EP1537194B1 - Verfahren zur herstellung von fettsäuren durch in situ hydrolysis von lipiden aus lipiden enthaltenden pflanzensamen - Google Patents
Verfahren zur herstellung von fettsäuren durch in situ hydrolysis von lipiden aus lipiden enthaltenden pflanzensamen Download PDFInfo
- Publication number
- EP1537194B1 EP1537194B1 EP03769562A EP03769562A EP1537194B1 EP 1537194 B1 EP1537194 B1 EP 1537194B1 EP 03769562 A EP03769562 A EP 03769562A EP 03769562 A EP03769562 A EP 03769562A EP 1537194 B1 EP1537194 B1 EP 1537194B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- hydrolysis
- lipase
- fatty acids
- homogenization
- seeds
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 150000002632 lipids Chemical class 0.000 title claims description 102
- 235000014113 dietary fatty acids Nutrition 0.000 title claims description 71
- 239000000194 fatty acid Substances 0.000 title claims description 71
- 229930195729 fatty acid Natural products 0.000 title claims description 71
- 150000004665 fatty acids Chemical class 0.000 title claims description 69
- 238000000034 method Methods 0.000 title claims description 56
- 238000011065 in-situ storage Methods 0.000 title claims description 21
- 230000003301 hydrolyzing effect Effects 0.000 title claims description 5
- 238000006460 hydrolysis reaction Methods 0.000 claims description 120
- 230000007062 hydrolysis Effects 0.000 claims description 112
- 102000004882 Lipase Human genes 0.000 claims description 106
- 108090001060 Lipase Proteins 0.000 claims description 106
- 239000004367 Lipase Substances 0.000 claims description 106
- 235000019421 lipase Nutrition 0.000 claims description 106
- 238000000265 homogenisation Methods 0.000 claims description 70
- 239000000839 emulsion Substances 0.000 claims description 66
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 30
- 239000012071 phase Substances 0.000 claims description 19
- 239000012736 aqueous medium Substances 0.000 claims description 18
- 239000000725 suspension Substances 0.000 claims description 17
- 239000002245 particle Substances 0.000 claims description 15
- 239000000243 solution Substances 0.000 claims description 15
- 239000002904 solvent Substances 0.000 claims description 14
- 238000001035 drying Methods 0.000 claims description 7
- 238000011084 recovery Methods 0.000 claims description 6
- 239000002253 acid Substances 0.000 claims description 5
- 150000001298 alcohols Chemical class 0.000 claims description 5
- 239000002960 lipid emulsion Substances 0.000 claims description 5
- 150000002148 esters Chemical class 0.000 claims description 4
- 239000007790 solid phase Substances 0.000 claims description 4
- 150000005677 organic carbonates Chemical class 0.000 claims description 3
- 150000002576 ketones Chemical class 0.000 claims description 2
- 150000007513 acids Chemical class 0.000 claims 1
- 239000002609 medium Substances 0.000 description 63
- 238000000527 sonication Methods 0.000 description 48
- 238000006243 chemical reaction Methods 0.000 description 32
- 230000008569 process Effects 0.000 description 26
- 238000000605 extraction Methods 0.000 description 25
- 240000002791 Brassica napus Species 0.000 description 24
- 102000004190 Enzymes Human genes 0.000 description 23
- 108090000790 Enzymes Proteins 0.000 description 23
- 238000012360 testing method Methods 0.000 description 23
- 210000004027 cell Anatomy 0.000 description 21
- 230000000694 effects Effects 0.000 description 21
- 239000007787 solid Substances 0.000 description 20
- 235000004977 Brassica sinapistrum Nutrition 0.000 description 19
- 238000005119 centrifugation Methods 0.000 description 19
- 239000003921 oil Substances 0.000 description 19
- 235000019198 oils Nutrition 0.000 description 19
- 239000007788 liquid Substances 0.000 description 18
- 230000009257 reactivity Effects 0.000 description 18
- 238000002604 ultrasonography Methods 0.000 description 18
- 238000000227 grinding Methods 0.000 description 16
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 14
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 14
- 241000222175 Diutina rugosa Species 0.000 description 12
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 12
- 230000003993 interaction Effects 0.000 description 12
- 238000002360 preparation method Methods 0.000 description 12
- 238000011282 treatment Methods 0.000 description 12
- 230000007071 enzymatic hydrolysis Effects 0.000 description 11
- 238000006047 enzymatic hydrolysis reaction Methods 0.000 description 11
- 239000000463 material Substances 0.000 description 11
- 235000018102 proteins Nutrition 0.000 description 11
- 102000004169 proteins and genes Human genes 0.000 description 11
- 108090000623 proteins and genes Proteins 0.000 description 11
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 10
- 229910052698 phosphorus Inorganic materials 0.000 description 10
- 239000011574 phosphorus Substances 0.000 description 10
- 239000012429 reaction media Substances 0.000 description 10
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 9
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 9
- 125000005456 glyceride group Chemical group 0.000 description 9
- 239000000523 sample Substances 0.000 description 9
- 239000000758 substrate Substances 0.000 description 9
- 239000000203 mixture Substances 0.000 description 8
- 241000196324 Embryophyta Species 0.000 description 7
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 7
- 239000003795 chemical substances by application Substances 0.000 description 7
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 210000002421 cell wall Anatomy 0.000 description 6
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 6
- 239000008188 pellet Substances 0.000 description 6
- 238000003756 stirring Methods 0.000 description 6
- 235000011293 Brassica napus Nutrition 0.000 description 5
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 5
- 230000009471 action Effects 0.000 description 5
- 238000004364 calculation method Methods 0.000 description 5
- 230000000052 comparative effect Effects 0.000 description 5
- 150000001875 compounds Chemical class 0.000 description 5
- 239000000470 constituent Substances 0.000 description 5
- 235000013305 food Nutrition 0.000 description 5
- 125000004383 glucosinolate group Chemical group 0.000 description 5
- 230000000670 limiting effect Effects 0.000 description 5
- 229910017604 nitric acid Inorganic materials 0.000 description 5
- 229910052757 nitrogen Inorganic materials 0.000 description 5
- 150000003626 triacylglycerols Chemical class 0.000 description 5
- 239000002956 ash Substances 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 239000007853 buffer solution Substances 0.000 description 4
- 238000004821 distillation Methods 0.000 description 4
- 230000005764 inhibitory process Effects 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- MEFBJEMVZONFCJ-UHFFFAOYSA-N molybdate Chemical compound [O-][Mo]([O-])(=O)=O MEFBJEMVZONFCJ-UHFFFAOYSA-N 0.000 description 4
- 150000003904 phospholipids Chemical class 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- LSGOVYNHVSXFFJ-UHFFFAOYSA-N vanadate(3-) Chemical compound [O-][V]([O-])([O-])=O LSGOVYNHVSXFFJ-UHFFFAOYSA-N 0.000 description 4
- 238000005303 weighing Methods 0.000 description 4
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 3
- 235000010469 Glycine max Nutrition 0.000 description 3
- 229910019142 PO4 Inorganic materials 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 239000008346 aqueous phase Substances 0.000 description 3
- 238000003556 assay Methods 0.000 description 3
- 230000033228 biological regulation Effects 0.000 description 3
- 230000033558 biomineral tissue development Effects 0.000 description 3
- 230000003197 catalytic effect Effects 0.000 description 3
- 230000001413 cellular effect Effects 0.000 description 3
- 239000012153 distilled water Substances 0.000 description 3
- 238000001704 evaporation Methods 0.000 description 3
- 230000008020 evaporation Effects 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 238000000386 microscopy Methods 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 239000004576 sand Substances 0.000 description 3
- 239000011734 sodium Substances 0.000 description 3
- 230000006641 stabilisation Effects 0.000 description 3
- 238000011105 stabilization Methods 0.000 description 3
- 238000009210 therapy by ultrasound Methods 0.000 description 3
- 238000004448 titration Methods 0.000 description 3
- 238000001238 wet grinding Methods 0.000 description 3
- 244000068988 Glycine max Species 0.000 description 2
- 238000007696 Kjeldahl method Methods 0.000 description 2
- 235000019484 Rapeseed oil Nutrition 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 238000002835 absorbance Methods 0.000 description 2
- 239000003513 alkali Substances 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- 238000004581 coalescence Methods 0.000 description 2
- 238000009833 condensation Methods 0.000 description 2
- 230000005494 condensation Effects 0.000 description 2
- 230000001186 cumulative effect Effects 0.000 description 2
- 238000010908 decantation Methods 0.000 description 2
- 235000021245 dietary protein Nutrition 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- 238000003760 magnetic stirring Methods 0.000 description 2
- 239000012074 organic phase Substances 0.000 description 2
- 230000008092 positive effect Effects 0.000 description 2
- 238000005070 sampling Methods 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 108010058651 thioglucosidase Proteins 0.000 description 2
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 2
- OOSZCNKVJAVHJI-UHFFFAOYSA-N 1-[(4-fluorophenyl)methyl]piperazine Chemical compound C1=CC(F)=CC=C1CN1CCNCC1 OOSZCNKVJAVHJI-UHFFFAOYSA-N 0.000 description 1
- WPDXAMRGYMDTOV-UHFFFAOYSA-N 3-bromo-2-methylphenol Chemical compound CC1=C(O)C=CC=C1Br WPDXAMRGYMDTOV-UHFFFAOYSA-N 0.000 description 1
- 241000588986 Alcaligenes Species 0.000 description 1
- 241000228212 Aspergillus Species 0.000 description 1
- FRPHFZCDPYBUAU-UHFFFAOYSA-N Bromocresolgreen Chemical compound CC1=C(Br)C(O)=C(Br)C=C1C1(C=2C(=C(Br)C(O)=C(Br)C=2)C)C2=CC=CC=C2S(=O)(=O)O1 FRPHFZCDPYBUAU-UHFFFAOYSA-N 0.000 description 1
- 241000222120 Candida <Saccharomycetales> Species 0.000 description 1
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 1
- 241001536352 Fraxinus americana Species 0.000 description 1
- 235000002918 Fraxinus excelsior Nutrition 0.000 description 1
- 241000447437 Gerreidae Species 0.000 description 1
- 241000223198 Humicola Species 0.000 description 1
- NHTMVDHEPJAVLT-UHFFFAOYSA-N Isooctane Chemical compound CC(C)CC(C)(C)C NHTMVDHEPJAVLT-UHFFFAOYSA-N 0.000 description 1
- 241000446313 Lamella Species 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 241000235395 Mucor Species 0.000 description 1
- 229910021204 NaH2 PO4 Inorganic materials 0.000 description 1
- 241000228143 Penicillium Species 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 241000589516 Pseudomonas Species 0.000 description 1
- 241000235527 Rhizopus Species 0.000 description 1
- 235000004443 Ricinus communis Nutrition 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 1
- 241000223257 Thermomyces Species 0.000 description 1
- 208000024799 Thyroid disease Diseases 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- OGBUMNBNEWYMNJ-UHFFFAOYSA-N batilol Chemical class CCCCCCCCCCCCCCCCCCOCC(O)CO OGBUMNBNEWYMNJ-UHFFFAOYSA-N 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- FFBHFFJDDLITSX-UHFFFAOYSA-N benzyl N-[2-hydroxy-4-(3-oxomorpholin-4-yl)phenyl]carbamate Chemical compound OC1=C(NC(=O)OCC2=CC=CC=C2)C=CC(=C1)N1CCOCC1=O FFBHFFJDDLITSX-UHFFFAOYSA-N 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 238000004061 bleaching Methods 0.000 description 1
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 1
- 239000004327 boric acid Substances 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 230000006037 cell lysis Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 230000007073 chemical hydrolysis Effects 0.000 description 1
- 239000011362 coarse particle Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 230000006957 competitive inhibition Effects 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 230000006837 decompression Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 238000001784 detoxification Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- BNIILDVGGAEEIG-UHFFFAOYSA-L disodium hydrogen phosphate Chemical compound [Na+].[Na+].OP([O-])([O-])=O BNIILDVGGAEEIG-UHFFFAOYSA-L 0.000 description 1
- 229910000397 disodium phosphate Inorganic materials 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000009837 dry grinding Methods 0.000 description 1
- 238000005265 energy consumption Methods 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 238000006911 enzymatic reaction Methods 0.000 description 1
- 238000011066 ex-situ storage Methods 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 235000021189 garnishes Nutrition 0.000 description 1
- 238000004817 gas chromatography Methods 0.000 description 1
- 230000035784 germination Effects 0.000 description 1
- 238000005469 granulation Methods 0.000 description 1
- 230000003179 granulation Effects 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- XLYOFNOQVPJJNP-ZSJDYOACSA-N heavy water Substances [2H]O[2H] XLYOFNOQVPJJNP-ZSJDYOACSA-N 0.000 description 1
- 238000005984 hydrogenation reaction Methods 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 230000004130 lipolysis Effects 0.000 description 1
- 239000000395 magnesium oxide Substances 0.000 description 1
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 1
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical compound [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- CEQFOVLGLXCDCX-WUKNDPDISA-N methyl red Chemical compound C1=CC(N(C)C)=CC=C1\N=N\C1=CC=CC=C1C(O)=O CEQFOVLGLXCDCX-WUKNDPDISA-N 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 238000003801 milling Methods 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- -1 on the one hand Chemical class 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 239000003973 paint Substances 0.000 description 1
- 230000003071 parasitic effect Effects 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 238000009877 rendering Methods 0.000 description 1
- 238000012958 reprocessing Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 230000035939 shock Effects 0.000 description 1
- 239000000344 soap Substances 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 229940074545 sodium dihydrogen phosphate dihydrate Drugs 0.000 description 1
- WZWGGYFEOBVNLA-UHFFFAOYSA-N sodium;dihydrate Chemical compound O.O.[Na] WZWGGYFEOBVNLA-UHFFFAOYSA-N 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 238000001256 steam distillation Methods 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 150000003464 sulfur compounds Chemical class 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 238000003809 water extraction Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11C—FATTY ACIDS FROM FATS, OILS OR WAXES; CANDLES; FATS, OILS OR FATTY ACIDS BY CHEMICAL MODIFICATION OF FATS, OILS, OR FATTY ACIDS OBTAINED THEREFROM
- C11C1/00—Preparation of fatty acids from fats, fatty oils, or waxes; Refining the fatty acids
- C11C1/02—Preparation of fatty acids from fats, fatty oils, or waxes; Refining the fatty acids from fats or fatty oils
- C11C1/04—Preparation of fatty acids from fats, fatty oils, or waxes; Refining the fatty acids from fats or fatty oils by hydrolysis
- C11C1/045—Preparation of fatty acids from fats, fatty oils, or waxes; Refining the fatty acids from fats or fatty oils by hydrolysis using enzymes or microorganisms, living or dead
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11B—PRODUCING, e.g. BY PRESSING RAW MATERIALS OR BY EXTRACTION FROM WASTE MATERIALS, REFINING OR PRESERVING FATS, FATTY SUBSTANCES, e.g. LANOLIN, FATTY OILS OR WAXES; ESSENTIAL OILS; PERFUMES
- C11B1/00—Production of fats or fatty oils from raw materials
- C11B1/02—Pretreatment
- C11B1/025—Pretreatment by enzymes or microorganisms, living or dead
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11B—PRODUCING, e.g. BY PRESSING RAW MATERIALS OR BY EXTRACTION FROM WASTE MATERIALS, REFINING OR PRESERVING FATS, FATTY SUBSTANCES, e.g. LANOLIN, FATTY OILS OR WAXES; ESSENTIAL OILS; PERFUMES
- C11B1/00—Production of fats or fatty oils from raw materials
- C11B1/02—Pretreatment
- C11B1/04—Pretreatment of vegetable raw material
Definitions
- the present invention relates to the field of the preparation of fatty acids from the lipids contained in the seeds of certain plants.
- Fatty acids are carboxylic acids with varying degrees of unsaturation and a wide variety of molecular weights. Fatty acids are used in the manufacture of a wide variety of products, such as soaps and surfactants, lubricants, paints and coatings, and candles, as well as in a wide variety of other consumer products. agriculture and industry, particularly in the agri-food sector.
- fatty acids are produced by chemical hydrolysis of an oil, for example by the action of heat and pressure in the presence of water, in order to break the bonds between the acid and the alcohol constituting the lipids contained in the oils.
- the US patent published under number US 5,932,458 discloses a method of hydrolyzing an oil with an immobilized lipase.
- the lipase can be obtained by grinding oat seeds and then removing the lipids by extraction with a solvent.
- the immobilized lipase is then placed in contact with soybean oil in a solvent. organic, 2,2,4-trimethylpentane.
- RAO et al. ( RAO KSVA et al., 1992, Res. Ind., Vol.37: 36 ) describe a method of hydrolysis using castor seed mill, in which endogenous lipase of the seed, which is released from intracellular spheronomas by seed milling, is used to hydrolyze the endogenous lipids of these milled seeds.
- JACHMANLAN et al. Jachmanian I et al., 1995, J Agric. Food Chem. flight. 43 (11): 2992-2996 ) studied the hydrolysis of lipids of a crushed seed rapeseed under the action of endogenous lipase.
- US Patent No. US 3,640,725 discloses a process for preparing a protein extract from soybeans.
- the method of this prior document comprises in particular a step of incubating a paste obtained from crushed soybeans with a proteolytic enzyme composition.
- the applicant has endeavored to develop an improved process for preparing fatty acids, by hydrolysis of the lipids contained in the seeds of a plant, which is simple, fast, and which allows obtaining a high yield hydrolysis.
- the applicant has endeavored to develop an improved process that does not require a step of extracting the oil prior to bringing the lipase into contact with the lipids whose hydrolysis, respectively in fatty acids and alcohols, is sought after.
- an exogenous lipase placed in a solid / liquid heterogeneous substrate reaction medium which reaction medium is subjected to a drastic homogenization treatment at high pressure or by ultrasound, retains its catalytic activity. hydrolysis of the lipids contained in the substrate solution thus treated.
- an exogenous lipase was capable of carrying out a complete or almost complete hydrolysis of the lipids present in this heterogeneous solid / liquid reaction medium in the form of an emulsion consisting of droplets of oil dispersed in an aqueous medium, and that the fatty acids resulting from the hydrolysis of the lipids contained in said emulsion could be easily recovered, for example by simple extraction.
- step a) grinding is carried out using a knife mill type "Waring Blendor" in an aqueous medium.
- obtaining solid particles with an average diameter of less than 500 ⁇ m can be obtained by a grinding time of the seeds in the aqueous medium of a few minutes, preferably between 1 and 10 minutes and very preferably between 2 and 7 minutes, for example for a period of 5 minutes.
- a buffer solution is not preferred because a buffer solution is likely to introduce undesirable contaminants compounds that are difficult or long to eliminate, in the following process.
- the seeds are added to the aqueous medium at a proportion of less than 20%, preferably less than 15%, by total weight of the aqueous medium containing the seeds.
- step a) of grinding is difficult or impossible to achieve because of a too high viscosity of the aqueous medium.
- step e) of recovery of fatty acids is difficult to achieve optimally, due to a proportion of solid phase too high.
- the proportion by weight of seeds, relative to the total weight of the aqueous medium containing the seeds is less than 15%.
- the exogenous lipase, added in step b) in the heterogeneous liquid / solid suspension obtained at the end of step a), may be of any kind. It may be a lipase obtained from microorganisms of the genera Rhizopus, Mucor, Alcaligenes, Candida, Aspergillus, Pseudomonas, Humicola, Thermomyces or Penicillium or from the lipases of plants.
- the lipase obtained from Candida rugosa can be used, as described in the examples.
- the exogenous lipase is added in a proportion of 100 to 500 units, preferably 150 to 400 units of lipase, per gram of the weight of the seeds treated in step a).
- One unit of lipase corresponds to the amount of lipase required to release 1 ⁇ mol of fatty acid from glyceride per minute under optimal conditions of catalytic activity of the enzyme.
- lipid hydrolysis For higher lipase / seed ratios, the kinetics of hydrolysis is further enhanced and the hydrolysis of lipids is almost complete. Thus, a degree of lipid hydrolysis greater than 90% is obtained with a lipase / seed ratio of 166 units of lipase per gram of weight of seeds treated in step a).
- step c) the homogenization of the liquid / solid suspension initially containing coarse particles of seeds less than 500 ⁇ m is carried out until a homogenate consisting of a lipid emulsion initially contained in the seeds, mixed with the solid particles of the seeds.
- Step c) of homogenizing the heterogeneous liquid / solid suspension and containing the exogenous lipase may be carried out by any means known per se.
- step c) of homogenization is carried out by homogenization under pressure or by homogenization by sonication.
- step c) by homogenization under pressure, it is possible to use a high-pressure homogenizer such as the Lab 1000 type homogenizer marketed by the APV Company (Evreux, France), according to the manufacturer's recommendations.
- a high-pressure homogenizer such as the Lab 1000 type homogenizer marketed by the APV Company (Evreux, France), according to the manufacturer's recommendations.
- the homogenization under pressure is carried out at a pressure of between 25 and 1000 bar, preferably between 100 and 500 bar, for example at 150 bar.
- the pressure jump caused in the middle produces a shock wave contributing to the disintegration of the seed cells and disperses the lipids contained in the seed.
- step c) of homogenization will be carried out by two successive cycles of homogenization under pressure.
- the homogenization step c) can be carried out by 1 to 4 cycles of passage in a high pressure homogenizer, optimal results are obtained with two cycles of high pressure homogenization. In this case, the extraction yield of the fatty acids at the end of the process is increased without drastically affecting the activity of the exogenous lipase.
- step c) of homogenizing the suspension containing the exogenous lipase may be carried out by sonication of said suspension until a homogenate consisting of an emulsion of the lipids initially contained in the seeds is obtained. , mixed with the solid particles of the seeds.
- the suspension solid particles to which the exogenous lipase has been added is treated with ultrasound at a power greater than 20 Watts.
- the frequency of ultrasound does not seem to be an essential parameter of treatment.
- ultrasonic power of between 20 and 200 Watts is used, and most preferably between 20 and 100 Watts.
- the duration of the homogenization step by sonication is advantageously between 10 seconds and 10 minutes, preferably between 30 seconds and 5 minutes.
- a sonication duration of 30 seconds at the power of 64 Watts average kinetics of lipid hydrolysis was observed leading to a degree of hydrolysis after 120 minutes of reaction greater than 85%.
- a sonication time of 1 minute considerably improves the reaction kinetics and a degree of hydrolysis greater than 90% is reached after 120 minutes of reaction.
- the degrees of hydrolysis of the lipids obtained at the end of the process are comparable. They are generally greater than 95% under the optimum conditions indicated above.
- the results obtained in the examples show that the final extraction yield of the fatty acids resulting from the hydrolysis of the lipids is substantially the same, that the step c) of homogenization was carried out by homogenization under pressure or else by homogenization by sonication.
- step c) shows that this homogenate is formed of lipid droplets which aggregate into small clusters forming an emulsion, the homogenate also containing many solid debris seeds, including seed shell debris as well as cellular debris.
- the size of the fat drops is on average between 5 and 15 ⁇ m and their surface appears thin and smooth, which suggests that the stabilization of the oil / water interface of the droplets does not involve solid particles.
- the homogenate contains the lipids initially contained in the seeds in the form of a liquid / liquid emulsion with the water present in the aqueous medium that constitutes said homogenate.
- Step d) of hydrolysis of the lipids contained in the homogenate obtained in step c) is carried out by incubating the homogenate at a temperature of between 15 ° C. and 55 ° C. for a period of time between 15 minutes and 5 hours.
- the exogenous lipase catalyzes the hydrolysis reaction of the lipids, in particular triglycerides, contained in the form of a liquid / liquid emulsion in the homogenate, respectively in the different constituent fatty acids of said lipids, on the one hand , and various alcohols, especially glycerol, with which said fatty acids were esterified in the spheroids of seeds.
- the method for preparing fatty acids by in situ hydrolysis of the invention can therefore be carried out at ambient temperature without requiring heating of the reaction medium consisting of the homogenate obtained in step c), which makes this process easy to implementation and also inexpensive.
- step d) of hydrolysis at low temperature, for example between 20 ° C. and 30 ° C., makes it possible to avoid, or at least reduce, the simultaneous hydrolysis of other compounds.
- other compounds such as glucosinolates, whose sulfur hydrolysis products cause thyroid disorders in humans and animals.
- myrosinase which is the enzyme responsible for the hydrolysis of glucosinolates, is not very active, the optimal catalytic activity of this enzyme being between 40 ° C and 70 ° C. ° C.
- step d) of hydrolysis makes the process of the invention particularly useful. simple to perform, since no precise regulation of the reaction temperature is required.
- the duration of step d) of hydrolysis of the lipids can be adapted as a function of the chosen reaction temperature. However, it can be optimally performed for a period of between 60 and 120 minutes at a reaction temperature equal to or greater than 27 ° C.
- the method for preparing fatty acids by in situ hydrolysis of the lipids contained in the seeds of a plant above is highly reproducible. It has been shown that the difference in degree of hydrolysis between 0 and 15 minutes of reaction varies linearly with the exogenous lipase / weight weight ratio of the seeds.
- Step c) homogenization makes it possible in fact to physically break the macroscopic structure of the seeds and to release into the aqueous medium lipids initially contained in the spherosomes, which are intracellular vesicles surrounded by a membrane.
- the lipase is located on the entire surface of the oil / water interface of the lipid droplets generated which allows a rapid and as complete hydrolysis of said lipid substrates as possible. which are easily accessible to the enzyme.
- Step e) of recovering the fatty acids resulting from the hydrolysis of the lipids made in step d) can be carried out by any lipid extraction method known to those skilled in the art.
- the fatty acids are separated from the other constituents of the homogenate obtained in step d) by obtaining a liquid medium with three phases, respectively a solid phase, a phase aqueous solution and a lipid emulsion phase, by centrifugation of the homogenate, and then recovery of the lipid emulsion phase.
- Obtaining the three-phase liquid medium is preferably carried out by centrifugation of the heterogeneous liquid / solid reaction medium at the end of the hydrolysis step d). If necessary, several successive centrifugations can be carried out in order to improve the yield of the process.
- a triphasic medium is obtained respectively comprising (i) a solid pellet essentially comprising solid debris from seeds, cell shells or debris, (ii) an aqueous phase and (iii) an oily phase under the shape of an emulsion.
- the oily phase in the form of an emulsion constitutes the phase enriched in fatty acids resulting from the hydrolysis of the lipids initially contained in the seeds.
- This oily phase represents about 7 to 10% by weight of the total weight of the reaction medium obtained at the end of the hydrolysis step d).
- the oily emulsion phase is separated from the other two phases respectively aqueous and solid, for example by simple sampling on the surface of the three-phase medium obtained after centrifugation.
- the oily emulsion fractions are combined in order to proceed to step e2) of extracting the fatty acids with a solvent.
- the extraction of the fatty acids is carried out with a solvent chosen from water, an alcohol, an ester, an organic carbonate or a ketone.
- Water is preferably used for the extraction of fatty acids in the form of an emulsion.
- Ethanol is preferably used for the extraction of fatty acids and the production of concentrates, as well as for the subsequent conversion of fatty acids to ethylenic esters, for various applications, in particular in solvents, lubricants and cosmetics. , curators, etc ...
- An ester or an organic carbonate is preferably used for the subsequent transformation of fatty acids, in situ or ex situ.
- the solvent precipitates the proteins and phospholipids contained in the emulsion and solubilizes the fatty acids.
- the fraction of precipitated compounds and the solubilized fatty acid fraction can be easily separated, for example by centrifugation.
- the solvent used is ethanol, which may for example be added to the emulsion containing the fatty acids in a proportion of 1.5 to 2 volumes of ethanol per volume of emulsion.
- Step e2) of extracting the fatty acids from the emulsion with the aid of a solvent is advantageously followed by a step e3) of drying the extraction solution containing the fatty acids.
- the drying step can be carried out by simple evaporation, at atmospheric pressure or under vacuum until complete drying of the fatty acids is achieved.
- the drying step e3) may be a moderate drying step in which only the solvent is removed, the residual aqueous medium being retained.
- step e3) of the process the quality of the fatty acids obtained at the end of the process is the best, because the water retains any impurities such as peptides soluble in alcohol. or traces of phospholipids.
- the rapeseeds are added with water in order to obtain the proportions 1: 8 in masses, respectively.
- This mixture is then poured into a household mixer type "Warring Blendor". The grinding is then carried out for 5 minutes, taking care to return to the bottom of the bowl the particles that adhere to the walls.
- the lipase resulting from Candida rugosa (Lipolyve, Lyven, Cagny, France) is solubilized in water so as to obtain a concentration of 10 mg / ml.
- Ninety grams of the previously obtained seed mill material is supplemented with 10 grams of enzyme solution in a 150 ml jacketed beaker. At this stage, the medium contains 10% by weight of rapeseeds and a quantity of lipase corresponding to 300 UL / g of seeds.
- the mixture obtained after dry grinding or in the presence of water is then treated with ultrasound (Vibracell, Bioblock Scientific, Illkirch, France) for 5 minutes at 59 W (70% of active period).
- the medium is cooled during the sonication by circulation of cold water in the wall of the beaker. After this step, the flow of cold water is immediately replaced by that coming from a bath thermostated at 37 ° C.
- the medium is then allowed to evolve under magnetic stirring (250 rpm) for 120 minutes.
- the reaction is monitored by a series of sampling made at predetermined times. In these, 1.5 ml of medium are transferred to a tube containing 1 ml of hydrochloric acid and 5 ml of chloroform.
- the lipase is added to the medium after grinding the seeds for 5 minutes in the presence of water.
- the sonication step is replaced by two homogenization cycles by a Lab 1000 homogenizer (APV, Evreux, France) equipped with two homogenization stages.
- the pressure of the first homogenization stage is set at 150 bar, that of the second stage is set at 20 bar, in agreement with the results published by Wäsche et al (1993) and with the supplier's recommendations.
- the seed content of the medium is 13.5% by weight and the lipase / seed ratio is set at 333 UL / g of seeds, in order to be able to compare the measured kinetics with those already obtained before with the use of ultrasound.
- the stopwatch is triggered at the addition of the lipase, just before the start of the passage of the medium in the homogenizer.
- Obtaining a DH of the order of 95% in 2 hours clearly shows that the homogenization is capable of generating adequate conditions for the enzymatic hydrolysis in situ of rapeseed lipids. It would have been possible that the violence of the treatment suffered by the medium degrades the lipase, rendering it inactive. Moreover, the tests of this new material were carried out without any optimization.
- the measured hydrolysis rate therefore does not a priori represent an optimum of reactivity, and the range of possible homogenization pressures for the apparatus used is fairly wide. Thus, it is possible to vary the homogenization pressure, but also the number of passes made or the pressure of the second homogenization stage.
- One liter of medium at 15% by mass is prepared by passage of 5 minutes in the 150g mixer of rapeseed in 850mL of H 2 O. After passing through the mixer, the amount of medium recovered is weighed in order to estimate the quantity seed (15% of the recovered mass).
- the hydrolysis is carried out with the enzyme "LIPOLYVE CC" whose concentration is 30 units / mg.
- the enzyme solution is prepared in water in order to obtain, after mixing with the medium, an enzyme concentration of the order of 300 units per gram of seeds and a concentration of seeds in the medium of the order of 13.5% by weight.
- the enzyme solution is added to the medium and the whole is passed to the homogenizer 2 cycles at 350 bars.
- the homogenate (medium passed to the homogenizer) thus recovered is placed in a reactor at 37 ° C. with stirring at 250 rpm for two hours.
- the hydrolyzate (homogenate after 2 hours of hydrolysis at 37 ° C.) is finally stored at 4 ° C.
- Emulsion recovery protocol :
- the emulsion is prepared from the hydrolyzate by centrifugation. After stirring, the hydrolyzate is centrifuged for 5 minutes at maximum speed. During the last emulsion preparation, the hydrolyzate was centrifuged for 5 minutes at 7600 G. During the centrifugation, the different phases are separated and it is possible to recover the emulsion by filtration on a fine mesh. The pellet is then resuspended in the aqueous phase before being centrifuged a second time under the same conditions. The emulsion formed is thus recovered two more times.
- the determination of the dry matter makes it possible to calculate the quantity of water contained in the emulsion.
- the amount of dry matter is calculated by weighing a certain volume of emulsion (about 4 ml) before and after passing 6 hours in an oven at 103 ° C. To avoid losses of emulsion by projections, the weighed emulsion volume is mixed with sand before being placed in an oven. To avoid losses, the weighing is carried out with the aluminum cup containing the emulsion, with the sand and with the spatula used to mix the sand and the emulsion.
- the amount remaining after 6 hours at 103 ° C represents the dry matter.
- the difference in weight gives us information on the quantity of water contained in the emulsion.
- the amount of fatty acids resulting from the hydrolysis of the triglycerides of the lipids of the crushed seeds is expected by the oil.
- the oil content of our emulsion is determined according to a "simplified method by hexane extraction" (Standard V 03-908). For safety reasons, cyclohexane and not hexane were used.
- This content is determined on the dry matter that allowed us to calculate the amount of water contained in the emulsion. To avoid distorting the results, the aluminum cup containing the dry matter and the spatula were placed in the soxhlet cartridge.
- Oil content m 1 - m 0 / m trial x 100 m 0 : tare of the balloon in grams m 1 : balloon mass after evaporation of cyclohexane in grams m test : mass of emulsion, in grams, used to obtain the dry matter
- Oil content d ⁇ e l ' ed ⁇ m ⁇ u ⁇ l ⁇ s ⁇ i ⁇ o ⁇ not 58 , 3 + ⁇ / - ⁇ 1 , 3 %
- the determination of the proteins contained in the emulsion is done indirectly by nitrogen determination according to the Kjeldahl method: mineralization and steam distillation.
- Phosphorus content PO 4 2 x 0.05 x 100 / m [PO 4 2- ]: Phosphorus concentration of dissolved ash in mg / mL m: mass of the test sample in grams Content p ⁇ h ⁇ o ⁇ s ⁇ p ⁇ h ⁇ o ⁇ r ⁇ e d ⁇ e l ' ed ⁇ m ⁇ u ⁇ l ⁇ s ⁇ i ⁇ o ⁇ not : 0 , 148 + ⁇ / - ⁇ 0 , 004 %
- the emulsion is composed of: ... 32.5% H 2 O + / - 0.6% 58.3% oil + / - 1.3% 7.24% protein + / - 0.47% 3.8% phospholipids + /. 0.1%
- the emulsion had a density of 0,943 + / - 0.004%
- the media used to carry out these tests are obtained by homogenization under pressure or by homogenization by sonication, as indicated in Examples 1 and 2.
- the homogenization by sonication was carried out by continuous sonication at the power of 82 W.
- the pellet is weighed before being completed with distilled water until the initial mass of treated medium. The whole is then homogenized by vigorous stirring for 30 seconds. This new suspension is then treated as the initial medium by centrifugation and then extraction of the fat from the higher phases to the solvent. In total, 4 cycles are carried out in this manner, the last pellet obtained being also extracted with chloroform and methanol after being resuspended in 15 ml of distilled water. A report on lipids can be made.
- the cumulative percentage of total lipids extracted after each wash cycle is presented on the figure 3 .
- the comparison of the results obtained for a hydrolysis involving sonication or homogenization shows us an identical lipid extractability during the first cycle.
- the total percentage of lipids extracted in subsequent cycles is significantly higher if the medium has undergone pressure homogenization treatment. Indeed, a difference of 6.5% is observed on the total yield.
- the preparation of the hydrolysis media by grinding followed by homogenization makes it possible to significantly improve the total yield of extraction of the fatty acids produced during the reaction.
- the difference measured after 4 cycles of washing / centrifugation is 6.5%.
- the extractibility observed after the first aqueous extraction cycle suggests that the interactions between the emulsion droplets and the cell debris of the pellet are of the same order in the case of the use of ultrasound or homogenization. under pressure.
- the proportion of seeds introduced into the medium directly conditions the degree of filling of the reactor with a glyceride substrate. Indeed, when increasing the seed content of the medium, it is logically enriched in glycerides and proteins, which represent respectively 46.5 and 18% of the fresh mass of rapeseeds used. the quantity of seeds in the medium has been varied within the limits of the possibilities of the material.
- the response measured during this study is the evolution of the degree of hydrolysis (DH) over time.
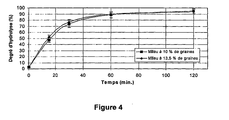
- the reaction kinetics measurement protocol is performed using wet milling as a pretreatment step prior to sonication.
- the ultrasonic probe has been set to its maximum power, ie 82 W with an active period of 100%. Two seed contents were tested: 10 and 13.5%. Beyond this, the ultrasound probe heats up, which presents a risk of deterioration of the equipment. As before, each test was performed three times to ensure reproducibility.
- the kinetics obtained, presented on the figure 4 are superimposable, to experimental errors close.
- the rate of hydrolysis is rapid during the first 30 minutes, then slow down gradually until reaching a plateau after 60 minutes of reaction, for an optimum value of 95% DH.
- lipase does not seem to be affected by this variation in dry matter content. This seems logical because if the proportion of seeds introduced into the medium differs, the enzyme / seed ratio does not vary. This means that neither the glyceride / lipase ratio nor the proportion of proteins relative to the enzyme has changed. Thus, the contribution of potentially inhibitory compounds is counterbalanced by the addition in parallel of an identical proportion of lipase. The only parameter that could have been limiting is the amount of water available for the reaction to take place. Experimental results show that this is not the case.
- a mixture containing 15% of rapeseeds in distilled water is milled for 5 minutes in a "Warring Blendor" type household blender.
- the lipase resulting from Candida rugosa (Lipolyve, Lyven, Cagny, France) is solubilized in water so as to obtain a concentration of 10 mg / ml.
- Ninety grams of the previously obtained seed mill material is supplemented with 10 grams of enzyme solution in a 150 ml jacketed beaker.
- the medium contains 13.5% by weight of rapeseeds and a quantity of lipase corresponding to 333 UL / g of seeds.
- the mixture is then treated with ultrasound (Vibracell, Bioblock Scientific, Illkirch, France) for 5 minutes at 12, 28, 47, 64 or 82 W (100% of active period) according to the tests.
- the medium is cooled during the sonication by circulation of cold water in the wall of the beaker. After this step, the flow of cold water is immediately replaced by that coming from a bath thermostated at 37 ° C.
- the medium is then allowed to evolve under magnetic stirring (250 rpm) for 120 minutes.
- the reaction is monitored by a series of samples taken at predetermined times. In these, 1.5 ml of medium are transferred to a tube containing 1 ml of hydrochloric acid and 5 ml of chloroform. After vigorous stirring, the organic phase is removed, dried with anhydrous Na 2 SO 4 and then diluted 10-fold before being injected with GPC for analysis. Each test was performed three times to determine the error in the experimental value
- EXAMPLE 7 Optimal conditions for homogenization by sonication - duration of sonication.
- the sonication period was set in turn at 30 sec, 1 min, 2 min, 3 min. and 5 min for an ultrasonic power set at 64 W.
- the evolution of DH as a function of time is used as the answer, each test being repeated three times to ensure reproducibility.
- the kinetics obtained during the hydrolysis of rapeseed lipids in situ with different sonication durations at 64W are represented on the figure 6 .
- the observed rates of hydrolysis are all the more rapid as the duration of the period of passage of the ultrasound medium is long.
- the curves obtained for 3 and 5 minutes of treatment of the medium by the ultrasound are superimposable, with the experimental errors close. According to these results, the duration of sonication of the The middle therefore plays a positive role to a certain extent. For higher processing times, a plateau is reached.
- the positive effect of the sonication duration seems logical in view of the results obtained in the context of the tests on the impact of the ultrasonic power dissipated in the medium.
- the increase in ultrasound passage time favors their action. This results in a more complete disruption of the cell walls, which improves the accessibility of the lipase to its lipid substrate.
- the increase in the duration of sonication favors interactions between the seed oil and the lipase, which explains an increase in reactivity.
- the palliation is justified as for him the result to a maximum of the process of cell lysis for which the sound power introduced into the medium becomes limiting for the continuation of the degradation of the biological structures of the seed. As a result, a maximum of reactivity is achieved for a given ultrasonic power.
- the duration of sonication of the medium is an important parameter, which plays a positive role on reactivity.
- the explanation of these observations is close to that advanced to justify the influence of the ultrasonic power applied to the medium.
- a maximum is observed for a duration of treatment greater than or equal to 3 minutes, beyond which there is no longer any improvement in the interactions between the lipase and its substrate.
- the ultrasonic power dissipated in the medium is 82 W and the temperature is set at 22, 27, 32, 37 or 42 ° C.
- the different kinetics of DH obtained are compared with each other. Each test is repeated 3 times and a standard deviation is calculated.
- the amount of lipase is a second determining factor for reactivity.
- the determination of this parameter thus makes it possible to determine the influence of the enzyme / seed ratio on the reactivity and on the efficiency of the lipase, in particular with regard to inhibitions of the enzyme by seed compounds.
- the protocol followed is identical to the previous examples, with an ultrasonic power set at 82 W, a temperature of 37 ° C.
- the amount of lipase is, for its part, variable according to the tests, the various lipase / seed ratios tested being 27.7, 55, 111, 222 and 333 UL / g of seeds. As in previous experiments, each point was done 3 times to determine the experimental error bar.
- the denominator represents total concentration of fatty acid radicals, whether in free form or bound to glycerol. This sum therefore remains constant, whatever the DH. The difference in DH between 0 and 15 minutes is therefore equal to the amount of fatty acids produced during this period, normalized by the amount of total fatty acid radicals.
- the amount of lipase introduced has a positive effect on the kinetics of hydrolysis of the lipids of the seed.
- the calculation of initial velocities allows us to show that this response varies linearly with the amount of enzyme added to the medium, the specific activity remaining relatively stable. It is deduced that despite inhibition caused by competition for binding at the interface, the amount of lipase introduced into the medium is a limiting factor of hydrolysis.
- the media observed are prepared according to the same protocol as during the study of the reactivity presented in the previous example.
- the lipase / seed ratio is set at 333 UL / g of seeds.
- the cell break is for its part ensured by a wet grinding 5 minutes followed by a period of 5 min. son of 82 W.
- the proportion of seed introduced is 13.5% by weight.
- the media thus obtained are allowed to react until complete hydrolysis.
- the fatty acids appear in the form of a granulation present over the entire surface observed.
- the lipid droplets do not seem to disperse but rather aggregate into small clusters. This phenomenon is important for the efficiency of the extraction step because it indicates that the surfactants of all kinds that garnish the fat globules do not create repulsion between them. In case of difficulties for the rupture of the emulsion, only the rigidity of the interface can be questioned.
- the photo of the Figure 11 demonstrates that the centrifugation of the medium allows a good separation of lipids and cell debris resulting from the breaking of the seed. Moreover, the trend already observed at lower magnification that the droplets associate to form aggregates is confirmed by this finer shooting.
- This coalescence may result either from the fusion of fat globules during the breakage treatment of native cell structures, or from a natural instability of the formed emulsion.
- the first hypothesis seems the most likely, however, because no visible change in the size of fat globules seems to have occurred during centrifugation.
- the figure 12 is a photograph taken at an intermediate magnification of 400 ⁇ , shows the detail of a group of cells that have undergone the hydrolysis process. In this photograph, it is possible to clearly distinguish fully emptied cells from their contents, as well as cells that still contain some of their constituents.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Microbiology (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Biochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
Claims (11)
- Verfahren zur Herstellung von Fettsäuren durch in-situ-Hydrolyse der in den Samen einer Pflanze enthaltenen Lipide, dadurch gekennzeichnet, dass es die folgenden Schritte umfasst:a) Mahlen der Samen in wässrigem Medium, bis man eine Suspension von festen Teilchen mit einem Durchmesser von unter 500 µm erhält;b) Zufügen einer exogenen Lipase zu der in Schritt a) erhaltenen Suspension;c) Homogenisierung der die Lipase enthaltenden Suspension, bis man ein Homogenisat erhält, das aus einer Emulsion der anfangs in den Samen enthaltenen Lipide im Gemisch mit den festen Teilchen der Samen erhält;d) Hydrolyse der in dem in Schritt c) erhaltenen Homogenisat enthaltenen Lipide bei einer Temperatur zwischen 15 °C und 55 °C während einer Zeit zwischen 15 Minuten und 5 Stunden;e) Gewinnung der Fettsäuren, die sich aus der in Schritt d) durchgeführten Hydrolyse der Lipide ergeben.
- Verfahren gemäß Anspruch 1, dadurch gekennzeichnet, dass die Samen in Schritt a) in einem Anteil von weniger als 20%, vorzugsweise weniger als 15%, bezogen auf das Gesamtgewicht des die Samen enthaltenden wässrigen Mediums, zu dem wässrigen Medium gegeben werden.
- Verfahren gemäß einem der Ansprüche 1 oder 2, dadurch gekennzeichnet, dass die Lipase in Schritt b) in einer Menge von 100 bis 500 Einheiten, vorzugsweise 150 bis 400 Einheiten, Lipase pro Gramm Gewicht der in Schritt a) behandelten Samen hinzugefügt wird.
- Verfahren gemäß einem der Ansprüche 1 bis 3, dadurch gekennzeichnet, dass der Homogenisierungsschritt c) durch Druckhomogenisierung oder durch Ultraschallhomogenisierung durchgeführt wird.
- Verfahren gemäß Anspruch 4, dadurch gekennzeichnet, dass die Druckhomogenisierung durch einen oder mehrere Homogenisierungszyklen unter einem Druck zwischen 25 und 1000 bar, vorzugsweise zwischen 100 und 500 bar, durchgeführt wird.
- Verfahren gemäß Anspruch 4, dadurch gekennzeichnet, dass die Druckhomogenisierung durch zwei Homogenisierungszyklen unter hohem Druck durchgeführt wird.
- Verfahren gemäß Anspruch 4, dadurch gekennzeichnet, dass die Ultraschallhomogenisierung mit einer Ultraschallleistung von über 20 Watt durchgeführt wird.
- Verfahren gemäß einem der Ansprüche 1 bis 7, dadurch gekennzeichnet, dass der Fettsäurengewinnungsschritt e) die folgenden Schritte umfasst:e1) Trennung der Fettsäuren von den anderen Bestandteilen des in Schritt d) erhaltenen Homogenisats in Form einer Emulsion;e2) Extraktion der Fettsäuren aus der Emulsion mit einem Lösungsmittel.
- Verfahren gemäß Anspruch 8, dadurch gekennzeichnet, dass die Fettsäuren in Schritt e1) dadurch von den anderen Bestandteilen des in Schritt d) erhaltenen Homogenisats getrennt werden, dass man durch Zentrifugation des Homogenisats ein flüssiges Medium mit drei Phasen erhält, nämlich einer festen Phase, einer wässrigen Phase und einer Lipidemulsionsphase, und die Lipidemulsionsphase gewinnt.
- Verfahren gemäß Anspruch 8, dadurch gekennzeichnet, dass die Fettsäuren in Schritt e2) durch Wasser oder durch ein Lösungsmittel, das aus einem Alkohol, einem Ester, einem organischen Carbonat und einem Keton ausgewählt ist, extrahiert werden.
- Verfahren gemäß einem der Ansprüche 8 bis 10, dadurch gekennzeichnet, dass auf den Fettsäurenextraktionsschritt e2) ein Schritt e3) der Trocknung der die Fettsäuren enthaltenden Lösung folgt.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR0210942A FR2843970B1 (fr) | 2002-09-04 | 2002-09-04 | Procede de preparation d'acides gras par hydrolyse in situ des lipides contenus dans les graines d'une plante. |
| FR0210942 | 2002-09-04 | ||
| PCT/FR2003/002636 WO2004022677A1 (fr) | 2002-09-04 | 2003-09-03 | Procede de preparation d'acides gras par hydrolyse in situ des lipides contenus dans les graines d'une plante |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1537194A1 EP1537194A1 (de) | 2005-06-08 |
| EP1537194B1 true EP1537194B1 (de) | 2012-10-31 |
Family
ID=31503105
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP03769562A Expired - Lifetime EP1537194B1 (de) | 2002-09-04 | 2003-09-03 | Verfahren zur herstellung von fettsäuren durch in situ hydrolysis von lipiden aus lipiden enthaltenden pflanzensamen |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP1537194B1 (de) |
| AU (1) | AU2003278252A1 (de) |
| FR (1) | FR2843970B1 (de) |
| WO (1) | WO2004022677A1 (de) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR3029738B1 (fr) * | 2014-12-10 | 2016-12-30 | Agronutrition | Procede de preparation d'une composition de traitement de plante, composition obtenue et ses utilisations |
| CN110564494A (zh) * | 2019-10-17 | 2019-12-13 | 东北农业大学 | 一种超声波预处理溶剂法提取大豆油脂的方法 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3640725A (en) * | 1969-07-02 | 1972-02-08 | Rohm & Haas | Soybean fractionation employing a protease |
| FR2283902A1 (fr) * | 1974-09-06 | 1976-04-02 | Cpc International Inc | Procede de degradation enzymatique d'une matiere riche en proteine |
| SE450927B (sv) * | 1983-12-22 | 1987-08-17 | Svenska Lantmennens Riksforbun | Sett och anordning for behandling av raps- eller rypsfro sa att mjolkavkastningen hos mjolkkor hojs samt anvendning av den erhallna produkten |
| DE3843027A1 (de) * | 1988-12-21 | 1990-06-28 | Battelle Institut E V | Biotechnisches verfahren zur gewinnung von oel und ggf. fettsaeuren aus oelhaltigen pflanzen |
| US5616215A (en) * | 1991-04-19 | 1997-04-01 | Novo Nordisk A/S | Method of making paper from pulp treated with lipase and an aluminum salt |
| EP0619950B1 (de) * | 1993-02-09 | 1998-10-28 | The Quaker Oats Company | Haferfraktionierungsverfahren und Produkt daraus |
| US6685975B2 (en) * | 2000-05-19 | 2004-02-03 | Biozyme Systems Inc. | Process for recovering bone and oil from animal byproducts |
-
2002
- 2002-09-04 FR FR0210942A patent/FR2843970B1/fr not_active Expired - Fee Related
-
2003
- 2003-09-03 EP EP03769562A patent/EP1537194B1/de not_active Expired - Lifetime
- 2003-09-03 AU AU2003278252A patent/AU2003278252A1/en not_active Abandoned
- 2003-09-03 WO PCT/FR2003/002636 patent/WO2004022677A1/fr not_active Ceased
Also Published As
| Publication number | Publication date |
|---|---|
| FR2843970A1 (fr) | 2004-03-05 |
| WO2004022677A1 (fr) | 2004-03-18 |
| FR2843970B1 (fr) | 2006-02-17 |
| AU2003278252A8 (en) | 2004-03-29 |
| AU2003278252A1 (en) | 2004-03-29 |
| EP1537194A1 (de) | 2005-06-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8557297B2 (en) | Method for processing crustaceans and products thereof | |
| CA2777738C (fr) | Procede d'extraction enzymatique en milieu aqueux d'huiles et de proteines a partir de matiere vegetale | |
| CN103635564B (zh) | 一种分离磷脂的方法 | |
| ES2593787T3 (es) | Desgomado de aceite libre de emulsificación | |
| WO2000036059A1 (en) | Two phase extraction of oil from biomass | |
| EP1261684A2 (de) | Verfahren zum fraktionieren eines speiseöls | |
| FR2984748A1 (fr) | Utilisation d'avocats mous entiers pour obtenir une huile d'avocat riche en insaponifiable | |
| CA2915320C (fr) | Nanoparticules de selenium elementaire et procede de preparation | |
| FR2930782A1 (fr) | Processus enzymatique pour l'obtention d'un ester d'acide gras | |
| Di Caprio et al. | Extraction of microalgal starch and pigments by using different cell disruption methods and aqueous two‐phase system | |
| Liu et al. | Effects of pretreatment on the yield of peanut oil and protein extracted by aqueous enzymatic extraction and the characteristics of the emulsion | |
| FR3098687A1 (fr) | Procede d’obtention de farines enrichies en proteines provenant de larves utilisees pour la bioconversion de dechets organiques | |
| EP1537194B1 (de) | Verfahren zur herstellung von fettsäuren durch in situ hydrolysis von lipiden aus lipiden enthaltenden pflanzensamen | |
| Zhang et al. | An innovative strategy of comprehensive utilization of tiger nuts (Cyperus esculentus L.): Simultaneous extraction of oil and glucose syrup by amylolysis-assisted aqueous extraction process | |
| WO1994008028A1 (fr) | Procede de preparation, par voie enzymatique, d'aromes, notamment des ionones et des aldehydes en c6 a c¿10? | |
| EP0785010B1 (de) | Verfahren zur Behandlung eines mit Kohlenwasserstoff verunreinigten wässrigen Mediums, und entschäumende und zerstreuende Zusammensetzung auf der Basis von Polyglycerolestern | |
| WO2017167914A1 (fr) | Procédé d'extraction de glycolipides et glycolipides obtenus | |
| FR2940112A1 (fr) | Procede d'extraction de composes presents au sein d'un materiau vegetal frais par cryobroyage et enzymolyse | |
| WO2023156727A1 (fr) | Methode de determination d'un parametre d'une composition d'origine naturelle permettant de determiner le traitement d'elimination en heteroatomes le plus approprie de cette composition | |
| JP7504915B2 (ja) | 捕捉剤を使用した水性分散液からの植物クチクラワックスの抽出および精製 | |
| EP1846570B1 (de) | Verfahren zum nachweis und/oder zur messung einer lipase- oder phospholipaseaktivität mit hoher geschwindigkeit | |
| Bao et al. | A novel efficient method for the simultaneous recycle of amygdalin and oil from bitter apricot kernels: Ultrasonication based on natural deep eutectic solvent | |
| EP4151741B1 (de) | Verfahren zur enzymatischen synthese eines biodiesels aus gebrauchten lipiden | |
| Cherstva et al. | Using of enzymes to extract of rapeseed oil by pressing | |
| FR3122808A1 (fr) | Co-extraction de composés actifs à caractères hydrophiles et hydrophobes issus de microalgues et macroalgues |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20050404 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL LT LV MK |
|
| DAX | Request for extension of the european patent (deleted) | ||
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: MECHLING, ERIC Inventor name: MOULOUNGUI, ZEPHIRIN |
|
| 17Q | First examination report despatched |
Effective date: 20071126 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 582045 Country of ref document: AT Kind code of ref document: T Effective date: 20121115 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: FRENCH |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 60342491 Country of ref document: DE Effective date: 20130103 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: AU Effective date: 20130205 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 582045 Country of ref document: AT Kind code of ref document: T Effective date: 20121031 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: VDEP Effective date: 20121031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121031 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130211 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121031 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121031 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121031 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130201 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130228 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121031 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20130131 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121031 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121031 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121031 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121031 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20130801 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 60342491 Country of ref document: DE Effective date: 20130801 |
|
| BERE | Be: lapsed |
Owner name: INSTITUT NATIONAL DE LA RECHERCHE AGRONOMIQUE (IN Effective date: 20130930 Owner name: INSTITUT NATIONAL POLYTECHNIQUE DE TOULOUSE (I.N. Effective date: 20130930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121031 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20130903 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 60342491 Country of ref document: DE Effective date: 20140401 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130930 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130930 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130903 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130930 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130903 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20140401 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130903 Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20030903 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20150925 Year of fee payment: 13 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20170531 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160930 |