EP0858495B1 - Use of a process operating a combustion plant of a coal-fired power station for the accelerated coal combustion in a smelt chamber - Google Patents
Use of a process operating a combustion plant of a coal-fired power station for the accelerated coal combustion in a smelt chamber Download PDFInfo
- Publication number
- EP0858495B1 EP0858495B1 EP96929184A EP96929184A EP0858495B1 EP 0858495 B1 EP0858495 B1 EP 0858495B1 EP 96929184 A EP96929184 A EP 96929184A EP 96929184 A EP96929184 A EP 96929184A EP 0858495 B1 EP0858495 B1 EP 0858495B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- coal
- titanium dioxide
- containing material
- combustion
- use according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L9/00—Treating solid fuels to improve their combustion
- C10L9/10—Treating solid fuels to improve their combustion by using additives
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F23—COMBUSTION APPARATUS; COMBUSTION PROCESSES
- F23J—REMOVAL OR TREATMENT OF COMBUSTION PRODUCTS OR COMBUSTION RESIDUES; FLUES
- F23J7/00—Arrangement of devices for supplying chemicals to fire
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L10/00—Use of additives to fuels or fires for particular purposes
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L10/00—Use of additives to fuels or fires for particular purposes
- C10L10/02—Use of additives to fuels or fires for particular purposes for reducing smoke development
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F23—COMBUSTION APPARATUS; COMBUSTION PROCESSES
- F23B—METHODS OR APPARATUS FOR COMBUSTION USING ONLY SOLID FUEL
- F23B5/00—Combustion apparatus with arrangements for burning uncombusted material from primary combustion
- F23B5/02—Combustion apparatus with arrangements for burning uncombusted material from primary combustion in main combustion chamber
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S44/00—Fuel and related compositions
- Y10S44/905—Method involving added catalyst
Definitions
- the invention relates to the use of a method for operating a combustion plant of a coal-fired power plant with smelting chamber firing.
- the combustion temperature in the combustion chamber which in this case is also referred to as the melting chamber, is above the melting temperature of the ash. Under normal operating conditions, this is approx. 1500 ° C.
- the ash melting temperature of the coal used for firing can vary widely and is essentially dependent on the content of aluminum oxide Al 2 O 3 and silicate SiO 2 .
- the majority of the ashes combine to form a melt flow at the bottom of the combustion chamber and are supplied to wet slag removers below through outlet openings. These are pools of water in which the leaking liquid ash is caught and quenched.
- the granulate is a popular material in road construction and is used, for example, as a bulk material but also as a grit or blasting agent.
- the fly ash entrained by the flue gas flow which can consist of up to 50% combustible material (carbon and / or semi-burned hydrocarbons), is separated in the electrostatic precipitators.
- the temperature of the combustion or melting chamber and the melting temperature of the ash must be coordinated for particularly effective melting chamber operation, ie complete burnout, rapid fuel conversion and avoidance of NO x formation.
- the composition of the coal (depending on the composition, the ash melting temperature varies between 1300 ° C and 1700 ° C) determines the design of the coal-fired power plant, such as the size of the combustion chamber. By adding limestone, however, it is possible to lower the melting temperature of the ash. Experience shows that by adding approx. 2% limestone to coal, the melting temperature of the ash can be reduced by approx. 100 ° C. This procedure provides a regulation for the operation of the furnace.
- the fly ash return makes it a perfect one Burnout of the fuel achieved, however, the increases average residence time of a coal or ash particle in the Feuerungsniklauf.
- the disadvantage is the maximum Throughput of coal and thus the possible performance of the Power plant limited.
- the invention is therefore based on the object of an inexpensive Method for operating a coal-fired power plant, which according to the process of melting chamber firing works, with which the throughput of fuel and thus the performance of the power plant can be increased.
- This is supposed to be with a incinerator suitable for carrying out the method can be achieved with particularly simple means.
- Titanium dioxide at most in a titanium dioxide: coal ratio of 3:97.
- the invention is based on the observation that titanium dioxide the burning out of the coal in the combustion chamber and thus can increase the throughput of coal, which in turn Performance increase of the power plant leads.
- the viscosity and the melting temperature of the ash is not essential to be changed.
- the addition of titanium the is present as titanium dioxide under the conditions of the melting chamber, slag-like approaches behind the combustion chamber, that stick to pipes and walls, do not favor. It has been shown that titanium dioxide has the melting point of Ash or slag lowers. From a sand-like, not melted and non-sticking dust could result in a viscous, flowing and sticky melt become the higher Cleaning costs and financial losses during maintenance of the coal-fired power plant. However, it was found that the titanium dioxide is largely found in the liquid ash.
- the titanium dioxide content is in the total amount of coal and materials containing titanium dioxide added at most 2.25%.
- titanium dioxide content is also lower in the mixture of coal and materials containing titanium dioxide lead at a coal-fired power plant with dry combustion system already to a considerable intensification of the slagging behind the combustion chamber and to a flowing Slag consistency.
- Such additives are titanium dioxide therefore especially for the operation of a coal-fired power plant Melting chamber firing suitable.
- the supplied titanium dioxide-containing material is advantageous more than 50% from titanium dioxide. This can even with a small additions an acceleration of coal burnout be achieved.
- a titanium dioxide: carbon ratio is advantageous here of at least 1:99.
- a power plant without fly ash return to the Melting chamber is made according to an embodiment of the invention the added titanium as titanium dioxide to a low Partly excreted via fly ash, but mostly via liquid ash. Since titanium dioxide is not toxic, it cannot only the liquid ash, but also the fly ash as usual continue to be used.
- the coal-fired power plant works with one Fly ash return, the resulting fly ash is in the furnace returned so that the titanium is practically exclusively as titanium dioxide together with the resulting Liquid ash is excreted.
- the material containing titanium dioxide advantageously becomes coal added, then it can be used in a coal mill of the power plant and ground over a coal belt the burners are inserted into the combustion chamber of the power plant.
- the material containing titanium dioxide can be particularly simple also pneumatically into the combustion chamber, preferably via the Fly ash return, to be blown in.
- liquid ash can also be beneficial to use the liquid ash to pass into a wet slag remover at the bottom of the combustion chamber and process it into granules. This allows aggregates in the admixed titanium dioxide-containing material safely into the resulting Granules are melted down.
- the incinerator 1 shown in Figure 1 is part of a coal-fired power plant, not shown. It comprises a high-temperature combustion chamber designed as a melting chamber 2 with at least one burner 2a, and with a feed 2b, for example a conveyor belt for the coal K, and a fresh air line 4 guided over a compressor 3. It further comprises a discharge line 5 for liquid ash F with one on it connected wet purifier 6. It also comprises a flue gas line 7 and in the flue gas line 7 connected in series a dust filter system 8 with a fly ash collector 9, a flue gas desulfurization system 10 and a catalytic denitrification system 11. The flue gas line 7 opens into a chimney 12.
- the feed 2b is connected to a Coal mill 13 connected, which is connected to a feed shaft 14 of a coal store 15 and to a separate feed line 16 for adding material M containing titanium dioxide.
- the burnout acceleration of the coal K in the combustion chamber 2 is set via the amount of titanium dioxide-containing material M.
- the coal K is conveyed from the coal store 15 via the feed shaft 14 into the coal mill 13.
- the titanium dioxide-containing material M is either introduced via the feed line 16 and the feed shaft 14 or directly into the coal mill 13 and there, together with the coal K, is ground very finely.
- Fuel B prepared in this way reaches the combustion chamber 2 via the feed 2b and the burner 2a, where it is burned with compressed air L supplied via the fresh air line 4.
- the resulting flue gas RG flows via the flue gas line 7 into the dust filter system 8, where fly ash or fly dust S entrained by the flue gas is intercepted and discharged via the fly ash collector 9.
- the now practically dust-free flue gas RG arrives at the flue gas desulfurization system 10 and into the chimney 12 via the denoxification system 11, which is generally referred to as a DeNO x system.
- the liquid ash F collecting on the combustion chamber bottom 2c becomes via the discharge line 5 to the wet slag remover 6 and processed into granules G.
- the fly ash S collected at the collector 9 can be recycled as usual become.
- Up to 3% material containing titanium dioxide is advantageous M used with a titanium dioxide content of more than 50%.
- This melting chamber granulate G can be used as a building material as usual.
- the incinerator 1 with Melting chamber firing a fly ash return 20 on This opens directly into the combustion chamber 2 of the melting chamber furnace.
- the in the dust filter system 8 via the collector 9 restrained fly ash S is pneumatically using a additional compressor 21 blown into the combustion chamber 2.
- titanium dioxide-containing Dust-finely ground material M is added to the fly ash S. and gets into the combustion chamber 2 by adding Titanium dioxide-containing material M in the combustion chamber 2 of the coal-fired power plant with melting chamber firing in combination with a Fly ash return 20 becomes particularly effective burnout with a simultaneous acceleration of the throughput Coal K achieved in the power plant. This increases the performance of the Power plant.
- Additives contained in the fly ash S, which are contaminated with heavy metal, and titanium dioxide are insolubly incorporated into the resulting melting chamber granules G. In this way, used DeNO x catalysts with more than 50% TiO 2 can be disposed of without any problems.
- Example 1 Used DeNO x catalysts are used as the titanium dioxide-containing material M and mixed with coal K.
- a highly decarburized, high-ballast hard coal is used as coal K, which, according to its degree of decarburization and the proportion of volatile constituents, belongs to lean coal and lies on the border between lean coal and anthracite coal.
- the ashes of this coal show normal melting behavior.
- the catalyst used consists of approximately 75% TiO 2 and contains further catalytic components (approx. 11% SiO 2 , approx. 8% WO 3 and approx. 1.8% V 2 O 5 ).
- combustion tests are carried out in a combustion chamber 2.
- the combustion chamber 2 is designed as a laboratory combustion chamber, each with a liquid ash extractor and a dry ash extractor.
- the composition of the ash, the influence of the slagging behavior of the coal by adding used catalyst, the influence of the catalyst fraction M K on the slagging intensity of the heating surfaces behind the combustion chamber and the distribution of the catalyst material in the combustion residues are examined. An X-ray fluorescence analysis of these combustion residues is carried out.
- FIGS. 3 to 7 show the test results as an example for the combustion chamber with liquid ash extraction.
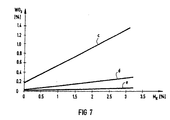
- Curves c, d and e of FIGS. 5 to 7 show the percentage of active catalyst substances TiO 2 (FIG. 5), V 2 O 5 (FIG. 6) and WO 3 (FIG. 7) in the slag F, in the fly ash S. or in the slag-like approaches.
- Another surprising result is that the catalyst is found primarily in the slag or liquid ash F (curve c, FIGS. 5 to 7) and partly in the fly ash S (curve d, FIGS. 5 to 7), but hardly in the slag-like approaches ( Curve e, Figures 5 to 7) takes place.
- M K (0 to 3%) in the fuel, only the proportions of TiO 2 (FIG. 5), V 2 O 5 (FIG. 6) and WO 3 (FIG. 7) in the slag F and in the fly ash S become clear to. In the slag-like approaches behind the combustion chamber, however, they remain practically unchanged.
- Example 2 Fly ash from an electrostatic precipitator of a coal-fired power plant with smelting chamber firing is mixed with calcium carbonate (CaCO 3 ) in a mass ratio of 100: 5. As a result, a melt can be obtained directly ("zero test”). The same mixture is mixed for comparison with dust-finely ground, used DeNO x catalyst in such a way that the catalyst content is 1%. The mixture is melted at 1550 ° C for 20 minutes and quenched in water ("comparative sample”). 5 g of the granules G obtained are eluted with 50 g of H 2 O for 24 hours and the eluate is examined for traces of vanadium V, tungsten W and arsenic as.
- CaCO 3 calcium carbonate
- the amount of active washed out from the comparative sample Catalyst substances (V, W) are below the detection limit ( ⁇ 0.1 mg / l).
- the arsenic content is in both samples in the same area.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Combustion & Propulsion (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Organic Chemistry (AREA)
- Mechanical Engineering (AREA)
- General Engineering & Computer Science (AREA)
- Thermal Sciences (AREA)
- Physics & Mathematics (AREA)
- Gasification And Melting Of Waste (AREA)
- Processing Of Solid Wastes (AREA)
- Exhaust Gas Treatment By Means Of Catalyst (AREA)
- Catalysts (AREA)
- Solid Fuels And Fuel-Associated Substances (AREA)
Abstract
Description
Die Erfindung bezieht sich auf eine Verwendung eines Verfahren zum Betreiben einer Verbrennungsanlage eines Kohlekraftwerkes mit Schmelzkammerfeuerung.The invention relates to the use of a method for operating a combustion plant of a coal-fired power plant with smelting chamber firing.
Zum Betreiben einer Verbrennungsanlage von Kohlekraftwerken gibt es im wesentlichen zwei unterschiedliche Feuerungstechniken, nämlich das Verfahren der Trockenfeuerung und das Verfahren der Schmelzkammerfeuerung. Bei der Trockenfeuerung liegt die Temperatur in der Brennkammer unterhalb der Schmelztemperatur der Asche. Die entstehende Asche wird deshalb nahezu vollständig vom Rauchgasstrom mitgerissen und setzt sich als Flugasche in nachgeschalteten Abscheidesystemen, wie z.B. Elektrofiltern, ab. Die Flugasche oder der Flugstaub kann als Zusatzstoff in der Bauindustrie eingesetzt werden.,Der DE 31 28 903 A1 zufolge ist bereits vorgeschlagen worden, zum Verbessern der Verbrennung bei Trockenfeuerung als ein Additiv verschiedene Metalloxide zu verwenden.To operate an incineration plant in coal-fired power plants there are essentially two different firing techniques, namely the process of dry firing and the process the melting chamber firing. With dry firing the temperature in the combustion chamber is below Melting temperature of the ashes. The resulting ash is therefore almost completely swept away by the flue gas flow and settles as fly ash in downstream separation systems, such as. Electrostatic precipitators. The fly ash or the Fly dust can be used as an additive in the construction industry are. According to DE 31 28 903 A1 has already been proposed has been used to improve combustion in dry firing to use various metal oxides as an additive.
Bei der Schmelzkammerfeuerung liegt die Verbrennungstemperatur in der Brennkammer, welche in diesem Fall auch als Schmelzkammer bezeichnet wird, oberhalb der Schmelztemperatur der Asche. Bei normalen Betriebsbedingungen sind dies ca. 1500°C. Die Ascheschmelztemperatur der zur Feuerung verwendeten Kohle kann stark variieren und ist im wesentlichen vom Gehalt an Aluminiumoxid Al2O3 und Silikat SiO2 abhängig. Der überwiegende Teil der Asche vereinigt sich zu einem Schmelzfluß am Brennkammerboden und wird über Auslaßöffnungen darunter befindlichen Naßentschlackern zugeführt. Dies sind Wasserbecken, in denen die auslaufende Flüssigasche aufgefangen und abgeschreckt wird. Das dabei entstehende Granulat (= Schmelzkammergranulat), welches im wesentlichen aus Aluminiumsilikat besteht, weist eine grobe Struktur auf. Das Granulat ist ein begehrter Stoff im Straßenbau und wird beispielsweise als Schüttgut aber auch als Streu- oder Strahlmittel verwendet. Die vom Rauchgasstrom mitgerissene Flugasche, die bis zu 50 % aus brennbarem Material (Kohlenstoff und/oder halbverbrannten Kohlenwasserstoffen) bestehen kann, wird in den Elektrofiltern abgeschieden.In melting chamber firing, the combustion temperature in the combustion chamber, which in this case is also referred to as the melting chamber, is above the melting temperature of the ash. Under normal operating conditions, this is approx. 1500 ° C. The ash melting temperature of the coal used for firing can vary widely and is essentially dependent on the content of aluminum oxide Al 2 O 3 and silicate SiO 2 . The majority of the ashes combine to form a melt flow at the bottom of the combustion chamber and are supplied to wet slag removers below through outlet openings. These are pools of water in which the leaking liquid ash is caught and quenched. The resulting granulate (= melting chamber granulate), which essentially consists of aluminum silicate, has a rough structure. The granulate is a popular material in road construction and is used, for example, as a bulk material but also as a grit or blasting agent. The fly ash entrained by the flue gas flow, which can consist of up to 50% combustible material (carbon and / or semi-burned hydrocarbons), is separated in the electrostatic precipitators.
Für einen besonders effektiven Schmelzkammerbetrieb, d.h. vollkommener Ausbrand,schneller Brennstoffumsatz und Vermeidung von NOx-Bildung, müssen die Temperatur der Brenn- oder Schmelzkammer und die Schmelztemperatur der Asche aufeinander abgestimmt sein. Die Zusammensetzung der Kohle (je nach Zusammensetzung variiert die Ascheschmelztemperatur zwischen 1300°C und 1700°C) bestimmt damit die Auslegung des Kohlekraftwerkes, wie z.B. die Brennkammerdimensionierung. Durch Zumischen von Kalkstein ist es jedoch möglich, die Schmelztemperaturen der Asche zu senken. Erfahrungen zeigen, daß durch eine Zumischung von ca. 2 % Kalkstein zur Kohle die Schmelztemperatur der Asche um ca. 100°C abgesenkt werden kann. Dieses Verfahren liefert für den Betrieb der Feuerung ein Regulativ.The temperature of the combustion or melting chamber and the melting temperature of the ash must be coordinated for particularly effective melting chamber operation, ie complete burnout, rapid fuel conversion and avoidance of NO x formation. The composition of the coal (depending on the composition, the ash melting temperature varies between 1300 ° C and 1700 ° C) determines the design of the coal-fired power plant, such as the size of the combustion chamber. By adding limestone, however, it is possible to lower the melting temperature of the ash. Experience shows that by adding approx. 2% limestone to coal, the melting temperature of the ash can be reduced by approx. 100 ° C. This procedure provides a regulation for the operation of the furnace.
Um einen hohen Wirkungsgrad durch vollkommenen Ausbrand des Brennstoffs zu erzielen, wird in modernen Kohlekraftwerken, welche nach dem Verfahren der Schmelzkammerfeuerung arbeiten, die Flugasche über eine separate Flugasche-Rückführung erneut in die Brennkammer eingeblasen. In diesem Fall fällt die gesamte Asche der Brenn- oder Schmelzkammer als Schlacke an und läßt sich auf die übliche Weise entsorgen.To achieve a high level of efficiency through complete burnout of the Obtaining fuel is used in modern coal-fired power plants, which work according to the melting chamber firing method, the fly ash again via a separate fly ash return blown into the combustion chamber. In this case, the entire falls Ash from the combustion or melting chamber as slag and can be disposed of in the usual way.
Durch die Flugasche-Rückführung wird zwar ein vollkommener Ausbrand des Brennstoffs erzielt, jedoch erhöht sich die mittlere Verweilzeit eines Kohle- bzw. Aschepartikels in dem Feuerungskreislauf. Als Nachteil wird damit die maximale Durchsatzmenge an Kohle und damit die mögliche Leistung des Kraftwerks limitiert.The fly ash return makes it a perfect one Burnout of the fuel achieved, however, the increases average residence time of a coal or ash particle in the Feuerungskreislauf. The disadvantage is the maximum Throughput of coal and thus the possible performance of the Power plant limited.
Der Erfindung liegt daher die Aufgabe zugrunde, ein günstiges Verfahren zum Betreiben eines Kohlekraftwerkes, welches nach dem Verfahren der Schmelzkammerfeuerung arbeitet, zu schaffen, mit dem der Durchsatz an Brennstoff und damit die Leistung des Kraftwerks erhöht werden kann. Dies soll mit einer zur Durchführung des Verfahrens geeigneten Verbrennungsanlage mit besonders einfachen Mitteln erreicht werden.The invention is therefore based on the object of an inexpensive Method for operating a coal-fired power plant, which according to the process of melting chamber firing works, with which the throughput of fuel and thus the performance of the power plant can be increased. This is supposed to be with a incinerator suitable for carrying out the method can be achieved with particularly simple means.
Bezüglich des Verfahrens wird diese Aufgabe erfindungsgemäß
gelöst, durch die Merkmale des Patentanspruchs 1.
Dabei sollte Titandioxid,
höchstens in einem Titandioxid:Kohle-Verhältnis von
3:97 vorliegen.With regard to the method, this object is achieved according to the invention
solved by the features of
Die Erfindung geht dabei von der Beobachtung aus, daß Titandioxid den Ausbrand der Kohle in der Brennkammer und damit den Durchsatz an Kohle erhöhen kann, was wiederum zu einer Leistungssteigerung des Kraftwerks führt.The invention is based on the observation that titanium dioxide the burning out of the coal in the combustion chamber and thus can increase the throughput of coal, which in turn Performance increase of the power plant leads.
Für einen effektiven Feuerungsbetrieb soll die Viskosität und die Schmelztemperatur der Asche, wie eingangs erwähnt, durch die Zugabemenge an titanhaltigen Materialien nicht wesentlich verändert werden. Insbesondere soll die Zugabe an Titan, das unter den Bedingungen der Schmelzkammer als Titandioxid vorliegt, verschlackungsartige Ansätze hinter der Brennkammer, die sich an Rohren und Wänden festsetzen, nicht begünstigen. Es hat sich gezeigt, daß Titandioxid den Schmelzpunkt der Asche bzw. der Schlacke senkt. Aus einem sandartigen, nicht geschmolzenen und nicht haftenden Staub könnte dadurch eine zähe, fließende und haftende Schmelze werden, die zu höheren Reinigungskosten und finanziellen Einbußen während der Wartung des Kohlekraftwerks führt. Es wurde jedoch gefunden, daß sich das Titandioxid weitgehend in der Flüssigasche wiederfindet. Bei Titandioxidgehalten unter etwa 3 % in der zugeführten Gesamtmenge an Kohle und titandioxidhaltigem Material wird erreicht, daß sich die Konsistenz der verschlackungsartigen Ansätze nicht ändert, da sich das Titandioxid nun praktisch nur in der Flüssigasche befindet. In vorteilhafter Ausgestaltung beträgt der Titandioxidanteil in der zugegebenen Gesamtmenge an Kohle und titandioxidhaltigen Materialien höchstens 2,25 %.For effective firing operation, the viscosity and the melting temperature of the ash, as mentioned at the beginning, by the addition amount of titanium-containing materials is not essential to be changed. In particular, the addition of titanium, the is present as titanium dioxide under the conditions of the melting chamber, slag-like approaches behind the combustion chamber, that stick to pipes and walls, do not favor. It has been shown that titanium dioxide has the melting point of Ash or slag lowers. From a sand-like, not melted and non-sticking dust could result in a viscous, flowing and sticky melt become the higher Cleaning costs and financial losses during maintenance of the coal-fired power plant. However, it was found that the titanium dioxide is largely found in the liquid ash. With titanium dioxide levels below about 3% in the total amount of coal and titanium dioxide Material is achieved that the consistency of the slag-like approaches do not change since the titanium dioxide now practically only in the liquid bag. In In an advantageous embodiment, the titanium dioxide content is in the total amount of coal and materials containing titanium dioxide added at most 2.25%.
Dieser Befund ist überraschend, denn auch geringere Titandioxidanteile in der Mischung aus Kohle und titandioxidhaltigen Materialien führen bei einem Kohlekraftwerk mit Trockenfeuerungsanlage bereits zu einer erheblichen Intensivierung der Verschlackung hinter der Brennkammer und zu einer fließenden Konsistenz der Schlacke. Solche titandioxidhaltigen Zusätze sind daher besonders für den Betrieb eines Kohlekraftwerkes mit Schmelzkammerfeuerung geeignet.This finding is surprising, because the titanium dioxide content is also lower in the mixture of coal and materials containing titanium dioxide lead at a coal-fired power plant with dry combustion system already to a considerable intensification of the slagging behind the combustion chamber and to a flowing Slag consistency. Such additives are titanium dioxide therefore especially for the operation of a coal-fired power plant Melting chamber firing suitable.
Vorteilhaft besteht das zugeführte titandioxidhaltige Material zu mehr als 50 % aus Titandioxid. Dadurch kann selbst bei einer kleinen Zugabemenge eine Beschleunigung des Kohleausbrandes erzielt werden. Vorteilhaft ist dabei ein Titandioxid:Kohle-Verhältnis von mindestens 1:99.The supplied titanium dioxide-containing material is advantageous more than 50% from titanium dioxide. This can even with a small additions an acceleration of coal burnout be achieved. A titanium dioxide: carbon ratio is advantageous here of at least 1:99.
Bei einer Kraftwerksanlage ohne Flugasche-Rückführung in die Schmelzkammer wird nach einem Ausführungsbeispiel der Erfindung das zugegebene Titan als Titandioxid zu einem geringen Teil über Flugasche, überwiegend aber über Flüssigasche ausgeschieden. Da Titandioxid nicht toxisch wirkt, kann nicht nur die Flüssigasche, sondern auch die Flugasche wie üblich weiter verwendet werden. Arbeitet das Kohlekraftwerk mit einer Flugasche-Rückführung, wird die entstehende Flugasche in die Feuerung zurückgeführt, so daß das Titan praktisch ausschließlich als Titandioxid zusammen mit der entstehenden Flüssigasche ausgeschieden wird. In a power plant without fly ash return to the Melting chamber is made according to an embodiment of the invention the added titanium as titanium dioxide to a low Partly excreted via fly ash, but mostly via liquid ash. Since titanium dioxide is not toxic, it cannot only the liquid ash, but also the fly ash as usual continue to be used. The coal-fired power plant works with one Fly ash return, the resulting fly ash is in the furnace returned so that the titanium is practically exclusively as titanium dioxide together with the resulting Liquid ash is excreted.
Das titandioxidhaltige Material wird vorteilhafterweise der Kohle beigemischt, anschließend kann es mit dieser in einer Kohlemühle des Kraftwerkes vermahlen und über ein Kohleband über die Brenner in die Brennkammer des Kraftwerks eingeführt werden. Besonders einfach kann das titandioxidhaltige Material aber auch pneumatisch in die Brennkammer, vorzugsweise über die Flugasche-Rückführung, eingeblasen werden.The material containing titanium dioxide advantageously becomes coal added, then it can be used in a coal mill of the power plant and ground over a coal belt the burners are inserted into the combustion chamber of the power plant. However, the material containing titanium dioxide can be particularly simple also pneumatically into the combustion chamber, preferably via the Fly ash return, to be blown in.
In vielen Fällen kann es auch vorteilhaft sein, die Flüssigasche am Brennkammerboden in einen Naßentschlacker zu leiten und zu Granulat zu verarbeiten. Dadurch können Zuschlagstoffe im beigemischten titandioxidhaltigen Material gefahrlos in das entstehende Granulat eingeschmolzen werden.In many cases it can also be beneficial to use the liquid ash to pass into a wet slag remover at the bottom of the combustion chamber and process it into granules. This allows aggregates in the admixed titanium dioxide-containing material safely into the resulting Granules are melted down.
Eine Gefahr für die Umwelt bei Verwendung des Granulats als Baumaterial besteht nicht, weil die eingeschmolzenen Zuschlagstoffe, wie z.B. Schwermetalle, unlöslich in das Granulat eingebunden sind.A danger to the environment when using the granulate as There is no building material because the melted aggregates, such as. Heavy metals, insoluble in the granules are involved.
Bei einer besonders vorteilhaften Variante des Verfahrens werden als titandioxidhaltiges Material gebrauchte, d.h. zu entsorgende DeNOx-Katalysatoren oder Abfallprodukte, z. B. der titanverarbeitenden Industrie, verwendet. Für gebrauchte DeNOx-Katalysatoren entsteht dabei ein billiger, umweltgerechter Entsorgungsweg, da ansonsten Kosten durch Deponierung oder teuere Wiederaufbereitungsmaßnahmen anfallen. Lediglich für bestimmte, weitgehend aus Titandioxid bestehende Katalysatoren, die 10 % Molybdän oder mehr enthalten, hat sich gezeigt, daß Schwermetalle (insbesondere Arsen) aus einem derart erzeugten Granulat in einem nachweisbaren Umfang ausgelaugt werden können. Bei einem DeNOx-Katalysator mit 4,5 % Molybdän wurde ein derartiges Auslaugen jedoch nicht gefunden, so daß sich lediglich für Katalysatoren mit derart hohem MolybdänGehalt Einschränkungen ergeben können.In a particularly advantageous variant of the process used as titanium dioxide-containing material, ie to be disposed of DeNO x catalysts or waste products, eg. B. the titanium processing industry used. For used DeNO x catalysts, this creates a cheaper, environmentally friendly disposal route, since otherwise costs are incurred through landfilling or expensive reprocessing measures. Only for certain catalysts, which consist largely of titanium dioxide and contain 10% molybdenum or more, has it been shown that heavy metals (in particular arsenic) can be leached out of a granulate produced in this way to a detectable extent. However, such leaching was not found in a DeNO x catalyst with 4.5% molybdenum, so that restrictions can only arise for catalysts with such a high molybdenum content.
Auch für die titanverarbeitende Industrie - in der BRD werden ca. 300.000 bis 400.000 Tonnen Titandioxid jährlich produziert - bietet sich das Verfahren als ein günstiger Entsorgungsweg für die Abfallprodukte, wie z.B. Titanschlacke, an.Also for the titanium processing industry - in the FRG approx. 300,000 to 400,000 tons of titanium dioxide are produced annually - The process offers itself as an inexpensive disposal route for the waste products, e.g. Titanium slag.
Ausführungsbeispiele werden anhand einer Zeichnung näher erläutert. Darin zeigen:
- FIG 1
- eine schematische Darstellung einer Verbrennungsanlage eines Kohlekraftwerks mit einer Schmelzkammer, einer Kohlemühle, einer DeNOx-Anlage und einer Granulaterzeugung;
- FIG 2
- ein Kohlekraftwerk gemäß
Figur 1 mit einer Flugasche-Rückführung; - FIG 3
- in einem ersten Diagramm die Masse an Flugasche bei steigender Zugabe von verbrauchtem Katalysatormaterial;
- FIG 4
- in einem zweiten Diagramm den brennbaren Anteil in der Flugasche als Funktion des Katalysatoranteils in der Kohlemischung; und
- FIG 5 - 7
- in einem dritten, vierten bzw. fünften Diagramm den Gehalt an Katalysatorbestandteilen (TiO2, V2O5, WO3) eines DeNOx-Katalysators in der Schlacke, in der Flugasche bzw. in den schlackeartigen Abscheidungen an der Brennkammer nachgeordneten Bauteilen, jeweils als Funktion des Katalysatoranteils in der Kohlemischung.
- FIG. 1
- a schematic representation of a combustion plant of a coal-fired power plant with a melting chamber, a coal mill, a DeNO x plant and a granulate production;
- FIG 2
- a coal-fired power plant according to Figure 1 with a fly ash return;
- FIG 3
- in a first diagram the mass of fly ash with increasing addition of spent catalyst material;
- FIG 4
- in a second diagram, the combustible fraction in the fly ash as a function of the catalyst fraction in the coal mixture; and
- FIG 5-7
- in a third, fourth or fifth diagram the content of catalyst components (TiO 2 , V 2 O 5 , WO 3 ) of a DeNO x catalyst in the slag, in the fly ash or in the slag-like deposits on the components downstream of the combustion chamber, in each case as a function of the proportion of catalyst in the coal mixture.
Die in Figur 1 dargestellte Verbrennungsanlage 1
ist Teil eines nicht näher
dargestellten Kohlekraftwerkes. Sie umfaßt eine als Schmelzkammer
2 ausgebildete Hochtemperaturbrennkammer mit mindestens
einem Brenner 2a, und mit einer Zuführung 2b, z.B. einem
Förderband für die Kohle K, sowie eine über einen Verdichter
3 geführte Frischluftleitung 4. Sie umfaßt weiter eine
Abzugsleitung 5 für Flüssigasche F mit einem daran angeschlossenen
Naßentschlacker 6. Sie umfaßt ferner eine Rauchgasleitung
7 und in der Rauchgasleitung 7 in Serie geschaltet
eine Staubfilteranlage 8 mit einem Flugaschesammler 9, eine
Rauchgasentschwefelungsanlage 10 und eine katalytische Entstickungsanlage
11. Die Rauchgasleitung 7 mündet in einen Kamin
12. Die Zuführung 2b ist an eine Kohlemühle 13 angeschlossen,
die einem Zuführschacht 14 eines Kohlelagers 15
und mit einer separaten Zuführungsleitung 16 zur Zugabe titandioxidhaltigen
Materials M verbunden ist. Über die zugeführte
Menge an titandioxidhaltigem Material M wird dabei die Ausbrandbeschleunigung
der Kohle K in der Brennkammer 2 eingestellt.
Beim Betrieb des Kohlekraftwerks wird die Kohle K vom Kohlelager
15 über den Zuführschacht 14 in die Kohlemühle 13 gefördert.
Das titandioxidhaltige Material M wird entweder über die
Zuführungsleitung 16 und den Zuführschacht 14 oder direkt in
die Kohlemühle 13 eingeführt und dort zusammen mit der Kohle
K staubfein zermahlen. Derart aufbereiteter Brennstoff B gelangt
über die Zuführung 2b und den Brenner 2a in die Brennkammer
2. Dort wird er mit über die Frischluftleitung 4 zugeführter
verdichteter Luft L verbrannt. Entstehendes Rauchgas
RG strömt über die Rauchgasleitung 7 in die Staubfilteranlage
8, wo vom Rauchgas mitgerissene Flugasche oder Flugstaub S
abgefangen und über den Flugaschesammler 9 abgeführt wird.
Das nun praktisch staubfreie Rauchgas RG gelangt zur Rauchgasentschwefelungsanlage
10 und über die allgemein als DeNOx-Anlage
bezeichnete Entstickungsanlage 11 in den Kamin 12. The
Die sich am Brennkammerboden 2c sammelnde Flüssigasche F wird
über die Abzugsleitung 5 dem Naßentschlacker 6 zugeführt und
zu Granulat G verarbeitet.The liquid ash F collecting on the
Die am Sammler 9 gesammelte Flugasche S kann wie üblich verwertet
werden. Vorteilhaft wird bis zu 3 % titandioxidhaltiges Material
M mit einem Titandioxidgehalt von mehr als 50 % verwendet.
In diesem Material M enthaltene Zuschlagstoffe oder
Verunreinigungen, wie z.B. Schwermetalle, werden unlöslich in
das gewonnene Granulat G eingeschmolzen. Dieses Schmelzkammergranulat
G kann wie üblich als Baumaterial verwendet werden.The fly ash S collected at the
Gemäß Figur 2 weist die Verbrennungsanlage 1 mit
Schmelzkammerfeuerung eine Flugasche-Rückführung 20 auf.
Diese mündet direkt in die Brennkammer 2 der Schmelzkammerfeuerung.
Die in der Staubfilteranlage 8 über den Sammler 9
zurückgehaltene Flugasche S wird pneumatisch mit Hilfe eines
zusätzlichen Verdichters 21 in die Brennkammer 2 eingeblasen.
Über eine separate Zuführungsleitung 22 wird titandioxidhaltiges,
staubfein gemahlenes Material M der Flugasche S beigemischt
und gelangt mit dieser in die Brennkammer 2. Durch Zugabe von
titandioxidhaltigem Material M in die Brennkammer 2 des Kohlekraftwerks
mit Schmelzkammerfeuerung in Kombination mit einer
Flugasche-Rückführung 20 wird besonders effektiver Ausbrand
bei einer gleichzeitigen Beschleunigung des Durchsatzes an
Kohle K im Kraftwerk erzielt. Dies steigert die Leistung des
Kraftwerks.According to Figure 2, the
In der Flugasche S enthaltene, mit Schwermetall belastete Zuschläge sowie Titandioxid werden unlöslich in das entstehende Schmelzkammergranulat G eingebunden. Auf diese Weise können verbrauchte DeNOx-Katalysatoren mit mehr als 50 % TiO2 problemlos entsorgt werden. Additives contained in the fly ash S, which are contaminated with heavy metal, and titanium dioxide are insolubly incorporated into the resulting melting chamber granules G. In this way, used DeNO x catalysts with more than 50% TiO 2 can be disposed of without any problems.
Im folgenden werden Untersuchungsergebnisse erläutert. Darin bedeuten Teile Massenanteile.Test results are explained below. In this parts mean parts by mass.
Beispiel 1: Als titandioxidhaltiges Material M werden verbrauchte DeNOx-Katalysatoren verwendet und mit Kohle K vermischt. Als Kohle K wird eine hochentkohlte, ballastreiche Steinkohle verwendet, die nach ihrem Entkohlungsgrad und dem Anteil an flüchtigen Bestandteilen zu den Magerkohlen gehört und an der Grenze zwischen Magerkohlen und Anthrazitkohlen liegt. Die Asche dieser Kohle zeigt ein normales Schmelzverhalten. Der verwendete Katalysator besteht zu etwa 75 % aus TiO2 und enthält weitere katalytische Komponenten (ca. 11 % SiO2, ca. 8 % WO3 und ca. 1,8 % V2O5).Example 1: Used DeNO x catalysts are used as the titanium dioxide-containing material M and mixed with coal K. A highly decarburized, high-ballast hard coal is used as coal K, which, according to its degree of decarburization and the proportion of volatile constituents, belongs to lean coal and lies on the border between lean coal and anthracite coal. The ashes of this coal show normal melting behavior. The catalyst used consists of approximately 75% TiO 2 and contains further catalytic components (approx. 11% SiO 2 , approx. 8% WO 3 and approx. 1.8% V 2 O 5 ).
Bei einem Katalysatoranteil MK von 0 %, 1 % und 3 % in der
Mischung aus Katalysatormaterial und Kohle werden Verbrennungsversuche
in einer Brennkammer 2 durchgeführt. Die Brennkammer
2 ist als Laborbrennkammer jeweils mit einem Flüssigascheabzug
und einem Trockenascheabzug ausgebildet. Untersucht
werden die Zusammensetzung der Asche, die Beeinflussung
des Verschlackungsverhaltens der Kohle durch Zusatz von verbrauchtem
Katalysator, der Einfluß des Katalysatoranteils MK
auf die Verschlackungsintensität der Heizflächen hinter der
Brennkammer sowie die Verteilung des Katalysatormaterials in
den Verbrennungsrückständen. Es wird eine Röntgen-Fluoreszenz-Analyse
dieser Verbrennungsrückstände durchgeführt.With a catalyst fraction M K of 0%, 1% and 3% in the mixture of catalyst material and coal, combustion tests are carried out in a
Figuren 3 bis 7 zeigen die Untersuchungsergebnisse beispielhaft für die Brennkammer mit Flüssigascheabzug. Figur 3 zeigt die bei der Verbrennung entstehende Masse an Flugasche SM pro Kilogramm Kohle als Funktion des zugeführten Katalysatoranteils MK. Es zeigt sich, daß bis zu einem Katalysatoranteil MK von 3 % sich die Masse der Flugasche SM nicht verändert (Kurve a). Überraschenderweise zeigt sich aber sehr deutlich, daß der Katalysatoranteil den Ausbrand der Kohle (gemessen an dem Anteil BS an Brennbarem in der Flugasche) verbessert (Kurve b in Figur 4). Bei einem Katalysatoranteil MK von 3 % in der Mischung aus Kohle und Katalysator verringert sich der Anteil BS an Brennbarem in der Flugasche gegenüber MK = 0 % von 50 % auf 30 %.FIGS. 3 to 7 show the test results as an example for the combustion chamber with liquid ash extraction. FIG. 3 shows the mass of fly ash S M produced per kilogram of coal as a function of the catalyst fraction M K supplied. It can be seen that the mass of the fly ash S M does not change up to a catalyst fraction M K of 3% (curve a). Surprisingly, however, it can be seen very clearly that the catalyst fraction improves the burnout of the coal (measured in terms of the fraction B S of combustibles in the fly ash) (curve b in FIG. 4). With a catalyst proportion M K of 3% in the mixture of coal and catalyst, the proportion B S of combustibles in the fly ash is reduced from 50% to 30% compared to M K = 0%.
Die Kurven c, d und e der Figuren 5 bis 7 zeigen den prozentualen Anteil der aktiven Katalysatorsubstanzen TiO2 (Figur 5), V2O5 (Figur 6) und WO3 (Figur 7) in der Schlacke F, in der Flugasche S bzw. in den verschlackungsartigen Ansätzen. Ein weiteres überraschendes Ergebnis ist, daß sich der Katalysator vor allem in der Schlacke oder Flüssigasche F (Kurve c, Figuren 5 bis 7) und teilweise in der Flugasche S (Kurve d, Figuren 5 bis 7), aber kaum in den verschlackungsartigen Ansätzen (Kurve e, Figuren 5 bis 7) findet. Mit wachsendem Katalysatoranteil MK (0 bis 3 %) im Brennstoff nehmen nur die Anteile an TiO2 (Figur 5), V2O5 (Figur 6) und WO3 (Figur 7) in der Schlacke F und in der Flugasche S deutlich zu. In den verschlackungsartigen Ansätzen hinter der Brennkammer bleiben sie aber praktisch unverändert.Curves c, d and e of FIGS. 5 to 7 show the percentage of active catalyst substances TiO 2 (FIG. 5), V 2 O 5 (FIG. 6) and WO 3 (FIG. 7) in the slag F, in the fly ash S. or in the slag-like approaches. Another surprising result is that the catalyst is found primarily in the slag or liquid ash F (curve c, FIGS. 5 to 7) and partly in the fly ash S (curve d, FIGS. 5 to 7), but hardly in the slag-like approaches ( Curve e, Figures 5 to 7) takes place. With increasing catalyst proportion M K (0 to 3%) in the fuel, only the proportions of TiO 2 (FIG. 5), V 2 O 5 (FIG. 6) and WO 3 (FIG. 7) in the slag F and in the fly ash S become clear to. In the slag-like approaches behind the combustion chamber, however, they remain practically unchanged.
In dem Abkühlungsbereich wird kein einziges Mal eine intensivere Verschlackung hinter der Brennkammer festgestellt (Tabelle 1). Die kleinen Mengen an verschlackungsartigen Ansätzen hinter der Brennkammer sind in jedem Fall weich, nicht geschmolzen und nicht haftend. Die Tatsache, daß der zusätzliche Katalysatoranteil bis 3 % hinter der Brennkammer mit flüssigem Ascheabzug keine Veränderung des Verschlackungsverhaltens verursacht, ist dadurch zu erklären, daß sich der Katalysator kaum in den Ansätzen wiederfinden läßt.In the cooling area, there is never a more intense one Slagging found behind the combustion chamber (Table 1). The small amounts of slag-like approaches behind the combustion chamber are definitely not soft melted and not sticky. The fact that the additional Catalyst share up to 3% behind the combustion chamber liquid ash removal no change in slagging behavior is caused by the fact that the catalyst can hardly be found in the beginning.
Die Untersuchungen, welche in der Laborbrennkammer mit trokkenem
Ascheabzug (Trockenfeuerung) durchgeführt werden, zeigen
deutlich, daß die Ansatzbildung bei wachsendem Katalysatoranteil
stark intensiviert wird (Tabelle 1). Die Ansätze
hinter der Brennkammer mit trockenem Ascheabzug weisen eine
harte geschmolzene Struktur auf und zeigen schon in der
Brennkammer ein deutliches Fließverhalten.
Beispiel 2: Flugasche aus einem Elektrofilter eines Kohlekraftwerks mit Schmelzkammerfeuerung wird mit Kalciumkarbonat (CaCO3) in einem Masseverhältnis von 100:5 gemischt. Dadurch kann direkt eine Schmelze erhalten werden ("Nullprobe"). Die gleiche Mischung wird zum Vergleich mit staubfein gemahlenem, gebrauchtem DeNOx-Katalysator in einer solchen Weise vermengt, daß der Katalysator-Anteil 1 % beträgt. Die Mischung wird bei 1550 °C 20 Minuten lang geschmolzen und in Wasser abgeschreckt ("Vergleichsprobe"). Jeweils 5 g des erhaltenen Granulats G werden 24 Stunden mit 50 g H2O eluiert und das Eluat auf Spuren von Vanadium V, Wolfram W und Arsen As untersucht.Example 2: Fly ash from an electrostatic precipitator of a coal-fired power plant with smelting chamber firing is mixed with calcium carbonate (CaCO 3 ) in a mass ratio of 100: 5. As a result, a melt can be obtained directly ("zero test"). The same mixture is mixed for comparison with dust-finely ground, used DeNO x catalyst in such a way that the catalyst content is 1%. The mixture is melted at 1550 ° C for 20 minutes and quenched in water ("comparative sample"). 5 g of the granules G obtained are eluted with 50 g of H 2 O for 24 hours and the eluate is examined for traces of vanadium V, tungsten W and arsenic as.
Die aus der Vergleichsprobe ausgewaschene Menge der aktiven Katalysatorsubstanzen (V, W) liegt unterhalb der Nachweisgrenze (< 0,1 mg/l). Der Arsengehalt liegt bei beiden Proben im gleichen Bereich.The amount of active washed out from the comparative sample Catalyst substances (V, W) are below the detection limit (<0.1 mg / l). The arsenic content is in both samples in the same area.
Claims (13)
- Use of a process for operating a combustion plant of a coal-fired power station working according to the smelt chamber firing process, in order to accelerate coal combustion in a smelt chamber (2) with coal (K), wherein a titanium dioxide-containing material (M) is added and is combusted together with the coal (K) as a fuel (B), so that the acceleration of the combustion of the coal (K) in the smelt chamber (2) is adjusted by the added amount of titanium dioxide-containing material (M).
- Use according to claim 1, characterised in that titanium dioxide TiO2 is present at most in a titanium dioxide:coal ratio of 3:97.
- Use according to claim 1 or 2, characterised in that the titanium dioxide mass fraction in the added total amount of coal (K) and titanium dioxide-containing material (M) is at most 2.25%.
- Use according to claims 1 to 3, characterised in that the titanium dioxide-containing material (M) consists of more than 50% by mass of titanium dioxide.
- Use according to claim 4, characterised in that the mass ratio of the predominantly titanium dioxide-containing material to coal is below 3:97.
- Use according to one of claims 1 to 5, characterised in that the titanium dioxide:coal mass ratio is at least 1:99.
- Use according to claims 1 to 6, characterised in that the titanium dioxide is separated on the one hand through flue ash (S) and on the other hand through molten ash (F).
- Use according to claims 1 to 7, characterised in that flue ash (S) formed in the combustion is returned to the smelt chamber (2) and the titanium dioxide is separated together with molten ash (F).
- Use according to claims 1 to 8, characterised in that the titanium dioxide-containing material (M) is mixed with the coal (K).
- Use according to claims 1 to 8, characterised in that the titanium dioxide-containing material (M) is blown pneumatically into the smelt chamber (2), preferably through a flue ash recycling line (20).
- Use according to claims 7 to 10, characterised in that the molten ash (F) is processed in a wet slag removal means (6) to form granular material (G), in which the titanium dioxide is fused.
- Use according to claims 1 to 11, characterised in that DeNOx catalysts to be disposed of are used as titanium dioxide-containing material (M).
- Use according to claims 1 to 11, characterised in that titanium dioxide-containing waste products are used as titanium dioxide-containing material (M).
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19534558A DE19534558C1 (en) | 1995-09-18 | 1995-09-18 | Operating combustion appts. of coal power station |
| DE19534558 | 1995-09-18 | ||
| PCT/DE1996/001721 WO1997011139A1 (en) | 1995-09-18 | 1996-09-12 | Process for operating a combustion plant of a coal-fired power station with slag tap firing and combustion plant operating thus |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0858495A1 EP0858495A1 (en) | 1998-08-19 |
| EP0858495B1 true EP0858495B1 (en) | 2003-07-02 |
Family
ID=7772466
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP96929184A Expired - Lifetime EP0858495B1 (en) | 1995-09-18 | 1996-09-12 | Use of a process operating a combustion plant of a coal-fired power station for the accelerated coal combustion in a smelt chamber |
Country Status (12)
| Country | Link |
|---|---|
| US (1) | US6067914A (en) |
| EP (1) | EP0858495B1 (en) |
| JP (1) | JP2989272B2 (en) |
| KR (1) | KR19990045747A (en) |
| CN (1) | CN1197477A (en) |
| AT (1) | ATE244292T1 (en) |
| CA (1) | CA2232476A1 (en) |
| DE (2) | DE19534558C1 (en) |
| ES (1) | ES2202461T3 (en) |
| RU (1) | RU2152428C1 (en) |
| TW (1) | TW301698B (en) |
| WO (1) | WO1997011139A1 (en) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4909296B2 (en) * | 2008-02-12 | 2012-04-04 | 三菱重工業株式会社 | Heavy fuel fired boiler system and operating method thereof |
| CN101524695B (en) * | 2009-04-03 | 2011-06-08 | 沈阳航空工业学院 | Method for utilizing flying ash in electric power plant to produce floating beads |
| WO2015060795A1 (en) * | 2013-10-21 | 2015-04-30 | Dora Teknolojik Bilgisayar Ürünleri Endüstrisi Anonim Şirketi | Process for the minimization/elimination of so2 and co2 emission emerging from the combustion of coal |
| CN106635242A (en) * | 2016-12-07 | 2017-05-10 | 江西稀有金属钨业控股集团有限公司 | Method and device for utilizing scheelite concentrate smelting slag and application of scheelite concentrate smelting slag |
| CN114574262B (en) * | 2022-03-04 | 2022-12-13 | 安徽工业大学 | Coal-fired catalyst produced by using titanium white waste acid and preparation method thereof |
Family Cites Families (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1947460A (en) * | 1927-01-31 | 1934-02-20 | Coutant Jay Gould | Pulverized fuel furnace and method of combustion |
| CH253222A (en) * | 1945-11-08 | 1948-02-29 | Rothenbach Walter Ing Dipl | Process for producing a fuel. |
| BE703706A (en) * | 1967-09-11 | 1968-02-01 | ||
| GB1402207A (en) * | 1972-03-03 | 1975-08-06 | Siemens Ag | Catalyst and its use in hydrocarbon cracking processes |
| US4057398A (en) * | 1976-02-24 | 1977-11-08 | Apollo Chemical Corporation | Process for reducing the fusion point of coal ash |
| US4388877A (en) * | 1981-07-07 | 1983-06-21 | Benmol Corporation | Method and composition for combustion of fossil fuels in fluidized bed |
| DE3128903C2 (en) * | 1981-07-22 | 1983-09-08 | L. & C. Steinmüller GmbH, 5270 Gummersbach | "Method for introducing additive into a reaction gas stream" |
| US4377118A (en) * | 1981-12-21 | 1983-03-22 | Nalco Chemical Company | Process for reducing slag build-up |
| US4577566A (en) * | 1982-04-01 | 1986-03-25 | Betz Laboratories, Inc. | Method of conditioning fireside fouling deposits using large particle size amorphous silica |
| DE3504122C2 (en) * | 1985-02-07 | 1994-02-03 | Kat Tec Ges Fuer Katalysatorte | Catalyst for the conversion of hydrocarbons, nitrogen oxides and fuel gases |
| JPS6348392A (en) * | 1986-08-15 | 1988-03-01 | Toa Netsuken Kk | Method of controlling clinker ash of coal exhaust gas dust |
| DE3635027A1 (en) * | 1986-10-15 | 1988-04-28 | Steinmueller Gmbh L & C | DIRECT DESOLFURATION PROCESS WITH FLUE DUST RETURN |
| US4771712A (en) * | 1987-06-24 | 1988-09-20 | A. Ahlstrom Corporation | Combustion of fuel containing alkalines |
| DE3741604C1 (en) * | 1987-12-09 | 1989-02-23 | Metallgesellschaft Ag | Process for separating the ash from the gas produced by burning coal |
| DE58904374D1 (en) * | 1988-01-14 | 1993-06-24 | Siemens Ag | METHOD AND DEVICE FOR PURIFYING SMOKE GASES. |
| US4836117A (en) * | 1988-01-15 | 1989-06-06 | The Standard Oil Company | Oxidation catalyst and processes using same |
| US4843980A (en) * | 1988-04-26 | 1989-07-04 | Lucille Markham | Composition for use in reducing air contaminants from combustion effluents |
| US4979447A (en) * | 1988-06-08 | 1990-12-25 | Velino Ventures Inc. | Combustion of carbon containing materials in a furnace |
| JPH03244692A (en) * | 1990-02-23 | 1991-10-31 | Taiho Ind Co Ltd | Fuel additive |
| DE4013720C2 (en) * | 1990-04-28 | 1994-05-19 | Huels Chemische Werke Ag | Process for recycling used DeNOx catalysts |
| DE4021362A1 (en) * | 1990-07-05 | 1992-01-09 | Siemens Ag | Disposal of solids loaded with pollutants - in slagging furnace with total ash recycle |
| DE4209166A1 (en) * | 1992-03-20 | 1993-09-23 | Siemens Ag | Catalytic cleaning of flue gas - involves introducing comminuted fresh, waste or spent catalytic material |
| US5309850A (en) * | 1992-11-18 | 1994-05-10 | The Babcock & Wilcox Company | Incineration of hazardous wastes using closed cycle combustion ash vitrification |
| DE4301814A1 (en) * | 1993-01-23 | 1994-07-28 | Steinmueller Gmbh L & C | Process for burning waste consisting essentially of plastic, in particular PVC waste |
| US5819672A (en) * | 1995-04-06 | 1998-10-13 | Addchem Systems | Treatment to enhance heat retention in coal and biomass burning furnaces |
-
1995
- 1995-09-18 DE DE19534558A patent/DE19534558C1/en not_active Expired - Fee Related
-
1996
- 1996-09-12 WO PCT/DE1996/001721 patent/WO1997011139A1/en not_active Application Discontinuation
- 1996-09-12 ES ES96929184T patent/ES2202461T3/en not_active Expired - Lifetime
- 1996-09-12 CA CA002232476A patent/CA2232476A1/en not_active Abandoned
- 1996-09-12 CN CN96197176A patent/CN1197477A/en active Pending
- 1996-09-12 JP JP9512311A patent/JP2989272B2/en not_active Expired - Fee Related
- 1996-09-12 KR KR1019980701990A patent/KR19990045747A/en not_active Application Discontinuation
- 1996-09-12 AT AT96929184T patent/ATE244292T1/en not_active IP Right Cessation
- 1996-09-12 DE DE59610578T patent/DE59610578D1/en not_active Expired - Lifetime
- 1996-09-12 EP EP96929184A patent/EP0858495B1/en not_active Expired - Lifetime
- 1996-09-12 RU RU98107258/12A patent/RU2152428C1/en not_active IP Right Cessation
- 1996-09-18 TW TW085111387A patent/TW301698B/zh active
-
1998
- 1998-03-18 US US09/040,970 patent/US6067914A/en not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| DE19534558C1 (en) | 1996-11-07 |
| EP0858495A1 (en) | 1998-08-19 |
| TW301698B (en) | 1997-04-01 |
| CN1197477A (en) | 1998-10-28 |
| CA2232476A1 (en) | 1997-03-27 |
| JP2989272B2 (en) | 1999-12-13 |
| ES2202461T3 (en) | 2004-04-01 |
| JPH11502897A (en) | 1999-03-09 |
| US6067914A (en) | 2000-05-30 |
| WO1997011139A1 (en) | 1997-03-27 |
| RU2152428C1 (en) | 2000-07-10 |
| DE59610578D1 (en) | 2003-08-07 |
| KR19990045747A (en) | 1999-06-25 |
| ATE244292T1 (en) | 2003-07-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0118931B1 (en) | Afterburning and cleaning proces of off-gases | |
| EP0437679B1 (en) | Process for the treatment of ash in incinerator plants and an incinerator plant for this process | |
| DE3915992A1 (en) | Process for the reduction of nitrogen oxides | |
| CH622082A5 (en) | ||
| EP0338103B1 (en) | Process for diminishing harmful emissions when operating coal combustion plants | |
| DE3836899C1 (en) | ||
| EP0372039A1 (en) | Process and device for processing residues from refuse incinerators. | |
| EP1007746A1 (en) | Method for operating a sintering plant, and sintering plant | |
| EP0862019B1 (en) | Method and device for thermal treatment of fly ash from grate incinerators | |
| EP0340644B1 (en) | Process for the removal and recycling of waste materials | |
| DE3247228C2 (en) | Process for the recovery of unburned coal from coal ash | |
| EP0324454B2 (en) | Process and apparatus for cleaning smoke | |
| EP0368962B1 (en) | Process and device for cleaning slag from refuse incinerators | |
| EP0858495B1 (en) | Use of a process operating a combustion plant of a coal-fired power station for the accelerated coal combustion in a smelt chamber | |
| DE3604318C2 (en) | Process for burning cow dung | |
| DE4013720C2 (en) | Process for recycling used DeNOx catalysts | |
| DE3520447A1 (en) | Process and plant for thermally treating a fine granular material such as cement raw meal, using fuel-containing wastes and/or low-grade fuels | |
| EP1359374B1 (en) | Process for treating residues of an incineration plant | |
| EP1647770B1 (en) | Method of influencing the properties of incineration residues of an incineration plant | |
| DE3733831C2 (en) | ||
| DE10341610B4 (en) | Process for the incineration of solid waste | |
| DE4209166A1 (en) | Catalytic cleaning of flue gas - involves introducing comminuted fresh, waste or spent catalytic material | |
| DE3841221A1 (en) | Process for purifying the flue gases of combustion plants | |
| EP1111305A1 (en) | Process for thermal processing of grate ashes from waste incinerators | |
| DE3324411A1 (en) | Process for the combustion of pulverulent fuel and steam generator for carrying out the process |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19980304 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT CH DE DK ES FI FR GB IT LI NL SE |
|
| 17Q | First examination report despatched |
Effective date: 19990121 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: STEAG ENCOTEC GMBH Owner name: SIEMENS AKTIENGESELLSCHAFT |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| RTI1 | Title (correction) |
Free format text: USE OF A PROCESS OPERATING A COMBUSTION PLANT OF A COAL-FIRED POWER STATION FOR THE ACCELERATED COAL COMBUSTION IN A SMELT CHAMBER |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): AT CH DE DK ES FI FR GB IT LI NL SE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20030702 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20030702 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REF | Corresponds to: |
Ref document number: 59610578 Country of ref document: DE Date of ref document: 20030807 Kind code of ref document: P |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20031002 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20031002 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: NV Representative=s name: DR. LUSUARDI AG |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 20031022 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2202461 Country of ref document: ES Kind code of ref document: T3 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20040405 |
|
| EN | Fr: translation not filed | ||
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PFA Owner name: SIEMENS AKTIENGESELLSCHAFT Free format text: SIEMENS AKTIENGESELLSCHAFT#WITTELSBACHERPLATZ 2#80333 MUENCHEN (DE) $ STEAG ENCOTEC GMBH#RUETTENSCHEIDER STRASSE 1-3#45128 ESSEN (DE) -TRANSFER TO- SIEMENS AKTIENGESELLSCHAFT#WITTELSBACHERPLATZ 2#80333 MUENCHEN (DE) $ STEAG ENCOTEC GMBH#RUETTENSCHEIDER STRASSE 1-3#45128 ESSEN (DE) |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20080923 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20080922 Year of fee payment: 13 Ref country code: IT Payment date: 20080925 Year of fee payment: 13 Ref country code: AT Payment date: 20080919 Year of fee payment: 13 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PUEA Owner name: STEAG ENCOTEC GMBH Free format text: SIEMENS AKTIENGESELLSCHAFT#WITTELSBACHERPLATZ 2#80333 MUENCHEN (DE) $ STEAG ENCOTEC GMBH#RUETTENSCHEIDER STRASSE 1-3#45128 ESSEN (DE) -TRANSFER TO- STEAG ENCOTEC GMBH#RUETTENSCHEIDER STRASSE 1-3#45128 ESSEN (DE) $ ARGILLON GMBH#BAHNHOFSTRASSE 43#96257 REDWITZ (DE) Ref country code: CH Ref legal event code: PFA Owner name: ARGILLON GMBH Free format text: STEAG ENCOTEC GMBH#RUETTENSCHEIDER STRASSE 1-3#45128 ESSEN (DE) $ ARGILLON GMBH#BAHNHOFSTRASSE 43#96257 REDWITZ (DE) -TRANSFER TO- ARGILLON GMBH#BAHNHOFSTRASSE 43#96257 REDWITZ (DE) $ EVONIK ENERGY SERVICES GMBH#RUETTENSCHEIDER STRASSE 1-3#45128 ESSEN (DE) |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E Free format text: REGISTERED BETWEEN 20090528 AND 20090603 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: PC2A |
|
| NLS | Nl: assignments of ep-patents |
Owner name: STEAG ENCOTEC GMBH Effective date: 20100112 Owner name: ARGILLON GMBH Effective date: 20100112 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: V1 Effective date: 20100401 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090912 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20100401 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090930 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090912 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20110923 Year of fee payment: 16 Ref country code: ES Payment date: 20110926 Year of fee payment: 16 Ref country code: GB Payment date: 20110920 Year of fee payment: 16 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 59610578 Country of ref document: DE Representative=s name: FDST PATENTANWAELTE FREIER DOERR STAMMLER TSCH, DE Effective date: 20111020 Ref country code: DE Ref legal event code: R081 Ref document number: 59610578 Country of ref document: DE Owner name: JOHNSON MATTHEY CATALYSTS (GERMANY) GMBH, DE Free format text: FORMER OWNERS: ARGILLON GMBH, 96257 REDWITZ, DE; EVONIK ENERGY SERVICES GMBH, 45128 ESSEN, DE Effective date: 20111020 Ref country code: DE Ref legal event code: R081 Ref document number: 59610578 Country of ref document: DE Owner name: STEAG ENERGY SERVICES GMBH, DE Free format text: FORMER OWNERS: ARGILLON GMBH, 96257 REDWITZ, DE; EVONIK ENERGY SERVICES GMBH, 45128 ESSEN, DE Effective date: 20111020 Ref country code: DE Ref legal event code: R081 Ref document number: 59610578 Country of ref document: DE Owner name: STEAG ENERGY SERVICES GMBH, DE Free format text: FORMER OWNER: ARGILLON GMBH, EVONIK ENERGY SERVICES GMBH, , DE Effective date: 20111020 Ref country code: DE Ref legal event code: R081 Ref document number: 59610578 Country of ref document: DE Owner name: JOHNSON MATTHEY CATALYSTS (GERMANY) GMBH, DE Free format text: FORMER OWNER: ARGILLON GMBH, EVONIK ENERGY SERVICES GMBH, , DE Effective date: 20111020 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: PC2A Owner name: JOHNSON MATTHEY CATALYSTS(GERMANY)GMBH Effective date: 20120409 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20120912 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130403 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120912 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 59610578 Country of ref document: DE Effective date: 20130403 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20131021 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120913 |