(N=C2C3=CC=CC=C3C(N=C3C4=CC=CC=C4C(=N4)N3)=N2)=C(C=CC=C2)C2=C1N=C1C2=CC=CC=C2C4=N1 IEQIEDJGQAUEQZ-UHFFFAOYSA-N 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 125000001453 quaternary ammonium group Chemical group 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 229910052938 sodium sulfate Inorganic materials 0.000 description 2
- 235000011152 sodium sulphate Nutrition 0.000 description 2
- 235000019832 sodium triphosphate Nutrition 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 229910021653 sulphate ion Inorganic materials 0.000 description 2
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 1
- 0 *N=N[C@]1c(cccc2)c2C(N=Nc(c2ccc3)cccc2c3NN=C(C=Cc2ccccc22)C2=O)=CC1 Chemical compound *N=N[C@]1c(cccc2)c2C(N=Nc(c2ccc3)cccc2c3NN=C(C=Cc2ccccc22)C2=O)=CC1 0.000 description 1
- BLFZMXOCPASACY-UHFFFAOYSA-N 1,4-bis(propan-2-ylamino)anthracene-9,10-dione Chemical compound O=C1C2=CC=CC=C2C(=O)C2=C1C(NC(C)C)=CC=C2NC(C)C BLFZMXOCPASACY-UHFFFAOYSA-N 0.000 description 1
- ZMLPKJYZRQZLDA-UHFFFAOYSA-N 1-(2-phenylethenyl)-4-[4-(2-phenylethenyl)phenyl]benzene Chemical group C=1C=CC=CC=1C=CC(C=C1)=CC=C1C(C=C1)=CC=C1C=CC1=CC=CC=C1 ZMLPKJYZRQZLDA-UHFFFAOYSA-N 0.000 description 1
- ITYXXSSJBOAGAR-UHFFFAOYSA-N 1-(methylamino)-4-(4-methylanilino)anthracene-9,10-dione Chemical compound C1=2C(=O)C3=CC=CC=C3C(=O)C=2C(NC)=CC=C1NC1=CC=C(C)C=C1 ITYXXSSJBOAGAR-UHFFFAOYSA-N 0.000 description 1
- ZNQIAQXHADXXQI-UHFFFAOYSA-N 1-anilino-4-hydroxyanthracene-9,10-dione Chemical compound C1=2C(=O)C3=CC=CC=C3C(=O)C=2C(O)=CC=C1NC1=CC=CC=C1 ZNQIAQXHADXXQI-UHFFFAOYSA-N 0.000 description 1
- HNUQMTZUNUBOLQ-UHFFFAOYSA-N 2-[2-[2-[2-[2-[2-[2-[2-[2-(2-octadecoxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethanol Chemical compound CCCCCCCCCCCCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO HNUQMTZUNUBOLQ-UHFFFAOYSA-N 0.000 description 1
- AMPCGOAFZFKBGH-UHFFFAOYSA-N 4-[[4-(dimethylamino)phenyl]-(4-methyliminocyclohexa-2,5-dien-1-ylidene)methyl]-n,n-dimethylaniline Chemical compound C1=CC(=NC)C=CC1=C(C=1C=CC(=CC=1)N(C)C)C1=CC=C(N(C)C)C=C1 AMPCGOAFZFKBGH-UHFFFAOYSA-N 0.000 description 1
- YGUMVDWOQQJBGA-VAWYXSNFSA-N 5-[(4-anilino-6-morpholin-4-yl-1,3,5-triazin-2-yl)amino]-2-[(e)-2-[4-[(4-anilino-6-morpholin-4-yl-1,3,5-triazin-2-yl)amino]-2-sulfophenyl]ethenyl]benzenesulfonic acid Chemical compound C=1C=C(\C=C\C=2C(=CC(NC=3N=C(N=C(NC=4C=CC=CC=4)N=3)N3CCOCC3)=CC=2)S(O)(=O)=O)C(S(=O)(=O)O)=CC=1NC(N=C(N=1)N2CCOCC2)=NC=1NC1=CC=CC=C1 YGUMVDWOQQJBGA-VAWYXSNFSA-N 0.000 description 1
- QYOVMAREBTZLBT-KTKRTIGZSA-N CCCCCCCC\C=C/CCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO Chemical compound CCCCCCCC\C=C/CCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO QYOVMAREBTZLBT-KTKRTIGZSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- JSFUMBWFPQSADC-UHFFFAOYSA-N Disperse Blue 1 Chemical compound O=C1C2=C(N)C=CC(N)=C2C(=O)C2=C1C(N)=CC=C2N JSFUMBWFPQSADC-UHFFFAOYSA-N 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical class CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 231100000766 Possible carcinogen Toxicity 0.000 description 1
- BGRWYDHXPHLNKA-UHFFFAOYSA-N Tetraacetylethylenediamine Chemical compound CC(=O)N(C(C)=O)CCN(C(C)=O)C(C)=O BGRWYDHXPHLNKA-UHFFFAOYSA-N 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 125000003158 alcohol group Polymers 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 239000003945 anionic surfactant Substances 0.000 description 1
- 239000001000 anthraquinone dye Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 238000004380 ashing Methods 0.000 description 1
- 239000000987 azo dye Substances 0.000 description 1
- 125000000751 azo group Chemical group [*]N=N[*] 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- 239000004305 biphenyl Substances 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 239000012876 carrier material Substances 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 239000003093 cationic surfactant Substances 0.000 description 1
- 238000004040 coloring Methods 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 230000003750 conditioning effect Effects 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- XWZDJOJCYUSIEY-UHFFFAOYSA-L disodium 5-[(4,6-dichloro-1,3,5-triazin-2-yl)amino]-4-hydroxy-3-phenyldiazenylnaphthalene-2,7-disulfonate Chemical compound [Na+].[Na+].Oc1c(N=Nc2ccccc2)c(cc2cc(cc(Nc3nc(Cl)nc(Cl)n3)c12)S([O-])(=O)=O)S([O-])(=O)=O XWZDJOJCYUSIEY-UHFFFAOYSA-L 0.000 description 1
- LARMRMCFZNGNNX-UHFFFAOYSA-L disodium 7-anilino-3-[[4-[(2,4-dimethyl-6-sulfonatophenyl)diazenyl]-2-methoxy-5-methylphenyl]diazenyl]-4-hydroxynaphthalene-2-sulfonate Chemical compound [Na+].[Na+].COc1cc(N=Nc2c(C)cc(C)cc2S([O-])(=O)=O)c(C)cc1N=Nc1c(O)c2ccc(Nc3ccccc3)cc2cc1S([O-])(=O)=O LARMRMCFZNGNNX-UHFFFAOYSA-L 0.000 description 1
- UWBXIFCTIZXXLS-UHFFFAOYSA-L disodium;2,3,4,5-tetrachloro-6-(2,4,5,7-tetraiodo-3-oxido-6-oxoxanthen-9-yl)benzoate Chemical compound [Na+].[Na+].[O-]C(=O)C1=C(Cl)C(Cl)=C(Cl)C(Cl)=C1C1=C2C=C(I)C(=O)C(I)=C2OC2=C(I)C([O-])=C(I)C=C21 UWBXIFCTIZXXLS-UHFFFAOYSA-L 0.000 description 1
- VUJGKADZTYCLIL-YHPRVSEPSA-L disodium;5-[(4-anilino-6-morpholin-4-yl-1,3,5-triazin-2-yl)amino]-2-[(e)-2-[4-[(4-anilino-6-morpholin-4-yl-1,3,5-triazin-2-yl)amino]-2-sulfonatophenyl]ethenyl]benzenesulfonate Chemical compound [Na+].[Na+].C=1C=C(\C=C\C=2C(=CC(NC=3N=C(N=C(NC=4C=CC=CC=4)N=3)N3CCOCC3)=CC=2)S([O-])(=O)=O)C(S(=O)(=O)[O-])=CC=1NC(N=C(N=1)N2CCOCC2)=NC=1NC1=CC=CC=C1 VUJGKADZTYCLIL-YHPRVSEPSA-L 0.000 description 1
- VTIIJXUACCWYHX-UHFFFAOYSA-L disodium;carboxylatooxy carbonate Chemical compound [Na+].[Na+].[O-]C(=O)OOC([O-])=O VTIIJXUACCWYHX-UHFFFAOYSA-L 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000004043 dyeing Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 230000031700 light absorption Effects 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- VMGAPWLDMVPYIA-HIDZBRGKSA-N n'-amino-n-iminomethanimidamide Chemical compound N\N=C\N=N VMGAPWLDMVPYIA-HIDZBRGKSA-N 0.000 description 1
- QRKGKRSGMAWUMO-UHFFFAOYSA-N n-[2-[(2-bromo-4,6-dinitrophenyl)diazenyl]-5-(diethylamino)-4-methoxyphenyl]acetamide Chemical compound C1=C(OC)C(N(CC)CC)=CC(NC(C)=O)=C1N=NC1=C(Br)C=C([N+]([O-])=O)C=C1[N+]([O-])=O QRKGKRSGMAWUMO-UHFFFAOYSA-N 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 239000011236 particulate material Substances 0.000 description 1
- 239000002304 perfume Substances 0.000 description 1
- GVKCHTBDSMQENH-UHFFFAOYSA-L phloxine B Chemical compound [Na+].[Na+].[O-]C(=O)C1=C(Cl)C(Cl)=C(Cl)C(Cl)=C1C1=C2C=C(Br)C(=O)C(Br)=C2OC2=C(Br)C([O-])=C(Br)C=C21 GVKCHTBDSMQENH-UHFFFAOYSA-L 0.000 description 1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 1
- 150000003219 pyrazolines Chemical class 0.000 description 1
- 238000006862 quantum yield reaction Methods 0.000 description 1
- KUIXZSYWBHSYCN-UHFFFAOYSA-L remazol brilliant blue r Chemical compound [Na+].[Na+].C1=C(S([O-])(=O)=O)C(N)=C2C(=O)C3=CC=CC=C3C(=O)C2=C1NC1=CC=CC(S(=O)(=O)CCOS([O-])(=O)=O)=C1 KUIXZSYWBHSYCN-UHFFFAOYSA-L 0.000 description 1
- HFIYIRIMGZMCPC-YOLJWEMLSA-J remazole black-GR Chemical compound [Na+].[Na+].[Na+].[Na+].[O-]S(=O)(=O)C1=CC2=CC(S([O-])(=O)=O)=C(\N=N\C=3C=CC(=CC=3)S(=O)(=O)CCOS([O-])(=O)=O)C(O)=C2C(N)=C1\N=N\C1=CC=C(S(=O)(=O)CCOS([O-])(=O)=O)C=C1 HFIYIRIMGZMCPC-YOLJWEMLSA-J 0.000 description 1
- BOLDJAUMGUJJKM-LSDHHAIUSA-N renifolin D Natural products CC(=C)[C@@H]1Cc2c(O)c(O)ccc2[C@H]1CC(=O)c3ccc(O)cc3O BOLDJAUMGUJJKM-LSDHHAIUSA-N 0.000 description 1
- 210000002345 respiratory system Anatomy 0.000 description 1
- 239000000344 soap Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 235000011083 sodium citrates Nutrition 0.000 description 1
- 229940045872 sodium percarbonate Drugs 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- AXMCIYLNKNGNOT-UHFFFAOYSA-N sodium;3-[[4-[(4-dimethylazaniumylidenecyclohexa-2,5-dien-1-ylidene)-[4-[ethyl-[(3-sulfophenyl)methyl]amino]phenyl]methyl]-n-ethylanilino]methyl]benzenesulfonate Chemical compound [Na+].C=1C=C(C(=C2C=CC(C=C2)=[N+](C)C)C=2C=CC(=CC=2)N(CC)CC=2C=C(C=CC=2)S([O-])(=O)=O)C=CC=1N(CC)CC1=CC=CC(S(O)(=O)=O)=C1 AXMCIYLNKNGNOT-UHFFFAOYSA-N 0.000 description 1
- MWNQXXOSWHCCOZ-UHFFFAOYSA-L sodium;oxido carbonate Chemical compound [Na+].[O-]OC([O-])=O MWNQXXOSWHCCOZ-UHFFFAOYSA-L 0.000 description 1
- 239000002689 soil Substances 0.000 description 1
- 230000003381 solubilizing effect Effects 0.000 description 1
- 239000002195 soluble material Substances 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- BDHFUVZGWQCTTF-UHFFFAOYSA-N sulfonic acid Chemical compound OS(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-N 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 231100000041 toxicology testing Toxicity 0.000 description 1
- 229910052723 transition metal Inorganic materials 0.000 description 1
- 150000003624 transition metals Chemical class 0.000 description 1
- AAAQKTZKLRYKHR-UHFFFAOYSA-N triphenylmethane Chemical compound C1=CC=CC=C1C(C=1C=CC=CC=1)C1=CC=CC=C1 AAAQKTZKLRYKHR-UHFFFAOYSA-N 0.000 description 1
- GPRLSGONYQIRFK-MNYXATJNSA-N triton Chemical compound [3H+] GPRLSGONYQIRFK-MNYXATJNSA-N 0.000 description 1
- 235000012711 vitamin K3 Nutrition 0.000 description 1
- 239000011652 vitamin K3 Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/40—Dyes ; Pigments
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D17/00—Detergent materials or soaps characterised by their shape or physical properties
- C11D17/0034—Fixed on a solid conventional detergent ingredient
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/02—Inorganic compounds ; Elemental compounds
- C11D3/12—Water-insoluble compounds
- C11D3/124—Silicon containing, e.g. silica, silex, quartz or glass beads
- C11D3/1246—Silicates, e.g. diatomaceous earth
- C11D3/1253—Layer silicates, e.g. talcum, kaolin, clay, bentonite, smectite, montmorillonite, hectorite or attapulgite
- C11D3/126—Layer silicates, e.g. talcum, kaolin, clay, bentonite, smectite, montmorillonite, hectorite or attapulgite in solid compositions
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/02—Inorganic compounds ; Elemental compounds
- C11D3/12—Water-insoluble compounds
- C11D3/124—Silicon containing, e.g. silica, silex, quartz or glass beads
- C11D3/1246—Silicates, e.g. diatomaceous earth
- C11D3/128—Aluminium silicates, e.g. zeolites
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/37—Polymers
- C11D3/3746—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- C11D3/3757—(Co)polymerised carboxylic acids, -anhydrides, -esters in solid and liquid compositions
- C11D3/3761—(Co)polymerised carboxylic acids, -anhydrides, -esters in solid and liquid compositions in solid compositions
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/66—Non-ionic compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/66—Non-ionic compounds
- C11D1/72—Ethers of polyoxyalkylene glycols
Definitions
- the present invention relates to laundry treatment compositions that comprise a dye.
- WO02/10327 discloses the use of sodium chloride to reduce staining of fabrics.
- US 3 748 093 discloses a spray dried granule compostion for increasing the apparent whiteness of laundry to a human eye.
- Dyes are used in detergent powders in order to provide colouring of the powder or shading benefits to white fabrics.
- One drawback with these powders is that under certain conditions localised spotting occurs on fabric treated with the detergent powder.
- non-ionic surfactants may be applied to non-ionic surfactant soluble dyes in order to reduce and/or prevent undesired spotting of fabrics by the dye under wash conditions.
- the present invention is applicable to dyes that are substantive to fabrics.
- the present invention provides a granule comprising:
- the granule comprises between 10 to 2.5 wt% of a non-ionic surfactant
- the binder is present in the range 2 to 10 wt%.
- the amount of dye dissolved in the non-ionic surfactant is in the range between 0.1 to 2 wt%.
- the dye has a solubility in the non-ionic surfactant of at least 0.1 wt%, more preferably 1 wt %, and even more preferably at least 5 wt%.
- the solubility of the dye referred to herein is that to be measured at 25 °C.
- the dye has a visual effect on the human eye as a single dye having a peak absorption wavelength on a textile of from 550nm to 650nm.
- the most preferred is a dye or a mixture thereof that have the visual appearance as blue or violet.
- the dyes are those substantive to a fabric, in particular cotton and polyester.
- the present invention provides a laundry composition comprising a granule as defined herein and a method of treating a textile.
- the present invention provides a method of granulation comprising the steps of:
- step (ii) and/or step (iii) it is preferred that a binding agent, other than the non-ionic surfactant, is used.
- a "unit dose” as used herein is a particular amount of the laundry treatment composition used for a type of wash, conditioning or requisite treatment step.
- the unit dose may be in the form of a defined volume of powder, granules or tablet or unit dose detergent liquid.
- the dye or mixture of dyes used in the granule of the present invention need to have a solubility % in the non-ionic surfactant, or mixture thereof, of at least 0.1 wt%.
- the dye individually or as a mixture of dyes preferably have the visual effect on the human eye as a single dye having a peak absorption wavelength on a textile of from 550nm to 650nm, most preferably from 570nm to 630nm. This visual effect provides the aesthetic appearance of blue to violet-blue which in turn the consumer perceives as whiteness.
- Preferred dyes for shading polyester are hydrophobic dyes and preferred dyes for shading cotton are: hydrolysed reactive dyes; acid dyes; and direct dyes.
- the dyes found below may be used individually or in mixture with the present invention and are provided, as example, but are preferred dyes.
- Hydrophobic dyes are defined as organic compounds with a maximum extinction coefficient greater than 1000 L/mol/cm in the wavelength range of 400 to 750 nm and that are uncharged in aqueous solution at a pH in the range from 7 to 11.
- the hydrophobic dyes are devoid of polar solubilizing groups. In particular the hydrophobic dye does not contain any sulphonic acid, carboxylic acid, or quaternary ammonium groups.
- the dye chromophore is preferably selected from the group comprising: azo; anthraquinone; phthalocyanine; benzodifuranes; quinophthalones; azothiophenes; azobenzothioazoles and, triphenylmethane chromophores. Most preferred are azo and anthraquinone dye chromophores.
- hydrophobic dyes are found in the classes of solvent and disperse dyes.
- Shading of white garments may be done with any colour depending on consumer preference. Blue and Violet are particularly preferred shades and consequently preferred dyes or mixtures of dyes are ones that give a blue or violet shade on white.
- suitable solvent and disperse dyes are available. However detailed toxicological studies have shown that a number of such dyes are possible carcinogens, for example disperse blue 1. Such dyes are not preferred. More suitable dyes may be selected from those solvent and disperse dyes used in cosmetics. For example as listed by the European Union in directive 76/768/EEC Annex IV part 1. For example disperse violet 27 and solvent violet 13.
- Preferred azo hydrophobic dyes for use in the present invention are: Disperse blue 10, 11, 12, 21, 30, 33, 36, 38, 42, 43, 44, 47,79, 79:1, 79:2, 79:3, 82, 85, 88, 90, 94, 96, 100, 101, 102, 106, 106:1, 121, 122, 124, 125, 128, 130, 133, 137, 138, 139, 142, 146, 148, 149, 165, 165:1, 165:2, 165:3, 171, 173, 174, 175, 177, 183, 187, 189, 193, 194, 200, 201, 202, 205, 206, 207, 209, 210, 211, 212, 219, 220, 222, 224, 225, 248, 252, 253, 254, 255, 256, 257, 258, 259, 260, 264, 265, 266, 267, 268, 269, 270, 278, 2
- Preferred anthraquinone hydrophobic dyes for use in the present invention are: Solvent Violet 11, 13, 14, 15, 15, 26, 28, 29, 30, 31, 32, 33, 34, 26, 37, 33, 40, 41, 42, 45, 48, 59; Solvent Blue 11, 12, 13, 14, 15, 17, 18, 19, 20, 21, 22, 35, 36, 40, 41, 45, 59, 59:1, 63, 65, 68, 69, 78, 90; Disperse Violet 1, 4, 8, 11, 11:1, 14, 15, 17, 22, 26, 27, 28, 29, 34, 35, 36, 38, 41, 44, 46, 47, 51, 56, 57, 59, 60, 61, 62, 64, 65, 67, 68, 70, 71, 72, 78, 79, 81, 83, 84, 85, 87, 89, 105; Disperse Blue 2, 3, 3:2, 8, 9, 13, 13:1, 14, 16, 17, 18, 19, 22, 23, 24, 26, 27.
- Non-azo hydrophobic dyes for use in the present invention are: Disperse Blue 250, 354, 364, 366, Solvent Violet 8, solvent blue 43,solvent blue 57, Lumogen F Blau 650, and Lumogen F Violet 570.
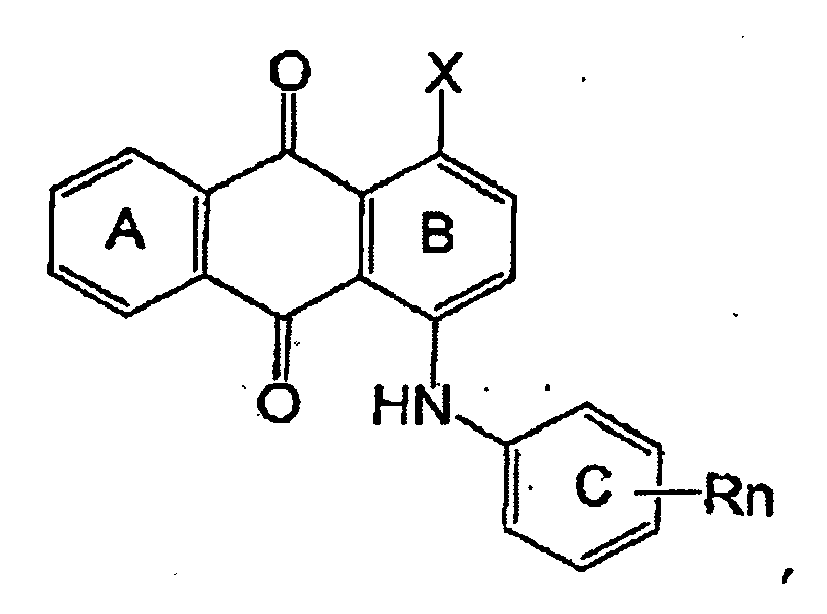
- the reactive dyes may be considered to be made up of a chromophore which is linked to an anchoring moiety,
- the chromophore may be linked directly to the anchor or via a bridging group.
- the chromophore serves to provide a colour and the anchor to bind to a textile substrate.
- a marked advantage of reactive dyes over direct dyes is that their chemical structure is much simpler, their absorption bands are narrower and the dyeing/shading are brighter; industrial Dyes, K. Hunger ed. Wiley-VCH 2003 ISBN 3-527-30426-6 .
- mammalian contact with reactive dyes results in irritation and/or sensitisation of the respiratory tract and/or skin.
- wash conditions are not ideal for deposition of dyes because the efficiency of deposition is low.
- each individual anchor group of each reactive dyes is hydrolysed such that the most reactive group(s) of anchor groups of the dye is/are hydrolysed.
- hydrolysed reactive dye encompasses both fully and partially hydrolysed reactive dyes.
- the reactive dye may have more than one anchor. If the dye has more than one anchor, then each and every anchor, that contributes to irritation and/or sensitisation, needs to be hydrolysed to the extent discussed above.
- the hydrolysed dyes comprise a chromophore and an anchor that are covalently bound and may be represented in the following manner: Chromophore-anchor.
- the hydrolysed reactive dye comprises a chromophore moiety covalently bound to an anchoring group, the anchoring group for binding to cotton, the anchoring group selected from the group consisting of: a heteroaromatic ring, preferably comprising a nitrogen heteroatom, having at least one -OH substituent covalently bound to the heteroaromatic ring, and
- anchor group is of the form: wherein:
- the chromophore is selected from the group consisting of: azo, anthraquinone, phthalocyanine, formazan and triphendioaxazine.
- hydrolysed reactive dyes are hydrolysed Reactive Red 2, hydrolysed Reactive Blue 4, hydrolysed Reactive Black 5, and hydrolysed Reactive Blue 19.
- dyes examples are direct violet 5, 9, 11, 31, and 51. Further examples of these dyes are also direct blue 34, 70, 71, 72, 75, 78, 82, and 120. Preferably the dye is direct violet 9.
- the granules may comprise different dyes or a mixture of dyes such that a laundry composition comprising the granules of the present invention comprise between 0.001 to 0.01 wt % of a hydrophobic dye for shading polyester and/or between 0.001 to 0.01 wt % of one or more other dyes selected from cotton substantive shading dyes of the group consisting of: hydrolysed reactive dye; acid dye; and direct dye.
- the level of dye found in the laundry composition is provided by the dye in the granule as defined herein.
- the total dye in the laundry composition is most preferably in the range between 0.001 to 0.01 wt %.
- the dye(s) has a maximum extinction coefficient greater than 1000 L/mol/cm in the wavelength range of 400 to 750 nm. Tuning of levels of the respective dyes in the composition will be such that dye deposition to the polyester and cotton will be aesthetically matched. It is preferred that the dyes have a peak absorption wavelength of from 550nm to 650nm, preferably from 570nm to 630nm. A combination of dyes may be used which together have the visual effect on the human eye as a single dye having a peak absorption wavelength on polyester or cotton of from 550nm to 650nm, preferably from 570nm to 630nm.
- This may be provided, for example by mixing a red and green-blue dye to yield a blue or violet shade.
- a specific example for the acid dyes is a mixture of acid red 17, acid red 88, acid red 51, and/or acid red 73 with acid black 1 and/or acid blue 25. The same spectral quantities are required for both the cotton and polyester substantive dyes.
- Preferred non-ionic surfactants are, for example, polyethoxylated alcohols, ethoxylated alkyl phenols, anhydrosorbitols, and alkoxylated anhydrosorbitol esters.
- An example of a preferred nonionic surfactant is a polyethoxylated alcohol manufactured and marketed by the Shell Chemical Company under the trademark "Neodol”.
- Neodol 25-7 which is a mixture of 12 to 15 carbon chain length alcohols with about 7 ethylene oxide groups per molecule; Neodol 23-65, a C12-13 mixture with about 6.5 moles of ethylene oxide; Neodol 25-9, a C12-13 mixture with about 9 moles of ethylene oxide, and Neodol 45-7, a C14-15 mixture with about seven moles of ethylene oxide.
- nonionic surfactants useful in the present invention include trimethyl nonyl polyethylene glycol ethers such as those, manufactured and marketed by Union Carbide Corporation under the Trademark Tergitol, octyl phenoxy polyethoxy ethanols sold by Rohm and Haas under the Trademark Triton, and polyoxyethylene alcohols, such as Brij 76 and Brij 97, trademarked products of Atlas Chemical Co.
- the hydrophilic lipophilic balance (HLB) is preferably below about 13, and more preferably below 10.
- a ratio of carrier to surfactant falls within the range of about 1:1 to 10:1, more preferably about 2:1 to 5:1. It is within the scope of the invention to use mixtures of non-ionic surfactants. Most preferably the non-ionic surfactant is an ethoxylated surfactant.
- the carrier may be water/surfactant soluble carrier or water/surfactant insoluble.
- water/surfactant soluble carriers are sodium carbonate, sodium sulphate, sodium chloride, and sodium citrate. It is however preferred that the carrier is water/surfactant insoluble and in this regard preferred carriers are zeolite (e.g., zeolite 4A and zeolite MAP), clay and minerals; most preferably clay. The preferred clay is bentonite.
- the granule is preferably 180 to 1000 microns in maximum width. This is deflected by the ability of the granule to pass through a graded sieve.
- the granule most preferably comprises a fluorescent agent(optical brightener).
- fluorescent agents are well known and many such fluorescent agents are available commercially. Usually, these fluorescent agents are supplied and used in the form of their alkali metal salts, for example, the sodium salts.
- the total amount of the fluorescent agent or agents used in laundry treatment composition is generally from 0.005 to 2 wt %, more preferably 0.01 to 0.1 wt %.
- Preferred classes of fluorescer are: Di-styryl biphenyl compounds, e.g. Tinopal (Trade Mark) CBS-X, Di-amine stilbene di-sulphonic acid compounds, e.g.
- Preferred fluorescers are: sodium 2 (4-styryl-3-sulfophenyl)-2H-napthol[1,2-d]trazole, disodium 4,4'-bis ⁇ [(4-anilino-6-(N methyl-N-2 hydroxyethyl) amino 1,3,5-triazin-2-yl)]amino ⁇ stilbene-2-2' disulfonate, disodium 4,4'-bis ⁇ [(4-anilino-6-morpholino-1,3,5-triazin-2-yl)]amino ⁇ stilbene-2-2' disulfonate, and disodium 4,4'-bis(2-sulfoslyryl)biphenyl.
- the granule may also comprise a photo-bleach which is a compounds that absorbs light in the range 290 to 750nm. On absorption of light the photobleach produces reactive species such as singlet oxygen or radicals, with high quantum yields (>0.05), that can bleach stains.
- a photo-bleach which is a compounds that absorbs light in the range 290 to 750nm. On absorption of light the photobleach produces reactive species such as singlet oxygen or radicals, with high quantum yields (>0.05), that can bleach stains.
- photobleaches are radical photoinitiators, such as vitamin K3 and singlet oxygen producing dyes such as metallated phthalocyanines (marketed by CIBA under the TINOLUX tradename).
- the granule of the present invention may be the laundry detergent composition per se. Conversely and preferably, the granule of the present invention may be mixed with other adjuncts and carriers to make up the laundry detergent composition. These other adjuncts and carriers may include, as will as components listed above, non-ionic, cationic and anionic surfactants, builders, enzymes, antifoam agents, soil release polymers, sodium percarbonate, activators, transition metal catalysts, chelants, dye transfer inhibition polymers and brighteners. It is preferred that a laundry detergent composition comprising the dye containing granule is such that the dye level contribution from the granule in the total detergent composition is between 0.00005 to 0.01 wt%, preferably 0.001 to 0.01 wt%.
- Acid Black 1 was dissolved in COCO 7EO nonionic surfactant to give a 1 wt%.
- the dye/NI solution (2.5 g) was added to 10g bentonite clay powder and mixed thoroughly. At this level the mixture is still a free-flowing powder.
- the resultant powder was then granulated with 3 g of a 40% solution of Sokalan CP5 polymer solution. The resultant granules were then dried in an oven at 80 C, and finally sieved to give granules in the range 180 to 1000 microns.
- the dry composition of these granules, granules A was: Component wt (g) % by weight Dye 0.025g 0.18 NI 2.475 18.1 Bentonite 10g 73.0 CP5 1.2 g 8.8
- Comparable granules without non-ionic were created by mixing 0.025 g of dye with 10 g bentonite and then granulating the mixture with 4 g of CP5 solution. The resulting granules were again dried at 80 C and finally sieved to 180 to 1000 microns.
- the dry composition of the granules, granules B are therefore: Component wt (g) % by weight Dye 0.025 0.215 Bentonite 10g 86.0 CP5 1.6g 13.8
- Example 1 The granules of Example 1 were separately added to a base washing powder and thoroughly mixed to give a powder with a final dye level of 0.004% by weight.
- the washing powder contained 18% NaLAS, 73% salts (silicate, sodium tri-poly-phosphate, sulphate, carbonate), 3% minors including perborate, fluorescer and enzymes, remainder impurities and water.
- a 20 x 20cm piece of white bleached woven non-mercerised cotton was placed in a solution of water, such that the cloth was flat and the liquor to cloth ration was 3:1. 10g of the powder was spread on the cloth and left for 30 minutes. Then the cloth was thoroughly rinsed, dried and the number of visible dye spots counted. The results are shown below.
- Powder with Granule A had 62 spots.
- Powder with Granule B had 385 spots.
- the dye granule with non-ionic showed substantially less spotting.
- Example 1 The experiment of Example 1 and 2 were repeated except using direct violet 51 as the dye.
- Powder with Granule A type had 2 spots.
- Powder with Granule B type had 78 spots.
- the dye granule with non-ionic (A type), showed substantially less spotting.
- Example 2 The experiment of Example 2 was repeated using the granules created in Example 5. Powder with Granule C type had 54 spots. Powder with Granule D type had 62 spots Powder with Granule E type had 123 spots.
- Granule C and D contain approximately twice the level of non-ionic as granule A (Example 1), but the spotting is similar.
- Granule D has double the concentration of dye compared to Granule C, (and hence is preferably be dosed in a laundry detergent composition at half the weight) but has similar loading.
- Granule E on zeolite shows less spotting than granule B, without non-ionic but more than the clay granules.
- the granules with the lower level of non-ionic, A and E function as well in terms of even colour delivery to the cloth as that for the granule without non-ionic (B).
- the higher level of non-ionic granules, C and D deliver less colour per weight dye.
- Solvent Violet 13 was dissolved in COCO 7EO nonionic surfactant to give a 1%wt solution.
- the dye/NI solution (2.5 g) was added to 10g bentonite clay powder and mixed thoroughly. At this level the mixture is still a free-flowing powder.
- the resultant powder was then granulated with 3 g of a 40% solution of Sokalan CP5 polymer solution as binder. The resultant granules were then dried in an oven at 80 C, and finally sieved to give granules in the range 180 to 1000 microns.
- the dry composition of these granules was: Component wt (g) % by weight Dye 0.025g 0.18 NI 2.475 18.1 Bentonite 10g 73.0 CP5 1.2 g 8.8
- the resultant granules were sieved to remove oversized materials (>1000um) and stored in sealed containers.
- the granules of examples 6 to 10 show low spotting and good delivery of dye to polyester.
- the granules of examples 7 to 10 were separately added to a base washing powder and thoroughly mixed to give a powder with a final dye level of 0.001 and 0.004% by weight.
- the washing powder contained 18% NaLAS, 73% salts (silicate, sodium tri-poly-phosphate, sulphate, carbonate), 3% minors including perborate, fluorescer and enzymes, remainder impurities and water.
- the granules of examples 7 to 10 were separately added to a base washing powder and thoroughly mixed to give a powder with a final dye level of 0.0005 and 0.002% by weight.
- the washing powder contained 10% NaLAS, 5% 7EO non-ionic, 1% soap, 17% zeolile A24, 12% percarbonate, 4% TAED, 40% salts (sodium sulphate, sodium carbonate), remainder, fluorescer, enzymes, anti-redep agents, moisture, perfume, sequesterants, anti-ashing agents, antifoam and dispersants.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Detergent Compositions (AREA)
Claims (18)
- Granulat, umfassend:(i) zwischen 5 und 40 Gewichts-% eines nicht-ionischen Tensids, das darin zwischen 0,0001 und 5 Gewichts-% eines Farbstoffs gelöst hat, wobei der Farbstoff eine Löslichkeit in dem nicht-ionischen Tensid von wenigstens 0,01 Gewichts-% hat;(ii) zwischen 20 und 90 Gewichts-% eines festen Trägers;(iii) zwischen 0 und 20 Gewichts-% eines Bindemittels und(iv) zwischen 0 und 1 Gewichts-% eines Fotobleichmittels, wobei der Farbstoff ausgewählt ist aus: einem hydrophoben Farbstoff; einem hydrolysierten Reaktivfarbstoff; einem Säurefarbstoff und einem Direktfarbstoff, und wobei der Säurefarbstoff ausgewählt ist aus:blauen und violetten Säurefarbstoffen der Struktur
roten Säurefarbstoffen der Struktur:
Gruppen der folgenden Strukturen:
das Naphthyl mit zwei SO3-Gruppen in einer der folgenden ausgewählten Orientierungen am Ring substituiert ist: 7,8; 6,8; 5,8; 4,8; 3,8; 7,6; 7,5; 7,4; 7,3; 6,5; 6,4; 5,4; 5,3, und 4,3;
B eine Aryl-Gruppe, ausgewählt aus Phenyl und Naphthyl, ist, wobei die Aryl-Gruppe mit einer Gruppe substituiert ist, die unabhängig ausgewählt ist aus: einer -NH2-Gruppe; einer -NH-Ph-Gruppe; einer -N=N-C6H5-Gruppe; einer -N=N-C10H7-Gruppe; einem oder mehreren -OMe und einem oder mehreren -Me;
Gruppen der folgenden Strukturen:
wobei der Direktfarbstoff ausgewählt ist aus:
blauen Triazo-Direktfarbstoffen der Formel: - Granulat nach Anspruch 1, wobei das Granulat zwischen 10 und 25 Gewichts-% eines nicht-ionischen Tensids umfasst.
- Granulat nach Anspruch 1 oder 2, wobei der feste Träger in Wasser und Tensiden unlöslich ist.
- Granulat nach einem vorangehenden Anspruch, wobei der Farbstoff substantiv für ein Gewebe ist und einen visuellen Effekt auf das menschliche Auge als ein Einzelfarbstoff hat, der eine Peak-Absorptionswellenlänge an einer Textilie von 550 nm bis 650 hat.
- Granulat nach einem vorangehenden Anspruch, wobei das Bindemittel im Bereich von 2 bis 10 Gewichts-% vorliegt.
- Granulat nach einem vorangehenden Anspruch, wobei der Träger aus Zeolith, Ton und Mineralien ausgewählt ist.
- Granulat nach Anspruch 6, wobei der Träger aus Bentonit, Zeolith 4A und Zeolith MAP ausgewählt ist.
- Granulat nach einem vorangehenden Anspruch, wobei das Bindemittel ausgewählt ist aus der Gruppe, bestehend aus einem Polyacrylat, Polyethylenglykol und Polyacrylat/Maleat-Copolymer.
- Granulat nach einem vorangehenden Anspruch, wobei das Verhältnis von Träger zu Tensid von etwa 1:1 bis 10:1 ist.
- Granulat nach Anspruch 9, wobei das Verhältnis von Träger zu Tensid von etwa 2:1 bis 5:1 ist.
- Granulat nach einem der Ansprüche 1 bis 10, wobei der Farbstoff ein Gemisch aus einem hydrophoben Farbstoff und einem Farbstoff ist, der ausgewählt ist aus der Gruppe, bestehend aus: einem hydrolysierten Reaktivfarbstoff; einem Säurefarbstoff und einem Direktfarbstoff.
- Granulat nach einem vorangehenden Anspruch, wobei der hydrophobe Farbstoff ein Lösungsmittel- oder Dispersionsfarbstoff ist.
- Granulat nach einem der Ansprüche 1 bis 10, wobei der hydrophobe Farbstoff ausgewählt ist aus: Dispersionsblau 79:1, Lösungsmittelschwarz 3; Lösungsmittelviolett 13, Lösungsmittelblau 59, Lösungsmittelblau 35, Lösungsmittelrot 24, Dispersionsrot 1, Dispersionsblau 3 und Dispersionsblau 106.
- Granulat nach einem der Ansprüche 1 bis 10, wobei der Farbstoff ausgewählt ist aus: Säureschwarz 24, Säureblau 25, Säureblau 29, Säureschwarz 1, Säureblau 113, Säurerot 17, Säurerot 51, Säurerot 73, Säurerot 88 und Säurerot 87, Säurerot 91, Säurerot 92, Säurerot 94, Direktviolett 9 und Säureviolett 17.
- Granulat nach Anspruch 14, wobei der Farbstoff Direktviolett 9 ist.
- Waschmittel, umfassend das Granulat, wie es in einem vorangehenden Anspruch definiert ist, wobei der Farbstoff-Konzentrationsbeitrag aus dem Granulat in dem gesamten Waschmittel zwischen 0,00005 und 0,01 Gewichts-% liegt.
- Waschmittel, umfassend das Granulat nach Anspruch 16, wobei der Farbstoff-Konzentrationsbeitrag aus dem Granulat in dem gesamten Waschmittel zwischen 0,001 und 0,01 Gewichts-% liegt.
- Granulierungsverfahren, umfassend die Schritte:(i) Lösen von zwischen 0,0001 und 1 Gewichts-% eines Farbstoffs in 5 bis 40 Gewichts-% eines nicht-ionixhen Tensids, wobei der Farbstoff in dem nicht-ionischen Tensid eine Löslichkeit von wenigstens 0,1 Gewichts-% hat;(ii) Mischen der Lösung von Farbstoff und nicht-ionischem Tensid mit zwischen 20 und 90 Gewichts-% eines festen Trägers und(iii) Granulieren des resultierenden Gemisches aus Schritt (ii).
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GBGB0425580.8A GB0425580D0 (en) | 2004-09-23 | 2004-11-22 | Laundry treatment compositions |
| PCT/EP2005/009518 WO2006053598A1 (en) | 2004-11-22 | 2005-09-05 | Laundry treatment compositions |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1814974A1 EP1814974A1 (de) | 2007-08-08 |
| EP1814974B1 true EP1814974B1 (de) | 2010-11-10 |
Family
ID=35431600
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP05790693A Revoked EP1814974B1 (de) | 2004-11-22 | 2005-09-05 | Wäschebehandlungsmittel |
Country Status (9)
| Country | Link |
|---|---|
| EP (1) | EP1814974B1 (de) |
| CN (1) | CN101068914A (de) |
| AR (1) | AR051963A1 (de) |
| AT (1) | ATE487784T1 (de) |
| CA (1) | CA2588068A1 (de) |
| DE (1) | DE602005024709D1 (de) |
| ES (1) | ES2354367T3 (de) |
| WO (1) | WO2006053598A1 (de) |
| ZA (1) | ZA200704091B (de) |
Families Citing this family (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB0519347D0 (en) † | 2005-09-22 | 2005-11-02 | Unilever Plc | Composition of enhanced stability and a process for making such a composition |
| WO2008017570A1 (en) * | 2006-08-10 | 2008-02-14 | Unilever Plc | Shading composition |
| BRPI0718690B1 (pt) * | 2006-11-10 | 2017-12-05 | The Procter & Gamble Company | A granular detergent composition for washing clothes, a process for their production and method for confering a designed tone to a fabric |
| DE102006054436A1 (de) | 2006-11-16 | 2008-05-21 | Henkel Kgaa | Feste, textil- und/oder hautpflegende Zusammensetzung |
| ES2388018T3 (es) * | 2008-01-10 | 2012-10-05 | Unilever N.V. | Gránulos |
| WO2009132870A1 (en) * | 2008-05-02 | 2009-11-05 | Unilever Plc | Reduced spotting granules |
| EP2382299B1 (de) | 2009-01-26 | 2013-03-13 | Unilever PLC | Einbringung von farbstoff in eine granulatwäschereizusammensetzung |
| EP2228429A1 (de) | 2009-03-13 | 2010-09-15 | Unilever PLC | Abschattungsfärbung und Katalysatorkombination |
| US8318652B2 (en) | 2009-08-25 | 2012-11-27 | Milliken & Company | Colored speckles comprising a porous carrier and a releasing agent layer |
| EP2343359A1 (de) * | 2010-01-07 | 2011-07-13 | Unilever PLC | Waschmittelformulierung, die sprühgetrocknetes Granulat enthält |
| WO2012059363A1 (en) | 2010-11-01 | 2012-05-10 | Unilever Nv | A detergent composition having shading dyes and lipase |
| ES2544539T3 (es) | 2011-05-26 | 2015-09-01 | Unilever N.V. | Composición líquida para lavandería |
| US20140371435A9 (en) | 2011-06-03 | 2014-12-18 | Eduardo Torres | Laundry Care Compositions Containing Thiophene Azo Dyes |
| JP5911996B2 (ja) | 2012-03-19 | 2016-04-27 | ザ プロクター アンド ギャンブル カンパニー | 染料を含むランドリーケア組成物 |
| US9540599B2 (en) * | 2012-05-09 | 2017-01-10 | Milliken & Company | Laundry detergent composition comprising a particle having hueing agent and clay |
| US9540600B2 (en) * | 2012-05-09 | 2017-01-10 | The Procter & Gamble Company | Laundry detergent composition comprising a particle having hueing agent and clay |
| BR112015029686A2 (pt) | 2013-05-28 | 2017-07-25 | Procter & Gamble | composições para o tratamento de superfícies compreendendo corantes fotocrômicos |
| CN103416397A (zh) * | 2013-08-19 | 2013-12-04 | 南通市通州区益君劳务有限公司 | 一种专用于清洁桉树树叶的喷雾及其制造方法 |
| CN105555936A (zh) | 2013-09-18 | 2016-05-04 | 宝洁公司 | 包含羧化物染料的衣物洗涤护理组合物 |
| CA2920901A1 (en) | 2013-09-18 | 2015-03-26 | The Procter & Gamble Company | Laundry care compositions containing thiophene azo carboxylate dyes |
| US9834682B2 (en) | 2013-09-18 | 2017-12-05 | Milliken & Company | Laundry care composition comprising carboxylate dye |
| CA2921433A1 (en) | 2013-09-18 | 2015-03-26 | The Procter & Gamble Company | Laundry care composition comprising carboxylate dye |
| WO2015112340A1 (en) | 2014-01-22 | 2015-07-30 | The Procter & Gamble Company | Method of treating textile fabrics |
| EP3097173B1 (de) | 2014-01-22 | 2020-12-23 | The Procter and Gamble Company | Gewebebehandlungszusammensetzung |
| WO2015112341A1 (en) | 2014-01-22 | 2015-07-30 | The Procter & Gamble Company | Fabric treatment composition |
| EP3097172A1 (de) | 2014-01-22 | 2016-11-30 | The Procter & Gamble Company | Verfahren zur behandlung von textilstoffen |
| JP2017518407A (ja) | 2014-05-06 | 2017-07-06 | ミリケン・アンド・カンパニーMilliken & Company | ランドリーケア組成物 |
| CN107532007B (zh) | 2015-05-04 | 2020-06-30 | 美利肯公司 | 在洗衣护理组合物中作为上蓝剂的隐色三苯甲烷着色剂 |
| US20180119056A1 (en) | 2016-11-03 | 2018-05-03 | Milliken & Company | Leuco Triphenylmethane Colorants As Bluing Agents in Laundry Care Compositions |
| CN111971372B (zh) | 2018-04-03 | 2022-03-11 | 联合利华知识产权控股有限公司 | 染料颗粒 |
| US20220098520A1 (en) | 2019-01-22 | 2022-03-31 | Conopco, Inc., D/B/A Unilever | Laundry detergent |
| WO2020151959A1 (en) | 2019-01-22 | 2020-07-30 | Unilever N.V. | Laundry detergent |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3755201A (en) | 1971-07-26 | 1973-08-28 | Colgate Palmolive Co | Laundry product containing mixed dye bluing agents |
| DE2632367C2 (de) * | 1975-07-23 | 1986-03-27 | The Procter & Gamble Co., Cincinnati, Ohio | Granulierte gefärbte Partikel |
| GB0018774D0 (en) | 2000-07-31 | 2000-09-20 | Unilever Plc | Coloured speckle composition and particulate laundry detergent compositions containing it |
| DE10048875A1 (de) * | 2000-09-29 | 2002-04-25 | Henkel Kgaa | Verfahren zur Herstellung von gefärbten Wasch- und Reinigungsmittelteilchen |
| DE60209804T2 (de) | 2001-08-20 | 2006-08-17 | Unilever N.V. | Photobleichsprenkel und sie enthaltende waschmittel |
-
2005
- 2005-09-05 DE DE602005024709T patent/DE602005024709D1/de not_active Expired - Lifetime
- 2005-09-05 WO PCT/EP2005/009518 patent/WO2006053598A1/en not_active Ceased
- 2005-09-05 AT AT05790693T patent/ATE487784T1/de not_active IP Right Cessation
- 2005-09-05 EP EP05790693A patent/EP1814974B1/de not_active Revoked
- 2005-09-05 ES ES05790693T patent/ES2354367T3/es not_active Expired - Lifetime
- 2005-09-05 CA CA002588068A patent/CA2588068A1/en not_active Withdrawn
- 2005-09-05 ZA ZA200704091A patent/ZA200704091B/xx unknown
- 2005-09-05 CN CNA2005800394905A patent/CN101068914A/zh active Pending
- 2005-11-17 AR ARP050104833A patent/AR051963A1/es not_active Application Discontinuation
Also Published As
| Publication number | Publication date |
|---|---|
| CA2588068A1 (en) | 2006-05-26 |
| DE602005024709D1 (de) | 2010-12-23 |
| EP1814974A1 (de) | 2007-08-08 |
| ZA200704091B (en) | 2008-09-25 |
| AR051963A1 (es) | 2007-02-21 |
| CN101068914A (zh) | 2007-11-07 |
| WO2006053598A1 (en) | 2006-05-26 |
| ATE487784T1 (de) | 2010-11-15 |
| ES2354367T3 (es) | 2011-03-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1814974B1 (de) | Wäschebehandlungsmittel | |
| EP1791940B1 (de) | Wäschebehandlungsmittel | |
| EP1902122A1 (de) | Farbstoffabgabegranulate | |
| EP1945747B1 (de) | Nuancierungsmittel | |
| US8062382B2 (en) | Shading composition | |
| EP1794274B1 (de) | Wäschebehandlungsmittel | |
| US10106762B2 (en) | Treating a textile garment with a hydrophobic dye solution | |
| EP2382299B1 (de) | Einbringung von farbstoff in eine granulatwäschereizusammensetzung | |
| US20080034511A1 (en) | Laundry Treatment Compositions | |
| EP2227534B1 (de) | Schattierungszusammensetzung | |
| CN103608446B (zh) | 染料向粒状洗衣组合物中的并入 | |
| EP3752589A1 (de) | Waschmittel | |
| EP3914682A1 (de) | Waschmittel |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20070417 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI SK TR |
|
| DAX | Request for extension of the european patent (deleted) | ||
| 17Q | First examination report despatched |
Effective date: 20080221 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: UNILEVER PLC Owner name: UNILEVER NAAMLOZE VENNOOTSCHAP |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: UNILEVER N.V. Owner name: UNILEVER PLC |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REF | Corresponds to: |
Ref document number: 602005024709 Country of ref document: DE Date of ref document: 20101223 Kind code of ref document: P |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: VDEP Effective date: 20101110 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Effective date: 20110302 |
|
| LTIE | Lt: invalidation of european patent or patent extension |
Effective date: 20101110 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20101110 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110210 Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20101110 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110310 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20101110 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20101110 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20101110 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110310 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20101110 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20101110 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20101110 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110211 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20101110 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20101110 |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20101110 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20101110 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20101110 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20101110 |
|
| PLAX | Notice of opposition and request to file observation + time limit sent |
Free format text: ORIGINAL CODE: EPIDOSNOBS2 |
|
| 26 | Opposition filed |
Opponent name: THE PROCTER & GAMBLE COMPANY Effective date: 20110810 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R026 Ref document number: 602005024709 Country of ref document: DE Effective date: 20110810 |
|
| PLAF | Information modified related to communication of a notice of opposition and request to file observations + time limit |
Free format text: ORIGINAL CODE: EPIDOSCOBS2 |
|
| PLBB | Reply of patent proprietor to notice(s) of opposition received |
Free format text: ORIGINAL CODE: EPIDOSNOBS3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110930 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110930 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110905 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110930 |
|
| RIC2 | Information provided on ipc code assigned after grant |
Ipc: C11D 17/06 20060101ALI20130128BHEP Ipc: C11D 17/00 20060101AFI20130128BHEP Ipc: C11D 3/12 20060101ALI20130128BHEP Ipc: C11D 3/40 20060101ALI20130128BHEP Ipc: C11D 3/37 20060101ALI20130128BHEP |
|
| APBM | Appeal reference recorded |
Free format text: ORIGINAL CODE: EPIDOSNREFNO |
|
| APBP | Date of receipt of notice of appeal recorded |
Free format text: ORIGINAL CODE: EPIDOSNNOA2O |
|
| APAH | Appeal reference modified |
Free format text: ORIGINAL CODE: EPIDOSCREFNO |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110905 |
|
| APBQ | Date of receipt of statement of grounds of appeal recorded |
Free format text: ORIGINAL CODE: EPIDOSNNOA3O |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20101110 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: TR Payment date: 20130902 Year of fee payment: 9 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20150626 Year of fee payment: 11 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R064 Ref document number: 602005024709 Country of ref document: DE Ref country code: DE Ref legal event code: R103 Ref document number: 602005024709 Country of ref document: DE |
|
| APBU | Appeal procedure closed |
Free format text: ORIGINAL CODE: EPIDOSNNOA9O |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20150922 Year of fee payment: 11 Ref country code: GB Payment date: 20150917 Year of fee payment: 11 Ref country code: ES Payment date: 20150916 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20150918 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20150924 Year of fee payment: 11 |
|
| RDAF | Communication despatched that patent is revoked |
Free format text: ORIGINAL CODE: EPIDOSNREV1 |
|
| RDAG | Patent revoked |
Free format text: ORIGINAL CODE: 0009271 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT REVOKED |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| 27W | Patent revoked |
Effective date: 20151014 |
|
| GBPR | Gb: patent revoked under art. 102 of the ep convention designating the uk as contracting state |
Effective date: 20151014 |
|
| R26 | Opposition filed (corrected) |
Opponent name: THE PROCTER & GAMBLE COMPANY Effective date: 20110810 |