EP0015396B1 - Procédé pour l'augmentation de la durabilité des revêtements réfractaires des convertisseurs à garniture basique - Google Patents
Procédé pour l'augmentation de la durabilité des revêtements réfractaires des convertisseurs à garniture basique Download PDFInfo
- Publication number
- EP0015396B1 EP0015396B1 EP80100604A EP80100604A EP0015396B1 EP 0015396 B1 EP0015396 B1 EP 0015396B1 EP 80100604 A EP80100604 A EP 80100604A EP 80100604 A EP80100604 A EP 80100604A EP 0015396 B1 EP0015396 B1 EP 0015396B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- melt
- inert gas
- slag
- vessel
- oxygen
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 239000001301 oxygen Substances 0.000 title claims description 28
- 229910052760 oxygen Inorganic materials 0.000 title claims description 28
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 title claims description 27
- 238000000034 method Methods 0.000 title claims description 17
- 239000000155 melt Substances 0.000 claims description 32
- 239000011261 inert gas Substances 0.000 claims description 23
- 229910052717 sulfur Inorganic materials 0.000 claims description 22
- 239000011593 sulfur Substances 0.000 claims description 22
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 claims description 21
- 239000002893 slag Substances 0.000 claims description 21
- YLUIKWVQCKSMCF-UHFFFAOYSA-N calcium;magnesium;oxygen(2-) Chemical compound [O-2].[O-2].[Mg+2].[Ca+2] YLUIKWVQCKSMCF-UHFFFAOYSA-N 0.000 claims description 18
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 claims description 16
- 235000008733 Citrus aurantifolia Nutrition 0.000 claims description 14
- 235000011941 Tilia x europaea Nutrition 0.000 claims description 14
- 239000004571 lime Substances 0.000 claims description 14
- 229910000831 Steel Inorganic materials 0.000 claims description 11
- 239000011575 calcium Substances 0.000 claims description 11
- 229910052791 calcium Inorganic materials 0.000 claims description 11
- 239000010959 steel Substances 0.000 claims description 11
- 229910052786 argon Inorganic materials 0.000 claims description 8
- 230000003993 interaction Effects 0.000 claims description 8
- 150000001875 compounds Chemical class 0.000 claims description 5
- 239000004615 ingredient Substances 0.000 claims description 5
- 238000007664 blowing Methods 0.000 claims description 4
- 238000004519 manufacturing process Methods 0.000 claims description 3
- CWYNVVGOOAEACU-UHFFFAOYSA-N Fe2+ Chemical compound [Fe+2] CWYNVVGOOAEACU-UHFFFAOYSA-N 0.000 claims description 2
- 244000089742 Citrus aurantifolia Species 0.000 description 12
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 6
- 239000007789 gas Substances 0.000 description 5
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 3
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 3
- CPLXHLVBOLITMK-UHFFFAOYSA-N Magnesium oxide Chemical compound [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 3
- 229910052799 carbon Inorganic materials 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 229910052710 silicon Inorganic materials 0.000 description 3
- 239000010703 silicon Substances 0.000 description 3
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 238000007792 addition Methods 0.000 description 2
- 238000013019 agitation Methods 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 239000000395 magnesium oxide Substances 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 238000007670 refining Methods 0.000 description 2
- -1 steam Chemical compound 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 1
- 235000019738 Limestone Nutrition 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- JGIATAMCQXIDNZ-UHFFFAOYSA-N calcium sulfide Chemical compound [Ca]=S JGIATAMCQXIDNZ-UHFFFAOYSA-N 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 239000010436 fluorite Substances 0.000 description 1
- 239000001307 helium Substances 0.000 description 1
- 229910052734 helium Inorganic materials 0.000 description 1
- SWQJXJOGLNCZEY-UHFFFAOYSA-N helium atom Chemical compound [He] SWQJXJOGLNCZEY-UHFFFAOYSA-N 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 229910052743 krypton Inorganic materials 0.000 description 1
- DNNSSWSSYDEUBZ-UHFFFAOYSA-N krypton atom Chemical compound [Kr] DNNSSWSSYDEUBZ-UHFFFAOYSA-N 0.000 description 1
- 239000006028 limestone Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 229910001338 liquidmetal Inorganic materials 0.000 description 1
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical compound [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229910052754 neon Inorganic materials 0.000 description 1
- GKAOGPIIYCISHV-UHFFFAOYSA-N neon atom Chemical compound [Ne] GKAOGPIIYCISHV-UHFFFAOYSA-N 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 238000009877 rendering Methods 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 229910052724 xenon Inorganic materials 0.000 description 1
- FHNFHKCVQCLJFQ-UHFFFAOYSA-N xenon atom Chemical compound [Xe] FHNFHKCVQCLJFQ-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21C—PROCESSING OF PIG-IRON, e.g. REFINING, MANUFACTURE OF WROUGHT-IRON OR STEEL; TREATMENT IN MOLTEN STATE OF FERROUS ALLOYS
- C21C5/00—Manufacture of carbon-steel, e.g. plain mild steel, medium carbon steel or cast steel or stainless steel
- C21C5/28—Manufacture of steel in the converter
- C21C5/36—Processes yielding slags of special composition
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21C—PROCESSING OF PIG-IRON, e.g. REFINING, MANUFACTURE OF WROUGHT-IRON OR STEEL; TREATMENT IN MOLTEN STATE OF FERROUS ALLOYS
- C21C5/00—Manufacture of carbon-steel, e.g. plain mild steel, medium carbon steel or cast steel or stainless steel
- C21C5/28—Manufacture of steel in the converter
- C21C5/42—Constructional features of converters
- C21C5/44—Refractory linings
- C21C5/441—Equipment used for making or repairing linings
- C21C5/443—Hot fettling; Flame gunning
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21C—PROCESSING OF PIG-IRON, e.g. REFINING, MANUFACTURE OF WROUGHT-IRON OR STEEL; TREATMENT IN MOLTEN STATE OF FERROUS ALLOYS
- C21C5/00—Manufacture of carbon-steel, e.g. plain mild steel, medium carbon steel or cast steel or stainless steel
- C21C5/28—Manufacture of steel in the converter
- C21C5/36—Processes yielding slags of special composition
- C21C2005/366—Foam slags
Definitions
- This invention relates to a method for increasing the life of the refractory lining of a basic refractory-lined vessel for the production of steel by blowing oxygen into a ferrous melt from above the surface of the melt, comprising introducing slag-forming ingredients into the vessel, including high-calcium lime and dolomitic lime.
- dolomitic lime is introduced in an amount which is at least equal to that of the high-calcium lime introduced, and that inert gas is introduced from above the melt surface into the melt in such a manner as to cause intensive interaction between the slag and the melt.
- inert gas as used throughout the present specification and claims is intended to means a gas other than oxygen having as many as possible of the following characteristics: low reactivity, low specific heat, absence of objectionable contaminants, and high density.
- the preferred inert gas is argon. However, if nitrogen contamination of the melt is not a problem, nitrogen or air may be used.
- Other possible inert gases for use in practicing the invention include helium, neon, krypton, xenon, carbon dioxide, steam, ammonia, and mixtures thereof.
- argon which. may be either commercially pure or crude argon is by far the most preferable inert gas.
- the preferred method of introducing inert gas is through the oxygen lance admixed with oxygen.
- the iron charged to a basic oxygen furnace typically contains carbon, silicon, sulfur, and other impurities.
- the main purpose of the oxygen is to remove carbon and silicon from the melt.
- the silicon is oxidized to silicon dioxide which floats on the surface of the melt.
- the carbon is oxidized to carbon monoxide gas which escapes from the mouth of the vessel.
- Slag forming ingredients typically including high-calcium lime, dolomitic lime, lime stone, and fluorspar, are added to the melt to form a basic slag.
- the high-calcium lime normally containing at least 90 percent by weight of CaO, also removes sulfur by reacting with it to form calcium sulfide.
- Dolomitic lime i.e.
- lime containing at least 30% by weight magnesium oxide is known to improve the life of a vessel's lining, but in addition, it increases the viscosity of the slag, thereby reducing the amount of interaction between the lime in the slag and the melt. The reduced interaction makes it difficult for the lime to remove sulfur from the melt. Since the conventional solution to the problem of obtaining long vessel lining life makes it difficult to make steel having a sufficiently low sulfur content, the amount of dolomitic lime charged to the vessel for conventional oxygen blowing must be limited.
- dolomitic lime is introduced to the slag in an amount which is at least equal to that of the high-calcium lime introduced.
- the slag forming compounds should be introduced in an amount sufficient to reduce the sulfur content of the melt to a desired level, based upon the stoichiometric and thermodynamic aspects of the reaction of sulfur with the compounds.
- dolomitic lime is essential to increasing the life of the vessel's refractory lining.
- the sulfur content of the steel can be reduced to meet the specification, even if the amount of dolomitic lime is equal to 2 to 3 times the amount of lime introduced.
- Extra agitation or mixing is required in the vessel because of the increased slag viscosity caused by the larger amount of dolomitic lime.
- the extra agitation is provided by introducing inert gas into the vessel in such manner as to cause intensive interaction between the slag and the melt.
- the present invention may be practiced in conjunction with the method disclosed in DE-Al-27 45 722 for using argon in the BOF or make low nitrogen and low oxygen steel.
- the inert gas must be introduced in such manner as to cause intensive interaction between the slag and the melt.
- the inert gas is introduced through the oxygen lance by metering it into the oxygen line.
- the inert gas may be introduced through a separate lance directed to impinge oxygen-free fluid against the surface of the melt.
- the sulfur content of the melt is one of the most difficult variables to control in the basic oxygen process, occasionally, even when the present invention is practiced the sulfur content of the melt at the end of a blow will be higher than desired.
- the sulfur content of the melt may be lowered in accordance with a further development of the present invention by adding at least one sulfur-removing compound, such as high-calcium lime, to the slag in the vessel and re-blowing the melt with inert gas alone in such a manner as to cause intensive interaction between the slag and the melt until the sulfur content is reduced to the desired level.
- at least one sulfur-removing compound such as high-calcium lime
- the melt with inert gas alone in the above described manner may be used to lower the sulfur content to the desired level.
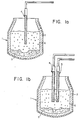
- Fig. 1A illustrates a basic oxygen refining vessel together with an oxygen lance through which inert gas likewise is introduced by metering it into the oxygen line.
- Fig. 1b illustrates a modified embodiment for practicing the method of the invention, wherein the inert gas is introduced through a separate lance.
- a basic oxygen vessel 1 is provided with a refractory lining 2.
- a lance 4 is used to inject oxygen and inert gas which is metered into the oxygen line connected to lance 4.
- the gases are blown into the melt 5 from above the melt surface through lance 4 whereby an emulsion 6 composed of a complex mixture of liquid oxides, gas bubbles, solid oxide particles and droplets of liquid metal is formed.
- a separate lance 3 is provided in addition to oxygen lance 4.
- the inert gas is introduced through lance 3 directed to impinge oxygen-free fluid against the surface of melt 5.
- the normal dolomitic lime charge for this vessel was 7,257 kg.
- the normal slag-forming ingredients were comprised of 7,257 kg of dolomitic lime and 10,886 kg of high-calcium lime, and 907 kg of flurospar.
- the dolomitic lime charge was increased to 15,876 kg and the high-calcium lime charge was reduced to 4,536 kg and argon was injected into the vessel in accordance with the method of addition disclosed in DE-Al-27 45 722 mentioned previously.
- the argon was injected at a constant rate of 99 standard m 3 /min during the latter portion of the oxygen blow.
- the life of the lining of this vessel was extended from a previous life of about 780 heats to about 1100 heats.
- dolomitic lime added as slag forming ingredients no difficulty was encountered making steel having sulfur content as low as desired. Furthermore, during this test flurospar, which is known to aid sulfur removal, was not used.
- Another advantage of the invention is a saving in the amount of flurospar charged to the vessel.
- the typical maximum sulfur content allowed by the specifications for steel made by this vessel was 0.025% sulfur.
Landscapes
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Manufacturing & Machinery (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Treatment Of Steel In Its Molten State (AREA)
- Carbon Steel Or Casting Steel Manufacturing (AREA)
- Furnace Housings, Linings, Walls, And Ceilings (AREA)
Claims (7)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US1031579A | 1979-02-07 | 1979-02-07 | |
| US10315 | 1979-02-07 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0015396A1 EP0015396A1 (fr) | 1980-09-17 |
| EP0015396B1 true EP0015396B1 (fr) | 1985-05-15 |
Family
ID=21745183
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP80100604A Expired EP0015396B1 (fr) | 1979-02-07 | 1980-02-06 | Procédé pour l'augmentation de la durabilité des revêtements réfractaires des convertisseurs à garniture basique |
Country Status (14)
| Country | Link |
|---|---|
| EP (1) | EP0015396B1 (fr) |
| JP (1) | JPS5952201B2 (fr) |
| AU (1) | AU5526080A (fr) |
| BR (1) | BR8000733A (fr) |
| CA (1) | CA1143947A (fr) |
| DE (1) | DE3070636D1 (fr) |
| ES (1) | ES488303A0 (fr) |
| FI (1) | FI800335A (fr) |
| IN (1) | IN153626B (fr) |
| MX (1) | MX154163A (fr) |
| NO (1) | NO800301L (fr) |
| PH (1) | PH15430A (fr) |
| RO (1) | RO79757A (fr) |
| ZA (1) | ZA80213B (fr) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2525633A1 (fr) * | 1982-04-22 | 1983-10-28 | Siderurgie Fse Inst Rech | Procede pour ameliorer la duree de vie d'elements refractaires permeables loges dans le fond des recipients metallurgiques d'affinage, notamment des convertisseurs d'acierie a soufflage d'oxygene par le haut |
| AT385771B (de) * | 1986-06-16 | 1988-05-10 | Voest Alpine Ag | Verfahren zum ueberziehen der feuerfesten auskleidung eines metallurgischen schmelzofens mit einem hitzebestaendigen material |
| DE3936715A1 (de) * | 1989-11-03 | 1991-05-08 | Kortec Ag | Verfahren zum einbringen von fliessfaehigen zuschlagsstoffen in ein metallurgisches gefaess und gefaess fuer dieses verfahren |
| CN1035017C (zh) * | 1994-04-21 | 1997-05-28 | 鞍山钢铁公司 | 炼钢中用轻烧镁球团造渣法 |
| GB2553342A (en) * | 2016-09-02 | 2018-03-07 | Materials Proc Institute | Producing steel |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3288592A (en) * | 1963-01-16 | 1966-11-29 | Pfizer & Co C | Process for reducing deterioration in equipment handling molten materials |
| FR1346148A (fr) * | 1963-01-31 | 1963-12-13 | Centre Nat Rech Metall | Procédé pour la protection du revêtement intérieur des fours métallurgiques |
| FR1536457A (fr) * | 1967-07-07 | 1968-08-16 | Siderurgie Fse Inst Rech | Procédé pour la protection des revêtements réfractaires des récipients métallurgiques d'affinage continu |
| US3726665A (en) * | 1969-10-15 | 1973-04-10 | C & W Corson H Inc | Slagging in basic steel-making process |
| US3915696A (en) * | 1970-01-08 | 1975-10-28 | Ferdinand Fink | Sintered preformed slag for the steel industry |
| BR7302595D0 (pt) * | 1972-05-09 | 1974-06-27 | Blanq Cazaux Morales A | Aperfeicoamentos em processo para fabricacao de ferro e acaperfeicoamentos em processo para fabricacao de ferro e acos os |
| FR2322202A1 (fr) * | 1975-08-29 | 1977-03-25 | Siderurgie Fse Inst Rech | Procede d'elaboration d'acier par soufflage d'oxygene |
| ZA775918B (en) * | 1977-01-11 | 1978-05-30 | Nat Steel Corp | The use of orgon to prepare low-carbon,low-nitrogen steels in the basic oxygen process |
| JPS545813A (en) * | 1977-06-17 | 1979-01-17 | Fuaizaa Kuiguree Kk | Method of extending useful life of refractory lining material in aod furnace |
-
1979
- 1979-12-19 CA CA000342245A patent/CA1143947A/fr not_active Expired
-
1980
- 1980-01-14 ZA ZA00800213A patent/ZA80213B/xx unknown
- 1980-01-21 IN IN40/DEL/80A patent/IN153626B/en unknown
- 1980-02-04 JP JP55011600A patent/JPS5952201B2/ja not_active Expired
- 1980-02-04 FI FI800335A patent/FI800335A/fi not_active Application Discontinuation
- 1980-02-05 NO NO800301A patent/NO800301L/no unknown
- 1980-02-05 PH PH23600A patent/PH15430A/en unknown
- 1980-02-06 MX MX181089A patent/MX154163A/es unknown
- 1980-02-06 EP EP80100604A patent/EP0015396B1/fr not_active Expired
- 1980-02-06 AU AU55260/80A patent/AU5526080A/en not_active Abandoned
- 1980-02-06 DE DE8080100604T patent/DE3070636D1/de not_active Expired
- 1980-02-06 BR BR8000733A patent/BR8000733A/pt unknown
- 1980-02-06 RO RO80100107A patent/RO79757A/fr unknown
- 1980-02-06 ES ES488303A patent/ES488303A0/es active Granted
Non-Patent Citations (1)
| Title |
|---|
| Stahl und Eisen 96 (1976), pages 878/879 * |
Also Published As
| Publication number | Publication date |
|---|---|
| IN153626B (fr) | 1984-07-28 |
| ES8100349A1 (es) | 1980-11-01 |
| EP0015396A1 (fr) | 1980-09-17 |
| DE3070636D1 (en) | 1985-06-20 |
| AU5526080A (en) | 1980-08-14 |
| MX154163A (es) | 1987-05-28 |
| ZA80213B (en) | 1980-12-31 |
| PH15430A (en) | 1983-01-18 |
| RO79757A (fr) | 1983-02-01 |
| FI800335A (fi) | 1980-08-08 |
| NO800301L (no) | 1980-08-08 |
| ES488303A0 (es) | 1980-11-01 |
| JPS55107714A (en) | 1980-08-19 |
| BR8000733A (pt) | 1980-10-21 |
| JPS5952201B2 (ja) | 1984-12-18 |
| CA1143947A (fr) | 1983-04-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4373949A (en) | Method for increasing vessel lining life for basic oxygen furnaces | |
| TW201938799A (zh) | 熔銑的脫磷方法 | |
| EP0152674A1 (fr) | Procédé de fabrication d'acier en convertisseur utilisant une grande quantité de ferrailles froides | |
| EP0015396B1 (fr) | Procédé pour l'augmentation de la durabilité des revêtements réfractaires des convertisseurs à garniture basique | |
| US4001012A (en) | Method of producing stainless steel | |
| EP0033780B2 (fr) | Procédé pour réduire des projections dans un convertisseur à soufflage par le fond | |
| EP0073274B1 (fr) | Procédé pour la désiliciation préliminaire de fer fondu par insufflation d'oxygène gazeux | |
| US4891064A (en) | Method of melting cold material including iron | |
| GB2057509A (en) | Steel making in top-blown converter | |
| JP2000129329A (ja) | 溶銑の脱りん方法 | |
| KR850001607B1 (ko) | 염기성 산소정련로에서 내화라이닝의 수명연장법 | |
| KR900002710B1 (ko) | 급속탈탄 제강공정 | |
| KR100423452B1 (ko) | 전로 취련중 용철의 탈황방법 | |
| JP2856106B2 (ja) | 溶銑の脱硫方法 | |
| EP0104841B1 (fr) | Déphosphoration du fer | |
| EP0097971B1 (fr) | Procédé permettant l'obtention de teneurs basses en hydrogène dans des acides produits par affinage pneumatique submergé | |
| KR0129035B1 (ko) | 크롬의 산화손실이 적은 함크롬 용선의 탈인(脫燐) 방법 | |
| SU1527278A1 (ru) | Способ регенерации конечного шлака | |
| JPH0353014A (ja) | 極低硫鋼の溶製方法 | |
| SU956572A1 (ru) | Способ выплавки стали в дуговых печах | |
| US2816018A (en) | Process for the production of steel from high phosphorus pig iron | |
| SU1315483A1 (ru) | Шлакообразующа смесь | |
| JPS6154081B2 (fr) | ||
| JPH111714A (ja) | 製鋼方法 | |
| JPH01252715A (ja) | 鉄浴式溶融還元炉の操業方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT BE DE FR GB IT LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19810316 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: NATIONAL STEEL CORPORATION Owner name: UNION CARBIDE CORPORATION |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): BE DE FR GB IT LU NL |
|
| ITF | It: translation for a ep patent filed | ||
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): BE DE FR GB IT LU NL |
|
| REF | Corresponds to: |
Ref document number: 3070636 Country of ref document: DE Date of ref document: 19850620 |
|
| ET | Fr: translation filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: LU Payment date: 19860122 Year of fee payment: 7 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19860228 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19870228 Year of fee payment: 8 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Effective date: 19890206 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Effective date: 19890901 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee | ||
| GBPC | Gb: european patent ceased through non-payment of renewal fee | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19891027 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19891101 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19910311 Year of fee payment: 12 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Effective date: 19920228 |
|
| BERE | Be: lapsed |
Owner name: NATIONAL STEEL CORP. Effective date: 19920228 Owner name: UNION CARBIDE CORP. Effective date: 19920228 |