EP0735181A2 - Multicolor dyeing with manganese compounds of fibrous materials containing polyamide fibres - Google Patents
Multicolor dyeing with manganese compounds of fibrous materials containing polyamide fibres Download PDFInfo
- Publication number
- EP0735181A2 EP0735181A2 EP96810166A EP96810166A EP0735181A2 EP 0735181 A2 EP0735181 A2 EP 0735181A2 EP 96810166 A EP96810166 A EP 96810166A EP 96810166 A EP96810166 A EP 96810166A EP 0735181 A2 EP0735181 A2 EP 0735181A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- manganese
- dyes
- dyeing
- compounds
- acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
Classifications
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P3/00—Special processes of dyeing or printing textiles, or dyeing leather, furs, or solid macromolecular substances in any form, classified according to the material treated
- D06P3/02—Material containing basic nitrogen
- D06P3/04—Material containing basic nitrogen containing amide groups
- D06P3/24—Polyamides; Polyurethanes
- D06P3/242—Polyamides; Polyurethanes using basic dyes
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P1/00—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed
- D06P1/44—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed using insoluble pigments or auxiliary substances, e.g. binders
- D06P1/64—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed using insoluble pigments or auxiliary substances, e.g. binders using compositions containing low-molecular-weight organic compounds without sulfate or sulfonate groups
- D06P1/642—Compounds containing nitrogen
- D06P1/647—Nitrogen-containing carboxylic acids or their salts
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P1/00—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed
- D06P1/44—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed using insoluble pigments or auxiliary substances, e.g. binders
- D06P1/653—Nitrogen-free carboxylic acids or their salts
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P1/00—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed
- D06P1/44—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed using insoluble pigments or auxiliary substances, e.g. binders
- D06P1/667—Organo-phosphorus compounds
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P1/00—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed
- D06P1/44—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed using insoluble pigments or auxiliary substances, e.g. binders
- D06P1/673—Inorganic compounds
- D06P1/67333—Salts or hydroxides
- D06P1/67341—Salts or hydroxides of elements different from the alkaline or alkaline-earth metals or with anions containing those elements
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P3/00—Special processes of dyeing or printing textiles, or dyeing leather, furs, or solid macromolecular substances in any form, classified according to the material treated
- D06P3/02—Material containing basic nitrogen
- D06P3/04—Material containing basic nitrogen containing amide groups
- D06P3/24—Polyamides; Polyurethanes
- D06P3/241—Polyamides; Polyurethanes using acid dyes
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P3/00—Special processes of dyeing or printing textiles, or dyeing leather, furs, or solid macromolecular substances in any form, classified according to the material treated
- D06P3/82—Textiles which contain different kinds of fibres
- D06P3/8204—Textiles which contain different kinds of fibres fibres of different chemical nature
- D06P3/8209—Textiles which contain different kinds of fibres fibres of different chemical nature mixtures of fibres containing amide groups
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P3/00—Special processes of dyeing or printing textiles, or dyeing leather, furs, or solid macromolecular substances in any form, classified according to the material treated
- D06P3/82—Textiles which contain different kinds of fibres
- D06P3/854—Textiles which contain different kinds of fibres containing modified or unmodified fibres, i.e. containing the same type of fibres having different characteristics, e.g. twisted and not-twisted fibres
Definitions
- the present invention relates to a process for achieving multicolor effects on mixtures of differently dyeable polyamide fiber materials in the presence of manganese compounds, which provides dyeings with improved light fastness, and the polyamide fiber material treated therewith.
- Polyamide fiber materials are thermally and / or photochemically sensitive.
- the polyamide fiber can be damaged by exposure to light and / or heat.
- the polyamide fiber material has to be matted, which is technically achieved by introducing titanium dioxide pigments.
- Pigmented polyamide fiber materials have proven to be particularly sensitive.
- the object of the present invention is to reduce the thermal and / or photochemical instability of polyamide fiber materials in order to achieve high light fastness, good tear resistance and good aging resistance.
- the invention therefore relates to a process for dyeing polyamide fiber mixtures which contain regularly dyeable polyamide fiber materials and acid-modified polyamide fiber materials, with dye mixtures comprising acid dyes and cationic dyes, which is characterized in that at least one manganese (II) before, during or after dyeing the bath. -Connection is added.
- II manganese
- the dyeings obtained with the process according to the invention show improved light fastness and improved tear resistance.
- All inorganic and organic salts and Mn (II) complex compounds can be considered as manganese (II) compounds in the process according to the invention.
- inorganic salts are: chlorides, acetates, phosphates, nitrates and sulfates.
- organic salts are: formates, oxalates and lactates.
- Mn (II) complex compounds are: Mn (II) complexes of citric acid, ethylenediaminetetraacetic acid or phosphonoalkane polycarboxylic acids.
- the Mn (II) compounds mentioned can also be used in any mixture with one another.
- the Mn (II) compounds can be added to the dye bath before, during or after the dyeing process.
- the Mn (II) compounds are advantageously added to the dyebath in amounts of 0.01 to 5 g / l, in particular 0.1 to 1 g / l, preferably 0.1 to 0.7 g / l.
- Suitable acid dyes for the process according to the invention are e.g. those in the Color Index, 3rd edition from 1971, Volume 1, under C.I. Acid Dyes on pages 1003 to 1561 as well as the dyes specified in the supplementary volumes.
- Suitable cationic dyes for the process according to the invention are e.g. those in the Color Index, 3rd edition from 1971, Volume 1, under C.I. Basic Dyes on pages 1611 to 1688 as well as the dyes specified in the supplementary volumes.
- the acid dyes and the cationic dyes which are used in the process according to the invention are known and can be prepared by known methods.
- the cationic dyes are chemically dyes which contain a colored (chromophore-containing) cation, in particular a quaternary nitrogen atom, and a colorless anion.
- the dyes are in the form of a salt with an inorganic or organic acid.
- sulfates, chlorides, acetates or Methyl sulfates of azo dyes such as monoazo, disazo and polyazo dyes, of anthraquinone dyes, phthalocyanine dyes, of diphenylmethane and triarylmethane dyes, of methine, polymethine, azomethine and azacyanine dyes, of thiazole, ketoneimine, acridine, cyanine, cyanine -, Quinoline, benzimidazole, xanthene, azine oxazine and thiazine dyes.
- Preferred cationic dyes are those which contain a quaternary nitrogen atom in a heterocyclic ring; however, cationic dyes can also be used which contain the quaternary nitrogen atom on an alkyl chain.
- cationic dyes which can be used in the process according to the invention are, for example:
- the acid dyes are water-soluble dyes, which usually contain one or more water-solubilizing groups, in particular sulfonic acid groups.
- the acid dyes are usually in the form of salts.
- alkali salts in particular sodium, potassium or lithium salts
- ammonium-containing salts e.g. as ammonium or tetraalkylammonium salts.
- the acid dyes can belong to a wide variety of classes, such as azo dyes such as monoazo, disazo and polyazo dyes, anthraquinone dyes, phthalocyanine dyes, diphenylmethane and triarylmethane dyes, methine, polymethine, azomethine dyes.
- azo dyes such as monoazo, disazo and polyazo dyes, anthraquinone dyes, phthalocyanine dyes, diphenylmethane and triarylmethane dyes, methine, polymethine, azomethine dyes.
- acid dyes which can be used in the process according to the invention are, for example:
- the dyeing according to the process according to the invention is generally carried out under the conditions customary for dyeings on synthetic polyamide with cationic dyes and acid dyes.
- the pH is usually between 3.5 and 7.0, it being particularly advantageous to change the pH towards acidic pH during dyeing.
- a preferred embodiment of the process according to the invention is characterized in that a complexing agent which can form a complex with the Mn 2+ ions is added to the dyebath.
- a complexing agent which can form a complex with the Mn 2+ ions is added to the dyebath.
- the following compounds have proven to be suitable as complexing agents: gluconic acid, polyaminopolycarboxylic acids, tripolyphosphates, orthophosphates, phosphonic acid, phosphonoalkane polycarboxylic acids, citric acid, ethylenediaminetetraacetic acid.
- leveling agents come e.g. commercially available leveling aids into consideration, such as those e.g. are given in EP-A-0 089 004.
- R is C 8 -C 22 alkyl, C 8 -C 22 alkenyl or C 8 -C 22 cycloalkenyl, in particular RN is the rest of the tallow fatty amine and m + n is 2 to 25, in particular 2 to 15; or the formula wherein R is C 8 -C 22 alkyl, C 8 -C 22 alkenyl or C 8 -C 22 cycloalkenyl, in particular RN is the rest of the tallow fatty amine, m + n is 2 to 25, in particular 2 to 15, and M is hydrogen, alkali metal or ammonium;
- the dyebath can also contain other conventional aids, e.g. Contain wetting agents, deaerating agents and / or anti-foaming agents etc.
- the manganese (II) compounds can be applied continuously or discontinuously.
- carpets of mixtures of acid-modified polyamide fiber material and regular polyamide fiber material are preferred, the latter being able to be used in types which can be dyed to different depths.
- the dyeing process takes place in a manner known per se.
- the liquor ratio can be selected within a wide range, e.g. 1: 2 to 1: 100, preferably 1:10 to 1:40. It is convenient to work at a temperature of 30 to 130 ° C, preferably 50 to 98 ° C.
- the liquor order is expediently 40-700, preferably 40-500% by weight, followed by a steaming process, for example at 100 ° C. for 5 minutes.
- the dyeings produced are washed and dried in the customary manner.
- the dyeings to be stabilized according to the invention are those which are produced by acid dyes and cationic dyes, in particular azo and anthraquinone dyes.
- polyamide fiber material is a mixture of synthetic polyamide fiber materials, in particular polyamide-6 or polyamide 66 in a mixture with acid-modified polyamide fiber material, i.e. understood basic dyeable polyamide.
- the polyamide material can be in a wide variety of processing forms, e.g. as fiber, yarn, fabric, knitted fabric, fleece or pile material. Polyamide carpets are preferred.
- the polyamide fiber material made from regular and acid-modified polyamide can be present in any desired mixing ratio. Mixtures of these polyamide fiber materials in a ratio of 10: 1 to 1:10, in particular 25:75 to 75:25 and advantageously 40:60 to 60:40, are preferred.
- Parts mean parts by weight and percentages percentages by weight. Temperatures are given in degrees Celsius. The percentages by weight refer to the fiber weight used.
- the dye liquor is halved.
- Fleet 1 remains unchanged, while fleet 2 is mixed with 0.5 g / l manganese (II) acetate.
- the carpet samples are placed in the liquors heated to 40 ° C. and dyeing is started.
- the mixture is first heated to 95 ° C. at 2 ° C./minute, then colored for 60 minutes at this temperature, finally cooled, rinsed cold, centrifuged and dried at 100 ° C.
- a carpet with an orange / blue pattern is obtained.
- the separation of the two colors is excellent on both patterns.
- the light fastness of the two samples is determined in accordance with ISO standard 105-B02 and AATCC 16E. The following results are obtained: Carpet colored with Lightfastness ISO 105-B02 after 300 hours Light fastness AATCC 16E after 150 hours Dyeing liquor 1 5 3rd Dyeing liquor 2 6 - 7 3-4
- the light fastness grades according to ISO 105-B02 are determined using the blue scale and the light fastness grades according to AATCC 16E are determined using the gray scale.
- Mn (II) ions improves the lightfastness.
- the dyebath 3 remains analogous to dyebath 1 without the addition of Mn (II) ions, while 3% by weight of the following compound is added to the dyebath 4: Mn (II) complex of the tetrasodium salt of ethylenediaminetetraacetic acid.
- the light fastness of the two samples is determined in accordance with ISO standard 105-B02 and AATCC 16E. The following results are obtained: Coloring with Lightfastness ISO 105-B02 after 300 hours Light fastness AATCC 16E after 150 hours Dyeing liquor 3 4-5 3rd Dyeing liquor 4 6 4th
- the light fastness grades according to ISO 105-B02 are determined using the blue scale and the light fastness grades according to AATCC 16E are determined using the gray scale.
Landscapes
- Engineering & Computer Science (AREA)
- Textile Engineering (AREA)
- Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Coloring (AREA)
Abstract
Description
Die vorliegende Erfindung betrifft ein Verfahren zur Erzielung von Mehrfarbeneffekten auf Mischungen verschieden anfärbbarer Polyamidfasermaterialien in Gegenwart von Mangan-Verbindungen, welches Färbungen mit verbesserter Lichtechtheit liefert, und das damit behandelte Polyamidfasermaterial.The present invention relates to a process for achieving multicolor effects on mixtures of differently dyeable polyamide fiber materials in the presence of manganese compounds, which provides dyeings with improved light fastness, and the polyamide fiber material treated therewith.
Polyamidfasermaterialien sind thermisch und/oder photochemisch sensibel. Durch Licht- und/oder Wärmeeinwirkung kann die Polyamidfaser geschädigt werden.Polyamide fiber materials are thermally and / or photochemically sensitive. The polyamide fiber can be damaged by exposure to light and / or heat.
Für viele Verwendungszwecke muss das Polyamidfasermaterial mattiert sein, was technisch durch Einbringen von Titandioxidpigmenten erzielt wird. Derartig pigmentierte Polyamidfasermaterialien haben sich als besonders sensibel erwiesen.For many uses, the polyamide fiber material has to be matted, which is technically achieved by introducing titanium dioxide pigments. Pigmented polyamide fiber materials have proven to be particularly sensitive.
Aufgabe der vorliegenden Erfindung ist es die thermische und/oder photochemische Instabilität von Polyamidfasermaterialien zu vermindern, um hohe Lichtechtheiten, eine gute Reissfestigkeit und eine gute Alterungsbeständigkeit zu erzielen.The object of the present invention is to reduce the thermal and / or photochemical instability of polyamide fiber materials in order to achieve high light fastness, good tear resistance and good aging resistance.
Es wurde nun gefunden, dass durch Färben von Polyamidfasermischungen, welche aus regulär anfärbbaren Polyamidfasermaterialien und sauer modifizierten Polyamidfasermaterialien bestehen, mit Farbstoffmischungen aus Säurefarbstoffen und kationischen Farbstoffen in Gegenwart von Mangan(II)-Verbindungen die thermische und/oder photochemische Instabilität ganz oder zumindest in grossem Masse beseitigt werden kann und eine verbesserte Lichtechtheit erreicht wird.It has now been found that by dyeing polyamide fiber mixtures, which consist of regularly dyeable polyamide fiber materials and acid-modified polyamide fiber materials, with dye mixtures of acid dyes and cationic dyes in the presence of manganese (II) compounds, the thermal and / or photochemical instability entirely or at least to a large extent Mass can be eliminated and improved lightfastness is achieved.
Die Erfindung betrifft daher ein Verfahren zum Färben von Polyamidfasermischungen, welche regulär anfärbbare Polyamidfasermaterialien und sauer modifizierte Polyamidfasermaterialien enthalten, mit Farbstoffmischungen aus Säurefarbstoffen und kationischen Farbstoffen, welches dadurch gekennzeichnet ist, dass vor, während oder nach dem Färben dem Bad mindestens eine Mangan (II)-Verbindung zugesetzt wird.The invention therefore relates to a process for dyeing polyamide fiber mixtures which contain regularly dyeable polyamide fiber materials and acid-modified polyamide fiber materials, with dye mixtures comprising acid dyes and cationic dyes, which is characterized in that at least one manganese (II) before, during or after dyeing the bath. -Connection is added.
Überraschenderweise zeigen die mit dem erfindungsgemässen Verfahren erhaltenen Färbungen eine verbesserte Lichtechtheit und eine verbesserte Reissfestigkeit.Surprisingly, the dyeings obtained with the process according to the invention show improved light fastness and improved tear resistance.
Als Mangan(II)-Verbindungen kommen in dem erfindungsgemässen Verfahren alle anorganischen und organischen Salze sowie Mn(II)-Komplexverbindungen in Betracht. Als Beispiele für anorganische Salze seien genannt: Chloride, Acetate, Phosphate, Nitrate und Sulfate. Als Beispiele für organische Salze seien genannt: Formiate, Oxalate und Lactate. Als Beispiele für Mn(II)-Komplexverbindungen seien genannt: Mn(II)-Komplexe der Zitronensäure, Äthylendiamintetraessigsäure oder Phosphono-alkan-polycarbonsäuren.All inorganic and organic salts and Mn (II) complex compounds can be considered as manganese (II) compounds in the process according to the invention. Examples of inorganic salts are: chlorides, acetates, phosphates, nitrates and sulfates. Examples of organic salts are: formates, oxalates and lactates. Examples of Mn (II) complex compounds are: Mn (II) complexes of citric acid, ethylenediaminetetraacetic acid or phosphonoalkane polycarboxylic acids.
Die genannten Mn(II)-Verbindungen können auch in beliebiger Mischung untereinander eingesetzt werden.The Mn (II) compounds mentioned can also be used in any mixture with one another.
Die Mn(II)-Verbindungen können dem Färbebad vor, während oder nach dem Färbevorgang zugesetzt werden.The Mn (II) compounds can be added to the dye bath before, during or after the dyeing process.
Es hat sich als besonders vorteilhaft erwiesen, die Mn(II)-Verbindungen während des Färbevorgangs dem Färbebad zuzusetzen. Die Mn(II)-Verbindungen werden vorteilhafterweise in Mengen von 0,01 bis 5 g/l, insbesondere 0,1 bis 1 g/l, vorzugsweise 0,1 bis 0,7 g/l dem Färbebad zugesetzt.It has proven particularly advantageous to add the Mn (II) compounds to the dye bath during the dyeing process. The Mn (II) compounds are advantageously added to the dyebath in amounts of 0.01 to 5 g / l, in particular 0.1 to 1 g / l, preferably 0.1 to 0.7 g / l.
Für das erfindungsgemässe Verfahren eignen sich als Säurefarbstoffe z.B. die im Colour Index, 3. Edition von 1971, Volume 1, unter C.I. Acid Dyes auf den Seiten 1003 bis 1561 sowie auch in den Ergänzungsbänden dazu angegebenen Farbstoffe.Suitable acid dyes for the process according to the invention are e.g. those in the Color Index, 3rd edition from 1971, Volume 1, under C.I. Acid Dyes on pages 1003 to 1561 as well as the dyes specified in the supplementary volumes.
Für das erfindungsgemässe Verfahren eignen sich als kationische Farbstoffe z.B. die im Colour Index, 3. Edition von 1971, Volume 1, unter C.I. Basic Dyes auf den Seiten 1611 bis 1688 sowie auch in den Ergänzungsbänden dazu angegebenen Farbstoffe.Suitable cationic dyes for the process according to the invention are e.g. those in the Color Index, 3rd edition from 1971, Volume 1, under C.I. Basic Dyes on pages 1611 to 1688 as well as the dyes specified in the supplementary volumes.
Die Säurefarbstoffe und die kationischen Farbstoffe, die in dem erfindungsgemässen Verfahren verwendet werden, sind bekannt und können nach bekannten Methoden hergestellt werden.The acid dyes and the cationic dyes which are used in the process according to the invention are known and can be prepared by known methods.
Es handelt sich bei den kationischen Farbstoffen dabei chemisch um Farbstoffe, die ein farbiges (chromophorhaltiges) Kation, insbesondere ein quaternäres Stickstoffatom, und ein farbloses Anion enthalten. Die Farbstoffe liegen in Form eines Salzes mit einer anorganischen oder organischen Säure vor. Sie gehören den verschiedensten chemischen Klassen an; beispielsweise handelt es sich um Sulfate, Chloride, Acetate oder Methylsulfate von Azofarbstoffen, wie Monoazo-, Disazo- und Polyazofarbstoffen, von Anthrachinonfarbstoffen, Phthalocyaninfarbstoffen, von Diphenylmethan- und Triarylmethanfarbstoffen, von Methin-, Polymethin-, Azomethin- und Azacyaninfarbstoffen, von Thiazol-, Ketonimin-, Acridin-, Cyanin-, Nitro-, Chinolin-, Benzimidazol-, Xanthen-, Azin- Oxazin- und Thiazinfarbstoffen. Bevorzugte kationische Farbstoffe sind solche, die ein quaternäres Stickstoffatom in einem heterocyclischen Ring enthalten; es können aber auch kationische Farbstoffe verwendet werden, die das quaternäre Stickstoffatom an einer Alkylkette enthalten.The cationic dyes are chemically dyes which contain a colored (chromophore-containing) cation, in particular a quaternary nitrogen atom, and a colorless anion. The dyes are in the form of a salt with an inorganic or organic acid. They belong to a wide variety of chemical classes; for example, sulfates, chlorides, acetates or Methyl sulfates of azo dyes, such as monoazo, disazo and polyazo dyes, of anthraquinone dyes, phthalocyanine dyes, of diphenylmethane and triarylmethane dyes, of methine, polymethine, azomethine and azacyanine dyes, of thiazole, ketoneimine, acridine, cyanine, cyanine -, Quinoline, benzimidazole, xanthene, azine oxazine and thiazine dyes. Preferred cationic dyes are those which contain a quaternary nitrogen atom in a heterocyclic ring; however, cationic dyes can also be used which contain the quaternary nitrogen atom on an alkyl chain.
Als Beispiele für kationische Farbstoffe, die in dem erfindungsgemässen Verfahren eingesetzt werden können, kommen z.B. in Betracht:
Es handelt sich bei den Säurefarbstoffen um wasserlösliche Farbstoffe, die meist eine oder mehrere wasserlöslichmachende Gruppen, insbesondere Sulfonsäuregruppen, enthalten.The acid dyes are water-soluble dyes, which usually contain one or more water-solubilizing groups, in particular sulfonic acid groups.
Die Säurefarbstoffe liegen in der Regel als Salze vor. Als Salze kommen insbesondere Alkalisalze, insbesondere Natrium-, Kalium- oder Lithiumsalze, und ammoniumhaltige Salze, z.B. als Ammonium- oder Tetraalkylammoniumsalze, in Betracht.The acid dyes are usually in the form of salts. In particular, alkali salts, in particular sodium, potassium or lithium salts, and ammonium-containing salts, e.g. as ammonium or tetraalkylammonium salts.
Die Säurefarbstoffe können den verschiedensten Klassen angehören, wie z.B. Azofarbstoffen, wie Monoazo-, Disazo- und Polyazofarbstoffen, Anthrachinonfarbstoffen, Phthalocyaninfarbstoffen, Diphenylmethan- und Triarylmethanfarbstoffen, Methin-, Polymethin-, Azomethinfarbstoffen.
Als Beispiele für Säurefarbstoffe, die in dem erfindungsgemässen Verfahren eingesetzt werden können, kommen z.B. in Betracht:
Examples of acid dyes which can be used in the process according to the invention are, for example:
Die Färbung gemäss dem erfindungsgemässen Verfahren erfolgt in der Regel unter den für Färbungen auf synthetischem Polyamid mit kationischen Farbstoffen und Säurefarbstoffen üblichen Bedingungen. Als Färbetemperatur hat sich eine Temperatur zwischen 30 und 130°C, insbesondere zwischen 50 und 98°C als geeignet erwiesen. Der pH-Wert liegt üblicherweise zwischen 3,5 und 7,0, wobei es besonders vorteilhaft ist, den pH-Wert während des Färbens in Richtung saueren pH-Wert zu ändern..The dyeing according to the process according to the invention is generally carried out under the conditions customary for dyeings on synthetic polyamide with cationic dyes and acid dyes. A temperature between 30 and 130 ° C., in particular between 50 and 98 ° C., has proven to be suitable as the dyeing temperature. The pH is usually between 3.5 and 7.0, it being particularly advantageous to change the pH towards acidic pH during dyeing.
Eine bevorzugte Ausführungsform des erfindungsgemässen Verfahrens ist dadurch gekennzeichnet, dass man dem Färbebad einen Komplexbildner, der mit den Mn2+-Ionen einen Komplex bilden kann, zusetzt. Als Komplexbildner haben sich die folgenden Verbindungen als geeignet erwiesen: Gluconsäure, Polyaminopolycarbonsäuren, Tripolyphosphate, ortho-Phosphate, Phosphonsäure, Phosphono-alkan-polycarbonsäuren, Zitronensäure, Äthylendiamintetraessigsäure.A preferred embodiment of the process according to the invention is characterized in that a complexing agent which can form a complex with the Mn 2+ ions is added to the dyebath. The following compounds have proven to be suitable as complexing agents: gluconic acid, polyaminopolycarboxylic acids, tripolyphosphates, orthophosphates, phosphonic acid, phosphonoalkane polycarboxylic acids, citric acid, ethylenediaminetetraacetic acid.
Ebenfalls bevorzugt ist eine Ausführungsform des erfindungsgemässen Verfahrens, welche dadurch gekennzeichnet ist, dass man dem Färbebad ein Egalisiermittel zusetzt. Als Egalisiermittel kommen z.B. handelsübliche Egalisierhilfsmittel in Betracht, wie sie z.B. in der EP-A-0 089 004 angegeben sind.Also preferred is an embodiment of the method according to the invention, which is characterized in that a leveling agent is added to the dyebath. As leveling agents come e.g. commercially available leveling aids into consideration, such as those e.g. are given in EP-A-0 089 004.
Vorzugsweise werden folgende Verbindungen als Egalisierhilfsmittel eingesetzt: Verbindungen der Formel
Verbindungen der Formel
Verbindungen der Formel
Ferner sind auch Mischungen der genannten Komponenten als Egalisierhilfsmittel von Bedeutung. Das Färbebad kann auch weitere übliche Hilfsmittel z.B. Netzmittel, Entlüftungsmittel und/oder Antischaummittel usw. enthalten.Mixtures of the components mentioned are also important as leveling aids. The dyebath can also contain other conventional aids, e.g. Contain wetting agents, deaerating agents and / or anti-foaming agents etc.
Die Applikation der Mangan(II)-Verbindungen kann kontinuierlich oder diskontinuierlich erfolgen.The manganese (II) compounds can be applied continuously or discontinuously.
Bevorzugt sind in dem erfindungsgemässen Verfahren Teppiche aus Mischungen von sauer modifiziertem Polyamidfasermaterial und regulärem Polyamidfasermaterial, wobei letzteres in verschieden tief anfärbbaren Typen eingesetzt werden kann.In the process according to the invention, carpets of mixtures of acid-modified polyamide fiber material and regular polyamide fiber material are preferred, the latter being able to be used in types which can be dyed to different depths.
Der Färbevorgang erfolgt in an sich bekannter Weise.The dyeing process takes place in a manner known per se.
Beim Färben nach Ausziehverfahren kann das Flottenverhältnis innerhalb eines weiten Bereiches gewählt werden, z.B. 1:2 bis 1:100, vorzugsweise 1:10 bis 1:40. Man arbeitet zweckmässig bei einer Temperatur von 30 bis 130°C, vorzugsweise 50 bis 98°C.When dyeing using the exhaust method, the liquor ratio can be selected within a wide range, e.g. 1: 2 to 1: 100, preferably 1:10 to 1:40. It is convenient to work at a temperature of 30 to 130 ° C, preferably 50 to 98 ° C.
Beim Färben nach Kontinueverfahren beträgt der Flottenauftrag zweckmässig 40-700, vorzugsweise 40-500 Gew.-%, wobei sich ein Dämpfprozess, z.B. bei 100°C während 5 Minuten anschliesst.When dyeing according to the continuous process, the liquor order is expediently 40-700, preferably 40-500% by weight, followed by a steaming process, for example at 100 ° C. for 5 minutes.
Nach Beendigung des Färbeprozesses werden die hergestellten Färbungen auf übliche Weise gewaschen und getrocknet.After the dyeing process has ended, the dyeings produced are washed and dried in the customary manner.
Man erhält nach der vorliegenden Erfindung Färbungen mit guter thermischer und/oder photochemischer Stabilität.According to the present invention, dyeings with good thermal and / or photochemical stability are obtained.
Als die erfindungsgemäss zu stabilisierenden Färbungen kommen solche in Betracht, die durch Säurefarbstoffe und kationische Farbstoffe, besonders Azo- und Anthrachinonfarbstoffe erzeugt werden.The dyeings to be stabilized according to the invention are those which are produced by acid dyes and cationic dyes, in particular azo and anthraquinone dyes.
Unter Polyamidfasermaterial wird in dem erfindungsgemässen Verfahren ein Gemisch synthetischer Polyamidfasermaterialien, insbesondere Polyamid-6 bzw. Polyamid 66 in Mischung mit sauer modifiziertem Polyamidfasermaterial, d.h. basisch anfärbbares Polyamid verstanden. Grundsätzlich kann das Polyamidmaterial in den verschiedensten Verarbeitungsformen vorliegen, wie z.B. als Faser, Garn, Gewebe, Gewirke, Vlies oder Flormaterial. Bevorzugt sind Polyamidteppiche. Das Polyamidfasermaterial aus regulärem und sauer modifiziertem Polyamid kann in jedem gewünschten Mischungsverhältnis vorliegen. Bevorzugt sind Mischungen dieser Polyamidfasermaterialien im Verhältnis 10:1 bis 1:10, insbesondere 25:75 bis 75:25 und vorteilhaft 40:60 bis 60:40.In the process according to the invention, polyamide fiber material is a mixture of synthetic polyamide fiber materials, in particular polyamide-6 or polyamide 66 in a mixture with acid-modified polyamide fiber material, i.e. understood basic dyeable polyamide. In principle, the polyamide material can be in a wide variety of processing forms, e.g. as fiber, yarn, fabric, knitted fabric, fleece or pile material. Polyamide carpets are preferred. The polyamide fiber material made from regular and acid-modified polyamide can be present in any desired mixing ratio. Mixtures of these polyamide fiber materials in a ratio of 10: 1 to 1:10, in particular 25:75 to 75:25 and advantageously 40:60 to 60:40, are preferred.
Die folgenden Beispiele veranschaulichen die Erfindung. Teile bedeuten Gewichtsteile und Prozente Gewichtsprozente. Temperaturen sind in Celsiusgraden angegeben. Die angegebenen Gewichtsprozente beziehen sich auf das eingesetzte Fasergewicht.The following examples illustrate the invention. Parts mean parts by weight and percentages percentages by weight. Temperatures are given in degrees Celsius. The percentages by weight refer to the fiber weight used.
Es werden 2 Muster zu je 20g eines vorgewaschenen Polyamidteppichs vorbereitet; der Pol hat ein Gewicht von 500 g/qm und besteht zu 50 Gew.-% aus regulär färbbarem Polyamidfasermaterial und zu 50 Gew.-% aus basisch färbbarem Polyamidfasermaterial von 1300 dtex. Diese Muster werden in Bomben [z.B. in einem Labomat® der Firma Mathis, Niederhasli, Schweiz] gefärbt, wobei das Flottenverhältnis 1:25 beträgt. Für beide Färbungen wird zunächst eine Flotte folgender Zusammensetzung zubereitet:
- 1g/l des Färbereihilfsmittels der Formel
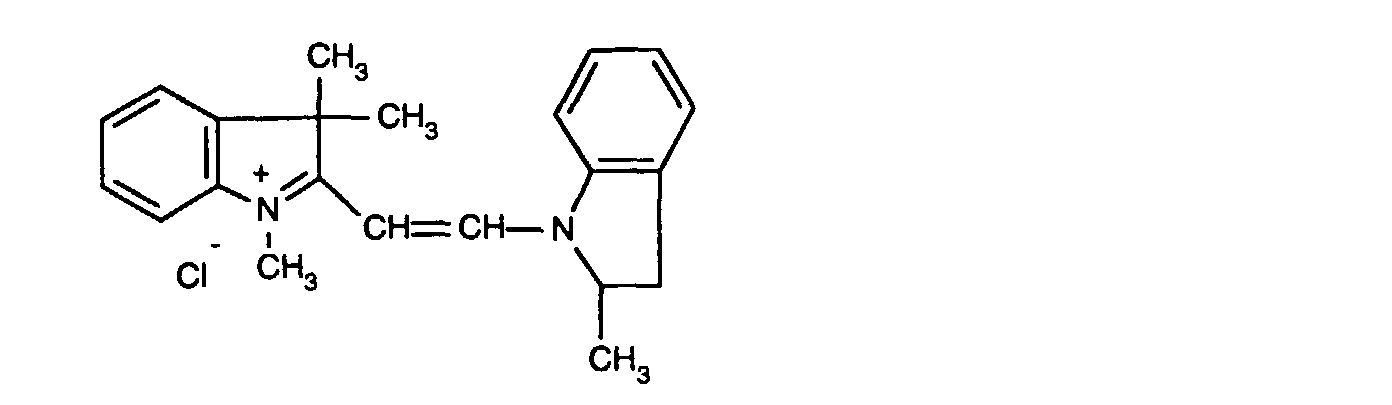
- 0,05 Gew.-% einer 1:1-Mischung aus den Farbstoffen der Formeln
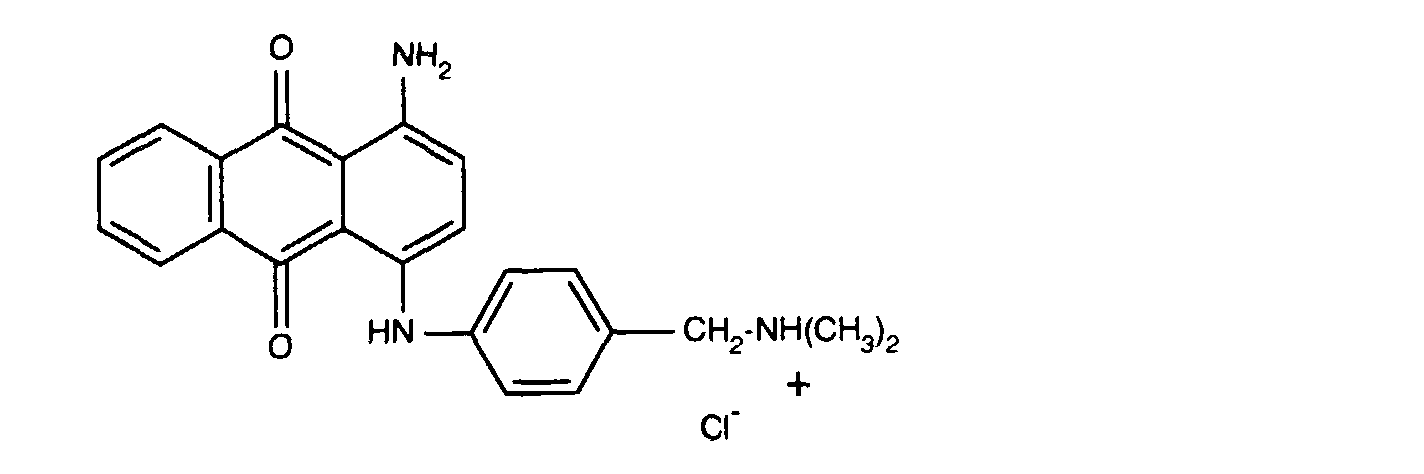
- 0,15 Gew.-% des Farbstoffes der Formel
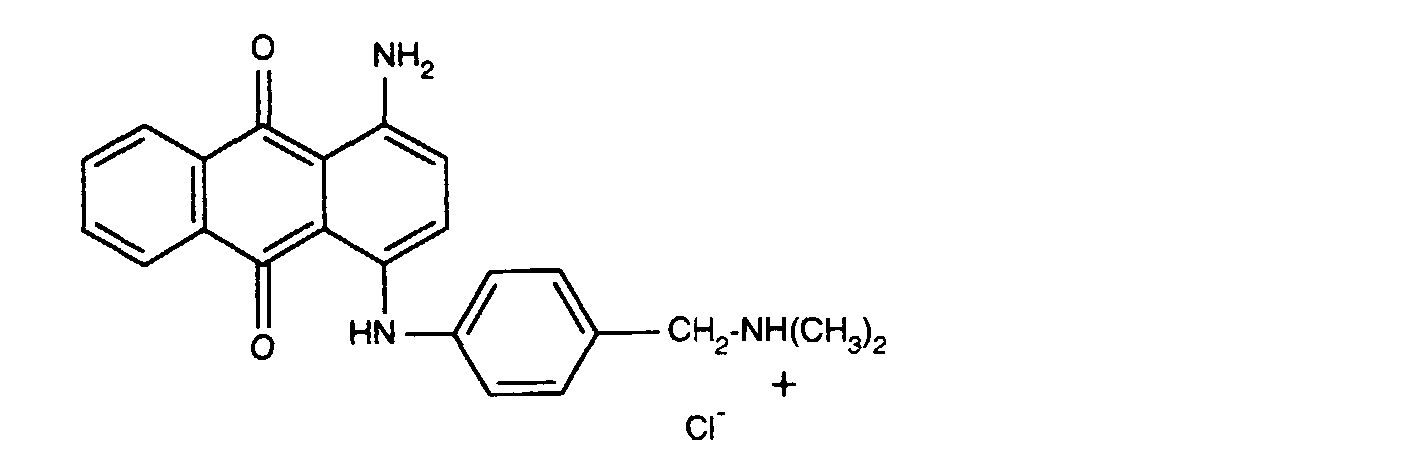
- 0,04Gew.-% des Farbstoffes der Formel
- 0,05 Gew.-% des Farbstoffes der Formel
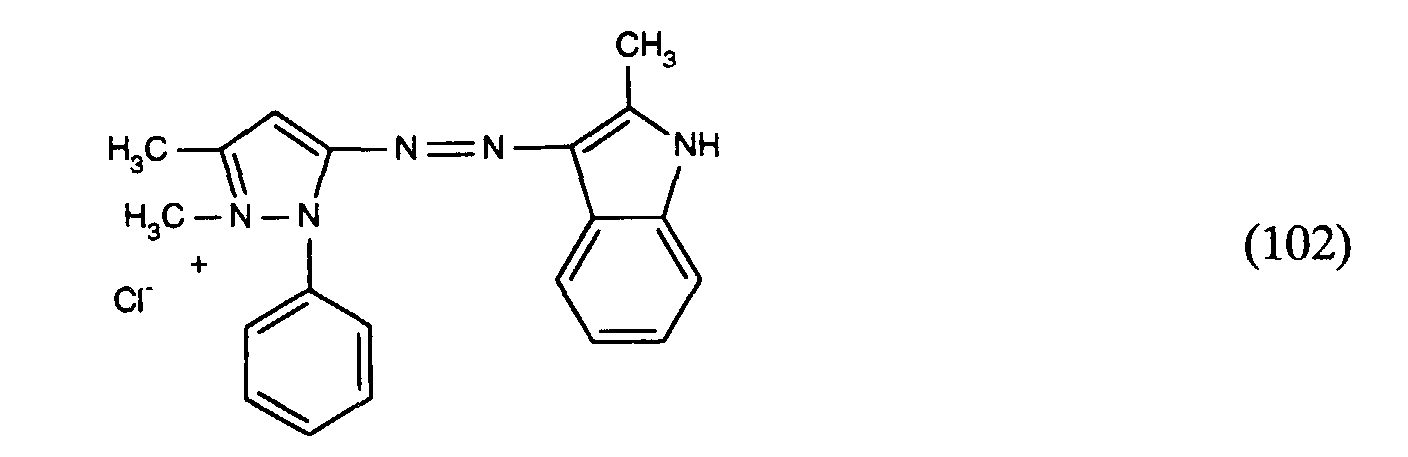
- und 0,15 Gew.-% der 3:1-Farbstoffmischung aus den Farbstoffen der Formeln
- 1g / l of dyeing aid of the formula
- 0.05% by weight of a 1: 1 mixture of the dyes of the formulas
- 0.15% by weight of the dye of the formula
- 0.04% by weight of the dye of the formula
- 0.05% by weight of the dye of the formula
- and 0.15% by weight of the 3: 1 dye mixture from the dyes of the formulas
Die Färbeflotte wird halbiert. Die Flotte 1 bleibt unverändert, Flotte 2 wird dagegen mit 0,5 g/l Mangan(II)acetat versetzt.The dye liquor is halved. Fleet 1 remains unchanged, while fleet 2 is mixed with 0.5 g / l manganese (II) acetate.
Die Teppichmuster werden in die auf 40°C erwärmten Flotten gegeben und mit dem Färben begonnen. Zunächst wird mit 2°C/Minute auf 95°C aufgeheizt, dann 60 Minuten bei dieser Temperatur gefärbt, schliesslich abgekühlt, kalt gespült, zentrifugiert und bei 100°C getrocknet.The carpet samples are placed in the liquors heated to 40 ° C. and dyeing is started. The mixture is first heated to 95 ° C. at 2 ° C./minute, then colored for 60 minutes at this temperature, finally cooled, rinsed cold, centrifuged and dried at 100 ° C.
Man erhält einen Teppich mit einer Orange/Blau-Musterung. Die Trennung der beiden Farben ist auf beiden Mustern ausgezeichnet.A carpet with an orange / blue pattern is obtained. The separation of the two colors is excellent on both patterns.
Die Ermittlung der Lichtechtheit der beiden Muster erfolgt nach ISO-Norm 105-B02 und AATCC 16E. Es werden folgende Ergebnisse erhalten:
Die Lichtechtheitsnoten gemäss ISO 105-B02 werden mit dem Blaumassstab und die Lichtechtheitsnoten gemäss AATCC 16E werden mit dem Graumassstab ermittelt.The light fastness grades according to ISO 105-B02 are determined using the blue scale and the light fastness grades according to AATCC 16E are determined using the gray scale.
Aus den belichteten Mustern ist zu ersehen, dass die Verbesserung der Lichtechtheit besonders den basisch anfärbbaren Teil betrifft, da dieser vom Farbton her (orange) das Dessin dominiert.From the exposed patterns it can be seen that the improvement in light fastness particularly affects the basic part that can be dyed because this (orange) dominates the design.
Durch den Zusatz von Mn(II)-Ionen wird eine Verbesserung der Lichtechtheit erzielt.The addition of Mn (II) ions improves the lightfastness.
Man bereitet 20 g eines vorgewaschenen Polyamidteppichs, dessen Flor aus mit basischen und sauren Farbstoffen anfärbbaren Fasern im Verhältnis 1:1 besteht. Man färbt wie in Beispiel 1 angegeben, verwendet jedoch die folgende Farbstoff-Kombination:
- 0,002 Gew.-% der 1:1-Farbstoffmischung der Farbstoffe der Formeln (101) und (102),
- 0,01 Gew.-% des Farbstoffes der Formel (103),
- 0,04 Gew.-% des Farbstoffes der Formel
- 0,22 Gew.-% des Farbstoffes der Formel (104),
- 0,18 Gew.-% des Farbstoffes der Formel (105), und
- 0.26 Gew.-% der 3:1-Farbstoffmischung der Farbstoffe der Formeln (106) und (107).
- 0.002% by weight of the 1: 1 dye mixture of the dyes of the formulas (101) and (102),
- 0.01% by weight of the dye of the formula (103),
- 0.04% by weight of the dye of the formula
- 0.22% by weight of the dye of the formula (104),
- 0.18% by weight of the dye of the formula (105), and
- 0.26% by weight of the 3: 1 dye mixture of the dyes of the formulas (106) and (107).
Das Färbebad 3 verbleibt analog Färbebad 1 ohne Zusatz von Mn(II)-Ionen, während dem Färbebad 4 3 Gew.-% der folgenden Verbindung zugesetzt werden: Mn(II)-Komplex des Tetranatriumsalzes der Äthylendiamintetraessigsäure.The dyebath 3 remains analogous to dyebath 1 without the addition of Mn (II) ions, while 3% by weight of the following compound is added to the dyebath 4: Mn (II) complex of the tetrasodium salt of ethylenediaminetetraacetic acid.
Die Fertigstellung und Trocknung der Färbung erfolgt wie in Beispiel 1 angegeben.The dyeing is completed and dried as described in Example 1.
Die Ermittlung der Lichtechtheit der beiden Muster erfolgt nach ISO-Norm 105-B02 und AATCC 16E. Es werden folgende Ergebnisse erhalten:
Die Lichtechtheitsnoten gemäss ISO 105-B02 werden mit dem Blaumassstab und die Lichtechtheitsnoten gemäss AATCC 16E werden mit dem Graumassstab ermittelt.The light fastness grades according to ISO 105-B02 are determined using the blue scale and the light fastness grades according to AATCC 16E are determined using the gray scale.
Aus den belichteten Mustern ist zu ersehen, dass sich die Verbesserung ausschliesslich auf den basisch anfärbbaren Anteil bezieht.From the exposed patterns it can be seen that the improvement relates exclusively to the basic colorable portion.
Wenn man wie in den Beispielen 1 und 2 angegeben verfährt und anstelle der dort angegebenen Farbstoffe solche der folgenden Formeln verwendet, erhält man ebenfalls Färbungen mit verbesserter Lichtechtheit:
Claims (12)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CH865/95 | 1995-03-27 | ||
| CH86595 | 1995-03-27 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0735181A2 true EP0735181A2 (en) | 1996-10-02 |
| EP0735181A3 EP0735181A3 (en) | 1998-04-15 |
Family
ID=4196988
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP96810166A Withdrawn EP0735181A3 (en) | 1995-03-27 | 1996-03-18 | Multicolor dyeing with manganese compounds of fibrous materials containing polyamide fibres |
Country Status (1)
| Country | Link |
|---|---|
| EP (1) | EP0735181A3 (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1069233A1 (en) * | 1999-07-08 | 2001-01-17 | E.I. Du Pont De Nemours & Company Incorporated | A method of imparting stain resistance to a differentially dyeable textile surface and the article produced thereby |
| WO2001004408A1 (en) * | 1999-07-08 | 2001-01-18 | E.I Du Pont De Nemours And Company | A method of imparting stain resistance to a differentially dyeable textile surface and the article produced thereby |
| EP1170414A1 (en) * | 2000-07-03 | 2002-01-09 | E.I. Du Pont De Nemours And Company | Method of after-treatment of a dyeable nylon textile surface with a stain resist and the article produced thereby |
| US6811574B2 (en) | 2000-07-03 | 2004-11-02 | Dupont Textiles & Interiors, Inc. | Method of after-treatment of a dyeable nylon textile surface with a stain resist and the article produced thereby |
| US6852134B2 (en) | 1999-07-08 | 2005-02-08 | Invista North America S.A.R.L. | Method of imparting stain resistance to a differentially dyeable textile surface and the article produced thereby |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0569793A1 (en) * | 1992-05-12 | 1993-11-18 | Bayer Ag | Process for dyeing of polyamides in the presence of manganese (II) salts |
| DE19610084A1 (en) * | 1995-03-17 | 1996-09-19 | Ciba Geigy Ag | Dyeing acid-modified polyamide fibre materials with cationic dyes |
-
1996
- 1996-03-18 EP EP96810166A patent/EP0735181A3/en not_active Withdrawn
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0569793A1 (en) * | 1992-05-12 | 1993-11-18 | Bayer Ag | Process for dyeing of polyamides in the presence of manganese (II) salts |
| DE19610084A1 (en) * | 1995-03-17 | 1996-09-19 | Ciba Geigy Ag | Dyeing acid-modified polyamide fibre materials with cationic dyes |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1069233A1 (en) * | 1999-07-08 | 2001-01-17 | E.I. Du Pont De Nemours & Company Incorporated | A method of imparting stain resistance to a differentially dyeable textile surface and the article produced thereby |
| WO2001004408A1 (en) * | 1999-07-08 | 2001-01-18 | E.I Du Pont De Nemours And Company | A method of imparting stain resistance to a differentially dyeable textile surface and the article produced thereby |
| US6852134B2 (en) | 1999-07-08 | 2005-02-08 | Invista North America S.A.R.L. | Method of imparting stain resistance to a differentially dyeable textile surface and the article produced thereby |
| EP1170414A1 (en) * | 2000-07-03 | 2002-01-09 | E.I. Du Pont De Nemours And Company | Method of after-treatment of a dyeable nylon textile surface with a stain resist and the article produced thereby |
| US6811574B2 (en) | 2000-07-03 | 2004-11-02 | Dupont Textiles & Interiors, Inc. | Method of after-treatment of a dyeable nylon textile surface with a stain resist and the article produced thereby |
Also Published As
| Publication number | Publication date |
|---|---|
| EP0735181A3 (en) | 1998-04-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0475905A1 (en) | Process for the photochemical stabilisation of wool | |
| DE4031650A1 (en) | Trichromatic dyeing or printing mixt., pref. for polyamide fibre | |
| EP0044483B1 (en) | Process for reactive dyeing | |
| EP0735181A2 (en) | Multicolor dyeing with manganese compounds of fibrous materials containing polyamide fibres | |
| DE2527962A1 (en) | METHOD FOR TREATMENT OF CELLULOSE FIBERS | |
| EP0593392B1 (en) | Process for dyeing of natural and synthetic polyamide fibers with dye mixtures | |
| DE2037554C3 (en) | Process for dyeing solidified nonwovens made of polyamide fibers by the indwelling process | |
| EP0302013A1 (en) | Process for dyeing polyamide textile fabrics | |
| DE1921827A1 (en) | Process for producing colors on fiber material made of polymeric or copolymeric acrylonitrile | |
| EP0059876B1 (en) | Process for colouring mixed polyester and keratinous fibre materials | |
| EP0189851B1 (en) | Pad-dyeing process for wool | |
| DE19610084A1 (en) | Dyeing acid-modified polyamide fibre materials with cationic dyes | |
| DE3506654A1 (en) | LOW TEMPERATURE COLORING PROCESS FOR WOOL FIBERS | |
| DE2052151C3 (en) | Process for dyeing acid-modified synthetic textile fibers | |
| DE3932869C2 (en) | Use of dialkyl monoamine alkoxylate compounds to reserve anionic dyeings on polyamide fiber material | |
| DE1917180C3 (en) | Process for the single bath dyeing of mixtures of cellulose, polyester and acid modified polyester fibers | |
| DE2128834C3 (en) | Process for creating multicolor effects on natural and synthetic polyamide fiber material | |
| DE3321658A1 (en) | METHOD FOR DYING POLYCAPRONAMIDE TEXTILE FABRICATION ITEMS | |
| EP0273300A2 (en) | Process for the one-bath single-stage dyeing of mixtures of carrier-free dyeable polyester fibres and cellulose fibres | |
| DE1801715C3 (en) | Process for the single bath dyeing of mixtures of cellulose, polyester and polyacrylonitrile fibers | |
| DE1769157C (en) | Process for the simultaneous heat setting of disperse and reactive dyes on mixtures of synthetic fibers and cellulose fibers | |
| EP0203890A1 (en) | Process for dyeing natural fibrous polyamide material with dye mixtures | |
| DE2235110B2 (en) | Process for the shortened and even high-temperature dyeing of textile materials made of hydrophobic fibers | |
| DE1963015A1 (en) | Process for the continuous fixing of prints and block coloring in melts | |
| DE2913718B2 (en) | Process for dyeing cellulose fibers and fiber blends containing cellulose fibers with reactive dyes |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): BE CH DE DK FR GB IT LI NL |
|
| AX | Request for extension of the european patent |
Free format text: LT PAYMENT 960320;LV PAYMENT 960320;SI PAYMENT 960320 |
|
| RAX | Requested extension states of the european patent have changed |
Free format text: LT PAYMENT 960320;LV PAYMENT 960320;SI PAYMENT 960320 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: CIBA SC HOLDING AG |
|
| RAP3 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: CIBA SPECIALTY CHEMICALS HOLDING INC. |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): BE CH DE DK FR GB IT LI NL |
|
| AX | Request for extension of the european patent |
Free format text: LT PAYMENT 960320;LV PAYMENT 960320;SI PAYMENT 960320 |
|
| 17P | Request for examination filed |
Effective date: 19980917 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION IS DEEMED TO BE WITHDRAWN |
|
| 18D | Application deemed to be withdrawn |
Effective date: 20001003 |