CN106170557B - 作为化疗应答标志物的微生物群组合物,以及微生物调节剂(益生元、益生菌或合生元)用于提高癌症治疗效力的用途 - Google Patents
作为化疗应答标志物的微生物群组合物,以及微生物调节剂(益生元、益生菌或合生元)用于提高癌症治疗效力的用途 Download PDFInfo
- Publication number
- CN106170557B CN106170557B CN201480063481.9A CN201480063481A CN106170557B CN 106170557 B CN106170557 B CN 106170557B CN 201480063481 A CN201480063481 A CN 201480063481A CN 106170557 B CN106170557 B CN 106170557B
- Authority
- CN
- China
- Prior art keywords
- ctx
- cells
- mice
- bacteria
- treatment
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 206010028980 Neoplasm Diseases 0.000 title claims abstract description 130
- 238000011282 treatment Methods 0.000 title claims abstract description 102
- 201000011510 cancer Diseases 0.000 title claims abstract description 65
- 239000006041 probiotic Substances 0.000 title claims abstract description 49
- 235000018291 probiotics Nutrition 0.000 title claims abstract description 49
- 239000000203 mixture Substances 0.000 title claims description 102
- 238000002512 chemotherapy Methods 0.000 title abstract description 78
- 230000004044 response Effects 0.000 title description 46
- 241000736262 Microbiota Species 0.000 title description 22
- 235000013406 prebiotics Nutrition 0.000 title description 7
- 230000000813 microbial effect Effects 0.000 title description 6
- 235000019722 synbiotics Nutrition 0.000 title description 6
- 230000000529 probiotic effect Effects 0.000 claims abstract description 38
- 230000007140 dysbiosis Effects 0.000 claims abstract description 24
- 208000027244 Dysbiosis Diseases 0.000 claims abstract description 21
- 241000894006 Bacteria Species 0.000 claims description 100
- 230000003115 biocidal effect Effects 0.000 claims description 78
- 230000000259 anti-tumor effect Effects 0.000 claims description 60
- 238000002560 therapeutic procedure Methods 0.000 claims description 48
- 241000894007 species Species 0.000 claims description 44
- 241001468157 Lactobacillus johnsonii Species 0.000 claims description 37
- 241000227342 Candidatus Arthromitus sp. SFB-mouse Species 0.000 claims description 31
- 241000193403 Clostridium Species 0.000 claims description 31
- 239000002246 antineoplastic agent Substances 0.000 claims description 29
- 241000194029 Enterococcus hirae Species 0.000 claims description 24
- 244000005700 microbiome Species 0.000 claims description 23
- 229940127089 cytotoxic agent Drugs 0.000 claims description 21
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 claims description 17
- 229960004397 cyclophosphamide Drugs 0.000 claims description 16
- 241000605894 Porphyromonas Species 0.000 claims description 14
- 239000002671 adjuvant Substances 0.000 claims description 13
- 241000194032 Enterococcus faecalis Species 0.000 claims description 12
- 238000004519 manufacturing process Methods 0.000 claims description 11
- 239000003814 drug Substances 0.000 claims description 10
- 239000012634 fragment Substances 0.000 claims description 10
- 230000002163 immunogen Effects 0.000 claims description 9
- 238000007920 subcutaneous administration Methods 0.000 claims description 8
- 229940022399 cancer vaccine Drugs 0.000 claims description 7
- 229940032049 enterococcus faecalis Drugs 0.000 claims description 5
- 241000862469 Holdemania Species 0.000 claims description 3
- 241000927512 Barnesiella Species 0.000 claims description 2
- 238000007918 intramuscular administration Methods 0.000 claims description 2
- 239000012646 vaccine adjuvant Substances 0.000 claims 1
- 229940124931 vaccine adjuvant Drugs 0.000 claims 1
- 230000001580 bacterial effect Effects 0.000 abstract description 56
- 238000000034 method Methods 0.000 abstract description 39
- 230000000968 intestinal effect Effects 0.000 abstract description 16
- 230000008901 benefit Effects 0.000 abstract description 9
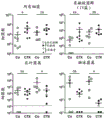
- 241000699670 Mus sp. Species 0.000 description 170
- 210000004027 cell Anatomy 0.000 description 96
- 210000001744 T-lymphocyte Anatomy 0.000 description 74
- 238000002474 experimental method Methods 0.000 description 55
- MYPYJXKWCTUITO-UHFFFAOYSA-N vancomycin Natural products O1C(C(=C2)Cl)=CC=C2C(O)C(C(NC(C2=CC(O)=CC(O)=C2C=2C(O)=CC=C3C=2)C(O)=O)=O)NC(=O)C3NC(=O)C2NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(CC(C)C)NC)C(O)C(C=C3Cl)=CC=C3OC3=CC2=CC1=C3OC1OC(CO)C(O)C(O)C1OC1CC(C)(N)C(O)C(C)O1 MYPYJXKWCTUITO-UHFFFAOYSA-N 0.000 description 55
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 52
- 108010059993 Vancomycin Proteins 0.000 description 52
- 229960003165 vancomycin Drugs 0.000 description 52
- MYPYJXKWCTUITO-LYRMYLQWSA-N vancomycin Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=C2C=C3C=C1OC1=CC=C(C=C1Cl)[C@@H](O)[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@H]3C(=O)N[C@H]1C(=O)N[C@H](C(N[C@@H](C3=CC(O)=CC(O)=C3C=3C(O)=CC=C1C=3)C(O)=O)=O)[C@H](O)C1=CC=C(C(=C1)Cl)O2)=O)NC(=O)[C@@H](CC(C)C)NC)[C@H]1C[C@](C)(N)[C@H](O)[C@H](C)O1 MYPYJXKWCTUITO-LYRMYLQWSA-N 0.000 description 52
- 244000005709 gut microbiome Species 0.000 description 48
- 230000000694 effects Effects 0.000 description 35
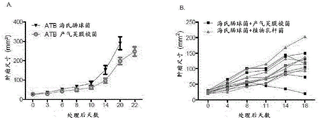
- 230000004614 tumor growth Effects 0.000 description 31
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 30
- 241001465754 Metazoa Species 0.000 description 30
- 210000004988 splenocyte Anatomy 0.000 description 29
- 210000000952 spleen Anatomy 0.000 description 28
- 241000192125 Firmicutes Species 0.000 description 27
- 239000011780 sodium chloride Substances 0.000 description 27
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 26
- 230000001093 anti-cancer Effects 0.000 description 25
- 210000004443 dendritic cell Anatomy 0.000 description 24
- 102000013691 Interleukin-17 Human genes 0.000 description 23
- 108050003558 Interleukin-17 Proteins 0.000 description 23
- 239000003242 anti bacterial agent Substances 0.000 description 23
- 241000699666 Mus <mouse, genus> Species 0.000 description 22
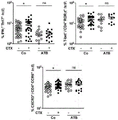
- 238000000684 flow cytometry Methods 0.000 description 22
- 241000194033 Enterococcus Species 0.000 description 21
- 238000004458 analytical method Methods 0.000 description 21
- 210000001035 gastrointestinal tract Anatomy 0.000 description 21
- 230000028993 immune response Effects 0.000 description 20
- 210000000813 small intestine Anatomy 0.000 description 20
- 108010078777 Colistin Proteins 0.000 description 18
- 229960003346 colistin Drugs 0.000 description 18
- 210000003405 ileum Anatomy 0.000 description 18
- JORAUNFTUVJTNG-BSTBCYLQSA-N n-[(2s)-4-amino-1-[[(2s,3r)-1-[[(2s)-4-amino-1-oxo-1-[[(3s,6s,9s,12s,15r,18s,21s)-6,9,18-tris(2-aminoethyl)-3-[(1r)-1-hydroxyethyl]-12,15-bis(2-methylpropyl)-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptazacyclotricos-21-yl]amino]butan-2-yl]amino]-3-h Chemical compound CC(C)CCCCC(=O)N[C@@H](CCN)C(=O)N[C@H]([C@@H](C)O)CN[C@@H](CCN)C(=O)N[C@H]1CCNC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCN)NC(=O)[C@H](CCN)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CCN)NC1=O.CCC(C)CCCCC(=O)N[C@@H](CCN)C(=O)N[C@H]([C@@H](C)O)CN[C@@H](CCN)C(=O)N[C@H]1CCNC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCN)NC(=O)[C@H](CCN)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CCN)NC1=O JORAUNFTUVJTNG-BSTBCYLQSA-N 0.000 description 18
- XDJYMJULXQKGMM-UHFFFAOYSA-N polymyxin E1 Natural products CCC(C)CCCCC(=O)NC(CCN)C(=O)NC(C(C)O)C(=O)NC(CCN)C(=O)NC1CCNC(=O)C(C(C)O)NC(=O)C(CCN)NC(=O)C(CCN)NC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(CCN)NC1=O XDJYMJULXQKGMM-UHFFFAOYSA-N 0.000 description 18
- KNIWPHSUTGNZST-UHFFFAOYSA-N polymyxin E2 Natural products CC(C)CCCCC(=O)NC(CCN)C(=O)NC(C(C)O)C(=O)NC(CCN)C(=O)NC1CCNC(=O)C(C(C)O)NC(=O)C(CCN)NC(=O)C(CCN)NC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(CCN)NC1=O KNIWPHSUTGNZST-UHFFFAOYSA-N 0.000 description 18
- 206010039491 Sarcoma Diseases 0.000 description 17
- 229940088710 antibiotic agent Drugs 0.000 description 17
- 108090000623 proteins and genes Proteins 0.000 description 17
- 238000003753 real-time PCR Methods 0.000 description 17
- 241000588724 Escherichia coli Species 0.000 description 16
- 241000186660 Lactobacillus Species 0.000 description 16
- 102100029424 Nucleotide-binding oligomerization domain-containing protein 1 Human genes 0.000 description 16
- 238000000338 in vitro Methods 0.000 description 16
- 229940039696 lactobacillus Drugs 0.000 description 16
- 101001125032 Homo sapiens Nucleotide-binding oligomerization domain-containing protein 1 Proteins 0.000 description 15
- 230000028327 secretion Effects 0.000 description 15
- 210000000068 Th17 cell Anatomy 0.000 description 14
- 229940100198 alkylating agent Drugs 0.000 description 14
- 239000002168 alkylating agent Substances 0.000 description 14
- 229960004679 doxorubicin Drugs 0.000 description 14
- 238000012175 pyrosequencing Methods 0.000 description 14
- 108020004465 16S ribosomal RNA Proteins 0.000 description 13
- 102000004127 Cytokines Human genes 0.000 description 13
- 108090000695 Cytokines Proteins 0.000 description 13
- 102100029441 Nucleotide-binding oligomerization domain-containing protein 2 Human genes 0.000 description 13
- 238000000692 Student's t-test Methods 0.000 description 13
- 230000005867 T cell response Effects 0.000 description 13
- 210000003608 fece Anatomy 0.000 description 13
- 210000004877 mucosa Anatomy 0.000 description 13
- 210000004400 mucous membrane Anatomy 0.000 description 13
- 229960005322 streptomycin Drugs 0.000 description 13
- 238000011740 C57BL/6 mouse Methods 0.000 description 12
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 description 12
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 description 12
- 239000012472 biological sample Substances 0.000 description 12
- 230000001413 cellular effect Effects 0.000 description 12
- 230000002950 deficient Effects 0.000 description 12
- 230000014509 gene expression Effects 0.000 description 12
- 230000001404 mediated effect Effects 0.000 description 12
- 238000003305 oral gavage Methods 0.000 description 12
- 239000000523 sample Substances 0.000 description 12
- 238000007619 statistical method Methods 0.000 description 12
- 238000012360 testing method Methods 0.000 description 12
- 238000012546 transfer Methods 0.000 description 12
- 241000605059 Bacteroidetes Species 0.000 description 11
- 206010009944 Colon cancer Diseases 0.000 description 11
- 108020004414 DNA Proteins 0.000 description 11
- 238000002965 ELISA Methods 0.000 description 11
- 101001046686 Homo sapiens Integrin alpha-M Proteins 0.000 description 11
- 101001125026 Homo sapiens Nucleotide-binding oligomerization domain-containing protein 2 Proteins 0.000 description 11
- 101000713602 Homo sapiens T-box transcription factor TBX21 Proteins 0.000 description 11
- 102100022338 Integrin alpha-M Human genes 0.000 description 11
- 238000003556 assay Methods 0.000 description 11
- 230000009467 reduction Effects 0.000 description 11
- 102100028990 C-X-C chemokine receptor type 3 Human genes 0.000 description 10
- 101000916050 Homo sapiens C-X-C chemokine receptor type 3 Proteins 0.000 description 10
- 229960000723 ampicillin Drugs 0.000 description 10
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 10
- 239000007924 injection Substances 0.000 description 10
- 238000002347 injection Methods 0.000 description 10
- 238000011081 inoculation Methods 0.000 description 10
- 230000002829 reductive effect Effects 0.000 description 10
- 241000186000 Bifidobacterium Species 0.000 description 9
- 241000160321 Parabacteroides Species 0.000 description 9
- 108091008778 RORγ2 Proteins 0.000 description 9
- 102100036840 T-box transcription factor TBX21 Human genes 0.000 description 9
- 239000002158 endotoxin Substances 0.000 description 9
- 230000012010 growth Effects 0.000 description 9
- 229920006008 lipopolysaccharide Polymers 0.000 description 9
- 230000010287 polarization Effects 0.000 description 9
- 238000000513 principal component analysis Methods 0.000 description 9
- 230000003393 splenic effect Effects 0.000 description 9
- 239000006228 supernatant Substances 0.000 description 9
- 238000001356 surgical procedure Methods 0.000 description 9
- 238000012353 t test Methods 0.000 description 9
- 230000008685 targeting Effects 0.000 description 9
- 229960005486 vaccine Drugs 0.000 description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 9
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 description 8
- 108050005493 CD3 protein, epsilon/gamma/delta subunit Proteins 0.000 description 8
- 102100022297 Integrin alpha-X Human genes 0.000 description 8
- 108090001005 Interleukin-6 Proteins 0.000 description 8
- 240000006024 Lactobacillus plantarum Species 0.000 description 8
- WKDDRNSBRWANNC-UHFFFAOYSA-N Thienamycin Natural products C1C(SCCN)=C(C(O)=O)N2C(=O)C(C(O)C)C21 WKDDRNSBRWANNC-UHFFFAOYSA-N 0.000 description 8
- 238000009825 accumulation Methods 0.000 description 8
- 238000001574 biopsy Methods 0.000 description 8
- 229940044683 chemotherapy drug Drugs 0.000 description 8
- 208000029742 colonic neoplasm Diseases 0.000 description 8
- 230000002550 fecal effect Effects 0.000 description 8
- 229960002182 imipenem Drugs 0.000 description 8
- ZSKVGTPCRGIANV-ZXFLCMHBSA-N imipenem Chemical compound C1C(SCC\N=C\N)=C(C(O)=O)N2C(=O)[C@H]([C@H](O)C)[C@H]21 ZSKVGTPCRGIANV-ZXFLCMHBSA-N 0.000 description 8
- 230000036039 immunity Effects 0.000 description 8
- 230000006698 induction Effects 0.000 description 8
- 230000000977 initiatory effect Effects 0.000 description 8
- 210000001165 lymph node Anatomy 0.000 description 8
- 230000001717 pathogenic effect Effects 0.000 description 8
- 210000001519 tissue Anatomy 0.000 description 8
- 229920001817 Agar Polymers 0.000 description 7
- 206010070545 Bacterial translocation Diseases 0.000 description 7
- 102100025074 C-C chemokine receptor-like 2 Human genes 0.000 description 7
- 241000605980 Faecalibacterium prausnitzii Species 0.000 description 7
- 241000282414 Homo sapiens Species 0.000 description 7
- 101000716068 Homo sapiens C-C chemokine receptor type 6 Proteins 0.000 description 7
- 102000002689 Toll-like receptor Human genes 0.000 description 7
- 108020000411 Toll-like receptor Proteins 0.000 description 7
- 239000008272 agar Substances 0.000 description 7
- 239000000427 antigen Substances 0.000 description 7
- 108091007433 antigens Proteins 0.000 description 7
- 102000036639 antigens Human genes 0.000 description 7
- 230000007375 bacterial translocation Effects 0.000 description 7
- 210000004369 blood Anatomy 0.000 description 7
- 239000008280 blood Substances 0.000 description 7
- 210000001198 duodenum Anatomy 0.000 description 7
- 238000001727 in vivo Methods 0.000 description 7
- 210000003071 memory t lymphocyte Anatomy 0.000 description 7
- 238000011002 quantification Methods 0.000 description 7
- 230000005945 translocation Effects 0.000 description 7
- 241000701474 Alistipes Species 0.000 description 6
- 241000193830 Bacillus <bacterium> Species 0.000 description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- 101000884271 Homo sapiens Signal transducer CD24 Proteins 0.000 description 6
- 241000186604 Lactobacillus reuteri Species 0.000 description 6
- 102000016943 Muramidase Human genes 0.000 description 6
- 108010014251 Muramidase Proteins 0.000 description 6
- 108010062010 N-Acetylmuramoyl-L-alanine Amidase Proteins 0.000 description 6
- 229930193140 Neomycin Natural products 0.000 description 6
- 102100038081 Signal transducer CD24 Human genes 0.000 description 6
- 210000000447 Th1 cell Anatomy 0.000 description 6
- 102100039360 Toll-like receptor 4 Human genes 0.000 description 6
- 229960004261 cefotaxime Drugs 0.000 description 6
- AZZMGZXNTDTSME-JUZDKLSSSA-M cefotaxime sodium Chemical compound [Na+].N([C@@H]1C(N2C(=C(COC(C)=O)CS[C@@H]21)C([O-])=O)=O)C(=O)\C(=N/OC)C1=CSC(N)=N1 AZZMGZXNTDTSME-JUZDKLSSSA-M 0.000 description 6
- 230000001419 dependent effect Effects 0.000 description 6
- 238000001514 detection method Methods 0.000 description 6
- 230000004069 differentiation Effects 0.000 description 6
- 238000009826 distribution Methods 0.000 description 6
- 230000002183 duodenal effect Effects 0.000 description 6
- 230000002349 favourable effect Effects 0.000 description 6
- 238000009169 immunotherapy Methods 0.000 description 6
- 210000005210 lymphoid organ Anatomy 0.000 description 6
- 229960004927 neomycin Drugs 0.000 description 6
- 208000002154 non-small cell lung carcinoma Diseases 0.000 description 6
- 230000002093 peripheral effect Effects 0.000 description 6
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 6
- 108090000765 processed proteins & peptides Proteins 0.000 description 6
- 238000010186 staining Methods 0.000 description 6
- 230000009885 systemic effect Effects 0.000 description 6
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 description 6
- 210000003171 tumor-infiltrating lymphocyte Anatomy 0.000 description 6
- 101000669447 Homo sapiens Toll-like receptor 4 Proteins 0.000 description 5
- 108010065637 Interleukin-23 Proteins 0.000 description 5
- 102000013264 Interleukin-23 Human genes 0.000 description 5
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 5
- 239000000556 agonist Substances 0.000 description 5
- 229940045799 anthracyclines and related substance Drugs 0.000 description 5
- 210000000612 antigen-presenting cell Anatomy 0.000 description 5
- 230000005975 antitumor immune response Effects 0.000 description 5
- 230000002238 attenuated effect Effects 0.000 description 5
- 210000001072 colon Anatomy 0.000 description 5
- 230000002596 correlated effect Effects 0.000 description 5
- 230000003247 decreasing effect Effects 0.000 description 5
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 5
- 230000002757 inflammatory effect Effects 0.000 description 5
- 239000007928 intraperitoneal injection Substances 0.000 description 5
- 238000011813 knockout mouse model Methods 0.000 description 5
- 208000020816 lung neoplasm Diseases 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 238000001840 matrix-assisted laser desorption--ionisation time-of-flight mass spectrometry Methods 0.000 description 5
- 238000012544 monitoring process Methods 0.000 description 5
- 229960001756 oxaliplatin Drugs 0.000 description 5
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 description 5
- 230000009261 transgenic effect Effects 0.000 description 5
- 210000004881 tumor cell Anatomy 0.000 description 5
- 235000013618 yogurt Nutrition 0.000 description 5
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 4
- 208000010507 Adenocarcinoma of Lung Diseases 0.000 description 4
- 108091093088 Amplicon Proteins 0.000 description 4
- 108700042778 Antimicrobial Peptides Proteins 0.000 description 4
- 102000044503 Antimicrobial Peptides Human genes 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 4
- 108010051219 Cre recombinase Proteins 0.000 description 4
- GQYIWUVLTXOXAJ-UHFFFAOYSA-N Lomustine Chemical compound ClCCN(N=O)C(=O)NC1CCCCC1 GQYIWUVLTXOXAJ-UHFFFAOYSA-N 0.000 description 4
- 101150053046 MYD88 gene Proteins 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N acetic acid Substances CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 4
- 230000003321 amplification Effects 0.000 description 4
- 230000009286 beneficial effect Effects 0.000 description 4
- 238000001815 biotherapy Methods 0.000 description 4
- 210000001185 bone marrow Anatomy 0.000 description 4
- 239000006285 cell suspension Substances 0.000 description 4
- 238000004132 cross linking Methods 0.000 description 4
- 230000029087 digestion Effects 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 239000012636 effector Substances 0.000 description 4
- 238000005516 engineering process Methods 0.000 description 4
- -1 flavonoid acetic acid derivatives Chemical class 0.000 description 4
- 235000013373 food additive Nutrition 0.000 description 4
- 239000002778 food additive Substances 0.000 description 4
- 235000013376 functional food Nutrition 0.000 description 4
- 210000002175 goblet cell Anatomy 0.000 description 4
- 230000002401 inhibitory effect Effects 0.000 description 4
- 230000005764 inhibitory process Effects 0.000 description 4
- 229940001882 lactobacillus reuteri Drugs 0.000 description 4
- 201000005249 lung adenocarcinoma Diseases 0.000 description 4
- 235000010335 lysozyme Nutrition 0.000 description 4
- 239000004325 lysozyme Substances 0.000 description 4
- 229960000274 lysozyme Drugs 0.000 description 4
- 201000001441 melanoma Diseases 0.000 description 4
- 238000011227 neoadjuvant chemotherapy Methods 0.000 description 4
- 238000003199 nucleic acid amplification method Methods 0.000 description 4
- 201000002528 pancreatic cancer Diseases 0.000 description 4
- 210000003134 paneth cell Anatomy 0.000 description 4
- 239000008188 pellet Substances 0.000 description 4
- 102000004196 processed proteins & peptides Human genes 0.000 description 4
- 238000001959 radiotherapy Methods 0.000 description 4
- 238000013207 serial dilution Methods 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 239000000725 suspension Substances 0.000 description 4
- 238000002255 vaccination Methods 0.000 description 4
- XAUDJQYHKZQPEU-KVQBGUIXSA-N 5-aza-2'-deoxycytidine Chemical compound O=C1N=C(N)N=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 XAUDJQYHKZQPEU-KVQBGUIXSA-N 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- 238000011746 C57BL/6J (JAX™ mouse strain) Methods 0.000 description 3
- 241000283707 Capra Species 0.000 description 3
- 201000009030 Carcinoma Diseases 0.000 description 3
- 241000193468 Clostridium perfringens Species 0.000 description 3
- 102000029816 Collagenase Human genes 0.000 description 3
- 108060005980 Collagenase Proteins 0.000 description 3
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 3
- 229920002307 Dextran Polymers 0.000 description 3
- 241000609971 Erysipelotrichaceae Species 0.000 description 3
- 241000986147 Faecalicoccus Species 0.000 description 3
- 101710113436 GTPase KRas Proteins 0.000 description 3
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 3
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 3
- 102100026122 High affinity immunoglobulin gamma Fc receptor I Human genes 0.000 description 3
- 101000913074 Homo sapiens High affinity immunoglobulin gamma Fc receptor I Proteins 0.000 description 3
- 102000000588 Interleukin-2 Human genes 0.000 description 3
- 108010002350 Interleukin-2 Proteins 0.000 description 3
- 241001134638 Lachnospira Species 0.000 description 3
- 241001112693 Lachnospiraceae Species 0.000 description 3
- 101100182715 Mus musculus Ly6c2 gene Proteins 0.000 description 3
- 102000010168 Myeloid Differentiation Factor 88 Human genes 0.000 description 3
- 108010077432 Myeloid Differentiation Factor 88 Proteins 0.000 description 3
- 238000002123 RNA extraction Methods 0.000 description 3
- 102100037886 Regenerating islet-derived protein 3-gamma Human genes 0.000 description 3
- 101710086144 Regenerating islet-derived protein 3-gamma Proteins 0.000 description 3
- 208000005718 Stomach Neoplasms Diseases 0.000 description 3
- 108091023040 Transcription factor Proteins 0.000 description 3
- 102000040945 Transcription factor Human genes 0.000 description 3
- 238000011226 adjuvant chemotherapy Methods 0.000 description 3
- 239000011324 bead Substances 0.000 description 3
- 235000013361 beverage Nutrition 0.000 description 3
- 239000006161 blood agar Substances 0.000 description 3
- 230000030833 cell death Effects 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 229960002424 collagenase Drugs 0.000 description 3
- 239000002299 complementary DNA Substances 0.000 description 3
- 238000011109 contamination Methods 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 239000003651 drinking water Substances 0.000 description 3
- 235000020188 drinking water Nutrition 0.000 description 3
- 230000008029 eradication Effects 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 230000002496 gastric effect Effects 0.000 description 3
- 230000036541 health Effects 0.000 description 3
- 238000001794 hormone therapy Methods 0.000 description 3
- 238000003384 imaging method Methods 0.000 description 3
- 210000000987 immune system Anatomy 0.000 description 3
- 230000003053 immunization Effects 0.000 description 3
- 238000002649 immunization Methods 0.000 description 3
- 238000003125 immunofluorescent labeling Methods 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 210000004347 intestinal mucosa Anatomy 0.000 description 3
- 230000003870 intestinal permeability Effects 0.000 description 3
- 230000003834 intracellular effect Effects 0.000 description 3
- 210000004072 lung Anatomy 0.000 description 3
- 208000037841 lung tumor Diseases 0.000 description 3
- 210000004698 lymphocyte Anatomy 0.000 description 3
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 3
- 238000004949 mass spectrometry Methods 0.000 description 3
- 201000006512 mast cell neoplasm Diseases 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 231100000590 oncogenic Toxicity 0.000 description 3
- 230000002246 oncogenic effect Effects 0.000 description 3
- 238000011275 oncology therapy Methods 0.000 description 3
- 208000008443 pancreatic carcinoma Diseases 0.000 description 3
- 244000052769 pathogen Species 0.000 description 3
- 239000006187 pill Substances 0.000 description 3
- 229910052697 platinum Inorganic materials 0.000 description 3
- 229940115272 polyinosinic:polycytidylic acid Drugs 0.000 description 3
- 239000003910 polypeptide antibiotic agent Substances 0.000 description 3
- 239000013641 positive control Substances 0.000 description 3
- 230000037452 priming Effects 0.000 description 3
- 230000001737 promoting effect Effects 0.000 description 3
- 102000004169 proteins and genes Human genes 0.000 description 3
- 238000011084 recovery Methods 0.000 description 3
- 210000003289 regulatory T cell Anatomy 0.000 description 3
- 238000003757 reverse transcription PCR Methods 0.000 description 3
- 210000002966 serum Anatomy 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 230000002195 synergetic effect Effects 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- 230000002103 transcriptional effect Effects 0.000 description 3
- GPRLSGONYQIRFK-MNYXATJNSA-N triton Chemical compound [3H+] GPRLSGONYQIRFK-MNYXATJNSA-N 0.000 description 3
- 230000002476 tumorcidal effect Effects 0.000 description 3
- 241000701161 unidentified adenovirus Species 0.000 description 3
- IAKHMKGGTNLKSZ-INIZCTEOSA-N (S)-colchicine Chemical compound C1([C@@H](NC(C)=O)CC2)=CC(=O)C(OC)=CC=C1C1=C2C=C(OC)C(OC)=C1OC IAKHMKGGTNLKSZ-INIZCTEOSA-N 0.000 description 2
- 239000012103 Alexa Fluor 488 Substances 0.000 description 2
- 206010006187 Breast cancer Diseases 0.000 description 2
- 208000026310 Breast neoplasm Diseases 0.000 description 2
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Chemical compound CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 2
- 102100032912 CD44 antigen Human genes 0.000 description 2
- GAGWJHPBXLXJQN-UORFTKCHSA-N Capecitabine Chemical compound C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](C)O1 GAGWJHPBXLXJQN-UORFTKCHSA-N 0.000 description 2
- GAGWJHPBXLXJQN-UHFFFAOYSA-N Capecitabine Natural products C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1C1C(O)C(O)C(C)O1 GAGWJHPBXLXJQN-UHFFFAOYSA-N 0.000 description 2
- 208000005623 Carcinogenesis Diseases 0.000 description 2
- 241000699800 Cricetinae Species 0.000 description 2
- 102000000541 Defensins Human genes 0.000 description 2
- 108010002069 Defensins Proteins 0.000 description 2
- 108010008532 Deoxyribonuclease I Proteins 0.000 description 2
- 102100030012 Deoxyribonuclease-1 Human genes 0.000 description 2
- 102000016911 Deoxyribonucleases Human genes 0.000 description 2
- 108010053770 Deoxyribonucleases Proteins 0.000 description 2
- MWWSFMDVAYGXBV-RUELKSSGSA-N Doxorubicin hydrochloride Chemical compound Cl.O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 MWWSFMDVAYGXBV-RUELKSSGSA-N 0.000 description 2
- 206010059866 Drug resistance Diseases 0.000 description 2
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 2
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 2
- WZUVPPKBWHMQCE-UHFFFAOYSA-N Haematoxylin Chemical compound C12=CC(O)=C(O)C=C2CC2(O)C1C1=CC=C(O)C(O)=C1OC2 WZUVPPKBWHMQCE-UHFFFAOYSA-N 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 101000868273 Homo sapiens CD44 antigen Proteins 0.000 description 2
- 101001109137 Homo sapiens Receptor-interacting serine/threonine-protein kinase 2 Proteins 0.000 description 2
- 101000733257 Homo sapiens Rho guanine nucleotide exchange factor 28 Proteins 0.000 description 2
- 101000946860 Homo sapiens T-cell surface glycoprotein CD3 epsilon chain Proteins 0.000 description 2
- 241000341655 Human papillomavirus type 16 Species 0.000 description 2
- 238000012404 In vitro experiment Methods 0.000 description 2
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 2
- 102100034343 Integrase Human genes 0.000 description 2
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 2
- 229930182816 L-glutamine Natural products 0.000 description 2
- 235000013965 Lactobacillus plantarum Nutrition 0.000 description 2
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 2
- 102000043131 MHC class II family Human genes 0.000 description 2
- 108091054438 MHC class II family Proteins 0.000 description 2
- 241001529936 Murinae Species 0.000 description 2
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 2
- ZRKWMRDKSOPRRS-UHFFFAOYSA-N N-Methyl-N-nitrosourea Chemical compound O=NN(C)C(N)=O ZRKWMRDKSOPRRS-UHFFFAOYSA-N 0.000 description 2
- 206010061309 Neoplasm progression Diseases 0.000 description 2
- 108700002045 Nod2 Signaling Adaptor Proteins 0.000 description 2
- 101150083031 Nod2 gene Proteins 0.000 description 2
- 208000008589 Obesity Diseases 0.000 description 2
- 206010030113 Oedema Diseases 0.000 description 2
- 108091034117 Oligonucleotide Proteins 0.000 description 2
- 206010033128 Ovarian cancer Diseases 0.000 description 2
- 206010061535 Ovarian neoplasm Diseases 0.000 description 2
- 241000192001 Pediococcus Species 0.000 description 2
- 229930182555 Penicillin Natural products 0.000 description 2
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 2
- 102100034539 Peptidyl-prolyl cis-trans isomerase A Human genes 0.000 description 2
- 241000425347 Phyla <beetle> Species 0.000 description 2
- 241000692843 Porphyromonadaceae Species 0.000 description 2
- 108010092799 RNA-directed DNA polymerase Proteins 0.000 description 2
- 238000011530 RNeasy Mini Kit Methods 0.000 description 2
- 238000011529 RT qPCR Methods 0.000 description 2
- 102100033204 Rho guanine nucleotide exchange factor 28 Human genes 0.000 description 2
- 241001501882 Rhodomonas Species 0.000 description 2
- 241000605947 Roseburia Species 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 102100035794 T-cell surface glycoprotein CD3 epsilon chain Human genes 0.000 description 2
- 230000029662 T-helper 1 type immune response Effects 0.000 description 2
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 2
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 2
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 2
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 2
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 2
- 238000001793 Wilcoxon signed-rank test Methods 0.000 description 2
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 2
- 208000009956 adenocarcinoma Diseases 0.000 description 2
- 125000000217 alkyl group Chemical group 0.000 description 2
- 230000005904 anticancer immunity Effects 0.000 description 2
- 238000011319 anticancer therapy Methods 0.000 description 2
- 229940034982 antineoplastic agent Drugs 0.000 description 2
- 229940045719 antineoplastic alkylating agent nitrosoureas Drugs 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 229960002756 azacitidine Drugs 0.000 description 2
- 150000001541 aziridines Chemical class 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 230000033228 biological regulation Effects 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 210000000481 breast Anatomy 0.000 description 2
- 230000036952 cancer formation Effects 0.000 description 2
- 238000009566 cancer vaccine Methods 0.000 description 2
- 229960004117 capecitabine Drugs 0.000 description 2
- 231100000504 carcinogenesis Toxicity 0.000 description 2
- 229960005243 carmustine Drugs 0.000 description 2
- 230000001364 causal effect Effects 0.000 description 2
- 210000004534 cecum Anatomy 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 239000012228 culture supernatant Substances 0.000 description 2
- 238000005520 cutting process Methods 0.000 description 2
- 230000009089 cytolysis Effects 0.000 description 2
- 230000001086 cytosolic effect Effects 0.000 description 2
- 231100000433 cytotoxic Toxicity 0.000 description 2
- 230000001472 cytotoxic effect Effects 0.000 description 2
- 230000002354 daily effect Effects 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- WVYXNIXAMZOZFK-UHFFFAOYSA-N diaziquone Chemical compound O=C1C(NC(=O)OCC)=C(N2CC2)C(=O)C(NC(=O)OCC)=C1N1CC1 WVYXNIXAMZOZFK-UHFFFAOYSA-N 0.000 description 2
- 235000005911 diet Nutrition 0.000 description 2
- 230000037213 diet Effects 0.000 description 2
- 235000015872 dietary supplement Nutrition 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 230000002708 enhancing effect Effects 0.000 description 2
- 230000003203 everyday effect Effects 0.000 description 2
- 229930003935 flavonoid Natural products 0.000 description 2
- 235000017173 flavonoids Nutrition 0.000 description 2
- ODKNJVUHOIMIIZ-RRKCRQDMSA-N floxuridine Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(F)=C1 ODKNJVUHOIMIIZ-RRKCRQDMSA-N 0.000 description 2
- 229960002949 fluorouracil Drugs 0.000 description 2
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 description 2
- 235000013305 food Nutrition 0.000 description 2
- 235000012041 food component Nutrition 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 206010017758 gastric cancer Diseases 0.000 description 2
- 230000007407 health benefit Effects 0.000 description 2
- 230000001900 immune effect Effects 0.000 description 2
- 238000010166 immunofluorescence Methods 0.000 description 2
- 230000005847 immunogenicity Effects 0.000 description 2
- 238000002513 implantation Methods 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 210000005024 intraepithelial lymphocyte Anatomy 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 230000002601 intratumoral effect Effects 0.000 description 2
- 238000010253 intravenous injection Methods 0.000 description 2
- PGHMRUGBZOYCAA-UHFFFAOYSA-N ionomycin Natural products O1C(CC(O)C(C)C(O)C(C)C=CCC(C)CC(C)C(O)=CC(=O)C(C)CC(C)CC(CCC(O)=O)C)CCC1(C)C1OC(C)(C(C)O)CC1 PGHMRUGBZOYCAA-UHFFFAOYSA-N 0.000 description 2
- PGHMRUGBZOYCAA-ADZNBVRBSA-N ionomycin Chemical compound O1[C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)/C=C/C[C@@H](C)C[C@@H](C)C(/O)=C/C(=O)[C@@H](C)C[C@@H](C)C[C@@H](CCC(O)=O)C)CC[C@@]1(C)[C@@H]1O[C@](C)([C@@H](C)O)CC1 PGHMRUGBZOYCAA-ADZNBVRBSA-N 0.000 description 2
- 150000002500 ions Chemical class 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 229940072205 lactobacillus plantarum Drugs 0.000 description 2
- 210000000265 leukocyte Anatomy 0.000 description 2
- 229960002247 lomustine Drugs 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 201000005202 lung cancer Diseases 0.000 description 2
- 208000006971 mastocytoma Diseases 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 230000001394 metastastic effect Effects 0.000 description 2
- 206010061289 metastatic neoplasm Diseases 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 239000003068 molecular probe Substances 0.000 description 2
- 210000005087 mononuclear cell Anatomy 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 239000002773 nucleotide Substances 0.000 description 2
- 125000003729 nucleotide group Chemical group 0.000 description 2
- 235000020824 obesity Nutrition 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 238000007427 paired t-test Methods 0.000 description 2
- 102000007863 pattern recognition receptors Human genes 0.000 description 2
- 108010089193 pattern recognition receptors Proteins 0.000 description 2
- 229940049954 penicillin Drugs 0.000 description 2
- 238000002733 pharmacodynamic assay Methods 0.000 description 2
- 230000035755 proliferation Effects 0.000 description 2
- 238000003906 pulsed field gel electrophoresis Methods 0.000 description 2
- 230000005855 radiation Effects 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 230000003362 replicative effect Effects 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 102200146742 rs2066844 Human genes 0.000 description 2
- 102200146596 rs2066845 Human genes 0.000 description 2
- 238000005070 sampling Methods 0.000 description 2
- 238000012163 sequencing technique Methods 0.000 description 2
- 230000001568 sexual effect Effects 0.000 description 2
- DAEPDZWVDSPTHF-UHFFFAOYSA-M sodium pyruvate Chemical compound [Na+].CC(=O)C([O-])=O DAEPDZWVDSPTHF-UHFFFAOYSA-M 0.000 description 2
- 238000001228 spectrum Methods 0.000 description 2
- 230000004936 stimulating effect Effects 0.000 description 2
- 201000011549 stomach cancer Diseases 0.000 description 2
- 239000007929 subcutaneous injection Substances 0.000 description 2
- 238000010254 subcutaneous injection Methods 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 150000004905 tetrazines Chemical class 0.000 description 2
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 2
- WYWHKKSPHMUBEB-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 2
- 230000005751 tumor progression Effects 0.000 description 2
- 241001148471 unidentified anaerobic bacterium Species 0.000 description 2
- 229960003048 vinblastine Drugs 0.000 description 2
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 2
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 2
- JVJGCCBAOOWGEO-RUTPOYCXSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-4-amino-2-[[(2s,3s)-2-[[(2s,3s)-2-[[(2s)-2-azaniumyl-3-hydroxypropanoyl]amino]-3-methylpentanoyl]amino]-3-methylpentanoyl]amino]-4-oxobutanoyl]amino]-3-phenylpropanoyl]amino]-4-carboxylatobutanoyl]amino]-6-azaniumy Chemical compound OC[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O)CC1=CC=CC=C1 JVJGCCBAOOWGEO-RUTPOYCXSA-N 0.000 description 1
- FPVKHBSQESCIEP-UHFFFAOYSA-N (8S)-3-(2-deoxy-beta-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol Natural products C1C(O)C(CO)OC1N1C(NC=NCC2O)=C2N=C1 FPVKHBSQESCIEP-UHFFFAOYSA-N 0.000 description 1
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 1
- FWBHETKCLVMNFS-UHFFFAOYSA-N 4',6-Diamino-2-phenylindol Chemical compound C1=CC(C(=N)N)=CC=C1C1=CC2=CC=C(C(N)=N)C=C2N1 FWBHETKCLVMNFS-UHFFFAOYSA-N 0.000 description 1
- AOJJSUZBOXZQNB-VTZDEGQISA-N 4'-epidoxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-VTZDEGQISA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- TVZGACDUOSZQKY-LBPRGKRZSA-N 4-aminofolic acid Chemical compound C1=NC2=NC(N)=NC(N)=C2N=C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 TVZGACDUOSZQKY-LBPRGKRZSA-N 0.000 description 1
- NMUSYJAQQFHJEW-UHFFFAOYSA-N 5-Azacytidine Natural products O=C1N=C(N)N=CN1C1C(O)C(O)C(CO)O1 NMUSYJAQQFHJEW-UHFFFAOYSA-N 0.000 description 1
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 description 1
- LJIRBXZDQGQUOO-KVTDHHQDSA-N 6-amino-3-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,4-dihydro-1,3,5-triazin-2-one Chemical compound C1NC(N)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 LJIRBXZDQGQUOO-KVTDHHQDSA-N 0.000 description 1
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 1
- 108010042708 Acetylmuramyl-Alanyl-Isoglutamine Proteins 0.000 description 1
- 241001156739 Actinobacteria <phylum> Species 0.000 description 1
- 239000012114 Alexa Fluor 647 Substances 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- 241000304886 Bacilli Species 0.000 description 1
- 241000606125 Bacteroides Species 0.000 description 1
- 206010004593 Bile duct cancer Diseases 0.000 description 1
- 241001474374 Blennius Species 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- FVLVBPDQNARYJU-XAHDHGMMSA-N C[C@H]1CCC(CC1)NC(=O)N(CCCl)N=O Chemical compound C[C@H]1CCC(CC1)NC(=O)N(CCCl)N=O FVLVBPDQNARYJU-XAHDHGMMSA-N 0.000 description 1
- 241000252983 Caecum Species 0.000 description 1
- 101100191768 Caenorhabditis elegans pbs-4 gene Proteins 0.000 description 1
- KLWPJMFMVPTNCC-UHFFFAOYSA-N Camptothecin Natural products CCC1(O)C(=O)OCC2=C1C=C3C4Nc5ccccc5C=C4CN3C2=O KLWPJMFMVPTNCC-UHFFFAOYSA-N 0.000 description 1
- 241000589876 Campylobacter Species 0.000 description 1
- 102100026089 Caspase recruitment domain-containing protein 9 Human genes 0.000 description 1
- 206010057248 Cell death Diseases 0.000 description 1
- 102000009410 Chemokine receptor Human genes 0.000 description 1
- 108050000299 Chemokine receptor Proteins 0.000 description 1
- PTOAARAWEBMLNO-KVQBGUIXSA-N Cladribine Chemical compound C1=NC=2C(N)=NC(Cl)=NC=2N1[C@H]1C[C@H](O)[C@@H](CO)O1 PTOAARAWEBMLNO-KVQBGUIXSA-N 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- FCKYPQBAHLOOJQ-UHFFFAOYSA-N Cyclohexane-1,2-diaminetetraacetic acid Chemical compound OC(=O)CN(CC(O)=O)C1CCCCC1N(CC(O)=O)CC(O)=O FCKYPQBAHLOOJQ-UHFFFAOYSA-N 0.000 description 1
- 108010072220 Cyclophilin A Proteins 0.000 description 1
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 1
- 102100039498 Cytotoxic T-lymphocyte protein 4 Human genes 0.000 description 1
- 238000007400 DNA extraction Methods 0.000 description 1
- 230000033616 DNA repair Effects 0.000 description 1
- 108010092160 Dactinomycin Proteins 0.000 description 1
- 241001143779 Dorea Species 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 241000305071 Enterobacterales Species 0.000 description 1
- 241001326278 Enterococcus faecalis JH2-2 Species 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- HTIJFSOGRVMCQR-UHFFFAOYSA-N Epirubicin Natural products COc1cccc2C(=O)c3c(O)c4CC(O)(CC(OC5CC(N)C(=O)C(C)O5)c4c(O)c3C(=O)c12)C(=O)CO HTIJFSOGRVMCQR-UHFFFAOYSA-N 0.000 description 1
- 241001288713 Escherichia coli MC1061 Species 0.000 description 1
- 108700039887 Essential Genes Proteins 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- 241001608234 Faecalibacterium Species 0.000 description 1
- 201000008808 Fibrosarcoma Diseases 0.000 description 1
- 241000605909 Fusobacterium Species 0.000 description 1
- 241000287828 Gallus gallus Species 0.000 description 1
- 101000609762 Gallus gallus Ovalbumin Proteins 0.000 description 1
- 206010061968 Gastric neoplasm Diseases 0.000 description 1
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 description 1
- 241001185600 Gemmiger Species 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- 206010053759 Growth retardation Diseases 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- 240000004153 Hibiscus sabdariffa Species 0.000 description 1
- 101000983508 Homo sapiens Caspase recruitment domain-containing protein 9 Proteins 0.000 description 1
- 101000889276 Homo sapiens Cytotoxic T-lymphocyte protein 4 Proteins 0.000 description 1
- 101001078158 Homo sapiens Integrin alpha-1 Proteins 0.000 description 1
- 101001018097 Homo sapiens L-selectin Proteins 0.000 description 1
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 1
- 101000831496 Homo sapiens Toll-like receptor 3 Proteins 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- XDXDZDZNSLXDNA-TZNDIEGXSA-N Idarubicin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XDXDZDZNSLXDNA-TZNDIEGXSA-N 0.000 description 1
- XDXDZDZNSLXDNA-UHFFFAOYSA-N Idarubicin Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XDXDZDZNSLXDNA-UHFFFAOYSA-N 0.000 description 1
- 108091008036 Immune checkpoint proteins Proteins 0.000 description 1
- 102000037982 Immune checkpoint proteins Human genes 0.000 description 1
- 206010062016 Immunosuppression Diseases 0.000 description 1
- 102100025323 Integrin alpha-1 Human genes 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- 108090000978 Interleukin-4 Proteins 0.000 description 1
- 108010002335 Interleukin-9 Proteins 0.000 description 1
- 102000015696 Interleukins Human genes 0.000 description 1
- 108010063738 Interleukins Proteins 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- 102100033467 L-selectin Human genes 0.000 description 1
- 239000005411 L01XE02 - Gefitinib Substances 0.000 description 1
- 239000005551 L01XE03 - Erlotinib Substances 0.000 description 1
- 102000016267 Leptin Human genes 0.000 description 1
- 108010092277 Leptin Proteins 0.000 description 1
- 102000013519 Lipocalin-2 Human genes 0.000 description 1
- 108010051335 Lipocalin-2 Proteins 0.000 description 1
- 206010025323 Lymphomas Diseases 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 238000007476 Maximum Likelihood Methods 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 229930191564 Monensin Natural products 0.000 description 1
- GAOZTHIDHYLHMS-UHFFFAOYSA-N Monensin A Natural products O1C(CC)(C2C(CC(O2)C2C(CC(C)C(O)(CO)O2)C)C)CCC1C(O1)(C)CCC21CC(O)C(C)C(C(C)C(OC)C(C)C(O)=O)O2 GAOZTHIDHYLHMS-UHFFFAOYSA-N 0.000 description 1
- 206010028116 Mucosal inflammation Diseases 0.000 description 1
- 201000010927 Mucositis Diseases 0.000 description 1
- MSFSPUZXLOGKHJ-UHFFFAOYSA-N Muraminsaeure Natural products OC(=O)C(C)OC1C(N)C(O)OC(CO)C1O MSFSPUZXLOGKHJ-UHFFFAOYSA-N 0.000 description 1
- 101001033286 Mus musculus Interleukin-1 beta Proteins 0.000 description 1
- 101000998145 Mus musculus Interleukin-17A Proteins 0.000 description 1
- OVBPIULPVIDEAO-UHFFFAOYSA-N N-Pteroyl-L-glutaminsaeure Natural products C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-UHFFFAOYSA-N 0.000 description 1
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 1
- 108700002046 Nod1 Signaling Adaptor Proteins 0.000 description 1
- 101150005821 Nod1 gene Proteins 0.000 description 1
- 108010075205 OVA-8 Proteins 0.000 description 1
- 240000007594 Oryza sativa Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- 108010058846 Ovalbumin Proteins 0.000 description 1
- 238000009004 PCR Kit Methods 0.000 description 1
- 101150056612 PPIA gene Proteins 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- 241000606210 Parabacteroides distasonis Species 0.000 description 1
- 108010013639 Peptidoglycan Proteins 0.000 description 1
- 101710111198 Peptidyl-prolyl cis-trans isomerase A Proteins 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 241000192142 Proteobacteria Species 0.000 description 1
- 241000589516 Pseudomonas Species 0.000 description 1
- 239000012979 RPMI medium Substances 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 101710141795 Ribonuclease inhibitor Proteins 0.000 description 1
- 229940122208 Ribonuclease inhibitor Drugs 0.000 description 1
- 102100037968 Ribonuclease inhibitor Human genes 0.000 description 1
- 108010079723 Shiga Toxin Proteins 0.000 description 1
- 231100000632 Spindle poison Toxicity 0.000 description 1
- 241001180364 Spirochaetes Species 0.000 description 1
- ZSJLQEPLLKMAKR-UHFFFAOYSA-N Streptozotocin Natural products O=NN(C)C(=O)NC1C(O)OC(CO)C(O)C1O ZSJLQEPLLKMAKR-UHFFFAOYSA-N 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 239000005864 Sulphur Substances 0.000 description 1
- 230000024932 T cell mediated immunity Effects 0.000 description 1
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 1
- 230000030429 T-helper 17 type immune response Effects 0.000 description 1
- 229940123237 Taxane Drugs 0.000 description 1
- BPEGJWRSRHCHSN-UHFFFAOYSA-N Temozolomide Chemical compound O=C1N(C)N=NC2=C(C(N)=O)N=CN21 BPEGJWRSRHCHSN-UHFFFAOYSA-N 0.000 description 1
- FOCVUCIESVLUNU-UHFFFAOYSA-N Thiotepa Chemical compound C1CN1P(N1CC1)(=S)N1CC1 FOCVUCIESVLUNU-UHFFFAOYSA-N 0.000 description 1
- 108010060804 Toll-Like Receptor 4 Proteins 0.000 description 1
- 102100024324 Toll-like receptor 3 Human genes 0.000 description 1
- IVTVGDXNLFLDRM-HNNXBMFYSA-N Tomudex Chemical compound C=1C=C2NC(C)=NC(=O)C2=CC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)S1 IVTVGDXNLFLDRM-HNNXBMFYSA-N 0.000 description 1
- 241000589886 Treponema Species 0.000 description 1
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Natural products NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 1
- 229940122803 Vinca alkaloid Drugs 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 241000186569 [Clostridium] leptum Species 0.000 description 1
- 229930183665 actinomycin Natural products 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 210000005006 adaptive immune system Anatomy 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 230000001464 adherent effect Effects 0.000 description 1
- 238000011360 adjunctive therapy Methods 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 230000009603 aerobic growth Effects 0.000 description 1
- 239000011543 agarose gel Substances 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 229960000548 alemtuzumab Drugs 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 230000007815 allergy Effects 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 229960000473 altretamine Drugs 0.000 description 1
- 229960003896 aminopterin Drugs 0.000 description 1
- XCPGHVQEEXUHNC-UHFFFAOYSA-N amsacrine Chemical compound COC1=CC(NS(C)(=O)=O)=CC=C1NC1=C(C=CC=C2)C2=NC2=CC=CC=C12 XCPGHVQEEXUHNC-UHFFFAOYSA-N 0.000 description 1
- 229960001220 amsacrine Drugs 0.000 description 1
- 230000009604 anaerobic growth Effects 0.000 description 1
- 230000006909 anti-apoptosis Effects 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 230000000118 anti-neoplastic effect Effects 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 229940045687 antimetabolites folic acid analogs Drugs 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- ATALOFNDEOCMKK-OITMNORJSA-N aprepitant Chemical compound O([C@@H]([C@@H]1C=2C=CC(F)=CC=2)O[C@H](C)C=2C=C(C=C(C=2)C(F)(F)F)C(F)(F)F)CCN1CC1=NNC(=O)N1 ATALOFNDEOCMKK-OITMNORJSA-N 0.000 description 1
- 229960001372 aprepitant Drugs 0.000 description 1
- 229940120638 avastin Drugs 0.000 description 1
- KLNFSAOEKUDMFA-UHFFFAOYSA-N azanide;2-hydroxyacetic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OCC(O)=O KLNFSAOEKUDMFA-UHFFFAOYSA-N 0.000 description 1
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 1
- LMEKQMALGUDUQG-UHFFFAOYSA-N azathioprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC=NC2=C1NC=N2 LMEKQMALGUDUQG-UHFFFAOYSA-N 0.000 description 1
- 229960002170 azathioprine Drugs 0.000 description 1
- 244000052616 bacterial pathogen Species 0.000 description 1
- 238000007681 bariatric surgery Methods 0.000 description 1
- 210000004082 barrier epithelial cell Anatomy 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 229960000397 bevacizumab Drugs 0.000 description 1
- 239000007621 bhi medium Substances 0.000 description 1
- 210000003445 biliary tract Anatomy 0.000 description 1
- 201000009036 biliary tract cancer Diseases 0.000 description 1
- 208000020790 biliary tract neoplasm Diseases 0.000 description 1
- 238000004166 bioassay Methods 0.000 description 1
- 229940126587 biotherapeutics Drugs 0.000 description 1
- 201000000053 blastoma Diseases 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- GXJABQQUPOEUTA-RDJZCZTQSA-N bortezomib Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)B(O)O)NC(=O)C=1N=CC=NC=1)C1=CC=CC=C1 GXJABQQUPOEUTA-RDJZCZTQSA-N 0.000 description 1
- 229960001467 bortezomib Drugs 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 229960002092 busulfan Drugs 0.000 description 1
- 229940127093 camptothecin Drugs 0.000 description 1
- VSJKWCGYPAHWDS-FQEVSTJZSA-N camptothecin Chemical compound C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-FQEVSTJZSA-N 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 235000013877 carbamide Nutrition 0.000 description 1
- 229960004562 carboplatin Drugs 0.000 description 1
- 238000012754 cardiac puncture Methods 0.000 description 1
- 230000022131 cell cycle Effects 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 230000036755 cellular response Effects 0.000 description 1
- 229960005395 cetuximab Drugs 0.000 description 1
- 239000013043 chemical agent Substances 0.000 description 1
- 230000035572 chemosensitivity Effects 0.000 description 1
- 238000009104 chemotherapy regimen Methods 0.000 description 1
- 235000013330 chicken meat Nutrition 0.000 description 1
- 229960004630 chlorambucil Drugs 0.000 description 1
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 1
- 208000037976 chronic inflammation Diseases 0.000 description 1
- 230000006020 chronic inflammation Effects 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- 229960002436 cladribine Drugs 0.000 description 1
- WDDPHFBMKLOVOX-AYQXTPAHSA-N clofarabine Chemical compound C1=NC=2C(N)=NC(Cl)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@@H]1F WDDPHFBMKLOVOX-AYQXTPAHSA-N 0.000 description 1
- 229960000928 clofarabine Drugs 0.000 description 1
- 229960001338 colchicine Drugs 0.000 description 1
- 230000004736 colon carcinogenesis Effects 0.000 description 1
- 230000000112 colonic effect Effects 0.000 description 1
- 230000001332 colony forming effect Effects 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 230000003750 conditioning effect Effects 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000010219 correlation analysis Methods 0.000 description 1
- 230000000875 corresponding effect Effects 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 229960000684 cytarabine Drugs 0.000 description 1
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 229960003901 dacarbazine Drugs 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- 229960000975 daunorubicin Drugs 0.000 description 1
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 229910003460 diamond Inorganic materials 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 229950002389 diaziquone Drugs 0.000 description 1
- 210000002249 digestive system Anatomy 0.000 description 1
- VSJKWCGYPAHWDS-UHFFFAOYSA-N dl-camptothecin Natural products C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)C5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-UHFFFAOYSA-N 0.000 description 1
- 239000003968 dna methyltransferase inhibitor Substances 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- 229960003668 docetaxel Drugs 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 229960002918 doxorubicin hydrochloride Drugs 0.000 description 1
- 238000002651 drug therapy Methods 0.000 description 1
- 235000006694 eating habits Nutrition 0.000 description 1
- BNFRJXLZYUTIII-UHFFFAOYSA-N efaproxiral Chemical compound CC1=CC(C)=CC(NC(=O)CC=2C=CC(OC(C)(C)C(O)=O)=CC=2)=C1 BNFRJXLZYUTIII-UHFFFAOYSA-N 0.000 description 1
- 229960000925 efaproxiral Drugs 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 201000008184 embryoma Diseases 0.000 description 1
- 210000001842 enterocyte Anatomy 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- YQGOJNYOYNNSMM-UHFFFAOYSA-N eosin Chemical compound [Na+].OC(=O)C1=CC=CC=C1C1=C2C=C(Br)C(=O)C(Br)=C2OC2=C(Br)C(O)=C(Br)C=C21 YQGOJNYOYNNSMM-UHFFFAOYSA-N 0.000 description 1
- 229960001904 epirubicin Drugs 0.000 description 1
- 230000004890 epithelial barrier function Effects 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- 229960001433 erlotinib Drugs 0.000 description 1
- AAKJLRGGTJKAMG-UHFFFAOYSA-N erlotinib Chemical compound C=12C=C(OCCOC)C(OCCOC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 AAKJLRGGTJKAMG-UHFFFAOYSA-N 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 239000003797 essential amino acid Substances 0.000 description 1
- 235000020776 essential amino acid Nutrition 0.000 description 1
- 230000017214 establishment of T cell polarity Effects 0.000 description 1
- 229960005542 ethidium bromide Drugs 0.000 description 1
- ZMMJGEGLRURXTF-UHFFFAOYSA-N ethidium bromide Chemical compound [Br-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CC)=C1C1=CC=CC=C1 ZMMJGEGLRURXTF-UHFFFAOYSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960000752 etoposide phosphate Drugs 0.000 description 1
- LIQODXNTTZAGID-OCBXBXKTSA-N etoposide phosphate Chemical compound COC1=C(OP(O)(O)=O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 LIQODXNTTZAGID-OCBXBXKTSA-N 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 230000028023 exocytosis Effects 0.000 description 1
- 210000001808 exosome Anatomy 0.000 description 1
- 235000021107 fermented food Nutrition 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 150000002215 flavonoids Chemical class 0.000 description 1
- 229960000961 floxuridine Drugs 0.000 description 1
- 229960000390 fludarabine Drugs 0.000 description 1
- GIUYCYHIANZCFB-FJFJXFQQSA-N fludarabine phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O GIUYCYHIANZCFB-FJFJXFQQSA-N 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- 150000005699 fluoropyrimidines Chemical class 0.000 description 1
- 229960000304 folic acid Drugs 0.000 description 1
- 235000019152 folic acid Nutrition 0.000 description 1
- 239000011724 folic acid Substances 0.000 description 1
- 150000002224 folic acids Chemical class 0.000 description 1
- 239000005417 food ingredient Substances 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 235000013350 formula milk Nutrition 0.000 description 1
- 229960004783 fotemustine Drugs 0.000 description 1
- YAKWPXVTIGTRJH-UHFFFAOYSA-N fotemustine Chemical compound CCOP(=O)(OCC)C(C)NC(=O)N(CCCl)N=O YAKWPXVTIGTRJH-UHFFFAOYSA-N 0.000 description 1
- 229960002584 gefitinib Drugs 0.000 description 1
- XGALLCVXEZPNRQ-UHFFFAOYSA-N gefitinib Chemical compound C=12C=C(OCCCN3CCOCC3)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 XGALLCVXEZPNRQ-UHFFFAOYSA-N 0.000 description 1
- 229960005277 gemcitabine Drugs 0.000 description 1
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 1
- 238000003633 gene expression assay Methods 0.000 description 1
- 238000001415 gene therapy Methods 0.000 description 1
- 230000009395 genetic defect Effects 0.000 description 1
- 229960002518 gentamicin Drugs 0.000 description 1
- 210000004602 germ cell Anatomy 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 231100000001 growth retardation Toxicity 0.000 description 1
- 210000005205 gut mucosa Anatomy 0.000 description 1
- 210000004209 hair Anatomy 0.000 description 1
- 201000010536 head and neck cancer Diseases 0.000 description 1
- 208000014829 head and neck neoplasm Diseases 0.000 description 1
- 230000005802 health problem Effects 0.000 description 1
- UUVWYPNAQBNQJQ-UHFFFAOYSA-N hexamethylmelamine Chemical compound CN(C)C1=NC(N(C)C)=NC(N(C)C)=N1 UUVWYPNAQBNQJQ-UHFFFAOYSA-N 0.000 description 1
- 230000013632 homeostatic process Effects 0.000 description 1
- 238000000265 homogenisation Methods 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 229960001001 ibritumomab tiuxetan Drugs 0.000 description 1
- 229960000908 idarubicin Drugs 0.000 description 1
- 229960001101 ifosfamide Drugs 0.000 description 1
- HOMGKSMUEGBAAB-UHFFFAOYSA-N ifosfamide Chemical compound ClCCNP1(=O)OCCCN1CCCl HOMGKSMUEGBAAB-UHFFFAOYSA-N 0.000 description 1
- 229960003685 imatinib mesylate Drugs 0.000 description 1
- YLMAHDNUQAMNNX-UHFFFAOYSA-N imatinib methanesulfonate Chemical compound CS(O)(=O)=O.C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 YLMAHDNUQAMNNX-UHFFFAOYSA-N 0.000 description 1
- 230000002519 immonomodulatory effect Effects 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 229940127121 immunoconjugate Drugs 0.000 description 1
- 238000002991 immunohistochemical analysis Methods 0.000 description 1
- 230000001506 immunosuppresive effect Effects 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 238000000099 in vitro assay Methods 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 230000015788 innate immune response Effects 0.000 description 1
- 210000005007 innate immune system Anatomy 0.000 description 1
- 102000006495 integrins Human genes 0.000 description 1
- 108010044426 integrins Proteins 0.000 description 1
- 229960003130 interferon gamma Drugs 0.000 description 1
- 229940047124 interferons Drugs 0.000 description 1
- 108010074108 interleukin-21 Proteins 0.000 description 1
- 229940047122 interleukins Drugs 0.000 description 1
- 210000005027 intestinal barrier Anatomy 0.000 description 1
- 230000007358 intestinal barrier function Effects 0.000 description 1
- 210000005026 intestinal epithelial barrier Anatomy 0.000 description 1
- 230000004674 intestinal mucosal integrity Effects 0.000 description 1
- 238000010212 intracellular staining Methods 0.000 description 1
- 229960005386 ipilimumab Drugs 0.000 description 1
- 229960004768 irinotecan Drugs 0.000 description 1
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 1
- 210000001630 jejunum Anatomy 0.000 description 1
- 210000001503 joint Anatomy 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 210000002429 large intestine Anatomy 0.000 description 1
- 229940039781 leptin Drugs 0.000 description 1
- NRYBAZVQPHGZNS-ZSOCWYAHSA-N leptin Chemical compound O=C([C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CC(C)C)CCSC)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CS)C(O)=O NRYBAZVQPHGZNS-ZSOCWYAHSA-N 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 231100000636 lethal dose Toxicity 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 238000007477 logistic regression Methods 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 230000004758 lung carcinogenesis Effects 0.000 description 1
- 210000003563 lymphoid tissue Anatomy 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 238000000816 matrix-assisted laser desorption--ionisation Methods 0.000 description 1
- 235000013372 meat Nutrition 0.000 description 1
- BAXLBXFAUKGCDY-UHFFFAOYSA-N mebendazole Chemical compound [CH]1C2=NC(NC(=O)OC)=NC2=CC=C1C(=O)C1=CC=CC=C1 BAXLBXFAUKGCDY-UHFFFAOYSA-N 0.000 description 1
- 229960003439 mebendazole Drugs 0.000 description 1
- 229960004961 mechlorethamine Drugs 0.000 description 1
- HAWPXGHAZFHHAD-UHFFFAOYSA-N mechlorethamine Chemical compound ClCCN(C)CCCl HAWPXGHAZFHHAD-UHFFFAOYSA-N 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 1
- 229960001428 mercaptopurine Drugs 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 208000030159 metabolic disease Diseases 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 230000003641 microbiacidal effect Effects 0.000 description 1
- 238000001000 micrograph Methods 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 229960004857 mitomycin Drugs 0.000 description 1
- 230000000394 mitotic effect Effects 0.000 description 1
- 229960001156 mitoxantrone Drugs 0.000 description 1
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 1
- QXYYYPFGTSJXNS-UHFFFAOYSA-N mitozolomide Chemical compound N1=NN(CCCl)C(=O)N2C1=C(C(=O)N)N=C2 QXYYYPFGTSJXNS-UHFFFAOYSA-N 0.000 description 1
- 229950005967 mitozolomide Drugs 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000009456 molecular mechanism Effects 0.000 description 1
- 229960005358 monensin Drugs 0.000 description 1
- GAOZTHIDHYLHMS-KEOBGNEYSA-N monensin A Chemical compound C([C@@](O1)(C)[C@H]2CC[C@@](O2)(CC)[C@H]2[C@H](C[C@@H](O2)[C@@H]2[C@H](C[C@@H](C)[C@](O)(CO)O2)C)C)C[C@@]21C[C@H](O)[C@@H](C)[C@@H]([C@@H](C)[C@@H](OC)[C@H](C)C(O)=O)O2 GAOZTHIDHYLHMS-KEOBGNEYSA-N 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 230000000877 morphologic effect Effects 0.000 description 1
- AARXZCZYLAFQQU-UHFFFAOYSA-N motexafin gadolinium Chemical compound [Gd].CC(O)=O.CC(O)=O.C1=C([N-]2)C(CC)=C(CC)C2=CC(C(=C2C)CCCO)=NC2=CN=C2C=C(OCCOCCOCCOC)C(OCCOCCOCCOC)=CC2=NC=C2C(C)=C(CCCO)C1=N2 AARXZCZYLAFQQU-UHFFFAOYSA-N 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 230000004682 mucosal barrier function Effects 0.000 description 1
- BSOQXXWZTUDTEL-ZUYCGGNHSA-N muramyl dipeptide Chemical compound OC(=O)CC[C@H](C(N)=O)NC(=O)[C@H](C)NC(=O)[C@@H](C)O[C@H]1[C@H](O)[C@@H](CO)O[C@@H](O)[C@@H]1NC(C)=O BSOQXXWZTUDTEL-ZUYCGGNHSA-N 0.000 description 1
- 230000003039 myelosuppressive effect Effects 0.000 description 1
- 238000011815 naïve C57Bl6 mouse Methods 0.000 description 1
- 229950007221 nedaplatin Drugs 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 208000004235 neutropenia Diseases 0.000 description 1
- 230000000683 nonmetastatic effect Effects 0.000 description 1
- 238000006384 oligomerization reaction Methods 0.000 description 1
- 230000005853 oncogenic activation Effects 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 229940092253 ovalbumin Drugs 0.000 description 1
- 230000002611 ovarian Effects 0.000 description 1
- MUTQTHUBSRXFQO-UHFFFAOYSA-L oxalate;platinum(2+) Chemical compound [Pt+2].[O-]C(=O)C([O-])=O MUTQTHUBSRXFQO-UHFFFAOYSA-L 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- 229960001972 panitumumab Drugs 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 229960005079 pemetrexed Drugs 0.000 description 1
- QOFFJEBXNKRSPX-ZDUSSCGKSA-N pemetrexed Chemical compound C1=N[C]2NC(N)=NC(=O)C2=C1CCC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 QOFFJEBXNKRSPX-ZDUSSCGKSA-N 0.000 description 1
- 229960002340 pentostatin Drugs 0.000 description 1
- FPVKHBSQESCIEP-JQCXWYLXSA-N pentostatin Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC[C@H]2O)=C2N=C1 FPVKHBSQESCIEP-JQCXWYLXSA-N 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 210000001986 peyer's patch Anatomy 0.000 description 1
- 230000003285 pharmacodynamic effect Effects 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 150000003057 platinum Chemical class 0.000 description 1
- 102000054765 polymorphisms of proteins Human genes 0.000 description 1
- 230000008092 positive effect Effects 0.000 description 1
- 238000012809 post-inoculation Methods 0.000 description 1
- CPTBDICYNRMXFX-UHFFFAOYSA-N procarbazine Chemical compound CNNCC1=CC=C(C(=O)NC(C)C)C=C1 CPTBDICYNRMXFX-UHFFFAOYSA-N 0.000 description 1
- 229960000624 procarbazine Drugs 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 238000013138 pruning Methods 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- WKSAUQYGYAYLPV-UHFFFAOYSA-N pyrimethamine Chemical compound CCC1=NC(N)=NC(N)=C1C1=CC=C(Cl)C=C1 WKSAUQYGYAYLPV-UHFFFAOYSA-N 0.000 description 1
- 229960000611 pyrimethamine Drugs 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 229960004432 raltitrexed Drugs 0.000 description 1
- 238000007637 random forest analysis Methods 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 230000007115 recruitment Effects 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 230000004043 responsiveness Effects 0.000 description 1
- 239000003161 ribonuclease inhibitor Substances 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- 229960004641 rituximab Drugs 0.000 description 1
- 102200146925 rs2066842 Human genes 0.000 description 1
- 102200098764 rs4986790 Human genes 0.000 description 1
- 102200098513 rs4986791 Human genes 0.000 description 1
- 229960005399 satraplatin Drugs 0.000 description 1
- 190014017285 satraplatin Chemical compound 0.000 description 1
- 239000012047 saturated solution Substances 0.000 description 1
- 229960003440 semustine Drugs 0.000 description 1
- FVLVBPDQNARYJU-UHFFFAOYSA-N semustine Chemical compound CC1CCC(NC(=O)N(CCCl)N=O)CC1 FVLVBPDQNARYJU-UHFFFAOYSA-N 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 230000011664 signaling Effects 0.000 description 1
- 229960000714 sipuleucel-t Drugs 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 229940054269 sodium pyruvate Drugs 0.000 description 1
- 210000001082 somatic cell Anatomy 0.000 description 1
- 230000000392 somatic effect Effects 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 229960001052 streptozocin Drugs 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- 230000008093 supporting effect Effects 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 230000007761 synergistic anti-cancer Effects 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 229960001603 tamoxifen Drugs 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- 229960004964 temozolomide Drugs 0.000 description 1
- NRUKOCRGYNPUPR-QBPJDGROSA-N teniposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 NRUKOCRGYNPUPR-QBPJDGROSA-N 0.000 description 1
- 229960001278 teniposide Drugs 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 229940021747 therapeutic vaccine Drugs 0.000 description 1
- 229960001196 thiotepa Drugs 0.000 description 1
- 210000000115 thoracic cavity Anatomy 0.000 description 1
- 210000001541 thymus gland Anatomy 0.000 description 1
- 210000002303 tibia Anatomy 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- 229950002376 tirapazamine Drugs 0.000 description 1
- QVMPZNRFXAKISM-UHFFFAOYSA-N tirapazamine Chemical compound C1=CC=C2[N+]([O-])=NC(=N)N(O)C2=C1 QVMPZNRFXAKISM-UHFFFAOYSA-N 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- 229960000303 topotecan Drugs 0.000 description 1
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 description 1
- 229960005267 tositumomab Drugs 0.000 description 1
- 231100000027 toxicology Toxicity 0.000 description 1
- 238000012549 training Methods 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 229960000575 trastuzumab Drugs 0.000 description 1
- 229960001612 trastuzumab emtansine Drugs 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- IEDVJHCEMCRBQM-UHFFFAOYSA-N trimethoprim Chemical compound COC1=C(OC)C(OC)=CC(CC=2C(=NC(N)=NC=2)N)=C1 IEDVJHCEMCRBQM-UHFFFAOYSA-N 0.000 description 1
- 229960001082 trimethoprim Drugs 0.000 description 1
- 190014017283 triplatin tetranitrate Chemical compound 0.000 description 1
- 229950002860 triplatin tetranitrate Drugs 0.000 description 1
- 229910021642 ultra pure water Inorganic materials 0.000 description 1
- 239000012498 ultrapure water Substances 0.000 description 1
- 241001113232 unclassified Lachnospiraceae Species 0.000 description 1
- 238000011870 unpaired t-test Methods 0.000 description 1
- 210000000689 upper leg Anatomy 0.000 description 1
- 229940045136 urea Drugs 0.000 description 1
- 210000004291 uterus Anatomy 0.000 description 1
- 229950008737 vadimezan Drugs 0.000 description 1
- XGOYIMQSIKSOBS-UHFFFAOYSA-N vadimezan Chemical compound C1=CC=C2C(=O)C3=CC=C(C)C(C)=C3OC2=C1CC(O)=O XGOYIMQSIKSOBS-UHFFFAOYSA-N 0.000 description 1
- 238000010200 validation analysis Methods 0.000 description 1
- 229960000653 valrubicin Drugs 0.000 description 1
- ZOCKGBMQLCSHFP-KQRAQHLDSA-N valrubicin Chemical compound O([C@H]1C[C@](CC2=C(O)C=3C(=O)C4=CC=CC(OC)=C4C(=O)C=3C(O)=C21)(O)C(=O)COC(=O)CCCC)[C@H]1C[C@H](NC(=O)C(F)(F)F)[C@H](O)[C@H](C)O1 ZOCKGBMQLCSHFP-KQRAQHLDSA-N 0.000 description 1
- 239000004066 vascular targeting agent Substances 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 229960004528 vincristine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 1
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 1
- NMDYYWFGPIMTKO-HBVLKOHWSA-N vinflunine Chemical compound C([C@@](C1=C(C2=CC=CC=C2N1)C1)(C2=C(OC)C=C3N(C)[C@@H]4[C@@]5(C3=C2)CCN2CC=C[C@]([C@@H]52)([C@H]([C@]4(O)C(=O)OC)OC(C)=O)CC)C(=O)OC)[C@H]2C[C@@H](C(C)(F)F)CN1C2 NMDYYWFGPIMTKO-HBVLKOHWSA-N 0.000 description 1
- 229960000922 vinflunine Drugs 0.000 description 1
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 230000004580 weight loss Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
- A61K35/741—Probiotics
- A61K35/744—Lactic acid bacteria, e.g. enterococci, pediococci, lactococci, streptococci or leuconostocs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/407—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil condensed with other heterocyclic ring systems, e.g. ketorolac, physostigmine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
- A61K35/741—Probiotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
- A61K35/741—Probiotics
- A61K35/744—Lactic acid bacteria, e.g. enterococci, pediococci, lactococci, streptococci or leuconostocs
- A61K35/747—Lactobacilli, e.g. L. acidophilus or L. brevis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/14—Peptides containing saccharide radicals; Derivatives thereof, e.g. bleomycin, phleomycin, muramylpeptides or vancomycin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/0216—Bacteriodetes, e.g. Bacteroides, Ornithobacter, Porphyromonas
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/09—Lactobacillales, e.g. aerococcus, enterococcus, lactobacillus, lactococcus, streptococcus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/39—Medicinal preparations containing antigens or antibodies characterised by the immunostimulating additives, e.g. chemical adjuvants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N1/00—Microorganisms, e.g. protozoa; Compositions thereof; Processes of propagating, maintaining or preserving microorganisms or compositions thereof; Processes of preparing or isolating a composition containing a microorganism; Culture media therefor
- C12N1/20—Bacteria; Culture media therefor
- C12N1/205—Bacterial isolates
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6883—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material
- C12Q1/6886—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material for cancer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55588—Adjuvants of undefined constitution
- A61K2039/55594—Adjuvants of undefined constitution from bacteria
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/106—Pharmacogenomics, i.e. genetic variability in individual responses to drugs and drug metabolism
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12R—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES C12C - C12Q, RELATING TO MICROORGANISMS
- C12R2001/00—Microorganisms ; Processes using microorganisms
- C12R2001/01—Bacteria or Actinomycetales ; using bacteria or Actinomycetales
- C12R2001/46—Streptococcus ; Enterococcus; Lactococcus
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Microbiology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Mycology (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Genetics & Genomics (AREA)
- Pathology (AREA)
- Analytical Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biophysics (AREA)
- Physics & Mathematics (AREA)
- Oncology (AREA)
- Hospice & Palliative Care (AREA)
- Gastroenterology & Hepatology (AREA)
- Biomedical Technology (AREA)
- Virology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP13306597.9 | 2013-11-21 | ||
| EP13306597.9A EP2876167A1 (en) | 2013-11-21 | 2013-11-21 | Microbiota composition, as a marker of responsiveness to chemotherapy, and use of microbial modulators (pre-,pro- or synbiotics) for improving the efficacy of a cancer treatment |
| PCT/IB2014/066249 WO2015075688A1 (en) | 2013-11-21 | 2014-11-21 | Microbiota composition, as a marker of responsiveness to chemotherapy, and use of microbial modulators (pre-, pro- or synbiotics) for improving the efficacy of a cancer treatment |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN106170557A CN106170557A (zh) | 2016-11-30 |
| CN106170557B true CN106170557B (zh) | 2020-08-21 |
Family
ID=49680948
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201480063481.9A Active CN106170557B (zh) | 2013-11-21 | 2014-11-21 | 作为化疗应答标志物的微生物群组合物,以及微生物调节剂(益生元、益生菌或合生元)用于提高癌症治疗效力的用途 |
Country Status (13)
| Country | Link |
|---|---|
| US (2) | US10646521B2 (enExample) |
| EP (2) | EP2876167A1 (enExample) |
| JP (2) | JP6662775B2 (enExample) |
| KR (1) | KR102464342B1 (enExample) |
| CN (1) | CN106170557B (enExample) |
| AU (1) | AU2014351348B2 (enExample) |
| CA (1) | CA2931363C (enExample) |
| DK (1) | DK3071709T3 (enExample) |
| ES (1) | ES2764282T3 (enExample) |
| IL (2) | IL245751B (enExample) |
| PT (1) | PT3071709T (enExample) |
| SG (1) | SG10201803877YA (enExample) |
| WO (1) | WO2015075688A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2835581C1 (ru) * | 2024-07-03 | 2025-02-28 | Федеральное государственное бюджетное научное учреждение "Институт экспериментальной медицины" (ФГБНУ "ИЭМ") | Способ подбора пробиотических и аутопробиотических штаммов микроорганизмов по устойчивости к цитостатикам при терапии колоректального рака |
Families Citing this family (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE112016002056T5 (de) | 2015-05-06 | 2018-02-08 | Snipr Technologies Limited | Verändern mikrobieller Populationen und Modifizieren von Mikrobiomen |
| KR102661603B1 (ko) * | 2015-06-01 | 2024-04-29 | 더 유니버서티 오브 시카고 | 공생 미생물총의 조작에 의한 암의 치료 |
| EP3130680A1 (en) * | 2015-08-11 | 2017-02-15 | Universitat de Girona | Method for the detection, follow up and/or classification of intestinal diseases |
| EP4592391A3 (en) | 2015-10-21 | 2025-10-08 | Ose Immunotherapeutics | Methods and compositions for modifying macrophage polarization into pro-inflammatory cells to treat cancer |
| MA45287A (fr) * | 2015-11-20 | 2018-08-22 | 4D Pharma Res Ltd | Compositions comprenant des souches bactériennes |
| GB201609811D0 (en) * | 2016-06-05 | 2016-07-20 | Snipr Technologies Ltd | Methods, cells, systems, arrays, RNA and kits |
| CN106011281B (zh) * | 2016-07-19 | 2019-04-23 | 浙江大学 | 肠道分节丝状菌鞭毛蛋白的检测方法 |
| CN109715059A (zh) * | 2016-09-16 | 2019-05-03 | 优比欧迈公司 | 用于组表征的方法和系统 |
| EP3518946A4 (en) | 2016-09-27 | 2020-09-09 | Board of Regents, The University of Texas System | METHODS TO IMPROVE IMMUNE CHECKPOINT BLOCKING TREATMENT BY MODULATING THE MICROBIOME |
| KR102644935B1 (ko) * | 2016-12-22 | 2024-03-07 | 인스티튜트 구스타브 루시 | 항-PD1/PD-L1/PD-L2 항체에 대한 반응성의 마커로서의 미생물총 조성물, 및 항-PD1/PD-L1/PD-L2 Ab-기반 치료의 효능을 개선하기 위한 미생물 조정제의 용도 |
| WO2018145082A1 (en) * | 2017-02-06 | 2018-08-09 | New York University | Methods and compositions for treating and diagnosing pancreatic cancers |
| EP3378949A1 (en) * | 2017-03-22 | 2018-09-26 | Assistance Publique - Hôpitaux de Paris | Method for determining the potential efficacy of anticancer treatment |
| MA51770A (fr) | 2017-07-05 | 2020-05-13 | Evelo Biosciences Inc | Compositions et méthodes de traitement du cancer à l'aide de bifidobacterium animalis ssp. lactis |
| MA49982A (fr) * | 2017-08-10 | 2020-06-17 | 4D Pharma Res Ltd | Compositions comprenant des souches bactériennes |
| EP3476396A1 (en) * | 2017-10-31 | 2019-05-01 | Institut Gustave Roussy | Bacterial and cell compositions for the treatment of colorectal cancer and methods for assessing a prognosis for patients having the same |
| CN107904298A (zh) * | 2017-12-29 | 2018-04-13 | 苏州普瑞森基因科技有限公司 | 一种用于分析肠道微生物的试剂盒及其应用 |
| EP3505177A1 (en) * | 2017-12-29 | 2019-07-03 | Institut Gustave Roussy | Immunogenic sequences from a phage tail length tape measure protein, bacteria expressing the same and their use in treating a cancer |
| CN111886333A (zh) * | 2018-02-09 | 2020-11-03 | 学校法人庆应义塾 | 诱导cd8+t细胞的组合物和方法 |
| US10760075B2 (en) | 2018-04-30 | 2020-09-01 | Snipr Biome Aps | Treating and preventing microbial infections |
| US11541105B2 (en) | 2018-06-01 | 2023-01-03 | The Research Foundation For The State University Of New York | Compositions and methods for disrupting biofilm formation and maintenance |
| EP3597202A1 (en) * | 2018-07-20 | 2020-01-22 | Maat Pharma | Fecal microbiota composition, for use in reducing treatment-induced inflammation |
| KR20210040997A (ko) * | 2018-08-05 | 2021-04-14 | 다 볼떼라 | 항암제 효능의 개선 방법 |
| US11331269B2 (en) * | 2018-09-06 | 2022-05-17 | Massachusetts Institute Of Technology | Methods and compositions targeting lung microbiota and its responding immune pathways for lung cancer treatment |
| EP3629023A1 (en) * | 2018-09-28 | 2020-04-01 | Institut Gustave Roussy | Microbiota composition, as a marker of responsiveness to anti-pd1/pd-l1/pd-l2 antibodies in renal cell cancer |
| IL311084A (en) * | 2018-11-30 | 2024-04-01 | Caris Mpi Inc | New generation molecular characterization |
| CN113166815B (zh) * | 2019-02-22 | 2024-06-11 | 深圳华大生命科学研究院 | 肠道宏基因组在筛选pd-1抗体阻断剂疗效方面的用途 |
| EP3935581A4 (en) | 2019-03-04 | 2022-11-30 | Iocurrents, Inc. | DATA COMPRESSION AND COMMUNICATION USING MACHINE LEARNING |
| CN113906130A (zh) * | 2019-03-28 | 2022-01-07 | 千实验室株式会社 | 粪杆菌属微生物及包含其的抗癌组合物 |
| CN111991427A (zh) * | 2019-05-07 | 2020-11-27 | 瑞微(深圳)生物科技有限公司 | 肠道细菌在制备用于促进TCR γδ+T细胞增殖的药物中的应用 |
| EP3965750A4 (en) * | 2019-05-08 | 2023-07-12 | Garvan Institute of Medical Research | Cancer stratification and treatment based on inhibition of nod-2 |
| KR20220116438A (ko) * | 2019-11-01 | 2022-08-23 | 유티아이 리미티드 파트너쉽 | 암 치료를 위한 미생물총 및 대사산물의 힘 활용 |
| WO2021102510A1 (en) * | 2019-11-26 | 2021-06-03 | South Australian Health And Medical Research Institute Limited | Methods and products for reducing side effects associated with use of immune agonist antibodies |
| WO2021112918A1 (en) * | 2019-12-02 | 2021-06-10 | Caris Mpi, Inc. | Pan-cancer platinum response predictor |
| CN114832088A (zh) * | 2022-05-19 | 2022-08-02 | 李臻 | 拉瑞唑来多肽在制备缓解阿霉素心脏毒性药物中的用途 |
| GB202209518D0 (en) | 2022-06-29 | 2022-08-10 | Snipr Biome Aps | Treating & preventing E coli infections |
| CN116287355A (zh) * | 2023-05-12 | 2023-06-23 | 中国医学科学院北京协和医院 | 上消化道肿瘤化疗疗效及不良反应的肠道菌群预测模型 |
| CN117237952B (zh) * | 2023-11-15 | 2024-02-09 | 山东大学 | 基于免疫地形图的染色病理切片细胞分布标注方法及系统 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102215836A (zh) * | 2008-09-19 | 2011-10-12 | 雀巢产品技术援助有限公司 | 预防或减轻在抗癌治疗期间的骨髓瘫痪或中性白细胞减少症的营养支持 |
| WO2011131472A1 (en) * | 2010-04-22 | 2011-10-27 | Institut Gustave Roussy | Compounds and uses thereof to induce an immunogenic cancer cell death in a subject |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH08295631A (ja) | 1995-02-27 | 1996-11-12 | Nichinichi Seiyaku Kk | 制癌増強剤 |
| JPH1029946A (ja) * | 1996-07-15 | 1998-02-03 | Nichinichi Seiyaku Kk | 液性免疫回復剤 |
| TW200904340A (en) | 2007-05-11 | 2009-02-01 | Mannatech Inc | Processing of natural polysaccharides by selected non-pathogenic microorganisms and methods of making and using the same |
| CA2736774C (en) | 2008-09-19 | 2017-08-22 | Institut Curie | Nutritional support to prevent and/or mitigate bone marrow toxicity from a cancerous tumor |
| JP5717116B2 (ja) * | 2008-12-22 | 2015-05-13 | 学校法人昭和大学 | 抗原特異的ヒトTh17細胞を調整する方法 |
-
2013
- 2013-11-21 EP EP13306597.9A patent/EP2876167A1/en not_active Withdrawn
-
2014
- 2014-11-21 ES ES14812648T patent/ES2764282T3/es active Active
- 2014-11-21 DK DK14812648.5T patent/DK3071709T3/da active
- 2014-11-21 KR KR1020167016068A patent/KR102464342B1/ko active Active
- 2014-11-21 PT PT148126485T patent/PT3071709T/pt unknown
- 2014-11-21 SG SG10201803877YA patent/SG10201803877YA/en unknown
- 2014-11-21 JP JP2016533172A patent/JP6662775B2/ja active Active
- 2014-11-21 US US15/038,073 patent/US10646521B2/en active Active
- 2014-11-21 AU AU2014351348A patent/AU2014351348B2/en active Active
- 2014-11-21 WO PCT/IB2014/066249 patent/WO2015075688A1/en not_active Ceased
- 2014-11-21 CN CN201480063481.9A patent/CN106170557B/zh active Active
- 2014-11-21 CA CA2931363A patent/CA2931363C/en active Active
- 2014-11-21 EP EP14812648.5A patent/EP3071709B1/en active Active
-
2016
- 2016-05-19 IL IL245751A patent/IL245751B/en active IP Right Grant
-
2019
- 2019-08-05 IL IL268526A patent/IL268526B/en unknown
- 2019-12-09 JP JP2019221925A patent/JP2020050664A/ja active Pending
-
2020
- 2020-04-21 US US16/854,117 patent/US11344586B2/en active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102215836A (zh) * | 2008-09-19 | 2011-10-12 | 雀巢产品技术援助有限公司 | 预防或减轻在抗癌治疗期间的骨髓瘫痪或中性白细胞减少症的营养支持 |
| WO2011131472A1 (en) * | 2010-04-22 | 2011-10-27 | Institut Gustave Roussy | Compounds and uses thereof to induce an immunogenic cancer cell death in a subject |
Non-Patent Citations (1)
| Title |
|---|
| Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study;P Osterlund et al.,;《British Journal of Cancer》;20071231;第97卷;1028-1034 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2835581C1 (ru) * | 2024-07-03 | 2025-02-28 | Федеральное государственное бюджетное научное учреждение "Институт экспериментальной медицины" (ФГБНУ "ИЭМ") | Способ подбора пробиотических и аутопробиотических штаммов микроорганизмов по устойчивости к цитостатикам при терапии колоректального рака |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2931363A1 (en) | 2015-05-28 |
| JP2020050664A (ja) | 2020-04-02 |
| KR102464342B1 (ko) | 2022-11-08 |
| KR20160079122A (ko) | 2016-07-05 |
| AU2014351348B2 (en) | 2020-12-03 |
| EP2876167A1 (en) | 2015-05-27 |
| US10646521B2 (en) | 2020-05-12 |
| IL245751B (en) | 2019-08-29 |
| JP6662775B2 (ja) | 2020-03-11 |
| EP3071709B1 (en) | 2019-12-25 |
| ES2764282T3 (es) | 2020-06-02 |
| US20160303172A1 (en) | 2016-10-20 |
| IL268526A (en) | 2019-09-26 |
| DK3071709T3 (da) | 2020-03-16 |
| CA2931363C (en) | 2023-07-18 |
| JP2016539119A (ja) | 2016-12-15 |
| US11344586B2 (en) | 2022-05-31 |
| PT3071709T (pt) | 2020-01-17 |
| CN106170557A (zh) | 2016-11-30 |
| IL268526B (en) | 2022-02-01 |
| SG10201803877YA (en) | 2018-06-28 |
| EP3071709A1 (en) | 2016-09-28 |
| WO2015075688A1 (en) | 2015-05-28 |
| AU2014351348A1 (en) | 2016-06-09 |
| IL245751A0 (en) | 2016-07-31 |
| US20200360449A1 (en) | 2020-11-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11344586B2 (en) | Microbiota composition, as a marker of responsiveness to chemotherapy, and use of microbial modulators (pre-, pro- or synbiotics) for improving the efficacy of a cancer treatment | |
| US20230263843A1 (en) | Microbiota composition, as a marker of responsiveness to anti-pd1/pd-l1/pd-l2 antibodies and use of microbial modulators for improving the efficacy of an anti-pd1/pd-l1/pd-l2 ab-based treatment | |
| AU2015334468B2 (en) | Methods and products for modulating microbiota composition for improving the efficacy of a cancer treatment with an immune checkpoint blocker | |
| Roberti et al. | Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer | |
| JP7441167B2 (ja) | 結腸直腸癌の治療のための細菌及び細胞組成物並びにそれを有する患者の予後を評価するための方法 | |
| US20160120915A1 (en) | Methods for manipulating immune responses by altering microbiota | |
| Zuo et al. | Bifidobacterium infantis attenuates colitis by regulating T cell subset responses | |
| US20240398876A1 (en) | Biological marker of intestinal dysbiosis, useful for predicting the response of a cancer patient to an anti-pdi drug | |
| EP3601614A1 (en) | Method for determining the potential efficacy of anticancer treatment | |
| KR20240093392A (ko) | 질병 치료를 위한 조성물 및 방법 | |
| D'Amico | The human gut microbiome in disease: Role in chemotherapy treatments and relationship with clinical outcomes | |
| Stendel | Assessing the Changes to the Gut Microbiome Following Radiation Therapy in a Bladder Cancer Context | |
| Geis | REGULATORY T CELLS PROMOTE IL-17-DEPENDENT CARCINOGENIC IMMUNITY TO ENTEROTOXIGENIC BACTEROIDES FRAGILIS | |
| Class et al. | Patent application title: METHODS FOR MANIPULATING IMMUNE RESPONSES BY ALTERING MICROBIOTA Inventors: Martin J. Blaser (New York, NY, US) Martin J. Blaser (New York, NY, US) Shingo Yamanishi (Bunkyo-Ku, JP) Laura M. Cox (Brooklyn, NY, US) Victoria E. Ruiz (Brooklyn, NY, US) Alexandra E. Livanos (New York, NY, US) Assignees: New York University | |
| Serrano Honeyman et al. | Downregulated Th17 responses are associated with reduced gastritis in Helicobacter pylori-infected children |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| TA01 | Transfer of patent application right |
Effective date of registration: 20191101 Address after: Fa Guobali Applicant after: National Health and Medicine Inst. Applicant after: Nanterre Applicant after: National Institute of Agronomique Address before: Fa Guoweileruifu Applicant before: Gustavoiros Institute |
|
| TA01 | Transfer of patent application right | ||
| TA01 | Transfer of patent application right |
Effective date of registration: 20200720 Address after: Fa Guoweileruifu Applicant after: Gustavus Institute Applicant after: INSERM (Institut National de la Sante et de la Recherche Medicale) Applicant after: University PARIS SUD Applicant after: INSTITUT NATIONAL DE LA RECHERCHE AGRONOMIQUE Address before: Fa Guobali Applicant before: INSERM (Institut National de la Sante et de la Recherche Medicale) Applicant before: University PARIS SUD Applicant before: INSTITUT NATIONAL DE LA RECHERCHE AGRONOMIQUE |
|
| TA01 | Transfer of patent application right | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant | ||
| CP01 | Change in the name or title of a patent holder |
Address after: Fa Guoweileruifu Patentee after: Gustavus Institute Patentee after: INSERM (Institut National de la Sante et de la Recherche Medicale) Patentee after: University PARIS SUD Patentee after: French National Academy of agriculture, food and environment Address before: Fa Guoweileruifu Patentee before: Gustavus Institute Patentee before: INSERM (Institut National de la Sante et de la Recherche Medicale) Patentee before: University PARIS SUD Patentee before: INSTITUT NATIONAL DE LA RECHERCHE AGRONOMIQUE |
|
| CP01 | Change in the name or title of a patent holder |