EP0056109B1 - Use of 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylic-acid methyl ester as a perfuming agent - Google Patents
Use of 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylic-acid methyl ester as a perfuming agent Download PDFInfo
- Publication number
- EP0056109B1 EP0056109B1 EP81109812A EP81109812A EP0056109B1 EP 0056109 B1 EP0056109 B1 EP 0056109B1 EP 81109812 A EP81109812 A EP 81109812A EP 81109812 A EP81109812 A EP 81109812A EP 0056109 B1 EP0056109 B1 EP 0056109B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- cyclohex
- trimethyl
- damascone
- ene
- methyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/50—Perfumes
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11B—PRODUCING, e.g. BY PRESSING RAW MATERIALS OR BY EXTRACTION FROM WASTE MATERIALS, REFINING OR PRESERVING FATS, FATTY SUBSTANCES, e.g. LANOLIN, FATTY OILS OR WAXES; ESSENTIAL OILS; PERFUMES
- C11B9/00—Essential oils; Perfumes
- C11B9/0026—Essential oils; Perfumes compounds containing an alicyclic ring not condensed with another ring
- C11B9/0034—Essential oils; Perfumes compounds containing an alicyclic ring not condensed with another ring the ring containing six carbon atoms
Definitions
- the invention relates to a new fragrance ingredient, in the specific case to an alicyclic ester of formula or methyl 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylate, also known as methyl a-cyclogeraniate.
- the subject of the invention is the use of said compound (I) as a perfuming ingredient, in particular its use in the pure state or as a mixture with at least one perfuming ingredient below: a-damascone, ⁇ -damascone, y-damascone, ⁇ -damascone, s-damascone, a-damascenone, ⁇ -damascenone, y-damascenone and 8-damascenone
- the subject of the invention is also a perfume composition containing said compound (1), in particular a perfume composition containing said compound (1) pure or in mixture with at least one of the perfume ingredients defined above.
- Methyl 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylate (1) is a known compound [see for example Helv. Chim. Acta 42, 2597 (1959)]; its olfactory properties have not been described so far, however.
- Compound (I) indeed develops an original olfactory note that can be described as both fruity and floral, accompanied by a fresh tone of the citrus genus. This fact is all the more remarkable since these olfactory characters only develop fully when the compound (I) is used in diluted form, in particular in the form of a 10, 5 or 1% solution, or even less.
- the compound (I) can be advantageously used for perfuming products such as soaps, liquid or solid detergents, cosmetic products or cleaning products for example. It can also be used just as advantageously in fine perfumery, in particular for the preparation of perfuming compositions of the fruity, floral, rosé, woody, spicy, chypre or citrus type, for example.
- the compound (I) can be used pure or in mixture with one or more perfuming ingredients, a solvent or a usual support.
- the compound (I) is preferably used in an amount of 0.1 to 5, or even 10% approximately relative to the weight of said composition. Proportions greater than 10% can also be considered, in particular when preparing bases or hearts for perfumes. Proportions less than 0.1% can also be envisaged, in particular when perfuming products such as soaps, detergents or cosmetic products for example.
- methyl 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylate (I) could have a particularly advantageous action in terms of the effects olfactory agents of certain perfume ingredients, in particular those of the series of damascones and damascenones.
- Damascones and damascenones are very popular ingredients both in fine perfumery and during the perfuming of technical products: they are characterized in particular by a remarkably radiant rosy and woody olfactory note, even fruity like apple, or mint depending on the case.
- compound (I) has, among other things, the advantage of combining in a very harmonious manner with said damascones and damascenones, contributing to give them even more richness and roundness.
- Compound (I) also has the particularity of significantly lowering the level of olfactory perception of the aforementioned ingredients: we can speak in this connection of synergistic effect.
- methyl 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylate (I) is used in mixture with one or more of said damascones or damascenones, olfactory effects such as those mentioned above can be obtained by using mixtures of compound (I) / damascone or damascenone in which the proportions of each of the constituents are most generally between 20: 1 and 1:20.
- the proportions given above are not, however, to be considered as absolute limits.
- Methyl 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxalate (I) can be easily obtained according to the methods described in the literature, for example by esterification of the corresponding cyclogeranic acid or by cyclization of methyl geraniate [see for example Helv. Chim. Acta 42, 2597 (1959) and Chem. Abstr. 57, 11239; (1962)].
- a basic perfuming composition was prepared as follows:
- the basic composition thus obtained has a relatively ill-defined floral-green odor.
- the new perfuming composition thus obtained is particularly suitable for perfuming products such as soaps or shampoos
- a basic "rose” type perfuming composition was prepared as follows:
- the base thus prepared has a "red rose” odor.
- a new perfuming composition is obtained, the odor of which has become fresher and more promising. and more elegant than that of said base; it can now be defined as "tea rose”.
- a basic "rose” type perfuming composition was prepared as follows:
- the base has a 'pink' type odor. This odor becomes clearly more characteristic after addition to said base of 15 parts of a 0.01% solution of ⁇ -damascenone in ethyl alcohol.
- talc 100 g was perfumed at 0.15% using a 50:50 mixture of p-damascone and 2,6,6-trimethyl methyl thyl-cyclohex-2-ene-1-yl-carboxylate. This gives a powder giving off a particularly pleasant rosé, fruity and fresh odor.
- a concentrated liquid detergent was perfumed at 0.15% using a 1:10 mixture of ⁇ -damascenone and 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylate. It now develops a particularly pleasant fruity and fresh rosé-like odor.
Description
L'invention se rapporte à un nouvel ingrédient parfumant, dans le cas précis à un ester alicyclique de formule
L'invention a pour objet l'utilisation dudit composé (I) à titre d'ingrédient parfumant, notamment son utilisation à l'etat pur ou en mélange avec au moins un ingrédient parfumant ci-après: a-damascone, β-damascone, y-damascone, δ-damascone, s-damascone, a-damascenone, β-damascenone, y-damascenone et 8-damascenoneThe subject of the invention is the use of said compound (I) as a perfuming ingredient, in particular its use in the pure state or as a mixture with at least one perfuming ingredient below: a-damascone, β-damascone, y-damascone, δ-damascone, s-damascone, a-damascenone, β-damascenone, y-damascenone and 8-damascenone
L'invention a également pour objet une composition parfumante contenant ledit composé (1), notamment une composition parfumante contenant ledit composé (1) pur ou en melange avec aumoins un des ingrédients parfumants définis plus haut.The subject of the invention is also a perfume composition containing said compound (1), in particular a perfume composition containing said compound (1) pure or in mixture with at least one of the perfume ingredients defined above.
Le 2,6,6-triméthyl-cyclohex-2-éne-1-yl-carboxylate de méthyle (1) est un composé connu [voir par exemple Helv. Chim. Acta 42, 2597 (1959)]; ses propriétés olfactives n'ont cependant pas été décrites jusqu'à maintenant.Methyl 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylate (1) is a known compound [see for example Helv. Chim. Acta 42, 2597 (1959)]; its olfactory properties have not been described so far, however.
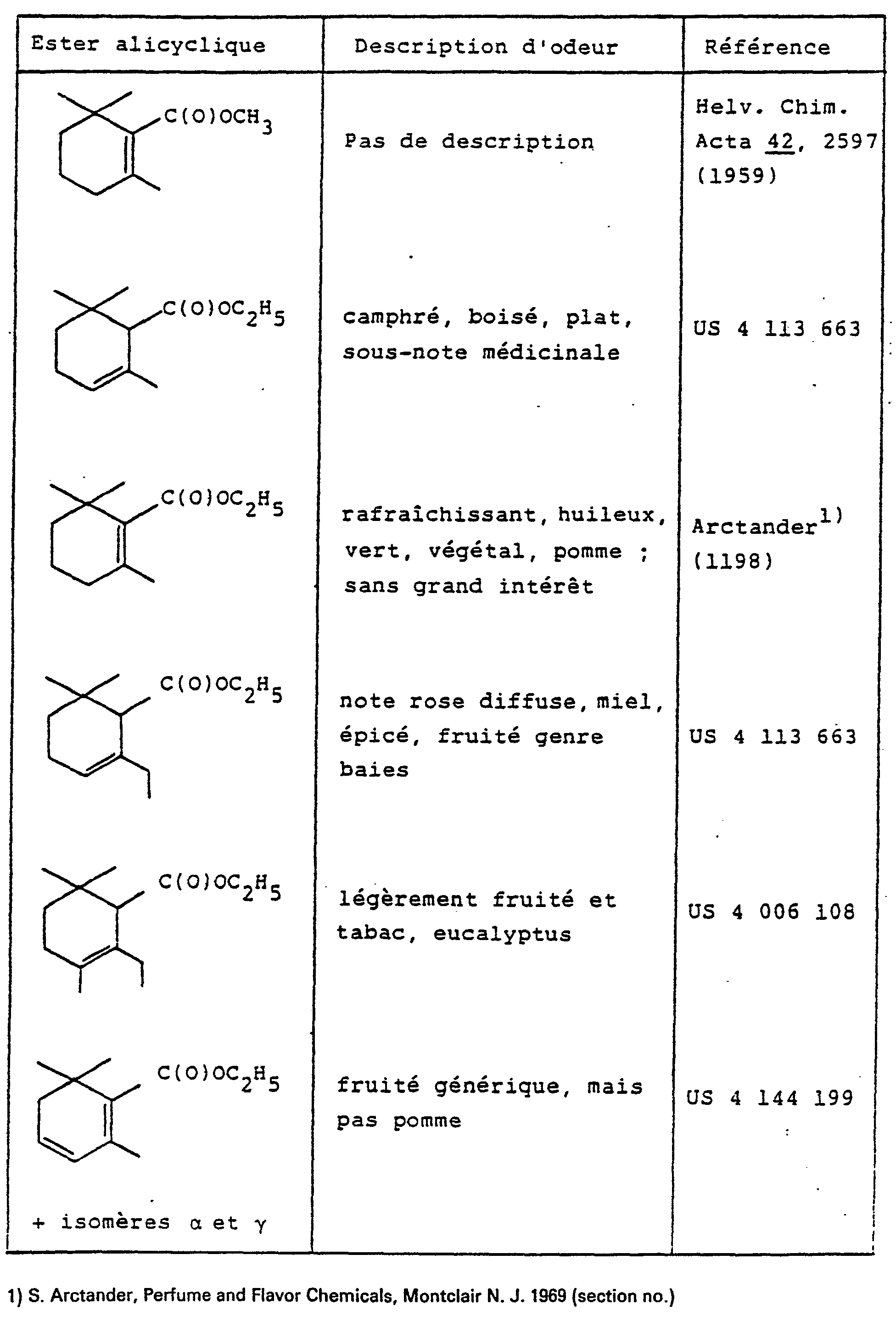
Lorsque l'on considère les esters alicycliques analogues ou homologues du composé (I), on parvient à définir l'état présent de la technique au moyen du tableu ci-après:
De l'état de la technique précité, on peut aisément déduire que seuls présentent un intérêt pour la parfumerie les esters homologues supérieurs du 2,6,6-triméthyl-cyclohex-2-ène-1-yl-carboxylate de méthyle (I), notamment les esters éthyliques comportant un groupe éthyle en position 2 du cycle à 6 membres, voire un groupe méthyle additionnel en position 3, ainsi que des mélanges isomériques de composés polyinsaturés correspondants, a-, β-et y-safranates d'éthyle plus précisément.From the aforementioned state of the art, it can easily be deduced that only the higher homologous esters of methyl 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylate are of interest for perfumery (I) , in particular ethyl esters comprising an ethyl group in position 2 of the 6-membered ring, or even an additional methyl group in position 3, as well as isomeric mixtures of corresponding polyunsaturated compounds, ethyl a-, β- and y-safranates plus precisely.
Les composés homologues inférieurs de la série définie ci-dessus, notamment le composé (I) et son isomère, le β-cyclogéraniate de méthyle, n'ont par contre jamais retenu l'attention des parfumeurs.The lower homologous compounds of the series defined above, in particular the compound (I) and its isomer, methyl β-cyclogeraniate, on the other hand have never caught the attention of perfumers.
Dans un tel contexte, il était donc d'autant plus surprenant de découvrir que le 2,6,6-triméthyl-cyclohex-2-ène-1-yl-carboxylate de méthyle (I) pouvait présenter un grand intérêt pour la parfumerie.In such a context, it was therefore all the more surprising to discover that methyl 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylate (I) could be of great interest for perfumery.
Le composé (I) développe en effet une note olfactive originale que l'on peut qualifier de tout à la fois fruitée et florale, accompagnée d'une tonalité fraîche du genre hespéridé. Ce fait est d'autant plus remarquable que ces caractères olfactifs ne se développent pleinement que lorsque le composé (I) est utilisé sous forme diluée, en particulier sous forme de solution à 10, 5 ou 1%, voire moins.Compound (I) indeed develops an original olfactory note that can be described as both fruity and floral, accompanied by a fresh tone of the citrus genus. This fact is all the more remarkable since these olfactory characters only develop fully when the compound (I) is used in diluted form, in particular in the form of a 10, 5 or 1% solution, or even less.
De ce fait, le composé (I) peut être avantageusement utilisé pour le parfumage de produits tels que savons, détergents liquides ou solides, produits cosmétiques ou produits d'entretien par exemple. Il peut être en outre utilisé tout aussi avantageusement en parfumerie fine, notamment pour la préparation de compositions parfumantes de type fruité, fleuri, rosé, boisé, épicé, chypre ou hespéridé par exemple.Therefore, the compound (I) can be advantageously used for perfuming products such as soaps, liquid or solid detergents, cosmetic products or cleaning products for example. It can also be used just as advantageously in fine perfumery, in particular for the preparation of perfuming compositions of the fruity, floral, rosé, woody, spicy, chypre or citrus type, for example.
Dans un tel cas, le composé (I) peut s'utiliser pur ou en mélange avec un ou plusieurs ingrédients parfumants, un solvant ou un support usuel. Lors de la préparation de compositions parfumantes, le composé (I) s'utilise de préférence à raison de 0,1 à 5, voire 10% environ par rapport au poids de ladite composition. Des proportions supérieures à 10% peuvent être également considérées, en particulier lors de la préparation de bases ou coeurs pour parfums. Des proportions inférieures à 0,1 % peuvent être également envisagées, notamment lors du parfumage de produits tels que savons, détergents ou produits cosmétiques par exemple.In such a case, the compound (I) can be used pure or in mixture with one or more perfuming ingredients, a solvent or a usual support. When preparing perfuming compositions, the compound (I) is preferably used in an amount of 0.1 to 5, or even 10% approximately relative to the weight of said composition. Proportions greater than 10% can also be considered, in particular when preparing bases or hearts for perfumes. Proportions less than 0.1% can also be envisaged, in particular when perfuming products such as soaps, detergents or cosmetic products for example.
Dans le cadre de la présente invention, il a été découvert en outre que le 2,6,6-triméthyl-cyclohex-2-ène-1-yl- carboxylate de méthyle (I) pouvait avoir une action particulièrement intéressante au niveau des effets olfactifs de certains ingrédients parfumants, notamment ceux de la série des damascones et damascénones. Damascones et damascénones sont des ingrédients très appréciés tant en parfumerie fine que lors du parfumage de produits techniques: ils se caractérisent notamment par une note olfactive rosée et boisée remarquablement radiante, voire fruitée de type pomme, ou menthée selon les cas.In the context of the present invention, it has also been discovered that methyl 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylate (I) could have a particularly advantageous action in terms of the effects olfactory agents of certain perfume ingredients, in particular those of the series of damascones and damascenones. Damascones and damascenones are very popular ingredients both in fine perfumery and during the perfuming of technical products: they are characterized in particular by a remarkably radiant rosy and woody olfactory note, even fruity like apple, or mint depending on the case.
Le tableau ci-après fait l'inventaire des représentants de cette importante série que l'on peut mettre à la disposition de l'industrie des parfums
Du point de vue olfactif, le composé (I) a entre autres l'avantage de se combiner de façon très harmonieuse auxdites damascones et damascénones, contribuant à leur donner encore plus de richesse et de rondeur. Le composé (I) a en outre la particularité d'abaisser sensiblement le niveau de perception olfactive des ingrédients sus-nommés: on peut parler à ce propos d'effet synergétique.From an olfactory point of view, compound (I) has, among other things, the advantage of combining in a very harmonious manner with said damascones and damascenones, contributing to give them even more richness and roundness. Compound (I) also has the particularity of significantly lowering the level of olfactory perception of the aforementioned ingredients: we can speak in this connection of synergistic effect.
Lorsque le 2,6,6-triméthyl-cyclohex-2-ène-1-yl-carboxylate de méthyle (I) s'utilise en mélange avec une ou plusieurs desdites damascones ou damascénones, des effet olfactifs tels que ceux mentionnés ci-dessus peuvent être obtenus par l'emploi de mélanges composé (I)/damascone ou damascénone dans lesquels les proportions de chacun des constituants sont le plus généralement comprises entre 20:1 et 1:20. Les proportions données ci-dessus ne sont cependant pas à considérer comme des limites absolues.When methyl 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylate (I) is used in mixture with one or more of said damascones or damascenones, olfactory effects such as those mentioned above can be obtained by using mixtures of compound (I) / damascone or damascenone in which the proportions of each of the constituents are most generally between 20: 1 and 1:20. The proportions given above are not, however, to be considered as absolute limits.
Le 2,6,6-triméthyl-cyclohex-2-ène-1-yl-carboxalate de méthyle (I) peut être aisément obtenu selon les méthodes décrites dans la littérature, par exemple par estérification de l'acide cyclogéranique correspondant ou par cyclisation de géraniate de méthyle [voir par exemple Helv. Chim. Acta 42, 2597 (1959) et Chem. Abstr. 57, 11239; (1962)].Methyl 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxalate (I) can be easily obtained according to the methods described in the literature, for example by esterification of the corresponding cyclogeranic acid or by cyclization of methyl geraniate [see for example Helv. Chim. Acta 42, 2597 (1959) and Chem. Abstr. 57, 11239; (1962)].
L'invention sera illustrée de façon plus détaillée à l'aide des exemples ci-après.The invention will be illustrated in more detail with the aid of the examples below.
Un détergent en poudre du commerce, olfactivement neutre, a été parfumé à raison de 0,05% à l'aide de 2,6,6-triméthyl-cyclohex-2-ène-1-yl-carboxylate de méthyle. Il acquiert ainsi une odeur agréable de type fruité, rappelant notamment celle de fruits mûrs.A commercially available powdered detergent, olfactory neutral, was scented at 0.05% using methyl 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylate. It thus acquires a pleasant fruity type smell, reminiscent of that of ripe fruit.
On a préparé une composition parfumante de base comme suit:
La composition de base ainsi obtenue possède une odeur de type fleuri-vert relativement peu définie. En ajoutant à 92 parties de ladite base 8 parties de 2,6,6- trimethyl-cyclohex-2-ène-1-yl-carboxylate de méthyle, on confère à celle-ci une note de type "pomme verte" très caractéristique.The basic composition thus obtained has a relatively ill-defined floral-green odor. By adding to 92 parts of said base 8 parts of methyl 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylate, this is given a very characteristic "green apple" type note.
La nouvelle composition parfumante ainsi obtenue convient particulièrement bien au parfumage de produits tels que savons ou shampoingsThe new perfuming composition thus obtained is particularly suitable for perfuming products such as soaps or shampoos
On a préparé une composition parfumante de base de type "rose" comme suit:
La base ainsi préparée possède une odeur de type "rose rouge". En ajoutant à 98 parties de ladite base 2 parties de 2,6,6-triméthyl-cyclohex-2-ène-1-yt-carboxylate de méthyle, on obtient une nouvelle composition parfumante dont l'odeur est devenue plus fraîche, plus montante et plus élégante que celle de ladite base; elle peut être maintenant définie de type "rose thé".The base thus prepared has a "red rose" odor. By adding 2 parts of methyl 2,6,6-trimethyl-cyclohex-2-ene-1-yt-carboxylate to 98 parts of said base, a new perfuming composition is obtained, the odor of which has become fresher and more promising. and more elegant than that of said base; it can now be defined as "tea rose".
On a préparé une composition parfumante de base de type "rose" comme suit:
Ainsi constituée, la base présente une odeur de type 'rose'. Cette odeur devient nettement plus caractéristique après addition à ladite base de 15 parties d'une solution à 0,01% de β-damascénone dans l'alcool éthylique.Thus formed, the base has a 'pink' type odor. This odor becomes clearly more characteristic after addition to said base of 15 parts of a 0.01% solution of β-damascenone in ethyl alcohol.
L'addition de 30 parties d'une solution de 2,6,6-triméthyl-cyclohex-2-ène-1-yl-carboxylate de méthyle à 0,1% dans l'alcool éthylique à la nouvelle composition ci-dessus confère à cette dernière une note de tête rosée et fruitée encore plus riche et plus montante.The addition of 30 parts of a solution of 2,6,6-trimethyl-cyclohex-2-ene-1-yl-methyl-carboxylate at 0.1% in ethyl alcohol to the new composition above gives to the latter a rosé and fruity top note even richer and more rising.
A 688 parties de la base parfumante préparée selon l'Exemple 4 on a ajouté 15 parties d'une solution à 0,01% de β-damascone dans l'alcool éthylique, pour obtenir une composition de type "rose" très caractéristique, à l'odeur fraîche et montanteTo 688 parts of the perfume base prepared according to Example 4, 15 parts of a 0.01% solution of β-damascone in ethyl alcohol were added, to obtain a very characteristic "pink" type composition, the fresh and rising smell
L'addition de 30 parties d'une solution de 2,6,6-triméthyl-cyclohex-2-ène-1-yl-carboxylate de méthyle à 0,1% dans l'alcool éthylique à la composition ci-dessus renforce encore le côté frais, fruité et montant typique de la β-damasconeThe addition of 30 parts of a solution of 2,6,6-trimethyl-cyclohex-2-ene-1-yl-methyl carboxylate at 0.1% in ethyl alcohol to the above composition further strengthens the fresh, fruity and typical amount of β-damascone
100 g de talc ont été parfumés à raison de 0,15% au moyen d'un mélange 50:50 de p-damascone et 2,6,6-trimé- thyl-cyclohex-2-ène-1-yl-carboxylate de méthyle. On obtien ainsi une poudre dégageant une odeur de type rosé, fruité et frais particulièrement plaisante.100 g of talc was perfumed at 0.15% using a 50:50 mixture of p-damascone and 2,6,6-trimethyl methyl thyl-cyclohex-2-ene-1-yl-carboxylate. This gives a powder giving off a particularly pleasant rosé, fruity and fresh odor.
Des effets olfactifs comparables, et tout aussi plaisants, ont été obtenus par l'emploi de mélange 50:50 d'a-damascone et 2,6,6-triméthyl-cyclohex-2-ène-1-yl-carboxylate de méthyle, respectivement de β-damascénone et 2,6,6-triméthyl-cyclohex-2-éne-1-yl-carboxylate de méthyleComparable and equally pleasant olfactory effects have been obtained by using a 50:50 mixture of a-damascone and methyl 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylate, respectively of methyl β-damascenone and 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylate
Un détergent liquide concentré a été parfumé à raison de 0,15% au moyen d'un mélange 1:10 de β-damascénone et 2,6,6-triméthyl-cyclohex-2-ène-1-yl-carboxylate de méthyle. Il développe désormais une odeur de type rosé fruité et frais particulièrement plaisante.A concentrated liquid detergent was perfumed at 0.15% using a 1:10 mixture of β-damascenone and 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylate. It now develops a particularly pleasant fruity and fresh rosé-like odor.
Des effets olfactifs comparables, et tout aussi plaisants, ont été obtenuspar l'emploi de mélanges 1:10 d'adamascone et 2,6,6-triméthyl-cyclohex-2-ène-1-yl-carboxylate de méthyle, respectivement de β-damascone et 2,6,6 triméthyl-cyclohex-2-ène-1-yl-carboxylate de méthyle.Comparable and equally pleasant olfactory effects were obtained by the use of 1:10 mixtures of adamascone and methyl 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylate, respectively of β -damascone and 2,6,6 trimethyl-cyclohex-2-ene-1-yl-methyl carboxylate.
Claims (4)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CH17781 | 1981-01-13 | ||
| CH177/81 | 1981-01-13 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0056109A2 EP0056109A2 (en) | 1982-07-21 |
| EP0056109A3 EP0056109A3 (en) | 1983-08-31 |
| EP0056109B1 true EP0056109B1 (en) | 1986-01-15 |
Family
ID=4181015
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP81109812A Expired EP0056109B1 (en) | 1981-01-13 | 1981-11-21 | Use of 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylic-acid methyl ester as a perfuming agent |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US4411829A (en) |
| EP (1) | EP0056109B1 (en) |
| JP (1) | JPS57139010A (en) |
| DE (1) | DE3173535D1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7196050B2 (en) | 2001-12-05 | 2007-03-27 | Firmenich Sa | Unsaturated ester as perfuming ingredient |
Families Citing this family (56)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE68916634T2 (en) * | 1989-01-18 | 1994-11-03 | Firmenich & Cie | Alicyclic esters and their use as fragrance components. |
| JP2840899B2 (en) * | 1991-03-26 | 1998-12-24 | 高砂香料工業株式会社 | Perfume composition containing optically active ethyl (1R, 6S) -2,2,6-trimethylcyclohexanecarboxylate and method for producing active ingredient thereof |
| EP0593917A1 (en) * | 1992-10-13 | 1994-04-27 | Firmenich Sa | Process for the preparation of optically active esters and thioesters |
| BR9307615A (en) * | 1992-12-11 | 1999-06-15 | Quest Int | Perfume scented products and process to prepare them |
| US6123935A (en) * | 1997-04-14 | 2000-09-26 | S. C. Johnson & Son, Inc. | Air freshener dispenser device with disposable heat-activated cartridge |
| US5903710A (en) * | 1997-04-14 | 1999-05-11 | S. C. Johnson & Son, Inc. | Air freshener dispenser device with disposable heat-promoted cartridge |
| US5945094A (en) * | 1997-04-14 | 1999-08-31 | S. C. Johnson & Son, Inc. | Disposable plug-in dispenser for use with air freshener and the like |
| US5976503A (en) * | 1997-04-14 | 1999-11-02 | S. C. Johnson & Son, Inc. | Disposable plug-in air freshener with heat activated cartridge |
| US5875968A (en) * | 1997-07-18 | 1999-03-02 | S. C. Johnson & Son, Inc. | Liquid air freshener dispenser device with nonporous capillary wicking function |
| US6019804A (en) * | 1997-11-25 | 2000-02-01 | S. C. Johnson & Son, Inc. | Compression-molded candle product |
| US6284007B1 (en) | 1998-08-12 | 2001-09-04 | Indiana Soybean Board, Inc. | Vegetable lipid-based composition and candle |
| US6569387B1 (en) | 1999-08-10 | 2003-05-27 | S.C. Johnson & Son, Inc. | Dual function dispenser |
| US6645261B2 (en) | 2000-03-06 | 2003-11-11 | Cargill, Inc. | Triacylglycerol-based alternative to paraffin wax |
| US6824572B2 (en) * | 2001-03-06 | 2004-11-30 | Cargill, Incorporated | Vegetable oil based wax compositions |
| US6503285B1 (en) * | 2001-05-11 | 2003-01-07 | Cargill, Inc. | Triacylglycerol based candle wax |
| US7128766B2 (en) | 2001-09-25 | 2006-10-31 | Cargill, Incorporated | Triacylglycerol based wax compositions |
| US6773469B2 (en) * | 2002-11-12 | 2004-08-10 | Cargill, Incorporated | Triacylglycerol based wax for use in candles |
| US6797020B2 (en) * | 2002-11-12 | 2004-09-28 | Cargill, Incorporated | Triacylglycerol based wax for use in container candles |
| BRPI0407009A (en) * | 2003-01-28 | 2006-01-10 | Ecosmart Technologies Inc | Herbicidal Compositions and Methods for Killing Weeds and Grasses |
| US7192457B2 (en) | 2003-05-08 | 2007-03-20 | Cargill, Incorporated | Wax and wax-based products |
| US7607250B2 (en) * | 2004-06-30 | 2009-10-27 | S.C. Johnson & Son, Inc. | Air freshener with picture frame |
| US7426799B2 (en) | 2004-06-30 | 2008-09-23 | S.C. Johnson & Son, Inc. | Air freshener with frame and refill holder |
| US7441360B2 (en) | 2004-06-30 | 2008-10-28 | S.C. Johnson & Son, Inc. | Air freshener with picture frame |
| ATE473264T1 (en) * | 2004-09-14 | 2010-07-15 | Firmenich & Cie | PERFUME INGREDIENTS WITH SAFFRON SMELL |
| US7510584B2 (en) * | 2004-10-13 | 2009-03-31 | Daniel S. Cap | Acetylated wax compositions and articles containing them |
| JP2008527110A (en) | 2005-01-10 | 2008-07-24 | カーギル,インコーポレイティド | Candles and candle waxes containing metathesis and metathesis-like products |
| US7588607B1 (en) | 2005-03-16 | 2009-09-15 | Daniel S. Cap | Candlewax compositions with improved scent-throw |
| US20060265233A1 (en) * | 2005-05-20 | 2006-11-23 | United Parcel Service Of America, Inc. | Systems and methods for facilitating stock product returns |
| US20060272199A1 (en) * | 2005-06-02 | 2006-12-07 | Bmc Manufacturing, Llc | Aqueous gel candle for use with a warming device |
| US20070006521A1 (en) * | 2005-07-11 | 2007-01-11 | Bmc Manufacturing,Llc | Multi-phase candle |
| US7416766B2 (en) * | 2005-08-16 | 2008-08-26 | S.C. Johnson & Son, Inc. | Bottles made from metallocene polypropylene for delivery of fragrances |
| US8192723B2 (en) * | 2006-03-31 | 2012-06-05 | Reckitt Benckiser (Uk) Limited | Aerosol composition |
| US7523577B2 (en) | 2006-04-03 | 2009-04-28 | S.C. Johnson & Son, Inc. | Air freshener with holder |
| US7665238B2 (en) | 2006-04-03 | 2010-02-23 | S.C. Johnson & Son, Inc. | Air freshener with holder |
| CN101563434B (en) | 2006-07-12 | 2012-01-25 | 埃莱文斯可更新科学公司 | Hot melt adhesive compositions comprising metathesized unsaturated polyol ester wax |
| EP2121846B1 (en) | 2007-02-16 | 2011-10-26 | Elevance Renewable Sciences, Inc. | Wax compositions and methods of preparing wax compositions |
| WO2008151064A1 (en) | 2007-05-30 | 2008-12-11 | Elevance Renewable Sciences, Inc. | Prilled waxes comprising small particles and smooth-sided compression candles made therefrom |
| WO2008157436A1 (en) | 2007-06-15 | 2008-12-24 | Elevance Renewable Sciences, Inc. | Hybrid wax compositions for use in compression molded wax articles such as candles |
| US20090212124A1 (en) * | 2007-09-12 | 2009-08-27 | Kevin Brian Kenny | Apparatus and method for dispensing a volatile composition |
| WO2009151726A1 (en) * | 2008-03-28 | 2009-12-17 | Securitypoint Holdings Llc | Methods and systems for efficient security screening |
| US9116513B2 (en) | 2008-03-28 | 2015-08-25 | Securitypoint Holdings, Inc. | Methods and systems for efficient security screening |
| US9516460B2 (en) | 2008-03-28 | 2016-12-06 | Securitypoint Holdings Llc | Systems and methods for security checkpoint condition information and sharing |
| US8500826B2 (en) | 2010-03-10 | 2013-08-06 | Elevance Renewable Sciences, Inc. | Lipid-based wax compositions substantially free of fat bloom and methods of making |
| CN102884143B (en) | 2010-05-12 | 2016-05-04 | 艾勒旺斯可再生科学公司 | The marking composition of natural oil base and manufacture method thereof |
| EP2590911B1 (en) | 2010-07-09 | 2014-05-14 | Elevance Renewable Sciences, Inc. | Waxes derived from metathesized natural oils and amines and methods of making |
| WO2012071306A1 (en) | 2010-11-23 | 2012-05-31 | Elevance Renewable Sciences, Inc. | Lipid-based wax compositions substantially free of fat bloom and methods of making |
| CA2841137A1 (en) | 2011-07-10 | 2013-01-17 | Elevance Renewable Sciences, Inc. | Metallic soap compositions for various applications |
| US9107969B2 (en) | 2012-06-08 | 2015-08-18 | Brandywine Product Group International Inc. | Air freshener |
| US20140230314A1 (en) | 2013-02-17 | 2014-08-21 | Elevance Renewable Sciences, Inc. | Wax compositions and the effect of metals on burn rates |
| CA2867008A1 (en) | 2013-10-11 | 2015-04-11 | Securitypoint Holdings, Inc. | Methods and systems for efficient security screening |
| US10960097B2 (en) | 2015-03-19 | 2021-03-30 | S. C. Johnson & Son, Inc. | Composite membrane |
| DE102015205068A1 (en) | 2015-03-20 | 2016-09-22 | Hoppel GmbH | Scented rod-shaped element and process for its preparation |
| EP3373898A1 (en) | 2015-11-09 | 2018-09-19 | Merial, Inc. | Pet care compositions |
| US10450256B2 (en) | 2017-10-06 | 2019-10-22 | Exxonmobil Research And Engineering Company | Renewable ketone waxes with unique carbon chain lengths and polarities |
| WO2023192504A1 (en) | 2022-03-30 | 2023-10-05 | Cargill, Incorporated | Candle wax compositions |
| WO2023192493A1 (en) | 2022-03-30 | 2023-10-05 | Cargill, Incorporated | Candle wax compositions |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0002110A1 (en) * | 1977-11-11 | 1979-05-30 | Motorola, Inc. | Fabrication of capacitive transducers by depositing a uniform glass insulating ring |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CH505773A (en) * | 1967-11-09 | 1971-04-15 | Firmenich & Cie | Cycloaliphatic unsatd. ketones contng. one double bond at one of posns. 1 and 2 or double bonds at posns. 1 and 3. (I) have organoleptic properties and ma |
| US4028278A (en) * | 1971-08-17 | 1977-06-07 | Firmenich S.A. | Cycloaliphatic unsaturated ketones as fragrance modifying agents |
| GB1456152A (en) * | 1974-02-22 | 1976-11-17 | Naarden International Nv | Cycloaliphatic unsaturated esters as flavou' and odour agents |
| GB1456151A (en) * | 1974-02-22 | 1976-11-17 | Naarden International Nv | Cycloaliphatic saturated and unsaturated ketones as flavour and odour agents |
| US4144199A (en) * | 1974-02-22 | 1979-03-13 | Naarden International, N.V. | Safranic acid ester perfume compositions |
| IT1034605B (en) * | 1974-04-19 | 1979-10-10 | Givaudan & Cie Sa | PERFUMES |
| CH600883A5 (en) * | 1974-04-19 | 1978-06-30 | Givaudan & Cie Sa | Perfumes contg crotonoyl gps |
| CH604717A5 (en) * | 1975-02-17 | 1978-09-15 | Givaudan & Cie Sa | Perfumes contg crotonoyl gps |
| CH615827A5 (en) * | 1975-10-09 | 1980-02-29 | Givaudan & Cie Sa | Use of ethyl 2-ethyl-6,6-dimethyl-2-cyclohexene-1-carboxylate as perfume |
| US4113663A (en) * | 1975-10-09 | 1978-09-12 | Givaudan Corporation | 2-Ethyl-6,6-dimethyl-2-cyclohexene-1-carboxylic acid ethyl ester perfume compositions |
| FR2422616A1 (en) * | 1977-11-15 | 1979-11-09 | Int Flavors & Fragrances Inc | TRANS, TRANS-D-DAMASCONE, AS WELL AS MIXTURES CONTAINING SIGNIFICANT PROPORTIONS OF THIS COMPOUND, THE PROCESSES FOR PREPARING THE SAME AND THE ORGANOLEPTIC USES OF THE LATTER |
| US4198309A (en) * | 1977-11-15 | 1980-04-15 | International Flavors & Fragrances Inc. | Use of trans, trans-Δ-damascone and mixtures containing 80% or more of trans, trans-Δ-damascone in augmenting or enhancing the aroma of a detergent |
| US4324704A (en) * | 1978-12-15 | 1982-04-13 | International Flavors & Fragrances Inc. | Process for hydrogenation of damascenone, products produced thereby and organoleptic uses of said products |
-
1981
- 1981-11-21 EP EP81109812A patent/EP0056109B1/en not_active Expired
- 1981-11-21 DE DE8181109812T patent/DE3173535D1/en not_active Expired
- 1981-12-15 US US06/331,013 patent/US4411829A/en not_active Expired - Lifetime
-
1982
- 1982-01-13 JP JP57002920A patent/JPS57139010A/en active Pending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0002110A1 (en) * | 1977-11-11 | 1979-05-30 | Motorola, Inc. | Fabrication of capacitive transducers by depositing a uniform glass insulating ring |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7196050B2 (en) | 2001-12-05 | 2007-03-27 | Firmenich Sa | Unsaturated ester as perfuming ingredient |
Also Published As
| Publication number | Publication date |
|---|---|
| JPS57139010A (en) | 1982-08-27 |
| EP0056109A2 (en) | 1982-07-21 |
| EP0056109A3 (en) | 1983-08-31 |
| US4411829A (en) | 1983-10-25 |
| DE3173535D1 (en) | 1986-02-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0056109B1 (en) | Use of 2,6,6-trimethyl-cyclohex-2-ene-1-yl-carboxylic-acid methyl ester as a perfuming agent | |
| US8883468B2 (en) | 1-hydroxy-octahydroazulenes as fragrances | |
| EP0180885B1 (en) | Use of a cycloaliphatic carbinol as a perfuming ingredient | |
| EP0482385B1 (en) | (2E,4Z,7Z)-ethyl decatrienoate and its use as a perfuming and flavouring ingredient | |
| EP0584477B1 (en) | Use of a cyclopentadecenone as a perfuming ingredient | |
| US5942272A (en) | Organoleptic compositions | |
| US4028279A (en) | Novel fragrance compositions containing 2,6,6 trimethyl-1-cyclohexen-1-yl acetaldehyde and phenyl C6 ketone | |
| US4036774A (en) | Fragrant soap compositions containing alpha-substituted acetaldehyde and ketone | |
| US4144199A (en) | Safranic acid ester perfume compositions | |
| EP0115278B1 (en) | Use of 1-cyclopentenylacetic acid as perfuming ingredient, perfuming composition containing it and perfumed products | |
| EP0080600B1 (en) | Use of 2-hydroxy-3,4,4-trimethyl-cyclopent-2-ene-1-one as odorant ingredient | |
| EP0809485B1 (en) | Use of 4-tert-butyl-1-cyclohexanol as an antioxidant | |
| EP0916650B1 (en) | Nitriles and aldehydes derived from 3-isopropenyl-1,2-dimethyl-1-cyclopentanol and their use in perfumery | |
| EP0052115B1 (en) | Bicyclic compounds and utilization thereof as perfuming agents | |
| EP1069176B1 (en) | Aliphatic esters and their use as perfume ingredients | |
| EP0081699B1 (en) | Alicyclic compounds, their use as perfume or flavouring agents and process for their preparation | |
| EP0045534B1 (en) | Use of phenyl-n-hexyl ketone as a perfuming agent, and perfume compositions containing the same | |
| EP0838215B1 (en) | Use of unsaturated aliphatic esters in perfumery | |
| EP0933420B1 (en) | Use of 2,5,6,-trimethyl-2-heptanol as a perfuming and a flavouring agent | |
| CH636009A5 (en) | USE OF A HYDROXY-ACETYLENIC DERIVATIVE AS A PERFUMING INGREDIENT. | |
| EP0181475B1 (en) | Unsaturated aliphatic ketone and its use as perfuming ingredient | |
| WO1992003402A1 (en) | Polycyclic ketone compound and use thereof as perfuming ingredient | |
| JP5317190B2 (en) | Fragrance material and fragrance composition containing ethyl saffronate | |
| EP0326869A2 (en) | Cycloaliphatic ketones, process for their preparation and their use as perfuming and aromatising ingredients | |
| CH586020A5 (en) | 1-Oxa-spiro-(4,5)-decane-6-ol and its esters - as perfumes and for modifying taste and aroma of foods, pharmaceuticals, tobacco etc. |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19811121 |
|
| AK | Designated contracting states |
Designated state(s): CH DE FR GB NL |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Designated state(s): CH DE FR GB LI NL |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): CH DE FR GB LI NL |
|
| REF | Corresponds to: |
Ref document number: 3173535 Country of ref document: DE Date of ref document: 19860227 |
|
| R20 | Corrections of a patent specification |
Effective date: 19860403 |
|
| D20 | Corrections of a patent specification (deleted) | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19911130 Year of fee payment: 11 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Effective date: 19930601 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20001009 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20001017 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20001020 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20001107 Year of fee payment: 20 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20011120 Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20011120 Ref country code: CH Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20011120 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 Effective date: 20011120 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |