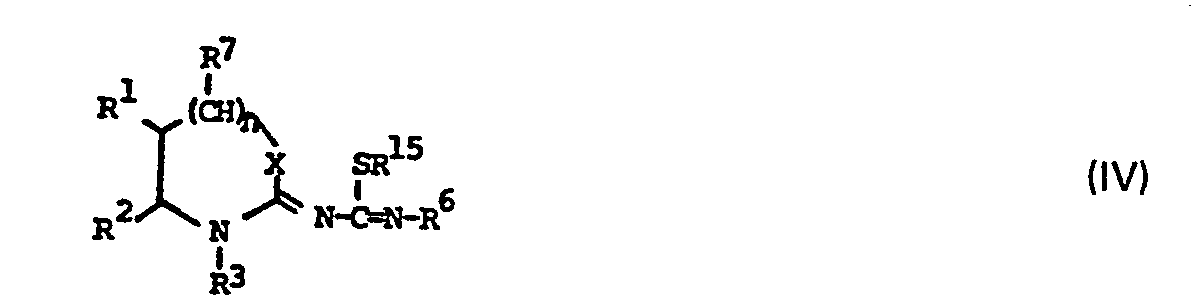EP0011963B1 - Heterocyclische Carboxamidin-Verbindungen, ihre Herstellung und diese enthaltende pharmazeutische Zusammensetzungen - Google Patents
Heterocyclische Carboxamidin-Verbindungen, ihre Herstellung und diese enthaltende pharmazeutische Zusammensetzungen Download PDFInfo
- Publication number
- EP0011963B1 EP0011963B1 EP79302564A EP79302564A EP0011963B1 EP 0011963 B1 EP0011963 B1 EP 0011963B1 EP 79302564 A EP79302564 A EP 79302564A EP 79302564 A EP79302564 A EP 79302564A EP 0011963 B1 EP0011963 B1 EP 0011963B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- alkyl
- formula
- phenyl
- hydrogen
- ylidene
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- PVOAHINGSUIXLS-UHFFFAOYSA-N CN1CCNCC1 Chemical compound CN1CCNCC1 PVOAHINGSUIXLS-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D265/00—Heterocyclic compounds containing six-membered rings having one nitrogen atom and one oxygen atom as the only ring hetero atoms
- C07D265/04—1,3-Oxazines; Hydrogenated 1,3-oxazines
- C07D265/06—1,3-Oxazines; Hydrogenated 1,3-oxazines not condensed with other rings
- C07D265/08—1,3-Oxazines; Hydrogenated 1,3-oxazines not condensed with other rings having one double bond between ring members or between a ring member and a non-ring member
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D263/00—Heterocyclic compounds containing 1,3-oxazole or hydrogenated 1,3-oxazole rings
- C07D263/02—Heterocyclic compounds containing 1,3-oxazole or hydrogenated 1,3-oxazole rings not condensed with other rings
- C07D263/08—Heterocyclic compounds containing 1,3-oxazole or hydrogenated 1,3-oxazole rings not condensed with other rings having one double bond between ring members or between a ring member and a non-ring member
- C07D263/16—Heterocyclic compounds containing 1,3-oxazole or hydrogenated 1,3-oxazole rings not condensed with other rings having one double bond between ring members or between a ring member and a non-ring member with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D263/28—Nitrogen atoms not forming part of a nitro radical
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D277/00—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings
- C07D277/02—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings
- C07D277/08—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings having one double bond between ring members or between a ring member and a non-ring member
- C07D277/12—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings having one double bond between ring members or between a ring member and a non-ring member with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D277/18—Nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D279/00—Heterocyclic compounds containing six-membered rings having one nitrogen atom and one sulfur atom as the only ring hetero atoms
- C07D279/04—1,3-Thiazines; Hydrogenated 1,3-thiazines
- C07D279/06—1,3-Thiazines; Hydrogenated 1,3-thiazines not condensed with other rings
Definitions
- This invention relates to a class of novel heterocyclic compounds which are useful in the treatment of diabetes.
- the invention also relates to a process for their preparation and to pharmaceutical compositions containing them.
- the compound of formula (I) has been reported to be useful in the treatment of diabetes in Belgian Patent No. 852,565 and in Diabetes, 27, 856 and 868 (1978).
- the present invention provides a compound of formula (II) or a pharmaceutically acceptable quaternary ammonium or acid addition salt thereof:
- Suitable quaternary salts of compound (II) include C 1-6 alkyl halides, di-C 1-6 alkyl sulphates and benzyl halides.
- Preferred quaternary salts are the C 1-6 alkyl halides; in particular the methylhalide, such as the methvliodide salt.
- Suitable acid addition salts of compound (II) include inorganic salts such as the sulphate, nitrate phosphate and borate, hydrohalides such as the hydrochloride, hydrobromide and hydroiodide, and organic acid addition salts such as acetate, oxalate, tartrate, maleate, citrate, succinate, benzoate, ascorbate, methanesulphonate and p-toluenesulphonate.
- Preferred salts are hydrohalide salts.
- the group X in compounds of formula (II) preferably represents sulphur and n is preferably zero.
- Examples of suitable C 1-6 alkyl groups which R' to R 5 and R 7 may represent include methyl, ethyl, n- and iso-propyl, and n-, sec-, iso-, and tert-butyl.
- cycloalkyl groups which R' and R 2 may represent include cyclopentyl and cyclohexyl.
- Suitable substituents for the phenyl and benzyl groups for R 5 and the phenyl group for R 6 include ortho-, meta- and para- methyl, methoxy, chloro and bromo.
- n is zero.
- R', R 2 and R 7 represent hydrogen, methyl, ethyl, or n-propyl.
- R' and R 2 are both hydrogen.
- n is 1, then preferably R 7 is hydrogen.
- R 3 is methyl, ethyl, n-propyl, or phenyl.
- R 3 represents hydrogen or C 1-6 alkyl, especially methyl or ethyl.
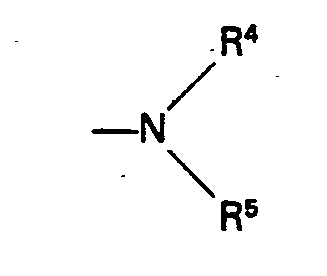
- R 4 is hydrogen, methyl, ethyl or n-propyl
- R 5 represents methyl, ethyl, n-propyl, phenyl or benzyl.
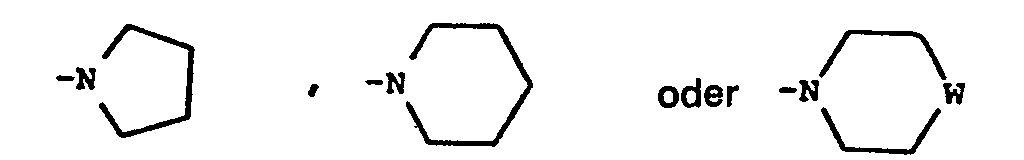
- suitable such rings include pyrrolidine, piperidine, morpholine, thiomorpholine, piperazine and 4-(C 1-6 alkyl)piperazine, for example 4-methylpiperazine rings.
- R 6 is phenyl
- Compounds of formula (11) include the following:
- Compounds of formula (II) may be prepared by reacting a compound of formula (IV) or a salt thereof: wherein R 1 , R 2 , R 3 , R 6 , R 7 , n and X are as defined with respect to formula (II) above and R 15 represents C 1-6 alkyl; with an amine of formula R 4 R 5 NH, wherein R 4 and R 5 are as defined with reference to formula (II) above and thereafter where desired converting a free base of formula (II) so obtained into a pharmaceutically acceptable salt or converting a salt of a compound of formula (II) so obtained into the free base.
- the reaction is conveniently carried out in polar organic solvent, the choice of which is not critical to the success of the reaction provided that it forms a homogeneous solution of the reagent and is substantially inert to the reagent and product. It has been found that lower alkanols such as iso- propanol are particularly convenient.
- the reaction is generally carried out at a moderate temperature i.e. greater than room temperature, the reflux temperature of the solvent being selected for convenience.
- the period for which the reaction is allowed to proceed depends upon the particular starting materials employed.
- the course of the reaction may be followed by conventional methods such as thin layer chromatography and terminated when an optimum quantity of product is present in the reaction mixture.
- Example of lower alkyl groups which R 15 may represent include methyl, ethyl, n-propyl or n-butyl but preferably R' 5 represents methyl.
- intermediates (IV) are prepared by alkylation of a thiourea (VI) using an alkylating agent R 15 .
- R 15 is as defined with reference to formula (IV) and Z is a leaving group such as chloride, bromide or iodide.
- the reaction is carried out in a polar organic solvent, the choice of which is not critical provided that the solvent is substantially inert to the reagents and product. Suitable solvents include lower alkanones and alcohols. The reaction is suitably carried out at the boiling point of the solvent.
- the thiourea (VI) is in turn prepared by reacting an iso-thiocyanate R 6 . NCS with a corresponding imino compound (V), where R', R 2 , R 3 , R 6 , R 7 n and X are as defined with reference to formula (II).
- This reaction is carried out in a solvent such as toluene, benzene, dioxane, tetrahydrofuran, methanol or ethanol.
- the reaction is carried out at non-extreme temperatures, i.e up to and including the reflux temperature of the solvent.
- Compounds of formula (II) may also be prepared by reacting a compound of formula (VII). wherein R 4 , R 5 and R 6 are as defined with respect to formula (II) above; with an imino compound (V), where R', R 2 , R 3 , R 7 , n and x are as defined with respect to formula (II) above and thereafter where desired converting a free base of formula (II) so obtained into a pharmaceutically acceptable salt or converting a salt of a compound of formula (II) so obtained into the free base.
- the reaction is conveniently carried out in a non-hydroxylic solvent system such as an ether, chlorinated hydrocarbon or a mixture thereof. Suitable solvent systems include mixtures of diethyl ether and chloroform.
- a non-hydroxylic solvent system such as an ether, chlorinated hydrocarbon or a mixture thereof.
- Suitable solvent systems include mixtures of diethyl ether and chloroform.
- the reaction is suitably carried out at ambient temperature. The period for which the reaction is allowed to proceed may be determined by methods as described hereinbefore; however, we have found it convenient to leave the reaction mixture to stand overnight.
- the reaction is carried out in ethereal solvent such as diethyl ether of tetrahydrofuran.
- the reaction is suitably carried out at ambient temperature.
- the period for which the reaction is allowed to proceed may be determined by methods as described hereinbefore; however, we have found a two hour reaction time to be sufficient.
- the quaternary ammonium salts of compounds of formula (II) may be prepared by reaction of the compounds of formula (II) with the corresponding quaternisation agent for example (C 1-6 ) alkyl, or benzyl halides such as methyl iodide, ethyl bromide, propyl bromide, or benzyl chloride, or sulphuric esters e.g. di(C,_ alkyl)sulphates such as dimethyl sulphate or diethyl sulphate.
- the corresponding quaternisation agent for example (C 1-6 ) alkyl, or benzyl halides such as methyl iodide, ethyl bromide, propyl bromide, or benzyl chloride, or sulphuric esters e.g. di(C,_ alkyl)sulphates such as dimethyl sulphate or diethyl sulphate.
- the quatemisation may be carried out in the presence or absence of a solvent, depending upon whether the quaternisation agent is or is not itself capable of acting as a solvent, at ambient temperature or under cooling, and under atmospheric pressure or under pressure in a sealed container.
- Organic solvents which are inert as regards the reaction and which are suitable for this purpose are ethers such as diethyl ether or tetrahydrofuran, hydrocarbons such as benzene or heptane, ketones such as acetone or butanone, and C '-6 alkanols such as ethanol, propanol or butanol.
- the anionic function of the quaternary salt can readily be exchanged by a traditional ion exchange technique.
- compositions in a variety of dosage forms.
- This invention therefore also includes a pharmaceutical composition which comprises a compound of formula (II) together with a pharmaceutically acceptable carrier or excipient.
- compositions may be formulated for administration by any route, although an oral administration is preferred.
- the compositions may be in the form of tablets, capsules, powders, granules, lozenges, or liquid preparations, such as oral or sterile parenteral solutions or suspensions.
- Tablets and capsules for oral administration may be in unit dose presentation form, and may contain conventional excipients such as binding agents, for example, acacia, gelatin, sorbitol, tragacanth, or polyvinyl-pyrollidone; fillers, for example calcium phosphate, sorbitol or glycine; tabletting lubricants, for example magnesium stearate, talc, polyethylene glycol, or silica; disintegrants, for example potato starch; or acceptable wetting agents such as sodium lauryl sulphate.
- the tablets may be coated according to methods well known in normal pharmaceutical practice.
- Oral liquid preparations may be in the form of, for example, aqueous or oily suspensions, solutions, emulsions, syrups, or elixirs, or may be presented as a dry product for reconstitution with water or other suitable vehicle before use.
- Such liquid preparations may contain conventional additives such as suspending agents, for example sorbitol, syrup, methyl cellulose, gelatin, hydroxyethylcellulose, carboxy-methyl cellulose, aluminium stearate gel or hydrogenated edible fats, emulsifying agents, for example lecithin, sorbitan monooleate, or acacia; non-aqueous vehicles (which may include edible oils), for example almond oil, fractionated coconut oil, oily esters such as glycerine, propylene glycol, or ethyl alcohol; preservatives, for example methyl or propyl p-hydroxy benzoate or sorbic acid, and if desired conventional flavouring or colouring agents.
- suspending agents for example sorbitol, syrup, methyl cellulose, gelatin, hydroxyethylcellulose, carboxy-methyl cellulose, aluminium stearate gel or hydrogenated edible fats, emulsifying agents, for example lecithin, sorbitan monooleate, or
- Suppositories will contain conventional suppository bases, e.g. cocoa butter or other glyceride.
- fluid unit dosage forms are prepared utilizing the compound and a sterile vehicle, water being preferred.
- the compound depending on the vehicle and concentration used, can be either suspended or dissolved in the vehicle.
- the compound can be dissolved in water for injection and filter sterilized before filling into a suitable vial or ampoule and sealing.
- adjuvants such as a local anaesthetic, preservative and buffering agents can be dissolved in the vehicle.
- the composition can be frozen after filling into the vial and the water removed under vacuum.
- Parenteral suspensions are prepared in substantially the same manner except that the compound is suspended in the vehicle instead of being dissolved and sterilization cannot be accomplished by filtration.
- the compound can be sterilized by exposure to ethylene oxide before suspending in the sterile vehicle.
- a surfactant or wetting agent is included in the composition to facilitate uniform distribution of the compound.
- compositions may contain from 0.1 % to 99% by weight, preferably from 10-60% by weight, of the active material, depending on the method of administration.
- the dosage employed for adult treatment will of course depend on the dose-response characteristics of the particular active ingredient but will normally be in the range 0.5 to 150 mg/kg/day.
- Mpt 130-2° was prepared by an analogous procedure to that described in Example (1b).
- N,N-diethyl-N'-(3-methylthiazolidin-2-ylidene)-N"-phenylguanidine as an oil, purified by column chromatography on silica using 5% of (33% dimethylamine in IMS) in CH 2 Cl 2 as eluent.
- N-(3-methyloxazolidin-2-ylidene)-N'-phenyl-1-pyrrolidine-carboxamidine as an oil, purified by chromatography using 1% of (33% dimethylamine in 1 MS) in CH 2 Cl 2 as eluent.
- mice were fasted for 24 hours before the experiment and then randomised so that each treatment group contained 8 mice.
- the compounds were dosed orally in 1% aqueous carboxy- methyl cellulose (10 ml/kg body weight), and 30 minutes later glucose (1 g/kg) was administered by the sub-cutaneous route.
- Blood samples for glucose analysis were taken from the tail 60 minutes after glucose administration; the results are shown in the table below.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Claims (29)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT79302564T ATE1339T1 (de) | 1978-11-29 | 1979-11-14 | Heterocyclische carboxamidin-verbindungen, ihre herstellung und diese enthaltende pharmazeutische zusammensetzungen. |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB4653178 | 1978-11-29 | ||
| GB7846531 | 1978-11-29 | ||
| GB7915737 | 1979-05-05 | ||
| GB7915737 | 1979-05-15 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0011963A1 EP0011963A1 (de) | 1980-06-11 |
| EP0011963B1 true EP0011963B1 (de) | 1982-07-14 |
Family
ID=26269786
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP79302564A Expired EP0011963B1 (de) | 1978-11-29 | 1979-11-14 | Heterocyclische Carboxamidin-Verbindungen, ihre Herstellung und diese enthaltende pharmazeutische Zusammensetzungen |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US4250173A (de) |
| EP (1) | EP0011963B1 (de) |
| AU (1) | AU5263779A (de) |
| DE (1) | DE2963350D1 (de) |
| DK (1) | DK485979A (de) |
| ES (2) | ES8101589A1 (de) |
| IL (1) | IL58681A0 (de) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4414211A (en) * | 1978-09-18 | 1983-11-08 | Mcneilab, Inc. | Heterocyclic derivatives of guanidine |
| ATE2075T1 (de) * | 1979-04-20 | 1983-01-15 | Beecham Group Plc | Oxazolin- und thiazolin-derivate, verfahren zu ihrer herstellung und sie enthaltende pharmazeutische zusammensetzungen. |
| US4342764A (en) * | 1979-05-29 | 1982-08-03 | Ciba-Geigy Corporation | Guanidine compounds, pharmaceutical compositions and use |
| EP0028883A3 (de) * | 1979-11-10 | 1981-06-03 | Beecham Group Plc | Benzimidazolin-Derivate, Verfahren zu ihrer Herstellung und sie enthaltende pharmazeutische Zusammensetzungen |
| US4421748A (en) * | 1982-07-13 | 1983-12-20 | Trager Seymour F | Artificial tear aid |
| US4704389A (en) * | 1986-03-05 | 1987-11-03 | Merrell Dow Pharmaceuticals Inc. | Aromatic omega-alkyl-imino-tetrahydro-6H-1,3-thiazin-6-one derivatives |
| GB8903592D0 (en) * | 1989-02-16 | 1989-04-05 | Boots Co Plc | Therapeutic agents |
| IT1277486B1 (it) * | 1994-12-14 | 1997-11-10 | Japan Tobacco Inc | Derivati di tiazina o di tiazepina |
| JP2001261652A (ja) | 2000-03-21 | 2001-09-26 | Suntory Ltd | 二置換イミノヘテロサイクリック化合物 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE1247318B (de) * | 1960-10-13 | 1967-08-17 | Albert Ag Chem Werke | Verfahren zur Herstellung von N-[5-Chlormethyloxazolinyl-(2)]-thioharnstoffderivaten |
| US3758491A (en) * | 1972-01-07 | 1973-09-11 | Parke Davis & Co | Novel anti infective agents and means of producing the same |
| DE2205744A1 (de) * | 1972-02-08 | 1973-08-09 | Thomae Gmbh Dr K | Neue durch einen guanidinylidenrest substituierte heterocyclen und verfahren zu ihrer herstellung |
| CS225804B2 (cs) | 1976-03-19 | 1984-02-13 | Mcneilab Inc | Způsob výroby heterocyklických derivátů guanidinu |
-
1979
- 1979-11-08 AU AU52637/79A patent/AU5263779A/en not_active Abandoned
- 1979-11-09 IL IL58681A patent/IL58681A0/xx unknown
- 1979-11-14 DE DE7979302564T patent/DE2963350D1/de not_active Expired
- 1979-11-14 EP EP79302564A patent/EP0011963B1/de not_active Expired
- 1979-11-14 US US06/094,100 patent/US4250173A/en not_active Expired - Lifetime
- 1979-11-15 DK DK485979A patent/DK485979A/da not_active Application Discontinuation
- 1979-11-28 ES ES79486422A patent/ES8101589A1/es not_active Expired
-
1980
- 1980-06-10 US US06/158,212 patent/US4282356A/en not_active Expired - Lifetime
- 1980-07-30 ES ES493859A patent/ES8200669A1/es not_active Expired
Also Published As
| Publication number | Publication date |
|---|---|
| EP0011963A1 (de) | 1980-06-11 |
| DK485979A (da) | 1980-05-30 |
| AU5263779A (en) | 1980-05-29 |
| ES493859A0 (es) | 1981-11-16 |
| ES486422A0 (es) | 1980-12-16 |
| US4250173A (en) | 1981-02-10 |
| IL58681A0 (en) | 1980-02-29 |
| US4282356A (en) | 1981-08-04 |
| ES8200669A1 (es) | 1981-11-16 |
| DE2963350D1 (en) | 1982-09-02 |
| ES8101589A1 (es) | 1980-12-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0040639B1 (de) | Isoxazol-derivate | |
| CS216544B2 (en) | Method of preparation of basic ethers | |
| EP0011963B1 (de) | Heterocyclische Carboxamidin-Verbindungen, ihre Herstellung und diese enthaltende pharmazeutische Zusammensetzungen | |
| US4518712A (en) | Piperazine derivative and analgesic composition containing the same | |
| US4159335A (en) | Substituted anilino-2-thiazolines | |
| US3927011A (en) | 2-Aminoalkyl-1-(pyridylcarbonylphenyl)imidazole compounds | |
| US3752810A (en) | Substituted n aminoalkyl arylamino imidazolines-(2) | |
| US4100292A (en) | 2-[N-phenyl-N-(cycloalkyl-methyl)-amino]-2-imidazolines and salts thereof | |
| US4379155A (en) | 3,5-Disubstituted-1H-1,2,4-triazole derivatives | |
| US4333929A (en) | Carboxamidine derivatives and hypoglycemical use | |
| US6288101B1 (en) | Imidazoles with serotonin receptor binding activity | |
| EP0018134B1 (de) | Dihydropyridin-Derivate, Verfahren zu ihrer Herstellung und sie enthaltende pharmazeutische Zusammensetzungen | |
| US4350685A (en) | Antiallergic imidodisulfamides | |
| US4355004A (en) | Benzimidazoline derivatives and pharmaceutical compositions containing them | |
| EP0018107B1 (de) | Oxazolin- und Thiazolin-Derivate, Verfahren zu ihrer Herstellung und sie enthaltende pharmazeutische Zusammensetzungen | |
| US4478838A (en) | 1-(3,4,5-Trimethoxycinnamoyl)-4-alkylaminocarbonylethyl piperazines | |
| US3965112A (en) | Imidazoline derivatives | |
| US3850947A (en) | 3-thiazol-4'-oxy-aminopropanol cardiovascular agents | |
| US3784545A (en) | 1-(5-phenyl-4-oxo-2-oxazolin-2-yl)-4-cinnamoylpiperazines | |
| US3852339A (en) | Aminoalkoxyphenylurea derivatives | |
| US4110376A (en) | Cyclopropylmethylamine derivatives | |
| CA1123852A (en) | Cyclododecane derivatives and a process for the preparation thereof | |
| US3538087A (en) | Oxadiazole derivatives | |
| US3758527A (en) | Esters of 1-aminoalkyl-cycloalkanols | |
| NO800697L (no) | Fremgangsmaate for fremstilling av heterocykliske forbindelser for anvendelse ved behandling av diabetes |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT NL SE |
|
| 17P | Request for examination filed |
Effective date: 19801121 |
|
| ITF | It: translation for a ep patent filed | ||
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT NL SE |
|
| REF | Corresponds to: |
Ref document number: 1339 Country of ref document: AT Date of ref document: 19820715 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 2963350 Country of ref document: DE Date of ref document: 19820902 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19821108 Year of fee payment: 4 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Effective date: 19821114 Ref country code: AT Effective date: 19821114 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Effective date: 19821115 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19821130 Year of fee payment: 4 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19821231 Year of fee payment: 4 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Effective date: 19831130 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Effective date: 19840601 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee | ||
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19840731 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19840801 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Effective date: 19881118 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 79302564.4 Effective date: 19850611 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |