CN107106723B - 具有吸收性粘合剂密封层的负压伤口敷料 - Google Patents
具有吸收性粘合剂密封层的负压伤口敷料 Download PDFInfo
- Publication number
- CN107106723B CN107106723B CN201580071925.8A CN201580071925A CN107106723B CN 107106723 B CN107106723 B CN 107106723B CN 201580071925 A CN201580071925 A CN 201580071925A CN 107106723 B CN107106723 B CN 107106723B
- Authority
- CN
- China
- Prior art keywords
- wound dressing
- porous layer
- adhesive gel
- layer
- absorbent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000000853 adhesive Substances 0.000 title claims abstract description 213
- 230000001070 adhesive effect Effects 0.000 title claims abstract description 213
- 239000002250 absorbent Substances 0.000 title claims abstract description 141
- 230000002745 absorbent Effects 0.000 title claims abstract description 141
- 238000007789 sealing Methods 0.000 title description 6
- 239000012530 fluid Substances 0.000 claims abstract description 18
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 21
- 239000006260 foam Substances 0.000 claims description 19
- 229910000831 Steel Inorganic materials 0.000 claims description 15
- 239000010959 steel Substances 0.000 claims description 15
- 230000005540 biological transmission Effects 0.000 claims description 14
- 239000004599 antimicrobial Substances 0.000 claims description 11
- 239000013464 silicone adhesive Substances 0.000 claims description 11
- 239000004745 nonwoven fabric Substances 0.000 claims description 9
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 claims description 8
- 239000002759 woven fabric Substances 0.000 claims description 5
- 239000012528 membrane Substances 0.000 claims description 3
- 239000010410 layer Substances 0.000 description 190
- 239000000499 gel Substances 0.000 description 126
- 206010052428 Wound Diseases 0.000 description 121
- 208000027418 Wounds and injury Diseases 0.000 description 121
- 230000008961 swelling Effects 0.000 description 28
- 239000003795 chemical substances by application Substances 0.000 description 26
- 229920000642 polymer Polymers 0.000 description 21
- 210000003491 skin Anatomy 0.000 description 21
- 238000012360 testing method Methods 0.000 description 21
- 239000000203 mixture Substances 0.000 description 19
- 239000000017 hydrogel Substances 0.000 description 18
- 239000007788 liquid Substances 0.000 description 17
- 229920002554 vinyl polymer Polymers 0.000 description 15
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 14
- 239000000463 material Substances 0.000 description 11
- -1 isooctyl acrylate ethylene oxide acrylate acrylic acid Chemical compound 0.000 description 10
- 210000000416 exudates and transudate Anatomy 0.000 description 9
- 238000000034 method Methods 0.000 description 9
- 239000000758 substrate Substances 0.000 description 8
- 239000004820 Pressure-sensitive adhesive Substances 0.000 description 7
- 239000000416 hydrocolloid Substances 0.000 description 7
- 238000005259 measurement Methods 0.000 description 7
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 6
- 229920001296 polysiloxane Polymers 0.000 description 6
- 239000000243 solution Substances 0.000 description 6
- 238000003860 storage Methods 0.000 description 6
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 5
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 5
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 5
- 239000002202 Polyethylene glycol Substances 0.000 description 5
- 235000011187 glycerol Nutrition 0.000 description 5
- 230000035876 healing Effects 0.000 description 5
- 229920001223 polyethylene glycol Polymers 0.000 description 5
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 4
- 244000007835 Cyamopsis tetragonoloba Species 0.000 description 4
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 4
- 238000001035 drying Methods 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 229920000191 poly(N-vinyl pyrrolidone) Polymers 0.000 description 4
- 238000002560 therapeutic procedure Methods 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- 239000004354 Hydroxyethyl cellulose Substances 0.000 description 3
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 3
- 229910052782 aluminium Inorganic materials 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 230000008878 coupling Effects 0.000 description 3
- 238000010168 coupling process Methods 0.000 description 3
- 238000005859 coupling reaction Methods 0.000 description 3
- 230000002209 hydrophobic effect Effects 0.000 description 3
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 description 3
- 238000005096 rolling process Methods 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 229920006395 saturated elastomer Polymers 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- VAZJLPXFVQHDFB-UHFFFAOYSA-N 1-(diaminomethylidene)-2-hexylguanidine Polymers CCCCCCN=C(N)N=C(N)N VAZJLPXFVQHDFB-UHFFFAOYSA-N 0.000 description 2
- 229920002785 Croscarmellose sodium Polymers 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- 229920002413 Polyhexanide Polymers 0.000 description 2
- 229920002367 Polyisobutene Polymers 0.000 description 2
- 229920005830 Polyurethane Foam Polymers 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 2
- 239000000654 additive Substances 0.000 description 2
- 239000012790 adhesive layer Substances 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 2
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 2
- 229920002678 cellulose Chemical class 0.000 description 2
- 235000010980 cellulose Nutrition 0.000 description 2
- YZIYKJHYYHPJIB-UUPCJSQJSA-N chlorhexidine gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O.OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O.C1=CC(Cl)=CC=C1NC(=N)NC(=N)NCCCCCCNC(=N)NC(=N)NC1=CC=C(Cl)C=C1 YZIYKJHYYHPJIB-UUPCJSQJSA-N 0.000 description 2
- 229960003333 chlorhexidine gluconate Drugs 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 239000004020 conductor Substances 0.000 description 2
- 229920001577 copolymer Polymers 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 230000008020 evaporation Effects 0.000 description 2
- 230000014509 gene expression Effects 0.000 description 2
- 150000004676 glycans Chemical class 0.000 description 2
- 230000036571 hydration Effects 0.000 description 2
- 238000006703 hydration reaction Methods 0.000 description 2
- 229920001477 hydrophilic polymer Polymers 0.000 description 2
- 229920001600 hydrophobic polymer Polymers 0.000 description 2
- 229940035535 iodophors Drugs 0.000 description 2
- 230000005865 ionizing radiation Effects 0.000 description 2
- 150000003951 lactams Chemical class 0.000 description 2
- 230000014759 maintenance of location Effects 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 230000035699 permeability Effects 0.000 description 2
- 229920001195 polyisoprene Polymers 0.000 description 2
- 229920001282 polysaccharide Polymers 0.000 description 2
- 239000005017 polysaccharide Substances 0.000 description 2
- 239000011496 polyurethane foam Substances 0.000 description 2
- 239000011148 porous material Substances 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 230000001681 protective effect Effects 0.000 description 2
- NDVLTYZPCACLMA-UHFFFAOYSA-N silver oxide Chemical compound [O-2].[Ag+].[Ag+] NDVLTYZPCACLMA-UHFFFAOYSA-N 0.000 description 2
- 239000008279 sol Substances 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- KIZJLJJOCNWOKK-UHFFFAOYSA-N 2-chloro-1-(diaminomethylidene)guanidine Chemical compound NC(N)=N\C(N)=N\Cl KIZJLJJOCNWOKK-UHFFFAOYSA-N 0.000 description 1
- OSDLLIBGSJNGJE-UHFFFAOYSA-N 4-chloro-3,5-dimethylphenol Chemical compound CC1=CC(O)=CC(C)=C1Cl OSDLLIBGSJNGJE-UHFFFAOYSA-N 0.000 description 1
- DXPPIEDUBFUSEZ-UHFFFAOYSA-N 6-methylheptyl prop-2-enoate Chemical compound CC(C)CCCCCOC(=O)C=C DXPPIEDUBFUSEZ-UHFFFAOYSA-N 0.000 description 1
- XVKLGCDWSRXKNR-UHFFFAOYSA-N 6-methylheptyl prop-2-enoate;prop-2-enamide Chemical compound NC(=O)C=C.CC(C)CCCCCOC(=O)C=C XVKLGCDWSRXKNR-UHFFFAOYSA-N 0.000 description 1
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 1
- 108010001478 Bacitracin Proteins 0.000 description 1
- 208000034309 Bacterial disease carrier Diseases 0.000 description 1
- XNCOSPRUTUOJCJ-UHFFFAOYSA-N Biguanide Chemical compound NC(N)=NC(N)=N XNCOSPRUTUOJCJ-UHFFFAOYSA-N 0.000 description 1
- 229940123208 Biguanide Drugs 0.000 description 1
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical compound [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- GHXZTYHSJHQHIJ-UHFFFAOYSA-N Chlorhexidine Chemical compound C=1C=C(Cl)C=CC=1NC(N)=NC(N)=NCCCCCCN=C(N)N=C(N)NC1=CC=C(Cl)C=C1 GHXZTYHSJHQHIJ-UHFFFAOYSA-N 0.000 description 1
- 229920001634 Copolyester Polymers 0.000 description 1
- 206010056340 Diabetic ulcer Diseases 0.000 description 1
- 206010063560 Excessive granulation tissue Diseases 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 229920002907 Guar gum Polymers 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 1
- 229930193140 Neomycin Natural products 0.000 description 1
- 229920005987 OPPANOL® Polymers 0.000 description 1
- 206010030124 Oedema peripheral Diseases 0.000 description 1
- 229920002614 Polyether block amide Polymers 0.000 description 1
- 108010093965 Polymyxin B Proteins 0.000 description 1
- 208000004210 Pressure Ulcer Diseases 0.000 description 1
- 229920002323 Silicone foam Polymers 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 206010040880 Skin irritation Diseases 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 208000031737 Tissue Adhesions Diseases 0.000 description 1
- XEFQLINVKFYRCS-UHFFFAOYSA-N Triclosan Chemical compound OC1=CC(Cl)=CC=C1OC1=CC=C(Cl)C=C1Cl XEFQLINVKFYRCS-UHFFFAOYSA-N 0.000 description 1
- 206010048038 Wound infection Diseases 0.000 description 1
- NJSSICCENMLTKO-HRCBOCMUSA-N [(1r,2s,4r,5r)-3-hydroxy-4-(4-methylphenyl)sulfonyloxy-6,8-dioxabicyclo[3.2.1]octan-2-yl] 4-methylbenzenesulfonate Chemical compound C1=CC(C)=CC=C1S(=O)(=O)O[C@H]1C(O)[C@@H](OS(=O)(=O)C=2C=CC(C)=CC=2)[C@@H]2OC[C@H]1O2 NJSSICCENMLTKO-HRCBOCMUSA-N 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 229920006322 acrylamide copolymer Polymers 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 239000002313 adhesive film Substances 0.000 description 1
- 239000002998 adhesive polymer Substances 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 238000000137 annealing Methods 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 229960003071 bacitracin Drugs 0.000 description 1
- 229930184125 bacitracin Natural products 0.000 description 1
- CLKOFPXJLQSYAH-ABRJDSQDSA-N bacitracin A Chemical compound C1SC([C@@H](N)[C@@H](C)CC)=N[C@@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]1C(=O)N[C@H](CCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2N=CNC=2)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)NCCCC1 CLKOFPXJLQSYAH-ABRJDSQDSA-N 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 230000000975 bioactive effect Effects 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 229910001424 calcium ion Inorganic materials 0.000 description 1
- 239000001913 cellulose Chemical class 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 229960003260 chlorhexidine Drugs 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 229910003460 diamond Inorganic materials 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 210000002615 epidermis Anatomy 0.000 description 1
- 150000002118 epoxides Chemical class 0.000 description 1
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 210000001126 granulation tissue Anatomy 0.000 description 1
- 230000037313 granulation tissue formation Effects 0.000 description 1
- 239000000665 guar gum Substances 0.000 description 1
- 235000010417 guar gum Nutrition 0.000 description 1
- 229960002154 guar gum Drugs 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- PNDPGZBMCMUPRI-UHFFFAOYSA-N iodine Chemical compound II PNDPGZBMCMUPRI-UHFFFAOYSA-N 0.000 description 1
- 210000000629 knee joint Anatomy 0.000 description 1
- 238000010030 laminating Methods 0.000 description 1
- 238000003475 lamination Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000011278 mitosis Effects 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 238000009581 negative-pressure wound therapy Methods 0.000 description 1
- 229960004927 neomycin Drugs 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 239000001814 pectin Substances 0.000 description 1
- 229920001277 pectin Polymers 0.000 description 1
- 235000010987 pectin Nutrition 0.000 description 1
- 150000002978 peroxides Chemical class 0.000 description 1
- 239000008177 pharmaceutical agent Substances 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 229920005597 polymer membrane Polymers 0.000 description 1
- 229920000024 polymyxin B Polymers 0.000 description 1
- 229960005266 polymyxin b Drugs 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 229920000098 polyolefin Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 229920006264 polyurethane film Polymers 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 150000003242 quaternary ammonium salts Chemical class 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 238000010526 radical polymerization reaction Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000036573 scar formation Effects 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 239000013514 silicone foam Substances 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 229910001923 silver oxide Inorganic materials 0.000 description 1
- 206010040872 skin infection Diseases 0.000 description 1
- 230000036556 skin irritation Effects 0.000 description 1
- 231100000475 skin irritation Toxicity 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 229910001415 sodium ion Inorganic materials 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 229920001897 terpolymer Polymers 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 230000000472 traumatic effect Effects 0.000 description 1
- 229960003500 triclosan Drugs 0.000 description 1
- 230000029663 wound healing Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/46—Deodorants or malodour counteractants, e.g. to inhibit the formation of ammonia or bacteria
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/00051—Accessories for dressings
- A61F13/00063—Accessories for dressings comprising medicaments or additives, e.g. odor control, PH control, debriding, antimicrobic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/02—Adhesive plasters or dressings
- A61F13/0203—Adhesive plasters or dressings having a fluid handling member
- A61F13/0206—Adhesive plasters or dressings having a fluid handling member the fluid handling member being absorbent fibrous layer, e.g. woven or nonwoven absorbent pad, island dressings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/02—Adhesive plasters or dressings
- A61F13/0203—Adhesive plasters or dressings having a fluid handling member
- A61F13/022—Adhesive plasters or dressings having a fluid handling member having more than one layer with different fluid handling characteristics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/02—Adhesive plasters or dressings
- A61F13/0246—Adhesive plasters or dressings characterised by the skin adhering layer
- A61F13/0253—Adhesive plasters or dressings characterised by the skin adhering layer characterized by the adhesive material
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/02—Adhesive plasters or dressings
- A61F13/0259—Adhesive plasters or dressings characterised by the release liner covering the skin adhering layer
- A61F13/0266—Adhesive plasters or dressings characterised by the release liner covering the skin adhering layer especially adapted for wound covering/occlusive dressings
-
- A61F13/05—
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/22—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing macromolecular materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/22—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing macromolecular materials
- A61L15/24—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/22—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing macromolecular materials
- A61L15/26—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/425—Porous materials, e.g. foams or sponges
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/44—Medicaments
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/58—Adhesives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/60—Liquid-swellable gel-forming materials, e.g. super-absorbents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B25/00—Layered products comprising a layer of natural or synthetic rubber
- B32B25/04—Layered products comprising a layer of natural or synthetic rubber comprising rubber as the main or only constituent of a layer, which is next to another layer of the same or of a different material
- B32B25/042—Layered products comprising a layer of natural or synthetic rubber comprising rubber as the main or only constituent of a layer, which is next to another layer of the same or of a different material of natural rubber or synthetic rubber
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B25/00—Layered products comprising a layer of natural or synthetic rubber
- B32B25/04—Layered products comprising a layer of natural or synthetic rubber comprising rubber as the main or only constituent of a layer, which is next to another layer of the same or of a different material
- B32B25/045—Layered products comprising a layer of natural or synthetic rubber comprising rubber as the main or only constituent of a layer, which is next to another layer of the same or of a different material of foam
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B25/00—Layered products comprising a layer of natural or synthetic rubber
- B32B25/04—Layered products comprising a layer of natural or synthetic rubber comprising rubber as the main or only constituent of a layer, which is next to another layer of the same or of a different material
- B32B25/08—Layered products comprising a layer of natural or synthetic rubber comprising rubber as the main or only constituent of a layer, which is next to another layer of the same or of a different material of synthetic resin
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B25/00—Layered products comprising a layer of natural or synthetic rubber
- B32B25/10—Layered products comprising a layer of natural or synthetic rubber next to a fibrous or filamentary layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B25/00—Layered products comprising a layer of natural or synthetic rubber
- B32B25/14—Layered products comprising a layer of natural or synthetic rubber comprising synthetic rubber copolymers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/06—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/06—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material
- B32B27/065—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material of foam
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/06—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material
- B32B27/08—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material of synthetic resin
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/12—Layered products comprising a layer of synthetic resin next to a fibrous or filamentary layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/28—Layered products comprising a layer of synthetic resin comprising synthetic resins not wholly covered by any one of the sub-groups B32B27/30 - B32B27/42
- B32B27/285—Layered products comprising a layer of synthetic resin comprising synthetic resins not wholly covered by any one of the sub-groups B32B27/30 - B32B27/42 comprising polyethers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/34—Layered products comprising a layer of synthetic resin comprising polyamides
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/36—Layered products comprising a layer of synthetic resin comprising polyesters
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/40—Layered products comprising a layer of synthetic resin comprising polyurethanes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B3/00—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar form; Layered products having particular features of form
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B3/00—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar form; Layered products having particular features of form
- B32B3/26—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar form; Layered products having particular features of form characterised by a particular shape of the outline of the cross-section of a continuous layer; characterised by a layer with cavities or internal voids ; characterised by an apertured layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B3/00—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar form; Layered products having particular features of form
- B32B3/26—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar form; Layered products having particular features of form characterised by a particular shape of the outline of the cross-section of a continuous layer; characterised by a layer with cavities or internal voids ; characterised by an apertured layer
- B32B3/266—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar form; Layered products having particular features of form characterised by a particular shape of the outline of the cross-section of a continuous layer; characterised by a layer with cavities or internal voids ; characterised by an apertured layer characterised by an apertured layer, the apertures going through the whole thickness of the layer, e.g. expanded metal, perforated layer, slit layer regular cells B32B3/12
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B5/00—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts
- B32B5/02—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by structural features of a fibrous or filamentary layer
- B32B5/022—Non-woven fabric
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B5/00—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts
- B32B5/02—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by structural features of a fibrous or filamentary layer
- B32B5/024—Woven fabric
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B5/00—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts
- B32B5/02—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by structural features of a fibrous or filamentary layer
- B32B5/026—Knitted fabric
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B5/00—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts
- B32B5/18—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by features of a layer of foamed material
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B5/00—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts
- B32B5/22—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by the presence of two or more layers which are next to each other and are fibrous, filamentary, formed of particles or foamed
- B32B5/24—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by the presence of two or more layers which are next to each other and are fibrous, filamentary, formed of particles or foamed one layer being a fibrous or filamentary layer
- B32B5/245—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by the presence of two or more layers which are next to each other and are fibrous, filamentary, formed of particles or foamed one layer being a fibrous or filamentary layer another layer next to it being a foam layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B5/00—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts
- B32B5/22—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by the presence of two or more layers which are next to each other and are fibrous, filamentary, formed of particles or foamed
- B32B5/24—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by the presence of two or more layers which are next to each other and are fibrous, filamentary, formed of particles or foamed one layer being a fibrous or filamentary layer
- B32B5/26—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by the presence of two or more layers which are next to each other and are fibrous, filamentary, formed of particles or foamed one layer being a fibrous or filamentary layer another layer next to it also being fibrous or filamentary
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B5/00—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts
- B32B5/22—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by the presence of two or more layers which are next to each other and are fibrous, filamentary, formed of particles or foamed
- B32B5/32—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by the presence of two or more layers which are next to each other and are fibrous, filamentary, formed of particles or foamed at least two layers being foamed and next to each other
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B7/00—Layered products characterised by the relation between layers; Layered products characterised by the relative orientation of features between layers, or by the relative values of a measurable parameter between layers, i.e. products comprising layers having different physical, chemical or physicochemical properties; Layered products characterised by the interconnection of layers
- B32B7/04—Interconnection of layers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B7/00—Layered products characterised by the relation between layers; Layered products characterised by the relative orientation of features between layers, or by the relative values of a measurable parameter between layers, i.e. products comprising layers having different physical, chemical or physicochemical properties; Layered products characterised by the interconnection of layers
- B32B7/04—Interconnection of layers
- B32B7/08—Interconnection of layers by mechanical means
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B7/00—Layered products characterised by the relation between layers; Layered products characterised by the relative orientation of features between layers, or by the relative values of a measurable parameter between layers, i.e. products comprising layers having different physical, chemical or physicochemical properties; Layered products characterised by the interconnection of layers
- B32B7/04—Interconnection of layers
- B32B7/12—Interconnection of layers using interposed adhesives or interposed materials with bonding properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B7/00—Layered products characterised by the relation between layers; Layered products characterised by the relative orientation of features between layers, or by the relative values of a measurable parameter between layers, i.e. products comprising layers having different physical, chemical or physicochemical properties; Layered products characterised by the interconnection of layers
- B32B7/04—Interconnection of layers
- B32B7/12—Interconnection of layers using interposed adhesives or interposed materials with bonding properties
- B32B7/14—Interconnection of layers using interposed adhesives or interposed materials with bonding properties applied in spaced arrangements, e.g. in stripes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/404—Biocides, antimicrobial agents, antiseptic agents
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2250/00—Layers arrangement
- B32B2250/44—Number of layers variable across the laminate
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2255/00—Coating on the layer surface
- B32B2255/02—Coating on the layer surface on fibrous or filamentary layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2255/00—Coating on the layer surface
- B32B2255/10—Coating on the layer surface on synthetic resin layer or on natural or synthetic rubber layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2255/00—Coating on the layer surface
- B32B2255/26—Polymeric coating
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2262/00—Composition or structural features of fibres which form a fibrous or filamentary layer or are present as additives
- B32B2262/02—Synthetic macromolecular fibres
- B32B2262/0276—Polyester fibres
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2262/00—Composition or structural features of fibres which form a fibrous or filamentary layer or are present as additives
- B32B2262/02—Synthetic macromolecular fibres
- B32B2262/0292—Polyurethane fibres
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2262/00—Composition or structural features of fibres which form a fibrous or filamentary layer or are present as additives
- B32B2262/04—Cellulosic plastic fibres, e.g. rayon
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2266/00—Composition of foam
- B32B2266/02—Organic
- B32B2266/0214—Materials belonging to B32B27/00
- B32B2266/0278—Polyurethane
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2266/00—Composition of foam
- B32B2266/06—Open cell foam
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2270/00—Resin or rubber layer containing a blend of at least two different polymers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/40—Properties of the layers or laminate having particular optical properties
- B32B2307/412—Transparent
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/50—Properties of the layers or laminate having particular mechanical properties
- B32B2307/51—Elastic
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/50—Properties of the layers or laminate having particular mechanical properties
- B32B2307/542—Shear strength
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/50—Properties of the layers or laminate having particular mechanical properties
- B32B2307/546—Flexural strength; Flexion stiffness
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/50—Properties of the layers or laminate having particular mechanical properties
- B32B2307/558—Impact strength, toughness
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/714—Inert, i.e. inert to chemical degradation, corrosion
- B32B2307/7145—Rot proof, resistant to bacteria, mildew, mould, fungi
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/718—Weight, e.g. weight per square meter
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/72—Density
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/724—Permeability to gases, adsorption
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/726—Permeability to liquids, absorption
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/726—Permeability to liquids, absorption
- B32B2307/7265—Non-permeable
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/728—Hydrophilic
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/73—Hydrophobic
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/732—Dimensional properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2535/00—Medical equipment, e.g. bandage, prostheses, catheter
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2556/00—Patches, e.g. medical patches, repair patches
Abstract
本公开提供了一种伤口敷料。所述敷料包括:湿气可透过的背衬层,所述湿气可透过的背衬层具有第一主表面、第二主表面、背衬层周边和第一开口;粘附到所述背衬层的所述第二主表面的吸收性粘合剂凝胶,所述吸收性粘合剂凝胶包括粘合剂凝胶周边;以及多孔层,所述多孔层具有第一侧面、第二侧面和多孔层周边。所述第二主表面具有设置于其上的第一粘合剂。所述多孔层的所述第一侧面粘附到所述吸收性粘合剂凝胶。所述多孔层被构造成有利于使流体通过,到达所述第一开口。所述粘合剂凝胶周边百分之百与所述背衬层重叠。所述多孔层周边的至少50%与所述吸收性粘合剂凝胶重叠。
Description
相关申请的交叉引用
本专利申请要求2014年12月30日提交的美国临时专利申请号62/098,058和2015年2月6日提交的美国临时专利申请号62/112,714的优先权,这些临时专利申请的公开内容全文以引用方式并入本文。
背景技术
伤口的治疗已经促使开发出多种方法以利于愈合。一种常用技术已采用负压疗法(“NPT”),该疗法也被称为抽吸或真空疗法。已经开发出各种NPT装置以使得在隔离伤口以保护伤口的同时能够移除渗出物(即身体分泌物),以便缩短其恢复时间。
最近开发的NPT形式被称为封闭式负压引流(“VAC”)技术。采用VAC技术治疗伤口基于这样的前提:当受控负压施加于伤口时会刺激有丝分裂,这导致形成新生血管并导致伤口闭合。
研究已经表明,这种疗法通过下列方式有助于伤口愈合:通过提供潮湿保护环境、通过减轻伤口附近的外周性水肿、通过刺激血液循环至创面床、通过减少细菌定植以及通过增大肉芽组织形成和上皮形成速率。
NPT可用于治疗多种类型的伤口,包括急性、亚急性、慢性、创伤、图形(graphs)、皮瓣、压迫溃疡和糖尿病性溃疡。已有研究表明,NPT也有利于深伤口或腔洞伤口(cavitywounds)的愈合。具体地讲,它使死亡组织、碎片和/或渗出物在真空压力下从伤口区域离开,从而提高愈合速率。此外,已有研究表明,使用NPT治疗手术闭合切口可减少并发症、增强组织-组织附着并减少疤痕形成。
这些方法通常包括在伤口上形成不透水密封。一般来讲,不透水密封粘附到围绕伤口区域的表皮的一部分上。一种类型的伤口敷料将多孔泡沫插入伤口中。有时,排水管、排水泵和敷料盖件与多孔泡沫插件组合以形成从伤口虹吸渗出物的系统。存在与这种类型的敷料相关的问题。已经开发出另一种类型的敷料,该敷料使用具有一体结构的柔性单片敷料,该一体结构结合排水管,作为外伤口盖件的整体部分。
虽然存在多种可用的伤口敷料(包括NPT伤口敷料),但仍需要可处理伤口渗出物并提供有利于组织愈合的环境的伤口敷料。
发明内容
本公开整体涉及伤口敷料。具体地讲,本公开涉及伤口敷料,该伤口敷料可连接至负压源以便去除伤口部位处不期望的和/或多余的流体,从而促使组织愈合。本公开的伤口敷料有利地提供了用于使液体移动离开伤口部位的两条途径:1)结合通向真空源的液体路径放置的岐管多孔层,以及2)可高度透过湿气的层合物,该层合物包括吸收性粘合剂凝胶和背衬层,这提供了蒸发水分通过伤口敷料损失的方式。此外,高度适形的吸收性粘合剂凝胶有利于使敷料密封在皮肤上,并从而帮助维持对伤口部位的负压,即使当敷料施加于相对运动的解剖部位诸如关节(如,膝关节)时也是如此。凝胶充当多余伤口渗出物的吸收性储液器,并且相对于敷料中的其他吸收性层具有高保湿性,这使得当敷料用于负压伤口治疗时可使用较小筒(即,收集储液器)。最后,吸收性粘合剂凝胶向干伤口周边皮肤提供温和粘附力,这样在移除时降低皮肤创伤,并且它还提供对伤口部位的增强的固定,防止不期望的剪切力。
在一个方面,本公开提供了一种伤口敷料。这种伤口敷料可包括:湿气可透过的背衬层,该湿气可透过的背衬层具有第一主表面、第二主表面、背衬层周边和第一开口;粘附到背衬层第二主表面的至少一部分上的吸收性粘合剂凝胶,该吸收性粘合剂凝胶包括粘合剂凝胶周边;以及多孔层,该多孔层具有第一侧面、第二侧面和多孔层周边。第二主表面具有设置于其上的靠近背衬层周边的第一粘合剂。多孔层的第一侧面粘附到吸收性粘合剂凝胶。多孔层被构造成有利于使流体通过,到达第一开口。粘合剂凝胶周边百分之百与背衬层重叠。多孔层周边的至少50%与吸收性粘合剂凝胶重叠。
任选地,在任何实施方案中,吸收性粘合剂凝胶具有第二开口。在这些实施方案中,第一开口的至少一部分与第二开口的至少一部分重叠。
在任何实施方案中,多孔层周边的至少50%与吸收性粘合剂凝胶重叠。在任何实施方案中,多孔层周边的超过50%与吸收性粘合剂凝胶重叠。
在上述任一实施方案中,吸收性粘合剂凝胶可具有约0.2mm至约4.0mm的厚度。在上述任一实施方案中,吸收性粘合剂凝胶可包含少于40%(w/w)的水。在上述任一实施方案中,该敷料还包括粘附到多孔层的第二侧面上的穿孔层。在上述任一实施方案中,穿孔层(当存在时)可具有面向多孔层第二侧面的第一主表面和与第一主表面相背对的第二主表面,其中第二主表面包含有机硅粘合剂。在上述任一实施方案中,其上粘附有吸收性粘合剂凝胶的背衬层可具有通过EN-13726-1:2002第3.3部分测得的≥1g/10cm2/24h的湿气透过率。在上述任一实施方案中,在24℃、1rad/s的剪切速率下,吸收性粘合剂凝胶可具有介于约0.5N/cm2和约5N/cm2之间的剪切模量G’。在上述任一实施方案中,在24℃、1rad/s的剪切速率下,吸收性粘合剂凝胶可具有介于约0.2N/cm2和约2N/cm2之间的损耗剪切模量G”。在上述任一实施方案中,多孔吸收层可包括开孔泡沫(优选地,非水溶胀性泡沫,诸如用于可购自美国德克萨斯州圣安东尼奥的动力学概念公司(Kinetic Concepts,Inc.,San Antonio,TX)的GRANUFOAMTM敷料中的泡沫,或用于可购自美国明尼苏达州圣保罗的3M公司(3MCompany,St.Paul,MN)的3MTM TEGADERMTM高性能泡沫粘合剂敷料中的泡沫)、织造织物或非织造织物。在上述任一实施方案中,当与等渗盐水溶液在37℃下接触24小时时,吸收性粘合剂凝胶能够吸收其干重的≥1.5倍。
如本文所用,“水凝胶”和“亲水性凝胶”是指在接触水和其他亲水性溶胀剂时能够溶胀的亲水性聚合物连续相。该术语的使用与水合状态无关。基于无水状态水凝胶的重量计,可用的水凝胶将吸收至少40重量%。水凝胶为亲水性聚合物,其特征在于其亲水性(即,能够吸收大量流体,诸如伤口渗出物)。水凝胶通常为透明或半透明的,而无论其水化程度如何。一般来讲,水凝胶与水性胶体的区别是明显的,该水性胶体通常包括包含分散的亲水性粒子的疏水性基质。
如本文所用,“水性胶体”是指疏水聚合物的连续相,该疏水聚合物包含分散于其中的15重量%至45重量%的吸收性亲水颗粒、聚合物或纤维。疏水聚合物可基于有机硅或聚烯烃。根据其干重计,可用的水性胶体(当用作吸收性粘合剂凝胶时)将吸收至少150重量%。
术语“包括”及其变型形式在说明书和权利要求中出现这些术语的地方不具有限制的含义。
如本文所用,“一个”、“一种”、“所述(该)”、“至少一个(种)”以及“一个(种)或多个(种)”可互换使用。因此,例如多孔层可解释为意指“一个或多个”多孔层。
术语“和/或”意指所列要素的一个或全部,或者所列要素的任何两个或更多个的组合。
另外,在本文中,通过端点表述的数值范围包括该范围内包含的所有数值(例如,1至5包括1、1.5、2、2.75、3、3.80、4、5等)。
本发明的上述发明内容并非意图描述本发明的每个公开的实施方案或每种实施方式。以下描述更具体地举例说明示例性实施方案。在本专利申请的全文的若干处,通过实施例列表提供了指导,这些实施例可以各种组合使用。在每种情况下,引用的列表都只用作代表性的组,而不应理解为排它性列表。
这些及其他实施方案的附加细节在下文附图和描述中示出。通过具体实施方式、附图和权利要求书,其它特征、对象和优点将变得显而易见。
附图说明
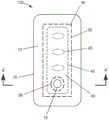
图1是根据本公开的具有任选穿孔层的伤口敷料的一个实施方案的示意性平面图。
图2是图1的伤口敷料的分解侧视图。
图3A和图3B是根据本公开的伤口敷料的替代实施方案的示意性平面图,其中吸收性粘合剂凝胶周边不与多孔层周边100%重叠。
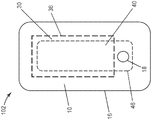
图4是施加至治疗部位的本公开伤口敷料的一个实施方案的示意性侧视图,该伤口敷料包括真空端口。
图5是用于测试根据本公开的伤口敷料的真空密封和液体管理特性的设备的示意性平面图。
具体实施方式
在详细解释本公开的任何实施方案之前,应当了解,本发明在其应用中不限于下文描述中所提及或下文附图中所示出的构造细节和部件布置方式。本发明能够具有其它实施方案,并且能够通过各种方式实践或进行。另外,应当理解,本文使用的措词和术语是用于说明目的而不应被视为限制性的。本文使用的“包括”、“包含”或“具有”及其变型形式意在涵盖其后列出的项目及其等同形式以及附加的项目。除非另外说明或限定,否则术语“连接”和“联接”及其变型形式均按广义使用,并且涵盖直接和间接这两方面的连接和联接。此外,“连接”和“联接”不限于物理或机械连接或联接。应当理解,在不偏离本公开范围的情况下,可采用其他实施方案并且可进行结构变化或逻辑变化。此外,术语例如“前面”“后面”“顶部”“底部”等仅用于描述与另一个元件相关的元件,但绝非意图说明装置的具体方位,表明或暗示装置必需或需要的方位,或指定在应用中如何使用、安装、展示或放置本文所述的发明。
本公开整体涉及负压型伤口敷料。这些敷料用于管理伤口部位处的流体积聚并用于在伤口表面和伤口覆盖物之间生成界面压力以刺激肉芽组织的形成和生长。本发明敷料提供了用于流体管理的两种不同机制(例如,排出途径)。此外,构造敷料的材料提供了这样的表面,其有利于伤口与解剖结构符合,并相对于其他负压敷料构造维持负压环境更长的时间段。
现在转到附图,图1是根据本公开的伤口敷料100的一个实施方案的分解侧视图,并且图2是其平面图。敷料100包括背衬层10、粘附到湿气可透过的背衬层10的至少一部分的吸收性粘合剂凝胶30,和多孔层40。在任何实施方案中,敷料100任选地包括穿孔层50。伤口敷料100可以各种形状形成,包括例如圆形、椭圆形、梯形、矩形和正方形,其对于各种形状中的每种可包括圆角。
背衬层10具有第一主表面12、与第一主表面相背对的第二主表面14、限定背衬层边缘的背衬层周边16、以及从第一主表面12延伸至第二主表面14的开口18。背衬层10由顺应性足以与皮肤表面的解剖轮廓相符的柔性材料(例如,聚合物弹性膜、弹性非织造织物或它们的组合)制成。背衬层周边16限定背衬层10的外边缘。
合适的背衬层10包括例如非织造纤维网、织造纤维网、机织材料、膜和其他熟悉的背衬材料。优选的背衬材料是半透明的或透明的弹性聚合物膜。最优选地,背衬为水蒸气透过度较高的膜背衬。美国专利号3,645,835描述了制备此类膜的方法和用于测试其湿气渗透性的方法,该专利的公开内容据此以引用方式并入。
在任何实施方案中,背衬层10具有约15μm至约250μm的厚度。
第一粘合剂20粘附到(例如,涂覆到)至少一部分(例如,沿着靠近整个背衬层周边16的第二主表面14延伸的连续部分)。在使用中,第一粘合剂20在背衬层10与施用敷料的皮肤之间形成密封(例如,围绕整个背衬层16的液体密封)。
优选地,背衬层/第一粘合剂复合物应当允许湿气以等于或大于人体皮肤的速率透过。优选地,根据EN-13726-1:2002第3.3部分测得,未涂覆的膜(即,其上未涂覆第一粘合剂的背衬层)以至少10g/10cm2/24h的速率透过湿气。甚至更优选地,未涂覆的背衬层以大于20g/10cm2/24h的速率透过湿气。在任何实施方案中,背衬层的被粘合剂涂覆的部分以至少0.8g/10cm2/24h,优选地大于1.6g/10cm2/24h的速率透过湿气。
背衬层10优选地适形于解剖表面。因此,当将背衬层施加到解剖表面上时,即使当该表面移动时它也适形于该表面。优选的背衬层10也适形于动物的解剖关节。当该关节弯曲并随后返回到其未弯曲位置时,该背衬层拉伸以适应该关节的弯曲,但其具有足够的回弹力以当该关节返回到其未弯曲状态时继续适形于该关节。优选地,背衬层具有大于200%的极限伸长率。更优选地,背衬层具有大于400%的极限伸长率。
在公布的美国专利号5,088,483和5,160,315中可找到优选用于本公开的伤口敷料的背衬层的该特性的描述,所述专利的公开内容据此全文以引用方式并入。特别优选的背衬层是弹性体聚氨酯、共聚酯或聚醚嵌段酰胺膜。这些膜结合了存在于优选背衬层中的回弹性、高湿气渗透性和透明性的理想性能。
虽然任何压敏粘合剂均可用于第一粘合剂20,但优选的粘合剂包括适度与皮肤相容的压敏粘合剂,诸如柔性有机硅凝胶粘合剂、基于的粘合剂、水性胶体粘合剂,或美国专利号RE 24,906所述的丙烯酸酯共聚物,该专利的公开内容据此全文以引用方式并入。特别优选的是97:3的丙烯酸异辛酯:丙烯酰胺共聚物。还优选的是70:15:15的丙烯酸异辛酯:环氧乙烷丙烯酸酯:丙烯酸的三元共聚物,如美国专利号4,737,410(实施例31)中所述的,该专利的公开内容全文以引用方式并入本文。用于第一粘合剂20的其他可用粘合剂在美国专利号3,389,827、4,112,213、4,310,509和4,323,557中有所描述,所述专利的公开内容据此全文以引用方式并入。也可设想到在粘合剂中添加药剂或抗菌剂,如美国专利号4,310,509和4,323,557中所述,这两个专利据此全文均以引用方式并入。
用于上述第一粘合剂20的优选压敏粘合剂优选地以大于或等于人体皮肤速率的速率传输湿气。虽然可以通过选择适当的粘合剂或通过使用非织造(例如,熔喷)粘合剂(如美国专利号6,171,985、美国专利号6,368,687和PCT公布号WO 99/27975所述,这些专利的全部内容全文以引用方式并入本文中)实现这样的特性,也可以设想在本公开的伤口敷料中可使用实现高湿气透过相对速率的其他方法,诸如对背衬层上的第一粘合剂进行图案涂覆(未示出)。另外,当在37摄氏度下的等渗盐水中浸没24小时时,该粘合剂具有低吸收性(即,小于其干重的25%,并且优选地小于其干重的10%)。
吸收性粘合剂凝胶30粘附到背衬层10的第二主表面14的至少一部分上。吸收性粘合剂凝胶30包括限定粘合剂凝胶周边的外边缘的吸收性粘合剂凝胶周边36。吸收性粘合剂凝胶周边36与背衬层周边16间隔开。因此,在使用中,当将背衬层10的第一粘合剂20施加到处理表面(未示出)时,背衬层10与吸收性粘合剂凝胶周边36 100%重叠。如本文所用,如果第一片(例如,背衬层)覆盖第二片(例如,吸收性粘合剂凝胶)的一部分或某个第三片的一部分(沿着其相对侧被第二片覆盖),则该第一片可以说是与第二片“重叠”或“叠置”。换句话讲,尽管由第三片隔开,但一片可与另一片“重叠”或“叠置”。
任选地,吸收性粘合剂凝胶包括第二开口38。当存在时,第二开口38优选地与吸收性粘合剂凝胶周边间隔开。背衬层10的第一开口18的至少一部分在其与多孔层之间不具有吸收性粘合剂凝胶30(例如,第一开口位于某一部分处,在该部分中在第一开口和多孔层之间不存在吸收性粘合剂凝胶,如图3A的伤口敷料102所示;或者第一开口与吸收性粘合剂凝胶中第二开口38的至少一部分重叠,如图3B所示)。在任何实施方案中,第一开口和第二开口可以共延。这种构造有利于液体从(皮肤)治疗表面流动通过多孔层40并经由第一开口18从背衬层10流出。吸收性粘合剂凝胶30可相对较薄。在任何实施方案中,吸收性粘合剂凝胶具有约0.2mm至约4.0mm的厚度。在任何实施方案中,吸收性粘合剂凝胶具有约0.4mm至约3mm的厚度。
吸收性粘合剂凝胶30能够吸收并保持具有与血液相似的离子强度的水性液体。因此,通过量化生理盐水的吸光度可容易地测试该特性。在任何实施方案中,当与等渗盐水溶液在37℃下接触24小时时,吸收性粘合剂凝胶能够吸收其干重的至少0.4倍;优选地,其干重的至少1.0倍;更优选地,大于或等于其干重的1.5倍;甚至更优选地,大于或等于其干重的4倍。凝胶也具有高保湿性,因为当利用40mm汞柱的压力从外部压缩该粘合剂时,该粘合剂将保持大于其吸收能力的50%(即,残余吸收能力)。
在任何实施方案中,吸收性粘合剂凝胶30可包括水凝胶,其包含聚甘油-3、交联的聚乙烯吡咯烷酮和/或羟丙基瓜尔胶。合适的吸收性粘合剂水凝胶的非限制性示例以及制备所述吸收性粘合剂水凝胶的方法在美国专利申请号2009/0187130中有所描述,该专利申请全文据此以引用方式并入。任选地,吸收性粘合剂凝胶可包含抗菌剂,诸如例如双胍类抗菌剂(例如,葡萄糖酸氯己定)。
合适的水凝胶组合物包括例如天然水凝胶,诸如果胶、明胶或羧甲基纤维素(CMC)(美国特拉华州威明顿的亚跨龙公司(Aqualon Corp.,Wilmington,Del.));半合成水凝胶,诸如交联的羧甲基纤维素X4ink CMC(例如,Ac-Di-Sol;美国宾夕法尼亚州费城的富曼实公司(FMC Corp.,Philadelphia,Pa.));合成水凝胶,诸如交联的聚丙烯酸(PAA)(例如,CARBOPOLTM No.974P;美国俄亥俄州布雷克斯维尔的古德里奇公司(B.F.Goodrich,Brecksville,Ohio)),或它们的组合。
在大多数实施方案中,水凝胶粘合剂包含可溶胀的交联聚(N-乙烯基内酰胺)、溶胀剂和改性聚合物,它们以足以形成如在美国专利公布号2004/0247655中进一步描述的内聚压敏粘合剂组合物的量存在。与交联的可溶胀的聚(N-乙烯基内酰胺)混合的溶胀剂的量可在组合物的约50重量%至约90重量%的范围内。因此,除了要添加到组合物中的任何生物相容性和/或治疗性和/或离子导电性材料之外,可溶胀的聚(N-乙烯基内酰胺)的重量百分比可为约10重量%至约50重量%。当聚(N-乙烯基内酰胺)为聚(N-乙烯基吡咯烷酮)时,聚(N-乙烯基吡咯烷酮)的重量百分比可在约15%至约45%的范围内。在特定实施方案中,聚(N-乙烯基吡咯烷酮)可在约18%至约35%的范围内。
在大多数实施方案中,本发明的水凝胶粘合剂组合物包含可溶胀的聚(N-乙烯基内酰胺),其通常在内酰胺处于固体形式时被辐射交联。在其他实施方案中,聚(N-乙烯基)内酰胺通过自由基聚合而在前体的本体或溶液中交联,所述前体包含N-乙烯基内酰胺单体,任选地其他单体以及如美国专利号4,931,282所述的交联化合物。可用于本公开的吸收性粘合剂中的聚(N-乙烯基内酰胺)可以易于被交联的任何形式提供,诸如美国专利号4,931,282、5,225,473和5,389,376所述的固体形式。通常,聚(N-乙烯基内酰胺)为N-乙烯基-2-吡咯烷酮的均聚物。
在暴露于电离辐射后,聚(N-乙烯基内酰胺)在水中的溶胀能力可为至少约15,通常至少约30,并且通常至少约40,如美国专利号5,409,966所述,该专利全文以引用方式并入本文中。任何固体形式的聚(N-乙烯基内酰胺)在经受来自高能量源的电离辐射时可交联以供使用。
改性聚合物存在于水凝胶粘合剂组合物中,以保持和/或增加内聚性,同时降低粘合性。当加入溶胀剂时,改性聚合物将溶解或悬浮在溶胀剂中。通常,当以1:9的改性聚合物对溶胀剂的比例将改性聚合物与溶胀剂混合时,改性聚合物将形成粘稠溶液或粘稠凝胶。
溶胀剂的选择通常将确定适当的改性聚合物以实现粘附性的降低,同时保持或改善粘合剂组合物的内聚力。难溶于一种溶胀剂中的改性聚合物可在用于本发明的不同溶胀剂中高度溶胀。在一些实施方案中,合适的改性可溶胀聚合物的示例包括但不限于多糖、多糖衍生物、丙烯酸酯、丙烯酸酯衍生物、纤维素、纤维素衍生物以及它们的组合。
在特定实施方案中,用于本发明的改性可溶胀聚合物为羟丙基瓜尔胶、瓜尔胶、羟乙基纤维素、羟丙基纤维素、羟丙基甲基纤维素、与三烷基铵取代的环氧化物反应的羟乙基纤维素的聚合季铵盐、羟乙基纤维素和二烯丙基二甲基氯化铵的共聚物,以及上述的衍生物及它们的组合。
改性聚合物的量可在组合物的最多约50重量%的范围内。因此,除了要添加到组合物中的任何生物相容性和/或治疗性的和/或离子导电性材料之外,改性聚合物的重量百分比可为约0.1重量%至约40重量%。当改性聚合物为羟丙基瓜尔胶时,羟丙基瓜尔胶的重量百分比可在约1%至约20%的范围内。
水凝胶粘合剂组合物还包含溶胀剂,该溶胀剂可溶胀交联的聚(N-乙烯基内酰胺)聚合物和改性聚合物并且与人体皮肤生物相容。可用于溶胀聚(N-乙烯基内酰胺)的溶胀剂的非限制性示例包括一元醇(例如,乙醇和异丙醇)、多元醇(例如,乙二醇、丙二醇、聚乙二醇(分子量在200和600之间)和甘油)、醚醇(例如,二醇醚)、不引起皮肤刺激或毒性反应的其他多元醇溶胀剂,和水。
根据水凝胶粘合剂组合物所需的最终用途,可使用非挥发性和/或挥发性溶胀剂。一种合适的溶胀剂可包含挥发性溶胀剂和非挥发性溶胀剂,诸如甘油或聚乙二醇与水的混合物。在一些实施方案中,非挥发性溶胀剂可单独使用,诸如例如甘油或聚乙二醇。同样,挥发性溶胀剂诸如水可在本发明的组合物中单独使用。对于本发明,“基本上不挥发”是指本发明中所使用的溶胀剂将使粘合剂聚合物诸如辐射的聚(N-乙烯基内酰胺)、充分内聚的压敏粘合剂,使得在暴露于加工或储存条件之后,小于给定体积的非挥发性溶胀剂的百分之十(10%)蒸发。
溶胀剂可以水凝胶粘合剂组合物的约50重量%至约90重量%范围内的量、优选地约60重量%至约80重量%范围内的量添加。在一些实施方案中,甘油和聚乙二醇被选择作为基本上不挥发的溶胀剂。甘油和聚乙二醇可占溶胀剂的最多100重量%。
吸收性粘合剂凝胶30可用于包含多种物质,任选地包含抗菌剂、用于透皮给药的药物、用于监控患者激素或其他物质的化学指示剂等。
吸收性粘合剂凝胶可将抗菌剂递送到皮肤,减少感染经皮器械的可能性,或者治疗皮肤或伤口的感染。在大多数实施方案中,抗菌剂以总组合物的最多10重量%的水平添加。
存在许多生物活性材料,其包括抗菌剂。抗菌剂的示例包括对氯间二甲苯酚、三氯生、氯己定及其盐诸如葡萄糖酸氯己定、聚六亚甲基双胍及其盐诸如聚六亚甲基双胍胺氯、碘、碘伏、脂肪酸单酯、聚-n-乙烯基吡咯烷酮碘伏、氧化银、银及其盐、过氧化物(例如,过氧化氢)、抗生素(例如,新霉素、杆菌肽和多粘菌素B)。其他合适的抗菌剂是美国专利公布号2004/0247655中所列出的那些。
制备本公开的水凝胶粘合剂组合物的方法包括将交联的聚(N-乙烯基内酰胺)与溶胀剂和改性聚合物以及溶剂中的其他添加剂(其在环境温度下或高于环境温度的温度下稍微挥发)混合。通常,溶胀剂、改性聚合物和其他添加剂诸如抗菌剂为基本上未经辐射的形态。合适的挥发性溶剂的示例包括水、乙醇、甲醇和异丙醇。然后将一定量的所得悬浮液浇铸到基材诸如剥离衬件或背衬材料的表面上,然后储存。挥发性溶剂通过加热诸如通过施加微波能量、红外能量,或通过对流空气流等而蒸发,以便在基材上形成内聚的压敏粘合剂组合物。通常,加热至约65℃的干燥烘箱可用于蒸发步骤。产品剥离衬件可任选地层合到组合物的暴露表面上方,以保护其免受污染。
在任何实施方案中,吸收性粘合剂凝胶30可包含水解胶体,该水解胶体含有聚异丁烯、基于聚异戊二烯的聚合物、可溶性吸收剂、不溶性吸收剂和增粘剂。合适的水性胶体组合物分别包括例如20重量%至40重量%的聚异丁烯(例如,来自巴斯夫公司(BASF)的OPPANOL)、15%至40%的基于聚异戊二烯的聚合物和15%至45%的可溶性和不溶性吸收剂诸如羧甲基纤维素(CMC)(美国特拉华州威明顿的亚跨龙公司(Aqualon Corp.,Wilmington,Del.))和交联的羧甲基纤维素X4ink CMC(例如,Ac-Di-Sol;美国宾夕法尼亚州费城的富曼实公司(FMC Corp.,Philadelphia,Pa.)),以及不超过15重量%的增粘剂诸如WINGTACK 95(美国宾夕法尼亚州埃克斯顿的沙多玛公司(Sartomer Co.,Exton,PA))。
在一些实施方案中,水解胶体包含交联的疏水有机硅凝胶,其中分散有可溶性和/或不溶性吸收剂。吸收剂含量应为15重量%至45重量%。
除了能够吸收水性液体之外,当粘附到背衬层10时,本公开的吸收性粘合剂凝胶30是湿气可透过的。因此,在任何实施方案中,粘附有吸收性粘合剂凝胶30的背衬层10具有通过EN-13726-1:2002第3.3部分测得的≥1g/10cm2/24h的湿气透过率(MVTR)。优选地,该湿气透过率大于3g/10cm2/24h。有利的是,由于伤口敷料100具有湿气可透过的背衬层和湿气可透过的吸收性粘合剂凝胶,因此湿气可通过两种方式远离伤口部位并离开敷料100:1)经由第一开口通过敷料(为液体状态)并离开敷料,以及2)通过吸收性粘合剂凝胶并离开完整的背衬层(为蒸汽状态)。通过凝胶和背衬层的高湿气透过率非常重要,因为它减小了当使用真空疗法治疗伤口时采集伤口渗出物所需筒的尺寸。高MVTR凝胶和背衬尤其可用于当使用恒力弹簧诸如在伤口护理系统(美国加利福尼亚州森尼韦尔的Spiracur公司(Spiracur,Inc.;Sunnyvale,CA))中使用的弹簧以机械方式产生真空源的实施方案中。高MVTR凝胶/背衬系统延长了机械真空系统的有效寿命,因为流体通过蒸发去除,所以不会填充筒或滤筒。
在任何实施方案中,在将本公开的伤口敷料施用到治疗部位之前,吸收性粘合剂凝胶包含少于40%(w/w)的水。在任何实施方案中,在将本公开的伤口敷料施用到治疗部位之前,吸收性粘合剂凝胶包含少于25%(w/w)的水。优选地,在将本公开的伤口敷料施用到处理部位之前,吸收性粘合剂凝胶包含少于10%(重量/重量)。使用包含<40%(w/w)的水、<25%(w/w)的水或<10%(w/w)的吸收性粘合剂凝胶减少了对专用包装的需求,在使用之前的储存期间本可能需要专用包装以保留凝胶中的水。
吸收性粘合剂凝胶的弹性性能(例如,通过储能模量(G’)表示)被选择为使得在吸收性粘合剂凝胶的厚度范围内,伤口敷料是适形的,但具有高完整性,从而在拉伸时不会轻易脱落。在任何实施方案中,在将本公开的伤口敷料施用于处理部位之前,在24℃、1rad/sec的剪切速率下,吸收性粘合剂凝胶具有介于约5,000帕斯卡和约50,000帕斯卡之间的储能剪切模量。该储能剪切模量优选介于10,000帕斯卡和约30,000帕斯卡之间。
另外,损耗模量(G”)表示吸收性粘合剂凝胶30的粘性响应。在任何实施方案中,在将本公开的伤口敷料施用于处理部位之前,在24℃、1rad/sec的剪切速率下,吸收性粘合剂凝胶具有介于约2,000帕斯卡和约20,000帕斯卡(包括端值在内)之间的损耗剪切模量。优选地,该损耗剪切模量介于约3,000帕斯卡和约15,000帕斯卡之间(包括端值在内)。有利的是,在24℃、1rad/s的剪切速率下损耗模量G”介于约2,000帕斯卡和约20,000帕斯卡之间(包括端值在内)的吸收性粘合剂凝胶充分柔性以在皮肤上形成良好的真空密封,但不是太柔性以至于轻易流动且容易失去其完整性。
回到附图,根据本公开的伤口敷料100包括多孔层40。多孔层40具有第一侧面42、第二侧面44和多孔层周边46,该多孔层周边限定多孔层的外边缘。这还限定多孔层区域。多孔层40的第一侧面42粘附到吸收性粘合剂凝胶30。在任何实施方案中,多孔层周边的至少50%与吸收性粘合剂凝胶重叠。在任何实施方案中,多孔层周边的超过50%与吸收性粘合剂凝胶重叠。在任何实施方案中,多孔层周边的至少60%与吸收性粘合剂凝胶重叠。在任何实施方案中,多孔层周边的至少70%与吸收性粘合剂凝胶重叠。在任何实施方案中,多孔层周边的至少75%与吸收性粘合剂凝胶重叠。在任何实施方案中,多孔层周边的至少80%与吸收性粘合剂凝胶重叠。在任何实施方案中,多孔层周边的至少85%与吸收性粘合剂凝胶重叠。在任何实施方案中,多孔层周边的至少90%与吸收性粘合剂凝胶重叠。在任何实施方案中,多孔层周边的至少95%与吸收性粘合剂凝胶重叠。在任何实施方案中,多孔层周边100%与吸收性粘合剂凝胶重叠。优选地,凝胶延伸经过多孔层周边(即,朝向背衬层周边)至少3mm,并且更优选至少5mm。
除了为皮肤提供良好的真空密封之外,吸收性粘合剂凝胶也起到流体储液器的作用,使得其从多孔层吸收湿气,这有利于保持多孔层不含液体。多孔层中的液体越少,在真空下敷料上的压降越低。当多孔层被液体饱和时,这种压降非常显著。
多孔层40被构造成便于流体(例如,从伤口部位诸如切口渗出的血液或其他流体)穿过多孔层到达背衬层10的第一开口18。在任何实施方案中,多孔层40可包括开孔泡沫、织造织物(例如,纱布)或非织造织物。多层多孔聚合物膜也将是可接受的。
在任何实施方案中,多孔层40可为材料层的组合。例如,层合(或者以其他方式附接)到泡沫的非织造织物可被认为是多孔层。在一个优选实施方案中,具有层合到其一侧或两侧上的多孔膜(大孔的或微孔的)然后层合到开孔泡沫上的非织造织物可用作良好的多孔层,因为多层使流体能够轻易穿过多孔层并且因为它在真空下更加抗塌陷。
在任何实施方案中,多孔层40任选地包括一个或多个开口区域43。在任何实施方案中,多孔层40任选地包括多个开口区域43。开口区域43是基本上大于多孔层40的孔的通孔。在任何实施方案中,开口区域43总计具有大于或等于多孔层区域的5%并小于或等于多孔层区域的50%的面积。在任何实施方案中,开口区域43总计具有大于或等于多孔层区域的5%并小于或等于多孔层区域的20%的面积。有利的是,开口区域43用于方便在不取下敷料的情况下观察伤口部位。
优选的是,开口区域应当足够大,使得粘合剂凝胶将适形于开口区域的两侧并在施加真空时接触皮肤或穿孔层。在任何实施方案中,这些孔为例如椭圆形、圆形、正方形、矩形或菱形形状,并且孔面积为至少0.4cm2,并优选大于0.75cm2,并更优选大于1.5cm2。单个孔的面积不应超过13cm2,因为太大的开口区域减少了多孔层用于将负压传送至伤口并用于使流体从伤口排出的可用区域。
开口区域43可呈现任何形状形式。图1和图2的伤口敷料100的所示实施方案包括多个椭圆形开口区域。适于开口区域43的形状的非限制性示例包括圆形、三角形、正方形、矩形和其他多边形。
在任何实施方案中,本公开的伤口敷料100任选地包括穿孔层50。穿孔层50包括具有第一表面52、第二表面53、穿孔层周边56和延伸穿过其中的多个穿孔54的基材51(例如,聚合物膜基材、泡沫、非织造织物、织造织物或者任何两种或更多种前述材料的组合)。穿孔54提供用于来自伤口的渗出物的穿过基材51并进入多孔层40中的液体通道。穿孔层50的第一表面51经由第二粘合剂56粘附到多孔层40的第二侧44,该第二粘合剂可涂覆在穿孔层50上。第二粘合剂可为将穿孔层50粘附到多孔层40上的任何合适粘合剂。任选地,穿孔层50可包含涂覆在其上的第三粘合剂58。第三粘合剂58可提供伤口敷料100与处理部位(皮肤)的附加固定。在任何实施方案中,第三粘合剂58可包含疏水柔性有机硅粘合剂。第二粘合剂56和第三粘合剂58可在对基材进行穿孔之前涂覆到穿孔层50上,并因此,第二粘合剂和第三粘合剂也包括穿孔。另选地或除此之外,第二粘合剂56和第三粘合剂58可以图案化地涂覆到基材51上。有利的是,穿孔层50中的穿孔54不仅提供用于湿气传输到多孔层40的通道,其还使穿孔层50与各种伤口部位的解剖表面高度适形。穿孔层不必与多孔层的周边或开口区域共延。
优选地,第三粘合剂58为柔性有机硅粘合剂,并且大于50微米厚,更优选大于75微米厚。第三粘合剂58提供了伤口敷料对组织的增强的固定,并提供了在靠近多孔层的皮肤/粘合剂接合处的改善的真空密封性能。优选地,具有柔性有机硅粘合剂层的该多孔层对钢的粘附力小于吸收性粘合剂凝胶和/或涂覆在背衬上的粘合剂层对钢的粘附力。此外,在37摄氏度下在等渗盐水中保持24小时之后,第三粘合剂具有低吸收性。(即,小于其干重的25%,并优选小于其干重的10%)。
在本公开的伤口敷料的任何实施方案中,敷料还包括真空端口(未在图1至图3中示出)。真空端口是允许将伤口敷料可操作地联接至负压源的结构,如图4所示。真空端口可为本领域中已知的可密封地附接(例如,经由压敏粘合剂、次级透明粘合剂/膜敷料或热密封)至背衬层的任何合适的真空端口。
虽然图1和图2所示的实施方案示出了其中吸收性粘合剂凝胶周边36完全与多孔层周边46重叠的伤口敷料100,但可以设想,在某些实施方案中,吸收性粘合剂凝胶周边36不与多孔层周边46 100%重叠,如上文所述。图3B示出了伤口敷料101的一个实施方案,其中吸收性粘合剂凝胶30的吸收性粘合剂凝胶周边36不完全与多孔层40的多孔层周边46重叠。在此实施方案中,背衬层10的背衬层周边16与吸收性粘合剂凝胶周边36和多孔层周边46完全重叠。同样在图3B中示出了背衬层10的第一开口18和吸收性粘合剂凝胶30的第二开口38。
在使用中,将伤口敷料施用到具有面向皮肤的背衬层的(即,第二主表面的)粘合剂侧的处理部位。图4示出了施用于皮肤5表面治疗部位处的伤口敷料103的一个实施方案的侧视图(局部剖视)。伤口敷料103与图1的伤口敷料100相同,不同之处在于除了包括具有第一开口(未示出)的背衬层10、包括第二开口38的吸收性粘合剂凝胶30、具有多个开口区域43的多孔层40、和穿孔层50之外,伤口敷料103还包括真空端口70。真空端口70经由管材82可操作地联接至负压源80(例如,真空泵(未示出))。第二主表面14上的第一粘合剂(未示出)在皮肤5上形成不透液密封。
在任何实施方案中,包括根据本公开的真空端口的伤口敷料可以可操作地连接至真空源。真空源可为例如机械泵。在任何实施方案中,真空源可为机械驱动、便携的真空泵。在任何实施方案中,机械驱动的真空源包括一个或多个恒力弹簧。
示例性实施方案
实施方案A为一种伤口敷料,包括:
湿气可透过的背衬层,该湿气可透过的背衬层具有第一主表面、第二主表面、背衬层周边和第一开口;
其中第二主表面具有设置于其上的靠近背衬层周边的第一粘合剂;
吸收性粘合剂凝胶,该吸收性粘合剂凝胶粘附到背衬层的第二主表面的至少一部分上,吸收性粘合剂凝胶包括粘合剂凝胶周边;和
多孔层,该多孔层具有第一侧面、第二侧面和多孔层周边;
其中多孔层的第一侧面粘附到吸收性粘合剂凝胶;
其中多孔层被构造成有利于使流体通过,到达第一开口;
其中粘合剂凝胶周边100%与背衬层重叠;
其中多孔层周边的至少50%与吸收性粘合剂凝胶重叠。
实施方案B为根据实施方案A所述的伤口敷料,其中吸收性粘合剂凝胶还包括第二开口。
实施方案C为根据实施方案B所述的伤口敷料,其中第一开口的至少一部分与第二开口的至少一部分重叠。
实施方案D为根据前述实施方案中任一项所述的伤口敷料,其中吸收性粘合剂凝胶具有约0.2mm至约4.0mm的厚度。
实施方案E为根据实施方案D所述的伤口敷料,其中吸收性粘合剂凝胶具有约0.5mm至约4.0mm的厚度。
实施方案F为根据实施方案E所述的伤口敷料,其中吸收性粘合剂凝胶具有约0.5mm至约3.0mm的厚度。
实施方案G为根据前述实施方案中任一项所述的伤口敷料,其中吸收性粘合剂凝胶包含少于或等于约40%(w/w)的水。
实施方案H为根据前述实施方案中任一项所述的伤口敷料,其中吸收性粘合剂凝胶包含少于或等于约25%(w/w)的水。
实施方案I为根据实施方案G或实施方案H所述的伤口敷料,其中吸收性粘合剂凝胶包含少于或等于约10%(w/w)的水。
实施方案J为根据前述实施方案中任一项所述的伤口敷料:
其中多孔层具有由多孔层周边限定的多孔层区域;
其中多孔层包括至少一个开口区域;
其中开口区域总计大于或等于多孔层区域的5%并小于或等于多孔层区域的50%。
实施方案K为根据前述实施方案中任一项所述的伤口敷料:
其中多孔层具有由多孔层周边限定的多孔层区域;
其中多孔层包括多个开口区域,每个开口区域大于或等于多孔层区域的5%并小于或等于多孔层区域的50%。
实施方案L为根据实施方案J或实施方案K所述的伤口敷料,其中开口区域总计大于或等于多孔层区域的5%并小于或等于多孔层区域的20%。
实施方案M为根据前述实施方案中任一项所述的伤口敷料,其中敷料还包括粘附到多孔层的第二侧面的穿孔层。
实施方案N为根据实施方案M所述的伤口敷料,其中穿孔层包括穿孔聚合物基材。
实施方案O为根据实施方案M或实施方案N所述的伤口敷料,其中穿孔层具有面向多孔层的第二侧面的第一主表面和与第一主表面相背对的第二主表面,其中第二主表面包含有机硅粘合剂。
实施方案P为根据实施方案O所述的伤口敷料,其中有机硅粘合剂具有大于或等于50微米的厚度。
实施方案Q为根据实施方案O所述的伤口敷料,其中有机硅粘合剂具有大于或等于75微米的厚度。
实施方案R为根据实施方案O至Q中任一项所述的伤口敷料,其中粘附到背衬层的吸收性粘合剂凝胶对钢的粘附力大于粘附到多孔层的有机硅粘合剂对钢的粘附力。
实施方案S为根据实施方案O至R中任一项所述的伤口敷料,其中粘附到背衬层的吸收性粘合剂凝胶对钢的粘附力大于粘附到背衬层的第一粘合剂对钢的粘附力。
实施方案T为根据前述实施方案中任一项所述的伤口敷料,其中背衬层包括聚合物弹性膜、弹性非织造织物或它们的组合。
实施方案U为根据实施方案T所述的伤口敷料,其中背衬层为约15μm至约250μm厚。
实施方案V为根据前述实施方案中任一项所述的伤口敷料,其中背衬层具有通过EN-13726-1:2002第3.3部分测得的≥10g/10cm2/24h的湿气透过率。
实施方案W为根据前述实施方案中任一项所述的伤口敷料,其中粘附有吸收性粘合剂凝胶的背衬层具有通过EN-13726-1:2002第3.3部分测得的≥1g/10cm2/24h的湿气透过率。
实施方案X为根据前述实施方案中任一项所述的伤口敷料,其中在24℃、1rad/s的剪切速率下,吸收性粘合剂凝胶具有介于约5,000帕斯卡和约50,000帕斯卡之间的剪切模量。
实施方案Y为根据前述实施方案中任一项所述的伤口敷料,其中在24℃、1rad/s的剪切速率下,吸收性粘合剂凝胶具有介于约2,000帕斯卡和约20,000帕斯卡之间的损耗剪切模量。
实施方案Z为根据前述实施方案中任一项所述的伤口敷料,其中吸收性粘合剂凝胶还包含抗菌剂。
实施方案AA为根据前述实施方案中任一项所述的伤口敷料,其中多孔吸收层包括开孔泡沫、织造织物、或非织造织物、多孔膜的多个层或上述任意两种或更多种材料的组合。
实施方案AB为根据前述实施方案中任一项所述的伤口敷料,其中当与等渗盐水溶液在37℃下接触24小时时,吸收性粘合剂凝胶能够吸收其干重的≥1.5倍。
实施方案AC为根据前述实施方案中任一项所述的伤口敷料,其还包括真空端口。
实施方案AD为根据实施方案AB所述的伤口敷料,其还包括机械驱动的真空源。
实施方案AE为根据实施方案AC所述的伤口敷料,其中机械驱动的真空源包括一个或多个恒力弹簧。
实施例
通过下面的实施例另外示出了本发明的目的和优点,但这些实施例中列举的具体材料及其量以及其他条件和细节不应被解释为是对本发明的不当限制。除非另外指明,否则所有的份数和百分比均以重量计,所有的水为蒸馏水,并且所有的分子量为重均分子量。
对钢的粘附力测试
通过光亮退火工艺使用304AISI不锈钢板进行对钢的粘附力测试。板的尺寸为51mm×127mm×1.6mm。在每次测试之前,使用经异丙醇饱和的一次性实验室擦拭物擦拭一次,并且使用经正庚烷饱和的一次性实验室擦拭物擦拭三次,以清洁测试表面。使表面干燥,然后将样品置于测试表面上。测试样品的长度为1”宽。在测量粘合力之前,将样品的粘合剂侧手动地压贴在钢板上,并且用辊子将样品的非粘合剂侧在每个方向上滚动一次以将粘合剂侧压贴在板上。辊的总直径为3.75"(95mm),表面宽度为1.75"(44mm),并且涂覆有约0.25"(6mm)厚、有效轧制重量为4.5磅或2043克、硬度计硬度为75至85的橡胶。轧制速度为约50毫米/秒。以305mm/min的速率使用180度剥离进行轧制后,立即测试样品。在25℃和50%相对湿度下的室内进行测试。
流体管理测试
测试设备被构造成模拟切口伤口。该设备的示意性顶视图示于图5中。该设备包括狭缝,液体通过三个间隔开的端口送入该狭缝中。该狭缝旨在模拟皮肤中在大约150mm长的切口型伤口部位处的开口。通过间隔开的端口送入的液体旨在模拟从切口型伤口流出或渗出的血液和/或浆液。
铝板(12mm厚板)具有平坦顶部表面90(图5),其中三个进液端口94在板顶部90中的狭槽92内等距间隔开。狭槽92为1mm宽、1.6mm深和152mm长,并且两个进液端口孔94与狭槽的相应端部向内间隔19mm,剩余进液端口孔94在狭槽中心。三个真空测量端口96位于距狭槽约2mm处。一个真空测量端口位于靠近狭槽一端处,并且另两个端口在狭槽的相背对侧面上,距其相应狭槽端部约50mm。所有端口完全延伸通过板,并且这些端口通过螺纹连接在板底部上。软管倒钩配件连接至板底部处的进液端口和真空测量端口,以便易于连接合适管材。
在使用过程中,流体经由布置在注射器泵(型号NE-1600;美国纽约州法明代尔的新世纪泵系统公司(New Era Pump Systems,Inc.;Farmingdale,NY))中的三个注射器送入铝板底部处的每个进液端口中。使用来自美国康涅狄格州斯坦福德的欧米茄工程公司(Omega Engineering,Stamford,Connecticut)的压力传感器测量真空。当使用这种设备测试实施例时,使用ATMOS WOUND RX 041真空单元(美国宾夕法尼亚州艾伦镇(Allentown,PA))将真空通过密封端口施加至实施例中所述的样品顶部。使用这种测试设备的所有实验均在环境温度(大约24摄氏度)和环境湿度(35%-65%相对湿度)下进行。所测量的真空为与大气环境的压差。例如,120mm Hg的读数意指系统或敷料中的压力低于大气环境压力120mm Hg。
按照实施例1制备的伤口敷料的粘合剂侧面压贴测试设备的表面以致伤口敷料的吸收性泡沫层与狭缝、进液端口和真空测量端口重叠。
实施例1
将涂覆有粘合剂的背衬层(诸如可购自美国明尼苏达州圣保罗的3M公司(3MCompany,St.Paul,MN)的3M TegadermTM透明膜卷16004)切成大约10.16cm×27.94cm的矩形。穿过粘合剂/膜背衬距敷料一边缘大约2.5"(6.35cm)并大约沿如图1所示粘合剂膜背衬宽度中心打出1.27cm直径孔。然后取下保护粘合剂的隔离衬件。
采用US2009/0187130的实施例E3中所述的方法制备大吸收性粘合剂凝胶,该专利全文以引用方式并入本文。将吸收性粘合剂凝胶切成57mm×229mm的矩形。在靠近吸收性粘合剂凝胶的边缘处打出38mm直径孔。然后将吸收性粘合剂凝胶层合至可呼吸背衬层的粘合剂侧面,以致粘合剂/膜背衬中的孔大约在吸收性粘合剂凝胶中的孔中心处。
使用一层多孔粘合剂(大约25%开口)将3.3mm厚吸收性聚氨酯泡沫(用于906423M TEGADERM有机硅泡沫边界敷料;购自美国明尼苏达州圣保罗的3M公司(3M Company,St.Paul,MN)的泡沫)层合至吸收性非织造层(用于购自美国明尼苏达州圣保罗的3M公司(3M Company,St.Paul,MN)的3M TEGADERM+垫产品系列中)。从该泡沫/非织造层合物中切下带圆角的八英寸(20.3cm)×二英寸(5.1cm)矩形以形成多孔层。从距多孔层一端大约12.5mm处开始,在该矩形中冲压出五个椭圆形(16mm×25mm)开口区域。然后将穿孔的(具有1.5mm孔的大约15%开口)粘合剂/聚氨酯膜/粘合剂层合至多孔层的泡沫侧面。再将多孔层的非织造侧面置于吸收性粘合剂凝胶的中心并层合至其上。
然后用粘合剂将真空端口和管材(从购自美国马萨诸塞州安多福的施乐辉公司(Smith&Nephew,Andover,MA)的PICO单用途负压装置的敷料中取下)附接至所构建样品的背衬层中的开口,以确保端口处无渗漏密封。
将敷料置于(上述)流体管理测试设备上,其中粘合剂侧面附接至铝板上且多孔层在所有端口和整个狭缝上延伸。该设备连接至真空系统。然后对敷料抽120mm Hg的真空。然后以1mL/h的总流速将含142mmol/L钠离子(来自氯化钠)和2.5mmol/L钙离子(来自氯化钙)的水溶液送入敷料。表1的数据示出了在三种相同敷料中所测量的真空水平随时间推移的变化。数据表明,当将敷料置于真空下然后保持长达约21 1/2小时时间段时,吸收性粘合剂凝胶的存在有助于最大程度降低敷料中的压降。
表1包括本文所述吸收性粘合剂凝胶的敷料中低于大气压的平均压力。测试是在本文所述的伤口敷料真空测试中所描述的条件下进行的。
比较例1
以与实施例1相同的方式构建和测试比较例1,不同的是不使用吸收性粘合剂凝胶。表2的数据示出了敷料中低于大气压的平均测量压力水平随时间推移的变化。
表2
不包括本文所述吸收性粘合剂凝胶的敷料中低于大气压的平均压力。测试是在本文的伤口敷料真空测试中所描述的条件下进行的。
表1和表2中的数据表明,吸收性粘合剂凝胶有利于去除多孔层中的液体,并从而最大程度降低敷料中压降同时允许液体流离开伤口并穿过多孔层。
实施例2
使用TA Instruments公司的ARES流变仪(美国特拉华州纽卡斯尔的德州仪器公司(Texas Instruments,New Castle,DE))测量实施例1的敷料中使用的吸收性粘合剂凝胶的流变学特性随干燥温度和时间的变化。在48.9℃下干燥吸收性粘合剂凝胶样品。在24℃和0.1至500rad/s频率范围下进行剪切测量。粘合剂凝胶样品是直径为25mm、厚度为1.6mm的圆。动态剪切粘度的结果示于表3中。储能剪切模量(G’)和损耗剪切模量(G”)测量的结果示于表4中。
表3:在不同剪切速率下随干燥时间的粘度(泊)变化。
表4:剪切模量(G’)和损耗剪切模量(G”)随干燥时间和剪切速率的变化。所有测量值均以帕斯卡示出。
实施例3
按照与实施例1中相同的方式制备和测试测试样品,不同之处在于1)除去真空端口中所包括的液体屏障;2)真空源为125mm Hg料筒;3)多孔层中未切出开口区域;以及4)背衬为层合有25微米厚丙烯酸酯粘合剂的23微米厚氨基甲酸酯膜。在层合至膜上之前除去丙烯酸酯粘合剂中的两个5cm×5cm切口,使得吸收性粘合剂凝胶直接接触这些区域中的氨基甲酸酯膜,从而形成敷料的高MVTR区域。使用来自美国俄亥俄州威克里夫的路博润公司(Lubrizol Co;Wickliffe,OH)的58237聚合物制备氨基甲酸酯膜。在测试24小时之后(即,24cm3溶液送入敷料中),真空料筒的体积仅改变了8cm3。24小时之后敷料中的平均压力低于大气压120mm Hg。该实施例示出了使用高MVTR吸收性凝胶和背衬来降低料筒尺寸并增强机械真空源或动力真空源的真空能力的优势。
实施例4
将Estane 58237的23微米厚膜层合至不连续(大约1mm直径孔模式,具有10%总开口区域)丙烯酸酯粘合剂层(如美国专利号RE 24,906所述;涂层重量为750mg/200cm2的丙烯酸异辛酯:丙烯酰胺(97:3)的共聚物)。将该膜粘合剂样品在25摄氏度和50%相对湿度下进行平衡。在4N/25mm下测量该样品对钢的粘附力值。
实施例5
将Estane 58237的23微米厚膜层合至1.75mm厚吸收性粘合剂的连续层上(如US2009/0187130的实施例E3所述)。将该膜粘合剂样品在25摄氏度和50%相对湿度下进行平衡。在5.8N/25mm下测量该样品对钢的粘附力值。
实施例6
将30微米厚氨基甲酸酯膜(来自拜耳公司(Bayer)的Texin 1209)挤出到涂层重量为550mg/200cm2的连续丙烯酸酯粘合剂上。然后以0.1mm厚度涂覆有机硅凝胶粘合剂到氨基甲酸酯膜/粘合剂层合物上并使其固化(如WO2010/056543中所述),并将保护衬件置于此有机硅粘合剂上。然后将该多层层合物机械穿孔(1.5mm直径孔,20%开口)。接着将该层合物的丙烯酸酯侧面层合到从90642 3MTM TegadermTM泡沫有机硅边界敷料切割出的聚氨酯泡沫上。然后在25摄氏度和50%相对湿度下测试该泡沫层合物的有机硅侧面对钢的粘附力。在2N/25mm下测量对钢的粘附力值。
本文引用的所有专利、专利申请和专利公开的全部公开内容以及可供使用的电子版材料均以引用方式并入。在本专利申请的公开内容和以引用方式并入本文的任何文献的一个或多个公开内容之间存在任何矛盾的情况下,应以本专利申请的公开内容为准。上述具体实施方式和实施例仅为清楚理解本发明而给出。但它们不应被理解为不必要的限制。本发明不限于示出的和描述的具体细节,对本领域的技术人员而言显而易见的变型形式将包括在由权利要求书所限定的本发明中。
所有的标题是为了阅读者方便,而不应该用于限制该标题后面的正文的含义,除非如此规定。
本文说明性地描述的本发明可在不存在本文未具体公开的任何元件的情况下进行适当地实践。因此,例如,在本文的每个实例中,术语“包括”、“基本上由......组成”和“由......组成”中的任一个可被替换为其它两个术语中的任一个。尽管将已采用的术语和表达用作描述而非限制术语,并且非旨在使用此类术语和表达而排出所示和所描述的特征或其部分的任何等同物,但是已经认识到,在所要求保护的本发明的范围内的各种修改是可能的。因此,应当理解,尽管本发明已通过优选的实施方式和任选的特征而具体公开,但是本领域的技术人员可推出本文所公开的概念的修改和变型,并且此类修改和变型被视为在由所附的权利要求所限定的本发明的范围内。
Claims (20)
1.一种伤口敷料,包括:
湿气可透过的背衬层,所述湿气可透过的背衬层具有第一主表面、第二主表面、背衬层周边和第一开口;
其中所述第二主表面具有设置于其上的靠近所述背衬层周边的第一粘合剂;
吸收性粘合剂凝胶,所述吸收性粘合剂凝胶粘附到所述背衬层的所述第二主表面的至少一部分上,所述吸收性粘合剂凝胶包括粘合剂凝胶周边;和
多孔层,所述多孔层具有第一侧面、第二侧面和多孔层周边;
其中所述多孔层的所述第一侧面粘附到所述吸收性粘合剂凝胶;
其中所述多孔层被构造成有利于使流体通过,到达所述第一开口;
其中所述粘合剂凝胶周边100%与所述背衬层重叠;
其中所述多孔层周边的至少50%与所述吸收性粘合剂凝胶重叠,
其中所述多孔层包括至少一个开口区域。
2.根据权利要求1所述的伤口敷料,其中所述吸收性粘合剂凝胶还包括第二开口。
3.根据权利要求1所述的伤口敷料,其中所述吸收性粘合剂凝胶具有0.2mm至4.0mm的厚度。
4.根据权利要求1所述的伤口敷料,其中所述吸收性粘合剂凝胶包含少于40%(w/w)的水。
5.根据权利要求1所述的伤口敷料:
其中所述多孔层具有由所述多孔层周边限定的多孔层区域;
其中所述至少一个开口区域大于或等于所述多孔层区域的5%并小于或等于所述多孔层区域的50%。
6.根据权利要求1所述的伤口敷料:
其中所述多孔层具有由所述多孔层周边限定的多孔层区域;
其中所述多孔层包括多个开口区域,每个开口区域大于或等于所述多孔层区域的5%并小于或等于所述多孔层区域的50%。
7.根据权利要求5所述的伤口敷料,其中所述开口区域中的至少一个大于或等于所述多孔层区域的5%并小于或等于所述多孔层区域的20%。
8.根据权利要求1所述的伤口敷料,其中所述敷料还包括粘附到所述多孔层的所述第二侧面的穿孔层。
9.根据权利要求8所述的伤口敷料,其中所述穿孔层具有面向所述多孔层的所述第二侧面的第一主表面和与所述第一主表面相背对的第二主表面,其中所述第二主表面包含有机硅粘合剂。
10.根据权利要求9所述的伤口敷料,其中粘附到所述背衬层的所述吸收性粘合剂凝胶对钢的粘附力大于粘附到所述多孔层的所述有机硅粘合剂对钢的粘附力。
11.根据权利要求9所述的伤口敷料,其中粘附到所述背衬层的所述吸收性粘合剂凝胶对钢的粘附力大于粘附到所述背衬层的所述第一粘合剂对钢的粘附力。
12.根据权利要求1所述的伤口敷料,其中所述背衬层包括聚合物弹性膜、弹性非织造织物或它们的组合。
13.根据权利要求1所述的伤口敷料,其中所述背衬层具有通过EN-13726-1:2002第3.3部分测得的≥10g/10cm2/24h的湿气透过率。
14.根据权利要求1所述的伤口敷料,其中粘附有所述吸收性粘合剂凝胶的所述背衬层具有通过EN-13726-1:2002第3.3部分测得的≥1g/10cm2/24h的湿气透过率。
15.根据权利要求1所述的伤口敷料,其中在24℃、1rad/s的剪切速率下,所述吸收性粘合剂凝胶具有介于5,000帕斯卡和50,000帕斯卡之间的剪切模量。
16.根据权利要求1所述的伤口敷料,其中在24℃、1rad/s的剪切速率下,所述吸收性粘合剂凝胶具有介于2,000帕斯卡和20,000帕斯卡之间的损耗剪切模量。
17.根据权利要求1所述的伤口敷料,其中所述吸收性粘合剂凝胶还包含抗菌剂。
18.根据权利要求1所述的伤口敷料,其中所述多孔层包括开孔泡沫、织造织物、非织造织物、多孔膜或它们的组合。
19.根据权利要求1所述的伤口敷料,其中当与等渗盐水溶液在37℃下接触24小时时,所述吸收性粘合剂凝胶能够吸收其干重的≥1.5倍。
20.根据前述权利要求中任一项所述的伤口敷料,其还包括真空端口。
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201462098058P | 2014-12-30 | 2014-12-30 | |
| US62/098,058 | 2014-12-30 | ||
| US201562112714P | 2015-02-06 | 2015-02-06 | |
| US62/112,714 | 2015-02-06 | ||
| PCT/US2015/067661 WO2016109420A1 (en) | 2014-12-30 | 2015-12-28 | Negative pressure wound dressing with absorbent adhesive sealant layer |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN107106723A CN107106723A (zh) | 2017-08-29 |
| CN107106723B true CN107106723B (zh) | 2020-10-23 |
Family
ID=55315699
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201580071938.5A Active CN107106334B (zh) | 2014-12-30 | 2015-12-28 | 具有多个粘合剂层的伤口敷料 |
| CN201580071925.8A Active CN107106723B (zh) | 2014-12-30 | 2015-12-28 | 具有吸收性粘合剂密封层的负压伤口敷料 |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201580071938.5A Active CN107106334B (zh) | 2014-12-30 | 2015-12-28 | 具有多个粘合剂层的伤口敷料 |
Country Status (5)
| Country | Link |
|---|---|
| US (3) | US10537478B2 (zh) |
| EP (2) | EP3240581B1 (zh) |
| JP (2) | JP6744315B2 (zh) |
| CN (2) | CN107106334B (zh) |
| WO (2) | WO2016109418A1 (zh) |
Families Citing this family (42)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB0808376D0 (en) | 2008-05-08 | 2008-06-18 | Bristol Myers Squibb Co | Wound dressing |
| GB0817796D0 (en) | 2008-09-29 | 2008-11-05 | Convatec Inc | wound dressing |
| GB201020236D0 (en) | 2010-11-30 | 2011-01-12 | Convatec Technologies Inc | A composition for detecting biofilms on viable tissues |
| WO2012078781A1 (en) | 2010-12-08 | 2012-06-14 | Convatec Technologies Inc. | Integrated system for assessing wound exudates |
| US9526816B2 (en) | 2010-12-08 | 2016-12-27 | Convatec Technologies Inc. | Wound exudate monitor accessory |
| GB201115182D0 (en) | 2011-09-02 | 2011-10-19 | Trio Healthcare Ltd | Skin contact material |
| GB2497406A (en) | 2011-11-29 | 2013-06-12 | Webtec Converting Llc | Dressing with a perforated binder layer |
| KR20150099776A (ko) | 2012-12-20 | 2015-09-01 | 컨바텍 테크놀러지스 인크 | 화학적 개질된 셀룰로스 섬유의 처리 |
| EP3240581B1 (en) * | 2014-12-30 | 2020-04-15 | 3M Innovative Properties Company | Wound dressing with multiple adhesive layers |
| JP6747457B2 (ja) * | 2016-01-29 | 2020-08-26 | 王子ホールディングス株式会社 | 絆創膏 |
| CN109310528B (zh) | 2016-03-30 | 2021-07-20 | 康沃特克科技公司 | 检测伤口中的微生物感染 |
| KR20190008199A (ko) | 2016-03-30 | 2019-01-23 | 시노보 게엠베하 | 상처에서 미생물 감염의 검출 방법 |
| ES2882336T3 (es) | 2016-07-08 | 2021-12-01 | Convatec Technologies Inc | Sistema flexible de presión negativa |
| EP3481360B1 (en) | 2016-07-08 | 2022-03-09 | ConvaTec Technologies Inc. | Fluid flow sensing |
| WO2018009873A1 (en) | 2016-07-08 | 2018-01-11 | Convatec Technologies Inc. | Fluid collection apparatus |
| EP3281617B1 (en) * | 2016-08-10 | 2020-09-23 | Advanced Medical Solutions Limited | Wound dressing |
| EP3315145B1 (en) * | 2016-10-28 | 2022-06-08 | BSN medical GmbH | Multi-layer wound care product with perforated collagen layer |
| JP7231227B2 (ja) | 2017-02-22 | 2023-03-01 | コーネル ユニヴァーシティー | 小さい切開創を機械式に管理、保護及び吸引するための機械式真空ドレッシング |
| CN107574575A (zh) * | 2017-08-02 | 2018-01-12 | 六安尚荣无纺布制品有限公司 | 一种医用抗菌无纺布的制备工艺 |
| TWI795430B (zh) | 2017-08-25 | 2023-03-11 | 美商3M新設資產公司 | 容許無損傷移除的黏著劑物品 |
| CA3073940A1 (en) | 2017-08-25 | 2019-02-28 | 3M Innovative Properties Company | Adhesive articles permitting damage free removal |
| EP3694457A1 (en) * | 2017-10-09 | 2020-08-19 | 3M Innovative Properties Company | Securement dressing with conformal border |
| EP3700598A1 (en) | 2017-10-23 | 2020-09-02 | KCI Licensing, Inc. | Low profile distribution components for wound therapy |
| US11432967B2 (en) * | 2017-10-23 | 2022-09-06 | Kci Licensing, Inc. | Fluid bridge for simultaneous application of negative pressure to multiple tissue sites |
| CN111432855B (zh) | 2017-12-06 | 2024-02-23 | 康奈尔大学 | 具有提高的泵效率、自动压力指示器和自动压力限制器的手动操作的负压伤口治疗(npwt)绷带 |
| US20190351094A1 (en) * | 2018-05-21 | 2019-11-21 | Milliken & Company | Wound care device having fluid transfer and adhesive properties |
| US20210205141A1 (en) * | 2018-06-15 | 2021-07-08 | Coloplast A/S | Wound dressing with electrode multiplexing and related methods |
| US10993708B2 (en) | 2018-07-31 | 2021-05-04 | Ethicon, Inc. | Skin closure devices with interrupted closure |
| WO2020035811A1 (en) * | 2018-08-17 | 2020-02-20 | 3M Innovative Properties Company | Wound dressing system |
| US11666680B2 (en) | 2018-08-28 | 2023-06-06 | Aatru Medical, LLC | Dressing |
| US11007083B2 (en) * | 2018-08-28 | 2021-05-18 | Aatru Medical, LLC | Dressing |
| GB2611267B (en) * | 2018-10-18 | 2023-06-28 | Smith & Nephew | Tissue treatment device |
| USD961782S1 (en) | 2018-10-23 | 2022-08-23 | Kci Licensing, Inc. | Low-profile fluid conductor for tissue therapy |
| WO2021124357A1 (en) * | 2019-12-19 | 2021-06-24 | Pankajkumar Kumanbhai Chhatrala | A mechanical barrier device for medically incorporated instruments |
| DK3838238T3 (da) * | 2019-12-19 | 2023-05-01 | Moelnlycke Health Care Ab | Dobbelt belægning til sårforbindinger |
| US11771819B2 (en) | 2019-12-27 | 2023-10-03 | Convatec Limited | Low profile filter devices suitable for use in negative pressure wound therapy systems |
| US11331221B2 (en) * | 2019-12-27 | 2022-05-17 | Convatec Limited | Negative pressure wound dressing |
| US20210244357A1 (en) * | 2020-02-07 | 2021-08-12 | Medtronic Minimed, Inc. | Systems for medical device breathability |
| GB202013871D0 (en) * | 2020-09-03 | 2020-10-21 | Brightwake Ltd | Wound dressing |
| CN112691230A (zh) * | 2020-12-23 | 2021-04-23 | 上海弘生医疗科技有限公司 | 一种硅胶敷料胶带及其制备工艺 |
| GB202107893D0 (en) * | 2021-06-02 | 2021-07-14 | Medtrade Products Ltd | Antimicrobial wound dressing |
| CN114712088B (zh) * | 2022-04-01 | 2023-05-16 | 浙江隆腾医用新材料有限公司 | 一种羧甲基纤维素创面敷料 |
Family Cites Families (64)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1032400A (en) | 1911-01-21 | 1912-07-16 | Wilhelm Guenther | Process of treating ores. |
| US1013052A (en) | 1911-03-22 | 1911-12-26 | Goldman & Company Inc E | Conveyer-chain. |
| IT610737A (zh) | 1955-11-18 | 1900-01-01 | ||
| US4112213A (en) | 1964-09-28 | 1978-09-05 | Johnson & Johnson | Pressure sensitive adhesive tapes and method of making same |
| US3389827A (en) | 1967-04-10 | 1968-06-25 | Minnesota Mining & Mfg | Easy-open container and sealing tape |
| NO134790C (no) | 1968-07-09 | 1984-03-22 | Smith & Nephew | Klebende,; trykkfoelsomt, vanndamp-permeabelt produkt for bruk paa hud hos mennesker. |
| US4323557A (en) | 1979-07-31 | 1982-04-06 | Minnesota Mining & Manufacturing Company | Pressure-sensitive adhesive containing iodine |
| US4310509A (en) | 1979-07-31 | 1982-01-12 | Minnesota Mining And Manufacturing Company | Pressure-sensitive adhesive having a broad spectrum antimicrobial therein |
| ATE17191T1 (de) | 1982-04-08 | 1986-01-15 | Smith & Nephew Ass | Chirurgisches heftpflaster. |
| US4737410A (en) | 1986-11-28 | 1988-04-12 | Minnesota Mining And Manufacturing Company | Polyalkyloxazoline-reinforced acrylic pressure-sensitive adhesive composition |
| US5225473A (en) | 1987-11-25 | 1993-07-06 | Minnesota Mining And Manufacturing Company | Pressure-sensitive adhesives |
| US4931282A (en) | 1987-11-25 | 1990-06-05 | Minnesota Mining And Manufacturing Company | Pressure-sensitive medical sealant |
| US5088483A (en) | 1988-11-04 | 1992-02-18 | Minnesota Mining And Manufacturing Co. | Adhesive frame bandage |
| US4969880A (en) | 1989-04-03 | 1990-11-13 | Zamierowski David S | Wound dressing and treatment method |
| US5261893A (en) | 1989-04-03 | 1993-11-16 | Zamierowski David S | Fastening system and method |
| US5527293A (en) | 1989-04-03 | 1996-06-18 | Kinetic Concepts, Inc. | Fastening system and method |
| US5160315A (en) | 1991-04-05 | 1992-11-03 | Minnesota Mining And Manufacturing Company | Combined adhesive strip and transparent dressing delivery system |
| US5276079A (en) | 1991-11-15 | 1994-01-04 | Minnesota Mining And Manufacturing Company | Pressure-sensitive poly(n-vinyl lactam) adhesive composition and method for producing and using same |
| US5244457A (en) * | 1992-03-23 | 1993-09-14 | The Kendall Company | Vented wound dressing |
| DE19727032A1 (de) | 1997-06-25 | 1999-01-07 | Hartmann Paul Ag | Pflaster |
| US6171985B1 (en) | 1997-12-01 | 2001-01-09 | 3M Innovative Properties Company | Low trauma adhesive article |
| US6071267A (en) | 1998-02-06 | 2000-06-06 | Kinetic Concepts, Inc. | Medical patient fluid management interface system and method |
| SE9801899L (sv) | 1998-05-28 | 1999-07-05 | Moelnlycke Health Care Ab | Sårförband eller fixeringstejp för hud innefattande ett laminat av en plastfilm och ett material med oregelbunden ytstruktur och som är belagt med en klibbig elastomer |
| US20020015726A1 (en) * | 2000-06-30 | 2002-02-07 | Scamilla Aledo Maria Aparecida De Carvalho | Dressings and bandages comprising same |
| US6566575B1 (en) * | 2000-02-15 | 2003-05-20 | 3M Innovative Properties Company | Patterned absorbent article for wound dressing |
| BR0017149B1 (pt) | 2000-03-10 | 2009-01-13 | curativo mÉdico possuindo superfÍcies de topo e de fundo. | |
| WO2003057485A1 (en) * | 2001-12-21 | 2003-07-17 | Avery Dennison Corporation | Process for the manufacture of multi-layered wound dressings |
| US7005143B2 (en) * | 2002-04-12 | 2006-02-28 | 3M Innovative Properties Company | Gel materials, medical articles, and methods |
| USD468548S1 (en) | 2002-05-25 | 2003-01-14 | H. Wayne Head, Jr. | Portable stadium seat |
| US6979324B2 (en) | 2002-09-13 | 2005-12-27 | Neogen Technologies, Inc. | Closed wound drainage system |
| US7520872B2 (en) | 2002-09-13 | 2009-04-21 | Neogen Technologies, Inc. | Closed wound drainage system |
| CN100448436C (zh) * | 2002-12-31 | 2009-01-07 | 奥苏尔公司 | 创伤敷料 |
| US9278155B2 (en) * | 2003-06-05 | 2016-03-08 | 3M Innovative Properties Company | Adhesive compositions, articles incorporating same and methods of manufacture |
| GB0606661D0 (en) | 2006-04-03 | 2006-05-10 | Brightwake Ltd | Improvements relating to dressings |
| ES2361025T3 (es) | 2006-11-07 | 2011-06-13 | Paul Hartmann Ag | Apósito absorbente de múltiples capas con una capa hidrófila de contacto con la herida. |
| US7947366B2 (en) | 2007-03-19 | 2011-05-24 | 3M Innovative Properties Company | Adhesive sheet article |
| EP2180837B1 (en) | 2007-08-21 | 2021-02-24 | 3M Innovative Properties Company | Reduced-pressure system employing a gasket |
| WO2009091682A2 (en) | 2008-01-18 | 2009-07-23 | 3M Innovative Properties Company | Hydrogels with tapered edge |
| CA2726815C (en) | 2008-05-27 | 2016-07-05 | Kalypto Medical, Inc. | Negative pressure wound therapy device |
| DE102008031183A1 (de) * | 2008-07-03 | 2010-01-07 | Paul Hartmann Ag | Wundauflage |
| EP2349154B1 (en) | 2008-10-24 | 2014-08-27 | 3M Innovative Properties Company | Wound dressing |
| JP5662329B2 (ja) | 2008-10-29 | 2015-01-28 | スリーエム イノベイティブ プロパティズ カンパニー | 電子ビーム硬化シリコーン材料 |
| EP2350195B1 (en) | 2008-10-29 | 2013-09-18 | 3M Innovative Properties Company | Electron beam cured, nonfunctionalized silicone pressure sensitive adhesives |
| US9421309B2 (en) | 2009-06-02 | 2016-08-23 | Kci Licensing, Inc. | Reduced-pressure treatment systems and methods employing hydrogel reservoir members |
| GB2470940A (en) | 2009-06-10 | 2010-12-15 | Systagenix Wound Man Ip Co Bv | Vacuum wound dressing with hydrogel layer |
| SE533841C2 (sv) * | 2009-06-15 | 2011-02-01 | Moelnlycke Health Care Ab | Sårförband med hög vätskehanteringskapacitet |
| US8690844B2 (en) * | 2009-08-27 | 2014-04-08 | Kci Licensing, Inc. | Re-epithelialization wound dressings and systems |
| KR20120091339A (ko) | 2009-11-09 | 2012-08-17 | 쓰리엠 이노베이티브 프로퍼티즈 컴파니 | 비혼화성 물질을 사용한 의료 용품 및 제조 방법 |
| US9358158B2 (en) * | 2010-03-16 | 2016-06-07 | Kci Licensing, Inc. | Patterned neo-epithelialization dressings, systems, and methods |
| GB201006986D0 (en) * | 2010-04-27 | 2010-06-09 | Smith & Nephew | Wound dressing |
| US9107990B2 (en) | 2011-02-14 | 2015-08-18 | Kci Licensing, Inc. | Reduced-pressure dressings, systems, and methods for use with linear wounds |
| GB201108229D0 (en) | 2011-05-17 | 2011-06-29 | Smith & Nephew | Tissue healing |
| CN107252383A (zh) | 2011-07-14 | 2017-10-17 | 史密夫及内修公开有限公司 | 伤口敷料和治疗方法 |
| GB2504872B (en) * | 2011-11-01 | 2015-07-01 | Brightwake Ltd | Wound dressings, and yarn useful therein |
| JP6089262B2 (ja) | 2012-03-01 | 2017-03-08 | アルケア株式会社 | 傷手当用品 |
| EP2863855B1 (en) | 2012-06-26 | 2019-09-25 | 3M Innovative Properties Company | Medical dressing with multiple adhesives |
| US10667955B2 (en) * | 2012-08-01 | 2020-06-02 | Smith & Nephew Plc | Wound dressing and method of treatment |
| ITMI20121899A1 (it) | 2012-11-07 | 2014-05-08 | Prysmian Spa | Cavo elettrico per un impianto solare per la generazione di energia elettrica e di energia termica ed impianto che lo comprende |
| RU2015143116A (ru) * | 2013-03-15 | 2017-04-20 | СМИТ ЭНД НЕФЬЮ ПиЭлСи | Герметик для раневой повязки и его использование |
| US20160120706A1 (en) | 2013-03-15 | 2016-05-05 | Smith & Nephew Plc | Wound dressing sealant and use thereof |
| WO2015134249A1 (en) | 2014-03-05 | 2015-09-11 | 3M Innovative Properties Company | Gentle to skin (meth)acrylate pressure-sensitive adhesive |
| EP3157484B1 (en) | 2014-06-18 | 2020-02-26 | Smith & Nephew plc | Wound dressing |
| EP3240581B1 (en) * | 2014-12-30 | 2020-04-15 | 3M Innovative Properties Company | Wound dressing with multiple adhesive layers |
| US10324007B2 (en) * | 2016-10-04 | 2019-06-18 | Ch2M Hill, Inc. | Passive device for vapor intrusion sampling |
-
2015
- 2015-12-28 EP EP15832851.8A patent/EP3240581B1/en active Active
- 2015-12-28 CN CN201580071938.5A patent/CN107106334B/zh active Active
- 2015-12-28 EP EP15832852.6A patent/EP3240580B1/en active Active
- 2015-12-28 JP JP2017534994A patent/JP6744315B2/ja active Active
- 2015-12-28 US US15/539,855 patent/US10537478B2/en active Active
- 2015-12-28 CN CN201580071925.8A patent/CN107106723B/zh active Active
- 2015-12-28 WO PCT/US2015/067655 patent/WO2016109418A1/en active Application Filing
- 2015-12-28 US US15/539,866 patent/US11007086B2/en active Active
- 2015-12-28 WO PCT/US2015/067661 patent/WO2016109420A1/en active Application Filing
- 2015-12-28 JP JP2017535053A patent/JP6838812B2/ja active Active
-
2019
- 2019-12-04 US US16/703,676 patent/US20200107967A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| CN107106334B (zh) | 2021-02-09 |
| US20170367896A1 (en) | 2017-12-28 |
| EP3240581B1 (en) | 2020-04-15 |
| JP2018508243A (ja) | 2018-03-29 |
| EP3240580A1 (en) | 2017-11-08 |
| WO2016109418A1 (en) | 2016-07-07 |
| JP6744315B2 (ja) | 2020-08-19 |
| EP3240581A1 (en) | 2017-11-08 |
| EP3240580B1 (en) | 2020-04-15 |
| US10537478B2 (en) | 2020-01-21 |
| US20200107967A1 (en) | 2020-04-09 |
| WO2016109420A1 (en) | 2016-07-07 |
| CN107106334A (zh) | 2017-08-29 |
| CN107106723A (zh) | 2017-08-29 |
| JP2018500137A (ja) | 2018-01-11 |
| US11007086B2 (en) | 2021-05-18 |
| US20170367895A1 (en) | 2017-12-28 |
| JP6838812B2 (ja) | 2021-03-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN107106723B (zh) | 具有吸收性粘合剂密封层的负压伤口敷料 | |
| US20180064843A1 (en) | Wound dressing | |
| US9168180B2 (en) | Conformable medical dressing with self supporting substrate | |
| HU225557B1 (en) | Absorbent wound dressing containing a hydrogel layer | |
| JP2001511396A (ja) | 改良創傷包帯 | |
| CN113507909A (zh) | 抗微生物敷料、敷料部件和方法 | |
| US20140228786A1 (en) | Absorbent body for the therapeutic treatment of a wound by means of negative pressure | |
| KR20240008975A (ko) | 상처 드레싱 | |
| US20140221946A1 (en) | Absorbent body for the therapeutic treatment of a wound by means of negative pressure | |
| GB2379392A (en) | Wound dressing sheet materials | |
| US9656000B2 (en) | Wound dressing | |
| KR200359390Y1 (ko) | 의료용 패치 | |
| WO2024064336A1 (en) | Transparent dressing with hydrogel layer | |
| WO2021239652A1 (en) | A negative pressure wound therapy (npwt) system |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant | ||
| TR01 | Transfer of patent right |
Effective date of registration: 20240329 Address after: U.S.A. Patentee after: Shuwanuo Intellectual Property Co. Country or region after: U.S.A. Address before: American Minnesota Patentee before: 3M INNOVATIVE PROPERTIES Co. Country or region before: U.S.A. |
|
| TR01 | Transfer of patent right |