EP0211595A2 - Devices for administering medicaments to patients - Google Patents
Devices for administering medicaments to patients Download PDFInfo
- Publication number
- EP0211595A2 EP0211595A2 EP86305807A EP86305807A EP0211595A2 EP 0211595 A2 EP0211595 A2 EP 0211595A2 EP 86305807 A EP86305807 A EP 86305807A EP 86305807 A EP86305807 A EP 86305807A EP 0211595 A2 EP0211595 A2 EP 0211595A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- container
- tray
- plunger
- housing
- support
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- SOWIQCZCJHSPMA-UHFFFAOYSA-N C1[O]2C1CCC2 Chemical compound C1[O]2C1CCC2 SOWIQCZCJHSPMA-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
- A61M15/0046—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier
- A61M15/0048—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier the dosages being arranged in a plane, e.g. on diskettes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/06—Solids
- A61M2202/064—Powder
Definitions
- This invention relates to devices by which a medicament in solid finely divided form can be administered to or by patients inhaling through the devices.
- Such devices are now quite well known for administering medicaments contained in capsules to patients suffering from bronchial conditions such as, for example; bronchial asthma.
- medicament in powder or other finely divided form to be supplied in capsules which are loaded by a patient into such a device. The medicament is then released from the capsule and inhaled by the patient, usually through the mouth, but sometimes through the nose.
- capsules which are made of gelatin; to contain medicaments.
- Gelatin is relatively unstable and is lacking in physical strength so that the capsules need to be protected by packaging; for example in glass bottles. Environmental degradation of both the capsules and their contents may occur in a relatively short time.
- the capsules are nounted in what is referred to therein as a blister pack, and is in fact a plurality of capsules mounted in a blister pack on a rotor which is designed to spin during exhalation by the patient to throw medicament out of an opened capsule, whereafter the patient inhales.
- This has a number of disadvantages, including the fact that the exhalation which is required is more difficult for some patients; for example asthma patients, than inhalation.
- GB-A-2129691 we provided a more convenient way of administering medicament to such patients than had been possible hitherto and which avoided the need to pack medicaments in capsules.
- the device there described makes use of the technique of packaging a medicament by loading the medicament directly into a blister pack comprising a sheet; which may be laminated, of foil or plastics material which acts as a carrier and which is provided with a number of breakable or openable containers called "blisters" incorporating a sheet secured on a first sheet to form a cover or lid.
- Such blister packs are in common use with tablets of one kind or another, but we have discovered that they can also be used with medicaments in finely divided solid form.
- GB-A-2129691 provides a device for administering to patients medicaments in blister pack form;
- a device for administering medicaments in solid finely divided form to patients comprising a housing; a tray mounted in the housing and movable between first and second positions relative to the housing; a support provided on the tray and adapted to receive, in use, a carrier provided with at least one medicament container; a plunger operable, in use, to penetrate a container registered therewith to open the container, movement of the tray from its first to its second position being such as to cause in use, the support to bring a container into registration with the plunger; an air inlet through which in use, air can enter the device, and an outlet through which a patient can inhale, whereby medicament will be released from an opened container and entrained in an air flow produced by the patient, air entering the air inlet and passing out through the outlet having entrained medicament therein.
- the support is rotatably mounted on the tray and the carrier has a plurality of medicament containers arranged in a circle.
- Indexing means are preferably provided so that movement of the tray from its first to its second position causes the support to be indexed to bring the next container into registration with the plunger.
- the housing of the device preferably has a base member and a lid pivotally mounted thereon for movement between a closed position and an open position.
- the plunger can then be carried by the lid and arranged to penetrate a container when the lid is moved to its open position.
- the device of the invention is suitable for administering a variety of medicaments such as, for example, salbutamol, beclomethasone dipropionate and sodium cromoglycate.
- a significant number of asthma patients suffer from asthma with a severity such that they need to take not one but two medicaments.
- a a-stimulant for example salbutamol or sodium cromoglycate
- an anti-inflammatory steroid for example beclomethasone dipropionate.
- a patient needing both these medicaments will take alternate doses of the two medicaments at prescribed intervals during the day.
- the two medicaments concerned may be a S-stimulant and an anti-inflammatory steroid respectively, or some other pair of medicaments used in treating asthma, or some other pair of medicaments inhaled for the purpose of treating some other condition.
- the reference to two medicaments is to be understood as including not only a pair of medicaments containing two different active ingredients, but also a pair of medicaments containing the same active ingredient in different dosages.
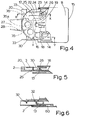
- the device shown in Figures 1 to 6 comprise four principal components, namely a housing 1, a tray 2, a rotatable support 3 and a cover 4.
- the support 3 is designed to receive a circular blister pack 5, as described in more detail below.
- the housing 1 comprises a base member 10 and a lid 11 hinged thereto by pivots 12.
- the base member 10 has a base wall 13, upstanding side and rear walls 14 and 15 and a top wall 16 which extends over only the forward portion of the base member to form a kind of bridge.
- the top wall 16 has an aperture 17 formed therein.
- Extending forwardly from the front edge of the lid 11 is an elongated plunger 18. This is so positioned that when the lid 11 is raised the plunger passes through the aperture 17 which also acts as an air inlet into the device.
- the plunger is conveniently tapered at the tip to form a relatively sharp point, but this is not essential and a blunter plunger would serve the intended purpose which is described below.
- the plunger 18 is protected from damage by upstanding walls 19 formed on the upper wall 16.
- the tray 2 defines a shallow chamber 20 for receiving the rotatable support 3.
- an upstanding lug 21 on which the support 3 is mounted for rotation.
- the lug 21 is shown as being cruciform in cross-section, but need not be; a lug of circular cross-section, for example, could be used instead.
- the tray 2 can be moved in the housing between an inward position, as shown in Figure 2, and an outward position, as shown in Figures 3 and 4. Further outward movement beyond the above mentioned outward position is normally prevented by a lug 23 which is formed on the end of an arm 22 and which engages behind an inwardly directed protrusion on one of the side walls 14 of the housing 1.
- the arm 22 is secured to the rest of the tray only at its forward end and is substantially separated from the rest of the tray by a slot 24.
- the arm 22 is resilient, and when it is desired to remove the tray completely from the housing this can be achieved by pressing the arm 22 inwardly and then withdrawing the tray. Withdrawal is assisted by the provision of thumb grips 33, in the form of ribs, on both sides of the tray.
- the tray also has a tongue 25 which can be depressed downwardly, as described below, and which has an open slot 26.
- a mouthpiece 27 Extending from the front of the tray 2 is a mouthpiece 27. It is through this mouthpiece that medicament leaves the device as it is inhaled by a patient. To improve airflow through the mouthpiece it may be provided with a pair of apertures 28.
- the rotatable support 3 is in the form of a disc in which is formed a circular array of circular openings 30.
- a central opening 31 enables the carrier to be mounted for rotation on the lug 21.
- a corresponding plurality of ribs 32 are formed on the underside of the support 3, with one rib extending between each two adjacent openings 30.
- the cover 4 is removed and the tray with the support 3 mounted thereon, is then removed completely from the housing 1 after the arm 22 has been depressed.
- a blister pack 5 is then mounted on the support 3 with one blister extending into each of the openings 30.
- the tray, support and blister pack are then inserted together into the housing.
- the cover 4 is then replaced.
- When a patient desires to inhale medicament he removes the cover and raises the lid 11 so as to cause the plunger 18 to pass through the aperture 17 and puncture a respective blister located immediately below the aperture 17.
- the lid is then lowered to withdraw the plunger from the blister, leaving a hole therein, and the patient inhales the medicament through the mouthpiece 27.
- the plunger is positively withdrawn from the blister by the patient rather than being left to withdraw under spring pressure (as in GB-A-2129691 mentioned above) which avoids any risk of the plunger remaining jammed in the blister.
- the support 3 is rotated to bring the next blister beneath the aperture 17. This is achieved as follows.
- the tray 2 is withdrawn to its outward position and then pushed back to its inward position.
- an arm 60 which extends forwardly in the casing 1 and is secured to the base wall 13 thereof engages one of the ribs on the underside of the support 3.
- the upper surface of the blister pack 5 carries a series of numbers, arranged in a circle, corresponding to the number of blisters in the pack (in this case the numbers 1 to 8).
- the top wall 16 of the housing 1 has an aperture 34 through which a respective one of the numbers is visible to indicate the number of the blister then aligned with the aperture 17 ; and hence to indicate how many blisters are left for use, or alternatively how many blisters have been used.
- the tray 2 is provided adjacent the mouthpiece with a pair of upstanding walls 35 which converge towards the centre of the tray, the radially inner ends of the walls 35 being interconnected by a wall 35a.
- the support 3 is in a position in which a blister is aligned with the plunger, two adjacent ribs 32 of the support are aligned with the wall 35 and in close contact therewith.
- the blister pack 5 is in close contact with the underside of the top wall 16 of the tray, at least in the vicinity of the aperture 17.
- the only air flow which is produced is one which passes through the aperture 17, through the hole formed in the blister aligned therewith, through a chamber defined by the wall 35 and the ribs 32 in contact therewith and thence through the mouthpiece 27, optionally supplemented by air flowing into the mouthpiece through the apertures 28 if these are provided.
- the device shown in Figures 1 to 6 is preferably provided with a recess, located inwardly of the rear wall 15 and extending parallel thereto, for removably receiving a brush which the patient can use to clean the device of powdered medicament spilt therein.
- the plunger 18 may be curved, as viewed in side elevation so that as it pierces a blister it produces in it a hole which is smaller and more nearly circular than that which is produced if the plunger is straight as illustrated. This provides for improved entrainment of powdered medicament in the air flow produced by inhalation and helps to avoid powder being trapped in the blister.
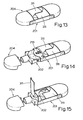
- Figures 7 and 8 illustrate a second embodiment of the invention.
- Figures 7 and 8 is broadly similar to that of Figures 1 to 6, and the reference numerals in Figures 7 and 8 are the same as in Figures 1 to 6, where appropriate, but with the addition of a prime. Because of the similarities between the two embodiments the following description deals only with features of Figures 7 and 8 which differ from the corresponding features of Figures 1 to 6.
- Figure 9 shows a second embodiment of the invention, which instead of using a blister pack comprising a plurality of blisters, uses a plurality of individual packs each containing a single blister.
- components which are comparable in function to those of Figures 1 to 6 are denoted by the same reference numerals plus 100.
- the device of Figure 9 comprises a housing 101, a tray 102 with an integral support 103, a cover 104, and a plurality of individual blister packs 105.
- the housing 101 comprises a base member 110 and a lid 111 hinged thereto by pivots 112.
- the base member 110 includes side walls 114 and a top wall 116 which extends over only the forward portion of the base member to form a kind of bridge.
- Extending forwardly from the lid 111 is an elongate plunger 118 which is so positioned that when the lid 111 is raised the plunger passes through an aperture 117 formed in the top wall 116. When the lid is in its lowered position the plunger 118 is protected from damage by upstanding walls 119 formed on the top wall 116.
- the blister packs are removably contained in a magazine 151 which is fixed or removable and is located at the rear of the housing 101 and normally covered by the lid 111.
- the magazine is arranged to contain four packs, but other sizes of magazine could be used instead.
- the tray 102 defines a recess 150 adapted to receive one of the blister packs 105.
- the recess communicates at its forward end with a mouthpiece 127.
- the tray is slidable between the outward position illustrated and an inward position in which a flange 152 thereof rests against the forward end of the housing 101. sliding movement is achieved by means of a pair of runners 153 which pass down the inside of the housing adjacent the side walls 114 thereof.
- the sides of the magazine 151 stop short of the side walls 114 to permit the runners to pass.
- the patient removes the cover 104 and, with the tray in either its inward or outward position, raises the lid 111, and removes a blister pack 105 from the magazine 151.
- the patient places the blister pack on the tray with blister thereof extending downwardly into the recess 150.
- the lid is then lowered.
- the tray is then pushed to its inward position and the lid raised, to cause the plunger 118 to puncture the blister, and then lowered.
- the patient then inhales through the mouthpiece 127 ; medicament from the blister being entrained in the air flow thus produced.
- the blister pack is in close contact with the underside of the top wall 116 so that substantially the only air flow is that which passes through the aperture 117, through the hole formed in the blister aligned therewith, through the recess 150 and thence through the mouthpiece 127.
- the mouthpiece 127 could be provided with apertures corresponding to the apertures 28 of Figure 1.
- the embodiment of Figures 10 to 12 comprises a pair of identical inhalation devices arranged back to back to form a single article.
- Each device comprises a housing 201, a tray 202, a rotatable support 203 and a cover 204.
- the support 203 is designed to receive a circular blister pack which, for use in the embodiment illustrated in Figures 10 to 12, comprises four blisters arranged in a circle. It is to be understood, however, that blister packs with other numbers of blisters could be used instead, given appropriate modification to the rotatable support 203.
- the housing 201 comprises a base member 210 which is common to each of the devices.
- the housing further comprises a lid 211 hinged to the base member 210 by pivots 212.
- Each device has its own lid.
- the lid 211 has a recess 211' in the upper surface thereof; the recess in one side making it easier for a patient to lift the other lid.
- the recesses of the two lids are offset from one another on opposite sides of the article.
- the base member 210 has a base wall (not visible in the drawings), upstanding side walls 214, and a pair of top walls 216 ; one in each device, each top wall 216 being arranged to form a bridge between the side walls.
- Each top wall 216 has an aperture 217 formed therein.
- Extending forwardly from the front edge of each lid 211 is an elongate plunger 218. This is so positioned that when the lid 211 is raised (see Figure 11) the plunger passes through the aperture 217 which also acts as an air inlet into the device.
- the plunger 218 is protected from damage by upstanding walls 219 formed on the upper wall 216.
- the plunger 218 is curved, as viewed in side elevation, for reasons set out above.
- the tray 202 defines a shallow chamber for receiving the rotatable support 203.
- the tray 202 can be moved in the housing between an inward position, as shown in Figure 12, and an outward position, as shown in Figure 11. Further outward movement beyond the above mentioned outward position is possible only on releasing a lug mechanism which, when released, makes it possible to remove the tray completely from the housing.
- the lug mechanism can be the same as that described above with reference to Figures 1 to 0; or Figures 7 and 8, and including a lug 23, as are details of the other internal components of the devices, and these are not therefore described in more detail here.
- a mouthpiece 227 Extending from the front of the tray 202 is a mouthpiece 227.
- the mouthpiece is provided with a pair of apertures 228,though these are optional.
- two separate blister packs may be held in the article, one in each of the two devices. These two blister packs may contain different medicaments, and thus a patient needing two different medicaments can use a single article without the problem of needing repeatedly to change over the blister pack from one medicament to the other.
- FIG. 1 The embodiment shown in Figuresl3 to 15 is identical to that shown in Figures 10 to 12 except as regards the lids.
- the lids denoted by reference numeral 311
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pulmonology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
- External Artificial Organs (AREA)
- Electrotherapy Devices (AREA)
- Motorcycle And Bicycle Frame (AREA)
Abstract
Description
- This invention relates to devices by which a medicament in solid finely divided form can be administered to or by patients inhaling through the devices. Such devices are now quite well known for administering medicaments contained in capsules to patients suffering from bronchial conditions such as, for example; bronchial asthma. It is well known for medicament in powder or other finely divided form to be supplied in capsules which are loaded by a patient into such a device. The medicament is then released from the capsule and inhaled by the patient, usually through the mouth, but sometimes through the nose.
- The specification of PCT Application Publication No. W082/01470, GB-A-1387954 and GB-A-2061735 all describe devices for dispensing medicament in finely divided form from capsules. In each of these previously described devices; the capsules are mounted on a rotatable support member on which each capsule in turn can be brought to a position in which it is opened to enable medicament to exit from the capsule to permit it to be inhaled by a patient inhaling through a mouthpiece of the device.
- There are disadvantages in the use of capsules; which are made of gelatin; to contain medicaments. Gelatin is relatively unstable and is lacking in physical strength so that the capsules need to be protected by packaging; for example in glass bottles. Environmental degradation of both the capsules and their contents may occur in a relatively short time.
- In the device described in UK Patent Specification 1387954 the capsules are nounted in what is referred to therein as a blister pack, and is in fact a plurality of capsules mounted in a blister pack on a rotor which is designed to spin during exhalation by the patient to throw medicament out of an opened capsule, whereafter the patient inhales. This has a number of disadvantages, including the fact that the exhalation which is required is more difficult for some patients; for example asthma patients, than inhalation.
- In our patent specification GB-A-2129691 we provided a more convenient way of administering medicament to such patients than had been possible hitherto and which avoided the need to pack medicaments in capsules. The device there described makes use of the technique of packaging a medicament by loading the medicament directly into a blister pack comprising a sheet; which may be laminated, of foil or plastics material which acts as a carrier and which is provided with a number of breakable or openable containers called "blisters" incorporating a sheet secured on a first sheet to form a cover or lid. Such blister packs are in common use with tablets of one kind or another, but we have discovered that they can also be used with medicaments in finely divided solid form. GB-A-2129691 provides a device for administering to patients medicaments in blister pack form;
- However, the embodiments described in GB-A-2129691 are more bulky than is desirable. It is an object of the present invention to provide a device which avoids or mitigates this problem.
- Attention is also directed to our specification GB-A-2142246 which describes various alternative devices for administering medicaments held in solid finely divided form in blister packs.
- According to the present invention there is provided a device for administering medicaments in solid finely divided form to patients, comprising a housing; a tray mounted in the housing and movable between first and second positions relative to the housing; a support provided on the tray and adapted to receive, in use, a carrier provided with at least one medicament container; a plunger operable, in use, to penetrate a container registered therewith to open the container, movement of the tray from its first to its second position being such as to cause in use, the support to bring a container into registration with the plunger; an air inlet through which in use, air can enter the device, and an outlet through which a patient can inhale, whereby medicament will be released from an opened container and entrained in an air flow produced by the patient, air entering the air inlet and passing out through the outlet having entrained medicament therein.
- In an embodiment of the invention the support is rotatably mounted on the tray and the carrier has a plurality of medicament containers arranged in a circle. Indexing means are preferably provided so that movement of the tray from its first to its second position causes the support to be indexed to bring the next container into registration with the plunger.
- The housing of the device preferably has a base member and a lid pivotally mounted thereon for movement between a closed position and an open position. The plunger can then be carried by the lid and arranged to penetrate a container when the lid is moved to its open position.
- The device of the invention is suitable for administering a variety of medicaments such as, for example, salbutamol, beclomethasone dipropionate and sodium cromoglycate.
- A significant number of asthma patients suffer from asthma with a severity such that they need to take not one but two medicaments. These are, respectively, a a-stimulant, for example salbutamol or sodium cromoglycate, and an anti-inflammatory steroid, for example beclomethasone dipropionate. Typically a patient needing both these medicaments will take alternate doses of the two medicaments at prescribed intervals during the day.
- It is an object of one aspect of the present invention to provide a single device from which two medicaments can be administered. The two medicaments concerned may be a S-stimulant and an anti-inflammatory steroid respectively, or some other pair of medicaments used in treating asthma, or some other pair of medicaments inhaled for the purpose of treating some other condition. The reference to two medicaments is to be understood as including not only a pair of medicaments containing two different active ingredients, but also a pair of medicaments containing the same active ingredient in different dosages.
- According to this aspect of the present invention there is provided a device according to the invention in tandem with another such device to form a single article.
- Some embodiments of the invention are illustrated in the accompanying schematic drawings in which:
- Figure 1 is an exploded perspective view of a device according to one embodiment of the invention;
- Figure 2 is a perspective view of the device of Figure 1 with a cover thereof removed:
- Figure 3 is a view similar to that of Figure 2 but with a tray portion thereof in an outward position;
- Figure 4 is a plan view of the device with a portion broken away;
- Figure 5 is a section taken'on line X-X in Figure 4;
- Figure 6 is a section taken on line Y-Y in Figure 4;
- Figure 7 is an exploded perspective view of a second embodiment of the invention;
- Figure 8 is an underplan view of the rotatable support used in the device of Figure 7;
- Figure 9 is a perspective view of a third embodiment of the invention with the cover removed;
- Figure 10 is a perspective view of a fourth embodiment of the invention, for use in dispensing two medicaments;
- Figure 11 is a perspective view of the article of Figure 10 with a cover thereof removed and with a tray portion thereof in an outward position;
- Figure 12 is a perspective view of the article of Figure 10 with one of the covers thereof removed and with a lid thereof in a raised position; and
- Figures 13 to 15 show a fourth embodiment of the invention, also for use in dispensing two medicaments, in positions corresponding to those Figures 10 to 12.
- The device shown in Figures 1 to 6 comprise four principal components, namely a
housing 1, atray 2, arotatable support 3 and acover 4. Thesupport 3 is designed to receive acircular blister pack 5, as described in more detail below. - Considering first the
housing 1; this comprises abase member 10 and alid 11 hinged thereto bypivots 12. Thebase member 10 has abase wall 13, upstanding side andrear walls top wall 16 which extends over only the forward portion of the base member to form a kind of bridge. Thetop wall 16 has anaperture 17 formed therein. Extending forwardly from the front edge of thelid 11 is anelongated plunger 18. This is so positioned that when thelid 11 is raised the plunger passes through theaperture 17 which also acts as an air inlet into the device. The plunger is conveniently tapered at the tip to form a relatively sharp point, but this is not essential and a blunter plunger would serve the intended purpose which is described below. When the lid is in its lowered position theplunger 18 is protected from damage byupstanding walls 19 formed on theupper wall 16. - The
tray 2 defines ashallow chamber 20 for receiving therotatable support 3. In the centre of thechamber 20 is anupstanding lug 21 on which thesupport 3 is mounted for rotation. Thelug 21 is shown as being cruciform in cross-section, but need not be; a lug of circular cross-section, for example, could be used instead. Thetray 2 can be moved in the housing between an inward position, as shown in Figure 2, and an outward position, as shown in Figures 3 and 4. Further outward movement beyond the above mentioned outward position is normally prevented by alug 23 which is formed on the end of anarm 22 and which engages behind an inwardly directed protrusion on one of theside walls 14 of thehousing 1. Thearm 22 is secured to the rest of the tray only at its forward end and is substantially separated from the rest of the tray by aslot 24. Thearm 22 is resilient, and when it is desired to remove the tray completely from the housing this can be achieved by pressing thearm 22 inwardly and then withdrawing the tray. Withdrawal is assisted by the provision ofthumb grips 33, in the form of ribs, on both sides of the tray. The tray also has atongue 25 which can be depressed downwardly, as described below, and which has anopen slot 26. - Extending from the front of the
tray 2 is amouthpiece 27. It is through this mouthpiece that medicament leaves the device as it is inhaled by a patient. To improve airflow through the mouthpiece it may be provided with a pair ofapertures 28. - The
rotatable support 3 is in the form of a disc in which is formed a circular array ofcircular openings 30. Acentral opening 31 enables the carrier to be mounted for rotation on thelug 21. A corresponding plurality ofribs 32 are formed on the underside of thesupport 3, with one rib extending between each twoadjacent openings 30. - In use, the
cover 4 is removed and the tray with thesupport 3 mounted thereon, is then removed completely from thehousing 1 after thearm 22 has been depressed. Ablister pack 5 is then mounted on thesupport 3 with one blister extending into each of theopenings 30. The tray, support and blister pack are then inserted together into the housing. Thecover 4 is then replaced. When a patient desires to inhale medicament he removes the cover and raises thelid 11 so as to cause theplunger 18 to pass through theaperture 17 and puncture a respective blister located immediately below theaperture 17. The lid is then lowered to withdraw the plunger from the blister, leaving a hole therein, and the patient inhales the medicament through themouthpiece 27. It should be noted that the plunger is positively withdrawn from the blister by the patient rather than being left to withdraw under spring pressure (as in GB-A-2129691 mentioned above) which avoids any risk of the plunger remaining jammed in the blister. Either before replacing the cover, or on the next occasion when the patient desires to use the device, thesupport 3 is rotated to bring the next blister beneath theaperture 17. This is achieved as follows. Thetray 2 is withdrawn to its outward position and then pushed back to its inward position. During the latter movement anarm 60 which extends forwardly in thecasing 1 and is secured to thebase wall 13 thereof engages one of the ribs on the underside of thesupport 3. This causes the support to rotate in a clockwise direction, as viewed in Figure 4; by an amount sufficient to bring the next blister beneath theaperture 17. During this rotational movement another of the ribs bears against, and progressively depresses, thetongue 25 until that rib engages in theslot 26 which retains the support in its desired position and prevents further rotation. It will be seen from Figure 5 that on either side of theslot 26 are a pair of sloping shoulders, the larger of which prevents anticlockwise rotation of thesupport 3, and the smaller of which is sufficient to normally retain the rib in theslot 26 but which is not such as to prevent the rib leaving the slot on the next occasion when thesupport 3 is rotated as described above. - The upper surface of the
blister pack 5 carries a series of numbers, arranged in a circle, corresponding to the number of blisters in the pack (in this case thenumbers 1 to 8). Thetop wall 16 of thehousing 1 has anaperture 34 through which a respective one of the numbers is visible to indicate the number of the blister then aligned with theaperture 17; and hence to indicate how many blisters are left for use, or alternatively how many blisters have been used. - In order to assist in providing maximum efficiency of powder entrainment, means are provided for ensuring an air flow path through the device which is substantially isolated from the surrounding environment. To this end, the
tray 2 is provided adjacent the mouthpiece with a pair ofupstanding walls 35 which converge towards the centre of the tray, the radially inner ends of thewalls 35 being interconnected by awall 35a. When thesupport 3 is in a position in which a blister is aligned with the plunger, twoadjacent ribs 32 of the support are aligned with thewall 35 and in close contact therewith. Also, theblister pack 5 is in close contact with the underside of thetop wall 16 of the tray, at least in the vicinity of theaperture 17. Thus when the patient inhales through themouthpiece 27 substantially the only air flow which is produced is one which passes through theaperture 17, through the hole formed in the blister aligned therewith, through a chamber defined by thewall 35 and theribs 32 in contact therewith and thence through themouthpiece 27, optionally supplemented by air flowing into the mouthpiece through theapertures 28 if these are provided. - There is thus no requirement for airtightness in other parts of the device, for example, airtightness between the
housing parts - Although not visible in the drawings, the device shown in Figures 1 to 6 is preferably provided with a recess, located inwardly of the
rear wall 15 and extending parallel thereto, for removably receiving a brush which the patient can use to clean the device of powdered medicament spilt therein. - The device shown in Figures 1 to 6 may be modified in various ways. For example, the
plunger 18 may be curved, as viewed in side elevation so that as it pierces a blister it produces in it a hole which is smaller and more nearly circular than that which is produced if the plunger is straight as illustrated. This provides for improved entrainment of powdered medicament in the air flow produced by inhalation and helps to avoid powder being trapped in the blister. This and other modifications are shown in Figures 7 and 8 which illustrate a second embodiment of the invention. - The embodiment of Figures 7 and 8 is broadly similar to that of Figures 1 to 6, and the reference numerals in Figures 7 and 8 are the same as in Figures 1 to 6, where appropriate, but with the addition of a prime. Because of the similarities between the two embodiments the following description deals only with features of Figures 7 and 8 which differ from the corresponding features of Figures 1 to 6.
- (a) The
arm 22 is replaced by a pair of resilient arms 22', one on either side. The symmetry thus achieved makes it easier to slide the tray 2' in and out. - (b) There is no
slot 26. Instead, the ribs 32' on the underside of the disc 3' engage behind the rear edge of tongue 25'. - (c) The
walls straight wall 35a is replaced by an arcuate wall portion the ends of which merge into the radially inner ends of thewalls 35. A corresponding modification is required in the ribs on the underside of the rotatable support, and Figure 8 is an underplan view of such a modified support. - (d) The lug 21' is circular in cross-section, rather than being cruciform as shown in Figure 1.
- (e) The thumb grips 33 are replaced by
thumb grips 33a' on the arms 22' and additional thumb grips 33b' are provided on the cover 4'. - (f) The
aperture 34, through which numbers on the blister pack are visible is replaced by a slot 34'. Most of the slot is covered by the lid 11', but the forward portion is not; and it is through this forward portion that the numbers are visible. - (g) The brush, which is referred to above but is not shown in Figures 1 to 6 is shown in Figure 7 and denoted by
reference numeral 40. - Figure 9 shows a second embodiment of the invention, which instead of using a blister pack comprising a plurality of blisters, uses a plurality of individual packs each containing a single blister. In Figure 9 components which are comparable in function to those of Figures 1 to 6 are denoted by the same reference numerals plus 100.
- The device of Figure 9 comprises a
housing 101, atray 102 with anintegral support 103, acover 104, and a plurality of individual blister packs 105. Thehousing 101 comprises abase member 110 and alid 111 hinged thereto bypivots 112. Thebase member 110 includesside walls 114 and atop wall 116 which extends over only the forward portion of the base member to form a kind of bridge. Extending forwardly from thelid 111 is anelongate plunger 118 which is so positioned that when thelid 111 is raised the plunger passes through anaperture 117 formed in thetop wall 116. When the lid is in its lowered position theplunger 118 is protected from damage byupstanding walls 119 formed on thetop wall 116. - The blister packs are removably contained in a
magazine 151 which is fixed or removable and is located at the rear of thehousing 101 and normally covered by thelid 111. In the illustrated embodiment the magazine is arranged to contain four packs, but other sizes of magazine could be used instead. - The
tray 102 defines arecess 150 adapted to receive one of the blister packs 105. The recess communicates at its forward end with amouthpiece 127. The tray is slidable between the outward position illustrated and an inward position in which aflange 152 thereof rests against the forward end of thehousing 101. sliding movement is achieved by means of a pair ofrunners 153 which pass down the inside of the housing adjacent theside walls 114 thereof. The sides of themagazine 151 stop short of theside walls 114 to permit the runners to pass. - In use, the patient removes the
cover 104 and, with the tray in either its inward or outward position, raises thelid 111, and removes ablister pack 105 from themagazine 151. With the tray in its outward position, the patient then places the blister pack on the tray with blister thereof extending downwardly into therecess 150. The lid is then lowered. The tray is then pushed to its inward position and the lid raised, to cause theplunger 118 to puncture the blister, and then lowered. The patient then inhales through themouthpiece 127; medicament from the blister being entrained in the air flow thus produced. The blister pack is in close contact with the underside of thetop wall 116 so that substantially the only air flow is that which passes through theaperture 117, through the hole formed in the blister aligned therewith, through therecess 150 and thence through themouthpiece 127. If desired, themouthpiece 127 could be provided with apertures corresponding to theapertures 28 of Figure 1. - The embodiment of Figures 10 to 12 comprises a pair of identical inhalation devices arranged back to back to form a single article. Each device comprises a
housing 201, atray 202, arotatable support 203 and acover 204. Thesupport 203 is designed to receive a circular blister pack which, for use in the embodiment illustrated in Figures 10 to 12, comprises four blisters arranged in a circle. It is to be understood, however, that blister packs with other numbers of blisters could be used instead, given appropriate modification to therotatable support 203. - Considering first the
housing 201, this comprises abase member 210 which is common to each of the devices. The housing further comprises alid 211 hinged to thebase member 210 bypivots 212. Each device has its own lid. Thelid 211 has a recess 211' in the upper surface thereof; the recess in one side making it easier for a patient to lift the other lid. - The recesses of the two lids are offset from one another on opposite sides of the article. The
base member 210 has a base wall (not visible in the drawings),upstanding side walls 214, and a pair oftop walls 216; one in each device, eachtop wall 216 being arranged to form a bridge between the side walls. Eachtop wall 216 has anaperture 217 formed therein. Extending forwardly from the front edge of eachlid 211 is anelongate plunger 218. This is so positioned that when thelid 211 is raised (see Figure 11) the plunger passes through theaperture 217 which also acts as an air inlet into the device. When the lid is in its lowered position theplunger 218 is protected from damage byupstanding walls 219 formed on theupper wall 216. As can be seen, theplunger 218 is curved, as viewed in side elevation, for reasons set out above. - The
tray 202 defines a shallow chamber for receiving therotatable support 203. Thetray 202 can be moved in the housing between an inward position, as shown in Figure 12, and an outward position, as shown in Figure 11. Further outward movement beyond the above mentioned outward position is possible only on releasing a lug mechanism which, when released, makes it possible to remove the tray completely from the housing. The lug mechanism can be the same as that described above with reference to Figures 1 to 0; or Figures 7 and 8, and including alug 23, as are details of the other internal components of the devices, and these are not therefore described in more detail here. - Extending from the front of the
tray 202 is amouthpiece 227. The mouthpiece is provided with a pair ofapertures 228,though these are optional. - The remaining constructional details and the manner of use of the devices shown in Figures 10to 12 can be ascertained from the above description of Figures 1 to 6, and Figures 7 and 8.
- It will be apparent that two separate blister packs may be held in the article, one in each of the two devices. These two blister packs may contain different medicaments, and thus a patient needing two different medicaments can use a single article without the problem of needing repeatedly to change over the blister pack from one medicament to the other.
- The embodiment shown in Figuresl3 to 15 is identical to that shown in Figures 10 to 12 except as regards the lids. In the embodiment of Figures 13 to 15 the lids, denoted by
reference numeral 311, each have the general shape of an L, with the stems of the two L's each occupying half the width of the article and being side by side with one another. This makes it possible for the distance between the distal end of each lid and the pivot thereof to be greater than is the case with the embodiment ofFigures 10to 12. This in turn means that for a given upward force applied by the patient to a lid the downward force of the tip of theplunger 218 will be greater in the embodiment of Figures 13 to 15 than it is in the embodiment of Figures 10to 12, This in turn makes it easier for a patient to puncture a blister, a fact which may be of considerable significance to some patients, particularly the elderly and infirm.
Claims (19)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB858519141A GB8519141D0 (en) | 1985-07-30 | 1985-07-30 | Administering medicaments to patients |
| GB8519141 | 1985-07-30 | ||
| GB858525067A GB8525067D0 (en) | 1985-10-10 | 1985-10-10 | Administering medicaments to patients |
| GB8525067 | 1985-10-10 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0211595A2 true EP0211595A2 (en) | 1987-02-25 |
| EP0211595A3 EP0211595A3 (en) | 1988-10-05 |
| EP0211595B1 EP0211595B1 (en) | 1991-11-13 |
Family
ID=26289568
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP86305807A Expired - Lifetime EP0211595B1 (en) | 1985-07-30 | 1986-07-29 | Devices for administering medicaments to patients |
Country Status (35)
| Country | Link |
|---|---|
| US (2) | US4811731A (en) |
| EP (1) | EP0211595B1 (en) |
| JP (1) | JPS6241668A (en) |
| KR (1) | KR940002247B1 (en) |
| AT (1) | AT396872B (en) |
| AU (1) | AU591152B2 (en) |
| BE (1) | BE905189A (en) |
| BR (1) | BR8603576A (en) |
| CA (1) | CA1272917A (en) |
| CH (1) | CH672600A5 (en) |
| CY (1) | CY1481A (en) |
| DE (2) | DE3682457D1 (en) |
| DK (1) | DK163640C (en) |
| DO (1) | DOP1989004707A (en) |
| ES (1) | ES2000781A6 (en) |
| FI (1) | FI88112C (en) |
| FR (1) | FR2585563B1 (en) |
| GB (1) | GB2178965B (en) |
| GR (1) | GR861995B (en) |
| HK (1) | HK67589A (en) |
| HU (1) | HU199306B (en) |
| IE (1) | IE59026B1 (en) |
| IL (1) | IL79550A (en) |
| IT (1) | IT1195984B (en) |
| KE (1) | KE3865A (en) |
| LU (1) | LU86534A1 (en) |
| MX (1) | MX171389B (en) |
| NL (1) | NL8601949A (en) |
| NO (1) | NO166268C (en) |
| NZ (1) | NZ217006A (en) |
| PH (1) | PH26882A (en) |
| PL (1) | PL149733B1 (en) |
| PT (1) | PT83094B (en) |
| SE (1) | SE8603252L (en) |
| SG (1) | SG8789G (en) |
Cited By (38)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2660550A1 (en) * | 1990-03-02 | 1991-10-11 | Glaxo Group Ltd | Medicament pack for inhalation device |
| WO1993009831A1 (en) * | 1991-11-18 | 1993-05-27 | Smithkline Beecham Plc | Oral inhaler |
| EP0547429A1 (en) * | 1991-12-14 | 1993-06-23 | ASTA Medica Aktiengesellschaft | Powder inhaler |
| US5388572A (en) * | 1993-10-26 | 1995-02-14 | Tenax Corporation (A Connecticut Corp.) | Dry powder medicament inhalator having an inhalation-activated piston to aerosolize dose and deliver same |
| US5388573A (en) * | 1993-12-02 | 1995-02-14 | Tenax Corporation | Dry powder inhalator medicament carrier |
| BE1007408A3 (en) * | 1993-08-13 | 1995-06-06 | Winters Joep | Method and device for checking medicine intake |
| US5429122A (en) * | 1990-09-26 | 1995-07-04 | Zanen; Pieter | Inhaler devices provided with a reservoir for several doses of medium for inhaling, transporting device, whirl chamber |
| US5460173A (en) * | 1993-03-03 | 1995-10-24 | Tenax Corporation | Dry powder inhaler medicament carrier |
| US5492112A (en) * | 1991-05-20 | 1996-02-20 | Dura Pharmaceuticals, Inc. | Dry powder inhaler |
| US5692496A (en) * | 1995-08-02 | 1997-12-02 | Innovative Devices, Llc | Dry powder medicament inhalator having an inhalation-activated flow diverting means for triggering delivery of medicament |
| WO1998004308A1 (en) | 1996-07-31 | 1998-02-05 | Glaxo Group Limited | Medicament carrier with agglomerated large medicament particles and related method of manufacture thereof |
| US5823183A (en) * | 1995-08-02 | 1998-10-20 | Innovative Devices | Dry powder medicament inhalator having an inhalation-activated flow diverting means for triggering delivery of medicament |
| US5881719A (en) * | 1995-06-30 | 1999-03-16 | Asta Medica Aktiengesellschaft | Inhaler for administering medicaments from blister packs |
| US5921237A (en) * | 1995-04-24 | 1999-07-13 | Dura Pharmaceuticals, Inc. | Dry powder inhaler |
| US5988163A (en) * | 1995-08-02 | 1999-11-23 | Innovative Devices | Dry powder medicament inhalator having an inhalation-activated flow diverting means for triggering delivery of delivery of medicament |
| US6076522A (en) * | 1996-05-23 | 2000-06-20 | Glaxo Wellcome Inc. | Metering apparatus |
| US6209538B1 (en) * | 1995-08-02 | 2001-04-03 | Robert A. Casper | Dry powder medicament inhalator having an inhalation-activated flow diverting means for triggering delivery of medicament |
| WO2002026302A1 (en) | 2000-09-27 | 2002-04-04 | Merck Patent Gmbh | Device for administering doses of particulate material |
| US6536427B2 (en) | 1990-03-02 | 2003-03-25 | Glaxo Group Limited | Inhalation device |
| US6792945B2 (en) | 1990-03-02 | 2004-09-21 | Glaxo Group Limited | Inhalation device |
| US6923178B2 (en) | 2000-05-10 | 2005-08-02 | Innovative Devices, Llc. | Medicament container with same side airflow inlet and outlet |
| US7225808B2 (en) | 1990-03-02 | 2007-06-05 | Glaxo Group Limited | Inhalation device |
| US8022082B2 (en) | 2002-04-09 | 2011-09-20 | Boehringer Ingelheim Pharma Gmbh & Co., Kg | Method for the administration of an anticholinergic by inhalation |
| US8357696B2 (en) | 2005-05-18 | 2013-01-22 | Mpex Pharmaceuticals, Inc. | Aerosolized fluoroquinolones and uses thereof |
| EP2594272A2 (en) | 2005-05-18 | 2013-05-22 | Mpex Pharmaceuticals, Inc. | Aerosolized fluoroquinolones and uses thereof |
| EP2666497A1 (en) | 2012-05-25 | 2013-11-27 | Arven Ilac Sanayi Ve Ticaret A.S. | An inhaler comprising a mouthpiece having an improved air channel |
| US8629139B2 (en) | 2008-10-07 | 2014-01-14 | Mpex Pharmaceuticals, Inc. | Topical use of Levofloxacin for reducing lung inflammation |
| US8815838B2 (en) | 2008-10-07 | 2014-08-26 | David C. Griffith | Aerosol fluoroquinolone formulations for improved pharmacokinetics |
| CN104984448A (en) * | 2015-07-30 | 2015-10-21 | 中山市美捷时包装制品有限公司 | Dry powder inhaler |
| US9700564B2 (en) | 2009-09-04 | 2017-07-11 | Horizon Orphan Llc | Use of aerosolized levofloxacin for treating cystic fibrosis |
| US10028966B2 (en) | 2014-01-10 | 2018-07-24 | Avalyn Pharma Inc. | Aerosol pirfenidone and pyridone analog compounds and uses thereof |
| US10583261B2 (en) | 2013-07-12 | 2020-03-10 | Liita Holdings Ltd | Inhaler |
| WO2020148276A1 (en) * | 2019-01-14 | 2020-07-23 | Alfred Von Schuckmann | Device for inhaling powder-type substances, substance container for a device of this type and method for filling a device of this type |
| EP3782604A1 (en) | 2013-07-31 | 2021-02-24 | Windward Pharma, Inc. | Aerosol tyrosine kinase inhibitor compounds and uses thereof |
| EP3459578B1 (en) | 2003-07-02 | 2021-03-03 | Pfizer Limited | Dispensing device |
| EP4059499A1 (en) | 2011-01-31 | 2022-09-21 | Avalyn Pharma Inc. | Aerosol pirfenidone and pyridone analog compounds and uses thereof |
| WO2022240897A1 (en) | 2021-05-10 | 2022-11-17 | Sepelo Therapeutics, Llc | Pharmaceutical composition comprising delafloxacin for administration into the lung |
| WO2023028364A1 (en) | 2021-08-27 | 2023-03-02 | Sepelo Therapeutics, Llc | Targeted compositions and uses therof |
Families Citing this family (478)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GR861995B (en) * | 1985-07-30 | 1986-11-04 | Glaxo Group Ltd | Devices for administering medicaments to patients |
| IT1222509B (en) * | 1987-08-17 | 1990-09-05 | Miat Spa | INSUFFLATOR FOR THE ADMINISTRATION OF DRUGS IN THE FORM OF PRE-DOSED POWDER IN OPERATIONS |
| US4817789A (en) * | 1987-09-23 | 1989-04-04 | Allergan, Inc. | Lens container assembly |
| FR2626500B1 (en) * | 1988-01-28 | 1990-04-27 | Valois Sa | VAPOR PUSH BUTTON AGENCY FOR MIXING AN ADDITIVE IN THE EMISSION OF THE JET |
| CH678151A5 (en) * | 1988-07-13 | 1991-08-15 | Heinz Hermann Weick | Self-medication nasal dispenser |
| GB8824804D0 (en) * | 1988-10-22 | 1988-11-30 | Fisons Plc | Device |
| SE466684B (en) * | 1989-03-07 | 1992-03-23 | Draco Ab | DEVICE INHALATOR AND PROCEDURE TO REGISTER WITH THE DEVICE INHALATOR MEDICATION |
| US5176132A (en) * | 1989-05-31 | 1993-01-05 | Fisons Plc | Medicament inhalation device and formulation |
| US5239991A (en) * | 1989-06-21 | 1993-08-31 | Fisons Plc | Disposable powder medicament inhalation device with peel-off cover |
| IT1230313B (en) * | 1989-07-07 | 1991-10-18 | Somova Spa | INHALER FOR CAPSULES MEDICATIONS. |
| DE3927170A1 (en) * | 1989-08-17 | 1991-02-21 | Boehringer Ingelheim Kg | INHALATOR |
| US5270305A (en) * | 1989-09-08 | 1993-12-14 | Glaxo Group Limited | Medicaments |
| US5208226A (en) * | 1989-09-08 | 1993-05-04 | Glaxo Group Limited | Medicaments |
| DK544589D0 (en) * | 1989-11-01 | 1989-11-01 | Novo Nordisk As | MANUALLY OPERATED DEVICE FOR DISPENSING A PRESCRIBED QUANTITY OF A POWDER-SHAPED SUBSTANCE |
| GB9012870D0 (en) * | 1990-06-08 | 1990-08-01 | Glaxo Group Ltd | Device |
| GB9015522D0 (en) * | 1990-07-13 | 1990-08-29 | Braithwaite Philip W | Inhaler |
| IT1243344B (en) * | 1990-07-16 | 1994-06-10 | Promo Pack Sa | MULTI-DOSE INHALER FOR POWDER MEDICATIONS |
| SE9002895D0 (en) * | 1990-09-12 | 1990-09-12 | Astra Ab | INHALATION DEVICES FOR DISPENSING POWDERS I |
| FR2667509B1 (en) * | 1990-10-04 | 1995-08-25 | Valois | POWDER INHALER, DEVICE FOR PACKAGING POWDER MICRODOSES IN THE FORM OF BANDS SUITABLE FOR USE IN A POWDER INHALER, AND METHOD FOR MANUFACTURING SUCH BANDS. |
| NL9002706A (en) * | 1990-12-10 | 1992-07-01 | Pharmachemie Bv | Apparatus for use when inhaling powdered materials packaged in rod capsules. |
| US5243970A (en) * | 1991-04-15 | 1993-09-14 | Schering Corporation | Dosing device for administering metered amounts of powdered medicaments to patients |
| US6681767B1 (en) | 1991-07-02 | 2004-01-27 | Nektar Therapeutics | Method and device for delivering aerosolized medicaments |
| JP3230056B2 (en) * | 1991-07-02 | 2001-11-19 | インヘイル・インコーポレーテッド | Device for forming an aerosolized dose of a drug |
| US5337740A (en) * | 1991-08-01 | 1994-08-16 | New England Pharmaceuticals, Inc. | Inhalation devices |
| US5161524A (en) * | 1991-08-02 | 1992-11-10 | Glaxo Inc. | Dosage inhalator with air flow velocity regulating means |
| US5287850A (en) * | 1991-08-20 | 1994-02-22 | Habley Medical Technology Corporation | Timing and velocity controlled powered pharmaceutical inhaler |
| DE4128295A1 (en) * | 1991-08-27 | 1993-03-04 | Pfeiffer Erich Gmbh & Co Kg | DISCHARGE DEVICE FOR FLOWABLE MEDIA |
| GB2264237A (en) * | 1992-02-05 | 1993-08-25 | Robert Edward Newell | An inhaler |
| GB9203761D0 (en) * | 1992-02-21 | 1992-04-08 | Innovata Biomed Ltd | Inhaler |
| EP0558879B1 (en) * | 1992-03-04 | 1997-05-14 | Astra Aktiebolag | Disposable inhaler |
| US5301664A (en) * | 1992-03-06 | 1994-04-12 | Sievers Robert E | Methods and apparatus for drug delivery using supercritical solutions |
| JPH0633935Y2 (en) * | 1992-03-27 | 1994-09-07 | サカセ化学工業株式会社 | Grouping Medicine Pool |
| DE9307115U1 (en) * | 1992-05-29 | 1993-09-02 | GGU Gesellschaft für Gesundheits- und Umweltforschung mbH & Co. Vertriebs KG, 65933 Frankfurt | Device for producing inhalable active substances |
| US5394868A (en) * | 1992-06-25 | 1995-03-07 | Schering Corporation | Inhalation device for powdered medicaments |
| US5785049A (en) * | 1994-09-21 | 1998-07-28 | Inhale Therapeutic Systems | Method and apparatus for dispersion of dry powder medicaments |
| US6673335B1 (en) * | 1992-07-08 | 2004-01-06 | Nektar Therapeutics | Compositions and methods for the pulmonary delivery of aerosolized medicaments |
| US6509006B1 (en) | 1992-07-08 | 2003-01-21 | Inhale Therapeutic Systems, Inc. | Devices compositions and methods for the pulmonary delivery of aerosolized medicaments |
| US6582728B1 (en) | 1992-07-08 | 2003-06-24 | Inhale Therapeutic Systems, Inc. | Spray drying of macromolecules to produce inhaleable dry powders |
| US7448375B2 (en) * | 1993-01-29 | 2008-11-11 | Aradigm Corporation | Method of treating diabetes mellitus in a patient |
| US6024090A (en) * | 1993-01-29 | 2000-02-15 | Aradigm Corporation | Method of treating a diabetic patient by aerosolized administration of insulin lispro |
| TW360548B (en) | 1993-04-08 | 1999-06-11 | Powderject Res Ltd | Products for therapeutic use |
| US5372128A (en) * | 1993-04-14 | 1994-12-13 | Habley Medical Technology Corporation | Fluidizing powder inhaler |
| US5533502A (en) * | 1993-05-28 | 1996-07-09 | Vortran Medical Technology, Inc. | Powder inhaler with aerosolization occurring within each individual powder receptacle |
| US5524613A (en) * | 1993-08-25 | 1996-06-11 | Habley Medical Technology Corporation | Controlled multi-pharmaceutical inhaler |
| PT101450B (en) * | 1994-02-02 | 1999-11-30 | Hovione Produtos Farmaceuticos | NEW INHALATION DEVICE |
| DK0748213T3 (en) | 1994-03-07 | 2004-08-02 | Nektar Therapeutics | Methods and compositions for pulmonary administration of insulin |
| US6051256A (en) | 1994-03-07 | 2000-04-18 | Inhale Therapeutic Systems | Dispersible macromolecule compositions and methods for their preparation and use |
| US6102036A (en) * | 1994-04-12 | 2000-08-15 | Smoke-Stop | Breath activated inhaler |
| CA2190502A1 (en) * | 1994-05-18 | 1995-11-23 | Robert M. Platz | Methods and compositions for the dry powder formulation of interferons |
| US5483954A (en) * | 1994-06-10 | 1996-01-16 | Mecikalski; Mark B. | Inhaler and medicated package |
| US6290991B1 (en) | 1994-12-02 | 2001-09-18 | Quandrant Holdings Cambridge Limited | Solid dose delivery vehicle and methods of making same |
| ES2302332T3 (en) * | 1994-09-21 | 2008-07-01 | Nektar Therapeutics | APPARATUS AND METHODS TO DISPERSE DRY POWDER DRUGS. |
| FR2725626A1 (en) * | 1994-10-18 | 1996-04-19 | Sofab | DEVICE FOR INHALING POWDERED PRODUCTS |
| DE19500764C2 (en) * | 1995-01-13 | 2001-09-27 | Sofotec Gmbh & Co Kg | Device for administering medication in solid form, finely distributed in an air stream |
| US5676643A (en) * | 1995-02-13 | 1997-10-14 | The Procter & Gamble Company | Dispenser for friably releasing dry particulate medicaments |
| US5780014A (en) * | 1995-04-14 | 1998-07-14 | Inhale Therapeutic Systems | Method and apparatus for pulmonary administration of dry powder alpha 1-antitrypsin |
| US5622166A (en) * | 1995-04-24 | 1997-04-22 | Dura Pharmaceuticals, Inc. | Dry powder inhaler delivery system |
| US5669973A (en) * | 1995-06-06 | 1997-09-23 | David Sarnoff Research Center, Inc. | Apparatus for electrostatically depositing and retaining materials upon a substrate |
| US5714007A (en) * | 1995-06-06 | 1998-02-03 | David Sarnoff Research Center, Inc. | Apparatus for electrostatically depositing a medicament powder upon predefined regions of a substrate |
| US20040237961A1 (en) * | 1995-06-08 | 2004-12-02 | Snow John Medlin | Inhalation actuated device for use with metered dose inhalers (MDIs) |
| US6672304B1 (en) | 1995-06-08 | 2004-01-06 | Innovative Devices, Llc | Inhalation actuated device for use with metered dose inhalers (MDIs) |
| US5642727A (en) * | 1995-07-25 | 1997-07-01 | David Sarnoff Research Center, Inc. | Inhaler apparatus using a tribo-electric charging technique |
| NL1001031C1 (en) * | 1995-08-23 | 1997-02-25 | Npk Ind Design B V | Device for dispensing pills from a blister pack. |
| US5649554A (en) * | 1995-10-16 | 1997-07-22 | Philip Morris Incorporated | Electrical lighter with a rotatable tobacco supply |
| US5827985A (en) * | 1995-12-19 | 1998-10-27 | Endress + Hauser Gmbh + Co. | Sensor apparatus for process measurement |
| US5669378A (en) * | 1995-12-21 | 1997-09-23 | Pera; Ivo | Inhaling device |
| JP2000503565A (en) | 1996-01-03 | 2000-03-28 | グラクソ、グループ、リミテッド | Inhaler |
| USD381416S (en) * | 1996-02-08 | 1997-07-22 | Astra Aktiebolag | Unit dose inhaler |
| US5743251A (en) | 1996-05-15 | 1998-04-28 | Philip Morris Incorporated | Aerosol and a method and apparatus for generating an aerosol |
| US5871010A (en) * | 1996-06-10 | 1999-02-16 | Sarnoff Corporation | Inhaler apparatus with modified surfaces for enhanced release of dry powders |
| US5857456A (en) * | 1996-06-10 | 1999-01-12 | Sarnoff Corporation | Inhaler apparatus with an electronic means for enhanced release of dry powders |
| AU6014098A (en) | 1996-12-31 | 1998-07-31 | Inhale Therapeutic Systems | Aerosolized hydrophobic drug |
| US20030203036A1 (en) * | 2000-03-17 | 2003-10-30 | Gordon Marc S. | Systems and processes for spray drying hydrophobic drugs with hydrophilic excipients |
| US5794613A (en) * | 1997-01-09 | 1998-08-18 | Sepracor, Inc. | Multiple-dose dispenser for dry powder inhalers |
| TW469832U (en) * | 1997-03-14 | 2001-12-21 | Astra Ab | Inhalation device |
| US6006747A (en) * | 1997-03-20 | 1999-12-28 | Dura Pharmaceuticals, Inc. | Dry powder inhaler |
| US20030035778A1 (en) * | 1997-07-14 | 2003-02-20 | Robert Platz | Methods and compositions for the dry powder formulation of interferon |
| US6237590B1 (en) * | 1997-09-18 | 2001-05-29 | Delsys Pharmaceutical Corporation | Dry powder delivery system apparatus |
| USD417731S (en) * | 1997-10-22 | 1999-12-14 | The Procter & Gamble Company | Container for nasal spray |
| US6116238A (en) * | 1997-12-02 | 2000-09-12 | Dura Pharmaceuticals, Inc. | Dry powder inhaler |
| DE69840876D1 (en) * | 1997-12-02 | 2009-07-16 | Valois Sas | dry powder inhaler |
| US6237591B1 (en) | 1998-11-02 | 2001-05-29 | Dura Pharmaceuticals, Inc. | Turbine dry powder inhaler |
| PL343276A1 (en) | 1998-03-16 | 2001-08-13 | Inhale Therapeutic Syst | Aerosolized active agent delivery |
| DE19817417A1 (en) | 1998-04-18 | 1999-10-21 | Pfeiffer Erich Gmbh & Co Kg | Dispenser for media, especially powder |
| US6257233B1 (en) | 1998-06-04 | 2001-07-10 | Inhale Therapeutic Systems | Dry powder dispersing apparatus and methods for their use |
| US6149774A (en) | 1998-06-10 | 2000-11-21 | Delsys Pharmaceutical Corporation | AC waveforms biasing for bead manipulating chucks |
| DE19831525A1 (en) * | 1998-07-14 | 2000-01-20 | Pfeiffer Erich Gmbh & Co Kg | Media Donor |
| KR100522910B1 (en) * | 1998-09-15 | 2005-12-21 | 주식회사 새 한 | Manufacturing method of polyester flammable fabric with excellent refreshing feeling |
| UA73924C2 (en) * | 1998-10-09 | 2005-10-17 | Nektar Therapeutics | Device for delivering active agent formulation to lungs of human patient |
| US6234167B1 (en) | 1998-10-14 | 2001-05-22 | Chrysalis Technologies, Incorporated | Aerosol generator and methods of making and using an aerosol generator |
| US6923979B2 (en) * | 1999-04-27 | 2005-08-02 | Microdose Technologies, Inc. | Method for depositing particles onto a substrate using an alternating electric field |
| CA2725731A1 (en) * | 1999-06-05 | 2000-12-14 | Innovata Biomed Limited | Delivery system |
| US9006175B2 (en) | 1999-06-29 | 2015-04-14 | Mannkind Corporation | Potentiation of glucose elimination |
| US6606992B1 (en) * | 1999-06-30 | 2003-08-19 | Nektar Therapeutics | Systems and methods for aerosolizing pharmaceutical formulations |
| US7305986B1 (en) | 1999-07-23 | 2007-12-11 | Mannkind Corporation | Unit dose capsules for use in a dry powder inhaler |
| US6730066B1 (en) | 1999-08-03 | 2004-05-04 | Pharmacia Ab | Liquid delivery container |
| GB9920839D0 (en) | 1999-09-04 | 1999-11-10 | Innovata Biomed Ltd | Inhaler |
| GB9924415D0 (en) * | 1999-10-16 | 1999-12-15 | Glaxo Group Ltd | Medicament pack |
| AU1752601A (en) * | 1999-11-11 | 2001-06-06 | Dura Pharmaceuticals, Inc. | Dry powder inhaler |
| US6810872B1 (en) | 1999-12-10 | 2004-11-02 | Unisia Jecs Corporation | Inhalant medicator |
| US20010029947A1 (en) | 1999-12-17 | 2001-10-18 | Steve Paboojian | Receptacles to facilitate the extraction of powders |
| US6883516B2 (en) * | 2000-04-27 | 2005-04-26 | Chrysalis Technologies Incorporated | Method for generating an aerosol with a predetermined and/or substantially monodispersed particle size distribution |
| MY136453A (en) * | 2000-04-27 | 2008-10-31 | Philip Morris Usa Inc | "improved method and apparatus for generating an aerosol" |
| GB0015034D0 (en) * | 2000-06-21 | 2000-08-09 | Glaxo Group Ltd | Inhalation device |
| AR028746A1 (en) * | 2000-06-23 | 2003-05-21 | Norton Health Care Ltd | DOSE CARTRIDGE PREVIOUSLY MEASURES FOR DRY POWDER INHALER OPERATED BY BREATHING, INHALER AND A METHOD OF PROVISION OF DOSE PREVIOUSLY DRY POWDER MEASURES |
| US7575761B2 (en) * | 2000-06-30 | 2009-08-18 | Novartis Pharma Ag | Spray drying process control of drying kinetics |
| EP1172122A1 (en) * | 2000-07-14 | 2002-01-16 | The Technology Partnership Public Limited Company | Dry powder inhaler |
| US6759398B2 (en) | 2000-08-05 | 2004-07-06 | Smithkline Beecham Corporation | Anti-inflammatory androstane derivative |
| BRPI0113042B8 (en) | 2000-08-05 | 2021-05-25 | Glaxo Group Ltd | compound of the formula or a physiologically acceptable solvate thereof, use thereof, pharmaceutical composition, pharmaceutical formulation, method of treating a human or animal subject with an inflammatory and/or allergic condition, and, process for preparing a compound or a solvate thereof |
| AU2001286518A1 (en) * | 2000-08-15 | 2002-02-25 | University Of Kentucky Research Foundation | Programmable multi-dose intranasal drug delivery device |
| SE517229C2 (en) * | 2000-09-25 | 2002-05-14 | Microdrug Ag | Continuous dry powder inhaler |
| US6595210B2 (en) | 2000-11-27 | 2003-07-22 | Unisia Jecs Corporation | Inhalator for administering powder composition |
| US6701921B2 (en) * | 2000-12-22 | 2004-03-09 | Chrysalis Technologies Incorporated | Aerosol generator having heater in multilayered composite and method of use thereof |
| US6501052B2 (en) | 2000-12-22 | 2002-12-31 | Chrysalis Technologies Incorporated | Aerosol generator having multiple heating zones and methods of use thereof |
| US7077130B2 (en) | 2000-12-22 | 2006-07-18 | Chrysalis Technologies Incorporated | Disposable inhaler system |
| US6491233B2 (en) | 2000-12-22 | 2002-12-10 | Chrysalis Technologies Incorporated | Vapor driven aerosol generator and method of use thereof |
| US6681998B2 (en) | 2000-12-22 | 2004-01-27 | Chrysalis Technologies Incorporated | Aerosol generator having inductive heater and method of use thereof |
| US6799572B2 (en) | 2000-12-22 | 2004-10-05 | Chrysalis Technologies Incorporated | Disposable aerosol generator system and methods for administering the aerosol |
| BR0207062A (en) * | 2001-02-06 | 2004-10-05 | Innovata Biomed Ltd | Bimodal pharmaceutical composition, dry powder inhaler, method of applying a therapeutically effective amount of a substantially fine active ingredient to a patient's lungs, method of treating a respiratory disorder, method of treating chronic obstructive pulmonary disease (COPD), use of an antiinflammatory agent, use of a bronchodilator, use of a mixture of an antiinflammatory agent and a bronchodilator, and process for the manufacture of a bimodal pharmaceutical composition |
| GB0103630D0 (en) | 2001-02-14 | 2001-03-28 | Glaxo Group Ltd | Chemical compounds |
| ES2296923T3 (en) | 2001-03-22 | 2008-05-01 | Glaxo Group Limited | FORMANILID DERIVATIVES AS AGONISTS OF THE BETA2 ADRENORRECEPTOR. |
| EP1381417A4 (en) | 2001-04-26 | 2009-12-30 | New England Pharm Inc | Metered dose delivery device for liquid and powder agents |
| BR0209271A (en) | 2001-04-30 | 2004-06-15 | Glaxo Group Ltd | Compound, use of a compound pharmaceutical composition, pharmaceutical aerosol formulation, method for treating a human or animal patient with an inflammatory and / or allergic condition, and process for preparing a compound |
| US6681768B2 (en) | 2001-06-22 | 2004-01-27 | Sofotec Gmbh & Co. Kg | Powder formulation disintegrating system and method for dry powder inhalers |
| US8061006B2 (en) * | 2001-07-26 | 2011-11-22 | Powderject Research Limited | Particle cassette, method and kit therefor |
| US7176278B2 (en) | 2001-08-30 | 2007-02-13 | Biorexis Technology, Inc. | Modified transferrin fusion proteins |
| US6540081B2 (en) * | 2001-09-06 | 2003-04-01 | Ecolab Inc. | Unit dose blister pack product dispenser |
| USRE44874E1 (en) | 2001-09-14 | 2014-04-29 | Glaxo Group Limited | Phenethanolamine derivatives for treatment of respiratory diseases |
| AU2002333644A1 (en) | 2001-09-17 | 2003-04-01 | Glaxo Group Limited | Dry powder medicament formulations |
| CA2460904C (en) | 2001-09-19 | 2011-03-22 | Advent Pharmaceuticals Pty Ltd | An inhaler for delivering metered doses of powdered medicament |
| US6640050B2 (en) | 2001-09-21 | 2003-10-28 | Chrysalis Technologies Incorporated | Fluid vaporizing device having controlled temperature profile heater/capillary tube |
| US6568390B2 (en) | 2001-09-21 | 2003-05-27 | Chrysalis Technologies Incorporated | Dual capillary fluid vaporizing device |
| US7931022B2 (en) * | 2001-10-19 | 2011-04-26 | Respirks, Inc. | Method and apparatus for dispensing inhalator medicament |
| WO2003041777A1 (en) * | 2001-11-14 | 2003-05-22 | Nektar Therapeutics | Aerosolization device with improved endpiece connection |
| GB0128148D0 (en) | 2001-11-23 | 2002-01-16 | Innovata Biomed Ltd | Assembly |
| US6681769B2 (en) | 2001-12-06 | 2004-01-27 | Crysalis Technologies Incorporated | Aerosol generator having a multiple path heater arrangement and method of use thereof |
| US6804458B2 (en) | 2001-12-06 | 2004-10-12 | Chrysalis Technologies Incorporated | Aerosol generator having heater arranged to vaporize fluid in fluid passage between bonded layers of laminate |
| US20030168057A1 (en) * | 2001-12-14 | 2003-09-11 | Inhale Therapeutic Systems, Inc. | Electronically controllable aerosol delivery |
| US6701922B2 (en) | 2001-12-20 | 2004-03-09 | Chrysalis Technologies Incorporated | Mouthpiece entrainment airflow control for aerosol generators |
| US7458373B2 (en) | 2002-01-15 | 2008-12-02 | Philip Morris Usa Inc. | Aerosol generator for drug formulation |
| GB0217199D0 (en) * | 2002-07-25 | 2002-09-04 | Glaxo Group Ltd | Medicament dispenser |
| WO2003061744A1 (en) * | 2002-01-25 | 2003-07-31 | Glaxo Group Limited | Medicament dispenser |
| GB0201677D0 (en) | 2002-01-25 | 2002-03-13 | Glaxo Group Ltd | Medicament dispenser |
| AU2003216401A1 (en) * | 2002-03-01 | 2003-09-16 | Glaxo Group Limited | Rotary blending apparatus and system |
| CA2477604A1 (en) | 2002-03-13 | 2003-09-25 | Signum Biosciences, Inc. | Modulation of protein methylation and phosphoprotein phosphate |
| DE10212264A1 (en) | 2002-03-20 | 2003-10-02 | Boehringer Ingelheim Pharma | Crystalline micronisate, process for its preparation and its use for the manufacture of a medicament |
| EP1894591B1 (en) | 2002-03-20 | 2013-06-26 | MannKind Corporation | Cartridge for an inhalation apparatus |
| WO2003095332A1 (en) * | 2002-03-26 | 2003-11-20 | Glaxo Group Limited | A method for forming a laminate assembly and products formed thereby |
| GR1004350B (en) * | 2002-03-29 | 2003-09-26 | Inhaler for dry powder | |
| DE10216429A1 (en) | 2002-04-12 | 2003-10-23 | Boehringer Ingelheim Pharma | Synergistic medicaments for treating inflammatory or obstructive respiratory tract diseases, containing quaternized scopine ester anticholinergic agent and steroid, e.g. budesonide |
| GB0208608D0 (en) * | 2002-04-13 | 2002-05-22 | Glaxo Group Ltd | Composition |
| GB0208609D0 (en) * | 2002-04-13 | 2002-05-22 | Glaxo Group Ltd | Compositions |
| GB0216562D0 (en) * | 2002-04-25 | 2002-08-28 | Bradford Particle Design Ltd | Particulate materials |
| AU2003222841A1 (en) | 2002-04-25 | 2003-11-10 | Glaxo Group Limited | Phenethanolamine derivatives |
| JP2005524134A (en) * | 2002-04-25 | 2005-08-11 | グラクソ グループ リミテッド | Magnetoacoustic sensor system and related method for detecting environmental conditions |
| US9339459B2 (en) | 2003-04-24 | 2016-05-17 | Nektar Therapeutics | Particulate materials |
| WO2003095005A1 (en) * | 2002-05-10 | 2003-11-20 | Chrysalis Technologies Incorporated | Aerosol generator for drug formulation and methods of generating aerosol |
| JP2005526261A (en) * | 2002-05-15 | 2005-09-02 | グラクソ グループ リミテッド | Microelectromechanical system and method for measuring temperature and moisture profiles in pharmaceutical packaging |
| US7185651B2 (en) * | 2002-06-18 | 2007-03-06 | Nektar Therapeutics | Flow regulator for aerosol drug delivery and methods |
| DE10230751A1 (en) | 2002-07-09 | 2004-01-22 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | New drug compositions based on new anticholinergics and EGFR kinase inhibitors |
| EP1707205A2 (en) | 2002-07-09 | 2006-10-04 | Boehringer Ingelheim Pharma GmbH & Co. KG | Pharmaceutical compositions of anticholinergics and p38 kinase inhibitors in the treatment of respiratory diseases |
| GB0217198D0 (en) * | 2002-07-25 | 2002-09-04 | Glaxo Group Ltd | Medicament dispenser |
| GB0217196D0 (en) * | 2002-07-25 | 2002-09-04 | Glaxo Group Ltd | Medicament dispenser |
| WO2004011067A1 (en) * | 2002-07-25 | 2004-02-05 | Glaxo Group Limited | Medicament dispenser |
| GB0217225D0 (en) | 2002-07-25 | 2002-09-04 | Glaxo Group Ltd | Medicinal compounds |
| WO2004011068A1 (en) * | 2002-07-25 | 2004-02-05 | Glaxo Group Limited | Medicament dispenser |
| DE60335401D1 (en) * | 2002-09-06 | 2011-01-27 | Philip Morris Usa Inc | AEROSOL PRODUCING DEVICES AND METHOD FOR PRODUCING AEROSOLS WITH CONTROLLED PARTICLE SIZES |
| US20070139442A1 (en) * | 2002-09-13 | 2007-06-21 | Robinson Karl E | Coated blending system |
| US6962266B2 (en) * | 2002-10-04 | 2005-11-08 | Ecolab Inc. | Method and apparatus for using a unit dose dispenser |
| ES2291733T3 (en) * | 2002-10-22 | 2008-03-01 | Glaxo Group Limited | MEDICAL ARYLETHANOLAMINE COMPOUNDS. |
| US6772757B2 (en) | 2002-10-25 | 2004-08-10 | Chrysalis Technologies Incorporated | Concentric controlled temperature profile fluid vaporizing device |
| SI1556342T1 (en) | 2002-10-28 | 2008-08-31 | Glaxo Group Ltd | Phenethanolamine derivative for the treatment of respiratory diseases |
| US7056916B2 (en) | 2002-11-15 | 2006-06-06 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Medicaments for the treatment of chronic obstructive pulmonary disease |
| US6941947B2 (en) * | 2002-12-18 | 2005-09-13 | Quadrant Technologies Limited | Unit dose dry powder inhaler |
| WO2004060260A2 (en) * | 2002-12-18 | 2004-07-22 | Glaxo Group Limited | Drug delivery system with vented mouthpiece |
| EP1587482A4 (en) * | 2003-01-31 | 2010-08-25 | Technion Res & Dev Foundation | Anti-inflammatory compositions and uses thereof |
| WO2004071522A1 (en) * | 2003-02-11 | 2004-08-26 | Boehringer Ingelheim International Gmbh | New pharmaceutical compositions based on anticholinergics and soluble tnf receptor fusion proteins |
| JP2006517214A (en) * | 2003-02-11 | 2006-07-20 | ベーリンガー インゲルハイム インターナショナル ゲゼルシャフト ミット ベシュレンクテル ハフツング | Novel pharmaceutical composition based on novel anticholinergic agent and TNFα synthesis or action inhibitor |
| PE20040950A1 (en) | 2003-02-14 | 2005-01-01 | Theravance Inc | BIPHENYL DERIVATIVES AS AGONISTS OF ß2-ADRENERGIC RECEPTORS AND AS ANTAGONISTS OF MUSCARINAL RECEPTORS |
| GB0303396D0 (en) | 2003-02-14 | 2003-03-19 | Glaxo Group Ltd | Medicinal compounds |
| DE10317461A1 (en) | 2003-04-16 | 2004-10-28 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Preparing microparticles labeled with technetium, useful as powders for inhalation, e.g. to study deposition of pharmaceuticals, such as anticholinergic agents, involves incubation with solution of technetium salt |
| US20040245279A1 (en) * | 2003-05-05 | 2004-12-09 | Bradley Tareasa L. | System for dispensing an active ingredient using a dispensable tablet, dispensable tablet and container for holding such dispensable tablets |
| TW200510298A (en) | 2003-06-13 | 2005-03-16 | Theravance Inc | Substituted pyrrolidine and related compounds |
| EP1488819A1 (en) * | 2003-06-16 | 2004-12-22 | Rijksuniversiteit te Groningen | Dry powder inhaler and method for pulmonary inhalation of dry powder |
| GB0316335D0 (en) * | 2003-07-11 | 2003-08-13 | Glaxo Group Ltd | Pharmaceutical formulations |
| PE20050250A1 (en) | 2003-07-17 | 2005-04-08 | Glaxo Group Ltd | ANTAGONISTS OF ACETYLCHOLIN MUSCARINAL RECEPTORS |
| GB0317374D0 (en) | 2003-07-24 | 2003-08-27 | Glaxo Group Ltd | Medicament dispenser |
| EP1803469A3 (en) | 2003-07-29 | 2011-10-26 | Boehringer Ingelheim Pharma GmbH & Co. KG | Medicaments for inhalation comprising betamimetics and an anticholinergic |
| US7367334B2 (en) | 2003-08-27 | 2008-05-06 | Philip Morris Usa Inc. | Fluid vaporizing device having controlled temperature profile heater/capillary tube |
| US6991131B2 (en) * | 2003-09-02 | 2006-01-31 | Ecolab, Inc. | Distributable container and system and method using distributable container |
| CA2540179A1 (en) * | 2003-09-24 | 2005-03-31 | Medi-Stream Pty Ltd | Medication holder |
| AU2004273547B2 (en) * | 2003-09-24 | 2006-07-06 | Tianda Pharmaceuticals (Australia) Pty Ltd | Medication holder |
| PL1677795T3 (en) | 2003-10-14 | 2011-05-31 | Glaxo Group Ltd | Muscarinic acetycholine receptor antagonists |
| GB2407042B (en) | 2003-10-17 | 2007-10-24 | Vectura Ltd | Inhaler |
| AP2006003575A0 (en) | 2003-10-17 | 2006-04-30 | Glaxo Group Ltd | Muscarinic acetylcholine receptor antagonists. |
| GB0324654D0 (en) * | 2003-10-22 | 2003-11-26 | Glaxo Group Ltd | Medicinal compounds |
| GB0324886D0 (en) * | 2003-10-24 | 2003-11-26 | Glaxo Group Ltd | Medicinal compounds |
| GB0324897D0 (en) * | 2003-10-24 | 2003-11-26 | Glaxo Group Ltd | Composition |
| WO2005044173A1 (en) * | 2003-10-27 | 2005-05-19 | Oriel Therapeutics, Inc. | Blister packages and associated methods of fabricating dry powder drug containment systems |
| WO2005044187A2 (en) * | 2003-10-28 | 2005-05-19 | Glaxo Group Limited | Inhalable pharmaceutical formulations employing lactose anhydrate and methods of administering the same |
| WO2005042528A1 (en) | 2003-11-03 | 2005-05-12 | Boehringer Ingelheim International Gmbh | Novel tiotropium salts, methods for the production thereof, and pharmaceutical formulations containing the same |
| GB0325627D0 (en) * | 2003-11-03 | 2003-12-10 | Glaxo Group Ltd | A hand-held capsule device |
| JP5165244B2 (en) | 2003-11-03 | 2013-03-21 | ベーリンガー インゲルハイム インターナショナル ゲゼルシャフト ミット ベシュレンクテル ハフツング | Method for producing tiotropium salt, tiotropium salt and pharmaceutical composition containing the same |
| AR046225A1 (en) | 2003-11-04 | 2005-11-30 | Glaxo Group Ltd | COMPOSITE OF 8-AZONIABICICLO (3.2.1) OCTOBER, PHARMACEUTICAL COMPOSITION FOR THE TREATMENT OF DISEASES MEDIATED BY MUSCARINIC ACETILCOLINE RECEPTORS THAT UNDERSTAND IT AND USE OF THE COMPOUND TO PREPARE SUCH COMPOSITION |
| GB0329182D0 (en) | 2003-12-17 | 2004-01-21 | Glaxo Group Ltd | Chemical compounds |
| US8147426B2 (en) * | 2003-12-31 | 2012-04-03 | Nipro Diagnostics, Inc. | Integrated diagnostic test system |
| GB2410947B (en) * | 2004-02-11 | 2008-09-17 | Cambridge Lab Ltd | Pharmaceutical compounds |
| TWI341836B (en) | 2004-03-11 | 2011-05-11 | Theravance Inc | Biphenyl compounds useful as muscarinic receptor antagonists |
| US7384946B2 (en) | 2004-03-17 | 2008-06-10 | Glaxo Group Limited | M3 muscarinic acetylcholine receptor antagonists |
| BRPI0509382A (en) * | 2004-04-02 | 2007-09-18 | Glaxo Group Ltd | process for the preparation of a compound monohydrochloride salt, crystalline monohydrochloride, process for obtaining crystalline monohydrochloride, method for the prophylaxis or treatment of a clinical condition in a mammal, use of crystalline monohydrochloride, pharmaceutical formulation, and, combination |
| CA2562386C (en) | 2004-04-21 | 2014-11-18 | Innovata Biomed Limited | Inhaler |
| SI1781298T1 (en) | 2004-04-22 | 2012-04-30 | Boehringer Ingelheim Int | Pharmaceutical combinations containing benzoxazine for treating respiratory diseases |
| US20050272726A1 (en) * | 2004-04-22 | 2005-12-08 | Boehringer Ingelheim International Gmbh | Novel medicaments for the treatment of respiratory diseases |
| US7861712B2 (en) * | 2004-04-23 | 2011-01-04 | Manta Product Development | Sealed capsule including an integrated puncturing mechanism |
| GB0409197D0 (en) | 2004-04-24 | 2004-05-26 | Innovata Biomed Ltd | Device |
| TWI363759B (en) | 2004-04-27 | 2012-05-11 | Glaxo Group Ltd | Muscarinic acetylcholine receptor antagonists |
| US7727962B2 (en) * | 2004-05-10 | 2010-06-01 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Powder comprising new compositions of oligosaccharides and methods for their preparation |
| US7723306B2 (en) * | 2004-05-10 | 2010-05-25 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Spray-dried powder comprising at least one 1,4 O-linked saccharose-derivative and methods for their preparation |
| US7611709B2 (en) * | 2004-05-10 | 2009-11-03 | Boehringer Ingelheim Pharma Gmbh And Co. Kg | 1,4 O-linked saccharose derivatives for stabilization of antibodies or antibody derivatives |
| EP1747219A4 (en) | 2004-05-13 | 2010-05-26 | Glaxo Group Ltd | Muscarinic acetylcholine receptor antagonists field of the invention |
| US20050256115A1 (en) * | 2004-05-14 | 2005-11-17 | Boehringer Ingelheim International Gmbh | Aerosol formulation for the inhalation of beta-agonists |
| US7220742B2 (en) | 2004-05-14 | 2007-05-22 | Boehringer Ingelheim International Gmbh | Enantiomerically pure beta agonists, process for the manufacture thereof and use thereof as medicaments |
| WO2005110519A1 (en) * | 2004-05-17 | 2005-11-24 | Sun Pharmaceutical Industries Limited | Dry powder inhaler |
| SE530006C2 (en) * | 2004-06-18 | 2008-02-05 | Mederio Ag | Inhaler using tub |
| US7896195B2 (en) * | 2004-06-08 | 2011-03-01 | Ecolab Inc. | Tablet dispenser with isolated product hopper |
| WO2006009776A2 (en) * | 2004-06-17 | 2006-01-26 | Signal Investment & Management Co. | Reusable support device with therapeutic inserts |
| US20060035893A1 (en) | 2004-08-07 | 2006-02-16 | Boehringer Ingelheim International Gmbh | Pharmaceutical compositions for treatment of respiratory and gastrointestinal disorders |
| US20060079544A1 (en) * | 2004-08-13 | 2006-04-13 | Boehringer Ingelheim International Gmbh | Medicaments for the prevention or treatment of alveolar pneumonia comprising an anticholinergic |
| GB0418278D0 (en) * | 2004-08-16 | 2004-09-15 | Glaxo Group Ltd | Medicament dispenser |
| DK1786784T3 (en) | 2004-08-20 | 2011-02-14 | Mannkind Corp | Catalysis of diketopiperazine synthesis |
| GB0418738D0 (en) * | 2004-08-23 | 2004-09-22 | 3M Innovative Properties Co | Medicinal aerosol formulation receptacle and production thereof |
| CN104436170B (en) | 2004-08-23 | 2018-02-23 | 曼金德公司 | Diketopiperazine salt for drug delivery |
| US8165460B2 (en) * | 2004-10-26 | 2012-04-24 | The University Of North Carolina At Chapel Hill | Coated filament for evaporation/condensation aerosol generation of therapeutic agents and methods for using |
| JP2006130143A (en) * | 2004-11-08 | 2006-05-25 | Hitachi Ltd | Inhalation administration device and medicine cartridge |
| US20060120972A1 (en) * | 2004-11-09 | 2006-06-08 | Peter Engels | 9-(N-methyl-piperidyliden-4)-thioxanthene for treatment of pulmonary hypertension |
| EP1812778A2 (en) * | 2004-11-12 | 2007-08-01 | Glaxo Group Limited | Sensor system with acoustic transducer |
| NZ555501A (en) | 2004-11-24 | 2010-01-29 | Medpointe Healthcare Inc | Compositions comprising azelastine and methods of use thereof |
| US8758816B2 (en) * | 2004-11-24 | 2014-06-24 | Meda Pharmaceuticals Inc. | Compositions comprising azelastine and methods of use thereof |
| US20070020330A1 (en) | 2004-11-24 | 2007-01-25 | Medpointe Healthcare Inc. | Compositions comprising azelastine and methods of use thereof |
| GB0427858D0 (en) * | 2004-12-20 | 2005-01-19 | Glaxo Group Ltd | Manifold for use in medicament dispenser |
| GB0427856D0 (en) * | 2004-12-20 | 2005-01-19 | Glaxo Group Ltd | Maniflod for use in medicament dispenser |
| GB0427853D0 (en) * | 2004-12-20 | 2005-01-19 | Glaxo Group Ltd | Manifold for use in medicament dispenser |
| KR20070097106A (en) * | 2005-01-11 | 2007-10-02 | 글락소 그룹 리미티드 | Cinnamate salts of a beta-2 adrenergic agonist |
| US7923041B2 (en) | 2005-02-03 | 2011-04-12 | Signum Biosciences, Inc. | Compositions and methods for enhancing cognitive function |
| WO2006084033A1 (en) * | 2005-02-03 | 2006-08-10 | Signum Biosciences, Inc. | Compositions and methods for enhancing cognitive function |
| CN104177448A (en) | 2005-02-10 | 2014-12-03 | 葛兰素集团有限公司 | Processes For Making Lactose Utilizing Pre-Classification Techniques And Pharmaceutical Formulations Formed Therefrom |
| WO2006086130A2 (en) * | 2005-02-10 | 2006-08-17 | Glaxo Group Limited | Process for crystallizing lactose particles for use in pharmaceutical formulations |
| MY145281A (en) | 2005-03-25 | 2012-01-13 | Glaxo Group Ltd | Novel compounds |
| AR053450A1 (en) | 2005-03-25 | 2007-05-09 | Glaxo Group Ltd | DERIVATIVES OF 3,4-DIHYDRO-PYRIMID (4,5-D) PYRIMIDIN-2- (1H) -ONA 1,5,7 TRISUSTITUTED AS INHIBITORS OF QUINASE P38 |
| GB0507711D0 (en) | 2005-04-15 | 2005-05-25 | Vectura Group Plc | Improved blister piercing |
| WO2007052154A2 (en) | 2005-04-29 | 2007-05-10 | University Of Medicine And Dentistry Of New Jersey | Erythropoietin-derived short peptide and its mimics as immuno/inflammatory modulators |
| US9345745B2 (en) | 2005-04-29 | 2016-05-24 | Bo Wang | Methods for treating inflammatory disorders and traumatic brain injury using stabilized non-hematopoietic EPO short peptides |
| US9585932B2 (en) | 2005-04-29 | 2017-03-07 | Peter C. Dowling | Use of EPO-derived peptide fragments for the treatment of neurodegenerative disorders |
| NZ563596A (en) | 2005-05-02 | 2011-01-28 | Boehringer Ingelheim Int | Crystalline form of tiotropium bromide anhydrate |
| EP1898894A1 (en) * | 2005-06-17 | 2008-03-19 | Boehringer Ingelheim International GmbH | Mrp iv inhibitors for the treatment of respiratory diseases |
| DE102005030733A1 (en) | 2005-07-01 | 2007-01-04 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | New drug combinations for the treatment of respiratory diseases containing long-acting beta-2 agonists and at least one other active ingredient |
| GB0514501D0 (en) * | 2005-07-14 | 2005-08-24 | Cambridge Lab Ireland Ltd | Pharmaceutical compounds |
| US20070012316A1 (en) * | 2005-07-14 | 2007-01-18 | Joann Truza | Disposable compact rescue inhaler |
| US8763605B2 (en) | 2005-07-20 | 2014-07-01 | Manta Devices, Llc | Inhalation device |
| GB0515584D0 (en) * | 2005-07-28 | 2005-09-07 | Glaxo Group Ltd | Medicament dispenser |
| GB0516168D0 (en) * | 2005-08-05 | 2005-09-14 | Cambridge Lab Ireland Ltd | Pharmaceutical compounds |
| MX2008001976A (en) * | 2005-08-15 | 2008-03-25 | Boehringer Ingelheim Int | Method for producing betamimetics. |
| EP1937068A4 (en) | 2005-08-18 | 2010-08-04 | Glaxo Group Ltd | Muscarinic acetylcholine receptor antagonists |
| WO2007024876A2 (en) * | 2005-08-24 | 2007-03-01 | The Board Of Trustees Of Leland Stanford Junior University | Methods for treating and monitoring inflammation and redox imbalance in cystic fibrosis |
| US20090192227A1 (en) * | 2005-08-24 | 2009-07-30 | Rabindra Tirouvanziam | N-Acetylcysteine Compositions and Methods for Treating Acute Exacerbations of Inflammatory Lung Disease |
| US20130131007A1 (en) | 2005-09-07 | 2013-05-23 | Bebaas, Inc. | Vitamin b12 compositions |
| US20070178141A1 (en) * | 2005-09-07 | 2007-08-02 | Bebaas, Inc. | Vitamin B12 compositions |
| US8227408B2 (en) * | 2005-09-07 | 2012-07-24 | Neurotez, Inc. | Leptin as an anti-amyloidogenic biologic and methods for delaying the onset and reducing Alzheimer's disease-like pathology |
| US7481331B2 (en) * | 2005-09-09 | 2009-01-27 | Manrex Limited | Dispensing container for a blister pack of medications |
| HUE028623T2 (en) | 2005-09-14 | 2016-12-28 | Mannkind Corp | Method of drug formulation based on increasing the affinity of active agents for crystalline microparticle surfaces |
| TWI396541B (en) | 2005-10-10 | 2013-05-21 | Boehringer Ingelheim Int | Novel combinations of medicaments for the treatment of respiratory diseases |
| US9107824B2 (en) | 2005-11-08 | 2015-08-18 | Insmed Incorporated | Methods of treating cancer with high potency lipid-based platinum compound formulations administered intraperitoneally |
| DE102005057685A1 (en) * | 2005-12-01 | 2007-06-06 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Inhaler and storage for a dry drug formulation and methods and use thereof |
| AR058289A1 (en) * | 2005-12-12 | 2008-01-30 | Glaxo Group Ltd | COLLECTOR TO BE USED IN MEDICINAL DISPENSER |
| AR058290A1 (en) * | 2005-12-12 | 2008-01-30 | Glaxo Group Ltd | MEDICINAL DISPENSER |
| DE102005059602A1 (en) | 2005-12-14 | 2007-06-21 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Micronization process |
| IN2015DN00888A (en) | 2006-02-22 | 2015-07-10 | Mannkind Corp | |
| US8127763B2 (en) * | 2006-03-03 | 2012-03-06 | Stc.Unm | Dry powder inhaler with aeroelastic dispersion mechanism |
| CN101437562A (en) * | 2006-03-03 | 2009-05-20 | Stc.Unm公司 | Dry powder inhaler with aeroelastic dispersion mechanism |
| DE102006016904A1 (en) * | 2006-04-11 | 2007-10-25 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | inhaler |
| EP1844808A1 (en) * | 2006-04-13 | 2007-10-17 | BOEHRINGER INGELHEIM PHARMA GMBH & CO. KG | Medicament delivery device |
| EP1844805A1 (en) * | 2006-04-13 | 2007-10-17 | Boehringer Ingelheim Pharma GmbH & Co.KG | Inhaler |
| PT103481B (en) | 2006-05-16 | 2008-08-01 | Hovione Farmaciencia S A | INHALER OF SIMPLE USE AND INHALATION METHOD |
| CA2653048C (en) | 2006-05-19 | 2014-12-09 | Valspar Sourcing, Inc. | Coating system for cement composite articles |
| US20090250058A1 (en) * | 2006-07-14 | 2009-10-08 | Astrazeneca Ab | Inhalation System and Delivery Device for the Administration of a Drug in the Form of Dry Powder |
| CA2660186A1 (en) | 2006-08-07 | 2008-02-14 | Boehringer Ingelheim International Gmbh | Pharmaceutical combinations for the treatment of respiratory diseases |
| UY30552A1 (en) * | 2006-08-22 | 2008-03-31 | Boehringer Ingelheim Int | PHARMACOLOGICAL COMBINATIONS BASED ON SUBSTITUTED DERIVATIVES OF THE N- (5- {2- [1,1-DIMETIL-PROPILAMINO] -1-HIDROXI-METIL} -2-HYDROXI-PHENYL) -METANSULPHONAMIDE AND APPLICATIONS |
| DE102006045788A1 (en) * | 2006-09-26 | 2008-03-27 | Alfred Von Schuckmann | Dispenser for powdery masses |
| AU2007309412B2 (en) | 2006-10-25 | 2011-10-20 | Novartis Ag | Powder dispersion apparatus, method of making and using the apparatus, and components that can be used on the apparatus and other devices |
| GB0622827D0 (en) * | 2006-11-15 | 2006-12-27 | Glaxo Group Ltd | Sheet driver for use in a drug dispenser |
| EP1925295A1 (en) | 2006-11-22 | 2008-05-28 | Boehringer Ingelheim Pharma GmbH & Co. KG | Stable powder formulation containing a new antichinolinergic agent |
| EP2155228B1 (en) | 2007-01-10 | 2014-04-02 | Purdue Research Foundation | Polypeptide inhibitors of hsp27 kinase and uses therefor |
| PE20081889A1 (en) | 2007-03-23 | 2009-03-05 | Smithkline Beecham Corp | INDOL CARBOXAMIDES AS INHIBITORS OF IKK2 |
| US20080237082A1 (en) * | 2007-03-29 | 2008-10-02 | R. P. Scherer Technologies, Inc. | Child resistant device for housing blister packs |
| AT505246B8 (en) * | 2007-06-01 | 2009-06-15 | Croma Pharma Gmbh | CONTAINER FOR A MULTIPLE OF INDIVIDUAL CANS AND APPLICATOR FOR SUCH CONTAINERS |
| US11224704B2 (en) | 2007-07-06 | 2022-01-18 | Manta Devices, Llc | Dose delivery device for inhalation |
| DK2898914T3 (en) | 2007-07-06 | 2018-09-03 | Manta Devices Llc | INHALATION DEVICES FOR STORAGE AND DELIVERY OF MEDICINES |
| JP5703466B2 (en) | 2007-08-07 | 2015-04-22 | パーデュー・リサーチ・ファウンデーションPurdue Research Foundation | Kinase inhibitors and uses thereof |
| EP2093219A1 (en) | 2008-02-22 | 2009-08-26 | Boehringer Ingelheim International Gmbh | Crystalline enantiomer free salt form of a betamimetic and its use as medicine |
| US8765661B2 (en) | 2008-03-20 | 2014-07-01 | Virun, Inc. | Compositions containing non-polar compounds |
| WO2009117152A1 (en) | 2008-03-20 | 2009-09-24 | Virun, Inc. | Emulsions including a peg-derivative of tocopherol |
| KR20110028457A (en) * | 2008-05-21 | 2011-03-18 | 뉴로테즈 인코포레이티드 | Methods for treating progressive cognitive disorders related to neurofibrillary tangles |
| US8485180B2 (en) | 2008-06-13 | 2013-07-16 | Mannkind Corporation | Dry powder drug delivery system |
| GB0810857D0 (en) * | 2008-06-13 | 2008-07-23 | Cambridge Lab Ireland Ltd | Pharmaceutical compounds |
| ES2570400T3 (en) | 2008-06-13 | 2016-05-18 | Mannkind Corp | A dry powder inhaler and a drug delivery system |
| JP5479465B2 (en) | 2008-06-20 | 2014-04-23 | マンカインド コーポレイション | Interactive device and method for profiling inhalation efforts in real time |
| US8337931B2 (en) * | 2008-06-23 | 2012-12-25 | Virun, Inc. | Compositions containing non-polar compounds |
| JP5339794B2 (en) * | 2008-07-04 | 2013-11-13 | キヤノン株式会社 | Inhaler |
| TWI532497B (en) | 2008-08-11 | 2016-05-11 | 曼凱公司 | Use of ultrarapid acting insulin |
| US20110053866A1 (en) * | 2008-08-12 | 2011-03-03 | Biovail Laboratories International (Barbados) S.R.L. | Pharmaceutical compositions |
| GB2462611A (en) * | 2008-08-12 | 2010-02-17 | Cambridge Lab | Pharmaceutical composition comprising tetrabenazine |
| GB2463451A (en) | 2008-09-08 | 2010-03-17 | Cambridge Lab | 3, 11b cis-dihydrotetrabenazine compounds for use in the treatment of dementia |
| GB2463452A (en) * | 2008-09-08 | 2010-03-17 | Cambridge Lab | Desmethyl derivatives of tetrabenazine and pharmaceutical compositions thereof |
| GB2463283A (en) * | 2008-09-08 | 2010-03-10 | Cambridge Lab | 3,11b-cis-dihydrotetrabenazine for use in treating asthma |
| CA2732826C (en) * | 2008-09-26 | 2017-08-22 | Oriel Therapeutics, Inc. | Inhaler mechanisms with radially biased piercers and related methods |
| MX2011003244A (en) | 2008-09-26 | 2011-04-28 | Oriel Therapeutics Inc | Inhalers with airway disks having discrete airway channels and related disks and methods. |
| MX2011003233A (en) | 2008-09-26 | 2011-04-28 | Oriel Therapeutics Inc | Dry powder inhalers with dual piercing members and related devices and methods. |
| WO2010039201A2 (en) * | 2008-09-30 | 2010-04-08 | Oriel Therapeutics, Inc. | Dry powder inhalers with multi-facet surface deagglomeration chambers and related devices and methods |
| WO2010039202A2 (en) * | 2008-10-01 | 2010-04-08 | Oriel Therapeutics, Inc. | Dry powder inhalers with rotating piercing mechanisms and related devices and methods |
| DK2349310T3 (en) * | 2008-10-20 | 2014-08-11 | Moerae Matrix Inc | POLYPEPTIME FOR TREATMENT OR PREVENTION OF ADHESIONS |
| JP2012508242A (en) * | 2008-11-04 | 2012-04-05 | ニコラオス テザプシディス | Leptin compositions and methods for treating progressive cognitive impairment resulting from neurofibrillary tangles and amyloid beta accumulation |
| CN105920582B (en) * | 2008-12-10 | 2020-04-14 | 普渡研究基金会 | Cell-penetrating peptide-based kinase inhibitors |
| US8314106B2 (en) | 2008-12-29 | 2012-11-20 | Mannkind Corporation | Substituted diketopiperazine analogs for use as drug delivery agents |
| US8550074B2 (en) * | 2009-01-15 | 2013-10-08 | Manta Devices, Llc | Delivery device and related methods |
| GB0901520D0 (en) * | 2009-01-30 | 2009-03-11 | Vectura Delivery Devices Ltd | Inhaler |
| WO2010091198A1 (en) | 2009-02-06 | 2010-08-12 | University Of Southern California | Therapeutic compositions comprising monoterpenes |
| JP2012517405A (en) | 2009-02-09 | 2012-08-02 | ベーリンガー インゲルハイム インターナショナル ゲゼルシャフト ミット ベシュレンクテル ハフツング | Novel pharmaceutical compositions for the treatment of respiratory and gastrointestinal diseases |
| PT3578169T (en) | 2009-02-26 | 2024-07-29 | Glaxo Group Ltd | Pharmaceutical formulations comprising 4-{(1 r)-2-[(6-{2-[(2,6-dichlorobenzyl)oxy]ethoxy}hexyl)amino]-1-hydroxyethyl}-2-(hydroxymethyl)phenol |
| EP2405963B1 (en) | 2009-03-11 | 2013-11-06 | MannKind Corporation | Apparatus, system and method for measuring resistance of an inhaler |
| CA2755543A1 (en) * | 2009-03-13 | 2010-09-16 | Nucitec S.A. De C.V. | Compositions and methods for treatment and prevention of cardiovascular disease |
| SG174345A1 (en) | 2009-03-18 | 2011-11-28 | Mannkind Corp | Inhaler adaptor for a laser diffraction apparatus and method for measuring particle size distribution |
| KR101875969B1 (en) | 2009-06-12 | 2018-07-06 | 맨카인드 코포레이션 | Diketopiperazine microparticles with defined specific surface areas |
| AR077101A1 (en) | 2009-06-16 | 2011-08-03 | Schering Corp | STEROIDS OF HETEROARILO (3,2-C), AS GLUCOCORTICOID RECEPTOR AGONISTS, COMPOSITIONS AND USES OF THE SAME |
| US20120142715A1 (en) | 2009-07-06 | 2012-06-07 | Boehringer Ingelheim International Gmbh | Polymorph of [4,6-bis(dimethylamino)-2-(4-benzyl)pyrimidin-5-yl] |
| PT2453894E (en) | 2009-07-15 | 2016-02-02 | Theravance Biopharma R&D Ip Llc | Crystalline freebase form of a biphenyl compound |
| EP3202399A1 (en) | 2009-07-24 | 2017-08-09 | Amazetis SA | Compounds, compositions and methods for protecting brain health in neurodegenerative disorders |
| WO2011017132A2 (en) * | 2009-07-27 | 2011-02-10 | Purdue Research, Foundation | Mk2 inhibitor compositions and methods to enhance neurite outgrowth, neuroprotection, and nerve regeneration |
| EP2477642A4 (en) * | 2009-09-17 | 2013-03-13 | Mutual Pharmaceutical Co | Method of treating asthma with antiviral agents |
| JP5784622B2 (en) | 2009-11-03 | 2015-09-24 | マンカインド コーポレ−ション | Apparatus and method for simulating inhalation activity |
| AU2010319328A1 (en) | 2009-11-12 | 2012-05-31 | Stc.Unm | Dry powder inhaler with flutter dispersion member |
| CN102666499A (en) | 2009-11-24 | 2012-09-12 | 贝林格尔.英格海姆国际有限公司 | Process for preparing a polymorph of the choline salt of a pyrimidin-5-yl acetic acid derivative |
| JP2013512239A (en) | 2009-11-24 | 2013-04-11 | ベーリンガー インゲルハイム インターナショナル ゲゼルシャフト ミット ベシュレンクテル ハフツング | Novel salt forms of pyrimidin-5-ylacetic acid derivatives |
| GB0921075D0 (en) | 2009-12-01 | 2010-01-13 | Glaxo Group Ltd | Novel combination of the therapeutic agents |
| US10210216B2 (en) * | 2009-12-18 | 2019-02-19 | Sybase, Inc. | Dynamic attributes for mobile business objects |
| BR112012022209A2 (en) | 2010-03-03 | 2017-06-06 | Neonc Tech Inc | pharmaceutical compositions comprising monoterpenes |
| WO2011116293A2 (en) | 2010-03-19 | 2011-09-22 | Manta Devices, Llc | Delivery device and related methods |
| CN103037708B (en) | 2010-03-23 | 2015-05-20 | 维尔恩公司 | Nanoemulsion including sucrose fatty acid ester |
| USD641076S1 (en) | 2010-03-26 | 2011-07-05 | Oriel Therapeutics, Inc. | Dry powder inhaler |
| USD635246S1 (en) | 2010-03-26 | 2011-03-29 | Oriel Therapeutics, Inc. | Dose disk for dry powder inhalers |
| US8741373B2 (en) | 2010-06-21 | 2014-06-03 | Virun, Inc. | Compositions containing non-polar compounds |
| MX359281B (en) | 2010-06-21 | 2018-09-21 | Mannkind Corp | Dry powder drug delivery system and methods. |
| US20160038600A1 (en) | 2012-08-03 | 2016-02-11 | Neonc Technologies Inc. | Pharmaceutical compositions comprising poh derivatives |
| EP2883543B1 (en) | 2010-08-27 | 2016-11-16 | Neonc Technologies Inc. | Pharmaceutical compositions comprising perillyl alcohol carbamates |
| JP5792307B2 (en) | 2010-08-31 | 2015-10-07 | グラクソ グループ リミテッドGlaxo Group Limited | Dry powder inhalation drug product exhibiting moisture control characteristics and method of administration thereof |
| JP5792308B2 (en) | 2010-08-31 | 2015-10-07 | グラクソ グループ リミテッドGlaxo Group Limited | Dry powder inhalation drug product exhibiting moisture control characteristics and method of administration thereof |
| WO2012064286A1 (en) | 2010-11-11 | 2012-05-18 | Agency For Science, Technology And Research | Targeting metabolic enzymes in human cancer |
| WO2012078804A1 (en) | 2010-12-07 | 2012-06-14 | Respira Therapeutics, Inc. | Dry powder inhaler |
| DK2651864T3 (en) | 2010-12-17 | 2016-09-05 | Neonc Tech Inc | Methods and devices for use of isoperillylalkohol |
| MX353285B (en) | 2011-04-01 | 2018-01-05 | Mannkind Corp | Blister package for pharmaceutical cartridges. |
| US9890200B2 (en) | 2011-04-12 | 2018-02-13 | Moerae Matrix, Inc. | Compositions and methods for preventing or treating diseases, conditions, or processes characterized by aberrant fibroblast proliferation and extracellular matrix deposition |
| CA3042808A1 (en) | 2011-04-12 | 2012-10-18 | Moerae Matrix, Inc. | Compositions and methods for preventing or treating diseases, conditions, or processes characterized by aberrant fibroblast proliferation and extracellular matrix deposition |
| KR101763195B1 (en) | 2011-05-19 | 2017-07-31 | 사바라 인코포레이티드 | Dry powder vancomycin compositions and associated methods |
| WO2012174472A1 (en) | 2011-06-17 | 2012-12-20 | Mannkind Corporation | High capacity diketopiperazine microparticles |
| US8528569B1 (en) | 2011-06-28 | 2013-09-10 | Kyle D. Newton | Electronic cigarette with liquid reservoir |
| US11103659B2 (en) | 2011-07-06 | 2021-08-31 | Manta Devices, Llc | Delivery device and related methods |
| GB201116641D0 (en) | 2011-09-27 | 2011-11-09 | Glaxo Group Ltd | Novel compounds |
| CN103945859A (en) | 2011-10-24 | 2014-07-23 | 曼金德公司 | Methods and compositions for treating pain |
| EP2589381B1 (en) | 2011-11-04 | 2016-08-31 | Rabindra Tirouvanziam | Compositions for improving or preserving lung function in a patient with a pulmonary disorder |
| US9603906B2 (en) | 2012-02-01 | 2017-03-28 | Protalix Ltd. | Inhalable liquid formulations of DNase I |
| SG11201404640YA (en) | 2012-02-10 | 2014-09-26 | Virun Inc | Beverage compositions containing non-polar compounds |
| US10463815B2 (en) | 2012-02-21 | 2019-11-05 | Respira Therapeutics, Inc. | Inhaler to deliver substances for prophylaxis or prevention of disease or injury caused by the inhalation of biological or chemical agents |
| US9452218B2 (en) | 2012-03-09 | 2016-09-27 | Purdue Research Foundation | Compositions and methods for delivery of kinase inhibiting peptides |
| GB201207406D0 (en) | 2012-04-27 | 2012-06-13 | Glaxo Group Ltd | Novel compounds |
| PE20142400A1 (en) | 2012-04-27 | 2015-02-04 | Glaxo Group Ltd | NOVEL COMPOUNDS |
| US9649454B2 (en) | 2012-05-03 | 2017-05-16 | Manta Devices, Llc | Delivery device and related methods |
| WO2014012069A2 (en) | 2012-07-12 | 2014-01-16 | Mannkind Corporation | Dry powder drug delivery systems and methods |
| CA2883703C (en) | 2012-09-04 | 2021-10-19 | Eleison Pharmaceuticals, Llc | Preventing pulmonary recurrence of cancer with lipid-complexed cisplatin |
| EP2911690A1 (en) | 2012-10-26 | 2015-09-02 | MannKind Corporation | Inhalable influenza vaccine compositions and methods |
| SG11201503697TA (en) | 2012-11-28 | 2015-06-29 | Intercept Pharmaceuticals Inc | Treatment of pulmonary disease |
| GB201301192D0 (en) | 2013-01-23 | 2013-03-06 | Vectura Delivery Devices Ltd | A blister piercing element for a dry powder inhaler |
| US9351517B2 (en) | 2013-03-15 | 2016-05-31 | Virun, Inc. | Formulations of water-soluble derivatives of vitamin E and compositions containing same |
| AU2014228415B2 (en) | 2013-03-15 | 2018-08-09 | Mannkind Corporation | Microcrystalline diketopiperazine compositions and methods |
| KR102321339B1 (en) | 2013-07-18 | 2021-11-02 | 맨카인드 코포레이션 | Heat-stable dry powder pharmaceutical compositions and methods |
| US11446127B2 (en) | 2013-08-05 | 2022-09-20 | Mannkind Corporation | Insufflation apparatus and methods |
| US9693574B2 (en) | 2013-08-08 | 2017-07-04 | Virun, Inc. | Compositions containing water-soluble derivatives of vitamin E mixtures and modified food starch |
| WO2015038644A2 (en) | 2013-09-10 | 2015-03-19 | Debrabander Jef | Therapeutics targeting truncated adenomatous polyposis coli (apc) proteins |
| US10039321B2 (en) | 2013-11-12 | 2018-08-07 | Vmr Products Llc | Vaporizer |
| US10058129B2 (en) | 2013-12-23 | 2018-08-28 | Juul Labs, Inc. | Vaporization device systems and methods |
| US10076139B2 (en) | 2013-12-23 | 2018-09-18 | Juul Labs, Inc. | Vaporizer apparatus |
| GB2560651B8 (en) | 2013-12-23 | 2018-12-19 | Juul Labs Uk Holdco Ltd | Vaporization device systems and methods |
| USD769438S1 (en) | 2014-01-08 | 2016-10-18 | Glaxo Group Limited | Inhaler device |
| TWI751467B (en) | 2014-02-06 | 2022-01-01 | 美商尤爾實驗室有限公司 | A device for generating an inhalable aerosol and a separable cartridge for use therewith |
| US10709173B2 (en) | 2014-02-06 | 2020-07-14 | Juul Labs, Inc. | Vaporizer apparatus |
| US10307464B2 (en) | 2014-03-28 | 2019-06-04 | Mannkind Corporation | Use of ultrarapid acting insulin |
| US10336788B2 (en) | 2014-04-17 | 2019-07-02 | Moerae Matrix, Inc. | Inhibition of cardiac fibrosis in myocardial infarction |
| AU2015250132B2 (en) * | 2014-04-24 | 2020-11-26 | Questa Corporation | Multi-size pill splitter and methods |
| US11147936B2 (en) * | 2014-05-02 | 2021-10-19 | Manta Devices, Llc | Dose delivery device with cover connected to dose chamber seal |
| EA201692111A1 (en) | 2014-05-12 | 2017-08-31 | Глаксосмитклайн Интеллекчуал Проперти (№ 2) Лимитед | PHARMACEUTICAL COMPOSITIONS CONTAINING DANIRYXIN FOR TREATING INFECTIOUS DISEASES |
| US10940279B2 (en) * | 2014-07-01 | 2021-03-09 | Jennifer K. Keener | Aromatherapy device |
| US10082496B2 (en) | 2014-09-10 | 2018-09-25 | Board Of Regents Of The University Of Texas System | Targeting emopamil binding protein (EBP) with small molecules that induce an abnormal feedback response by lowering endogenous cholesterol biosynthesis |
| US9861611B2 (en) | 2014-09-18 | 2018-01-09 | Virun, Inc. | Formulations of water-soluble derivatives of vitamin E and soft gel compositions, concentrates and powders containing same |
| US10016363B2 (en) | 2014-09-18 | 2018-07-10 | Virun, Inc. | Pre-spray emulsions and powders containing non-polar compounds |
| US10561806B2 (en) | 2014-10-02 | 2020-02-18 | Mannkind Corporation | Mouthpiece cover for an inhaler |
| US20170304459A1 (en) | 2014-10-10 | 2017-10-26 | Alnylam Pharmaceuticals, Inc. | Methods and compositions for inhalation delivery of conjugated oligonucleotide |
| EP3212212B1 (en) | 2014-10-31 | 2020-09-23 | Monash University | Powder formulation |
| BR112017010238A2 (en) | 2014-11-17 | 2018-02-06 | Moerae Matrix, Inc. | compositions and methods for preventing or treating diseases, conditions or processes characterized by fibroblast proliferation and aberrant extracellular matrix deposition |
| WO2016092378A1 (en) | 2014-12-10 | 2016-06-16 | Angelo Luigi Vescovi | Methods and compositions for reducing growth, migration and invasiveness of brain cancer stem cells and improving survival of patients with brian tumors |
| BR112017014737A2 (en) | 2015-01-08 | 2018-01-16 | Moerae Matrix Inc | formulation of mk2 inhibitor peptides |
| MX2017009112A (en) | 2015-01-14 | 2018-06-15 | Respira Therapeutics Inc | Powder dispersion methods and devices. |
| US9522918B2 (en) | 2015-02-12 | 2016-12-20 | Neonc Technologies, Inc. | Pharmaceutical compositions comprising perillyl alcohol derivatives |
| EP3061501A1 (en) | 2015-02-27 | 2016-08-31 | Rottapharm Ltd. | Composition for the treatment of acne |
| TW201643179A (en) | 2015-03-11 | 2016-12-16 | 葛蘭素史克智慧財產發展有限公司 | TSLP binding proteins |
| AU2016229017A1 (en) | 2015-03-12 | 2017-09-28 | Moerae Matrix, Inc. | Use of MK2 inhibitor peptide-containing compositions for treating non-small cell lung cancer with same |
| US10179215B2 (en) | 2015-03-19 | 2019-01-15 | Altria Client Services Llc | Vaporizer for vaporizing a constituent of a plant material |
| AU2016201131B2 (en) * | 2015-03-30 | 2020-04-02 | Manrex Limited | Dispensing container for blister pack of medication |
| CA2987071A1 (en) | 2015-06-10 | 2016-12-15 | Hackensack University Medical Center | Use of telmisartan to prevent and treat graft versus host disease and other alloimmune and autoimmune diseases |
| CA2988374A1 (en) | 2015-06-15 | 2016-12-22 | Glaxosmithkline Intellectual Property Development Limited | Nrf2 regulators |
| MX2017016405A (en) | 2015-06-15 | 2018-05-22 | Glaxosmithkline Ip Dev Ltd | Nrf2 regulators. |
| CA2988373A1 (en) | 2015-06-15 | 2016-12-22 | Glaxosmithkline Intellectual Property Development Limited | Nrf2 regulators |
| WO2017007634A1 (en) | 2015-07-06 | 2017-01-12 | The Board Of Regents Of The University Of Texas System | Benzamide or benzamine compounds useful as anticancer agents for the treatment of human cancers |
| EP3322417B1 (en) | 2015-07-15 | 2023-08-23 | The Board of Regents of The University of Texas System | Targeting emopamil binding protein (ebp) with small molecules that induce an abnormal feedback response by lowering endogenous cholesterol biosynthesis |
| EP3117825A1 (en) | 2015-07-16 | 2017-01-18 | Rottapharm S.p.A. | Oral formulation comprising berberine and morus alba extract |
| CN105013054B (en) * | 2015-07-30 | 2018-11-09 | 中山市美捷时包装制品有限公司 | A kind of more capsule dry-powder inhaling devices |
| UY36851A (en) | 2015-08-16 | 2017-03-31 | Glaxosmithkline Ip Dev Ltd | COMPOUNDS FOR USE IN ANTIBACTERIAL APPLICATIONS |
| EP3371167A1 (en) | 2015-10-06 | 2018-09-12 | GlaxoSmithKline Intellectual Property Development Limited | Arylcyclohexyl pyrazoles as nrf2 regulators |
| WO2017060854A1 (en) | 2015-10-06 | 2017-04-13 | Glaxosmithkline Intellectual Property Development Limited | Biaryl pyrazoles as nrf2 regulators |
| JP2018535984A (en) | 2015-11-18 | 2018-12-06 | グラクソスミスクライン、インテレクチュアル、プロパティー、(ナンバー2)、リミテッドGlaxosmithkline Intellectual Property (No.2) Limited | Ribavirin pharmaceutical composition |
| US11833118B2 (en) | 2016-01-20 | 2023-12-05 | Flurry Powders, Llc | Encapsulation of lipophilic ingredients in dispersible spray dried powders suitable for inhalation |
| WO2017127641A1 (en) | 2016-01-20 | 2017-07-27 | Flurry Powders | Encapsulation of lipophilic ingredients in dispersible spray dried powders suitable for inhalation |
| USD861975S1 (en) | 2016-02-08 | 2019-10-01 | Juul Labs, Inc. | Vaporizer device with cartridges |
| MX2018009703A (en) | 2016-02-11 | 2019-07-08 | Juul Labs Inc | Securely attaching cartridges for vaporizer devices. |
| UA125687C2 (en) | 2016-02-11 | 2022-05-18 | Джуул Лебз, Інк. | Fillable vaporizer cartridge and method of filling |
| EP3419707B1 (en) | 2016-02-24 | 2023-01-25 | Boehringer Ingelheim International GmbH | Inhaler |
| BR112018067606A2 (en) | 2016-02-25 | 2019-01-08 | Juul Labs Inc | vaporization device control methods and systems |
| WO2018055527A1 (en) | 2016-09-20 | 2018-03-29 | Glaxosmithkline Intellectual Property (No.2) Limited | Trpv4 antagonists |
| TW201825458A (en) | 2016-09-20 | 2018-07-16 | 英商葛蘭素史克智慧財產(第二)有限公司 | TRPV 4 antagonists |
| CA3036933A1 (en) | 2016-09-20 | 2018-03-29 | Glaxosmithkline Intellectual Property (No.2) Limited | Trpv4 antagonists |
| CN110267538B (en) | 2016-11-30 | 2022-04-19 | 尼昂克技术公司 | Perilla alcohol-3-bromopyruvate conjugates and methods of treating cancer |
| CN108779108A (en) | 2016-12-06 | 2018-11-09 | 葛兰素史密斯克莱知识产权发展有限公司 | 3- (2,3- dihydro -1H- indenes -5- bases) propanoic derivatives and their purposes as NRF2 conditioning agents |
| US10426698B2 (en) * | 2016-12-08 | 2019-10-01 | Breeden Brothers, LLC | Pill container with cap |
| JP2020500918A (en) | 2016-12-12 | 2020-01-16 | グラクソスミスクライン、インテレクチュアル、プロパティー、ディベロップメント、リミテッドGlaxosmithkline Intellectual Property Development Limited | N-arylpyrazoles as NRF2 regulators |
| WO2018109641A1 (en) | 2016-12-12 | 2018-06-21 | Glaxosmithkline Intellectual Property Development Limited | 3-carboxylic acid pyrroles as nrf2 regulators |
| US11117905B2 (en) | 2016-12-14 | 2021-09-14 | Glaxosmithkline Intellectual Property Development Limited | Bisaryl heterocycles as NRF2 activators |
| US20220002272A1 (en) | 2016-12-14 | 2022-01-06 | Glaxosmithkline Intellectual Property Development Limited | Bisaryl lactams as nrf2 activators |
| US11078216B2 (en) | 2016-12-14 | 2021-08-03 | Glaxosmithkline Intellectual Property Development Limited | Bisaryl amides as NRF2 activators |
| JP2020502152A (en) | 2016-12-15 | 2020-01-23 | グラクソスミスクライン、インテレクチュアル、プロパティー、ディベロップメント、リミテッドGlaxosmithkline Intellectual Property Development Limited | Ether-linked triazoles as NRF2 activators |
| JP7110197B2 (en) | 2016-12-15 | 2022-08-01 | グラクソスミスクライン、インテレクチュアル、プロパティー、ディベロップメント、リミテッド | NRF2 activator |
| WO2018174938A1 (en) | 2017-03-23 | 2018-09-27 | Virun, Inc. | Stable dry powders and emulsions containing probiotics and mucoadhesive protein |
| US11014941B2 (en) | 2017-04-24 | 2021-05-25 | Cocrystal Pharma, Inc. | Pyrrolopyrimidine derivatives useful as inhibitors of influenza virus replication |
| WO2019116231A1 (en) | 2017-12-11 | 2019-06-20 | Glaxosmithkline Intellectual Property Development Limited | Nrf2 activator for the treatment of acute lung injury, acute respiratory distress syndrome and multiple organ dysfunction syndrome |
| US20210177861A1 (en) | 2017-12-11 | 2021-06-17 | Glaxosmithkline Intellectual Property Development Limited | Nrf2 activator for the treatment of acute lung injury, acute respiratory distress syndrome and multiple organ dysfunction syndrome |
| GB201720989D0 (en) | 2017-12-15 | 2018-01-31 | Glaxosmithkline Ip Dev Ltd | Chemical compounds |
| CA3088347A1 (en) | 2018-01-17 | 2019-07-25 | Glaxosmithkline Intellectual Property Development Limited | Pi4kiii.beta. inhibitors |
| WO2019157195A1 (en) | 2018-02-08 | 2019-08-15 | Neonc Technologies, Inc | Methods of permeabilizing the blood brain barrier |
| WO2019224667A1 (en) | 2018-05-23 | 2019-11-28 | Glaxosmithkline Intellectual Property Development Limited | Indanes as nrf2 activators |
| MX2021000917A (en) | 2018-07-27 | 2021-06-23 | Cocrystal Pharma Inc | Pyrrolo[2,3-b]pyridin derivatives as inhibitors of influenza virus replication. |
| CN113286793B (en) | 2018-09-10 | 2024-04-05 | 共结晶制药公司 | Inhibitors of influenza virus replication of pyrrolopyrazines and pyridotriazines |
| MX2021004215A (en) | 2018-10-17 | 2021-07-15 | Cocrystal Pharma Inc | Combinations of inhibitors of influenza virus replication. |
| EA202191327A1 (en) | 2018-11-13 | 2021-08-09 | Кокристал Фарма, Инк. | DOSAGE FORMS OF THERAPEUTIC PREPARATIONS FOR INFLUENZA |
| EP3887355A1 (en) | 2018-11-26 | 2021-10-06 | Cocrystal Pharma, Inc. | Inhibitors of influenza virus replication |
| DE102019134285A1 (en) | 2019-01-15 | 2020-07-16 | Nuuvera Deutschland GmbH | Device and method for extracting and aspirating active substances, in particular from the cannabis plant |
| KR20210141546A (en) | 2019-03-14 | 2021-11-23 | 옴 파르마 에스아 | Stable bacterial extract manufacturing process and use thereof as a medicament |
| CN113613665A (en) | 2019-03-14 | 2021-11-05 | Om药物公司 | Methods of treating and/or preventing asthma, exacerbation of asthma, allergic asthma and/or a condition associated with microbiota associated with a respiratory condition |
| GB201908536D0 (en) | 2019-06-13 | 2019-07-31 | Glaxosmithkline Ip Dev Ltd | Compounds |
| USD943161S1 (en) | 2019-11-14 | 2022-02-08 | Juul Labs, Inc. | Vaporizer device |
| USD943160S1 (en) | 2019-11-14 | 2022-02-08 | Juul Labs, Inc. | Vaporizer device |
| USD943159S1 (en) | 2019-11-14 | 2022-02-08 | Juul Labs, Inc. | Component for a vaporizer cartridge |
| USD943158S1 (en) | 2019-11-14 | 2022-02-08 | Juul Labs, Inc. | Vaporizer cartridge |
| WO2021188620A1 (en) | 2020-03-17 | 2021-09-23 | Cocrystal Pharma Inc. | Peptidomimetic n5-methyl-n2-(nonanoyl-l-leucyl)-l-glutaminate derivatives, triazaspiro[4.14]nonadecane derivatives and similar compounds as inhibitors of norovirus and coronavirus replication |
| KR20230008079A (en) | 2020-04-10 | 2023-01-13 | 코크리스탈 파마, 아이엔씨. | Norovirus and coronavirus replication inhibitors |
| EP4138884A1 (en) | 2020-04-20 | 2023-03-01 | Sorrento Therapeutics, Inc. | Pulmonary administration of ace2 polypeptides |
| WO2022175425A1 (en) | 2021-02-22 | 2022-08-25 | Glaxosmithkline Intellectual Property Development Limited | Inhaled mtor kinase inhibitors for use in the treatment or the prevention of a respiratory rna virus infection |
| WO2022179967A1 (en) | 2021-02-23 | 2022-09-01 | Glaxosmithkline Intellectual Property (No.2) Limited | Vadadustat for treating covid-19 in a hospitalized subject |
| AU2022258576A1 (en) | 2021-04-14 | 2023-11-02 | Research Triangle Institute | Hot porous-solid metering systems and methods for generation of therapeutic aerosols by evaporation/condensation |
| CA3227602A1 (en) | 2021-08-03 | 2023-02-09 | Irina C. Jacobson | Inhibitors for coronaviruses |
| WO2023205389A1 (en) | 2022-04-22 | 2023-10-26 | Quench Medical Inc. | Dry powder inhalation delivery of pharmaceuticals |
| WO2023232976A1 (en) | 2022-06-03 | 2023-12-07 | Ags Therapeutics Sas | Extracellular vesicles from genetically-modified microalgae containing endogenously-loaded cargo, their preparation, and uses |
| WO2024095105A1 (en) * | 2022-11-04 | 2024-05-10 | Skietta Ag | Vaporiser for vaporising an inhalation medium |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2061735A (en) * | 1979-10-30 | 1981-05-20 | Riker Laboratories Inc | Breath actuated device for administration of powdered medicaments by inhalation |
| GB2129691A (en) * | 1982-10-08 | 1984-05-23 | Glaxo Group Ltd | Devices for administering medicaments to patients |
| EP0129985A1 (en) * | 1983-05-24 | 1985-01-02 | Glaxo Group Limited | Inhalation device |
Family Cites Families (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB190707525A (en) * | 1906-04-07 | 1907-11-28 | Eugene Joseph Decoll | Impovements relating to Appliances for Administering Anestetics |
| GB190706553A (en) * | 1907-03-19 | 1907-11-28 | Hume Purdie | Surgical Apparatus for the Administration of Anaesthetics |
| US1383270A (en) * | 1918-10-02 | 1921-06-28 | Henning Albert | Means for cooling internal-combustion engines and other machinery |
| US2386877A (en) * | 1943-07-27 | 1945-10-16 | Jr William E Neilson | Box opener and dispenser |
| US3949751A (en) * | 1970-03-03 | 1976-04-13 | Fisons Limited | Method and device for dispensing medicament to the body |
| US3888253A (en) * | 1972-08-04 | 1975-06-10 | Beecham Group Ltd | Device for administration of medicines |
| GB1392945A (en) * | 1972-08-23 | 1975-05-07 | Fisons Ltd | Inhalation device |
| GB1387954A (en) * | 1973-05-08 | 1975-03-19 | Miles Lab | Insufflator |
| US4014336A (en) * | 1975-01-13 | 1977-03-29 | Syntex Puerto Rico, Inc. | Inhalation device |
| GB1562732A (en) * | 1976-02-10 | 1980-03-12 | Allen & Hanburys Ltd | Device for dispensing medicaments |
| DE2834135C3 (en) * | 1978-08-03 | 1981-11-26 | SIEMENS AG AAAAA, 1000 Berlin und 8000 München | Method for manufacturing a traveling wave tube |
| DE2835135C3 (en) * | 1978-08-10 | 1982-03-04 | Bosch-Siemens Hausgeräte GmbH, 7000 Stuttgart | Active substance storage for an inhalation device |
| IT1116047B (en) * | 1979-04-27 | 1986-02-10 | Sigma Tau Ind Farmaceuti | DEVICE FOR THE QUICK INHALATION OF POWDER DRUGS BY PERSONS SUFFERING FROM ASTHMA |
| AU545574B2 (en) * | 1979-10-30 | 1985-07-18 | Riker Laboratories, Inc. | Breath actuated devices for adminstering powdered medicaments |
| DE3167567D1 (en) * | 1980-06-06 | 1985-01-17 | Fisons Plc | Inhalation device for powdered medicaments |
| ES8206980A1 (en) * | 1980-10-30 | 1982-09-01 | Riker Laboratories Inc | Powder inhalation device. |
| US4384649A (en) * | 1980-12-11 | 1983-05-24 | E. R. Squibb & Sons, Inc. | Dispensing package |
| CA1198065A (en) * | 1981-03-27 | 1985-12-17 | Roland Torterotot | Method for the manufacture and application of a closure for a container |
| ZA837318B (en) * | 1982-10-08 | 1985-06-26 | Glaxo Group Ltd | Device for administering medicaments to patients |
| DE3345722A1 (en) * | 1983-12-17 | 1985-06-27 | Boehringer Ingelheim KG, 6507 Ingelheim | INHALATOR |
| GR861995B (en) * | 1985-07-30 | 1986-11-04 | Glaxo Group Ltd | Devices for administering medicaments to patients |
| US4690279A (en) * | 1986-03-13 | 1987-09-01 | Charles Hochberg | Birth control pill dispenser in the form of a hair brush |
-
1986
- 1986-07-29 GR GR861995A patent/GR861995B/en unknown
- 1986-07-29 DE DE8686305807T patent/DE3682457D1/en not_active Expired - Lifetime
- 1986-07-29 SE SE8603252A patent/SE8603252L/en not_active Application Discontinuation
- 1986-07-29 US US06/891,536 patent/US4811731A/en not_active Expired - Lifetime
- 1986-07-29 NL NL8601949A patent/NL8601949A/en not_active Application Discontinuation
- 1986-07-29 DK DK360986A patent/DK163640C/en not_active IP Right Cessation
- 1986-07-29 NZ NZ217006A patent/NZ217006A/en unknown
- 1986-07-29 CA CA000514908A patent/CA1272917A/en not_active Expired - Lifetime
- 1986-07-29 IL IL79550A patent/IL79550A/en not_active IP Right Cessation
- 1986-07-29 GB GB08618466A patent/GB2178965B/en not_active Expired
- 1986-07-29 FI FI863094A patent/FI88112C/en not_active IP Right Cessation
- 1986-07-29 CH CH3025/86A patent/CH672600A5/de not_active IP Right Cessation
- 1986-07-29 ES ES8600683A patent/ES2000781A6/en not_active Expired
- 1986-07-29 NO NO863062A patent/NO166268C/en not_active IP Right Cessation
- 1986-07-29 BR BR8603576A patent/BR8603576A/en not_active IP Right Cessation
- 1986-07-29 BE BE0/216987A patent/BE905189A/en not_active IP Right Cessation
- 1986-07-29 IE IE202286A patent/IE59026B1/en not_active IP Right Cessation
- 1986-07-29 AU AU60655/86A patent/AU591152B2/en not_active Expired
- 1986-07-29 EP EP86305807A patent/EP0211595B1/en not_active Expired - Lifetime
- 1986-07-29 PT PT83094A patent/PT83094B/en not_active IP Right Cessation
- 1986-07-29 PH PH34078A patent/PH26882A/en unknown
- 1986-07-29 FR FR8610955A patent/FR2585563B1/en not_active Expired - Lifetime
- 1986-07-29 IT IT48322/86A patent/IT1195984B/en active
- 1986-07-29 AT AT0204086A patent/AT396872B/en not_active IP Right Cessation
- 1986-07-30 MX MX003326A patent/MX171389B/en unknown
- 1986-07-30 HU HU863243A patent/HU199306B/en not_active IP Right Cessation
- 1986-07-30 JP JP61177917A patent/JPS6241668A/en active Granted
- 1986-07-30 DE DE19863625685 patent/DE3625685A1/en active Granted
- 1986-07-30 KR KR1019860006254A patent/KR940002247B1/en not_active IP Right Cessation
- 1986-07-30 LU LU86534A patent/LU86534A1/en unknown
- 1986-07-30 PL PL1986260858A patent/PL149733B1/en unknown
-
1988
- 1988-12-27 US US07/290,628 patent/US5035237A/en not_active Expired - Lifetime
-
1989
- 1989-02-14 SG SG87/89A patent/SG8789G/en unknown
- 1989-02-24 KE KE3865A patent/KE3865A/en unknown
- 1989-08-24 HK HK675/89A patent/HK67589A/en not_active IP Right Cessation
- 1989-08-29 DO DO1989004707A patent/DOP1989004707A/en unknown
- 1989-12-08 CY CY1481A patent/CY1481A/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2061735A (en) * | 1979-10-30 | 1981-05-20 | Riker Laboratories Inc | Breath actuated device for administration of powdered medicaments by inhalation |
| GB2129691A (en) * | 1982-10-08 | 1984-05-23 | Glaxo Group Ltd | Devices for administering medicaments to patients |
| EP0129985A1 (en) * | 1983-05-24 | 1985-01-02 | Glaxo Group Limited | Inhalation device |
Cited By (63)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5590645A (en) * | 1990-03-02 | 1997-01-07 | Glaxo Group Limited | Inhalation device |
| US5860419A (en) * | 1990-03-02 | 1999-01-19 | Glaxo Group Limited | Inhalation device |
| US5873360A (en) * | 1990-03-02 | 1999-02-23 | Glaxo Group Limited | Inhalation device |
| FR2660550A1 (en) * | 1990-03-02 | 1991-10-11 | Glaxo Group Ltd | Medicament pack for inhalation device |
| US6792945B2 (en) | 1990-03-02 | 2004-09-21 | Glaxo Group Limited | Inhalation device |
| US6536427B2 (en) | 1990-03-02 | 2003-03-25 | Glaxo Group Limited | Inhalation device |
| US6032666A (en) * | 1990-03-02 | 2000-03-07 | Glaxo Group Limited | Inhalation device |
| US7225808B2 (en) | 1990-03-02 | 2007-06-05 | Glaxo Group Limited | Inhalation device |
| US7389775B2 (en) | 1990-03-02 | 2008-06-24 | Glaxo Group Limited | Inhalation device |
| US5429122A (en) * | 1990-09-26 | 1995-07-04 | Zanen; Pieter | Inhaler devices provided with a reservoir for several doses of medium for inhaling, transporting device, whirl chamber |
| US5765552A (en) * | 1990-09-26 | 1998-06-16 | Pharmachemie B.V. | Inhaler devices provided with a reservoir for several doses of medium for inhaling, transporting device, whirl chamber |
| US5492112A (en) * | 1991-05-20 | 1996-02-20 | Dura Pharmaceuticals, Inc. | Dry powder inhaler |
| WO1993009831A1 (en) * | 1991-11-18 | 1993-05-27 | Smithkline Beecham Plc | Oral inhaler |
| EP0547429A1 (en) * | 1991-12-14 | 1993-06-23 | ASTA Medica Aktiengesellschaft | Powder inhaler |
| US5460173A (en) * | 1993-03-03 | 1995-10-24 | Tenax Corporation | Dry powder inhaler medicament carrier |
| BE1007408A3 (en) * | 1993-08-13 | 1995-06-06 | Winters Joep | Method and device for checking medicine intake |
| US5388572A (en) * | 1993-10-26 | 1995-02-14 | Tenax Corporation (A Connecticut Corp.) | Dry powder medicament inhalator having an inhalation-activated piston to aerosolize dose and deliver same |
| US5388573A (en) * | 1993-12-02 | 1995-02-14 | Tenax Corporation | Dry powder inhalator medicament carrier |
| US5921237A (en) * | 1995-04-24 | 1999-07-13 | Dura Pharmaceuticals, Inc. | Dry powder inhaler |
| US5881719A (en) * | 1995-06-30 | 1999-03-16 | Asta Medica Aktiengesellschaft | Inhaler for administering medicaments from blister packs |
| US6209538B1 (en) * | 1995-08-02 | 2001-04-03 | Robert A. Casper | Dry powder medicament inhalator having an inhalation-activated flow diverting means for triggering delivery of medicament |
| US5823183A (en) * | 1995-08-02 | 1998-10-20 | Innovative Devices | Dry powder medicament inhalator having an inhalation-activated flow diverting means for triggering delivery of medicament |
| US5692496A (en) * | 1995-08-02 | 1997-12-02 | Innovative Devices, Llc | Dry powder medicament inhalator having an inhalation-activated flow diverting means for triggering delivery of medicament |
| US6550477B1 (en) | 1995-08-02 | 2003-04-22 | Innovative Devices, Llc | Dry powder medicament inhalator having an inhalation-activated flow diverting means for triggering delivery of medicament |
| US6561186B2 (en) | 1995-08-02 | 2003-05-13 | Innovative Devices | Dry powder medicament inhalator having an inhalation-activated flow diverting means for triggering delivery of medicament |
| US5988163A (en) * | 1995-08-02 | 1999-11-23 | Innovative Devices | Dry powder medicament inhalator having an inhalation-activated flow diverting means for triggering delivery of delivery of medicament |
| US6076522A (en) * | 1996-05-23 | 2000-06-20 | Glaxo Wellcome Inc. | Metering apparatus |
| WO1998004308A1 (en) | 1996-07-31 | 1998-02-05 | Glaxo Group Limited | Medicament carrier with agglomerated large medicament particles and related method of manufacture thereof |
| US7318436B2 (en) | 2000-05-10 | 2008-01-15 | Innovative Devices, Llc | Medicament container with same side airflow inlet and outlet and method of use |
| US6948494B1 (en) | 2000-05-10 | 2005-09-27 | Innovative Devices, Llc. | Medicament container with same side airflow inlet and outlet and method of use |
| US6923178B2 (en) | 2000-05-10 | 2005-08-02 | Innovative Devices, Llc. | Medicament container with same side airflow inlet and outlet |
| WO2002026302A1 (en) | 2000-09-27 | 2002-04-04 | Merck Patent Gmbh | Device for administering doses of particulate material |
| US7032594B2 (en) | 2000-09-27 | 2006-04-25 | Merck Patent Gmbh | Device for administering doses of particulate material |
| US8022082B2 (en) | 2002-04-09 | 2011-09-20 | Boehringer Ingelheim Pharma Gmbh & Co., Kg | Method for the administration of an anticholinergic by inhalation |
| US11116917B2 (en) | 2003-07-02 | 2021-09-14 | Pfizer Limited | Dispensing device |
| EP3459578B1 (en) | 2003-07-02 | 2021-03-03 | Pfizer Limited | Dispensing device |
| EP2594272A2 (en) | 2005-05-18 | 2013-05-22 | Mpex Pharmaceuticals, Inc. | Aerosolized fluoroquinolones and uses thereof |
| US8524734B2 (en) | 2005-05-18 | 2013-09-03 | Mpex Pharmaceuticals, Inc. | Aerosolized fluoroquinolones and uses thereof |
| US8546423B2 (en) | 2005-05-18 | 2013-10-01 | Mpex Pharmaceuticals, Inc. | Aerosolized fluoroquinolones and uses thereof |
| US8524735B2 (en) | 2005-05-18 | 2013-09-03 | Mpex Pharmaceuticals, Inc. | Aerosolized fluoroquinolones and uses thereof |
| US10987357B2 (en) | 2005-05-18 | 2021-04-27 | Horizon Orphan, LLC | Aerosolized fluoroquinolones and uses thereof |
| US8357696B2 (en) | 2005-05-18 | 2013-01-22 | Mpex Pharmaceuticals, Inc. | Aerosolized fluoroquinolones and uses thereof |
| US10722519B2 (en) | 2008-10-07 | 2020-07-28 | Horizon Orphan Llc | Aerosol fluoroquinolone formulations for improved pharmacokinetics |
| US11020481B2 (en) | 2008-10-07 | 2021-06-01 | Horizon Orphan Llc | Topical use of levofloxacin for reducing lung inflammation |
| US9326936B2 (en) | 2008-10-07 | 2016-05-03 | Raptor Pharmaceuticals, Inc. | Aerosol fluoroquinolone formulations for improved pharmacokinetics |
| US8629139B2 (en) | 2008-10-07 | 2014-01-14 | Mpex Pharmaceuticals, Inc. | Topical use of Levofloxacin for reducing lung inflammation |
| US9717738B2 (en) | 2008-10-07 | 2017-08-01 | Horizon Orphan Llc | Aerosol fluoroquinolone formulations for improved pharmacokinetics |
| US8815838B2 (en) | 2008-10-07 | 2014-08-26 | David C. Griffith | Aerosol fluoroquinolone formulations for improved pharmacokinetics |
| US10149854B2 (en) | 2008-10-07 | 2018-12-11 | Horizon Orphan Llc | Aerosol fluoroquinolone formulations for improved pharmacokinetics |
| US10231975B2 (en) | 2009-09-04 | 2019-03-19 | Horizon Orphan Llc | Use of aerosolized levofloxacin for treating cystic fibrosis |
| US10792289B2 (en) | 2009-09-04 | 2020-10-06 | Horizon Orphan Llc | Use of aerosolized levofloxacin for treating cystic fibrosis |
| US9700564B2 (en) | 2009-09-04 | 2017-07-11 | Horizon Orphan Llc | Use of aerosolized levofloxacin for treating cystic fibrosis |
| EP4059499A1 (en) | 2011-01-31 | 2022-09-21 | Avalyn Pharma Inc. | Aerosol pirfenidone and pyridone analog compounds and uses thereof |
| EP2666497A1 (en) | 2012-05-25 | 2013-11-27 | Arven Ilac Sanayi Ve Ticaret A.S. | An inhaler comprising a mouthpiece having an improved air channel |
| WO2013176638A1 (en) | 2012-05-25 | 2013-11-28 | Sanovel Ilac Sanayi Ve Ticaret Anonim Sirketi | An inhaler comprising a mouthpiece having an improved air channel |
| US10583261B2 (en) | 2013-07-12 | 2020-03-10 | Liita Holdings Ltd | Inhaler |
| EP3782604A1 (en) | 2013-07-31 | 2021-02-24 | Windward Pharma, Inc. | Aerosol tyrosine kinase inhibitor compounds and uses thereof |
| US10028966B2 (en) | 2014-01-10 | 2018-07-24 | Avalyn Pharma Inc. | Aerosol pirfenidone and pyridone analog compounds and uses thereof |
| CN104984448A (en) * | 2015-07-30 | 2015-10-21 | 中山市美捷时包装制品有限公司 | Dry powder inhaler |
| CN104984448B (en) * | 2015-07-30 | 2018-05-01 | 中山市美捷时包装制品有限公司 | A kind of Diskus |
| WO2020148276A1 (en) * | 2019-01-14 | 2020-07-23 | Alfred Von Schuckmann | Device for inhaling powder-type substances, substance container for a device of this type and method for filling a device of this type |
| WO2022240897A1 (en) | 2021-05-10 | 2022-11-17 | Sepelo Therapeutics, Llc | Pharmaceutical composition comprising delafloxacin for administration into the lung |
| WO2023028364A1 (en) | 2021-08-27 | 2023-03-02 | Sepelo Therapeutics, Llc | Targeted compositions and uses therof |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0211595B1 (en) | Devices for administering medicaments to patients | |
| EP2398537B1 (en) | Inhaler | |
| DK172541B1 (en) | Apparatus for administering medications to patients and a drug package for use in the apparatus | |
| EP0129985B1 (en) | Inhalation device | |
| GB2169265A (en) | Pack for medicament | |
| CZ288921B6 (en) | Blister disk for inhaler | |
| DK173079B1 (en) | Hand-held inhaler for powder or liq. medical inhalants - which are in dose sized blisters of circular disc blister pack | |
| IE56060B1 (en) | Medicament-containing pack |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): DE GB NL SE |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): DE GB NL SE |
|
| 17P | Request for examination filed |
Effective date: 19890314 |
|
| 17Q | First examination report despatched |
Effective date: 19900928 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): DE NL SE |
|
| REF | Corresponds to: |
Ref document number: 3682457 Country of ref document: DE Date of ref document: 19911219 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| EAL | Se: european patent in force in sweden |
Ref document number: 86305807.9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20050616 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20050704 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20050729 Year of fee payment: 20 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20060729 |
|
| EUG | Se: european patent has lapsed | ||
| NLV7 | Nl: ceased due to reaching the maximum lifetime of a patent |
Effective date: 20060729 |