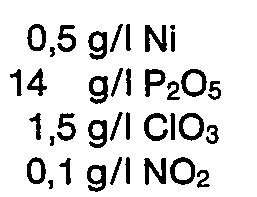EP0154367B1 - Process for phosphatizing metals - Google Patents
Process for phosphatizing metals Download PDFInfo
- Publication number
- EP0154367B1 EP0154367B1 EP85200192A EP85200192A EP0154367B1 EP 0154367 B1 EP0154367 B1 EP 0154367B1 EP 85200192 A EP85200192 A EP 85200192A EP 85200192 A EP85200192 A EP 85200192A EP 0154367 B1 EP0154367 B1 EP 0154367B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- phosphating
- metal surfaces
- steel
- phosphatizing
- phosphate
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 238000000034 method Methods 0.000 title claims abstract description 24
- 229910052751 metal Inorganic materials 0.000 title claims description 12
- 239000002184 metal Substances 0.000 title claims description 12
- 150000002739 metals Chemical class 0.000 title claims description 4
- 239000011701 zinc Substances 0.000 claims abstract description 15
- 229910052725 zinc Inorganic materials 0.000 claims abstract description 12
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 claims abstract description 10
- 239000012190 activator Substances 0.000 claims abstract description 8
- 238000000576 coating method Methods 0.000 claims abstract description 6
- MGFYIUFZLHCRTH-UHFFFAOYSA-N nitrilotriacetic acid Chemical compound OC(=O)CN(CC(O)=O)CC(O)=O MGFYIUFZLHCRTH-UHFFFAOYSA-N 0.000 claims abstract description 5
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 claims abstract description 4
- 229910019142 PO4 Inorganic materials 0.000 claims description 17
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 claims description 17
- 239000010452 phosphate Substances 0.000 claims description 17
- 229910000831 Steel Inorganic materials 0.000 claims description 13
- 239000010959 steel Substances 0.000 claims description 13
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 claims description 8
- -1 tetrafluoroborate Chemical compound 0.000 claims description 5
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 claims description 4
- 239000002253 acid Substances 0.000 claims description 4
- 229910052759 nickel Inorganic materials 0.000 claims description 4
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 claims description 3
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 claims description 3
- KRHYYFGTRYWZRS-UHFFFAOYSA-M Fluoride anion Chemical compound [F-] KRHYYFGTRYWZRS-UHFFFAOYSA-M 0.000 claims description 3
- 229910001335 Galvanized steel Inorganic materials 0.000 claims description 3
- AEMRFAOFKBGASW-UHFFFAOYSA-M Glycolate Chemical compound OCC([O-])=O AEMRFAOFKBGASW-UHFFFAOYSA-M 0.000 claims description 3
- 239000008397 galvanized steel Substances 0.000 claims description 3
- 150000004761 hexafluorosilicates Chemical class 0.000 claims description 3
- 239000007921 spray Substances 0.000 claims description 3
- 229940095064 tartrate Drugs 0.000 claims description 3
- 238000002360 preparation method Methods 0.000 claims description 2
- 239000011248 coating agent Substances 0.000 claims 2
- 238000004070 electrodeposition Methods 0.000 abstract description 7
- 229940066528 trichloroacetate Drugs 0.000 abstract description 3
- YNJBWRMUSHSURL-UHFFFAOYSA-N trichloroacetic acid Chemical compound OC(=O)C(Cl)(Cl)Cl YNJBWRMUSHSURL-UHFFFAOYSA-N 0.000 abstract description 3
- 239000003973 paint Substances 0.000 description 8
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 7
- 229910052742 iron Inorganic materials 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- IOVCWXUNBOPUCH-UHFFFAOYSA-M Nitrite anion Chemical compound [O-]N=O IOVCWXUNBOPUCH-UHFFFAOYSA-M 0.000 description 3
- 239000003513 alkali Substances 0.000 description 3
- 238000005452 bending Methods 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 229940071106 ethylenediaminetetraacetate Drugs 0.000 description 3
- 230000003647 oxidation Effects 0.000 description 3
- 238000007254 oxidation reaction Methods 0.000 description 3
- 238000005507 spraying Methods 0.000 description 3
- 230000003213 activating effect Effects 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- UJJUJHTVDYXQON-UHFFFAOYSA-N nitro benzenesulfonate Chemical compound [O-][N+](=O)OS(=O)(=O)C1=CC=CC=C1 UJJUJHTVDYXQON-UHFFFAOYSA-N 0.000 description 2
- 238000010422 painting Methods 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 229910000838 Al alloy Inorganic materials 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- 229910000611 Zinc aluminium Inorganic materials 0.000 description 1
- 229910001297 Zn alloy Inorganic materials 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 150000001447 alkali salts Chemical class 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 239000011260 aqueous acid Substances 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 229940005740 hexametaphosphate Drugs 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 239000004922 lacquer Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 229910052748 manganese Inorganic materials 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000004575 stone Substances 0.000 description 1
- 238000004381 surface treatment Methods 0.000 description 1
- JUWGUJSXVOBPHP-UHFFFAOYSA-B titanium(4+);tetraphosphate Chemical compound [Ti+4].[Ti+4].[Ti+4].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O JUWGUJSXVOBPHP-UHFFFAOYSA-B 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C22/00—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals
- C23C22/05—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions
- C23C22/06—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6
- C23C22/07—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6 containing phosphates
- C23C22/23—Condensed phosphates
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C22/00—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals
- C23C22/05—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions
- C23C22/06—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6
- C23C22/07—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6 containing phosphates
- C23C22/08—Orthophosphates
- C23C22/12—Orthophosphates containing zinc cations
- C23C22/17—Orthophosphates containing zinc cations containing also organic acids
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C22/00—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals
- C23C22/05—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions
- C23C22/06—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6
- C23C22/34—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6 containing fluorides or complex fluorides
- C23C22/36—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6 containing fluorides or complex fluorides containing also phosphates
- C23C22/362—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6 containing fluorides or complex fluorides containing also phosphates containing also zinc cations
Definitions
- the invention relates to a method for phosphating metals, in particular steel and galvanized steel, by spraying using an aqueous, acid accelerator, zinc and optionally nickel-containing phosphating solution and its use before electrocoating.
- the reduced zinc content compared to the usual phosphating baths leads to improved thin and uniform phosphate coatings, which are very adhesive and durable and are particularly suitable as a basis for the subsequent electrocoating.
- PCT publication WO 84/00386 discloses phosphating solutions with 0.2 to 0.6 g / l of Zn and Ni in a ratio of 5.2 to 16 mol of Ni to 1 mol of Zn, the phosphate layers having a particularly high resistance to Have resolution in alkali.
- the Japanese patent publication (according to Chemical Abstracts 99/216843 u) 58 144 477 describes spray phosphating solutions for steel with a content of 0.1 to 0.5 g / l Zn, 15 to 30 g / l phosphate and 0.01 to 0.2 g / l nitrite.
- the phosphate layers produced in this way are particularly suitable as pretreatment for a subsequent cathodic electrocoating.
- a disadvantage of the known processes with zinc concentrations in the phosphating bath of 0.1 to 0.6 g / l is that when used in spraying on steel they often lead to uneven phosphate layers with partly gray, partly greenish-bluish iridescent color. These irregularities can mark themselves in the subsequently applied electro-dip lacquer and cause expensive rework. Furthermore, paint adhesion is sufficient when subjected to e.g. Bending or prolonged contact with water does not always meet the requirements.
- the object of the invention is to provide a process which does not have the disadvantages of the known processes and which forms uniform, uniform phosphate layers with improved paint adhesion.
- the object is achieved by designing the method of the type mentioned at the outset in accordance with the invention in such a way that the metal surfaces are brought into contact with an aqueous acidic phosphating solution which and contains at least one activator from the group formate, nitrilotriacetate, trichloroacetate and ethylenediaminetetraacetate.
- the method according to the invention is intended in particular for the treatment of iron, steel and galvanized steel. However, it is also suitable for the phosphating of zinc alloys, aluminum and aluminum alloys.
- the treatment is carried out by spraying, the contact times for iron, steel and aluminum being 90 to 240 seconds, for example, and zinc 5 to 240 seconds.
- the method according to the invention is mostly used at bath temperatures between 30 and 60 ° C.
- the addition of nickel to the phosphating bath generally has a favorable effect on the phosphating speed, the formation of layers on steel surfaces that are difficult to phosphate, on the phosphating of zinc surfaces and on the corrosion protection properties.
- the phosphating solutions to be used in the process according to the invention preferably contain alkali ions, e.g. B. Na, K, Li, NH 4 , and if necessary further anions, for. B. NO 3 , CI, S0 4 .
- the baths can furthermore contain cations known in phosphating technology, such as Co, Cu, Mn, Ca, Mg and Fe.
- nitrite and / or nitrobenzenesulfonate are used as oxidation accelerators.
- further oxidation accelerators e.g. Clorat is possible and can be beneficial.
- An advantageous embodiment of the method according to the invention is to bring the metal surface into contact with a phosphating solution which contains at least one further activator from the group consisting of fluoride, hexafluorosilicate, tetrafluoroborate, glycolate, citrate, tartrate and condensed phosphate.
- the activators to be used in the method according to the invention have an accelerating and comparative effect on the layer formation and control the weight per unit area of the phosphate layers.
- the addition can e.g. over the corresponding acids or alkali salts.
- concentrations in the phosphating solution apply to the activators: 0.5 to 3 g / l fluoride; 1 to 5 g / l hexafluorosilicate; 3 to 10 g / l tetrafluoroborate; 5 to 15 g / l formate; 0.3 to 5 g / l glycolate; 0.3 to 3 g / l nitrilotriacetate; 2 to 15 g / l trichloroacetate; 0.1 to 3 g / l of ethylenediaminetetraacetate; 0.01 to 0.5 g / l citrate; 0.03 to 0.8 g / l tartrate; 0.03 to 0.3 g / l of conden
- the weight per unit area of the phosphate layers produced on steel using the method according to the invention is usually between 0.8 and 2.5 g / m 2 .
- activating agents e.g. B. on titanium phosphate basis, in the pre-rinse bath or the last cleaning stage.
- the phosphate layers produced with the method according to the invention are suitable in principle for all purposes for which phosphate layers are suitable.
- the layers bring about an unusually strong improvement in the resistance of the paint film against paint infiltration under corrosive stress as well as a significant increase in paint adhesion to the metallic surface under scratch, impact, and bending stress.
- electrocoating in particular in cathodic electrocoating, which is why the method is preferably used as a preparation for this type of painting.
- the method according to the invention finds practical application, for. B. for the phosphating of car bodies.
- Body steel sheets degreased with mildly alkaline, activating cleaner and then rinsed with water were sprayed with the following phosphating solutions for two minutes at 55 ° C, then rinsed with water, passively rinsed with dilute Cr (VI) / Cr (III) solution, rinsed with demineralized water and partly dried and partly cathodically electro-coated.
- the phosphating solutions according to 1 to 7 contained identical as well as the zinc contents indicated in the table.
- the phosphating solutions according to 8 to 11 contained as well as the activator levels listed in the table.
- the phosphating solutions mentioned were adjusted to the phosphating equilibrium with alkali.
- Examples 8 and 11 clearly show the layer evaluation of the advantages of the procedure according to the invention in comparison to examples 1 to 6 according to the prior art. Due to the different chemical surface activity of steel sheets from different rolled batches, the absolute value of the visual assessment is subject to certain fluctuations, but without significantly influencing the relative differences.
- the steel sheets phosphated according to Examples 1 to 6 led to a paint film with an uneven surface structure, while uniform paint films had to be deposited on the steel sheets phosphated according to Examples 7 to 11.
- cathodically coated metal sheets were provided with a total of about 100 11m automotive paint and tested using various methods.
- the results of the sheets treated according to the invention with regard to VW stone chips with a salt spray test, cross-cut after exposure to condensation and bending over the conical mandrel are very good and behave significantly better than Examples 4 and 7 of the prior art.
Landscapes
- Chemical & Material Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Chemical Treatment Of Metals (AREA)
- Paints Or Removers (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Electrolytic Production Of Metals (AREA)
- Catalysts (AREA)
- Glass Compositions (AREA)
- Secondary Cells (AREA)
- Materials For Medical Uses (AREA)
- Superconductors And Manufacturing Methods Therefor (AREA)
Abstract
Description
Die Erfindung betrifft ein Verfahren zur Phosphatierung von Metallen, insbesondere von Stahl und verzinktem Stahl, im Spritzverfahren mittels einer wäßrigen, sauren Beschleuniger, Zink und gegebenenfalls Nickel enthaltenden Phosphatierungslösung und dessen Anwendung vor der Elektrotauchlackierung.The invention relates to a method for phosphating metals, in particular steel and galvanized steel, by spraying using an aqueous, acid accelerator, zinc and optionally nickel-containing phosphating solution and its use before electrocoating.
In der DE-OS 22 32 067 werden wäßrige saure Phosphatierungslösungen mit einem Gewichtsverhältnis von Zn : P04 = 1 : (12 bis 110) zur Oberflächenbehandlung von Metallen, insbesondere Eisen und Stahl, beschrieben. Der gegenüber den üblichen Phosphatierbädern verringerte Zinkgehalt führt zu verbesserten dünnen und gleichmäßigen Phosphatüberzügen, die sehr haftfest und beständig und als Grundlage für die anschließende Elektrotauchlackierung besonders geeignet sind.DE-OS 22 32 067 describes aqueous acid phosphating solutions with a weight ratio of Zn: P0 4 = 1: (12 to 110) for the surface treatment of metals, in particular iron and steel. The reduced zinc content compared to the usual phosphating baths leads to improved thin and uniform phosphate coatings, which are very adhesive and durable and are particularly suitable as a basis for the subsequent electrocoating.
Aus der PCT-Publikation WO 84/00386 sind Phosphatierungslösungen mit 0,2 bis 0,6 g/I Zn und Ni in einem Verhältnis von 5,2 bis 16 Mol Ni zu 1 Mol Zn bekannt, die Phosphatschichten mit einer besonders hohen Beständigkeit gegen Auflösung in Alkali aufweisen.PCT publication WO 84/00386 discloses phosphating solutions with 0.2 to 0.6 g / l of Zn and Ni in a ratio of 5.2 to 16 mol of Ni to 1 mol of Zn, the phosphate layers having a particularly high resistance to Have resolution in alkali.
Die japanische Patentpublikation (gemäß Chemical Abstracts 99/216843 u) 58 144 477 beschreibt Spritzphosphatierlösungen für Stahl mit einem Gehalt an 0,1 bis 0,5 g/I Zn, 15 bis 30 g/I Phosphat und 0,01 bis 0,2 g/I Nitrit. Die damit erzeugten Phosphatschichten sind insbesondere als Vorbehandlung für eine anschließende kathodische Elektrotauchlackierung gut geeignet.The Japanese patent publication (according to Chemical Abstracts 99/216843 u) 58 144 477 describes spray phosphating solutions for steel with a content of 0.1 to 0.5 g / l Zn, 15 to 30 g / l phosphate and 0.01 to 0.2 g / l nitrite. The phosphate layers produced in this way are particularly suitable as pretreatment for a subsequent cathodic electrocoating.
Nachteilig an den bekannten Verfahren mit Zinkkonzentrationen im Phosphatierbad von 0,1 bis 0,6 g/I ist, daß sie bei der Anwendung im Spritzen auf Stahl häufig zu ungleichmäßigen Phosphatschichten mit teils grauer, teils grünlich-bläulich irisierender Farbe führen. Diese Ungleichmäßigkeiten können sich im anschließend aufgebrachten Elektrotauchlack markieren und teure Nacharbeiten verursachen. Ferner genügt die Lackhaftung bei Beanspruchung durch z.B. Biegung oder durch längeren Kontakt mit Wasser nicht immer den gestellten Anforderungen.A disadvantage of the known processes with zinc concentrations in the phosphating bath of 0.1 to 0.6 g / l is that when used in spraying on steel they often lead to uneven phosphate layers with partly gray, partly greenish-bluish iridescent color. These irregularities can mark themselves in the subsequently applied electro-dip lacquer and cause expensive rework. Furthermore, paint adhesion is sufficient when subjected to e.g. Bending or prolonged contact with water does not always meet the requirements.
Aufgabe der Erfindung ist es, ein Verfahren bereitzustellen, das die Nachteile der bekannten Verfahren nicht aufweist und gleichmäßige, einheitliche Phosphatschichten mit verbesserter Lackhaftung ausbildet.The object of the invention is to provide a process which does not have the disadvantages of the known processes and which forms uniform, uniform phosphate layers with improved paint adhesion.
Die Aufgabe wird gelöst, indem man das Verfahren der eingangs genannten Art entsprechend der Erfindung derart ausgestaltet, daß die Metalloberflächen mit einer wäßrigen sauren Phosphatierungslösung in Berührung gebracht werden, die
Zwar ist es aus der FR-1 086 955 bekannt, Lösungen zur Erzeugung von Phosphatüberzügen auf Metalloberflächen, die neben Phosphat Oxidationsbeschleuniger und z. B. Zink enthalten, alpha-Aminopolycarbonsäure, wie Ethylendiamintetraacetat oder Nitrilotriacetat, zuzusetzen. Jedoch handelt es sich dabei um hochzinkhaltige Lösungen mit z. B. 2,5 bis 5 g/I Zink. Darüber hinaus dient der Zusatz der Erhöhung des Schichtgewichtes.It is known from FR-1 086 955, solutions for the production of phosphate coatings on metal surfaces, which in addition to phosphate oxidation accelerators and z. B. contain zinc, alpha-aminopolycarboxylic acid such as ethylenediaminetetraacetate or nitrilotriacetate. However, these are high-zinc solutions with z. B. 2.5 to 5 g / l zinc. In addition, the addition serves to increase the layer weight.
Das erfindungsgemäße Verfahren ist insbesondere für die Behandlung von Eisen, Stahl und verzinktem Stahl gedacht. Es eignet sich jedoch außerdem für die Phosphatierung von Zinklegierungen, Aluminium und Aluminiumlegierungen.The method according to the invention is intended in particular for the treatment of iron, steel and galvanized steel. However, it is also suitable for the phosphating of zinc alloys, aluminum and aluminum alloys.
Die Behandlung erfolgt im Spritzverfahren, wobei die Kontaktzeiten für Eisen, Stahl und Aluminium beispielsweise 90 bis 240 sec, die für Zink beispielsweise 5 bis 240 sec betragen.The treatment is carried out by spraying, the contact times for iron, steel and aluminum being 90 to 240 seconds, for example, and zinc 5 to 240 seconds.
Das erfindungsgemäße Verfahren wird meist bei Badtemperaturen zwischen 30 und 60 °C eingesetzt.The method according to the invention is mostly used at bath temperatures between 30 and 60 ° C.
Die Zugabe von Nickel zum Phosphatierbad wirkt sich in der Regel günstig auf die Phosphatiergeschwindigkeit, die Schichtbildung auf schwerer phosphatierbaren Stahloberflächen, auf die Phosphatierung von Zinkoberflächen und auf die Korrosionsschutzeigenschaften aus.The addition of nickel to the phosphating bath generally has a favorable effect on the phosphating speed, the formation of layers on steel surfaces that are difficult to phosphate, on the phosphating of zinc surfaces and on the corrosion protection properties.
Zur Einstellung des Phosphatiergleichgewichtes enthalten die im erfindungsgemäßen Verfahren einzusetzenden Phosphatierungslösungen vorzugsweise Alkaliionen, z. B. Na, K, Li, NH4, und erforderlichenfalls weitere Anionen, z. B. NO3, CI, S04. Die Bäder können ferner weiter in der Phosphatiertechnik bekannte Kationen, wie Co, Cu, Mn, Ca, Mg und Fe, enthalten.To adjust the phosphating balance, the phosphating solutions to be used in the process according to the invention preferably contain alkali ions, e.g. B. Na, K, Li, NH 4 , and if necessary further anions, for. B. NO 3 , CI, S0 4 . The baths can furthermore contain cations known in phosphating technology, such as Co, Cu, Mn, Ca, Mg and Fe.
Als Oxidationsbeschleuniger werden beim erfinsungsgemäßen Verfahren Nitrit und/oder Nitrobenzolsulfonat verwendet. Die Mitverwendung weiterer Oxidationsbeschleuniger, z.B. Clorat, ist möglich und kann vorteilhaft sein.In the process according to the invention, nitrite and / or nitrobenzenesulfonate are used as oxidation accelerators. The use of further oxidation accelerators, e.g. Clorat is possible and can be beneficial.
Eine vorteilhafte Ausgestaltung des erfindungsgemäßen Verfahrens besteht darin, die Metalloberfläche mit einer Phosphatierungslösung in Berührung zu bringen, die mindestens einen weiteren Aktivator aus der Gruppe Fluorid, Hexafluorosilikat, Tetrafluoroborat, Glykolat, Citrat, Tartrat und kondensiertes Phosphat enthält.An advantageous embodiment of the method according to the invention is to bring the metal surface into contact with a phosphating solution which contains at least one further activator from the group consisting of fluoride, hexafluorosilicate, tetrafluoroborate, glycolate, citrate, tartrate and condensed phosphate.
Die beim erfindungsgemäßen Verfahren einzusetzenden Aktivatoren wirken beschleunigend und vergleichsmäßigend auf die Schichtbildung und steuern das Flächengewicht der Phosphatschichten. Die Zugabe kann z.B. über die entsprechenden Säuren oder auch Alkalisalze erfolgen. Für die Aktivatoren gelten folgende vorzugsweise Konzentrationen in der Phosphatierungslösung: 0,5 bis 3 g/I Fluorid; 1 bis 5 g/I Hexafluorosilikat; 3 bis 10 g/I Tetrafluoroborat; 5 bis 15 g/I Formiat; 0,3 bis 5 g/I Glykolat; 0,3 bis 3 g/I Nitrilotriacetat; 2 bis 15 g/I Trichloracetat; 0,1 bis 3 g/I Ethylendiamintetraacetat; 0,01 bis 0,5 g/I Citrat; 0,03 bis 0,8 g/I Tartrat; 0,03 bis 0,3 g/I kondensiertes Phosphat, wie Pyro-, Tripoly-und Hexametaphosphat.The activators to be used in the method according to the invention have an accelerating and comparative effect on the layer formation and control the weight per unit area of the phosphate layers. The addition can e.g. over the corresponding acids or alkali salts. The following preferably concentrations in the phosphating solution apply to the activators: 0.5 to 3 g / l fluoride; 1 to 5 g / l hexafluorosilicate; 3 to 10 g / l tetrafluoroborate; 5 to 15 g / l formate; 0.3 to 5 g / l glycolate; 0.3 to 3 g / l nitrilotriacetate; 2 to 15 g / l trichloroacetate; 0.1 to 3 g / l of ethylenediaminetetraacetate; 0.01 to 0.5 g / l citrate; 0.03 to 0.8 g / l tartrate; 0.03 to 0.3 g / l of condensed phosphate, such as pyro-, tripoly- and hexametaphosphate.
Das Flächengewicht der mit dem erfindungsgemäßen Verfahren auf Stahl erzeugten Phosphatschichten liegt meist zwischen 0,8 und 2,5 g/m2. Um die Ausbildung besonders dünner feinkristalliner Phosphatschichten weiter zu unterstützen, empfiehlt sich die Anwendung von Aktivierungsmitteln, z. B. auf Titanphosphatbasis, im Vorspülbad oder der letzten Reinigerstufe.The weight per unit area of the phosphate layers produced on steel using the method according to the invention is usually between 0.8 and 2.5 g / m 2 . For the formation of particularly thin fine crystalline To further support phosphate layers, the use of activating agents, e.g. B. on titanium phosphate basis, in the pre-rinse bath or the last cleaning stage.
Die mit dem erfindungsgemäßen Verfahren erzeugten Phosphatschichten eignen sich prinzipiell für alle Zwecke, für die Phosphatschichten geeignet sind. In Verbindung mit einer Lackierung, bewirken die Schichten eine ungewöhnlich starke Verbesserung der Beständigkeit des Lackfilmes gegen Lackunterwanderung bei korrosiver Beanspruchung sowie eine erhebliche Erhöhung der Lackhaftung zum metallischen Untergrund bei Kratz-, Schlag-, und Biegebeanspruchung. Diese Vorteile werden besonders bei der Elektrotauchlackierung, insbesondere bei der kathodischen Elektrotauchlackierung, deutlich, weshalb des Verfahren vorzugsweise als Vorbereitung für diese Lackierart angewendet wird. Praktische Anwendung findet das erfindungsmemäße Verfahren z. B. für die Phosphatierung von Autokarosserien.The phosphate layers produced with the method according to the invention are suitable in principle for all purposes for which phosphate layers are suitable. In conjunction with painting, the layers bring about an unusually strong improvement in the resistance of the paint film against paint infiltration under corrosive stress as well as a significant increase in paint adhesion to the metallic surface under scratch, impact, and bending stress. These advantages are particularly evident in electrocoating, in particular in cathodic electrocoating, which is why the method is preferably used as a preparation for this type of painting. The method according to the invention finds practical application, for. B. for the phosphating of car bodies.
Das erfindungsgemäße Verfahren wird anhand der folgenden Beispiele beispielsweise und näher erläutert.The process according to the invention is explained, for example and in more detail, using the following examples.
Mit mildalkalischem, aktivierendem Reiniger im Spritzverfahren entfettete und anschließend mit Wasser gespülte Karosseriestahlbleche wurden mit den nachstehenden Phosphatierungslösungen zwei min bei 55 °C im Spritzen behandelt, danach mit Wasser gespült, mit verdünnter Cr(VI)/Cr(III)-Lösung passivierend nachgespült, mit vollentsalztem Wasser abgebraust und zum Teil getrocknet und zum Teil kathodisch elektrotauchlackiert. Die Phosphatierungslösungen gemäß 1 bis 7 enthielten übereinstimmend
Die Phosphatierungslösungen gemäß 8 bis 11 enthielten
Die Ergebnisse der Phosphatierbehandlung sind in Form der Schichtbeurteilung nachfolgend zusammengestellt.
Die Schichtbeurteilung zeigt an den Beispielen 8 und 11 deutlich die Vorteile der erfindungsgemäßen Arbeitsweise im Vergleich zu den Beispielen 1 bis 6 nach dem Stand der Technik. Aufgrund unterschiedlicher chemischer Oberflächenaktivität von Stahlblechen aus verschiedenen Walzchargen unterliegt der Absolutwert der visuellen Beurteilung gewissen Schwankungen, ohne jedoch die relativen Unterschiede wesentlich zu beeinflussen.Examples 8 and 11 clearly show the layer evaluation of the advantages of the procedure according to the invention in comparison to examples 1 to 6 according to the prior art. Due to the different chemical surface activity of steel sheets from different rolled batches, the absolute value of the visual assessment is subject to certain fluctuations, but without significantly influencing the relative differences.
Auch in nitrithaltigen, chloratfreien Phosphatierungslösungen sowie solchen mit Nitrobenzolsulfonat, gegebenenfalls zusammen mit Nitrit, ergaben sich ähnliche Schichtbeurteilungen.Similar layer assessments were also found in nitrate-containing, chlorate-free phosphating solutions and those with nitrobenzenesulfonate, possibly together with nitrite.
Die gemäß Beispiel 1 bis 6 phosphatierten Stahlbleche führten nach der kathodischen Elektrotauchlackierung zu einem Lackfilm mit unruhiger Oberflächenstruktur, während auf den gemäß Beispiel 7 bis 11 phosphatierten Stahlblechen gleichmäßige Lackfilme abzuscheiden waren.After the cathodic electrocoating, the steel sheets phosphated according to Examples 1 to 6 led to a paint film with an uneven surface structure, while uniform paint films had to be deposited on the steel sheets phosphated according to Examples 7 to 11.
Ein Teil der kathodisch lackierten Bleche wurde mit einem Automobillackaufbau von insgesamt etwa 100 11m versehen und nach verschiedenen Methoden geprüft. Die Ergebnisse der erfindungsgemäß behandelten Bleche hinsichtlich VW-Steinschlag mit Salzsprühtest, Gitterschnitt nach Schwitzwasserbeanspruchung und Biegung über den konischen Dorn sind sehr gut und verhalten sich deutlich besser als die Beispiele 4 und 7 des Standes der Technik.Some of the cathodically coated metal sheets were provided with a total of about 100 11m automotive paint and tested using various methods. The results of the sheets treated according to the invention with regard to VW stone chips with a salt spray test, cross-cut after exposure to condensation and bending over the conical mandrel are very good and behave significantly better than Examples 4 and 7 of the prior art.
Claims (4)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT85200192T ATE37203T1 (en) | 1984-03-09 | 1985-02-15 | PROCESSES FOR THE PHOSPHATION OF METALS. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE3408577 | 1984-03-09 | ||
| DE19843408577 DE3408577A1 (en) | 1984-03-09 | 1984-03-09 | METHOD FOR PHOSPHATING METALS |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0154367A2 EP0154367A2 (en) | 1985-09-11 |
| EP0154367A3 EP0154367A3 (en) | 1986-08-20 |
| EP0154367B1 true EP0154367B1 (en) | 1988-09-14 |
Family
ID=6229954
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP85200192A Expired EP0154367B1 (en) | 1984-03-09 | 1985-02-15 | Process for phosphatizing metals |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US4637838A (en) |
| EP (1) | EP0154367B1 (en) |
| CN (1) | CN85101297A (en) |
| AT (1) | ATE37203T1 (en) |
| AU (1) | AU575380B2 (en) |
| CA (1) | CA1224121A (en) |
| DE (2) | DE3408577A1 (en) |
| ES (1) | ES541015A0 (en) |
| GB (1) | GB2155960A (en) |
| ZA (1) | ZA851761B (en) |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB8527833D0 (en) * | 1985-11-12 | 1985-12-18 | Pyrene Chemicals Services Ltd | Phosphate coating of metals |
| DE3630246A1 (en) * | 1986-09-05 | 1988-03-10 | Metallgesellschaft Ag | METHOD FOR PRODUCING PHOSPHATE COVER AND ITS APPLICATION |
| DE3871031D1 (en) * | 1987-08-19 | 1992-06-17 | Metallgesellschaft Ag | METHOD FOR PHOSPHATING METALS. |
| JPH01100281A (en) * | 1987-10-13 | 1989-04-18 | Nippon Parkerizing Co Ltd | Chemical conversion coating liquid for surface of metal |
| JPH0730455B2 (en) * | 1988-09-27 | 1995-04-05 | 日本パーカライジング株式会社 | Phosphate chemical treatment liquid |
| JP2781844B2 (en) * | 1988-10-20 | 1998-07-30 | 日本ペイント株式会社 | Undercoating agent for painting |
| JP2636919B2 (en) * | 1989-01-26 | 1997-08-06 | 日本パーカライジング株式会社 | Lubrication treatment method for cold plastic working of steel |
| DE3916498A1 (en) * | 1989-05-20 | 1990-11-22 | Kolbenschmidt Ag | METHOD FOR APPLYING A PHOSPHATE RUNNING LAYER TO A BEARING METAL LAYER |
| DE4409306A1 (en) * | 1994-03-18 | 1995-09-21 | Basf Ag | Process for modifying metal surfaces |
| DE4417965A1 (en) * | 1994-05-21 | 1995-11-23 | Henkel Kgaa | Iron phosphating using substituted monocarboxylic acids |
| US6716192B1 (en) * | 1997-09-30 | 2004-04-06 | Charles F. Schroeder | Medical needle having a visibly marked tip |
| US6551417B1 (en) | 2000-09-20 | 2003-04-22 | Ge Betz, Inc. | Tri-cation zinc phosphate conversion coating and process of making the same |
| DE102005047424A1 (en) * | 2005-09-30 | 2007-04-05 | Henkel Kgaa | Phosphating solution used as a pre-treatment for metal surfaces contains zinc irons, phosphate ions, hydrogen peroxide or an equivalent amount of a hydrogen peroxide-splitting substance and aliphatic chelate-forming carboxylic acid |
| CN102839374A (en) * | 2012-09-05 | 2012-12-26 | 业纮企业股份有限公司 | Surface treatment method of carbon steel sphere for ball valve |
| CN103695881B (en) * | 2013-12-19 | 2016-08-24 | 湖南金裕化工有限公司 | Room temperature is without slag fast bonderizing liquor and preparation method thereof |
| CN103668149B (en) * | 2013-12-19 | 2016-08-24 | 湖南金裕化工有限公司 | Ordinary-temp fast bonderizing liquor and preparation method thereof |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE1072055B (en) * | 1952-08-28 | |||
| US2884351A (en) * | 1956-01-25 | 1959-04-28 | Parker Rust Proof Co | Method of cold rolling ferrous strip stock |
| GB865497A (en) * | 1958-10-03 | 1961-04-19 | Pyrene Co Ltd | Improvements relating to the cleaning and phosphate coating of metallic surfaces |
| GB1414484A (en) * | 1972-05-03 | 1975-11-19 | Pyrene Chemical Services Ltd | Treatment of zinc surfaces |
| SU800240A1 (en) * | 1977-03-05 | 1981-01-30 | Научно-Исследовательский Институтхимии Саратовского Государственногоордена Трудового Красного Знамениуниверситета Им.H.Г.Чернышевского | Solution for cadmium parkerizing |
| GB1591039A (en) * | 1977-05-03 | 1981-06-10 | Pyrene Chemical Services Ltd | Processes and compositions for coating metal surfaces |
| JPS5811513B2 (en) * | 1979-02-13 | 1983-03-03 | 日本ペイント株式会社 | How to protect metal surfaces |
| DE2907094A1 (en) * | 1979-02-23 | 1980-09-04 | Metallgesellschaft Ag | PHOSPHATION SOLUTIONS |
| GB2072225B (en) * | 1980-03-21 | 1983-11-02 | Pyrene Chemical Services Ltd | Process and composition for coating metal surfaces |
| DE3016576A1 (en) * | 1980-04-30 | 1981-11-05 | Metallgesellschaft Ag, 6000 Frankfurt | METHOD FOR PHOSPHATING METAL SURFACES AND THE USE THEREOF |
| DE3118375A1 (en) * | 1981-05-09 | 1982-11-25 | Metallgesellschaft Ag, 6000 Frankfurt | METHOD FOR PHOSPHATING METALS AND ITS APPLICATION FOR PRE-TREATMENT FOR ELECTRO DIP PAINTING |
| US4498935A (en) * | 1981-07-13 | 1985-02-12 | Parker Chemical Company | Zinc phosphate conversion coating composition |
| US4486241A (en) * | 1981-09-17 | 1984-12-04 | Amchem Products, Inc. | Composition and process for treating steel |
| JPS58144477A (en) * | 1982-02-20 | 1983-08-27 | Nippon Paint Co Ltd | Phosphating process of metal surface |
| DE3311738A1 (en) * | 1983-03-31 | 1984-10-04 | Metallgesellschaft Ag, 6000 Frankfurt | METHOD FOR PHOSPHATING METAL SURFACES |
-
1984
- 1984-03-09 DE DE19843408577 patent/DE3408577A1/en not_active Withdrawn
-
1985
- 1985-02-15 AT AT85200192T patent/ATE37203T1/en not_active IP Right Cessation
- 1985-02-15 EP EP85200192A patent/EP0154367B1/en not_active Expired
- 1985-02-15 DE DE8585200192T patent/DE3564967D1/en not_active Expired
- 1985-03-05 US US06/708,463 patent/US4637838A/en not_active Expired - Fee Related
- 1985-03-06 AU AU39574/85A patent/AU575380B2/en not_active Ceased
- 1985-03-07 CA CA000475958A patent/CA1224121A/en not_active Expired
- 1985-03-07 ES ES541015A patent/ES541015A0/en active Granted
- 1985-03-08 GB GB08506049A patent/GB2155960A/en not_active Withdrawn
- 1985-03-08 ZA ZA851761A patent/ZA851761B/en unknown
- 1985-04-01 CN CN198585101297A patent/CN85101297A/en active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| ATE37203T1 (en) | 1988-09-15 |
| DE3564967D1 (en) | 1988-10-20 |
| EP0154367A3 (en) | 1986-08-20 |
| ES8602152A1 (en) | 1985-12-01 |
| ZA851761B (en) | 1985-11-27 |
| CN85101297A (en) | 1987-01-24 |
| AU575380B2 (en) | 1988-07-28 |
| ES541015A0 (en) | 1985-12-01 |
| GB8506049D0 (en) | 1985-04-11 |
| DE3408577A1 (en) | 1985-09-12 |
| GB2155960A (en) | 1985-10-02 |
| EP0154367A2 (en) | 1985-09-11 |
| CA1224121A (en) | 1987-07-14 |
| US4637838A (en) | 1987-01-20 |
| AU3957485A (en) | 1985-09-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE3118375C2 (en) | ||
| DE69737195T2 (en) | Solution and method for the production of protective layers on metals | |
| EP0154367B1 (en) | Process for phosphatizing metals | |
| EP0056881B1 (en) | Method of phosphating metals | |
| EP0796356B1 (en) | Method of applying phosphate coatings to metal surfaces | |
| EP1200641A1 (en) | Pretreatment of aluminum surfaces with chrome-free solutions | |
| EP0015021B1 (en) | Process for the pretreatment of metal surfaces for electrophoretic dip painting | |
| EP0578670B1 (en) | Process for phosphatizing metallic surfaces | |
| DE1955002C3 (en) | Process for applying zinc phosphate conversion coatings to metal surfaces | |
| EP0261704A1 (en) | Process for producing phosphate coatings on metal surfaces | |
| DE3932006A1 (en) | METHOD FOR APPLYING PHOSPHATE | |
| EP0410497B1 (en) | Process for the passivate rinsing of phosphate coatings | |
| DE2232067C3 (en) | Process for preparing metal surfaces for electrophoretic dip painting | |
| DE2406411A1 (en) | PROCESS FOR INCREASING THE CORROSION RESISTANCE OF METALS | |
| EP0359296A1 (en) | Phosphating process | |
| DE3245411C2 (en) | ||
| EP0039093B1 (en) | Method of phosphating the surfaces of metals, and its use | |
| EP0662164B1 (en) | Process for phosphating galvanised steel surfaces | |
| WO2020064548A1 (en) | Method for improving the phosphatability of metal surfaces which are provided with a temporary pre- or post-treatment | |
| DE3226239A1 (en) | DOUBLE-LAYER ELECTROPLATED STEEL SHEET WITH CORROSION RESISTANCE AFTER PAINTING AND A GOOD WETNESS OF THE COATING FILM | |
| DE3734596A1 (en) | METHOD FOR PRODUCING PHOSPHATO | |
| EP0096753B1 (en) | Process for the electroless production of corrosion-inhibiting layers on structural parts of aluminium | |
| EP0127204B1 (en) | Phosphating process for composite metals | |
| EP0477204A1 (en) | Process for producing manganese-containing phosphate coatings on metal surfaces | |
| DE1521879B2 (en) | Process for applying phosphate coatings to iron and steel |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR IT LI NL SE |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): AT BE CH DE FR IT LI NL SE |
|
| 17P | Request for examination filed |
Effective date: 19861008 |
|
| 17Q | First examination report despatched |
Effective date: 19871030 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE FR IT LI NL SE |
|
| REF | Corresponds to: |
Ref document number: 37203 Country of ref document: AT Date of ref document: 19880915 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 3564967 Country of ref document: DE Date of ref document: 19881020 |
|
| ET | Fr: translation filed | ||
| ITF | It: translation for a ep patent filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19890228 Year of fee payment: 5 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Effective date: 19900901 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee | ||
| ITTA | It: last paid annual fee | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19910313 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 19911213 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19911217 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19920115 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19920226 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19920323 Year of fee payment: 8 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19921103 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Effective date: 19930215 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Effective date: 19930216 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Effective date: 19930228 Ref country code: CH Effective date: 19930228 Ref country code: BE Effective date: 19930228 |
|
| BERE | Be: lapsed |
Owner name: SOC. CONTINENTALE PARKER Effective date: 19930228 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Effective date: 19931029 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| EUG | Se: european patent has lapsed |
Ref document number: 85200192.4 Effective date: 19930912 |